Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - BeiGene, Ltd. | a18-3293_1ex99d1.htm |
| EX-99.3 - EX-99.3 - BeiGene, Ltd. | a18-3293_1ex99d3.htm |
| 8-K - 8-K - BeiGene, Ltd. | a18-3293_18k.htm |
Company Overview
We are a commercial-stage biopharmaceutical company rooted in China that is dedicated to becoming a global leader in the discovery, development and commercialization of innovative, molecularly targeted and immuno-oncology drugs for the treatment of cancer.
We have three internally-developed late-stage clinical drug candidates:
· Zanubrutinib (BGB-3111) — an investigational small molecule inhibitor of Bruton’s tyrosine kinase, or BTK, that is currently being evaluated in a broad registrational clinical program globally and in China as a monotherapy and in combination with other therapies to treat various lymphomas;
· Tislelizumab (BGB-A317) — an investigational humanized monoclonal antibody against the immune checkpoint receptor PD-1 that is currently being evaluated in a broad registrational clinical program globally and in China as a monotherapy and in combination with other therapies to treat various solid and hematological cancers; and
· Pamiparib (BGB-290) — an investigational small molecule inhibitor of PARP1 and PARP2 that is being evaluated as a potential monotherapy and in combination for various solid tumors. It is currently in a pivotal clinical trial in China and is expected to enter late-stage development globally in 2018.
In 2017, we entered into a strategic collaboration with Celgene Corporation, or Celgene, in which we granted Celgene exclusive rights to develop and commercialize tislelizumab for solid tumors in the United States, Europe, Japan, and the rest of the world outside of Asia. We retained rights to tislelizumab for solid tumors in Asia (ex-Japan) and for hematological malignancies and internal combinations globally.
In addition, Celgene granted us an exclusive license to market its approved cancer therapies ABRAXANE®, REVLIMID®, and VIDAZA® in China excluding Hong Kong, Macau and Taiwan and also transferred its commercial operations and personnel in China to us in connection with our acquisition of 100% of the equity interests of Celgene Pharmaceutical (Shanghai) Co., Ltd., which has allowed us to generate product revenue in China since September 2017 and which we expect to expand in preparation for the potential launch of our internally developed drug candidates and our other in-licensed drug candidates in China.
As of January 1, 2018, we have a global team of over 850 employees, including more than 400 scientists and clinicians, in China, the United States and Australia. Our offices are located in Beijing; Shanghai; Cambridge, MA; Fort Lee, NJ; and the San Francisco Bay Area, CA; including a research and development center in Beijing, manufacturing sites in Suzhou and Guangzhou, and commercial operations in Shanghai.
The following table summarizes the status of our product portfolio and pipeline as of the date of this prospectus supplement:
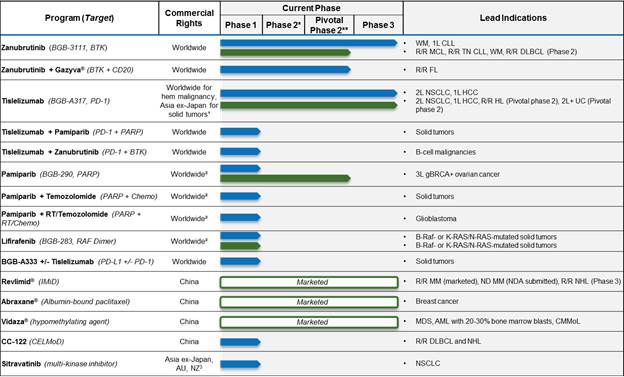
![]()
Abbreviations: WM = Waldenstrom’s macroglobulinemia; CLL = chronic lymphocytic leukemia; MCL = mantle cell lymphoma; FL = follicular lymphoma; NSCLC = non-small cell lung cancer; HCC = hepatocellular carcinoma; MM = multiple myeloma; HL = Hodgkin’s lymphoma; NHL = non-Hodgkin’s lymphoma; DLBCL = diffuse large B-cell lymphoma; MDS = Myelodysplastic syndrome; AML = acute myeloid leukemia; UC = urothelial carcinoma; CMMoL = chronic myelomonocytic leukemia; 1L/2L/3L = first, second or third line; R/R = relapsed/refractory; ND = newly diagnosed.
* Some indications will not require a non-pivotal Phase 2 clinical trial prior to beginning pivotal Phase 2 or 3 clinical trials.
** Confirmatory clinical trials post-approval are required for accelerated approvals.
(1) Celgene has the right to develop and commercialize tislelizumab in solid tumors in the United States, European Union, Japan and the rest-of-world outside of Asia.
(2) Limited collaboration with Merck KGaA.
(3) Partnership with Mirati Therapeutics, Inc.
Recent Developments
On January 9, 2018, we announced that we had entered into a commercial supply agreement with Boehringer Ingelheim Biopharmaceuticals (China) Ltd., or BI, for tislelizumab, which will be manufactured at BI’s facility in Shanghai.
On January 8, 2018, we announced that we had entered into an exclusive license agreement with Mirati Therapeutics, Inc., or Mirati, for the development, manufacturing and commercialization of Mirati’s investigational tyrosine kinase inhibitor sitravatinib in Asia (excluding Japan), Australia, and New Zealand.
We may be a passive foreign investment company in future taxable years, which may have adverse U.S. federal income tax consequences for U.S. shareholders.
U.S. investors should be aware that we determined that we were a passive foreign investment company, within the meaning of Section 1297 of the Internal Revenue Code of 1986, as amended, or PFIC, for 2016. Based on the composition of our assets and income in 2017, we believe we were not a PFIC for 2017 and based on the expected composition of our assets and income, we do not expect to be a PFIC for 2018. However, as our PFIC status must be determined annually with respect to each taxable year and will depend on the composition and character of our assets and income and the value of our assets (which may be determined, in part, by reference to the market value of our ADSs, which may be volatile) over the course of such taxable year and as we currently hold and expect to continue to hold a substantial amount of cash and cash equivalents, we may be a PFIC in any taxable year. If we are a PFIC for any taxable year during a U.S. shareholder’s holding period of the ADSs or ordinary shares, then, regardless of whether we cease to meet the threshold requirements for PFIC status, such U.S. shareholder generally will be required to treat any gain realized upon a disposition of the ADSs or ordinary shares, or any “excess distribution” received on the ADSs or ordinary shares, as ordinary income earned over the U.S. shareholder’s holding period for the ADSs or ordinary shares, and to pay the applicable taxes on such ordinary income along with an interest charge at the rate applicable to underpayments of tax on a portion of the resulting tax liability. In addition, the U.S. shareholder would be subject to the same adverse U.S. federal income tax consequences on (i) certain distributions by any of our subsidiaries treated as PFICs (“lower-tier PFICs”), and (ii) a disposition of shares of a lower-tier PFIC, in each case as if the U.S. shareholder owned the shares of the relevant lower-tier PFIC directly, even though the U.S. shareholder has not received the proceeds of those distributions or dispositions. For further information, U.S. shareholders should read the discussion under “Taxation—Material United States Federal Income Tax Considerations—Passive Foreign Investment Company” in the accompanying prospectus. Each U.S. shareholder should consult its own tax advisors regarding the PFIC rules and the U.S. federal income tax consequences of the acquisition, ownership and disposition of the ADSs or ordinary shares.
Risks Related to Obtaining Regulatory Approval for Our Drug Candidates
Our drug candidates may cause undesirable adverse events or have other properties that could interrupt, delay or halt clinical trials, delay or prevent regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following any regulatory approval.
Undesirable adverse events, or AEs, caused by our drug candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA, CFDA, EMA or other comparable regulatory authority. Results of our trials could reveal a high and unacceptable severity or prevalence of AEs. In such an event, our trials could be suspended or terminated and the FDA, CFDA, EMA or other comparable regulatory authorities could order us to cease further development of, or deny approval of, our drug candidates for any or all targeted indications.
Treatment-related serious adverse events, or SAEs, that have been reported in our monotherapy clinical trials include but not are limited to the following: (i) for BGB-3111, petechiae (spots that appear on the skin as a result of bleeding), purpura (subcutaneous bleeding), bruising, other serious hemorrhage (grade 3 hemorrhage or central nervous system, or CNS, hemorrhage of any grade), atrial fibrillation, diarrhea, haemothorax, colitis, febrile neutropenia, neutropenia, anemia, thrombocytopenia, pneumonia, renal hematoma, urinary tract infection, pneumonitis, leukocytosis, lymphocytosis, toxic epidermal necrolsysis, septic shock, cardiac arrest and headache; (ii) for BGB-A317, colitis, hypotension, diarrhea, diabetes mellitus, diabetic ketoacidosis, dyspnea, hypoxia, pneumonitis, fatigue, alanine aminotransferase, or ALT, increase, aspartate aminotransferase, or AST, increase, gamma-glutamyl transferase, or GGT, increase, autoimmune pancreatitis, back pain, dermatitis, hyperglycaemia, hyperthyroidism, nausea, proteinuria, stomatitis, bilirubin increase, leukopenia, neutropenia, pyrexia, mucosal inflammation and hepatitis; (iii) for BGB-290, anemia, neutropenia, nausea, vomiting, thrombocytopenia, diarrhea, fatigue, neutropenia and acute myeloid leukemia / myelodysplastic syndrome; and (iv) for BGB-283, thrombocytopenia, fatigue, nausea, anemia, neutropenia, vomiting, hepatitis, ALT increase, AST increase, GGT increase, pyrexia, decreased appetite, hypophosphataemia, hand-foot syndrome, hypertension, weight decrease, lymphopenia, leukopenia, and constipation. Some of these events have led to patient death.
In addition, treatment-related SAEs that have been reported in our combination clinical trials include the following: (i) for the BGB-3111 and obinutuzumab combination, neutropenia, thrombocytopenia, pneumonia, infusion-related reaction, and serious hemorrhage, including one report of a grade 3 intracranial hemorrhage SAE, which is possibly drug related, in one Diffuse Large B-Cell Lymphoma patient that caused the patient’s treatment with BGB-3111 to be interrupted; (ii) for the BGB-3111 and BGB-A317 combination, haemolytic anaemia, pneumonia, pneumonitis, anemia, autoimmune encephalitis, dyspnea, ALT increase, GGT increase, infusion-related reaction, peripheral edema, pyrexia, thrombocytopenia, limb abscess, ulcerative keratitis, catheter site hemorrhage, hemolytic transfusion reaction, nausea, lymph gland infection and eczema; and (iii) for the BGB-290 and BGB-A317 combination, nausea, vomiting, hepatitis, ALT increase, AST increase, GGT increase, fatigue, anemia, liver injury, hypophysitis, and neutropenia. Some of these events have led to patient death.
Drug-related AEs or SAEs could affect patient recruitment or the ability of enrolled subjects to complete the trial, and could result in potential product liability claims. Any of these occurrences may harm our reputation, business, financial condition and prospects significantly.
Additionally, if we or others identify undesirable side effects caused by our drugs or any future approved drug candidates, a number of potentially significant negative consequences could result, including:
· we may suspend marketing of the drug;
· regulatory authorities may withdraw approvals or revoke licenses of the drug;
· regulatory authorities may require additional warnings on the label;
· we may be required to develop a REMS for the drug, as is the case with REVLIMID®, or, if a REMS is already in place, to incorporate additional requirements under the REMS, or to develop a similar strategy as required by a comparable regulatory authority;
· we may be required to conduct post-market studies;
· we could be sued and held liable for harm caused to subjects or patients; and
· our reputation may suffer.
Any of these events could prevent us from achieving or maintaining market acceptance of the particular drug or drug candidate, and could significantly harm our business, results of operations and prospects.
Further, combination therapy, such as using our wholly-owned drug candidates as well as third-party products, involves unique AEs that could be exacerbated compared to AEs from monotherapies. These types of AEs could be caused by our drug candidates and could also cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA, CFDA, EMA or other comparable regulatory authority. Results of our trials could reveal a high and unacceptable severity or prevalence of AEs.
