Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Teligent, Inc. | form8k.htm |

JP Morgan Healthcare Conference
January 10, 2018
Jason Grenfell-Gardner │CEO
Nasdaq Global Select: TLGT

Safe Harbor
1
Except for historical facts, the statements in this presentation, as well as oral statements
or other written statements made or to be made by Teligent, Inc., are forward-looking
statements within the meaning of the Private Securities Litigation Reform Act of 1995 and
involve risks and uncertainties. For example, without limitation, statements about the
Company’s anticipated growth and future operations, the current or expected market size
for its products, the success of current or future product offerings and the research and
development efforts and the Company’s ability to file for and obtain U.S. Food and Drug
Administration (FDA) approvals for future products, are forward-looking statements.
Forward-looking statements are merely the Company’s current predictions of future
events. The statements are inherently uncertain, and actual results could differ materially
from the statements made herein. There is no assurance that the Company will achieve
the sales levels that will make its operations profitable or that FDA filings and approvals
will be completed and obtained as anticipated. For a description of additional risks and
uncertainties, please refer to the Company’s filings with the Securities and Exchange
Commission, including its latest Annual Report on Form 10–K and its latest Quarterly
Report on Form 10-Q. The Company assumes no obligation to update its forward-looking
statements to reflect new information and developments.

Key Investment Highlights
Teligent is a “market disruptor” in alternative dosage form generics
Focused on Topical, Injectable, Complex and Ophthalmic (TICO) markets
Led by a proven and dynamic management team
Significant investment in R&D has resulted in a high-value pipeline
State of the art topical and injectable manufacturing infrastructure
Strong historical sales growth
Solid foundation to support sustainable, profitable growth
1
2
3
4
5
6
7
2

Teligent at-a-glance
3
Teligent is a high-growth generics company that develops and markets a diversified
product portfolio focused on alternative dosage forms
Diversified Product
Portfolio
• 58 products sold
throughout North
America
− 28 injectables and
topicals in US
− 30 injectables in
Canada
Deep Development
Pipeline
• 32 ANDAs pending at
FDA representing
~$2B IMS opportunity
• >40 products in active
development
State of the Art
Manufacturing
• ~$55M invested in
facility expansion
and upgrade
−High-speed topical
filling
−New sterile
injectable capability
Skilled and Dedicated
Employees
• 200+ employees
worldwide
• Presence in New
Jersey, Toronto, and
Estonia
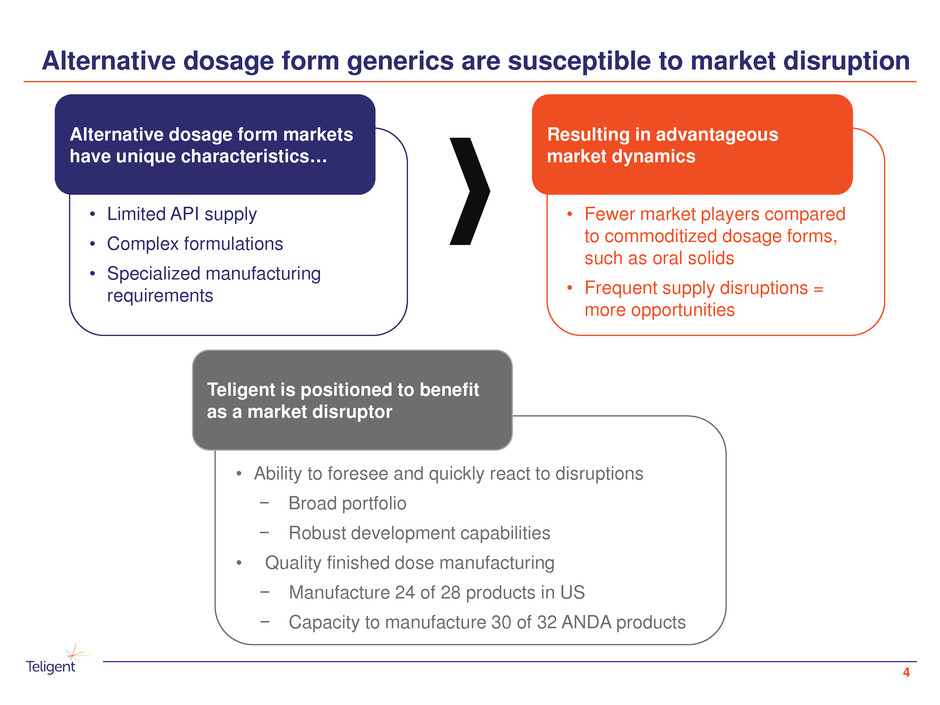
• Limited API supply
• Complex formulations
• Specialized manufacturing
requirements
• Fewer market players compared
to commoditized dosage forms,
such as oral solids
• Frequent supply disruptions =
more opportunities
• Ability to foresee and quickly react to disruptions
− Broad portfolio
− Robust development capabilities
• Quality finished dose manufacturing
− Manufacture 24 of 28 products in US
− Capacity to manufacture 30 of 32 ANDA products
Alternative dosage form generics are susceptible to market disruption
4
Alternative dosage form markets
have unique characteristics…
Resulting in advantageous
market dynamics
Teligent is positioned to benefit
as a market disruptor

Teligent is focused on Topical, Injectable, Complex and Ophthalmic
(TICO) markets
5
Dosage Form Description Current Teligent Strength
Topical • Core strength
• Established development and manufacturing capabilities
• 24 commercial products; 30 filed ANDAs
Injectable • Investing in manufacturing – first commercial product out of
new facility in 2019
• Expanded development capabilities
• 34 products commercialized in the US and Canada
Complex • Leverages existing development and commercial know-
how
• First product, representing >$200M IMS market
opportunity, filed in 2017
Ophthalmic • Leverages sterile injectable expertise and retail commercial
channel presence
• First ophthalmic filed in 2017, and three additional products
under active development with CMO

Led by a proven and dynamic executive leadership team
6
Jason Grenfell-Gardner
President & Chief Executive Officer
Jenniffer Collins
Chief Financial Officer
Steve Richardson
Chief Scientific Officer
Martin Wilson
General Counsel
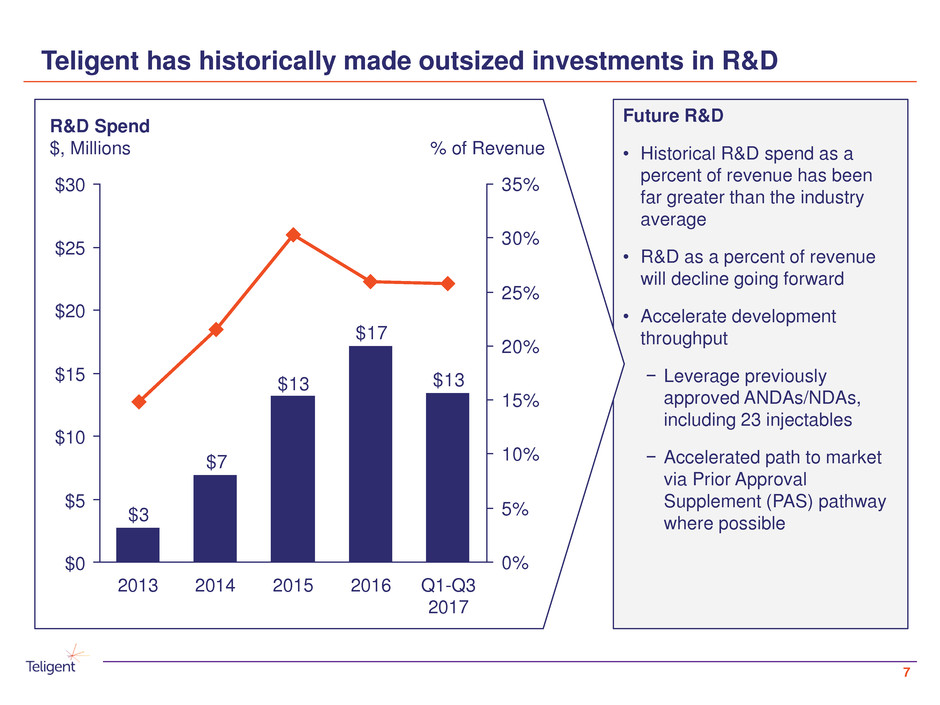
Future R&D
• Historical R&D spend as a
percent of revenue has been
far greater than the industry
average
• R&D as a percent of revenue
will decline going forward
• Accelerate development
throughput
− Leverage previously
approved ANDAs/NDAs,
including 23 injectables
− Accelerated path to market
via Prior Approval
Supplement (PAS) pathway
where possible
Teligent has historically made outsized investments in R&D
7
$13
$17
$13
$7
$3
0%
5%
10%
15%
20%
25%
30%
35%$30
$0
$10
$20
$15
$5
$25
% of Revenue
R&D Spend
$, Millions
2014 20162013 2015 Q1-Q3
2017

R&D investment has resulted in a deep, high-value pipeline
8
Cumulative US ANDA
Submissions and Approvals
As of December 31, 2017
8
13
19
31
36
32
1911
2012 2013 20172016
2
2014
1
2015
Pending ANDAsApproved ANDAs
% Total Addressable Market of
US Submissions
100% = ~$2.0 billion*
GDUFA Year 3-5
10%
Pre-GDUFA Year 3
90%
* IQVIA TAM as of November 2017
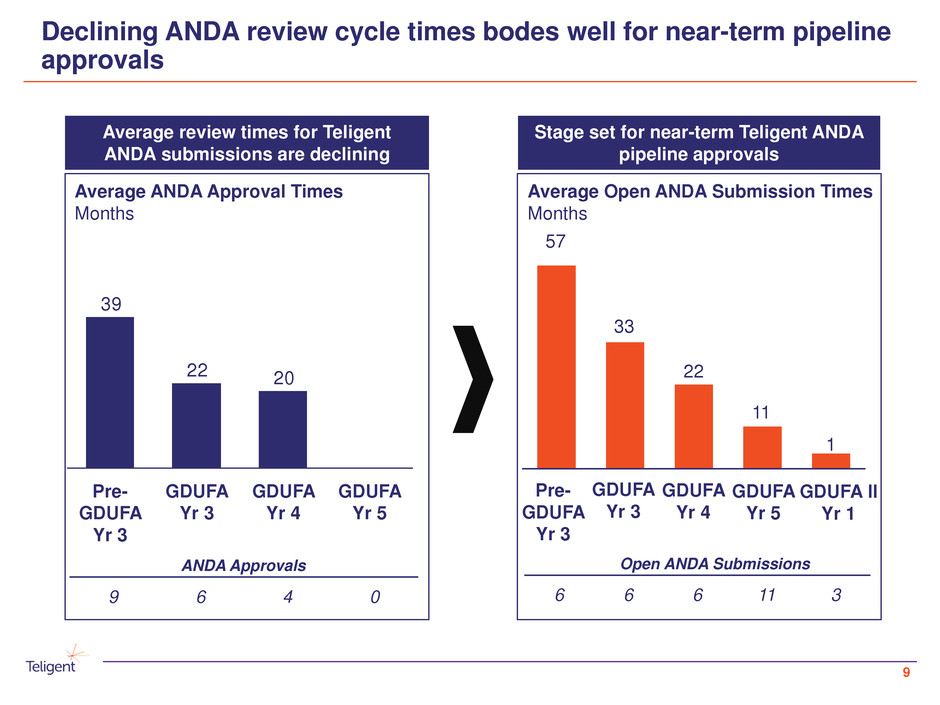
Declining ANDA review cycle times bodes well for near-term pipeline
approvals
9
2022
39
GDUFA
Yr 5
GDUFA
Yr 3
GDUFA
Yr 4
Pre-
GDUFA
Yr 3
57
33
22
11
1
GDUFA
Yr 5
Pre-
GDUFA
Yr 3
GDUFA
Yr 3
GDUFA
Yr 4
Average ANDA Approval Times
Months
Average Open ANDA Submission Times
Months
Average review times for Teligent
ANDA submissions are declining
Stage set for near-term Teligent ANDA
pipeline approvals
ANDA Approvals
9 6 4 0
Open ANDA Submissions
6 6 6 11 3
GDUFA II
Yr 1

Robust topical and injectable manufacturing infrastructure
10
Investing
~$55M
in the facility
expansion
Background
• Located in Buena, New Jersey
• Currently manufacture 24 Teligent-
label topical products
• Facility expansion >3x original plant
− Now almost 110,000 square feet
− Increased topical capacity
− New sterile injectable capability
Expansion Milestones
2016 – Commenced construction
2017 – Construction completed
Mid-2018 – File first injectable product
2H2018 – FDA prior approval inspection
2019 – Launch first injectable product
~4-8M
initial injectable unit
capacity with
increase to
~40M
with addition of
high-speed line
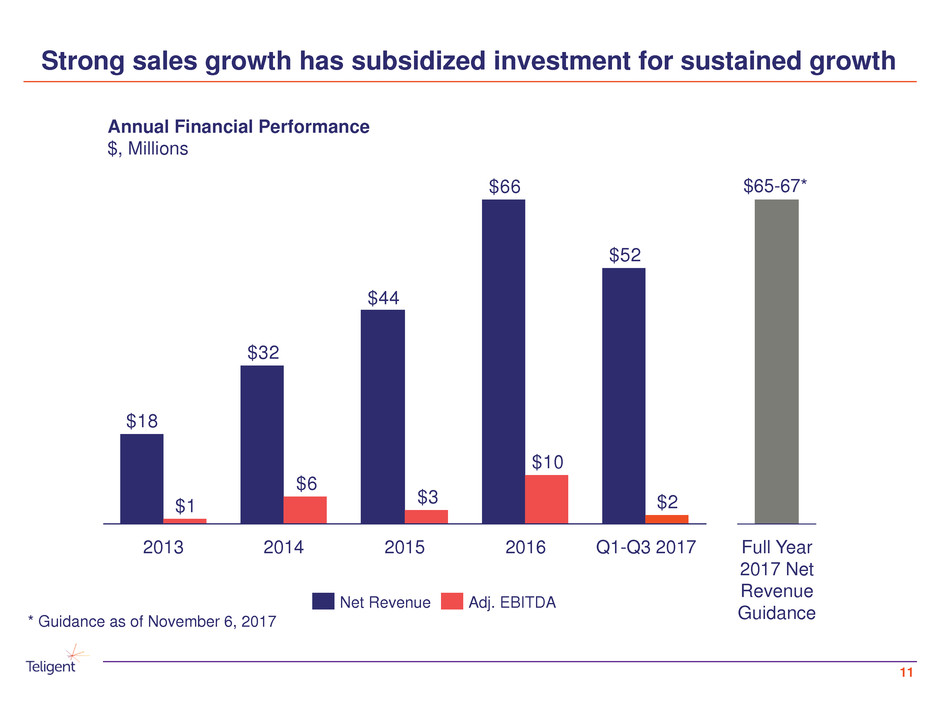
Strong sales growth has subsidized investment for sustained growth
11
$52
$66
$44
$32
$18
$2
$10
$3
$6
$1
2013 20152014 Q1-Q3 20172016
Annual Financial Performance
$, Millions
Adj. EBITDANet Revenue
Full Year
2017 Net
Revenue
Guidance
$65-67*
* Guidance as of November 6, 2017

Teligent-label product sales have exhibited strong growth
12
49
32
20
7
2014 20152013 2016
CAGR +88%
Annual Teligent-Label Product Net Sales
$, Millions
Key Growth Drivers:
• 19 Teligent-label products launched in
US since 2012
− Launched first Teligent-label product
in December 2012
• Acquired econazole nitrate cream in
2013
• Launched TICO strategy in 2014
• Acquired 3 injectable products (Fortaz®,
Zantac® and Zinacef®) in 2015
• Acquired Alveda Pharmaceuticals,
which was generating ~$16M CAD in
net sales, in 2015

Solid foundation to support sustainable, profitable growth
Focus on Investment
2012 - 2017
Focus on Profitability
2018 and beyond
• Invested >$150M* over the past 5
years
• Transitioned from contract services to
Teligent-label products
• Strengthened development
capabilities and grew product pipeline
• Expanded manufacturing capabilities
beyond topicals into injectables
• Harvest pipeline and accelerate new
product launches
• Strengthen commercial capabilities
and improve margins
• Focus on lean operations and
enhancing profitability
• Accelerate product development by
leveraging R&D and enhanced
manufacturing capabilities
* Investment defined as R&D, CapEx and M&A

Key 2018 Objectives
14
• Enhance profitability
− Disciplined cost control
• Increase development throughput
− Leverage previously launched ANDAs/NDAs
• Accelerate ANDA approvals
− 11 ANDAs with GDUFA goal dates in 1Q and 2Q
• Strengthen commercial presence
− Expand commercial team – enhance retail and
build institutional presence
• Operationalize new injectable facility
− First injectable filing, triggering PAI
