Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SYNERGY PHARMACEUTICALS, INC. | a18-6057_28k.htm |
SAFE HARBOR STATEMENT This presentation and any statements made for and during any presentation or meeting contain forward-looking statements related to Synergy Pharmaceuticals Inc. under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995 and are subject to risks and uncertainties that could cause actual results to differ materially from those projected. These statements may be identified by the use of forward-looking words such as "anticipate," "planned," "believe," "forecast," "estimated," "expected," and "intend," among others. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, the development, launch, introduction and commercial potential of TRULANCE™; growth and opportunity, including peak sales and the potential demand for TRULANCE, as well as its potential impact on applicable markets; market size; substantial competition; our ability to continue as a going concern; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payer reimbursement; dependence upon third parties; our financial performance and results, including the risk that we are unable to manage our operating expenses or cash use for operations, or are unable to commercialize our products, within the guided ranges or otherwise as expected; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. There are no guarantees that future clinical trials discussed in this presentation will be completed or successful or that any product will receive regulatory approval for any indication or prove to be commercially successful. Investors should read the risk factors set forth in our most recent periodic reports filed with the Securities and Exchange Commission, including our Form 10-K for the year ended December 31, 2016. While the list of factors presented here is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Forward-looking statements included herein are made as of the date hereof, and we do not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances except as required by law. The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation. 2

2018 KEY BUSINESS PRIORITIES 3 Optimizing the value of Trulance Continuing to grow market share in CIC Expanding Trulance use with new IBS-C indication Pulling-through market access wins and expanding coverage Ensuring a strong financial foundation Achieving cost efficiencies and prioritizing investments Access to additional capital Continuing to evaluate all strategic and business development opportunities Exploring all options to maximize the value of Trulance and dolcanatide Leveraging commercial infrastructure and GI expertise

TRULANCE PRESCRIPTION VOLUME GROWING CONSISTENTLY SINCE LAUNCH 4 TRX - 30-Count Packs Source: IQVIA, Mar – Dec 2017 0 2000 4000 6000 8000 10000 12000 14000 16000 18000 TRx Extended Unit TRx Trulance 2017 Monthly TRx Volume
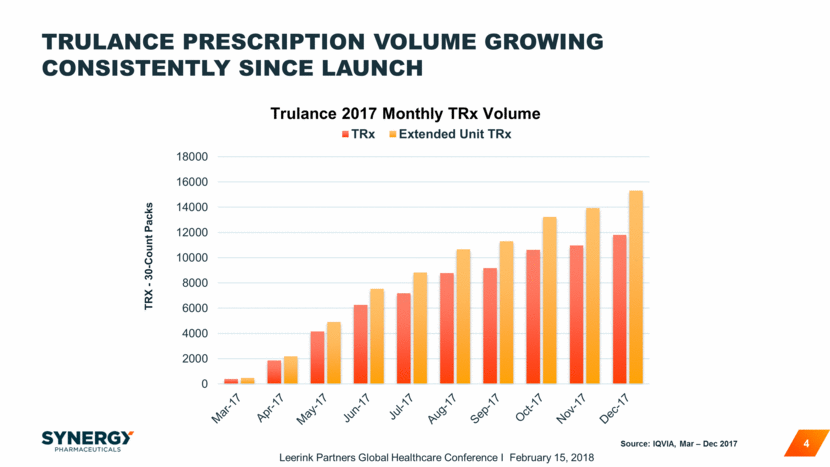
Trulance ‘pills per Rx’ increasing steadily since launch 5 Source: IQVIA, Mar – Dec 2017 30.0 31.0 32.0 33.0 34.0 35.0 36.0 37.0 38.0 39.0 40.0 Mar 2017 Apr 2017 May 2017 Jun 2017 Jul 2017 Aug 2017 Sep 2017 Oct 2017 Nov 2017 Dec 2017 Pills per Prescription Trulance TRx Size
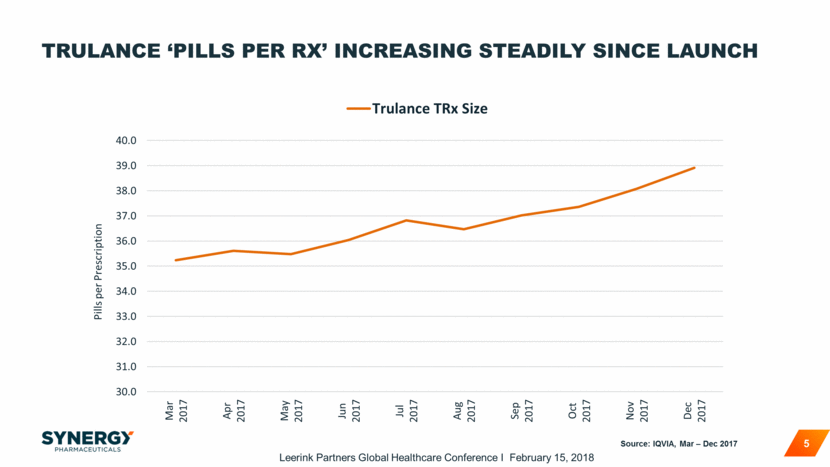
TRULANCE PRESCRIBER BASE EXPANDING SINCE LAUNCH 6 Source: IQVIA, Mar – Dec 2017 257 1,145 2,427 3,768 4,949 6,121 7,043 7,974 8,868 9,644 0 2,000 4,000 6,000 8,000 10,000 Cumulative Trulance Prescribers
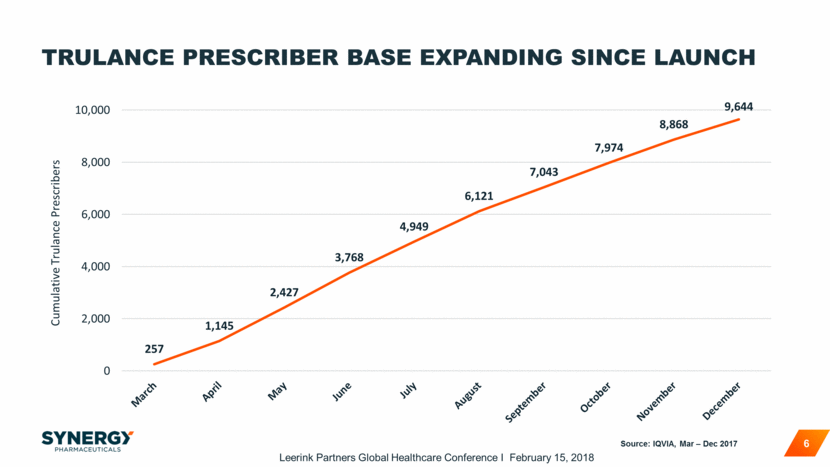
MAJORITY OF TRULANCE GROWTH COMING FROM PATIENTS NEW TO BRANDED PRESCRIPTION TREATMENTS 7 Nearly 50% of Trulance NRx were new branded Rx therapy starts 43% of Trulance patients have converted from other Rx treatments IQVIA - XPD, weeks ending 3/17 – 12/1. Add on 8% Switch To 43% New Therapy Starts 49% Trulance NRx Breakdown
NEXT STEPS CRITICAL TO ACCELERATING GROWTH IN 2018: Build off momentum from strong 2017 launch and continue to drive the uptake of Trulance Expand opportunity with new IBS-C label - ability to promote important abdominal pain component Leverage strong foundation established with healthcare providers to broaden Trulance prescriber base Continue to educate and activate the “Rx ready” patient Pull-through market access wins and expand coverage in 2018 8


