Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Corium International, Inc. | f8-k.htm |
Exhibit 99.1
|
|
Corium Investor Update 17th Annual Needham Healthcare Conference Nasdaq: CORI March 2018 Corium |
|
|
Forward-looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our business strategy, future financial and operating performance, business plans and objectives, potential growth and market opportunities, financing plans, competitive position, industry environment, product pipeline, clinical trial timing and plans, cash and resource requirements, clinical and regulatory pathways for our development programs, the achievement of clinical and commercial milestones, the advancement of our technologies and our proprietary, co-developed and partnered products and product candidates. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors including, but not limited to, those related to our future financial performance, market acceptance of our proprietary technology platforms for transdermal drug delivery, our ability to develop and maintain partnerships, our ability to identify, develop and market new products in a timely manner, our ability to maintain, protect and enhance our brand and intellectual property, and our ability to continue to stay in compliance with applicable laws and regulations. Moreover, we operate in a very competitive and rapidly changing environment, and new risks may emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that may be described in greater detail in our filings with the Securities and Exchange Commission (“SEC”), may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. Moreover, neither we, nor any other person, assume responsibility for the accuracy and completeness of the forward-looking statements. These forward-looking statements speak only as of the date of this presentation, and we undertake no obligation to publicly update any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. 2 |
|
|
Transdermal solutions that transform care Leading Transdermal Product Innovation Vision Building a robust pipeline of novel CNS products Streamlined path for donepezil Alzheimer’s program Approach Lead Product 15+ years of development & manufacturing expertise Core Strengths 3 corium |
|
|
Proven Leadership Team Peter Staple Chief Executive Officer Robert Breuil Chief Financial Officer Joseph Sarret, MD Chief Business Officer Bobby Singh, PhD Chief Technology Officer Tim Sweemer, CPA Chief Accounting Officer Dan Arsulowicz VP Operations Grand Rapids Christina Dickerson VP Corporate Development Great Pacific Capital 4 corium alza depomed chiron cetus bioseek Johnson & Johnson aerigen codexis solazyme latham & Watkins llp ucsf Novartis vyteris ciba-geigy mcc microelectonic modules corporation kpmg amway jpmorgan great pacific capital salomon brothers corium 4 |
|
|
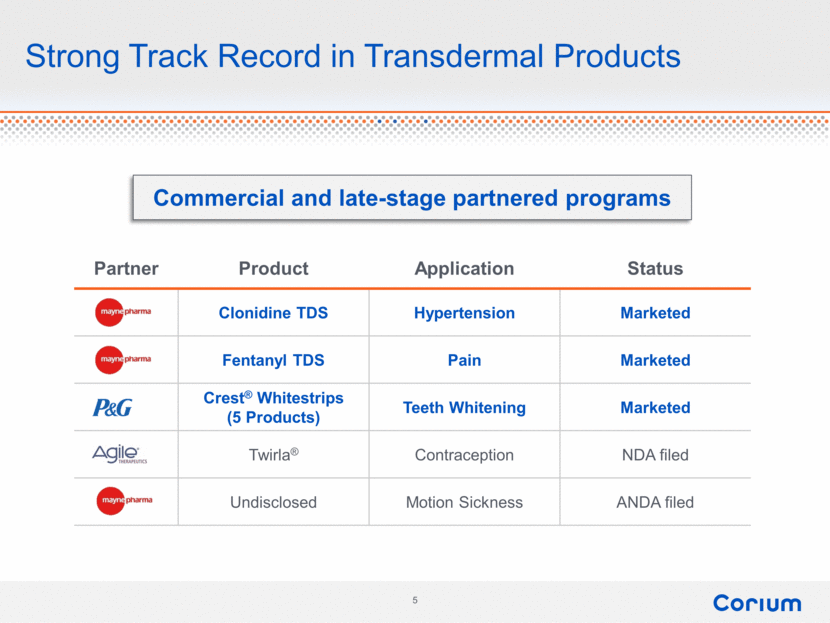
Partner Product Application Status Clonidine TDS Hypertension Marketed Fentanyl TDS Pain Marketed Crest® Whitestrips (5 Products) Teeth Whitening Marketed Twirla® Contraception NDA filed Undisclosed Motion Sickness ANDA filed Strong Track Record in Transdermal Products Commercial and late-stage partnered programs 5 maynepharma p&g agile therapeutics 5 corium |
|
|
Enabling Sustained Delivery: Corplex™ Technology Can adhere to both dry and wet surfaces Sustained drug delivery up to 7 days Load large amounts of drug/liquid Uniquely well-suited for poorly soluble drugs Enables first-in-patch products Overcomes limitations of other technologies Novel combinations of pressure-sensitive and bioadhesive polymers new crest 3D white whitestrips professional effects 6 corium |
|
|
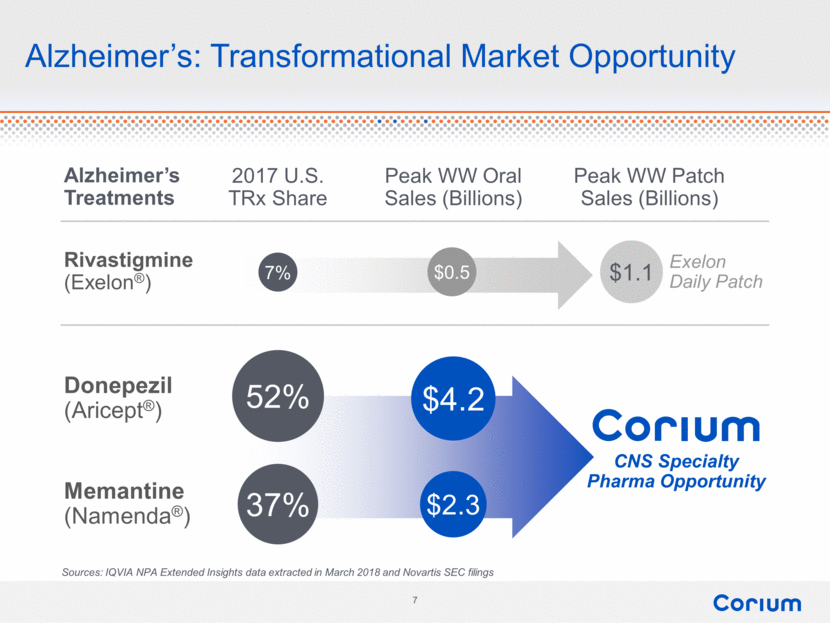
Exelon Daily Patch Alzheimer’s: Transformational Market Opportunity 7% 52% 37% $0.5 $4.2 $2.3 $1.1 Rivastigmine (Exelon®) Donepezil (Aricept®) Memantine (Namenda®) 2017 U.S. TRx Share Peak WW Oral Sales (Billions) Peak WW Patch Sales (Billions) CNS Specialty Pharma Opportunity Alzheimer’s Treatments Sources: IQVIA NPA Extended Insights data extracted in March 2018 and Novartis SEC filings corium 7 |
|
|
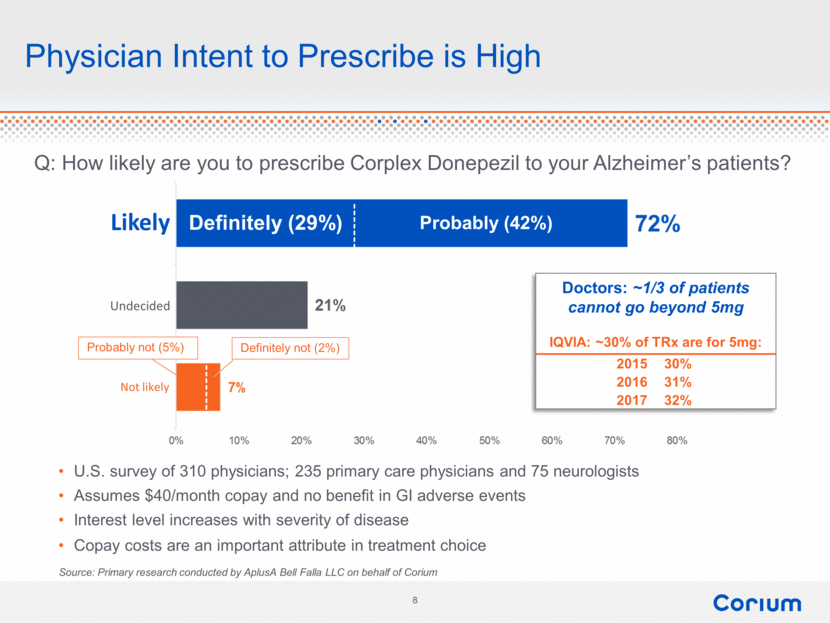
Physician Intent to Prescribe is High Q: How likely are you to prescribe Corplex Donepezil to your Alzheimer’s patients? Probably (42%) Definitely (29%) Undecided Probably not (5%) Definitely not (2%) Likely Not likely U.S. survey of 310 physicians; 235 primary care physicians and 75 neurologists Assumes $40/month copay and no benefit in GI adverse events Interest level increases with severity of disease Copay costs are an important attribute in treatment choice Source: Primary research conducted by AplusA Bell Falla LLC on behalf of Corium Doctors: ~1/3 of patients cannot go beyond 5mg IQVIA: ~30% of TRx are for 5mg: 2015 30% 2016 31% 2017 32% 8 corium 72% 21% 0% 10% 20% 30% 40% 50% 60% 70% 80% |
|
|
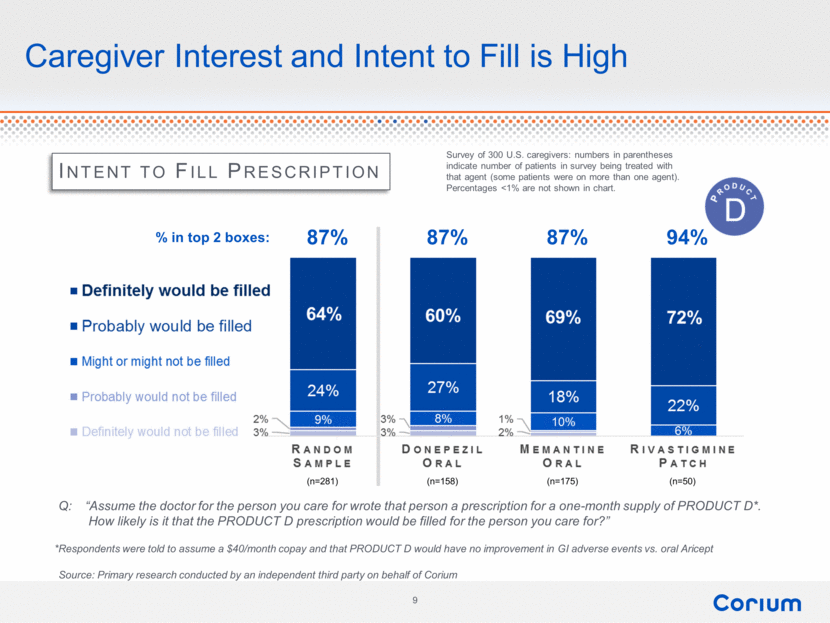
Intent to Fill Prescription Q: “Assume the doctor for the person you care for wrote that person a prescription for a one-month supply of PRODUCT D*. How likely is it that the PRODUCT D prescription would be filled for the person you care for?” 87% 87% 87% 94% (n=281) (n=158) (n=175) (n=50) Survey of 300 U.S. caregivers: numbers in parentheses indicate number of patients in survey being treated with that agent (some patients were on more than one agent). Percentages <1% are not shown in chart. Caregiver Interest and Intent to Fill is High % in top 2 boxes: *Respondents were told to assume a $40/month copay and that PRODUCT D would have no improvement in GI adverse events vs. oral Aricept Source: Primary research conducted by an independent third party on behalf of Corium 9 % in top 2 boxes definitely would be filled probably would be filled might or might not be filled probably would not be filled definitely would not be filled random sample 2% 3% 1% donepezil oral memantine oral rivastigmine patch 87% 94% product D 9 |
|
|
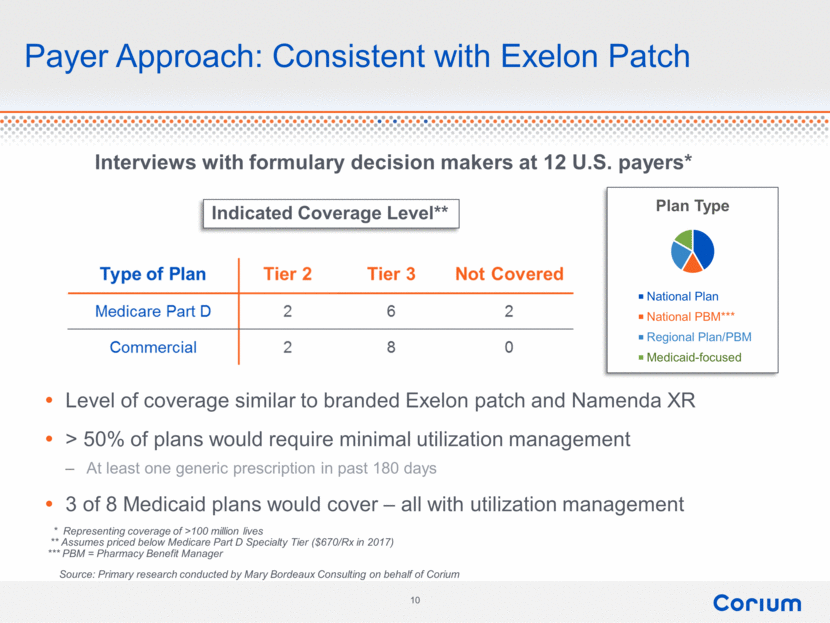
Payer Approach: Consistent with Exelon Patch Interviews with formulary decision makers at 12 U.S. payers* Level of coverage similar to branded Exelon patch and Namenda XR > 50% of plans would require minimal utilization management At least one generic prescription in past 180 days 3 of 8 Medicaid plans would cover – all with utilization management * Representing coverage of >100 million lives ** Assumes priced below Medicare Part D Specialty Tier ($670/Rx in 2017) *** PBM = Pharmacy Benefit Manager Indicated Coverage Level** Source: Primary research conducted by Mary Bordeaux Consulting on behalf of Corium Plan Type National Plan National PBM*** Regional Plan/PBM Medicaid-focused 10 corium type of plan medicare part D commercial 2 2 6 8 6 0 tier 2 tier 3 not covered |
|
|
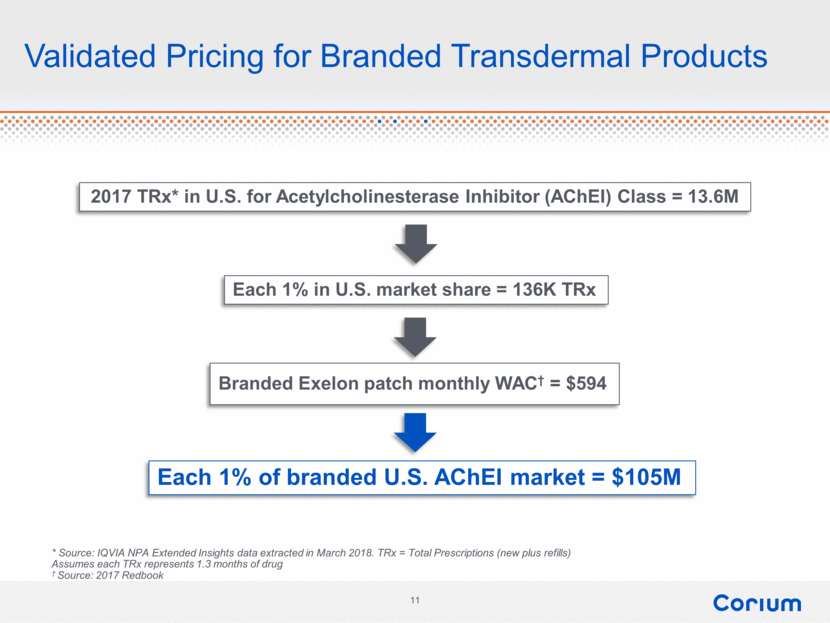
Validated Pricing for Branded Transdermal Products 2017 TRx* in U.S. for Acetylcholinesterase Inhibitor (AChEI) Class = 13.6M Each 1% in U.S. market share = 136K TRx Each 1% of branded U.S. AChEI market = $105M * Source: IQVIA NPA Extended Insights data extracted in March 2018. TRx = Total Prescriptions (new plus refills) Assumes each TRx represents 1.3 months of drug † Source: 2017 Redbook Branded Exelon patch monthly WAC† = $594 11 corium |
|
|
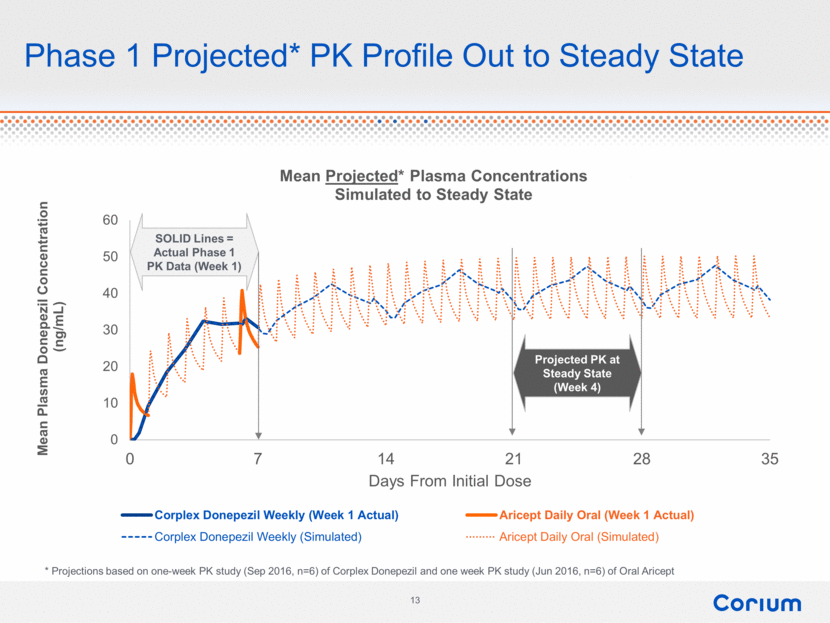
Phase 1 Projected* PK Profile Out to Steady State Mean Plasma Donepezil Concentration (ng/mL) SOLID Lines = Actual Phase 1 PK Data (Week 1) Projected PK at Steady State (Week 4) 0 10 20 30 40 50 60 0 7 14 21 28 35 Corplex Donepezil Weekly (week 1 actual) Corplex Donepezil Weekly (Simulated) Aricept Days From Initial Dose * Projections based on one-week PK study (Sep 2016, n=6) of Corplex Donepezil and one week PK study (Jun 2016, n=6) of Oral Aricept 13 corium |
|
|
Phase 1 Projected* PK Profile Out to Steady State SOLID Lines = Actual Phase 1 PK Data (Week 1) Projected PK at Steady State (Week 4) * Projections based on one-week PK study (Sep 2016, n=6) of Corplex Donepezil and one week PK study (Jun 2016, n=6) of Oral Aricept |
|
|
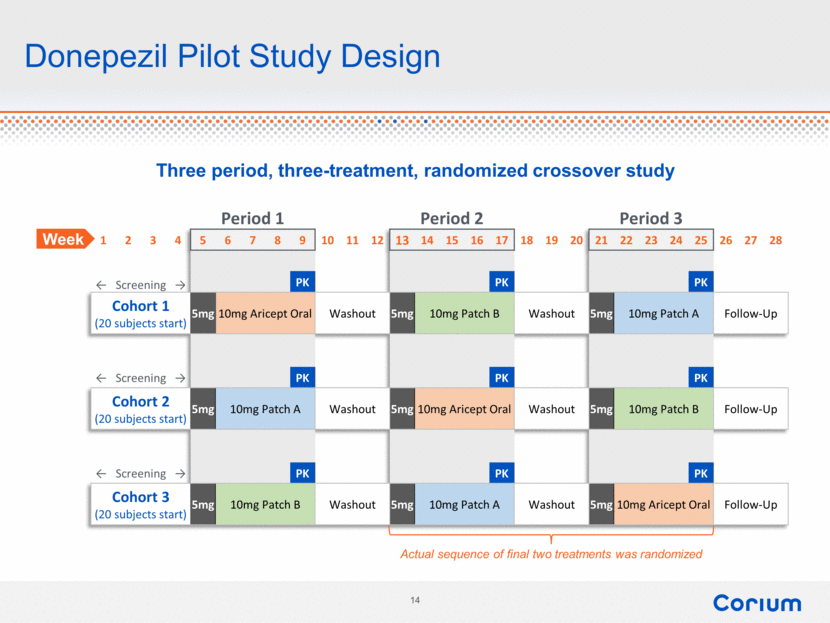
Donepezil Pilot Study Design Three period, three-treatment, randomized crossover study Period 1 Period 2 Period 3 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 ← Screening → PK PK PK Cohort 1 (20 subjects start) 5mg 10mg Aricept Oral Washout 5mg 10mg Patch B Washout 5mg 10mg Patch A Follow-Up ← Screening → PK PK PK Cohort 2 (20 subjects start) 5mg 10mg Patch A Washout 5mg 10mg Aricept Oral Washout 5mg 10mg Patch B Follow-Up ← Screening → PK PK PK Cohort 3 (20 subjects start) 5mg 10mg Patch B Washout 5mg 10mg Patch A Washout 5mg 10mg Aricept Oral Follow-Up Actual sequence of final two treatments was randomized Week 14 corium |
|
|
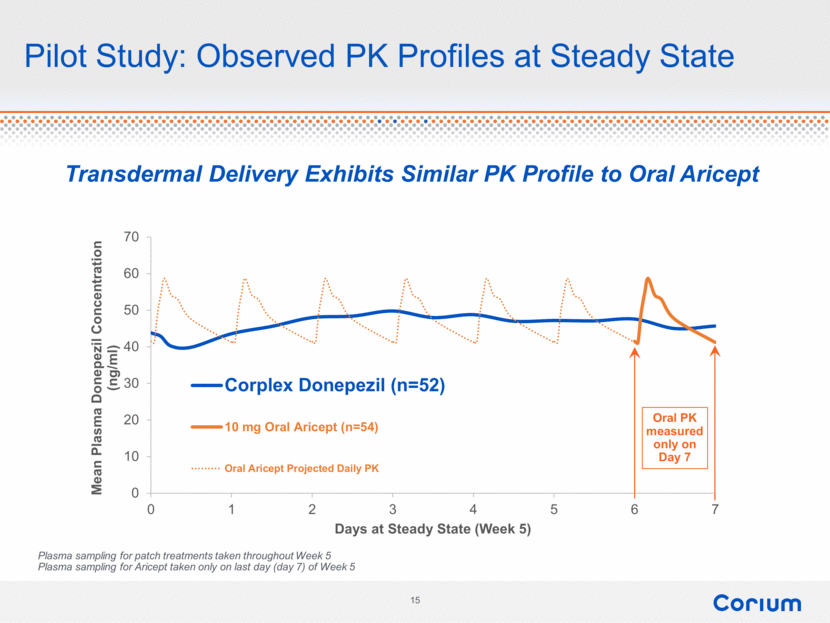
Pilot Study: Observed PK Profiles at Steady State Oral PK measured only on Day 7 Plasma sampling for patch treatments taken throughout Week 5 Plasma sampling for Aricept taken only on last day (day 7) of Week 5 Transdermal Delivery Exhibits Similar PK Profile to Oral Aricept 0 10 20 30 40 50 60 70 0 1 2 3 4 5 6 7 Mean Plasma Donepezil Concentration (ng/ml) Days at Steady State (Week 5) Corplex Donepezil (n=52) 10 mg Oral Aricept (n=54) Oral Aricept Projected Daily PK 15 corium |
|
|
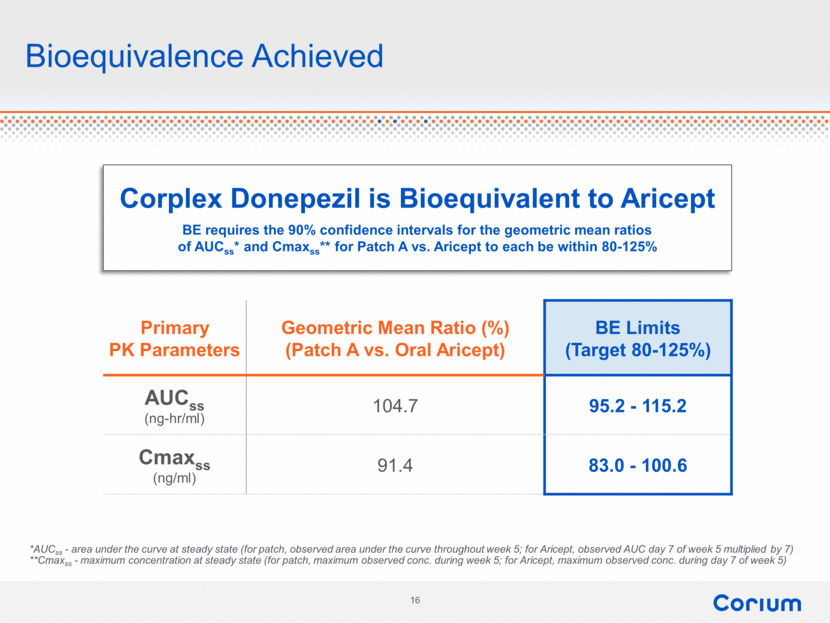
Bioequivalence Achieved Primary PK Parameters Geometric Mean Ratio (%) (Patch A vs. Oral Aricept) BE Limits (Target 80-125%) AUCss (ng-hr/ml) 104.7 95.2 - 115.2 Cmaxss (ng/ml) 91.4 83.0 - 100.6 *AUCss - area under the curve at steady state (for patch, observed area under the curve throughout week 5; for Aricept, observed AUC day 7 of week 5 multiplied by 7) **Cmaxss - maximum concentration at steady state (for patch, maximum observed conc. during week 5; for Aricept, maximum observed conc. during day 7 of week 5) Corplex Donepezil is Bioequivalent to Aricept BE requires the 90% confidence intervals for the geometric mean ratios of AUCss* and Cmaxss** for Patch A vs. Aricept to each be within 80-125% Corium 16 |
|
|
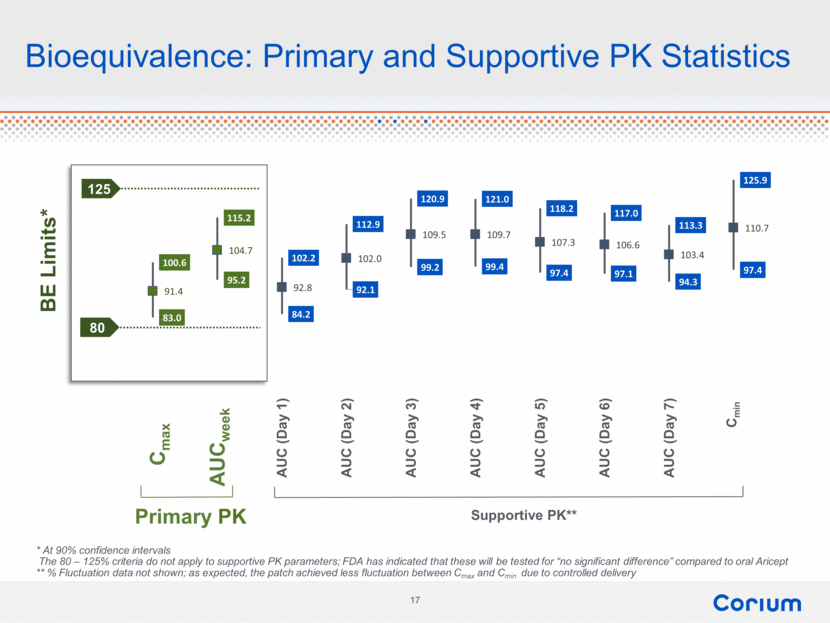
Bioequivalence: Primary and Supportive PK Statistics Primary PK Supportive PK** 125 80 Cmax AUCweek * At 90% confidence intervals The 80 – 125% criteria do not apply to supportive PK parameters; FDA has indicated that these will be tested for “no significant difference” compared to oral Aricept ** % Fluctuation data not shown; as expected, the patch achieved less fluctuation between Cmax and Cmin due to controlled delivery Cmin 100.6 115.2 102.2 112.9 120.9 121.0 118.2 117.0 113.3 125.9 83.0 95.2 84.2 92.1 99.2 99.4 97.4 97.1 94.3 97.4 91.4 104.7 92.8 102.0 109.5 109.7 107.3 106.6 103.4 110.7 Cmax AUC (week) AUC (Day 1) AUC (Day 2) AUC (Day 3) AUC (Day 4) AUC (Day 5) AUC (Day 6) AUC (Day 7) Cmin BE Limits* Corium 17 |
|
|
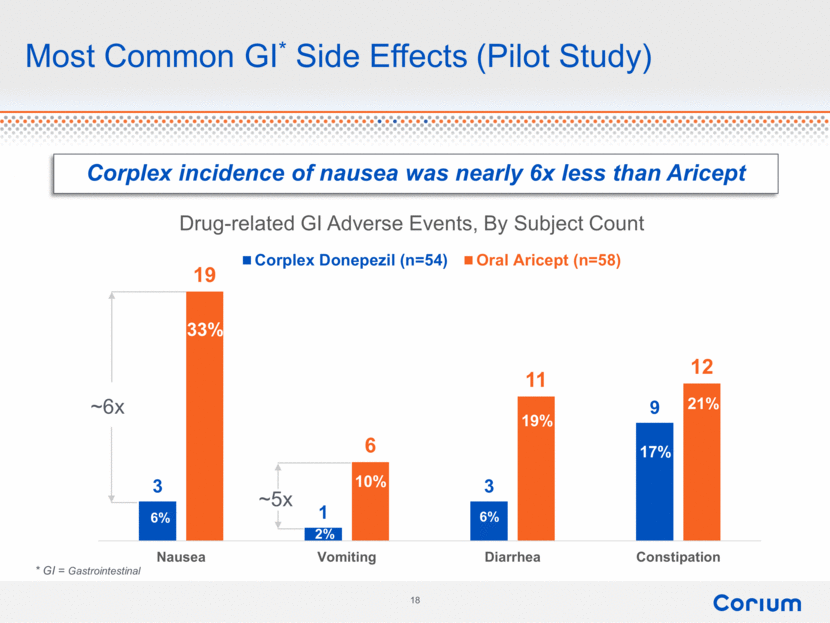
Most Common GI* Side Effects (Pilot Study) * GI = Gastrointestinal Corplex incidence of nausea was nearly 6x less than Aricept 29% 7% 4% 7% 19% 20% 9% 33% 6% 2% 6% 17% 21% 10% 19% ~6x ~5x 3 1 3 9 19 6 11 12 Nausea Vomiting Diarrhea Constipation Drug - related GI Adverse Events, By Subject Count Corplex Donepezil (n=54) Oral Aricept (n=58) Corium 18 |
|
|
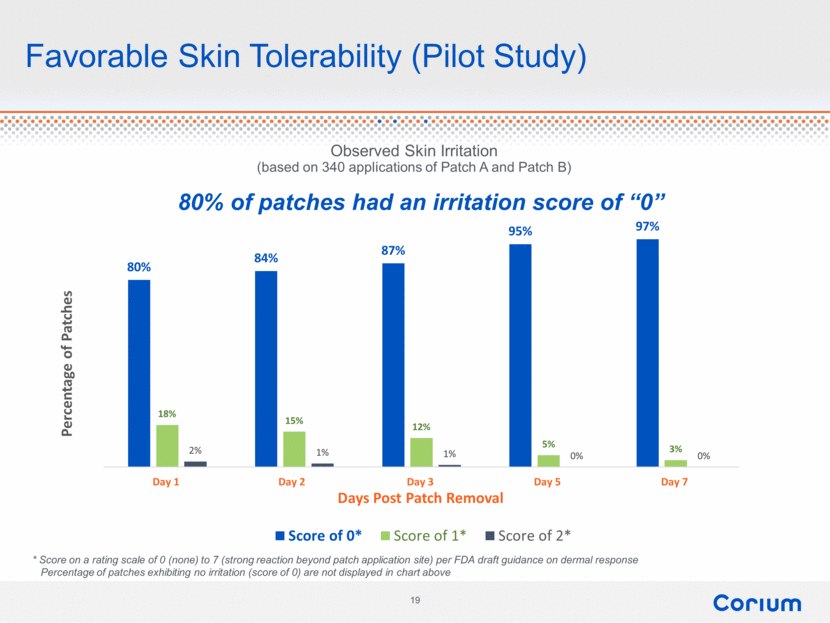
Favorable Skin Tolerability (Pilot Study) * Score on a rating scale of 0 (none) to 7 (strong reaction beyond patch application site) per FDA draft guidance on dermal response Percentage of patches exhibiting no irritation (score of 0) are not displayed in chart above Observed Skin Irritation (based on 340 applications of Patch A and Patch B) 80% of patches had an irritation score of “0” 80% 84% 87% 95% 97% 18% 15% 12% 5% 3% 2% 1% 1% 0% 0% Day 1 Day 2 Day 3 Day 5 Day 7 Percentage of Patches Days Post Patch Removal Score of 0* Score of 1* Score of 2* Corium 19 |
|
|
Recent FDA Feedback (February 2018) Based on its preliminary review, FDA advised that the results of the pilot study may, on their face, suffice as the sole basis for demonstrating bioequivalence between the Corplex Donepezil Transdermal System and Aricept. Corium 20 |
|
|
NDA Submission Strategy NDA to be submitted using pilot bioequivalence (BE) data “Pilot” PK data will serve as the sole basis for establishing BE Full Pilot PK data (>2,500 pages) submitted for FDA review/comment Agency was aware of “pivotal” study being conducted Reviewed strategy with external experts Consensus that feedback is as strong as possible prior to NDA review Potential partner feedback consistent with expert recommendations No longer planning to analyze blood samples from “pivotal” study Plasma samples are secured and stable Available for analysis at a later date, if necessary Targeting PDUFA date in Q4 CY2019 Due to stability timing, NDA submission expected in Q1 CY2019 |
|
|
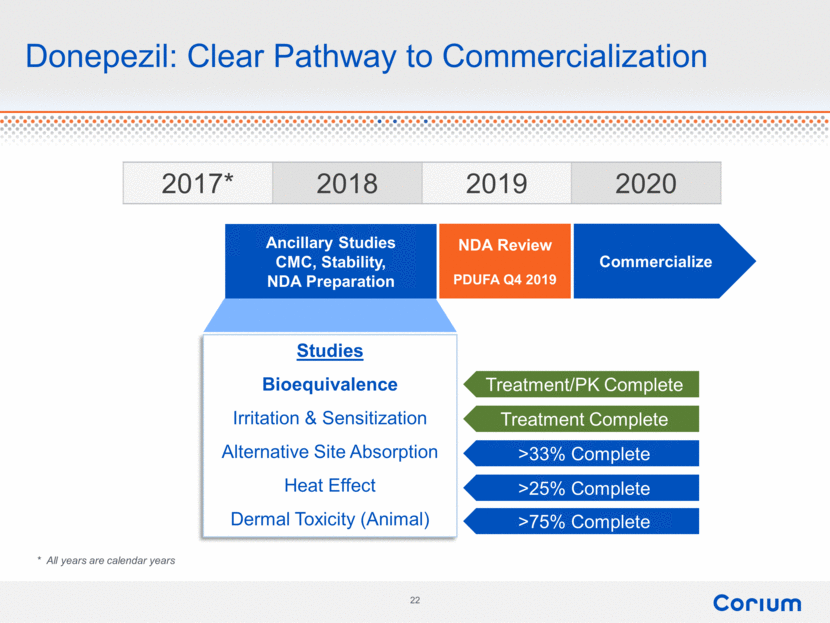
Donepezil: Clear Pathway to Commercialization 2017* 2018 2019 2020 Ancillary Studies CMC, Stability, NDA Preparation NDA Review PDUFA Q4 2019 Commercialize * All years are calendar years Studies Bioequivalence Irritation & Sensitization Alternative Site Absorption Heat Effect Dermal Toxicity (Animal) Treatment/PK Complete >33% Complete >75% Complete Treatment Complete >25% Complete Corium 22 |
|
|
FY2018 Q1 Total Revenues of $9.3M (+34% YoY) $5.9M in Product Revenues $3.2M Contract R&D Revenues Sole supplier of Crest Whitestrips for P&G 5-year extension signed 2017 $45.2M in unrestricted cash at December 31, 2017 $52.3M in long-term debt (primarily with CRG, due June 2019) 36.1M shares outstanding as of December 31, 2017 >10% holders: Essex Woodlands (26%) and Perceptive Advisors (18%) Financial Highlights at December 31, 2017 Corium 23 |
|
|
$120M gross proceeds; 7-year term; 5% semi-annual coupon $56M used to retire existing CRG 15% debt maturing in 2019 $60M in net proceeds to fund operations Strong demand drove placement of full shoe and overnight pricing Unlike similar offerings, demand was predominantly long-onlys Overnight pricing yielded a true conversion premium Closing price of $13 + 32.5% premium = $17.22 conversion price Significant operational advantages over term loans Unsecured, no restrictions/liens on IP No covenants, no one controlling stakeholder Lower interest rate, no fees Convertible Notes Offering (March 1, 2018) Corium 24 |
|
|
High-value CNS pipeline opportunity and strong IP Corplex Memantine complements Alzheimer’s portfolio New Phase 1 programs anticipated in 2H CY2018 Corium: Growth and Momentum in 2018 Building on 15+ years of development and commercial manufacturing Twirla has potential to drive significant upside in partner business An integrated transdermal products engine Our Approach: Commercialize Transformational CNS Products Lower-risk bioequivalence path tracking toward approval by late 2019 Major clinical de-risking achieved in pilot study; filing in 1Q CY2019 Corplex Donepezil – lead product in Alzheimer’s disease |
|
|
Thank You Corium Nasdaq: CORI |