Attached files
| file | filename |
|---|---|
| EX-21 - EX-21 - SYNERGY PHARMACEUTICALS, INC. | a15-23224_1ex21.htm |
| EX-10.12 - EX-10.12 - SYNERGY PHARMACEUTICALS, INC. | a15-23224_1ex10d12.htm |
| EX-32.1 - EX-32.1 - SYNERGY PHARMACEUTICALS, INC. | a15-23224_1ex32d1.htm |
| EX-31.1 - EX-31.1 - SYNERGY PHARMACEUTICALS, INC. | a15-23224_1ex31d1.htm |
| EX-10.10 - EX-10.10 - SYNERGY PHARMACEUTICALS, INC. | a15-23224_1ex10d10.htm |
| EX-10.13 - EX-10.13 - SYNERGY PHARMACEUTICALS, INC. | a15-23224_1ex10d13.htm |
| EX-10.14 - EX-10.14 - SYNERGY PHARMACEUTICALS, INC. | a15-23224_1ex10d14.htm |
| EX-32.2 - EX-32.2 - SYNERGY PHARMACEUTICALS, INC. | a15-23224_1ex32d2.htm |
| EX-10.8 - EX-10.8 - SYNERGY PHARMACEUTICALS, INC. | a15-23224_1ex10d8.htm |
| EX-31.2 - EX-31.2 - SYNERGY PHARMACEUTICALS, INC. | a15-23224_1ex31d2.htm |
| EX-10.11 - EX-10.11 - SYNERGY PHARMACEUTICALS, INC. | a15-23224_1ex10d11.htm |
| EX-23 - EX-23 - SYNERGY PHARMACEUTICALS, INC. | a15-23224_1ex23.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
x ANNUAL REPORT UNDER SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE FISCAL YEAR ENDED: DECEMBER 31, 2015
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number: 001-35268
SYNERGY PHARMACEUTICALS INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
33-0505269 |
|
(State or Other Jurisdiction of |
|
(I.R.S. Employer |
|
Incorporation or Organization) |
|
Identification No.) |
420 Lexington Avenue, Suite 2012, New York, New York 10170
(Address of principal executive offices) (Zip Code)
(212) 297-0020
(Registrant’s telephone number)
(Former Name, Former Address and Former Fiscal Year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Name of each exchange on which registered |
|
Units, each consisting of two shares of Common Stock and one Warrant to purchase one share of Common Stock |
|
The NASDAQ Capital Market |
|
|
|
|
|
Common Stock, $0.0001 par value |
|
The NASDAQ Global Select Market |
|
|
|
|
|
Warrants to purchase Common Stock |
|
The NASDAQ Capital Market |
Securities registered pursuant to section 12(g) of the Act:
Title of class: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 (the “Exchange Act”) during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer x |
|
Accelerated filer o |
|
|
|
|
|
Non-accelerated filer o |
|
Smaller reporting company o |
|
(Do not check if a smaller reporting company) |
|
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was $797,177,435 as of June 30, 2015, based upon the closing price on the NASDAQ Global Select market reported for such date.
The number of the registrant’s shares of common stock outstanding was 113,694,606 as of February 25, 2016.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the definitive proxy statement for our 2016 Annual Meeting of Stockholders are incorporated by reference into Part III of this report.
FORM 10-K
This Report on Form 10-K for Synergy Pharmaceuticals Inc. may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such forward-looking statements are characterized by future or conditional verbs such as “may,” “will,” “expect,” “intend,” “anticipate,” believe,” “estimate” and “continue” or similar words. You should read statements that contain these words carefully because they discuss future expectations and plans, which contain projections of future results of operations or financial condition or state other forward-looking information. Such statements are only predictions and our actual results may differ materially from those anticipated in these forward-looking statements. We believe that it is important to communicate future expectations to investors. However, there may be events in the future that we are not able to accurately predict or control. Factors that may cause such differences include, but are not limited to, those discussed under Item 1A. Risk Factors and elsewhere in this Form 10-K for the year ended December 31, 2015, as filed with the Securities and Exchange Commission, including the uncertainties associated with product development, the risk that products that appeared promising in early clinical trials do not demonstrate safety and efficacy in larger-scale clinical trials, the risk that we will not obtain approval to market our products, the risks associated with dependence upon key personnel and the need for additional financing. We do not assume any obligation to update forward-looking statements as circumstances change.
BUSINESS
Unless the context requires otherwise, the words “Synergy,” “the Company,” “we,” “us,” refer to Synergy Pharmaceuticals Inc. and Subsidiaries.
Business Overview
We are a biopharmaceutical company focused on the development and commercialization of novel gastrointestinal (GI) therapies. Our proprietary GI platform includes two lead product candidates — plecanatide and dolcanatide. Since the company’s inception in 2008, we have pioneered discovery, research and development efforts involving uroguanylin analogs for the treatment of functional gastrointestinal (GI) disorders and inflammatory bowel disease. Plecanatide is our first uroguanylin analog currently being evaluated for use as a once-daily tablet for two functional GI disorders, chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C). Plecanatide is a 16-amino acid peptide that is structurally identical to uroguanylin with the exception of a single amino acid change. Plecanatide is designed to mimic the function of uroguanylin by working locally in the upper GI tract to stimulate digestive fluid movement and support regular bowel function. In 2015, we announced positive phase 3 data in two CIC clinical trials and on January 29, 2016 we filed a new drug application (NDA) with the U.S. Food and Drug Administration (FDA) with plecanatide for CIC. We are continuing to progress two phase 3 clinical trials with plecanatide for IBS-C. We expect top-line results from the first phase 3 IBS-C trial in 1H 2016 and results from the second phase 3 IBS-C trial are expected in 2H 2016. We intend to file our second NDA with plecanatide for IBS-C by year-end 2016. Dolcanatide is our second uroguanylin analog currently being explored for inflammatory bowel disease (IBD). Dolcanatide is designed to be an analog of uroguanylin with enhanced resistance to standard digestive breakdown by proteases in the intestine. In January 2016, we announced positive phase 1b data with dolcanatide in patients with mild-to-moderate ulcerative colitis and we are presently evaluating plans for further clinical development in IBD.
Recent Developments
· On February 16, 2016, we announced that we have entered into a research collaboration agreement with BIND Therapeutics to engineer ACCURINS® decorated with our proprietary uroguanylin analogs to explore the potential targeting of guanylate cyclase-C (GC-C) receptors expressed on tumors, specifically GI malignancies. Upon achievement of proof-of-concept, we anticipate expanding the collaboration to enhance the potential effect of uroguanylin-based ACCURINS® by incorporating therapeutic payloads. This early research collaboration is not expected to have a material financial impact on us or BIND Therapeutics.
· On January 29, 2016, we announced that we had filed with the FDA our first NDA for plecanatide in CIC. The plecanatide NDA for CIC is supported by positive results from two phase 3 clinical trials which we completed in 2015. If approved, we plan to launch plecanatide with the CIC indication in the first quarter of 2017.
· On January 11, 2016, we announced positive phase 1b data with dolcanatide in a double-blind, placebo-controlled, four-week study evaluating 28 patients with mild-to-moderate ulcerative colitis. Analysis of the data indicates clear signals of improvement in dolcanatide-treated patients compared with placebo-treated patients. Dolcanatide was also safe and well-tolerated. We are currently evaluating how best to move dolcanatide forward in clinical development to treat ulcerative colitis.
· We have completed patient enrollment in our ongoing open-label, long-term safety trial with plecanatide for CIC. Patients who completed either of the two 12-week phase 3 CIC trials were allowed to enroll and receive either 3.0 mg or 6.0 mg plecanatide, once-daily, for one year or more. The objective of this trial is to evaluate the long-term safety and tolerability of plecanatide in patients with CIC.
· We are continuing to advance our two pivotal phase 3 clinical trials with plecanatide for IBS-C. Each randomized, 12-week, double-blind placebo-controlled trial is evaluating the efficacy and safety of both 3.0 mg and 6.0 mg plecanatide treatment doses, taken as a tablet once-a-day, in approximately 1,050 adult patients with IBS-C. The IBS-C program is designed to support regulatory submission in the U.S. and we intend to file our second NDA with plecanatide in the IBS-C indication by year-end 2016.
Plecanatide
Plecanatide is our first novel uroguanylin analog currently being evaluated for use as a once-daily tablet for chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C). Plecanatide is designed to mimic the function of uroguanylin by working locally in the upper GI tract to stimulate digestive fluid movement and support regular bowel function.
Plecanatide Phase 3 CIC Program
Design
The plecanatide phase 3 CIC program includes two randomized, 12-week, double-blind, placebo-controlled pivotal trials evaluating the efficacy and safety of two different plecanatide treatment doses (3.0 mg and 6.0 mg), taken as a tablet once-a-day, in patients with CIC. Both trials included a two-week pre-treatment baseline period, a 12-week treatment period, and a two-week post-treatment period.
The first phase 3 CIC trial was conducted in North America and assessed 1,346 adult patients (19.2% males and 80.8% females) that were randomly assigned to take 3.0 mg or 6.0 mg plecanatide or placebo once-a-day during the 12 week treatment period (453 patients in the 3 mg dose group, 441 patients in the 6.0 mg dose group and 452 patients in the placebo group).
The second phase 3 CIC trial was conducted in the United States and assessed 1,337 adult patients (21.6% males and 78.4% females) that were randomly assigned to take 3.0 mg or 6.0 mg plecanatide or placebo once-a-day during the 12 week treatment period (443 patients in the 3.0 mg dose group, 449 patients in the 6.0 mg dose group and 445 patients in the placebo group).
Primary Endpoint
The primary endpoint for both trials was the proportion of durable overall complete spontaneous bowl movement (CSBM) responders (%), which is the current regulatory endpoint required for U.S. approval in CIC. The FDA has defined an overall responder as a patient who fulfills both > 3 CSBMs per week plus an increase of > 1 CSBM from baseline in the same week, for 9 out of the 12 treatment weeks. In addition, the same patient must be a weekly responder for at least 3 of the last 4 treatment weeks in order to be considered a durable overall responder. Plecanatide has the potential to be the first drug approved for CIC using the more stringent regulatory requirement for durability of the overall response.
Patient Population
Patients were selected using the modified Rome 3 criteria for CIC and had (1) fewer than 3 defecations per week, (2) loose stools occurring rarely without laxatives, (3) inadequate criteria for irritable bowel syndrome with constipation (IBS-C), and (4) at least two of the following applied to at least 25% of defecations: (a) straining during evacuation, (b) lumpy or hard stools, (c) sensation of anorectal obstruction or blockage. Rome 3 requires patients to fulfill the criteria over the last 3 months with symptom onset at least 6 months prior to diagnosis.
First Phase 3 CIC Trial Top-line Results
On June 17, 2015 we announced positive top-line results from the first of two pivotal phase 3 clinical trials of plecanatide in 1,346 adult patients with CIC. Preliminary analysis of the data indicates that both plecanatide 3.0 mg and 6.0 mg doses met the study’s primary endpoint and demonstrated statistical significance in the proportion of patients in the intention-to-treat population who were durable overall CSBM responders compared to placebo during the 12-week treatment period (21.0% in 3.0 mg and 19.5% in 6.0 mg dose groups compared to 10.2% in placebo; p<0.001 for both doses). Plecanatide was safe and well tolerated at both doses; the most common adverse event was diarrhea, which occurred in 5.9% of patients in 3.0 mg and 5.5% of patients in 6.0 mg dose groups compared to 1.3% of placebo-treated patients. Stool consistency was the key secondary endpoint reported with top-line analyses; both 3.0 mg and 6.0 mg plecanatide doses showed statistically significant improvement from baseline in Bristol Stool Form Scale (BSFS) scores compared to placebo (mean increase of 1.53 in 3.0 mg and 1.52 in 6.0 mg dose groups compared to a mean increase of 0.77 in placebo; p<0.001 for both doses). The observed improvements began at Week 1, continued throughout the 12-week treatment period, and returned towards baseline with no indication of an exaggerated or rebound effect following discontinuation of treatment. 15 patients in the trial (1.1%) experienced serious adverse events but there was no imbalance across treatment groups in either incidences or individual serious adverse events. Overall, the rates of withdrawal from treatment because of an adverse event were low (5.1 % in 3.0 mg and 5.0% in 6.0 mg dose groups compared to 1.3% in placebo) and discontinuations due to diarrhea were infrequent (2.7% in 3.0 mg and 2.4% in 6.0 mg dose groups compared to 0.4% in placebo). No clinically relevant abnormalities were observed in serum chemistries, hematology, urinalysis, ECG or vital signs measurements.
Second Phase 3 CIC Trial Top-line Results
On July 30, 2015 we announced positive top-line results from the second of two pivotal phase 3 clinical trials of plecanatide in 1,337 adult patients with CIC. Preliminary analysis of the data indicates that both plecanatide 3.0 mg and 6.0 mg doses met the study’s primary endpoint and demonstrated statistical significance in the proportion of patients in the intention-to-treat population who were durable overall CSBM responders compared to placebo during the 12-week treatment period (20.1% in 3.0 mg and 20.0% in 6.0 mg dose groups compared to 12.8% in placebo; p=0.004 for both doses). Importantly, plecanatide was safe and well tolerated at both doses; the most common adverse event was diarrhea, which occurred in 3.2% of patients in 3.0 mg and 4.5% of patients in 6.0 mg dose groups compared to 1.3% of placebo-treated patients. Stool consistency was the key secondary endpoint reported with top-line analyses; both 3.0 mg and 6.0 mg plecanatide doses showed statistically significant improvement from baseline in Bristol Stool Form Scale (BSFS) scores compared to placebo (mean increase of 1.49 in 3.0 mg and 1.50 in 6.0 mg dose groups compared to a mean increase of 0.87 in placebo; p<0.001 for both doses). The observed improvements began at Week 1, continued throughout the 12-week treatment period, and returned towards baseline with no indication of an exaggerated or rebound effect following discontinuation of treatment. 20 patients in the trial (1.4%) experienced serious adverse events but there was no imbalance across treatment groups in either incidences or individual serious adverse events. Overall, the rates of withdrawal from treatment because of an adverse event were low (3.2% in 3.0 mg and 3.8% in 6.0 mg dose groups compared to 3.0% in placebo) and discontinuations due to diarrhea were infrequent (1.1% in 3.0 mg and 1.1% in 6.0 mg dose groups compared to 0.4% in placebo). No clinically relevant abnormalities were observed in serum chemistries, hematology, urinalysis, ECG or vital signs measurements.
Long-term Safety Study
We have completed enrollment into our ongoing CIC open-label, long-term safety trial with plecanatide. Patients who completed either of the two 12-week phase 3 CIC trials were allowed to enroll and receive either 3.0 mg or 6.0 mg plecanatide, once-daily, for one year or more. The objective of this trial is to evaluate the long-term safety and tolerability of plecanatide in patients with CIC.
We plan to present additional data results from both phase 3 CIC trials at appropriate scientific conferences.
Plecanatide Phase 3 IBS-C Program
Design
The plecanatide phase 3 IBS-C program includes two randomized, 12-week, double-blind, placebo-controlled pivotal trials evaluating the efficacy and safety of two different plecanatide treatment doses (3.0 mg and 6.0 mg), taken as a tablet once-a-day, in patients with IBS-C. Each phase 3 trial is expected to enroll approximately 1,050 patients with IBS-C and includes a two-week pre-treatment baseline period, a 12-week treatment period, and a two-week post-treatment period. The phase 3 IBS-C program was designed to support regulatory submissions in the U.S.
Primary Endpoint
The primary efficacy endpoint for both trials is the percentage of patients who are Overall Responders during the 12 week treatment period. An Overall Responder, as defined by the FDA, is a patient who is a weekly responder (i.e. meets both a 30% abdominal pain intensity reduction and stool frequency increase criteria in the same week) for at least 6 of the 12 treatment weeks.
Patient Population
Patients with IBS-C are defined by Rome III Criteria as having a history of constipation and abdominal pain for at least 6 months, including hard or lumpy stools for 25% or more of defecations, loose or watery stools for 25% or less of defecations, and abdominal pain or discomfort for 3 days or more per month for the last 3 months.
We announced the start of the first phase 3 IBS-C trial with plecanatide in December 2014 and the second trial was initiated in June 2015. We intend to file our second NDA with plecanatide in the IBS-C indication by year-end 2016.
Dolcanatide (formerly called SP-333)
Dolcanatide, our second uroguanylin analog, is currently being explored for inflammatory bowel disease (IBD). Dolcanatide is designed to be an analog of uroguanylin with enhanced resistance to standard digestive breakdown by proteases in the intestine. We have demonstrated the potential anti-inflammatory role of uroguanylin and uroguanylin analogs in a number of preclinical colitis models. In these earlier animal studies, oral treatment with dolcanatide was shown to ameliorate DSS- and TNBS-induced acute colitis in murine models and ameliorate spontaneous colitis in T-cell receptor alpha knockout mice.
On January 11, 2016, we announced positive phase 1b data with dolcanatide in a double-blind, placebo-controlled, four-week study evaluating 28 patients with mild-to-moderate ulcerative colitis. Analysis of the data indicates clear signals of improvement in dolcanatide-treated patients compared with placebo-treated patients. Dolcanatide was also safe and well-tolerated. We are currently evaluating how best to move dolcanatide forward in clinical development to treat ulcerative colitis.
On November 19, 2014 we announced positive top-line results from a phase 2 trial assessing safety, efficacy and dose-response of three different once-daily oral dolcanatide tablets (1.0, 3.0 and 6.0 mg) compared with placebo in 289 patients with Opioid Induced Constipation (OIC). Analysis of the data indicates dolcanatide met the study’s primary endpoint and demonstrated statistically significant improvement in mean change from baseline in the number of spontaneous bowel movements (SBMs) during Week 4 of the treatment period. Dolcanatide was safe and well tolerated at all doses. We will continue to monitor the OIC market opportunity but we currently have no plans to continue dolcanatide development in OIC.
Competition
The biopharmaceutical industry is characterized by rapidly evolving technology and intense competition. Our competitors include major pharmaceutical and biotechnology companies focusing on GI such as Ironwood Pharmaceuticals, Inc., Allergan plc, Takeda Pharmaceuticals America, Inc., Sucampo Pharmaceuticals, Inc., AstraZeneca, Valeant Pharmaceuticals International, Inc. and Shire, Plc. Most of our competitors have financial, technical and marketing resources significantly greater than our resources. Academic institutions, governmental agencies and other public and private research organizations are also conducting research activities and seeking patent protection and may commercialize products on their own or through joint ventures. We are aware of certain development projects for products to prevent or treat certain diseases targeted by us. The existence of these potential products or other products or treatments of which we are not aware, or products or treatments that may be developed in the future, may adversely affect our ability to market the products we develop.
Research and Development Expenses
Research and development costs include expenditures in connection with operating an in-house research and development laboratory, salaries and staff costs, application and filing for regulatory approval of proposed products, purchased in-process research and development, regulatory and scientific consulting fees, as well as contract research, patient costs, drug formulation and tableting, data collection, monitoring, clinical trial insurance. Research and development expenses for the year ended December 31, 2015 were approximately $78 million, as compared to approximately $83.3 million and $50.6 million for the year ended December 31, 2014 and 2013, respectively.
In accordance with FASB ASC Topic 730-10-55, Research and Development, we recorded prepaid research and development costs of approximately $3.1 million as of December 31, 2015 and $3.7 million as of December 31, 2014, for nonrefundable pre-payments for production of drug substance, analytical testing services and clinical trial costs for our drug candidates. In accordance with this guidance, we expense deferred research and development costs when drug compound is delivered or services are performed.
Patents and Proprietary Rights
We are able to protect our technology from unauthorized use by third parties only to the extent that it is covered by valid and enforceable patents or is effectively maintained as a trade secret or is protected by confidentiality agreements. Accordingly, patents or other proprietary rights are an essential element of our business.
As of December 31, 2015 we have 16 issued United States patents, and two allowed applications related to guanylate cyclase agonists. Two of these patents cover the composition-of-matter of plecanatide and were issued on May 9, 2006 and September 21, 2010; they will expire in 2023 and 2022, respectively. The patent that issued on May 9, 2006 has claims directed to the species of plecanatide, whereas the patent that issued on September 21, 2010 has claims directed to a genus of peptides that are identical in length to plecanatide and is inclusive of plecanatide. A third patent covers the composition-of-matter of dolcanatide issued on February 1, 2011 and expires in 2028. A fourth patent granted October 11, 2011 covers the composition-of-matter of certain analogs related to plecanatide and dolcanatide and will expire in 2029. A fifth patent granted February 14, 2012 covers certain methods of treating inflammatory bowel disease using plecanatide and will expire in 2022. A sixth patent granted January 28, 2014 covers methods of stimulating water transport in the gastrointestinal tract using plecanatide and will expire in 2022. A seventh patent granted June 26, 2012 covers an additional composition-of-matter related to certain analogs of plecanatide and dolcanatide and will expire in 2029. An eighth patent granted on January 22, 2013 covers another composition-of-matter related to certain analogs of plecanatide and will expire in 2029. A ninth patent granted on July 30, 2013 covers another composition-of-matter related to certain analogs of plecanatide and will expire in 2029. Another three patents that also cover compositions-of-matter related to certain analogs of plecanatide were issued on February 5, 2013, October 29, 2013, and March 4, 2014 and will expire in 2029. A thirteenth patent granted May 6, 2014 covers certain methods of treating a variety of gastrointestinal and other disorders using dolcanatide and will expire in 2029. A fourteenth patent granted December 2, 2014 and covers composition-of-matter of dolcanatide and expires in 2028. A fifteenth patent granted on March 3, 2015 and covers methods of use of plecanatide and expires in 2030. A sixteenth patent granted July 28, 2015 covers an additional composition-of-matter related to certain analogs of plecanatide and dolcanatide and will expire in 2028. One allowed patent application covers composition-of-matter of dolcanatide and is projected to expire in 2028. Another allowed patent application covers an additional composition-of-matter related to certain analogs of plecanatide and dolcanatide and is projected to expire in 2034.
In addition, we have seven granted foreign patents which cover the composition-of-matter of plecanatide and expire in 2022. These foreign patents cover Austria, Belgium, Switzerland, Cyprus, Germany, Denmark, Spain, Finland, France, the United Kingdom, Greece, Hong Kong, Ireland, Italy, Liechtenstein, Luxembourg, Monaco, the Netherlands, Portugal, Sweden, Turkey, Armenia, Azerbaijan, Belarus, Kazakhstan, the Kyrgyz Republic, Moldova, the Russian Federation, Tajikistan, Turkmenistan, Canada, China and Japan. We also have seven granted foreign patents that cover the composition-of-matter related to dolcanatide that expire in 2028. These patents cover Switzerland, Germany, Denmark, Spain, France, the United Kingdom, Ireland, Italy, the Netherlands, Hong Kong, Armenia, Azerbaijan, Belarus, Kazakhstan, the Kyrgyz Republic, Moldova, the Russian Federation, Tajikistan, Turkmenistan, China, Australia, Japan and Mexico. We also have three foreign patents that cover composition-of-matter of certain analogs related to plecanatide and methods of use to treat Ulcerative Colitis that expire in 2029. These patents cover Australia, Hong Kong, France, Germany, Italy, Spain and the United Kingdom. We also have two foreign patents that cover composition-of-matter of certain analogs related to dolcanatide and methods of use to treat gastrointestinal disorders and expires in 2029. These patents cover Australia, France, Germany, Italy, Spain and the United Kingdom. We also have three foreign patents related to plecanatide that cover treatment and prevention of Hypercholesterolemia and expire in 2030. These patents cover Japan, Mexico, France, Germany, Italy, Spain and the United Kingdom.
Additionally, as of the date of this report on Form 10-K, we have 17 pending United States utility patent applications; 3 pending U.S. provisional applications; and 79 pending foreign patent applications relating to plecanatide and dolcanatide, various derivatives and analogs of plecanatide and dolcanatide, and their uses and manufacture.
In April 2010, two parties filed an opposition to one of our granted European patents with the European Patent Office. An opposition hearing was held December 14, 2011, which resulted in the European Patent Office issuing the following statement: “Account being taken of the amendments made by the patent proprietor during the opposition proceedings, the patent and the invention to which it relates are found to meet the requirements of the European Patent Convention (Art.101(3)(a)EPC).” In particular, the composition-of-matter claim covering plecanatide was upheld.
On September 14, 2012 we entered into a binding letter of intent with Ironwood Pharmaceuticals, Inc. (“Ironwood”), pursuant to which we and Ironwood agreed to enter into a definitive license agreement giving us an exclusive worldwide license to Ironwood’s method of use patents on plecanatide. The letter of intent contemplates a low single digit royalty on net sales of plecanatide and both parties agreed not to challenge each other’s patents covering certain GC-C agonists, except that we retain the right to challenge Ironwood’s method of use patents on plecanatide.
During 2013, we transferred ownership of all FV-100 intellectual property rights we acquired from Bristol-Myers Squibb Company (“BMS”), in August 2012, to ContraVir Pharmaceuticals, Inc., our former majority-owned subsidiary which we spun off to our shareholders on February 18, 2014. The FV-100 assets acquired by ContraVir from us were licensed from University College Cardiff Consultants Limited (“Cardiff”) pursuant to the terms of that certain Patent and Technology License Agreement, dated as of February 2, 2005, between Cardiff and Contravir Research Incorporated, an entity with no prior relationship with us (“CRI”), as amended March 27, 2007, which ContraVir assumed from us (the “Cardiff Agreement”). Cardiff and Rega Foundation (“Rega”) were originally the joint owners of the patent rights. Pursuant to the terms of an agreement, dated September 24, 1998, as amended December 23, 2004, Cardiff received from Rega an exclusive, irrevocable worldwide license to manufacture, use, sell, or otherwise deal in or with products utilizing the patent rights, including the right to grant sublicenses thereunder. We assumed the obligations under the Cardiff Agreement from BMS pursuant to the terms of the BMS Agreement. BMS assumed the obligations under the Cardiff Agreement from Inhibitex Inc. (“Inhibitex”) upon its acquisition of Inhibitex in January 2012. Inhibitex assumed the obligations under the Cardiff Agreement upon its acquisition of FermaVir Pharmaceuticals, Inc. (“FermaVir”) in September 2010. FermaVir was the successor to CRI in a merger consummated in August 2005.
Patents extend for varying periods according to the date of patent filing or grant and the legal term of patents in the various countries where patent protection is obtained. The actual protection afforded by a patent, which can vary from country to country, depends on the type of patent, the scope of its coverage and the availability of legal remedies in the country.
While trade secret protection is an essential element of our business and we have taken security measures to protect our proprietary information and trade secrets, we cannot give assurance that our unpatented proprietary technology will afford us significant commercial protection. We seek to protect our trade secrets by entering into confidentiality agreements with third parties, employees and consultants. Our employees and consultants also sign agreements requiring that they assign to us their interests in intellectual property arising from their work for us. All employees sign an agreement not to engage in any conflicting employment or activity during their employment with us and not to disclose or misuse our confidential information. However, it is possible that these agreements may be breached or invalidated, and if so, there may not be an adequate corrective remedy available. Accordingly, we cannot ensure that employees, consultants or third parties will not breach the confidentiality provisions in our contracts, infringe or misappropriate our trade secrets and other proprietary rights or that measures we are taking to protect our proprietary rights will be adequate.
In the future, third parties may file claims asserting that our technologies or products infringe on their intellectual property. We cannot predict whether third parties will assert such claims against us or against the licensors of technology licensed to us, or whether those claims will harm our business. If we are forced to defend ourselves against such claims, whether they are with or without merit and whether they are resolved in favor of, or against, our licensors or us, we may face costly litigation and the diversion of management’s attention and resources. As a result of such disputes, we may have to develop costly non-infringing technology or enter into licensing agreements. These agreements, if necessary, may be unavailable on terms acceptable to us, or at all.
Government Regulation
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food and Drug Administration, and Cosmetic Act and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. The FDA has very broad enforcement authority
and failure to abide by applicable regulatory requirements can result in administrative or judicial sanctions being imposed on us, including warning letters, refusals of government contracts, clinical holds, civil penalties, injunctions, restitution, disgorgement of profits, recall or seizure of products, total or partial suspension of production or distribution, withdrawal of approval, refusal to approve pending applications, and criminal prosecution.
FDA Approval Process
Our product candidates are regulated by the FDA as drugs. No manufacturer may market a new drug until it has submitted a New Drug Application, or NDA, to the FDA, and the FDA has approved it. The steps required before the FDA may approve an NDA generally include:
· preclinical laboratory tests and animal tests conducted in compliance with FDA’s good laboratory practice requirements;
· development, manufacture and testing of active pharmaceutical product and dosage forms suitable for human use in compliance with current good manufacturing practices, or GMP;
· the submission to the FDA of an investigational new drug application, or IND, for human clinical testing, which must become effective before human clinical trials may begin;
· adequate and well-controlled human clinical trials to establish the safety and efficacy of the product for its specific intended use(s);
· the submission to the FDA of a New Drug Application, or NDA; and
· FDA review and approval of the NDA.
Preclinical tests include laboratory evaluation of the product candidate, as well as animal studies to assess the potential safety and efficacy of the product candidate. The conduct of the pre-clinical tests must comply with federal regulations and requirements including good laboratory practices. We must submit the results of the preclinical tests, together with manufacturing information, analytical data and a proposed clinical trial protocol to the FDA as part of an IND, which must become effective before we may commence human clinical trials. The IND will automatically become effective 30 days after its receipt by the FDA, unless the FDA raises concerns or questions before that time about the conduct of the proposed trials. In such a case, we must work with the FDA to resolve any outstanding concerns before clinical trials can proceed. We cannot be sure that submission of an IND will result in the FDA allowing clinical trials to begin, or that, once begun, issues will not arise that suspend or terminate such trials. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board for approval. An institutional review board may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the institutional review board’s requirements or may impose other conditions.
Clinical trials involve the administration of the product candidate to humans under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control. Clinical trials are typically conducted in three sequential phases, though the phases may overlap or be combined. In Phase 1, the initial introduction of the drug into healthy human subjects, the drug is usually tested for safety (adverse effects), dosage tolerance and pharmacologic action, as well as to understand how the drug is taken up by and distributed within the body. Phase 2 usually involves studies in a limited patient population (individuals with the disease under study) to:
· evaluate preliminarily the efficacy of the drug for specific, targeted conditions;
· determine dosage tolerance and appropriate dosage as well as other important information about how to design larger Phase 3 trials; and
· identify possible adverse effects and safety risks.
Phase 3 trials generally further evaluate clinical efficacy and test for safety within an expanded patient population. The conduct of the clinical trials is subject to extensive regulation, including compliance with good clinical practice regulations and guidance.
The FDA may order the temporary or permanent discontinuation of a clinical trial at any time or impose other sanctions if it believes that the clinical trial is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. We may also suspend clinical trials at any time on various grounds.
The results of the preclinical and clinical studies, together with other detailed information, including the manufacture and composition of the product candidate, are submitted to the FDA in the form of an NDA requesting approval to market the drug. FDA approval of the NDA is required before marketing of the product may begin in the U.S. If the NDA contains all pertinent information and data, the FDA will “file” the application and begin review. The FDA may “refuse to file” the NDA if it does not contain all pertinent information and data. In that case, the applicant may resubmit the NDA when it contains the missing information and data.
Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of new drug applications. Most such applications for non-priority drug products are reviewed within 10 months. The review process, however, may be extended by FDA requests for additional information, preclinical or clinical studies, clarification regarding information already provided in the submission, or submission of a risk evaluation and mitigation strategy. The FDA may refer an application to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. Before approving an NDA, the FDA will typically inspect the facilities at which the product candidate is manufactured and will not approve the product candidate unless GMP compliance is satisfactory. FDA also typically inspects facilities responsible for performing animal testing, as well as clinical investigators who participate in clinical trials. The FDA may refuse to approve an NDA if applicable regulatory criteria are not satisfied, or may require additional testing or information. The FDA may also limit the indications for use and/or require post-marketing testing and surveillance to monitor the safety or efficacy of a product. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
The testing and approval process requires substantial time, effort and financial resources, and our product candidates may not be approved on a timely basis, if at all. The time and expense required to perform the clinical testing necessary to obtain FDA approval for regulated products can frequently exceed the time and expense of the research and development initially required to create the product. The results of preclinical studies and initial clinical trials of our product candidates are not necessarily predictive of the results from large-scale clinical trials, and clinical trials may be subject to additional costs, delays or modifications due to a number of factors, including difficulty in obtaining enough patients, investigators or product candidate supply. Failure by us to obtain, or any delay in obtaining, regulatory approvals or in complying with requirements could adversely affect the commercialization of product candidates and our ability to receive product or royalty revenues.
Other Regulatory Requirements
After approval, drug products are subject to extensive continuing regulation by the FDA, which include company obligations to manufacture products in accordance with Good Manufacturing Practice, or GMP, maintain and provide to the FDA updated safety and efficacy information, report adverse experiences with the product, keep certain records and submit periodic reports, obtain FDA approval of certain manufacturing or labeling changes, and comply with FDA promotion and advertising requirements and restrictions. Failure to meet these obligations can result in various adverse consequences, both voluntary and FDA-imposed, including product recalls, withdrawal of approval, restrictions on marketing, and the imposition of civil fines and criminal penalties against the NDA holder. In addition, later discovery of previously unknown safety or efficacy issues may result in restrictions on the product, manufacturer or NDA holder.
We and any manufacturers of our products are required to comply with applicable FDA manufacturing requirements contained in the FDA’s GMP regulations. GMP regulations require among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation. The manufacturing facilities for our products must meet GMP requirements to the satisfaction of the FDA pursuant to a pre-approval inspection before we can use them to manufacture our products. We and any third-party manufacturers are also subject to periodic inspections of facilities by the FDA and other authorities, including procedures and operations used in the testing and manufacture of our products to assess our compliance with applicable regulations.
With respect to post-market product advertising and promotion, the FDA imposes a number of complex regulations on entities that advertise and promote pharmaceuticals, which include, among others, standards for direct-to-consumer advertising, promoting drugs for uses or in patient populations that are not described in the drug’s approved labeling (known as “off-label use”), industry- sponsored scientific and educational activities, and promotional activities involving the internet. Failure to comply with FDA requirements can have negative consequences, including adverse publicity, enforcement letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties. Although physicians may prescribe legally available drugs for off-label uses, manufacturers may not market or promote such off-label uses.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
Adverse event reporting and submission of periodic reports is required following FDA approval of an NDA. The FDA also may require post-marketing testing, known as Phase 4 testing, risk minimization action plans and surveillance to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product.
Outside the United States, our ability to market a product is contingent upon receiving marketing authorization from the appropriate regulatory authorities. The requirements governing marketing authorization, pricing and reimbursement vary widely from jurisdiction to jurisdiction. At present, foreign marketing authorizations are applied for at a national level, although within the European Union registration procedures are available to companies wishing to market a product in more than one European Union member state.
We are also subject to various environmental, health and safety regulations including those governing laboratory procedures and the handling, use, storage, treatment, and disposal of hazardous materials. From time to time, and in the future, our operations may involve the use of hazardous materials.
Employees
As of February 25, 2016, we had 44 full-time employees. We believe our employee relations are satisfactory.
Our Website
Our website address is www.synergypharma.com. Information found on our website is not incorporated by reference into this report. We make available free of charge through our website our Securities and Exchange Commission, or SEC, filings furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
The risks described below are not the only ones we face. Additional risks we are not presently aware of or that we currently believe are immaterial may also impair our business operations. Our business could be harmed by any of these risks. The trading price of our common stock could decline due to any of these risks, and you may lose all or part of your investment. In assessing these risks, you should also refer to the other information contained or incorporated by reference into this Form 10-K, including our financial statements and related notes.
Risks Related to Our Business
We are at an early stage of development as a company and currently have no source of revenue and may never become profitable. Currently, we have no products approved for commercial sale and, to date, we have not generated any revenue.
Our ability to generate revenue depends heavily on:
· our ability to raise additional capital on a timely basis to continue to fund our clinical trials and preparing for the commercial launch of plecanatide in the United States;
· demonstration in current and future clinical trials that our product candidates, plecanatide for the treatment of CIC and IBS-C and dolcanatide, are safe and effective;
· our ability to seek and obtain regulatory approvals, including with respect to the indications we are seeking;
· successful manufacture and commercialization of our product candidates; and
· market acceptance of our products.
All of our existing product candidates are in various stages of development and will require extensive additional clinical evaluation, regulatory review and approval, significant marketing efforts and substantial investment before they could provide us with any revenue. As a result, even if we successfully develop, achieve regulatory approval and commercialize our products, we may be unable to generate revenue for many years, if at all. We do not anticipate that we will generate revenue until 2017, at the earliest, or that we will achieve profitability for at least several years after generating material revenue, if at all. If we are unable to generate revenue, we will not become profitable, and we may be unable to continue our operations.
We will need to raise substantial additional capital to fund our operations, and our failure to obtain funding when needed may force us to delay, reduce or eliminate our development programs or commercialization efforts or even discontinue or curtail our operations.
During the year ended December 31, 2015, our operating activities used net cash of approximately $101 million. During the year ended December 31, 2014 and December 31, 2013, our operating activities used net cash of approximately $89.1 million and $52.6 million, respectively. Our available-for-sale securities as of December 31, 2015 and December 31, 2014 were $50.1 million and $49.9 million, respectively, which consists of U.S. Government securities with maturities of less than one year. In addition, as of December 31, 2015 and December 31, 2014 our cash and cash equivalents was $61.7 million and $146.5 million, respectively, consisting of checking accounts and short-term money market mutual funds.
We expect to continue to spend substantial amounts to:
· continue clinical and commercial development of plecanatide to treat GI disorders;
· finance our selling, general and administrative expenses;
· make interest payments on Senior Convertible Notes;
· prepare regulatory approval applications and seek approvals for plecanatide and other product candidates, including dolcanatide;
· license or acquire additional technologies;
· manufacture product for clinical trials and initial launch stock; and
· prepare for the launch and commercialization of our product candidates.
We will need to raise additional capital to fund our future operations and we cannot be certain that funding will be available on acceptable terms on a timely basis, or at all. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants that may impact our ability to conduct our business. If we are unable to raise additional capital when required or on acceptable terms, we may have to significantly
delay, scale back or discontinue the development and/or commercialization of our product candidates or our commercialization efforts. We also may be required to:
· seek collaborators for our product candidates at an earlier stage than otherwise would be desirable and on terms that are less favorable than might otherwise be available; and/or
· relinquish license or otherwise dispose of rights to technologies, product candidates or products that we would otherwise seek to develop or commercialize ourselves on unfavorable terms.
Our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern, which may hinder our ability to obtain future financing.
Our consolidated financial statements as of December 31, 2015 have been prepared under the assumption that we will continue as a going concern for the next twelve months. Our independent registered public accounting firm has issued a report that includes an explanatory paragraph referring to our recurring and continuing losses from operations and expressing substantial doubt in our ability to continue as a going concern without additional capital becoming available. Our ability to continue as a going concern is dependent upon our ability to obtain additional equity or debt financing, attain further operating efficiencies, reduce expenditures, and, ultimately, to generate revenue. Our consolidated financial statements as of December 31, 2015 did not include any adjustments that might result from the outcome of this uncertainty.
We do not have any products that are approved for commercial sale and therefore do not expect to generate any revenues from product sales in the foreseeable future, if ever.
We currently do not have any products that are approved for commercial sale. To date, we have funded our operations primarily from sales of our equity and debt securities. We have not received, and do not expect to receive for at least the next year, if at all, any revenues from the commercialization of our product candidates. To obtain revenues from sales of our product candidates, we must succeed, either alone or with third parties, in developing, obtaining regulatory approval for, manufacturing and marketing drugs with commercial potential. We may never succeed in these activities, and may not generate sufficient revenues to continue our business operations or achieve profitability.
We have incurred significant losses since inception and anticipate that we will incur continued losses for the foreseeable future.
As of December 31, 2015, we had an accumulated deficit of approximately $384.4 million. We expect to incur significant and increasing operating losses for the next several years as we expand our research and development, continue our clinical trials of plecanatide for the treatment of GI disorders, acquire or license technologies, advance other product candidates into clinical development, including dolcanatide, complete clinical trials, seek regulatory approval, prepare for the launch of plecanatide and, if we receive FDA approval, commercialize our products. Because of the numerous risks and uncertainties associated with product development efforts, we are unable to predict the extent of any future losses or when we will become profitable, if at all. If we are unable to achieve and then maintain profitability, the market value of our common stock will likely experience significant decline.
We are largely dependent on the success of our lead product candidate, plecanatide, and we cannot be certain that this product candidate will receive regulatory approval or be successfully commercialized.
We currently have no products for sale, and we cannot guarantee that we will ever have any drug products approved for sale. We and our product candidates are subject to extensive regulation by the FDA and comparable regulatory authorities in other countries governing, among other things, research, testing, clinical trials, manufacturing, labeling, promotion, selling, adverse event reporting and recordkeeping. We are not permitted to market any of our product candidates in or outside the United States until we receive approval of a new drug application, or NDA, for a product candidate from the FDA or the equivalent approval from a foreign regulatory authority. Obtaining FDA approval is a lengthy, expensive and uncertain process. We currently have one lead product candidate, plecanatide for the treatment of GI disorders, and the success of our business currently depends on our successful development, approval and commercialization.
The clinical development program for plecanatide may not lead to commercial products for a number of reasons, including if we fail to obtain necessary approvals from the FDA or foreign regulatory authorities because our clinical trials fail to demonstrate to their satisfaction that this product candidate is safe and effective. The FDA and any foreign regulatory authority might also approve
plecanatide for fewer or more limited indications than we request, or may grant approval contingent on the performance of costly post-approval clinical trials. In addition, the FDA and any foreign regulatory authority may not approve the labeling claims that we believe are necessary or desirable for the successful commercialization of plecanatide. Any failure to obtain regulatory approval of plecanatide would significantly limit our ability to generate revenues, and any failure to obtain such approval for all of the indications and labeling claims we deem desirable could reduce our potential revenue.
We may also fail to obtain future approvals if we have inadequate financial or other resources to advance our product candidates through the clinical trial process. Any failure or delay in completing clinical trials or obtaining regulatory approval for plecanatide in a timely manner would have a material adverse impact on our business and our stock price.
Clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results.
Our product candidates may not prove to be safe and efficacious in clinical trials and may not meet all the applicable regulatory requirements needed to receive regulatory approval. In order to receive regulatory approval for the commercialization of our product candidates, we must conduct, at our own expense, extensive preclinical testing and clinical trials to demonstrate safety and efficacy of these product candidates for the intended indication of use. Clinical testing is expensive, can take many years to complete, if at all, and its outcome is uncertain. Failure can occur at any time during the clinical trial process.
The results of preclinical studies and early clinical trials of new drugs do not necessarily predict the results of later-stage clinical trials. The design of our clinical trials is based on many assumptions about the expected effects of our product candidates, and if those assumptions are incorrect may not produce statistically significant results. Preliminary results may not be confirmed on full analysis of the detailed results of an early clinical trial. Product candidates in later stages of clinical trials may fail to show safety and efficacy sufficient to support intended use claims despite having progressed through initial clinical testing. The data collected from clinical trials of our product candidates may not be sufficient to support the filing of an NDA or to obtain regulatory approval in the United States or elsewhere. Because of the uncertainties associated with drug development and regulatory approval, we cannot determine if or when we will have an approved product for commercialization or achieve sales or profits.
Delays in clinical testing could result in increased costs to us and delay our ability to generate revenue.
We may experience delays in clinical testing of our product candidates. We do not know whether planned clinical trials will begin on time, will need to be redesigned or will be completed on schedule, if at all. Clinical trials can be delayed for a variety of reasons, including delays in obtaining regulatory approval to commence a clinical trial, in securing clinical trial agreements with prospective sites with acceptable terms, in obtaining institutional review board approval to conduct a clinical trial at a prospective site, in recruiting patients to participate in a clinical trial or in obtaining sufficient supplies of clinical trial materials. Many factors affect patient enrollment, including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the clinical trial, competing clinical trials and new drugs approved for the conditions we are investigating. Clinical investigators will need to decide whether to offer their patients enrollment in clinical trials of our product candidates versus treating these patients with commercially available drugs that have established safety and efficacy profiles. Any delays in completing our clinical trials will increase our costs, slow down our product development and timeliness and approval process and delay our ability to generate revenue.
The FDA’s expectations for clinical trials may change over time, complicating the process of obtaining evidence to support approval of our product candidates.
In May 2012, the FDA’s Center for Drugs Evaluation and Research, or CDER, released guidance entitled: “Irritable Bowel Syndrome—Clinical Evaluation of Products for Treatment” to assist the product sponsors developing new drugs for the treatment of IBS. In pertinent part, this document provides recommendations for IBS clinical trial design and endpoints, and describes the need for the future development of patient-reported outcome, or PRO, instruments for use in IBS clinical trials. The clinical trials we have planned for plecanatide are designed to follow the recommendations included in this guidance. The guidance document represents the FDA’s thinking on the clinical evaluation of products for the treatment of IBS. FDA guidance documents, however, do not establish legally enforceable requirements, should be viewed only as recommendations, and may be changed at any time. Therefore, even insofar as we intend to follow the recommendations provided in the guidance document, we cannot be sure that the FDA will accept the results of our clinical research even if such research follows the recommendations in the guidance document.
We may be required to suspend or discontinue clinical trials due to unexpected side effects or other safety risks that could preclude approval of our product candidates.
Our clinical trials may be suspended at any time for a number of reasons. For example, we may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to the clinical trial patients. In addition, the FDA or other regulatory agencies may order the temporary or permanent discontinuation of our clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements or that they present an unacceptable safety risk to the clinical trial patients.
Administering any product candidate to humans may produce undesirable side effects. These side effects could interrupt, delay or halt clinical trials of our product candidates and could result in the FDA or other regulatory authorities denying further development or approval of our product candidates for any or all targeted indications. Ultimately, some or all of our product candidates may prove to be unsafe for human use. Moreover, we could be subject to significant liability if any volunteer or patient suffers, or appears to suffer, adverse health effects as a result of participating in our clinical trials. Any of these events could prevent us from achieving or maintaining market acceptance of plecanatide and could substantially increase commercialization costs.
If we fail to comply with healthcare regulations, we could face substantial enforcement actions, including civil and criminal penalties and our business, operations and financial condition could be adversely affected.
As a developer of pharmaceuticals, even though we do not intend to make referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payors, certain federal and state healthcare laws and regulations pertaining to fraud and abuse, false claims and patients’ privacy rights are and will be applicable to our business. We could be subject to healthcare fraud and abuse laws and patient privacy laws of both the federal government and the states in which we conduct our business.
The laws include:
· the federal healthcare program anti-kickback law, which prohibits, among other things, persons from soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual, for an item or service or the purchasing or ordering of a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs;
· federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, and which may apply to entities like us which provide coding and billing information to customers;
· the federal Health Insurance Portability and Accountability Act of 1996, which prohibits executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information;
· the Federal Food, Drug, and Cosmetic Act, which among other things, strictly regulates drug manufacturing and product marketing, prohibits manufacturers from marketing drug products for off-label use and regulates the distribution of drug samples; and
· state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by federal laws, thus complicating compliance efforts.
If our operations are found to be in violation of any of the laws described above or any governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable federal and state privacy, security and fraud laws may prove costly.
If we are unable to satisfy regulatory requirements, we may not be able to commercialize our product candidates.
We need FDA approval prior to marketing our product candidates in the United States. If we fail to obtain FDA approval to market our product candidates, we will be unable to sell our product candidates in the United States and we will not generate any revenue.
The FDA’s review and approval process, including among other things, evaluation of preclinical studies and clinical trials of a product candidate as well as the manufacturing process and facility, is lengthy, expensive and uncertain. To receive approval, we must, among other things, demonstrate with substantial evidence from well-designed and well-controlled pre- clinical testing and clinical trials that the product candidate is both safe and effective for each indication for which approval is sought. Satisfaction of these requirements typically takes several years and the time needed to satisfy them may vary substantially, based on the type, complexity and novelty of the pharmaceutical product. We cannot predict if or when we will submit future NDAs for approval for our product candidates currently under development. Any approvals we may obtain may not cover all of the clinical indications for which we are seeking approval or may contain significant limitations on the conditions of use. If any of these events occur it could prevent us from achieving or maintaining market acceptance of our products and could substantially increase commercialization costs.
The FDA has substantial discretion in the NDA review process and may either refuse to file our NDA for substantive review or may decide that our data is insufficient to support approval of our product candidates for the claimed intended uses. Following any regulatory approval of our product candidates, we will be subject to continuing regulatory obligations such as safety reporting, required and additional post marketing obligations, and regulatory oversight of promotion and marketing. Even if we receive regulatory approvals, the FDA may subsequently seek to withdraw approval of our NDA if we determine that new data or a reevaluation of existing data show the product is unsafe for use under the conditions of use upon the basis of which the NDA was approved, or based on new evidence of adverse effects or adverse clinical experience, or upon other new information. If the FDA does not approve our NDA or withdraws approval of our NDA, the FDA may require that we conduct additional clinical trials, preclinical trials or manufacturing studies and submit that data before it will reconsider our application. Depending on the extent of these or any other requested studies, approval of any applications that we submit may be delayed by several years, may require us to expend more resources than we have available, or may never be obtained at all.
We may also be subject to a wide variety of foreign regulations governing the development, manufacture and marketing of our products. Whether or not FDA approval has been obtained, approval of a product by the comparable regulatory authorities of foreign countries must still be obtained prior to marketing the product in those countries. The approval process varies and the time needed to secure approval in any region such as the European Union or in a country with an independent review procedure may be longer or shorter than that required for FDA approval. We cannot assure you that clinical trials conducted in one country will be accepted by other countries or that an approval in one country or region will result in approval elsewhere.
If our product candidates are unable to compete effectively with marketed drugs targeting similar indications as our product candidates, our commercial opportunity will be reduced or eliminated.
We face competition generally from established pharmaceutical and biotechnology companies, as well as from academic institutions, government agencies and private and public research institutions. Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Small or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. Our commercial opportunity will be reduced or eliminated if our competitors develop and commercialize GI drugs that are safer, more effective, have fewer side effects or are less expensive than our product candidates. These potential competitors compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient enrollment for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business.
If approved and commercialized, plecanatide will compete with at least two currently approved prescription therapies for the treatment of CIC and IBS-C, Amitiza and Linzess. In addition, over-the-counter products are also used to treat certain symptoms of CIC and IBS-C. We believe other companies are developing products that will compete with plecanatide should they be approved by the FDA. To our knowledge, other potential competitors are in earlier stages of development. If potential competitors are successful in completing drug development for their product candidates and obtain approval from the FDA, they could limit the demand for plecanatide. We expect that our ability to compete effectively will depend upon our ability to:
· successfully and rapidly complete clinical trials and submit for and obtain all requisite regulatory approvals in a cost-effective manner;
· maintain a proprietary position for our products and manufacturing processes and other related product technology;
· attract and retain key personnel;
· ensure competitive patient access to our products in the U.S. based on any required discounts and rebates to payors;
· develop relationships with physicians prescribing these products; and
· build an adequate sales and marketing infrastructure for our product candidates.
Because we will be competing against significantly larger companies with established track records, we will have to demonstrate that, based on clinical data, side-effect profiles and other factors, our products, if approved, are competitive with other products. If we are unable to compete effectively in the GI drug market and differentiate our products from other marketed GI drugs, we may never generate meaningful revenue.
If we fail to attract and keep senior management and key scientific personnel, we may be unable to successfully develop our product candidates, conduct our clinical trials and commercialize our product candidates.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management, clinical and scientific personnel. We are highly dependent upon our senior management and scientific staff. The loss one or more of our senior management could delay or prevent the successful completion of our clinical trials or the commercialization of our product candidates.
The competition for qualified personnel in the biotechnology and pharmaceuticals field is intense. We will need to hire additional personnel as we expand our commercial and supply chain activities. We may not be able to attract and retain quality personnel on acceptable terms given the competition for such personnel among biotechnology, pharmaceutical and other companies.
We will need to increase the size of our organization, and we may experience difficulties in managing growth.
We are a small company with 42 employees as of December 31, 2015. To continue our clinical trials and commercialize our product candidates, we will need to expand our employee base for managerial, operational, financial and other resources. Future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate additional employees. Over the next 12 months depending on the progress of our planned clinical trials and status of the plecanatide NDA, we plan to add additional employees to assist us with our commercial programs. Our future financial performance and our ability to commercialize our product candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to:
· manage development efforts effectively;
· manage our commercialization activities effectively;
· integrate additional management, administrative, manufacturing and sales and marketing personnel;
· maintain sufficient administrative, accounting and management information systems and controls; and
· hire and train additional qualified personnel.
We may not be able to accomplish these tasks, and our failure to accomplish any of them could harm our financial results and impact our ability to achieve development milestones.
We are currently building our commercial organization. If we are unable to establish a direct sales force in the United States to promote our products, the commercial opportunity for our products may be diminished.
We are currently building our commercial organization. If any of our product candidates are approved by the FDA, we may market that product through our own sales force. We will incur significant additional expenses and commit significant additional management resources to establish our own sales force. We may not be able to establish these capabilities despite these additional expenditures. We will also have to compete with other pharmaceutical and biotechnology companies to recruit, hire and train sales and marketing personnel. If we elect to rely on third parties to sell our product candidates in the United States, we may receive less revenue than if we sold our products directly. In addition, although we would intend to use due diligence in monitoring their activities, we may have little or no control over the sales efforts of those third parties. In the event we are unable to develop our own
sales force or collaborate with a third party to sell our product candidates, we may not be able to commercialize our product candidates which would negatively impact our ability to generate revenue.
We may need to rely on third parties to market and commercialize our product candidates in international markets.
Currently, we do not have any plans to enter international markets. In the future, if appropriate regulatory approvals are obtained, we may commercialize our product candidates in international markets. However, we have not decided how to commercialize our product candidates in those markets. We may decide to build our own sales force or sell our products through third parties. If we decide to sell our product candidates in international markets through a third party, we may not be able to enter into any marketing arrangements on favorable terms or at all. In addition, these arrangements could result in lower levels of income to us than if we marketed our product candidates entirely on our own. If we are unable to enter into a marketing arrangement for our product candidates in international markets, we may not be able to develop an effective international sales force to successfully commercialize those products in international markets. If we fail to enter into marketing arrangements for our products and are unable to develop an effective international sales force, our ability to generate revenue would be limited.
If the manufacturers upon whom we rely fail to produce plecanatide and our other product candidates, including dolcanatide, in the volumes that we require on a timely basis, or fail to comply with stringent regulations applicable to pharmaceutical drug manufacturers, we may face delays in the development and commercialization of our product candidates.
We do not currently possess internal manufacturing capacity. We currently utilize the services of contract manufacturers to manufacture our clinical supplies and commercial products. With respect to the manufacturing of plecanatide, we have executed supply agreements with contract manufacturers sufficient to meet our foreseeable clinical trial requirements. If any of our suppliers were to limit or terminate production or otherwise fail to meet the quality or delivery requirements needed to satisfy the supply commitments, the process of locating and qualifying alternate sources could require up to several months, during which time our production could be delayed. Any curtailment in the availability of plecanatide could have a material adverse effect on our business, financial position and results of operations. In addition, because regulatory authorities must generally approve raw material sources for pharmaceutical products, changes in raw material suppliers may result in production delays or higher raw material costs.
Since the commercial manufacturing process is single sourced Active Pharmaceutical Ingredient (“API”) and Drug Product, we are at risk during launch and after launch until we establish secondary suppliers. We continue to pursue additional active pharmaceutical ingredients, or API, and drug product supply agreements with other manufacturers. We may be required to agree to minimum volume requirements, exclusivity arrangements or other restrictions with the contract manufacturers. We may not be able to enter into long-term agreements on commercially reasonable terms, or at all. If we change or add manufacturers, the FDA and comparable foreign regulators may require approval of the changes. Approval of these changes could require new testing by the manufacturer and compliance inspections to ensure the manufacturer is conforming to all applicable laws and regulations, including good manufacturing practices, or GMP. In addition, the new manufacturers would have to be educated in or independently develop the processes necessary for the production of our product candidates. Peptide manufacturing is a highly specialized manufacturing business. While we believe we will have long term arrangements with a sufficient number of contract manufacturers, if we lose a manufacturer, it would take us a substantial amount of time to identify and develop a relationship, and seek regulatory approval, where necessary, for an alternative manufacturer.
The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products may encounter difficulties in production, particularly in scaling up production. These problems include difficulties with production costs and yields, quality control, including stability of the product and quality assurance testing, shortages of qualified personnel, as well as compliance with federal, state and foreign regulations. In addition, any delay or interruption in the supply of clinical trial supplies could delay the completion of our clinical trials, increase the costs associated with conducting our clinical trials and, depending upon the period of delay, require us to commence new clinical trials at significant additional expense or to terminate a clinical trial.
We are responsible for ensuring that each of our contract manufacturers comply with the GMP requirements of the FDA and other regulatory authorities from which we seek to obtain product approval. These requirements include, among other things, quality control, quality assurance and the maintenance of records and documentation. The approval process for NDAs includes a review of the manufacturer’s compliance with GMP requirements. We are responsible for regularly assessing a contract manufacturer’s compliance with GMP requirements through record reviews and periodic audits and for ensuring that the contract manufacturer takes responsibility and corrective action for any identified deviations. Manufacturers of plecanatide and other product candidates,
including dolcanatide, may be unable to comply with these GMP requirements and with other FDA and foreign regulatory requirements, if any.
Pre-Approval inspection at all of the external manufacturing need to pass by the FDA for approval to move forward with manufacturing for commercial distribution
While we will oversee compliance by our contract manufacturers, ultimately we will not have control over our manufacturers’ compliance with these regulations and standards. A failure to comply with these requirements may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval. If the safety of plecanatide or other product candidates is compromised due to a manufacturers’ failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for or successfully commercialize plecanatide or other product candidates, and we may be held liable for any injuries sustained as a result. Any of these factors could cause a delay of clinical trials, regulatory submissions, approvals or commercialization of plecanatide or other product candidates, entail higher costs or result in us being unable to effectively commercialize plecanatide or other product candidates. Furthermore, if our manufacturers fail to deliver the required commercial quantities on a timely basis and at commercially reasonable prices, we may be unable to meet demand for any approved products and would lose potential revenues.
We may not be able to manufacture our product candidates in commercial quantities, which would prevent us from commercializing our product candidates.
To date, our product candidates have been manufactured in small quantities for preclinical studies and clinical trials. If any of our product candidates is approved by the FDA or comparable regulatory authorities in other countries for commercial sale, we will need to manufacture such product candidate in larger quantities. We may not be able to increase successfully the manufacturing capacity for any of our product candidates in a timely or economic manner, or at all. Significant scale-up of manufacturing may require additional validation studies, which the FDA must review and approve. If we are unable to increase successfully the manufacturing capacity for a product candidate, the clinical trials as well as the regulatory approval or commercial launch of that product candidate may be delayed or there may be a shortage in supply. Our product candidates require precise, high quality manufacturing. Our failure to achieve and maintain these high quality manufacturing standards in collaboration with our third-party manufacturers, including the incidence of manufacturing errors, could result in patient injury or death, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could harm our business, financial condition and results of operations.
Materials necessary to manufacture our product candidates may not be available on commercially reasonable terms, or at all, which may delay the development and commercialization of our product candidates.
We rely on the third-party manufacturers of our product candidates to purchase from third-party suppliers the materials necessary to produce the bulk APIs and product candidates for our clinical trials, and we will rely on such manufacturers to purchase such materials to produce the APIs and finished products for any commercial distribution of our products if we obtain marketing approval. Suppliers may not sell these materials to our manufacturers at the time they need them in order to meet our required delivery schedule or on commercially reasonable terms, if at all. We do not have any control over the process or timing of the acquisition of these materials by our manufacturers. Moreover, we currently do not have any agreements for the production of these materials. If our manufacturers are unable to obtain these materials for our clinical trials, testing of the affected product candidate would be delayed, which may significantly impact our ability to develop the product candidate. If we, or our manufacturers, are unable to purchase these materials after regulatory approval has been obtained for one of our products, the commercial launch of such product would be delayed or there would be a shortage in supply of such product, which would harm our ability to generate revenues from such product and achieve or sustain profitability.
Product validation will be the first time plecanatide will be run at full scale commercial batch and this product will be used for commercial distribution. If the validation batches are not executed flawlessly our commercial launch will be jeopardized.
Our product candidates, if approved for sale, may not gain acceptance among physicians, patients and the medical community, thereby limiting our potential to generate revenues.
If one of our product candidates is approved for commercial sale by the FDA or other regulatory authorities, the degree of market acceptance of any approved product by physicians, healthcare professionals and third-party payors and our profitability and growth will depend on a number of factors, including:
· demonstration of safety and efficacy;
· changes in the practice guidelines and the standard of care for the targeted indication;
· relative convenience and ease of administration;
· the prevalence and severity of any adverse side effects;
· budget impact of adoption of our product on relevant drug formularies
· the availability, cost and potential advantages of alternative treatments, including less expensive generic drugs;
· pricing, reimbursement and cost effectiveness, which may be subject to regulatory control;
· effectiveness of our or any of our partners’ sales and marketing strategies;
· the product labeling or product insert required by the FDA or regulatory authority in other countries; and
· the availability of adequate third-party insurance coverage or reimbursement.
If any product candidate that we develop does not provide a treatment regimen that is as beneficial as, or is perceived as being as beneficial as, the current standard of care or otherwise does not provide patient benefit, that product candidate, if approved for commercial sale by the FDA or other regulatory authorities, likely will not achieve market acceptance. Our ability to effectively promote and sell any approved products will also depend on pricing and cost-effectiveness, including our ability to produce a product at a competitive price and our ability to obtain sufficient third-party coverage or reimbursement. If any product candidate is approved but does not achieve an adequate level of acceptance by physicians, patients and third-party payors, our ability to generate revenues from that product would be substantially reduced. In addition, our efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources, may be constrained by FDA rules and policies on product promotion, and may never be successful.
Guidelines and recommendations published by various organizations can impact the use of our products candidates.
Government agencies promulgate regulations and guidelines directly applicable to us and to our product candidates. In addition, professional societies, practice management groups, private health and science foundations and organizations involved in various diseases from time to time may also publish guidelines or recommendations to the health care and patient communities. Recommendations of government agencies or these other groups or organizations may relate to such matters as usage, dosage, route of administration and use of concomitant therapies. Recommendations or guidelines suggesting the reduced use of our product candidates or the use of competitive or alternative products that are followed by patients and health care providers could result in decreased use of our proposed product candidates.
If product liability lawsuits are successfully brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability lawsuits related to the testing of our product candidates, and will face an even greater risk if we sell our product candidates commercially. Currently, we are not aware of any anticipated product liability claims with respect to our product candidates. In the future, an individual may bring a liability claim against us if one of our product candidates causes, or merely appears to have caused, an injury. If we cannot successfully defend ourselves against the product liability claim, we may incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
· decreased demand for our product candidates;
· injury to our reputation;
· withdrawal of clinical trial participants;
· costs of related litigation;
· initiation of investigations by regulators;
· substantial monetary awards to patients or other claimants;
· distraction of management’s attention from our primary business;
· product recalls;
· loss of revenue; and
· the inability to commercialize our product candidates.
We have clinical trial liability insurance with a $5,000,000 aggregate limit. We intend to expand our insurance coverage to include the sale of commercial products if marketing approval is obtained for our product candidates. Our current insurance coverage may prove insufficient to cover any liability claims brought against us. In addition, because of the increasing costs of insurance coverage, we may not be able to maintain insurance coverage at a reasonable cost or obtain insurance coverage that will be adequate to satisfy liabilities that may arise.
Even if our product candidates receive regulatory approval, they may still face future development and regulatory difficulties.
Even if U.S. regulatory approval is obtained, the FDA may still impose significant restrictions on a product’s indicated uses or impose ongoing requirements for potentially costly post-approval studies. Plecanatide and our other product candidates, including dolcanatide, would also be subject to ongoing FDA requirements governing the labeling, packaging, storage, advertising, promotion, recordkeeping and submission of safety and other post-market information. In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with GMP, regulations. If we or a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions on that product or the manufacturer, including requiring withdrawal of the product from the market or suspension of manufacturing. If we, our product candidates or the manufacturing facilities for our product candidates fail to comply with applicable regulatory requirements, a regulatory agency may:
· issue warning letters;
· impose civil or criminal penalties;
· suspend regulatory approval;
· suspend any ongoing clinical trials;
· refuse to approve pending applications or supplements to applications filed by us;
· impose restrictions on operations, including costly new manufacturing requirements;
· seize or detain products or request us to initiate a product recall; or
· pursue and obtain an injunction.
Our failure to successfully discover, acquire, develop and market additional product candidates or approved products could impair our ability to grow.
As part of our growth strategy, we intend to develop and market additional products and product candidates. We are pursuing various therapeutic opportunities through our pipeline. We may spend several years completing our development of any particular current or future internal product candidate, and failure can occur at any stage. The product candidates to which we allocate our resources may not end up being successful. In addition, because our internal research capabilities are limited, we may be dependent upon pharmaceutical and biotechnology companies, academic scientists and other researchers to sell or license products or technology to us. The success of this strategy depends partly upon our ability to identify, select, discover and acquire promising pharmaceutical product candidates and products. Failure of this strategy would impair our ability to grow.
The process of proposing, negotiating and implementing a license or acquisition of a product candidate or approved product is lengthy and complex. Other companies, including some with substantially greater financial, marketing and sales resources, may compete with us for the license or acquisition of product candidates and approved products. We have limited resources to identify and execute the acquisition or in-licensing of third-party products, businesses and technologies and integrate them into our current infrastructure. Moreover, we may devote resources to potential acquisitions or in-licensing opportunities that are never completed, or we may fail to realize the anticipated benefits of such efforts. We may not be able to acquire the rights to additional product candidates on terms that we find acceptable, or at all. In addition, future acquisitions may entail numerous operational and financial risks, including:
· disruption of our business and diversion of our management’s time and attention to develop acquired products or technologies;
· incurrence of substantial debt, dilutive issuances of securities or depletion of cash to pay for acquisitions;
· higher than expected acquisition and integration costs;
· difficulty in combining the operations and personnel of any acquired businesses with our operations and personnel;
· increased amortization expenses;
· assumption of known and unknown liabilities;
· impairment of relationships with key suppliers or customers of any acquired businesses due to changes in management and ownership; and
· inability to motivate key employees of any acquired businesses.
Further, any product candidate that we acquire may require additional development efforts prior to commercial sale, including extensive clinical testing and approval by the FDA and applicable foreign regulatory authorities. All product candidates are prone to risks of failure typical of pharmaceutical product development, including the possibility that a product candidate will not be shown to be sufficiently safe and effective for approval by regulatory authorities.
Drugs approved to treat CIC and IBS have been subject to considerable post- market scrutiny, with consequences up to and including voluntary withdrawal of approved products from the market. This may heighten FDA scrutiny of our product candidates before or following market approval.
Products approved for the treatment of IBS have been subject to considerable post-market scrutiny. For example, in 2007, Novartis voluntarily discontinued marketing Zelnorm (tegaserod), a product approved for the treatment of women with IBS-C, after the FDA found an increased risk of serious cardiovascular events associated with the use of the drug. Earlier, in 2000, GlaxoWellcome withdrew Lotronex (alosetron), which was approved for women with severe diarrhea-prominent IBS, after the manufacturer received numerous reports of adverse events or AEs, including ischemic colitis, severely obstructed or ruptured bowel, or death. In 2002, the FDA approved the manufacturer’s application to make Lotronex available again, on the condition that the drug only be made available through a restricted marketing program.
Although plecanatide is being investigated for IBS, plecanatide is from a different pharmacologic class than Zelnorm or Lotronex, and would not be expected to share the same clinical risk profile as those agents. Nevertheless, because these products are in the same or related therapeutic classes, it is possible that the FDA will have heightened scrutiny of plecanatide or any other agent under development for IBS. This could delay product approval, increase the cost of our clinical development program, or increase the cost of post- market study commitments for our IBS product candidates, including plecanatide.
Even if our product candidates receive regulatory approval in the United States, we may never receive approval to commercialize them outside of the United States.
In the future, we may seek to commercialize plecanatide and/or our other product candidates, including dolcanatide, in foreign countries outside of the United States. In order to market any products outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other jurisdictions regarding safety and efficacy. Approvals procedures vary among jurisdictions and can involve product testing and administrative review periods different from, and greater than, those in the United States. The time required to obtain approval in other jurisdictions might differ from that required to obtain FDA approval. The regulatory approval process in other jurisdictions may include all of the risks detailed above regarding FDA approval in the United States as well as other risks. Regulatory approval in one jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory processes in others. Failure to obtain regulatory approvals in other jurisdictions or any delay or setback in obtaining such approvals could have the same adverse effects detailed above regarding FDA approval in the United States. As described above, such effects include the risks that plecanatide or our other product candidates may not be approved for all indications for use included in proposed labeling or for any indications at all, which could limit the uses of plecanatide or other product candidates and have an adverse effect on our products’ commercial potential or require costly post-marketing studies.
We rely on third parties to conduct our clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to seek or obtain regulatory approval for or commercialize our product candidates.
We have agreements with third-party contract research organizations, or CROs, under which we have delegated to the CROs the responsibility to coordinate and monitor the conduct of our clinical trials and to manage data for our clinical programs. We, our CROs and our clinical sites are required to comply with current Good Clinical Practices, or cGCPs, regulations and guidelines issued by the FDA and by similar governmental authorities in other countries where we are conducting clinical trials. We have an ongoing
obligation to monitor the activities conducted by our CROs and at our clinical sites to confirm compliance with these requirements. In the future, if we, our CROs or our clinical sites fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA may require us to perform additional clinical trials before approving our marketing applications. In addition, our clinical trials must be conducted with product produced under cGMP regulations, and will require a large number of test subjects. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process.
If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to their failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As a result, our financial results and the commercial prospects for our product candidates would be harmed, our costs could increase, and our ability to generate revenue could be delayed.
Reimbursement may not be available for our product candidates, which would impede sales.
Market acceptance and sales of our product candidates may depend on coverage and reimbursement policies and health care reform measures. Decisions about formulary coverage as well as levels at which government authorities and third-party payers, such as private health insurers and health maintenance organizations, reimburse patients for the price they pay for our products as well as levels at which these payors pay directly for our products, where applicable, could affect whether we are able to commercialize these products. We cannot be sure that reimbursement will be available for any of these products. Also, we cannot be sure that coverage or reimbursement amounts will not reduce the demand for, or the price of, our products. We have not commenced efforts to have our product candidates reimbursed by government or third party payors. If coverage and reimbursement are not available or are available only at limited levels, we may not be able to commercialize our products.
In recent years, officials have made numerous proposals to change the health care system in the United States. These proposals include measures that would limit or prohibit payments for certain medical treatments or subject the pricing of drugs to government control. In addition, in many foreign countries, particularly the countries of the European Union, the pricing of prescription drugs is subject to government control. If our products are or become subject to government regulation that limits or prohibits payment for our products, or that subjects the price of our products to governmental control, we may not be able to generate revenue, attain profitability or commercialize our products.
As a result of legislative proposals and the trend towards managed health care in the United States, third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement of new drugs. They may also impose strict prior authorization requirements and/or refuse to provide any coverage of uses of approved products for medical indications other than those for which the FDA has granted market approvals. As a result, significant uncertainty exists as to whether and how much third-party payors will reimburse patients for their use of newly-approved drugs, which in turn will put pressure on the pricing of drugs.
Healthcare reform measures could hinder or prevent our product candidates’ commercial success.
The U.S. government and other governments have shown significant interest in pursuing continued healthcare reform. Any government-adopted reform measures could adversely impact the pricing of healthcare products and services in the United States or internationally and the amount of reimbursement available from governmental agencies or other third party payors. The continuing efforts of the U.S. and foreign governments, insurance companies, managed care organizations and other payors of health care services to contain or reduce health care costs may adversely affect our ability to set prices for our products which we believe are fair, and our ability to generate revenues and achieve and maintain profitability.
New laws, regulations and judicial decisions, or new interpretations of existing laws, regulations and decisions, that relate to healthcare availability, methods of delivery or payment for products and services, or sales, marketing or pricing, may limit our potential revenue, and we may need to revise our research and development programs. The pricing and reimbursement environment may change in the future and become more challenging due to several reasons, including policies advanced by the current executive administration in the United States, new healthcare legislation or fiscal challenges faced by government health administration authorities. Specifically, in both the United States and some foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the health care system in ways that could affect our ability to sell our products profitably.
For example, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or the PPACA has substantially changed the way healthcare is financed by both government health plans and private insurers, and significantly impacts the pharmaceutical industry. The PPACA contains a number of provisions that are expected to impact our business and operations in ways that may negatively affect our potential revenues in the future. For example, the PPACA imposes a non-deductible excise tax on pharmaceutical manufacturers or importers that sell branded prescription drugs to U.S. government programs which we believe will increase the cost of our products. In addition, as part of the PPACA’s provisions closing a funding gap that currently exists in the Medicare Part D prescription drug program (commonly known as the “donut hole”), we will be required to provide a discount on branded prescription drugs equal to 50% of the government-negotiated price, for drugs provided to certain beneficiaries who fall within the donut hole. Similarly, PPACA increases the level of Medicaid rebates payable by manufacturers of brand-name drugs from 15.1% to 23.1% and requires collection of rebates for drugs paid by Medicaid managed care organizations. The PPACA also includes significant changes to the 340B drug discount program including expansion of the list of eligible covered entities that may purchase drugs under the program. At the same time, the expansion in eligibility for health insurance benefits created under PPACA is expected to increase the number of patients with insurance coverage who may receive our products. While it is too early to predict all the specific effects the PPACA or any future healthcare reform legislation will have on our business, they could have a material adverse effect on our business and financial condition.
Congress periodically adopts legislation like the PPACA and the Medicare Prescription Drug, Improvement and Modernization Act of 2003, that modifies Medicare reimbursement and coverage policies pertaining to prescription drugs. Implementation of these laws is subject to ongoing revision through regulatory and sub regulatory policies. Congress also may consider additional changes to Medicare policies, potentially including Medicare prescription drug policies, as part of ongoing budget negotiations. While the scope of any such legislation is uncertain at this time, there can be no assurances that future legislation or regulations will not decrease the coverage and price that we may receive for our proposed products. Other third-party payors are increasingly challenging the prices charged for medical products and services. It will be time consuming and expensive for us to go through the process of seeking coverage and reimbursement from Medicare and private payors. Our proposed products may not be considered cost-effective, and coverage and reimbursement may not be available or sufficient to allow us to sell our proposed products on a profitable basis. Further federal and state proposals and health care reforms are likely which could limit the prices that can be charged for the product candidates that we develop and may further limit our commercial opportunities. Our results of operations could be materially adversely affected by proposed healthcare reforms, by the Medicare prescription drug coverage legislation, by the possible effect of such current or future legislation on amounts that private insurers will pay and by other health care reforms that may be enacted or adopted in the future.
In September 2007, the Food and Drug Administration Amendments Act of 2007 was enacted, giving the FDA enhanced post-marketing authority, including the authority to require post-marketing studies and clinical trials, labeling changes based on new safety information, and compliance with risk evaluations and mitigation strategies approved by the FDA. The FDA’s exercise of this authority could result in delays or increased costs during product development, clinical trials and regulatory review, increased costs to assure compliance with post-approval regulatory requirements, and potential restrictions on the sale and/or distribution of approved products.
Security breaches and other disruptions could compromise our information and expose us to liability, which would cause our business and reputation to suffer.
In the ordinary course of our business, we collect and store sensitive data, including intellectual property, our proprietary business information and that of our suppliers and business partners, as well as personally identifiable information of clinical trial participants and employees. Similarly, our business partners and third party providers possess certain of our sensitive data. The secure maintenance of this information is critical to our operations and business strategy. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Any such breach could compromise our networks and the information stored there could be accessed, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information, including our data being breached at our business partners or third-party providers, could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, disrupt our operations, and damage our reputation which could adversely affect our business.
It is difficult and costly to protect our proprietary rights, and we may not be able to ensure their protection.
Our commercial success will depend in part on obtaining and maintaining patent protection and trade secret protection of our product candidates, and the methods used to manufacture them, as well as successfully defending these patents against third-party challenges. We will only be able to protect our product candidates from unauthorized making, using, selling and offering to sell or
importation by third parties to the extent that we have rights under valid and enforceable patents or trade secrets that cover these activities.
For example:
· others may be able to make compounds that are competitive with our products but that are not covered by the claims of our patents;
· we may not have been the first to make the inventions covered by our pending patent applications;
· we may not have been the first to file patent applications for these inventions;
· others may independently develop similar or alternative technologies or duplicate any of our technologies;
· it is possible that our pending patent applications will not result in issued patents
· it is possible that our issued patents could be narrowed in scope, invalidated, held to be unenforceable, or circumvented;
· we may not develop additional proprietary technologies that are patentable; or
· the patents of others may have an adverse effect on our business.
We also may rely on trade secrets to protect our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. While we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, outside scientific collaborators and other advisors may unintentionally or willfully disclose our information to competitors. Enforcing a claim that a third party illegally obtained and is using our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights and we may be unable to protect our rights to, or use, our technology.
If we choose to go to court to stop someone else from using the inventions claimed in our patents, that individual or company has the right to ask the court to rule that these patents are invalid and/or should not be enforced against that third party. These lawsuits are expensive and would consume time and other resources even if we were successful in stopping the infringement of these patents. In addition, there is a risk that the court will decide that these patents are not valid and that we do not have the right to stop the other party from using the inventions. There is also the risk that, even if the validity of these patents is upheld, the court will refuse to stop the other party on the ground that such other party’s activities do not infringe our rights to these patents.
Furthermore, a third party may claim that we are using inventions covered by the third party’s patent rights and may go to court to stop us from engaging in our normal operations and activities, including making or selling our product candidates. These lawsuits are costly and could affect our results of operations and divert the attention of managerial and technical personnel. There is a risk that a court would decide that we are infringing the third party’s patents and would order us to stop the activities covered by the patents. In addition, there is a risk that a court will order us to pay the other party damages for having violated the other party’s patents. The biotechnology industry has produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we are sued for patent infringement, we would need to demonstrate that our products or methods of use either do not infringe the patent claims of the relevant patent and/or that the patent claims are invalid, and we may not be able to do this. Proving invalidity, in particular, is difficult since it requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents.
Because some patent applications in the United States may be maintained in secrecy until the patents are issued, patent applications in the United States and many foreign jurisdictions are typically not published until eighteen months after filing, and publications in the scientific literature often lag behind actual discoveries, we cannot be certain that others have not filed patent applications for technology covered by our issued patents or our pending applications or that we were the first to invent the technology. Our competitors have filed, and may in the future file, patent applications covering technology similar to ours. Any such patent application may have priority over our patent applications and could further require us to obtain rights to issued patents covering such technologies. If another party has filed a United States patent application on inventions similar to ours, we may have to participate in an interference proceeding declared by the PTO, to determine priority of invention in the United States. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful, resulting in a loss of our United States patent position with respect to such inventions.
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submissions, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The PTO and various foreign governmental patent agencies require compliance with a number of procedural, documentaries, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case.
We have not yet registered trademarks for plecanatide in our potential markets, and failure to secure those registrations could adversely affect our ability to market our product candidate and our business.
We have not yet registered trademarks for plecanatide in any jurisdiction. Our trademark applications in the United States, when filed, and any other jurisdictions where we may file may not be allowed for registration, and our registered trademarks may not be maintained or enforced. During trademark registration proceedings, we may receive rejections. Although we are given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in the PTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. Failure to secure such trademark registrations in the United States and in foreign jurisdictions could adversely affect our ability to market our product candidates and our business.
Confidentiality agreements with employees and others may not adequately prevent disclosure of our trade secrets and other proprietary information and may not adequately protect our intellectual property, which could limit our ability to compete.
Because we operate in the highly technical field of research and development of small molecule drugs, we rely in part on trade secret protection in order to protect our proprietary trade secrets and unpatented know-how. However, trade secrets are difficult to protect, and we cannot be certain that others will not develop the same or similar technologies on their own. We have taken steps, including entering into confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers, manufacturers and other advisors, to protect our trade secrets and unpatented know-how. These agreements generally require that the other party keep confidential and not disclose to third parties all confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. We also typically obtain agreements from these parties that provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, these agreements may not be honored and may not effectively assign intellectual property rights to us. Enforcing a claim that a party illegally obtained and is using our trade secrets or know-how is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets or know-how. The failure to obtain or maintain trade secret protection could adversely affect our competitive position.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology and pharmaceutical industry, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks Related to the Convertible Senior Notes
The indenture for our senior convertible notes, or the Notes, contains covenants limiting our financial and operating flexibility.
The indenture for the Notes contains covenants that will restrict our ability and the ability of certain of our subsidiaries to:
· incur or guarantee additional indebtedness, other than subordinated debt;
· declare or pay any dividends on our or our subsidiaries’ capital stock;
· redeem or repurchase capital stock or prepay or repurchase subordinated debt; or
· sell or license rights in North America to or otherwise encumber any of the intellectual property related to plecanatide.
These restrictive covenants could limit our ability to pursue our growth plans, restrict our flexibility in planning for, or reacting to, changes in our business and industry and increase our vulnerability to general adverse economic and industry conditions. We may enter into additional financing arrangements in the future, which could further restrict our flexibility.
Any defaults of covenants contained in the Notes may lead to an event of default under the Notes and the indenture. We may not be able to pay any amounts due to holders of the Notes in the event of such default, and such default may significantly impair our ability to satisfy our obligations under the Notes.
We will not make any adjustment to the conversion rate for Notes converted in connection with a fundamental change, and noteholders will not be compensated for any lost value of their Notes as a result of such transaction.
We will not increase or make any other adjustment to the conversion rate upon a conversion of Notes in connection with a fundamental change or similar event. Therefore, noteholders will not be compensated for any lost value of their Notes as a result of such transaction.
The Notes are effectively subordinated to any of our future secured debt and any liabilities of our subsidiaries.
The Notes will rank senior in right of payment to our future indebtedness that is expressly subordinated in right of payment to the Notes; equal in right of payment to our trade payables and other future unsecured indebtedness that is not so subordinated; effectively junior to any of our secured indebtedness to the extent of the value of the assets securing such indebtedness; and structurally junior to all future indebtedness (including trade payables) incurred by our subsidiaries. In the event of our bankruptcy, liquidation, reorganization or other winding up, our assets that secure debt ranking senior or equal in right of payment to the Notes will be available to pay obligations on the Notes only after the secured debt has been repaid in full. There may not be sufficient assets remaining to pay amounts due on any or all of the Notes then outstanding.
Servicing our debt will require a significant amount of cash, and we may not have sufficient cash flow from our business to pay our debt.
Our ability to make scheduled payments of the principal of, to pay interest on or to refinance our indebtedness, including the Notes, depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not generate cash flow from operations in the future sufficient to service our debt and make necessary capital expenditures. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt or obtaining additional equity capital on terms that may be onerous or highly dilutive. Our ability to refinance our indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
Recent regulatory actions may adversely affect the trading price and liquidity of the Notes.
We expect that investors in, and potential purchasers of, the Notes may employ, or seek to employ, an arbitrage strategy with respect to the Notes. Investors that employ an arbitrage strategy with respect to the Notes typically implement that strategy by selling short the common stock underlying the Notes and dynamically adjusting their short position while they hold the Notes. Investors may also implement this hedging strategy by entering into swaps on our common stock in lieu of or in addition to short selling the common stock.
The Securities and Exchange Commission (“SEC”) and other regulatory and self-regulatory authorities have implemented various rules and may adopt additional rules in the future that may impact those engaging in short selling activity involving equity securities (including our common stock), including Rule 201 of SEC regulation SHO, the Financial Industry Regulatory Authority, Inc.’s “Limit Up-Limit Down” program, market-wide circuit breaker systems that halt trading of stock for certain periods
following specific market declines, and rules stemming from the enactment and implementation of the Dodd-Frank Wall Street Reform and Consumer Protection Act. Past regulatory actions, including emergency actions or regulations, have had a significant impact on the trading prices and liquidity of equity-linked instruments. Any governmental action that similarly restricts the ability of investors in, or potential purchasers of, the Notes to effect short sales of our common stock could similarly adversely affect the trading price and the liquidity of the Notes.
Volatility in the market price and trading volume of our common stock could adversely impact the trading price of the Notes.
The stock market in recent years has experienced significant price and volume fluctuations that have often been unrelated to the operating performance of companies. The market price of our common stock could fluctuate significantly for many reasons, including in response to the risks described in this section and elsewhere in this Annual Report on Form 10-K or the documents we have incorporated by reference in this Annual Report on Form 10-K or for reasons unrelated to our operations, such as reports by industry analysts, investor perceptions or negative announcements by our customers, competitors or suppliers regarding their own performance, as well as industry conditions and general financial, economic and political instability. A decrease in the market price of our common stock would likely adversely impact the trading price of the Notes. The market price of our common stock could also be affected by possible sales of our common stock by investors who view the Notes as a more attractive means of equity participation in us and by hedging or arbitrage trading activity that we expect to develop involving our common stock. This trading activity could, in turn, affect the trading prices of the Notes. This may result in greater volatility in the trading price of the Notes than would be expected for non-convertible debt securities.
Subject to certain limitations, we continue to have the ability to incur debt; if we incur substantial additional debt, these higher levels of debt may affect our ability to pay the principal of and interest on the Notes.
Subject to certain limitations, we and our subsidiaries may be able to incur substantial additional debt in the future, some of which may be secured debt. The indenture governing the Notes does not restrict our ability to incur additional subordinated indebtedness or require us to maintain financial ratios or specified levels of net worth or liquidity. If we incur substantial additional indebtedness in the future, these higher levels of indebtedness may affect our ability to pay the principal of and interest on the Notes, or any fundamental change purchase price, and our creditworthiness generally.
We may not have the ability to raise the funds necessary to purchase the Notes as required upon a fundamental change, and our future debt may contain limitations on our ability to purchase the Notes.
Following a fundamental change as defined, holders of Notes will have the right to require us to purchase their Notes for cash. A fundamental change may also constitute an event of default or prepayment under, and result in the acceleration of the maturity of, our then-existing indebtedness. We cannot assure noteholders that we will have sufficient financial resources, or will be able to arrange financing, to pay the fundamental change purchase price in cash with respect to any Notes surrendered by holders for purchase upon a fundamental change. In addition, restrictions in our then existing credit facilities or other indebtedness, if any, may not allow us to purchase the Notes upon a fundamental change. Our failure to purchase the Notes upon a fundamental change when required would result in an event of default with respect to the Notes which could, in turn, constitute a default under the terms of our other indebtedness, if any. If the repayment of the related indebtedness were to be accelerated after any applicable notice or grace periods, we may not have sufficient funds to repay the indebtedness and purchase the Notes.
Some significant restructuring transactions may not constitute a fundamental change, in which case we would not be obligated to offer to purchase the Notes.
Upon the occurrence of a fundamental change as defined, noteholders have the right to require us to purchase their Notes. However, the fundamental change provisions will not afford protection to holders of Notes in the event of certain transactions that could adversely affect the Notes. For example, transactions such as leveraged recapitalizations, refinancings, restructurings or acquisitions initiated by us would not constitute a fundamental change requiring us to repurchase the Notes. In addition, holders will not be entitled to require us to purchase their Notes upon a significant change in the composition of our board. In the event of any such transaction, holders of the Notes would not have the right to require us to purchase their Notes, even though each of these transactions could increase the amount of our indebtedness, or otherwise adversely affect our capital structure or any credit ratings, thereby adversely affecting holders of the Notes.
Future sales of our common stock in the public market could lower the market price for our common stock and adversely impact the trading price of the Notes.
In the future, we may sell additional shares of our common stock to raise capital. In addition, a substantial number of shares of our common stock are reserved for issuance upon the exercise of stock options and warrants and upon conversion of the Notes. We cannot predict the size of future issuances or the effect, if any, that they may have on the market price for our common stock. The issuance and sale of substantial amounts of common stock, or the perception that such issuances and sales may occur, could adversely affect the trading price of the Notes and the market price of our common stock and impair our ability to raise capital through the sale of additional equity securities.
The Notes may not have an active market, and the price may be volatile, so noteholders may be unable to sell their Notes at the price they desire or at all.
The Notes are a new issue of securities for which there is currently no active trading market. We cannot be certain that a liquid market will develop for the Notes, that noteholders will be able to sell any of the Notes at a particular time (if at all) or that the prices they receive if or when noteholders sell the Notes will be above their initial offering price. In addition, we do not intend to apply to list the Notes on any securities exchange or for inclusion of the Notes on any automated dealer quotation system. The initial purchasers have advised us that they intend to make a market in the Notes, but they are not obligated to do so and may discontinue any market-making in the Notes at any time in their sole discretion and without notice. Future trading prices of the Notes on any market that may develop will depend on many factors, including our operating performance and financial condition, prevailing interest rates, the market for similar securities and general economic conditions.
Moreover, even if noteholders are able to sell their Notes, they may not receive a favorable price for their Notes. Future trading prices of the Notes will depend on many factors, including, among other things, prevailing interest rates, our operating results, the price of our common stock and the market for similar securities. Historically, the market for convertible debt has been subject to disruptions that have caused volatility in prices. It is possible that the market for the Notes will be subject to disruptions that may have a negative effect on the holders of the Notes, regardless of our prospects or financial performance.
Any adverse rating of the Notes may negatively affect the trading price and liquidity of the Notes and the price of our common stock.
We do not intend to seek a rating on the Notes. However, if a rating service were to rate the Notes and if such rating service were to assign the Notes a rating lower than the rating expected by investors or were to lower its rating on the Notes below the rating initially assigned to the Notes or otherwise announce its intention to put the Notes on credit watch, the trading price or liquidity of the Notes and the price of our common stock could decline.
The conversion rate of the Notes may not be adjusted for all dilutive events.
The conversion rate of the Notes is subject to adjustment for certain events, including, but not limited to, the issuance to all or substantially all holders of our common stock of stock dividends, certain rights, options or warrants, capital stock, indebtedness, assets or cash, and subdivisions and combinations of our common stock, and certain issuer tender or exchange offers as defined. However, the conversion rate will not be adjusted for other events, such as a third-party tender or exchange offer or an issuance of common stock for cash (as proposed in this prospectus supplement), that may adversely affect the trading price of the Notes or the common stock. An event that adversely affects the value of the Notes may occur, and that event may not result in an adjustment to the conversion rate.
The Notes are protected by restrictive covenants only to a limited extent.
The indenture governing the Notes does not contain any financial or operating covenants or restrictions on the payment of dividends, the incurrence of indebtedness or the issuance or repurchase of securities by us or any of our subsidiaries. The indenture does not contain covenants or other provisions to afford protection to holders of the Notes in the event of a fundamental change except as defined. We could engage in many types of transactions, such as acquisitions, refinancings or recapitalizations that could substantially affect our capital structure and the value of the Notes and shares of our common stock but may not constitute a fundamental change that permits holders to require us to purchase their Notes. For these reasons, noteholders should not consider the covenants in the indenture or the fundamental change purchase feature of the Notes as significant factors in evaluating whether to invest in the Notes.
The issuance of shares of common stock upon conversions of the Notes will dilute the ownership interest of our existing stockholders, including holders who had previously converted their Notes.
The issuance of shares of common stock upon the conversion of some or all of the Notes will dilute the ownership interests of our existing stockholders. Any sales in the public market of such shares of our common stock could adversely affect prevailing market prices of our common stock. In addition, the existence of the Notes may encourage short selling by market participants because the conversion of the Notes could depress the price of our common stock.
Noteholders are not entitled to any rights with respect to our common stock, but are subject to all changes made with respect to our common stock to the extent noteholders convert their Notes and receive shares of our common stock.
Holders who convert their Notes will not be entitled to any rights with respect to our common stock (including, without limitation, voting rights and rights to receive any dividends or other distributions on our common stock) until the conversion date relating to such Notes, but holders of Notes will be subject to all changes affecting our common stock. For example, if an amendment is proposed to our second amended and restated certificate of incorporation, as amended or our amended and restated by-laws requiring stockholder approval and the record date for determining the stockholders of record entitled to vote on the amendment occurs prior to the conversion date with respect to any Notes surrendered for conversion, then the holder surrendering such Notes will not be entitled to vote on the amendment, although such holder will nevertheless be subject to any changes affecting our common stock.
Upon conversion of the Notes, holders may receive less valuable consideration than expected because the value of our common stock may decline after they exercise their conversion right but before we settle our conversion obligation.
Under the Notes, a converting holder will be exposed to fluctuations in the value of our common stock during the period from the date such holder surrenders Notes for conversion until the date we settle our conversion obligation.
Upon conversion of the Notes, we will be required to deliver the shares of our common stock, together with cash for any fractional share, on the third business day following the relevant conversion date. Accordingly, if the price of our common stock decreases during this period, the value of the shares that you receive will be adversely affected and would be less than the conversion value of the Notes on the conversion date.
The fundamental change purchase feature of the Notes may delay or prevent an otherwise beneficial attempt to take over our company.
The terms of the Notes require us to offer to purchase the Notes for cash in the event of a fundamental change, as defined. A non-stock takeover of our company may trigger the requirement that we purchase the Notes. This feature may have the effect of delaying or preventing a takeover of our company that would otherwise be beneficial to investors.
Risks Related to Our Common Stock
The market price of our common stock may be volatile and adversely affected by several factors.
The market price of our common stock could fluctuate significantly in response to various factors and events, including:
· our ability to integrate operations, technology, products and services;
· our ability to execute our business plan;
· operating results below expectations;
· announcements concerning product development results, including clinical trial results, or intellectual property rights of others;
· litigation or public concern about the safety of our potential products;
· our issuance of additional securities, including debt or equity or a combination thereof, necessary to fund our operating expenses;
· announcements of technological innovations or new products by us or our competitors;
· loss of any strategic relationship;
· industry developments, including, without limitation, changes in healthcare policies or practices or third-party reimbursement policies;
· economic and other external factors effecting U.S. or Global equity markets;
· period-to-period fluctuations in our financial results; and
· whether an active trading market in our common stock develops and is maintained.
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our common stock.
We have not paid cash dividends in the past and do not expect to pay cash dividends in the foreseeable future. Any return on investment in shares of common stock may be limited to the value of our common stock.
We have never paid cash dividends on our common stock and do not anticipate paying cash dividends on our common stock in the foreseeable future. The payment of dividends on our capital stock will depend on our earnings, financial condition and other business and economic factors affecting us at such time as the board of directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on any investment in shares of our common stock will only occur if the common stock price appreciates.
A sale of a substantial number of shares of the common stock may cause the price of our common stock to decline.
If our stockholders sell, or the market perceives that our stockholders intend to sell for various reasons, substantial amounts of our common stock in the public market it may make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
The stock markets have from time to time experienced significant price and volume fluctuations that have affected the market prices for the common stock of biotechnology and biopharmaceutical companies. These broad market fluctuations may cause the market price of our common stock to decline. In the past, securities class action litigation has often been brought against a company following a decline in the market price of our securities. This risk is especially relevant for us because biotechnology and biopharmaceutical companies have experienced significant stock price volatility in recent years. We may become involved in this type of litigation in the future. Litigation often is expensive and diverts management’s attention and resources, which could adversely affect our business.
Our quarterly operating results may fluctuate significantly.
We expect our operating results to be subject to quarterly fluctuations. Our net loss and other operating results will be affected by numerous factors, including:
· variations in the level of expenses related to our commercialization and development programs;
· initiation or completion of clinical trials;
· any intellectual property infringement lawsuit in which we may become involved;
· regulatory developments affecting our product candidates;
· our execution of any collaborative, licensing or similar arrangements, and the timing of payments under these arrangements;
· if plecanatide receives regulatory approval, the level of underlying demand for that product and wholesalers’ buying patterns; and
· interest payments on our Convertible Debt.
If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of our common stock to fluctuate substantially.
Our ability to use our net operating loss carry forwards may be subject to limitation.
Generally, a change of more than 50% in the ownership of a company’s stock, by value, over a three-year period constitutes an ownership change for U.S. federal income tax purposes. An ownership change may limit our ability to use our net operating loss carryforwards attributable to the period prior to the change. As a result, if we earn net taxable income, our ability to use our pre-change net operating loss carryforwards to offset U.S. federal taxable income may become subject to limitations, which could potentially result in increased future tax liability for us. At December 31, 2015, we had net operating loss carryforwards aggregating approximately $384.3 million. We have determined that an ownership change occurred as of April 30, 2003 pursuant to Section 382 of the Internal Revenue Code of 1986, as amended, or the Code. In addition, the shares of our common stock that we issued from July 14, 2008 through July 8, 2010 have resulted in an additional ownership change. As a result of these events, our ability to utilize our operating loss carry forwards is limited.
If we fail to comply with the rules under the Sarbanes-Oxley Act of 2002 related to accounting controls and procedures, or if we discover material weaknesses and deficiencies in our internal control and accounting procedures, our stock price could decline significantly and raising capital could be more difficult.
If we fail to comply with the rules under the Sarbanes-Oxley Act of 2002 related to disclosure controls and procedures, or, if we discover material weaknesses and other deficiencies in our internal control and accounting procedures, our stock price could decline significantly and raising capital could be more difficult. Section 404 of the Sarbanes-Oxley Act requires annual management assessments of the effectiveness of our internal control over financial reporting and a report by our independent auditors addressing these assessments. If material weaknesses or significant deficiencies are discovered or if we otherwise fail to achieve and maintain the adequacy of our internal control, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act. Moreover, effective internal controls are necessary for us to produce reliable financial reports and are important to helping prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock could drop significantly.
Our certificate of incorporation and bylaws and Delaware law may have anti-takeover effects that could discourage, delay or prevent a change in control, which may cause our stock price, and the value of the Notes, to decline.
Our second amended and restated certificate of incorporation, as amended and our amended and restated bylaws and Delaware law could make it more difficult for a third party to acquire us, even if closing such a transaction would be beneficial to our stockholders or holders of the Notes. We are authorized to issue up to 20,000,000 shares of preferred stock. This preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our board of directors without further action by stockholders. The terms of any series of preferred stock may include voting rights (including the right to vote as a series on particular matters), preferences as to dividend, liquidation, conversion and redemption rights and sinking fund provisions. No preferred stock is currently outstanding. The issuance of any preferred stock could materially adversely affect the rights of the holders of our common stock, and therefore, reduce the value of our common stock and the Notes. In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell our assets to, a third party and thereby preserve control by the present management.
Provisions of our second amended and restated certificate of incorporation, as amended and our amended and restated bylaws and Delaware law also could have the effect of discouraging potential acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a stockholder or holder of the Notes might consider favorable. Such provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management. In particular, our second amended and restated certificate of incorporation, as amended and amended and restated bylaws and Delaware law, as applicable, among other things:
· provide the board of directors with the ability to alter the bylaws without stockholder approval;
· place limitations on the removal of directors; and
· provide that vacancies on the board of directors may be filled by a majority of directors in office, although less than a quorum.
We are subject to Section 203 of the Delaware General Corporation Law which, subject to certain exceptions, prohibits “business combinations” between a publicly-held Delaware corporation and an “interested stockholder,” which is generally defined as a stockholder who becomes a beneficial owner of 15% or more of a Delaware corporation’s voting stock for a three-year period following the date that such stockholder became an interested stockholder. These provisions are expected to discourage certain types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of us to first negotiate with our board. These provisions may delay or prevent someone from acquiring or merging with us, which may cause the market price of our common stock and the value of the Notes to decline.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None
Our corporate headquarters is located at 420 Lexington Avenue, New York, NY 10170. On June 30, 2014, we entered into a Lease Amendment of our New York office, (a) adding approximately 1,800 square feet of contiguous office to our existing lease of approximately 6,700 square feet and (b) extending our existing lease for additional three years to March 31, 2022, to be coterminous with our new space. This lease amendment results in total monthly rent of approximately $62,000 on straight line basis, prospectively.
In addition, we lease office space for operations in Wayne, Pennsylvania under a lease through November 30, 2017, at a monthly rate of approximately $10,000.
We also maintain a research and development laboratory and several offices in the Bucks County Biotechnology Center in Doylestown, Pennsylvania under a lease through December 31, 2016, at a monthly rate of approximately $3,700.
Rent expense for the yearended December 31, 2015, 2014 and 2013 totaled approximately $909,000, $651,000 and $575,000, respectively.
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm business.
There are currently no pending legal proceedings to which we or any of our subsidiaries is a party or of which any of its property is the subject that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition or operating results. As far as we are aware, no governmental authority is contemplating any such proceeding.
ITEM 4. MINE SAFETY DISCLOSURES
Not Applicable.
ITEM 5. MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS, AND ISSUER PURCHASES OF EQUITY SECURITES
Market Prices
From August 11, 2008 until February 18, 2011, our common stock was quoted on the Over the Counter Bulletin Board under the symbol “SGYP.OB.” From February 22, 2011 until November 30, 2011 our common stock was traded on the OTC QB under the symbol “SGYP.” Since December 1, 2011 our common stock has been traded on The NASDAQ Capital Market under the symbol “SGYP”. On February 21, 2013 our common stock began trading on The NASDAQ Global Market under the symbol “SGYP”. On January 2, 2014 our common stock began trading on the NASDAQ Global Select Market under the symbol “SGYP”.
The following table shows the reported high and low closing prices per share for our common stock as reported on The NASDAQ Global Market, and NASDAQ Global Select Market during the periods indicated.
|
|
|
High |
|
Low |
| ||
|
Year ended December 31, 2014 |
|
|
|
|
| ||
|
First quarter |
|
$ |
6.51 |
|
$ |
4.78 |
|
|
Second quarter |
|
$ |
5.28 |
|
$ |
3.58 |
|
|
Third quarter |
|
$ |
4.24 |
|
$ |
2.79 |
|
|
Fourth quarter |
|
$ |
3.58 |
|
$ |
2.57 |
|
|
Year ended December 31, 2015 |
|
|
|
|
| ||
|
First quarter |
|
$ |
4.68 |
|
$ |
2.75 |
|
|
Second quarter |
|
$ |
9.52 |
|
$ |
3.35 |
|
|
Third quarter |
|
$ |
9.56 |
|
$ |
5.20 |
|
|
Fourth quarter |
|
$ |
6.66 |
|
$ |
5.62 |
|
Holders of Common Stock
As of February 25, 2016, we had 635 holders of record of our common stock.
Dividends
Historically, we have not declared or paid any cash dividends to the holders of our common stock and we do not expect to pay any such dividends in the foreseeable future as we expect to retain our future earnings for use in the operation and expansion of our business.
On January 28, 2014, our Board of Directors declared a stock dividend of .0986 ContraVir shares for each share of our common stock held as of the record date of February 6, 2014, which was distributed on February 18, 2014.
As of June 30, 2015, we became a large accelerated filer per Rule 12b-2 of the Exchange Act of 1934.
Corporate Performance Graph
The following performance graph and related information shall not be deemed to be “soliciting material” or to be “filed” with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act or the Exchange Act, except to the extent that we specifically incorporate it by reference into such filing.
The following graph compares the performance of our common stock to the NASDAQ Stock Market (U.S.), the NASDAQ Pharmaceutical Index, the Russell 3000 index and the Russell 3000 Biotechnology Index from August 11, 2008 (the first date that shares of our common stock were publicly traded) through December 31, 2015. The comparison assumes $100 was invested after the market closed on August 11, 2008 in our common stock and in each of the foregoing indices, and it assumes reinvestment of dividends, if any.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN
Among the NASDAQ Stock Market (U.S.),
the NASDAQ Pharmaceutical Index, the Russell 3000 Index, Russell 3000 Biotechnology Index,
and Synergy Pharmaceuticals, Inc.
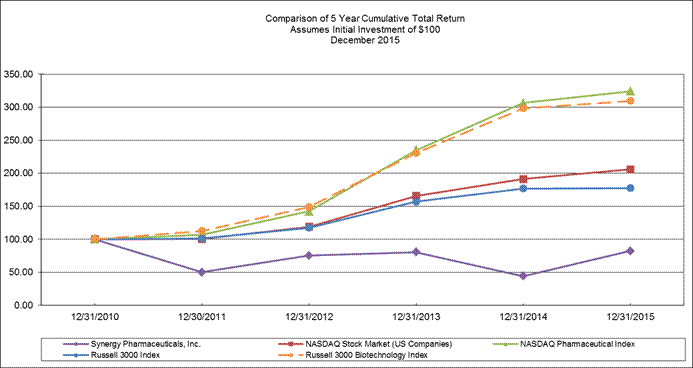
Equity Compensation Information
The following table summarizes information about our equity compensation plans as of December 31, 2015.
|
Plan Category |
|
(a) |
|
Weighted-Average |
|
Number of Options |
| |
|
Equity Compensation Plans Approved by Stockholders |
|
19,193,271 |
|
$ |
4.17 |
|
11,306,729 |
|
|
Equity Compensation Plans Not Approved by Stockholders (1) |
|
1,760,104 |
|
$ |
0.50 |
|
— |
|
|
Total |
|
20,953,375 |
|
|
|
11,306,729 |
| |
(1) Consists of options issued in conjunction with sales of our common stock as well as for consulting and professional services.
On June 8, 2015, our stockholders approved an increase in the number of our common stock shares reserved for issuance under the Plan from 15,000,000 to 30,000,000. As of December 31, 2015, there were 18,869,771 stock options outstanding under the 2008 Equity Compensation Incentive Plan, or Plan, and 323,500 options outstanding under the 2009 Directors Option Plan, or Directors Plan, with 11,130,229 stock options available for future issuance under the Plan and 176,500 stock options available under the Directors Plan.
ITEM 6. SELECTED FINANCIAL DATA
The following table sets forth our selected consolidated financial data and has been derived from our audited consolidated financial statements. Consolidated balance sheets as of December 31, 2015 and 2014, as well as consolidated statements of operations for the years ended December 31, 2015, 2014 and 2013, and the reports thereon are included elsewhere in this Annual Report on Form 10-K. The information below should be read in conjunction with our audited consolidated financial statements and the notes to such statements, included below in Item 8, and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included below in Item 7. Historical results are not necessarily indicative of the results to be expected in the future (in thousands except share and per share data).
|
|
|
Year Ended |
| |||||||||||||
|
|
|
December 31, |
| |||||||||||||
|
|
|
2015 |
|
2014 |
|
2013 |
|
2012 |
|
2011 |
| |||||
|
Revenues |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Costs and Expenses: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Research and development |
|
78,028 |
|
83,274 |
|
50,630 |
|
29,294 |
|
13,419 |
| |||||
|
Purchased in-process research and development |
|
— |
|
— |
|
— |
|
1,000 |
|
— |
| |||||
|
Selling, general and administrative |
|
21,794 |
|
11,004 |
|
11,681 |
|
7,941 |
|
6,745 |
| |||||
|
Loss from Operations |
|
(99,822 |
) |
(94,278 |
) |
(62,311 |
) |
(38,235 |
) |
(20,164 |
) | |||||
|
Other Income /(Loss): |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Amortization of deferred financing costs |
|
(4,566 |
) |
(411 |
) |
— |
|
— |
|
— |
| |||||
|
Interest and investment income/(expense) |
|
(12,718 |
) |
(2,464 |
) |
38 |
|
218 |
|
78 |
| |||||
|
Tax credits |
|
— |
|
83 |
|
— |
|
506 |
|
362 |
| |||||
|
Change in fair value of derivative instruments—warrants |
|
(394 |
) |
1,362 |
|
149 |
|
(1,933 |
) |
5,257 |
| |||||
|
Total Other (Loss)/ Income |
|
(17,678 |
) |
(1,430 |
) |
187 |
|
(1,209 |
) |
5,697 |
| |||||
|
Net loss |
|
$ |
(117,500 |
) |
$ |
(95,708 |
) |
$ |
(62,124 |
) |
$ |
(39,444 |
) |
$ |
(14,467 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Weighted Average Common Shares Outstanding |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Basic and diluted |
|
105,570,960 |
|
94,276,178 |
|
85,220,458 |
|
61,702,277 |
|
47,598,240 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Net Loss per Common Share, Basic and Diluted |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Net loss per common share, basic and diluted |
|
$ |
(1.11 |
) |
$ |
(1.02 |
) |
$ |
(0.73 |
) |
$ |
(0.64 |
) |
$ |
(0.30 |
)(*) |
(*) Weighted average shares outstanding reflects a one for two (1:2) reverse stock split effective on November 30, 2011.
|
|
|
December 31, |
| |||||||||||||
|
|
|
2015 |
|
2014 |
|
2013 |
|
2012 |
|
2011 |
| |||||
|
Consolidated Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Cash and cash equivalents and available-for-sale securities |
|
$ |
111,750 |
|
$ |
196,367 |
|
$ |
68,157 |
|
$ |
32,502 |
|
$ |
13,245 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Working capital |
|
$ |
95,476 |
|
$ |
181,872 |
|
$ |
56,199 |
|
$ |
26,734 |
|
$ |
11,561 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Total assets |
|
$ |
115,929 |
|
$ |
201,008 |
|
$ |
72,558 |
|
$ |
37,405 |
|
$ |
15,870 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Total stockholders’ equity/(deficit) |
|
$ |
(55,213 |
) |
$ |
(5,159 |
) |
$ |
55,348 |
|
$ |
24,832 |
|
$ |
9,797 |
|
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read this discussion together with the Financial Statements, related Notes and other financial information included elsewhere in this Report on Form 10-K. The following discussion contains assumptions, estimates and other forward-looking statements that involve a number of risks and uncertainties, including those discussed under “Risk Factors,” and elsewhere in this Form 10-K. To the extent that this Report contains forward-looking statements regarding the financial condition, operating results, business prospects or any other aspect of our Company, please be advised that our actual financial condition, operating results and business performance may differ materially from that projected or estimated by us in forward-looking statements and thus you should not unduly rely on these statements.
Business Overview
We are a biopharmaceutical company focused on the development and commercialization of novel gastrointestinal (GI) therapies. Our proprietary GI platform is based on uroguanylin and includes two lead product candidates — plecanatide and dolcanatide. Since the company’s inception in 2008, we have pioneered discovery, research and development efforts involving uroguanylin analogs for the treatment of functional gastrointestinal (GI) disorders and inflammatory bowel disease. Plecanatide is our first uroguanylin analog currently being evaluated for use as a once-daily tablet for two functional GI disorders, chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C). Plecanatide is a 16-amino acid peptide that is structurally identical to uroguanylin with the exception of a single amino acid change. Plecanatide is designed to mimic the function of uroguanylin by working locally in the upper GI tract to stimulate digestive fluid movement and support regular bowel function. In 2015, we announced positive phase 3 data in two CIC clinical trials and on January 29, 2016 we filed a new drug application (NDA) with the U.S. Food and Drug Administration (FDA) with plecanatide for CIC. We are continuing to progress two phase 3 clinical trials with plecanatide for IBS-C. We expect top-line results from the first phase 3 IBS-C trial in 1H 2016 and results from the second phase 3 IBS-C trial are expected in 2H 2016. We intend to file our second NDA with plecanatide for IBS-C by year-end 2016. Dolcanatide is our second uroguanylin analog currently being explored for inflammatory bowel disease (IBD). Dolcanatide is designed to be an analog of uroguanylin with enhanced resistance to standard digestive breakdown by proteases in the intestine. In January 2016, we announced positive phase 1b data with dolcanatide in patients with mild-to-moderate ulcerative colitis and we are presently evaluating plans for further clinical development in IBD.
RESULTS OF OPERATIONS
YEARS ENDED DECEMBER 31, 2015 AND DECEMBER 31, 2014
We had no revenues during the year ended December 31, 2015 and 2014 because we do not have any commercial biopharmaceutical products and we do not expect to have such products until 2017 at the earliest.
Research and development expenses for the year ended December 31, 2015 (“Current Year”) decreased by approximately $5.3 million or 6%, to approximately $78 million from approximately $83.3 million for the year ended December 31, 2014 (“Prior Year”). This decrease in research and development expenses was largely attributable to a reduction in clinical trial activities in dolcanatide. The following table sets forth our research and development expenses related to our product candidates for the year ended December 31, 2015 and 2014. Direct expenses are external costs associated with chemistry, manufacturing and controls including costs of drug substance and product formulation, as well as preclinical studies and clinical trial costs.
|
|
|
($ in thousands) |
|
($ in thousands) |
| ||
|
|
|
Year Ended |
|
Year Ended |
| ||
|
Drug candidates |
|
December 31, 2015 |
|
December 31, 2014 |
| ||
|
Plecanatide |
|
$ |
65,170 |
|
$ |
64,871 |
|
|
Dolcanatide |
|
5,026 |
|
10,944 |
| ||
|
Total direct cost |
|
$ |
70,196 |
|
$ |
75,815 |
|
|
Total indirect cost |
|
7,832 |
|
7,459 |
| ||
|
Total Research and Development |
|
$ |
78,028 |
|
$ |
83,274 |
|
Indirect research and development costs are comprised of in-house staff compensation, facilities, depreciation, stock-based compensation and research and development support services are not directly allocated to specific drug candidates. Indirect costs were approximately $7.8 million in the Current Year, as compared to approximately $7.5 million during the Prior Year representing an increase of $0.3 million which were primarily due to higher stock based compensation expenses and scientific consulting fees.
Selling, general and administrative expenses increased approximately $10.8 million or 98%, to approximately $21.8 million for the Current Year from approximately $11.0 million for the Prior Year. These increased expenses were primarily the result of higher employee stock based compensation of approximately $7.3 million for the Current Year, as compared to $2.8 million for the Prior Year, as well as an increase in expenses related to commercial activities including marketing, advertising and sales, of approximately $5.0 million for the Current Year as compared to the Prior Year.
Net loss for the Current Year was approximately $117.5 million as compared to a net loss of approximately $95.7 million incurred for the Prior Year. This increase in our net loss of approximately $21.8 million or 23% was a result of the increases in operating expenses discussed above, plus higher interest expense and amortization of financing costs of approximately $17.3 million for the Current Year, as compared to $2.9 million for the Prior Year, and changes in fair value of derivative instruments-warrants of $0.4 million during the Current Year, as compared to a gain on derivative instruments-warrants of approximately $1.4 million during the Prior Year, offset by a reduction of $5.3 million in Research and development expenses for the Current Year as compared to the Prior Year.
YEARS ENDED DECEMBER 31, 2014 AND DECEMBER 31, 2013
We had no revenues during the year ended December 31, 2014 and 2013 because we do not have any commercial biopharmaceutical products and we do not expect to have such products for several years, if at all.
Research and development expenses for the year ended December 31, 2014 increased approximately $32.7 million or 64%, to approximately $83.3 million from approximately $50.6 million for the year ended December 31, 2013. This increase in research and development expenses was largely attributable to ongoing development of our plecanatide product candidate. The following table sets forth our research and development expenses directly related to our product candidates for the year ended December 31, 2014 and 2013. These direct expenses were external costs associated with chemistry, manufacturing and controls including costs of drug substance and product formulation, as well as preclinical studies and clinical trial costs, as follows:
|
|
|
($ in thousands) |
|
($ in thousands) |
| ||
|
|
|
Year Ended |
|
Year Ended |
| ||
|
Drug candidates |
|
December 31, 2014 |
|
December 31, 2013 |
| ||
|
Plecanatide |
|
$ |
64,871 |
|
$ |
32,422 |
|
|
Dolcanatide |
|
10,944 |
|
12,013 |
| ||
|
Total direct cost |
|
$ |
75,815 |
|
$ |
44,435 |
|
|
Total indirect cost |
|
7,459 |
|
6,195 |
| ||
|
Total Research and Development |
|
$ |
83,274 |
|
$ |
50,630 |
|
Indirect research and development costs related to in-house staff compensation, facilities, depreciation, stock-based compensation and research and development support services are not directly allocated to specific drug candidates. Indirect costs were approximately $7.5 million for the year ended December 31, 2014, as compared to approximately $6.2 million for the year ended December 31, 2013 primarily due to higher stock based compensation expenses.
Selling, general and administrative expenses decreased approximately $0.7 million or 5.8 %, to approximately $11 million for the year ended December 31, 2014 from approximately $11.7 million for the year ended December 31, 2013. These decreased expenses were primarily the result of lower
corporate legal of approximately $1.1 million for the year ended December 31, 2014, as compared to $2.0 million for the year ended December 31, 2013, partially offset by higher facility expenses of $2.2 million for the year ended December 31, 2014, as compared to $1.9 million for the year ended December 31, 2013.
Net loss for the year ended December 31, 2014 was approximately $95.7 million as compared to a net loss of approximately $62.1 million incurred for the year ended December 31, 2013. This increase in our net loss of approximately $33.6 million or 54% was a result of the increases in operating expenses discussed above, plus loss from changes in fair value of derivative instruments-warrants of $1.4 million during the year ended December 31, 2014, as compared to a loss on derivative instruments-warrants of approximately $0.2 million during the year ended December 31, 2013.
LIQUIDITY AND CAPITAL RESOURCES
As of December 31, 2015, we had approximately $61.7 million of cash and cash equivalents and approximately $50.1 million in available- for-sale-securities. Net cash used in operating activities was approximately $101 million for the year ended December 31, 2015, as compared to approximately $89.1 million during the year ended December 31, 2014 and $52.6 million during the year ended December 31, 2013. Net cash provided by financing activities for the year ended December 31, 2015 was approximately $16.4 million, as compared to approximately $217 million and $89.2 million provided during the year ended December 31, 2014 and 2013, respectively.
As of December 31, 2015 we had working capital of approximately $95.5 million as compared to working capital of approximately $181.9 million on December 31, 2014.
On November 3, 2014 we announced the closing of a private offering of $200 million aggregate principal amount of 7.50% Convertible Senior Notes due 2019 (including the full exercise of the over-allotment option granted to the initial purchasers to purchase an additional $25 million aggregate principal amount of 7.50% Convertible Senior Notes due 2019). The notes are unsecured, senior obligations and bear interest at a rate of 7.50% per year, payable semiannually in arrears on May 1 and November 1 of each year, beginning on May 1, 2015. The notes will mature on November 1, 2019, unless earlier purchased or converted. The holders of the notes have the ability to require us to repurchase the notes in whole or in part for cash in the event of a fundamental change, as defined in debenture agreement. In such case, the repurchase price would generally be 100% of the principal amount of the notes plus any accrued and unpaid interest. The notes are convertible, at any time, into shares of our common stock at an initial conversion rate of 321.5434 shares per $1,000 principal amount of notes, which is equivalent to an initial conversion price of $3.11 per share. The net proceeds from this offering were approximately $187.3 million, after deducting estimated expenses and the initial purchasers’ discount.
On March 5, 2014, we entered into Amendment No. 1 (the “Amendment”) to our Controlled Equity Offering Sales (“ATM”) Agreement, dated June 21, 2012 (as amended, the “Agreement”), with Cantor Fitzgerald & Co., as sales agent (“Cantor”), pursuant to which the we may offer and sell, from time to time, through Cantor shares of our common stock, par value $0.0001 per share (the “Shares”), up to an additional aggregate offering price of $50.0 million. We will pay Cantor a selling agent fee of up to 3.0% of the gross sales price per share sold and has agreed to provide Cantor with customary indemnification and contribution rights. As of July 10, 2015, the Form S-3 registration statement related to the Agreement expired, effectively terminating our ATM program.
From January 1, 2014 through December 31, 2014, we sold 6,417,650 shares of common stock, pursuant to the ATM Agreement with Cantor, yielding gross proceeds of $30.7 million, at an average selling price of $4.78 per share. Selling agent fees related to above financings from January 1, 2014 through December 31, 2014 were $0.8 million.
From January 1, 2015 through December 31, 2015, we sold 3,435,998 shares of common stock, pursuant to the ATM Agreement with Cantor, yielding gross proceeds of $14.7 million, at an average selling price of $4.27 per share. Selling agent fees related to above financings from January 1, 2015 through December 31, 2015 were $0.4 million.
From January 1, 2015 through December 31, 2015, $41.0 million aggregate principal amount of the Notes was converted into approximately 13.2 million shares of our common stock.
On June 8, 2015, we amended its Articles of Incorporation and increased the number of shares of its common stock authorized for issuance from 200,000,000 to 350,000,000 shares.
On July 2, 2015, we filed a “shelf” registration statement on Form S-3 to offer and sell, from time to time in one or more offerings, any combination of common stock, preferred stock, debt securities, warrants to purchase common stock, preferred stock or debt securities, or any combination of the foregoing, either individually or as units comprised of one or more of the other securities,
having an aggregate initial offering price not exceeding $250,000,000. The registration statement was declared effective by the SEC on July 15, 2015. This shelf registration does not currently encompass a Controlled Equity Sales (ATM) program.
From January 1, 2015 through December 31, 2015 warrants to purchase 189,412 shares of common stock were exercised, yielding proceeds to us of $1.0 million. In addition employee stock options to purchase 269,720 shares of common stock were exercised yielding proceeds of $1.1 million.
Our working capital requirements will depend upon numerous factors including but not limited to the nature, cost and timing of pharmaceutical commercialization efforts along with research and development programs. We will be required to raise additional capital within the next year to complete the development and commercialization of current product candidates and to continue to fund operations at our current cash expenditure levels. To date, our sources of cash have been primarily limited to the sale of equity and debt securities. We cannot be certain that additional funding will be available on acceptable terms, or at all. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants that impact our ability to conduct business. If we are unable to raise additional capital when required or on acceptable terms, we may have to (i) significantly delay, scale back or discontinue the development and/or commercialization of one or more of product candidates; (ii) seek collaborators for product candidates at an earlier stage than otherwise would be desirable and on terms that are less favorable than might otherwise be available; or (iii) relinquish or otherwise dispose of rights to technologies, product candidates or products that we would otherwise seek to develop or commercialize ourselves on unfavorable terms.
Our consolidated financial statements as of December 31, 2015 have been prepared under the assumption that we will continue as a going concern for the next twelve months. Our independent registered public accounting firm has issued a report that includes an explanatory paragraph referring to our recurring and continuing losses from operations and expressing substantial doubt in our ability to continue as a going concern without additional capital becoming available. Our ability to continue as a going concern is dependent upon our ability to obtain additional equity or debt financing, attain further operating efficiencies, reduce expenditures, and, ultimately, to generate revenue. Our consolidated financial statements as of December 31, 2015 did not include any adjustments that might result from the outcome of this uncertainty.
CONTRACTUAL OBLIGATIONS AND COMMITMENTS
The following table is a summary of contractual obligations for the periods indicated that existed as of December 31, 2015, and is based on information appearing in the notes to Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K.
|
|
|
|
|
Less than |
|
|
|
|
|
More than |
| |||||
|
($ in thousands) |
|
Total |
|
1 Year |
|
1-3 Years |
|
3-5 Years |
|
5 years |
| |||||
|
Long Term debt Obligations (1) |
|
$ |
206,700 |
|
$ |
11,925 |
|
$ |
23,850 |
|
$ |
170,925 |
|
$ |
— |
|
|
Operating leases |
|
6,936 |
|
1,038 |
|
2,061 |
|
2,309 |
|
1,528 |
| |||||
|
Purchase obligations—principally employment and consulting services(2) |
|
5,104 |
|
3,192 |
|
1,912 |
|
— |
|
— |
| |||||
|
Purchase Obligations—Major Vendors(3) |
|
108,902 |
|
91,400 |
|
17,502 |
|
— |
|
— |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Total obligations |
|
$ |
327,642 |
|
$ |
107,555 |
|
$ |
45,325 |
|
$ |
173,234 |
|
$ |
1,528 |
|
(1) Represents Senior Convertible Notes, including interest. See Note 4 to our Consolidated Financial Statements.
(2) Represents salary, bonus, and benefits for employment and consulting agreements with remaining terms greater than one year.
(3) Represents amounts that will become due upon future delivery of supplies, drug substance and test results from various suppliers, under open purchase orders as of December 31, 2015.
OFF-BALANCE SHEET ARRANGEMENTS
We had no off-balance sheet arrangements as of December 31, 2015.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Financial Reporting Release No. 60 requires all companies to include a discussion of critical accounting policies or methods used in the preparation of financial statements. Our accounting policies are described in Item 8. Financial Statements—Note 2 Basis of Presentation and Accounting Policies. The preparation of financial statements in conformity with U.S. GAAP requires
management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates. We believe that the following discussion represents our critical accounting policies.
Financial Instruments - Cash, Cash Equivalents and Marketable Securities
All highly liquid investments with maturities of three months or less at the date of purchase are classified as cash equivalents. Our marketable securities consist solely of investments in US Treasury and Government Agency Notes and have been classified and accounted for as available-for-sale. Management determines the appropriate classification of our investments at the time of purchase and reevaluates the available-for-sale designations as of each balance sheet date. Cash equivalents and marketable securities are carried at amounts that approximate fair value due to their short-term maturities. We consider the declines in market value of our marketable securities investment portfolio to be temporary in nature. Fair values were determined for each individual security in the investment portfolio. When evaluating the investments for other-than-temporary impairment, we reviews factors such as the length of time and extent to which fair value has been below cost basis, the financial condition of the issuer and any changes thereto, and our intent to sell, or whether it is more likely than not we will be required to sell, the investment before recovery of the investment’s amortized cost basis. For the year ended December 31, 2015 and 2014, we did not consider any of our investments to be other-than-temporarily impaired.
Research and Development
We do not currently have any commercial biopharmaceutical products and therefore our research and development costs are expensed as incurred. These include expenditures in connection with an in-house research and development laboratory, salaries and staff costs, application and filing for regulatory approval of proposed products, regulatory and scientific consulting fees, as well as contract research, patient costs, drug formulation and tableting, data collection, monitoring, and clinical trial insurance. While certain of our research and development costs may have future benefits, our policy of expensing all research and development expenditures is predicated on the fact that we have no history of successful commercialization of biopharmaceutical products to base any estimate of the number of future periods that would be benefited.
In June 2007, the EITF of the FASB reached a consensus on ASC Topic 730, Research and Development (“ASC Topic 730”). This guidance requires that non-refundable advance payments for goods or services that will be used or rendered for future research and development activities should be deferred and capitalized. As the related goods are delivered or the services are performed, or when the goods or services are no longer expected to be provided, the deferred amounts are recognized as an expense. We adopted ASC Topic 730 on January 1, 2008 and the adoption did not have a material effect on our consolidated financial position, results of operations or cash flows. As of December 31, 2015 and 2014 we had $3.1 million and $3.6 million respectively, of such deferred amounts, which are included in Prepaid expenses and other current assets on our consolidated balance sheets.
Share-Based Compensation
We rely heavily on incentive compensation in the form of stock options to recruit, retain and motivate directors, executive officers, employees and consultants. Incentive compensation in the form of stock options and restricted stock units is designed to provide long-term incentives, develop and maintain an ownership stake and conserve cash during our development stage.
ASC Topic 718 “Compensation—Stock Compensation” requires companies to measure the cost of employee services received in exchange for the award of equity instruments based on the estimated fair value of the award at the date of grant. The expense is to be recognized over the period during which an employee is required to provide services in exchange for the award.
Share-based compensation is recognized as an expense in the financial statements based on the grant date fair value. Upon adoption of ASC Topic 718 “Compensation—Stock Compensation”, we selected the Black-Scholes option pricing model as the most appropriate model for determining the estimated fair value for stock-based awards. Use of a valuation model requires management to make certain assumptions with respect to selected model inputs. Expected volatility is based on the historical volatility of our stock. The risk-free interest rate is based on observed interest rate appropriate for the expected term of our employee stock options. Forfeiture rates and option term are estimated based on our historical experience plus management’s judgment, at the time of grant.
Fair value of financial instruments
In accordance with Accounting Standards Codification (“ASC”) Subtopic 820-10, we measure certain assets and liabilities at fair value on a recurring basis using the three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The three tiers include:
· Level 1, defined as observable inputs such as quoted prices for identical assets in active markets;
· Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable; and
· Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring management to develop its own assumptions based on best estimates of what market participants would use in pricing an asset or liability at the reporting date.
Financial instruments consist of cash and cash equivalents, marketable securities, accounts payable and derivative instruments. These financial instruments are stated at their respective historical carrying amounts, which approximate fair value due to their short term nature, except for derivative instruments which are marked to market at the end of each reporting period.
The Senior Convertible Notes are stated at their carrying value at December 31, 2015 and 2014. Carrying value approximates fair value because the Company believes it could obtain borrowings at December 31, 2015 at comparable interest rates as these November 2014 Senior Notes.
Warrants
We have issued common stock warrants in connection with the execution of certain equity financings. The fair value of certain warrants, deemed to be derivative instruments, is recorded as a derivative liability under the provisions of FASB ASC 815 Derivatives and Hedging (“ASC 815”) upon issuance. Subsequently the liability is adjusted to fair value as of each reporting period and the changes in fair value of derivative liabilities are recorded in the consolidated statement of operations under the caption “Change in fair value of derivative liabilities.”
The fair value of warrants deemed to be derivative instruments is determined using the Black-Scholes or Binomial option-pricing models using varying assumptions regarding volatility of our common share price, remaining life of the warrant, and risk-free interest rates at each period end. We thus use model-derived valuations where significant value drivers are unobservable to third parties to determine the fair value and accordingly classify such warrants in Level 3 per ASC 820. At December 31, 2015 and 2014 the fair value of such warrants was approximately $322,000 and $172,000, respectively, which we classified as a long term derivative liability on our balance sheets.
As of December 31, 2015 and 2014 our available-for-sale securities are classified as Level 1 per ASC 820.
RECENT ACCOUNTING PRONOUNCEMENTS
In January 2016, the FASB issued ASU No. 2016-01, Financial Instruments - Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities. The updated guidance enhances the reporting model for financial instruments, and requires entities to use the exit price notion when measuring the fair value of financial instruments for disclosure purposes, and the separate presentation of financial assets and financial liabilities by measurement category and form of financial asset (i.e., securities or loans and receivables) on the balance sheet or the accompanying notes to the financial statements. The guidance is effective for annual and interim reporting periods beginning after December 15, 2017. We are currently evaluating the effect the guidance will have on the Consolidated Financial Statements.
In November 2015, the FASB issued ASU No. 2015-17, Balance Sheet Classification of Deferred Taxes (“ASU 2015-17”), which requires entities to present deferred tax assets and deferred tax liabilities as noncurrent in a classified balance sheet. The ASU simplifies the current guidance in ASC Topic 740, Income Taxes, which requires entities to separately present deferred tax assets and liabilities as current and noncurrent in a classified balance sheet. ASU 2015-17 is effective for fiscal years beginning after December 15, 2016, and interim periods within those annual periods. Early adoption is permitted for all entities as of the beginning of an interim or annual reporting period. We expect that this guidance will have no effect on the Consolidated Financial Statements.
In April 2015, the FASB issued ASU No. 2015-03, Simplifying the Presentation of Debt Issuance Costs, which changes the presentation of debt issuance costs in the financial statements. Under the standard, debt issuance costs are presented in the balance sheet as a direct deduction from the related debt liability rather than as an asset. In addition amortization of debt issuance costs are to be combined with interest expense in the statement of operations. The guidance is effective for annual and interim reporting periods
beginning after December 15, 2015, with early adoption permitted. We adopted this guidance during the quarter ended June 30, 2015. (See Note 5 to the financial statements.)
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Risk
Our cash, cash equivalents and available for sale securities primarily consist of securities issued by the U.S. government, deposits, and money market mutual funds. The goals of our investment policy are preservation of capital, fulfillment of liquidity needs and fiduciary control of cash and investments. We also seek to maximize income from our investments without assuming significant risk.
Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of interest rates, particularly because our investments are in short-term money marketable funds and US treasury and U.S. government sponsored entity securities. Due to the short-term maturities of our investment portfolio and the relatively low risk profile of our investments, we do not believe a sudden change in interest rates would have a material effect on the fair market value of our portfolio, nor our operating results or cash flows.
We do not believe our cash, cash equivalents investments, and available for sale securities have significant risk of default or illiquidity, however, we maintain significant amounts of cash and cash equivalents at one or more financial institutions that are in excess of federally insured limits. Given the current instability of financial institutions, we cannot provide assurance that we will not experience losses on these deposits.
Foreign Currency Risk
We have no operations outside the U.S. and do not hold any foreign currency denominated financial instruments.
Effects of Inflation
We do not believe that inflation and changing prices during the years ended December 31, 2015, 2014 and 2013 had a significant impact on our results of operations.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The full text of our audited consolidated financial statements as of December 31, 2015 and 2014 and for the years ended December 31, 2015, 2014 and 2013 begins on page F-1 of this Annual Report on Form 10-K.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
N/A
ITEM 9A. CONTROLS AND PROCEDURES
a) Disclosure Controls and Procedures
Our chief executive officer and principal financial officer evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2015. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Securities Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Securities Exchange Act is accumulated and communicated to the company’s management, including our principal executive and principal financial officer, as appropriate to
allow timely decisions regarding required disclosure. Based on that evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures are effective, at the reasonable assurance level, in ensuring that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms.
b) Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting for our company. Internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) promulgated under the Exchange Act, as a process designed by, or under the supervision of, a company’s principal executive and principal financial officer and effected by the our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
(1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company;
(2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made in accordance with authorizations of management and directors of the company; and
(3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible enhancements to controls and procedures.
We conducted an evaluation of the effectiveness of internal control over financial reporting based on the framework in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our principal executive officer and principal financial officer conclude that, at December 31, 2015, our internal control over financial reporting was effective.
The effectiveness of our internal control over financial reporting at December 31, 2015 has been audited by BDO USA, LLP, an independent registered public accounting firm, as stated in their report which appears herein.
CHANGES IN INTERNAL CONTROL OVER FINANCIAL REPORTING
As required by Rule 13a-15(d) of the Exchange Act, our management, including our principal executive officer and our principal financial officer conducted an evaluation of the internal control over financial reporting to determine whether any changes occurred during the quarter ended December 31, 2015 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. Based on that evaluation, our principal executive officer and principal financial officer concluded there were no such changes during the quarter ended December 31, 2015.
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Synergy Pharmaceuticals Inc.
New York, New York
We have audited Synergy Pharmaceuticals Inc. and Subsidiaries’ (the “Company”) internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Synergy Pharmaceuticals Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Item 9A, Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Synergy Pharmaceuticals Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on the COSO criteria .
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Synergy Pharmaceuticals Inc. as of December 31, 2015 and 2014, and the related consolidated statements of operations, changes in stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2015 and our report dated February 25, 2016 expressed an unqualified opinion thereon.
/s/ BDO USA, LLP
New York, New York
February 25, 2016
None.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
We have adopted a formal Code of Business Conduct and Ethics applicable to all Board members, executive officers and employees. A copy of that code is available on our corporate website at http://www.synergypharma.com. A copy of our Code of Business Conduct and Ethics will also be provided free of charge upon request to: Secretary, Synergy Pharmaceuticals Inc. 420 Lexington Avenue, Suite 2012, New York, NY 10170. The content on our website is not incorporated by reference into this Annual Report on Form 10-K.
Information required by this item is incorporated by reference from our proxy statement for our 2016 Annual Meeting of Stockholders.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item is incorporated by reference from our proxy statement for our 2016 Annual Meeting of Stockholders.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item is incorporated by reference from our proxy statement for our 2016 Annual Meeting of Stockholders.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this item is incorporated by reference from our proxy statement for our 2016 Annual Meeting of Stockholders.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this item is incorporated by reference from our proxy statement for our 2016 Annual Meeting of Stockholders.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) List of Documents Filed as a Part of This Report:
(b) Index to Financial Statement Schedules:
All schedules have been omitted because the required information is included in the consolidated financial statements or the notes thereto, or is not applicable or required.
(c) Index to Exhibits
The Exhibits listed below are identified by numbers corresponding to the Exhibit Table of Item 601 of Regulation S-K. The Exhibits designated by an asterisk (*) are management contracts or compensatory plans or arrangements required to be filed pursuant to Item 15.
|
Exhibit No. |
|
Description |
|
1.2 |
|
Controlled Equity Offering Sales Agreement dated June 21, 2012 between Synergy Pharmaceuticals Inc. and Cantor Fitzgerald & Co. (incorporated by reference to Exhibit 1.2 to Form S-3 filed June 21, 2012). |
|
|
|
|
|
1.3 |
|
Amendment No. 1 to Controlled Equity Offering SM Sales Agreement dated June 21, 2012 with Cantor Fitzgerald & Co., as sales agent (incorporated by reference to Exhibit 10.1 to Form 8-K filed March 6, 2014). |
|
|
|
|
|
3.1 |
|
Second Amended and Restated Certificate of Incorporation of Synergy Pharmaceuticals Inc. (incorporated by reference to Exhibit 3.1 to Form 8-K filed June 19, 2015). |
|
|
|
|
|
3.2 |
|
Amendment to the Second Amended and Restated Certificate of Incorporation of Synergy Pharmaceuticals Inc. (incorporated by reference to Exhibit 3.1 to Form 8-K filed January 17, 2013). |
|
|
|
|
|
3.3 |
|
Second Amendment to the Second Amended and Restated Certificate of Incorporation of Synergy Pharmaceuticals Inc. (incorporated by reference to Exhibit 3.1 to Form 10-K filed March 15, 2012). |
|
|
|
|
|
3.4 |
|
Amended and Restated Bylaws (incorporated by reference to Exhibit 3.2 to Form 10-K filed March 15, 2012). |
|
|
|
|
|
4.1 |
|
2008 Equity Compensation Incentive Plan (incorporated by reference to Exhibit 4.1 to Form 8-K filed July 18, 2008)* |
|
|
|
|
|
4.2 |
|
2009 Directors Stock Option Plan (incorporated by reference to Exhibit 4.2 to Form 10-K filed March 15, 2010)* |
|
|
|
|
|
4.3 |
|
Form of Stock Certificate of the Registrant (incorporated by reference to Exhibit 4.6 to Form S-3 filed November 24, 2009). |
|
|
|
|
|
4.4 |
|
Form of Warrant in connection with June 30, 2010 financing (incorporated by reference to Exhibit 4.1 to Form 8-K filed July 7, 2010). |
|
|
|
|
|
4.5 |
|
Form of Warrant in connection with October 1, 2010 financing (incorporated by reference to Exhibit 4.1 to |
|
|
|
Form 8-K filed October 5, 2010). |
|
|
|
|
|
4.6 |
|
Form of Warrant in connection with March 4, 2011 financing (incorporated by reference to Exhibit 4.1 to Form 8-K filed March 10, 2011). |
|
|
|
|
|
4.7 |
|
Form of Warrant in connection with October 4, 2011 financing (incorporated by reference to Exhibit 4.1 to Form 8-K filed October 6, 2011). |
|
|
|
|
|
4.8 |
|
Form of Warrant in connection with October 14, 2011 financing (incorporated by reference to Exhibit 4.1 to Form 8-K filed October 14, 2011). |
|
|
|
|
|
4.9 |
|
Form of Warrant in connection with November 17, 2011 financing (incorporated by reference to Exhibit 4.1 to Form 8-K filed November 15, 2011). |
|
|
|
|
|
4.10 |
|
Amended and Restated Synergy Pharmaceuticals, Inc. Warrant Agency Agreement dated as of December 15, 2011 (incorporated by reference to Exhibit 4.1 to Form 8-K filed December 16, 2011). |
|
|
|
|
|
4.11 |
|
Amended and Restated Synergy Pharmaceuticals, Inc. Unit Agency Agreement dated as of December 15, 2011 (incorporated by reference to Exhibit 4.2 to Form 8-K filed December 16, 2011). |
|
|
|
|
|
4.12 |
|
Indenture related to the 7.50% Convertible Senior Notes due 2019, dated as of November 3, 2014, by and between Synergy Pharmaceuticals Inc. and Wells Fargo Bank, National Association, as trustee (incorporated by reference to Exhibit 4.1 to Form 8-K filed November 3, 2014). |
|
|
|
|
|
4.13 |
|
Form of 7.50% Convertible Senior Note due 2019 (incorporated by reference to Exhibit 4.2 to Form 8-K filed November 3, 2014). |
|
|
|
|
|
10.1 |
|
Form of Executive Non-statutory Stock Option Agreement (incorporated by reference to Exhibit 10.4 to Form 8-K filed July 18, 2008)* |
|
|
|
|
|
10.2 |
|
Form of Non-Executive Non-statutory Stock Option Agreement (incorporated by reference to Exhibit 10.5 to Form 8-K filed July 18, 2008)* |
|
|
|
|
|
10.3 |
|
Fourth Amended and Restated Executive Employment Agreement dated as of January 7, 2015 between Synergy Pharmaceuticals, Inc. and Gary S. Jacob (incorporated by reference to Exhibit 10.3 to Form 10-K filed March 16, 2015)* |
|
|
|
|
|
10.4 |
|
Third Amended and Restated Executive Employment Agreement dated as of January 7, 2015 between Synergy Pharmaceuticals, Inc. and Kunwar Shailubhai (incorporated by reference to Exhibit 10.4 to Form 10-K filed March 16, 2015)* |
|
|
|
|
|
10.5 |
|
Master Services Agreement dated July 20, 2010 (incorporated by reference to Exhibit 10.1 to Form 10-Q filed November 9, 2010)** |
|
|
|
|
|
10.6 |
|
Master Services Agreement dated August 5, 2010 (incorporated by reference to Exhibit 10.2 to Form 10-Q filed November 9, 2010)** |
|
|
|
|
|
10.7 |
|
Asset Purchase Agreement dated August 17, 2012 between Synergy Pharmaceuticals Inc. and Bristol-Myers Squibb Company (incorporated by reference to Exhibit 10.7 to Form 10-K filed March 18, 2013)** |
|
|
|
|
|
10.8 |
|
Executive Employment Agreement dated as of November 10, 2015 between Synergy Pharmaceuticals, Inc. and Gary L. Sender* |
|
|
|
|
|
10.9 |
|
Amended and Restated Executive Employment Agreement dated as of July 12, 2013 between Synergy Pharmaceuticals Inc. and Patrick H. Griffin, M.D., FACP (incorporated by reference to Exhibit 10.9 to Form 10-K filed March 16, 2015)* |
|
|
|
|
|
10.10 |
|
Executive Employment Agreement dated as of May 29, 2015 between Synergy Pharmaceuticals Inc. and Troy Hamilton* |
|
|
|
|
|
10.11 |
|
Amendment to Amended and Restated Executive Employment Agreement dated January 22, 2016 by and between Synergy Pharmaceuticals Inc. and Gary S. Jacob* |
|
|
|
|
|
10.12 |
|
Amendment to the Amended and Restated Executive Employment Agreement dated as of January 18, 2016 by and between Synergy Pharmaceuticals Inc. and Patrick H. Griffin, M.D., FACP* |
|
|
|
|
|
10.13 |
|
Amendment to Third Amended and Restated Executive Employment Agreement dated January 18, 2016 by and between Synergy Pharmaceuticals Inc. and Kunwar Shailubhai* |
|
|
|
|
|
10.14 |
|
Amendment to Executive Employment Agreement dated January 18, 2016 by and between Synergy Pharmaceuticals Inc. and Troy Hamilton.* |
|
|
|
|
|
14 |
|
Code of Business Conduct and Ethics (incorporated by reference to Exhibit 14 to Form 10-K filed April 15, 2009) |
|
|
|
|
|
21 |
|
List of Subsidiaries |
|
|
|
|
|
23 |
|
Consent of BDO USA, LLP |
|
|
|
|
|
31.1 |
|
Certification of Chief Executive Officer required under Rule 13a-14(a)/15d-14(a) under the Exchange Act |
|
|
|
|
|
31.2 |
|
Certification of Principal Financial Officer required under Rule 13a-14(a)/15d-14(a) under the Exchange Act |
|
|
|
|
|
32.1 |
|
Certification of Chief Executive Officer pursuant to 18 U.S.C Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
|
|
|
|
|
32.2 |
|
Certification of Principal Financial Officer pursuant to 18 U.S.C Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
|
|
|
|
|
101 |
|
Financial statements from the annual report on Form 10-K of Synergy for the year ended December 31, 2015, filed on February 25, 2016, formatted in Extensible Business Reporting Language (XBRL): (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Operations, (iii) the Consolidated Statement of Stockholders Equity (Deficit) (iv) the Consolidated Statements of Cash Flows and (v) the Notes to Consolidated Financial Statements tagged as blocks of text. |
* Indicates a management contract or compensatory plan or arrangement.
** Portions of this exhibit were omitted and filed separately with the U.S. Securities and Exchange Commission pursuant to a request for confidential treatment.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
SYNERGY PHARMACEUTICALS INC. | |
|
|
(Registrant) | |
|
|
| |
|
Date: February 25, 2016 |
By: |
/s/ GARY S. JACOB |
|
|
|
Gary S. Jacob, |
|
|
|
President, Chairman of the Board, and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ GARY S. JACOB |
|
President, Chairman of the Board, and Chief Executive Officer |
|
February 25, 2016 |
|
Gary S. Jacob |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
/s/ GARY SENDER |
|
Executive Vice President and Chief Financial Officer |
|
February 25, 2016 |
|
Gary Sender |
|
(Principal Financial and Accounting Officer) |
|
|
|
|
|
|
|
|
|
/s/ MELVIN K. SPIGELMAN |
|
Director |
|
February 25, 2016 |
|
Melvin K. Spigelman |
|
|
|
|
|
|
|
|
|
|
|
/s/ ALAN JOSLYN |
|
Director |
|
February 25, 2016 |
|
Alan Joslyn |
|
|
|
|
|
|
|
|
|
|
|
/s/ THOMAS H. ADAMS |
|
Director |
|
February 25, 2016 |
|
Thomas H. Adams |
|
|
|
|
|
|
|
|
|
|
|
/s/ JOHN BRANCACCIO |
|
Director |
|
February 25, 2016 |
|
John Brancaccio |
|
|
|
|
|
|
|
|
|
|
|
/s/ CHRISTOPHER P. MCGUIGAN |
|
Director |
|
February 25, 2016 |
|
Christopher P. McGuigan |
|
|
|
|
|
|
|
|
|
|
|
/s/ TIMOTHY CALLAHAN |
|
Director |
|
February 25, 2016 |
|
Timothy Callahan |
|
|
|
|
|
|
|
|
|
|
|
/s/ RICHARD DALY |
|
Director |
|
February 25, 2016 |
|
Richard Daly |
|
|
|
|
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
SYNERGY PHARMACEUTICALS, INC.
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Synergy Pharmaceuticals Inc.
New York, New York
We have audited the accompanying consolidated balance sheets of Synergy Pharmaceuticals Inc. and Subsidiaries (the “Company”) as of December 31, 2015 and 2014 and the related consolidated statements of operations, changes in stockholders’ equity (deficit) and cash flows for each of the three years in the period ended December 31, 2015. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Synergy Pharmaceuticals Inc. and Subsidiaries at December 31, 2015 and 2014, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2015, in conformity with accounting principles generally accepted in the United States of America.
As described in Note 2, the Company has early adopted the provisions of ASU No. 2015-03, “Simplifying the Presentation of Debt Issuance Costs”.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses from operations and will continue to have large losses in the future that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Synergy Pharmaceuticals Inc.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated February 25, 2016 expressed an unqualified opinion thereon.
/s/ BDO USA, LLP
New York, New York
February 25, 2016
SYNERGY PHARMACEUTICALS INC.
(In thousands, except Share Data)
|
|
|
December 31, 2015 |
|
December 31, 2014 |
| ||
|
ASSETS |
|
|
|
|
| ||
|
Current Assets: |
|
|
|
|
| ||
|
Cash and cash equivalents |
|
$ |
61,653 |
|
$ |
146,470 |
|
|
Available-for-sale securities |
|
50,097 |
|
49,897 |
| ||
|
Prepaid expenses and other current assets |
|
3,305 |
|
3,836 |
| ||
|
Total Current Assets |
|
115,055 |
|
200,203 |
| ||
|
Property and equipment, net |
|
531 |
|
642 |
| ||
|
Security deposits |
|
343 |
|
163 |
| ||
|
Total Assets |
|
$ |
115,929 |
|
$ |
201,008 |
|
|
|
|
|
|
|
| ||
|
LIABILITIES AND STOCKHOLDERS’ DEFICIT |
|
|
|
|
| ||
|
Current Liabilities: |
|
|
|
|
| ||
|
Accounts payable |
|
$ |
13,263 |
|
$ |
13,869 |
|
|
Accrued expenses |
|
4,328 |
|
1,962 |
| ||
|
Interest payable on Senior Convertible Notes |
|
1,988 |
|
2,500 |
| ||
|
Total Current Liabilities |
|
19,579 |
|
18,331 |
| ||
|
Senior Convertible Notes, net of deferred financing costs of $7,769 and $12,336 as of December 31, 2015 and December 31, 2014, respectively |
|
151,241 |
|
187,664 |
| ||
|
Derivative financial instruments, at estimated fair value-warrants |
|
322 |
|
172 |
| ||
|
Total Liabilities |
|
171,142 |
|
206,167 |
| ||
|
|
|
|
|
|
| ||
|
Commitments and contingencies (Note 9) |
|
|
|
|
| ||
|
|
|
|
|
|
| ||
|
Stockholders’ Deficit: |
|
|
|
|
| ||
|
Preferred stock, Authorized 20,000,000 shares and none outstanding, at December 31, 2015 and December 31, 2014 |
|
— |
|
— |
| ||
|
Common stock, par value of $.0001 authorized 350,000,000 shares at December 31, 2015. Issued and outstanding 113,694,606 and 96,609,764 shares at December 31, 2015 and 2014, respectively |
|
11 |
|
11 |
| ||
|
Additional paid-in capital |
|
329,161 |
|
261,715 |
| ||
|
Accumulated deficit |
|
(384,385 |
) |
(266,885 |
) | ||
|
Total Stockholders’ Deficit |
|
(55,213 |
) |
(5,159 |
) | ||
|
Total Liabilities and Stockholders’ Deficit |
|
$ |
115,929 |
|
$ |
201,008 |
|
The accompanying notes are an integral part of these consolidated financial statements.
SYNERGY PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except Share and Per Share Data)
|
|
|
Year Ended |
| |||||||
|
|
|
December 31, |
| |||||||
|
|
|
2015 |
|
2014 |
|
2013 |
| |||
|
Revenues |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
|
|
|
|
|
|
|
| |||
|
Costs and Expenses: |
|
|
|
|
|
|
| |||
|
Research and development |
|
78,028 |
|
83,274 |
|
50,630 |
| |||
|
Selling , general and administrative |
|
21,794 |
|
11,004 |
|
11,681 |
| |||
|
Loss from Operations |
|
(99,822 |
) |
(94,278 |
) |
(62,311 |
) | |||
|
Other Income /(Loss): |
|
|
|
|
|
|
| |||
|
Interest and investment income/(expense), net |
|
(17,284 |
) |
(2,875 |
) |
38 |
| |||
|
Tax credits |
|
— |
|
83 |
|
— |
| |||
|
Change in fair value of derivative instruments—warrants |
|
(394 |
) |
1,362 |
|
149 |
| |||
|
Total Other (Loss)/ Income |
|
(17,678 |
) |
(1,430 |
) |
187 |
| |||
|
Net loss |
|
$ |
(117,500 |
) |
$ |
(95,708 |
) |
$ |
(62,124 |
) |
|
|
|
|
|
|
|
|
| |||
|
Weighted Average Common Shares Outstanding |
|
|
|
|
|
|
| |||
|
Basic and diluted |
|
105,570,960 |
|
94,276,178 |
|
85,220,458 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Net Loss per Common Share, Basic and Diluted |
|
|
|
|
|
|
| |||
|
Net loss per common share, basic and diluted |
|
$ |
(1.11 |
) |
$ |
(1.02 |
) |
$ |
(0.73 |
) |
The accompanying notes are an integral part of these consolidated financial statements.
SYNERGY PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
($ in thousands, except share amount)
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
| |||||
|
|
|
|
|
Common |
|
Additional |
|
|
|
|
|
Stockholders’ |
| |||||
|
|
|
Common |
|
Stock, |
|
Paid in |
|
Deficit |
|
Non-Controlling |
|
Equity |
| |||||
|
|
|
Shares |
|
Par Value |
|
Capital |
|
Accumulated |
|
Interest |
|
(Deficit) |
| |||||
|
Balance, January 1, 2013 |
|
66,621,832 |
|
$ |
7 |
|
$ |
133,878 |
|
$ |
(109,053 |
) |
— |
|
$ |
24,832 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Common stock issued via registered direct offering |
|
17,133,093 |
|
2 |
|
94,732 |
|
— |
|
— |
|
94,734 |
| |||||
|
Fees and expenses related to financing transactions |
|
— |
|
— |
|
(5,623 |
) |
— |
|
— |
|
(5,623 |
) | |||||
|
Cancellation of unregistered shares owned by former controlling shareholder (Callisto) |
|
(22,294,976 |
) |
(2 |
) |
2 |
|
— |
|
— |
|
— |
| |||||
|
Common stock issued to former Callisto shareholders |
|
28,605,379 |
|
3 |
|
(3 |
) |
— |
|
— |
|
— |
| |||||
|
Fair value of warrants reclassified to additional paid in capital |
|
— |
|
— |
|
3,575 |
|
— |
|
— |
|
3,575 |
| |||||
|
Recapitalization of Synergy |
|
— |
|
— |
|
(4,904 |
) |
— |
|
— |
|
(4,904 |
) | |||||
|
Common stock issued for services rendered |
|
55,000 |
|
— |
|
250 |
|
— |
|
— |
|
250 |
| |||||
|
Exercise of stock options |
|
61,787 |
|
— |
|
119 |
|
— |
|
— |
|
119 |
| |||||
|
Stock based compensation expense |
|
— |
|
— |
|
4,489 |
|
— |
|
— |
|
4,489 |
| |||||
|
Net loss for the period |
|
— |
|
— |
|
— |
|
(62,124 |
) |
— |
|
(62,124 |
) | |||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Balance, December 31, 2013 |
|
90,182,115 |
|
10 |
|
226,515 |
|
(171,177 |
) |
— |
|
55,348 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Common stock issued pursuant to a controlled equity “at-the-market” sales agreement |
|
6,417,650 |
|
1 |
|
30,699 |
|
— |
|
— |
|
30,700 |
| |||||
|
Fees and expenses related to controlled equity sales |
|
— |
|
— |
|
(846 |
) |
— |
|
— |
|
(846 |
) | |||||
|
Stock based compensation expense |
|
— |
|
— |
|
4,722 |
|
— |
|
— |
|
4,722 |
| |||||
|
Exercise of stock options |
|
9,999 |
|
— |
|
36 |
|
— |
|
— |
|
36 |
| |||||
|
Private placement of ContraVir common stock |
|
— |
|
— |
|
3,224 |
|
— |
|
— |
|
3,224 |
| |||||
|
Fees and expenses associated with ContraVir Private Placement |
|
— |
|
— |
|
(15 |
) |
— |
|
— |
|
(15 |
) | |||||
|
Fair value of ContraVir warrants issued in connection with private placement |
|
— |
|
— |
|
(880 |
) |
— |
|
— |
|
(880 |
) | |||||
|
Noncontrolling interest of ContraVir |
|
— |
|
— |
|
— |
|
— |
|
(1,622 |
) |
(1,622 |
) | |||||
|
Distribution of ContraVir common stock to Synergy shareholders |
|
— |
|
— |
|
(1,740 |
) |
— |
|
— |
|
(1,740 |
) | |||||
|
Elimination of noncontrolling interest of ContraVir upon distribution |
|
— |
|
— |
|
— |
|
— |
|
1,622 |
|
1,622 |
| |||||
|
Net loss for the period |
|
— |
|
— |
|
— |
|
(95,708 |
) |
— |
|
(95,708 |
) | |||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Balance, December 31, 2014 |
|
96,609,764 |
|
11 |
|
261,715 |
|
(266,885 |
) |
— |
|
(5,159 |
) | |||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Common stock issued pursuant to a controlled equity “at-the-market” sales agreement |
|
3,435,998 |
|
— |
|
14,672 |
|
— |
|
— |
|
14,672 |
| |||||
|
Fees and expenses related to controlled equity sales |
|
— |
|
— |
|
(404 |
) |
— |
|
— |
|
(404 |
) | |||||
|
Common stock issued in connection with exercise of stock options |
|
269,720 |
|
— |
|
1,142 |
|
— |
|
— |
|
1,142 |
| |||||
|
Common stock issued in connection with exercise of warrants |
|
189,412 |
|
— |
|
1,012 |
|
— |
|
— |
|
1,012 |
| |||||
|
Shares issued in connection with conversion of Senior Convertible Debentures |
|
13,179,712 |
|
— |
|
40,989 |
|
— |
|
— |
|
40,989 |
| |||||
|
Change in fair value of warrants due to expiration of certain warrants |
|
— |
|
— |
|
244 |
|
— |
|
— |
|
244 |
| |||||
|
Stock based compensation expense |
|
— |
|
— |
|
9,724 |
|
— |
|
— |
|
9,724 |
| |||||
|
Stock issues in exchange for certain intellectual property |
|
10,000 |
|
— |
|
67 |
|
— |
|
— |
|
67 |
| |||||
|
Net loss for the period |
|
— |
|
— |
|
— |
|
(117,500 |
) |
— |
|
(117,500 |
) | |||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Balance, December 31, 2015 |
|
113,694,606 |
|
$ |
11 |
|
$ |
329,161 |
|
$ |
(384,385 |
) |
$ |
— |
|
$ |
(55,213 |
) |
The accompanying notes are an integral part of these consolidated financial statements.
SYNERGY PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
|
|
Year Ended |
| |||||||
|
|
|
December 31, |
| |||||||
|
|
|
2015 |
|
2014 |
|
2013 |
| |||
|
Cash Flows From Operating Activities: |
|
|
|
|
|
|
| |||
|
Net loss |
|
$ |
(117,500 |
) |
$ |
(95,708 |
) |
$ |
(62,124 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
| |||
|
Depreciation and amortization |
|
163 |
|
120 |
|
56 |
| |||
|
Amortization of deferred financing costs |
|
4,566 |
|
411 |
|
— |
| |||
|
Value of common stock issued for patent license |
|
67 |
|
— |
|
— |
| |||
|
Stock-based compensation expense |
|
9,724 |
|
4,722 |
|
4,614 |
| |||
|
Accretion of discount/premium on available for sale securities |
|
(109 |
) |
130 |
|
59 |
| |||
|
Change in fair value of derivative instruments—warrants |
|
394 |
|
(1,362 |
) |
(149 |
) | |||
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
| |||
|
Security deposit |
|
(180 |
) |
(69 |
) |
— |
| |||
|
Accounts payable and accrued expenses |
|
1,867 |
|
316 |
|
7,084 |
| |||
|
Prepaid expenses and other current assets |
|
531 |
|
(124 |
) |
(2,171 |
) | |||
|
Accrued interest expense on Senior Convertible Debentures |
|
(512 |
) |
2,500 |
|
— |
| |||
|
Total Adjustments |
|
16,511 |
|
6,644 |
|
9,493 |
| |||
|
Net Cash used in Operating Activities |
|
(100,989 |
) |
(89,064 |
) |
(52,631 |
) | |||
|
Cash Flows From Investing Activities: |
|
|
|
|
|
|
| |||
|
Loans to related parties |
|
— |
|
— |
|
(270 |
) | |||
|
Net purchases of available-for-sale securities |
|
(200 |
) |
— |
|
(30,000 |
) | |||
|
Additions to property and equipment |
|
(50 |
) |
(173 |
) |
(615 |
) | |||
|
Repayment on ContraVir loan receivable |
|
— |
|
455 |
|
— |
| |||
|
Net Cash provided by/(used in) Investing Activities |
|
(250 |
) |
282 |
|
(30,885 |
) | |||
|
Cash Flows From Financing Activities: |
|
|
|
|
|
|
| |||
|
Proceeds of sale of common stock |
|
14,672 |
|
30,700 |
|
94,734 |
| |||
|
Proceeds of sale of common stock — ContraVir |
|
— |
|
3,224 |
|
— |
| |||
|
Issuance of Senior Convertible Debentures |
|
— |
|
200,000 |
|
— |
| |||
|
Payment for debt financing costs |
|
— |
|
(12,747 |
) |
— |
| |||
|
Fees and expenses related to sale of common stock |
|
(404 |
) |
(861 |
) |
(5,623 |
) | |||
|
Proceeds from exercise of stock options |
|
1,142 |
|
36 |
|
119 |
| |||
|
Proceeds from exercise of warrants |
|
1,012 |
|
— |
|
— |
| |||
|
Distribution associated with ContraVir Spinoff |
|
— |
|
(3,230 |
) |
— |
| |||
|
Net Cash provided by Financing Activities |
|
16,422 |
|
217,122 |
|
89,230 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Net increase/(decrease) in cash and cash equivalents |
|
(84,817 |
) |
128,340 |
|
5,714 |
| |||
|
Cash and cash equivalents at beginning of period |
|
146,470 |
|
18,130 |
|
12,416 |
| |||
|
Cash and cash equivalents at end of period |
|
$ |
61,653 |
|
$ |
146,470 |
|
$ |
18,130 |
|
|
|
|
|
|
|
|
|
| |||
|
Supplementary disclosure of cash flow information: |
|
|
|
|
|
|
| |||
|
Cash paid for interest on Senior Convertible Debentures |
|
$ |
13,379 |
|
$ |
— |
|
$ |
— |
|
|
Cash paid for taxes |
|
$ |
258 |
|
$ |
55 |
|
$ |
81 |
|
|
Supplementary disclosure of non-cash investing and financing activities: |
|
|
|
|
|
|
| |||
|
Distribution of net assets of ContraVir |
|
$ |
— |
|
$ |
84 |
|
$ |
— |
|
|
Conversion of Senior Convertible Debentures to Synergy Common Stock |
|
$ |
40,989 |
|
$ |
— |
|
$ |
— |
|
The accompanying notes are an integral part of these consolidated financial statements.
SYNERGY PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Business Overview
Synergy Pharmaceuticals Inc. (the “Company” or “Synergy”) is a biopharmaceutical company focused on the development of novel therapies to treat gastrointestinal (GI) diseases and disorders. The Company’s proprietary platform technology is based on the naturally occurring human GI peptide — uroguanylin - a key regulator of normal GI physiology. Synergy has created two unique analogs of uroguanylin - plecanatide and dolcanatide — both designed to mimic uroguanylin’s natural activity and target a variety of GI conditions. Plecanatide is currently in phase 3 clinical development for chronic idiopathic constipation and irritable bowel
syndrome with constipation. Dolcanatide has successfully completed a phase 2 study in patients with opioid-induced constipation and is presently being evaluated for the treatment of ulcerative colitis.
2. Basis of Presentation, Accounting Policies and Going Concern
These consolidated financial statements include Synergy Pharmaceuticals Inc., a Delaware corporation, and subsidiaries: (1) Synergy Advanced Pharmaceuticals, Inc. (2) IgX, Ltd (Ireland—inactive) (henceforth “Synergy”), and (3) ContraVir Pharmaceuticals, Inc. (“ContraVir”) through February 18, 2014. All intercompany balances and transactions have been eliminated.
Net cash used in operating activities was approximately $101 million for the year ended December 31, 2015. As of December 31, 2015, Synergy had approximately $61.7 million of cash and cash equivalents and $50.1 million in available for sale securities. During the year ended December 31, 2015, Synergy incurred net losses from operations of $117.5 million. To date, Synergy’s sources of cash have been primarily limited to the sale of common stock and debt. Net cash provided by financing activities for the year ended December 31, 2015 was approximately $16.4 million. As of December 31, 2015 Synergy had a working capital of approximately $95.5 million.
Synergy will be required to raise additional capital within the next year to continue the development and commercialization of current product candidates and to continue to fund operations at the current cash expenditure levels. Synergy cannot be certain that additional funding will be available on acceptable terms, or at all. To the extent that Synergy raises additional funds by issuing equity securities, Synergy’s stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants that impact Synergy’s ability to conduct business. If Synergy is unable to raise additional capital when required or on acceptable terms, Synergy may have to (i) significantly delay, scale back or discontinue the development and/or commercialization of one or more product candidates; (ii) seek collaborators for product candidates at an earlier stage than otherwise would be desirable and on terms that are less favorable than might otherwise be available; or (iii) relinquish or otherwise dispose of rights to technologies, product candidates or products that Synergy would otherwise seek to develop or commercialize ourselves on unfavorable terms.
Our consolidated financial statements as of December 31, 2015 have been prepared under the assumption that we will continue as a going concern for the next twelve months. Our independent registered public accounting firm has issued a report that includes an explanatory paragraph referring to our recurring and continuing losses from operations and expressing substantial doubt in our ability to continue as a going concern without additional capital becoming available. Our ability to continue as a going concern is dependent upon our ability to obtain additional equity or debt financing, attain further operating efficiencies, reduce expenditures, and, ultimately, to generate revenue. Our consolidated financial statements as of December 31, 2015 did not include any adjustments that might result from the outcome of this uncertainty.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Changes in estimates and assumptions are reflected in reported results in the period in which they become known. Actual results could differ from those estimates.
Cash, Cash Equivalents and Marketable Securities
All highly liquid investments with maturities of three months or less at the date of purchase are classified as cash equivalents. As of December 31, 2015, the amount of cash and cash equivalents was approximately $61.7 million and consists of checking accounts and short-term money market mutual funds. As of December 31, 2014, the amount of cash and cash equivalents was approximately $146.5 million and consisted of checking accounts and short-term money market funds with U.S. commercial banks. At any point in time, the Company’s balance of cash and cash equivalents may exceed federally insured limits.
The Company’s marketable securities as of December 31, 2015 and 2014 consist of approximately $50.1 million and $49.9 million, respectively, in U.S. Treasury and U.S. government sponsored entity securities with maturities of less than one year, and have been classified and accounted for as available-for-sale. Management determines the appropriate classification of its investments at the time of purchase and reevaluates the available-for-sale designations as of each balance sheet date. As of December 31, 2015 and 2014, gross unrealized losses were not material. The Company recognized no net realized gains or losses for the year ended December 31, 2015 and 2014. Fair values were determined for each individual security in the investment portfolio. When evaluating the investments for other-than-temporary impairment, the Company reviews factors such as the length of time and extent to which fair value has been below cost basis, the financial condition of the issuer and any changes thereto, and the Company’s intent to sell, or whether it is more likely than not it will be required to sell, the investment before recovery of the investment’s amortized cost basis. During the year ended December 31, 2015 and 2014, the Company did not recognize any impairment charges.
Derivative Instruments
The Company’s derivative liabilities are related to warrants issued in connection with financing transactions and are therefore not designated as hedging instruments. All derivatives are recorded on the Company’s balance sheet at fair value in accordance with current accounting guidelines for such complex financial instruments. Changes in fair value are recorded in the Company’s statement of operations.
Fair Value of Financial Instruments
In accordance with Accounting Standards Codification (“ASC”) Subtopic 820-10, the Company measures certain assets and liabilities at fair value on a recurring basis using the three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The three tiers include:
· Level 1, defined as observable inputs such as quoted prices for identical assets in active markets;
· Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable; and
· Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring management to develop its own assumptions based on best estimates of what market participants would use in pricing an asset or liability at the reporting date.
Financial instruments consist of cash and cash equivalents, marketable securities, accounts payable and derivative instruments. These financial instruments are stated at their respective historical carrying amounts, which approximate fair value due to their short term nature, except for derivative instruments which are marked to market at the end of each reporting period.
The value of Senior Convertible Notes are stated at their carrying value at December 31, 2015 and 2014. Carrying value approximates fair value because the Company believes it could obtain borrowings at December 31, 2015 at comparable interest rates as these November 2014 Senior Notes, therefore, the carrying value approximates fair value.
Property, equipment and depreciation
Expenditures for additions, renewals and improvements are capitalized at cost. Depreciation is computed on a straight-line method based on the estimated useful lives of the related assets. The estimated useful lives of the major classes of depreciable assets are 2 to 5 years for equipment and furniture and fixtures. Leasehold improvements are depreciated over the remaining useful life of the lease. Expenditures for repairs and maintenance are charged to operations as incurred. Synergy periodically evaluates whether current events or circumstances indicate that the carrying value of its depreciable assets may not be recoverable.
Income Taxes
Income taxes have been determined using the asset and liability approach of accounting for income taxes. Under this approach, deferred taxes represent the future tax consequences expected to occur when the reported amounts of assets and liabilities are recovered or paid. Deferred taxes result from differences between the financial statement and tax bases of Synergy’s assets and liabilities and are adjusted for changes in tax rates and tax laws when changes are enacted. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized. The assessment of whether or not a valuation allowance is required often requires significant judgment.
Contingencies
In the normal course of business, Synergy is subject to loss contingencies, such as legal proceedings and claims arising out of its business, that cover a wide range of matters, including, among others, government investigations, shareholder lawsuits, product and environmental liability, and tax matters. In accordance with FASB ASC Topic 450, Accounting for Contingencies (“ASC Topic 450”), Synergy records accruals for such loss contingencies when it is probable that a liability will be incurred and the amount of loss can be reasonably estimated. Synergy, in accordance with this guidance, does not recognize gain contingencies until realized. For a discussion of contingencies, see Note 8, Commitments and Contingencies below.
Research and Development
Research and development costs include expenditures in connection with an in-house research and development laboratory, salaries and staff costs, application and filing for regulatory approval of proposed products, regulatory and scientific consulting fees, as well as contract research, patient costs, drug formulation and tableting, data collection, monitoring, and clinical trial insurance.
In accordance with FASB ASC Topic 730-10-55, Research and Development, Synergy recorded prepaid research and development costs of approximately $3.1 million and $3.7 million as of December 31, 2015 and December 31, 2014, respectively, of pre-payments for production of drug substance, analytical testing services and clinical trial monitoring for its drug candidates. In accordance with this guidance, Synergy expenses these costs when drug substance is delivered and/or services are performed.
Loss Per Share
Basic and diluted net loss per share is presented in conformity with ASC Topic 260, Earnings per Share, (“ASC Topic 260”) for all periods presented. In accordance with this guidance, basic and diluted net loss per common share was determined by dividing net loss applicable to common stockholders by the weighted-average common shares outstanding during the period. Diluted weighted-average shares are the same as basic weighted-average shares because shares issuable pursuant to the exercise of stock options would have been antidilutive. For the years ended December 31, 2015, 2014 and 2013, the effect of 20,953,375, 16,567,020 and 11,324,049, respectively, outstanding stock options and 4,726,823, 5,647,203 and 5,647,203, respectively, outstanding warrants were excluded from the calculation of diluted loss per share because the effect was antidilutive.
The Senior Convertible Notes carrying value of $159 million is convertible into 51,128,938 shares of common stock at December 31, 2015, the effect of which was excluded from the calculation of diluted loss per share because it was antidilutive. As of December 31, 2014, the carrying value of these notes was $200 million and was convertible into, 64,308,680 shares of common stock, the effect of which was excluded from the calculation of diluted loss per share because it was antidilutive.
Recent Accounting Pronouncements
In January 2016, the FASB issued ASU No. 2016-01, Financial Instruments - Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities. The updated guidance enhances the reporting model for financial instruments, and requires entities to use the exit price notion when measuring the fair value of financial instruments for disclosure purposes, and the separate presentation of financial assets and financial liabilities by measurement category and form of financial asset (i.e., securities or loans and receivables) on the balance sheet or the accompanying notes to the financial statements. The guidance is effective for annual and interim reporting periods beginning after December 15, 2017. The Company is currently evaluating the effect the guidance will have on the Consolidated Financial Statements.
In November 2015, the FASB issued ASU No. 2015-17, Balance Sheet Classification of Deferred Taxes (“ASU 2015-17”), which requires entities to present deferred tax assets and deferred tax liabilities as noncurrent in a classified balance sheet. The ASU simplifies the current guidance in ASC Topic 740, Income Taxes, which requires entities to separately present deferred tax assets and liabilities as current and noncurrent in a classified balance sheet. ASU 2015-17 is effective for fiscal years beginning after December 15, 2016, and interim periods within those annual periods. Early adoption is permitted for all entities as of the beginning of an interim or annual reporting period. The Company expects that this guidance will have no effect on the Consolidated Financial Statements.
In April 2015, the FASB issued ASU No. 2015-03, Simplifying the Presentation of Debt Issuance Costs, which changes the presentation of debt issuance costs in the financial statements. Under the standard, debt issuance costs are presented in the balance sheet as a direct deduction from the related debt liability rather than as an asset. In addition amortization of debt issuance costs are to be combined with interest expense in the statement of operations. The guidance is effective for annual and interim reporting periods beginning after December 15, 2015, with early adoption permitted. The Company adopted this guidance during the quarter ended June 30, 2015. (See Note 5 to the financial statements.)
3. Acquisitions and Stockholders’ Equity (Deficit)
On January 28, 2014, our board of directors approved the distribution of 9,000,000 shares of the issued and outstanding shares of common stock of ContraVir Pharmaceuticals, Inc., our subsidiary (“ContraVir”), on the basis of 0.0986 shares of ContraVir common stock for each share of our common stock held on the record date, February 6, 2014 (the “Distribution”). (See below.)
As a result of the Distribution, an adjustment was made to the exercise price of all outstanding warrants in accordance with their terms and accordingly the exercise price decreased approximately $0.011 per share on the record date. As of December 31, 2015 there were 4,726,823 warrants outstanding with a weighted average exercise price of $5.15 per share pre-Distribution and $5.13 per share as adjusted.
On March 5, 2014, Synergy entered into Amendment No. 1 (the “Amendment”) to its Controlled Equity Offering Sales (“ATM”) Agreement, dated June 21, 2012 (as amended, the “Agreement”), with Cantor Fitzgerald & Co., as sales agent (“Cantor”), pursuant to which the Company may offer and sell, from time to time, through Cantor shares of the Company’s common stock, par value $0.0001 per share (the “Shares”), up to an additional aggregate offering price of $50.0 million. The Company will pay Cantor a selling agent fee of up to 3.0% of the gross sales price per share sold and has agreed to provide Cantor with customary indemnification and contribution rights. As of July 10, 2015, the Form S-3 registration statement related to the Agreement expired, effectively terminating the Company’s ATM program.
From January 1, 2014 through December 31, 2014, Synergy sold 6,417,650 shares of common stock, pursuant to the ATM Agreement with Cantor, yielding gross proceeds of $30.7 million, at an average selling price of $4.78 per share. Selling agent fees related to above financings from January 1, 2014 through December 31, 2014 were $0.8 million.
From January 1, 2015 through December 31, 2015, Synergy sold 3,435,998 shares of common stock, pursuant to the ATM Agreement with Cantor, yielding gross proceeds of $14.7 million, at an average selling price of $4.27 per share. Selling agent fees related to above financings from January 1, 2015 through December 31, 2015 were $0.4 million.
From January 1, 2015 through December 31, 2015, $41.0 million aggregate principal amount of the Notes was converted into approximately 13.2 million shares of Synergy common stock.
On June 8, 2015, Synergy amended its Articles of Incorporation and increased the number of shares of its common stock authorized for issuance from 200,000,000 to 350,000,000 shares.
On July 2, 2015, Synergy filed a “shelf” registration statement on Form S-3 to offer and sell, from time to time in one or more offerings, any combination of common stock, preferred stock, debt securities, warrants to purchase common stock, preferred stock or debt securities, or any combination of the foregoing, either individually or as units comprised of one or more of the other securities, having an aggregate initial offering price not exceeding $250,000,000. The registration statement was declared effective by the SEC on July 15, 2015. This shelf registration does not currently encompass a Controlled Equity Sales (ATM) program.
From January 1, 2015 through December 31, 2015 warrants to purchase 189,412 shares of common stock were exercised, yielding proceeds to the Company of $1.0 million. In addition employee stock options to purchase 269,720 shares of common stock were exercised yielding proceeds of $1.1 million.
ContraVir
Private Placement
On February 4, 2014, Synergy’s wholly owned subsidiary, ContraVir Pharmaceuticals, Inc. (ContraVir) entered into a securities purchase agreement with accredited investors to sell securities and raise gross proceeds of approximately $3.2 million in a private placement and incurred expenses of $15,000 related to this placement. ContraVir sold 9,485,294 units to the investors with each unit consisting of one share of ContraVir’s common stock and one warrant to purchase an additional one half share of ContraVir’s common stock. The purchase price paid by the investors was $0.34 for each unit. The 4.7 million warrants expire after six years and are exercisable at $0.37 per share. Based upon the ContraVir’s analysis of the criteria contained in ASC Topic 815-40, “Derivatives and Hedging—Contracts in Entity’s Own Equity” ContraVir recorded approximately $0.88 million of derivative liability on the warrants issued in connection with this transaction.
Spin-off
On February 18, 2014, Synergy completed the distribution of the ContraVir common stock (its previous wholly-owned subsidiary) to Synergy’s stockholders on a pro rata basis with a stock dividend of .0986 ContraVir shares for each Synergy common stock share held as of the record date of February 6, 2014.
Synergy accounted for this distribution according to FASB ASC Topic 505-60, Spinoffs and reverse spinoffs by eliminating ContraVir’s net assets of approximately $1.7 million, with a corresponding decrease in additional paid in capital and the non-controlling interest of $1.6 million.
Net assets of ContraVir eliminated in connection with this spin-off was as follows:
|
|
|
Balance |
| |
|
($ in thousands) |
|
February 18, 2014 |
| |
|
Assets |
|
|
| |
|
Cash |
|
$ |
3,230 |
|
|
Prepaid expense |
|
6 |
| |
|
Total assets |
|
3,236 |
| |
|
Accounts payable and other liabilities |
|
(107 |
) | |
|
Note Payable to Synergy |
|
(455 |
) | |
|
Due to Synergy |
|
(54 |
) | |
|
Derivative financial instruments, at estimated fair value-warrants |
|
(880 |
) | |
|
|
|
|
| |
|
Total Liabilities |
|
(1,496 |
) | |
|
Net assets |
|
$ |
1,740 |
|
As a result of the ContraVir distribution, an adjustment was made to the exercise price of all our outstanding warrants in accordance with their terms. Accordingly the exercise price decreased approximately $0.011 per share on the record date. As of December 31, 2015, there were 4,726,823 of our non-public warrants outstanding with a weighted average exercise price of $5.15 per share pre-Distribution and $5.13 per share as adjusted.
4. Cash, Cash Equivalents and Available-for-sale Securities
All highly liquid investments with maturities of three months or less at the date of purchase are classified as cash equivalents. As of December 31, 2015 and December 31, 2014, the amount of cash and cash equivalents was $61.7 million and $146.5 million, respectively and consists of checking accounts and short-term money market mutual funds held at U.S. commercial banks. Our bank balances were in excess of the FDIC insurance limit.
The Company’s available-for-sale securities as of December 31, 2015 and December 31, 2014 consist of $50.1 million and $49.9 million, respectively, in U.S. Treasury securities with maturities of less than one year and U.S. government sponsored entity securities, and have been classified and accounted for as available-for-sale. Management determines the appropriate classification of its investments at the time of purchase and reevaluates the available-for-sale designations as of each balance sheet date. As of December 31, 2015 and 2014, there were no unrealized losses on available-for-sale securities.
5. Convertible Senior Notes
On November 3, 2014, Synergy closed a private offering of $200 million aggregate principal amount of 7.50% Convertible Senior Notes due 2019 (including the full exercise of the over-allotment option granted to the initial purchasers to purchase an
additional $25 million aggregate principal amount of 7.50% Convertible Senior Notes due 2019, (the “Notes”), interest payable semiannually in arrears on May 1 and November 1 of each year, beginning on May 1, 2015. The net proceeds from the offering were $187.3 million after deducting the initial purchasers’ discounts and offering expenses.
A summary of quarterly interest activity related to the loan is listed below (dollars in thousands):
|
Interest payable at September 30, 2014 |
|
$ |
— |
|
|
Accrued interest expense from November 3, 2014 to December 31, 2014 |
|
2,500 |
| |
|
Interest payable at January 1, 2015 |
|
2,500 |
| |
|
Accrued interest expense during the 3 months ended March 31, 2015 |
|
3,750 |
| |
|
Interest payable at March 31, 2015 |
|
6,250 |
| |
|
Interest payment at May 1, 2015 |
|
(7,416 |
) | |
|
Accrued interest expense during the 3 months ended June 30, 2015 |
|
3,388 |
| |
|
Interest payable as of June 30, 2015 |
|
2,222 |
| |
|
Accrued interest expense during the 3 months ended September 30, 2015 |
|
2,747 |
| |
|
Interest payable as of September 30, 2015 |
|
4,969 |
| |
|
Interest payment at November 1, 2015 |
|
(5,963 |
) | |
|
Accrued interest expense during the 3 months ended December 31, 2015 |
|
2,982 |
| |
|
Interest payable as of December 31, 2015 |
|
$ |
1,988 |
|
The Notes will mature on November 1, 2019, unless earlier purchased or converted. The Notes are convertible, at any time, into shares of Synergy’s common stock at an initial conversion rate of 321.5434 shares per $1,000 principal amount of notes, which is equivalent to an initial conversion price of $3.11 per share. During the year ended December 31, 2015, $41.0 million aggregate principal amount of the Notes was converted into 13.2 million shares of Synergy common stock. This brings the principal balance of the Notes to $159.0 million at December 31, 2015 as compared to $200.0 million at December 31, 2014. All conversions were noteholder initiated with no inducement or solicitation on the part of the Company. Transaction costs associated with the Notes of $12.7 million have been deferred and are being recognized as expense over the expected term of the Notes, calculated using the effective interest rate method. Amortization expense, including amortization associated with reduction of the principal due to the conversion of the debentures on a prorated basis for the year ended December 31, 2015 was $4.6 million. Amortization expense for the year ended December 31, 2014 was $0.4 million which represented only two months from the time the notes were issued on
November 1, 2014. The remaining transaction costs have been presented as a reduction of the Notes in accordance with the newly adopted Accounting Standards Update (“ASU”) No. 2015-03 “Simplifying the Presentation of Debt Issuance Costs”.
A summary of quarterly activity and balances associated with the Notes and related deferred transaction costs is presented below ($ in thousands):
|
|
|
|
|
|
|
Notes, net of |
| |||
|
|
|
Notes |
|
Transaction |
|
Transaction |
| |||
|
|
|
Balance |
|
Costs |
|
Costs |
| |||
|
Balance at issuance November 1, 2014 |
|
$ |
200,000 |
|
$ |
12,747 |
|
$ |
187,253 |
|
|
Less: amortization two months ended December 31, 2014 |
|
$ |
— |
|
$ |
(411 |
) |
$ |
411 |
|
|
Balance December 31, 2014 |
|
$ |
200,000 |
|
$ |
12,336 |
|
$ |
187,664 |
|
|
|
|
|
|
|
|
|
| |||
|
Less: amortization three months ended March 31, 2015 |
|
$ |
— |
|
$ |
(617 |
) |
$ |
617 |
|
|
Balance March 31, 2015 |
|
$ |
200,000 |
|
$ |
11,719 |
|
$ |
188,281 |
|
|
|
|
|
|
|
|
|
| |||
|
Less: amortization three months ended June 30, 2015 (1) |
|
$ |
— |
|
$ |
(1,899 |
) |
$ |
1,899 |
|
|
Conversions |
|
$ |
(22,213 |
) |
$ |
— |
|
$ |
(22,213 |
) |
|
Balance June 30, 2015 |
|
$ |
177,787 |
|
$ |
9,820 |
|
$ |
167,967 |
|
|
|
|
|
|
|
|
|
| |||
|
Less: amortization three months ended September 30, 2015 (1) |
|
$ |
— |
|
$ |
(1,544 |
) |
$ |
1,544 |
|
|
Conversions |
|
$ |
(18,776 |
) |
$ |
— |
|
$ |
(18,776 |
) |
|
Balance September 30, 2015 |
|
$ |
159,011 |
|
$ |
8,276 |
|
$ |
150,735 |
|
|
|
|
|
|
|
|
|
| |||
|
Less: amortization three months ended December 31, 2015 |
|
$ |
— |
|
$ |
(506 |
) |
$ |
506 |
|
|
Balance December 31, 2015 |
|
$ |
159,011 |
|
$ |
7,770 |
|
$ |
151,241 |
|
(1) Includes accelerated amortization of deferred financing costs attributable to conversions.
6. Accounting for Share-Based Payments
Stock Options
ASC Topic 718 “Compensation—Stock Compensation” requires companies to measure the cost of employee services received in exchange for the award of equity instruments based on the estimated fair value of the award at the date of grant. The expense is to be recognized over the period during which an employee is required to provide services in exchange for the award. Synergy accounts for shares of common stock, stock options and warrants issued to non-employees based on the fair value of the stock, stock option or warrant, if that value is more reliably measurable than the fair value of the consideration or services received. The Company accounts for stock options issued and vesting to non-employees in accordance with ASC Topic 505-50 “Equity -Based Payment to Non-Employees” and accordingly the value of the stock compensation to non-employees is based upon the measurement date as determined at either a) the date at which a performance commitment is reached, or b) at the date at which the necessary performance to earn the equity instruments is complete. Accordingly the fair value of these options is being “marked to market” quarterly until the measurement date is determined.
Synergy adopted the 2008 Equity Compensation Incentive Plan (the “Plan”) during the quarter ended September 30, 2008. Stock options granted under the Plan typically vest after three years of continuous service from the grant date and have a contractual term of ten years. Synergy did not issue stock options prior to the quarter ended September 30, 2008.
On January 17, 2013, Synergy amended its 2008 Equity Compensation Incentive Plan and increased the number of shares of its common stock reserved for issuance under the Plan from 7,500,000 to 15,000,000.
On June 8, 2015, our stockholders approved an increase in the number of our common stock shares reserved for issuance under the Plan from 15,000,000 to 30,000,000. As of December 31, 2015, there were 18,869,771 stock options outstanding under the 2008 Equity Compensation Incentive Plan, or Plan, and 323,500 options outstanding under the 2009 Directors Option Plan, or Directors Plan, with 11,130,229 stock options available for future issuance under the Plan and 176,500 stock options available under the Directors Plan.
Stock-based compensation expense related to Synergy options and restricted stock units have been recognized in operating results as follows:
|
|
|
Year Ended |
| |||||||
|
|
|
December 31, |
| |||||||
|
($ in thousands) |
|
2015 |
|
2014 |
|
2013 |
| |||
|
Included in research and development expense |
|
$ |
2,452 |
|
$ |
1,914 |
|
$ |
1,465 |
|
|
Included in selling, general and administrative |
|
7,272 |
|
2,808 |
|
3,149 |
| |||
|
Total stock-based compensation expense |
|
$ |
9,724 |
|
$ |
4,722 |
|
$ |
4,614 |
|
The unrecognized compensation cost related to non-vested stock options outstanding at December 31, 2015, net of expected forfeitures, was approximately $17.6 million to be recognized over a weighted-average remaining vesting period of approximately 2.17 years. This unrecognized compensation cost does not include amounts related to 2,159,500 shares of stock options which vest only upon a change of control. At December 31, 2014, the unrecognized compensation cost related to non-vested stock options outstanding, net of expected forfeitures, was approximately $9.1 million to be recognized over a weighted-average remaining vesting period of approximately 2 years. This unrecognized compensation cost does not include amounts related to the 2,159,500 shares of stock options which vest only upon a change of control.
The estimated fair value of stock option awards was determined on the date of grant using the Black-Scholes option valuation model with the following weighted-average assumptions during the periods indicated.
|
|
|
Year Ended |
| ||||
|
|
|
December 31, |
| ||||
|
($ in thousands) |
|
2015 |
|
2014 |
|
2013 |
|
|
Risk-free interest rate |
|
1.46%-2.02% |
|
1.78%-2.30% |
|
0.66%-2.75% |
|
|
Dividend yield |
|
— |
|
— |
|
— |
|
|
Expected volatility |
|
50%-80% |
|
52%-60% |
|
60 |
% |
|
Expected term (in years) |
|
6.0 years |
|
6.0 years |
|
6.0 years |
|
Risk-free interest rate —Based on the daily yield curve rates for U.S. Treasury obligations with maturities which correspond to the expected term of the Company’s stock options.
Dividend yield —Synergy has not paid any dividends on common stock since its inception and does not anticipate paying dividends on its common stock in the foreseeable future.
Expected volatility —Based on the historical volatility of Synergy stock.
Expected term —Synergy has had minimal stock options exercised since inception. The expected option term represents the period that stock-based awards are expected to be outstanding based on the simplified method provided in Staff Accounting Bulletin (“SAB”) No. 107, Share-Based Payment , (“SAB No. 107”), which averages an award’s weighted-average vesting period and expected term for “plain vanilla” share options. Under SAB No. 107, options are considered to be “plain vanilla” if they have the following basic characteristics: (i) granted “at-the-money”; (ii) exercisability is conditioned upon service through the vesting date; (iii) termination of service prior to vesting results in forfeiture; (iv) limited exercise period following termination of service; and (v) options are non-transferable and non-hedgeable.
In December 2007, the SEC issued SAB No. 110, Share-Based Payment, (“SAB No. 110”). SAB No. 110 was effective January 1, 2008 and expresses the views of the Staff of the SEC with respect to extending the use of the simplified method, as discussed in SAB No. 107, in developing an estimate of the expected term of “plain vanilla” share options in accordance with ASC Topic 718. The Company will continue to use the simplified method until it has the historical data necessary to provide a reasonable
estimate of expected life in accordance with SAB No. 107, as amended by SAB No. 110. For the expected term, the Company has “plain-vanilla” stock options, and therefore used a simple average of the vesting period and the contractual term for options granted subsequent to January 1, 2006 as permitted by SAB No. 107.
Forfeitures —ASC Topic 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Synergy estimated future unvested option forfeitures based on its historical experience.
The weighted-average fair value per share of all options granted for the years ended December 31, 2015, 2014 and 2013 estimated as of the grant date using the Black-Scholes option valuation model was $3.79, $1.98 and $2.68 per share respectively.
A summary of stock option activity and of changes in stock options outstanding under Synergy’s plans is presented below:
|
|
|
|
|
|
|
Weighted Average |
|
Intrinsic |
|
Weighted Average |
| |||
|
|
|
Number of |
|
Exercise Price |
|
Exercise Price |
|
Value |
|
Remaining |
| |||
|
|
|
Options |
|
Per Share |
|
Per Share |
|
($ in thousands) |
|
Contractual Term |
| |||
|
Balance outstanding, December 31, 2012 |
|
9,734,268 |
|
$ |
0.50-5.20 |
|
$ |
2.75 |
|
$ |
24,482 |
|
8.10 years |
|
|
Granted |
|
2,545,965 |
|
$ |
0.44 -20.01 |
|
$ |
6.41 |
|
— |
|
|
| |
|
Exercised |
|
(61,787 |
) |
$ |
0.50-4.28 |
|
$ |
1.91 |
|
$ |
221 |
|
|
|
|
Forfeited |
|
(894,397 |
) |
$ |
4.42-13.90 |
|
$ |
6.05 |
|
— |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |||
|
Balance outstanding, December 31, 2013 |
|
11,324,049 |
|
$ |
0.44-20.01 |
|
$ |
3.31 |
|
$ |
37,521 |
|
6.94 years |
|
|
Granted |
|
5,528,000 |
|
$ |
2.83-4.24 |
|
$ |
3.47 |
|
— |
|
|
| |
|
Exercised |
|
(9,999 |
) |
$ |
3.40-3.95 |
|
$ |
3.58 |
|
$ |
25 |
|
|
|
|
Forfeited |
|
(275,030 |
) |
$ |
4.24-20.01 |
|
$ |
13.42 |
|
— |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |||
|
Balance outstanding, December 31, 2014 |
|
16,567,020 |
|
$ |
0.44-17.79 |
|
$ |
3.20 |
|
$ |
8,949 |
|
7.29 years |
|
|
Granted |
|
4,961,112 |
|
$ |
2.94-9.33 |
|
$ |
6.51 |
|
— |
|
|
| |
|
Exercised |
|
(269,720 |
) |
$ |
2.98-6.28 |
|
$ |
4.24 |
|
$ |
904 |
|
|
|
|
Forfeited |
|
(305,037 |
) |
$ |
2.98-9.45 |
|
$ |
6.22 |
|
— |
|
|
| |
|
Balance outstanding, December 31, 2015 (1) |
|
20,953,375 |
|
$ |
0.44-9.12 |
|
$ |
3.86 |
|
$ |
42,438 |
|
7.15 years |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||
|
Exercisable at December 31, 2015 |
|
9,858,717 |
|
$ |
0.44-7.91 |
|
$ |
3.28 |
|
$ |
23,668 |
|
6.02 years |
|
(1) The number of options represented above includes 2,159,500 options with vesting contingent upon change of control granted on November 20, 2009. The fair value at the date of grant was approximately $28,564,103 determined using the Black-Scholes option valuation model assumptions discussed above. No stock based compensation expense associated with these options was recognized since the grant date.
ASC Topic 718 requires that cash flows resulting from tax deductions in excess of the cumulative compensation cost recognized for options exercised (excess tax benefits) be classified as cash inflows from financing activities and cash outflows from operating activities. Due to Synergy’s accumulated deficit position, no excess tax benefits have been recognized. Synergy accounts for common stock, stock options, and warrants granted to employees and non-employees based on the fair market value of the instrument, using the Black-Scholes option pricing model based on assumptions for expected stock price volatility, term of the option, risk-free interest rate and expected dividend yield, at the grant date.
7. Income Taxes
At December 31, 2015, Synergy has net operating loss carry forwards (“NOLs”) aggregating approximately $384.3 million, which, if not used, expire beginning in 2018 through 2035. The utilization of these NOLs is subject to limitations based on past and future changes in ownership of Synergy pursuant to Internal Revenue Code Section 382. The Company has determined that ownership changes have occurred for Internal Revenue Code Section 382 purposes and therefore, the ability of the Company to utilize its NOLs is limited. The Company has no other material deferred tax items. Synergy records a valuation allowance against deferred tax assets to the extent that it is more likely than not that some portion, or all of, the deferred tax assets will not be realized. As a result of this valuation allowance there are no income tax benefits reflected in the accompanying consolidated statements of operations to offset pre-tax losses.
The provisions of FASB ASC Topic 740-10-30-7, Accounting for Income Taxes were adopted by Synergy on January 1, 2007 and had no effect on Synergy’s financial position, cash flows or results of operations upon adoption, as Synergy did not have any unrecognized tax benefits. Synergy’s practice is to recognize interest and/or penalties related to income tax matters in income tax expense, and none have been incurred to date. Synergy has no uncertain tax positions subject to examination by the relevant tax authorities as of December 31, 2015 and December 31, 2014. Synergy files U.S. and state income tax returns in jurisdictions with varying statutes of limitations. The 2012 through 2015 tax years generally remain subject to examination by federal and most state tax authorities.
Synergy periodically files for and receives certain state and local research and development tax credits. As of December 31, 2015 the Company had no outstanding refundable tax credits due. During the year ended December 31, 2014 Synergy reported $83,000 as other income in the Company’s statement of operations.
8. Commitments and Contingencies
Lease agreements
On June 30, 2014, Synergy entered into a Lease Amendment of our New York office, (a) adding office space to our existing lease and (b) extending our existing lease for additional three years to March 2022, to be coterminous with our new space. This lease amendment results in total monthly rent of approximately $62,000 on straight line basis, prospectively.
Synergy also maintains a research and development laboratory and several offices in the Bucks County Biotechnology Center in Doylestown, Pennsylvania under a lease through December 31, 2016, at a monthly rate of approximately $3,700.
In addition, we lease office space for operations in Wayne, Pennsylvania under a lease through November 30, 2017, at a monthly rate of approximately $10,000.
Rent expense was $909,000, $651,000 and $575,000 for the years ended December 31, 2015, 2014 and 2013 respectively.
Change in Control and Severance Agreements
Certain employees have agreements which provide for payouts in the event that the Company consummates a change in control. At December 31, 2015, the amount of compensation for which the Company would be liable as a result of this event is approximately $5.1 million, as set forth in the agreements. These employees are also entitled to full vesting of their outstanding equity awards. These agreements also provide for routine severance compensation. As of December 31, 2015 and 2014, no amounts have been accrued.
Other Commitments
The Company has entered into certain licensing and collaboration agreements for products currently under development. The Company may be obligated in future periods to make additional payments, which would become due and payable only upon the
achievement of certain research and development, regulatory, and approval milestones. The specific timing of such milestones cannot be predicted and depend upon future discretionary research and clinical developments, as well as, regulatory agency actions. Further, under the terms of certain agreements the Company may be obligated to pay commercial milestones contingent upon the realization of sales revenues and sublicense revenues. Due to the long range nature of such commercial milestones, they are neither probable at this time nor predictable, and consequently are not considered contingent milestone payment amounts.
Contractual obligations and commitments
The following table is a summary of contractual obligations for the periods indicated that existed as of December 31, 2015.
|
|
|
|
|
Less than |
|
|
|
|
|
More than |
| |||||
|
($ in thousands) |
|
Total |
|
1 Year |
|
1-3 Years |
|
3-5 Years |
|
5 years |
| |||||
|
Long Term debt Obligations (1) |
|
$ |
206,700 |
|
$ |
11,925 |
|
$ |
23,850 |
|
$ |
170,925 |
|
$ |
— |
|
|
Operating leases |
|
6,936 |
|
1,038 |
|
2,061 |
|
2,309 |
|
1,528 |
| |||||
|
Purchase obligations—principally employment and consulting services(2) |
|
5,104 |
|
3,192 |
|
1,912 |
|
— |
|
— |
| |||||
|
Purchase Obligations—Major Vendors(3) |
|
108,902 |
|
91,400 |
|
17,502 |
|
— |
|
— |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Total obligations |
|
$ |
327,642 |
|
$ |
107,555 |
|
$ |
45,325 |
|
$ |
173,234 |
|
$ |
1,528 |
|
(1) Represents Senior Convertible Notes, including interest. See Note 5 to our Consolidated Financial Statements.
(2) Represents salary, bonus, and benefits for employment and consulting agreements with remaining terms greater than one year.
(3) Represents amounts that will become due upon future delivery of supplies, drug substance and test results from various suppliers, under open purchase orders.
Litigation
There are currently no pending legal proceedings to which Synergy or any of our subsidiaries is a party, or of which any of its property is the subject, that the Company believes will have, individually or in the aggregate, a material adverse effect on our business, financial condition or operating results. As far as the Company is aware, no governmental authority is contemplating any such proceeding.
9. Research and Development Expense
Research and development costs include expenditures in connection with an in-house research and development laboratory, salaries and staff costs, application and filing for regulatory approval of proposed products, purchased in-process research and development, regulatory and scientific consulting fees, as well as contract research, patient costs, drug formulation and tableting, data collection, monitoring and clinical trial insurance.
In accordance with FASB ASC Topic 730-10-55, Research and Development, Synergy recorded prepaid research and development costs of approximately $3.1 million and $3.7 million as of December 31, 2015 and December 31, 2014, respectively, for nonrefundable pre-payments for production of drug substance, analytical testing services for its drug candidates and clinical trials. In accordance with this guidance, Synergy expenses deferred research and development costs when drug compound is delivered and/or services are performed.
10. Derivative Financial Instruments
Effective January 1, 2009, the Company adopted provisions of ASC Topic 815-40, “Derivatives and Hedging: Contracts in Entity’s Own Equity” (“ASC Topic 815-40”). ASC Topic 815-40 clarifies the determination of whether an instrument issued by an
entity (or an embedded feature in the instrument) is indexed to an entity’s own stock, which would qualify as a scope exception under ASC Topic 815-10.
Based upon the Company’s analysis of the criteria contained in ASC Topic 815-40, Synergy has determined that certain warrants issued in connection with sale of its common stock must be classified as derivative instruments. In accordance with ASC Topic 815-40, these warrants are also being re-measured at each balance sheet date based on estimated fair value, and any resultant changes in fair value is being recorded in the Company’s statement of operations. The Company estimates the fair value of certain warrants using the Black-Scholes option pricing model in order to determine the associated derivative instrument liability and change in fair value described above. The range of assumptions used to determine the fair value of the warrants during each period was:
|
|
|
Year Ended |
|
Year Ended |
|
Year Ended |
|
|
|
|
December 31, 2015 |
|
December 31, 2014 |
|
December 31, 2013 |
|
|
Fair value of Synergy common stock |
|
$5.67 |
|
$3.05 |
|
$5.55 |
|
|
Expected warrant term |
|
2.2 years |
|
0.49-3.9 years |
|
1.8-4.4 years |
|
|
Risk-free interest rate |
|
1.18% |
|
0.08%-1.32% |
|
0.26%-1.76% |
|
|
Expected volatility |
|
50%-80% |
|
52%-60% |
|
60% |
|
|
Dividend yield |
|
— |
|
— |
|
— |
|
The fair value of stock is the closing market price of the Company’s common stock on the date of warrant issuance and at the end of each reporting period when the derivative instruments are marked to market. Expected volatility is a management estimate of
future volatility, over the expected warrant term, based on historical volatility of Synergy’s common stock. The warrants have a transferability provision and based on guidance provided in SAB 107 for instruments issued with such a provision, Synergy used the full contractual term as the expected term of the warrants. The risk free rate is based on the U.S. Treasury security rates for maturities consistent with the expected remaining term of the warrants at the date of grant or quarterly revaluation.
The following table sets forth the components of changes in the Synergy’s derivative financial instruments liability balance for the quarterly periods indicated:
|
|
|
|
|
|
|
Derivative |
| |
|
|
|
|
|
|
|
Instrument |
| |
|
|
|
|
|
|
|
Liability |
| |
|
Date |
|
Description |
|
Warrants |
|
(in thousands) |
| |
|
12/31/2013 |
|
Balance of derivative financial instruments liability |
|
858,469 |
|
1,534 |
| |
|
|
|
|
|
|
|
|
| |
|
3/31/2014 |
|
Fair value of new warrants issued during the quarter |
|
— |
|
— |
| |
|
3/31/2014 |
|
Change in fair value of warrants during the quarter |
|
— |
|
(223 |
) | |
|
6/30/2014 |
|
Fair value of new warrants issued during the quarter |
|
— |
|
— |
| |
|
6/30/2014 |
|
Change in fair value of warrants during the quarter |
|
— |
|
(756 |
) | |
|
9/30/2014 |
|
Fair value of new warrants issued during the quarter |
|
— |
|
— |
| |
|
9/30/2014 |
|
Change in fair value of warrants during the quarter |
|
— |
|
(425 |
) | |
|
12/31/2014 |
|
Fair value of new warrants issued during the quarter |
|
— |
|
— |
| |
|
12/31/2014 |
|
Change in fair value of warrants during the quarter |
|
— |
|
42 |
| |
|
12/31/2014 |
|
Balance of derivative financial instruments liability |
|
858,469 |
|
172 |
| |
|
|
|
|
|
|
|
|
| |
|
3/31/2015 |
|
Change in fair value of warrants during the quarter |
|
— |
|
268 |
| |
|
6/30/2015 |
|
Change in fair value of warrants during the quarter |
|
— |
|
1,541 |
| |
|
6/30/2015 |
|
Expiration of warrants |
|
(324,000 |
) |
— |
| |
|
9/30/2015 |
|
Change in fair value of warrants during the quarter |
|
— |
|
(1,445 |
) | |
|
9/30/2015 |
|
Exercise of warrants |
|
(30,000 |
) |
— |
| |
|
9/30/2015 |
|
Expiration of warrants |
|
(2,469 |
) |
(3 |
) | |
|
9/30/2015 |
|
Balance of derivative financial instruments liability |
|
502,000 |
|
533 |
| |
|
|
|
|
|
|
|
|
| |
|
12/31/2015 |
|
Change in fair value of warrants during the quarter |
|
— |
|
30 |
| |
|
12/31/2015 |
|
Exercise of warrants |
|
— |
|
— |
| |
|
12/31/2015 |
|
Expiration of warrants |
|
(292,000 |
) |
(241 |
) | |
|
12/31/2015 |
|
Balance of derivative financial instruments liability |
|
210,000 |
|
$ |
322 |
|
11. Fair Value Measurements
The following table presents the Company’s liabilities that are measured and recognized at fair value on a recurring basis classified under the appropriate level of the fair value hierarchy as of December 31, 2014 and December 31, 2015:
|
|
|
Quoted |
|
|
|
|
|
|
|
Quoted |
|
|
|
|
|
|
| ||||||||
|
|
|
Prices |
|
|
|
|
|
|
|
Prices |
|
|
|
|
|
|
| ||||||||
|
|
|
in |
|
|
|
|
|
|
|
in |
|
|
|
|
|
|
| ||||||||
|
|
|
Active |
|
|
|
|
|
|
|
Active |
|
|
|
|
|
|
| ||||||||
|
|
|
Markets |
|
Significant |
|
|
|
|
|
Markets |
|
Significant |
|
|
|
|
| ||||||||
|
|
|
for Identical |
|
Other |
|
Significant |
|
Balance |
|
for Identical |
|
Other |
|
Significant |
|
Balance |
| ||||||||
|
|
|
Assets and |
|
Observable |
|
Unobservable |
|
as of |
|
Assets and |
|
Observable |
|
Unobservable |
|
as of |
| ||||||||
|
|
|
Liabilities |
|
Inputs |
|
Inputs |
|
December |
|
Liabilities |
|
Inputs |
|
Inputs |
|
December |
| ||||||||
|
|
|
(Level 1) |
|
(Level 2) |
|
(Level 3) |
|
31, 2014 |
|
(Level 1) |
|
(Level 2) |
|
(Level 3) |
|
31, 2015 |
| ||||||||
|
Derivative liabilities related to Warrants |
|
$ |
— |
|
$ |
— |
|
$ |
172 |
|
$ |
172 |
|
$ |
— |
|
$ |
— |
|
$ |
322 |
|
$ |
322 |
|
The following table sets forth a summary of changes in the fair value of the Company’s Level 3 liabilities for the year ended December 31, 2014 and December 31, 2015.
|
|
|
|
|
(Gain) or |
|
|
|
(Gain) or |
|
|
|
|
| ||||||
|
|
|
|
|
loss |
|
|
|
loss |
|
|
|
|
| ||||||
|
|
|
|
|
recognized |
|
|
|
recognized |
|
|
|
|
| ||||||
|
|
|
|
|
in |
|
|
|
in |
|
|
|
|
| ||||||
|
|
|
Balance |
|
earnings |
|
Balance |
|
earnings |
|
|
|
Balance |
| ||||||
|
|
|
as of |
|
from |
|
as of |
|
from |
|
Expiration |
|
as of |
| ||||||
|
|
|
December |
|
Change in |
|
December |
|
Change in |
|
of |
|
December |
| ||||||
|
|
|
31, 2013 |
|
Fair Value |
|
31, 2014 |
|
Fair Value |
|
warrants |
|
31, 2015 |
| ||||||
|
Derivative liabilities related to Warrants |
|
$ |
1,534 |
|
$ |
(1,362 |
) |
$ |
172 |
|
$ |
394 |
|
$ |
(244 |
) |
$ |
322 |
|
The unrealized gains or losses on the derivative liabilities are recorded as a change in fair value of derivative liabilities in the Company’s statement of operations. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. At each reporting period, the Company reviews the assets and liabilities that are subject to ASC Topic 815-40. At each reporting period, all assets and liabilities for which the fair value measurement is based on significant unobservable inputs or instruments which trade infrequently and therefore have little or no price transparency are classified as Level 3.
As of December 31, 2015 and 2014, our available-for-sale securities are classified as Level 1 per ASC 820. The value of Senior convertible debt is stated at its carrying value at December 31, 2015 and 2014. The Company believes it could obtain borrowings at
December 31, 2015 at comparable interest rates as the November 2014 debentures, therefore, the carrying value approximates fair value.
12. Property and Equipment
Property and equipment consists of leasehold improvements, which are recorded at cost. The Company depreciates the costs when the property placed into service, using the straight-line method over the shorter of the life of asset and the remaining term of the underlying lease.
Furniture and equipment includes laboratory, testing and computer equipment and furniture and fixtures. All are stated at cost, with useful lives ranging from 2 - 5 years, depreciated on a straight line basis. Leasehold improvements are primarily related to Synergy’s corporate headquarters in New York City and are being amortized over the life of the lease. Depreciation and amortization expense for the years ended December 31, 2015, 2014 and 2013 were approximately $163,000, $120,000 and $56,000, respectively.
|
($ in thousands) |
|
December 31, 2015 |
|
December 31, 2014 |
| ||
|
Furniture and equipment |
|
$ |
243 |
|
$ |
220 |
|
|
Leasehold improvement |
|
674 |
|
645 |
| ||
|
Less accumulated depreciation |
|
(386 |
) |
(223 |
) | ||
|
Property and equipment, net |
|
$ |
531 |
|
$ |
642 |
|
13. Quarterly Consolidated Financial Data (Unaudited)
|
|
|
Quarter Ended |
| ||||||||||
|
|
|
March 31, |
|
June 30, |
|
September 30, |
|
December 31, |
| ||||
|
|
|
2015 |
|
2015 |
|
2015 |
|
2015 |
| ||||
|
|
|
(dollars in thousands, except share and per share data) |
| ||||||||||
|
Revenues |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Costs and Expenses: |
|
|
|
|
|
|
|
|
| ||||
|
Research and development |
|
18,198 |
|
19,525 |
|
20,424 |
|
19,881 |
| ||||
|
Purchased in-process research and development |
|
— |
|
— |
|
— |
|
— |
| ||||
|
Selling, general and administrative |
|
4,606 |
|
7,394 |
|
2,728 |
|
7,066 |
| ||||
|
Loss from Operations |
|
(22,804 |
) |
(26,919 |
) |
(23,152 |
) |
(26,947 |
) | ||||
|
Other Income /(Loss): |
|
|
|
|
|
|
|
|
| ||||
|
Interest and investment income/(expense), net |
|
(4,317 |
) |
(5,207 |
) |
(4,291 |
) |
(3,469 |
) | ||||
|
Tax credits |
|
— |
|
— |
|
— |
|
— |
| ||||
|
Change in fair value of derivative instruments—warrants |
|
(268 |
) |
(1,542 |
) |
1,446 |
|
(30 |
) | ||||
|
Total Other (Loss)/ Income |
|
(4,585 |
) |
(6,749 |
) |
(2,845 |
) |
(3,499 |
) | ||||
|
Net loss |
|
$ |
(27,389 |
) |
$ |
(33,668 |
) |
$ |
(25,997 |
) |
$ |
(30,446 |
) |
|
|
|
|
|
|
|
|
|
|
| ||||
|
Weighted Average Common Shares Outstanding—basic and diluted (a) |
|
96,683,525 |
|
100,343,637 |
|
111,328,339 |
|
113,678,306 |
| ||||
|
|
|
|
|
|
|
|
|
|
| ||||
|
Net Loss per Common Share—basic and diluted (a) |
|
$ |
(0.28 |
) |
$ |
(0.34 |
) |
$ |
(0.23 |
) |
$ |
(0.27 |
) |
(a) Basic and diluted EPS are computed independently for each of the periods presented. Accordingly, the sum of the quarterly EPS amounts may not agree to the total for the year.
|
|
|
Quarter Ended |
| ||||||||||
|
|
|
March 31, |
|
June 30, |
|
September 30, |
|
December 31, |
| ||||
|
|
|
(dollars in thousands, except share and per share data) |
| ||||||||||
|
Revenues |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
Costs and expenses: |
|
|
|
|
|
|
|
|
| ||||
|
Research and Development |
|
13,299 |
|
24,479 |
|
20,946 |
|
24,550 |
| ||||
|
Purchased in-process research and development |
|
— |
|
— |
|
— |
|
|
| ||||
|
General and administrative |
|
3,178 |
|
2,279 |
|
2,506 |
|
3,041 |
| ||||
|
Loss from operations |
|
(16,477 |
) |
(26,758 |
) |
(23,452 |
) |
(27,591 |
) | ||||
|
|
|
|
|
|
|
|
|
|
| ||||
|
Other income |
|
|
|
|
|
|
|
|
| ||||
|
Amortization of deferred financing costs |
|
— |
|
— |
|
— |
|
(411 |
) | ||||
|
Interest and investment income/(expense), net(includes interest expense of $2,500 on Senior Convertible Debentures for year 2014) |
|
29 |
|
(1 |
) |
19 |
|
(2,511 |
) | ||||
|
Tax credits |
|
— |
|
83 |
|
— |
|
— |
| ||||
|
Change in fair value of derivative instruments—warrants |
|
223 |
|
756 |
|
425 |
|
(42 |
) | ||||
|
Total other income/(loss) |
|
252 |
|
838 |
|
444 |
|
(2,964 |
) | ||||
|
Net Loss |
|
$ |
(16,225 |
) |
$ |
(25,920 |
) |
$ |
(23,008 |
) |
(30,555 |
) | |
|
|
|
|
|
|
|
|
|
|
| ||||
|
Weighted average common shares outstanding—basic and diluted (a) |
|
92,056,124 |
|
94,069,703 |
|
94,738,048 |
|
96,190,332 |
| ||||
|
Net loss per Common Share, basic and diluted (a): |
|
$ |
(0.18 |
) |
$ |
(0.28 |
) |
$ |
(0.24 |
) |
(0.32 |
) | |
(a) Basic and diluted EPS are computed independently for each of the periods presented. Accordingly, the sum of the quarterly EPS amounts may not agree to the total for the year.
