Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - HERON THERAPEUTICS, INC. /DE/ | d601588dex992.htm |
| EX-99.1 - EX-99.1 - HERON THERAPEUTICS, INC. /DE/ | d601588dex991.htm |
| 8-K/A - FORM 8-K/A - HERON THERAPEUTICS, INC. /DE/ | d601588d8ka.htm |
 HTX-011 Postoperative Pain Program Topline Results from Phase 2b Studies June 21, 2018 Exhibit 99.3 |
 2 Forward-Looking Statements This presentation contains "forward-looking statements" as defined by the Private Securities Litigation Reform Act
of 1995. We caution investors that forward-looking statements are based on
management’s expectations and assumptions as of the date of this
presentation and involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those
expressed or implied by the forward-looking statements. These risks and
uncertainties include, but are not limited to, those associated with: the
potential market opportunity for HTX-011; the timing of the NDA filing for HTX-011; the timing of completion and results of clinical studies for HTX-011; and other risks and uncertainties identified in
the Company's filings with the Securities and Exchange Commission. Forward-looking
statements reflect our analysis only on their stated date, and we take no
obligation to update or revise these statements except as may be required
by law. |
      3 Preclinical Clinical NDA Approved SUSTOL ® (granisetron) extended- release injection CINVANTI ® (aprepitant) injectable emulsion HTX-011 bupivacaine
+ meloxicam
ER Local Administration HTX-011 bupivacaine
+ meloxicam
ER Nerve Block Approved by U.S. FDA for CINV Prevention Status of Product Portfolio Approved by U.S. FDA for CINV Prevention Postoperative Pain with Local Administration • Fast Track and Breakthrough Therapy designations granted • Positive Phase 2, 2b and 3 results CINV Pain Positive Phase 2b results in breast augmentation Postoperative Pain with Nerve Block |
 4 HTX-011 for Postoperative Pain Management Has Received Breakthrough Therapy Designation • Breakthrough Therapy designation designed to expedite development and review of drugs: – Intended to treat serious conditions; and – For which preliminary clinical evidence indicates substantial improvement over
available therapies on clinically significant endpoint(s)
• Designation granted based on results of Phase 2 studies and two recently completed Phase 3 studies – HTX-011 produced significant reductions in both pain intensity and need for
opioids through 72 hours post-surgery compared to placebo and bupivacaine
solution, the standard of care
• HTX-011 was also granted Fast Track designation in November 2017 |
 5 Postoperative Opioids Are a Gateway to Addiction AS MANY AS 2.6 MILLION patients that take opioids to manage pain after surgery may become persistent opioid users each year . MORE THAN 40 MILLION patients undergoing surgical procedures are prescribed opioids for pain management in the United States each year UP TO 440,000 patients will become addicted to opioids each year In addition >1 BILLION OPIOID PILLS are taken home from the hospital after surgery each year 70% of all these
opioid pills go unused 90% of these pills remain inside
the home in unsecured locations
>$15 BILLION of the annual healthcare costs associated with addiction can be attributed to postoperative pain management 32% of all opioid addicts report
first opioid exposure
through leftover pills |
 6 HTX-011 ACHIEVED STATISTICALLY SIGNIFICANT REDUCTIONS IN PAIN AND THE NEED FOR OPIOIDS VS. BUPIVACAINE IN EVERY PHASE 2 STUDY AND BOTH PHASE 3 STUDIES |
 7 Seven Positive Controlled Studies to Be Included in HTX-011 New Drug Application (NDA) Study Phase Surgical Model Tissue Type Significant for Pain Reduction vs. PBO Significant for Pain Reduction vs. BPV Significant Reduction in Opioid Use PK – PD Relation- ship 202 2 Hernia Repair Soft 203 2 Abdominoplasty Soft 208 2 Bunionectomy Bony 209 2b TKA Bony 211 2b Breast Augmentation Soft 301 3 Bunionectomy Bony 302 3 Hernia Repair Soft NDA, planned in 2H 2018, will request broad label for reduction of postoperative pain
and opioid analgesics for 72 hours after surgery
PBO = placebo; BPV = bupivacaine solution; PK = pharmacokinetic; PD = pharmacodynamics;
TKA = total knee arthroplasty |
 8 RECENTLY COMPLETED PHASE 2B STUDIES |
 9 Study 209: Phase 2b Total Knee Arthroplasty (TKA) Study Design HTX-011 400 mg Instillation N=58 HTX-011 400 mg Instillation + Ropivacaine Injection N=56 Saline Placebo Injection N=53 Bupivacaine 125 mg Injection N=55 Cohort 1 HTX-011 200 mg Instillation N=20 HTX-011 200 mg Instillation + Injection N=22 Saline Placebo Injection N=11 Bupivacaine 125 mg Injection N=10 IRC Cohort 2 Stand-alone, adequate and well- controlled study cohort |
 10 Study 209: Subject Demographics (ITT) Saline Placebo (N=53) Bupivacaine Solution 125 mg (N=55) HTX-011 400 mg (N=114) Total (N=222) Age (years) – mean (SD) 61.5 (8.3) 61.4 (9.4) 62.8 (9.0) 62.1 (8.9) Sex – n (%) Female 25 (47.2%) 35 (63.6%) 53 (46.5%) 113 (50.9%) Male 28 (52.8%) 20 (36.4%) 61 (53.5%) 109 (49.1%) Race – n (%) Asian 0 1 (1.8%) 1 (0.9%) 2 (0.9%) American Indian or Alaska Native 0 0 2 (1.8%) 2 (0.9%) Black or African Descent 8 (15.1%) 7 (12.7%) 11 (9.6%) 26 (11.7%) White 45 (84.9%) 47 (85.5%) 100 (87.7%) 192 (86.5%) Ethnicity – n (%) Hispanic or Latino 12 (22.6%) 16 (29.1%) 25 (21.9%) 53 (23.9%) Not Hispanic or Latino 41 (77.4%) 39 (70.9%) 89 (78.1%) 169 (76.1%) |
 11 Study 209: Phase 2b TKA Results Hierarchy AUC 0-48 HTX-011 400 mg vs. Placebo AUC 0-72 HTX-011 400 mg + Ropivacaine vs. Placebo AUC 0-48 HTX-011 400 mg + Ropivacaine vs. Placebo p < 0.0001 AUC 0-72 HTX-011 400 mg vs. Placebo p = 0.0002 p < 0.0001 p = 0.0004 HTX-011 via instillation achieved primary and key secondary endpoints for pain |
 12 Study 209: Pain Reduction from HTX-011 at Rest Approximately Double that of Bupivacaine Windowed-worst observation carried-forward (wWOCF) for use of opioid rescue medication and last-observation carried-forward
(LOCF) for missing pain data HTX-011 achieved primary endpoint for
AUC 0-48 |
 13 Study 209: Both HTX-011 Arms Significantly Reduce Pain at Rest Compared to Placebo through 72 Hours wWOCF for use of opioid rescue medication and LOCF for missing pain data |
 14 Study 209: Significant Separation between HTX-011 Arms and Placebo through 72 Hours wWOCF for use of opioid rescue medication and LOCF for missing pain data |
 15 Study 209: Both HTX-011 Arms Reduce Pain with Activity Significantly Better than Placebo and Bupivacaine through 48 Hours wWOCF for use of opioid rescue medication and LOCF for missing pain data |
 16 Study 209: HTX-011 plus Ropivacaine Significantly Reduces Opioid Use vs. Placebo through 72 Hours Opioid consumption is presented in mean milligrams of morphine equivalents |
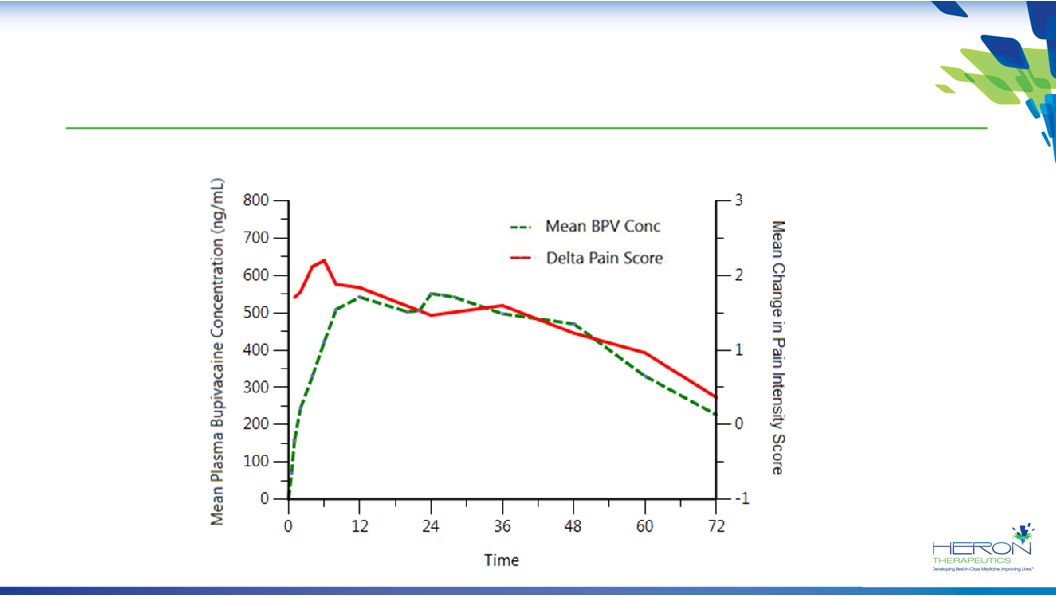 17 Study 209: Strong Correlation between Pain Reduction and Pharmacokinetics of HTX-011 in TKA HTX-011 400 mg Via Instillation |
 18 Study 209: HTX-011 Well Tolerated in TKA HTX-011 was well tolerated, with a safety profile comparable to placebo and bupivacaine solution: • No clinically meaningful differences in overall adverse events • No difference in the incidence of serious adverse events • No difference in premature discontinuations due to adverse events • No deaths • No clinically meaningful differences in potential local anesthetic systemic toxicity (LAST) adverse events in this highly vascular model • No increase in potential LAST when given with another local anesthetic, ropivacaine • No difference in wound healing |
 19 Study 209: TKA Summary • HTX-011 achieved primary and key secondary endpoints • Both HTX-011 arms achieved significant reductions in pain at rest vs. placebo
through 48 hours – Also significantly reduced pain at rest through 72 hours vs. placebo • Both HTX-011 arms achieved significant reductions in pain with activity (the
most conservative assessment) vs. placebo and bupivacaine through 48
hours –
HTX-011 plus ropivacaine
maintained superiority to both placebo and bupivacaine
through 72
hours • There was significant separation of the HTX-011 mean pain curves vs. placebo through 72 hours • Strong correlation between PK and PD in TKA • HTX-011 with or without ropivacaine was generally well tolerated
in TKA |
 20 HTX-011 60 mg Nerve Block N=12 Saline Placebo Nerve Block N=6 Study 211: Phase 2b Breast Augmentation Study Design Cohort 1 Cohort 2 Interim Review Committee (IRC) Cohort 2 dose decision HTX-011 120 mg Nerve Block N=27 Saline Placebo Nerve Block N=12 Bupivacaine 50 mg Nerve Block N=11 HTX-011 240 mg Nerve Block N=25 Saline Placebo Nerve Block N=11 IRC Cohort 3 dose decision IRC Cohort 4 dose decision Bupivacaine 50 mg Nerve Block N=12 HTX-011 400 mg Nerve Block N=47 HTX-011 400 mg Instillation N=50 Saline Placebo Nerve Block N=12 Bupivacaine 50 mg Nerve Block N=12 Cohort 4 Cohort 3 Protocol includes additional optional cohorts to evaluate other doses and administration techniques
Bupivacaine 50 mg Nerve Block N=6 |
 21 Study 211: Subject Demographics (ITT) Saline Placebo (N=41) Bupivacaine HCL 50 mg (N=41) HTX-011 (N=161) Total (N=243) Age (years) – mean (SD) 31.3 (9.0) 30.4 (7.8) 31.4 (7.8) 31.2 (8.0) Sex – n (%) Female 41 (100%) 41 (100%) 161 (100%) 243 (100%) Race – n (%) Asian 2 (4.9%) 2 (4.9%) 7 (4.3%) 11 (4.5%) Black or African Descent 7 (17.1%) 3 (7.3%) 26 (16.1%) 36 (14.8%) White 32 (78.0%) 35 (85.4%) 127 (78.9%) 194 (79.8%) Multiple 0 1 (2.4%) 1 (0.6%) 2 (0.8%) Ethnicity – n (%) Hispanic or Latino 16 (39.0%) 17 (41.5%) 61 (37.9%) 94 (38.7%) Not Hispanic or Latino 25 (61.0%) 24 (58.5%) 100 (62.1%) 149 (61.3%) |
 22 Study 211: Pain Reduction from HTX-011 at Rest Approximately Triple that of Bupivacaine HTX-011 achieved primary endpoint for AUC 0-24 |
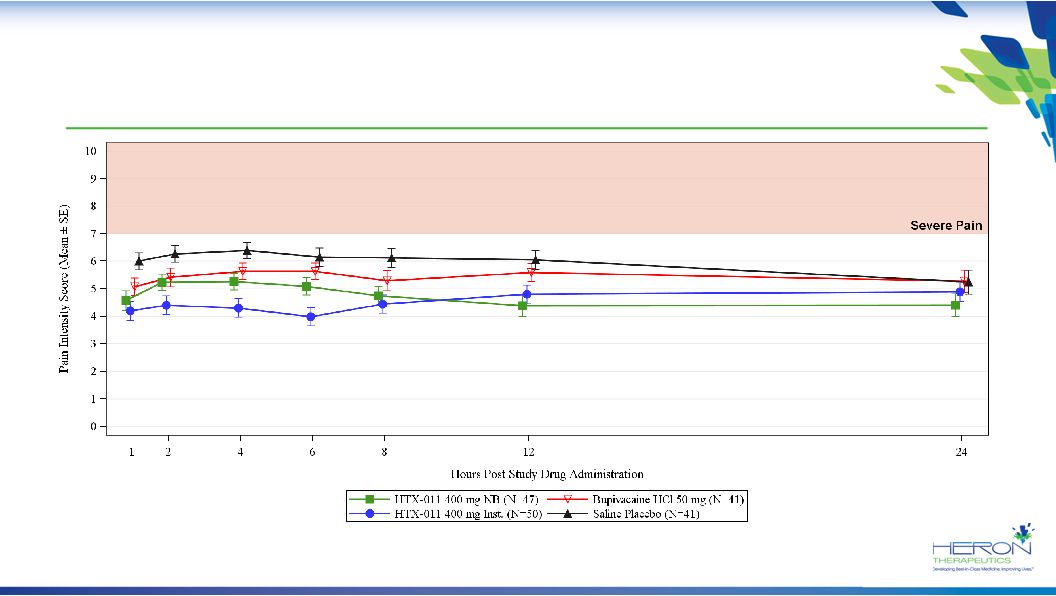 23 Study 211: HTX-011 Instillation Shows Superior Reduction in Pain Intensity Early and HTX-011 Nerve Block Shows Durable Response Notes: Pain intensity collected at rest wWOCF, windowed-worst observation carried-forward for use of opioid rescue medication and LOCF for missing pain data
|
 24 Study 211: Raw Pain Scores in All Arms Drop Quickly; Difficult to Discriminate between Arms after 24 Hours Notes: Raw pain intensity collected at rest Scores not adjusted for opioid use |
 25 Study 211: Both HTX-011 Arms Reduce Pain with Activity Significantly Better than Placebo through 24 Hours |
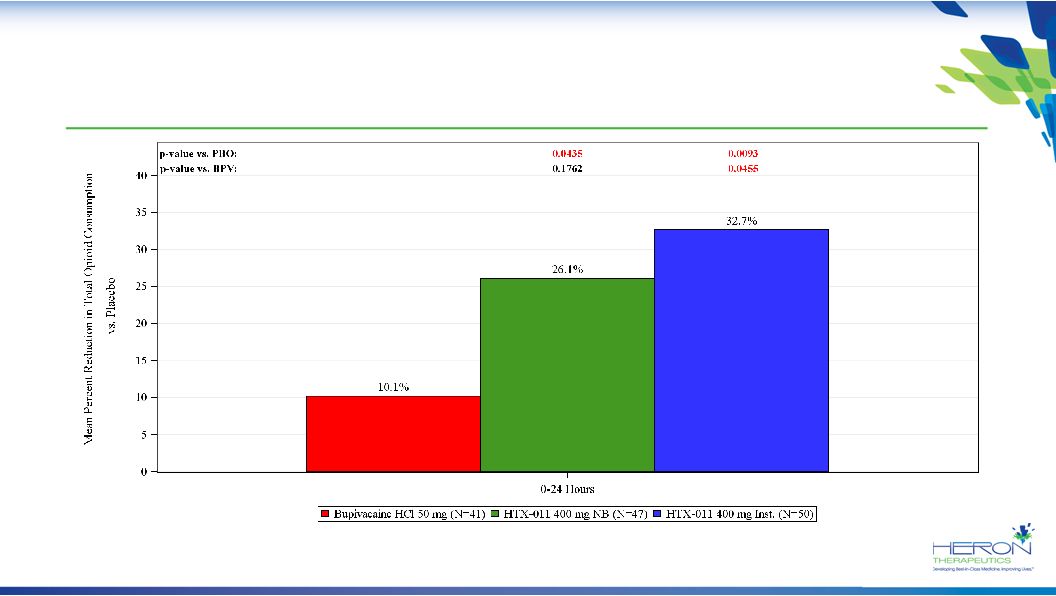 26 Study 211: Both HTX-011 Arms Significantly Reduce Opioid Use vs. Placebo through 24 Hours Opioid consumption is presented in mean milligrams of morphine equivalents |
 27 Study 211: Strong Correlation between Pain Reduction and Pharmacokinetics of HTX-011 Instillation in Breast Augmentation |
 28 HTX-011 Well Tolerated in Breast Augmentation HTX-011 was well tolerated, with a safety profile comparable to placebo and bupivacaine solution: • No clinically meaningful differences in overall adverse events • No difference in the incidence of serious adverse events • No premature discontinuations due to adverse events • No deaths • No clinically meaningful differences in potential LAST adverse events • No evidence of wound healing issues with local administration into the breast pocket |
 29 Study 211: Breast Augmentation Summary • HTX-011 achieved the primary endpoint • Both HTX-011 arms achieved significant reductions in pain at rest vs. placebo through 24 hours • Both HTX-011 arms achieved significant reductions in pain with activity (the most conservative assessment) vs. placebo through 24 hours • HTX-011 instillation (the most commercially relevant route for cosmetic surgery) produced the greatest reduction in pain and opioid use, beating both placebo and bupivacaine nerve block • Strong correlation between PK and PD in breast augmentation with instillation • HTX-011 administered via instillation or as a nerve block was generally well tolerated in breast augmentation with no wound healing issues |
 30 CONSISTENT PHARMACOKINETICS |
 31 HTX-011 Pharmacokinetics Across 5 Diverse Surgical Models Are More Dose-Linear than Bupivacaine Solution R² = 0.8103 0 100 200 300 400 500 600 700 800 0 100 200 300 400 Bupivacaine C max (ng/mL) Bupivacaine Dose (mg) Bupivacaine C max Bunionectomy Herniorrhaphy Abdominoplasty Total Knee Arthroplasty Augmentation Mammoplasty R² = 0.0349 0 100 200 300 400 500 600 700 25 50 75 100 125 Bupivacaine C max (ng/mL) Bupivacaine Dose (mg) Bupivacaine C max Bunionectomy Herniorrhaphy Abdominoplasty Total Knee Arthroplasty Augmentation Mammoplasty Bupivacaine Cmax With HTX-011 Bupivacaine Cmax With Bupivacaine HCl |
 32 Phase 2b Conclusions • HTX-011 plus ropivacaine was significantly superior to both placebo and bupivacaine in TKA • HTX-011 demonstrated significant activity via both instillation and nerve block in breast augmentation • Pharmacokinetics of HTX-011 remained consistent across 5 diverse surgical models with consistent correlation between PK and PD • HTX-011 has been generally well tolerated up to 400 mg by instillation and as a nerve block • Results from 7 positive Phase 2/3 studies across 5 surgical models are intended to support broad use of HTX-011 across a full range of surgical procedures |
 33 Large US Market Opportunity Target Market Opportunity Initial Targets Higher volume procedures across 4 major specialties ~6.5M Orthopedic procedures ~4.3M General Surgery procedures ~3.3 M OB/GYN procedures ~1.1M Plastic Surgery procedures Secondary Targets Higher volume procedures in non-core specialties (e.g., ENT, urology, hand, others) Tertiary Targets Lower volume procedures and procedures where local anesthetics are not widely used today ~28M Annual US Surgical Procedures Requiring Postoperative Pain Management That Were Considered Potentially Suited for HTX-011 ~15M procedures ~6M procedures ~7M procedures Target Market Opportunity Size* $4.9B $1.9B $2.3B *Based on the current WAC of Exparel |
 34 HTX-011 Has Demonstrated Significant Clinical Benefit in Several of the High-Value Procedures in Initial Target Market Completed studies Procedure Annual Volume (‘000s, US, 2015) Overall % Local Anesthetic Use HTX-011 Significantly Superior to Bupivacaine Claims Survey Ortho Surgery Knee arthroplasty 815 85% YES Hip arthroplasty 337 78% Shoulder arthroplasty 107 98% Rotator cuff repair 550 90% Spine procedures 750 100% General Surgery Hernia repair 1,096 67% YES Hemorrhoidectomy 504 86% Colon and small bowel resection 483 69% Plastic Surgery Abdominoplasty 160 73% YES Mammoplasty >300 86% YES OB/GYN C-Section 1,285 TBD |
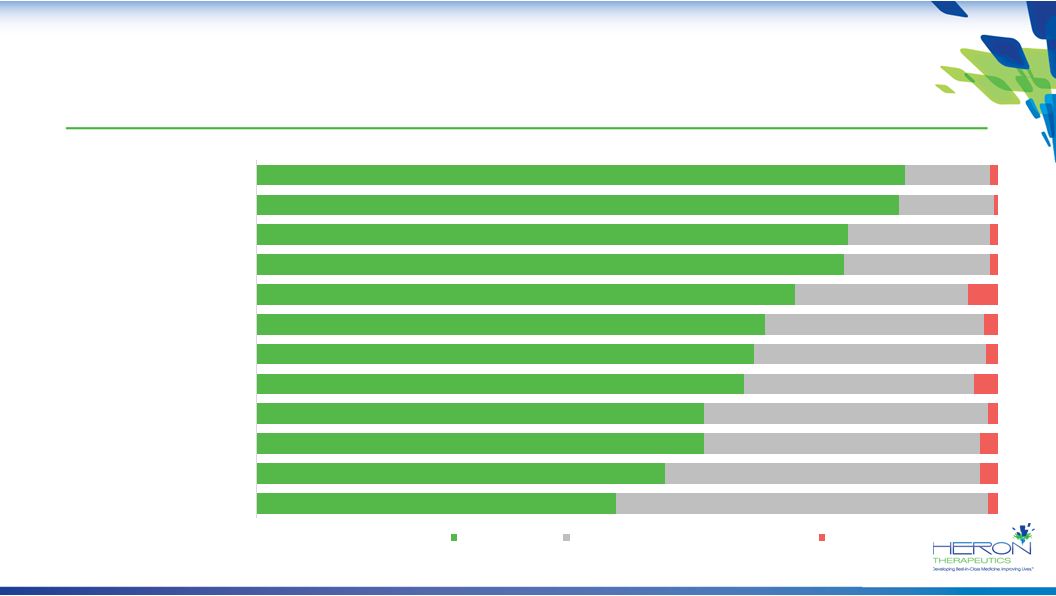 35 Positive Response by Physicians and Pharmacists to HTX-011’s Target Product Profile 48% 55% 60% 60% 66% 67% 69% 73% 79% 80% 87% 88% 50% 43% 37% 38% 31% 31% 30% 23% 20% 19% 13% 11% 1% Phase 3 Procedures Dosing Regimen Overall Safety No Impact on Implantables Compatability with NSAIDs Indication No Impact on Wound Healing Mechanism of Action Analgesia Duration Reduction in Opioid AEs* Reduction in Pain Score Reduction in Opioid Consumption Strength Neither a Strength Nor Weakness Weakness HTX-011 Target Product Profile: Strengths N = 376 total (101 anesthesiologists, 51 general surgeons, 122 orthopedic surgeons, 50 plastic surgeons, 52 pharmacy directors)
*Opioid AE’s are assumed to be reduced with significant reduction in
use |
