Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Fibrocell Science, Inc. | fcsc51818form8-k.htm |

FCX-007 Interim Data Update

Forward-Looking Statements This presentation and our accompanying remarks contain “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. All statements that are not historical facts are hereby identified as forward- looking statements for this purpose and include, among others, statements relating to: the potential advantages of our product candidates; the initiation, design and timing of pre-clinical studies and clinical trials and activities and the reporting of the results thereof; the timing of regulatory submissions and actions; expected milestones; and all other statements relating to our future operations, future financial performance, future financial condition, prospects or other future events. Forward-looking statements are based upon our current expectations and assumptions and are subject to a number of known and unknown risks, uncertainties and other factors that could cause actual results to differ materially and adversely from those expressed or implied by such statements. Factors that could cause or contribute to such differences include, among others: the impact of the announcement of the Board of Directors’ review of strategic alternatives, as well as any strategic transaction or alternative that may be pursued, on the Company's business, including its financial and operating results and its employees; that interim clinical trial results are not necessarily indicative of final clinical results and final clinical trial results may not be positive with regard to safety or efficacy of FCX-007 or FCX-013; uncertainties and delays relating to the initiation, enrollment and completion of pre-clinical studies and clinical trials, including with respect to FCX- 007, FCX-013 and our other product candidates; whether pre-clinical study and clinical trial results will validate and support the safety and efficacy of our product candidates; the risk that results seen in pre-clinical studies may not be replicated in humans; varying interpretation of pre-clinical and clinical data; unanticipated or excess costs relating to the development of our gene therapy product candidates; our ability to obtain additional capital to continue to fund operations; our ability to maintain our collaboration with Intrexon Corporation; and the other factors discussed under the caption “Item 1A. Risk Factors” in our most recent Form 10-K and Form 10-Qs which are available through the “Investors—SEC Filings” page of our website at www.fibrocell.com. As a result, you should not place undue reliance on forward-looking statements. The forward-looking statements made in connection with this presentation represent our views only as of the date of this presentation (or any earlier date indicated in such statement). While we may update certain forward-looking statements from time to time, we specifically disclaim any obligation to do so, even if new information becomes available in the future. 2

Investment Highlights • Medical breakthroughs for rare diseases of the skin and connective tissue Focus on Rare Skin • Unmet needs with no approved therapies Diseases • Significant mortality and morbidity impact on pediatric populations Proprietary Ex-Vivo Gene • Autologous fibroblasts derived from skin are the vehicle to deliver target proteins Modified Fibroblast locally to the site of disease Platform • Extensive experience culturing dermal fibroblasts, including commercial scale • FCX-007 for the treatment of Recessive Dystrophic Epidermolysis Bullosa (RDEB) . Obtained FDA allowance to initiate enrollment of pediatric patients in Phase 2 . Two Clinical Trial Programs Well tolerated safety and positive early trends noted in pharmacology and wound healing • FCX-013 for the treatment of moderate to severe Localized Scleroderma . IND allowed by FDA, expect enrollment to begin 3Q2018 • Regulatory advantages including more frequent communications with FDA, eligibility Multiple FDA Designations for Accelerated Approval and Priority Review, and Rolling Review • Two Rare Pediatric Disease Designations to potentially receive Priority Review Vouchers (PRVs) upon market authorization • In-house 13,000 square foot cGMP cell therapy manufacturing facility supporting both Internal Manufacturing FCX-007 and FCX-013 Infrastructure • Existing capacity to serve the U.S. RDEB market 3
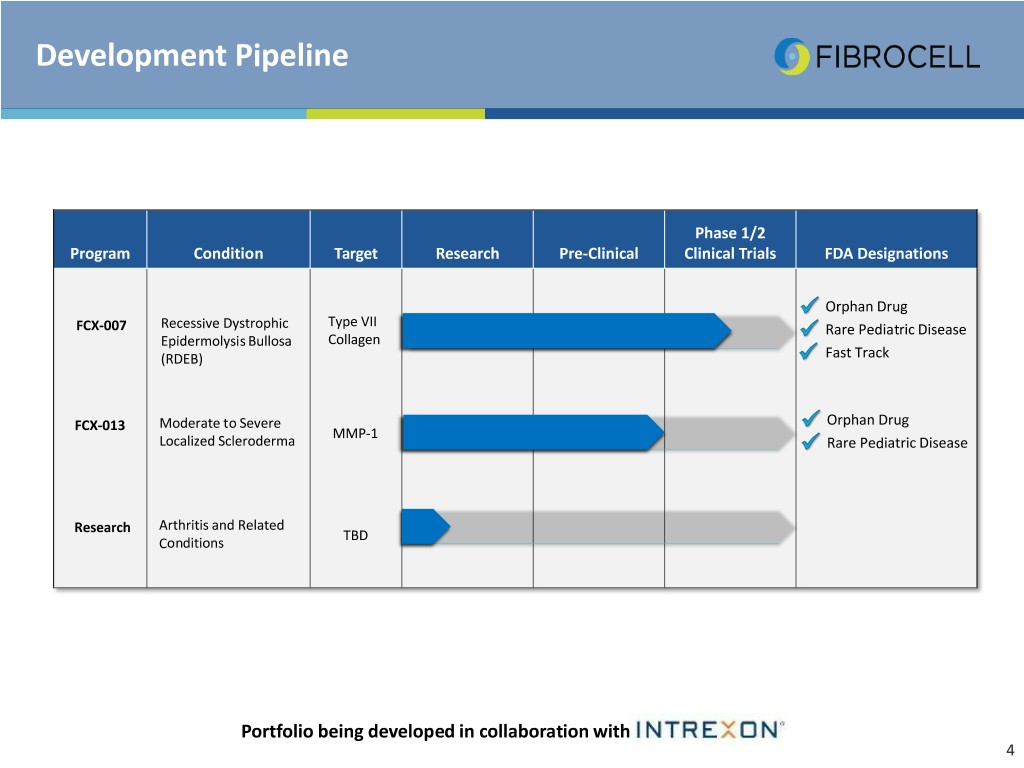
Development Pipeline Phase 1/2 Program Condition Target Research Pre-Clinical Clinical Trials FDA Designations Orphan Drug Type VII FCX-007 Recessive Dystrophic Rare Pediatric Disease Epidermolysis Bullosa Collagen (RDEB) Fast Track FCX-013 Moderate to Severe Orphan Drug MMP-1 Localized Scleroderma Rare Pediatric Disease Research Arthritis and Related TBD Conditions Portfolio being developed in collaboration with 4
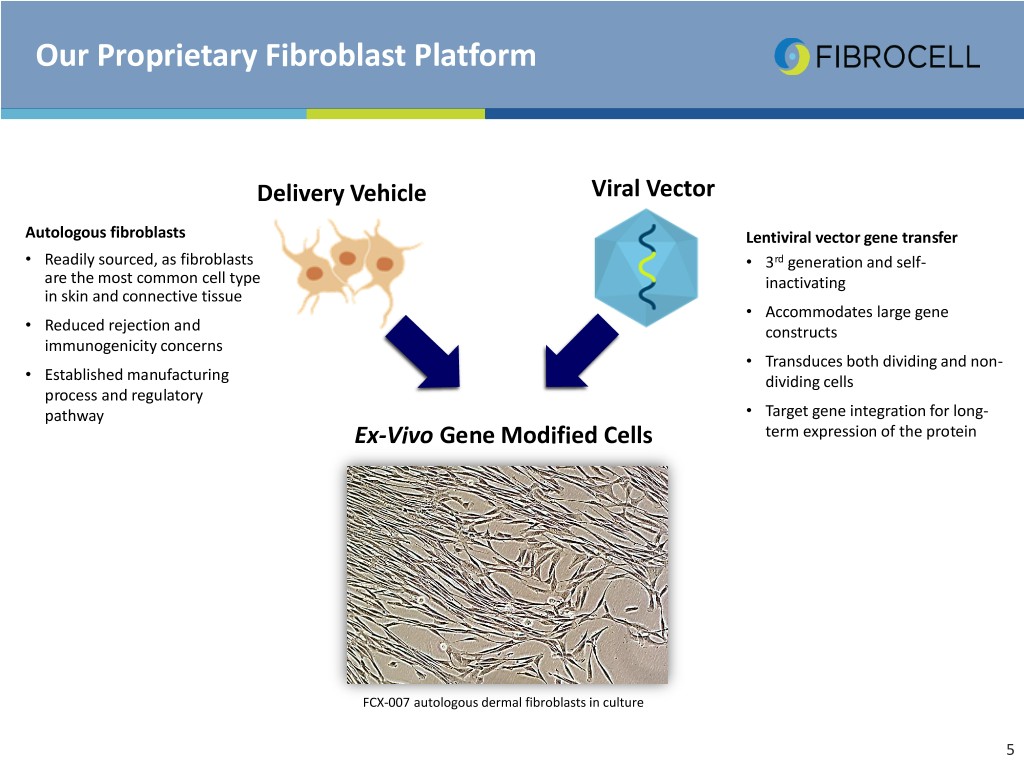
Our Proprietary Fibroblast Platform Viral Vector Delivery Vehicle Vector Autologous fibroblasts Lentiviral vector gene transfer • Readily sourced, as fibroblasts • 3rd generation and self- are the most common cell type inactivating in skin and connective tissue • Accommodates large gene • Reduced rejection and constructs immunogenicity concerns • Transduces both dividing and non- • Established manufacturing dividing cells process and regulatory Ex-vivo Gene pathway • Target gene integration for long- Ex-VivoModifiedGene Modified Cells Cells term expression of the protein FCX-007 autologous dermal fibroblasts in culture 5
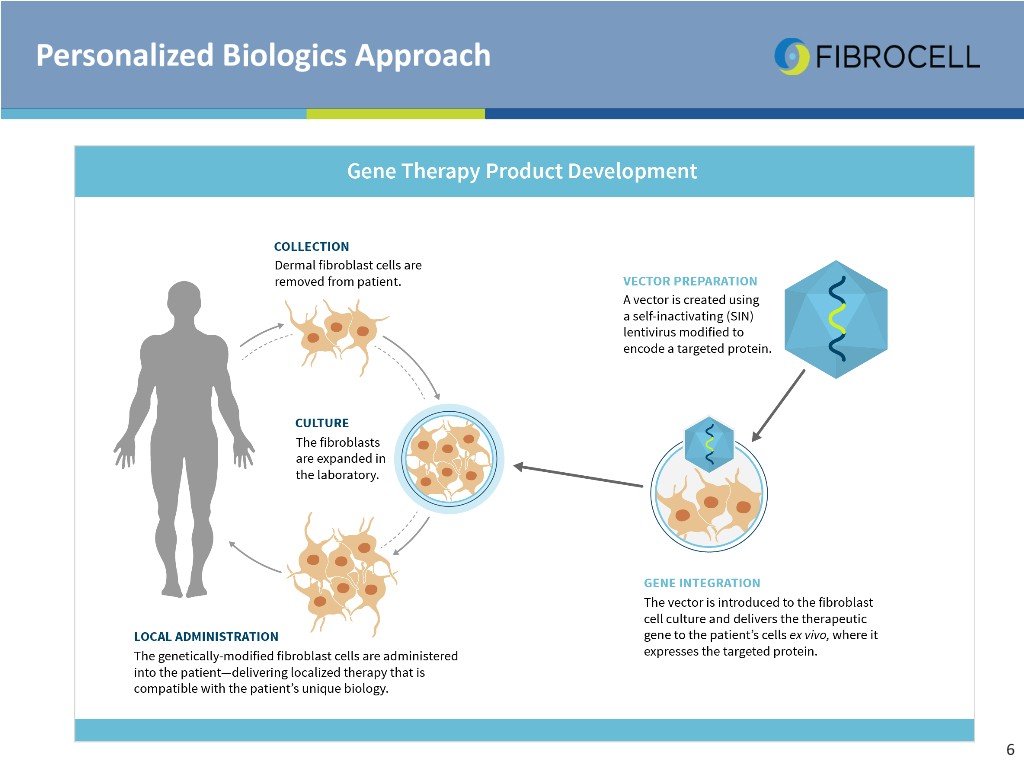
Personalized Biologics Approach 6

FCX-007 – Recessive Dystrophic Epidermolysis Bullosa FCX-007 is a dermal fibroblast transduced with a lentiviral (LV) vector encoded with the gene for type VII collagen (COL7) Orphan Drug Designation Rare Pediatric Disease Designation Fast Track Designation 7
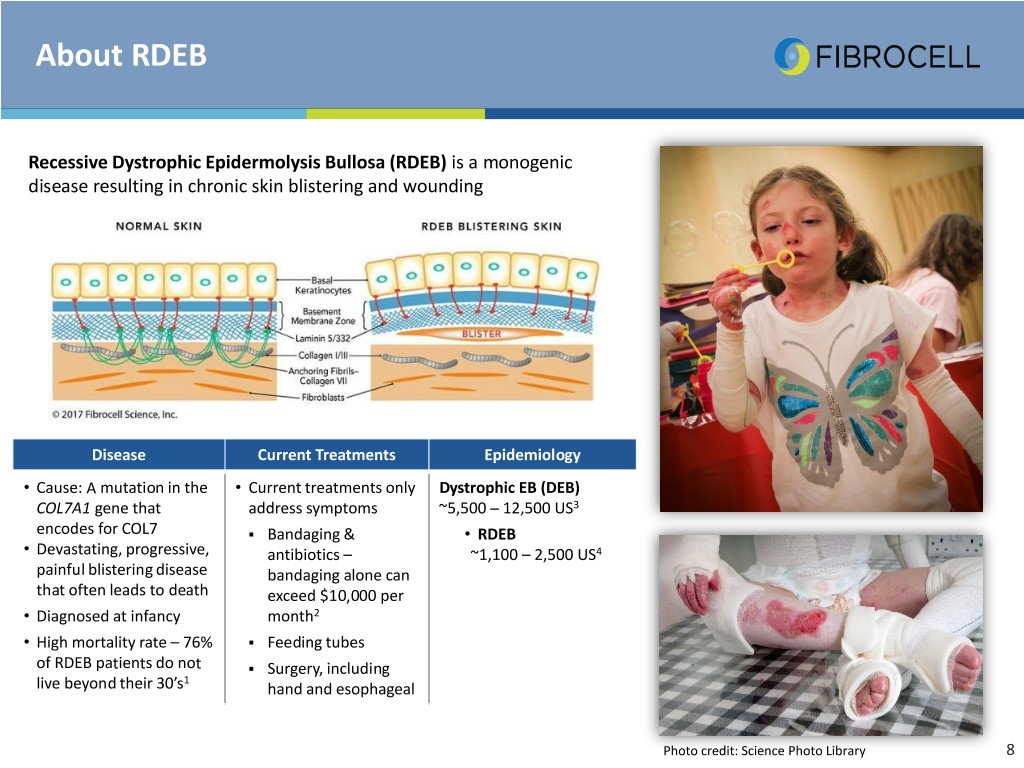
About RDEB Recessive Dystrophic Epidermolysis Bullosa (RDEB) is a monogenic disease resulting in chronic skin blistering and wounding Disease Current Treatments Epidemiology • Cause: A mutation in the • Current treatments only Dystrophic EB (DEB) COL7A1 gene that address symptoms ~5,500 – 12,500 US3 encodes for COL7 . Bandaging & • RDEB • Devastating, progressive, antibiotics – ~1,100 – 2,500 US4 painful blistering disease bandaging alone can that often leads to death exceed $10,000 per • Diagnosed at infancy month2 • High mortality rate – 76% . Feeding tubes of RDEB patients do not . Surgery, including 1 live beyond their 30’s hand and esophageal Photo credit: Science Photo Library 8
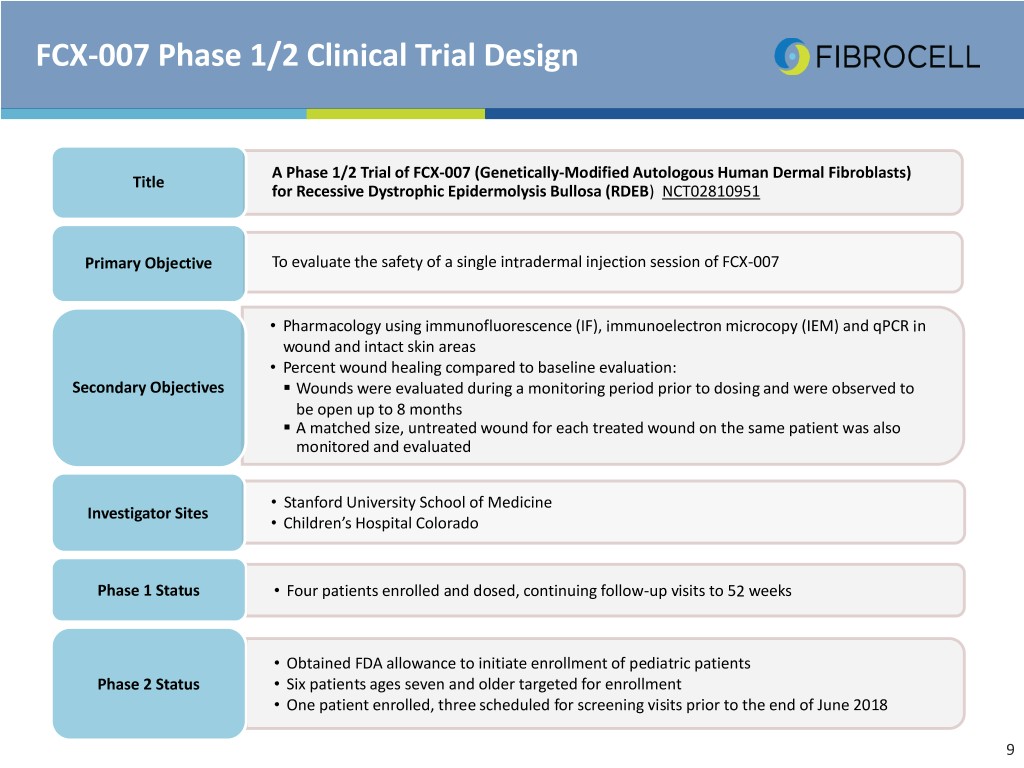
FCX-007 Phase 1/2 Clinical Trial Design A Phase 1/2 Trial of FCX-007 (Genetically-Modified Autologous Human Dermal Fibroblasts) Title for Recessive Dystrophic Epidermolysis Bullosa (RDEB) NCT02810951 Primary Objective To evaluate the safety of a single intradermal injection session of FCX-007 • Pharmacology using immunofluorescence (IF), immunoelectron microcopy (IEM) and qPCR in wound and intact skin areas • Percent wound healing compared to baseline evaluation: Secondary Objectives . Wounds were evaluated during a monitoring period prior to dosing and were observed to be open up to 8 months . A matched size, untreated wound for each treated wound on the same patient was also monitored and evaluated • Stanford University School of Medicine Investigator Sites • Children’s Hospital Colorado Phase 1 Status • Four patients enrolled and dosed, continuing follow-up visits to 52 weeks • Obtained FDA allowance to initiate enrollment of pediatric patients Phase 2 Status • Six patients ages seven and older targeted for enrollment • One patient enrolled, three scheduled for screening visits prior to the end of June 2018 9

Interim Update • Selected to present data at the International Investigative Dermatology (IID) meeting in Orlando on May 19, 2018 Dosing Parameters Number of adult patients dosed 4 Patient age range 20 - 37 Number of wounds treated 7 Wound size range (cm2) 4.4 - 23.2 Cell dose concentration range (cells/mL) 1.4 x 107 - 2.5 x 107 • Administered a single intradermal injection session at baseline to assess safety prior to increasing dose regimen . One patient received a second injection session in the remaining unhealed areas of the wound 25 weeks post-administration after the baseline dose • Performed on an outpatient basis using conscious sedation rather than in a surgical suite under general anesthesia • FCX-007 continues to be well tolerated up to 52 weeks post-administration . No serious adverse events or product related adverse events . No replication competent lentivirus (RCL) detected . No autoantibody response detected, including one null genotype patient (NC-1-) 10

Pharmacology: Immunofluorescence (IF) Patient A Patient B 332 - 1 1 Laminin - COL7 NC COL7 Merged with Merged stain nuclear 25 weeks 52 weeks 4 weeks 12 weeks 25 weeks 52 weeks* *following second injection at 25 weeks • COL7 expression continues to be detected in wounded and intact samples out to Week 52 • Increased expression of COL7 over time • Laminin-332, a basement membrane zone (BMZ) marker, displays linear expression in the BMZ and is a binding partner of COL7 • Linear COL7 formation observed along the BMZ (white arrows) 11

Pharmacology: Immunoelectron Microscopy (IEM) All images 25 weeks Post-Administration Samples labeled with LH24 mAb, specific to the NC-2 domain of the COL7 molecule COL7 Structure • COL7 expression continues to be detected in wounded and intact samples out to Week 52 • Anchoring fibrils detected (AF with arrow) • Black spots represent detectable COL7 in skin samples • Detection of the NC-2 domain indicates full length COL7 is expressed Reference: Bruckner-Tuderman, Leena. Can Type VII Collagen Injections Cure Dystrophic Epidermolysis Bullosa? Molecular Therapy (2008) 17 1, 6–7. 12

FCX-007 Interim Readout: Wound Healing Wounds Treated with FCX-007 Post-Baseline Visit Percent (Number) of Wounds > 50% Percent (Number) of Wounds > 75% Healed Compared to Baseline Healed Compared to Baseline 4 weeks 100% (7/7) 86% (6/7) 12 weeks 86% (6/7) 71% (5/7) 25/32 weeks 67% (2/3) 67% (2/3) 52 weeks 100% (1/1) 0 (0%) Untreated Control Wounds Post-Baseline Visit Percent (Number) of Wounds > 50% Healed Compared to Baseline 4 weeks 14% (1/7) 12 weeks 17% (1/6) 25/32 weeks 0% (0/2) 52 weeks 0% (0/1) 13

Phase 1 Observations • Product is well-tolerated up to 52 weeks post-administration, with no evidence of circulating autoantibodies to COL7 • Pharmacology: . Variable pharmacology signals continue to be detected throughout the data set out to 52 weeks post-administration, including COL7 expression using IF and IEM, and anchoring fibrils using IEM . Increased expression of COL7 over time noted using IF . Patients dosed with higher cell concentrations resulted in more robust COL7 expression signals . LV-COL7 vector was detected in skin 25 weeks post-administration, indicating FCX-007 cells are still present in the skin • Wound healing: . Positive healing trends maintained at 4 and 12 weeks post-administration . The majority of untreated control wounds are unhealed out to 12 weeks post-administration . A higher healing percentage is noted 25 weeks and beyond post-administration of FCX-007 compared to untreated control, but a larger data set is required to comment on trends . A patient’s wound receiving second injection at 25 weeks exhibited continued healing effect at 32 weeks . Based on observations, the addition of a second dose at 12 weeks post-administration may contribute to effect durability . Wound selection and classification is important for evaluation success 14

Moving Forward with Phase 2 • Based on safety, pharmacology and wound healing data, we plan to continue exploring dose range and administration to ensure robust information in support of future clinical study, including: . Addition of another injection session in the dose series . Increase in cells administered per injection session . Higher cell concentration targets • Three additional patients are scheduled for screening visits prior to the end of June 2018 . On track to achieve milestone of completing enrollment in 3Q2018 • 2018-2019 FCX-007 anticipated milestones: January 2018 2Q2018 3Q2018 1Q2019 FDA allowance of Interim Phase 1 data Complete enrollment Interim data readout pediatric patient readout and trial of Phase 2 patients and trial update enrollment in Phase 2 update Dose first Phase 2 patient 15

Manufacturing Experience and Solutions • Fibrocell’s existing cGMP cell therapy manufacturing facility in Exton, PA has been designated as the production site for FCX-007 and FCX-013 . ~13,000 square foot facility includes cleanroom manufacturing, cryogenic storage, QC laboratories and shipping/receiving areas . Multiple site inspections by FDA . Site for remaining clinical and future commercial manufacture of FCX-007 and FCX-013 . Existing capacity to serve the U.S. RDEB market commercially • Working towards a more efficient manufacturing process . Targeting the large scale culture portion of the process with new technology . Potentially reduces time in culture by ~50% . Aids scalability and site capacity due to smaller culture footprint without sacrificing yield . The new technology is already in use as part of our FCX-013 IND • Vector supply on hand for remaining clinical analysis and launch of FCX-007 16

References 1 Fine, J. et. al. (ed.). Epidermolysis Bullosa: Clinical, Epidemiologic, and Laboratory Advances and the Findings of the National Epidermolysis Bullosa Registry. The John Hopkins University Press, Baltimore, MD, 1999. 2 The Dystrophic Epidermolysis Research Association of America (DebRA). EB brochure, page 6: http://www.debra.org/DebraBrochure; accessed 11/10/17. 3 DEBRA International. What is EB Infographic: http://www.debra-international.org/what-is-eb.html; accessed 11/10/17. 4 Murauer, E, Koller, U, Pellegrini, G, De Luca, M, Bauer, J. Advances in Gene/Cell Therapy in Epidermolysis Bullosa. The Keio Journal of Medicine. 2015; 64. 17
