Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PRECIGEN, INC. | d525790d8k.htm |

J.P. Morgan Healthcare Conference January 10, 2018 Exhibit 99.1

Construction of powerful gene expression programs drives efficacy Control of gene expression and regulation drives safety Delivery through cellular and non-viral based approaches increases applicability Enabling Targeted and Controlled Gene-Based Therapies Combinations of these three key variables enables cost-effective, powerful and precise gene therapies for patients
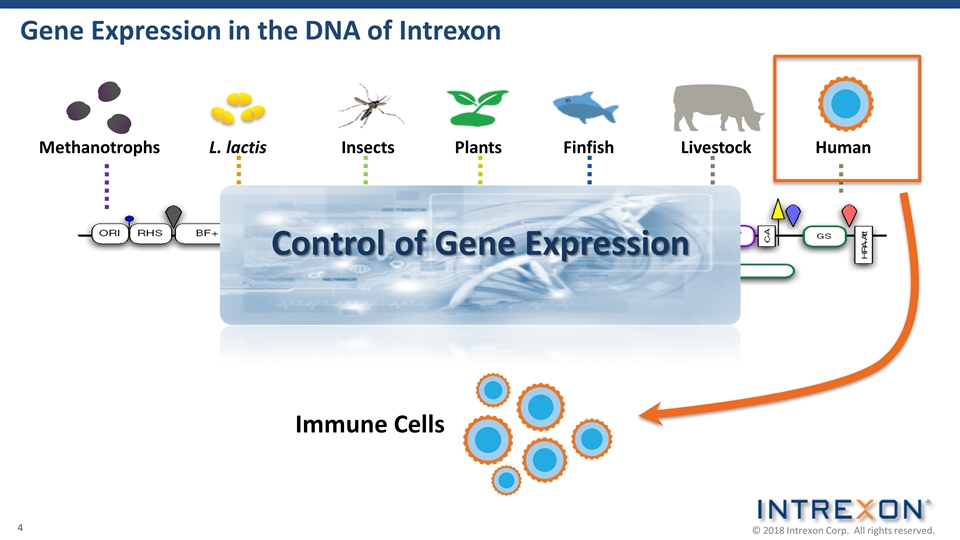
Gene Expression in the DNA of Intrexon Control of Gene Expression L. lactis Insects Methanotrophs Plants Finfish Livestock Immune Cells Human
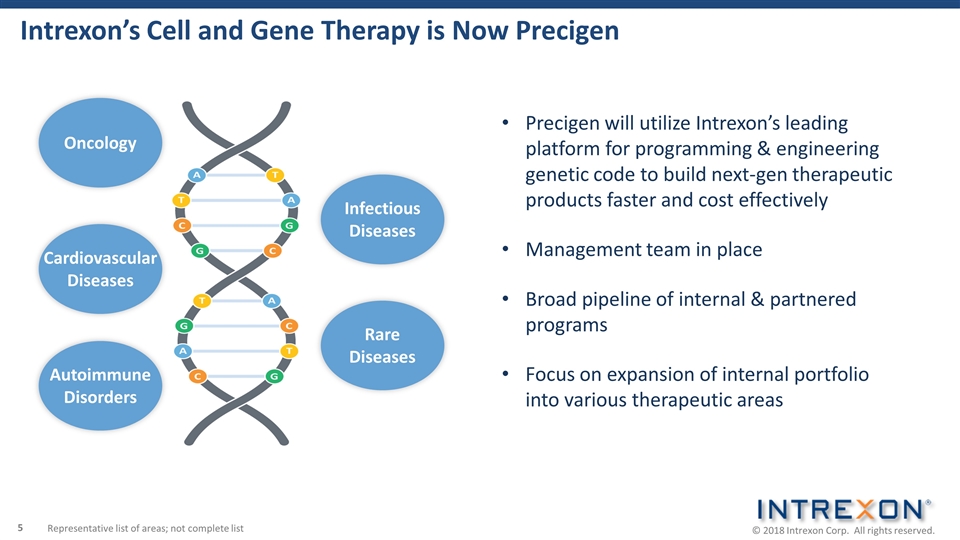
Intrexon’s Cell and Gene Therapy is Now Precigen Precigen will utilize Intrexon’s leading platform for programming & engineering genetic code to build next-gen therapeutic products faster and cost effectively Management team in place Broad pipeline of internal & partnered programs Focus on expansion of internal portfolio into various therapeutic areas Oncology Cardiovascular Diseases Representative list of areas; not complete list Rare Diseases Infectious Diseases Autoimmune Disorders
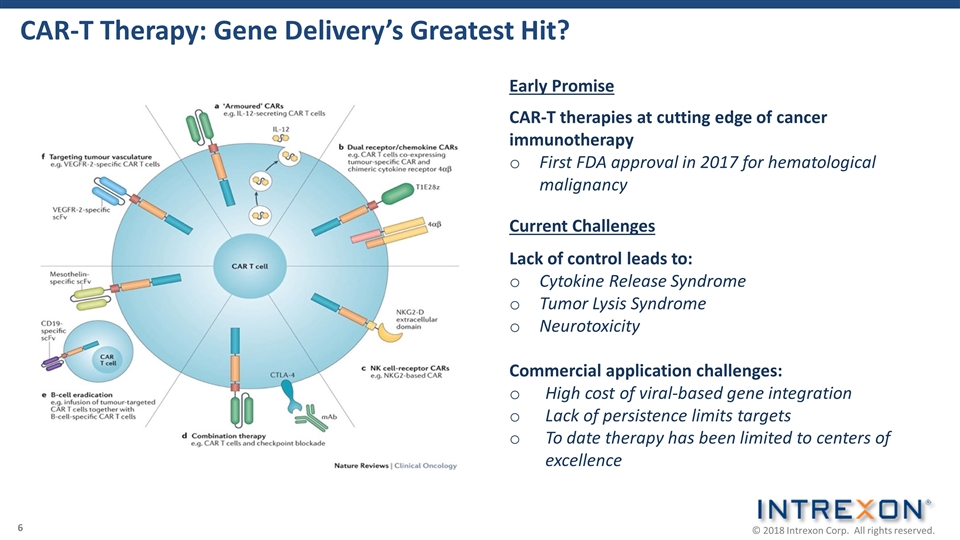
CAR-T Therapy: Gene Delivery’s Greatest Hit? Current Challenges Lack of control leads to: Cytokine Release Syndrome Tumor Lysis Syndrome Neurotoxicity Commercial application challenges: High cost of viral-based gene integration Lack of persistence limits targets To date therapy has been limited to centers of excellence CAR-T therapies at cutting edge of cancer immunotherapy First FDA approval in 2017 for hematological malignancy Early Promise
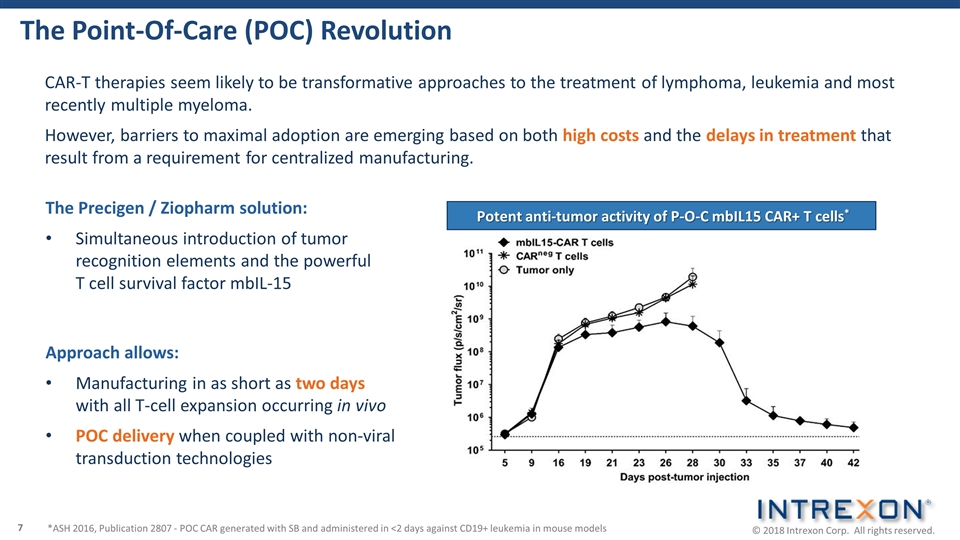
The Point-Of-Care (POC) Revolution CAR-T therapies seem likely to be transformative approaches to the treatment of lymphoma, leukemia and most recently multiple myeloma. However, barriers to maximal adoption are emerging based on both high costs and the delays in treatment that result from a requirement for centralized manufacturing. Approach allows: Manufacturing in as short as two days with all T-cell expansion occurring in vivo POC delivery when coupled with non-viral transduction technologies The Precigen / Ziopharm solution: Simultaneous introduction of tumor recognition elements and the powerful T cell survival factor mbIL-15 Potent anti-tumor activity of P-O-C mbIL15 CAR+ T cells* *ASH 2016, Publication 2807 - POC CAR generated with SB and administered in <2 days against CD19+ leukemia in mouse models
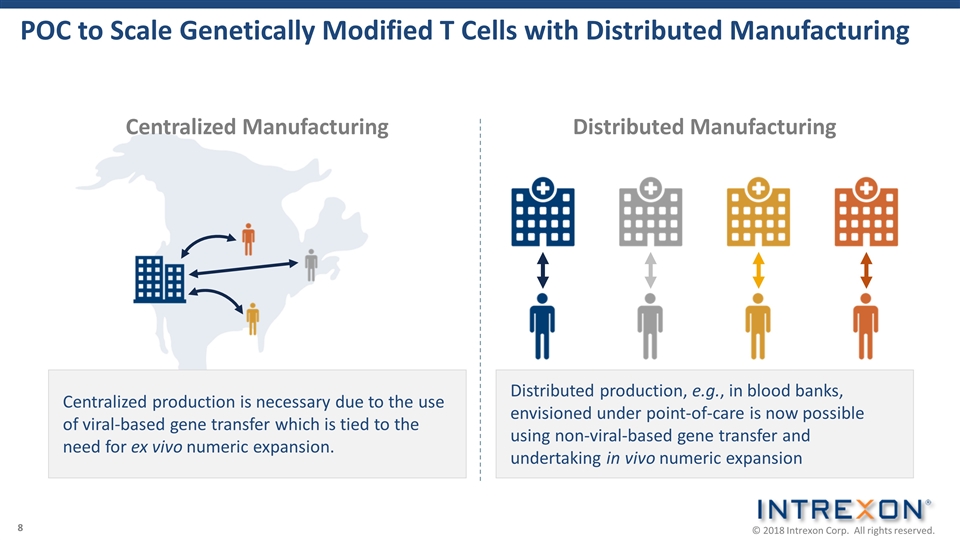
POC to Scale Genetically Modified T Cells with Distributed Manufacturing Centralized Manufacturing Distributed Manufacturing Centralized production is necessary due to the use of viral-based gene transfer which is tied to the need for ex vivo numeric expansion. Distributed production, e.g., in blood banks, envisioned under point-of-care is now possible using non-viral-based gene transfer and undertaking in vivo numeric expansion
POC Cells Potentiate a New Business Model No centralized manufacturing allows for a unique business model Partner with major medical centers. Hospitals hold exclusive regional license to Intrexon technology Intrexon receives per patient and yearly licensing fees and double digit royalties Expected safety advantages as cell growth is more gradual in practical models making outpatient use more probable Much lower therapeutic costs reduce overall cost significantly First dosing expected in Q2 of 2018 First Partner Signed January 2018
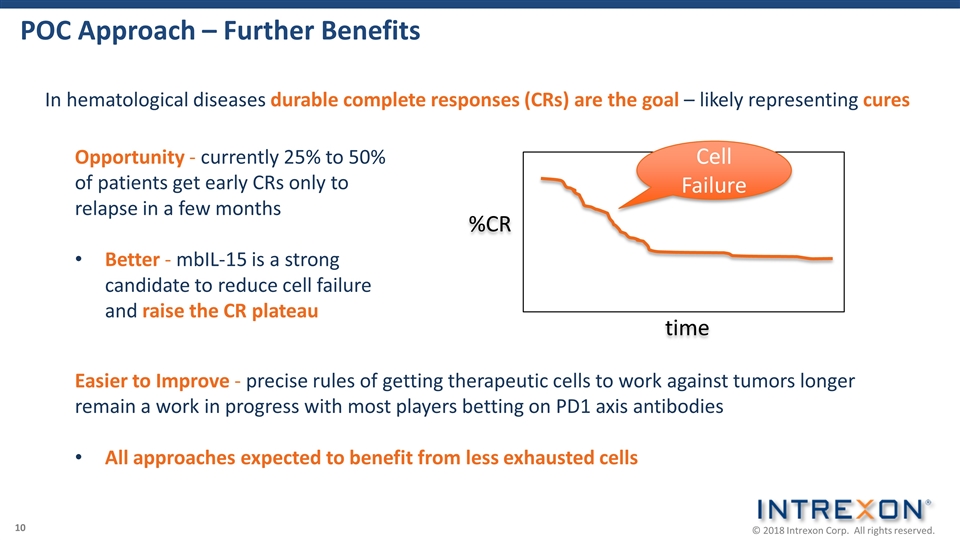
POC Approach – Further Benefits In hematological diseases durable complete responses (CRs) are the goal – likely representing cures Opportunity - currently 25% to 50% of patients get early CRs only to relapse in a few months Better - mbIL-15 is a strong candidate to reduce cell failure and raise the CR plateau %CR time Easier to Improve - precise rules of getting therapeutic cells to work against tumors longer remain a work in progress with most players betting on PD1 axis antibodies All approaches expected to benefit from less exhausted cells Cell Failure
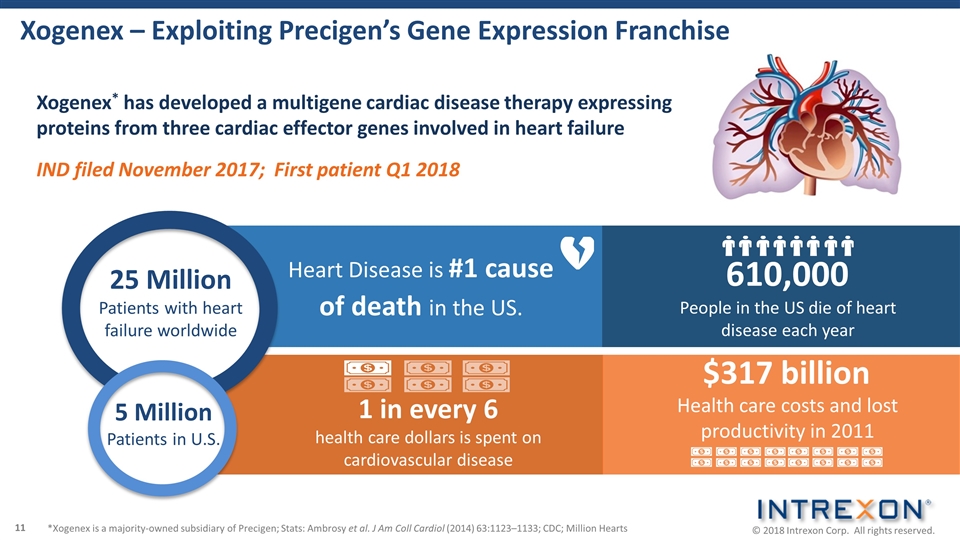
Xogenex – Exploiting Precigen’s Gene Expression Franchise Heart Disease is #1 cause of death in the US. 610,000 People in the US die of heart disease each year 1 in every 6 health care dollars is spent on cardiovascular disease $317 billion Health care costs and lost productivity in 2011 Xogenex* has developed a multigene cardiac disease therapy expressing proteins from three cardiac effector genes involved in heart failure IND filed November 2017; First patient Q1 2018 25 Million Patients with heart failure worldwide 5 Million Patients in U.S. *Xogenex is a majority-owned subsidiary of Precigen; Stats: Ambrosy et al. J Am Coll Cardiol (2014) 63:1123–1133; CDC; Million Hearts
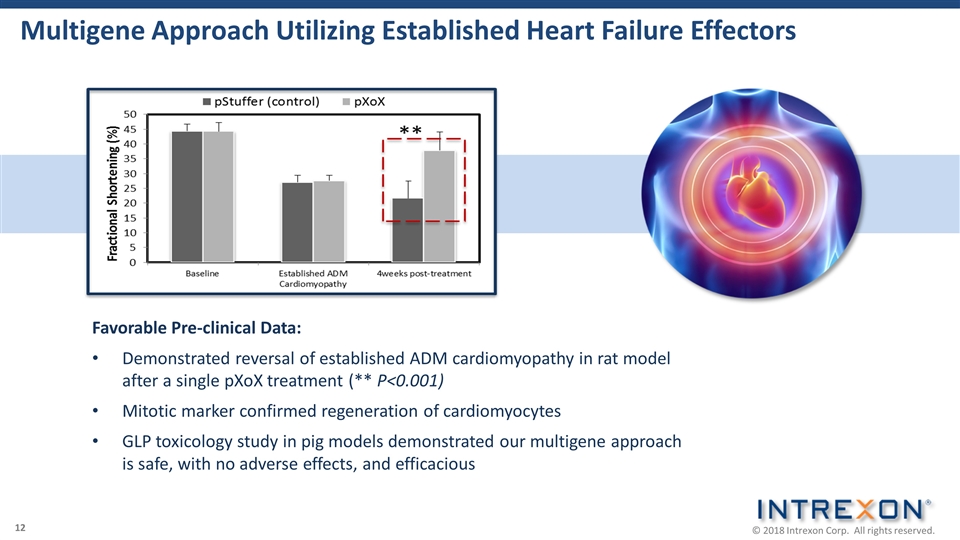
Multigene Approach Utilizing Established Heart Failure Effectors Favorable Pre-clinical Data: Demonstrated reversal of established ADM cardiomyopathy in rat model after a single pXoX treatment (** P<0.001) Mitotic marker confirmed regeneration of cardiomyocytes GLP toxicology study in pig models demonstrated our multigene approach is safe, with no adverse effects, and efficacious
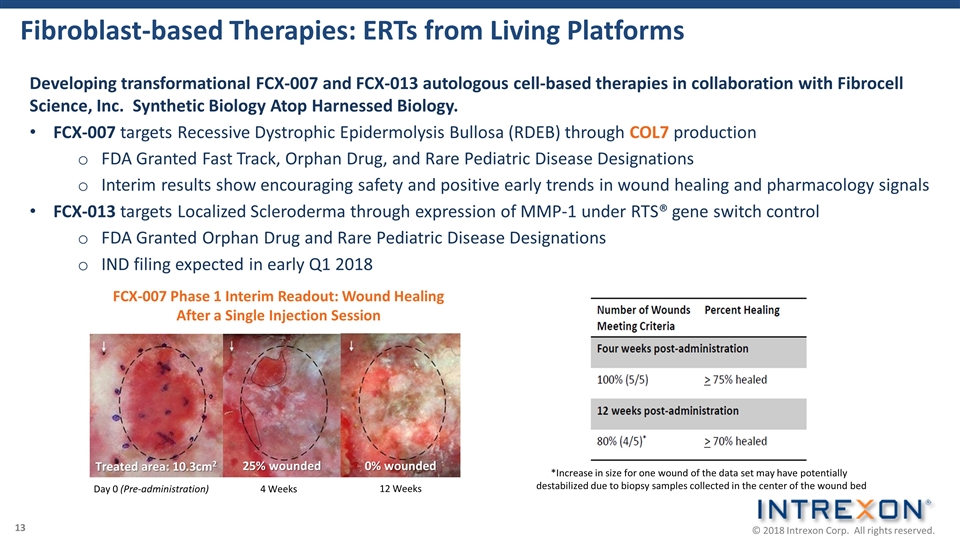
Fibroblast-based Therapies: ERTs from Living Platforms Developing transformational FCX-007 and FCX-013 autologous cell-based therapies in collaboration with Fibrocell Science, Inc. Synthetic Biology Atop Harnessed Biology. FCX-007 targets Recessive Dystrophic Epidermolysis Bullosa (RDEB) through COL7 production FDA Granted Fast Track, Orphan Drug, and Rare Pediatric Disease Designations Interim results show encouraging safety and positive early trends in wound healing and pharmacology signals FCX-013 targets Localized Scleroderma through expression of MMP-1 under RTS® gene switch control FDA Granted Orphan Drug and Rare Pediatric Disease Designations IND filing expected in early Q1 2018 *Increase in size for one wound of the data set may have potentially destabilized due to biopsy samples collected in the center of the wound bed 4 Weeks FCX-007 Phase 1 Interim Readout: Wound Healing After a Single Injection Session Day 0 (Pre-administration) 12 Weeks Treated area: 10.3cm2 25% wounded 0% wounded
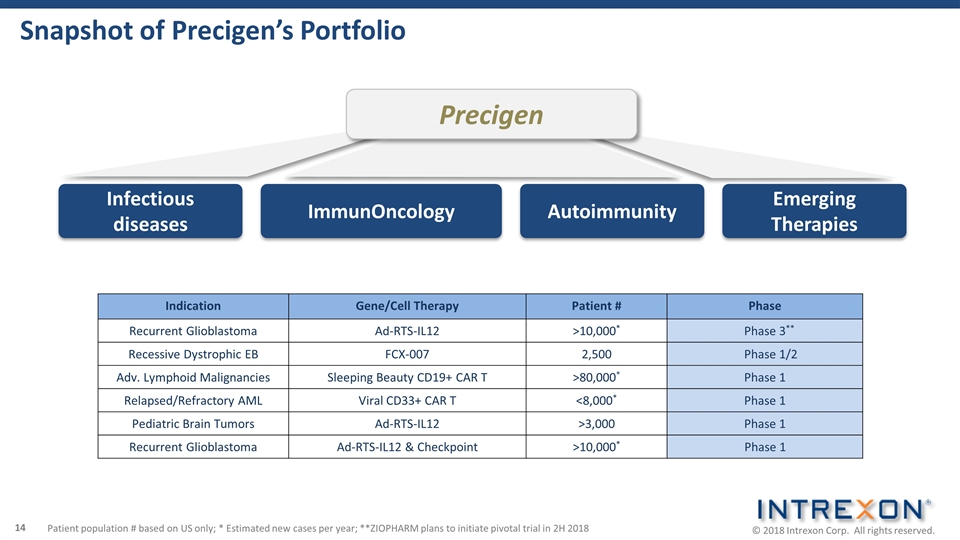
Infectious diseases ImmunOncology Precigen Emerging Therapies Autoimmunity Snapshot of Precigen’s Portfolio Indication Gene/Cell Therapy Patient # Phase Recurrent Glioblastoma Ad-RTS-IL12 >10,000* Phase 3** Recessive Dystrophic EB FCX-007 2,500 Phase 1/2 Adv. Lymphoid Malignancies Sleeping Beauty CD19+ CAR T >80,000* Phase 1 Relapsed/Refractory AML Viral CD33+ CAR T <8,000* Phase 1 Pediatric Brain Tumors Ad-RTS-IL12 >3,000 Phase 1 Recurrent Glioblastoma Ad-RTS-IL12 & Checkpoint >10,000* Phase 1 Patient population # based on US only; * Estimated new cases per year; **ZIOPHARM plans to initiate pivotal trial in 2H 2018
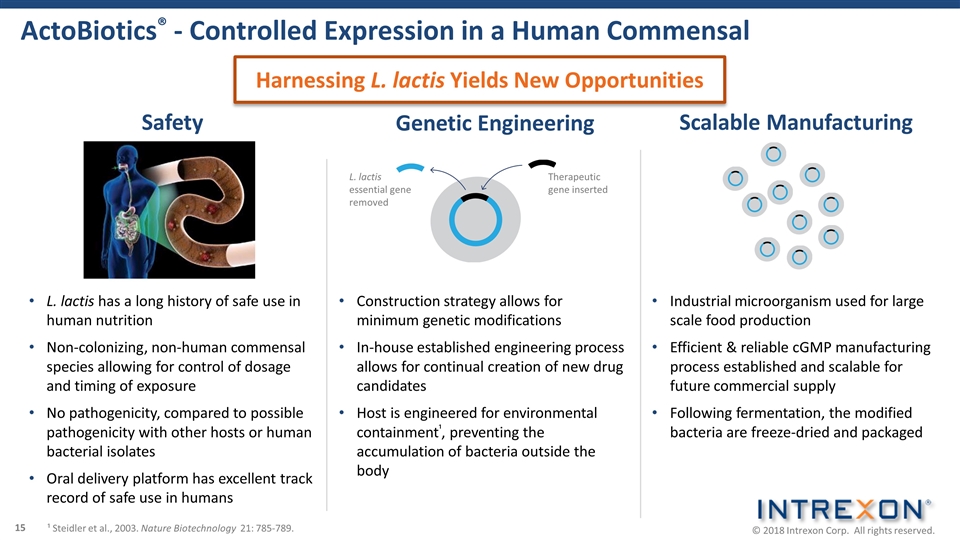
ActoBiotics® - Controlled Expression in a Human Commensal L. lactis essential gene removed Therapeutic gene inserted Construction strategy allows for minimum genetic modifications In-house established engineering process allows for continual creation of new drug candidates Host is engineered for environmental containment¹, preventing the accumulation of bacteria outside the body Industrial microorganism used for large scale food production Efficient & reliable cGMP manufacturing process established and scalable for future commercial supply Following fermentation, the modified bacteria are freeze-dried and packaged Genetic Engineering Scalable Manufacturing ¹ Steidler et al., 2003. Nature Biotechnology 21: 785-789. L. lactis has a long history of safe use in human nutrition Non-colonizing, non-human commensal species allowing for control of dosage and timing of exposure No pathogenicity, compared to possible pathogenicity with other hosts or human bacterial isolates Oral delivery platform has excellent track record of safe use in humans Safety Harnessing L. lactis Yields New Opportunities
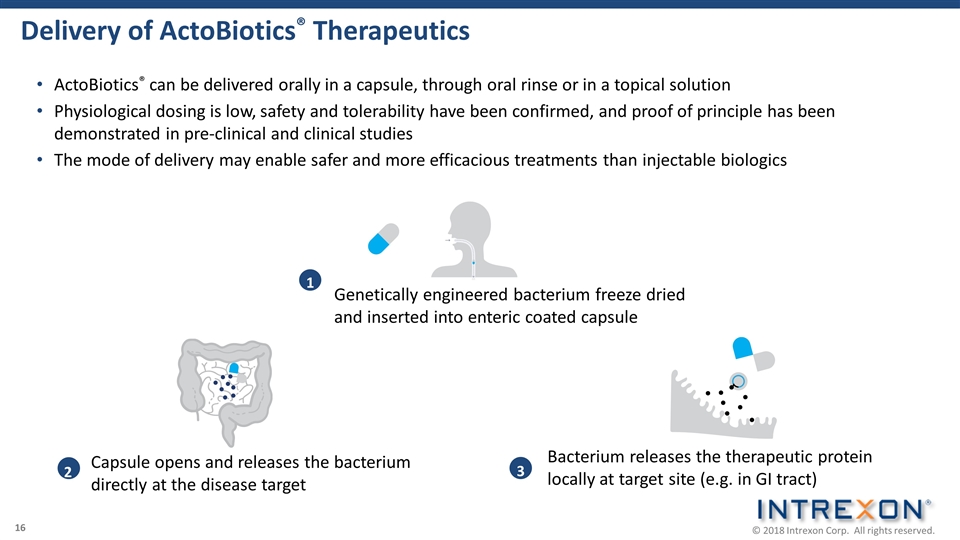
Delivery of ActoBiotics® Therapeutics 1 Genetically engineered bacterium freeze dried and inserted into enteric coated capsule 2 Capsule opens and releases the bacterium directly at the disease target 3 Bacterium releases the therapeutic protein locally at target site (e.g. in GI tract) ActoBiotics® can be delivered orally in a capsule, through oral rinse or in a topical solution Physiological dosing is low, safety and tolerability have been confirmed, and proof of principle has been demonstrated in pre-clinical and clinical studies The mode of delivery may enable safer and more efficacious treatments than injectable biologics
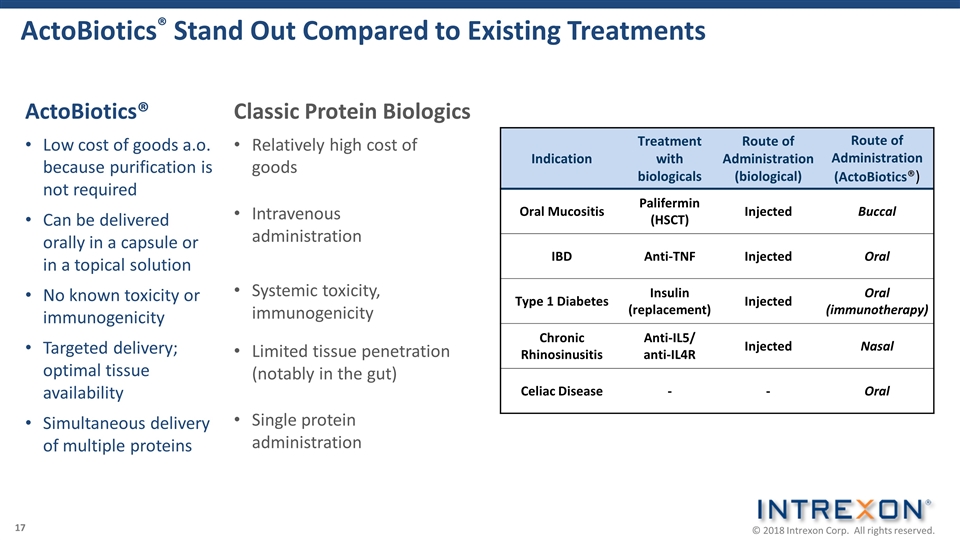
ActoBiotics® Stand Out Compared to Existing Treatments ActoBiotics® Low cost of goods a.o. because purification is not required Can be delivered orally in a capsule or in a topical solution No known toxicity or immunogenicity Targeted delivery; optimal tissue availability Simultaneous delivery of multiple proteins Classic Protein Biologics Relatively high cost of goods Intravenous administration Systemic toxicity, immunogenicity Limited tissue penetration (notably in the gut) Single protein administration Indication Treatment with biologicals Route of Administration (biological) Route of Administration (ActoBiotics®) Oral Mucositis Palifermin (HSCT) Injected Buccal IBD Anti-TNF Injected Oral Type 1 Diabetes Insulin (replacement) Injected Oral (immunotherapy) Chronic Rhinosinusitis Anti-IL5/ anti-IL4R Injected Nasal Celiac Disease - - Oral

Non-Healthcare Programs Enjoy High Technical Synergies
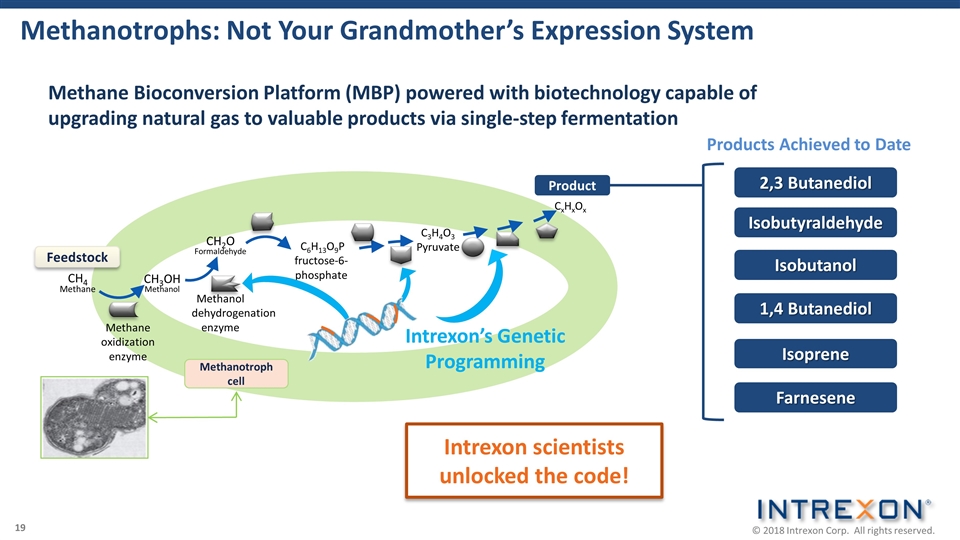
Methanotrophs: Not Your Grandmother’s Expression System CH3OH Methanol Methane oxidization enzyme CH2O Formaldehyde Methanol dehydrogenation enzyme C6H13O9P fructose-6-phosphate C3H4O3 Pyruvate CxHxOx Feedstock Product Methanotroph cell CH4 Methane Intrexon’s Genetic Programming Intrexon scientists unlocked the code! Methane Bioconversion Platform (MBP) powered with biotechnology capable of upgrading natural gas to valuable products via single-step fermentation Isobutanol 2,3 Butanediol Isobutyraldehyde 1,4 Butanediol Isoprene Farnesene Products Achieved to Date
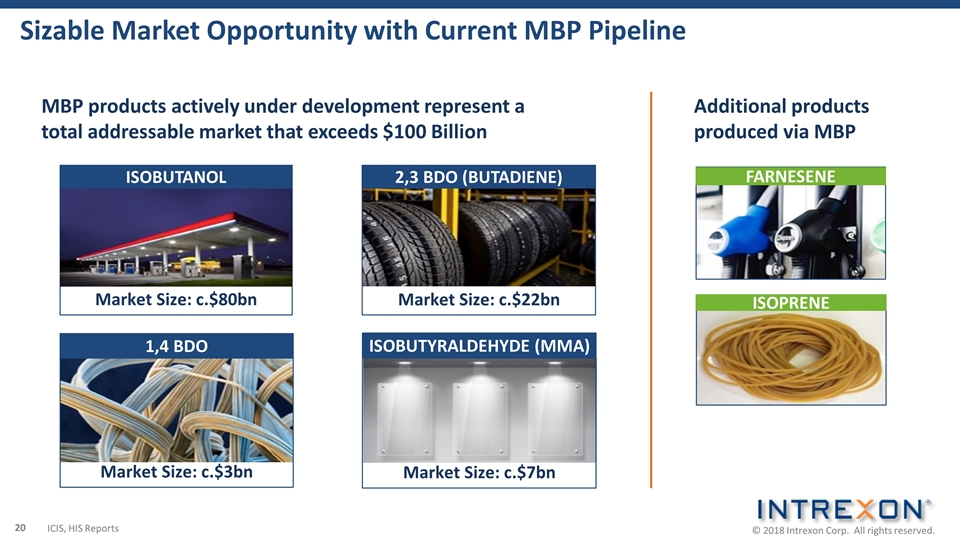
Sizable Market Opportunity with Current MBP Pipeline Market Size: c.$80bn ISOBUTANOL Market Size: c.$22bn 2,3 BDO (BUTADIENE) Market Size: c.$3bn 1,4 BDO Market Size: c.$7bn ISOBUTYRALDEHYDE (MMA) ISOPRENE MBP products actively under development represent a total addressable market that exceeds $100 Billion Additional products produced via MBP FARNESENE ICIS, HIS Reports
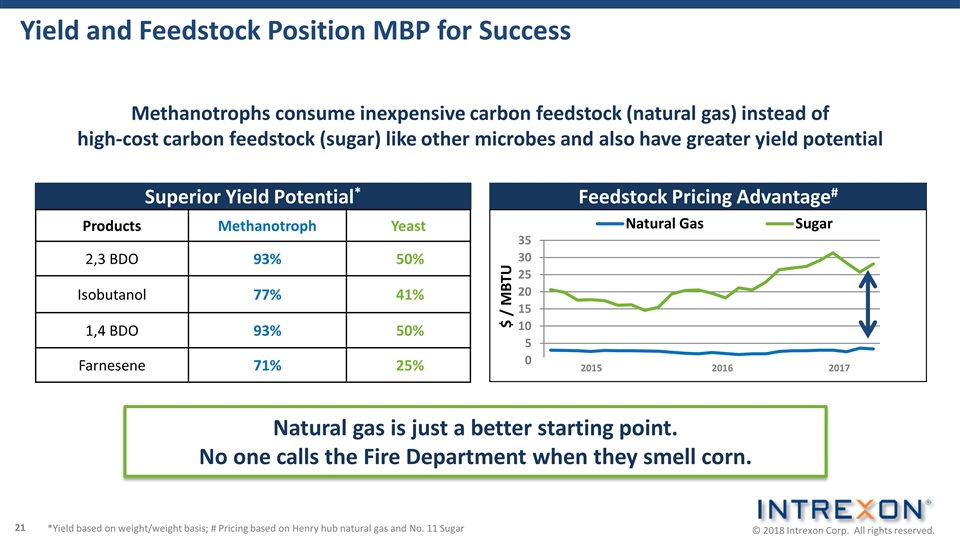
Feedstock Pricing Advantage# Superior Yield Potential* Yield and Feedstock Position MBP for Success Products Methanotroph Yeast 2,3 BDO 93% 50% Isobutanol 77% 41% 1,4 BDO 93% 50% Farnesene 71% 25% 2015 2016 2017 *Yield based on weight/weight basis; # Pricing based on Henry hub natural gas and No. 11 Sugar $ / MBTU Methanotrophs consume inexpensive carbon feedstock (natural gas) instead of high-cost carbon feedstock (sugar) like other microbes and also have greater yield potential Natural gas is just a better starting point. No one calls the Fire Department when they smell corn.

Current approaches using chemicals or anti-oxidants to stop browning impact flavor and cost of sliced apples 40% of apples are currently wasted, much from superficial bruising / browning Arctic® Apples – Simple Science Via RNAi technology, we reduced the levels of polyphenol oxidase (PPO), the enzyme that initiates browning PPO reduction can be applied to existing apple varieties Same composition & nutrition as conventional counterparts Grows exactly the same as conventional apples in the orchard Limitations within Current Addressable Market for Apples Ag Bio - Okanagan Specialty Fruits™ Conventional Golden Arctic® Golden Conventional Granny Arctic® Granny
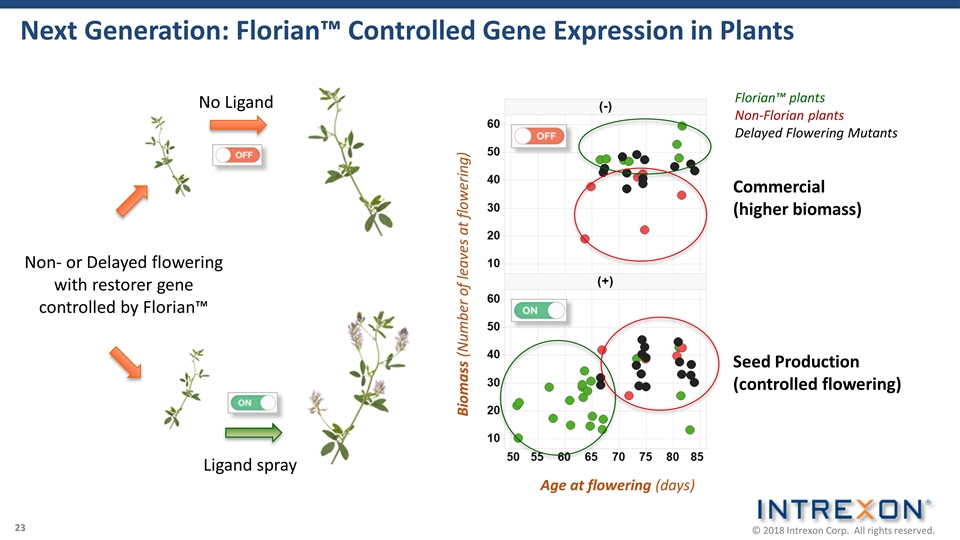
Next Generation: Florian™ Controlled Gene Expression in Plants Non- or Delayed flowering with restorer gene controlled by Florian™ Ligand spray No Ligand Age at flowering (days) Biomass (Number of leaves at flowering) Seed Production (controlled flowering) Commercial (higher biomass) Florian™ plants Non-Florian plants Delayed Flowering Mutants

J.P. Morgan Healthcare Conference January 10, 2018
