Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - FIVE PRIME THERAPEUTICS, INC. | d524382d8k.htm |
Exhibit 99.1
As used in this Exhibit 99.1, “Five Prime,” “the company,” “we,” “us” and “our” refer to Five Prime Therapeutics, Inc. The Five Prime logo is our registered trademark. All other registered marks, trademarks and trade names included in this Exhibit 99.1 are the property of their respective owners.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement and the accompanying prospectus, including the documents incorporated by reference herein and therein, and any free writing prospectus that we have authorized for use in connection with this offering contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, which we refer to as the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, which we refer to as the Exchange Act, that involve substantial risks and uncertainties. In some cases you can identify these statements by forward-looking words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “would,” “project,” “plan,” “expect” or similar expressions, or the negative or plural of these words or expressions. Discussions containing these forward-looking statements may be found, among other places, in “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” incorporated by reference from our most recent Annual Report on Form 10-K and in our most recent Quarterly Report on Form 10-Q filed with the SEC, as well as any amendments thereto reflected in subsequent filings with the SEC. These statements involve risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. These forward-looking statements include, but are not limited to, statements concerning the following:
| • | our planned uses for the net proceeds we expect to receive from this offering; |
| • | our estimates regarding our expenses, revenues, anticipated capital requirements and our needs for additional financing; |
| • | our potential receipt of future milestone payments or royalties, and the timing of such payments; |
| • | our or our partners’ ability to timely advance drug candidates into and through clinical data readouts and successful completion of clinical trials; |
| • | the timing of the initiation, progress and results of preclinical studies and research and development programs; |
| • | our expectations regarding the potential safety, efficacy or clinical utility of our product candidates; |
| • | the implementation, timing and likelihood of success of our plans to develop companion diagnostics for our product candidates; |
| • | our ability to establish and maintain collaborations and necessary licenses; |
| • | the implementation of our business model and strategic plans for our business, product candidates and technology; |
| • | the scope of protection we establish and maintain for intellectual property rights covering our product candidates and technology; |
| • | the size of patient populations targeted by products we or our partners develop and market adoption of such products by physicians and patients; |
| • | the timing or likelihood of regulatory filings and approvals; |
| • | the ability to negotiate adequate reimbursement and pricing for our product candidates by third parties and government authorities; |
| • | developments relating to our competitors and our industry; and |
| • | our expectations regarding licensing, acquisitions and strategic operations. |
In addition, you should refer to the “Risk Factors” section in any free writing prospectus we may authorize for use in connection with this offering for a discussion of other important factors, risks and uncertainties that
1
may cause our actual results to differ materially from those expressed or implied by these forward-looking statements. Given these other important factors, risks and uncertainties, you should not place undue reliance on these forward-looking statements. Also, these forward-looking statements represent our estimates and assumptions only as of the date such forward-looking statements are made. You should carefully read this prospectus supplement and the accompanying prospectus, together with the information incorporated by reference herein and therein completely and with the understanding that our actual future results may be materially different from what we expect. We can give no assurances that any of the events anticipated by the forward-looking statements will occur or, if any of them do, what impact they will have on our business, results of operations and financial condition.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this prospectus supplement, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.
You should rely only on information contained or incorporated by reference in this prospectus supplement, the accompanying prospectus, the registration statement of which this prospectus supplement is a part, including the exhibits that we have filed with the registration statement, and in any free writing prospectus we may authorize for use in connection with this offering. You should understand that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in the foregoing documents by these cautionary statements.
Except as required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or developments. You should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. Before deciding to invest, you should carefully consider the risk factors discussed and incorporated by reference in this prospectus supplement and the accompanying prospectus or in any free writing prospectus that we may have authorized for use in connection with this offering.
2
Our Company
We are a clinical-stage biotechnology company focused on discovering and developing innovative protein therapeutics to improve the lives of patients with serious diseases. Each of our product candidates has an innovative mechanism of action and addresses patient populations for which better therapies are needed. We have an emphasis in immuno-oncology, an area in which we have clinical, preclinical and discovery programs and product and discovery collaborations. In addition, we plan to use companion diagnostics where appropriate to allow us to select patients most likely to benefit from treatment with our product candidates. Our most advanced product candidates are identified below.
| • | Cabiralizumab (FPA008) is an antibody that inhibits colony stimulating factor-1, or CSF1, receptor, or CSF1R, that we are studying in clinical trials as a monotherapy in tenosynovial giant cell tumor, also known as diffuse pigmented villonodular synovitis, or PVNS, and in multiple cancers in combination with Bristol-Myers Squibb Company’s, or BMS, PD-1 immune checkpoint inhibitor, Opdivo® (nivolumab). In October 2015, we entered into a license and collaboration agreement, or the cabiralizumab collaboration agreement, with BMS, pursuant to which we granted BMS an exclusive worldwide license for the development and commercialization of cabiralizumab. |
| • | Bemarituzumab (FPA144) is an antibody that inhibits fibroblast growth factor receptor 2b, or FGFR2b, that we are initially developing to treat patients with gastric (stomach) or gastroesophageal junction, or GEJ, cancer and bladder cancer. In December 2017, we entered into a license and collaboration agreement, or the bemarituzumab collaboration agreement, with Zai Lab (Shanghai) Co., Ltd., or Zai Lab, pursuant to which we granted Zai Lab an exclusive license for the development and commercialization of bemarituzumab in China, Hong Kong, Macau and Taiwan. |
| • | FPA150 is a CD8 T cell checkpoint inhibitor antibody that targets B7-H4 that we are initially developing as a monotherapy in multiple cancers. We plan to begin a Phase 1 clinical trial for FPA150 in the first half of 2018. |
We have a differentiated target discovery platform and extensive libraries of extracellular soluble proteins that we believe encompass substantially all of the body’s medically important targets for protein therapeutics. We have identified approximately 700 of these proteins, which we refer to as the immunome, that we believe modulate immune cell interactions and may be important in understanding and treating cancer in patients using immuno-oncology therapeutics. Our target discovery platform and capabilities position us well to explore pathways in cancer and inflammation and their intersection in immuno-oncology, an area of oncology with significant therapeutic potential and the focus of our research activities. We are applying our biologics discovery platform, including cell-based screening, immunome-by-immunome biophysical interaction screening, in vivo screening, receptor-ligand matching technologies and bioinformatics, in our immuno-oncology research program. We have identified several targets that we believe could be useful in immuno-oncology that we are actively validating, and we are also conducting research to discover additional targets. We generate and preclinically test
3
therapeutic proteins, including antibodies and fusion proteins containing or directed to the targets we identify. We plan to continue to advance selected therapeutic candidates into clinical development.
We have no products approved for commercial sale and have not generated any revenue from product sales to date. We continue to incur significant research and development and other expenses related to our ongoing operations. We expect that our expenses will increase as we advance our product candidates into later stages of clinical development and increase the number of product candidates in clinical development. We have incurred losses in each period since our inception in 2002, with the exception of the fiscal year ended December 31, 2015, due primarily to the $350.0 million upfront payment we received from BMS under our cabiralizumab collaboration agreement, and the fiscal year ended December 31, 2011, due primarily to the $50.0 million upfront payment we received from GlaxoSmithKline from our license and collaboration agreement for FP-1039, our FGFR1-Fc fusion protein. For the nine months ended September 30, 2017 and 2016, we reported a net loss of $121.0 million and $45.6 million, respectively.
Recent Events
Cabiralizumab
In November 2017, we completed enrollment in the Phase 1b portion of our Phase 1a/1b clinical trial to evaluate the safety, tolerability and preliminary efficacy of combining cabiralizumab with Opdivo as a potential treatment for a variety of cancers.
In November 2017, we presented preliminary safety, tolerability and efficacy data from patients from the Phase 1a/1b clinical trial at the Society for Immunotherapy of Cancer 32nd Annual Meeting, or the SITC presentation. As of the August 1, 2017 data cutoff for the SITC presentation, we had tested cabiralizumab as monotherapy in advanced solid tumors at escalating doses of cabiralizumab in 24 patients, in combination with Opdivo in advanced solid tumors at escalating doses of cabiralizumab in 10 patients, and in combination with Opdivo in advanced solid tumors in disease-specific cohorts at a dose of 4 mg/kg of cabiralizumab every two weeks in 195 patients. We observed a tolerable safety profile of cabiralizumab monotherapy and of cabiralizumab in combination with Opdivo. The most common treatment-related laboratory abnormalities were elevations in creatine kinase and serum liver enzymes without an associated elevation in bilirubin levels or other clinical sequelae. These treatment-related adverse abnormalities are believed to be secondary to cabiralizumab’s depletion of Kupffer cells and have been observed with other CSF1R-targeting agents. The most common treatment-related adverse events were: periorbital edema (20.8%), fatigue (29.2%), nausea (12.5%) and pruritus (8.3%). Grade 5 treatment-related adverse events in the trial occurred in three (1.3%) patients treated with a combination of cabiralizumab and Opdivo. The Grade 5 events were pneumonitis in a patient with thyroid cancer and respiratory distress and acute respiratory distress in two patients with lung cancer.
Among the other data, we observed preliminary evidence of a durable clinical benefit of the combination therapy in the cohort of patients with advanced pancreatic cancer. Based on radiographic assessments of anti-tumor activity in the 31 second- or later-line patients who had advanced pancreatic cancer, we observed, as of the August 1, 2017 data cutoff date:
| • | five patients with durable clinical benefit (16%); |
| • | four confirmed objective responses (13%); and |
| • | disease control for at least five to over nine months. |
All four confirmed objective responses were in patients with microsatellite stable tumors who had received an average of three prior therapies. In addition, the responses were accompanied by steep declines in levels of the pancreatic tumor marker CA-19-9 over the baseline.
4
The data suggest that a combination therapy of cabiralizumab with Opdivo may benefit patients with pancreatic cancer, including those with microsatellite stable tumors, and support further study of cabiralizumab in combination with Opdivo in pancreatic cancer.
Based on the clinical data we observed in the cohort of patients with pancreatic cancer in the Phase 1a/1b trial, we are in the process of enrolling approximately 30 patients with second- or later-line pancreatic cancer in the Phase 1a portion of our Phase 1a/1b clinical trial to further evaluate the combination of cabiralizumab and Opdivo in this patient population. We are collecting pre- and on-treatment tumor biopsy samples from these patients, and are conducting comprehensive biomarker analyses to evaluate potential biomarker signatures that may predict responsiveness to this therapeutic combination and to assess changes that occur in the tumor microenvironment following treatment.
BMS is currently recruiting patients for a randomized, multi-arm Phase 2 clinical trial to determine the efficacy of cabiralizumab in combination with Opdivo, with and without chemotherapy, as a treatment for patients with second-line pancreatic cancer (NCT03336216). BMS plans to enroll approximately 160 patients with pancreatic cancer in the study, who will be randomized to one of four study arms based on the patient’s prior therapy. The dosing of the first patient in the trial by BMS will trigger a $25 million milestone payable to us under the license and collaboration agreement between the companies established in 2015.
Bemarituzumab (FPA144)
Phase 1 Clinical Trial of Bemarituzumab (FPA144) in Gastric and GEJ Cancer
We are conducting a Phase 1 clinical trial of bemarituzumab (FPA144) to evaluate the safety, pharmacokinetics, or PK, and efficacy of bemarituzumab as a monotherapy in patients with metastatic gastric and GEJ cancer and bladder cancer whose tumors overexpress the FGFR2b protein. In June 2017, we presented updated safety and efficacy data from the Phase 1 clinical trial in a clinical poster at the 2017 ASCO Annual Meeting, or the ASCO presentation. As of the March 20, 2017 data cut-off date for the ASCO presentation, we had tested bemarituzumab in advanced solid tumors at doses of up to 15 mg/kg given as monotherapy every two weeks, including in patients with gastric or GEJ cancer and one patient with bladder cancer. We did not observe any dose-limiting toxicities or a maximum-tolerated dose. In addition, unlike small molecule FGF receptor kinase inhibitors, which block signaling through a broad number of FGF receptors and can lead to hyperphosphatemia, we did not observe any treatment-related hyperphosphatemia in patients treated with bemarituzumab. All treatment-related adverse events were Grades 1, 2 or 3. All treatment-related ocular adverse events were Grades 1 or 2, and no retinal toxicity was reported.
With respect to the patients with gastric or GEJ cancer, we observed preliminary anti-tumor activity with bemarituzumab monotherapy in late-line patients who had a median of three prior therapies and whose tumors overexpress the FGFR2b protein. Based on radiographic assessments by RECIST 1.1 of anti-tumor activity in the 21 patients who had high FGFR2b+ overexpressing gastric or GEJ cancer, we observed, as of the March 20, 2017 data cut-off date:
| • | four confirmed partial responses (one each at the 6 mg/kg and 10 mg/kg dose levels and two at the 15 mg/kg dose levels); one unconfirmed partial response (at the 15 mg/kg dose level); |
| • | an objective response rate, or ORR, of 19.0%; |
| • | a median duration of response of 15.4 weeks; and |
| • | a disease control rate, or DCR, at 6 weeks of 57.1%. |
In September 2017, we closed enrollment in the expansion cohorts of metastatic gastric and GEJ cancer in our Phase 1 clinical trial to focus efforts on preparing for our global Phase 1/3 registrational trial of
5
bemarituzumab in combination with 5-fluorouracil (5-FU), leucovorin, and oxaliplatin, or mFOLFOX6, as front-line treatment of patients with gastric and GEJ cancer that overexpresses FGFR2b, or the FIGHT trial. We continue to enroll patients in the bladder cancer expansion cohort in our Phase 1 trial.
Phase 1/3 FIGHT Clinical Trial
We designed our initial Phase 1 clinical trial testing bemarituzumab as monotherapy to evaluate the safety and tolerability of bemarituzumab as well as to gain early evidence of effectiveness, including by evaluating ORR, DCR and duration of response of patients with gastric or GEJ cancer that overexpresses FGFR2b. We have generated ORR, DCR and duration of response and safety data in our initial Phase 1 clinical trial that we believe support the evaluation of bemarituzumab in a registrational trial. Because patients with gastric or GEJ cancer that overexpresses FGFR2b have a worse prognosis as compared to those patients that do not overexpress FGFR2b, we believe that patients with FGFR2b-overexpressing disease progress more rapidly and that such patients are less likely to survive and become third- or even second-line patients. As a result, we believe testing bemarituzumab as a front-line treatment would increase the pool of patients that would be eligible to enroll in the trial and would result in faster enrollment and completion of a registrational trial than had we decided to test bemarituzumab as a second- or third-line treatment. In addition, because of the heterogeneity of advanced gastric and GEJ cancer, and because our preclinical data show additive efficacy against FGFR2b-overexpressing gastric cancer when adding bemarituzumab to chemotherapy, we believe that testing bemarituzumab in combination with chemotherapy may increase the extent and duration of response as compared to treatment with bemarituzumab alone. Moreover, we believe that bemarituzumab’s safety profile allows for the combination of bemarituzumab with chemotherapy while maintaining an acceptable safety profile. Based on the foregoing, we designed the FIGHT trial to test bemarituzumab in combination with mFOLFOX6 as front-line treatment of patients with gastric or GEJ cancer that overexpresses FGFR2b.
Because we have not yet clinically tested bemarituzumab in combination with mFOLFOX6, we included a Phase 1 safety lead-in for the FIGHT trial. During this Phase 1 safety lead-in portion of the FIGHT trial, we will evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of bemarituzumab in combination with mFOLFOX6 in patients with any type of gastrointestinal cancer to identify a recommended dose of bemarituzumab to use in the Phase 3 portion of the trial. In December 2017, we initiated dosing in the Phase 1 safety lead-in portion of the FIGHT trial. We expect to initiate the Phase 3 portion of the trial in mid-2018.
Exclusive License Agreement with Zai Lab
In December 2017, we entered into the bemarituzumab collaboration agreement with Zai Lab, pursuant to which we granted Zai Lab an exclusive license to develop and commercialize bemarituzumab, and all fragments, conjugates, derivatives and modifications thereof, or the licensed antibody, in China, Hong Kong, Macau, and Taiwan, each a region, and collectively, the territory.
Under the terms of the bemarituzumab collaboration agreement, Zai Lab will be responsible, at its expense, for (i) developing and commercializing products containing the licensed antibody, each, a licensed product, under a territory development plan and (ii) performing certain development activities to support our global development and registration of licensed products, including the Phase 3 portion of the FIGHT trial, in the territory, under a global development plan.
Pursuant to the bemarituzumab collaboration agreement, we earned a $5 million upfront payment from Zai Lab in December 2017. Additionally, with respect to each licensed product, we are eligible to receive up to $39 million in specified development and regulatory milestone payments.
Zai Lab will also be obligated to pay us a royalty, on a licensed product-by-licensed product and region-by-region basis, in the high teens or low twenties, depending on the number of patients Zai Lab enrolls in
6
the FIGHT trial, subject to reduction in certain circumstances, on net sales of each licensed product in the territory until the latest of (i) the 11th anniversary of the first commercial sale of such licensed product in such region, (ii) the expiration of certain patents covering such licensed product in such region, and (iii) the date on which any applicable regulatory, pediatric, orphan drug or data exclusivity with respect to such licensed product expires in such region. We cannot determine the date on which Zai Lab’s potential royalty payment obligations to us would expire because Zai Lab has not yet developed any licensed products under the bemarituzumab collaboration agreement, and we therefore cannot at this time identify the date of the first commercial sale or any related patents covering or regulatory exclusivity periods with respect to such licensed product.
Under the agreement, provided that Zai Lab enrolls and treats a specified number of patients in the FIGHT trial in China, Zai Lab is eligible to receive a low single-digit percentage royalty, on a licensed product-by-licensed product basis on net sales of a licensed product outside the territory until the 10th anniversary of the first commercial sale of each such licensed product outside the territory.
Unless earlier terminated by either party, the bemarituzumab collaboration agreement will expire on a licensed product-by-licensed product and region-by-region basis upon the expiration of Zai Lab’s payment obligations with respect to each licensed product under the agreement. Zai Lab may terminate the agreement in its entirety at any time with advance written notice. Either party may terminate the agreement in its entirety with written notice for the other party’s material breach if such party fails to cure the breach. We may terminate the agreement in its entirety with written notice for Zai Lab’s material breach of its diligence obligations with respect to development and obtaining marketing approval, and may terminate the agreement on a region-by-region basis for Zai Lab’s breach of its diligence obligations with respect to timely commercialization of a licensed product in a region following marketing approval. We may terminate the agreement in its entirety if Zai Lab or its affiliates or sublicensees commences a legal action challenging the validity, enforceability or scope of any of our patents in the territory. Either party also may terminate the agreement in its entirety upon certain insolvency events involving the other party.
FPA150
In December 2017, we filed an IND to initiate a Phase 1a/1b clinical trial to evaluate the safety, tolerability and preliminary efficacy of FPA150 monotherapy as a potential therapy in patients with a variety of cancers. In January 2018, we received clearance from the FDA to proceed with the clinical development of FPA150.
BMS Discovery Collaboration Agreement
In December 2017, we earned a $5 million milestone payment under our March 2014 discovery collaboration agreement with BMS in connection with BMS’s filing of an IND for its fully-human monoclonal antibody targeting TIM-3 (T-cell immunoglobulin and mucin domain-3), an immune checkpoint receptor that is known to limit the duration and magnitude of T-cell responses. This antibody is BMS’s first clinical candidate arising from the collaboration.
In addition, BMS exercised its option to extend the research term of the collaboration to March 2019. BMS will provide us with funding for the additional research we will conduct during the extended term. This is the second extension to the original collaboration term under the agreement.
7
Clinical Pipeline
The following table shows the stage of development of our most advanced product candidates:
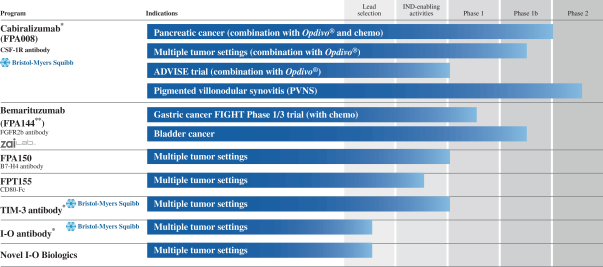
| * | Partnered with BMS—see “Part I—Item 1. Collaborations” of our most recent Annual Report on Form 10-K for a description of the commercial arrangement with BMS. |
| ** | Partnered with Zai Lab—see our Current Report on Form 8-K filed with the SEC on December 19, 2017 for a description of the bemarituzumab collaboration agreement with Zai Lab. |
8
