Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - ReShape Lifesciences Inc. | a18-5092_1ex99d3.htm |
| EX-99.2 - EX-99.2 - ReShape Lifesciences Inc. | a18-5092_1ex99d2.htm |
| 8-K - 8-K - ReShape Lifesciences Inc. | a18-5092_18k.htm |
NASDAQ: ETRM Company Presentation 2017 NASDAQ: RSLS Company Presentation January 2018 Confidential information. Do not distribute.

Safe Harbor Statement and Risk Factors 2 This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements generally can be identified by the use of words such as expect,” “plan,” “anticipate,” “could,” “may,” “intend,” “will,” “continue,” “future,” other words of similar meaning and the use of future dates. Forward-looking statements in this presentation include statements about the company’s total addressable market and annual procedures; competitive companies, technologies and procedures; the company’s ability to implement its business model and strategic plan, including its commercialization and reimbursement strategies; expansion of the company’s sales team; and implementation and completion of clinical trials. These forward-looking statements are based on the current expectations of our management and involve known and unknown risks and uncertainties that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others: our limited history of operations; our losses since inception and for the foreseeable future; our limited commercial sales experience with our vBloc® System for the treatment of obesity in the United States or in any foreign market other than Australia and the European Community; the competitive industry in which we operate; our ability to maintain compliance with the Nasdaq continued listing requirements; our ability to commercialize our vBloc® System; our dependence on third parties to initiate and perform our clinical trials; the need to obtain regulatory approval for any modifications to our vBloc® System; physician adoption of our vBloc® System and vBloc® Neurometabolic Therapy; our ability to obtain third party coding, coverage or payment levels; ongoing regulatory compliance; our dependence on third party manufacturers and suppliers; the successful development of our sales and marketing capabilities; our ability to raise additional capital when needed; international commercialization and operation; our ability to attract and retain management and other personnel and to manage our growth effectively; potential product liability claims; the cost and management time of operating a public company; potential healthcare fraud and abuse claims; healthcare legislative reform; our ability to obtain and maintain intellectual property protection for our technology and products; our acquisition of BarioSurg, Inc. may involve unexpected costs or liabilities; the ability to recognize benefits of the acquisition; and risks that the acquisition disrupts current plans and operations. These and additional risks and uncertainties are described more fully in the Company's filings with the Securities and Exchange Commission, particularly those factors identified as "risk factors" in the annual report on Form 10-K filed March 8, 2017 and quarterly report on Form 10-Q filed May 15, 2017. We are providing this information as of the date of this presentation and do not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise.

Addressing $1.5 billion/year surgical device market¹ Safe, minimally invasive, anatomy preserving solutions with durable outcomes Providing a comprehensive toolbox of solutions for obesity continuum of care 1. Global Bariatric Surgery Devices Market Analysis and Trends, Industry Forecast to 2025, Accuray Research 3 A medical technology company serving the growing obesity epidemic
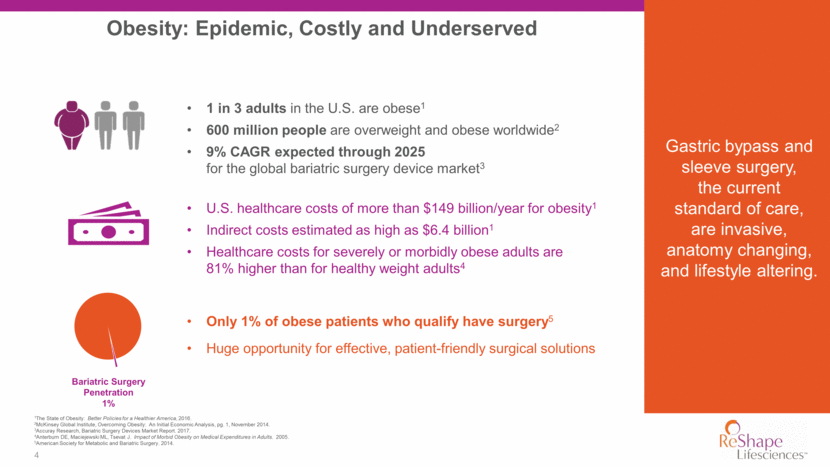
Obesity: Epidemic, Costly and Underserved 4 1The State of Obesity: Better Policies for a Healthier America, 2016. 2McKinsey Global Institute, Overcoming Obesity: An Initial Economic Analysis, pg. 1, November 2014. 3Accuray Research, Bariatric Surgery Devices Market Report. 2017. 4Anterburn DE, Maciejewski ML, Tsevat J. Impact of Morbid Obesity on Medical Expenditures in Adults. 2005. 5American Society for Metabolic and Bariatric Surgery. 2014. 1 in 3 adults in the U.S. are obese1 600 million people are overweight and obese worldwide2 9% CAGR expected through 2025 for the global bariatric surgery device market3 U.S. healthcare costs of more than $149 billion/year for obesity1 Indirect costs estimated as high as $6.4 billion1 Healthcare costs for severely or morbidly obese adults are 81% higher than for healthy weight adults4 Only 1% of obese patients who qualify have surgery5 Huge opportunity for effective, patient-friendly surgical solutions Bariatric Surgery Penetration 1% Gastric bypass and sleeve surgery, the current standard of care, are invasive, anatomy changing, and lifestyle altering.
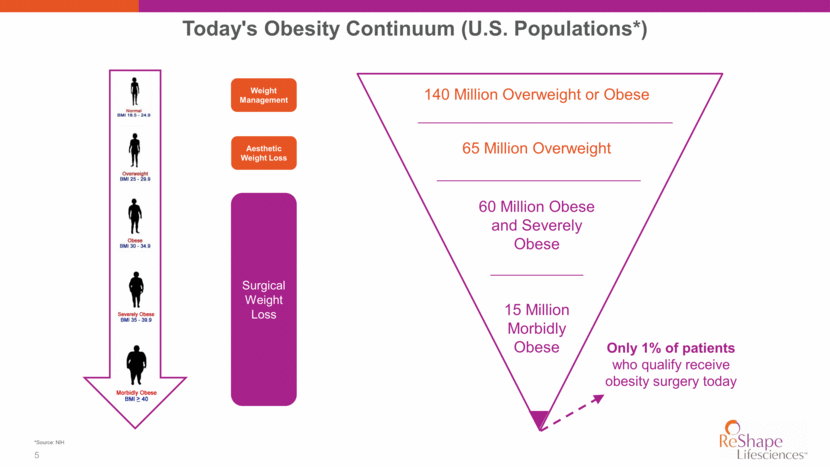
Today's Obesity Continuum (U.S. Populations*) 140 Million Overweight or Obese 65 Million Overweight 60 Million Obese and Severely Obese 15 Million Morbidly Obese Weight Management Surgical Weight Loss Only 1% of patients who qualify receive obesity surgery today Aesthetic Weight Loss *Source: NIH 5
2015 – 2025 Growth Drivers More interventions with new procedures Anatomy-friendly Out-patient Improved coverage by payers Therapies proven to address comorbidities Data supporting health economic savings Cost efficiencies vs. drugs 6 Growth of U.S. Obesity and Bariatric Procedures (2011 – 2025E) https://www.cdc.gov/nchs/data/databriefs/db288_table.pdf https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers Global Bariatric Surgery Devices Market Analysis and Trends, Industry Forecast to 2025, Accuray Research Canaccord Genuity Equity Research 2014 0 200 400 600 800 1,000 15% 20% 25% 30% 35% 40% 45% 2011 - 2012 2013 - 2014 2015 - 2016 2016 - 2017 2020E 2025E Obese U.S. Adults 34.90% 37.70% 39.60% 41.88% 44.15% Obese U.S. Youth 16.90% 17.20% 18.50% 20.21% 22.49% Surgical Procedures 158,000 179,000 196,000 216,000 800,000 Bariatric Procedures (Thousands) % of Population with Obesity
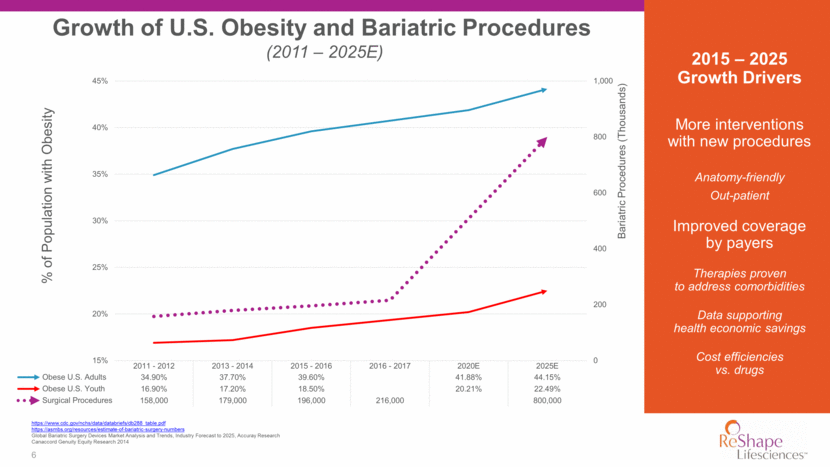
7 ReShape: Minimally Invasive Offerings for the Full Continuum of Care *ReShape Vest is for investigational use only and not approved for use. Vagal nerve stimulation blocks hunger signals Takes up room in the stomach so there is less space for food * Restriction creates feelings of fullness
ReShape Products Meet Patient Requirements 8 MINIMALLY INVASIVE LIFESTYLE ENHANCING DURABLE WEIGHT LOSS ReShape products help patients lose weight and live a more comfortable life, while also reducing co-morbidities associated with excess weight ReShape products do not change anatomy and are removable or reversible ReShape offers sustainable solutions that help patients achieve long term success Current surgical options do not satisfy patient needs; ReShape products will drive market penetration
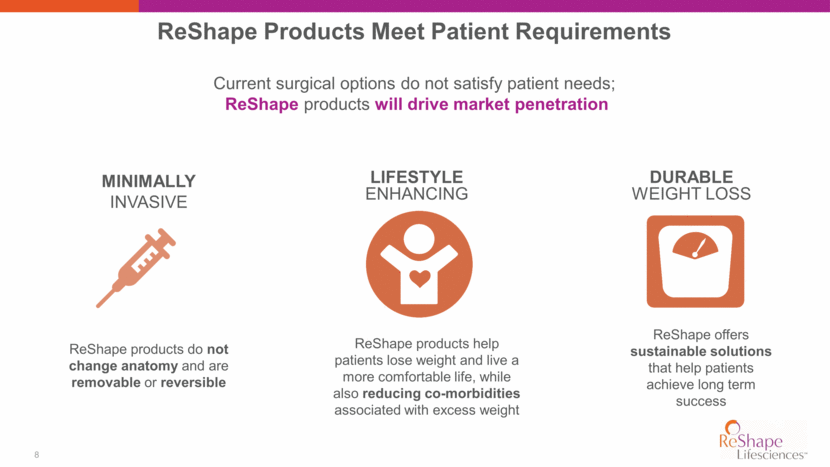
9 2015 2025E ~198,000 Procedures $1.5 Billion ~800,000 Procedures $3.8 Billion *Global Bariatric Surgery Devices Market Analysis and Trends, Industry Forecast to 2025, Accuray Research; Canaccord Genuity Equity Research 2014 U.S. Obesity Procedure Mix (2015 – 2025E)* 9% CAGR Gastric Bypass 22% Balloon, vBloc 1% Revisions 14% Lap - Band 6% Sleeve 53% Other 4% Anatomy Altering Surgery 25% Anatomy Friendly Procedures 60 % Revisions 15%
ReShape Intragastric Dual Balloon Technology 10 30 – 40 >4,000 Patients Implanted 10.5% 200+ Physicians Implanting 60% Maintained or continued to lose weight (1 year) Acquired in October 2017 FDA approved Endoscopic procedure through the mouth Not anatomy altering Six Month Balloon Implant fills volume in stomach to make patient feel full Patient BMI Range* Total Weight Loss (6 months)** * The ReShape Dual Balloon is approved for patients with a BMI of 30-40 with one or more health-related comorbidities. ** Shawn Garber, Spencer Holover, John Angstadt, Eric Sommer, Nikilesh Sekhar, Jeffrey Chiao,” Intragastric Balloon: 342 Patients Treated at a Multicenter Bariatric Practice” 30-40 30 - 40
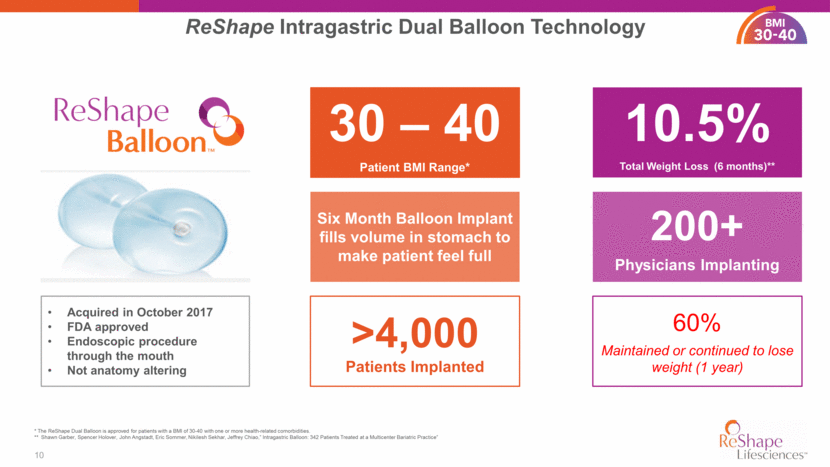
PATIENT RESULTS AT 48 MONTHS Patients demonstrated improvements in outcomes and comorbidities HbA1c (%) reduction of 0.2 points Systolic blood pressure decreased 6.6 mm Hg Diastolic blood pressure decreased 4.4 mm Hg Improvements in co-morbidities yield significant savings to the health care system ReShape Results* 11 Waist circumference reduced by 2.2" 30 - 40 * Shawn Garber, Spencer Holover, John Angstadt, Eric Sommer, Nikilesh Sekhar, Jeffrey Chiao,” Intragastric Balloon: 342 Patients Treated at a Multicenter Bariatric Practice” Decrease in LDL of 4.6 units
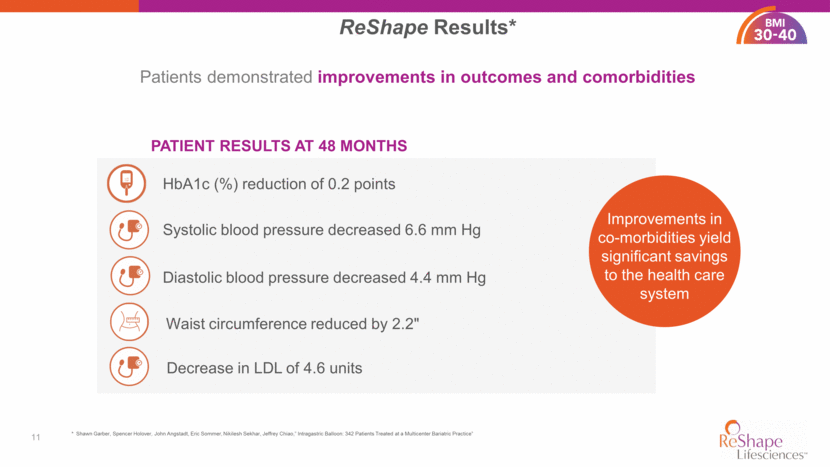
vBloc® Bioelectronic Neuroblocking Technology 12 35 - 45 Intermittently blocks signals of hunger on the vagus nerve >700 Patients Implanted 4.3” 16 Centers Implanting 25% Implanted subcutaneously Reversible or removable Not anatomy altering FDA Approved EWL at 1 Year** Patient BMI Range* * vBloc Therapy is approved for use in patients with a BMI of 35 – 40 along with one related comorbid condition and for use in patients with a BMI of 40 – 45. ** Shikora, Scott, et al. “Vagal Blocking Improves Glycemic Control and Elevated Blood Pressure in Obese Subjects with Type 2 Diabetes Mellitus.” Journal of Obesity, (2013) Article ID 245683. DOI: 10.1155/2013/245683. Waist Reduction at 1 Year** 35 - 45
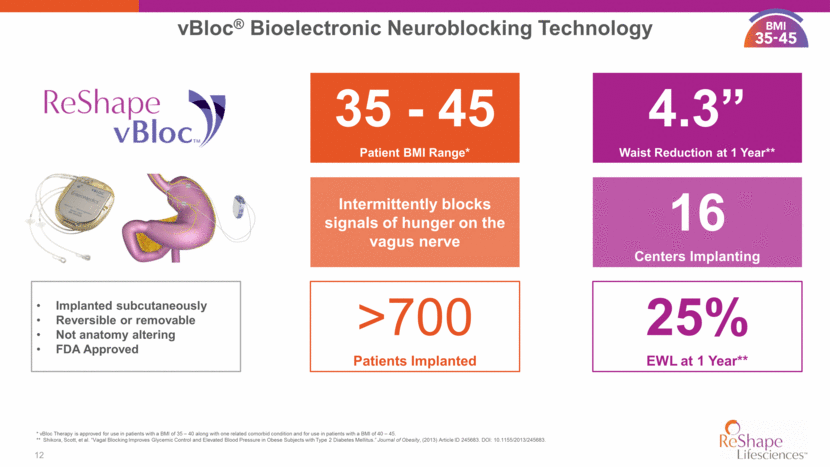
50% remittance of metabolic syndrome Systolic blood pressure reduced by 12 mmHg in patients with elevated BP Reduction in LDL “bad” cholesterol of 6.2 mg/dL HbA1c (%) reduction of 1.0 point Highly competitive with leading diabetes drugs 13 vBloc Clinical Evidence CLINICAL STUDY PATIENTS AT 1 YEAR vBloc Therapy patients achieved meaningful and sustainable weight loss and experienced a reduction in comorbidities and improvements in overall cardiovascular health CLINICAL STUDY PATIENTS AT 2 YEARS Reductions in co-morbidities yield significant savings to the health care system 50% remittance of pre-diabetes 11. Shikora, Scott, et al. “Vagal Blocking Improves Glycemic Control and Elevated Blood Pressure in Obese Subjects with Type 2 Diabetes Mellitus.” Journal of Obesity, (2013) Article ID 245683. DOI: 10.1155/2013/245683. 7.9% total body weight 35 - 45
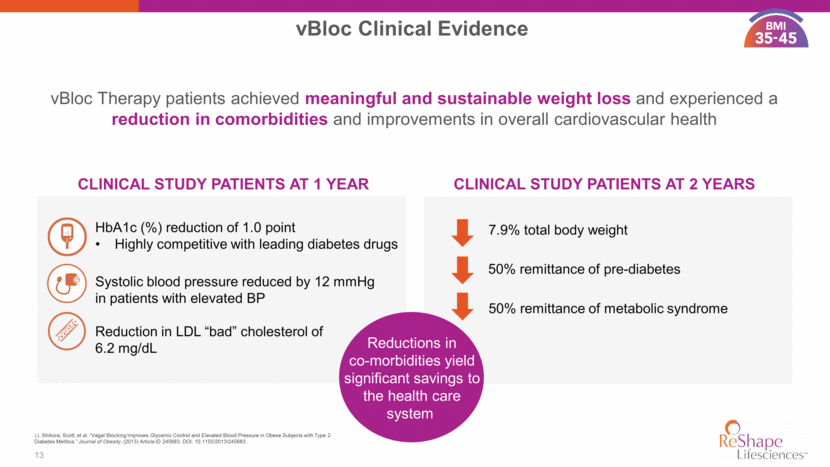
14 11. Shikora, Scott, et al. “Vagal Blocking Improves Glycemic Control and Elevated Blood Pressure in Obese Subjects with Type 2 Diabetes Mellitus.” Journal of Obesity, (2013) Article ID 245683. DOI: 10.1155/2013/245683. Study Design Status Kaiser Permanente Diabetes Study vBloc Now Patient Registry vBloc ReNEW Post Approval Study Prior Authorizations Submitted Covered Veterans with Obesity ? ? Balloon Post Approval Study Real world outcomes for reimbursement 125 patients w/ comorbidity data 3 year, 60 patients Demonstrate vBloc advantage vs meds for diabetes 5 years, 15 sites, 200 patients Safety and efficacy weeks, 15 sites, 250 patients Safety and efficacy Initiated June 2017 66 patients enrolled Study protocol completed Enrollment initiation Q1 2018 4 Sites activated 1 Enrolled with 4 scheduled 15 active sites 124 subjects enrolled Strategy to Collect Outcomes Data for Reimbursement
Provide A Compelling, Differentiated Offering Equip Practices For Success Reach & Convert Motivated & Qualified Consumers 15 The ReShape sales team is comprised of 13 direct reps, growing to 16 in 2018 All team members rep all products Three dedicated teams focus on VA, International and U.S./non-VA Only company to offer a complete toolbox of minimally invasive, patient-friendly devices for bariatric surgery Field-based marketing personnel partner with practices to drive ReShape Balloon and ReShape vBloc patient acquisition and integration Agreement with VA enables national ReShape vBloc coverage for veterans CarePlus coverage of ReShape Balloon sets precedent for corporate coverage policies Sales Strategy for vBloc and ReShape
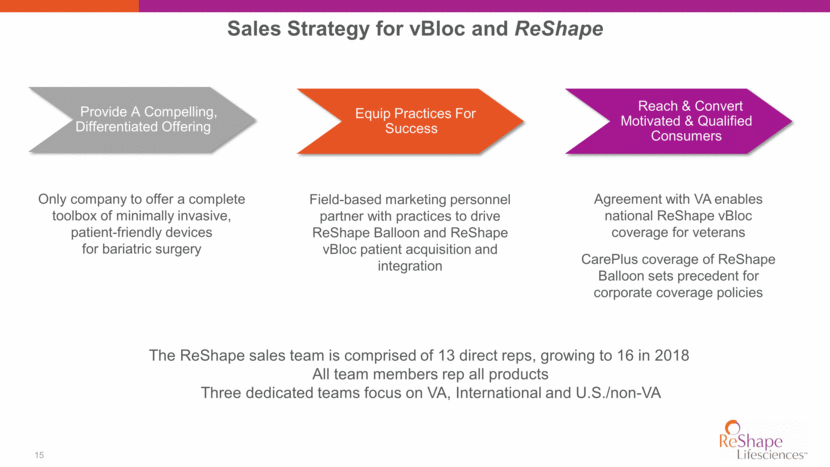
16 ReShape Vest Wraps around stomach to restrict amount of food eaten; emulates conventional weight loss surgery 85.5% 15” CE Mark Study 1H / 2018 IDE Planned 2H / 2018 Patients Implanted Excess Weight Loss (1 Year)* Acquired May 2017 Not anatomy altering Intended for morbidly obese needing rapid weight loss Currently investigational use >40 Patient BMI Range * Juan López-Corvalá MD, Fernando Guzmán-Cordero MD, Cleysa Hermosillo-Valdez MD, Janine Rosales-Landgrave MD, “Gastric Vest System: Initial Results of a Novel Restrictive Bariatric Procedure” Waist Reduction** > 40
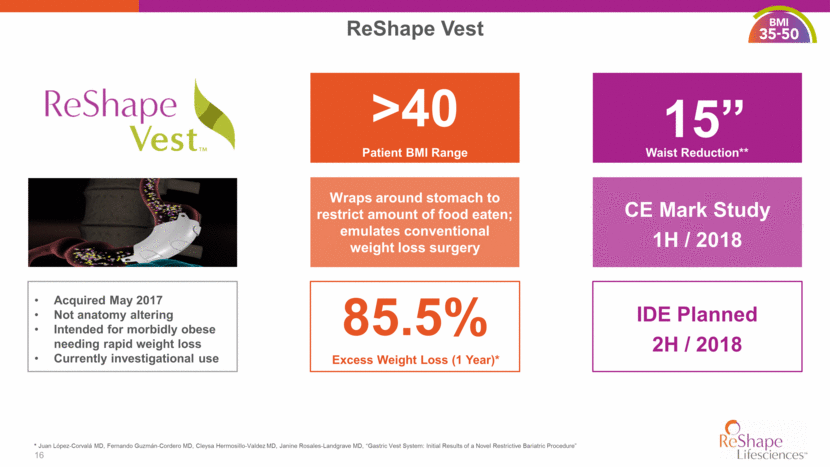
17 Gastric Vest System Pilot Study Outcomes Efficacious Pilot study patients achieved gold standard of weight loss at 12 months Minimally Invasive Vest is laparoscopic and a removable, anatomy friendly procedure Transformative Technology No other device has TBWL near the standard of bariatric surgery 30.2% 12. Schauer et al. N.E.J.M. 366: 1567, 2012. * Juan López-Corvalá MD, Fernando Guzmán-Cordero MD, Cleysa Hermosillo-Valdez MD, Janine Rosales-Landgrave MD, “Gastric Vest System: Initial Results of a Novel Restrictive Bariatric Procedure” 27.6 24.9 0 5 10 15 20 25 30 35 Month 1 Month 3 Month 6 Month 9 Month 12 Percent Total Body Weight Loss (%TBWL) Gastric Bypass Sleeve Gastrectomy Gastric Vest System™
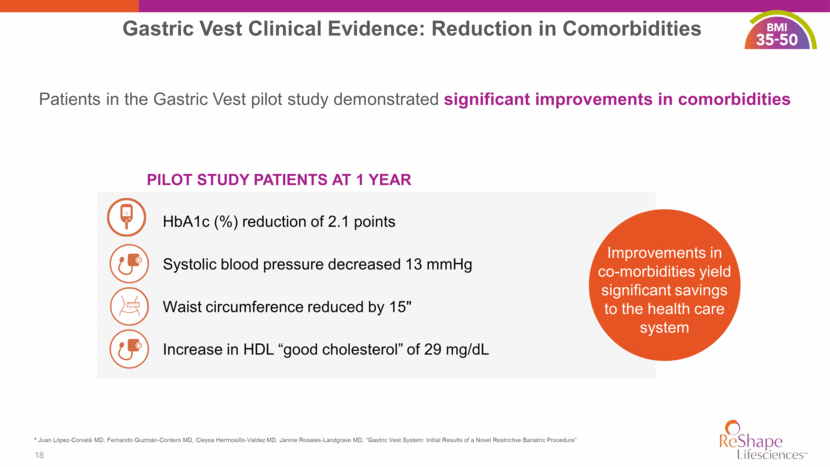
PILOT STUDY PATIENTS AT 1 YEAR Patients in the Gastric Vest pilot study demonstrated significant improvements in comorbidities HbA1c (%) reduction of 2.1 points Systolic blood pressure decreased 13 mmHg Increase in HDL “good cholesterol” of 29 mg/dL Improvements in co-morbidities yield significant savings to the health care system 18 Gastric Vest Clinical Evidence: Reduction in Comorbidities Waist circumference reduced by 15" > 40 * Juan López-Corvalá MD, Fernando Guzmán-Cordero MD, Cleysa Hermosillo-Valdez MD, Janine Rosales-Landgrave MD, “Gastric Vest System: Initial Results of a Novel Restrictive Bariatric Procedure”
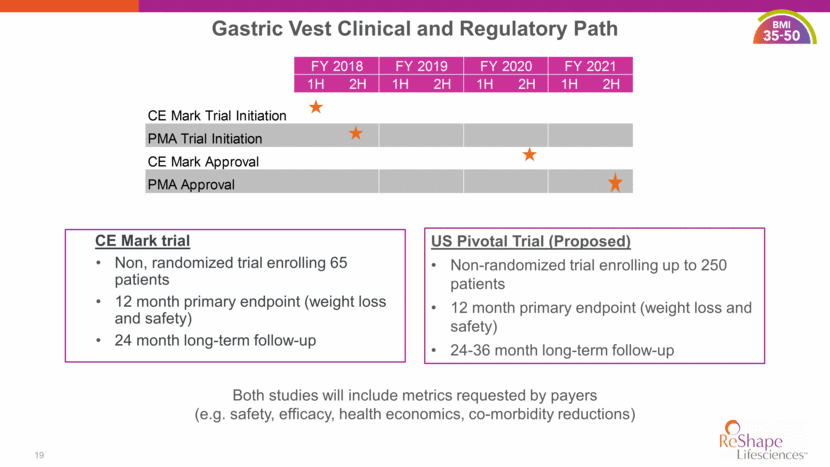
CE Mark trial Non, randomized trial enrolling 65 patients 12 month primary endpoint (weight loss and safety) 24 month long-term follow-up 19 Gastric Vest Clinical and Regulatory Path US Pivotal Trial (Proposed) Non-randomized trial enrolling up to 250 patients 12 month primary endpoint (weight loss and safety) 24-36 month long-term follow-up Both studies will include metrics requested by payers (e.g. safety, efficacy, health economics, co-morbidity reductions) 1H 2H 1H 2H 1H 2H 1H 2H CE Mark Trial Initiation PMA Trial Initiation CE Mark Approval PMA Approval FY 2018 FY 2019 FY 2020 FY 2021
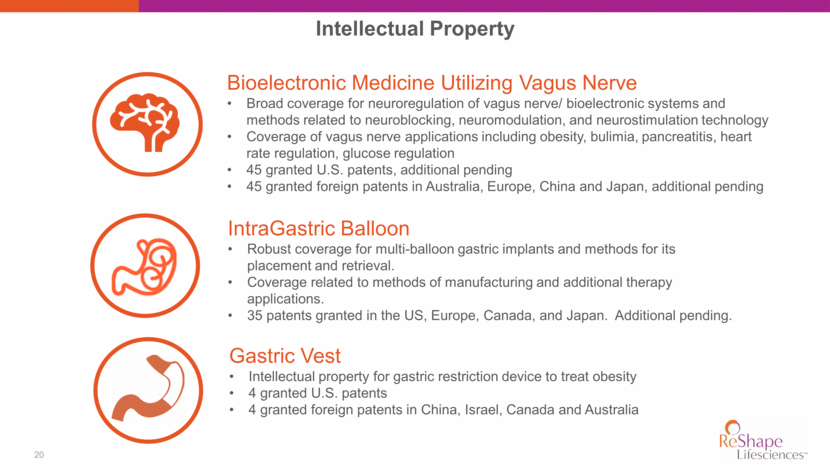
20 Intellectual Property Bioelectronic Medicine Utilizing Vagus Nerve Broad coverage for neuroregulation of vagus nerve/ bioelectronic systems and methods related to neuroblocking, neuromodulation, and neurostimulation technology Coverage of vagus nerve applications including obesity, bulimia, pancreatitis, heart rate regulation, glucose regulation 45 granted U.S. patents, additional pending 45 granted foreign patents in Australia, Europe, China and Japan, additional pending Gastric Vest Intellectual property for gastric restriction device to treat obesity 4 granted U.S. patents 4 granted foreign patents in China, Israel, Canada and Australia IntraGastric Balloon Robust coverage for multi-balloon gastric implants and methods for its placement and retrieval. Coverage related to methods of manufacturing and additional therapy applications. 35 patents granted in the US, Europe, Canada, and Japan. Additional pending.
21 Strategic Partnership for Neuroregulators and the Vagus Nerve Collaboration Agreement (for pre-clinical research) Funded co-development of new modified ReShape Lifesciences‘ products Future opportunities (continued IP + technology platform co-development) Intellectual property licensing ReShape Lifesciences will receive payments for its development work and supply under this agreement

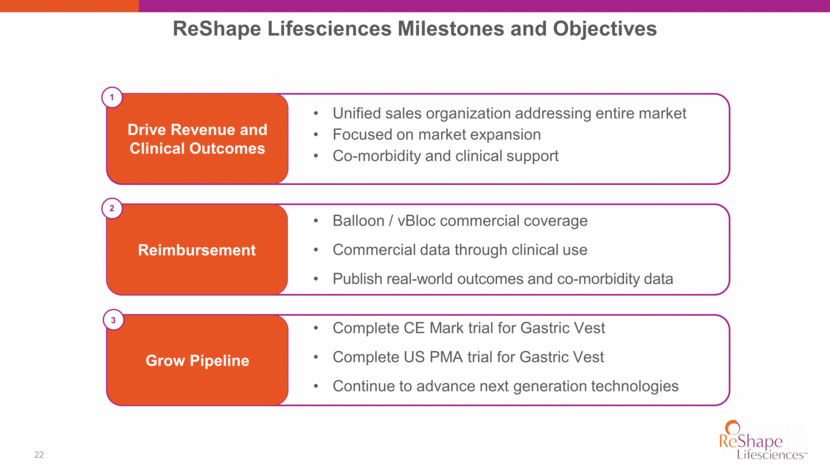
22 ReShape Lifesciences Milestones and Objectives Reimbursement Grow Pipeline Drive Revenue and Clinical Outcomes Balloon / vBloc commercial coverage Commercial data through clinical use Publish real-world outcomes and co-morbidity data Complete CE Mark trial for Gastric Vest Complete US PMA trial for Gastric Vest Continue to advance next generation technologies Unified sales organization addressing entire market Focused on market expansion Co-morbidity and clinical support 3 1 2
23 Management Team Dan w. Gladney – Chief Executive officer Health care executive with over 25 years of experience leading a variety of medical device companies Lanx, Heart Leaflet Technologies, ACIST Medical, Compex Technologies; Norwest Equity Partners Scott P. Youngstrom – Chief financial officer Financial executive focused on creating shareholder value with 25 years of strategic financial and operational experience in a variety of medical device companies Galil Medical, Anulex, Enpath Medical, Compex Technologies Raj Nihalami, MD – CHIEF Technology officer and business devlopment Medical device executive and physician entrepreneur with 20 + years of experience in small and large medtech companies Founder and CEO of BarioSurg; Onciomed, Medtronic, Endologix, Acufocus, Rox Medical, Mdnook DEBORAH SCHMALZ – VICE PRESIDENT, CLINICAL AND REGULATORY Over 25 years of executive leadership experience in medical device regulatory affairs, clinical research, compliance and reimbursement Medpace, Veniti, Entellus Medical, Vascular Solutions, Empi BOB HAGGERTY – VICE PRESIDENT, SALES Proven background in creating distribution, launching new technologies, and developing long-term relationships with customers Ceterix Orthopaedics, Coviden/GI Solutions, BARRX Medical, Gyrus/ ACMI, Boston Scientific Amy Scott – vice president, marketing Over 20 years of marketing and commercialization experience with medical technologies Edwards Lifesciences, WaveTec Vision Systems, DJO Global Sharon Whalen – vice president, REIMBURSEMENT Leader in medical device and managed care. Evidence development, health technology reviews, new product reimbursement, and advocacy expertise. Acelity, Edwards Lifesciences, World Heart, Endocare, PacticCare.

ReShape Lifesciences Inc. (NASDAQ:RSLS) 1001 Calle Amanecer San Clemente, CA 92673 Dan Gladney President, Chief Executive Officer & Chairman of the Board 651-634-3089 dwgladney@reshapelifesci.com

APPENDIX

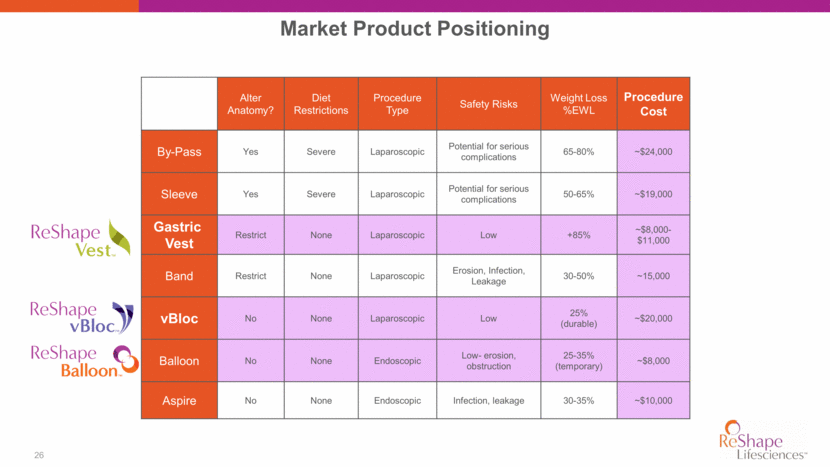
26 Market Product Positioning Alter Anatomy? Diet Restrictions Procedure Type Safety Risks Weight Loss %EWL Procedure Cost By-Pass Yes Severe Laparoscopic Potential for serious complications 65-80% ~$24,000 Sleeve Yes Severe Laparoscopic Potential for serious complications 50-65% ~$19,000 Gastric Vest Restrict None Laparoscopic Low +85% ~$8,000-$11,000 Band Restrict None Laparoscopic Erosion, Infection, Leakage 30-50% ~15,000 vBloc No None Laparoscopic Low 25% (durable) ~$20,000 Balloon No None Endoscopic Low- erosion, obstruction 25-35% (temporary) ~$8,000 Aspire No None Endoscopic Infection, leakage 30-35% ~$10,000