Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SYNERGY PHARMACEUTICALS, INC. | a18-8734_18k.htm |
SAFE HARBOR STATEMENT This presentation and any statements made for and during any presentation or meeting contain forward-looking statements related to Synergy Pharmaceuticals Inc. under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995 and are subject to risks and uncertainties that could cause actual results to differ materially from those projected. These statements may be identified by the use of forward-looking words such as "anticipate," "planned," "believe," "forecast," "estimated," "expected," and "intend," among others. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, the development, launch, introduction and commercial potential of TRULANCE ®; growth and opportunity, including peak sales and the potential demand for TRULANCE, as well as its potential impact on applicable markets; market size; substantial competition; our ability to continue as a going concern; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payer reimbursement; dependence upon third parties; our financial performance and results, including the risk that we are unable to manage our operating expenses or cash use for operations, or are unable to commercialize our products, within the guided ranges or otherwise as expected; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. There are no guarantees that future clinical trials discussed in this presentation will be completed or successful or that any product will receive regulatory approval for any indication or prove to be commercially successful. Investors should read the risk factors set forth in our most recent periodic reports filed with the Securities and Exchange Commission, including our Form 10-K for the year ended December 31, 2017. While the list of factors presented here is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Forward-looking statements included herein are made as of the date hereof, and we do not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances except as required by law. The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation. 2 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018
KEY HIGHLIGHTS • • Developing and commercializing novel gastrointestinal (GI) therapies Launching our first product, Trulance®, in the U.S. for the treatment of adults with Chronic Idiopathic Constipation (CIC) and Irritable Bowel Syndrome with Constipation (IBS-C) OUR FOCUS • Proprietary uroguanylin based GI platform with two wholly owned assets – Trulance and dolcanatide Strong patent portfolio with long life span OUR PLATFORM • • • Proven leadership team with significant GI and launch experience Newly integrated sales force executing on a focused commercial strategy to support successful U.S. launch of Trulance OUR TEAM 3 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018
CIC AND IBS-C REPRESENT A SIGNIFICANT MEDICAL AND SOCIAL BURDEN at all satisfied with their care4 1. Suares NC, Ford AC. Am J Gastroenterology. 2011;106(9):1582-1591; quiz 1581, 1592. 2. Doshi JA et al. J Manag Care Spec Pharm. 2014;20(4):382-390. 3. Saito YA et al. Am J Gastroenterology. 2002;97(8):1910-1915. 4. Heidelbaugh JJ et al. Am J Gastroenterol. 2015;110(4):580-587. 5. Ford AC, Suares NC. Gut. 2011;60(2):209-218. 6. Ford AC et al. Am J Gastroenterol. 2014;109(suppl 1):S2-S26; quiz S27. 7. Belsey J et al. Aliment Pharmacol Ther. 2010;31(9):938-949. 8. Lee C et al. J Neurogastroenterol Motil. 2017;23(3):349-362. 9. Polster A et al. Aliment Pharmacol Ther. 2017;46(5):529-539. Oppenheimer 28th Annual Healthcare Conference I March 20, 2018 4 ApproximatelyApproximately 35 million10 million US adultsUS adults have CIC1 have IBS-C2,3 Medications for CIC and IBS-C5,6: • Not all patients respond to therapy • Some patients experience adverse events Nearly twice as common in women compared with men1,4 • CIC and IBS-C adversely affect physical and emotional aspects of life4,7-9 • Many patients are little or not
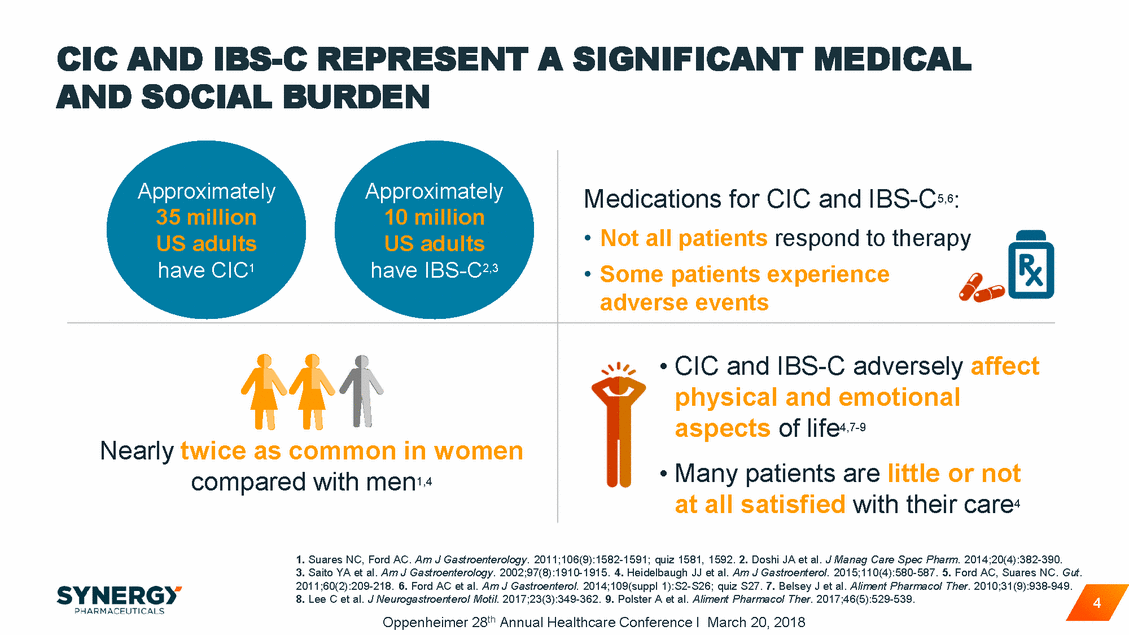
BURDEN–CIC AND BURDEN–IBS-C: 2 ROBUST STUDIES OF PATIENT AND HCP PERSPECTIVES ON LIVING WITH AND MANAGING CIC AND IBS-C 1,2 The BURDEN designed to: Better Understand and Recognize Disconnects, Studies were Study Design: • • Peer-reviewed design, IRB approved BURDEN-CIC1: >1200 patients and >325 HCPs BURDEN–IBS-C2: >1300 patients and >325 HCPs Evaluated potential gaps/needs, quality of life, and treatment satisfaction among HCPs and patients with CIC or IBS-C • • Experiences, and Needs of patients and the HCPs who treat them HCP=health care provider; IRB=institutional review board. 1. Harris LA et al. [published online November 6, 2017.] Adv Ther. doi:10.1007/s12325-017-0633-5. 2. Quigley EMM et al. Poster presented at: World Congress of Gastroenterology at ACG 2017; October 13-18, 2017; Orlando, FL. Poster P2019. Oppenheimer 28th Annual Healthcare Conference I March 20, 2018 5
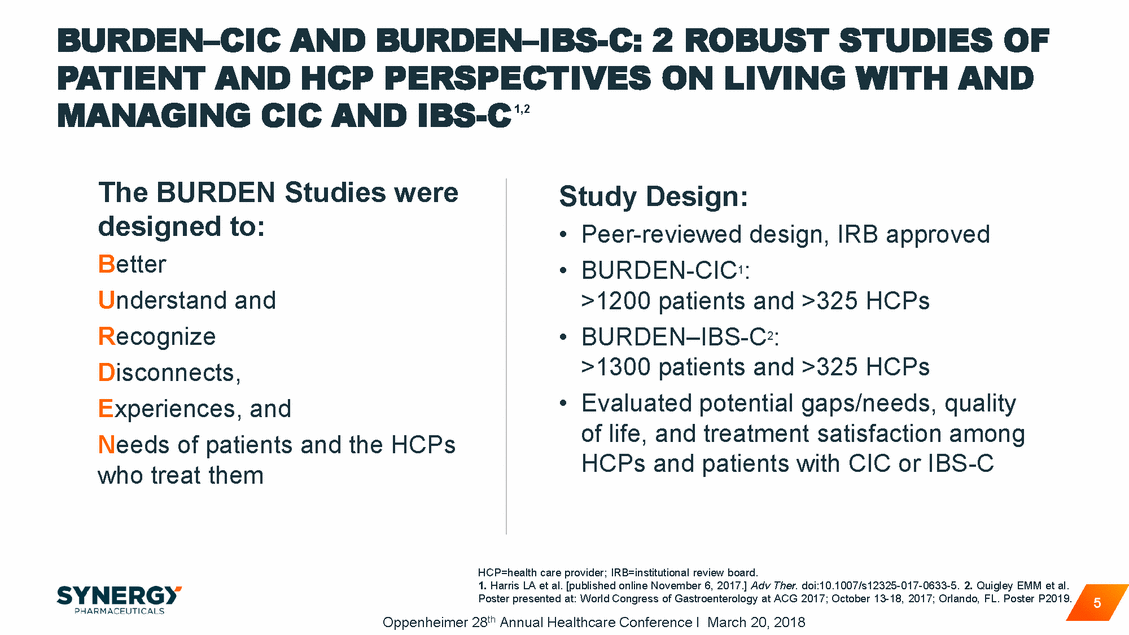
PATIENTS WERE NOT SATISFIED* WITH CURRENT OPTIONS1,2 CHRONIC PRESCRIPTION† TREATMENT Not Satisfied For patients not completely satisfied, top reasons were efficacy 59% of CIC patients and tolerability 66% of IBS-C patients †Linzess®/Amitiza® *Among chronic Rx patients stating not satisfied or not completely satisfied 1. Harris LA et al. [published online November 6, 2017.] Adv Ther. doi:10.1007/s12325-017-0633-5. 2. Quigley EMM et al. Poster presented at: World Congress of Gastroenterology at ACG 2017; October 13-18, 2017; Orlando, FL. Poster P2019. Oppenheimer 28th Annual Healthcare Conference I March 20, 2018 6
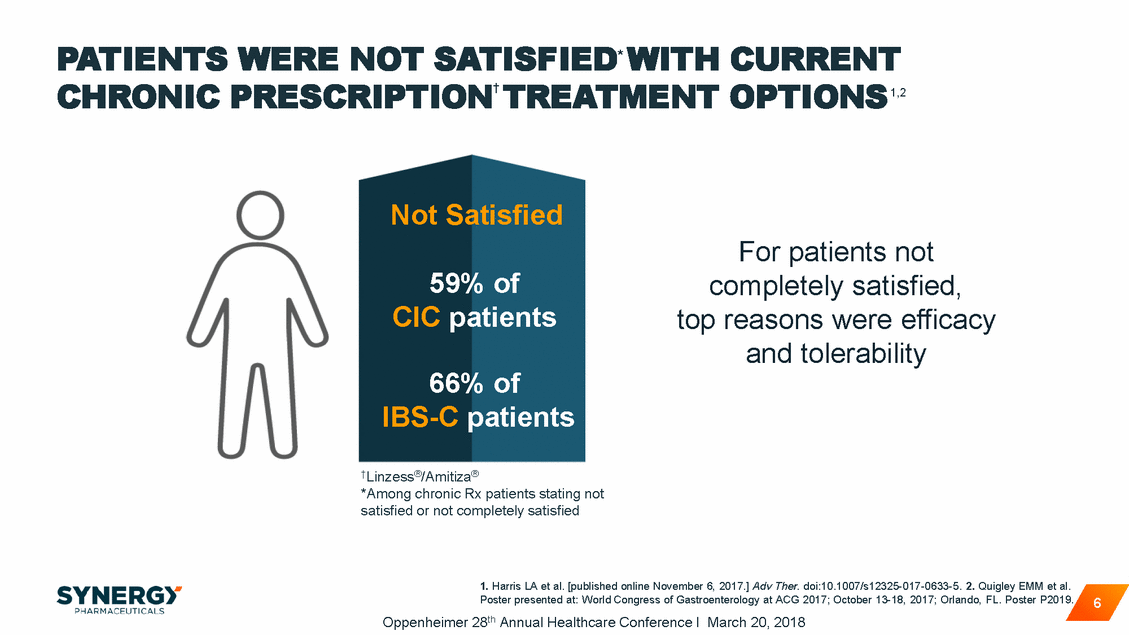
~80% OF HCPs SURVEYED WERE NOT SATISFIED* WITH ALL CURRENT RX TREATMENTS FOR EITHER CIC OR Top rated challenges for HCPs include efficacy, compliance, and treatment-related diarrhea1,2 Compliance IBS-C1,2 IBS-C1 CIC2 58 Inadequate Response 55 55 Diarrhea 41 Lack of Options 40 Side Effects Other Than Diarrhea 32 *Not satisfied or not completely satisfied 1. Data on file. Synergy Pharmaceuticals Inc. 2. Harris LA et al. [published online November 6, 2017.] Adv Ther. doi:10.1007/s12325-017-0633-5. 7 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018 25 34 34 55
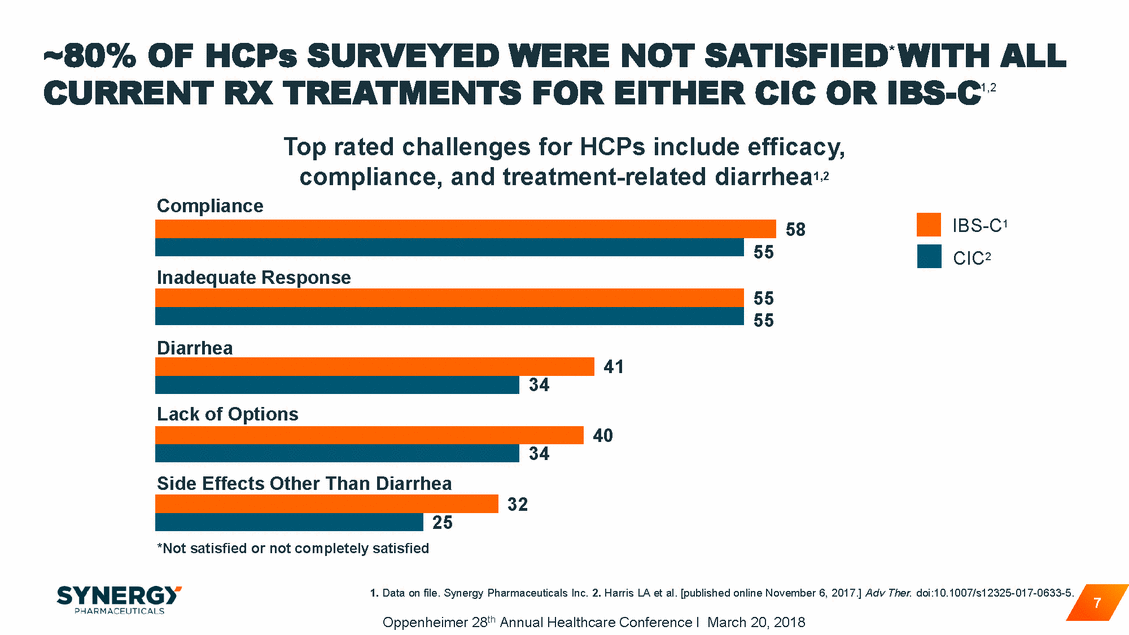
STILL AN UNTAPPED US MARKET OPPORTUNITY ~ 45MM U.S. Adults With CIC / IBS-C Growth Drivers • • • Increasing awareness of “gut health” Aging population 70% of patients dissatisfied with OTC treatment2 Growing education of health care professionals and consumers – HCP promotional efforts – Direct to consumer/patient initiatives Availability of new prescription treatment options such as Trulance > 95% currently not treated with branded Rx therapies1 Branded Rx market serving < 5% • • 1. IQVIA, NPA - December 2017; 2. Lieberman “GI Patient Landscape Survey” 2010. 8 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018
2017 MARKED BY SOLID LAUNCH EXECUTION – LAYING THE FOUNDATION FOR FUTURE SUCCESS • Drove strong customer demand with TRx volume up 70% on average month-over-month • Established strong base with top gastroenterologists key for fueling future growth • Activated the Rx-ready patient with almost half of new prescriptions coming from patients new to branded Rx treatment • Significant increases in commercial, Medicare Part D, and Medicaid coverage since launch 9 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018
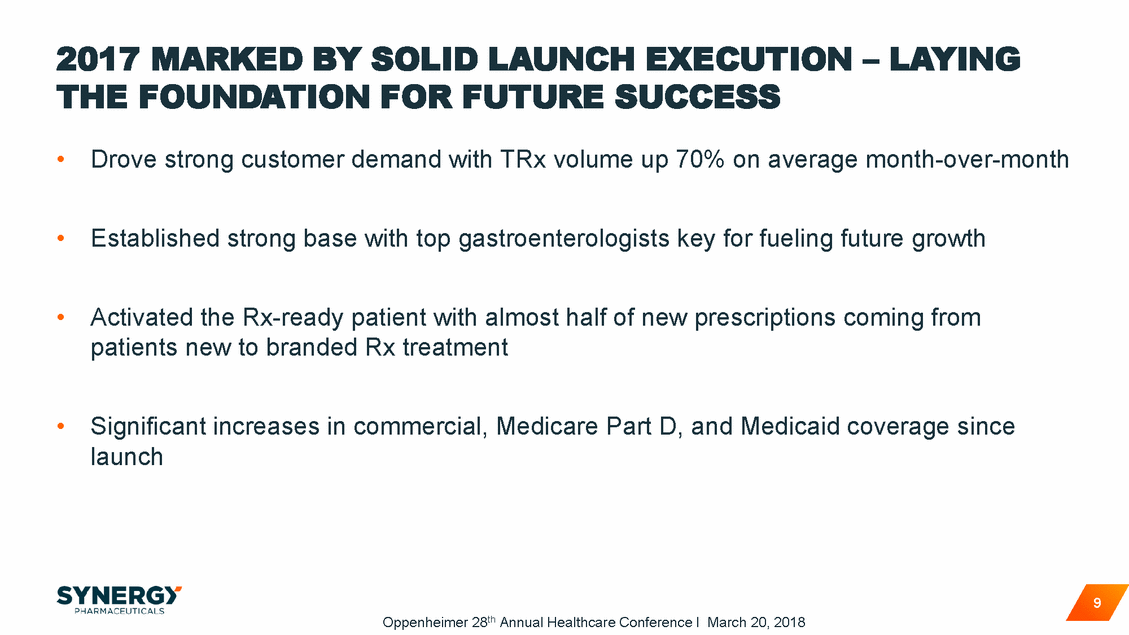
TRULANCE TOTAL PRESCRIPTION VOLUME GREW CONSISTENTLY IN 2017 Monthly TRx (IQVIA) Monthly Normalized / Extended Unit TRx 16000 15321 14000 12000 10000 8000 6000 4000 2000 0 Mar-17 Apr-17 May-17 Jun-17 Jul-17 Aug-17 Sep-17 Oct-17 Nov-17 Dec-17 Source: IQVIA, Mar. – Dec. 2017 Note: Trulance launched March 20, 2017 10 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018 Trulance 30-count packs 11813
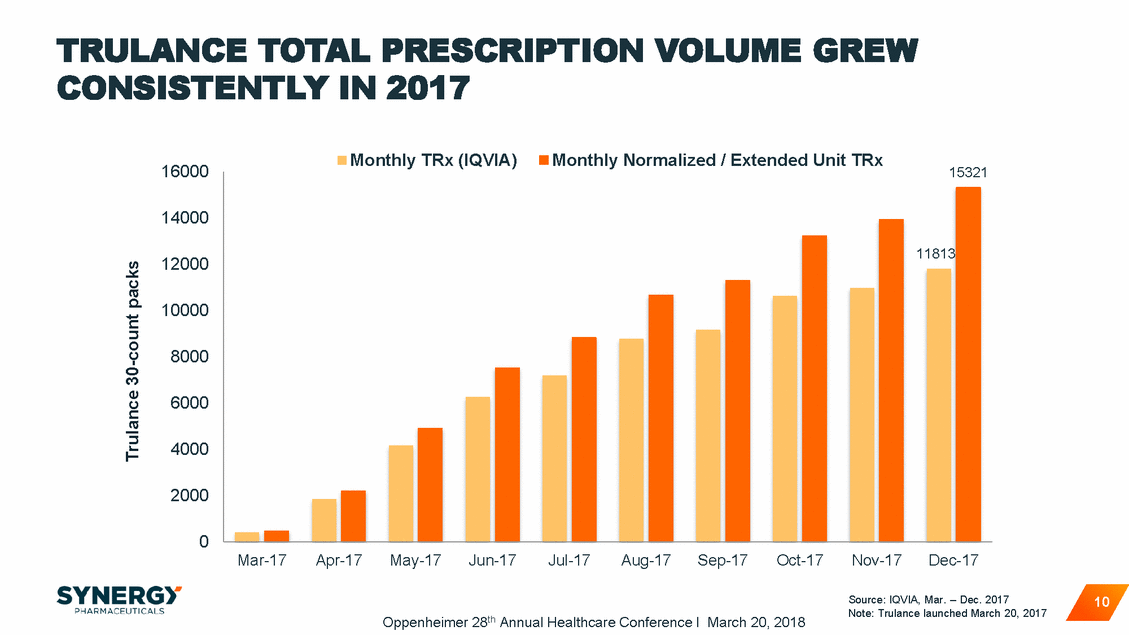
TRULANCE PRESCRIBER BASE SHOWED STEADY GROWTH 9,644 10,000 8,868 7,974 8,000 7,043 6,121 6,000 4,949 3,768 4,000 2,427 2,000 1,145 257 0 Source: IQVIA, Mar. – Dec. 2017 Note: Trulance launched March 20, 2017 11 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018 Cumulative Trulance Prescribers
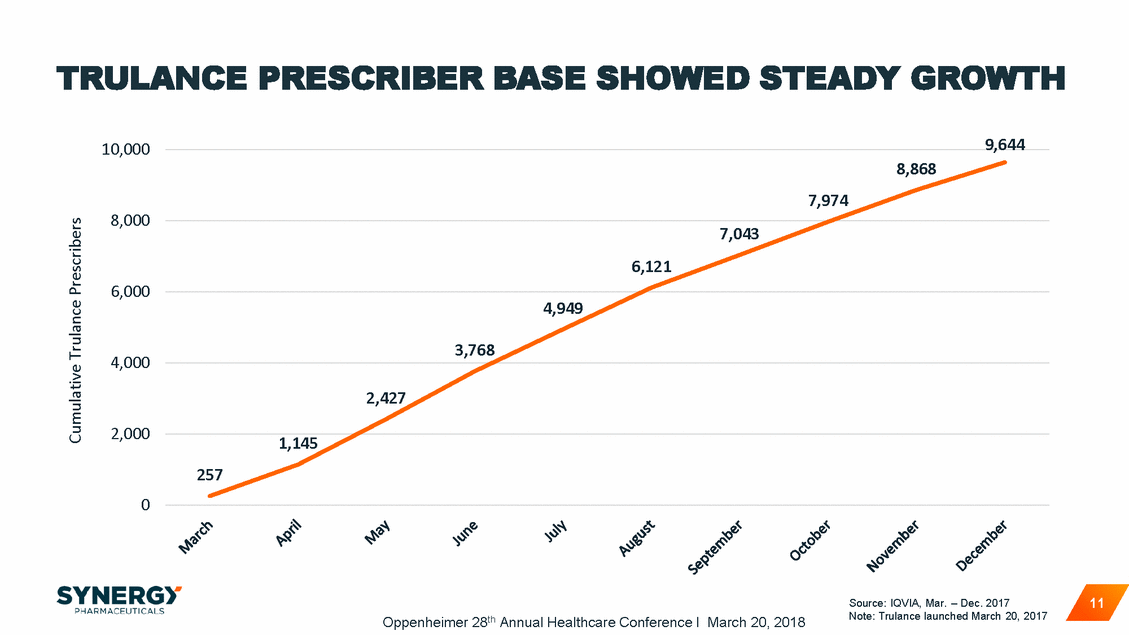
TRULANCE OUTPERFORMED LAUNCH PENETRATION OF HIGH DECILE WRITERS VS. SIMILAR LAUNCH ANALOGS Analog Penetration Trulance Penetration 80% 70% 60% 50% 40% 30% 20% 10% 0% 10 9 Market Decile 8 Source: IQVIA, Mar. – Dec. 2017 12 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018 % Penetration 75% 65% 66% 56% 53% 49%
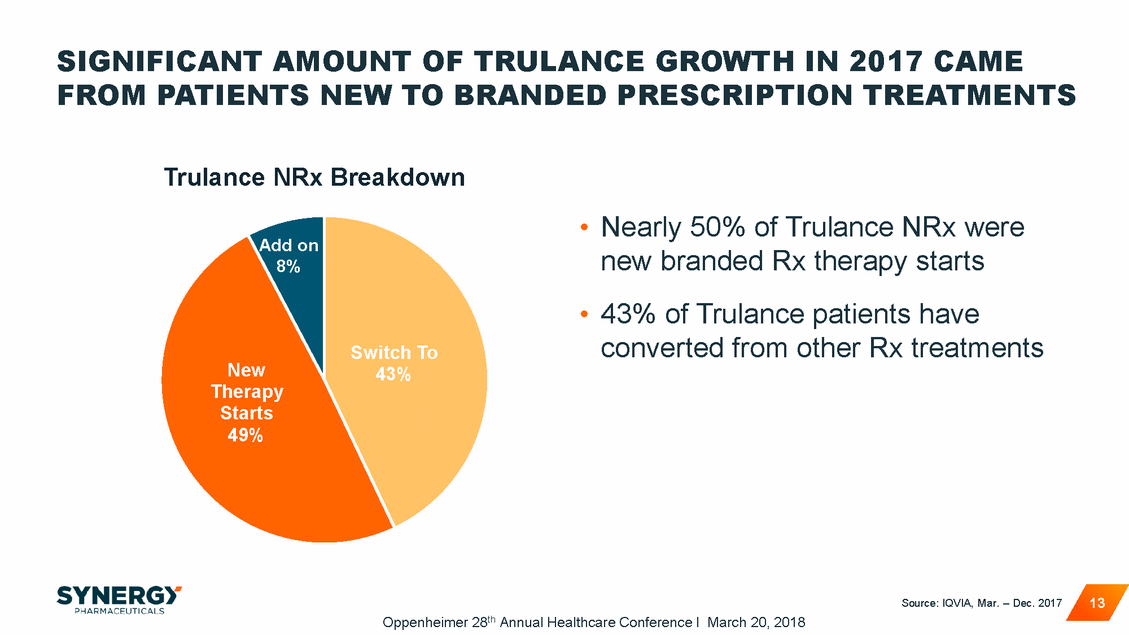
SIGNIFICANT AMOUNT OF TRULANCE GROWTH IN 2017 CAME FROM PATIENTS NEW TO BRANDED PRESCRIPTION TREATMENTS Trulance NRx Breakdown • Nearly 50% of Trulance NRx were new branded Rx therapy starts 43% of Trulance patients have Add on 8% • converted from other Rx treatments Sw h To Therapy Starts 49% Source: IQVIA, Mar. – Dec. 2017 13 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018
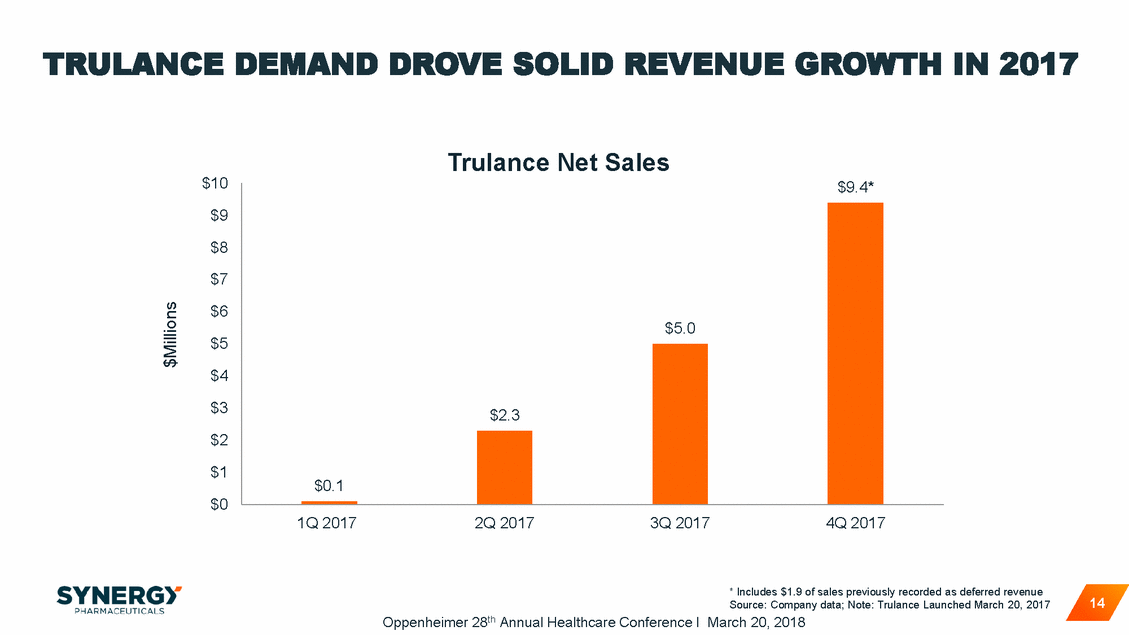
TRULANCE DEMAND DROVE SOLID REVENUE GROWTH IN 2017 Trulance Net Sales $10 $9 $8 $7 $6 $5 $4 $3 $2 $1 $0 1Q 2017 2Q 2017 3Q 2017 4Q 2017 * Includes $1.9 of sales previously recorded as deferred revenue Source: Company data; Note: Trulance Launched March 20, 2017 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018 14 $Millions $9.4* $5.0 $2.3 $0.1
2018 KEY BUSINESS PRIORITIES • OPTIMIZING THE VALUE OF TRULANCE • ENSURING A STRONG FINANCIAL FOUNDATION • EXPLORING ALL STRATEGIC AND BUSINESS DEVELOPMENT OPPORTUNITIES 15 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018

OPTIMIZING THE VALUE OF TRULANCE • Continuing to grow market share in CIC • Accelerating uptake with new IBS-C indication • Leveraging strong foundation established with healthcare broaden prescriber base providers to • Continuing to educate and activate the Rx ready patient • Pulling-through market access wins and expanding coverage 16 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018
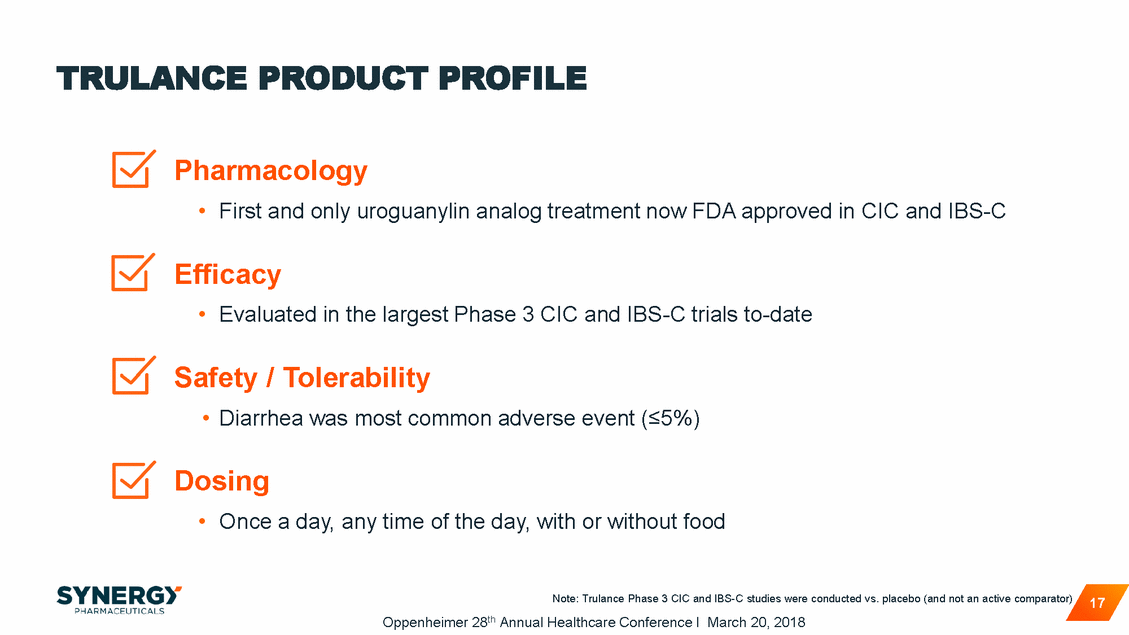
TRULANCE PRODUCT PROFILE Pharmacology • First and only uroguanylin analog treatment now FDA approved in CIC and IBS-C Efficacy • Evaluated in the largest Phase 3 CIC and IBS-C trials to-date Safety / Tolerability • Diarrhea was most common adverse event (<5%) Dosing • Once a day, any time of the day, with or without food Note: Trulance Phase 3 CIC and IBS-C studies were conducted vs. placebo (and not an active comparator) Oppenheimer 28th Annual Healthcare Conference I March 20, 2018 17
TRULANCE PROVIDES SIMPLE PACKAGING DOSING WITH CONVENIENT Simple Dosing & Administration Once a day Any time of day Convenient & Innovative Packaging With or without food Source: Company Data 18 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018
KEY CUSTOMERS AND CORE STRATEGIES Drive Awareness of Trulance and Trial and Adoption Stimulate HCPs Activate and Support the Rx Ready Patient PATIENTS Ensure Access PAYERS 19 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018 Core Strategies
EDUCATING HCPs WITH STRONG PERSONAL SELLING, NON-PERSONAL PROMOTION AND PEER-TO-PEER MARKETING • Highly experienced sales force continuing to educate top prescribers on Trulance for CIC and now IBS-C HCPs • Drive Awareness of Trulance and Stimulate Trial / Adoption • Distributed nearly half a million one-week sample packs to the field since launch • Completed >850 peer-to-peer educational speaker programs • Activate and Support the Rx Ready Patient • Launched IBS-C promotional campaign in February • Ensure Access Source: Company Data 20 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018
DIRECT-TO-PATIENT PLAN FOCUSED ON ACTIVATING AND SUPPORTING THE RX READY PATIENT • Trulance Branded Campaign PATIENTS – – – – Point-of-Care Promotion: ~20,000 offices Web Sponsorships/Display Ads: >215,500,000 impressions Search Marketing: >20,400,000 impressions Trulance.com (consumer site): > 1,200,000 visits • Drive Awareness of Trulance and Stimulate Trial / Adoption • Activate and Support the Rx Ready Patient • Confront Constipation Campaign (disease awareness) – – – 34 original media placements > 310,668,000 media impressions > 100,000 Emoji App downloads • Ensure Access 21 Source: Company Data Oppenheimer 28th Annual Healthcare Conference I March 20, 2018
PAYER STRATEGY FOCUSED ON ENSURING BROAD ACCESS PAYERS • Optimizing current coverage by pulling through market access wins Focused on improving national Commercial and Medicare Part D coverage Identifying key regional Commercial and Medicaid plans to expand coverage and coordinate sales activities to drive share • Drive Awareness of Trulance and Stimulate Trial / Adoption • • Activate and Support the Rx Ready Patient • • Ensure Access 22 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018
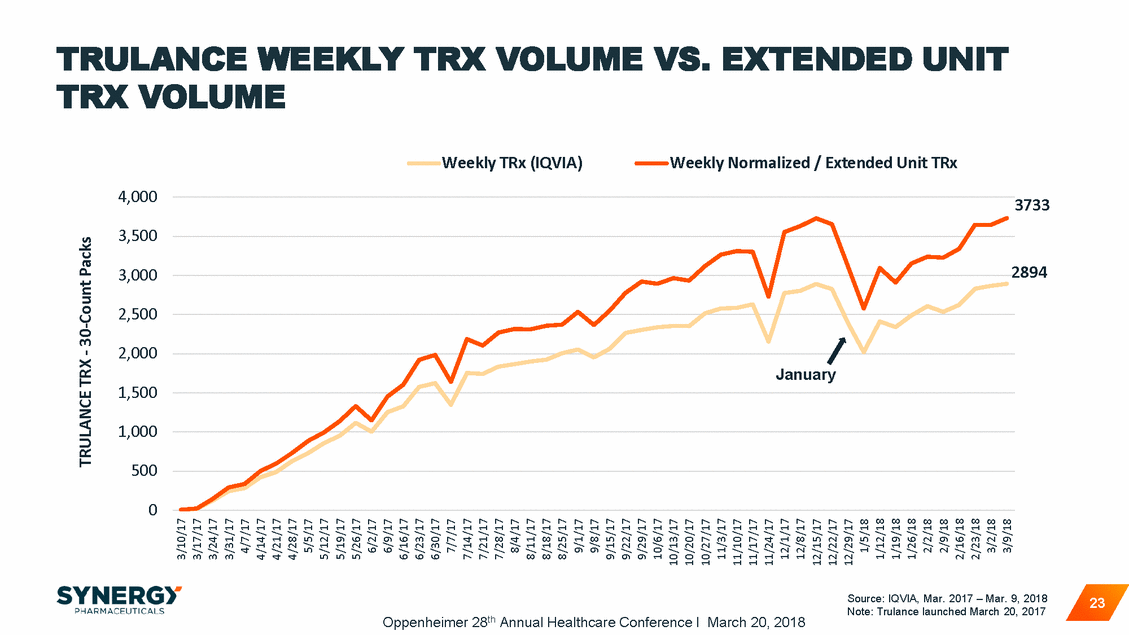
TRULANCE WEEKLY TRX VOLUME TRX VOLUME VS. EXTENDED UNIT Weekly TRx (IQVIA) Weekly Normalized / Extended Unit TRx 4,000 3733 3,500 2894 3,000 2,500 2,000 January 1,500 1,000 500 0 Source: IQVIA, Mar. 2017 – Mar. 9, 2018 Note: Trulance launched March 20, 2017 23 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018 TRULANCE TRX - 30-Count Packs 3/10/17 3/17/17 3/24/17 3/31/17 4/7/17 4/14/17 4/21/17 4/28/17 5/5/17 5/12/17 5/19/17 5/26/17 6/2/17 6/9/17 6/16/17 6/23/17 6/30/17 7/7/17 7/14/17 7/21/17 7/28/17 8/4/17 8/11/17 8/18/17 8/25/17 9/1/17 9/8/17 9/15/17 9/22/17 9/29/17 10/6/17 10/13/17 10/20/17 10/27/17 11/3/17 11/10/17 11/17/17 11/24/17 12/1/17 12/8/17 12/15/17 12/22/17 12/29/17 1/5/18 1/12/18 1/19/18 1/26/18 2/2/18 2/9/18 2/16/18 2/23/18 3/2/18 3/9/18
ENSURING A STRONG FINANCIAL FOUNDATION • Achieving cost efficiencies and maintaining disciplined expense management – – Reduced Q4 2017 OPEX by 13% from Q3 2017 Projecting total OPEX in range of $175-185 million for 2018, a reduction of 10-15% from 2017 • Prioritizing key commercial investments in high-return top-line growth drivers – Successfully integrated sales force to coincide with IBS-C approval and launch • Ensuring continued access to capital and financial flexibility – – $137.0 million of cash and cash equivalents on-hand as of 12/31/17 Ability to access up to an additional $100 million in 2018 through CRG debt facility Note: OPEX excludes stock based compensation 24 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018
EXPLORING ALL STRATEGIC AND BUSINESS DEVELOPMENT OPPORTUNITIES • Maximizing Trulance through partnerships in U.S. and ex-U.S. – Completed Canadian licensing deal • Continuing to evaluate opportunities to advance dolcanatide development • Actively exploring opportunities that leverage our commercial infrastructure and GI expertise 25 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018

2018 KEY BUSINESS PRIORITIES • OPTIMIZING THE VALUE OF TRULANCE – – – Continuing to grow market share in CIC Accelerating Trulance uptake with new IBS-C indication and newly integrated field force Pulling-through market access wins and expanding coverage • ENSURING STRONG FINANCIAL FOUNDATION – – – Achieving cost efficiencies and maintaining disciplined expense management Prioritizing key commercial investments in high-return top-line growth drivers Ensuring continued access to capital and financial flexibility • EXPLORING ALL STRATEGIC AND BUSINESS DEVELOPMENT OPPORTUNITIES – – Evaluating all options to maximize Trulance and dolcanatide Leveraging commercial infrastructure and GI expertise 26 Oppenheimer 28th Annual Healthcare Conference I March 20, 2018
OPPENHEIMER 28TH ANNUAL HEALTHCARE CONFERENCE March 20, 2018