Attached files
| file | filename |
|---|---|
| EX-32 - EXHIBIT 32.1 - AKCEA THERAPEUTICS, INC. | exhibit32_1.htm |
| EX-31.2 - EXHIBIT 31.2 - AKCEA THERAPEUTICS, INC. | exhibit31_2.htm |
| EX-31.1 - EXHIBIT 31.1 - AKCEA THERAPEUTICS, INC. | exhibit31_1.htm |
| EX-23.1 - EXHIBIT 23.1 - AKCEA THERAPEUTICS, INC. | exhibit23_1.htm |
| EX-21.1 - EXHIBIT 21.1 - AKCEA THERAPEUTICS, INC. | exhibit21_1.htm |
| EX-14.1 - EXHIBIT 14.1 - AKCEA THERAPEUTICS, INC. | exhibit14_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
| ☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the fiscal year ended December 31, 2017
| ☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the transition period from ___________ to ___________
Commission file number 001-38137
Akcea Therapeutics, Inc.
(Exact name of Registrant as specified in its charter)
|
Delaware
|
47-2608175
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(IRS Employer Identification No.)
|
|
55 Cambridge Parkway, Suite 100, Cambridge, MA
|
02142
|
|
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
617-207-0202
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Name of each exchange on which registered
|
|
|
Common Stock, $.001 Par Value
|
The Nasdaq Stock Market, LLC
|
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer," "smaller reporting company" and "emerging growth company" in Rule 12b-2 of the Exchange Act:
|
Large accelerated filer ☐
|
Accelerated filer ☐
|
|
Non-accelerated filer ☒
|
Smaller reporting company ☐
|
|
(Do not check if a smaller reporting company)
|
|
|
Emerging growth company ☒
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12(b)-2 of the Securities Exchange Act of 1934). Yes ☐ No ☒
As of June 30, 2017, the last business day of the registrant's most recently completed second fiscal quarter, the registrant's common stock was not listed on any exchange or over-the-counter market. The registrant's common stock began trading on The NASDAQ Global Select Market on July 19, 2017.
The number of shares of common stock outstanding as of February 20, 2018 was 66,630,687.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant's definitive Information Statement with the Securities and Exchange Commission in connection with the Registrant's annual meeting of stockholders are incorporated by reference into Part III of this Report. Such information statement will be filed with the Securities and Exchange Commission not later than 120 days following the end of the Registrant's fiscal year ended December 31, 2017.
FORWARD-LOOKING STATEMENTS
This report on Form 10-K and the information incorporated herein by reference includes forward-looking statements regarding our business. Any statement describing our goals, expectations, financial or other projections, intentions or beliefs, is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties, particularly those inherent in the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs. Our forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause our results to differ materially from those expressed or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in this report on Form 10-K, including those identified in Item 1A entitled "Risk Factors". Although our forward-looking statements reflect the good faith judgment of our management, these statements are based only on facts and factors currently known by us. As a result, you are cautioned not to rely on these forward-looking statements.
In this report, unless the context requires otherwise, "Akcea," "Company," "we," "our," and "us" refers to Akcea Therapeutics, Inc. and its subsidiaries.
TRADEMARKS
"Akcea," the Akcea logo, and other trademarks or service marks of Akcea Therapeutics, Inc. appearing in this report are the property of Akcea Therapeutics, Inc. This report contains additional trade names, trademarks and service marks of others, which are the property of their respective owners. Solely for convenience, trademarks and trade names referred to in this report may appear without the ® or TM symbols.
CORPORATE INFORMATION
We incorporated in Delaware in December 2014. Our principal offices are in Cambridge, Massachusetts. We make available, free of charge, on our website, www.akceatx.com, our reports on Forms 10-K, 10-Q, 8-K and amendments thereto, as soon as reasonably practical after we file such materials with the Securities and Exchange Commission. Any information that we include on or link to our website is not a part of this report or any registration statement that incorporates this report by reference. You may also read and copy our filings at the SEC's Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-732-0330. The SEC also maintains a website that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is www.sec.gov.
2
AKCEA THERAPEUTICS, INC.
ANNUAL REPORT ON FORM 10-K FOR THE YEAR ENDED DECEMBER 31, 2017
INDEX
|
PART I
|
||
|
Page
|
||
|
Item 1.
|
Business
|
4 |
|
Item 1A.
|
Risk Factors
|
45 |
|
Item 1B.
|
Unresolved Staff Comments
|
65 |
|
Item 2.
|
Properties
|
65 |
|
Item 3.
|
Legal Proceedings
|
65 |
|
Item 4.
|
Mine Safety Disclosures
|
65 |
|
PART II
|
||
|
Item 5.
|
Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
66 |
|
Item 6.
|
Selected Financial Data
|
68 |
|
Item 7.
|
Management's Discussion and Analysis of Financial Condition and Results of Operations
|
69 |
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
86 |
|
Item 8.
|
Financial Statements and Supplementary Data
|
86 |
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
86 |
|
Item 9A.
|
Controls and Procedures
|
86 |
|
Item 9B.
|
Other Information
|
87 |
|
PART III
|
||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
88 |
|
Item 11.
|
Executive Compensation
|
88 |
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
88 |
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
88 |
|
Item 14.
|
Principal Accounting Fees and Services
|
88 |
|
PART IV
|
||
|
Item 15.
|
Exhibits, Financial Statement Schedules
|
89 |
|
Item 16.
|
Form 10-K Summary
|
91 |
|
Signatures
|
92 |
3
PART I
Item 1. Business
Overview
We are a late-stage biopharmaceutical company focused on developing and commercializing drugs to treat patients with serious cardiometabolic diseases caused by lipid disorders. Our goal is to become the premier company offering treatments for these inadequately treated diseases. We are advancing a mature pipeline of four novel drugs with the potential to treat multiple diseases. Our drugs, volanesorsen, AKCEA-APO(a)-LRx, AKCEA-ANGPTL3-LRx and AKCEA-APOCIII-LRx, are all based on antisense technology developed by Ionis Pharmaceuticals, Inc., or Ionis, which owns approximately 68% of our outstanding common stock. Our most advanced drug, volanesorsen, is currently under review by regulatory agencies in the United States, or U.S., European Union, or EU, and Canada for the treatment of people with familial chylomicronemia syndrome, or FCS. In the U.S., the Food and Drug Administration, or FDA, assigned a Prescription Drug User Fee Act, or PDUFA, goal date of August 30, 2018 and scheduled advisory committee meeting for May 10, 2018. In Canada, our New Drug Submission, or NDS, was granted Priority Review by Health Canada. FCS is a severe, rare, genetically defined lipid disorder characterized by extremely elevated levels of triglycerides. FCS has life-threatening consequences such as acute pancreatitis and the lives of patients with these diseases are impacted daily by the associated symptoms. In our clinical program, we have observed consistent and substantial (>70%) decreases in triglycerides and improvements in other manifestations of FCS, including pancreatitis attacks and abdominal pain. We believe the safety and efficacy data from the volanesorsen program demonstrate a favorable risk-benefit profile for patients with FCS. We are preparing for approval and launch of volanesorsen in mid-2018. Volanesorsen is also in Phase 3 clinical development for the treatment of familial partial lipodystrophy, or FPL. Our other three drugs are currently in Phase 2 clinical development.
We have made substantial progress in assembling the infrastructure to commercialize our drugs globally for rare disease indications with an initial focus on lipid specialists as the primary call point. A key element of our commercial strategy is to provide the specialized, patient-centric support required to successfully address rare disease patient populations. We believe our focus on treating patients with inadequately addressed lipid disorders will allow us to partner efficiently and effectively with the specialized medical community that supports these patients. In the future, this global infrastructure may support commercialization of additional drugs within and outside the cardiometabolic arena.
To maximize the commercial potential of two of the drugs in our pipeline, we initiated a strategic collaboration with Novartis Pharma AG, or Novartis, for the development and commercialization of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. We believe Novartis brings significant resources and expertise to the collaboration that can accelerate our ability to deliver these potential therapies to the large populations of patients who have high cardiovascular risk due to inadequately treated lipid disorders. As part of our collaboration, we received $75.0 million in an upfront option payment, of which we retained $60.0 million and paid $15.0 million to Ionis as a sublicense fee. Under our agreement with Novartis, after we complete Phase 2 development of each of AKCEA-APO(a)-LRx (data planned for the second half of 2018) and AKCEA-APOCIII-LRx (data planned for 2019), and if, on a drug-by-drug basis, Novartis exercises its option to license a drug and pays us the $150.0 million license fee to do so, Novartis would conduct and pay for a Phase 3 cardiovascular outcome study in high-risk patients and, if approved, commercialize each such licensed drug worldwide. Novartis will have 60 days following the end of the applicable end-of-Phase 2 meeting to exercise its option for each of these drugs. We plan to co-commercialize any licensed drug commercialized by Novartis in selected markets, under terms and conditions that we plan to negotiate with Novartis in the future, through the specialized sales force we are building to commercialize volanesorsen. Overall, we are eligible to receive license fees, milestone payments and royalties on sales of each drug Novartis licenses if and when it meets the development, regulatory and sales milestones specified in our agreement. We will share any license fees, milestone payments and royalties equally with Ionis.
Cardiometabolic disease, which includes cardiovascular diseases and metabolic diseases, is the number one cause of death globally. According to the American Heart Association, or AHA, cardiovascular disease, or CVD, alone accounts for 17.3 million deaths per year globally, a number that the AHA expects to grow to more than 23.6 million by 2030. Further, between 2010 and 2030, total direct medical costs of CVD in the United States alone are projected to triple from $272.5 billion to $818.1 billion, according to the AHA. In addition, the number of individuals with metabolic diseases, including diabetes, is rising dramatically. According to a 2010 study published in Population Health Metrics, the number of people in the United States with diabetes is projected to grow from approximately 20 million in 2010 to between 37 million and 56 million by 2030. Cardiometabolic risk factors include metabolic syndrome, dyslipidemia, hypertension, obesity and insulin resistance. Lipid risk factors driven by abnormalities in lipid molecules or the processing of lipid molecules contribute to cardiometabolic diseases, with elevated low-density lipoprotein cholesterol, or LDL-C, being the most widely recognized. Despite the availability of powerful drugs to lower LDL-C, many people remain at significant risk due to other lipid disorders that are not adequately addressed with current therapies. We believe this treatment gap beyond LDL lowering represents a significant commercial opportunity both in rare and in broader patient populations.
4
Each of the four drugs in our pipeline targets the specific ribonucleic acid, or RNA, that encodes for a unique protein associated with lipid dysfunction, robustly and selectively inhibiting the production of such protein. These drugs were designed and developed at Ionis, and use Ionis' proprietary antisense technology, which is a potent and specific way of reducing expression of disease-causing proteins. Specifically, our drugs utilize Ionis' generation 2+ antisense technology, which is designed for increased potency and enhanced safety characteristics relative to Ionis' generation 2.0 technology. Additionally, AKCEA-APO(a)-LRx, AKCEA-ANGPTL3-LRx and AKCEA-APOCIII-LRx utilize Ionis' advanced Ligand Conjugated Antisense, or LICA, technology. LICA technology conjugates specific chemical structures or molecules to antisense drugs to increase the efficiency of drug uptake in a particular tissue. We believe the enhancements from LICA technology have the potential to allow for less frequent administration and significantly lower doses, providing greater patient convenience. Phase 1 studies of all three of Akcea's LICA drugs have shown that doses up to 30-fold lower than non-LICA drugs result in consistent target reductions and a favorable safety and tolerability profile. Our current pipeline includes drugs with the potential to treat patients with a wide range of lipid disorders associated with cardiometabolic disease that other technologies, such as small molecules and antibodies, have not been able to adequately address. Our development approach and commercialization strategy include:
| ● |
transforming the lives of patients with serious diseases that are currently inadequately addressed;
|
| ● |
addressing the root cause of each disease;
|
| ● |
maximizing near-term and long-term commercial opportunities; and
|
| ● |
optimizing the efficiency of our sales, marketing and patient support infrastructure by focusing on rare and specialty diseases.
|
Our clinical pipeline contains novel drugs with the potential to treat inadequately addressed lipid disorders beyond elevated LDL-C that are contributing to the dramatic increase in the incidence of cardiometabolic disease, such as elevated triglycerides, oxidized phospholipids and other lipoproteins such as lipoprotein(a), or Lp(a).
| ● |
Volanesorsen. In the third quarter of 2017, we filed for marketing authorization for volanesorsen to treat patients with FCS in the U.S., EU and Canada. FCS is characterized by extremely elevated triglyceride levels and a high risk of life-threatening pancreatitis. Patients with FCS live with daily and chronic manifestations of their disease that negatively affect their lives, including severe, recurrent abdominal pain, cognitive impairment and fatigue. Volanesorsen is also in Phase 3 clinical development for the treatment of FPL. We plan to report data from the FPL Phase 3 study, called the BROADEN study, in 2019. Volanesorsen acts to reduce triglyceride levels by inhibiting the production of apolipoprotein C-III, or apoC-III, a protein that is a key regulator of triglyceride clearance. If approved, we plan to globally commercialize volanesorsen ourselves for both FCS and FPL.
|
In 2017, we completed the Phase 3 program for volanesorsen to treat patients with FCS. The Phase 3 program consisted of two studies, the APPROACH study and the COMPASS study. The APPROACH study, a one-year randomized, placebo-controlled study in 66 patients with FCS (average incoming triglycerides of 2,209 mg/dL), achieved its primary endpoint of reduction in triglycerides at three months, with a 77% mean reduction in triglycerides, which translated into a 1,712 mg/dL mean absolute triglyceride reduction in volanesorsen-treated patients. We observed that 50% of treated patients achieved triglyceride levels below 500 mg/dL, a commonly accepted threshold for pancreatitis risk. In addition, in the APPROACH study, treatment with volanesorsen was associated with a statistically significant reduced rate of pancreatitis attacks in the group of patients who had the highest incidence of pre-study pancreatitis, and reduced abdominal pain in patients reporting pain before treatment in the study. The triglyceride-lowering effects we observed were maintained throughout the 12-month study period.
The COMPASS study, a six-month randomized placebo-controlled study in 113 patients with very high triglycerides (>500 mg/dL), also achieved its primary endpoint of reduction in triglycerides at three months, with a 71% mean reduction in triglycerides. In the COMPASS study, treatment with volanesorsen was associated with a statistically significant reduction in pancreatitis attacks. The data from the COMPASS and APPROACH studies is consistent with and supports the robust triglyceride lowering we observed in the Phase 2 program for volanesorsen.
The most common adverse event in patients in the studies was injection site reactions, which were mostly mild. In addition, declines in platelet counts were observed in many patients and some patients discontinued the study because of platelet declines. These platelet declines were not clinically significant in most patients and were generally well managed with monitoring and dose adjustment. Some patients also discontinued participation in the APPROACH study due to other non-serious adverse events, including sweating and chills, severe fatigue, rash and injection site reaction. In the APPROACH study and the open label extension study, the potentially treatment-related serious adverse events, or SAEs, observed were serious platelet events (grade 4 thrombocytopenia). These events resolved without complication after cessation of dosing. Enhanced monitoring was implemented during the study to support early detection and management of these issues. Since implementation of the enhanced monitoring, serious platelet events have been infrequent. In the COMPASS study, the most common adverse event in the volanesorsen-treated group of patients was injection site reactions, which were mostly mild, and the potentially treatment-related SAE of serum sickness reaction where the patient fully recovered. There have been no deaths and no treatment-related bleeding or cardiovascular events in any volanesorsen clinical study.
5
We recently initiated a global early access program, or EAP, for people with FCS. Early access, sometimes referred to as "compassionate use", is the use of an investigational medicinal product outside of a clinical trial that is intended to treat a serious or life-threatening condition. Our EAP program is being initiated on a country-by-country basis globally and is currently available in select countries in Europe.
The remainder of our pipeline incorporates Ionis' LICA technology.
| ● |
AKCEA-APO(a)-LRx. We are developing AKCEA-APO(a)-LRx for patients who are at significant risk of CVD because of their elevated levels of Lp(a). AKCEA-APO(a)-LRx inhibits the production of the apolipoprotein(a), or apo(a), protein, thereby reducing Lp(a). Apo(a) is a form of low-density lipoprotein, or LDL, that is very atherogenic (promoting the formation of plaques in the arteries) and very thrombogenic (promoting the formation of blood clots). We have started a Phase 2b dose-ranging study of AKCEA-APO(a)-LRx in patients with hyperlipoproteinemia(a), which in this clinical study we have defined as individuals with levels of Lp(a) greater than 60 mg/dL, and established CVD. We expect data from this study in the second half of 2018. We have initiated a strategic collaboration with Novartis for this drug. In this collaboration, we intend to complete the above-referenced Phase 2b study. Following completion of this study, Novartis has an option to license the drug. If Novartis exercises its option to license AKCEA-APO(a)-LRx, Novartis plans to conduct and pay for a Phase 3 cardiovascular outcome study in high-risk patients and, if approved, to commercialize AKCEA-APO(a)-LRx worldwide.
|
| ● |
AKCEA-ANGPTL3-LRx. We are developing AKCEA-ANGPTL3-LRx to treat multiple lipid disorders. In preclinical studies, an analog of AKCEA-ANGPTL3-LRx inhibited the production of the angiopoietin-like 3, or ANGPTL3, protein in the liver, inhibiting liver fat accumulation and lowering blood levels of triglycerides, LDL-C and very-low-density lipoprotein cholesterol, or VLDL-C. We have completed a Phase 1/2 program for AKCEA-ANGPTL3-LRx in people with elevated triglycerides. We reported results for the initial cohort from this study at the AHA meeting in November 2016 and published the data in the New England Journal of Medicine. In the fourth quarter of 2017, we initiated a study of AKCEA-ANGPTL3-LRx in patients with nonalcoholic fatty liver disease, or NAFLD, with metabolic complications which include hypertriglyceridemia, type 2 diabetes or nonalcoholic steatohepatitis, or NASH. We expect data from this study in 2019. Further, in the fourth quarter of 2017, we initiated a study of AKCEA-ANGPTL3-LRx in patients with rare hyperlipidemias, including patients with FCS. If we find that AKCEA-ANGPTL3-LRx can effectively lower triglyceride levels in patients with rare hyperlipidemias, including patients with FCS, through a different mechanism of action from volanesorsen, it may represent an opportunity to expand our FCS franchise. We expect data from this study in 2018. As part of our exploratory rare hyperlipidemia clinical program, we are also studying AKCEA-ANGPTL3-LRx in patients with FPL and in homozygous familial hypercholesterolemia, or HoFH.
|
| ● |
AKCEA-APOCIII-LRx. We are developing AKCEA-APOCIII-LRx to inhibit the production of apoC-III, the same protein inhibited by volanesorsen, for the broad population of patients who have cardiometabolic disease due to their elevated triglyceride levels. We believe that the enhancements offered by Ionis' LICA technology can provide greater patient convenience by allowing for significantly lower doses and less frequent administration, compared to volanesorsen. We conducted a Phase 1/2 study of AKCEA-APOCIII-LRx in people with elevated triglycerides and reported positive results from this study in the second half of 2017. We have initiated a strategic collaboration with Novartis for this drug. In this collaboration, we intend to complete the Phase 2 program required to define the appropriate dose and regimen to support a planned cardiovascular outcome study. We recently initiated a Phase 2b dose-ranging study of AKCEA-APOCIII-LRx in patients with hypertriglyceridemia and established CVD and plan to report data from this study in 2019. At the completion of Phase 2 development, Novartis has an option to license the drug. If Novartis exercises its option to license AKCEA-APOCIII-LRx, Novartis plans to conduct and pay for a Phase 3 cardiovascular outcome study in high-risk patients and, if approved, to commercialize AKCEA-APOCIII-LRx worldwide.
|
6
Our Strategy
Our goal is to become the premier company offering treatments for previously inadequately treated lipid disorders. The critical components of our business strategy to achieve this goal include the following:
| ● |
Successfully complete development, obtain regulatory approval and commercialize volanesorsen in two orphan indications. We are focused on rapidly and efficiently developing and commercializing volanesorsen for the treatment of patients with FCS and FPL. There are limited therapeutic options available for these patients, who suffer from serious health issues including heightened risk of premature death. Volanesorsen has completed a Phase 3 clinical program for the treatment of FCS and is currently being investigated in the Phase 3 BROADEN clinical study for the treatment of FPL. We announced data from the APPROACH study in FCS patients in March 2017, and the COMPASS study in patients with high triglycerides in December 2016. We filed for regulatory approval in the U.S., EU and Canada in this indication in the third quarter of 2017 and are preparing for commercialization in mid-2018. Enrollment in the BROADEN study is ongoing and we plan to report data in 2019.
|
| ● |
Pursue indications that drive the greatest near and long-term value. We seek to maximize near-term and long-term commercial opportunities through development paths in both orphan and broader patient populations. We are developing our first drug for the treatment of orphan lipid disorders, which may provide a more rapid path to marketing authorization, nearer-term commercial value and more immediate clinical benefit for the patients with the greatest need and their physicians. We are pursuing this strategy with our pipeline as well, in particular with AKCEA-ANGPTL3-LRx, which we are developing for both broad and rare diseases.
|
| ● |
Advance multiple novel clinical-stage drugs to commercialization and further grow our pipeline. Our pipeline of antisense drugs currently contains four clinical-stage novel therapies that we plan to develop and commercialize by ourselves or in conjunction with a partner, such as Novartis, for multiple indications driven by lipid disorders. To sustain our goal of being the premier lipid disorder company, we also plan to actively replenish our pipeline as our current drugs advance through development. For example, we will have the opportunity to potentially license antisense drugs that Ionis advances to treat rare cardiometabolic and rare inherited metabolic diseases under our right of first negotiation that Ionis granted us.
|
| ● |
Build a leading, fully integrated, independent development and commercialization organization with a specialized and focused global team centered around a high touch patient and physician experience. As our drug pipeline and commercialization efforts mature, we plan to strategically expand our internal development and regulatory capabilities. Further, we are currently building our own global commercial organization. We are starting with a small, highly focused commercial organization for volanesorsen. This organization will work closely with the same specialists who are participating in developing our drugs, including lipid specialists, specialized endocrinologists and pancreatologists. We plan to efficiently manage this organization to access additional markets as our commercial opportunities for both volanesorsen and our other drugs expand into additional patient populations. We plan to provide high touch patient and healthcare provider support through dedicated case management providing reimbursement assistance, as well as by establishing partnerships with specialty pharmacies, injection training, routine platelet monitoring and dietary counseling, which we believe will enable strong integration with treating physicians and facilitate patient uptake and compliance.
|
| ● |
Create value through strategic collaborations, such as our strategic collaboration with Novartis, to drive drugs to their fullest potential. We believe that each of the drugs in our pipeline can be developed for multiple lipid disorders, some of which have very large patient populations. In these patient populations, large, costly, late-stage clinical development programs, as well as large sales forces, are required to maximize a drug's commercial potential. As a result, in some cases, partnering with a large organization with global scale may be the optimal approach for maximizing the potential of drugs in these indications. As an example, we have initiated a strategic collaboration with Novartis for AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx, to provide us with an opportunity to move rapidly to Phase 3 cardiovascular outcome studies with both drugs, which should enhance the commercial potential of each drug. We also plan to co-commercialize these two drugs in selected markets, under terms and conditions that we plan to negotiate with Novartis in the future, through the specialized sales force we are building to commercialize volanesorsen. We believe Novartis brings significant resources and expertise to the collaboration that can accelerate our ability to deliver these potential therapies to the large populations of patients who have high cardiovascular risk due to inadequately treated lipid disorders.
|
7
Commercial Approach
We plan to commercialize volanesorsen ourselves globally, with a specialized and comprehensive patient-centric approach. Our orphan-focused commercial model will include a small highly focused salesforce in each country that we are targeting, complemented by medical affairs and patient and healthcare provider services. We plan to provide high touch patient and healthcare provider support through reimbursement assistance, partnerships with specialty pharmacies, injection training, routine platelet monitoring and dietary counseling, which we believe will enable strong integration with treating physicians and facilitate patient uptake and compliance. Reimbursement assistance may include activities such as a reimbursement hotline, patient assistance, co-pay assistance through foundations and insurance verification. We plan to include dedicated case managers as part of our support team who will work directly with patients, caregivers and healthcare providers to help patients start and stay on therapy. Our global commercial organization is initially focused on our nearest-term opportunity with volanesorsen to treat patients with FCS. Our initial plan is to focus on lipid specialists, specialized endocrinologists and pancreatologists as our primary call points. At the outset, we plan to focus our commercial efforts in the U.S., Canada and EU, and intend to expand over time to other relevant geographies. We believe the relatively small number of specialized physicians treating FCS patients will allow us to address this market with a nimble, scalable organization. We are currently identifying patients and having them referred to specialists for treatment, which we believe will facilitate successful commercialization. Building awareness of this orphan disease among not only lipid specialists, but also referring physicians, is a key element of our pre-commercial and commercial plans. We are focused on disease education and market access, with the goal of ensuring that identified patients can most effectively obtain our drugs once commercialized. We are also creating the specialized support required to potentially address other rare disease patient populations.
We plan to commercialize by ourselves any approved drugs with a rare disease or specialty focus. We may enter into additional strategic relationships to commercialize certain of our drugs, particularly in indications with large patient populations, as evidenced by our collaboration with Novartis. We believe Novartis brings significant resources and expertise to the collaboration that can accelerate our ability to deliver AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx to the large populations of patients who have high cardiovascular risk due to inadequately treated lipid disorders. We also plan to co-commercialize any such drug in selected markets, under terms and conditions that we plan to negotiate with Novartis in the future, through the specialized sales force we are building to commercialize volanesorsen.
Integrated Development and Commercial Opportunities
Our drugs are designed to target a variety of lipid disorders, present in both orphan and broad patient populations, which available therapies do not adequately address. We are initially focused on developing volanesorsen and AKCEA-ANGPTL3-LRx for orphan indications that will not require large cardiovascular outcome studies. The smaller, orphan-size populations allow a potentially rapid path to commercialization and we believe will allow us to address the commercial market with a nimble, scalable organization. At the same time, we initiated a strategic collaboration with Novartis for AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx, allowing development of these drugs in larger populations with the potential to expand the commercial opportunity.
While preparing to commercialize volanesorsen, we are building relationships with specialist physicians. These specialists influence and drive treatment practice across lipid disorders. Accordingly, we believe that we will be able to leverage these relationships in commercializing all of the drugs in our pipeline.
Technology Overview
Antisense Technology
Ionis discovered each of the drugs in our pipeline using its innovative antisense technology platform. Antisense technology is based on the use of synthetic nucleic acid sequences, which are primarily used to interrupt the production of a specified protein by targeting the specific corresponding messenger RNA, or mRNA, that encodes that protein. In this way, antisense drugs can be used to reduce the level of proteins that cause, or contribute to, the progression of various diseases. Because there are virtually no undruggable mRNA targets, we believe antisense technology may have broader potential than small molecule- and antibody-based technologies that target proteins. Furthermore, antisense technology has the potential to target a growing number of disease-related genes more directly and efficiently than other protein-directed modalities. We believe this technology represents an important advance in treating diseases.
The production of a protein starts with a process called transcription, where the instructions for making a protein are transcribed from a gene, or DNA, into mRNA. The cell's protein production process is called translation, and antisense drugs can be designed to interrupt this process by causing the destruction of the targeted mRNA and therefore preventing the production of a protein of interest. The graphic below further illustrates the impact of antisense drugs on the production of proteins via this mechanism of action:
8
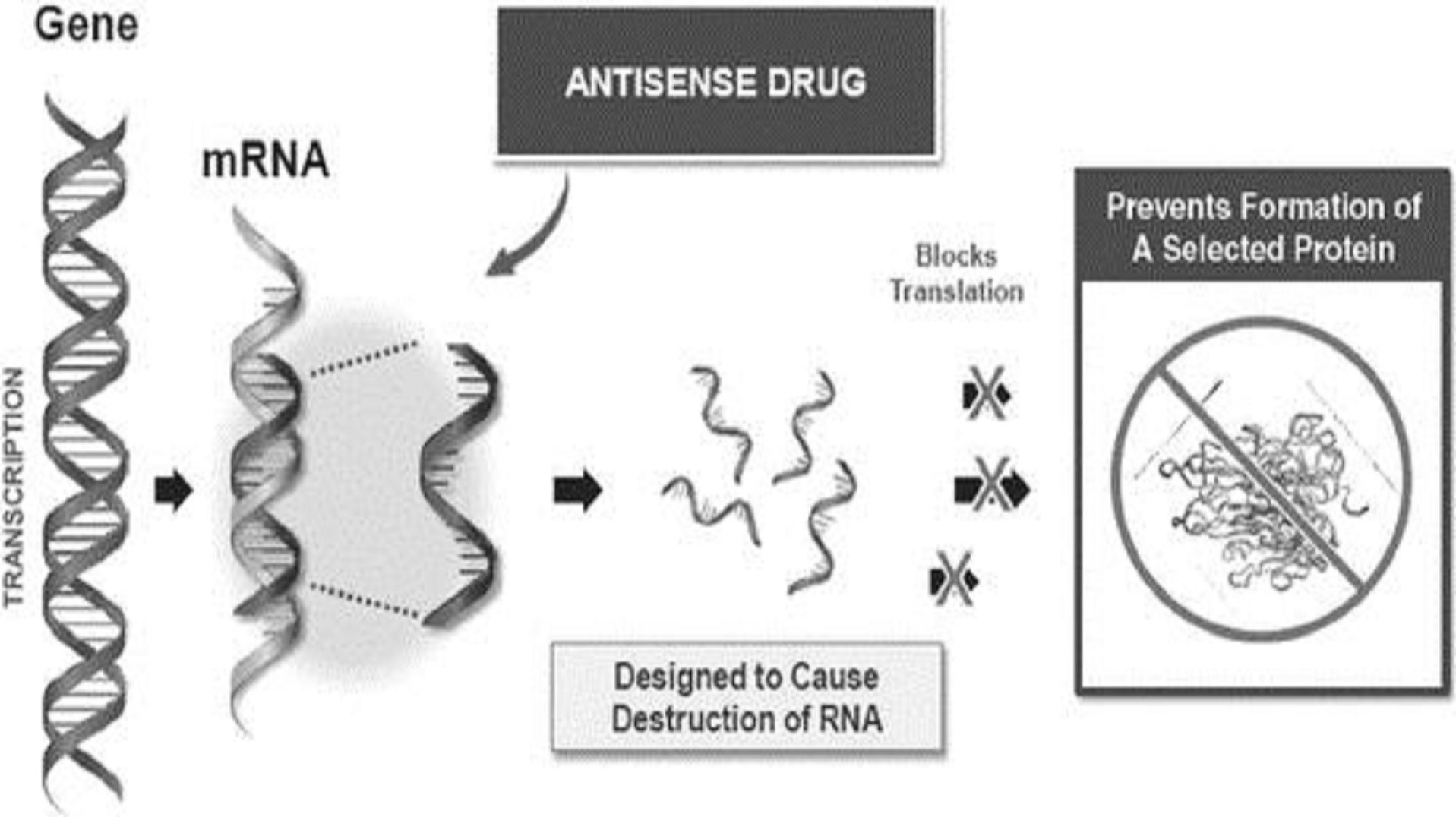
Ionis has made significant improvements in its antisense drug technology in recent years. These include improving the discovery screening processes, which resulted in second-generation drugs, or generation 2+ drugs, with better properties. In addition, Ionis observed lower incidences of injection site reactions and flu-like symptoms compared to Ionis' generation 2.0 drugs.
The unique properties of our antisense drugs provide several potential advantages over traditional drug modalities. These advantages include:
| ● |
Precise specificity. Our antisense drugs are designed using Ionis' generation 2+ screening processes to target single mRNAs, which minimizes or eliminates the possibility of our drugs binding to unintended genetic targets that can cause unwanted side effects.
|
| ● |
Favorable dosing properties. We believe our drugs have predictable safety, pharmacokinetic and pharmacodynamic properties based on Ionis' experience with dosing over 6,000 people with antisense drugs to date. Further, our drugs have a relatively long half-life of two to four weeks, which enables volanesorsen to be dosed once weekly and other drugs in our pipeline, which incorporate Ionis' LICA technology, to potentially be dosed once monthly or less frequently. Upon dosing, our drugs distribute well throughout the body, eliminating the need for special formulations or delivery vehicles.
|
| ● |
No anticipated drug-to-drug interactions. Because they are nucleic acid based, we believe our drugs can be used in combination with virtually any existing treatment modality without the risk of drug-to-drug interactions or susceptibility to traditional enzyme degradation or metabolism pathways.
|
| ● |
Broad applications to multiple disease targets, multiple tissues and multiple mechanisms. There are virtually no "undruggable" targets with antisense technology.
|
| ● |
Efficient discovery and early development. Because of the efficiency of antisense technology, drug discovery and early development costs and success rates compare favorably to small molecule or antibody drug discovery and development.
|
LICA Technology
Ionis' LICA technology conjugates specific chemical structures or molecules to antisense drugs to increase the efficiency of drug uptake in a particular tissue. For drugs that target proteins primarily expressed in the liver, including the three drugs in our pipeline beyond volanesorsen, Ionis' design uses N acetyl galactosamine, or GalNAc, LICA that interact specifically with receptors present on the surface of important liver cells. Phase 1 studies of all three of Akcea's LICA drugs have shown that doses up to 30-fold lower than non-LICA drugs result in consistent and clinically meaningful target reductions and a favorable safety and tolerability profile. We believe the enhancements from LICA technology have the potential to allow for less frequent administration and significantly lower doses providing greater patient convenience as compared to non-LICA forms.
Lipid Biology
Lipids are a group of organic compounds that, together with carbohydrates and proteins, constitute the primary structural material of living cells. Lipids include fatty acids and cholesterol, as well as triglycerides, which are lipids that contain three fatty acid molecules and are a major source of energy. Triglycerides are made in the liver or in the intestine after a person eats foods containing fat.
9
Because lipids are relatively insoluble in water, they must be transported from one site in the body to another in the form of lipoproteins. Lipoproteins package the lipids in a soluble form and also contain special proteins, known as apolipoproteins, that help regulate the metabolism of the lipids and direct their sub-cellular delivery. Commonly recognized lipoproteins are LDL, which transports cholesterol made in the liver to other tissues and is associated with elevated CVD risk, and high-density lipoprotein, or HDL, which transports cholesterol from the body back to the liver. Another important lipoprotein is an aggressive form of LDL known as Lp(a). Lp(a) not only carries the risks of LDL, but also has attached a protein, known as apo(a), which carries highly inflammatory oxidized phospholipids. When levels of these lipoproteins are high, they can more aggressively accumulate in the walls of blood vessels, leading to cholesterol accumulation, plaque formation and inflammation. This cholesterol deposition and inflammation can profoundly damage the arteries and, if continued, cause CVD, which includes heart attacks, strokes and disease of the peripheral arteries in the legs. Lp(a) both accumulates in the artery with higher affinity and has a longer residence time than LDL, causing more thrombogenesis (process of formation of a blood clot) and atherosclerosis (buildup of a waxy plaque on the inside of blood vessels).
Triglycerides are also transported by lipoproteins known as triglyceride-rich lipoproteins, such as chylomicrons and VLDL. High levels of these lipoproteins can cause metabolic complications such as pancreatitis, insulin resistance and diabetes. When triglyceride levels are too high, remnants, which are cholesterol-containing breakdown products of the triglyceride-rich lipoproteins, can also enter the artery wall and, in a similar manner as LDL, lead to atherosclerosis. Further, the release of excess fatty acids can promote insulin resistance, fatty liver, and diabetes.
Statistical Significance
In the description of our clinical trials below, n represents the number of patients in a particular group and p or p-values represent the probability that random chance caused the result (e.g., a p-value = 0.001 means that there is a 0.1% probability that the difference between the placebo group and the treatment group is purely due to random chance). A p-value ≤0.05 is a commonly used criterion for statistical significance, and may be supportive of a finding of efficacy by regulatory authorities. However, regulatory authorities, including the U.S. Food and Drug Administration, or FDA and the European Medicines Agency, or EMA, do not rely on strict statistical significance thresholds as criteria for market approval and maintain the flexibility to evaluate the overall risks and benefits of a treatment.
10
Clinical Pipeline
Cardiometabolic disease, which includes cardiovascular diseases and metabolic diseases such as diabetes, is the number one cause of death globally. According to the AHA, CVD alone accounts for 17.3 million deaths per year globally, a number that the AHA expects to grow to more than 23.6 million by 2030. Further, the number of individuals with metabolic diseases, including diabetes, is also rising dramatically. According to a 2010 study published in Population Health Metrics, the number of people in the United States with diabetes is projected to grow from approximately 20 million in 2010 to between 37 million and 56 million by 2030. Cardiometabolic risk factors include metabolic syndrome, dyslipidemia, hypertension, obesity and insulin resistance.
Lipid risk factors driven by abnormalities in lipid molecules contribute to cardiometabolic diseases, with elevated LDL-C being the most widely recognized. Despite the availability of powerful drugs to lower LDL-C, many people remain at significant risk due to other lipid disorders that are not adequately addressed with current therapies. This treatment gap represents a significant commercial opportunity both in orphan and in broader diseases, with new therapies needed.
The following figure illustrates our pipeline:
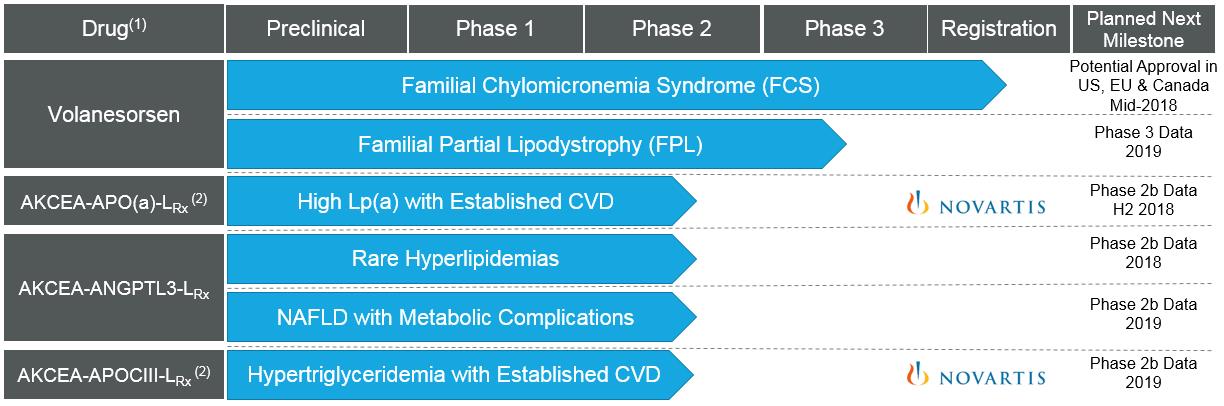
|
(1)
|
We have used alternate names for our drugs:
|
| ● |
Volanesorsen also has been known as IONIS-APOCIIIRx, ISIS-APOCIIIRx and ISIS 304801.
|
| ● |
AKCEA-APO(a)-LRx also has been known as IONIS-APO(a)-LRx, ISIS-APO(a)-LRx and ISIS 681257.
|
| ● |
AKCEA-ANGPTL3-LRx also has been known as IONIS-ANGPTL3-LRx, ISIS-ANGPTL3-LRx and ISIS 703802.
|
| ● |
AKCEA-APOCIII-LRx also has been known as IONIS-APOCIII-LRx, ISIS-APOCIII-LRx and ISIS 678354.
|
|
(2)
|
We have initiated a strategic collaboration with Novartis for AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx.
|
| Note: |
The arrows designate the current phase of development for each drug and indication, and do not represent the extent of completion of the activities we are currently conducting within the phase.
|
| Note: |
The "L" designation indicates drugs that use Ionis' LICA technology.
|
Volanesorsen
Overview
We have filed for marketing authorization for volanesorsen to treat patients with FCS in the U.S., EU and Canada. FCS is an orphan disease characterized by extremely elevated triglyceride levels and a high risk of life-threatening pancreatitis. Patients with FCS live with daily and chronic manifestations of their disease that negatively affect their lives, including severe, recurrent abdominal pain and cognitive impairment. Volanesorsen acts to reduce triglyceride levels by inhibiting the production of apoC-III, a protein that is a key regulator of triglyceride clearance. People who have low levels of apoC-III or reduced apoC-III function have lower levels of triglycerides and a lower incidence of CVD.
We believe volanesorsen has the potential to significantly improve the lives of patients with FCS. We demonstrated in Phase 2 studies that volanesorsen robustly reduced apoC-III and triglycerides in patients, including in FCS patients, and also had a beneficial impact on insulin sensitivity. Further, in a Phase 2 study, the triglyceride levels in all patients with FCS treated with volanesorsen were reduced to levels below 500 mg/dL, which is a commonly accepted level associated with reduced risk of pancreatitis. We published our findings from the Phase 2 studies with volanesorsen in two publications in the New England Journal of Medicine.
11
We filed our marketing applications in multiple jurisdictions based on positive data from volanesorsen's Phase 3 program to treat patients with FCS. The Phase 3 program consisted of two studies, the APPROACH study and the COMPASS study. The APPROACH study, a one-year randomized, placebo-controlled study in 66 patients with FCS (average incoming triglycerides of 2,209 mg/dL), achieved its primary endpoint of reduction in triglycerides at three months, with a 77% mean reduction in triglycerides (p<0.0001), which translated into a 1,712 mg/dL mean absolute triglyceride reduction in volanesorsen-treated patients (p<0.0001). In the study, we observed that more than 75% of treated patients achieved triglyceride levels below 750 mg/dL, the level at which chylomicron formation begins to become significant, and 50% of treated patients achieved triglyceride levels below 500 mg/dL, a commonly accepted threshold for pancreatitis risk. Each of these results was statistically significant compared to placebo-treated patients, none of whom achieved triglyceride levels below 500 mg/dL. In addition, in the APPROACH study, treatment with volanesorsen was associated with a statistically significant reduced rate of pancreatitis attacks in the group of patients who had a documented history of recurrent pancreatitis attacks in the five years prior to the study (p=0.02). Patients treated with volanesorsen who had reported abdominal pain before treatment in the study, also experienced reduced and less frequent pain than their placebo-treated counterparts, a difference that was more evident as the study progressed. The triglyceride lowering effects we observed were maintained throughout the 12-month study period. The COMPASS study, a six-month randomized placebo-controlled study in 113 patients with very high triglycerides (>500 mg/dL), also achieved its primary endpoint of reduction in triglycerides at three months, with a 71% mean reduction in triglycerides. In the COMPASS study, treatment with volanesorsen was associated with a statistically significant reduction in pancreatitis attacks (p=0.036). The data from the COMPASS and APPROACH studies is consistent with and supports the robust triglyceride lowering we observed in the Phase 2 program for volanesorsen.
The most common adverse event in the studies was injection site reactions, which were mostly mild. In addition, declines in platelet counts were observed in many patients and some patients discontinued the study because of platelet declines. These platelet declines were not clinically significant in most patients and were generally well managed with monitoring and dose adjustment. Some patients also discontinued participation in the APPROACH study due to other non-serious adverse events, including sweating and chills, severe fatigue, rash and injection site reaction. In the APPROACH study and the open label extension study, the potentially treatment-related serious adverse events, or SAEs, observed were serious platelet events (grade 4 thrombocytopenia). These events resolved without complication after cessation of dosing. Enhanced monitoring was implemented during the study to support early detection and management of these issues. Since implementation of the enhanced monitoring, serious platelet events have been infrequent. In the COMPASS study, the most common adverse event in the volanesorsen-treated group of patients was injection site reactions, which were mostly mild, and the potentially treatment-related SAE of serum sickness reaction where the patient fully recovered. There have been no deaths and no treatment-related cardiovascular events in any volanesorsen clinical study. We believe our greater involvement with physicians and patients, which will be a core focus of the education and support provided by our patient-centric commercial approach, should allow us to better maintain patients on volanesorsen therapy.
We have filed for marketing authorization in the U.S., EU and Canada for volanesorsen to treat patients with FCS based on what we believe is a favorable risk-benefit profile supported by data from both APPROACH and COMPASS. We are preparing to globally commercialize volanesorsen ourselves for FCS and, if approved, we will launch quickly thereafter in each geography.
Volanesorsen is also in Phase 3 clinical development for the treatment of FPL. The Phase 3 BROADEN study is currently enrolling patients with FPL. We plan to report data from this study in 2019. Patients with FPL have elevated triglyceride levels associated with a rare genetic disorder characterized by selective, progressive loss of body fat (adipose tissue) from various areas of the body. The FDA and EMA have granted orphan drug designation to volanesorsen for the treatment of patients with FCS. The EMA has granted orphan drug designation to volanesorsen for the treatment of patients with FPL.
Disease Background
Familial chylomicronemia syndrome
FCS is an inherited orphan disorder and includes type 1 hyperlipoproteinemia, Fredrickson type 1 hyperlipidemia and lipoprotein lipase, or LPL, deficiency. Patients with FCS lack the ability to produce enzymes to clear triglycerides, normally due to one or more loss-of-function mutations in genes related to triglyceride metabolism, which often results in triglyceride levels higher than 2,000 mg/dL—more than 10 times the normal level. As a result, patients with FCS may suffer from many health issues including severe, recurrent abdominal pain, fatigue and a high risk of life-threatening pancreatitis. In addition, they also suffer from daily conditions that can negatively impact their quality of life including neuropsychiatric symptoms such as memory loss, dementia, mild depression and cognitive impairment (described as brain fog and forgetfulness), as well as gastrointestinal symptoms including nausea and vomiting. There are no approved therapeutic options for patients with FCS. Standard triglyceride lowering agents, including niacin, fish oils and fibrates, are generally not effective in this patient population. Patients are required to adhere to a very strict, low-fat diet, which is extremely burdensome and difficult to maintain, and many patients still experience symptoms even if they are compliant with the diet. By inhibiting the production of apoC-III, volanesorsen is able to increase triglyceride clearance in FCS patients, reducing their triglyceride levels.
12
Familial Partial Lipodystrophy
People with FPL lack subcutaneous adipose tissue and have abnormal subcutaneous fat distribution. Because FPL patients are unable to store fat properly, they may have triglyceride levels higher than 1,000 mg/dL—more than five times the normal level. Additionally, because FPL patients cannot store excess triglycerides, their triglyceride levels may be extremely elevated after meals. These triglycerides deposit in organs other than normal fat tissue, known as ectopic fat. Ectopic fat accumulation may affect many organs, but primarily leads to health issues in the liver, pancreas and skeletal muscles. As a result, patients with FPL experience an increased incidence of potentially life-threatening pancreatitis, diabetes and extreme insulin resistance, as well as the accumulation of harmful fat in the liver, known as hepatic steatosis. Without enough fat tissue, an FPL patient's metabolic system, which regulates energy use, also falls out of balance. We believe that the robust triglyceride reduction and the improvements in glucose control and insulin sensitivity we observed in our Phase 2 program support development of volanesorsen for patients with FPL.
Burden of Disease
Familial Chylomicronemia Syndrome
Due to the high levels of triglycerides in their blood, patients with FCS may suffer from many chronic health issues including severe, recurrent abdominal pain, fatigue, high risk of life-threatening pancreatitis and abnormal enlargement of the liver or spleen. When triglyceride levels are very high (greater than 750 mg/dL), they form chylomicrons, which are large particles that can block pancreatic ducts causing an inflammatory cascade that ultimately results in pancreatitis, which is when the organ begins to digest itself. The presence of excess chylomicrons results in blood that is milky-white in appearance due to the excess of these fat particles. Patients with FCS may also experience organ failure and pancreatic necrosis. Some of these debilitating conditions may also result in lengthy hospitalization stays, including time in the intensive care unit. In addition, they also suffer from daily conditions that can negatively impact their quality of life, including neuropsychiatric symptoms such as memory loss, dementia, mild depression and cognitive impairment (described as brain fog and forgetfulness), as well as gastrointestinal symptoms, including nausea and vomiting. In addition, patients with FCS have to adhere to a very low-fat diet, which is extremely burdensome. Generally, patients try to consume no more than the equivalent of approximately one tablespoon of olive oil per day. As a result of these factors, patients with FCS are often unable to work, adding to the burden of the disease.
In order to quantify the burden of FCS on patients and the healthcare system, we, in conjunction with patients and clinicians, developed and conducted a global FCS patient survey called IN-FOCUS. We recruited approximately 170 patients, from multiple countries, to take this survey. We completed an interim analysis on the first 60 respondents which showed that pain, fatigue and chronic pancreatitis impact employment and productivity. Final analysis of the study was consistent with the interim analysis. The final analysis has been submitted for publication.
Other key findings from the interim analysis were:
| ● |
Age: The majority of respondents were between 20 and 40 years of age, with an average age of 36.
|
| ● |
Diagnosis: Respondents saw an average of five physicians (range: 1-30) before receiving a diagnosis of FCS.
|
| ● |
Symptoms: All of the symptoms described below occurred daily to several times per week and were moderate to very severe in magnitude.
|
| ● |
Physical symptoms: generalized abdominal pain, bloating, indigestion and lack of appetite as well as generalized weakness and fatigue.
|
| ● |
Emotional symptoms: anxiety about their overall health due to FCS, constant uncertainty about having an attack of pain or acute pancreatitis at any time, embarrassment about always thinking about and planning for food and anxiety about eating food prepared by someone else.
|
| ● |
22% of patients reported feeling depressed, as compared to a 6.7% rate of depression diagnosis in the general adult U.S. population.
|
| ● |
33% of patients reported constant anxiety, fear or worry about having an attack of pain or acute pancreatitis at any time.
|
| ● |
Cognitive symptoms: difficulty concentrating, "brain fog" (i.e. lack of thought clarity), impaired judgment, forgetfulness and recent memory loss.
|
| ● |
Acute Pancreatitis: Lifetime number of pancreatitis attacks ranged from 1-31+, with a mean of 12. Additionally, over their lifetimes, 40% of those hospitalized for acute pancreatitis were readmitted within 30 days of their discharge.
|
| ● |
Diet: The average fat intake of respondents was 20-21g/day. 87% of the respondents noted that managing their diet was extremely challenging.
|
| ● |
Employment: Only 22% of patients reported full time employment and 20% were unemployed.
|
| ● |
Absences: Time off from work due to FCS ranged from 0-61+ days with a mean of 30 days in the past 12 months, compared with the U.S. Bureau of Labor Statistics reported average absence of four to five days per calendar year.
|
Davidson et. al. The burden of familial chylomicronemia syndrome: interim results from the IN-FOCUS study. Expert Rev Cardiovasc Ther. May; 15(5): 415-423.
While all the complications of FCS cause patients to have a lower quality of life, pancreatitis is the most serious consequence of the disease. The mortality rate of acute pancreatitis ranges between six and eight percent. Some FCS patients have multiple episodes of acute pancreatitis in a year. Further, pancreatitis attacks generally become more frequent from the teenage years through patients' 30s and 40s. Patients are often admitted to the intensive care unit for further monitoring and hospitalization can last for multiple days. In severe cases, patients can have bleeding into the pancreas, serious tissue damage, infection and cyst formation, as well as damage to other vital organs such as the heart, lungs and kidneys. Further, even a single episode of acute pancreatitis can permanently damage the pancreas, potentially leading to pancreatic deficiency, which can cause digestive issues, oily stool and insulin-dependent diabetes. The persistent and often severe abdominal pain that FCS patients may experience may also be indicative of episodes of undiagnosed pancreatitis. There is no specific drug treatment for acute pancreatitis and typically physicians manage pancreatitis with intravenous fluids and pain medications in the hospital.
13
Acute pancreatitis caused by high triglycerides can result in more days in the hospital, with a risk of irreversible organ damage and premature death. For example, a 2015 study published by Nawaz et. al. in The American Journal of Gastroenterology demonstrated that acute pancreatitis caused by high triglycerides (triglycerides >1000 mg/dL) can have serious manifestations, and can be substantially worse than pancreatitis from other causes (triglycerides <150 mg/dL), leading to longer median hospital stays, increased need for intensive care, a higher rate of pancreatic necrosis, more frequent persistent (i.e. >48 hr.) organ failure and higher rates of mortality, as illustrated in the figure below:
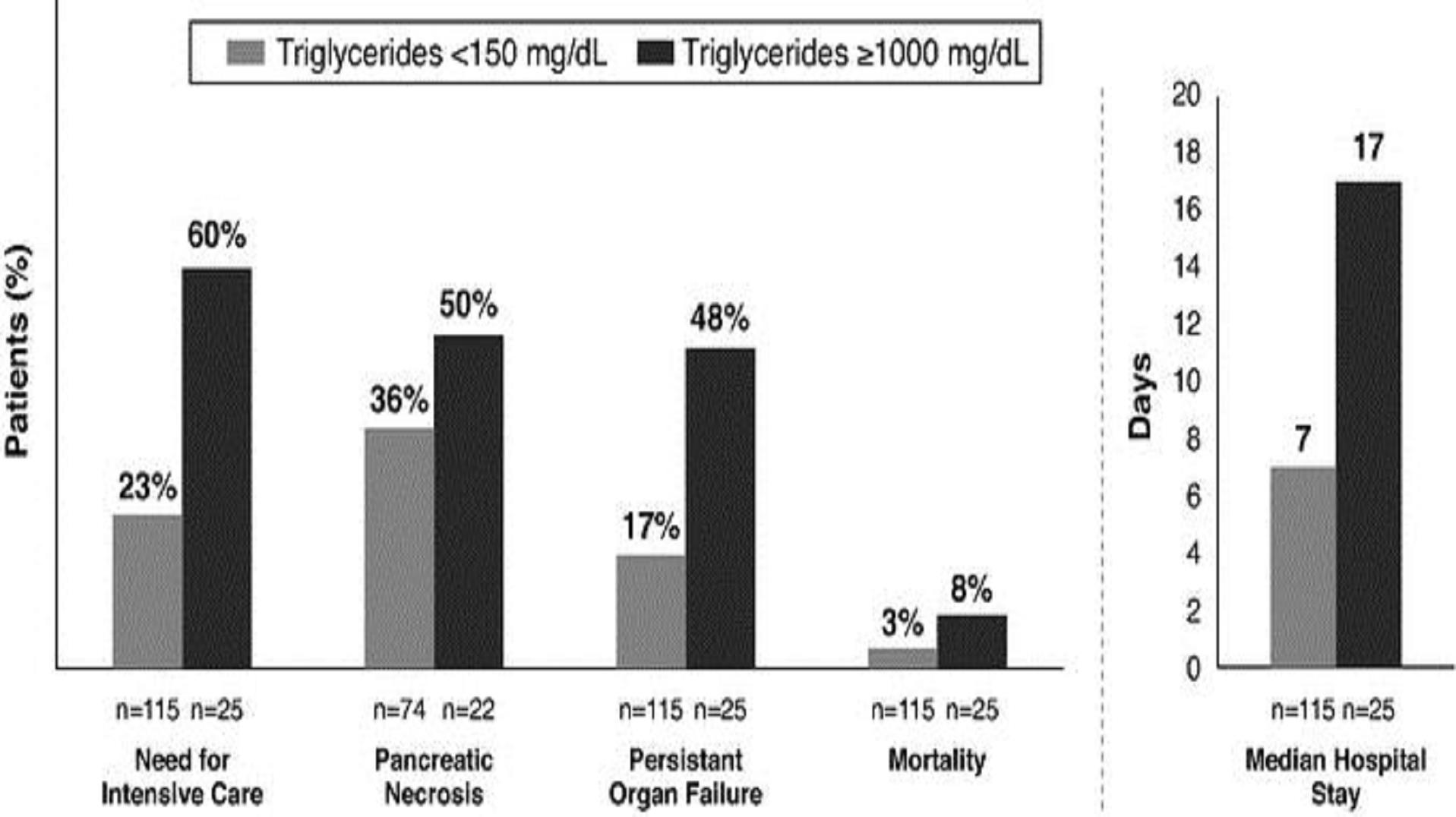
Adapted from Nawaz et. al. 2015
Familial Partial Lipodystrophy
FPL is a rare lipid disorder characterized by abnormal fat distribution across the body and a range of metabolic abnormalities, including severe insulin resistance, dyslipidemia and hypertriglyceridemia, hepatic steatosis and, in affected women, features of hyperandrogenism. People with FPL often present with polycystic ovarian syndrome or unusually insulin-resistant diabetes and are at increased risk of acute pancreatitis in addition to long-term, progressive consequences including premature cardiovascular disease and liver disease, resulting in cirrhosis. They are unable to store fat or triglycerides in normal fat stores, so excess triglycerides are stored in the liver and muscle and accumulate at high levels in the bloodstream. In order to quantify the burden of FPL on patients and the healthcare system, we, in conjunction with patients and clinicians, developed and are currently conducting a global FPL patient survey called REVEAL.
Volanesorsen Clinical Development
Phase 2 studies
We conducted a randomized, double-blind, placebo-controlled, dose-ranging, Phase 2 study to evaluate volanesorsen in both untreated patients with fasting triglyceride levels between 350 mg/dL and 2,000 mg/dL (volanesorsen monotherapy cohort) and in patients receiving stable fibrate therapy who had fasting triglyceride levels between 225 mg/dL and 2,000 mg/dL (volanesorsen—fibrate cohort). We randomly assigned eligible patients to receive either volanesorsen, at doses ranging from 100 to 300 mg, or placebo, once weekly for 13 weeks. The primary endpoint was percent change in apoC-III level from baseline. We designed the secondary endpoints to evaluate the effects of volanesorsen on additional lipid parameters, including triglyceride, LDL and HDL levels. We also investigated pharmacokinetic and pharmacodynamic effects of volanesorsen in these patients. We published the results of this study, which was the first to support the role of apoC-III as a key regulator of triglyceride metabolism in a wide variety of patients with hypertriglyceridemia, in the New England Journal of Medicine in 2015.
A total of 57 patients were treated in the volanesorsen monotherapy cohort (41 received volanesorsen and 16 received placebo), and 28 patients were treated in the volanesorsen—fibrate cohort (20 received volanesorsen and 8 received placebo). The mean baseline triglyceride levels in the two cohorts were 581 mg/dL and 376 mg/dL, respectively. Treatment with volanesorsen resulted in dose-dependent, highly consistent and prolonged decreases in plasma apoC-III and in triglyceride levels when clinicians administered the drug as a single agent and as an add-on to fibrates.
14
The tables below illustrate the triglyceride changes in aggregate across the study cohorts:
Monotherapy Cohort
|
Dose (mg) / Patients (1)
|
Mean Baseline
Triglyceride Level
(mg/dL)
|
Average of Day 85 and
92 Triglyceride Level
(mg/dL)
|
Mean Change (%)
|
p-value
|
||||
|
100 (n=11)
|
591
|
312
|
-31.3
|
p = 0.015
|
||||
|
200 (n=13)
|
642
|
235
|
-57.7
|
p = 0.001
|
||||
|
300 (n=11)
|
559
|
139
|
-70.9
|
p < 0.001
|
||||
|
Placebo (n=16)
|
523
|
547
|
20.1
|
Volanesorsen-Fibrate Cohort
|
Dose (mg) / Patients (1)
|
Mean Baseline
Triglyceride Level
(mg/dL)
|
Average of Day 85 and
92 Triglyceride Level
(mg/dL)
|
Mean Change (%)
|
p-value
|
||||
|
200 (n=8)
|
282
|
141
|
-51.0
|
p = 0.008
|
||||
|
300 (n=10)
|
394
|
134
|
-63.9
|
p = 0.002
|
||||
|
Placebo (n=8)
|
457
|
372
|
-7.7
|
| (1) |
The number of patients in the tables above represent those who completed the study. Additional patients received at least one dose of volanesorsen, but discontinued treatment prior to completing the study.
|
15
The graphics below illustrate certain changes as seen in the study published in the New England Journal of Medicine in 2015:
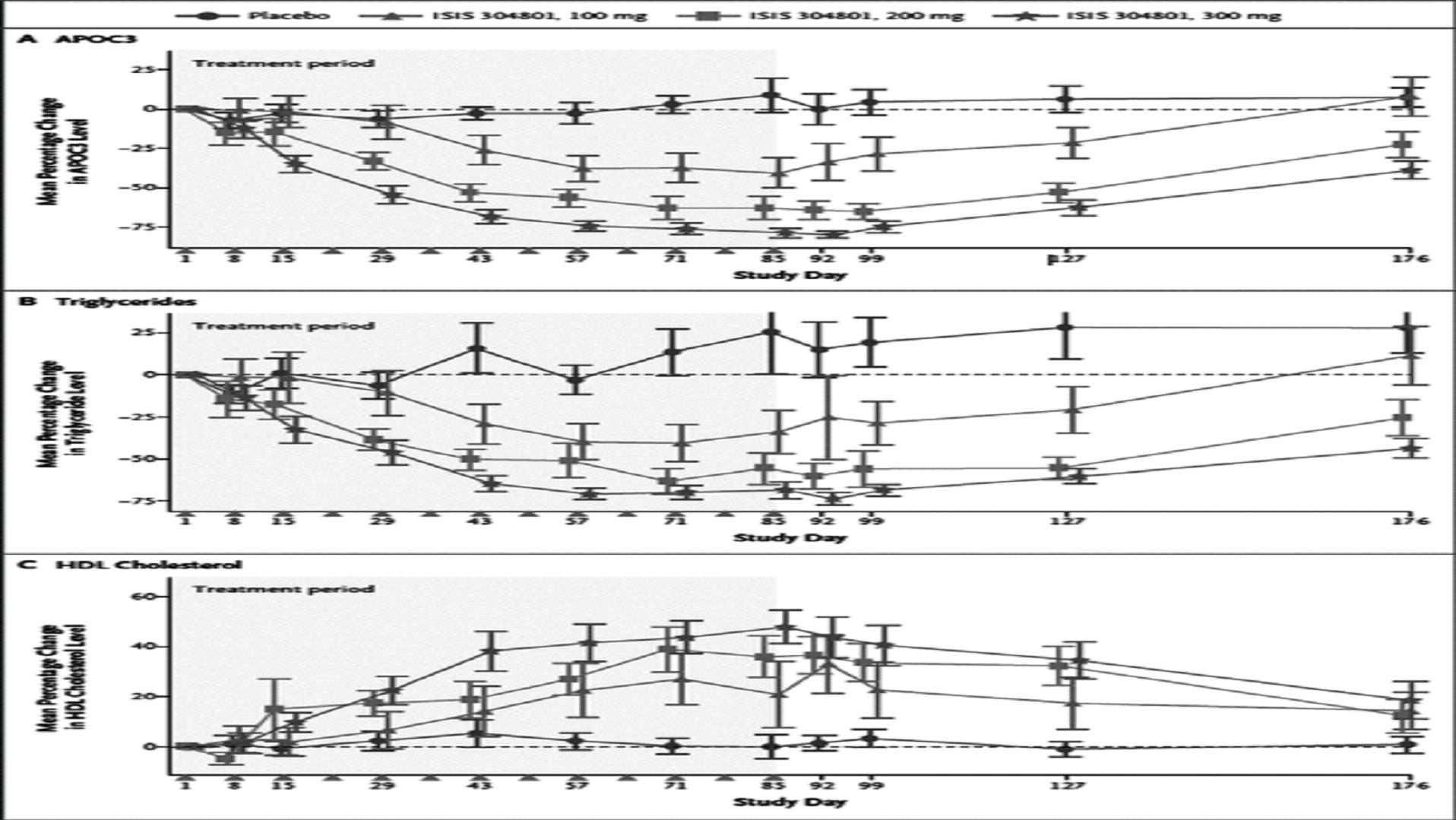
| * |
The graphics above show the mean percent changes from baseline over time in levels of apoC-III, triglycerides and HDL cholesterol in the cohort that received ISIS 304801 (which we refer to as volanesorsen) monotherapy or placebo. Triangles indicate dosing days. I bars indicate standard errors. N Engl J Med 2015; 373:438-447.
|
As part of our Phase 2 study, we included an open label cohort using a 300 mg dose of volanesorsen in three FCS patients. At baseline, apoC-III levels were elevated to two to three times normal levels in all three patients. The patients' apoC-III levels fell dramatically during the first two weeks of treatment with volanesorsen with reductions in apoC-III from baseline ranging from approximately 70% to 90%. Baseline triglyceride levels in the three patients varied, but were all above 1,000 mg/dL, and fell rapidly during the first two weeks of treatment in parallel with decreases in apoC-III, with triglyceride levels in all patients dropping below 500 mg/dL during the study. Triglyceride levels at day 85, the time of the primary analysis, were 56% to 86% lower than at baseline, with absolute reductions of 790 mg/dL to 1,796 mg/dL. The triglyceride levels of patients two and three, who had baseline triglyceride levels greater than 2,000 mg/dL, dropped to as low as 251 mg/dL and 234 mg/dL, respectively, during the treatment period. After cessation of dosing on day 85, triglycerides slowly began to return to pre-treatment levels.
16
The figures below further illustrate these results:
Fasting apoC-III levels in FCS patients treated with 300 mg of volanesorsen
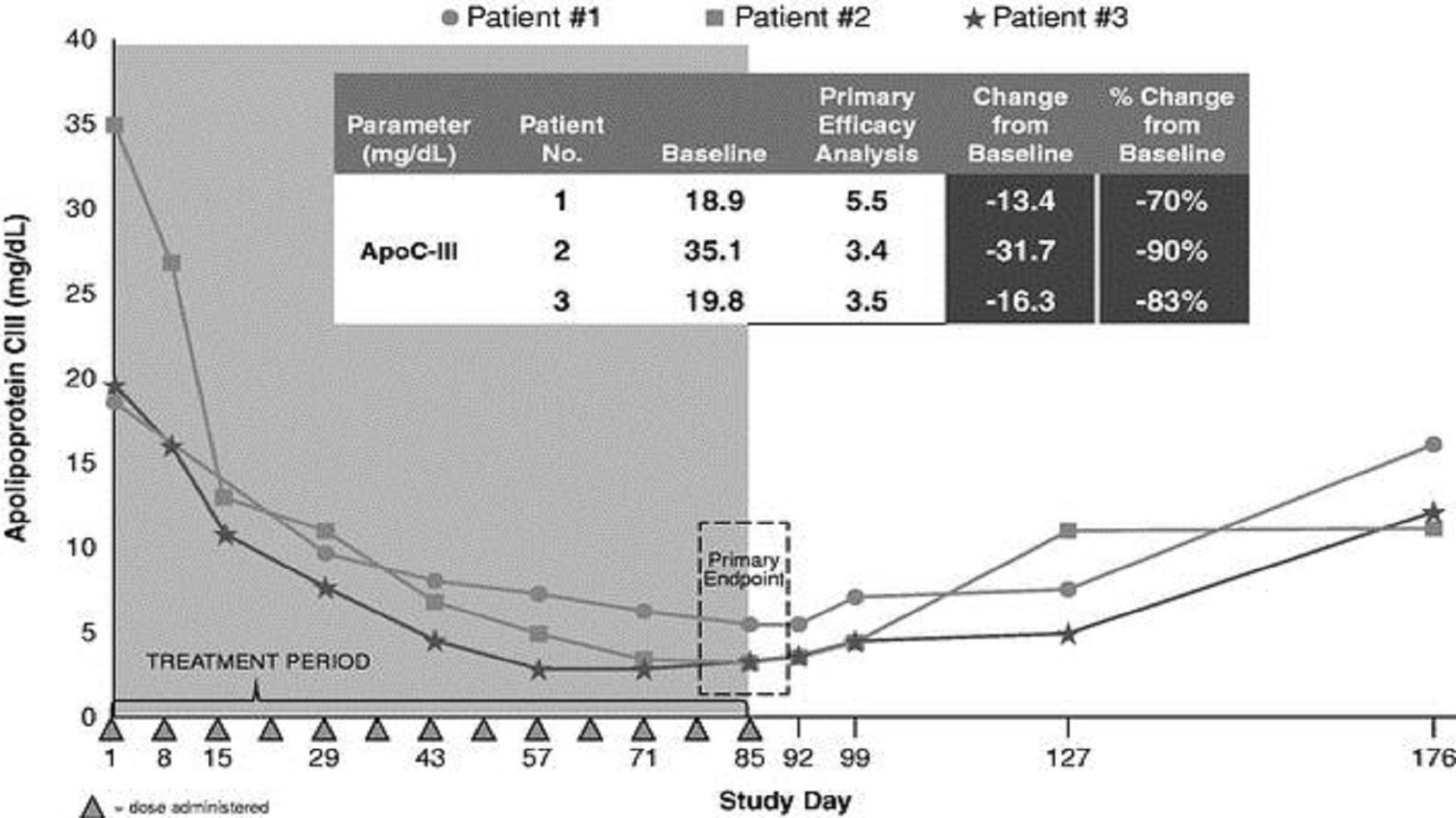
Fasting triglyceride levels in FCS patients treated with 300 mg of volanesorsen
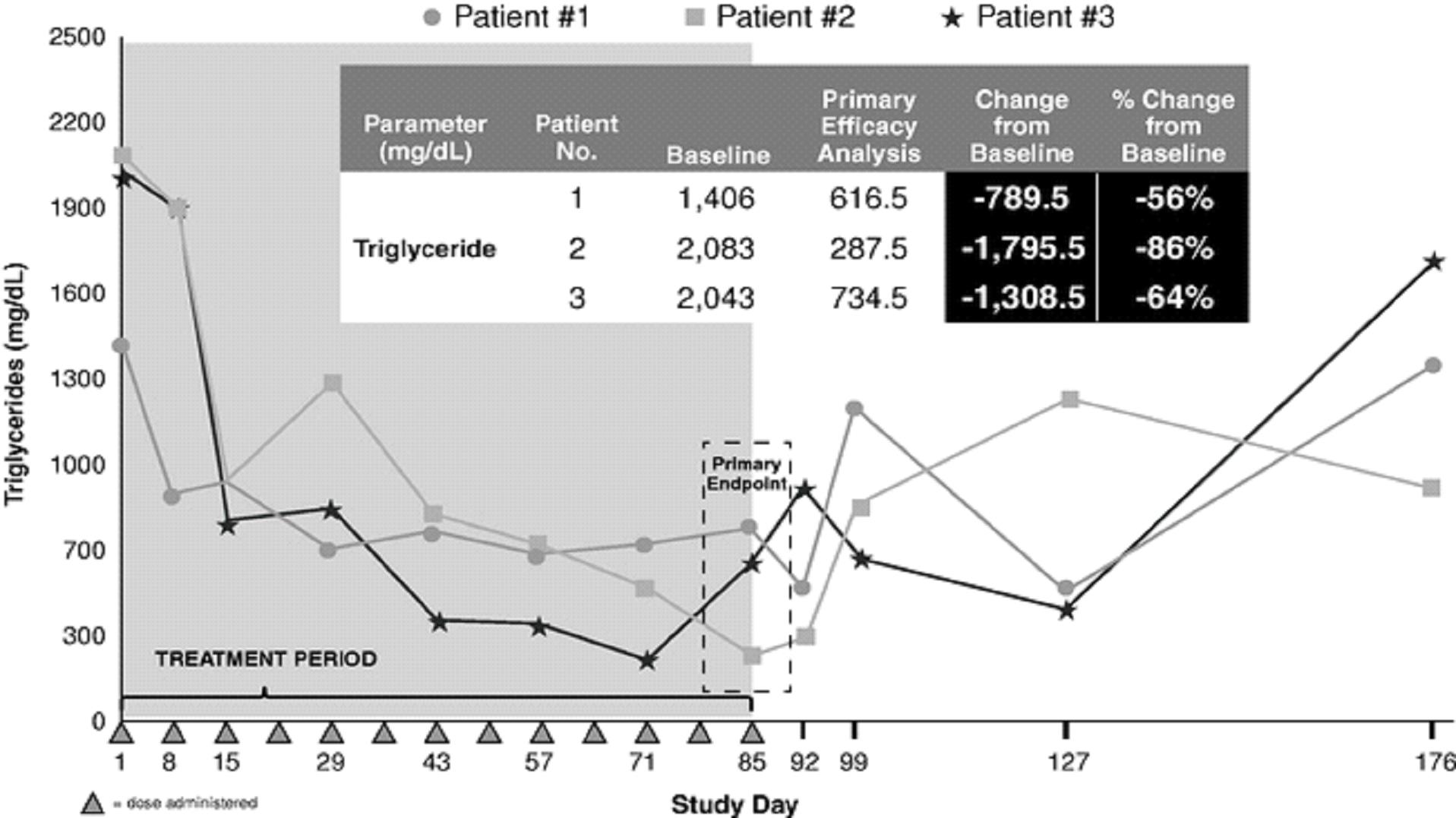
We published the results of the FCS patient cohort in the New England Journal of Medicine in 2014 because they revealed for the first time that apoC-III raised triglycerides by two different pathways, one dependent on LPL and one independent of LPL. It was previously thought that apoC-III raised triglycerides primarily by inhibiting LPL, which breaks down triglycerides in the blood. Patients with FCS lack LPL activity and yet inhibiting apoC-III with volanesorsen nevertheless dramatically lowered triglyceride levels.
Additionally, in a separate Phase 2 clinical study, patients with high triglycerides and type 2 diabetes treated with volanesorsen achieved significant reductions in apoC-III and triglyceride levels and exhibited improvements in measures of glucose control and insulin sensitivity. We observed a 57% improvement in insulin sensitivity in patients treated with volanesorsen compared to patients receiving placebo, as measured by a hyperinsulinemic euglycemic clamp, which assesses how well the body uses insulin to remove sugar, in the form of glucose, from the blood and maintain normal blood sugar levels.
17
No safety concerns were identified in our Phase 2 study that included the monotherapy group, the volanesorsen-fibrate group and the FCS patient group. Injection site reactions occurred across all three groups. These reactions were typically mild redness or pain, did not get worse or lead to other issues and resolved spontaneously. Other safety assessments, including vital signs, electrocardiographic findings and urinalysis results, were clinically unremarkable. In addition, there was no clinical or laboratory evidence of drug-to-drug interactions in patients receiving concomitant medications, including statins, fibrates and glucose-lowering agents.
A similar safety profile was seen in the study involving patients with high triglycerides and type 2 diabetes. No patients treated in this study with volanesorsen discontinued treatment with the study drug because of adverse events. Across the Phase 2 studies, a potentially treatment-related SAE was serum sickness-like reaction where the patient fully recovered.
Phase 3 program and regulatory approach
Volanesorsen has completed a Phase 3 clinical program for the treatment of FCS and is currently in Phase 3 clinical development for the treatment of FPL. The Phase 3 FCS program includes the APPROACH and COMPASS studies. The Phase 3 FPL program includes these same two studies, as well as the BROADEN study.
APPROACH Study
APPROACH is a randomized, double-blind, placebo-controlled study of 300 mg of volanesorsen administered by a subcutaneous injection in patients with FCS. Patients in the study were treated once a week for a period of one year. The primary endpoint in this study was percent change, relative to baseline, in fasting triglycerides at three months. In addition, we designed the secondary endpoints to allow us to further evaluate changes in triglycerides, changes in frequency and severity of abdominal pain and pancreatitis, and levels of hepatosplenomegaly, which is abnormal swelling of the spleen and liver. The patients were randomized 1:1, receiving either volanesorsen or placebo. After one year of dosing, patients were eligible to roll over into an open label extension study, in which all patients receive volanesorsen. APPROACH closed enrollment in December 2015 with a total of 66 patients. We dosed the last patient with his/her last dose in the study in January 2017. We reported top-line data from this study in March 2017.
The average incoming triglyceride level of patients in the study was 2,209 mg/dL. Patients treated with volanesorsen experienced clinically meaningful benefits in triglyceride levels, consistent with the Phase 2 experience described above as well as additional disease benefits, as summarized below. For the primary endpoint of the study, volanesorsen-treated patients (n=33) achieved a statistically significant (p<0.0001) mean reduction in triglycerides of 77% from baseline after three months of treatment, compared to a mean increase of 18% in placebo-treated patients (n=33). This represented a mean absolute reduction of 1,712 mg/dL in treated patients.
| ● |
The treatment effect was maintained over the 52-week treatment period.
|
| ● |
50% of the treated patients achieved triglyceride levels less than 500 mg/dL after three months of treatment, a commonly accepted threshold for pancreatitis risk. By comparison, none of the placebo-treated patients achieved this level at the analysis time points (p<0.003). Additionally, 76.7% of the treated patients, as compared to 9.7% of the placebo-treated patients (p=0.0001), achieved triglyceride levels less than 750 mg/dL after three months of treatment, a level above which chylomicron formation begins.
|
| ● |
A statistically significant reduction in abdominal pain was observed in volanesorsen-treated patients compared to placebo-treated patients (p=0.02) who reported abdominal pain before treatment in the study.
|
| ● |
Volanesorsen-treated patients who had a documented history of recurrent pancreatitis attacks in the five years prior to the study experienced no attacks during the 52-week treatment period (p=0.02) as compared to the placebo. Further details are shown in the figure below:
|
|
Placebo
|
Volanesorsen
|
||||||
|
Patients
|
Events
|
Patients
|
Events
|
||||
|
Patients with Multiple Adjudicated Events in Past 5 Years
|
4
|
17
|
7
|
24
|
|||
|
Events During Study
|
3
|
4
|
0
|
0
|
|||
|
p-value
|
p = 0.02
|
||||||
The most common adverse event in the volanesorsen-treated group of patients was injection site reactions, which were mostly mild. In addition, declines in platelet counts were observed in many patients and some patients discontinued the study because of platelet declines. These platelet declines were not clinically significant in most patients and were generally well managed with monitoring and dose adjustment. Some patients also discontinued participation in the APPROACH study due to other non-serious adverse events, including sweating and chills, severe fatigue, rash and injection site reaction. In the APPROACH and the open label extension studies, the potentially treatment-related SAEs observed were serious platelet events (grade 4 thrombocytopenia). These events resolved without complication after cessation of dosing. Enhanced monitoring was implemented during the study to support early detection and management of these issues. Since implementation of the enhanced monitoring, serious platelet events have been infrequent. Platelet monitoring is occurring once weekly in our open label extension study and is planned to occur once every other week in the commercial setting for all patients on volanesorsen. In the study, there were no treatment-related liver adverse events, including no increases in liver fat. There were no treatment-related renal adverse events. There were no deaths and no treatment-related cardiovascular events in the study. We believe our greater involvement with physicians and patients, which will be a core focus of the education and support provided by our patient-centric commercial approach, should allow us to better maintain patients on volanesorsen therapy.
18
COMPASS Study
COMPASS is a randomized, double-blind, placebo-controlled Phase 3 study of 300 mg of volanesorsen administered by subcutaneous injection in patients with elevated triglyceride levels (greater than 500 mg/dL). Patients in the study were dosed once a week for a period of six months. The primary endpoint is percent change, relative to baseline, in fasting triglycerides at week 13. We designed the secondary endpoints to allow us to further evaluate changes in triglycerides, incidence of pancreatitis and parameters associated with insulin resistance and diabetes. The patients were randomized 2:1, receiving either volanesorsen or placebo. We completed enrollment in this study in May 2016 with 113 patients dosed. We dosed the last patient with his/her last dose in the study in November 2016.
In December 2016, we announced that the COMPASS study met its primary endpoint. The average incoming triglyceride level of patients in the study was 1,261 mg/dL. Patients treated with volanesorsen experienced clinically meaningful benefits in triglyceride levels, consistent with the Phase 2 experience described above, and as summarized below:
| ● |
For the primary endpoint of the study, volanesorsen-treated patients (n=75) achieved a statistically significant (p<0.0001) mean reduction in triglycerides of 71% from baseline after 13 weeks of treatment, compared with a mean reduction of 0.9% in placebo-treated patients (n=38). This represented a mean absolute reduction of 869 mg/dL in treated patients. The treatment effect observed was maintained through the end of the 26-week treatment period.
|
| ● |
In a subset of seven patients with FCS, whose average incoming triglyceride level was 2,280 mg/dL, volanesorsen-treated patients (n=5) achieved a mean reduction in triglycerides of 73% from baseline after 13 weeks of treatment, compared with a mean increase of 70% in placebo-treated patients (n=2). This represented a mean absolute reduction of 1,511 mg/dL in treated patients. The treatment effect observed was maintained through the end of the 26-week treatment period. None of the reductions in triglyceride levels in the FCS group were statistically significant.
|
| ● |
In addition, 82% of patients treated with volanesorsen, including three of the volanesorsen-treated FCS patients, achieved triglyceride levels less than 500 mg/dL, a commonly accepted threshold for pancreatitis risk, after 13 weeks of treatment, compared to 14% of placebo-treated patients (p<0.0001).
|
| ● |
Further, we observed a statistically significant reduction in pancreatitis events with volanesorsen treatment compared to placebo (p=0.036), with five pancreatitis events in the placebo-treated cohort and no pancreatitis events in the volanesorsen-treated cohort during the treatment period.
|
The most common adverse event in the volanesorsen-treated group of patients was injection site reactions, which were mostly mild. In this study, patients discontinued treatment due to injection site reactions and other adverse events. None of the FCS patients in the study discontinued. There were no deaths or cardiovascular events in the study. In addition, there were no serious platelet events in the study. Serum sickness was reported as a potentially related serious adverse event in the volanesorsen arm. The patient fully recovered.
BROADEN Study
BROADEN is a randomized, double-blind, placebo-controlled study of 300 mg of volanesorsen administered by a subcutaneous injection in patients with FPL. Patients in the study will be dosed once weekly for a period of one year. The primary endpoint is the percent change, relative to baseline, in fasting triglycerides at week 13. We designed the secondary endpoints to allow us to further evaluate changes in triglycerides, rates of pancreatitis, parameters associated with insulin resistance and diabetes, and changes in liver fat. The patients are randomized 1:1, receiving either volanesorsen or placebo. After one year of dosing, patients are eligible to roll over into an open label extension period in which all patients will receive volanesorsen. We plan to report results from the BROADEN study in 2019.
Volanesorsen Clinical Data Summary
In both APPROACH and COMPASS, we saw reductions in rates of pancreatitis, one of the most important and impactful symptoms of FCS, in patients treated with volanesorsen. The table below describes the number of pancreatitis attacks in each study and also shows the combined rate of pancreatitis across both studies. While the reduction in the total number of pancreatitis attacks in APPROACH was not statistically significant due to the small sample size, both COMPASS (p=0.036) and the combined data (p=0.019) showed statistically significant reductions in pancreatitis attacks.
|
Incidence of Pancreatitis
|
Placebo
|
Volanesorsen
|
||||
|
APPROACH (n)
|
33
|
33
|
||||
|
# of pancreatitis events
|
4
|
1
|
||||
|
COMPASSS (n)
|
38
|
75
|
||||
|
# of pancreatitis events
|
5
|
0
|
(p = 0.036)
|
|||
|
COMPASS + APPROACH (n)
|
71
|
108
|
||||
|
# of pancreatitis events
|
9
|
1
|
(p = 0.019)
|
Based on what we believe is a favorable risk-benefit profile, supported by data from both APPROACH and COMPASS, we filed for marketing authorization in the U.S., Canada and EU in the third quarter of 2017 for volanesorsen to treat patients with FCS. We plan to file marketing applications to treat patients with FCS in regions outside of the U.S., Canada and EU as soon as practical starting in 2018.
19
Volanesorsen Commercial Opportunity
If we are successful in obtaining regulatory approval to commercialize volanesorsen to treat patients with FCS and FPL on our anticipated timeline, we believe volanesorsen could be the only drug on the market in the United States specifically approved for these indications. We also believe volanesorsen could be the only specifically approved drug for these indications on the market in Europe. We believe that volanesorsen could, over time, be used in a significant proportion of FCS and FPL patients, given that there are no currently approved, effective treatments for FCS or FPL.
We estimate there are 3,000 to 5,000 FCS patients in treatable markets and an additional 3,000 to 5,000 FPL patients globally. As with many orphan diseases, however, patients with FCS and FPL are underdiagnosed and, as a result, we believe the population sizes may be underestimated. Based on our work so far, we believe that the number of patients in treatable markets is closer to the higher end of the range for FCS. We have underway an epidemiology project to gain insight into the FCS population. In addition, we believe that our efforts to raise awareness of these diseases and improve diagnosis with simplified clinical diagnosis criteria, plus the availability of a drug, could significantly improve identification of patients and result in larger identified patient populations. We are building a database of identified patients by working with physicians and patient organizations and through improved diagnosis and referrals. In order to protect patient confidentiality, we do not include patient-specific information in the database. We add blinded patient information to our database through communication with physicians, patient organizations and other tools, such as surveys conducted in partnership with medical societies and electronic medical record database searches. We plan to use our database to help us engage with physicians who may have patients who could potentially benefit from our drugs.
Due to the specialized nature of managing FCS and FPL, there are a limited number of treating physicians.
| ● |
In the United States, there are approximately:
|
● 45 lipid treatment hubs; and
● 200 to 300 lipid specialists, with an additional 300 to 400 endocrinologists specializing in lipids.
| ● |
In Europe, there are approximately:
|
● 75 specialized lipid treatment hubs; and
● 400 to 600 physician specialists who treat lipid disorders.
AKCEA-APO(a)-LRx
Overview
We are developing AKCEA-APO(a)-LRx for patients who are at significant risk of CVD because of their elevated levels of Lp(a). AKCEA-APO(a)-LRx inhibits the production of the apo(a) protein, thereby reducing Lp(a). Lp(a) is a very atherogenic and thrombogenic form of LDL. Elevated Lp(a) is recognized as an independent, genetic cause of coronary artery disease, heart attack, stroke and peripheral arterial disease. Inhibiting the production of apo(a) in the liver reduces the level of Lp(a) in blood, potentially slowing down or reversing cardiovascular disease in patients with hyperlipoproteinemia(a), a condition in which individuals have levels of Lp(a) greater than 60 mg/dL. Lp(a) is difficult to inhibit using other technologies, such as small molecules and antibodies; there are multiple genetically determined forms of the apo(a) molecule and creating a small molecule or antibody that can interact with multiple targets is difficult. We believe antisense technology is particularly well suited to address hyperlipoproteinemia(a) because it specifically targets the RNA that codes for all forms of the apo(a) molecule. As a result, it can stop the production of all the forms of the protein. Furthermore, we believe addressing elevated Lp(a) is the next important horizon in lipid-focused treatment and, through our collaboration with Novartis, we plan to develop AKCEA-APO(a)-LRx to treat patients with established cardiovascular disease in whom hyperlipoproteinemia(a) likely plays a causal role.
We have completed a Phase 1/2 study with AKCEA-APO(a)-LRx in patients with hyperlipoproteinemia(a) and we reported the results at the AHA meeting in November 2015. In this clinical study, we observed significant and sustained reductions in Lp(a) of up to 97% with a mean reduction of 79% after only a single, small volume dose of AKCEA-APO(a)-LRx. With multiple doses of AKCEA-APO(a)-LRx, we observed even greater reductions of Lp(a) of up to 99% with a mean reduction of 92%. Based on these results, we started a Phase 2b dose-ranging study of AKCEA-APO(a)-LRx in patients with hyperlipoproteinemia(a) and established CVD. We completed enrollment in this study in January 2018 and expect to report data from this study in the second half of 2018. We have initiated a strategic collaboration with Novartis for this drug. See "—Our Strategic Collaboration with Novartis" for additional information.
Disease Background
Despite the management of LDL-C with statins and other therapies, the incidence of CVD continues to rise dramatically. Lipid disorders are a cause of this continuing rise. Hyperlipoproteinemia(a), which is present in approximately 20% of the general population, causes CVD.
20
Currently, there is no effective drug therapy to specifically and robustly lower elevated levels of Lp(a). Lp(a) levels are determined at birth and, therefore, lifestyle modification, including diet and exercise, do not impact Lp(a) levels. Statins do not have significant effects on Lp(a) levels. Further, a new class of drugs that lower LDL-C and modestly lower Lp(a) levels, known as PCSK9 inhibitors, inactivate a protein in the plasma that regulates the number of LDL receptors on the liver cell surface, thereby capturing and removing additional LDL particles from the blood. While PCSK9 inhibitors reduce Lp(a) by approximately 25%, we believe this level of reduction is unlikely to materially reduce the risk of cardiovascular events related to hyperlipoproteinemia(a). The only currently known effective way to significantly reduce plasma Lp(a) is to physically remove the particles from blood through a process called apheresis. In this process, the patient's blood is filtered through a machine where the LDL-C and Lp(a) particles are removed and the blood is returned to the patient's body. Since 2010, apheresis has been an approved therapy in Germany to treat patients with hyperlipoproteinemia(a), but it is expensive, time consuming and only performed by a small number of centers worldwide. Lp(a) apheresis has been shown to lower the rate of cardiovascular events, providing support that lowering Lp(a) can provide therapeutic benefit.
A number of expert groups, including the National Institutes for Health, European Society of Cardiology and the National Lipid Association, and publications have stated that Lp(a) is an independent cause of cardiovascular risk. The authors of three such publications evaluated data from over 180,000 participants and used statistical and genetic approaches to evaluate the correlation between Lp(a) levels and cardiovascular risk. The specific techniques the authors used were epidemiological/meta-analyses, Mendelian randomization and genome wide associations. In each technique used, the authors demonstrated a clear relationship between elevated levels of Lp(a) and increased cardiovascular risk.
The graphics below further illustrate these correlations:
AKCEA-APO(a)-LRx Clinical Development
Phase 1/2a studies
We conducted a Phase 1/2a single and multiple ascending dose study with AKCEA-APO(a)-LRx in 58 people with elevated levels of Lp(a). Individuals treated with AKCEA-APO(a)-LRx achieved significant dose-dependent reductions in Lp(a), with the largest reductions at day 30. The results of this study were published in the Lancet in 2016. In the single dose portion of the study, we investigated five dose levels of AKCEA-APO(a)-LRx ranging from 10 mg to 120 mg, compared to placebo.
The results from this portion of the study are illustrated in the tables below:
Single Dose; Randomized 3:1
|
Dose
|
10mg
|
20mg
|
40mg
|
80mg
|
120mg
|
placebo
|
||||||
|
Number of people
|
3
|
3
|
3
|
6
|
6
|
7
|
||||||
|
Mean change from baseline at day 30 (%)
|
-26%
|
-33%
|
-44%
|
-79%
|
-85%
|
3%
|
In the multiple ascending dose portion of the study, we investigated three dose levels of AKCEA-APO(a)-LRx, compared to placebo. At each dose level, people received three doses on alternate days during the first week and then a single dose once a week for the next three weeks.
The results from this portion of the study are illustrated in the tables below:
Multiple Ascending Dose; Randomized 4:1
|
Dose
|
10mg
|
20mg
|
40mg
|
placebo
|
||||
|
Number of people
|
8
|
8
|
8
|
6
|
||||
|
Mean change from baseline at day 30 (%)
|
-66%
|
-80%
|
-92%
|
-9%
|
21
The graphic below shows the mean percent change in Lp(a) in the multiple ascending dose groups:
| * |
The asterisks represent p-values as follows: *p<0.05; **p≤0.01; ***p≤0.001. Arrows indicate dosing at days 1, 3, 5, 8, 15, and 22. P-values are for the primary efficacy endpoint at day 36 as determined by the Exact Wilcoxon Rank Sum comparing AKCEA-APO(a)-LRx versus placebo.
|
AKCEA-APO(a)-LRx was generally safe and well tolerated in the study, which supported continued development. There were no injection site reactions or flu-like symptoms reported. Additionally, in the multiple-dose cohorts, there were no clinically relevant changes in electrocardiograms or vital signs, or in safety laboratory parameters including liver and kidney markers, hematology (including platelet count), coagulation (including activated partial thromboplastin time), inflammation (high sensitivity C Reactive Protein), complement and urinalysis.
Phase 2b study
We are conducting a Phase 2b study of AKCEA-APO(a)-LRx in patients with hyperlipoproteinemia(a) and established CVD. The goal of the study is to determine the dose level and frequency for use in a future cardiovascular outcome study. The study is a randomized, double-blind, placebo-controlled, dose-ranging study of AKCEA-APO(a)-LRx administered by a subcutaneous injection. Patients in the study will be dosed for a period of six months, and a portion of the patients will continue for up to one year. We are testing multiple different dosing levels and regimens. The primary endpoint is the percent change in plasma Lp(a) from baseline at six months. The secondary endpoints will be changes in LDL-C, responder analyses, and other key lipid parameters. Further, we are evaluating safety and pharmacokinetics of the different doses. We enrolled over 270 patients, randomized 5:1, to receive AKCEA-APO(a)-LRx or a matched quantity of placebo. We completed enrollment into the study in January 2018 and plan to report topline data in the second half of 2018.
22
AKCEA-APO(a)-LRx Commercial Opportunity
Elevated levels of Lp(a) are associated with increased cardiovascular risk and lowering Lp(a) may reduce the risk. We estimate the eligible population to be 8.5 to 11 million people globally. We believe that positive results from a large cardiovascular outcome study will be required to support marketing authorization for the treatment of these patients. If Novartis exercises its option, it would conduct, at its expense, such a study pursuant to our strategic collaboration and, if approved, to commercialize AKCEA-APO(a)-LRx for these patients.
AKCEA-ANGPTL3-LRx
Overview
We are developing AKCEA-ANGPTL3-LRx to treat multiple lipid disorders. Studies have shown that elevated levels of the ANGPTL3 protein are associated with an increased risk of premature heart attacks, increased arterial wall thickness and multiple metabolic disorders, such as insulin resistance. In contrast, people with lower levels of ANGPTL3 have lower LDL-C and triglyceride levels and thus lower risk of heart attacks and multiple metabolic disorders. In preclinical studies, an analog of AKCEA-ANGPTL3-LRx inhibited the production of the ANGPTL3 protein in the liver, inhibiting liver fat accumulation and lowering blood levels of triglycerides, LDL-C and very low-density lipoprotein cholesterol, or VLDL-C. In addition, our preclinical data and initial Phase 1 data suggest that inhibiting the production of ANGPTL3 could improve other lipid parameters, including triglyceride levels and total cholesterol.
We conducted a Phase 1/2 program for AKCEA-ANGPTL3-LRx in people with elevated triglycerides. We reported results for the initial cohort from this study at the AHA meeting in November 2016. We observed that the people with elevated triglycerides achieved dose-dependent, statistically significant mean reductions in ANGPTL3 of up to 83%. Treatment with AKCEA-ANGPTL3-LRx was also associated with statistically significant mean reductions in triglycerides of up to 66%, in LDL-C of up to 35% and in total cholesterol of up to 36%. In this study, AKCEA-ANGPTL3-LRx was reported to be well tolerated. The most common adverse events in the AKCEA-ANGPTL3-LRx treated group of patients were mild headaches and dizziness that were approximately equal to the rate observed in the placebo group. In the fourth quarter of 2017, we initiated a study of AKCEA-ANGPTL3-LRx in patients with NAFLD with metabolic complications which include hypertriglyceridemia, type 2 diabetes or NASH. We expect data from this study in 2019. Further, in the fourth quarter of 2017, we also initiated a study of AKCEA-ANGPTL3-LRx in patients with rare hyperlipidemias, including patients with FCS, FPL and HoFH. We plan to report data from this program in 2018. We may consider developing AKCEA-ANGPTL3-LRx for additional indications including other rare hyperlipidemias and lipodystrophies. If we find that AKCEA-ANGPTL3-LRx can effectively lower triglyceride levels through a different mechanism of action from volanesorsen, it may represent an opportunity to expand our FCS franchise.
Disease Background
Fatty liver disease
While some fat in the liver is normal, a significant percentage of individuals have elevated levels of liver fat. Individuals with excessive fat accumulation in the liver also have elevated risk of developing insulin resistance and metabolic syndrome, type 2 diabetes and CVD. These risks are further elevated in patients with hyperlipidemia, especially those with elevated triglyceride levels. The most common form of fatty liver disease is NAFLD, which is associated with obesity-related disorders even in patients who drink little or no alcohol, and is characterized by the gradual accumulation of fat in the liver, or steatosis. One of the key causes of this condition is the Western diet, which is rich in processed foods with high fat and sugar content. In the early stages of NAFLD, patients typically experience steatosis that is slow progressing. Over time, a subset of these patients progresses to steatohepatitis, a more severe and progressive form of NAFLD characterized by chronic inflammation and liver-cell damage, called NASH. Over time, the chronic inflammation caused by NASH can lead to the formation of scar tissue in the liver, known as fibrosis. As scar tissue gradually replaces healthy liver tissue, blood flow is restricted, which can lead to the loss of normal liver function, cirrhosis, portal hypertension, liver cancer and ultimately liver failure. Currently, there are no approved treatments specifically for NAFLD or NASH. If the disease ultimately progresses beyond NASH, the only alternative is a liver transplant.
Rare Hyperlipidemias
Rare hyperlipidemias are genetic diseases characterized by high levels of lipids or lipoproteins in the blood. Function or levels of various lipid clearing enzymes, like LPL, LDL receptor and hepatic lipase, are decreased in patients with rare hyperlipidemias. These patients may also have a reduced ability to clear other lipids, including LDL, leading to very high lipid levels. Examples of diseases in this category include FCS and familial hypercholesterolemia. Despite existing and emerging therapies, there remains an unmet need to reduce multiple lipid parameters in these patients, including LDL and triglycerides.
23
Lipodystrophies
Congenital and acquired forms of lipodystrophy are diseases characterized by abnormal or degenerative conditions of the body's adipose tissue. Patients with various forms of lipodystrophy may have difficulties in normal processing of lipids resulting in high LDL-C, triglycerides and fatty liver disease.
AKCEA-ANGPTL3-LRx Clinical Development
Preclinical and other related studies
Our preclinical data suggest that reducing ANGPTL3 could improve lipid parameters, including LDL-C, triglycerides, and total cholesterol. In a mouse model of increased liver fat, we observed that treatment with an analog of AKCEA-ANGPTL3-LRx reduced liver fat concentrations by more than 50%.
Further, in a Phase 1 study that Ionis conducted with a non-LICA version of AKCEA-ANGPTL3-LRx, healthy volunteers experienced significant reductions of up to 93% in ANGPTL3, up to 63% in triglycerides and up to 46% in total cholesterol.
Phase 1/2 program
We conducted a placebo-controlled, dose escalation Phase 1/2 program to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of single and multiple doses of AKCEA-ANGPTL3-LRx administered by a subcutaneous injection to people with elevated triglycerides. We evaluated single doses of AKCEA-ANGPTL3-LRx at 20 mg, 40 mg, 80 mg and 120 mg and randomized healthy people with elevated triglycerides 3:1, active to placebo, where three participants received AKCEA-ANGPTL3-LRx and one participant received placebo. The multiple dose cohorts used lower doses with eight participants in each cohort. We reported initial results for the initial group of people with elevated triglycerides from this study at the AHA meeting in November 2016 and published in the New England Journal of Medicine in June 2017.
People who received multiple doses of 10 mg, 20 mg, 40 mg, or 60 mg of AKCEA-ANGPTL3-LRx achieved dose-dependent, statistically significant mean reductions at Day 37 in ANGPTL3 of up to 83% (p ≤0.001). These subjects also experienced statistically significant mean reductions in triglycerides of up to 66% (p ≤0.001), in LDL-C of up to 35% (p ≤0.001) and in total cholesterol of up to 36% (p ≤0.001). In this study, AKCEA-ANGPTL3-LRx was reported to be well tolerated. The most common adverse events in the AKCEA-ANGPTL3-LRx treated group of patients were mild headaches and dizziness that were approximately equal to the rate observed in the placebo group. There were no discontinuations due to adverse events and no platelet declines. The graphic below further summarizes these results.
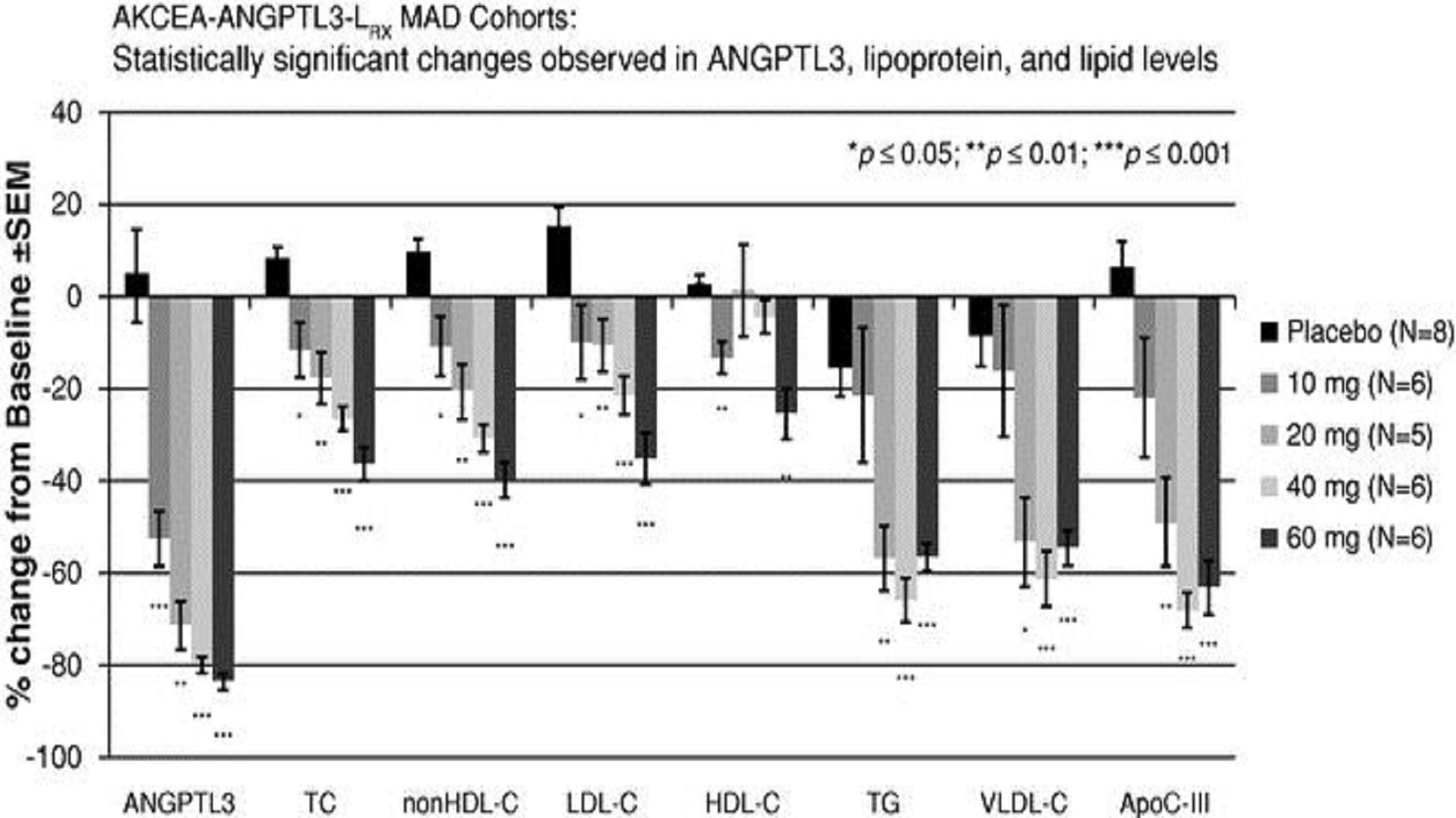
24
Phase 2 studies
In the second half of 2017, we began a study of AKCEA-ANGPTL3-LRx in patients with NAFLD with metabolic complications which include hypertriglyceridemia, type 2 diabetes or NASH. The goal of the study is to evaluate dose levels, frequencies and markers of liver fat. The study is a randomized, double-blind, placebo-controlled, dose-ranging study of AKCEA-ANGPTL3-LRx administered by a subcutaneous injection. The study is designed to dose patients over a period of at least six months. We are testing multiple doses and plan to evaluate safety and efficacy to support dose selection in future trials. The primary endpoint is the change in serum triglyceride levels at six months. We are also evaluating changes in key lipid parameters including LDL-C, as well as measures of liver fat, markers of liver inflammation and fibrosis and other metabolic parameters. We plan to enroll approximately 144 patients in the study. We plan to report data from this study in 2019.
We are also conducting exploratory studies of AKCEA-ANGPTL3-LRx in patients with three different rare hyperlipidemias, including patients with FCS, FPL and HoFH. The primary goal of these studies is to evaluate the ability of AKCEA-ANGPTL3-LRx to lower triglyceride levels, LDL levels and/or other important lipid markers in patients with rare hyperlipidemias, and markers of insulin sensitivity and liver fat in FPL. We plan to test multiple doses and expect to evaluate safety and efficacy to support dose selection in future trials. We plan to report data from this program in 2018. If we demonstrate that we can successfully lower key lipid parameters, reduce liver fat, insulin sensitivity and body fat composition in these patients, we plan to begin the initiation process of our Phase 3 study in rare hyperlipidemias in the second half of 2018.
AKCEA-ANGPTL3-LRx Commercial Opportunity
NAFLD is the most common chronic liver disease worldwide and more than 75 million patients are affected in the United States alone. Approximately 30% of patients with NAFLD will eventually progress to NASH. In the United States, the most recent epidemiological studies show that approximately 3% to 5% of the general population has NASH. We believe there are a comparable number of patients in Europe and the rest of the world. While there are a number of treatments currently in development for the treatment of NAFLD and NASH, none are currently approved and we believe there will continue to be a significant unmet medical need in this population.
Rare hyperlipidemia contains multiple diseases, including FCS and familial hypercholesterolemia. We believe that these populations are all orphan sized.
There are several types of lipodystrophies, congenital generalized, acquired generalized, familial partial, acquired partial, mandibuloacral dysplasia associated, and HIV associated. We believe these populations are all orphan sized.
AKCEA-APOCIII-LRx
Overview
We are developing AKCEA-APOCIII-LRx to inhibit the production of apoC-III, the same protein inhibited by volanesorsen, for the broad population of patients who have cardiometabolic disease due to their elevated triglyceride levels. ApoC-III impacts triglyceride levels and may also increase inflammatory processes. This combination of effects makes apoC-III a promising target for patients with LDL-C already controlled on statin therapy, but for whom triglycerides remain poorly controlled. We believe that the enhancements offered by Ionis' LICA technology can provide greater patient convenience by allowing for significantly lower doses and less frequent administration, compared to volanesorsen. We conducted a Phase 1/2 study of AKCEA-APOCIII-LRx in people with elevated triglycerides and reported results from this study in the fourth quarter of 2017. We initiated a Phase 2b dose-ranging study of AKCEA-APOCIII-LRx in patients with hypertriglyceridemia and established CVD in the first quarter of 2018 and plan to report data from this study in 2019. At the completion of Phase 2 development, Novartis has an option to license the drug. As part of our Novartis collaboration, we intend to complete the Phase 2 program required to define the appropriate dose and regimen to support a planned cardiovascular outcome study. If Novartis exercises its option to license AKCEA-APOCIII-LRx, it would pay us $150.0 million, of which $75.0 million would go to Ionis. If exercised, Novartis plans to conduct and pay for a Phase 3 cardiovascular outcome study in high-risk patients and, if approved, to commercialize AKCEA-APOCIII-LRx worldwide. We plan to co-commercialize AKCEA-APOCIII-LRx with Novartis in selected markets, under terms and conditions that we plan to negotiate with Novartis in the future, through the specialized sales force we are building to commercialize volanesorsen. We believe Novartis brings significant resources and expertise to the collaboration that can accelerate our ability to deliver this potential therapy to patients at significant cardiovascular risk due to their elevated triglyceride levels.
25
Disease Background
ApoC-III is an important emerging target linking hypertriglyceridemia with CVD. Several studies have found that apoC-III levels are an independent risk factor for CVD. Further, its presence on lipoproteins may increase their atherogenicity. A study in the New England Journal of Medicine reported that out of a sample of over 100,000 people, individuals with an apoC-III gene loss of function mutation had a reduced risk of clinical coronary heart disease. Each decrease of 1 mg/dL in plasma levels of apoC-III was associated with a 4% decrease in the risk of incident coronary heart disease. Triglycerides may also play a role in cardiovascular risk. As shown in the figure below, in two separate studies encompassing nearly 20,000 patients, as triglyceride levels increased, so did the risk of a cardiovascular event. In summary, apoC-III impacts triglyceride levels and may also increase inflammatory processes, and this combination of effects makes apoC-III a promising target for reducing the residual CVD risk in patients already on statin therapy, but for whom triglycerides are poorly controlled.
AKCEA-APOCIII-LRx Clinical Development
Phase 1/2 program
We conducted a placebo-controlled, dose escalation Phase 1/2 program to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of single and multiple doses of AKCEA-APOCIII-LRx administered by a subcutaneous injection to people with elevated triglycerides. We evaluated single doses of AKCEA-APOCIII-LRx at 10 mg, 20 mg, 40 mg, 80 mg and 120 mg and randomized healthy people with elevated triglycerides 3:1, active to placebo, where three participants received AKCEA-APOCIII-LRx and one participant received placebo. The multiple dose cohorts used lower doses with eight participants in each cohort. We reported initial results for the initial group of people with elevated triglycerides from this study in late 2017.
People who received multiple doses of 10 mg, 20 mg, 40 mg, or 60 mg of AKCEA-APOCIII-LRx achieved dose-dependent, statistically significant mean reductions at Day 37 in apoCIII of up to 84% (p ≤ 0.001) after six weeks of treatment. These subjects also experienced statistically significant mean dose-dependent reductions in triglycerides of up to 71% (p ≤0.001). Significant dose-dependent reductions of up to 30% in apolipoprotein B, or apoB, and increases of up to 100% in high-density lipoprotein cholesterol, or HDL-C, were also observed. Both decreased levels of apoB and increased levels of HDL-C are associated with decreased cardiovascular risk. AKCEA-APOCIII-LRx was well tolerated in the study. We saw no platelet reductions, including among patients who had four monthly doses, and no adverse events leading to treatment discontinuation were observed.
26
Phase 2 studies
In the first quarter of 2018, we began a study of AKCEA-APOCIII-LRx in patients with hypertriglyceridemia and established CVD. The multicenter, randomized, double-blind, placebo-controlled, dose-ranging Phase 2b study of AKCEA-APOCIII-LRx in approximately 100 patients with hypertriglyceridemia and established CVD. The primary endpoint of the study is to evaluate the safety and efficacy of AKCEA-APOCIII-LRx and assess different doses and dosing regimens for the reduction of serum of triglyceride levels to support dose selection in future trials. We anticipate reporting top-line data from this study in 2019.
We have initiated a strategic collaboration with Novartis for this drug. See "Our Strategic Collaboration with Novartis" for additional information.
AKCEA-APOCIII-LRx Commercial Opportunity
ApoC-III levels and elevated triglycerides have been linked to increased cardiovascular risk and lowering apoC-III and triglycerides may reduce this risk. We estimate the eligible population to be 8.5 to 14.5 million people globally. We believe that positive results from a large cardiovascular outcome study will be required to support marketing authorization for the treatment of these patients. If Novartis exercises its option, it plans to conduct, at its expense, such a study pursuant to our strategic collaboration to conduct this study and to commercialize AKCEA-APOCIII-LRx for these patients.
Sales and Marketing
Our goal is to become the premier company offering treatments for previously inadequately treated lipid disorders. We are assembling the global infrastructure to develop the drugs in our pipeline and to commercialize them with a focus on lipid specialists, specialized endocrinologists and pancreatologists as our primary call points. We are also creating the specialized support required to potentially address other rare disease patient populations. We are building a small, highly focused salesforce to support the commercialization of volanesorsen, if approved, which would serve as the foundation of our sales, marketing and patient support efforts for all of the drugs in our pipeline, including our co-commercialization activities with Novartis for AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx, if and when approved under terms and conditions that we plan to negotiate with Novartis in the future.
Global Commercialization Infrastructure
We plan to commercialize volanesorsen ourselves globally and we have made significant progress toward building a global commercial organization with leadership in place overseeing 13 initial countries. We are doing this using a specialized and comprehensive patient-centric approach. Our orphan-focused commercial model will include a small, highly focused salesforce in each country that we are targeting, complemented by medical affairs and a high touch support program to assist both patients and healthcare providers. We are preparing for approval and launch of volanesorsen in mid-2018.
Our global commercial organization is initially focused on our nearest term opportunities with volanesorsen to treat patients with FCS and FPL. Our initial plan is to focus on lipid specialists, specialized endocrinologists and pancreatologists as our primary call points. We believe the relatively small number of specialized physicians treating FCS and FPL patients will allow us to address this market with a nimble, scalable organization. We are currently identifying patients and having them referred to specialists for treatment, which we believe will facilitate successful commercialization. Building awareness of this orphan disease among not only lipid specialists, but also referring physicians, is a key element of our pre-commercial and commercial plans. We are focused on disease education and market access, with the goal of ensuring that identified patients can most effectively obtain our drugs once commercialized. We are also creating the specialized support required to potentially address other rare disease patient populations.
Our approach will vary by market based on local health care practices and needs, but we are actively building services that can include reimbursement assistance, partnerships with specialty pharmacies, injection training and routine platelet monitoring, which we believe will enable strong integration with treating physicians and facilitate patient uptake and compliance. Reimbursement assistance may include activities such as a reimbursement hotline, patient assistance and insurance verification. We plan to include dedicated case managers as part of our support team who will work directly with patients, caregivers and healthcare providers to help patients start and stay on therapy.
27
Due to the specialized nature of managing FCS and FPL, there are a limited number of treating physicians.
| ● |
In the United States, there are approximately:
|
| ● |
45 lipid treatment hubs; and
|
| ● |
200 to 300 lipid specialists, with an additional 300 to 400 endocrinologists specializing in lipid disorders.
|
| ● |
In Europe, there are approximately:
|
| ● |
75 specialized lipid treatment hubs; and
|
| ● |
400 to 600 physician specialists who treat lipid disorders.
|
In North America and Europe, we are planning for an overall field force size of between 40 and 50 individuals in the U.S. and between 40 and 50 in the EU and Canada for the initial launch of volanesorsen in FCS, which we expect to be sufficient to target substantially all of the potential volanesorsen prescribers. This field force would include sales representatives, medical liaisons and personnel for reimbursement assistance and patient support. We formed wholly owned subsidiaries to support our initial pre-commercialization activities in the UK in 2016 and in Canada, France and Germany in 2017.
We expect to market our drugs to the same specialist call point as volanesorsen, enabling us to leverage this commercial organization as the core global infrastructure for all of our drugs. We plan to commercialize by ourselves any approved drugs with a rare disease or specialty focus. We may enter into strategic relationships to commercialize certain of our drugs, particularly in indications with large patient populations, as evidenced by our collaboration with Novartis. We believe Novartis brings significant resources and expertise to the collaboration that can accelerate our ability to deliver AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx to the large populations of patients who have high cardiovascular risk due to inadequately treated lipid disorders. We also plan to co-commercialize any such drug in selected markets, under terms and conditions that we plan to negotiate with Novartis in the future, through the specialized sales force we are building to commercialize volanesorsen.
Preparing for Successful Commercialization
A key aspect of successfully commercializing therapies for orphan diseases is to provide education on disease diagnosis to assist physicians in identifying eligible patients. Patient populations are frequently very small and sometimes heterogeneous. Our management team is experienced in maximizing patient identification for both clinical development and commercial purposes in orphan diseases. We also have significant experience in establishing the burden of disease in support of securing orphan pricing and reimbursement.
Our commercial organization is focused on the following priorities to prepare for the launch of volanesorsen:
| ● |
Improve diagnosis by working with a small number of specialist physician experts to advance the understanding of the signs and symptoms of FCS and then communicate that simplified clinical diagnosis criteria to the broader physician and patient community.
|
| ● |
Build a database of patients by working with physicians and patient organizations and through improved diagnosis and referrals. In order to protect patient confidentiality, we do not include patient-specific information in the database. We add blinded patient information to our database through communication with physicians, patient organizations and other tools, such as electronic medical record database searches. We plan to use our database to help us engage with physicians who may have patients who could potentially benefit from our drugs.
|
| ● |
Build an integrated high touch patient support team to help patients start and stay on therapy. We plan to provide support for patients to access our drug and to provide injection training, platelet monitoring and dietary support, as well as establish partnerships with specialty pharmacies, to help patients remain on therapy over the long term. We plan to include dedicated case managers as part of our support team who will work directly with patients, caregivers and healthcare providers to help patients start and stay on therapy.
|
| ● |
Prepare for successful market access through payors and other reimbursement authorities by establishing and quantifying the burden of disease associated with living with FCS.
|
28
Our Relationship with Ionis
We founded our operations in 2015 as a wholly owned subsidiary of Ionis to develop and commercialize Ionis' drugs to treat lipid disorders. On July 19, 2017, we completed our initial public offering, or IPO. Ionis funded our expenses up until our IPO in July 2017. As of December 31, 2017, Ionis is our majority shareholder with approximately 68% ownership of our common stock. Although we expect Ionis to remain our principal stockholder for the foreseeable future, we are an independent company building a focus and excellence in development and commercialization.
As we prepare to launch volanesorsen and continue to build out our corporate functions, we expect to rely less on Ionis for support services. We currently have three main agreements that govern our relationship with Ionis: a development, commercialization and license agreement; a service agreement; and an investor rights agreement. Through our relationship with Ionis, we benefit in the following ways:
| ● |
We have access to Ionis' innovative generation 2+ antisense and LICA technologies for use in our drugs. These technologies allow for precise specificity, favorable dosing properties and no anticipated drug-to-drug interactions.
|
| ● |
We obtained exclusive rights to globally commercialize a robust, mature pipeline of drugs, including volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development. Our licensed rights also include access to Ionis' intellectual property and expertise to develop, manufacture and commercialize these drugs.
|
| ● |
We have a joint development program that provides us access to Ionis' development and regulatory organization, which has significant expertise in developing drugs to treat patients with lipid disorders. Ionis also provides resources to support our nonclinical and clinical studies.
|
| ● |
We contract with Ionis for support in areas such as legal, finance and human resources, which allows us to be more capital efficient than a typical company of our size and stage of development. This support allows us to focus our efforts and resources on developing and preparing to commercialize our drugs.
|
| ● |
We are not required to make any upfront or pre-commercialization payments to Ionis for drugs we are developing under our development, commercialization and license agreement, as would be typical in a drug license. Our agreement allows us to more efficiently invest our capital in developing and preparing to commercialize our drugs, as we are only required to make milestone and royalty payments post-commercialization or if we grant a sublicense to Ionis' technology.
|
| ● |
As a result of our relationship with Ionis, we may have the opportunity to evaluate additional antisense drugs that may complement our efforts in becoming the premier lipid disease company. For example, Ionis has granted us a right of first negotiation with respect to Ionis development candidates that are designed to treat a rare cardiometabolic disease or a rare inherited metabolic disease.
|
While we and Ionis intend our relationship to enhance our capabilities, certain terms of our relationship may limit our ability to achieve this expected benefit, including:
| ● |
Some of our directors may have a conflict of interest because of their positions with Ionis.
|
| ● |
A Joint Steering Committee, or JSC, sets the development and regulatory strategy for our drugs by mutual agreement. If the JSC cannot come to a mutual agreement, it could delay our ability to develop and commercialize our drugs in development.
|
| ● |
We will need to mutually agree with Ionis on the terms of any additional sublicense to a third party for our drugs in development. If we cannot mutually agree, it could delay or prevent our ability to develop and commercialize our drugs.
|
| ● |
Our agreements prevent Ionis from developing and commercializing drugs targeting apoC-III, apo(a) or ANGPTL3 RNA. However, our agreements do not prevent Ionis from developing and commercializing other drugs to pursue the same indications we are pursuing with our drugs.
|
Exclusive Rights to Development Pipeline and Intellectual Property; Right of First Negotiation
Ionis is the leading company researching and developing antisense drugs. Under our agreements with Ionis, we have rights to Ionis' proprietary technologies for use with our drugs. Specifically, we obtained an exclusive license from Ionis to globally commercialize our development pipeline of drugs, including volanesorsen, AKCEA-APO(a)-LRx, AKCEA-ANGPTL3-LRx and AKCEA-APOCIII-LRx. Ionis also agreed that it would not work on its own or with other parties to develop or commercialize antisense drugs that target the same gene targets as the drugs we are developing and commercializing. Under our agreements with Ionis, we have a license to use Ionis' technology platform with our drugs. We also have access to future improvements Ionis may make to its antisense technology platform, such as improved manufacturing technologies.
In addition, Ionis has granted us a right of first negotiation with respect to Ionis development candidates that are designed to treat a rare cardiometabolic disease or a rare inherited metabolic disease.
29
Access to Ionis' Development, Regulatory and Manufacturing Expertise
Development
We receive access to Ionis' infrastructure and expertise in developing antisense drugs and, in particular, drugs to treat lipid disorders. We have a Joint Steering Committee comprised of Akcea and Ionis representatives who set the development strategy for each of our drugs. In addition, a team of Akcea and Ionis employees run each clinical study for our drugs. This way we can stay focused on developing our drugs and preparing for commercialization rather than immediately building an extensive development organization. Over time as we strategically expand our internal development capabilities, we plan to assume more and more responsibility for each development program. Because of our relationship with a much larger company like Ionis, we can use Ionis' relationships and negotiating power with clinical research organizations and other vendors to obtain lower pricing and better resourcing from these vendors than we otherwise could achieve on our own as a relatively new and smaller company. These benefits help us manage our development costs.
Regulatory
We take a similar approach to regulatory affairs as we do for drug development. We have a Joint Regulatory Committee comprised of Akcea and Ionis representatives that sets the regulatory strategy for each of our drugs. Because Ionis has filed over 30 investigational new drug applications, or INDs, for antisense drugs, and supported the approval of three new drug applications, or NDAs, for antisense drugs, Ionis understands how to successfully work with the FDA and other regulatory agencies. We can also benefit from Ionis' more than 25 years of knowledge regarding antisense drugs to prepare important sections of an IND or NDA. These are important benefits we can immediately access as we continue to build our own regulatory organization and assume more regulatory responsibility for our drugs.
Manufacturing
As the leader in antisense technology, Ionis has discovered many of the breakthroughs that have made it possible to manufacture antisense drugs at commercial scale and at a commercially feasible price. Through our relationship with Ionis, we enjoy the benefit of Ionis' historical and continuing investment in antisense drug manufacturing. Specifically, Ionis has supplied the active pharmaceutical ingredient, or API, and, through its outside vendors, the finished drug product for the clinical studies for each of the drugs in our pipeline through the completion of the on-going studies. Ionis also has agreed to supply and has supplied the API and the finished drug product, which we believe is adequate for at least the first two years of volanesorsen's launch. Ionis has long-standing and strong relationships with third-party vendors who can supply us with both API and finished drug product and are currently supplying API and finished drug product to other of Ionis' partners. Ionis also has long-standing and strong relationships with the vendors who supply the key raw materials to Ionis to make our drugs and to the other major oligonucleotide contract manufacturers. We plan to use these relationships to establish our own long term raw material and drug supplies at competitive prices.
Infrastructure
When Ionis formed our company, a key premise was to initially utilize Ionis' existing infrastructure in areas like business development, finance, patents, legal, human resources, benefits and other general and administrative areas, so that we could remain critically focused on developing and preparing to commercialize our drugs and could more efficiently utilize our capital. By taking advantage of Ionis' existing infrastructure in these areas, we can spend very little of our management and financial resources building these functions ourselves. As we commercialize our drugs, we expect to be able to expand this infrastructure with a relatively modest additional investment. We can use Ionis' extensive corporate partnering expertise and resources to help us if and when we choose to partner our drugs for the larger indications.
Payment Structure Under our Agreements with Ionis
We have agreed to pay Ionis for the services it provides us. We intentionally designed our agreements with Ionis to allow us to invest our initial capital to develop and prepare to commercialize our drugs. We were not required to make an upfront cash payment to license Ionis' drugs and technology. In addition, other than paying Ionis the cost of the support services Ionis provides to us, we are not required to make significant payments to Ionis until we successfully commercialize or partner our drugs. For drug development Ionis conducts on our behalf, we reimburse Ionis for its out-of-pocket expenses and for the cost of Ionis' employees who conduct the research and development activities for our drugs. For general and administrative services, we pay Ionis for our share of internal and external expenses for each of the functions they provide based on our relative use of each function, plus an allocation of facility-related expenses.
For the drugs we commercialize ourselves, we will pay Ionis royalties from the mid-teens to the mid-twenty percent rage on sales related to those drugs. If we sell a drug for an orphan disease indication, defined in our agreement as less than 500,000 patients worldwide, or an indication that required a Phase 3 program of less than 1,000 patients and less than two years of treatment, we pay a higher royalty rate to Ionis than we do if we sell a drug for a disease having more than 500,000 patients worldwide or an indication that required a Phase 3 program of 1,000 or more patients and two or more years of treatment. Other than with respect to the drugs licensed to Novartis under the strategic collaboration, option and license agreement, if our annual sales reach $500.0 million, $1.0 billion and $2.0 billion, we will pay Ionis sales milestones in the amount of $50.0 million for each sales milestone reached by each drug, spread in equal installments over the 12 quarters following the milestone event.
30
For drugs we sublicense to a commercial partner, we will share 50% of any revenue from the commercial partner with Ionis, excluding money received from our partner specifically designated to fund future development costs and money we are obligated to spend to co-commercialize a drug. Regarding our Novartis collaboration, we paid Ionis $15.0 million of the $75.0 million upfront option payment we received from Novartis. We will pay Ionis 50% of any additional payments we receive from Novartis, excluding money received specifically designated to fund future development costs and money we are obligated to spend to co-commercialize a drug.
Line of Credit Agreement
In January 2017, we entered into a line of credit agreement with Ionis for up to $150.0 million. We had $106.0 million outstanding as of June 30, 2017, prior to our IPO. We used a portion of the $106.0 million to pay our intercompany expenses. The amounts we borrowed under the line of credit bore interest at an annual interest rate of 4%, compounded monthly. The outstanding principal and accrued interest under our line of credit converted into 13,438,339 shares of our common stock in connection with the closing of our IPO. We no longer have access to this line of credit following the closing of our IPO. After the IPO through the date of this filing, there has been no line of credit or any other financing arrangement with Ionis.
Our Strategic Collaboration with Novartis
In January 2017, we initiated a strategic collaboration with Novartis for the development and commercialization of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Under the strategic collaboration, option and license agreement, Novartis has an exclusive option to develop and commercialize these drugs. We are responsible for completing a Phase 2 program and conducting an end-of-Phase 2 meeting with the FDA for each drug and delivering API. Following the successful completion of each Phase 2 program, and prior to initiation of the Phase 3 study, Novartis will be able to exercise its option to license and commercialize each drug. Novartis will have 60 days following the end of the applicable end-of-Phase 2 meeting to exercise its option for each of these drugs. If Novartis exercises its option for a drug, Novartis will be responsible, at its expense, to use commercially reasonable efforts to develop and commercialize that drug. We received $75.0 million in an upfront option payment, of which we retained $60.0 million and paid Ionis $15.0 million as a sublicense fee under our license agreement with Ionis. In conjunction with this collaboration, Novartis purchased $100.0 million of Ionis' common stock at a premium. During 2017, we recognized $55.2 million of revenue related to our Novartis collaboration.
If Novartis exercises its option for a drug, Novartis will pay us a license fee equal to $150.0 million for each drug licensed by Novartis. In addition, for AKCEA-APO(a)-LRx, we are eligible to receive up to $600.0 million in substantive milestone payments, including $25.0 million for the achievement of a development milestone, up to $290.0 million for the achievement of regulatory milestones and up to $285.0 million for the achievement of commercialization milestones. In addition, for AKCEA-APOCIII-LRx, we are eligible to receive up to $530.0 million in substantive milestone payments, including $25.0 million for the achievement of a development milestone, up to $240.0 million for the achievement of regulatory milestones and up to $265.0 million for the achievement of commercialization milestones. We will earn the next milestone payment of $25.0 million under this collaboration if Novartis advances the Phase 3 study for either drug. We are also eligible to receive tiered royalties in the mid-teens to low-twenty percent range on net sales of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Novartis will reduce these royalties upon the expiration of certain patents or if a generic competitor negatively impacts the product in a specific country. We will pay 50% of these license fees, milestone payments and royalties to Ionis as a sublicense fee.
For each product Novartis commercializes under this agreement, we will have the right to co-commercialize the product with Novartis in selected markets, through the specialized sales force we are building to commercialize volanesorsen, on terms and conditions that we plan to negotiate with Novartis in the future.
If Novartis does not exercise its option, or stops developing or commercializing after exercising its option with respect to a particular drug, we will have all rights to develop or commercialize the drug (including the right to sublicense these rights to a third party) at our sole expense. If Novartis stops developing or commercializing a drug after exercising its option, and we subsequently commercialize the drug on our own or with another party, we are required to negotiate in good faith and mutually agree with Novartis the terms of a royalty payable to Novartis on the returned drug.
The agreement with Novartis will continue until the earlier of the date that all of Novartis' options to obtain the exclusive licenses under the agreement expire unexercised or, if Novartis exercises its options, until the expiration of all payment obligations under the agreement. In addition, the agreement as a whole or with respect to any drug under the agreement may terminate early under the following situations:
| ● |
Novartis may terminate the agreement as a whole or with respect to any drug at any time by providing written notice to us;
|
| ● |
Either we or Novartis may terminate the agreement with respect to any drug by providing written notice to the other party in good faith that we or Novartis have determined that the continued development or commercialization of the drug presents safety concerns that pose an unacceptable risk or threat of harm in humans or would violate any applicable law, ethical principles or principles of scientific integrity;
|
| ● |
Either we or Novartis may terminate the agreement for a drug by providing written notice to the other party upon the other party's uncured failure to perform a material obligation related to the drug under the agreement, or the entire agreement if the other party becomes insolvent; and
|
| ● |
We may terminate the agreement if Novartis disputes or assists a third party to dispute the validity of any of our or Ionis' patents.
|
31
Additionally, in January 2017, we and Ionis entered into a Stock Purchase Agreement, or SPA, with Novartis. Under the SPA, in July 2017, Novartis purchased $50.0 million of our common stock in a separate private placement concurrent with the completion of our IPO at a price per share equal to the IPO price.
Competition
The commercialization of new drugs is competitive, and we may face worldwide competition from major pharmaceutical companies, specialty pharmaceutical companies, biotechnology companies, nutraceutical companies and ultimately generic companies. Our competitors may develop or market therapies that are more effective, safer, more convenient to use, or less costly than any that we are commercializing, or may obtain regulatory or reimbursement approval for their therapies more rapidly than we may obtain approval for ours. Many of our competitors have substantially greater financial, technical and human resources than we have. Additionally, mergers and acquisitions in the pharmaceutical industry may result in even more resources being concentrated in our competitors. Competition may increase further as a result of advances made in the commercial applicability of technologies and greater availability of capital for investment in these fields.
With respect to volanesorsen to treat patients with FCS, we believed Glybera, which is a gene therapy made by uniQure N.V., could compete with volanesorsen. However, in October 2017, uniQure announced that Glybera's marketing authorization in Europe was not renewed and Glybera was taken off the market. UniQure has previously announced it is not pursuing approval in the Unites States. Gemphire Therapeutics' Gemcabene is being studied in patients with severe hypertriglyceridemia, defined as triglycerides above 500 mg/dL and Gemphire expects to report top-line results from its Phase 2 study in the second quarter of 2018.
Metreleptin is in a Phase 3 trial for FPL patients who also have NASH. Metreleptin is currently approved for use in generalized lipodystrophy, or GL, patients. The FDA denied initial approval in FPL patients, not all of whom are leptin deficient, the mechanism by which metreleptin works. In December 2016, Novelion Therapeutics, Inc. submitted a marketing authorization application to the EMA seeking approval for Metreleptin as replacement therapy to treat complications of leptin deficiency in a small subset of FPL patients and in patients with GL, and for which decision is expected in the first half of 2018. An investigator-sponsored study is currently ongoing with the support of Novelion to evaluate Metreleptin in FPL patients who also have NASH. Metreleptin does not affect apoC-III levels. ApoC-III levels have been shown to be elevated in FPL patients and directly correlated to triglyceride levels. To our knowledge, there are currently no other direct competitors for lowering apoC-III in clinical development.
In addition, many patients with FCS and FPL use diet, niacin, fish oils and/or fibrates to reduce their elevated triglycerides. Niacin, fish oils and fibrates are generally not effective in patients with FCS. The ultra-low-fat diet that patients with FCS and FPL are required to maintain is extremely burdensome to patients and their families. Based on our volanesorsen clinical experience, including in individuals with FCS, we believe that volanesorsen will work equally well as a single agent or in combination with other triglyceride-lowering drugs or approaches.
With respect to AKCEA-APO(a)-LRx, we are not aware of any other drugs currently in clinical development specifically for the treatment of hyperlipoproteinemia(a) and associated cardiovascular disease. In September 2016, Arrowhead Pharmaceuticals, Inc. and Amgen Inc. announced a license and collaboration for development of Arrowhead's preclinical program, ARO-LPA, which uses an RNAi drug conjugated with a GalNAc for the same target as AKCEA-APO(a)-LRx. It is possible that other competitors may produce, develop and commercialize drugs, or utilize other approaches, to treat patients with CVD. Under its strategic collaboration agreement with Alnylam Pharmaceuticals, Inc., or Alnylam, Ionis received an exclusive, royalty-bearing license to Alnylam's chemistry, RNA targeting mechanism and target-specific intellectual property for oligonucleotides against apo(a), which means that Alnylam agreed not to use the exclusively licensed technology to develop or commercialize an oligonucleotide against apo(a).
AKCEA-ANGPTL3-LRx may compete with a monoclonal antibody that binds to ANGPTL3 that Regeneron Pharmaceuticals, Inc. is developing, currently in Phase 2 development for the treatment of HoFH and severe forms of hyperlipidemia. Additionally, many patients with familial hyperlipidemias are treated using diet and statins, which have limited effect in these patients.
AKCEA-APOCIII-LRx may compete with gemcabene, an oral small molecule that reduces apoC-III, that Gemphire Therapeutics, Inc. is developing to treat patients with triglycerides above 500 mg/dL. Gemphire is conducting a Phase 2 study of gemcabene in patients with severely high triglycerides and expects to report top-line results in the second quarter of 2018. We are aware of other approaches such as RNA interference, or RNAi, that are in preclinical development for apoC-III-driven cardiometabolic disease.
32
Intellectual Property
We have in-licensed numerous patents and patent applications and possess substantial know-how and trade secrets relating to volanesorsen, AKCEA-APO(a)-LRx, our other drugs in development and, more generally, the development and commercialization of oligonucleotide therapeutics. Our objective is to continue to develop and strengthen our proprietary position to further protect our drugs.
We obtained our rights to the patents covering volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development and our rights in Ionis' proprietary technology platform and know-how under our development, commercialization and license agreement with Ionis. We seek to expand our portfolio of patents and patent applications by filing and prosecuting existing patent rights and filing additional patent applications.
We seek patent protection in significant markets and/or countries for each drug in development. We also seek to maximize patent term. The patent exclusivity period for a drug will prevent generic drugs from entering the market. Patent exclusivity depends on a number of factors, including initial patent term and available patent term extensions based upon delays caused by the regulatory approval process.
ApoC-III, Volanesorsen and AKCEA-APOCIII-LRx Intellectual Property
We have an exclusive license under Ionis' apoC-III patent estate to develop and commercialize volanesorsen and the LICA follow-on drug AKCEA-APOCIII-LRx. The apoC-III patent estate includes patent claims in the United States drawn to the use of antisense compounds complementary to the mRNA of human apoC-III, including compounds designed to the region targeted by volanesorsen and AKCEA-APOCIII-LRx (US 7,598,227) which, excluding any additional term adjustments or patent term extensions, expires in 2023. Similar claims covering compounds complementary to any site on human apoC-III have granted in Australia.
The apoC-III patent estate also includes issued patent claims to the specific antisense sequence and chemical composition of volanesorsen in the United States (US 7,750,141), Australia and Europe (EP1622597). The issued claims in the United States should protect volanesorsen from generic competition in the United States until at least 2023. In addition, depending upon the timing, duration and specifics of FDA regulatory review, this patent may be eligible for patent term restoration to recapture a portion of the term lost during such review. We are also pursuing additional patent applications directed to methods of using volanesorsen and other apoC-III compounds for treating various disorders, including FCS in jurisdictions worldwide. Claims drawn to methods of using apoC-III specific inhibitors, and specifically compounds designed to target the same sequence as volanesorsen and AKCEA-APOCIII-LRx, for treating FCS have issued in the United States (US 9,593,333) and will expire in 2034, excluding any additional term adjustments or patent term extensions.
The apoC-III patent estate also includes issued patent claims covering the specific chemical composition of AKCEA-APOCIII-LRx in the United States (US 9,163,239). The claims should protect AKCEA-APOCIII-LRx from generic competition until at least 2034. We are pursuing additional patent coverage for AKCEA-APOCIII-LRx in jurisdictions worldwide.
Apo(a) and AKCEA-APO(a)-LRx Intellectual Property
We have an exclusive license under Ionis' apo(a) patent estate to develop and commercialize AKCEA-APO(a)-LRx. The apo(a) patent estate includes issued patent claims to the specific antisense sequence and chemical composition of AKCEA-APO(a)-LRx in the United States (US 9,181,550). The issued claims directed to the composition should protect AKCEA-APO(a)-LRx from generic competition in the United States until at least 2034. In addition, patent term restoration may be available to recapture a portion of the term lost during FDA regulatory review. We are also pursuing additional patent applications designed to protect the AKCEA-APO(a)-LRx composition and additional dosing and methods of use in jurisdictions worldwide.
ANGPTL3 and AKCEA-ANGPTL3-LRx Intellectual Property
We have an exclusive license under Ionis' ANGPTL3 patent estate to develop and commercialize AKCEA-ANGPTL3-LRx. The ANGPTL3 patent estate includes issued patent claims drawn to the use of antisense compounds complementary to ANGPTL3 RNA for inhibiting the production of ANGPTL3 (US 8,653,047). The ANGPTL3 patent estate also includes issued patent claims covering the specific antisense sequence and chemical composition of AKCEA-ANGPTL3-LRx in the United States (US 9,382,540). The issued claims directed to the chemical composition should protect AKCEA-ANGPTL3-LRx from generic competition until at least 2035. We are pursuing additional patent claims designed to protect the sequence and chemical composition of AKCEA-ANGPTL3-LRx in jurisdictions worldwide.
33
Trade Secrets
In addition to the protections afforded by patents and other regulatory protections, we may rely, in some circumstances, on trade secrets to protect our technology. Trade secrets may be useful to protect proprietary know-how that is not patentable or which we elect not to patent. Trade secrets may also be useful for processes or improvements for which patents are difficult to enforce. We also protect our drugs and the proprietary technology platform by confidentiality agreements with employees, consultants, advisors, contractors and collaborators. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems.
Manufacturing
We believe we have sufficient manufacturing capacity, through Ionis, to meet our current development needs, including the Phase 3 clinical study for volanesorsen and Phase 2 studies for AKCEA-APO(a)-LRx, AKCEA-ANGPTL3-LRx and AKCEA-APOCIII-LRx. We believe that we have, or will be able to develop or acquire, sufficient supply capacity to meet our anticipated future needs. Ionis has supplied the API and the finished drug product for the clinical studies for each of the drugs in our pipeline through the completion of the on-going studies. Ionis also has agreed to supply and has supplied the API and the finished drug product, which we believe is adequate for at least the first two years of volanesorsen's launch. Ionis has long-standing and strong relationships with third-party vendors who can supply us with both API and finished drug product, and are currently supplying API and finished drug product to other of Ionis' partners. Ionis also has long-standing and strong relationships with the vendors who supply the key raw materials to Ionis to make our drugs and to the other major oligonucleotide contract manufacturers. We also believe that with anticipated benefits from increases in scale and improvements in chemistry, through Ionis or third parties, we will be able to manufacture our antisense drugs at commercially reasonable prices.
In the past, except for small quantities, it was generally expensive and difficult to produce chemically modified oligonucleotides like antisense drugs. As a result, Ionis dedicated significant resources to develop ways to improve manufacturing efficiency and capacity. Since Ionis can use variants of the same nucleotide building blocks and the same type of equipment to produce their oligonucleotide drugs, they found that the same techniques used to efficiently manufacture one oligonucleotide drug could help improve the manufacturing processes for many other oligonucleotide drugs. By developing several proprietary chemical processes to scale up their manufacturing capabilities, Ionis has greatly reduced the cost of producing oligonucleotide drugs. For example, Ionis has significantly reduced the cost of raw materials through improved yield efficiency, while at the same time increasing its capacity to make the drugs. Through both Ionis' internal research and development programs and collaborations with outside vendors, we may benefit from even greater efficiency and further cost reductions. In addition, if Novartis exercises its option to license AKCEA-APO(a)-LRx or AKCEA-APOCIII-LRx, Novartis will be responsible for the long-term supply of drug substance and finished drug product for the licensed drug.
For LICA-conjugated drugs, to date, Ionis has manufactured itself or through a contract manufacturing organization only limited supplies of LICA for their own and our own nonclinical and clinical studies. LICA enables lower doses than unconjugated oligonucleotides. Along with Ionis' expertise in optimizing manufacturing of oligonucleotides, we believe this will enable the development of new processes to scale up manufacturing of these LICA conjugated drugs at commercially competitive prices.
Government Regulation and Approval
United States—FDA Process
In the United States, the FDA regulates drugs. The Federal Food, Drug and Cosmetic Act, or FDCA, and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling and import and export of drugs. To obtain regulatory approvals in the United States and in foreign countries, and subsequently comply with applicable statutes and regulations, we will need to spend substantial time and financial resources.
34
Approval Process
The FDA must approve any new unapproved drug or a drug with certain changes to a previously approved drug before a manufacturer can market it in the United States. If a company does not comply with applicable United States requirements, it may be subject to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending applications, Warning or Untitled Letters, clinical holds, drug recalls, drug seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution. The steps we must complete before we can market a drug include:
| ● |
completion of preclinical laboratory tests, animal studies, and formulation studies, all performed in accordance with the FDA's Good Laboratory Practice, or GLP, regulations;
|
| ● |
submission to the FDA of an IND application for human clinical testing, which must become effective before human clinical studies start. The sponsor must update the IND annually;
|
| ● |
approval of the study by an independent institutional review board, or IRB, or ethics committee representing each clinical site before each clinical study begins;
|
| ● |
performance of adequate and well-controlled human clinical studies to establish the safety and efficacy of the drug for each indication to the FDA's satisfaction;
|
| ● |
submission to the FDA of an NDA;
|
| ● |
potential review of the drug application by an FDA advisory committee, where appropriate and if applicable;
|
| ● |
satisfactory completion of an FDA inspection of the manufacturing facility or facilities to assess compliance with current good manufacturing practices, cGMP, or regulations; and
|
| ● |
FDA review and approval of the NDA.
|
It generally takes companies many years to satisfy the FDA approval requirements, but this varies substantially based upon the type, complexity and novelty of the drug or disease. Preclinical tests include laboratory evaluation of a drug's chemistry, formulation, and toxicity, as well as animal trials to assess the characteristics and potential safety and efficacy of the drug. The conduct of the preclinical tests must comply with federal regulations and requirements, including GLP. The company submits the results of the preclinical testing to the FDA as part of an IND along with other information, including information about the drug's chemistry, manufacturing and controls, and a proposed clinical study protocol. Long term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after submitting the initial IND.
The FDA requires a 30-day waiting period after the submission of each IND before a company can begin clinical testing in humans in the United States. If the FDA has neither commented on nor questioned the IND within this 30-day period, the IND sponsor may begin the proposed clinical study. However, the FDA may, within the 30-day time period, raise concerns or questions relating to one or more proposed clinical studies and place the clinical study on a clinical hold. In such a case, the company and the FDA must resolve any outstanding concerns before the company begins the clinical study. Accordingly, the submission of an IND may or may not be sufficient for the FDA to permit the sponsor to start a clinical study. The company must also make a separate submission to an existing IND for each successive clinical study conducted during drug development.
Clinical Studies
Clinical studies involve administering the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. The company must conduct clinical studies:
| ● |
in compliance with federal regulations;
|
| ● |
in compliance with good clinical practice, or GCP, an international standard meant to protect the rights and health of patients and to define the roles of clinical study sponsors, administrators and monitors; and
|
| ● |
under protocols detailing the objectives of the trial, the safety monitoring parameters and the effectiveness criteria.
|
The company must submit each protocol involving testing on United States patients and subsequent protocol amendments to the FDA as part of the IND. The FDA may order the temporary, or permanent, discontinuation of a clinical study at any time, or impose other sanctions, if it believes that the sponsor is not conducting the clinical study in accordance with FDA requirements or presents an unacceptable risk to the clinical study patients. The sponsor must also submit the study protocol and informed consent information for patients in clinical studies to an IRB for approval. An IRB may halt the clinical study, either temporarily or permanently, for failure to comply with the IRB's requirements, or may impose other conditions.
35
Companies generally divide the clinical investigation of a drug into three or four phases.
| ● |
Phase 1. The company evaluates the drug in healthy human subjects or patients with the target disease or condition. These studies typically evaluate the safety, dosage tolerance, metabolism and pharmacologic actions of the investigational new drug in humans, the side effects associated with increasing doses and, if possible, gain early evidence on effectiveness.
|
| ● |
Phase 2. The company administers the drug to a limited patient population to evaluate dosage tolerance and optimal dosage, identify possible adverse side effects and safety risks and preliminarily evaluate efficacy.
|
| ● |
Phase 3. The company administers the drug to an expanded patient population, generally at geographically dispersed clinical study sites, to generate enough data to statistically evaluate dosage, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the investigational drug and to provide an adequate basis for product approval.
|
| ● |
Phase 4. In some cases, the FDA may condition approval of an NDA for a drug on the company's agreement to conduct additional clinical studies after approval. In other cases, a sponsor may voluntarily conduct additional clinical studies after approval to gain more information about the drug. We typically refer to such post-approval studies as Phase 4 clinical studies.
|
While companies usually conduct these phases sequentially, they are sometimes overlapped or combined. A combined phase trial, such as a Phase 1/2 or a Phase 2/3 trial, is one that combines elements of objectives from two ordinarily sequential phases of development. For example, in a Phase 1/2 trial, the objectives may include both dose-finding and initial efficacy. In a Phase 2/3 trial, dosing regimen or population selection objectives are combined with confirmation of the safety and efficacy of the administration schedule in the intended population.
A pivotal study is a clinical study that adequately meets regulatory agency requirements to evaluate a drug's efficacy and safety to justify the approval of the drug. Generally, pivotal studies are Phase 3 studies, but the FDA may accept results from Phase 2 studies if the study design provides a well-controlled and reliable assessment of clinical benefit, particularly in situations in which there is an unmet medical need and the results are sufficiently robust.
The FDA, the IRB or the clinical study sponsor may suspend or terminate a clinical study at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Additionally, an independent group of qualified experts organized by the clinical study sponsor, known as a data safety monitoring board or committee, may oversee some clinical studies. This group provides authorization for whether or not a study may move forward at designated check points based on access to certain data from the study. We may also suspend or terminate a clinical study based on evolving business objectives and the competitive climate.
Submission of an NDA
After we complete the required clinical testing, we can prepare and submit an NDA to the FDA, which must approve the NDA before we can start marketing the drug in the United States. An NDA must include all relevant data available from pertinent preclinical and clinical studies, including negative or ambiguous results as well as positive findings, together with detailed information relating to the drug's chemistry, manufacturing, controls and proposed labeling, among other things. Data can come from company-sponsored clinical studies on a drug, or from a number of alternative sources, including studies initiated by investigators. To support marketing authorization, the data we submit must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational drug to the FDA's satisfaction.
The cost of preparing and submitting an NDA is substantial. The submission of most NDAs is additionally subject to a substantial application user fee, and the manufacturer and/or sponsor under an approved new drug application are also subject to annual drug and establishment user fees. The FDA typically increases these fees annually. Orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical study costs, tax advantages and user-fee waivers.
The FDA has 60 days from its receipt of an NDA to determine whether it will accept the application for filing based on the agency's threshold determination that the application is sufficiently complete to permit substantive review. Once the FDA accepts the filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of NDAs. Under the Prescription Drug User Fee Act, the FDA has a goal of responding to standard review NDAs within ten months after the 60-day filing review period, but this timeframe is often extended. The FDA reviews most applications for standard review drugs within ten to 12 months and most applications for priority review drugs within six to eight months. Priority review can be applied to drugs that the FDA determines offer major advances in treatment or provide a treatment where no adequate therapy exists.
The FDA may also refer applications for novel drugs that present difficult questions of safety or efficacy to an advisory committee. This is typically a panel that includes clinicians and other experts that will review, evaluate and recommend whether the FDA should approve the application. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. Before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP and will inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the drug unless compliance with cGMP is satisfactory and the NDA contains data that provide evidence that the drug is safe and effective in the indication studied.
36
The FDA's Decision on an NDA
After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter indicates that the FDA has completed its review of the application and the agency has determined that it will not approve the application in its present form. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional clinical data and/or other significant, expensive and time-consuming requirements related to clinical studies, preclinical studies and/or manufacturing. The FDA has committed to reviewing resubmissions of the NDA addressing such deficiencies in two or six months, depending on the type of information included. Even if we submit such data, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Also, the government may establish additional requirements, including those resulting from new legislation, or the FDA's policies may change, which could delay or prevent regulatory approval of our drugs under development.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require a risk evaluation and mitigation strategy, or REMS, to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring and the use of patient registries. The requirement for REMS can materially affect the potential market and profitability of the drug. Moreover, the FDA may condition approval on substantial post-approval testing and surveillance to monitor the drug's safety or efficacy. Once granted, the FDA may withdraw drug approvals if the company fails to comply with regulatory standards or identifies problems following initial marketing.
Changes to some of the conditions established in an approved application, including changes in indications, labeling or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before we can implement the change. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing new NDAs. As with new NDAs, the FDA often significantly extends the review process with requests for additional information or clarification.
Expedited review and accelerated approval programs
A sponsor may seek approval of its drug candidate under programs designed to accelerate the FDA's review and approval of NDAs. For example, the FDA may grant Fast Track Designation to a drug intended for treatment of a serious or life-threatening disease or condition that has potential to address unmet medical needs for the disease or condition. The key benefits of Fast Track Designation are the eligibility for priority review, rolling review (submission of portions of an application before the complete marketing application is submitted), and accelerated approval, if the application meets relevant criteria. Based on results of the Phase 3 clinical study(ies) submitted in an NDA, upon the request of an applicant, the FDA may grant the NDA a priority review designation, which sets the target date for FDA action on the application at six months after the FDA accepts the application for filing. The FDA grants priority review where there is evidence that the proposed drug would be a significant improvement in the safety or effectiveness of the treatment, diagnosis, or prevention of a serious condition. If the criteria for priority review are not met, the application is subject to the standard FDA review period of ten months after the FDA accepts the application for filing. Priority review designation does not change the scientific/medical standard for approval or the quality of evidence necessary to support approval.
Under the accelerated approval program, the FDA may approve an NDA on the basis of either a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. The FDA generally requires post-marketing studies or completion of ongoing studies after marketing authorization to verify the drug's clinical benefit in relationship to the surrogate endpoint or ultimate outcome in relationship to the clinical benefit. In addition, a sponsor may seek FDA designation of its drug candidate as a breakthrough therapy if the drug can, alone or in combination with one or more other drugs, treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development.
Post-approval Requirements
The FDA regulates drugs that we manufacture or distribute pursuant to FDA approvals and has specific requirements pertaining to recordkeeping, periodic reporting, drug sampling and distribution, advertising and promotion and reporting of adverse experiences with the drug. After approval, the FDA must provide review and approval for most changes to the approved drug, such as adding new indications or other labeling claims. There also are continuing, annual user fee requirements for any marketed drugs and the establishments who manufacture our drugs, as well as new application fees for supplemental applications with clinical data.
37
In some cases, the FDA may condition approval of an NDA for a drug on the sponsor's agreement to conduct additional clinical studies after approval. In other cases, a sponsor may voluntarily conduct additional clinical studies after approval to gain more information about the drug. We typically refer to such post-approval studies as Phase 4 clinical studies.
Drug manufacturers are subject to periodic unannounced inspections by the FDA and state agencies for compliance with cGMP requirements. There are strict regulations regarding changes to the manufacturing process, and depending on the significance of the change, it may require prior FDA approval before we can implement it. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
We rely, and expect to continue to rely, on third parties for the production of clinical quantities of our drugs and we expect to rely in the future on third parties for the production of commercial quantities. Future FDA and state inspections may identify compliance issues at our facilities or at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of previously unknown problems with a drug or the failure to comply with applicable requirements may result in restrictions on a drug, manufacturer or holder of an approved NDA, including withdrawal or recall of the drug from the market or other voluntary, FDA-initiated or judicial actions that could delay or prohibit further marketing.
The FDA may withdraw approval if a company does not comply with regulatory requirements and maintain standards or if problems occur after the drug reaches the market. If a company or the FDA discovers previously unknown problems with a drug, including adverse events of unanticipated severity or frequency, issues with manufacturing processes or the company's failure to comply with regulatory requirements, the FDA may require revisions to the approved labeling to add new safety information; impose post-marketing studies or other clinical studies to assess new safety risks; or impose distribution or other restrictions under a REMS program. Other potential consequences may include:
| ● |
restrictions on the marketing or manufacturing of the drug, complete withdrawal of the drug from the market or drug recalls;
|
| ● |
fines, warning letters or holds on post-approval clinical studies;
|
| ● |
the FDA refusing to approve pending NDAs or supplements to approved NDAs, or suspending or revoking drug license approvals;
|
| ● |
drug seizure or detention, or refusal to permit the import or export of drugs; or
|
| ● |
injunctions or the imposition of civil or criminal penalties.
|
The FDA strictly regulates marketing, labeling, advertising and promotion of drugs that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. We could be subject to significant liability if we violated these laws and regulations.
Orphan Drug Designation
The FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition that affects fewer than 200,000 individuals in the United States, or if it affects more than 200,000 individuals in the United States and there is no reasonable expectation that the cost of developing and making the drug for this type of disease or condition will be recovered from sales in the United States.
Orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical study costs, tax advantages and user-fee waivers. In addition, if a drug receives FDA approval for the indication for which it has orphan designation, the drug has orphan drug exclusivity, which means the FDA may not approve any other application to market the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the drug with orphan exclusivity.
Pediatric Information
Under the Pediatric Research Equity Act, or PREA, NDAs or supplements to NDAs must contain data to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the drug is safe and effective. The FDA may grant full or partial waivers, or deferrals, for submission of data. Unless otherwise required by regulation, PREA does not apply to any drug for an indication for which the FDA has granted an orphan designation.
38
U.S. Patent Term Restoration
Patent term can also be extended based on the amount of time the patented drug spends in regulatory review for drug approval. The length of time between drug launch and patent expiration is significantly less than the full 20-year patent term because companies often obtain the patents relating to a drug early in development and the development path for regulatory approval is long. In the United States, The Drug Price Competition and Patent Term Restoration Act of 1984 (commonly known as the Hatch-Waxman Act) permits a patent holder to seek a patent extension, commonly called patent term restoration, for a patent on a drug governed by the FDCA. The length of patent term restoration is related to the length of time the drug is under regulatory review. Patent term restoration can be a maximum of 5 years and cannot extend the remaining term of a patent beyond a total of 14 years from the date of drug approval. Only one patent applicable to an approved drug may be extended. Similar provisions are available in Europe and certain other foreign jurisdictions to extend the term of a patent that covers an approved drug in that jurisdiction.
Abbreviated New Drug Applications for Generic Drugs
In 1984, with passage of the Hatch-Waxman Act, Congress authorized the FDA to approve generic drugs that are the same as drugs previously approved by the FDA under the NDA provisions of the statute. To obtain approval of a generic drug, an applicant must submit an abbreviated new drug application, or ANDA, to the agency. In support of such applications, a generic manufacturer may rely on the preclinical and clinical testing previously conducted for a drug previously approved under an NDA, known as the reference listed drug, or RLD.
Specifically, in order to approve an ANDA, the FDA must find that the generic version is identical to the RLD with respect to the active ingredients, the route of administration, the dosage form and the strength of the drug. At the same time, the FDA must also determine that the generic drug is bioequivalent to the RLD. Under the statute, a generic drug is bioequivalent to an RLD if "the rate and extent of absorption of the generic drug do not show a significant difference from the rate and extent of absorption of the listed drug…"
Upon approval of an ANDA, the FDA indicates that the generic drug is "therapeutically equivalent" to the RLD and it assigns a therapeutic equivalence rating to the approved generic drug in its publication "Approved Drug Products with Therapeutic Equivalence Evaluations," also referred to as the "Orange Book." Physicians and pharmacists consider an "AB" therapeutic equivalence rating to mean that a generic drug is fully substitutable for the RLD. In addition, by operation of certain state laws and numerous health insurance programs, the FDA's designation of an "AB" rating often results in substitution of the generic drug without the knowledge or consent of either the prescribing physician or patient.
The FDCA provides a period of five years of non-patent exclusivity for a new drug containing a new chemical entity. In cases where such exclusivity has been granted, an ANDA may not be filed with the FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification, in which case the applicant may submit its application four years following the original product approval. The FDCA also provides for a period of three years of exclusivity if the NDA includes reports of one or more new clinical investigations that were conducted by or for the applicant and are essential to the approval of the application, and are not bioavailability or bioequivalence studies. This three-year exclusivity period often protects changes to a previously approved drug, such as a new dosage form, route of administration, combination or indication.
Hatch-Waxman Patent Certification and the 30-month Stay
Upon approval of an NDA or a supplement thereto, NDA sponsors must list with the FDA each patent with claims that cover the applicant's drug or a method of using the drug. Each of the patents listed by the NDA sponsor is published in the Orange Book. When an ANDA applicant files its application with the FDA, the applicant is required to certify to the FDA concerning any patents listed for the reference drug in the Orange Book, except for patents covering methods of use for which the ANDA applicant is not seeking approval. Specifically, the applicant must certify with respect to each patent that:
| ● |
the required patent information has not been filed;
|
| ● |
the listed patent has expired;
|
| ● |
the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or
|
| ● |
the listed patent is invalid, unenforceable or will not be infringed by the new drug.
|
A certification that the new drug will not infringe the already approved drug's listed patents or that such patents are invalid or unenforceable is called a Paragraph IV certification. If the applicant does not challenge the listed patents or indicates that it is not seeking approval of a patented method of use, the FDA will not approve the ANDA application until all the listed patents claiming the referenced drug have expired.
If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days after the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months after the receipt of the Paragraph IV notice, expiration of the patent, or a decision in the infringement case that is favorable to the ANDA applicant.
39
Disclosure of Clinical Study Information
Sponsors of clinical studies of FDA-regulated products, including drugs, are required to register and disclose certain clinical study information. Information related to the product, patient population, phase of investigation, trial sites and investigators, and other aspects of the clinical study is then made public as part of the registration. Sponsors are also obligated to discuss the results of their clinical studies after completion. Disclosure of the results of these studies can be delayed until the new drug or new indication being studied has been approved. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs.
Healthcare Reform
In the United States and foreign jurisdictions, the legislative landscape continues to evolve. There have been a number of legislative and regulatory changes to the healthcare system that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the United States federal and state levels that seek to reduce healthcare costs. In March 2010, the Patient Protection and Affordable Care Act, or PPACA, was enacted, which includes measures that have significantly changed health care financing by both governmental and private insurers. The provisions of the PPACA of importance to the pharmaceutical and biotechnology industry are, among others, the following:
| ● |
an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs agents, apportioned among these entities according to their market share in certain government healthcare programs;
|
| ● |
an increase in the rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13% of the average manufacturer price for branded and generic drugs, respectively;
|
| ● |
a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts to negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer's outpatient drugs to be covered under Medicare Part D;
|
| ● |
extension of manufacturers' Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations, unless the drug is subject to discounts under the 340B drug discount program;
|
| ● |
expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the Federal Poverty Level, thereby potentially increasing manufacturers' Medicaid rebate liability;
|
| ● |
expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;
|
| ● |
expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government investigative powers and enhanced penalties for noncompliance;
|
| ● |
new requirements under the federal Physician Payments Sunshine Act for drug manufacturers to report information related to payments and other transfers of value made to physicians and teaching hospitals as well as ownership or investment interests held by physicians and their immediate family members; and
|
| ● |
a new requirement to annually report certain drug samples that manufacturers and distributors provide to licensed practitioners, or to pharmacies of hospitals or other healthcare entities.
|
Since its enactment there have been judicial and Congressional challenges to or proposals to amend certain aspects of PPACA. We expect there will be additional challenges and amendments to it in the future.
In addition, other health reform measures have been proposed and adopted in the United States since PPACA was enacted. For example, as a result of the Budget Control Act of 2011, providers are subject to Medicare payment reductions of 2% per fiscal year through 2025 unless additional Congressional action is taken. Further, the American Taxpayer Relief Act of 2012 reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments from providers from three to five years. More recently, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which have resulted in several recent Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs.
European Union—EMA Process
In the European Union, drugs follow a similar demanding process as that we described above for the United States and the ICH Common Technical Document is the basis for applications. Prior to submitting a European Marketing Authorization Application, or MAA, it is necessary to gain approval of a detailed Pediatric Investigation Plan, or PIP, with the European Medicines Agency's Pediatric Committee, or PDCO. After gaining PIP approval, EU regulatory authorities can authorize the drug using either the centralized authorization procedure or national authorization procedures.
40
Centralized Procedure
Under the centralized procedure, after the EMA issues an opinion, the European Commission issues a single marketing authorization valid across the European Union, as well as Iceland, Liechtenstein and Norway. The centralized procedure is compulsory for human drugs that: are derived from biotechnology processes, such as genetic engineering; contain a new active substance indicated for the treatment of certain diseases, such as HIV/AIDS, cancer, diabetes, neurodegenerative disorders or autoimmune diseases and other immune dysfunctions; and are officially designated orphan drugs. For drugs that do not fall within these categories, an applicant has the option of submitting an application for a centralized marketing authorization to the EMA, as long as the drug concerned is a significant therapeutic, scientific or technical innovation, or if its authorization would be in the interest of public health. Volanesorsen has been granted a Promising Innovative Medicine, or PIM, Designation by the United Kingdom's Medicines and Healthcare products Regulatory Agency, or MHRA, for the treatment of people with FCS. A PIM Designation is an early indication that a medicinal product is a promising candidate for the Early Access to Medicines Scheme, or EAMS, in the UK, intended for the treatment, diagnosis or prevention of a life-threatening or seriously debilitating condition, with the potential to address an unmet medical need.
National Authorization Procedures
There are also two other possible routes to authorize medicinal products in several countries, which are available for products that fall outside the scope of the centralized procedure:
| ● |
Decentralized procedure. Using the decentralized procedure, an applicant may apply for simultaneous authorization in more than one European Union country of a medicinal product that has not yet been authorized in any European Union country and that does not fall within the mandatory scope of the centralized procedure.
|
| ● |
Mutual recognition procedure. In the mutual recognition procedure, a medicine is first authorized in one European Union Member State, in accordance with the national procedures of that country. Thereafter, further marketing authorizations can be sought from other European Union countries in a procedure whereby the countries concerned agree to recognize the validity of the original, national marketing authorization.
|
Good Manufacturing Practices
Like the FDA, the EMA, the competent authorities of the European Union Member States, and other regulatory agencies regulate and inspect equipment, facilities and processes used in the manufacturing of drugs prior to approving a drug. If, after receiving clearance from regulatory agencies, a company makes a material change in manufacturing equipment, location or process, additional regulatory review and approval may be required. Once we or our partners commercialize drugs, we will be required to comply with cGMP and drug-specific regulations enforced by the European Commission, the EMA and the competent authorities of European Union Member States following drug approval. Also like the FDA, the EMA, the competent authorities of the European Union Member States, and other regulatory agencies also conduct regular, periodic visits to re-inspect equipment, facilities and processes following the initial approval of a drug. If, as a result of these inspections, the regulatory agencies determine that our or our partners' equipment, facilities, or processes do not comply with applicable regulations and conditions of drug approval, they may seek civil, criminal or administrative sanctions and/or remedies against us, including the suspension of our manufacturing operations or the withdrawal of our drug from the market.
Data and Market Exclusivity
Similar to the United States, there is a process to authorize generic versions of innovative drugs in the European Union. Generic competitors can submit abridged applications to authorize generic versions of drugs authorized by EMA through a centralized procedure referencing the innovator's data and demonstrating bioequivalence to the reference drug, among other things. New drugs in the European Union can receive eight years of data exclusivity coupled with two years of market exclusivity, and a potential one-year extension, if the marketing authorizations holder obtains an authorization for one or more new therapeutic indications that demonstrates "significant clinical benefit" in comparison with existing therapies. This system is usually referred to as "8+2." Abridged applications cannot rely on an innovator's data until after expiry of the eight-year date exclusivity term, meaning that a competitor can file an application for a generic drug, but the drug cannot be marketed until the end of the market exclusivity term.
Other International Markets—Drug Approval Process
In some international markets (such as China or Japan), although data generated in United States or European Union studies may be submitted in support of a marketing authorization application, regulators may require additional clinical studies conducted in the host territory, or studying people of the ethnicity of the host territory, prior to the filing or approval of marketing applications within the country.
41
Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drugs for which we may obtain regulatory approval. In the United States and in other countries, sales of any drugs for which we receive regulatory approval for commercial sale will depend in part on the availability of coverage and reimbursement from third-party payors. Third-party payors include government authorities, managed care providers, private health insurers and other organizations. The process for determining whether a payor will provide coverage for a drug may be separate from the process for setting the reimbursement rate that the payor will pay for the drug. Third-party payors may limit coverage to specific drugs on an approved list, or formulary, which might not include all of the FDA-approved drugs for a particular indication. Moreover, a payor's decision to provide coverage for a drug does not imply that an adequate reimbursement rate will be approved. Additionally, coverage and reimbursement for drugs can differ significantly from payor to payor. One third-party payor's decision to cover a particular drug does not ensure that other payors will also provide coverage for the drug, or will provide coverage at an adequate reimbursement rate. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in drug development.
Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of drugs and services, in addition to their safety and efficacy. To obtain coverage and reimbursement for any drug that might be approved for sale, we may need to conduct expensive pharmacoeconomic studies to demonstrate the medical necessity and cost-effectiveness of our drug. These studies will be in addition to the studies required to obtain regulatory approvals. If third-party payors do not consider a drug to be cost-effective compared to other available therapies, they may not cover the drug after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow a company to sell its drugs at a profit.
The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost containment programs to limit the growth of government-paid health care costs, including price controls, restrictions on reimbursement and requirements for substitution of generic drugs for branded prescription drugs. By way of example, the PPACA contains provisions that may reduce the profitability of drugs, including, for example, increased rebates for drugs sold to Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies' share of sales to federal health care programs. Adoption of government controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for our drugs.
In the European community, governments influence the price of drugs through their pricing and reimbursement rules and control of national health care systems that fund a large part of the cost of those drugs to consumers. Some jurisdictions operate positive and negative list systems under which drugs may only be marketed once a reimbursement price has been agreed to by the government. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical studies that compare the cost effectiveness of a particular drug candidate to currently available therapies. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new drugs. In addition, in some countries, cross border imports from low-priced markets exert a commercial pressure on pricing within a country.
The marketability of any drugs for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, the focus on cost containment measures in the United States and other countries has increased and we expect will continue to increase the pressure on pharmaceutical pricing. Coverage policies and third-party reimbursement rates may change at any time. Even if we attain favorable coverage and reimbursement status for one or more drugs for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Sales and Marketing
Numerous regulatory authorities in addition to the FDA, including, in the United States, the Centers for Medicare & Medicaid Services, other divisions of the U.S. Department of Health and Human Services, the U.S. Department of Justice, and similar foreign, state and local government authorities, regulate sales, promotion and other activities following drug approval. As described above, the FDA regulates all advertising and promotion activities for drugs under its jurisdiction both prior to and after approval. Only those claims relating to safety and efficacy that the FDA has approved may be used in labeling. Physicians may prescribe legally available drugs for uses that are not described in the drug's labeling and that differ from those we tested and the FDA approved. Such off-label uses are common across medical specialties and often reflect a physician's belief that the off-label use is the best treatment for the patients. The FDA does not regulate the behavior of physicians in their choice of treatments, but FDA regulations do impose stringent restrictions on manufacturers' communications regarding off-label uses. If we do not comply with applicable FDA requirements, we may face adverse publicity, enforcement action by the FDA, corrective advertising, consent decrees and the full range of civil and criminal penalties available to the FDA. Promotion of off-label uses of drugs can also implicate the false claims laws described below.
42
In the United States sales, marketing and scientific/educational programs must also comply with various federal and state laws pertaining to healthcare "fraud and abuse," including anti-kickback laws and false claims laws. Anti-kickback laws make it illegal for a prescription drug manufacturer to solicit, offer, receive, or pay any remuneration in exchange for, or to induce, the referral of business, including the purchase or prescription of a particular drug. Due to the breadth of the statutory provisions, limited statutory exceptions and regulatory safe harbors, and the absence of guidance in the form of regulations and very few court decisions addressing industry practices, it is possible that our practices might be challenged under anti-kickback or similar laws. Moreover, recent healthcare reform legislation has strengthened these laws. For example, the PPACA among other things, amends the intent requirement of the federal anti-kickback and criminal healthcare fraud statutes to clarify that a person or entity does not need to have actual knowledge of this statute or specific intent to violate it. In addition, PPACA clarifies that the government may assert that a claim that includes items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes. False claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented for payment, to third-party payors (including Medicare and Medicaid) claims for reimbursed drugs or services that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. Our activities relating to the sale and marketing of our drugs may be subject to scrutiny under these laws. Violations of fraud and abuse laws may be punishable by criminal and civil sanctions, including fines and civil monetary penalties, the possibility of exclusion from federal healthcare programs (including Medicare and Medicaid) and corporate integrity agreements, which impose, among other things, rigorous operational and monitoring requirements on companies. Similar sanctions and penalties also can be imposed upon executive officers and employees, including criminal sanctions against executive officers under the so-called "responsible corporate officer" doctrine, even in situations where the executive officer did not intend to violate the law and was unaware of any wrongdoing.
Given the significant penalties and fines that can be imposed on companies and individuals if convicted, allegations of such violations often result in settlements even if the company or individual being investigated admits no wrongdoing. Settlements often include significant civil sanctions, including fines and civil monetary penalties, and corporate integrity agreements. If the government were to allege or convict us or our executive officers of violating these laws, our business could be harmed. In addition, private individuals can bring similar actions. Our activities could be subject to challenge for the reasons discussed above and due to the broad scope of these laws and the increasing attention being given to them by law enforcement authorities. Other healthcare laws that may affect our ability to operate include the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, which governs the conduct of certain electronic healthcare transactions and protects the security and privacy of protected health information; analogous state laws governing the privacy and security of health information, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, and the Physician Payments Sunshine Act, which requires manufacturers of drugs, devices, biologics, and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians and teaching hospitals, and ownership and investment interests held by physicians and their immediate family members. Further, there are an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information. Many of these laws contain ambiguities as to what is required to comply with the laws. Given the lack of clarity in laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state authorities.
Similar rigid restrictions are imposed on the promotion and marketing of drugs in the European Union and other countries. Even in those countries where we may not be directly responsible for the promotion and marketing of our drugs, if our potential international distribution partners engage in inappropriate activity it can have adverse implications for us.
The Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act, or FCPA, prohibits any U.S. individual or business from paying, offering, or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party, or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations. Activities that violate the FCPA, even if they occur wholly outside the United States, can result in criminal and civil fines, imprisonment, disgorgement, oversight, and debarment from government contracts.
Employees
As of February 20, 2018, we employed 100 people. A significant number of our management and professional employees have had prior experience with pharmaceutical, biotechnology or medical product companies. Collective bargaining agreements do not cover any of our employees and management considers relations with our employees to be good.
43
Executive Officers of Akcea
The following sets forth certain information regarding our executive officers as of February 20, 2018:
|
Name
|
Age
|
Position
|
||
|
Paula Soteropoulos
|
50
|
President and Chief Executive Officer
|
||
|
Michael MacLean
|
52
|
Chief Financial Officer
|
||
|
Jeffrey M. Goldberg
|
45
|
Chief Operating Officer
|
||
|
Louis St. L. O'Dea, MB BCh BAO, FRCP(C)
|
67
|
Executive Vice President and Chief Medical Officer
|
PAULA SOTEROPOLOUS
President and Chief Executive Officer
Ms. Soteropoulos joined Akcea as its President and Chief Executive Officer and as a member of our board of directors in January 2015. Prior to joining Akcea, Ms. Soteropoulos was a member of the executive leadership team of Moderna Therapeutics Inc., a private biotechnology company, serving as the Cardiometabolic Business Unit General Manager and Senior Vice President of Strategic Alliances from July 2013 to December 2014. Prior to Moderna, Ms. Soteropoulos spent 21 years at Genzyme Corporation in various leadership positions driving strategy, sales and marketing, business development, manufacturing process development, strategic capacity planning, and supply chain development. Since July 2013, Ms. Soteropoulos has served on the supervisory board of uniQure N.V., a public biotechnology company. Ms. Soteropoulos also serves on the advisory board of the Tufts University Chemical and Biological Engineering Department. Our board of directors believes that Ms. Soteropoulos is uniquely suited to serve on our board of directors because of her experience in the biotechnology industry and her daily insight into corporate matters as our President and Chief Executive Officer.
MICHAEL MACLEAN
Chief Financial Officer
Mr. MacLean has served as its Chief Financial Officer of Akcea since September 2017. Prior to joining Akcea, from September 2015 to September 2017, Mr. MacLean was Chief Financial Officer and Executive Vice President for PureTech Health, an advanced, clinical-stage, public biopharmaceutical company focusing on diseases caused by dysfunctions in the nervous, gastrointestinal and immune systems. Previously, Mr. MacLean served as Senior Vice President of Finance and Chief Accounting Officer of Biogen Inc. where he led the Company's worldwide finance organization.
JEFFREY M. GOLDBERG
Chief Operating Officer
Mr. Goldberg joined Akcea as its Chief Operating Officer in January 2015. Prior to joining Akcea, from December 2012 to September 2014, Mr. Goldberg was a member of the executive leadership team at Proteostasis Therapeutics, Inc., a now public biotechnology company focusing on neurology and orphan diseases, where he served as Vice President of Business Operations. Prior to that, Mr. Goldberg spent over 11 years in positions of increasing responsibility with Genzyme and Sanofi S.A., most recently as Associate Vice President, Project Head, within Sanofi Oncology.
LOUIS ST. L. O'DEA
Chief Medical Officer
Dr. O'Dea joined Akcea as its Executive Vice President and Chief Medical Officer in January 2016. Prior to joining Akcea, Dr. O'Dea was Chief Medical Officer at Oxford Immunotec Global PLC, a private diagnostics company, from June 2014 to January 2016, overseeing medical affairs and clinical development. Prior to Oxford, Dr. O'Dea was Chief Medical Officer and Head of Regulatory Affairs at Moderna from January 2012 to June 2014. Before Moderna, Dr. O'Dea held positions including Chief Medical Officer at Radius Health, Inc., a public biopharmaceuticals company, an academic position at McGill University, and worldwide Head of Clinical Development for Endocrine and Metabolic products at Serono.
44
Item 1A. RISK FACTORS
Investing in our securities involves a high degree of risk. You should consider carefully the following information about the risks described below, together with the other information contained in this report and in our other public filings in evaluating our business. If any of the following risks actually occur, our business could be materially harmed, and our financial condition and results of operations could be materially and adversely affected. As a result, the trading price of our securities could decline, and you might lose all or part of your investment. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial may also adversely affect our business.
Risks Related to Our Financial Condition and Need for Additional Capital
We have a limited operating history and may never become profitable.
Ionis Pharmaceuticals, Inc., or Ionis, incorporated us as a Delaware corporation in December 2014, and we have operated as an affiliate of Ionis since that time. As such, we have limited experience as a company, and no experience operating independently from Ionis, and have not yet demonstrated that we can successfully overcome many of the risks and uncertainties frequently encountered in new and rapidly evolving fields, particularly the biotechnology and pharmaceutical fields.
As a company, we have never obtained regulatory approval for, or commercialized, any product. Our ability to generate substantial revenue and achieve profitability depends on our ability, alone or with strategic partners, to successfully develop our drugs, and obtain the regulatory approvals necessary to commercialize our drugs, including volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development. If volanesorsen is approved, we anticipate receiving our first revenue from product sales in 2018. Even if we achieve profitability in the future, we may not sustain profitability in subsequent periods. Our ability to generate revenue from product sales depends heavily on our and our current and future strategic partners' success in:
| ● |
completing clinical development of volanesorsen for additional indications and nonclinical and clinical development of AKCEA-APO(a)-LRx, AKCEA-ANGPTL3-LRx and AKCEA-APOCIII-LRx;
|
| ● |
seeking and obtaining regulatory and marketing authorization for our drugs, including volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development;
|
| ● |
establishing and maintaining supply and manufacturing relationships with third parties that can provide the amount and quality of products and services we need to continue to develop and, if approved, commercialize volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development;
|
| ● |
launching and commercializing volanesorsen and AKCEA-ANGPTL3-LRx by establishing a sales, marketing and distribution infrastructure;
|
| ● |
launching and co-commercializing AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx through our collaboration with Novartis Pharma AG, or Novartis, under terms that we plan to negotiate with Novartis in the future;
|
| ● |
educating physicians about our target patient populations, including patients with familial chylomicronemia syndrome, or FCS, and patients with familial partial lipodystrophy, or FPL;
|
| ● |
obtaining market acceptance of volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development as viable treatment options;
|
| ● |
obtaining and maintaining adequate coverage and reimbursement from third-party payors for volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development;
|
| ● |
addressing any competing technological and market developments;
|
| ● |
implementing additional internal systems and infrastructure, as needed, to ultimately operate without reliance on Ionis;
|
| ● |
negotiating favorable terms in any partnership, licensing or other arrangements into which we may enter;
|
| ● |
maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, product trademarks and know-how;
|
| ● |
developing and commercializing volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development without infringing others' intellectual property rights; and
|
| ● |
attracting, hiring and retaining qualified personnel.
|
We may not successfully develop any products, generate product revenue or achieve profitability. If we cannot achieve or maintain profitability, it would depress the market price of our common stock and could impair our ability to raise capital, expand our business, diversify our product offerings or continue our operations. If the market price of our common stock declined, you could lose all or part of your investment.
We have incurred losses since our inception.
Because drug discovery and development require substantial lead-time and funding prior to commercialization, we have incurred expenses while generating limited revenue from our operating activities since our formation. Our net losses were $109.8 million, $83.2 million and $61.4 million for the years ended December 31, 2017, December 31, 2016 and December 31, 2015, respectively. As of December 31, 2017, we had an accumulated deficit of approximately $284.4 million. Most of the losses resulted from costs incurred in connection with our development programs and from general and administrative costs associated with our operations. We expect to incur additional operating losses for the foreseeable future, and these losses may increase if we cannot generate substantial revenue.
45
We will require substantial additional funding to achieve our goals. If we fail to obtain timely funding, we may need to curtail or abandon some of our programs.
All of our drugs are undergoing clinical studies. All of our drug programs will require additional nonclinical and/or clinical testing and/or marketing authorization prior to commercialization. We will need to spend significant additional resources to conduct these activities. Our expenses could increase beyond expectations if the U.S. Food and Drug Administration, or FDA, the European Medicines Agency, or EMA, or other regulatory authorities require us to perform clinical studies and other studies in addition to those that we currently anticipate. As of December 31, 2017, we had cash, cash equivalents and short-term investments equal to $260.1 million. Our operating expenses were $163.9 million, $83.5 million and $61.4 million for the years ended December 31, 2017, December 31, 2016 and December 31, 2015, respectively.
Prior to our IPO, we funded our operating activities through a $100.0 million cash contribution we received from Ionis in 2015, $75.0 million we received from initiating our collaboration with Novartis and $106.0 million in drawdowns under our line of credit with Ionis. The line of credit converted to our common stock when we closed our IPO. We no longer have access to the line of credit following the closing of our IPO and we do not have any firm commitment from Ionis to fund our cash flow deficits or provide other direct or indirect financial assistance to us. Additionally, in July 2017 we received $182.3 million in net proceeds from our IPO including $25.0 million Ionis invested in our IPO and the Novartis concurrent private placement of $50 million. Based on our existing cash, cash equivalents and short-term investments and the proceeds from our IPO and the concurrent private placement with Novartis, we will need to raise additional funding to continue developing the drugs in our pipeline and to seek regulatory approval for and to commercialize volanesorsen and other drugs in our pipeline.
Even if we obtain marketing authorizations to sell volanesorsen or AKCEA-ANGPTL3-LRx, we will incur significant costs to commercialize the approved product. Even if we generate revenue from the sale of any approved products, we may not become profitable and would need to obtain additional funding to continue operations.
Risks Related to Clinical Development, Regulatory Review and Approval of Our Drugs
If the results of clinical testing indicate that any of our drugs are not suitable for commercial use, we may need to abandon one or more of our drug development programs.
Drug discovery and development has inherent risks and the historical failure rate for drugs is high. Antisense drugs are a relatively new approach to therapeutics. If we cannot demonstrate that our drugs are safe and effective for human use in the intended indication, we may need to abandon one or more of our drug development programs.
If any of our drugs in clinical studies, including volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, do not show sufficient safety and efficacy in patients with the targeted indication, it would negatively affect our development and commercialization goals for the drug and we would have expended significant resources with little or no benefit to us.
Even if our drugs are successful in preclinical and earlier-stage clinical studies, the drugs may not be successful in later-stage clinical studies.
Successful results in preclinical or initial clinical studies, including the results of earlier studies for our drugs in development, may not predict the results of subsequent clinical studies, including the Phase 3 study of volanesorsen for the treatment of FPL. There are a number of factors that could cause a clinical study to fail or be delayed, including:
| ● |
the clinical study may produce negative or inconclusive results;
|
| ● |
regulators may require that we hold, suspend or terminate clinical research for noncompliance with regulatory requirements;
|
| ● |
we, our partners, the FDA or foreign regulatory authorities could suspend or terminate a clinical study due to adverse side effects of a drug on people in the study;
|
| ● |
we or our partners may decide, or regulators may require us, to conduct additional preclinical testing or clinical studies;
|
| ● |
we or our partners may not identify, recruit and train suitable clinical investigators at a sufficient number of study sites;
|
| ● |
the institutional review board for a prospective site might withhold or delay its approval for the study;
|
| ● |
enrollment in our clinical studies may be slower than we anticipate;
|
| ● |
patients who enroll in the clinical study may later drop out due to adverse events, a perception they are not benefiting from participating in the study, fatigue with the clinical study process or personal issues;
|
| ● |
a clinical study site may deviate from the protocol for the study;
|
| ● |
the cost of our clinical studies may be greater than we anticipate;
|
| ● |
we or our partners may require additional capital to fund the clinical study; and
|
| ● |
the supply or quality of our drugs or other materials necessary to conduct the clinical studies may be insufficient, inadequate or delayed.
|
46
In addition, volanesorsen and AKCEA-APOCIII-LRx have the same mechanism of action, and all of our current drugs, including volanesorsen, AKCEA-APO(a)-LRx, AKCEA-ANGPTL3-LRx and AKCEA-APOCIII-LRx, are chemically similar to each other and the drugs Ionis and other companies are developing separately. As a result, a safety observation we, Ionis or other companies encounter with one of our or their drugs could have or be perceived by a regulatory authority to have an impact on a different drug we are developing. This could cause the FDA and other regulators to ask questions or take actions that could harm or delay our ability to develop and commercialize our drugs or increase our costs. For example, the FDA or other regulatory agencies could request, among other things, any of the following regarding one of our drugs: additional information or commitments before we can start or continue a clinical study, protocol amendments, increased safety monitoring, additional product labeling information, and post-approval commitments. Similarly, we have an ongoing Phase 3 study of volanesorsen in patients with FPL, an ongoing open label extension study of volanesorsen in patients with FCS, and an early access program, or EAP, for volanesorsen. Adverse events or results from these studies or the EAP could negatively impact our planned marketing approval applications for volanesorsen in patients with FCS or the commercial opportunity for volanesorsen.
Any failure or delay in the clinical studies for any of our drugs in development could reduce the commercial potential or viability of our drugs.
We may not have appropriately designed the planned and ongoing clinical studies for volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development to support submission of a marketing application to the FDA and foreign regulatory authorities or demonstrate safety or efficacy at the level required by the FDA and foreign regulatory authorities for product approval.
We recently completed a Phase 3 clinical program for volanesorsen for the treatment of FCS and have an ongoing Phase 3 study of volanesorsen in patients with FPL. We are also conducting or plan to conduct additional clinical studies for AKCEA-APO(a)-LRx, AKCEA-ANGPTL3-LRx and AKCEA-APOCIII-LRx.
Even if we achieve positive results on the endpoints for these clinical studies or any future clinical studies, the FDA or foreign regulatory authorities may believe the clinical studies do not show the appropriate balance of safety and efficacy in the indication being sought or may interpret the data differently than we do, and deem the results insufficient to demonstrate the appropriate balance of safety and efficacy at the level required for product approval. For example, the FDA or foreign regulatory authorities could claim that we have not tested volanesorsen in a sufficient number of patients to demonstrate volanesorsen is safe and effective in patients with FCS or FPL to support an application for marketing authorization. In such a case, we may need to conduct additional clinical studies before obtaining marketing authorization, which would be expensive and delay these development programs. These risks are more likely to occur since we are developing our drugs against therapeutic targets or to treat diseases in which there is little or no clinical experience. In addition, these risks may be more likely to occur for volanesorsen since there were some patients in the Phase 3 program that experienced serious platelet events (grade 4 thrombocytopenia), a condition in which the patient has very low platelet levels, and additional patients experienced other adverse events in the program, including patients who discontinued participation in the APPROACH study due to platelet count declines. We believe that the enhanced monitoring we have implemented to support early detection and management of these issues can help manage these safety issues so that patients can continue treatment. Since implementation of the enhanced monitoring, serious platelet events have been infrequent.
We may make modifications to the clinical study protocols or designs of our ongoing clinical studies that delay enrollment or completion of such clinical studies and could delay regulatory approval of volanesorsen and our other drugs in development. Any failure to obtain approval for volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development on the timeline that we currently anticipate, or at all, would have a material and adverse impact on our business, prospects, financial condition and results of operations and could cause our stock price to decline.
If we or our partners fail to obtain regulatory approval for our drugs, including volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, we or our partners cannot sell them in the applicable markets.
We cannot guarantee that any of our drugs, including volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, will be safe and effective, or will be approved for commercialization. We and our partners must conduct time-consuming, extensive and costly clinical studies to demonstrate the safety and efficacy of each of our drugs, including volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, before they can be approved for sale. We and our partners must conduct these studies in compliance with FDA regulations and with comparable regulations in other countries.
We or our partners may not obtain necessary regulatory approvals on a timely basis, if at all, for any of our drugs. It is possible that regulatory authorities will not approve any of our drugs, including volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, for marketing. If the FDA or another regulatory authority believes that we or our partners have not sufficiently demonstrated the safety or efficacy of any of our drugs, including volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, the authority will not approve the specific drug or will require additional studies, which can be time consuming and expensive and which will delay or harm our ability to successfully commercialize the drug. For example, since some patients in the Phase 3 program for volanesorsen experienced serious platelet events (grade 4 thrombocytopenia), a condition in which the patient has very low platelet levels, and additional patients experienced other adverse events in the program, some of whom discontinued participation in the studies, including patients who discontinued participation in the APPROACH study due to platelet count declines, the FDA or another regulatory authority may require us to conduct additional studies of volanesorsen before considering an application for marketing approval. We believe that the enhanced monitoring we have implemented to support early detection and management of these issues can help manage these safety issues so that patients can continue treatment. Since implementation of the enhanced monitoring, serious platelet events have been infrequent.
47
The FDA or other comparable foreign regulatory authorities can delay, limit or deny approval of a drug for many reasons, including:
| ● |
such authorities may disagree with the design or implementation of our clinical studies;
|
| ● |
we or our partners may be unable to demonstrate to the satisfaction of the FDA or other regulatory authorities that a drug is safe and effective for any indication;
|
| ● |
such authorities may not accept clinical data from studies conducted at clinical facilities that have deficient clinical practices or that are in countries where the standard of care is potentially different from the United States;
|
| ● |
we or our partners may be unable to demonstrate that our drug's clinical and other benefits outweigh its safety risks to support approval;
|
| ● |
such authorities may disagree with the interpretation of data from preclinical or clinical studies;
|
| ● |
such authorities may find deficiencies in the manufacturing processes or facilities of third-party manufacturers who manufacture clinical and commercial supplies for our drugs; and
|
| ● |
the approval policies or regulations of such authorities or their prior guidance to us or our partners during clinical development may significantly change in a manner rendering our clinical data insufficient for approval.
|
Failure to successfully develop volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, or to receive marketing authorization for these drugs or delays in these authorizations would prevent or delay the commercial launch of the drug, and, as a result, would negatively affect our ability to generate revenue.
The FDA's Division of Metabolism and Endocrinology Products advisory committee will discuss and advise FDA on the risk-benefit profile of volanesorsen for the treatment of FCS tentatively scheduled for May 10, 2018. In advance of this advisory committee meeting, we and the FDA will submit briefing documents for the committee's review, and these briefing documents will be made available to the public and may include information from the volanesorsen development program that have not previously been disclosed. Historically, for some companies, disclosure of information in this manner has led to increased volatility in their stock price. The advisory committee and FDA may interpret nonclinical and clinical data differently than we and our experts have. Press coverage and public scrutiny of the materials that will be discussed at the advisory committee meeting may negatively affect the potential for our volanesorsen NDA to receive approval or the trading price of our securities. Even if we ultimately obtain approval of the volanesorsen NDA, the matters discussed at the advisory committee meeting could limit our ability to successfully commercialize volanesorsen.
We may not be able to benefit from orphan drug designation for volanesorsen, or any of our other drugs.
The FDA and EMA have granted orphan drug designation to volanesorsen for the treatment of patients with FCS. The EMA has granted orphan drug designation to volanesorsen for the treatment of patients with FPL and we are in the process of applying for orphan drug status for FPL in the United States. In the United States, under the Orphan Drug Act, the FDA may designate a drug as an orphan drug if it is intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals in the United States. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process, but it can provide financial incentives, such as tax advantages and user-fee waivers, as well as longer regulatory exclusivity periods.
We may lose orphan drug exclusivity if the FDA determines that the request for designation was materially defective or if we cannot assure sufficient quantity of the applicable drug to meet the needs of patients with the rare disease or condition.
Even if we maintain orphan drug exclusivity for volanesorsen or obtain orphan drug exclusivity for our other drugs, the exclusivity may not effectively protect the drug from competition because regulatory authorities still may authorize different drugs for the same condition.
We may expend our limited resources to pursue a particular drug or indication and fail to capitalize on drugs or indications that may be more profitable or for which there is a greater likelihood of success.
We are dedicating a substantial amount of our resources to develop and seek regulatory approval for volanesorsen to treat patients with FCS and FPL. As a result, we may forego or delay pursuit of opportunities with our other drugs or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial drugs or profitable market opportunities. Our spending on current and future research and development programs and drugs for specific indications may not yield any commercially viable drugs.
48
Our drugs, including volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, could be subject to regulatory limitations following approval.
Following approval of a drug, we and our partners must comply with comprehensive government regulations regarding the manufacture, marketing and distribution of drug products. Promotional communications regarding prescription drugs must be consistent with the information in the product's approved labeling. We and our partners may not obtain the labeling claims necessary or desirable to successfully commercialize our drug products, including volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development.
The FDA and foreign regulatory authorities can impose significant restrictions on an approved drug product through the product label and on advertising, promotional and distribution activities.
In addition, when approved, the FDA or a foreign regulatory authority may condition approval on the performance of post-approval clinical studies or patient monitoring, which could be time consuming and expensive. If the results of such post-marketing studies are not satisfactory, the FDA or a foreign regulatory authority may withdraw marketing authorization or may condition continued marketing on commitments from us or our partners that may be expensive and/or time consuming to fulfill.
In addition, if we or others identify side effects after any of our drug products are on the market, if manufacturing problems occur subsequent to regulatory approval, or if we, our manufacturers or our partners fail to comply with regulatory requirements, we or our partners could be subject to:
| ● |
restrictions on our ability to conduct clinical studies, including full or partial clinical holds on ongoing or planned clinical studies;
|
| ● |
restrictions on such products' manufacturing processes;
|
| ● |
changes to the product label;
|
| ● |
restrictions on the marketing of a product;
|
| ● |
restrictions on product distribution;
|
| ● |
requirements to conduct post-marketing clinical studies;
|
| ● |
Untitled or Warning Letters;
|
| ● |
withdrawal of the products from the market;
|
| ● |
refusal to approve pending applications or supplements to approved applications that we submit;
|
| ● |
recall of products;
|
| ● |
fines, restitution or disgorgement of profits or revenue;
|
| ● |
suspension or withdrawal of regulatory approvals;
|
| ● |
refusal to permit the import or export of our products;
|
| ● |
product seizure;
|
| ● |
injunctions; or
|
| ● |
imposition of civil or criminal penalties.
|
Any one or a combination of these events could prevent us from achieving or maintaining market acceptance of the affected drug product or could substantially increase the costs and expenses of commercializing such drug product, which in turn could delay or prevent us from generating any revenue or profit from the sale of the drug product.
Risks Related to Commercialization of Our Drugs
If we cannot establish effective marketing and sales capabilities or enter into agreements with third parties to market and sell our drug products, we may not generate product revenue.
We currently have a limited commercial infrastructure to market, sell or distribute our drugs. If approved, to commercialize our products, we must build our marketing, sales and distribution capabilities or make arrangements with third parties to perform these services. We may not be successful in doing so. To commercialize volanesorsen and AKCEA-ANGPTL3-LRx in the initial indications we plan to pursue, we plan to build a specialty sales force in each global region we expect to market the applicable drug, supported by case managers, reimbursement specialists, partnerships with specialty pharmacies, injection training, routine platelet monitoring, dietary counseling and a medical affairs team. We may seek to further penetrate markets by expanding our sales force or through strategic partnerships with other pharmaceutical or biotechnology companies or third-party sales organizations, such as our strategic collaboration with Novartis.
Even though certain members of our management team and other employees have significant experience commercializing drug products, as a company we have no prior experience marketing, selling or distributing drug products, and there are significant risks involved in building and managing a commercial infrastructure. It will be expensive and time consuming for us to build and establish our own sales force and related compliance protocols to market any drug products. We may never successfully develop this capability and any failure could delay or preclude a product launch. We and our partners, will have to compete with other companies to recruit, hire, train, manage and retain marketing and sales personnel.
49
We will incur expenses prior to product launch to develop a marketing and sales infrastructure. If regulatory requirements or other factors cause a delay in the commercial launch of volanesorsen, or our other drugs in development, we would incur additional expenses for having developed these capabilities earlier than required and prior to realizing any revenue from sales of volanesorsen and our other drugs in development. Even if we can effectively hire a sales force and develop a marketing and sales infrastructure, our sales force and marketing teams may not successfully commercialize volanesorsen or our other drugs in development.
If we cannot hire a sales force or collaborate with a third-party marketing and sales organization to globally commercialize any approved drug product, our ability to generate product revenue may be limited. To the extent we rely on third parties to commercialize any drug products, such as would be the case if Novartis exercises its option for AKCEA-APO(a)-LRx or AKCEA-APOCIII-LRx, we may receive less revenue than if we commercialized these drug products ourselves. In addition, we would have less control over the sales efforts of any other third parties involved in our commercialization efforts.
We plan to rely on third-party specialty channels to distribute volanesorsen, and our other drugs to patients. If we cannot effectively establish and manage this distribution process, it could harm or delay the commercial launch and sales of volanesorsen and our other drugs in development.
We and our strategic partners may contract with, and rely on, third-party specialty pharmacies to distribute volanesorsen, and our other drugs to patients. A specialty pharmacy is a pharmacy that specializes in dispensing medications for complex or chronic conditions, a process that requires a high level of patient education and ongoing management. Our management team will need to devote a significant amount of its attention to building and managing this distribution network. If we cannot effectively build and manage this distribution process, the commercial launch and sales of volanesorsen and AKCEA-ANGPTL3-LRx will be delayed or less successful, which would harm our results of operations.
In addition, the use of specialty pharmacies involves certain risks, including, but not limited to, risks that these organizations will:
| ● |
not provide us with accurate or timely information regarding their inventories, the number of patients who are using our drugs or complaints regarding our drugs;
|
| ● |
not effectively sell or support volanesorsen, AKCEA-ANGPTL3-LRx and our other drugs;
|
| ● |
reduce or discontinue their efforts to sell or support volanesorsen, AKCEA-ANGPTL3-LRx or our other drugs;
|
| ● |
not devote the resources necessary to sell volanesorsen, AKCEA-ANGPTL3-LRx or our other drugs in the volumes and within the time frames that we expect;
|
| ● |
not satisfy financial obligations to us or others; or
|
| ● |
cease operations.
|
Any such events may result in decreased sales and lower revenue, which could have a material adverse effect on our business, prospects, financial condition and results of operations.
If the market does not accept our drugs, including volanesorsen and our other drugs in development, we are not likely to generate substantial product revenue or become profitable.
Even if we or our strategic partners obtain a marketing authorization for volanesorsen and our other drugs in development, our success will depend upon the medical community, patients and third-party payors accepting our drugs as medically useful, cost-effective, safe and convenient. Even if the FDA or foreign regulatory authorities authorize our drugs for commercialization, doctors may not prescribe our drugs to treat patients. We and our partners may not successfully commercialize additional drugs.
Additionally, in many of the markets where we or our partners may sell our drugs in the future, if we cannot agree with the government or other third-party payors regarding the price we can charge for our drugs, then we may not be able to sell our drugs in that market. Similarly, cost control initiatives by governments or third-party payors could decrease the price received for our drugs or increase patient coinsurance to a level that makes commercializing volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development economically unviable.
The degree of market acceptance for volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development depends upon a number of factors, including the:
| ● |
receipt and scope of marketing authorizations;
|
| ● |
establishment and demonstration in the medical and patient community of the efficacy and safety of our drugs and their potential advantages over competing products;
|
| ● |
cost and effectiveness of our drugs compared to other available therapies;
|
| ● |
patient convenience of the dosing regimen for our drugs; and
|
| ● |
reimbursement by government and third-party payors.
|
Based on the profile of our drugs, physicians, patients, patient advocates, payors or the medical community in general may not accept and/or use any drugs that we may develop. For example, in the clinical studies with volanesorsen, declines in platelet counts were observed in many patients and some patients discontinued the study because of platelet declines. Therefore, we expect volanesorsen's product label will require periodic platelet monitoring, which could negatively affect our ability to attract and retain patients for volanesorsen. We believe that the enhanced monitoring we have implemented to support early detection and management of these issues can help manage these safety issues so that patients can continue treatment. Since implementation of the enhanced monitoring, serious platelet events have been infrequent. While we believe we can better maintain patients on volanesorsen through our patient-centric commercial approach where we plan to have greater involvement with physicians and patients, if we cannot effectively maintain patients on volanesorsen, we may not be able to generate substantial revenue from volanesorsen sales.
50
The patient populations suffering from FCS and FPL are small and have not been established with precision. If the actual number of patients is smaller than we estimate, or if we cannot raise awareness of these diseases and diagnosis is not improved, our revenue and ability to achieve profitability may be adversely affected.
We estimate there are 3,000 to 5,000 FCS patients and an additional 3,000 to 5,000 FPL patients globally. Our estimates of the sizes of the patient populations are based on published studies as well as internal analyses. If the results of these studies or our analyses of them do not accurately reflect the number of patients with FCS and FPL, our assessment of the market potential for volanesorsen may be inaccurate, making it difficult or impossible for us to meet our revenue goals, or to obtain and maintain profitability. In addition, as is the case with most orphan diseases, if we cannot successfully raise awareness of these diseases and improve diagnosis, it will be more difficult or impossible to achieve profitability.
In addition, since the patient populations for FCS and FPL are small, the per-patient drug pricing must be high in order to recover our development and manufacturing costs, fund adequate patient support programs and achieve profitability. For these initial indications, we may not maintain or obtain sufficient sales volume at a price high enough to justify our product development efforts and our sales and marketing and manufacturing expenses.
If we or our partners fail to compete effectively, volanesorsen and our other drugs in development will not contribute significant revenue.
Our competitors engage in drug discovery throughout the world, are numerous and include, among others, major pharmaceutical companies and specialized biopharmaceutical firms. Our competitors may succeed in developing drugs that are:
| ● |
safer than our drugs;
|
| ● |
more effective than our drugs;
|
| ● |
priced lower than our drugs;
|
| ● |
reimbursed more favorably by government and other third-party payors than our drugs; or
|
| ● |
more convenient to use than our drugs.
|
These competitive developments could make our drugs, including volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, obsolete or non-competitive. Further, all of our drugs are delivered by injection, which may render them less attractive to patients than non-injectable products offered by our current or future competitors.
Many of our competitors have substantially greater financial, technical and human resources than we do. In addition, many of these competitors have significantly greater experience than we do in conducting preclinical testing and human clinical studies, in obtaining FDA and other regulatory authorizations and in commercializing pharmaceutical products. Accordingly, our competitors may succeed in obtaining regulatory authorization for products earlier than we do. Marketing and sales capability is another factor relevant to the competitive position of our drugs, and many of our competitors will have greater marketing and sales capabilities than our capabilities.
There are several pharmaceutical and biotechnology companies engaged in the development or commercialization of products against targets that are also targets of drugs in our development pipeline. For example, if approved, volanesorsen could face competition from drugs like metreleptin. Metreleptin, produced by Novelion Therapeutics, Inc., is currently approved for use in generalized lipodystrophy patients. In September 2016, Arrowhead Pharmaceuticals, Inc. and Amgen Inc. announced a license and collaboration for development of Arrowhead's preclinical program which uses an RNAi conjugated with a GalNAc for the same target as AKCEA-APO(a)-LRx. AKCEA-APOCIII-LRx may compete with gemcabene, an oral small molecule that reduces apoC-III, that Gemphire Therapeutics, Inc. is developing to treat patients with triglycerides above 500 mg/dL. If volanesorsen or the other drugs in our pipeline cannot compete effectively with these and other products with common or similar indications to the drugs in our pipeline, we may not be able to generate substantial revenue from our product sales.
If government or other third-party payors fail to provide adequate coverage and payment rates for volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, our revenue and prospects for profitability will be limited.
In both domestic and foreign markets, sales of our future products will depend in part upon the availability of coverage and reimbursement from third-party payors. The majority of patients in the United States who would fit within our target patient populations for our drugs have their healthcare supported by a combination of Medicare coverage, other government health programs such as Medicaid, managed care providers, private health insurers and other organizations. Coverage decisions may depend upon clinical and economic standards that disfavor new drug products when more established or lower cost therapeutic alternatives are already available or subsequently become available. Assuming coverage is approved, the resulting reimbursement payment rates might not be enough to make our drugs affordable. Accordingly, volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, if approved, will face competition from other therapies and drugs for limited financial resources. We may need to conduct post-marketing studies to demonstrate the cost-effectiveness of any future products to satisfy third-party payors. These studies might require us to commit a significant amount of management time and financial and other resources. Third-party payors may never consider our future products as cost-effective. Adequate third-party coverage and reimbursement might not be available to enable us to maintain price levels sufficient to realize an appropriate return on investment in product development.
Third-party payors, whether foreign or domestic, or governmental or commercial, are developing increasingly sophisticated methods of controlling healthcare costs. In addition, in the United States, no uniform policy of coverage and reimbursement for drug products exists among third-party payors. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. Further, we believe that future coverage and reimbursement will likely be subject to increased restrictions both in the United States and in international markets. For example, in the United States, recent health reform measures have resulted in reductions in Medicare and other healthcare funding, and there have been several recent U.S. Congressional inquiries and proposed federal legislation designed to, among other things, reform government program reimbursement methodologies for drug products and bring more transparency to drug pricing. Third-party coverage and reimbursement for our products or drugs may not be available or adequate in either the United States or international markets, which would negatively affect the potential commercial success of our products, our revenue and our profits.
51
If we are found in violation of federal or state "fraud and abuse" laws or other healthcare laws and regulations, we may be required to pay a penalty and/or be suspended from participation in federal or state healthcare programs, which may adversely affect our business, financial condition and results of operation.
We may be subject to various federal and state laws pertaining to healthcare "fraud and abuse," including anti-kickback laws and false claims laws. Anti-kickback laws, among other things, make it illegal for a prescription drug manufacturer to pay, or offer to pay, a healthcare provider to refer, purchase or prescribe a particular drug. Due to the breadth of the statutory and regulatory provisions, it is possible that government authorities and others might challenge our practices under anti-kickback or other fraud and abuse laws. Moreover, recent healthcare reform legislation has strengthened these laws. In addition, false claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented for payment, to government third-party payors, including Medicare and Medicaid claims for reimbursed drugs that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. Our activities relating to the sale and marketing of our products may be subject to scrutiny under these laws. If we violated fraud and abuse laws, we could face a combination of:
| ● |
criminal and civil sanctions, including fines and civil monetary penalties;
|
| ● |
the possibility of exclusion from federal healthcare programs, including Medicare and Medicaid; and
|
| ● |
corporate integrity agreements, which could impose rigorous operational and monitoring requirements on us.
|
Given the significant penalties and fines that the government can impose on companies and individuals if convicted, allegations of violations often result in settlements even if the company or individual being investigated admits no wrongdoing. Settlements often include significant civil sanctions, including fines and civil monetary penalties, and corporate integrity agreements. If the government were to allege or convict us or our executive officers of violating these laws, our business could be harmed. In addition, private individuals may bring similar actions under the False Claims Act. Our activities could be subject to challenge for the reasons discussed above and due to the broad scope of these laws and the increasing focus on these laws by law enforcement authorities. To the extent we have access to protected health information we could be subject to federal and state health information privacy and security laws, including without limitation, the Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, which governs the conduct of certain electronic healthcare transactions and protects the security and privacy of protected health information. State health information privacy and security laws in certain circumstances are more stringent than HIPAA and many of the state laws differ from each other in significant ways and may not have the same effect, thus complicating compliance. Our failure to comply with applicable federal and state health information privacy and security laws could subject us to significant fines and multi-year corrective action plans. Once we have a commercialized drug, we will be required to report annually to Centers for Medicare and Medicaid Services certain information related to payments and other transfers of value we may provide to physicians and teaching hospitals. Further, an increasing number of state laws require manufacturers to make reports to states on pricing and marketing information. Many of these laws are unclear as to what is required to comply with the laws. Given the lack of clarity in laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state authorities.
Similar rigid restrictions related to anti-kickbacks and promoting and marketing medicinal products apply in the European Union and other countries. Authorities in these countries strictly enforce these restrictions. Even in those countries where we will not be directly responsible for promoting and marketing our products, inappropriate activity by any of our international commercialization partners we may have could harm us.
Risks Related to Dependence on Third Parties
We plan to substantially depend on our collaboration with Novartis to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx.
We have granted Novartis an exclusive option to exclusively license each of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx pursuant to our strategic collaboration, option and license agreement with Novartis. We plan to substantially depend on Novartis to further develop and commercialize these drugs. We initiated this collaboration primarily to have Novartis:
| ● |
conduct the cardiovascular outcome studies that are likely to be required for approval of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx;
|
| ● |
seek and obtain regulatory approvals for AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx; and
|
| ● |
globally commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx.
|
If Novartis exercises its option to license one or both of these drugs, we would rely on Novartis to further develop, obtain regulatory approvals for, and commercialize the licensed drug. In general, we cannot control the amount and timing of resources that Novartis devotes to our strategic collaboration. If Novartis fails to use commercially reasonable efforts to further develop, obtain regulatory approvals for, or commercialize these drugs, or if Novartis' efforts are not effective, our business may be negatively affected. Novartis could pursue other technologies or develop other drugs either on its own or in collaboration with others to treat the same diseases as we and Novartis plan to treat with AKCEA-APO(a)-LRx or AKCEA-APOCIII-LRx. Novartis could pursue these technologies and develop these other drugs at the same time as it is developing or commercializing AKCEA-APO(a)-LRx or AKCEA-APOCIII-LRx, and Novartis is not required to inform us of such activities.
52
Our strategic collaboration with Novartis may not continue for various reasons. Novartis can terminate our agreement at any time and is under no obligation to exercise the options we granted them. If Novartis does not exercise its option, or following option exercise stops developing or commercializing a drug, we will have to seek additional sources for funding and may have to delay or reduce our development and commercialization plans for AKCEA-APO(a)-LRx or AKCEA-APOCIII-LRx.
In addition, if Novartis exercises its option to license AKCEA-APO(a)-LRx or AKCEA-APOCIII-LRx, Novartis would be responsible for the long-term supply of drug substance and finished drug product for the licensed drug.
Our strategic collaboration with Novartis may not result in the successful commercialization of AKCEA-APO(a)-LRx or AKCEA-APOCIII-LRx. If Novartis does not successfully develop, manufacture or commercialize AKCEA-APO(a)-LRx or AKCEA-APOCIII-LRx, we may receive limited or no revenues for these drugs.
AKCEA-APOCIII-LRx and AKCEA-ANGPTL3-LRx may compete with volanesorsen, which could reduce our expected revenues for volanesorsen.
Volanesorsen and AKCEA-APOCIII-LRx both inhibit the production of the same protein. We believe the enhancements we incorporated into AKCEA-APOCIII-LRx can provide greater patient convenience by allowing for significantly lower doses and less frequent administration compared to volanesorsen. As such, if Novartis exercises its option and successfully commercializes AKCEA-APOCIII-LRx while we are commercializing volanesorsen, to the extent physicians and patients elect to use AKCEA-APOCIII-LRx instead of volanesorsen, it will reduce the revenue we derive from volanesorsen. In addition, while AKCEA-ANGPTL3-LRx and volanesorsen use different mechanisms of action, if AKCEA-ANGPTL3-LRx can effectively lower triglyceride levels in FCS patients, it may likewise reduce the revenue we derive from volanesorsen.
If we cannot manufacture our drugs or contract with a third party to manufacture our drugs at costs that allow us to charge competitive prices to buyers, we will not be able to operate profitably.
To successfully commercialize volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, we will need to establish large-scale commercial manufacturing capabilities either on our own or through a third-party manufacturer. In addition, as our drug development pipeline matures, we will have a greater need for clinical study and commercial manufacturing capacity. We have no direct experience manufacturing pharmaceutical products of the chemical class represented by our drugs, called oligonucleotides, on a commercial scale for the systemic administration of a drug. We currently rely and expect to rely for the foreseeable future on Ionis' manufacturing capacity and efficiency to produce our oligonucleotide drugs, and our business could be negatively affected if Ionis ceased to provide us with this capability for any reason. In addition, there are a small number of suppliers for certain raw materials that we use to manufacture our drugs, and some of these suppliers will need to increase their scale of production to meet our projected needs for commercial manufacturing. Further, if we cannot continue to acquire raw materials from these suppliers on commercially reasonable terms or at all, we may be required to find alternative suppliers, which could be expensive and time consuming and negatively affect our ability to develop or commercialize our drugs in a timely manner or at all. We may not be able to manufacture our drugs at a cost or in quantities necessary to make commercially successful products.
We do not have long-term supply agreements for our drugs. We cannot guarantee that we will have a steady supply of drug to complete clinical studies, make registration batches for approval or satisfy market demand if commercialized at prices that are commercially acceptable. In addition, if we need to change manufacturers for any reason, we will need to verify that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations and guidelines. The delays associated with verifying a new manufacturer could negatively affect our ability to develop drugs in a timely manner or within budget.
Also, manufacturers must adhere to the FDA's current Good Manufacturing Practices regulations and similar regulations in foreign countries, which the applicable regulatory authorities enforce through facilities inspection programs. Our contract manufacturers may not comply or maintain compliance with Good Manufacturing Practices, or similar foreign regulations. Non-compliance could significantly delay or prevent receipt of marketing authorization for our drugs, including authorizations for volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, or result in enforcement action after authorization that could limit the commercial success of our drugs, including volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development.
We depend on third parties to conduct our clinical studies for our drugs and any failure of those parties to fulfill their obligations could adversely affect our development and commercialization plans.
We depend on independent clinical investigators, contract research organizations and other third-party service providers to conduct the clinical studies for our drugs and expect to continue to do so in the future. For example, we use clinical research organizations for the clinical studies for volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development. We rely heavily on these parties for successful execution of our clinical studies, but do not control many aspects of their activities. For example, the investigators are not our employees. However, we are responsible for ensuring that these third parties conduct each of our clinical studies in accordance with the general investigational plan, approved protocols for the study and applicable regulations. Third parties may not complete activities on schedule or may not conduct our clinical studies in accordance with regulatory requirements or our stated protocols. The failure of these third parties to carry out their obligations or a termination of our relationship with these third parties could delay or prevent the development, marketing authorization and commercialization of our drugs, including authorizations for volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development.
53
We may seek to form additional partnerships in the future with respect to volanesorsen, and our other drugs in development, and we may not realize the benefits of such partnerships.
Although we intend to develop and commercialize volanesorsen for patients with FCS and FPL ourselves, we may form partnerships, create joint ventures or collaborations or enter into licensing arrangements with third parties for the development and commercialization of our drugs in development. For example, we have granted Novartis an exclusive option to exclusively license AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. We face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Any delays in entering into new strategic partnership agreements related to our drugs could delay the development and commercialization of our drugs and reduce their competitiveness even if they reach the market. Moreover, we may not be successful in our efforts to establish a strategic partnership or other collaborative arrangement for any additional drugs because the potential partner may consider that our development pipeline is not advanced enough to justify a collaborative effort, or that volanesorsen and our other drugs in development do not have the requisite potential to demonstrate safety and efficacy in the target populations. In addition, we will need to mutually agree with Ionis on the terms of any sublicense to a third party for volanesorsen and our other drugs in development. If we cannot mutually agree on terms for a sublicense to a third party or if Ionis does not agree to a sublicense at all, it could delay our ability to develop and commercialize volanesorsen and our other drugs in development. Even if we are successful in establishing such a strategic partnership or collaboration, we cannot be certain that, following such a strategic transaction or collaboration, we will be able to progress the development and commercialization of the applicable drugs as envisioned, or that we will achieve the revenue that would justify such transaction. If we do not accurately evaluate the commercial potential or target market for a particular drug, we may relinquish valuable rights to that drug through future collaboration, licensing or other arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights.
Risks Related to Our Relationship with Ionis
Ionis controls the direction of our business, and the concentrated ownership of our common stock will prevent you and other stockholders from influencing significant decisions.
Ionis owns approximately 68% of the economic interest and voting power of our outstanding common stock. As long as Ionis beneficially controls a majority of the voting power of our outstanding common stock, it will generally be able to determine the outcome of all corporate actions requiring stockholder approval, including the election and removal of directors. Even if Ionis were to control less than a majority of the voting power of our outstanding common stock, it may influence the outcome of such corporate actions so long as it owns a significant portion of our common stock. If Ionis continues to hold its shares of our common stock, it could remain our controlling stockholder for an extended period of time or indefinitely.
Ionis' interests may not be the same as, or may conflict with, the interests of our other stockholders. You will not be able to affect the outcome of any stockholder vote while Ionis controls the majority of the voting power of our outstanding common stock. As a result, Ionis can control, directly or indirectly and subject to applicable law, all matters affecting us, including:
| ● |
any determination with respect to our business strategy and policies, including the appointment and removal of officers and directors;
|
| ● |
any determinations with respect to mergers, business combinations or disposition of assets;
|
| ● |
our financing and dividend policy;
|
| ● |
compensation and benefit programs and other human resources policy decisions;
|
| ● |
termination of, changes to or determinations under our development, commercialization and license agreement, which we refer to as the license agreement, and services agreement with Ionis;
|
| ● |
changes to any other agreements that may adversely affect us; and
|
| ● |
determinations with respect to our tax returns.
|
Because Ionis' interests may differ from ours or yours, actions that Ionis takes with respect to us, as our controlling stockholder, may not be favorable to us or you.
If Ionis sells a controlling interest in our company to a third party in a private transaction, you may not realize a change of control premium on shares of our common stock, and we may become subject to the control of a presently unknown third party.
Ionis owns a significant equity interest in our company. This means that Ionis could choose to sell some or all of its shares of our common stock in a privately negotiated transaction, which, if sufficient in size, could result in a change of control of our company.
Ionis' ability to privately sell its shares of our common stock, with no requirement for a concurrent offer to be made to acquire your shares of our common stock, could prevent you from realizing any change of control premium on your shares of our common stock that may otherwise accrue to Ionis on its private sale of our common stock. Additionally, if Ionis privately sells its significant equity interest in our company, we may become subject to the control of a presently unknown third party. Such third party may have conflicts of interest with those of other stockholders. In addition, if Ionis sells a controlling interest in our company to a third party, such a sale could negatively impact or accelerate any future indebtedness we may incur, and negatively impact any other commercial agreements and relationships, all of which may adversely affect our ability to run our business as described herein and may have a material adverse effect on our operating results and financial condition.
54
Certain of our directors and officers may have actual or potential conflicts of interest because of their positions with Ionis.
Stanley T. Crooke, Chairman of the Board and Chief Executive Officer for Ionis, and B. Lynne Parshall, Senior Strategic Advisor and board member for Ionis, serve on our board of directors and retain their positions or engagements with Ionis. In addition, these individuals own Ionis equity and Ionis equity awards. Ionis common stock, options to purchase Ionis common stock and other Ionis equity awards represent a significant portion of these individuals' net worth. Their position at Ionis and the ownership of any Ionis equity or equity awards creates, or may create the appearance of, conflicts of interest when we ask these individuals to make decisions that could have different implications for Ionis than the decisions have for us. In addition, our certificate of incorporation provides for the allocation of certain corporate opportunities between us and Ionis. Under these provisions, neither Ionis or its other affiliates, nor any of their officers, directors, agents or stockholders, will have any obligation to present to us certain corporate opportunities. For example, a director of our company who also serves as a director, officer or employee of Ionis or any of its other affiliates may present to Ionis certain acquisitions, in-licenses, potential development programs or other opportunities that may be complementary to our business and, as a result, such opportunities may not be available to us. To the extent attractive corporate opportunities are allocated to Ionis or its other affiliates instead of to us, we may not be able to benefit from these opportunities.
The resources Ionis provides us under the license agreement and the services agreement may not be sufficient for us to operate as a standalone company, and we may experience difficulty in separating our resources from Ionis.
Because we have not operated separately from Ionis in the past, we may have difficulty doing so. We will need to acquire resources in addition to, and eventually in lieu of, those provided by Ionis to our company, and may also face difficulty in separating our resources from Ionis' resources and integrating newly acquired resources into our business. In addition, Ionis may prioritize its own research, development, manufacturing and other needs ahead of the services Ionis has agreed to provide us, or Ionis employees who conduct services for us may prioritize Ionis' interests over our interests. Our business, financial condition and results of operations could be harmed if we have difficulty operating as a standalone company, fail to acquire resources that prove to be important to our operations or incur unexpected costs in separating our resources from Ionis' resources or integrating newly acquired resources.
We will incur incremental costs as a standalone company.
Ionis currently performs or supports many important corporate functions for our company. Our consolidated financial statements reflect charges for these services on an allocation basis. Under our services agreement with Ionis we can use these Ionis services for a fixed term established on a service-by-service basis. However, we generally will have the right to terminate a service earlier if we give notice to Ionis. Partial reduction in the provision of any service requires Ionis' consent. In addition, either party will be able to terminate the agreement due to a material breach of the other party, upon prior written notice, subject to limited cure periods.
We will pay Ionis mutually agreed upon fees for these services, based on Ionis' costs of providing the services. Since we negotiated the services agreement in the context of a parent subsidiary relationship, the terms of the agreement, including the fees charged for the services, may be higher or lower than those that would be agreed to by parties bargaining at arm's length for similar services and may be higher or lower than the costs reflected in the allocations in our historical consolidated financial statements. Ionis will pass third party costs through to us at Ionis' cost. In addition, while Ionis provides us these services, our operational flexibility to modify or implement changes with respect to such services or the amounts we pay for them will be limited.
We may not be able to replace these services or enter into appropriate third-party agreements on terms and conditions, including cost, comparable to those that we will receive from Ionis under our services agreement. Additionally, after the agreement terminates, we may not sustain the services at the same levels or obtain the same benefits as when we were receiving such services and benefits from Ionis. When we begin to operate these functions separately, if we do not have our own adequate systems and business functions in place, or cannot obtain them from other providers, we may not operate our business effectively or at comparable costs, and our business may suffer. In addition, we have historically received informal support from Ionis, which may not be addressed in our services agreement. The level of this informal support will diminish and could end in the future.
We may not be able to fully realize the expected benefits of our license agreement with Ionis.
We have a development, commercialization and license agreement with Ionis. Pursuant to the license agreement, subject to certain restrictions, we and Ionis will share development responsibilities for volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development. We are paying for research and development costs and reimbursing Ionis for Ionis' employees supporting our development activities. Until we build or acquire our own capabilities to replace those Ionis is providing to us, particularly development, regulatory and manufacturing services, we will be heavily dependent on Ionis.
55
While we and Ionis intend the license agreement to bolster our capabilities, certain terms of the license agreement may limit our ability to achieve this expected benefit, including:
| ● |
a Joint Steering Committee, or JSC, comprising two senior members from our company and two senior members from Ionis, sets the development strategy for our drugs by mutual agreement. A Regulatory Sub-committee, established by the JSC and having equal membership from our company and Ionis, will set the regulatory strategy for each of our drugs by mutual agreement. If the JSC or the Regulatory Sub-committee cannot come to a mutual agreement, it could delay our ability to develop and commercialize volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development;
|
| ● |
we will need to mutually agree with Ionis on the terms of any additional sublicense to a third party for volanesorsen and our other drugs in development. If we cannot mutually agree on terms for a sublicense to a third party or if Ionis does not agree to a sublicense at all, it could delay or prevent our ability to develop and commercialize volanesorsen and our other drugs in development;
|
| ● |
we will need to obtain Ionis' approval to in-license a product, acquire a product or acquire another company, until the earlier of (i) 5 years following our IPO or (ii) when Ionis no longer is required to record its share of our profits and losses from an accounting perspective; and
|
| ● |
there is nothing in our agreements with Ionis to prevent Ionis from developing and commercializing drugs targeting RNAs that are not apoC-III, Apo(a) or ANGPTL3 to pursue the same indications we are pursuing with our drugs.
|
Each of the foregoing terms and Ionis' other rights under the license agreement, could limit our ability to realize the expected benefits of the license agreement or otherwise limit our ability to pursue transactions or development efforts other stockholders may view as beneficial. Further, if Ionis does not continue to own a significant portion of our equity, Ionis' incentive to help us would be diminished. If we fail to achieve the expected benefits of our agreements with Ionis, it may be more difficult, time consuming or expensive for us to develop and commercialize volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, or may result in our drugs being later to market than those of our competitors or prevent them from ever getting to market. If these events cause delays in new product development we could lose the first in class products in a given therapeutic area.
Risks Related to Our Intellectual Property
If we breach our obligations under our license agreement with Ionis, we could lose our rights to volanesorsen and our other drugs in development.
We obtained our rights to volanesorsen and our other drugs in development under our license agreement with Ionis. If we breach our obligations under this license agreement and, as a result, Ionis subsequently exercises its right to terminate it, we generally would not be able to continue to develop or commercialize volanesorsen, and our other drugs in development that incorporate Ionis' intellectual property, and Ionis would receive a royalty-free, nonexclusive license to our improvements to those programs, meaning we would lose the benefits of our investment in these programs. If we breach our obligations under this license agreement with respect to AKCEA-APO(a)-LRx or AKCEA-APOCIII-LRx and, as a result, Ionis exercises its right to terminate it, then our strategic collaboration with Novartis would convert into a direct strategic collaboration between Novartis and Ionis, and Ionis would receive all of the revenue and other benefits associated with that strategic collaboration.
If we cannot protect our patent rights or our other proprietary rights, others may compete more effectively against us.
Our success depends to a significant degree upon whether we can continue to secure and maintain intellectual property rights that protect volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development. However, patents may not issue from any of our pending patent applications in the United States or in other countries and we may not be able to obtain, maintain or enforce our owned or licensed patents and other intellectual property rights which could impact our ability to compete effectively. In addition, the scope of any of our owned or licensed patents may not be sufficiently broad to provide us with a competitive advantage. Furthermore, other parties may successfully challenge, invalidate or circumvent our issued patents or patents licensed to us so that our patent rights do not create an effective competitive barrier or revenue source.
Composition of matter patents on the active pharmaceutical ingredient for a product are generally considered to be the strongest form of intellectual property protection for pharmaceutical products, as such patents provide protection without regard to any method of use. Our volanesorsen patent portfolio currently includes:
| ● |
issued patent claims to the specific antisense sequence and chemical composition of volanesorsen in the United States, Australia, and Europe;
|
| ● |
issued patent claims in the United States and Australia drawn to the use of antisense compounds complementary to an active region of human apoC-III messenger ribonucleic acid, including the site targeted by volanesorsen;
|
| ● |
additional patent applications designed to protect the volanesorsen composition in Canada; and
|
| ● |
additional methods of use in jurisdictions worldwide for volanesorsen.
|
The natural term of the issued U.S. patent covering the volanesorsen composition of matter will expire in 2023, but we plan to seek to extend the U.S. patent expiration beyond 2023 based upon the development and regulatory review period in the United States. The natural term of the granted European and Australian patents covering volanesorsen will expire in 2024, but we plan to seek to extend each of these patents beyond 2024 based upon the development and regulatory review periods in Europe and Australia.
56
We cannot be certain that the U.S. Patent and Trademark Office, or U.S. PTO, and courts in the United States or the patent offices and courts in foreign countries will consider the claims in our owned or licensed patents and applications covering volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development as patentable. Method-of-use patents protect the use of a product for the specified method. This type of patent does not prevent a competitor from making and marketing a product that is identical to our product for an indication that is outside the scope of the patented method. Moreover, even if competitors do not actively promote their product for our targeted indications, physicians may prescribe these products off-label. Although off-label prescriptions may infringe or contribute to the infringement of method-of-use patents, the practice is common and such infringement is difficult to prevent, including through legal action.
If we or any licensor partner loses or cannot obtain patent protection for volanesorsen, AKCEA-APO(a)-LRx or our other drugs in development it could have a material adverse impact on our business.
Intellectual property litigation could cause us to spend substantial resources and prevent us from pursuing our programs.
From time to time we may have to defend our intellectual property rights. If we are involved in an intellectual property dispute, we may need to litigate to defend our rights or assert them against others. Disputes can involve arbitration, litigation or proceedings declared by the U.S. PTO or the International Trade Commission or foreign patent authorities. Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability and the ability of our strategic partners to develop, manufacture, market and sell our drugs and use our proprietary technologies without infringing the proprietary rights and intellectual property of third parties. Extensive litigation regarding patents and other intellectual property rights is common in the biotechnology and pharmaceutical industries. We may in the future become party to, or threatened with, adversarial proceedings or litigation regarding intellectual property rights with respect to our drugs and technology, including interference, derivation, reexamination, post-grant review, opposition, cancellation or similar proceedings before the U.S. PTO or its foreign counterparts. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. We may not be aware of all such intellectual property rights potentially relating to our drugs and their uses. If a third party claims that volanesorsen, AKCEA-APO(a)-LRx, our other drugs in development or our technology infringe its patents or other intellectual property rights, we or our partners may have to discontinue an important product or product line, alter our products and processes, pay license fees or cease certain activities. We may not be able to obtain a license to needed intellectual property on favorable terms, if at all. There are many patents issued or applied for in the biotechnology industry, and we may not be aware of patents or patent applications held by others that relate to our business. This is especially true since patent applications in the United States are filed confidentially for the first 18 months. Moreover, the validity and breadth of biotechnology patents involve complex legal and factual questions for which important legal issues remain. Thus, we do not know with certainty that our drugs or our intended commercialization thereof, does and will not infringe or otherwise violate any third party's intellectual property.
We will not seek to protect our intellectual property rights in all jurisdictions throughout the world and we may not be able to adequately enforce our intellectual property rights even in the jurisdictions where we seek protection.
Filing, prosecuting and defending patents on drugs in all countries and jurisdictions throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States could be less extensive than those we could obtain in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions.
Competitors may use our technologies in jurisdictions where we do not pursue and obtain patent protection to develop their own products. In addition, competitors may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patent rights or other intellectual property rights may not be effective or sufficient to prevent them from competing. Even if we pursue and obtain issued patents in particular jurisdictions, our patent claims or other intellectual property rights may not be effective or sufficient to prevent third parties from so competing.
57
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to biotechnology. This could make it difficult for us to stop competitors from infringing our patent rights or misappropriating our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, many countries limit our right to enforce our patent rights against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit. We must ultimately seek patent protection on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries.
In addition, proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patent rights at risk of being invalidated or interpreted narrowly, could put our owned or licensed patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
If we do not obtain additional protection under the Hatch-Waxman Amendments and similar foreign legislation by extending the patent protection for volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, our business may be materially harmed.
Depending upon the timing, duration and specifics of the first FDA marketing authorization of volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, a United States patent that we own or license may be eligible for limited patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments allow the owner of an approved product to extend patent protection for up to five years as compensation for patent term lost during product development and the FDA regulatory review process. During this period of extension, the scope of protection is limited to the approved product and approved uses.
Although we plan on seeking patent term restoration for our products, we may not succeed if, for example, we fail to apply within applicable deadlines, fail to apply prior to expiration of relevant patents or otherwise fail to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we cannot obtain patent term restoration or the term of any such patent restoration is less than we request, our competitors may enter the market and compete against us sooner than we anticipate, and our ability to generate revenue could be materially adversely affected.
Changes in United States patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
Recent United States Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the United States Congress, the federal courts, and the U.S. PTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
If we and our partners do not adequately protect the trademarks and trade names for our products, then we and our partners may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our competitors or other third parties may challenge, infringe or circumvent the trademarks or trade names for our products. We and our partners may not be able to protect these trademarks and trade names. In addition, if the trademarks or trade names for one of our products infringe the rights of others, we or our partners may be forced to stop using the trademarks or trade names, which we need for name recognition in our markets of interest. If we cannot establish name recognition based on our trademarks and trade names, we and our partners may not be able to compete effectively and our business may be adversely affected.
58
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
| ● |
others may make compounds that are similar to our drugs but that are not covered by the claims of the patents that we own or have exclusively licensed;
|
| ● |
we, or our license partners or current or future strategic partners, might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed;
|
| ● |
we, or our license partners or current or future strategic partners, might not have been the first to file patent applications covering our inventions;
|
| ● |
others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights;
|
| ● |
our pending licensed patent applications or those that we own in the future may not lead to issued patents;
|
| ● |
issued patents that we hold rights to may be held invalid or unenforceable, including as a result of legal challenges by our competitors;
|
| ● |
our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
|
| ● |
we may not develop additional proprietary technologies that are patentable; and
|
| ● |
the patents of others may have an adverse effect on our business.
|
Should any of these events occur, they could significantly harm our business, results of operations and prospects.
Risks Related to Our Business and Industry
We will need to significantly increase the size of our organization, and we may experience difficulties in managing growth.
We are currently a small company. To commercialize volanesorsen, and our other drugs in development that we are responsible for commercializing, we will need to increase our operations and expand our use of third-party contractors. We plan to continue to build our compliance, financial and operating infrastructure to ensure the maintenance of a well-managed company including hiring additional staff within our regulatory, clinical and medical affairs groups and an in-house commercial organization initially focused on marketing and selling volanesorsen, if approved. We currently have limited sales and marketing capability and therefore intend to recruit a specialty sales force in anticipation of volanesorsen's potential approval.
Future growth will impose significant added responsibilities on our management, including the need to identify, recruit, maintain and integrate additional employees. In addition, to meet our obligations as a public company, we will need to increase our general and administrative capabilities. Our current management, personnel and systems may not be adequate to support this future growth. Our future financial performance and our ability to commercialize our drugs and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to:
| ● |
manage our clinical studies and the regulatory process effectively;
|
| ● |
manage the manufacturing of our drugs for clinical and commercial use;
|
| ● |
integrate current and additional management, administrative, financial and sales and marketing personnel;
|
| ● |
develop a marketing and sales infrastructure;
|
| ● |
hire new personnel necessary to effectively commercialize volanesorsen and our other drugs in development;
|
| ● |
develop our administrative, accounting and management information systems and controls; and
|
| ● |
hire and train additional qualified personnel.
|
Our staff, financial resources, systems, procedures or controls may be inadequate to support our operations and our management may be unable to successfully manage future market opportunities or our relationships with customers and other third parties.
If we do not progress in our programs as anticipated, the price of our securities could decrease.
For planning purposes, we estimate and may disclose the timing of a variety of clinical, regulatory and other milestones, such as when we anticipate a certain drug will enter into clinical trials, when we anticipate completing a clinical study, when we anticipate filing an application for marketing authorization, or when we or our partners plan to commercially launch a drug. We base our estimates on present facts and a variety of assumptions. Many underlying assumptions are outside of our control. If we do not achieve milestones in accordance with our or our investors' or securities analysts' expectations, including milestones related to volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development, the price of our securities could decrease.
59
The loss of key personnel, or if we cannot attract and retain highly skilled personnel, could make it more difficult to run our business and reduce our likelihood of success.
We are dependent on the principal members of our management and scientific staff. We do not have employment agreements with any of our executive officers that would prevent them from leaving us. The loss of management and key scientific employees might slow the achievement of important research and development goals. It is also critical to our success that we recruit and retain qualified scientific personnel to perform development work and marketing, sales and commercial support personnel to perform commercialization activities. We may not be able to attract and retain skilled and experienced scientific and commercial personnel on acceptable terms because of intense competition for experienced personnel among many pharmaceutical and health care companies, universities and non-profit research institutions. In addition, failure to successfully complete clinical studies, obtain regulatory approvals or effectively commercialize drugs may make it more challenging to recruit and retain qualified personnel.
We are exposed to potential product liability claims, and insurance against these claims may not be available to us at a reasonable rate in the future or at all.
Our business exposes us to potential product liability risks that are inherent in the testing, manufacturing, marketing and sale of therapeutic products, including potential product liability claims related to volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development. We have clinical study insurance coverage and commercial product liability insurance coverage. In addition, Novartis has agreed to indemnify us against specific claims arising from Novartis' development and commercialization of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. However, this insurance coverage and indemnities may not be adequate to cover claims against us. Insurance may not be available to us at an acceptable cost, if at all. Regardless of their merit or eventual outcome, products liability claims may result in decreased demand for our drug products, injury to our reputation, withdrawal of clinical study volunteers and loss of revenue. Thus, whether or not we are insured or indemnified, a product liability claim or product recall may result in losses that could be material.
Because we use biological materials, hazardous materials, chemicals and radioactive compounds, if we do not comply with laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
Our development and manufacturing activities involve the use of potentially harmful biological materials as well as materials, chemicals and various radioactive compounds that could be hazardous to human health and safety or the environment. We cannot completely eliminate the risk of contamination, which could cause:
| ● |
interruption of our development, manufacturing and distribution efforts;
|
| ● |
injury to our employees and others;
|
| ● |
environmental damage resulting in costly clean up; and
|
| ● |
liabilities under federal, state and local laws and regulations governing health and human safety, as well as the use, storage, handling and disposal of these materials and resultant waste products.
|
In such an event, we may be held liable for any resulting damages, and any liability could exceed our resources. Although we carry insurance in amounts and types that we consider commercially reasonable, we do not have insurance coverage for losses relating to an interruption of our development, manufacturing or commercialization efforts caused by contamination, and the coverage or coverage limits of our insurance policies may not be adequate. If our losses exceed our insurance coverage, our financial condition would be adversely affected.
A variety of risks associated with operating our business and, following approval, marketing our drugs internationally could materially adversely affect our business.
In addition to our U.S. operations, we plan to establish operations and, following approval, commercialize our products in Europe and other countries globally. We face risks associated with our current and planned international operations, including possible unfavorable regulatory, pricing and reimbursement, political, tax and labor conditions, which could harm our business. Once we establish international operations we will be subject to numerous risks associated with international business activities, including:
| ● |
compliance with differing or unexpected regulatory requirements for our drugs and foreign employees;
|
| ● |
complexities associated with managing multiple payor reimbursement regimes, government payors or patient self-pay systems;
|
| ● |
difficulties in staffing and managing foreign operations;
|
| ● |
in certain circumstances, increased dependence on the commercialization efforts and regulatory compliance of third-party distributors or strategic partners;
|
| ● |
foreign government taxes, regulations and permit requirements;
|
| ● |
U.S. and foreign government tariffs, trade restrictions, price and exchange controls and other regulatory requirements;
|
| ● |
anti-corruption laws, including the Foreign Corrupt Practices Act, or the FCPA, and its equivalent in foreign jurisdictions;
|
| ● |
economic weakness, including inflation, natural disasters, war, events of terrorism or political instability in particular foreign countries;
|
| ● |
fluctuations in currency exchange rates, which could result in increased operating expenses and reduced revenue, and other obligations related to doing business in another country;
|
| ● |
compliance with tax, employment, privacy, immigration and labor laws, regulations and restrictions for employees living or traveling abroad;
|
| ● |
workforce uncertainty in countries where labor unrest is more common than in the United States; and
|
| ● |
changes in diplomatic and trade relationships.
|
The UK's anticipated exit from the European Union could increase these risks.
Our business activities outside of the United States are subject to the FCPA and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we operate, including the U.K.'s Bribery Act 2010. In many other countries, the healthcare providers who prescribe pharmaceuticals are employed by their government, and the purchasers of pharmaceuticals are government entities; therefore, any dealings with these prescribers and purchasers may be subject to regulation under the FCPA. There is no certainty that all employees and third-party business partners (including our distributors, wholesalers, agents, contractors and other partners) will comply with anti-bribery laws. In particular, we do not control the actions of manufacturers and other third-party agents, although we may be liable for their actions. Violation of these laws may result in civil or criminal sanctions, which could include monetary fines, criminal penalties, and disgorgement of past profits, which could have a material adverse impact on our business and financial condition.
60
If a natural or man-made disaster strikes our development or manufacturing facilities or otherwise affects our business, it could delay our progress developing and commercializing our drugs.
We currently rely on Ionis to manufacture our clinical supplies in a manufacturing facility located in Carlsbad, California. The facilities and the equipment required to develop and manufacture our drugs would be costly to replace and could require substantial lead time to repair or replace. Natural or man-made disasters, including, without limitation, earthquakes, floods, fires and acts of terrorism may harm these facilities. If a disaster affects these facilities, our and our partners' development and commercialization efforts would be delayed. Although we possess insurance for damage to our property and the disruption of our business from casualties, this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all. In addition, a shutdown of the U.S. government, including the FDA could harm or delay our development and commercialization activities.
Our business and operations would suffer in the event of computer system failures.
Despite the implementation of security measures, our internal computer systems, and those of our CROs, manufacturers and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. If issues were to arise and cause interruptions in our operations, it could result in a material disruption of our drug programs. For example, the loss of clinical study data from completed or ongoing or planned clinical studies could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development could be delayed.
Risks Related to Our Common Stock
We are an "emerging growth company" and as a result of the reduced disclosure and governance requirements applicable to emerging growth companies, our common stock may be less attractive to investors.
We are an "emerging growth company," as defined in the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not "emerging growth companies" including, but not limited to, the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive if we choose to rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
An active public trading market for our common stock may not be sustained.
Prior to the completion of our IPO in July 2017, no public market for our common stock existed. An active public trading market for our common stock may not be sustained. The lack of an active market may impair your ability to sell your shares at the time you wish to sell them or at a price that you consider reasonable. The lack of an active market may also reduce the fair value of your shares. An inactive market may also impair our ability to raise capital to continue to fund operations by selling shares. Additionally, Ionis owns approximately 68% of our outstanding common stock. Ionis intends to hold its shares of our common stock for the foreseeable future, which could reduce the public market for our stock.
The market price for our common stock may be volatile, which could contribute to the loss of your investment.
Fluctuations in the price of our common stock could contribute to the loss of all or part of your investment. There has been a public market for our common stock for a limited period of time. The trading price of our common stock is likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. Any of the factors listed below could have a material adverse effect on your investment in our common stock and our common stock may trade at prices significantly below your purchase price. In such circumstances the trading price of our common stock may not recover and may experience a further decline.
Factors affecting the trading price of our common stock may include:
| ● |
our failure to effectively develop and commercialize volanesorsen and our other drugs in development;
|
| ● |
Novartis' failure to exercise its option and/or effectively develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx to the extent it exercises its option to license those drugs from us;
|
| ● |
changes in the market's expectations about our operating results;
|
61
| ● |
adverse results or delays in preclinical or clinical studies;
|
| ● |
our decision to initiate a clinical study, not to initiate a clinical study or to terminate an existing clinical study;
|
| ● |
adverse regulatory decisions, including failure to receive regulatory approval for volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development;
|
| ● |
success or failure of competitive products or antisense drugs more generally;
|
| ● |
adverse developments concerning our manufacturers or our strategic partnerships;
|
| ● |
inability to obtain adequate product supply for any drug for clinical studies or commercial sale or inability to do so at acceptable prices;
|
| ● |
the termination of a strategic partnership or the inability to establish additional strategic partnerships;
|
| ● |
unanticipated serious safety concerns related to the use of volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development;
|
| ● |
adverse safety or other clinical results, such as those that have occurred in the past or that may occur in the future, related to drugs being developed by Ionis or other companies that are or may be perceived to be similar to our drugs;
|
| ● |
our ability to effectively manage our growth;
|
| ● |
the size and growth, if any, of the targeted market;
|
| ● |
our operating results do not meet the expectation of securities analysts or investors in a particular period;
|
| ● |
actual or anticipated fluctuations in our quarterly financial results or the quarterly financial results of companies perceived to be similar to us;
|
| ● |
securities analysts do not publish reports about us or our business or publish negative reports;
|
| ● |
changes in financial estimates and recommendations by securities analysts concerning our company, our market opportunity, or the biotechnology and pharmaceutical industries in general;
|
| ● |
operating and stock price performance of other companies that investors deem comparable to us;
|
| ● |
overall performance of the equity markets;
|
| ● |
announcements by us or our competitors of acquisitions, new drugs or programs, significant contracts, commercial relationships or capital commitments;
|
| ● |
our and our strategic partners' ability to successfully market volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development;
|
| ● |
changes in laws and regulations affecting our business, including but not limited to clinical study requirements for approvals;
|
| ● |
disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain and maintain patent protection for volanesorsen, AKCEA-APO(a)-LRx and our other drugs in development;
|
| ● |
commencement of, or involvement in, litigation involving our company, our general industry, or both;
|
| ● |
changes in our capital structure, such as future issuances of securities or the incurrence of additional debt;
|
| ● |
the volume of shares of our common stock available for public sale;
|
| ● |
additions or departures of key scientific or management personnel;
|
| ● |
any major change in our board or management;
|
| ● |
changes in accounting practices;
|
| ● |
ineffectiveness of our internal control over financial reporting;
|
| ● |
significant changes in our relationship with Ionis;
|
| ● |
sales of substantial amounts of common stock by our directors, executive officers or significant stockholders or the perception that such sales could occur; and
|
| ● |
general economic and political conditions such as recessions, interest rates, fuel prices, elections, drug pricing policies, international currency fluctuations and acts of war or terrorism.
|
Broad market and industry factors may materially harm the market price of our common stock irrespective of our operating performance. The stock market in general, and NASDAQ and the market for biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of the particular companies affected. The trading prices and valuations of these stocks, and of ours, may not be predictable. A loss of investor confidence in the market for biotechnology or pharmaceutical stocks or the stocks of other companies which investors perceive to be similar to us, the opportunities in the biotechnology and pharmaceutical market or the stock market in general, could depress our stock price regardless of our business, prospects, financial conditions or results of operations.
Sales of a substantial number of shares of our common stock by our existing stockholders in the public market may cause our stock price to decline.
Sales of our common stock in the public market, or the perception that these sales may occur, could cause the market price of our common stock to decline. Immediately following our IPO and concurrent private placement we had 66,541,629 shares of common stock outstanding. Of these, only 14,843,750 shares of our common stock sold in our IPO are freely transferable without restriction or additional registration under the Securities Act. Novartis has agreed that it will not sell any of the shares it purchased in the concurrent private placement until the earlier of January 5, 2020 or six months after we stop developing a drug under our agreement with Novartis. Thereafter, Novartis may only sell a limited number of shares each day. Up to an additional 45,447,879 shares of common stock held by Ionis are eligible for sale in the public market, all of which will be subject to volume limitations under Rule 144 under the Securities Act. In addition, 9,000,000 shares of common stock that are either subject to outstanding options or reserved for future issuance under our employee benefit plans are eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules, the lock-up agreements and Rule 144 and Rule 701 under the Securities Act. To the extent the holders of these shares sell them into the market or our stockholders believe these sales might occur, the market price of our common stock could decline.
We cannot predict with certainty whether or when Ionis will sell a substantial number of shares of our common stock. Ionis' sale of a substantial number of shares, or a perception that such sales could occur, could significantly reduce the market price of our common stock.
62
We do not expect to pay any cash dividends for the foreseeable future.
You should not rely on an investment in our common stock to provide dividend income. We do not anticipate that we will pay any cash dividends to holders of our common stock in the foreseeable future. Instead, we plan to retain any earnings to maintain and expand our operations. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any return on their investment. As a result, investors seeking cash dividends should not purchase our common stock.
The United States recently passed a comprehensive tax reform bill that could adversely affect our financial performance.
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cut and Jobs Act of 2017, or the Tax Act. The Tax Act makes broad and complex changes to the U.S. tax code. The changes include, but are not limited to, reduction of the corporate tax rate from 35% to 21%, limitation of the tax deduction for interest expense, limitation on the utilization of net operating losses to 80% of taxable income and elimination of net operating loss carrybacks, a mandatory one-time transition tax on certain unrepatriated earnings of foreign subsidiaries, introduction of bonus depreciation that allows for full expensing of qualified property, and modifying or repealing many business tax deductions and credits. Notwithstanding the reduction in the corporate income tax rate, the overall impact of the new federal tax law is uncertain, and our financial performance could be adversely affected. In addition, it is uncertain if, and to what extent various states will conform to the new tax law and foreign countries may react by adopting tax legislation or taking other actions that could adversely affect our business.
Uncertainties in the interpretation and application of the 2017 Tax Cuts and Jobs Act could materially affect our tax obligations, effective tax rate and operating results.
The Tax Act was enacted on December 22, 2017 and significantly affected U.S. tax law by changing how the U.S. imposes income tax on multinational corporations. The U.S. Department of Treasury has broad authority to issue regulations and interpretative guidance that may significantly impact our tax obligations, effective tax rate and our results of operations. The Tax Act will likely be subject to ongoing technical guidance and accounting interpretation, which we will continue to monitor and assess. It is not possible to fully measure the potential impact on our business, prospects or results of operations at this time.
We could be subject to additional tax liabilities.
We are subject to U.S. federal, state, local and sales taxes in the United States and foreign income taxes, withholding taxes and transaction taxes in foreign jurisdictions. Significant judgment is required in evaluating our tax positions and our worldwide provision for taxes. During the ordinary course of business, there are many activities and transactions for which the ultimate tax determination is uncertain. In addition, our tax obligations and effective tax rates could be adversely affected by changes in the relevant tax, accounting and other laws, regulations, principles and interpretations, including those relating to income tax nexus, by recognizing tax losses or lower than anticipated earnings in jurisdictions where we have lower statutory rates and higher than anticipated earnings in jurisdictions where we have higher statutory rates, by changes in foreign currency exchange rates, or by changes in the valuation of our deferred tax assets and liabilities. We may be audited in various jurisdictions, and such jurisdictions may assess additional taxes, sales taxes and value-added taxes against us. Although we believe our tax estimates are reasonable, the final determination of any tax audits or litigation could be materially different from our historical tax provisions and accruals, which could have a material adverse effect on our operating results or cash flows in the period for which a determination is made.
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management's attention and resources, which could harm our business.
63
Provisions in our amended and restated certificate of incorporation, our amended and restated bylaws and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders, and may prevent attempts by our stockholders to replace or remove our current management.
Our amended and restated certificate of incorporation, amended and restated bylaws and Delaware law contain provisions that may have the effect of delaying or preventing a change in control of us or changes in our management. Our amended and restated certificate of incorporation and bylaws include provisions that:
| ● |
authorize "blank check" preferred stock, which could be issued by our board of directors without stockholder approval and may contain voting, liquidation, dividend and other rights superior to our common stock;
|
| ● |
specify that only board of directors or holders of greater than 10% of our common stock can call special meetings of our stockholders;
|
| ● |
prohibit stockholder action by written consent once Ionis no longer holds a majority of our voting power;
|
| ● |
establish an advance notice procedure for stockholder approvals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to our board of directors;
|
| ● |
provide that a majority of directors then in office, even though less than a quorum, may fill vacancies on our board of directors;
|
| ● |
specify that no stockholder is permitted to cumulate votes at any election of directors;
|
| ● |
expressly authorize our board of directors to modify, alter or repeal our amended and restated bylaws; and
|
| ● |
require supermajority votes of the holders of our common stock to amend specified provisions of our amended and restated certificate of incorporation and amended and restated bylaws.
|
These provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our management. Further, Novartis has agreed that until Novartis holds less than 7.5% of our outstanding common stock, Novartis will vote the Novartis Private Placement Shares consistent with the recommendation of our board of directors. Although Novartis has retained the right to vote the Novartis Private Placement Shares in its sole discretion in connection with certain enumerated matters, including any transaction which would result in our change of control, our agreement with Novartis may nevertheless delay or prevent changes in our management or board of directors.
In addition, because we are incorporated in the State of Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which limits the ability of stockholders owning in excess of 15% of our outstanding voting stock to merge or combine with us.
Any provision of our amended and restated certificate of incorporation or amended and restated bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit your opportunity to receive a premium for your shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
Our bylaws designate the Court of Chancery of the State of Delaware and federal court within the State of Delaware as the exclusive forum for certain types of actions and proceedings that our stockholders may initiate, which could limit our stockholders' ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our bylaws provide that, subject to limited exceptions, the Court of Chancery of the State of Delaware and federal court within the State of Delaware will be exclusive forums for any:
| ● |
derivative action or proceeding brought on our behalf;
|
| ● |
action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders;
|
| ● |
action asserting a claim against us arising pursuant to any provision of the Delaware General Corporation Law, our amended and restated certificate of incorporation or our amended and restated bylaws; or
|
| ● |
other action asserting a claim against us that is governed by the internal affairs doctrine.
|
Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock shall be deemed to have notice of and to have consented to the provisions of our bylaws described above. This choice of forum provision may limit a stockholder's ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and employees. Alternatively, if a court were to find these provisions of our amended and restated certificate of incorporation inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business and financial condition.
64
Item 1B. Unresolved Staff Comments
Not applicable.
Item 2. Properties
Our corporate headquarters is located in Cambridge, Massachusetts. We currently occupy approximately 9,200 square feet of office space. Our lease expires for 6,100 square feet at the end of July 2018 and the remaining 3,100 square feet expires in April 2020. We will need additional space in the future as we continue to build our development, commercial and support teams. We are currently searching for a new office facility and believe we can find suitable additional space in the future on commercially reasonable terms.
Item 3. Legal Proceedings
From time to time, we may be involved in various claims and legal proceedings relating to claims arising out of our operations. We are not currently a party to any legal proceedings that, in the opinion of our management, are likely to have a material adverse effect on our business. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Item 4. Mine Safety Disclosures
Not applicable.
65
PART II
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock is traded publicly through The NASDAQ Global Select Market under the symbol "AKCA." The following table presents quarterly information on the price range of our common stock since our initial public offering, or IPO, on July 19, 2017. Prior to our IPO, there was no public trading market for our common stock. Our initial public offering was priced at $8.00 per share on July 17, 2017. This information indicates the high and low sale prices reported by The NASDAQ Global Select Market. These prices do not include retail markups, markdowns or commissions.
|
HIGH
|
LOW
|
|||||||
|
Year Ended December 31, 2017
|
||||||||
|
Third Quarter (from July 19, 2017 through September 30, 2017)
|
$
|
31.23
|
$
|
8.10
|
||||
|
Fourth Quarter
|
$
|
30.20
|
$
|
15.20
|
||||
As of February 20, 2018, there were 6 stockholders of record of our common stock. Because many of our shares are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of stockholders represented by these record holders.
We have never paid dividends and do not anticipate paying any dividends in the foreseeable future. Any future determination as to the declaration and payment of dividends, if any, will be at the discretion of our board of directors, subject to applicable laws, and will depend on then existing conditions, including our financial condition, operating results, contractual restrictions, capital requirements, business prospects, and other factors our board of directors may deem relevant.
Set forth below is a table and chart comparing the total return on an indexed basis of $100 invested on July 19, 2017, which is the date our shares began trading, in our common stock, the NASDAQ Composite Index (total return) and the NASDAQ Biotechnology Index. The total return assumes reinvestment of dividends. Historical stockholder return is not necessarily indicative of the performance to be expected for any future periods.
66
Performance Graph (1)
Comparison of Cumulative Total Return*
Among Akcea Therapeutics, Inc., the NASDAQ Composite Index and the NASDAQ Biotechnology Index
*$100 invested on July 19, 2017 in stock or index, including reinvestment of dividends. Fiscal year ending December 31.
___________________________________________________________________________________
| (1) |
This section is not "soliciting material," is not deemed "filed" with the SEC, is not subject to the liabilities of Section 18 of the Exchange Act and is not to be incorporated by reference in any of our filings under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
|
Recent Sale of Unregistered Securities
During the year ended December 31, 2017, we did not issue or sell any unregistered securities not previously disclosed in a Quarterly Report on Form 10-Q or in a Current Report on Form 8-K.
Use of Proceeds from Public Offering of Our Common Stock
On July 19, 2017, we closed our IPO of 17,968,750 shares of common stock at an offering price of $8.00 per share, resulting in gross proceeds to us of approximately $143.8 million. All of the shares issued and sold in our IPO were registered under the Securities Act pursuant to a registration statement on Form S-1 (File No. 333-216949), which was declared effective by the SEC on July 13, 2017. Cowen and Company, LLC, Stifel, Nicolaus & Company, Incorporated and Wells Fargo Securities, LLC acted as joint book-running managers for our initial public offering and BMO Capital Markets Corp. acted as lead manager for our initial public offering. The offering commenced on June 20, 2017 and did not terminate before all of the securities registered in the registration statement were sold.
The net proceeds to us, after deducting underwriting discounts and commissions of approximately $8.4 million and offering expenses of approximately $3.1 million, were approximately $132.3 million. No offering expenses were paid directly or indirectly to any of our directors or officers (or their associates) or persons owning ten percent or more of any class of our equity securities or to any other affiliates. We have invested a portion of the net offering proceeds in debt instruments of the U.S. Treasury, financial institutions, corporations and U.S. government agencies. There has been no material change in the planned use of proceeds from our IPO from those disclosed in the final prospectus for our IPO dated July 13, 2017 and filed with the SEC pursuant to Rule 424(b)(4).
As of December 31, 2017, all expenses incurred in connection with our initial public offering had been paid.
Purchase of Equity Securities by the Issuer and Affiliated Purchasers
None.
67
Item 6. Selected Financial Data
This selected financial data should be read in conjunction with our audited consolidated financial statements and accompanying notes and Management's Discussion and Analysis of Financial Condition and Results of Operations included elsewhere in this Annual Report on Form 10-K. Our consolidated financial information may not be indicative of our future performance. Set forth below are our selected consolidated financial data (in thousands, except per share amounts):
|
|
Years Ended December 31,
|
|||||||||||||||
|
|
2017
|
2016
|
2015
|
2014
|
||||||||||||
|
Consolidated Statement of Operations Data:
|
||||||||||||||||
|
Research and development revenue under collaborative agreements
|
$
|
55,209
|
$
|
—
|
$
|
—
|
$
|
—
|
||||||||
|
Research and development expenses
|
$
|
126,890
|
$
|
68,459
|
$
|
50,885
|
$
|
29,028
|
||||||||
|
General and administrative expenses
|
$
|
36,981
|
$
|
15,053
|
$
|
10,553
|
$
|
995
|
||||||||
|
Net loss
|
$
|
(109,751
|
)
|
$
|
(83,217
|
)
|
$
|
(61,422
|
)
|
$
|
(30,023
|
)
|
||||
|
Basic and diluted net loss per share of preferred stock
|
$
|
(1.55
|
)
|
$
|
(2.88
|
)
|
$
|
(2.13
|
)
|
$
|
(1.04
|
)
|
||||
|
Shares used in computing basic and diluted net loss per share of preferred stock
|
15,748
|
28,885
|
28,885
|
28,885
|
||||||||||||
|
Basic and diluted net loss per share of common stock
|
$
|
(2.82
|
)
|
—
|
—
|
—
|
||||||||||
|
Shares used in computing basic and diluted net loss per share of common stock
|
30,263
|
—
|
—
|
—
|
||||||||||||
|
As of December 31,
|
||||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Consolidated Balance Sheet:
|
||||||||||||
|
Cash, cash equivalents and short-term investments
|
$
|
260,130
|
$
|
7,857
|
$
|
64,310
|
||||||
|
Working capital
|
$
|
185,992
|
$
|
(19,344
|
)
|
$
|
53,761
|
|||||
|
Total assets
|
$
|
268,804
|
$
|
10,684
|
$
|
66,067
|
||||||
|
Payable to Ionis
|
$
|
14,365
|
$
|
24,355
|
$
|
9,198
|
||||||
|
Series A convertible preferred stock
|
$
|
—
|
$
|
100,000
|
$
|
100,000
|
||||||
|
Common stock and additional paid-in capital
|
$
|
464,497
|
$
|
—
|
$
|
—
|
||||||
|
Accumulated deficit
|
$
|
(284,413
|
)
|
$
|
(174,662
|
)
|
$
|
(91,445
|
)
|
|||
|
Stockholders' equity (deficit)
|
$
|
179,633
|
$
|
(17,747
|
)
|
$
|
55,267
|
|||||
68
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
This financial review presents our operating results for each of the three years in the period ended December 31, 2017, and our financial condition at December 31, 2017. Except for the historical information contained herein, the following discussion contains forward-looking statements which are subject to known and unknown risks, uncertainties and other factors that may cause our actual results to differ materially from those expressed or implied by such forward-looking statements. We discuss such risks, uncertainties and other factors throughout this report and specifically under Item 1A of Part I of this report, "Risk Factors." In addition, the following review should be read in connection with the information presented in our consolidated financial statements and the related notes to our consolidated financial statements as indexed on page F-1.
Overview
We are a late-stage biopharmaceutical company focused on developing and commercializing drugs to treat patients with serious cardiometabolic diseases caused by lipid disorders. Our goal is to become the premier company offering treatments for inadequately treated lipid disorders. We are advancing a mature pipeline of four novel drugs with the potential to treat multiple diseases. Our drugs, volanesorsen, AKCEA-APO(a)-LRx, AKCEA-ANGPTL3-LRx and AKCEA-APOCIII-LRx, are all based on antisense technology developed by Ionis Pharmaceuticals, Inc., or Ionis, which owns approximately 68% of our common stock. Our most advanced drug, volanesorsen, is currently under review by regulatory agencies in the U.S., EU, and Canada for the treatment of people with familial chylomicronemia syndrome, or FCS. In the U.S., the Food and Drug Administration, or FDA, assigned a Prescription Drug User Fee Act, or PDUFA, goal date of August 30, 2018 and scheduled advisory committee meeting for May 10, 2018. In Canada, our New Drug Submission, or NDS, was granted Priority Review by Health Canada. FCS is a severe, rare, genetically defined lipid disorder characterized by extremely elevated levels of triglycerides. FCS has life-threatening consequences and the lives of patients with this disease are impacted daily by the associated symptoms. In our clinical program, we have observed consistent and substantial (>70%) decreases in triglycerides and improvements in other manifestations of FCS, including pancreatitis attacks and abdominal pain. We believe the safety and efficacy data from the volanesorsen program demonstrate a favorable risk-benefit profile for patients with FCS. Volanesorsen is also currently in Phase 3 clinical development for the treatment of familial partial lipodystrophy, or FPL.
We have made substantial progress in assembling the infrastructure to commercialize our drugs globally with a focus on lipid specialists as the primary call point. We have established operations in the U.S., UK, France, Canada and Germany as well as sales team members and additional field medical directors to further FCS disease education prior to our volanesorsen launch. A key element of our commercial strategy is to provide the specialized, patient-centric support required to successfully address rare disease patient populations. We believe our focus on treating patients with inadequately addressed lipid disorders will allow us to partner efficiently and effectively with the specialized medical community that supports these patients. Most recently, we worked with experts to potentially help simplify diagnosis criteria, resulting in a streamlined patient journey to lipid specialists. Through our FCSFocus.com website, we provide a disease education book and a care toolkit to help patients understand their disease and organize all of their medical records to enable smoother communication with physicians.
To maximize the commercial potential of two of the drugs in our pipeline, we initiated a strategic collaboration with Novartis Pharma AG, or Novartis, for the development and commercialization of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. We believe Novartis brings significant resources and expertise to the collaboration that can accelerate our ability to deliver these potential therapies to the large populations of patients who have high cardiovascular risk due to inadequately treated lipid disorders. Under our agreement with Novartis, after we complete Phase 2 development of each of AKCEA-APO(a)-LRx (planned for the second half of 2018) and AKCEA-APOCIII-LRx (planned for 2019), and if, on a drug-by-drug basis, Novartis exercises its option to license a drug and pays us the $150.0 million license fee to do so, Novartis would conduct and pay for a Phase 3 cardiovascular outcome study in high-risk patients and, if approved, commercialize each such licensed drug worldwide. We plan to co-commercialize any licensed drug commercialized by Novartis in selected markets, under terms and conditions that we plan to negotiate with Novartis in the future, through the specialized sales force we are building to commercialize volanesorsen.
Our strategic collaboration with Novartis has a potential aggregate transaction value of over $1.0 billion, plus royalties, which we would generally be required to share equally with Ionis. The calculation of potential aggregate transaction value assumes that Novartis licenses, successfully develops and achieves regulatory approval for both AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx in the United States, Europe and Japan, and that Novartis achieves pre-specified sales targets with respect to both drugs. As part of our collaboration, we received $75.0 million in an upfront option payment, of which we retained $60.0 million and paid $15.0 million to Ionis as a sublicense fee. In addition, for AKCEA-APO(a)-LRx we are eligible to receive up to $600.0 million in milestone payments, including $25.0 million for the achievement of a development milestone, up to $290.0 million for the achievement of regulatory milestones and up to $285.0 million for the achievement of commercialization milestones. In addition, for AKCEA-APOCIII-LRx we are eligible to receive up to $530.0 million in milestone payments, including $25.0 million for the achievement of a development milestone, up to $240.0 million for the achievement of regulatory milestones and up to $265.0 million for the achievement of commercialization milestones. We are also eligible to receive tiered royalties in the mid-teens to low twenty percent range on net sales of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Novartis will reduce these royalties upon the expiration of certain patents or if a generic competitor negatively impacts the product in a specific country. We will pay 50% of these license fees, milestone payments and royalties to Ionis as a sublicense fee. See Note 8, Strategic Collaboration with Novartis, to our consolidated financial statements for additional information.
69
Through 2016, we did not generate revenue and we have incurred net losses in each period since inception. In January 2017, we initiated our strategic collaboration with Novartis and began recognizing revenue under this collaboration. Our revenue for the year ended December 31, 2017 was $55.2 million, solely related to our strategic collaboration with Novartis. Our net losses have resulted from costs incurred in developing volanesorsen and the other drugs in our pipeline, preparing to commercialize volanesorsen and general and administrative activities associated with our operations. We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future as we continue to develop volanesorsen and our other drugs, and seek regulatory approval for and prepare to commercialize volanesorsen. We expect to incur significant expenses to continue to build the infrastructure to support volanesorsen's commercialization, including manufacturing, marketing, sales and distribution functions. Further, we expect to incur additional costs associated with operating as a public company and in building our internal resources to become less reliant on Ionis.
As of December 31, 2017, we had cash, cash equivalents and short-term investments of $260.1 million. We have funded our operating activities through a $100.0 million cash contribution that we received from Ionis in 2015, $75.0 million from initiating our collaboration with Novartis that we received in the first quarter of 2017 and $106.0 million in drawdowns under our line of credit with Ionis that we received in the first and second quarters of 2017. In July 2017 we completed our IPO and raised $182.3 million in net proceeds including $50 million from the Novartis concurrent private placement and $25 million from Ionis. We plan to further advance our drugs and commercialization efforts with our cash on hand.
We believe that our existing cash, cash equivalents and short-term investments will be sufficient to fund our operations for at least the next 12 months. However, we expect to raise additional funds in the future to continue developing the drugs in our pipeline and to further commercialize any approved drugs, including volanesorsen. We may seek to obtain additional financing in the future through the issuance of our common stock, through other equity or debt financings or through collaborations or partnerships with other companies. We may not be able to raise additional capital on terms acceptable to us, or at all, and any failure to raise capital as and when needed could compromise our ability to execute on our current business plan.
Our Relationship with Ionis
Prior to January 2015, the drugs we licensed from Ionis were part of Ionis' broad pipeline of antisense drugs. Ionis' employees performed all of the development, regulatory and manufacturing activities for these drugs either themselves or through third-party providers. As such, Ionis incurred all of the expenses associated with these activities and reported them in its consolidated financial statements. Ionis formed Akcea as a wholly owned subsidiary to complete development of and commercialize Ionis' drugs to treat lipid disorders. We began business operations in January 2015.
We exclusively licensed our pipeline of four novel drugs from Ionis effective in January 2015. Prior to then, Ionis had been advancing these drugs in development and incurring the expenses for those activities. Under our license agreement with Ionis, Ionis continued and is continuing to conduct development, regulatory and manufacturing activities for our drugs and charge us for this work. In this way, we benefit from Ionis' more than 25 years of experience developing and manufacturing antisense drugs. As we are building our development, regulatory and manufacturing capabilities and capacity, we expect to assume increasing responsibility for these functions and Ionis' responsibilities will decrease. We expect that our collaborative approach will allow us to build these capabilities and capacity while still working closely with Ionis as we transition our drug development activities. Moreover, because Ionis has been conducting the majority of the development activities for our drugs, we have been able to focus on building the commercial organization and conducting the pre-commercialization activities necessary to support the launch of volanesorsen, if approved for marketing.
We pay Ionis for the research and development expenses it incurs on our behalf, which include both external and internal expenses in accordance with our license agreement with Ionis. External research and development expenses include costs for contract research organizations, or CROs, costs to conduct nonclinical and clinical studies on our drugs, costs to acquire and evaluate clinical study data such as investigator grants, patient screening fees and laboratory work, and fees paid to consultants. Internal development expenses include costs for the work that Ionis' development employees perform for us. Ionis charges us a full-time equivalent rate that covers personnel-related expenses, including salaries and benefits, plus an allocation of facility-related expenses, including rent, utilities, insurance and property taxes, for those research and development employees who work either directly or indirectly on the development of our drugs. In accordance with the license agreement, we pay Ionis for external research and development expenses and internal research and development expenses. We also pay Ionis for the active pharmaceutical ingredient, or API, and drug product we use in our nonclinical and clinical studies for all of our drugs. Ionis manufactures the API for us and charges us a price per gram consistent with the price Ionis charges its pharmaceutical partners, which includes the cost for direct materials, direct labor and overhead required to manufacture the API. If we need the API filled in vials or pre-filled syringes for our clinical studies, Ionis will contract with a third party to perform this work and Ionis will charge us for the resulting cost.
Under the services agreement, Ionis also provides us certain services, including, without limitation, general and administrative support services and development support services. We pay Ionis for our share of the internal and external expenses for each of these functions based on our relative use of each function, plus an allocation of facility-related expenses. As our business grows and we assume increasing responsibility from Ionis, we are assuming direct responsibility for procuring and financing the services we currently receive from Ionis and Ionis' responsibility to provide us with these services is decreasing.
70
We do not pay a mark-up or profit on the external or internal expenses Ionis bills to us or on the cost of the drugs Ionis manufactures for us. Moreover, Ionis only charges us for the portion of its resources that we use. For example, we do not have to pay for a full-time person if we only need the person's skills for 50% of the time. In this way, we can increase our headcount as our requirements grow and as we assume increasing responsibility for our drugs from Ionis, rather than building capabilities and capacity in advance of full utilization. We believe that our expenses reasonably reflect the expenses we would have incurred if we had the capabilities and capacity in place to perform this work ourselves. Further, we do not believe that our expenses will increase significantly as we assume development, regulatory, manufacturing and administrative responsibilities from Ionis because we will only assume these functions when we believe we can do so in a cost-efficient manner. See Note 4, Development, Commercialization and License Agreement and Services Agreement with Ionis, to our consolidated financial statements for more information on our agreements with Ionis.
In addition, Ionis has helped fund our operations through a line of credit agreement that we entered into in January 2017 under which we borrowed $106.0 million. The outstanding principal and accrued interest automatically converted upon closing of our IPO into an aggregate of 13,438,339 shares of our common stock. Following the closing of our IPO, we no longer have access to the line of credit with Ionis.
Basis of Presentation
We have derived the consolidated financial statements for the year ended December 31, 2014 presented in this annual report by carving out the expenses associated with our drugs from Ionis' consolidated financial statements in accordance with applicable accounting standards and SEC regulations. These results reflect amounts specifically attributable to our business, including the costs Ionis incurred for the drugs we exclusively licensed from Ionis under our license agreement with Ionis. We also have a services agreement with Ionis that became effective in January 2015 that provides us with certain general and administrative and development support services.
We consider our expense methodology and results to be reasonable for all periods we present. However, our allocations may not be indicative of the actual expenses we would have incurred had we operated as an independent, publicly traded company for the periods we present.
We discuss our agreements with Ionis in Note 4, Development, Commercialization and License Agreement and Services Agreement with Ionis, to our consolidated financial statements.
Critical Accounting Policies
We prepare our consolidated financial statements in conformity with accounting principles generally accepted in the United States, or GAAP. As such, we make certain estimates, judgments and assumptions that we believe are reasonable, based upon the information available to us. These judgments involve making estimates about the effect of matters that are inherently uncertain and may significantly impact our quarterly or annual results of operations and financial condition. In the following paragraphs, we describe our most significant accounting policies, which we believe are the most critical to aid in fully understanding and evaluating our reported financial results. As described below, there are specific risks associated with these critical accounting policies and we caution that future events rarely develop exactly as one may expect, and that best estimates may require adjustment.
The significant accounting policies, which we believe are the most critical to aid in fully understanding and evaluating our reported financial results, require the following:
| ● |
Assessing the propriety of revenue recognition and associated deferred revenue;
|
| ● |
Determining the appropriate cost estimates for unbilled preclinical and clinical development activities;
|
| ● |
Determining the stock-based compensation expense and valuation assumptions;
|
| ● |
Determining the fair value of our common stock prior to our IPO; and
|
| ● |
Determining the valuation allowance for net deferred tax assets.
|
Descriptions of these critical accounting policies follow.
71
Revenue Recognition
We will recognize revenue when we have satisfied all contractual obligations and we are reasonably assured of collecting the resulting receivable. We may be entitled to bill our customers and receive payment from our customers in advance of recognizing the revenue. In the instances in which we receive payment from our customers in advance of recognizing revenue, we will include the amounts in deferred revenue on our consolidated balance sheet and will recognize such amounts as revenue over time as they are earned.
Research and development revenue under collaborative agreements
Arrangements with multiple deliverables
Our strategic collaboration, option and license agreement, or collaboration agreement, with Novartis, which we entered into in January 2017, contains multiple elements, or deliverables, including options to obtain licenses to drugs, research and development services, and manufacturing services. Therefore, we accounted for the collaboration under the multiple deliverables guidance.
Multiple agreements
When we enter into separate agreements at or near the same time with the same partner, we must first evaluate such agreements to determine whether they should be accounted for individually as distinct arrangements or whether the separate agreements are, in substance, a single multiple element arrangement. We evaluate whether the negotiations are conducted jointly as part of a single negotiation, whether the deliverables are interrelated or interdependent, whether fees in one arrangement are tied to performance in another arrangement, and whether elements in one arrangement are essential to another arrangement. Our evaluation involves significant judgment to determine whether a group of agreements might be so closely related that they are, in effect, part of a single arrangement. For example, in the first quarter of 2017, we and Ionis entered into two separate agreements with Novartis at the same time: a collaboration agreement and a stock purchase agreement, or SPA.
We entered into the collaboration agreement with Novartis to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Under the collaboration agreement, we received a $75.0 million upfront payment. For each drug, we are responsible for completing a Phase 2 program, conducting an end-of-Phase 2 meeting with the FDA and delivering active pharmaceutical ingredient, or API. Under the collaboration agreement, Novartis has an exclusive option to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. If Novartis exercises an option for one of these drugs, it will pay us a license fee and will assume all further global development, regulatory and commercialization activities for the licensed drug. We are also eligible to receive a development milestone payment, milestone payments if Novartis achieves pre-specified regulatory milestones, commercial milestone payments and tiered royalties on net sales from each drug under the collaboration.
Under the SPA, Novartis purchased 1.6 million shares of Ionis' common stock for $100.0 million in the first quarter of 2017 and paid a premium over the weighted average trading price at the time of purchase. Additionally, Novartis agreed to purchase up to $50.0 million of our common stock in a separate private placement concurrent with the completion of our IPO at a price per share equal to the initial public offering price, subject to a number of conditions. If we did not complete our IPO or a similar offering by the 15-month anniversary of the SPA, or if we completed an offering that did not meet the specified criteria for Novartis to invest, then Novartis would have been required to purchase $50.0 million of Ionis' common stock at a premium over the weighted average trading price of Ionis' common stock at the time of purchase.
We evaluated the Novartis agreements to determine whether we should treat the agreements separately or as a single arrangement. We considered that the agreements were negotiated concurrently and in contemplation of one another. Additionally, the same individuals were involved in the negotiations of both agreements. Based on these facts and circumstances, we concluded that we should treat both agreements as a single arrangement, which we refer to as the Novartis collaboration. We evaluated the provisions of the agreements on a combined basis.
Identifying deliverables and units of accounting
We evaluate the deliverables in a collaboration agreement to determine whether they meet the criteria to be accounted for as separate units of accounting or whether they should be combined with other deliverables and accounted for as a single unit of accounting. When the delivered items in an arrangement have 'stand-alone value' to the customer, we will account for the deliverables as separate units of accounting. Delivered items have stand-alone value if they are sold separately by any vendor or the customer could resell the delivered items on a stand-alone basis. For example, our Novartis collaboration and SPA have multiple elements. We evaluated the deliverables in the Novartis collaboration when we entered into the agreements and determined that certain deliverables have stand-alone value.
72
We identified the following four separate units of accounting under the collaboration, each with stand-alone value:
| ● |
Development activities for AKCEA-APO(a)-LRx;
|
| ● |
Development activities for AKCEA-APOCIII-LRx;
|
| ● |
API for AKCEA-APO(a)-LRx; and
|
| ● |
API for AKCEA-APOCIII-LRx.
|
The development activities and the supply of API each have stand-alone value because Novartis or another third party could provide these items without our assistance.
Measurement and allocation of arrangement consideration
Our Novartis collaboration provides for various types of payments to us including upfront payments, milestone payments, licensing fees, royalties on product sales and payments for the purchase of common stock. We first evaluated the total consideration under both the collaboration agreement and SPA and determined how much of the total consideration was attributable to elements that we are delivering under the collaboration.
We determined that our portion of the allocable arrangement consideration for the Novartis collaboration was $108.4 million, comprised of the following:
| ● |
$75.0 million from the upfront payment received;
|
| ● |
$28.4 million for the premium paid by Novartis, which represents the excess of the fair value Ionis received from Novartis' purchase of Ionis' stock at a premium in the first quarter of 2017; and
|
| ● |
$5.0 million for the potential premium Novartis would have paid if they had been required to purchase Ionis' stock in the future at a premium.
|
We are recognizing the $75.0 million upfront payment plus the premium paid by Novartis from its purchase of Ionis' stock and the premium associated with Novartis' obligation to purchase Ionis' stock if we did not complete our IPO because we are the party providing the services and API under the collaboration agreement.
We initially allocate the amount of consideration that is fixed or determinable at the time the agreement is entered into and exclude contingent consideration. We allocate the consideration to each unit of accounting based on the relative selling price of each deliverable. We use the following hierarchy of values to estimate the selling price of each deliverable: (i) vendor-specific objective evidence of fair value; (ii) third-party evidence of selling price; and (iii) best estimate of selling price, or BESP. BESP reflects our best estimate of what the selling price would be if we regularly sold the deliverable on a stand-alone basis. We recognize the revenue allocated to each unit of accounting as we deliver the related goods or services. If we determine that we should treat certain deliverables as a single unit of accounting, then we will recognize the revenue ratably over our estimated period of performance.
We allocated the consideration based on the relative BESP of each unit of accounting. We estimated the selling price of the development services over the expected period during which we will perform these services. The significant inputs we used to determine the selling price of the development services included:
| ● |
The number of internal hours we will spend performing these services;
|
| ● |
The estimated cost of the work we will perform;
|
| ● |
The estimated cost of work that we will contract with third parties to perform; and
|
| ● |
The estimated cost of API we will use.
|
For purposes of determining BESP of the services we perform and the API we deliver under our Novartis collaboration, accounting guidance required us to include a markup for a reasonable profit margin.
Based on the units of accounting under the Novartis collaboration, we allocated the $108.4 million of allocable consideration as follows:
| ● |
$64.0 million for development services for AKCEA-APO(a)-LRx;
|
| ● |
$40.1 million for development services for AKCEA-APOCIII-LRx;
|
| ● |
$1.5 million for the delivery of AKCEA-APO(a)-LRx API; and
|
| ● |
$2.8 million for the delivery of AKCEA-APOCIII-LRx API.
|
73
Timing of revenue recognition
We recognize revenue as we deliver each item under our Novartis collaboration as we provide services and the related revenue is realizable and earned. We also recognize revenue over time. Our Novartis collaboration agreement includes a development project plan outlining the activities the agreement requires each party to perform during the collaboration. We estimated our period of performance when the agreement was entered into because the agreement did not clearly define such information. We then recognize revenue from development services ratably over such period. We made estimates of our time to complete our obligations under our Novartis collaboration agreement, and in certain instances the timing of satisfying these obligations may change as the development plans for our drugs progress. If our estimates and judgments change over the course of the Novartis collaboration agreement, it may affect the timing and amount of revenue that we will recognize in future periods. Any changes in estimates are recognized on a prospective basis.
The following are the periods over which we are recognizing revenue for each of our units of accounting under the Novartis collaboration:
| ● |
We are recognizing the amount attributed to the development services for AKCEA-APO(a)-LRx over the period of time we are performing the services, currently estimated to be through November 2018;
|
| ● |
We are recognizing the amount attributed to the development services for AKCEA-APOCIII-LRx over the period of time we are performing the services, currently estimated to be through June 2019;
|
| ● |
We recognize the amount attributed to the AKCEA-APO(a)-LRx API supply when we deliver API to Novartis; and
|
| ● |
We will recognize the amount attributed to the AKCEA-APOCIII-LRx API supply when we deliver API to Novartis.
|
Milestone payments
Our Novartis collaboration contains contractual milestone payments that relate to the achievement of pre-specified development, regulatory and commercialization events. These three categories of milestone events reflect the three stages of the life-cycle of our drugs, which we describe in more detail in the following paragraphs.
The designation of a development candidate is the first stage in the life-cycle of our drugs. A development candidate is a chemical compound that has demonstrated the necessary safety and efficacy in preclinical animal studies to warrant further study in humans.
During the first step of the development stage, we or our partner study our drugs in Investigational New Drug, or IND-enabling studies, which are animal studies intended to support an IND application, and/or the foreign equivalent. An approved IND allows us or our partners to study our development candidate in humans. If the regulatory agency approves the IND, we or our partners initiate Phase 1 clinical trials in which we typically enroll a small number of healthy volunteers to ensure the development candidate is safe for use in patients. If we or our partners determine that a development candidate is safe based on the Phase 1 data, we or our partners initiate Phase 2 studies that are generally larger scale studies in patients with the primary intent of determining the efficacy of the development candidate.
The final step in the development stage is Phase 3 studies to gather the necessary safety and efficacy data to request marketing authorization from the FDA and/or foreign equivalents. The Phase 3 studies typically involve large numbers of patients and can take up to several years to complete. If the data gathered during the trials demonstrates acceptable safety and efficacy results, we or our partners will submit an application to the FDA and/or its foreign equivalents for marketing authorization. This stage of the drug's life-cycle is the regulatory stage.
If a drug achieves marketing authorization, it moves into the commercialization stage, during which we or our partners will market and sell the drug to patients. Although our partner may ultimately be responsible for marketing and selling the partnered drug, our efforts to develop a drug that is safe, effective and reliable contributes significantly to our partner's ability to successfully sell the drug. The FDA and its foreign equivalents have the authority to impose significant restrictions on an approved drug through the product label and on advertising, promotional and distribution activities. Therefore, our efforts designing and executing the necessary animal and human studies are critical to obtaining claims in the product label from the regulatory agencies that would allow us or our partners to successfully commercialize our drug. Further, the patent protection afforded our drugs as a result of our initial patent applications and related prosecution activities in the United States and foreign jurisdictions are critical to our or our partner's ability to sell our drugs without competition from generic drugs. The potential sales volume of an approved drug is dependent on several factors including the size of the patient population, market penetration of the drug and the price charged for the drug.
74
The milestone events contained in our Novartis collaboration agreement coincide with the progression of our drugs from development to marketing authorization and then to commercialization. The process of successfully discovering a new development candidate, having it approved and ultimately sold for a profit is highly uncertain. As such, the milestone payments we may earn from our partners involve a significant degree of risk to achieve. Therefore, as a drug progresses through the stages of its life-cycle, the value of the drug generally increases.
Development milestones in our Novartis collaboration agreement or potential future collaborations may include the following types of events:
| ● |
Designation of a development candidate. Following the designation of a development candidate, IND-enabling animal studies for a new development candidate generally take 12 to 18 months to complete;
|
| ● |
Initiation of a Phase 1 clinical trial. Generally, Phase 1 clinical trials take one to two years to complete;
|
| ● |
Initiation or completion of a Phase 2 clinical trial. Generally, Phase 2 clinical trials take one to three years to complete; and
|
| ● |
Initiation or completion of a Phase 3 clinical trial. Generally, Phase 3 clinical trials take two to four years to complete.
|
Regulatory milestones in our Novartis collaboration agreement or potential future collaborations may include the following types of events:
| ● |
Filing of regulatory applications for marketing authorization such as a New Drug Application, or NDA, in the United States or a Marketing Authorization Application, or MAA, in Europe. Generally, it takes six to twelve months to prepare and submit regulatory filings.
|
| ● |
Marketing authorization in a major market, such as the United States, Europe or Japan. Generally, it takes one to two years after an application is submitted to obtain authorization from the applicable regulatory agency.
|
Commercialization milestones in our Novartis agreement or potential future collaborations may include the following types of events:
| ● |
First commercial sale in a particular market, such as in the United States or Europe.
|
| ● |
Product sales in excess of a pre-specified threshold, such as annual sales exceeding $1 billion. The amount of time to achieve this type of milestone depends on several factors including but not limited to the dollar amount of the threshold, the pricing of the product and the pace at which customers begin using the product.
|
We will assess whether a substantive milestone exists at the inception of the collaboration agreement. When a substantive milestone is achieved, we will recognize revenue related to the milestone payment immediately. In evaluating if a milestone is substantive we will consider whether:
| ● |
Substantive uncertainty exists as to the achievement of the milestone event at the inception of the arrangement;
|
| ● |
The achievement of the milestone involves substantive effort and can only be achieved based in whole or in part on the performance or the occurrence of a specific outcome resulting from our performance;
|
| ● |
The amount of the milestone payment appears reasonable either in relation to the effort expended or to the enhancement of the value of the delivered items;
|
| ● |
There is no future performance required to earn the milestone payment; and
|
| ● |
The consideration is reasonable relative to all deliverables and payment terms in the arrangement.
|
If any of these conditions are not met, we will not consider the milestone to be substantive and we will defer recognition of the milestone payment and recognize it as revenue over the estimated period of performance, if any. We have determined that all milestones under our Novartis collaboration are substantive milestones.
Option to license
When we have a multiple element arrangement that includes an option to obtain a license, we will evaluate if the option is a deliverable at the inception of the arrangement. We do not consider the option to be a deliverable if we conclude that it is substantive and not priced at a significant and incremental discount. We will consider an option substantive if, at the inception of the arrangement, we are at risk as to whether the collaboration partner will choose to exercise its option to obtain the license. In those circumstances, we do not include the associated license fee in the allocable consideration at the inception of the agreement. Rather, we account for the license fee when our partner exercises its option. Under the Novartis collaboration, we concluded that the option to license is a substantive option. Therefore, we did not include any amounts in the initial allocable consideration at the inception of the collaboration. We will recognize any future exercise of an option to license a drug under the Novartis agreement in full in the period in which the option is exercised.
75
Estimated Liability for Research and Development Costs
We record accrued liabilities related to expenses for which vendors or service providers have not yet billed us. These liabilities are for products or services that we have received and primarily relate to ongoing nonclinical studies and clinical studies. These costs primarily include third-party clinical management costs, laboratory and analysis costs, toxicology studies and investigator grants. We have drugs in concurrent, nonclinical and clinical studies at several sites throughout the world. To ensure that we have adequately provided for ongoing nonclinical and clinical research and development costs during the period in which we incur such costs, we maintain an accrual to cover these costs. We update our estimate for this accrual on at least a quarterly basis. The assessment of these costs is a subjective process that requires judgment. Upon settlement, these costs may differ materially from the amounts accrued in our consolidated financial statements. Our historical accrual estimates have not been materially different from our actual amounts.
Stock-Based Compensation Expense and Valuation Assumptions
We measure stock-based compensation expense for equity-classified stock option awards based on the estimated fair value of the award on the date of grant. We recognize the value of the portion of the award that we ultimately expect to vest as stock-based compensation expense over the requisite service period in our statements of operations. We reduce stock-based compensation expense for estimated forfeitures at the time of grant and revise in subsequent periods if actual forfeitures differ from those estimates.
Prior to December 2015, Ionis granted our employees options to purchase shares of Ionis' common stock, or Ionis options. In December 2015, we granted our employees holding Ionis options additional options to purchase shares of our common stock, or Akcea options.
We determined the stock-based compensation expense for the Ionis options at the date of grant and recognized compensation expense over the vesting period of the Ionis options. In December 2015, we accounted for the issuance of the Akcea options as a modification to the original grant of the Ionis options because the grant of the Ionis options and Akcea options essentially represented a single stock award as the exercisability provisions of the Ionis options and Akcea options were interrelated and mutually exclusive. The total compensation expense measured on the modification date was the sum of the grant date fair value of the Ionis options plus any incremental compensation expense resulting from the grant of the Akcea options.
In 2016, we began concurrently granting Ionis options and Akcea options to our employees. Because the exercisability provisions of the awards are interrelated and mutually exclusive as described above, the fair values of the Ionis options and the Akcea options were determined on the date of grant and the option with the greater fair value is recognized over the vesting period of the awards. In 2017, we no longer concurrently granted Ionis options and Akcea options to our employees.
Following our IPO, we no longer grant Ionis options to our employees. Under the terms of the Ionis options, when we completed our IPO, the Ionis options our employees were holding were terminated. The termination of the Ionis options was determined not to be a modification, as the options were terminated based upon the existing contractual terms of the option agreements. As such, we will continue to recognize stock-based compensation expense based upon the valuation that we determined at the grant date for options issued in 2016 or the modification date for options issued in 2015 and 2017.
We recognize compensation expense for option awards using the accelerated multiple-option approach. Under the accelerated multiple-option approach, also known as the graded-vesting method, an entity recognizes compensation expense over the requisite service period for each separately vesting tranche of the award as though the award were in substance multiple awards, which results in the expense being front-loaded over the vesting period.
We and Ionis value our stock option awards using the Black-Scholes option pricing model. The determination of the grant date fair value of options using an option pricing model is affected principally by the estimated common stock fair value and requires management to make a number of other assumptions, including: the risk-free interest rate, expected dividend yield, expected volatility, expected term, rate of forfeiture and fair value of common stock.
Ionis considered the following factors in valuing options for its common stock granted to our employees:
| ● |
Risk-free interest rate. Ionis bases the risk-free interest rate assumption on the yields of U.S. Treasury securities with maturities that correspond to the term of the award.
|
| ● |
Expected dividend yield. Ionis bases the dividend yield assumption on its history and expectation of dividend payouts. Ionis has not paid dividends in the past and it does not expect to do so in the foreseeable future.
|
| ● |
Expected volatility. Ionis uses an average of the historical stock price volatility of Ionis' stock. Ionis computes its historical stock volatility based on the expected term of its awards.
|
76
| ● |
Expected term. The expected term of stock options Ionis has granted represents the period of time that it expects them to be outstanding. Ionis estimates the expected term of options it has granted based on actual and projected exercise patterns.
|
| ● |
Rate of forfeiture. Ionis estimates forfeitures at the time of grant and revises its estimates, if necessary, in subsequent periods if actual forfeitures differ from those estimates. It estimates forfeitures based on historical experience. Ionis' historical forfeiture estimates have not been materially different from its actual forfeitures.
|
| ● |
Fair value of common stock. Ionis uses the market closing price for its common stock on the date of grant as reported on NASDAQ to determine the fair value of Ionis' common stock on the date of grant.
|
We considered the following factors in valuing options for our common stock:
| ● |
Risk-free interest rate. We determine the risk-free interest rate assumption based on the yields of U.S. Treasury securities with maturities that correspond to the term of the award.
|
| ● |
Expected dividend yield. We assume a dividend yield of zero as we have not paid dividends in the past and do not expect we will pay dividends on our common stock for the foreseeable future.
|
| ● |
Expected volatility. We do not have sufficient history to estimate the volatility of our common stock. We calculate expected volatility based on reported data from selected publicly traded peer companies for which historical information is available. We plan to continue to use a peer group to calculate our volatility until the historical volatility of our common stock is sufficient to measure expected volatility for future option grants.
|
| ● |
Expected term. Our expected term estimates represent the period of time that we expect the options to be outstanding. As we do not have historical information, we use the simplified method for estimating the expected term. Under the simplified method, we calculate the expected term as the average of the time-to-vesting and the contractual life of the options. As we gain additional historical information, we will transition to calculating our expected term based on our exercise patterns.
|
| ● |
Rate of forfeiture. We estimate forfeitures based on Ionis' historical rates of forfeiture as we do not have similar historical information for ourselves. We and Ionis are engaged in similar businesses and we believe this is a good estimate of expected forfeitures. As we gain additional historical information, we will transition to using our historical forfeiture rate.
|
| ● |
Fair value of common stock. Prior to our IPO, we estimated the fair value of our common stock as our common stock has not historically been publicly traded. See "Fair Value of Common Stock Prior to Initial Public Offering" below. Upon completion of our IPO in July 2017, we use the market closing price for our common stock on the date of grant as reported on NASDAQ to determine the fair value of our common stock on the date of grant.
|
Fair Value of Common Stock Prior to Initial Public Offering
We granted all options to purchase shares of our common stock with an exercise price per share no less than the fair value per share of our common stock underlying those options on the date of grant. Historically, for all periods prior to our IPO, the fair values of the shares of common stock underlying our stock options were estimated on each grant date by our board of directors. Given the absence of a public trading market of our common stock, our board of directors exercised reasonable judgment and considered a number of objective and subjective factors to determine the best estimate of the fair value of our common stock. To determine the fair value of our common stock, our board of directors considered, among other things, contemporaneous valuations of our common stock prepared by an unrelated third-party valuation firm in accordance with the guidance provided by the American Institute of Certified Public Accountants 2013 Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, or the Practice Aid. Our board of directors also considered various objective and subjective factors in estimating the fair value of our common stock on the date of grant, including:
| ● |
the prices, rights, preferences and privileges of our preferred stock relative to our common stock;
|
| ● |
our business, financial condition and results of operations, including related industry trends affecting our operations;
|
| ● |
the likelihood of achieving a liquidity event, such as an initial public offering or sale of our company, given prevailing market conditions;
|
| ● |
the lack of marketability of our common stock;
|
| ● |
the market performance of comparable publicly traded companies; and
|
| ● |
U.S. and global economic and capital market conditions and outlook.
|
Enterprise Valuation Methodologies
Our third-party valuation firm prepared our valuations in accordance with the guidelines in the Practice Aid, which prescribes several valuation approaches for setting the value of an enterprise, such as the cost, income and market approaches, and various methodologies for allocating the value of an enterprise to its common stock. The cost approach establishes the value of an enterprise based on the cost of reproducing or replacing the property less depreciation and functional or economic obsolescence, if present. The income approach establishes the value of an enterprise based on the present value of future cash flows that are reasonably reflective of a company's future operations, discounting to the present value with an appropriate risk adjusted discount rate or capitalization rate. The market approach is based on the assumption that the value of an asset is equal to the value of a substitute asset with the same characteristics. Our third-party valuation firm used the income and market valuation approaches to determine our stock price prior to completion of our IPO. When applying the income approach, our third-party valuation firm uses a discounted cash flow analysis based on our projections. When applying the market approach, our third-party valuation firm used the guideline publicly traded companies method choosing pharmaceutical companies whose business descriptions, including products and/or stage of development, are similar to ours. Our third-party valuation firm calculated our enterprise value under each of the income and market approaches and then used an equal weighting of these two approaches to arrive at our enterprise value.
77
Methods Used to Allocate Our Enterprise Value to Classes of Securities
In accordance with the Practice Aid, our third-party valuation firm considered the various methods for allocating the enterprise value across our classes and series of capital stock to determine the fair value of our common stock at each valuation date. The methods the third-party valuation firm considered consisted of the following:
Current Value Method
Under the current value method, once the fair value of the enterprise is established, the value is allocated to the various series of preferred and common stock based on their respective seniority, liquidation preference or conversion values, whichever is greatest. This method was considered, but not used in any of the valuations discussed below.
Option Pricing Method
The option pricing method, or OPM, treats common stock and preferred stock as call options on the total equity value of a company, with exercise prices based on the liquidation preference of the preferred stock. Under this method, the common stock has value only if the funds available for distribution to the stockholders exceed the value of the liquidation preference at the time of a liquidity event such as a merger, sale, or initial public offering, assuming the enterprise has funds available to make a liquidation preference meaningful and collectible by the stockholders. The common stock is modeled as a call option on the underlying equity value at a predetermined exercise price. In the model, the exercise price is based on a comparison with the total equity value, rather than, as in the case of a regular call option, a comparison with a per share stock price. The OPM uses the Black-Scholes option-pricing model to price the call option.
The valuation of our common stock as of January 1, 2015 used the OPM. We applied a discount to the valuation due to the lack of marketability of our stock. We calculated the discount for lack of marketability using the Finnerty model and applied it as applicable to each allocation.
Probability-Weighted Expected Return Method
The probability-weighted expected return method, or PWERM, considers various potential liquidity outcomes, including in our case an initial public offering, the sale of our company, dissolution and staying private, and assigns probabilities to each outcome to arrive at a weighted equity value.
We performed an updated valuation analysis of our common stock as of January 1, 2017 using a hybrid of the OPM and the PWERM, consistent with how such hybrid method is described in the Practice Aid. We calculated the discount for lack of marketability using the Finnerty model and applied it as applicable to each allocation.
Income Taxes
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act of 2017 (the "Tax Act"). The Tax Act makes broad and complex changes to the U.S. tax code. The changes include, but are not limited to, reducing the U.S. federal corporate tax rate from 35% to 21%, imposing a mandatory one-time transition tax on certain unrepatriated earnings of foreign subsidiaries, introducing bonus depreciation that will allow for full expensing of qualified property, eliminating the corporate alternative minimum tax ("AMT") and changing how existing AMT credits can be realized.
The SEC staff issued Staff Accounting Bulletin No. 118 ("SAB 118") to address the application of U.S. GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the Tax Act.
In accordance with SAB 118, we provided our best estimate of the impact of the Tax Act in the period ending December 31, 2017 based on our understanding of the Tax Act and guidance available as of the date of this filing. We remeasured our existing net U.S. deferred tax assets using the enacted tax rate and other known significant changes to the tax code. This resulted in a total decrease in these assets by $17.5 million which was fully offset by the decrease in the valuation allowance. In addition, we recorded a $0.5 million long-term income tax receivable related to our estimated 2017 AMT liability because under the Tax Act, AMT credits are refundable from 2018 through 2021.
Prior to the completion of our IPO we filed our tax returns on a consolidated and combined basis with Ionis for federal and state income tax purposes, respectively. For financial statement purposes when we are required to file on a consolidated or combined basis, we calculate our income tax amounts, including net operating losses and tax credit carryforwards, using a separate return methodology which determines income taxes as if we were a separate taxpayer from Ionis. Effective July 19, 2017, the date of our IPO, we are no longer included in the consolidated federal income tax return with Ionis. We determined the amount of federal tax attributes, primarily net operating losses and tax credit carryforwards that transferred to us upon deconsolidation from Ionis.
78
We are still required to file most of our state tax returns on a consolidated or combined basis with Ionis. Therefore, for financial statement purposes we calculated our state income tax amounts using the separate return method. We have not yet determined the amount of state tax attributes, primarily net operating losses and tax credit carryforwards, which we would retain if we were to deconsolidate for state tax purposes from Ionis.
We account for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in our financial statements or tax returns. In addition, deferred tax assets are recorded for the future benefit of utilizing net operating losses and research and development credit carryforwards. Valuation allowances are provided when necessary to reduce deferred tax assets to the amount expected to be realized.
We apply the authoritative accounting guidance prescribing a threshold and measurement attribute for the financial recognition and measurement of a tax position taken or expected to be taken in a tax return. We recognize liabilities for uncertain tax positions based on a two-step process. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step requires us to estimate and measure the tax benefit as the largest amount that is more than 50% likely to be realized upon ultimate settlement.
Significant judgment is required in evaluating our uncertain tax positions and determining our provision for income taxes. Although we believe our reserves are reasonable, no assurance can be given that the final tax outcome of these matters will not be different from that which is reflected in our historical income tax provisions and accruals. We adjust these reserves for changing facts and circumstances, such as the closing of a tax audit or the refinement of an estimate. To the extent that the final tax outcome of these matters is different than the amounts recorded, such differences may impact the provision for income taxes in the period in which such determination is made.
We recognize interest and penalties related to unrecognized tax benefits within the income tax expense line in the accompanying consolidated statements of operations. Accrued interest and penalties are included within other long-term liabilities in the consolidated balance sheets.
Significant judgment is also required in determining any valuation allowance recorded against deferred tax assets. In assessing the need for a valuation allowance, we consider all available evidence, including scheduled reversal of deferred tax liabilities, past operating results, the feasibility of tax planning strategies and estimates of future taxable income. Estimates of future taxable income are based on assumptions that are consistent with our plans. Assumptions represent management's best estimates and involve inherent uncertainties and the application of management's judgment. Should actual amounts differ from our estimates, the amount of our tax expense and liabilities could be materially impacted.
We record a valuation allowance to reduce the balance of our net deferred tax assets to the amount we believe is more-likely-than-not to be realized. We have incurred financial statement losses since inception and as a result we have a full valuation allowance recorded against our net deferred tax assets. We regularly assess the future realization of our net deferred tax assets and will reduce the valuation allowance in any such period in which we determine that all, or a portion, of our deferred tax assets are more-likely-than-not to be realized.
We do not provide for a U.S. income tax liability and foreign withholding taxes on undistributed foreign earnings of our foreign subsidiaries. The earnings of non-U.S. subsidiaries are currently expected to be indefinitely reinvested in non-U.S. operations.
79
JOBS Act and Emerging Growth Company Status
Under Section 107(b) of the Jumpstart our Business Startups Act of 2012, or the JOBS Act, an emerging growth company, or EGC, can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
We are in the process of evaluating the benefits of relying on other exemptions and reduced reporting requirements under the JOBS Act. Subject to certain conditions, as an EGC, we intend to rely on certain of these exemptions, including exemptions from the requirement to provide an auditor's attestation report on our system of internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act of 2002. We will remain an EGC until the earlier to occur of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of our IPO, (b) in which we have total annual gross revenue of at least $1.07 billion or (c) in which we are deemed to be a "large accelerated filer" under the rules of the SEC, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
Components of Results of Operations
Revenue
Through 2016, we did not generate any revenue. In January 2017, we initiated a strategic collaboration with Novartis and began recognizing revenue under this collaboration. For the year ended December 31, 2017, we recognized $55.2 million in research and development revenue from our collaboration with Novartis.
Operating Expenses
Our operating expenses consist of research and development expenses and general and administrative expenses, which are described below.
Research and Development Expenses
Since our inception, we have focused on developing our lead drug, volanesorsen, and the other drugs in our pipeline. Our research and development expenses primarily consist of:
| ● |
salaries and related expenses for research and development personnel, including expenses related to stock-based compensation granted to personnel in development functions;
|
| ● |
fees paid to clinical study sites and vendors, including contractor research organizations, or CROs, in connection with our clinical studies, costs of acquiring and evaluating clinical study data such as investigator grants, patient screening fees, laboratory work and statistical compilation and analysis, and fees paid to clinical consultants;
|
| ● |
expenses to acquire clinical study materials, including fees paid to Ionis;
|
| ● |
other consulting fees paid to third parties;
|
| ● |
expenses related to compliance with drug development regulatory requirements;
|
| ● |
travel, facilities, depreciation and amortization, insurance and other expenses; and
|
| ● |
sublicense expenses related to partnered drugs that we licensed from Ionis.
|
As described above, Ionis charges us for many of the expenses listed above because it is performing many of the development activities for our drugs on our behalf. As we assume increasing responsibility for developing and manufacturing our drugs, we will also increase the amount of expenses that we directly incur. As Ionis' responsibilities decrease, the expenses Ionis charges us will also decrease. We do not expect our overall research and development expenses to change significantly as we transition work from Ionis to us. However, we expect our overall development expenses to increase as we advance our pipeline. This increase will be driven by external costs associated with larger clinical studies as the pipeline moves into the later stages of development, costs of manufacturing drug product to be used in clinical studies and for validation and regulatory purposes, regulatory costs associated with seeking approval for our drugs and costs associated with expanding our internal development organization to support our pipeline as it advances into the later stages of development.
We expense our research and development costs as we incur them. We do not track research and development expenses by project, with the exception of costs related to volanesorsen. We typically use our employees, consultants and infrastructure resources across all of our projects. Thus, some of our research and development expenses are not attributable to an individual project, but are included in other research and development projects in our results of operations.
80
Our expenses related to clinical studies are based on estimates of patient enrollment and related expenses at clinical investigator sites as well as estimates for the services received and efforts expended pursuant to contracts with CROs that we may use to conduct and manage our clinical studies on our behalf. We generally accrue expenses related to clinical studies based on contracted amounts applied to the level of patient enrollment and activity. If we modify timelines or contracts based upon changes in the clinical study protocol or scope of work to be performed, we modify our estimates of accrued expenses accordingly on a prospective basis.
Development activities are central to our business model. We cannot determine with certainty the timing of initiation, the duration or the costs to complete current or future clinical studies of our drugs, including volanesorsen. Clinical development timelines, the probability of success and development costs can differ materially from expectations. The cost of clinical studies may vary significantly over the life of a project as a result of differences arising during clinical development, including, among others:
| ● |
per patient study costs;
|
| ● |
the number of studies required for approval;
|
| ● |
the number of sites included in the studies;
|
| ● |
the length of time required to enroll suitable patients;
|
| ● |
the number of doses that patients receive;
|
| ● |
the number of patients that participate in the studies;
|
| ● |
the drop-out or discontinuation rates of patients;
|
| ● |
the duration of patient follow-up;
|
| ● |
potential additional safety monitoring or other studies requested by regulatory agencies;
|
| ● |
the number and complexity of analyses and tests performed during the study;
|
| ● |
the phase of development of the drug; and
|
| ● |
the efficacy and safety profile of the drug.
|
In addition, we expect to incur substantial expenses beyond our present and planned nonclinical and clinical studies to file for marketing authorization for our drugs in development, assuming the data are supportive.
We cannot forecast which drugs may be subject to future collaborations, when we will complete such arrangements, if at all, and to what degree such arrangements would affect our development plans and capital requirements.
General and Administrative Expenses
Our general and administrative expenses consist of salaries and personnel-related costs, including stock-based compensation, for our employees in executive, sales and marketing and administrative functions. Significant external general and administrative expenses also include costs associated with the pre-commercialization activities we are performing to prepare to launch volanesorsen, if approved, for marketing. Our general and administrative expenses also include professional fees for accounting, auditing and consulting services, legal services, investor relations, travel and facilities. As described above, Ionis charges us for many of the expenses associated with these functions, including, among others, accounting, human resources, legal and investor relations. We expect to assume responsibility from Ionis for these general and administrative functions as our business grows and we build our internal development and commercialization capabilities. As Ionis' efforts on our behalf decrease, so will the expenses Ionis charges us for those efforts. We expect the increase in expenses we will incur for performing the work ourselves will be largely offset by the decrease in expenses Ionis charges us. We do not expect our overall general and administrative expenses to change significantly as we transition work from Ionis to us.
We anticipate our general and administrative expenses to increase in the future to support our continued development and potential commercialization of volanesorsen and the continued development of the other drugs in our pipeline. In addition, we expect to incur increased expenses associated with expanding our sales and marketing team and commercialization infrastructure to support the launch of volanesorsen. Increases over and above the level of work Ionis is currently performing on our behalf will result in an increase in general and administrative expenses and could include costs related to hiring additional personnel, increased office space, implementing new IT systems and other costs associated with expanding our general and administrative functions.
81
Results of Operations
Comparison of the Years Ended December 31, 2017 and December 31, 2016
Revenue
For the year ended December 31, 2017, we recognized $55.2 million in research and development revenue from our collaboration with Novartis, which we initiated in January 2017. For the year ended December 31, 2016, we did not generate any revenue.
Operating Expenses
Operating expenses for the year ended December 31, 2017 were $163.9 million and increased compared to $83.5 million for the year ended December 31, 2016. Our operating expenses increased in part due to sublicensing expenses due to Ionis of $48.4 million related to our collaboration with Novartis, of which $33.4 million was non-cash and development activities including the initiation of a Phase 2b dose-ranging study of AKCEA-APO(a)-LRx.
In order to analyze and compare our results of operations to other similar companies, we believe it is important to exclude non-cash compensation expense related to equity awards from our operating expenses. We believe non-cash compensation expense is not indicative of our operating results or cash flows from our operations. Further, we internally evaluate the performance of our operations excluding it.
Research and Development Expenses
The following table sets forth our research and development expenses for the periods presented:
|
Years Ended December 31,
|
||||||||
|
(in thousands)
|
2017
|
2016
|
||||||
|
External volanesorsen expenses
|
$
|
26,505
|
$
|
38,403
|
||||
|
Other external research and development project expenses
|
21,789
|
11,567
|
||||||
|
Research and development personnel and overhead expenses
|
21,572
|
13,913
|
||||||
|
Sublicensing expenses
|
48,394
|
—
|
||||||
|
Total research and development expenses, excluding non-cash stock-based compensation expense
|
118,260
|
63,883
|
||||||
|
Non-cash stock-based compensation expense
|
8,630
|
4,576
|
||||||
|
Total research and development expenses
|
$
|
126,890
|
$
|
68,459
|
||||
Research and development expenses were $118.3 million for 2017 and increased compared to $63.9 million for 2016. The increase in expenses was primarily due to sublicensing expenses related to our collaboration with Novartis, which we incurred in the first quarter of 2017, the majority of which were non-cash. The progression of our other drugs in development, including AKCEA-APO(a)-LRx, AKCEA-APOCIII-LRx and AKCEA-ANGPTL3-LRx, during 2017 also contributed to the increase in our expenses. In particular we commenced four Phase 2 trials in 2017. This increase in research and development expenses was offset in part by a decrease in external volanesorsen expenses primarily related to the completion of the COMPASS and APPROACH studies. All amounts exclude non-cash compensation expense related to equity awards.
General and Administrative Expenses
The following table sets forth our general and administrative expenses for the periods presented:
|
Years Ended December 31,
|
||||||||
|
(in thousands)
|
2017
|
2016
|
||||||
|
General and administrative support expenses
|
$
|
9,426
|
$
|
5,591
|
||||
|
Pre-commercialization expenses for volanesorsen
|
18,646
|
3,889
|
||||||
|
Total general and administrative expenses, excluding non-cash stock-based compensation expense
|
28,072
|
9,480
|
||||||
|
Non-cash stock-based compensation expense
|
8,909
|
5,573
|
||||||
|
Total general and administrative expenses
|
$
|
36,981
|
$
|
15,053
|
||||
General and administrative expenses were $28.1 million for 2017 and increased compared to $9.5 million for 2016. Our general and administrative expenses increased due to the ongoing buildout of our commercial organization and advancement of pre-commercialization activities necessary to launch volanesorsen, if approved for marketing in the US, Canada and certain EU countries. All amounts exclude non-cash compensation expense related to equity awards.
Investment Income
Investment income for 2017 totaled $1.8 million compared to $0.3 million for 2016. The increase in investment income was primarily due to a higher average short-term investment balance and an increase in the interest rates on high quality debt and U.S. government agencies investments during 2017 compared to 2016.
Interest Expense
Interest expense is comprised entirely of interest incurred under our line of credit agreement with Ionis. Interest expense for 2017 totaled $1.7 million. We incurred no interest expense for 2016. The outstanding principal and accrued interest under our line of credit converted into 13,438,339 shares of our common stock in connection with the closing of our IPO in July 2017 and we no longer have access to this line of credit following the closing of our IPO.
Net Loss and Net Loss Per Share
Net loss for 2017 was $109.8 million compared $83.2 million for 2016. Basic and diluted net loss per preferred share for the year ended December 31, 2017 was $1.55 compared to $2.88 for 2016. Basic and diluted net loss per common share for the year ended December 31, 2017 was $2.82. We had no outstanding common stock at December 31, 2016. We incurred a higher net loss in 2017 compared to 2016 primarily due to the increase in expenses related to pre-commercialization and development activities for our drugs, sublicensing expenses related to our collaboration with Novartis, the ongoing global expansion of our company and becoming and operating as a public company.
Comparison of the Years Ended December 31, 2016 and December 31, 2015
Revenue
Through 2016, we did not generate any revenue.
Operating Expenses
Operating expenses for 2016 were $83.5 million and increased compared to $61.4 million for 2015 as a result of the following:
| ● |
We were conducting more and later-stage clinical studies in 2016 than we were in 2015, including the continuation of our Phase 3 studies for volanesorsen in patients with FCS and FPL.
|
| ● |
Our operating expenses also increased in 2016 as we continued to build our organization and advance the pre-commercialization activities necessary to launch volanesorsen, if approved for marketing.
|
Research and Development Expenses
The following table sets forth our research and development expenses for the periods presented:
|
Years Ended December 31,
|
||||||||
|
(in thousands)
|
2016
|
2015
|
||||||
|
External volanesorsen expenses
|
$
|
38,403
|
$
|
23,137
|
||||
|
Other external research and development project expenses
|
11,567
|
19,199
|
||||||
|
Research and development personnel and overhead expenses
|
13,913
|
7,722
|
||||||
|
Total research and development expenses, excluding non-cash stock-based compensation expense
|
63,883
|
50,058
|
||||||
|
Non-cash stock-based compensation expense
|
4,576
|
827
|
||||||
|
Total research and development expenses
|
$
|
68,459
|
$
|
50,885
|
||||
Research and development expenses were $63.9 million for 2016 and increased compared to $50.1 million for 2015. The increase in expenses was primarily due to our Phase 3 studies for volanesorsen, which continued to advance, and the progression of our other drugs, including AKCEA-APO(a)-LRx and AKCEA-ANGPTL3-LRx. All amounts exclude non-cash compensation expense related to equity awards.
82
General and Administrative Expenses
The following table sets forth our general and administrative expenses for the periods presented:
|
Years Ended December 31,
|
||||||||
|
(in thousands)
|
2016
|
2015
|
||||||
|
General and administrative support expenses
|
$
|
5,591
|
$
|
3,424
|
||||
|
Pre-commercialization expenses for volanesorsen
|
3,889
|
1,460
|
||||||
|
Total general and administrative expenses, excluding non-cash stock-based compensation expense
|
9,480
|
4,884
|
||||||
|
Non-cash stock-based compensation expense
|
5,573
|
5,669
|
||||||
|
Total general and administrative expenses
|
$
|
15,053
|
$
|
10,553
|
||||
General and administrative expenses were $9.5 million for 2016 and compared to $4.9 million for 2015. Our general and administrative expenses increased primarily because we were continuing to build the organization and advance pre-commercialization activities necessary to launch volanesorsen, if approved for marketing. All amounts exclude non-cash compensation expense related to equity awards.
Investment Income
Investment income for 2016 totaled $0.3 million compared to $16,000 for 2015. The increase in investment income was due to a higher average cash balance.
Net Loss and Net Loss Per Share
Net loss for 2016 was $83.2 million compared $61.4 million for 2015. Basic and diluted net loss per preferred share for the year ended December 31, 2016 was $2.88 compared to $2.13 for 2015. We had a higher net loss in 2016 compared to 2017 primarily due to the increase in expenses related to development activities for our drugs.
Liquidity and Capital Resources
At December 31, 2017 we had cash, cash equivalents and short-term investments of $260.1 million and accumulated deficit of $284.4 million.
We have funded our operating activities through a $100.0 million cash contribution that we received from Ionis in 2015, $75.0 million from initiating our collaboration with Novartis that we received in the first quarter of 2017 and $106.0 million in drawdowns under our line of credit with Ionis that we received in the first and second quarters of 2017. Our borrowings under our line of credit agreement with Ionis converted into shares of our common stock at the IPO price in connection with the closing of our IPO in July 2017. We no longer have access to the line of credit. Additionally, in July 2017 we received $182.3 million in net proceeds from our IPO including $25.0 million Ionis invested in our IPO and the Novartis concurrent private placement of $50 million.
At December 31, 2017, we had working capital of $186.0 million, compared to a working capital deficit of $19.3 million at December 31, 2016. Working capital increased in 2017 primarily due to the increase in our cash and short-term investments from proceeds related to our IPO and concurrent private placement with Novartis and a decrease in our cash payable to Ionis under our development, commercialization and license agreement and services agreement. As of December 31, 2017, our outstanding payable to Ionis was $14.4 million. In January 2017, we initiated a strategic collaboration with Novartis and we received $75.0 million in an upfront option payment, of which we retained $60.0 million and paid Ionis $15.0 million as a sublicense fee under our license agreement with Ionis, in May 2017.
We do not currently have any approved drugs and, therefore, we do not expect to generate significant revenue from drug sales unless and until we or our partners obtain regulatory approval for and commercialize volanesorsen or one of our other drugs in development. We anticipate that we will continue to incur losses for the foreseeable future, and we expect the losses to increase as we continue to develop, seek regulatory approval for, and begin to commercialize our drugs. We are subject to all of the risks incident in developing and commercializing new drugs and we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business.
83
Future Funding Requirements
We will need to raise additional funding in the future to continue developing the drugs in our pipeline and to commercialize any approved drug, including expanding our commercial efforts around volanesorsen. We believe that our existing cash, cash equivalents and short-term investments will be sufficient to fund our operations for at least the next 12 months. Until such time, if ever, as we can generate substantial product revenue, we may finance our cash needs through additional financing in the future through the issuance of our common stock, through other equity or debt financings or through collaborations or partnerships with other companies. In any event, we may not generate significant revenue from product sales prior to the use of our existing cash, cash equivalents and short-term investments. We do not have any committed external source of funds and we no longer have access to our line of credit with Ionis. Additional capital may not be available on reasonable terms, if at all. To the extent that we raise additional capital through the sale of stock or convertible debt securities, the ownership interest of our stockholders will be diluted and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders. Debt financing, if available, may involve agreements that include increased fixed payment obligations and covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, declaring dividends, selling or licensing intellectual property rights and other operating restrictions that could adversely affect our ability to conduct our business. If we raise additional funds through collaborations or licensing arrangements with third parties, we may have to relinquish valuable rights to our drugs or grant licenses on terms that may not be favorable to us. If we cannot raise additional funds through stock offerings or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and commercialize our drugs even if we would otherwise prefer to develop and commercialize the drugs ourselves.
Our forecast of the period of time through which our financial resources will be adequate to support our operations involves risks and uncertainties, and actual results could vary as a result of a number of factors. We have based this estimate on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we currently expect. The amount and timing of future funding requirements, both near- and long-term, will depend on many factors, including, but not limited to:
| ● |
the design, initiation, progress, size, timing, costs and results of our clinical and nonclinical studies;
|
| ● |
the outcome, timing and cost of regulatory approvals by the FDA and comparable foreign regulatory authorities, including the potential for the FDA or comparable foreign regulatory authorities to require that we perform more studies than, or evaluate clinical endpoints other than, those that we currently expect;
|
| ● |
the number and characteristics of drugs that we may pursue;
|
| ● |
our need to expand our development activities, including our need and ability to hire additional employees;
|
| ● |
the effect of competing technological and market developments;
|
| ● |
the cost of establishing sales, marketing, manufacturing and distribution capabilities for our drugs;
|
| ● |
our strategic collaborators' success in developing and commercializing our drugs;
|
| ● |
our need to add infrastructure, implement internal systems and hire additional employees to operate as a public company; and
|
| ● |
the revenue, if any, generated from commercial sales of our drugs for which we receive marketing authorization, which may be affected by market conditions, including obtaining coverage and adequate reimbursement of our drugs from third-party payors, including government programs and managed care organizations, and competition within the therapeutic class to which our drugs are assigned.
|
If we cannot expand our operations or otherwise capitalize on our business opportunities because we lack sufficient capital, our business, financial condition and results of operations could be materially adversely affected.
Contractual Obligations and Commitments
The following table summarizes our contractual obligations as of December 31, 2017, which consist of our operating leases for our office facility. The table provides a breakdown of when our operating lease obligations become due (in thousands):
|
Contractual obligations
|
Total
|
Less than
1 year
|
1 - 3 years
|
3 - 5 years
|
||||||||||||
|
Operating lease obligations
|
$
|
820
|
$
|
486
|
$
|
334
|
$
|
—
|
||||||||
We have not included potential milestone payments, sublicense fees and royalties that we may be required to pay Ionis for the license of intellectual property. We have not included these potential obligations in the table above because they are contingent upon the occurrence of future events and we do not know the timing and likelihood of such potential obligations with certainty.
The table above does not include certain general and administrative and development support services for which we will pay Ionis under our services agreement or obligations under agreements that we can cancel without a significant penalty. We describe our agreements with Ionis in more detail in Note 4, Development, Commercialization and License Agreement and Services Agreement with Ionis, to our consolidated financial statements.
84
Due to the uncertainty with respect to the timing of future cash flows associated with our unrecognized tax benefits, we are unable to make reasonably reliable estimates of the period of cash settlement with the respective taxing authorities. Therefore, we have excluded $5 million of gross unrecognized tax benefits from our contractual obligations table above.
In addition to contractual obligations, we had outstanding purchase orders as of December 31, 2017 and 2016 for the purchase of services and materials as part of our normal course of business.
Recently Issued Accounting Pronouncements
We describe the recently issued accounting pronouncements that apply to us in Note 1 to our consolidated financial statements, Organization and Significant Accounting Policies.
Off-Balance Sheet Arrangements
We did not have any off-balance sheet arrangements during the period presented, as defined in the rules and regulations of the SEC.
85
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We are exposed to changes in interest rates primarily from our investments in certain short-term investments. We place our cash equivalents and short-term investments with reputable financial institutions. We primarily invest our excess cash in commercial paper and debt instruments of the U.S. Treasury, financial institutions, corporations, and U.S. government agencies with strong credit ratings and an investment grade rating at or above A-1, P-1 or F-1 by Moody's, Standard & Poor's, or Fitch, respectively. We have established guidelines relative to diversification and maturities that are designed to maintain safety and liquidity. We periodically review and modify these guidelines to maximize trends in yields and interest rates without compromising safety and liquidity. We typically hold our investments for the duration of the term of the respective instrument. We do not utilize derivative financial instruments, derivative commodity instruments or other market risk sensitive instruments, positions or transactions to manage exposure to interest rate changes. Accordingly, we believe that, while the securities we hold are subject to changes in the financial standing of the issuer of such securities, we are not subject to any material risks arising from changes in interest rates, foreign currency exchange rates, commodity prices, equity prices or other market changes that affect market risk sensitive instruments.
Our results of operations are subject to foreign currency exchange rate fluctuations as we have foreign subsidiaries, Akcea Therapeutics UK Ltd., or Akcea UK, Akcea Therapeutics Canada, Inc., or Akcea Canada, Akcea Therapeutics France SAS, or Akcea France, and Akcea Therapeutics Germany GmbH, or Akcea Germany, with functional currencies other than the U.S. dollar. We created these foreign subsidiaries to support our initial pre-commercialization activities in North America and Europe and to serve as potential entities for future North American and European operations. We translate the foreign subsidiaries' functional currencies to our reporting currency, the U.S. dollar. As a result, our financial position, results of operations and cash flows can be affected by market fluctuations in the foreign currencies to U.S. dollar exchange rate which are difficult to predict. However, because the Akcea foreign subsidiaries currently have limited operations, the effect of fluctuations of the foreign currencies to U.S. dollar exchange rate on our consolidated results is immaterial to our consolidated financial statements. Our business strategy incorporates potentially significant international expansion, particularly in anticipation of approval of volanesorsen, therefore we expect that the impact of foreign currency exchange rate fluctuations may become more substantial in the future.
Item 8. Financial Statements and Supplementary Data
We filed our consolidated financial statements and supplementary data required by this item as exhibits hereto, and listed them under Item 15(a)(1) and (2), and incorporate them herein by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or Exchange Act) that are designed to ensure that information we are required to disclose in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. We designed and evaluate our disclosure controls and procedures recognizing that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance and not absolute assurance of achieving the desired control objectives.
As of the end of the period covered by this report on Form 10-K, we carried out an evaluation of our disclosure controls and procedures under the supervision of, and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer. Based on our evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of December 31, 2017.
Management's Report on Internal Control Over Financial Reporting
This annual report does not include a report of management's assessment regarding internal control over financial reporting due to a transition period established by rules of the Securities and Exchange Commission for newly public companies.
86
Changes in Internal Control Over Financial Reporting
The above assessment did not identify any change in our internal control over financial reporting that occurred during our latest fiscal quarter and that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
87
PART III
Item 10. Directors, Executive Officers and Corporate Governance
We incorporate by reference the information required by this Item with respect to directors and the Audit Committee from the information under the caption "ELECTION OF DIRECTORS," including in particular the information under "Nominating, Governance and Review Committee" and "Audit Committee," contained in our definitive information statement (the "Information Statement"), to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2017.
We incorporate by reference the required information concerning our Code of Ethics from the information under the caption "Code of Ethics and Business Conduct" contained in the Information Statement. Our Code of Ethics and Business Conduct is posted on our website at www.akceatx.com(1) and is available in print free of charge to any stockholder upon request. We intend to disclose future amendments to, or waivers from, our Code of Ethics and Business Conduct on our website. No such waivers have been issued during fiscal 2017.
Item 1, Part I of this Report contains information concerning our executive officers. We incorporate by reference the information required by this Item concerning compliance with Section 16(a) of the Exchange Act from the information under the caption "Section 16(a) Beneficial Ownership Reporting Compliance" contained in the Information Statement to be filed within 120 days after the end of the fiscal year ended December 31, 2017.
________________
| (1) |
Any information that is included on or linked to our website is not part of this Form 10-K.
|
Item 11. Executive Compensation
We incorporate by reference the information required by this item to the information under the caption "EXECUTIVE COMPENSATION," "Compensation Committee Interlocks and Insider Participation" and "COMPENSATION COMMITTEE REPORT" contained in the Information Statement to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2017.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
We incorporate by reference the information required by this item to the information under the captions "SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT" contained in the Information Statement to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2017.
Securities Authorized for Issuance under Equity Compensation Plans
The following table sets forth information regarding outstanding options and shares reserved for future issuance under our equity compensation plans as of December 31, 2017.
|
Plan Category
|
Number of Shares
to be Issued
Upon Exercise of
Outstanding Options
|
Weighted Average
Exercise Price of
Outstanding Options
|
Number of Shares
Remaining
Available for
Future Issuance
|
|
|||||||||
|
Equity compensation plans approved by stockholders (a)
|
7,905,110
|
$
|
9.64
|
1,061,070
|
(b)
|
||||||||
|
Total
|
7,905,110
|
$
|
9.64
|
1,061,070
|
|
||||||||
________________
| (a) |
Consists of two Akcea plans: 2015 Equity Incentive Plan and 2017 Employee Stock Purchase Plan, or ESPP.
|
| (b) |
Of these shares, 500,000 remained available for purchase under the ESPP as of December 31, 2017. The ESPP incorporates an evergreen formula pursuant to which on January 1 of each year, we automatically increase the aggregate number of shares reserved for issuance under the plan by an amount equal to the lesser of (i) 1% of the total number of shares of Common Stock outstanding on December 31st of the preceding calendar year, and (ii) 500,000 shares of Common Stock shares.
|
Item 13. Certain Relationships and Related Transactions, and Director Independence
We incorporate by reference the information required by this item to the information under the captions "Independence of the Board of Directors" and "Certain Relationships and Related Transactions" contained in the Information Statement to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2017.
Item 14. Principal Accounting Fees and Services
We incorporate by reference the information required by this item to the information under the caption "Ratification of Selection of Independent Auditors" contained in the Information Statement to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2017.
88
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a)(1) Index to Financial Statements
We submitted the consolidated financial statements required by this item in a separate section beginning on page F-1 of this Report.
(a)(2) Index to Financial Statement Schedules
We omitted these schedules because they are not required, or are not applicable, or the required information is shown in the consolidated financial statements or notes thereto.
(a)(3) Index to Exhibits
89
INDEX TO EXHIBITS
|
Exhibit
Number
|
Description of Document
|
|
|
3.1*
|
Amended and Restated Certificate of Incorporation of the Registrant. – Filed as an exhibit to the Registrant's Current Report on Form 8-K filed July 19, 2017 and incorporated herein by reference.
|
|
|
3.2*
|
Amended and Restated Bylaws of the Registrant - Filed as an exhibit to the Registrant's current Report on Form 8-K filed July 19, 2017 and incorporated herein by reference.
|
|
|
4.1*
|
Specimen Common Stock Certificate. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
4.2*
|
Investor Rights Agreement, dated December 18, 2015, between the Registrant and Ionis Pharmaceuticals, Inc. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.1+*
|
Form of Indemnification Agreement. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.2+*
|
2015 Equity Incentive Plan, as amended, and Form of Award Agreements. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.3+*
|
Form of 2017 Employee Stock Purchase Plan. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.4**
|
Development, Commercialization and License Agreement, dated December 18, 2015, between the Registrant and Ionis Pharmaceuticals, Inc. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.5**
|
Services Agreement, dated December 18, 2015, between the Registrant and Ionis Pharmaceuticals, Inc. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.6*
|
Senior Unsecured Line of Credit Agreement, dated January 18, 2017, between the Registrant and Ionis Pharmaceuticals, Inc. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.7**
|
Strategic Collaboration, Option and License Agreement, dated January 5, 2017, between the Registrant and Novartis Pharma AG. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.8*
|
Stock Purchase Agreement, dated January 5, 2017, between the Registrant, Ionis Pharmaceuticals, Inc. and Novartis Pharma AG. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.9*
|
Office Lease Agreement dated March 25, 2015 between the Registrant and 55 Cambridge Parkway, LLC. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.10*
|
Amendment of Lease dated February 1, 2016 between the Registrant and 55 Cambridge Parkway, LLC. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.11+*
|
Non-Employee Director Compensation Plan. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.12+*
|
Offer Letter Agreement, dated November 17, 2014, between the Registrant and Paula Soteropoulos. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.13+*
|
Offer Letter Agreement, dated January 5, 2015, between the Registrant and Jeffrey M. Goldberg. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.14+*
|
Offer Letter Agreement, dated January 18, 2016, between the Registrant and Louis St. L. O'Dea. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.15**
|
Letter Agreement regarding Development, Commercialization and License Agreement, dated January 16, 2017, between the Registrant and Ionis Pharmaceuticals, Inc. – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.16*
|
Amendment of Lease dated March 16, 2017 between the Registrant and 55 Cambridge Parkway, LLC. - – Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 333-216949) or amendments thereto and incorporated herein by reference.
|
|
|
10.17+*
|
Form of Severance Benefit Agreement. – Filed as an exhibit to the Registrant's Current Report on Form 8-K filed November 30, 2017 and incorporated herein by reference.
|
|
|
10.18+*
|
Offer Letter Agreement, dated July 31, 2017, between the Registrant and Michael MacLean.
|
|
|
Registrant's Code of Ethics and Business Conduct.
|
90
|
List of Subsidiaries.
|
||
|
Consent of Independent Registered Public Accounting Firm.
|
||
|
24.1
|
Power of Attorney (included in the signature page to this Report).
|
|
|
Certification by Chief Executive Officer pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as amended.
|
||
|
Certification by Chief Financial Officer pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as amended.
|
||
|
32.1o
|
Certification Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101
|
The following financial statements from the Akcea Therapeutics, Inc. Annual Report on Form 10-K for the year ended December 31, 2017, formatted in Extensive Business Reporting Language (XBRL): (i) consolidated balance sheets, (ii) consolidated statements of operations, (iii) consolidated statements of comprehensive loss, (iv) consolidated statements of stockholders' equity, (v) consolidated statements of cash flows and (vi) notes to consolidated financial statements (detail tagged).
|
| (*) |
Previously filed
|
| (+) |
Indicates management contract or compensatory plan.
|
| (**) |
Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portion have been filed separately with the Securities and Exchange Commission.
|
| (°) |
This certification is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended.
|
(b) Exhibits
We listed the exhibits required by this Item under Item 15(a)(3).
(c) Financial Statement Schedules
None.
Item 16. Form 10-K Summary
Not Applicable.
91
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on the 28th day of February 2018.
|
|
AKCEA THERAPEUTICS, INC.
|
|
|
|
||
|
|
By:
|
/s/ PAULA SOTEROPOULOS
|
|
|
Paula Soteropoulos
|
|
|
|
Chief Executive Officer, President and Director
(Principal executive officer)
|
|
92
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Paula Soteropoulos and Michael MacLean, or any of them, his or her attorney-in-fact, each with the power of substitution, for him or her in any and all capacities, to sign any amendments to this Report, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his or her substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
|
Signatures
|
Title
|
Date
|
||
|
/s/ PAULA SOTEROPOULOS
|
Chief Executive Officer, President and Director
|
February 28, 2018
|
||
|
Paula Soteropoulos
|
(Principal executive officer)
|
|||
|
/s/ MICHAEL MACLEAN
|
Chief Financial Officer
|
February 28, 2018
|
||
|
Michael MacLean
|
(Principal financial and accounting officer)
|
|||
|
/s/ CHRISTOPHER GABRIELI
|
Chairman of the Board
|
February 28, 2018
|
||
|
Christopher Gabrieli
|
||||
|
/s/ STANLEY T. CROOKE
|
Director
|
February 28, 2018
|
||
|
Stanley T. Crooke, M.D., Ph.D.
|
||||
|
/s/ EDWARD M. FITZGERALD
|
Director
|
February 28, 2018
|
||
|
Edward M. Fitzgerald
|
||||
|
/s/ ELAINE HOCHBERG
|
Director
|
February 28, 2018
|
||
|
Elaine Hochberg
|
||||
|
/s/ B. LYNNE PARSHALL
|
Director
|
February 28, 2018
|
||
|
B. Lynne Parshall, J.D.
|
||||
|
/s/ SANDFORD D. SMITH
|
Director
|
February 28, 2018
|
||
|
Sandford D. Smith
|
93
AKCEA THERAPEUTICS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Page
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
Consolidated Balance Sheets at December 31, 2017 and 2016
|
F-3
|
|
Consolidated Statements of Operations for the years ended December 31, 2017, 2016 and 2015
|
F-4
|
|
Consolidated Statements of Comprehensive Loss for the years ended December 31, 2017, 2016 and 2015
|
F-5
|
|
Consolidated Statements of Stockholders' Equity for the years ended December 31, 2017, 2016 and 2015
|
F-6
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2017, 2016 and 2015
|
F-7
|
|
Notes to Consolidated Financial Statements
|
F-8
|
F-1
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Akcea Therapeutics, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Akcea Therapeutics, Inc. (the Company) as of December 31, 2017 and 2016, and the related consolidated statements of operations, comprehensive loss, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2017, and the related notes (collectively referred to as the "consolidated financial statements"). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company's auditor since 2016
San Diego, California
February 28, 2018
F-2
AKCEA THERAPEUTICS, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
|
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
58,367
|
$
|
7,857
|
||||
|
Short-term investments
|
201,763
|
—
|
||||||
|
Contracts receivable
|
5,413
|
—
|
||||||
|
Other current assets
|
1,302
|
1,209
|
||||||
|
Total current assets
|
266,845
|
9,066
|
||||||
|
Property, plant and equipment, net
|
77
|
177
|
||||||
|
Licenses, net
|
1,221
|
1,341
|
||||||
|
Deposits and other assets
|
661
|
100
|
||||||
|
Total assets
|
$
|
268,804
|
$
|
10,684
|
||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT)
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$
|
2,381
|
$
|
476
|
||||
|
Payable to Ionis Pharmaceuticals, Inc.
|
14,365
|
24,355
|
||||||
|
Accrued compensation
|
4,083
|
2,505
|
||||||
|
Accrued liabilities
|
7,570
|
1,041
|
||||||
|
Current portion of deferred revenue
|
50,579
|
—
|
||||||
|
Other current liabilities
|
1,875
|
33
|
||||||
|
Total current liabilities
|
80,853
|
28,410
|
||||||
|
Long-term portion of deferred rent
|
12
|
21
|
||||||
|
Long-term portion of deferred revenue
|
8,306
|
—
|
||||||
|
Total liabilities
|
89,171
|
28,431
|
||||||
|
Stockholders' equity (deficit):
|
||||||||
|
Series A convertible preferred stock, $0.001 par value; no and 28,884,540 shares authorized, no and 28,884,540 shares issued and outstanding at December 31, 2017 and 2016, respectively; aggregate liquidation value of $0 and $610,304 as of December 31, 2017 and 2016, respectively
|
—
|
100,000
|
||||||
|
Common stock, $0.001 par value; 100,000,000 shares authorized, 66,541,629 and no shares issued and outstanding at December 31, 2017 and 2016, respectively
|
67
|
—
|
||||||
|
Additional paid-in capital
|
464,430
|
56,936
|
||||||
|
Accumulated other comprehensive loss
|
(451
|
)
|
(21
|
)
|
||||
|
Accumulated deficit
|
(284,413
|
)
|
(174,662
|
)
|
||||
|
Total stockholders' equity (deficit)
|
179,633
|
(17,747
|
)
|
|||||
|
Total liabilities and stockholders' equity (deficit)
|
$
|
268,804
|
$
|
10,684
|
||||
See accompanying notes.
F-3
AKCEA THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except for share and per share data)
|
Years Ended December 31,
|
||||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Revenue:
|
||||||||||||
|
Research and development revenue under collaborative agreements
|
$
|
55,209
|
$
|
—
|
$
|
—
|
||||||
|
Total revenue
|
55,209
|
—
|
—
|
|||||||||
|
Expenses:
|
||||||||||||
|
Research and development
|
126,890
|
68,459
|
50,885
|
|||||||||
|
General and administrative
|
36,981
|
15,053
|
10,553
|
|||||||||
|
Total operating expenses
|
163,871
|
83,512
|
61,438
|
|||||||||
|
Loss from operations
|
(108,662
|
)
|
(83,512
|
)
|
(61,438
|
)
|
||||||
|
Other income (expense):
|
||||||||||||
|
Investment income
|
1,813
|
295
|
16
|
|||||||||
|
Interest expense
|
(1,731
|
)
|
—
|
—
|
||||||||
|
Other income
|
104
|
—
|
—
|
|||||||||
|
Loss before income tax expense
|
(108,476
|
)
|
(83,217
|
)
|
(61,422
|
)
|
||||||
|
Income tax expense
|
(1,275
|
)
|
—
|
—
|
||||||||
|
Net loss
|
$
|
(109,751
|
)
|
$
|
(83,217
|
)
|
$
|
(61,422
|
)
|
|||
|
Net loss per share of preferred stock, basic and diluted
|
$
|
(1.55
|
)
|
$
|
(2.88
|
)
|
$
|
(2.13
|
)
|
|||
|
Weighted-average shares of preferred stock outstanding, basic and diluted
|
15,748,009
|
28,884,540
|
28,884,540
|
|||||||||
|
Net loss per share of common stock, basic and diluted
|
$
|
(2.82
|
)
|
$
|
—
|
$
|
—
|
|||||
|
Weighted-average shares of common stock outstanding, basic and diluted
|
30,262,768
|
—
|
—
|
|||||||||
See accompanying notes.
F-4
AKCEA THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(in thousands)
|
Years Ended December 31,
|
||||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Net loss
|
$
|
(109,751
|
)
|
$
|
(83,217
|
)
|
$
|
(61,422
|
)
|
|||
|
Unrealized gains (losses) on investments, net of tax
|
(337
|
)
|
75
|
(75
|
)
|
|||||||
|
Currency translation adjustment
|
(93
|
)
|
(21
|
)
|
—
|
|||||||
|
Comprehensive loss
|
$
|
(110,181
|
)
|
$
|
(83,163
|
)
|
$
|
(61,497
|
)
|
|||
See accompanying notes.
F-5
AKCEA THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
Years Ended December 31, 2017, 2016 and 2015
(In thousands)
|
Convertible
Preferred Stock
|
Common Stock
|
Additional
Paid In
|
Accumulated
Other
Comprehensive
|
Accumulated
|
Total
Stockholders'
|
|||||||||||||||||||||||||||
|
Description
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
Loss
|
Deficit
|
Equity (Deficit)
|
||||||||||||||||||||||||
|
Balance at December 31, 2014
|
—
|
$
|
—
|
—
|
$
|
—
|
$
|
31,602
|
$
|
—
|
$
|
(30,023
|
)
|
$
|
1,579
|
|||||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
—
|
—
|
(61,422
|
)
|
(61,422
|
)
|
||||||||||||||||||||||
|
Ionis investment in Akcea
|
—
|
—
|
—
|
—
|
8,689
|
—
|
—
|
8,689
|
||||||||||||||||||||||||
|
Change in unrealized losses, net of tax
|
—
|
—
|
—
|
—
|
—
|
(75
|
)
|
—
|
(75
|
)
|
||||||||||||||||||||||
|
Issuance of Series A convertible preferred stock
|
28,885
|
100,000
|
—
|
—
|
—
|
—
|
—
|
100,000
|
||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
—
|
—
|
6,496
|
—
|
—
|
6,496
|
||||||||||||||||||||||||
|
Balance at December 31, 2015
|
28,885
|
$
|
100,000
|
—
|
$
|
—
|
$
|
46,787
|
$
|
(75
|
)
|
$
|
(91,445
|
)
|
$
|
55,267
|
||||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
—
|
—
|
(83,217
|
)
|
(83,217
|
)
|
||||||||||||||||||||||
|
Change in unrealized gains, net of tax
|
—
|
—
|
—
|
—
|
—
|
75
|
—
|
75
|
||||||||||||||||||||||||
|
Currency translation adjustment
|
—
|
—
|
—
|
—
|
—
|
(21
|
)
|
—
|
(21
|
)
|
||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
—
|
—
|
10,149
|
—
|
—
|
10,149
|
||||||||||||||||||||||||
|
Balance at December 31, 2016
|
28,885
|
$
|
100,000
|
—
|
$
|
—
|
$
|
56,936
|
$
|
(21
|
)
|
$
|
(174,662
|
)
|
$
|
(17,747
|
)
|
|||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
—
|
—
|
(109,751
|
)
|
(109,751
|
)
|
||||||||||||||||||||||
|
Change in unrealized gains (losses), net of tax
|
—
|
—
|
—
|
—
|
—
|
(337
|
)
|
—
|
(337
|
)
|
||||||||||||||||||||||
|
Currency translation adjustment
|
—
|
—
|
—
|
—
|
—
|
(93
|
)
|
—
|
(93
|
)
|
||||||||||||||||||||||
|
Conversion of convertible preferred stock to common stock
|
(28,885
|
)
|
(100,000
|
)
|
28,885
|
29
|
99,971
|
—
|
—
|
—
|
||||||||||||||||||||||
|
Initial public offering of common stock, net of commissions, underwriting discounts and offering costs
|
—
|
—
|
17,969
|
18
|
132,273
|
—
|
—
|
132,291
|
||||||||||||||||||||||||
|
Issuance of common stock in connection with conversion of line of credit with Ionis Pharmaceuticals Inc. together with accrued interest
|
—
|
—
|
13,438
|
14
|
107,717
|
—
|
—
|
107,731
|
||||||||||||||||||||||||
|
Issuance of common stock in connection with private placement
|
—
|
—
|
6,250
|
6
|
49,994
|
—
|
—
|
50,000
|
||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
—
|
—
|
17,539
|
—
|
—
|
17,539
|
||||||||||||||||||||||||
|
Balance at December 31, 2017
|
—
|
$
|
—
|
66,542
|
$
|
67
|
$
|
464,430
|
$
|
(451
|
)
|
$
|
(284,413
|
)
|
$
|
179,633
|
||||||||||||||||
See accompanying notes.
F-6
AKCEA THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Operating activities:
|
||||||||||||
|
Net loss
|
$
|
(109,751
|
)
|
$
|
(83,217
|
)
|
$
|
(61,422
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||||||
|
Depreciation
|
108
|
12
|
—
|
|||||||||
|
Amortization of licenses
|
120
|
119
|
119
|
|||||||||
|
Amortization of premium on investments, net
|
499
|
170
|
18
|
|||||||||
|
Non-cash interest expense for line of credit with Ionis Pharmaceuticals, Inc.
|
1,731
|
—
|
—
|
|||||||||
|
Non-cash sublicensing expense
|
33,394
|
—
|
—
|
|||||||||
|
Stock-based compensation expense
|
17,539
|
10,149
|
6,496
|
|||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Contracts receivable
|
(5,413
|
)
|
—
|
—
|
||||||||
|
Other current and long-term assets
|
(1,761
|
)
|
64
|
(286
|
)
|
|||||||
|
Accounts payable
|
1,905
|
(54
|
)
|
239
|
||||||||
|
Payable to Ionis Pharmaceuticals, Inc.
|
(43,385
|
)
|
15,157
|
9,198
|
||||||||
|
Accrued compensation
|
1,578
|
1,582
|
923
|
|||||||||
|
Deferred rent
|
(15
|
)
|
20
|
35
|
||||||||
|
Accrued liabilities
|
6,587
|
637
|
405
|
|||||||||
|
Income taxes payable
|
1,789
|
—
|
—
|
|||||||||
|
Deferred revenue
|
58,885
|
—
|
—
|
|||||||||
|
Net cash used in operating activities
|
(36,190
|
)
|
(55,361
|
)
|
(44,275
|
)
|
||||||
|
Investing activities:
|
||||||||||||
|
Purchases of short-term investments
|
(301,377
|
)
|
(16,638
|
)
|
(35,975
|
)
|
||||||
|
Proceeds from sale of short-term investments
|
98,778
|
51,464
|
960
|
|||||||||
|
Purchases of property, plant and equipment
|
(9
|
)
|
(179
|
)
|
(10
|
)
|
||||||
|
Net cash (used in) provided by investing activities
|
(202,608
|
)
|
34,647
|
(35,025
|
)
|
|||||||
|
Financing activities:
|
||||||||||||
|
Proceeds from issuance of Series A convertible preferred stock to Ionis Pharmaceuticals, Inc.
|
—
|
—
|
100,000
|
|||||||||
|
Capital contribution from Ionis Pharmaceuticals, Inc.
|
—
|
—
|
8,689
|
|||||||||
|
Proceeds from issuance of common stock, net of underwriters' discounts
|
135,438
|
—
|
—
|
|||||||||
|
Proceeds from sale of common stock to Novartis in private placement
|
50,000
|
—
|
—
|
|||||||||
|
Proceeds from line of credit from Ionis Pharmaceuticals, Inc.
|
106,000
|
—
|
—
|
|||||||||
|
Offering costs paid
|
(2,037
|
)
|
(818
|
)
|
—
|
|||||||
|
Net cash provided by (used in) financing activities
|
289,401
|
(818
|
)
|
108,689
|
||||||||
|
Effect of exchange rates on cash
|
(93
|
)
|
—
|
—
|
||||||||
|
Net increase (decrease) in cash and cash equivalents
|
50,510
|
(21,532
|
)
|
29,389
|
||||||||
|
Cash and cash equivalents at beginning of period
|
7,857
|
29,389
|
—
|
|||||||||
|
Cash and cash equivalents at end of period
|
$
|
58,367
|
$
|
7,857
|
29,389
|
|||||||
|
Supplemental disclosures of non-cash financing activities:
|
||||||||||||
|
Unpaid deferred offering costs
|
$
|
—
|
$
|
291
|
—
|
|||||||
|
Conversion of preferred stock to common stock upon initial public offering
|
$
|
100,000
|
—
|
—
|
||||||||
|
Conversion of line of credit from Ionis Pharmaceuticals, Inc. into common stock
|
$
|
107,731
|
$
|
—
|
—
|
|||||||
In conjunction with our initial public offering (Note 9), the line of credit with Ionis Pharmaceuticals Inc., together with accrued interest, totaling $107.7 million was converted into 13,438,339 shares of our common stock and all of the Series A convertible preferred stock was converted into 28,884,540 shares of our common stock.
See accompanying notes.
F-7
AKCEA THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
| 1. |
Organization and Significant Accounting Policies
|
Basis of Presentation
The consolidated financial statements include the accounts of Akcea Therapeutics, Inc. ("we," "our," and "us") and our wholly owned subsidiaries: Akcea Therapeutics UK Ltd., or Akcea UK (formed in August 2016), Akcea Intl Ltd., or Akcea Intl (formed in February 2017), Akcea Therapeutics Canada, Inc., or Akcea Canada (formed in May 2017), Akcea Therapeutics France SAS, or Akcea France (formed in September 2017), Akcea Therapeutics Germany GmbH, or Akcea Germany (formed in December 2017), and Akcea Therapeutics Securities Corporation, or Akcea Securities Corp. (formed in December 2017). All intercompany transactions and balances were eliminated in consolidation.
Organization and Business Activity
We were incorporated in Delaware in December 2014. We were organized by Ionis Pharmaceuticals, Inc., or Ionis, to focus on developing and commercializing drugs to treat patients with serious cardiometabolic diseases caused by lipid disorders. On July 19, 2017, we completed our initial public offering, or IPO. As of December 31, 2017, Ionis owns approximately 68% of our common stock and is our majority shareholder. Prior to our IPO, we were wholly owned by Ionis.
Revenue Recognition
We recognize revenue when we have satisfied all contractual obligations and we are reasonably assured of collecting the resulting receivable. We may be entitled to bill our customers and receive payment from our customers in advance of recognizing the revenue. In the instances in which we receive payment from our customers in advance of recognizing revenue, we will include the amounts of unrecognized revenue in deferred revenue on our consolidated balance sheet.
Research and development revenue under collaborative agreements
Arrangements with multiple deliverables
Our strategic collaboration, option and license agreement, or collaboration agreement, with Novartis Pharma AG, or Novartis, which we entered into in January 2017, contains multiple elements, or deliverables, including options to obtain licenses to drugs, research and development services and manufacturing services. Therefore, we accounted for the collaboration under the multiple deliverables guidance.
Multiple agreements
When we enter into separate agreements at or near the same time with the same partner, we must first evaluate such agreements to determine whether they should be accounted for individually as distinct arrangements or whether the separate agreements are, in substance, a single multiple element arrangement. We evaluate whether the negotiations are conducted jointly as part of a single negotiation, whether the deliverables are interrelated or interdependent, whether fees in one arrangement are tied to performance in another arrangement, and whether elements in one arrangement are essential to another arrangement. Our evaluation involves significant judgment to determine whether a group of agreements might be so closely related that they are, in effect, part of a single arrangement. For example, in the first quarter of 2017, we and Ionis entered into two separate agreements with Novartis at the same time: a collaboration agreement and a stock purchase agreement, or SPA.
We entered into the collaboration agreement with Novartis to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Under the collaboration agreement, we received a $75.0 million upfront payment. For each drug, we are responsible for completing a Phase 2 program, conducting an end-of-Phase 2 meeting with the U.S. Food and Drug Administration, or FDA, and delivering active pharmaceutical ingredient, or API. Under the collaboration agreement, Novartis has an exclusive option to further develop and commercialize each of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. If Novartis exercises an option for one of these drugs, it will pay us a license fee and will assume all further global development, regulatory and commercialization activities for the licensed drug. We are also eligible to receive a development milestone payment, milestone payments if Novartis achieves pre-specified regulatory milestones, commercial milestones and tiered royalties on net sales from each drug under the collaboration.
Under the SPA, Novartis purchased 1.6 million shares of Ionis' common stock for $100.0 million in the first quarter of 2017 and paid a premium over the weighted average trading price at the time of purchase. Additionally in July 2017, Novartis purchased $50.0 million of our common stock in a separate private placement concurrent with the completion of our IPO. Our IPO is discussed in Note 9, Initial Public Offering.
F-8
We evaluated the Novartis agreements to determine whether we should treat the agreements separately or as a single arrangement. We considered that the agreements were negotiated concurrently and in contemplation of one another. Additionally, the same individuals were involved in the negotiations of both agreements. Based on these facts and circumstances, we concluded that we should treat both agreements as a single arrangement, which we refer to as the Novartis collaboration. We evaluated the provisions of the agreements on a combined basis.
Identifying deliverables and units of accounting
We evaluate the deliverables in a collaboration agreement to determine whether they meet the criteria to be accounted for as separate units of accounting or whether they should be combined with other deliverables and accounted for as a single unit of accounting. When the delivered items in an arrangement have "stand-alone value" to the customer, we will account for the deliverables as separate units of accounting. Delivered items have stand-alone value if they are sold separately by any vendor or the customer could resell the delivered items on a stand-alone basis. For example, our Novartis collaboration and SPA have multiple elements. We evaluated the deliverables in the Novartis collaboration when we entered into the agreements and determined that certain deliverables have stand-alone value.
We identified the following four separate units of accounting under the collaboration, each with stand-alone value:
| ● |
Development activities for AKCEA-APO(a)-LRx;
|
| ● |
Development activities for AKCEA-APOCIII-LRx;
|
| ● |
API for AKCEA-APO(a)-LRx; and
|
| ● |
API for AKCEA-APOCIII-LRx.
|
The development activities and the supply of API each have stand-alone value because Novartis or another third party could provide these items without our assistance.
Measurement and allocation of arrangement consideration
Our Novartis collaboration provides for various types of payments to us including upfront payments, milestone payments, licensing fees, royalties on product sales and payments for the purchase of common stock. We first evaluated the total consideration under both the collaboration agreement and SPA and determined how much of the total consideration was attributable to elements that we are delivering under the collaboration.
We determined that our portion of the allocable arrangement consideration for the Novartis collaboration was $108.4 million, comprised of the following:
| ● |
$75.0 million from the upfront payment we received;
|
| ● |
$28.4 million for the premium paid by Novartis, which represents the excess of the fair value Ionis received from Novartis' purchase of Ionis' stock at a premium in the first quarter of 2017; and
|
| ● |
$5.0 million for the premium Novartis would have paid to purchase Ionis' stock if we did not complete our IPO within 15 months of the inception of the agreement.
|
We are recognizing the $75.0 million upfront payment plus the premium paid by Novartis from its purchase of Ionis' stock and the premium associated with Novartis' obligation to purchase Ionis' stock if we did not complete our IPO because we are the party providing the services and API under the collaboration agreement.
We initially allocated the amount of consideration that was fixed or determinable at the time the agreement was entered into and excluded contingent consideration. We allocated the consideration to each unit of accounting based on the relative selling price of each deliverable. We used the following hierarchy of values to estimate the selling price of each deliverable: (i) vendor-specific objective evidence of fair value; (ii) third-party evidence of selling price; and (iii) best estimate of selling price, or BESP. BESP reflects our best estimate of what the selling price would be if we regularly sold the deliverable on a stand-alone basis. We are recognizing the revenue allocated to each unit of accounting as we deliver the related goods or services. If we determine that we should treat certain deliverables as a single unit of accounting, then we will recognize the revenue ratably over our estimated period of performance.
We allocated the consideration based on the relative BESP of each unit of accounting. We estimated the selling price of the development services over the expected period during which we will perform these services. The significant inputs we used to determine the selling price of the development services included:
| ● |
The number of internal hours we will spend performing these services;
|
| ● |
The estimated cost of the work we will perform;
|
| ● |
The estimated cost of work that we will contract with third parties to perform; and
|
| ● |
The estimated cost of API we will use.
|
F-9
For purposes of determining BESP of the services we will perform and the API we will deliver under our Novartis collaboration, accounting guidance required us to include a markup for a reasonable profit margin.
Based on the units of accounting under the Novartis collaboration, we allocated the $108.4 million of allocable consideration as follows:
| ● |
$64.0 million for development services for AKCEA-APO(a)-LRx;
|
| ● |
$40.1 million for development services for AKCEA-APOCIII-LRx;
|
| ● |
$1.5 million for the delivery of AKCEA-APO(a)-LRx API; and
|
| ● |
$2.8 million for the delivery of AKCEA-APOCIII-LRx API.
|
Timing of revenue recognition
We recognize revenue as we deliver each item under our Novartis collaboration as we provide services and the related revenue is realizable and earned. We also recognize revenue over time. Our Novartis collaboration agreement includes a development project plan outlining the activities the agreement requires each party to perform during the collaboration. We estimated our period of performance when the agreement was entered into because the agreement did not clearly define such information. We then recognize revenue for development services ratably over such period. We made estimates of our time to complete our obligations under our Novartis collaboration agreement and in certain instances the timing of satisfying these obligations may change as the development plans for our drugs progress. If our estimates and judgments change over the course of the Novartis collaboration agreement, it may affect the timing and amount of revenue that we will recognize in future periods. Any changes in estimates are recognized on a prospective basis.
The following are the periods over which we are recognizing revenue for each of our units of accounting under the Novartis collaboration:
| ● |
We are recognizing the amount attributed to the development services for AKCEA-APO(a)-LRx over the period of time we are performing the services, currently estimated to be through November 2018;
|
| ● |
We are recognizing the amount attributed to the development services for AKCEA-APOCIII-LRx over the period of time we are performing the services, currently estimated to be through June 2019;
|
| ● |
We will recognize the amount attributed to the AKCEA-APO(a)-LRx API supply when we deliver API to Novartis; and
|
| ● |
We recognized the amount attributed to the AKCEA-APOCIII-LRx API supply when we delivered API to Novartis in 2017.
|
Milestone payments
Our Novartis collaboration agreement contains contractual milestone payments that relate to the achievement of pre-specified development, regulatory and commercialization events. These three categories of milestone events - development, regulatory and commercialization - reflect the three stages of the life-cycle of our drugs, which we describe in more detail in the following paragraphs.
The designation of a development candidate is the first stage in the life-cycle of our drugs. A development candidate is a chemical compound that has demonstrated the necessary safety and efficacy in preclinical animal studies to warrant further study in humans.
During the first step of the development stage, we or our partner study our drugs in Investigational New Drug, or IND-enabling studies, which are animal studies intended to support an IND application and/or the foreign equivalent. An approved IND allows us or our partners to study our development candidate in humans. If the regulatory agency approves the IND, we or our partners initiate Phase 1 clinical trials in which we typically enroll a small number of healthy volunteers to ensure the development candidate is safe for use in patients. If we or our partners determine that a development candidate is safe based on Phase 1 data, we or our partners initiate Phase 2 studies that are generally larger studies in patients with the primary intent of determining the preliminary efficacy and safety of the development candidate.
The final step in the development stage is Phase 3 studies to gather the necessary safety and efficacy data to request marketing authorization from the FDA and/or foreign equivalents. Phase 3 studies typically involve large numbers of patients and can take up to three to five years to complete.
If the data gathered during the trials demonstrates acceptable safety and efficacy results, we or our partners will submit an application to the FDA and/or its foreign equivalents for marketing authorization. This stage of the drug's life-cycle is the regulatory stage.
F-10
If a drug achieves marketing authorization, it moves into the commercialization stage, during which we or our partners will market and sell the drug to patients. Even in those situations in which our partner is ultimately responsible for marketing and selling the partnered drug, our efforts to develop a drug that is safe, effective and reliable contributes significantly to our partner's ability to successfully sell the drug. The FDA and its foreign equivalents have the authority to impose significant restrictions on an approved drug through the product label and on advertising, promotional and distribution activities. Therefore, our efforts designing and executing the necessary animal and human studies are critical to obtaining claims in the product label from the regulatory agencies that would allow us or our partners to successfully commercialize our drug. Further, the patent protection afforded our drugs as a result of our initial patent applications and related prosecution activities in the United States and foreign jurisdictions are critical to our partner's ability to sell our drugs without competition from generic drugs. The potential sales volume of an approved drug is dependent on several factors including the size of the patient population, the market penetration of the drug and the price charged for the drug.
The milestone events contained in our Novartis collaboration agreement coincide with the progression of our drugs from development, to marketing authorization and then to commercialization. The process of successfully discovering a new development candidate, having it approved and ultimately sold for a profit is highly uncertain. As such, the milestone payments we may earn from our partners involve a significant degree of risk to achievement. Therefore, as a drug progresses through the stages of its life-cycle, the value of the drug generally increases.
Development milestones in our Novartis collaboration agreement or potential future collaborations may include the following types of events:
| ● |
Designation of a development candidate. Following the designation of a development candidate, IND-enabling animal studies for a new development candidate generally take 12 to 18 months to complete;
|
| ● |
Initiation of a Phase 1 clinical trial. Generally, Phase 1 clinical trials take one to two years to complete;
|
| ● |
Initiation or completion of a Phase 2 clinical trial. Generally, Phase 2 clinical trials take one to three years to complete; and
|
| ● |
Initiation or completion of a Phase 3 clinical trial. Generally, Phase 3 clinical trials take three to five years to complete.
|
Regulatory milestones in our Novartis collaboration agreement or potential future collaborations may include the following types of events:
| ● |
Filing of regulatory applications for marketing authorization such as a New Drug Application, or NDA, in the United States or a Marketing Authorization Application, or MAA, in Europe. Generally, it takes six to twelve months to prepare and submit regulatory filings.
|
| ● |
Marketing authorization in a major market, such as the United States, Europe or Japan. Generally it takes one to two years after an application is submitted to obtain authorization from the applicable regulatory agency.
|
Commercialization milestones in our Novartis agreement or potential future collaborations may include the following types of events:
| ● |
First commercial sale in a particular market, such as in the United States or Europe.
|
| ● |
Product sales in excess of a pre-specified threshold, such as annual sales exceeding $1 billion. The amount of time to achieve this type of milestone depends on several factors including, but not limited to, the dollar amount of the threshold, the pricing of the product and the pace at which customers begin using the product.
|
We will assess whether a substantive milestone exists at the inception of the collaboration agreement. When a substantive milestone is achieved, we will recognize revenue related to the milestone payment immediately. In evaluating if a milestone is substantive, we will consider whether:
| ● |
Substantive uncertainty exists as to the achievement of the milestone event at the inception of the arrangement;
|
| ● |
The achievement of the milestone involves substantive effort and can only be achieved based in whole or in part on the performance or the occurrence of a specific outcome resulting from its performance;
|
| ● |
The amount of the milestone payment appears reasonable either in relation to the effort expended or to the enhancement of the value of the delivered items;
|
| ● |
There is no future performance required to earn the milestone payment; and
|
| ● |
The consideration is reasonable relative to all deliverables and payment terms in the arrangement.
|
If any of these conditions are not met, we will not consider the milestone to be substantive and we will defer recognition of the milestone payment and recognize it as revenue over the estimated period of performance, if any. We have determined that all milestones under our Novartis collaboration are substantive milestones.
F-11
Option to license
When we have a multiple element arrangement that includes an option to obtain a license, we will evaluate if the option is a deliverable at the inception of the arrangement. We do not consider the option to be a deliverable if we conclude that it is substantive and not priced at a significant and incremental discount. We will consider an option substantive if, at the inception of the arrangement, we are at risk as to whether the collaboration partner will choose to exercise its option to obtain the license. In those circumstances, we do not include the associated license fee in the allocable consideration at the inception of the agreement. Rather, we account for the license fee when our partner exercises its option. Under the Novartis collaboration, we concluded that the option to license is a substantive option. Therefore, we did not include any amounts in the initial allocable consideration at the inception of the collaboration. We will recognize any future consideration for the exercise of an option to license a drug under our Novartis agreement in full in the period in which the option is exercised.
Refer to Note 8, Strategic Collaboration with Novartis, where we discuss our Novartis collaboration agreement in more detail.
Research and Development Expenses
Our research and development expenses include wages, benefits, facilities, supplies, external services, clinical study and manufacturing costs and other expenses that are directly related to our research and development activities. We expense research and development costs as we incur them. We do not conduct research activities and no such costs are included in these amounts.
If we make payments for research and development services prior to the services being rendered, we record those amounts as prepaid assets on our balance sheet and we expense them as the services are provided.
Sublicensing Expenses
We incur sublicense expenses under our development, commercialization and license agreement and services agreement with Ionis related to the drugs we have licensed under the agreement. We include our sublicense fee expenses in our research and development expenses on our consolidated results of operations since the applicable drugs are not yet approved for marketing. We recognize sublicense fee expenses in the period they are incurred. For example, in the first quarter of 2017, we incurred $48.4 million of sublicense fee expenses related to our collaboration with Novartis, of which $33.4 million of these expenses were non-cash and were related to the premium Novartis paid and the potential premium Novartis would have paid on Ionis' stock if we did not complete our IPO. Under the Novartis collaboration, we will recognize $108.4 million of revenue over the period of our performance, which began in February 2017. In 2017, we recognized $48.4 million of sublicensing expense, all of which was recognized in the first quarter of 2017. The $48.4 million is comprised of the following:
| ● |
$15.0 million for the portion of the $75.0 million upfront payment we received upon initiating the Novartis collaboration that we paid in cash to Ionis;
|
| ● |
$28.4 million for the premium paid by Novartis for its purchase of Ionis' stock in the first quarter of 2017, which is a non-cash expense. We determined the fair value of the premium by calculating the stated premium and applying a discount for lack of marketability because Ionis initially issued unregistered shares to Novartis; and
|
| ● |
$5.0 million for the premium associated with Novartis' obligation to purchase Ionis' stock if we did not complete our IPO, which is a non-cash expense. We determined the fair value of the potential premium at the inception of the collaboration by calculating the value of the future premium based upon the stated premium, adjusting for the probability of us completing an IPO by the 15-month anniversary of the SPA and applying a discount for lack of marketability because Ionis would have issued unregistered shares to Novartis if it purchased Ionis' common stock.
|
We will pay 50% of all future license fees, milestone payments and royalties we receive to Ionis as a sublicense fee.
Estimated Liability for Research and Development Costs
We record accrued liabilities related to expenses for which vendors or service providers have not yet billed us. These liabilities are for products or services that we have received and primarily relate to ongoing nonclinical and clinical studies. These costs primarily include third-party clinical management costs, laboratory and analysis costs, toxicology studies and investigator grants. We have drugs in concurrent nonclinical and clinical studies at several sites throughout the world. To ensure that we have adequately provided for ongoing nonclinical and clinical research and development costs during the period in which we incur such costs, we maintain an accrual to cover these costs. We update our estimate for this accrual on at least a quarterly basis. The assessment of these costs is a subjective process that requires judgment. Upon settlement, these costs may differ materially from the amounts accrued in our consolidated financial statements. Our historical accrual estimates have not been materially different from our actual amounts.
F-12
License
As part of our founding in 2015, we obtained an exclusive license from Ionis for specific patents that Ionis owns and maintains related to our drug pipeline. We recorded our license from Ionis as a capital contribution using the carryover basis of Ionis' historical cost for the related patents. For comparative purposes, we have assumed that we obtained the license as of January 1, 2014. We are amortizing our capitalized license over its estimated useful life, which is the term of the underlying individual patents owned by Ionis. The weighted average remaining amortizable life of our license from Ionis is 12.2 years at December 31, 2017. The gross value of the license recorded on our consolidated balance sheet at December 31, 2017 and 2016 was $1.7 million. Accumulated amortization related to this license was $478,000, and $358,000 for the periods ended December 31, 2017 and 2016, respectively. Amortization expense related to this license was $120,000, $119,000 and $119,000 for the years ended December 31, 2017, 2016 and 2015, respectively.
We estimated amortization expense for our license from Ionis in each of the next five years is as follows:
|
Years Ending December 31, (in thousands)
|
Amortization
|
|||
|
2018
|
$
|
120
|
||
|
2019
|
$
|
120
|
||
|
2020
|
$
|
120
|
||
|
2021
|
$
|
118
|
||
|
2022
|
$
|
112
|
||
For additional detail of Akcea's license agreement with Ionis see Note 4, Development, Commercialization and License Agreement and Services Agreement with Ionis.
Concentration of Credit Risk
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash, cash equivalents, short-term investments and receivables. We place our cash, cash equivalents and short-term investments with reputable financial institutions. We primarily invest our excess cash in commercial paper and debt instruments of the U.S. Treasury, financial institutions, corporations and U.S. government agencies with strong credit ratings and an investment grade rating at or above A-1, P-1 or F-1 by Moody's, Standard & Poor's, or S&P, or Fitch, respectively. We have established guidelines relative to diversification and maturities that maintain safety and liquidity. We periodically review and modify these guidelines to maximize trends in yields and interest rates without compromising safety and liquidity.
Cash Equivalents and Short-Term Investments
We consider all liquid investments with maturities of three months or less when we purchase them to be cash equivalents. Our short-term investments have initial maturities of greater than three months from date of purchase. We classify our short-term investments as available-for-sale and we carry them at fair market value based upon prices for identical or similar items on the last day of the fiscal period. We record unrealized gains and losses as a separate component of comprehensive income (loss) and we include net realized gains and losses in investment income (expense) on our consolidated statement of operations. We use the specific identification method to determine the cost of securities sold.
Property, Plant and Equipment
We carry our leasehold improvements and equipment at cost and depreciate it using the straight-line method over its estimated useful life. At December 31, 2017 and 2016, our leasehold improvements consisted of improvements to our office facility that we are amortizing over the shorter of the lease term or the estimated useful life of the asset. At December 31, 2017 and 2016, our equipment consisted of computer equipment that we are depreciating over three years.
Fair Value of Financial Instruments
We have estimated the fair value of our financial instruments. The amounts reported for cash equivalents, accounts payable and accrued expenses approximate fair value because of their short maturities. We report our investment securities at their estimated fair value based on quoted market prices for identical or similar instruments.
F-13
Operating Leases
We lease our office space in a building in Cambridge, Massachusetts under a non-cancelable operating lease, which commenced in April 2015 and was subsequently amended and expanded in February 2016 and March 2017. A portion of our lease currently expires in July 2018 and a portion of it expires in April 2020.
Annual future minimum payments under our operating lease for our office space in Cambridge, Massachusetts are as follows (in thousands) for each year indicated:
|
Cambridge
Office Space
Operating Lease
|
||||
|
2018
|
$
|
486
|
||
|
2019
|
250
|
|||
|
2020
|
84
|
|||
|
Total minimum payments
|
$
|
820
|
||
Rent expense for the year ended December 31, 2017, 2016 and 2015 was $677,000, $435,000 and $183,000, respectively. We recognize rent expense on a straight-line basis over the lease term for the lease of our office space, which resulted in a deferred rent balance of $39,000 and $54,000 at December 31, 2017 and 2016, respectively.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires our management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ from those estimates.
Translation of Foreign Currency
For our foreign subsidiaries that report in a functional currency other than United States dollars, we translate their assets and liabilities into United States dollars using the exchange rate at the balance sheet date. We translate revenue and expenses at the monthly average exchange rates for the period. We translate capital accounts at the historical exchange rate in effect at the date of the transaction. We include foreign currency translation adjustments as a component of accumulated other comprehensive loss within the consolidated statements of comprehensive loss.
Segment Information
We operate as a single segment because our chief decision maker reviews operating results on an aggregate basis and manages our operations as a single operating segment.
Stock-Based Compensation Expense
We measure stock-based compensation expense for equity-classified awards, principally related to stock options, restricted stock units, or RSUs, and stock purchase rights under our employee stock purchase plan, or ESPP, based on the estimated fair value of the award on the date of grant. We recognize the value of the portion of the award that we ultimately expect to vest as stock-based compensation expense over the requisite service period in our consolidated statements of operations. We reduce stock-based compensation expense for estimated forfeitures at the time of grant and revise the expense in subsequent periods if actual forfeitures differ from those estimates.
We value our stock option awards and stock purchase rights under our ESPP using the Black-Scholes model. The determination of the grant date fair value of options using an option pricing model is affected principally by our estimated common stock fair value and requires us to make a number of other assumptions, including: the expected life of the option, the volatility of the underlying stock, the risk-free interest rate and expected dividends.
The fair value of RSUs is based on the market price of our common stock on the date of grant. The RSUs we have granted vest annually over a four-year period.
Prior to December 2015, Ionis granted our employees options to purchase shares of Ionis' common stock, or Ionis options. In December 2015, we granted our employees holding Ionis options additional options to purchase shares of our common stock, or Akcea options.
F-14
We determined the stock-based compensation expense for the Ionis options at the date of grant and recognized compensation expense over the vesting period of the Ionis options. In December 2015, we accounted for the issuance of the Akcea options as a modification to the original grant of the Ionis options because the grant of the Ionis options and Akcea options essentially represented a single stock award as the exercisability provisions of the Ionis options and Akcea options grants were interrelated and mutually exclusive. The total compensation expense measured on the modification date was the sum of the grant date fair value of the Ionis options plus any incremental compensation cost resulting from the grant of the Akcea options.
In 2016, we began concurrently granting Ionis options and Akcea options to our employees. Because the exercisability provisions of the awards are interrelated and mutually exclusive as described above, the fair values of the Ionis options and the Akcea options were determined on the date of grant and the option with the greater fair value was recognized over the vesting period of the awards. In 2017, we no longer concurrently granted Ionis and Akcea options. Our board of directors only receive grants under the Akcea option plan.
Following our IPO, we no longer grant Ionis options to our employees. Under the terms of the Ionis options, when we completed our IPO, the Ionis options our employees were holding were terminated. The termination of the Ionis options was determined not to be a modification, as the options were terminated based upon the existing contractual terms of the option agreements. As such, we will continue to recognize expense based on the valuation that was determined upon the grant date for options issued in 2016 or the modification date for options issued in 2015 and 2017.
The fair value of stock options granted under our 2015 Equity Incentive Plan is based on the fair value of our common stock on the date of grant. The fair value of stock options granted under the Ionis 2011 Equity Incentive Plan is based on the fair value of Ionis' common stock on the date of grant. Options granted to employees vest over a four-year period, with 25 percent exercisable at the end of one year from the date of the grant and the balance vesting ratably, on a monthly basis, thereafter and have a term of ten years. Options granted to directors vest annually over a four-year period and have a term of ten years.
See Note 6, Stockholders' Equity (Deficit), for additional information regarding our stock-based compensation plans.
Accumulated Other Comprehensive Loss
Accumulated other comprehensive loss is comprised of unrealized gains and losses on investments, net of taxes and currency translation adjustments. The following table summarizes changes in accumulated other comprehensive loss for the years ended December 31, 2017, 2016 and 2015 (in thousands):
|
Years Ended December 31,
|
||||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Beginning balance accumulated other comprehensive loss
|
$
|
(21
|
)
|
$
|
(75
|
)
|
$
|
—
|
||||
|
Unrealized gains (losses) on investments, net of tax (1)
|
(337
|
)
|
75
|
(75
|
)
|
|||||||
|
Currency translation adjustment
|
(93
|
)
|
(21
|
)
|
—
|
|||||||
|
Net other comprehensive income (loss)
|
(430
|
)
|
54
|
(75
|
)
|
|||||||
|
Ending balance accumulated other comprehensive loss
|
$
|
(451
|
)
|
$
|
(21
|
)
|
$
|
(75
|
)
|
|||
| (1) |
There was no tax benefit for other comprehensive income (loss) for the years ended December 31, 2017, 2016 and 2015.
|
Income Taxes
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act of 2017 (the "Tax Act"). The Tax Act makes broad and complex changes to the U.S. tax code. The changes include, but are not limited to, reducing the U.S. federal corporate tax rate from 35% to 21%, imposing a mandatory one-time transition tax on certain unrepatriated earnings of foreign subsidiaries, introducing bonus depreciation that will allow for full expensing of qualified property, eliminating the corporate alternative minimum tax ("AMT") and changing how existing AMT credits can be realized.
The SEC staff issued Staff Accounting Bulletin No. 118 ("SAB 118") to address the application of U.S. GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the Tax Act.
Prior to the completion of our IPO we filed our tax returns on a consolidated and combined basis with Ionis for federal and state income tax purposes, respectively. For financial statement purposes when we are required to file on a consolidated or combined basis, we calculate our income tax amounts, including net operating losses and tax credit carryforwards, using a separate return methodology which determines income taxes as if we were a separate taxpayer from Ionis. Effective July 19, 2017, the date of our IPO, we are no longer included in the consolidated federal income tax return with Ionis. We determined the amount of federal tax attributes, primarily net operating losses and tax credit carryforwards that transferred to us upon deconsolidation from Ionis. We are still required to file most of our state tax returns on a consolidated or combined basis with Ionis. Therefore, for financial statement purposes we calculated our state income tax amounts using the separate return method. We have not yet determined the amount of state tax attributes, primarily net operating losses and tax credit carryforwards, which we would retain if we were to deconsolidate for state tax purposes from Ionis.
F-15
We account for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in our financial statements or tax returns. In addition, deferred tax assets are recorded for the future benefit of utilizing net operating losses and research and development credit carry forwards. Valuation allowances are provided when necessary to reduce deferred tax assets to the amount expected to be realized.
We apply the authoritative accounting guidance prescribing a threshold and measurement attribute for the financial recognition and measurement of a tax position taken or expected to be taken in a tax return. We recognize liabilities for uncertain tax positions based on a two-step process. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation settlement. The second step is to estimate and measure the tax benefit as the largest amount that is more than 50% likely to be realized upon ultimate settlement.
We recognize interest and penalties related to unrecognized tax benefits within the income tax expense line in the accompanying consolidated statements of operations. Accrued interest and penalties are included within other long-term liabilities in the consolidated balance sheets.
Significant judgment is required in evaluating our uncertain tax positions and determining our provision for income taxes. Although we believe our reserves are reasonable, no assurance can be given that the final tax outcome of these matters will not be different from that which is reflected in our historical income tax provisions and accruals. We adjust these reserves for changing facts and circumstances, such as the closing of a tax audit or the refinement of an estimate. To the extent that the final tax outcome of these matters is different than the amounts recorded, such differences may impact the provision for income taxes in the period in which such determination is made.
Significant judgment is also required in determining any valuation allowance recorded against deferred tax assets. In assessing the need for a valuation allowance, we consider all available evidence, including scheduled reversal of deferred tax liabilities, past operating results, the feasibility of tax planning strategies and estimates of future taxable income. Estimates of future taxable income are based on assumptions that are consistent with our plans. Assumptions represent management's best estimates and involve inherent uncertainties and the application of management's judgment. Should actual amounts differ from our estimates, the amount of our tax expense and liabilities could be materially impacted.
We record a valuation allowance to reduce the balance of our net deferred tax assets to the amount we believe is more-likely-than-not to be realized. We have incurred financial statement losses since inception and as a result we have a full valuation allowance recorded against our net deferred tax assets. We regularly assess the future realization of our net deferred tax assets and will reduce the valuation allowance in any such period in which we determine that all, or a portion, of our deferred tax assets are more-likely-than-not to be realized.
We do not provide for a U.S. income tax liability and foreign withholding taxes on undistributed foreign earnings of our foreign subsidiaries. The earnings of non-U.S. subsidiaries are currently expected to be indefinitely reinvested in non-U.S. operations.
Impact of Recently Issued Accounting Standards
In May 2014, the Financial Accounting Standards Board, or FASB, issued accounting guidance on the recognition of revenue from customers. Under this guidance, an entity will recognize revenue when it transfers promised goods or services to customers in an amount that reflects what the entity expects to receive in exchange for the goods or services. This new guidance also requires more detailed disclosures to enable users of the financial statements to understand the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. The guidance as originally issued is effective for annual and interim periods, beginning after December 15, 2016. In July 2015, the FASB issued updated accounting guidance to allow for an optional one-year deferral from the original effective date. As a result, we will adopt this guidance beginning on January 1, 2018. Prior to 2017, we had not generated revenue. In January 2017, we entered into a strategic collaboration agreement with Novartis and began recognizing revenue. We will adopt this guidance on January 1, 2018 under the full retrospective approach, which requires us to recast our prior period amounts in the period of adoption. We have completed an analysis of our collaboration agreement with Novartis and have determined there will not be any material changes to the revenue we have already recognized in our consolidated financial statements.
In January 2016, the FASB issued amended accounting guidance related to the recognition, measurement, presentation and disclosure of certain financial instruments. The amended guidance requires us to measure and record equity investments at fair value, except those accounted for under the equity method of accounting that have a readily determinable fair value, and for us to recognize the changes in fair value in our net income (loss) instead of recognizing unrealized gains and losses through accumulated other comprehensive income, as we currently do under the existing guidance. The amended guidance also changes several disclosure requirements for financial instruments, including the methods and significant assumptions we use to estimate fair value. The guidance is effective for annual and interim periods beginning after December 15, 2017. We will adopt this guidance on January 1, 2018. The adoption of this guidance will not have a material impact on our financial results.
F-16
In February 2016, the FASB issued amended accounting guidance related to lease accounting, which requires us to record all leases with a term longer than one year on our balance sheet. When we record leases on our balance sheet under the new guidance, we will record a liability with a value equal to the present value of payments we will make over the life of the lease and an asset representing the underlying leased asset. The new accounting guidance requires us to determine if any lease we have is an operating or financing lease, similar to current accounting guidance. We will record expense for an operating type lease on a straight-line basis as an operating expense and we will record expense for a finance type lease as interest expense. The new lease standard is effective for annual and interim periods beginning after December 15, 2018, with early adoption permitted. We must adopt the new standard on a modified retrospective basis, which requires us to reflect any leases we have on our consolidated balance sheet for the earliest comparative period presented. We are currently assessing the timing of adoption as well as the effects it will have on our consolidated financial statements and disclosures.
In March 2016, the FASB issued amended guidance to simplify certain aspects of stock-based payment accounting. Under the amended guidance, we will recognize excess tax benefits and tax deficiencies as income tax expense or benefit in our consolidated statement of operations on a prospective basis. As we have a valuation allowance, this change will impact our net operating loss carryforward and the valuation allowance disclosures. Additionally, we will classify excess tax benefits as an operating activity and classify amounts we withhold in shares for the payment of employee taxes as a financing activity on the consolidated statement of cash flows for each period presented. Lastly, the amended guidance allows us to account for forfeitures when they occur or continue to estimate them. We will continue to estimate our forfeitures. The amended stock-based payment standard is effective for annual and interim periods beginning after December 15, 2016, with early adoption permitted in any interim or annual period. We adopted this guidance on January 1, 2017. The amended guidance did not impact our financial results.
In June 2016, the FASB issued guidance that changes the measurement of credit losses for most financial assets and certain other instruments. If we have credit losses, this updated guidance requires us to record allowances for these instruments under a new expected credit loss model. This model requires us to estimate the expected credit loss of an instrument over its lifetime, which represents the portion of the amortized cost basis we do not expect to collect. This change will result in us remeasuring our allowance in each reporting period we have credit losses. The new standard is effective for annual and interim periods beginning after December 15, 2019. Early adoption is permitted for periods beginning after December 15, 2018. When we adopt the new standard, we will make any adjustments to beginning balances through a cumulative-effect adjustment to accumulated deficit on that date. We are currently assessing the timing of adoption as well as the effects it will have on our consolidated financial statements and disclosures.
In May 2017, the FASB issued clarifying guidance related to the accounting for modifications of stock-based payment awards. The new guidance is meant to clarify when modification accounting is required. We early adopted this guidance in our financial statements for the quarter ended June 30, 2017 and it did not have an effect on our consolidated financial statements and disclosures.
| 2. |
Investments
|
As of December 31, 2017, we primarily invested our excess cash in debt instruments of the U.S. Treasury, financial institutions, corporations and U.S. government agencies with strong credit ratings and an investment grade rating at or above A-1, P-1 or F-1 by Moody's, S&P or Fitch, respectively. We have established guidelines relative to diversification and maturities that maintain safety and liquidity. We periodically review and modify these guidelines to maximize trends in yields and interest rates without compromising safety and liquidity.
All of our available-for-sale securities are available to us for use in our current operations. As a result, we categorized all of these securities as current assets even though the stated maturity of some individual securities may be one year or more beyond the balance sheet date.
As of December 31, 2016, we only invested in money market funds which were classified in cash and cash equivalents on our balance sheet.
F-17
The following is a summary of our investments at December 31, 2017 (in thousands):
|
|
Gross Unrealized
|
Estimated
|
||||||||||||||
|
|
Cost
|
Gains
|
Losses
|
Fair Value
|
||||||||||||
|
Available-for-sale securities (1):
|
||||||||||||||||
|
Corporate debt securities
|
$
|
132,434
|
$
|
—
|
$
|
(206
|
)
|
$
|
132,228
|
|||||||
|
Debt securities issued by U.S. government agencies
|
38,135
|
—
|
(59
|
)
|
38,076
|
|||||||||||
|
Total securities with a maturity of one year or less
|
170,569
|
—
|
(265
|
)
|
170,304
|
|||||||||||
|
Corporate debt securities
|
8,267
|
—
|
(35
|
)
|
8,232
|
|||||||||||
|
Debt securities issued by U.S. government agencies
|
23,264
|
—
|
(37
|
)
|
23,227
|
|||||||||||
|
Total securities with a maturity of one to two years
|
31,531
|
—
|
(72
|
)
|
31,459
|
|||||||||||
|
Total available-for-sale securities
|
$
|
202,100
|
$
|
—
|
$
|
(337
|
)
|
$
|
201,763
|
|||||||
_______________________________
| (1) |
Our available-for-sale securities are held at amortized cost.
|
We believe that the decline in value of these securities is temporary and primarily related to the change in market interest rates since purchase. We believe it is more likely than not that we will be able to hold our debt securities to maturity. Therefore, we anticipate a full recovery of our debt securities' amortized cost basis at maturity.
| 3. |
Fair Value Measurements
|
We use a three-tier fair value hierarchy to prioritize the inputs used in our fair value measurements. These tiers include: Level 1, defined as observable inputs such as quoted prices in active markets for identical assets, which includes our money market funds and treasury securities classified as available-for-sale securities; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable, which includes our fixed income securities and commercial paper classified as available-for-sale securities; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring us to develop our own assumptions. We have not historically held any Level 3 investments. Our securities have been classified as Level 1 or Level 2. We obtain the fair value of our Level 2 investments from our custodian bank and from a professional pricing service. We validate the fair value of our Level 2 investments by understanding the pricing model used by the custodian banks or professional pricing service provider and comparing that fair value to the fair value based on observable market prices. We recognize transfers between levels of the fair value hierarchy on the date of the event or change in circumstances that caused the transfer. We did not have any Level 3 investments or liabilities at December 31, 2017 and 2016. For the years ended December 31, 2017 and 2016, there were no transfers between our Level 1 and Level 2 investments.
The following table presents the major security types we held at December 31, 2017 that we regularly measured and carried at fair value. The table segregates each security by the level within the fair value hierarchy of the valuation techniques we utilized to determine the respective securities' fair value (in thousands):
|
|
At
December 31, 2017
|
Quoted Prices
in Active Markets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
|||||||||
|
Cash equivalents (1)
|
$
|
48,430
|
$
|
48,430
|
$
|
—
|
||||||
|
Corporate debt securities (2)
|
140,460
|
—
|
140,460
|
|||||||||
|
Debt securities issued by U.S. government agencies (2)
|
61,303
|
—
|
61,303
|
|||||||||
|
Total
|
$
|
250,193
|
$
|
48,430
|
$
|
201,763
|
||||||
| (1) |
Included in cash and cash equivalents on our consolidated balance sheets.
|
| (2) |
Included in short-term investments on our consolidated balance sheets.
|
At December 31, 2016, the Company held $7.1 million of money market fund investments which are Level 1 investments and are considered cash equivalents.
F-18
4. Development, Commercialization and License Agreement and Services Agreement with Ionis
We entered into a development, commercialization and license agreement and a services agreement in December 2015 with Ionis. The following section summarizes these related party agreements with Ionis.
Development, Commercialization and License Agreement
Our development, commercialization and license agreement, or the license agreement, with Ionis granted exclusive rights to us to develop and commercialize volanesorsen, AKCEA-APO(a)-LRx, AKCEA-APOCIII-LRx, and AKCEA-ANGPTL3-LRx, which are collectively referred to as the Lipid Drugs. As a part of the grant to us from Ionis, Ionis granted an exclusive license to certain patents to develop and commercialize products containing the Lipid Drugs. Ionis also granted us a non-exclusive license to the Ionis antisense platform technology for us to develop and commercialize products containing the Lipid Drugs. Ionis also granted us non-exclusive rights under its manufacturing technology to manufacture the Lipid Drugs in our own facility or at a contract manufacturer. As a part of this agreement both companies agreed not to work with any other parties to develop or commercialize other drugs that are designed to inhibit any of the Lipid Drug targets so long as we are developing or commercializing the Lipid Drugs.
We and Ionis share development responsibilities for the Lipid Drugs. We pay Ionis for the research and development expenses it incurs on our behalf, which include both external and internal expenses. External research and development expenses include costs for contract research organizations, or CROs, costs to conduct nonclinical and clinical studies on our drugs, costs to acquire and evaluate clinical study data, such as investigator grants, patient screening fees and laboratory work, and fees paid to consultants. Internal research and development expenses include costs for the work that Ionis' research and development employees perform for us. Ionis charges us a full-time equivalent rate that covers personnel-related expenses, including salaries and benefits, plus an allocation of facility-related expenses, including rent, utilities, insurance and property taxes, for those development employees who work either directly or indirectly on the development of our drugs. We also pay Ionis for the active pharmaceutical ingredient, or API, and drug product we use in our nonclinical and clinical studies for all of our drugs. Ionis manufactures the API for us and charges us a price per gram consistent with the price Ionis charges its pharmaceutical partners, which includes the cost for direct materials, direct labor and overhead required to manufacture the API. If we need the API filled in vials for our clinical studies and Ionis contracts with a third party to perform this work, Ionis will charge us for the resulting cost.
As we commercialize each of the Lipid Drugs, we will pay Ionis royalties from the mid-teens to the mid-twenty percent range on sales related to the Lipid Drugs that we sell. If we sell a Lipid Drug for a Rare Disease Indication (defined in the agreement as less than 500,000 patients worldwide or an indication that required a Phase 3 program of less than 1,000 patients and less than two years of treatment), we will pay a higher royalty rate to Ionis than if we sell a Lipid Drug for a Broad Disease Patient Population (defined in the agreement as more than 500,000 patients worldwide or an indication that required a Phase 3 program of 1,000 or more patients and two or more years of treatment). Other than with respect to the drugs licensed to Novartis under the collaboration agreement, if our annual sales reach $500.0 million, $1.0 billion and $2.0 billion, we will be obligated to pay Ionis sales milestones in the amount of $50.0 million for each sales milestone reached by each Lipid Drug. If and when triggered, we will pay Ionis each of these sales milestones over the subsequent 12 quarters in equal payments.
We may terminate this agreement if Ionis is in material breach of the agreement. Ionis may terminate this agreement if we are in material breach of the agreement. In each circumstance the party that is in breach will have an opportunity to cure the breach prior to the other party terminating this agreement.
In the first quarter of 2017, we entered into letter agreements with Ionis to reflect the agreed upon payment terms with respect to the upfront option payment that we received from Novartis and to allocate the premium that Novartis paid for Ionis' common stock in connection with our strategic collaboration with Novartis. For additional detail regarding our strategic collaboration with Novartis see Note 8, Strategic Collaboration with Novartis.
Services Agreement
Our services agreement with Ionis is designed to be flexible to adjust for our increasing capabilities in various functions. Under the services agreement, Ionis provides us certain services, including, without limitation, general and administrative support services and development support services. Ionis allocated a certain percentage of personnel to perform the services that it provides to us based on its good faith estimate of the required services. We pay Ionis for these allocated costs, which reflect the Ionis full-time equivalent, or FTE, rate for the applicable personnel, plus out-of-pocket expenses such as occupancy costs associated with the FTEs allocated to providing us these services. We do not pay a mark-up or profit on the external or internal expenses Ionis bills to us. Ionis invoices us quarterly for all amounts due under the services agreement and payments are due within 30 days of the receipt of an invoice.
In addition, as long as Ionis continues to consolidate our financials, we will comply with Ionis' policies and procedures and internal controls. As long as we are consolidated into Ionis' financial statements under U.S. GAAP, we may continue to access the following services from Ionis:
| ● |
investor relations services,
|
| ● |
human resources and personnel services,
|
| ● |
risk management and insurance services,
|
| ● |
tax related services,
|
| ● |
corporate record keeping services,
|
| ● |
financial and accounting services,
|
| ● |
credit services, and
|
| ● |
COO/CFO/CBO oversight.
|
F-19
However, if we wanted to provide for our own human resources and personnel services, and doing so would not negatively impact Ionis' internal controls and procedures for financial reporting, we can negotiate in good faith with Ionis for a reduced scope of services related to human resources and personnel services. When Ionis determines it should no longer consolidate our financials, we may mutually agree with Ionis in writing to extend the term in six-month increments.
We can establish our own benefits programs or can continue to use Ionis' benefits, however we must provide Ionis a minimum advance notice to opt-out of using Ionis' benefits. We do not currently plan to establish our own benefits program at this time or in the near future.
As of December 31, 2017 and 2016, we owed Ionis $14.4 million and $24.4 million, respectively.
The following table summarizes the amounts included in our operating expenses that were generated by transactions with Ionis for the following periods (in thousands):
|
Years Ended December 31,
|
||||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Services performed by Ionis
|
$
|
9,742
|
$
|
8,599
|
$
|
7,162
|
||||||
|
Active pharmaceutical ingredient manufactured by Ionis
|
6,012
|
12,648
|
5,620
|
|||||||||
|
Sublicensing expenses
|
48,394
|
—
|
—
|
|||||||||
|
Out-of-pocket expenses paid by Ionis
|
37,426
|
42,367
|
40,771
|
|||||||||
|
Total expenses generated by transactions with Ionis
|
101,574
|
63,614
|
53,553
|
|||||||||
|
Payable balance to Ionis at the beginning of the period
|
24,355
|
9,198
|
—
|
|||||||||
|
Less: amounts contributed by Ionis in the form of capital
|
—
|
—
|
(8,689
|
)
|
||||||||
|
Less: total amounts paid to Ionis during the period
|
(78,170
|
)
|
(48,457
|
)
|
(35,666
|
)
|
||||||
|
Less: non-cash sublicensing expenses
|
(33,394
|
)
|
—
|
—
|
||||||||
|
Total amount payable to Ionis at period end
|
$
|
14,365
|
$
|
24,355
|
$
|
9,198
|
||||||
| 5. |
Line of Credit Agreement with Ionis
|
In January 2017, we entered into a line of credit agreement with Ionis for up to $150.0 million. We had $106.0 million outstanding as of June 30, 2017. We used a portion of the $106.0 million to pay our intercompany expenses. The amounts we borrowed under the line of credit bore interest at an annual interest rate of 4%, compounded monthly. The outstanding principal and accrued interest under our line of credit converted into 13,438,339 shares of our common stock in connection with the closing of our IPO. We no longer have access to this line of credit following the closing of our IPO. Our IPO is discussed in Note 9, Initial Public Offering. For the year ended December 31, 2017, interest expense was $1.7 million. For the years ended December 31, 2016 and 2015, we incurred no interest expense.
| 6. |
Stockholders' Equity (Deficit)
|
Series A Convertible Preferred Stock
In December 2015, we issued and sold to Ionis an aggregate of 28,884,540 shares of Series A convertible preferred stock for a total purchase price of $100.0 million plus the grant of the rights and licenses we received under the development, commercialization and license agreement with Ionis. The $100.0 million of proceeds we received was recorded in Series A convertible preferred stock on our consolidated balance sheet. We had 28,884,540 shares of Series A convertible preferred stock authorized, issued and outstanding as of December 31, 2016, of which all was held by Ionis.
Conversion
Shares of our Series A convertible preferred stock were convertible 1:1 into common stock, subject to certain adjustments for reorganizations, reclassifications, stock splits, stock dividends and dilutive issuances. All shares of Series A convertible preferred stock automatically converted into common stock upon completion of the IPO in July 2017. As of December 31, 2017, we had no shares of Series A convertible preferred stock issued or outstanding. Our IPO is discussed in Note 9, Initial Public Offering.
Preferred Stock
In July 2017, our board of directors approved an amendment and restatement of our certificate of incorporation to, among other things, change the authorized shares of our preferred stock to 10,000,000 shares with a par value of $0.001, all of which are undesignated. Our board of directors may establish the rights, preference and privileges of the preferred stock from time to time. The amended and restated certificate of incorporation was approved by our stockholders and became effective upon the completion of our IPO and the filing of the amended and restated certificate of incorporation with the State of Delaware in July 2017. As of December 31, 2017, there were no shares of Preferred Stock outstanding.
F-20
Common Stock
At December 31, 2017 and 2016, we had 100,000,000 shares of common stock authorized, of which 66,541,629 and none were issued and outstanding as of December 31, 2017 and 2016, respectively.
In May 2017, our board of directors approved an amendment to our certificate of incorporation to (1) effect a reverse stock split on outstanding shares of our common stock and preferred stock on a one-for-2.555 basis, (2) change the authorized shares of our preferred stock to 40,000,000 and (3) modify the threshold for automatic conversion of our preferred stock into shares of our common stock in connection with an IPO to eliminate the price per share threshold and only require that we raise at least $50.0 million in gross proceeds (collectively, the "Charter Amendment"). The par values of the common stock and preferred stock were not adjusted as a result of the reverse stock split. The amendment to our certificate of incorporation was approved by our stockholder and became effective upon the filing with the State of Delaware in June 2017. All issued and outstanding common stock and preferred stock and related share and per share amounts contained in these consolidated financial statements have been retroactively adjusted to reflect the reverse stock split for all periods presented.
Stock Plans
2015 Equity Incentive Plan
In December 2015, our board of directors and stockholder adopted and approved our 2015 Equity Incentive Plan, or the 2015 Plan. In May 2017 and June 2017, our board of directors and stockholder, respectively, approved an amendment to our 2015 Equity Incentive Plan in order to, among other things, increase the number of shares of common stock reserved for issuance thereunder to 8,500,000 shares of common stock in conjunction with the IPO.
As of December 31, 2017, the aggregate number of shares of common stock that may be issued pursuant to stock awards under the 2015 Plan was 8,500,000 shares, not including an additional 5,000,000 shares approved by the Board of Directors in December 2017, subject to shareholder approval. The 2015 Plan also provides for the grant of nonstatutory stock options, or NSOs, incentive stock options, or ISOs, stock appreciation rights, restricted stock awards and restricted stock unit awards. At December 31, 2017, a total of 7,905,110 options were outstanding, of which 2,964,262 were exercisable, 33,820 restricted stock unit awards were outstanding, and 561,070 shares were available for future grant under the 2015 Plan.
2017 Employee Stock Purchase Plan
In May 2017 and June 2017, our board of directors and stockholder, respectively, approved our 2017 Employee Stock Purchase Plan, or 2017 ESPP, which became effective upon the completion of our IPO, and the reservation for issuance thereunder of 500,000 shares of common stock. In addition, the number of shares of common stock that may be issued under the ESPP will automatically increase commencing on January 1, 2018 and ending on (and including) January 1, 2027 in an amount equal to the lesser of (i) 1% of the total number of shares of Common Stock outstanding on December 31st of the preceding calendar year, and (ii) 500,000 shares of Common Stock. During the year ended December 31, 2017, no shares were issued under our 2017 ESPP. At December 31, 2017, accrued liabilities included $175,000 of ESPP contributions related to our first enrollment period for which the related shares were issued on January 2, 2018.
Stock Option Activity
The following table summarizes the stock option activity for the year ended December 31, 2017 (in thousands, except per share and contractual life data) for the 2015 Plan:
|
Number of
Shares
|
Weighted
Average Exercise
Price per Share
|
Average
Remaining
Contractual Term
(Years)
|
Aggregate
Intrinsic
Value
|
|||||||||||||
|
Outstanding at December 31, 2016
|
5,064
|
$
|
6.48
|
9.11
|
—
|
|||||||||||
|
Granted
|
2,890
|
$
|
15.14
|
|||||||||||||
|
Cancelled/forfeited/expired
|
(49
|
)
|
$
|
7.70
|
||||||||||||
|
Outstanding at December 31, 2017
|
7,905
|
$
|
9.64
|
8.51
|
$
|
63,971
|
||||||||||
|
Exercisable at December 31, 2017
|
2,964
|
$
|
6.48
|
7.84
|
$
|
32,251
|
||||||||||
The weighted-average estimated fair value of options granted were $10.40, $4.13 and $4.01 for the years ended December 31, 2017, 2016 and 2015, respectively. For the year ended December 31, 2017, no stock options were exercised. As of December 31, 2017, total unrecognized estimated non-cash stock-based compensation expense related to non-vested stock options was $27.9 million. We will adjust total unrecognized compensation cost for future forfeitures. We expect to recognize the cost of non-cash stock-based compensation expense related to non-vested stock options over a weighted average amortization period of 1.31 years.
F-21
The following table summarizes the stock option activity for the year ended December 31, 2017 (in thousands, except per share and contractual life data) for options granted to our employees under the Ionis 2011 Equity Incentive:
|
Number of
Shares
|
Weighted
Average Exercise
Price per Share
|
Average
Remaining
Contractual Term
(Years)
|
Aggregate
Intrinsic
Value
|
|||||||||||||
|
Outstanding at December 31, 2016
|
801
|
$
|
54.92
|
5.72
|
2,203
|
|||||||||||
|
Granted
|
628
|
$
|
45.76
|
|||||||||||||
|
Cancelled/forfeited/expired
|
(1,429
|
)
|
$
|
50.90
|
||||||||||||
|
Outstanding at December 31, 2017
|
—
|
$
|
—
|
—
|
$
|
—
|
||||||||||
|
Exercisable at December 31, 2017
|
—
|
$
|
—
|
—
|
$
|
—
|
||||||||||
The weighted average grant-date fair value of options to purchase Ionis common stock granted to Akcea employees were $24.23, $23.02 and $27.99 for the years ended December 31, 2017, 2016 and 2015, respectively.
Stock-based Compensation Expense and Valuation Information
The following table summarizes stock-based compensation expense for the years ended December 31, 2017, 2016 and 2015 (in thousands):
|
Years Ended December 31,
|
||||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Research and development expenses
|
$
|
8,630
|
$
|
4,576
|
$
|
827
|
||||||
|
General and administrative expenses
|
8,909
|
5,573
|
5,669
|
|||||||||
|
Total
|
$
|
17,539
|
$
|
10,149
|
$
|
6,496
|
||||||
Determining Fair Value
Valuation. We measure stock-based compensation expense for equity-classified awards related to stock options and stock purchase rights under the ESPP at the grant date, based on the estimated fair value of the award and we recognize the expense over the employee's requisite service period.
We use the Black-Scholes model to estimate the fair value of stock options granted and stock purchase rights under our ESPP. The expected term of stock options granted represents the period of time that we expect them to be outstanding. We estimate the expected term of options granted based on actual and projected exercise patterns. We recognize compensation expense for stock options granted and stock purchase rights under the ESPP using the accelerated multiple-option approach. Under the accelerated multiple-option approach (also known as the graded-vesting method), an entity recognizes compensation expense over the requisite service period for each separately vesting tranche of the award as though the award were in substance multiple awards, which results in the expense being front-loaded over the vesting period.
In valuing our options, we made a number of assumptions, including the risk-free interest rate, expected dividend yield, expected volatility, expected term, rate of forfeitures and fair value of common stock. We considered the following factors in applying these assumptions:
Risk-Free Interest Rate. We determine the risk-free interest rate assumption based on the yields of U.S. Treasury securities with maturities that correspond to the term of the award.
Expected Dividend Yield. We assume a dividend yield of zero as we have not paid dividends in the past and do not expect to pay dividends on our common stock for the foreseeable future.
Expected Volatility. We do not have sufficient history to estimate the volatility of our common stock. We calculate expected volatility based on reported data from selected publicly traded peer companies for which historical information is available. We plan to continue to use a peer group to calculate our volatility until the historical volatility of our common stock is sufficient to measure expected volatility for future option grants.
Expected Term. The expected term estimates represent the period of time that we expect the options to be outstanding. As we do not have historical information, we use the simplified method for estimating the expected term. Under the simplified method we calculate the expected term as the average time-to-vesting and the contractual life of the options. As we gain additional historical information, we will transition to calculating our expected term based on our exercise patterns.
F-22
Rate of Forfeiture. We estimate forfeitures based on Ionis' historical rates of forfeiture as we do not have similar historical information for ourselves. We and Ionis are engaged in similar businesses and we believe this is a good estimate of expected forfeitures. As we gain additional historical information, we will transition to using our historical forfeiture rate.
Fair Value of Common Stock. Prior to our IPO our board of directors estimated the fair value of our common stock considering, among other things, contemporaneous valuations of our common stock prepared by an unrelated third-party valuation firm in accordance with the guidance provided by the American Institute of Certified Public Accountants 2013 Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation. Subsequent to the IPO, we use the market closing price for our common stock on the date of grant as reported on Nasdaq to determine the fair value of our common stock on the date of grant.
For the years ended December 31, 2017, 2016 and 2015, we used the following weighted-average assumptions in our Black-Scholes calculations for stock option grants under our 2015 Equity Incentive Plan:
Employee Stock Options:
|
Years Ended December 31,
|
||||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Risk-free interest rate
|
1.9 | % |
1.6
|
%
|
2.0
|
%
|
||||||
|
Dividend yield
|
0.0
|
%
|
0.0
|
%
|
0.0
|
%
|
||||||
|
Volatility
|
79.5
|
%
|
71.4
|
%
|
67.9
|
%
|
||||||
|
Expected life
|
6.06 years
|
6.08 years
|
6.08 years
|
|||||||||
Board of Director Stock Options:
|
Years Ended December 31,
|
||||||||
|
|
2017
|
2016
|
||||||
|
Risk-free interest rate
|
1.9
|
%
|
2.0
|
%
|
||||
|
Dividend yield
|
0.0
|
%
|
0.0
|
%
|
||||
|
Volatility
|
79.4
|
%
|
79.6
|
%
|
||||
|
Expected life
|
6.25 years
|
6.08 years
|
||||||
In valuing options for Ionis common stock, Ionis made a number of assumptions, including the risk-free interest rate, expected dividend yield, expected volatility, expected term, rate of forfeiture and fair value of common stock. Ionis considered the following factors in applying these assumptions:
Risk-Free Interest Rate. Ionis bases the risk-free interest rate assumption on the yields of U.S. Treasury securities with maturities that correspond to the term of the award.
Expected Dividend Yield. Ionis bases the dividend yield assumption on its history and expectation of dividend payouts. Ionis has not paid dividends in the past and it does not expect to pay dividends for the foreseeable future.
Expected Volatility. Ionis uses an average of the historical stock price volatility of Ionis' stock. It computed the historical stock volatility based on the expected term of the awards.
Expected Term. The expected term of stock options Ionis has granted represents the period of time that it expects them to be outstanding. Ionis estimated the expected term of options Ionis has granted based on actual and projected exercise patterns.
Rate of Forfeiture. Ionis estimates forfeitures at the time of grant and revises, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Ionis estimates forfeitures based on historical experience. Ionis' historical forfeiture estimates have not been materially different from its actual forfeitures.
Fair Value of Common Stock. Ionis uses the market closing price for its common stock on the date of grant as reported on Nasdaq to determine the fair value of Ionis' common stock on the date of grant.
For the years ended December 31, 2017, 2016 and 2015, Ionis used the following weighted-average assumptions in its Black-Scholes calculations for stock option grants under the Ionis 2011 Equity Incentive Plan:
F-23
Employee Stock Options:
|
Years Ended December 31,
|
||||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Risk-free interest rate
|
1.9
|
%
|
1.5
|
%
|
1.5
|
%
|
||||||
|
Dividend yield
|
0.0
|
%
|
0.0
|
%
|
0.0
|
%
|
||||||
|
Volatility
|
65.8
|
%
|
59.4
|
%
|
54.1
|
%
|
||||||
|
Expected life
|
4.5 years
|
4.5 years
|
4.5 years
|
|||||||||
Restricted Stock Units:
In October 2017, we issued 33,820 RSUs to certain employees. The expense recognized for these awards is based on the grant date fair value of our common stock multiplied by the number of units granted. We recognized $75,000 of related expense during the year ended December 31, 2017 related to RSUs. We did not incur stock-based compensation expense for the years ended December 31, 2016 and 2015 related to RSUs.
The following table presents a summary of our RSU activity and related information (in thousands, except per share data):
|
|
Number of
Shares
|
Weighted Average
Grant Date Fair
Value per Share
|
||||||
|
Unvested restricted stock units at December 31, 2016
|
—
|
$
|
—
|
|||||
|
Granted
|
34
|
23.04
|
||||||
|
Vested
|
—
|
—
|
||||||
|
Forfeited
|
—
|
—
|
||||||
|
Unvested restricted stock units at December 31, 2017
|
34
|
$
|
23.04
|
|||||
The weighted-average grant date fair values of RSUs granted during the year ended December 31, 2017 was $23.04 per share. There were no awards of RSUs during the years ended December 31, 2016 and 2015. No RSUs vested during the year ended December 31, 2017. As of December 31, 2017, total unrecognized estimated non-cash stock-based compensation expense related to unvested RSUs was $0.6 million. We expect to recognize the cost of non-cash stock-based compensation expense related to the unvested RSUs over a remaining weighted-average period of approximately 2.3 years.
In addition to granting RSUs, we issued cash awards to certain employees, which will vest annually over four years starting on the employee's hire date, provided that the employee continues to remain employed through each vesting date. The target payment amount totals $1.0 million of which we recognized expense of $124,000 during the year ended December 31, 2017.
| 7. |
Income Taxes
|
Loss before income taxes is comprised of (in thousands):
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
United States
|
$
|
(96,883
|
)
|
$
|
(83,217
|
)
|
$
|
(61,422
|
)
|
|||
|
Foreign
|
(11,593
|
)
|
—
|
—
|
||||||||
|
Loss before income tax expense
|
$
|
(108,476
|
)
|
$
|
(83,217
|
)
|
$
|
(61,422
|
)
|
|||
The provision (benefit) for income taxes is comprised of (in thousands):
|
Years Ended December 31,
|
||||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Current:
|
||||||||||||
|
Federal
|
$
|
—
|
$
|
—
|
$
|
—
|
||||||
|
State
|
1,041
|
—
|
—
|
|||||||||
|
Foreign
|
234
|
—
|
—
|
|||||||||
|
Total current
|
1,275
|
—
|
—
|
|||||||||
|
|
||||||||||||
|
Deferred:
|
||||||||||||
|
Federal
|
—
|
—
|
—
|
|||||||||
|
State
|
—
|
—
|
—
|
|||||||||
|
Foreign
|
—
|
—
|
—
|
|||||||||
|
Total deferred
|
—
|
—
|
—
|
|||||||||
|
Income tax expense
|
$
|
1,275
|
$
|
—
|
$
|
—
|
||||||
There is no provision for income taxes for the years ended December 31, 2016 and 2015 because we have historically incurred net operating losses and we maintain a full valuation allowance against our net deferred tax assets.
F-24
We have taxable income for the year ending December 31, 2017 primarily due to the income we recognized from our Novartis collaboration. We recorded income tax expense of $1.3 million for the year ended December 31, 2017, which primarily consists of state and foreign income tax.
The reconciliation between our effective tax rate on loss from continuing operations and the statutory U.S. tax rate is as follows (in thousands):
|
|
Years Ended December 31,
|
|||||||||||||||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||||||||||||||
|
Pre-tax loss
|
$
|
(108,476
|
)
|
$
|
(83,217
|
)
|
$
|
(61,422
|
)
|
|||||||||||||||
|
Statutory rate
|
(37,964
|
)
|
35.0
|
%
|
(29,126
|
)
|
35.0
|
%
|
(21,498
|
)
|
35.0
|
%
|
||||||||||||
|
State income tax net of federal benefit
|
(2,070
|
)
|
1.9
|
%
|
(4,099
|
)
|
4.9
|
%
|
(3,194
|
)
|
5.2
|
%
|
||||||||||||
|
Impact of foreign tax rate differential
|
4,072
|
(3.8
|
)%
|
—
|
0.0
|
%
|
—
|
0.0
|
%
|
|||||||||||||||
|
Net change in valuation allowance
|
(22,103
|
)
|
20.4
|
%
|
43,438
|
(52.1
|
)%
|
30,857
|
(50.2
|
)%
|
||||||||||||||
|
Tax credits
|
4,189
|
(3.9
|
)%
|
(11,007
|
)
|
13.2
|
%
|
(6,187
|
)
|
10.0
|
%
|
|||||||||||||
|
IPO/Deconsolidation adjustment
|
37,911
|
(34.9
|
)%
|
—
|
0.0
|
%
|
—
|
0.0
|
%
|
|||||||||||||||
|
Tax Cut and Jobs Act
|
17,518
|
(16.1
|
)%
|
—
|
0.0
|
%
|
—
|
0.0
|
%
|
|||||||||||||||
|
Nondeductible items and other
|
(278
|
)
|
0.3
|
%
|
794
|
(1.0
|
)%
|
22
|
0.0
|
%
|
||||||||||||||
|
Effective rate
|
$
|
1,275
|
(1.1
|
)%
|
$
|
—
|
0.0
|
%
|
$
|
—
|
0.0
|
%
|
||||||||||||
Significant components of our deferred tax assets and liabilities as of December 31, 2017 and 2016 are as follows (in thousands):
|
|
December 31,
|
|||||||
|
|
2017
|
2016
|
||||||
|
Deferred Tax Assets:
|
||||||||
|
Net operating loss carryovers
|
$
|
1,157
|
$
|
48,813
|
||||
|
Tax credits
|
29,334
|
30,057
|
||||||
|
Stock-based compensation
|
7,515
|
6,620
|
||||||
|
Deferred revenue
|
26,070
|
—
|
||||||
|
Other
|
240
|
1,251
|
||||||
|
Total deferred tax assets
|
$
|
64,316
|
$
|
86,741
|
||||
|
|
||||||||
|
Deferred Tax Liabilities:
|
||||||||
|
Intangible assets
|
(125
|
)
|
(281
|
)
|
||||
|
Total deferred tax liabilities
|
$
|
(125
|
)
|
$
|
(281
|
)
|
||
|
Valuation allowance
|
(64,191
|
)
|
(86,460
|
)
|
||||
|
|
||||||||
|
Net deferred tax assets and liabilities
|
$
|
—
|
$
|
—
|
||||
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act of 2017, or Tax Act. The Tax Act makes broad and complex changes to the U.S. tax code. The changes include, but are not limited to, reducing the U.S. federal corporate tax rate from 35% to 21%, imposing a mandatory one-time transition tax on certain unrepatriated earnings of foreign subsidiaries, introducing bonus depreciation that will allow for full expensing of qualified property, eliminating the corporate alternative minimum tax, or AMT, and changing how existing AMT credits can be realized, and modifying or repealing many business tax deductions and credits.
As a result of the tax rate reduction, we remeasured our existing net U.S. deferred tax assets using the enacted rate and other known existing changes to the tax code. This resulted in a total decrease in these assets by $17.5 million, the tax effect of which was fully offset by a decrease in the valuation allowance.
As a result of the repeal of the corporate AMT, we recorded a $0.5 million long-term income tax receivable related to our 2017 estimated AMT liability because under the Tax Act, AMT tax credits are now refundable from 2018 through 2021. The net effect of the repeal of the corporate AMT on our income tax provision is zero.
In accordance with the Securities and Exchange Commission Staff Accounting Bulletin No. 118, we provided our best estimate of the impact of the Tax Act in the period ended December 31, 2017 based on our understanding of the Tax Act and guidance available as of the date of this filing. We have recognized provisional tax impacts related to deemed repatriated earnings, the revaluation of our deferred tax assets and the impact of the repeal of the corporate AMT. Our preliminary analysis resulted in no deemed repatriation amount under Section 965(a) and no net financial statement impact from the revaluation of our deferred tax assets due to the change in the corporate tax rate and no net financial statement impact due to the repeal of the corporate AMT. The ultimate impact may differ materially from these provisional amounts due to, among other things, additional analysis, changes in our interpretations and assumptions, additional regulatory guidance that may be issued, and other actions we may take as a result of the Tax Act.
F-25
Prior to the completion of our IPO we filed our tax returns on a consolidated and combined basis with Ionis for federal and state income tax purposes, respectively. For financial statement purposes when we are required to file on a consolidated or combined basis, we calculate our income tax amounts, including net operating losses and tax credit carryforwards, using a separate return methodology which determines income taxes as if we were a separate taxpayer from Ionis. Effective July 19, 2017, the date of our IPO, we are no longer included in the consolidated federal income tax return with Ionis. We determined the amount of federal tax attributes, primarily net operating losses and tax credit carryforwards, that transferred to us upon deconsolidation from Ionis.
We are still required to file most of our state tax returns on a consolidated or combined basis with Ionis. Therefore, for financial statement purposes we calculated our state income tax amounts using the separate return method. We have not yet determined the amount of state tax attributes, primarily net operating losses and tax credit carryforwards, which we would retain if we were to deconsolidate for state tax purposes from Ionis.
At December 31, 2017, we had federal and state tax net operating loss carry forwards on a separate basis of approximately $5.3 million and $1.2 million, respectively, available to reduce future taxable income, if any. If not realized, the federal and state loss carryforwards will begin to expire in years 2034 and 2027, respectively. We also have federal research and development tax credit carry forwards of approximately $33.3 million that will begin to expire in 2034.
Utilization of the net operating loss carry forwards and credits may be subject to an annual limitation due to the ownership change limitations provided by the Internal Revenue Code of 1986, as amended, and similar state provisions. The annual limitation may result in the expiration of net operating losses and credits before utilization.
We record a valuation allowance to reduce the balance of our net deferred tax assets to the amount we believe is more-likely-than-not to be realized. We have incurred financial statement losses since inception and as a result we have a full valuation allowance recorded against our net deferred tax assets. We regularly assess the future realization of our net deferred tax assets and will reduce the valuation allowance in any such period in which we determine that all, or a portion, of our deferred tax assets are more-likely-than-not to be realized.
Our valuation allowance decreased by $22.3 million from December 31, 2016 to December 31, 2017. The decrease relates primarily to the remeasurement of our net deferred tax assets as required by the Tax Act and the impact from our deconsolidation from Ionis, offset partially by increases from current year activity.
Historically, we recognized excess tax benefits associated with stock-based compensation to stockholders' equity only when realized. We followed the with and without approach excluding any indirect effects of the excess tax deductions to determine when we should realize excess tax benefits relating to stock-based compensation. Under this approach, we do not realize our excess tax benefits related to stock-based compensation until after we utilize all our other tax benefits available to us.
In March 2016, the FASB issued amended guidance to simplify certain aspects of stock-based payment accounting. Under the amended guidance, we will recognize excess tax benefits and tax deficiencies as income tax expense or benefit in our consolidated statement of operations on a prospective basis. As we have a valuation allowance, this change will impact our net operating loss carryforward and the valuation allowance disclosures.
We analyze our filing positions in all the U.S. federal, state and foreign jurisdictions where we are required to file income tax returns to determine if we have any uncertain tax positions on any income tax returns. We recognize the impact of an uncertain tax position on an income tax return at the largest amount that the relevant taxing authority is more-likely-than not to sustain upon audit. We do not recognize a tax benefit if the position has a less than 50 percent likelihood of being sustained upon examination.
The following table summarizes our gross unrecognized tax benefits (in thousands):
|
Years Ended December 31,
|
||||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Beginning balance of unrecognized tax benefits
|
$
|
5,012
|
$
|
1,766
|
$
|
138
|
||||||
|
Additions related to the current year
|
1,723
|
3,246
|
1,628
|
|||||||||
|
Decreases related to prior year tax positions
|
(1,734
|
)
|
—
|
—
|
||||||||
|
Ending balance of unrecognized tax benefits
|
$
|
5,001
|
$
|
5,012
|
$
|
1,766
|
||||||
Due to our valuation allowance, there are no unrecognized tax benefits at December 31, 2017 that would impact our effective tax rate, if recognized.
F-26
We do not foresee any material changes to our gross unrecognized tax benefits within the next twelve months.
We recognize interest and/or penalties related to income tax matters in income tax expense. We did not recognize any accrued interest and penalties related to gross unrecognized tax benefits during the year ended December 31, 2017.
We are subject to taxation in the United States and various state and foreign jurisdictions. The tax years for 2014 through 2016 are subject to examination by the U.S. federal, state and foreign tax authorities.
| 8. |
Strategic Collaboration with Novartis
|
In January 2017, we initiated a strategic collaboration with Novartis for the development and commercialization of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Under the Novartis collaboration, Novartis has an exclusive option to further develop and commercialize these drugs. We are responsible for completing a Phase 2 program, conducting an end-of-Phase 2 meeting with the FDA and providing API for each drug. If Novartis exercises an option for one of these drugs, Novartis will be responsible for all further global development, regulatory and co-commercialization activities and costs for such drug.
We received a $75.0 million upfront payment in the first quarter of 2017, of which we retained $60.0 million and we paid Ionis $15.0 million as a sublicense fee under our license agreement with Ionis. If Novartis exercises its option for a drug, Novartis will pay us a license fee equal to $150.0 million for each drug licensed by Novartis. In addition, for AKCEA-APO(a)-LRx, we are eligible to receive up to $600.0 million in substantive milestone payments, including $25.0 million for the achievement of a development milestone, up to $290.0 million for the achievement of regulatory milestones and up to $285.0 million for the achievement of commercialization milestones. In addition, for AKCEA-APOCIII-LRx, we are eligible to receive up to $530.0 million in substantive milestone payments, including $25.0 million for the achievement of a development milestone, up to $240.0 million for the achievement of regulatory milestones and up to $265.0 million for the achievement of commercialization milestones. We will earn the next milestone payment of $25.0 million under this collaboration if Novartis advances the Phase 3 study for either drug. We are also eligible to receive tiered royalties in the mid-teens to low twenty percent range on net sales of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Novartis will reduce these royalties upon the expiration of certain patents or if a generic competitor negatively impacts the product in a specific country. We will pay 50% of these license fees, milestone payments and royalties to Ionis as a sublicense fee. We plan to co-commercialize any licensed drug commercialized by Novartis in selected markets under terms and conditions that we plan to negotiate with Novartis in the future, through the specialized sales force we are building to commercialize volanesorsen.
The agreement with Novartis will continue until the earlier of the date that all of Novartis' options to obtain the exclusive licenses under the agreement expire unexercised or, if Novartis exercises its options, until the expiration of all payment obligations under the agreement. In addition, the agreement as a whole or with respect to any drug under the agreement may terminate early under the following situations:
| ● |
Novartis may terminate the agreement as a whole or with respect to any drug at any time by providing written notice to us;
|
| ● |
Either we or Novartis may terminate the agreement with respect to any drug by providing written notice to the other party in good faith that we or Novartis have determined that the continued development or commercialization of the drug presents safety concerns that pose an unacceptable risk or threat of harm in humans or would violate any applicable law, ethical principles or principles of scientific integrity;
|
| ● |
Either we or Novartis may terminate the agreement for a drug by providing written notice to the other party upon the other party's uncured failure to perform a material obligation related to the drug under the agreement, or the entire agreement if the other party becomes insolvent; and
|
| ● |
We may terminate the agreement if Novartis disputes or assists a third party to dispute the validity of any of our or Ionis' patents.
|
Additionally, we and Ionis entered into a SPA with Novartis. Under the SPA, in July 2017, as part of our IPO, Novartis purchased $50.0 million of our common stock in a separate private placement concurrent with the completion of our IPO at a price per share equal to the IPO price. Our IPO is discussed in Note 9, Initial Public Offering.
During the year ended December 31, 2017, we earned revenue of $55.2 million from our relationship with Novartis, representing 100% of our revenue. Through December 31, 2016, we did not generate revenue. Our consolidated balance sheet at December 31, 2017 included deferred revenue of $58.9 million related to our relationship with Novartis.
F-27
9. Initial Public Offering
On July 19, 2017, we completed our IPO. Total net proceeds were $182.3 million, including the following:
| ● |
$132.3 million from the sale of 17,968,750 shares of our common stock in our IPO of which $25 million was invested by Ionis; and
|
| ● |
$50.0 million from the purchase of 6,250,000 shares by Novartis in a concurrent private placement.
|
In addition, both of the following occurred in connection with the completion of our IPO on July 19, 2017:
| ● |
the conversion of all outstanding shares of Series A convertible preferred stock into 28,884,540 shares of our common stock; and
|
| ● |
the conversion of $106.0 million of outstanding principal plus accrued interest from the line of credit into 13,438,339 shares of common stock.
|
| 10. |
Employment Benefits
|
We have an employee 401(k) salary deferral plan covering all employees. Employees may make contributions by withholding a percentage of their salary up to the IRS annual limit $18,000 and $24,000 in 2017 for employees under 50 years old and employees 50 years old or over, respectively. We made approximately $299,000, $211,000 and $28,000 in matching contributions for the years ended December 31, 2017, 2016 and 2015, respectively.
| 11. |
Basic and Diluted Net Loss Per Share
|
We issued 28,884,540 shares of Series A convertible preferred stock in December 2015. Prior to the completion of our IPO, we used the Series A convertible preferred stock to calculate basic net loss per share because there was no common stock outstanding during these periods, and the Series A convertible preferred stock represented the lowest subordinated form of outstanding equity that would have been required to absorb our losses. For purposes of calculating diluted net loss per share, we considered the conversion of the Series A convertible preferred stock using its 1:1 conversion ratio and the potential dilutive effect of employee stock options.
The Series A convertible preferred stock converted into common stock in conjunction with the IPO in July 2017. As a result there were 66,541,629 shares of common stock issued and outstanding and there were no longer any outstanding shares of Series A convertible preferred stock. We determined that the Series A convertible preferred stock was in substance common stock during the period that it was outstanding because the Series A convertible preferred stock was the lowest form of subordinated equity outstanding during that period and therefore this class of stock would have been required to absorb our losses. Accordingly, we are using the two-class method for computing EPS.
The two-class method is an earnings allocation formula that determines EPS for each class of common stock and participating security according to dividends declared (or accumulated) and participation rights in undistributed earnings. For the purposes of calculating EPS under the two-class method, we have allocated the net loss between the common stock and the Series A convertible preferred stock.
Basic EPS for each class of stock is computed by dividing total distributable losses applicable to preferred and common stock, including the 6% cumulative dividend contractually due to Series A convertible preferred shareholders, by the weighted-average of preferred and common shares outstanding during the requisite period. The cumulative preferred stock dividend was not paid upon completion of the IPO because the IPO was not a liquidation event or a change in control. Prior to the IPO, the 6% cumulative Series A convertible preferred stock dividend was considered as required under the two-class method regardless of whether those dividends were actually distributed.
The following table summarizes the distributable losses for the years ended December 31, 2017, 2016 and 2015 (in thousands):
|
Years Ended December 31,
|
||||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Net loss
|
$
|
(109,751
|
)
|
$
|
(83,217
|
)
|
$
|
(61,422
|
)
|
|||
|
Preferred stock dividend
|
(20,100
|
)
|
—
|
—
|
||||||||
|
Distributable losses
|
$
|
(129,851
|
)
|
$
|
(83,217
|
)
|
$
|
(61,422
|
)
|
|||
F-28
The following table summarizes the reconciliation of weighted-average shares outstanding used in the calculation of basic EPS for the years ended December 31, 2017, 2016 and 2015:
|
|
Years Ended December 31,
|
|||||||||||
|
Determination of shares:
|
2017
|
2016
|
2015
|
|||||||||
|
Weighted-average preferred shares outstanding
|
15,748,009
|
28,884,540
|
28,884,540
|
|||||||||
|
Weighted-average common shares outstanding
|
30,262,768
|
—
|
—
|
|||||||||
|
Total weighted-average shares outstanding
|
46,010,777
|
28,884,540
|
28,884,540
|
|||||||||
The following table summarizes the calculation of basic EPS for the years ended December 31, 2017, 2016 and 2015 (in thousands, except per share amounts):
|
|
Years Ended December 31,
|
|||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Losses attributable to preferred shares
|
$
|
(44,444
|
)
|
$
|
(83,217
|
)
|
$
|
(61,422
|
)
|
|||
|
Less: Assumed dividend to preferred shares
|
20,100
|
—
|
—
|
|||||||||
|
Income (losses) allocated to preferred shares
|
$
|
(24,344
|
)
|
$
|
(83,217
|
)
|
$
|
(61,422
|
)
|
|||
|
Weighted-average preferred shares outstanding
|
15,748
|
28,884,540
|
28,884,540
|
|||||||||
|
Basic loss per preferred share
|
$
|
(1.55
|
)
|
$
|
(2.88
|
)
|
$
|
(2.13
|
)
|
|||
|
|
||||||||||||
|
Losses allocated to common shares
|
$
|
(85,407
|
)
|
$
|
—
|
$
|
—
|
|||||
|
Weighted-average common shares outstanding
|
30,263
|
—
|
—
|
|||||||||
|
Basic loss per common share
|
$
|
(2.82
|
)
|
$
|
—
|
$
|
—
|
|||||
The following table presents amounts that were excluded from the calculation of diluted net loss per share, due to their anti-dilutive effect:
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Options to purchase common stock
|
7,905,110
|
5,063,585
|
2,905,006
|
|||||||||
|
Unvested restricted stock
|
33,820
|
—
|
—
|
|||||||||
|
Employee stock purchase plan
|
9,488
|
—
|
—
|
|||||||||
F-29
12. Quarterly Financial Data (Unaudited)
The following financial information reflects all normal recurring adjustments, which are, in the opinion of management, necessary for a fair statement of the results of the interim periods. Summarized quarterly data for the years ended December 31, 2017 and 2016 are as follows (in thousands, except per share data):
|
2017 Quarters
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
||||||||||||
|
Revenue
|
$
|
9,597
|
$
|
14,128
|
$
|
13,449
|
$
|
18,035
|
||||||||
|
Operating expenses
|
$
|
69,470
|
$
|
25,402
|
$
|
26,013
|
$
|
42,986
|
||||||||
|
Income (loss) from operations
|
$
|
(59,873
|
)
|
$
|
(11,274
|
)
|
$
|
(12,564
|
)
|
$
|
(24,951
|
)
|
||||
|
Net income (loss)
|
$
|
(60,353
|
)
|
$
|
(11,944
|
)
|
$
|
(14,094
|
)
|
$
|
(23,360
|
)
|
||||
|
Net (loss) income per preferred share – basic and diluted (1) (2) (3)
|
$
|
(2.09
|
)
|
$
|
(0.41
|
)
|
$
|
0.05
|
$
|
—
|
||||||
|
Net loss per common share – basic and diluted (1) (2) (3)
|
—
|
—
|
$
|
(0.27
|
)
|
$
|
(0.35
|
)
|
||||||||
|
2016 Quarters
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
||||||||||||
|
Revenue
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
||||||||
|
Operating expenses
|
$
|
16,049
|
$
|
13,706
|
$
|
19,902
|
$
|
33,855
|
||||||||
|
Income (loss) from operations
|
$
|
(16,049
|
)
|
$
|
(13,706
|
)
|
$
|
(19,902
|
)
|
$
|
(33,855
|
)
|
||||
|
Net income (loss)
|
$
|
(15,962
|
)
|
$
|
(13,615
|
)
|
$
|
(19,809
|
)
|
$
|
(33,831
|
)
|
||||
|
Net loss per preferred share – basic and diluted (1) (3)
|
$
|
(0.55
|
)
|
$
|
(0.47
|
)
|
$
|
(0.69
|
)
|
$
|
(1.17
|
)
|
||||
_______________________________________________________________________
| (1) |
We computed net loss per share independently for each of the quarters presented. Therefore, the sum of the quarterly net loss per share will not necessarily equal the total for the year.
|
| (2) |
For the purposes of calculating EPS under the two-class method since our IPO in July 2017, we have allocated the net loss between the common stock and the Series A convertible preferred stock for the three-month period ended September 30, 2017. We determined it was appropriate to allocate losses to the Series A convertible preferred stock because it was the lowest form of subordinated equity during such period and because Ionis, the sole holder of the Series A convertible preferred stock, was absorbing our losses during such period. Basic EPS for each class of stock is computed by dividing total distributable losses applicable to preferred and common stock, including the 6% cumulative dividend contractually due to Series A convertible preferred shareholders, by the weighted-average of preferred and common shares outstanding during the requisite period. The cumulative preferred stock dividend was not paid upon completion of the IPO because the IPO was not a liquidation event or a change in control. Prior to the IPO, the 6% cumulative Series A convertible preferred stock dividend was considered as required under the two-class method regardless of whether those dividends were actually distributed.
|
The following table summarizes the distributable losses for the quarter ended September 30, 2017 (in thousands):
|
|
September 30, 2017
|
|||
|
Net loss
|
$
|
(14,094
|
)
|
|
|
Preferred stock dividend
|
(1,791
|
)
|
||
|
Distributable losses
|
$
|
(15,885
|
)
|
|
The following table summarizes the reconciliation of weighted-average shares outstanding used in the calculation of basic EPS for the quarter ended September 30, 2017:
|
Determination of shares:
|
September 30, 2017
|
|||
|
Weighted-average preferred shares outstanding
|
5,651,323
|
|||
|
Weighted-average common shares outstanding
|
53,522,615
|
|||
|
Total weighted-average shares outstanding
|
59,173,938
|
|||
The following table summarizes the calculation of basic EPS for the quarter ended September 30, 2017 (in thousands, except per share amounts):
|
|
September 30, 2017
|
|||
|
Losses attributable to preferred shares
|
$
|
(1,517
|
)
|
|
|
Less: Assumed dividend to preferred shares
|
1,791
|
|||
|
Income (losses) allocated to preferred shares
|
$
|
274
|
||
|
Weighted-average preferred shares outstanding
|
5,651,323
|
|||
|
Basic income (loss) per preferred share
|
$
|
0.05
|
||
|
|
||||
|
Losses allocated to common shares
|
$
|
(14,368
|
)
|
|
|
Weighted-average common shares outstanding
|
53,522,615
|
|||
|
Basic loss per common share
|
$
|
(0.27
|
)
|
|
| (3) |
We did not include dilutive common equivalent shares in the computation of diluted net loss per share because the effect would have been antidilutive.
|
F-30
