Exhibit 99.1


This presentation includes statements relating to the company's Acuitas® MDRO and Acuitas Lighthouse® clinical laboratory services, FDA cleared QuickFISH® products, and commercialization plans for these products and services.
These statements and other statements regarding our future plans and goals constitute "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934,
and are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act
of 1995. Such statements are subject to risks and uncertainties that are often difficult to predict, are beyond our control, and which may cause results to differ materially from expectations. Factors that could cause our results to differ materially from those described include, but are not limited to, our successful development of new products and services, the rate of adoption of our products and services by hospitals and other healthcare providers, the success of our commercialization efforts, the effect on our business of existing and new regulatory requirements, and other economic and competitive factors. For a discussion of the most significant risks and uncertainties associated with OpGen's business, please review
our filings with the Securities and Exchange Commission (SEC). You are cautioned not to place undue reliance on these forward-looking statements, which are based on our expectations as of the date of this presentation and speak only as of the date of this presentation. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
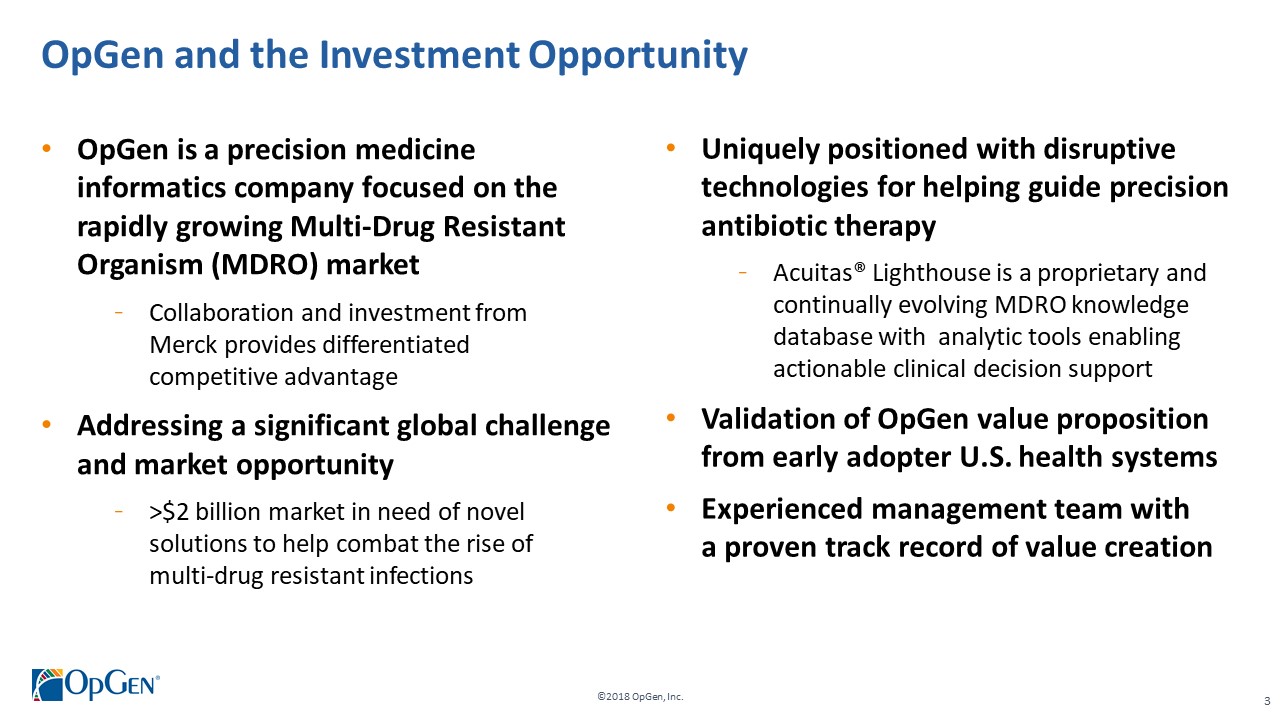
•OpGen is a precision medicine informatics company focused on the rapidly growing Multi-Drug Resistant Organism (MDRO) market -Collaboration and investment from Merck provides differentiated competitive advantage •Addressing a significant global challenge and market opportunity
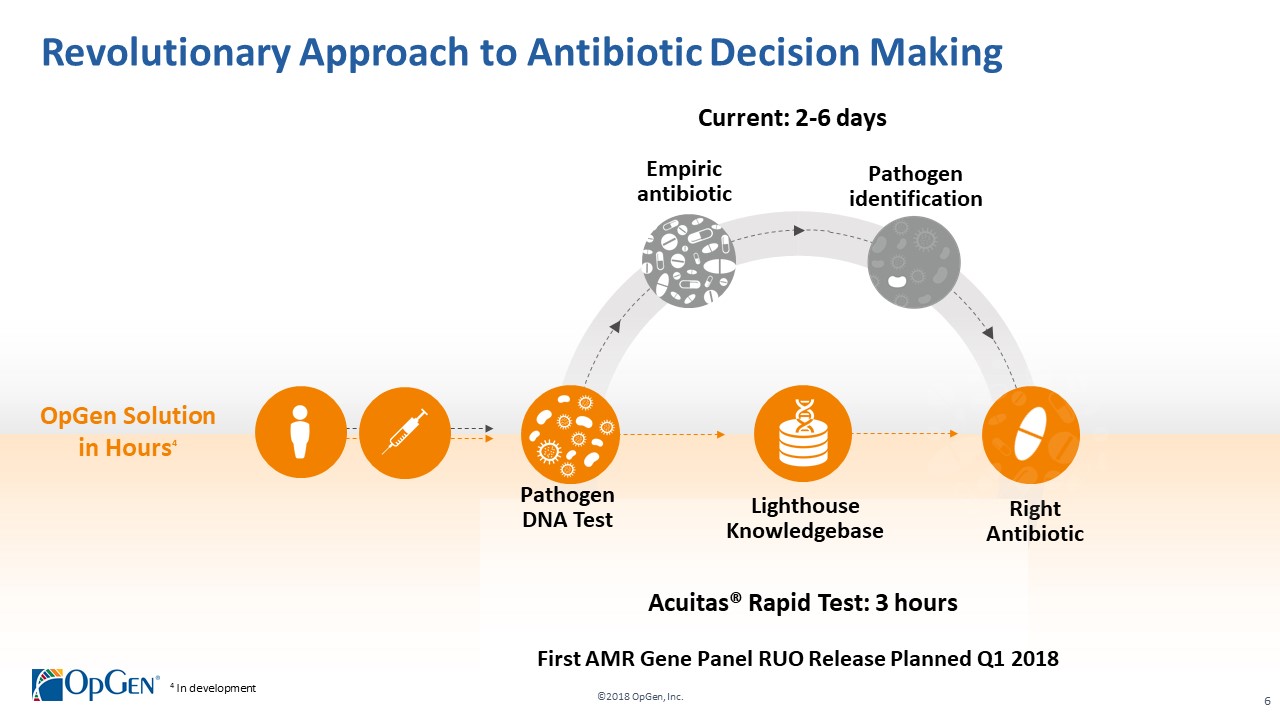
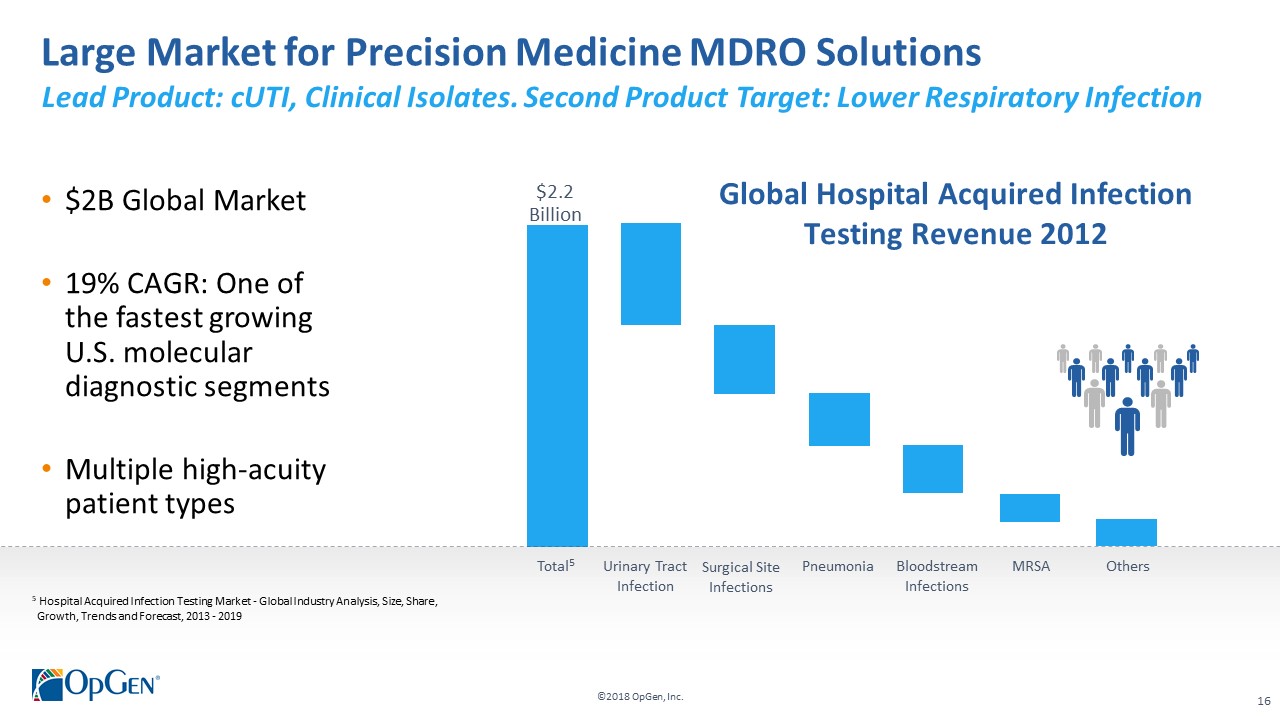
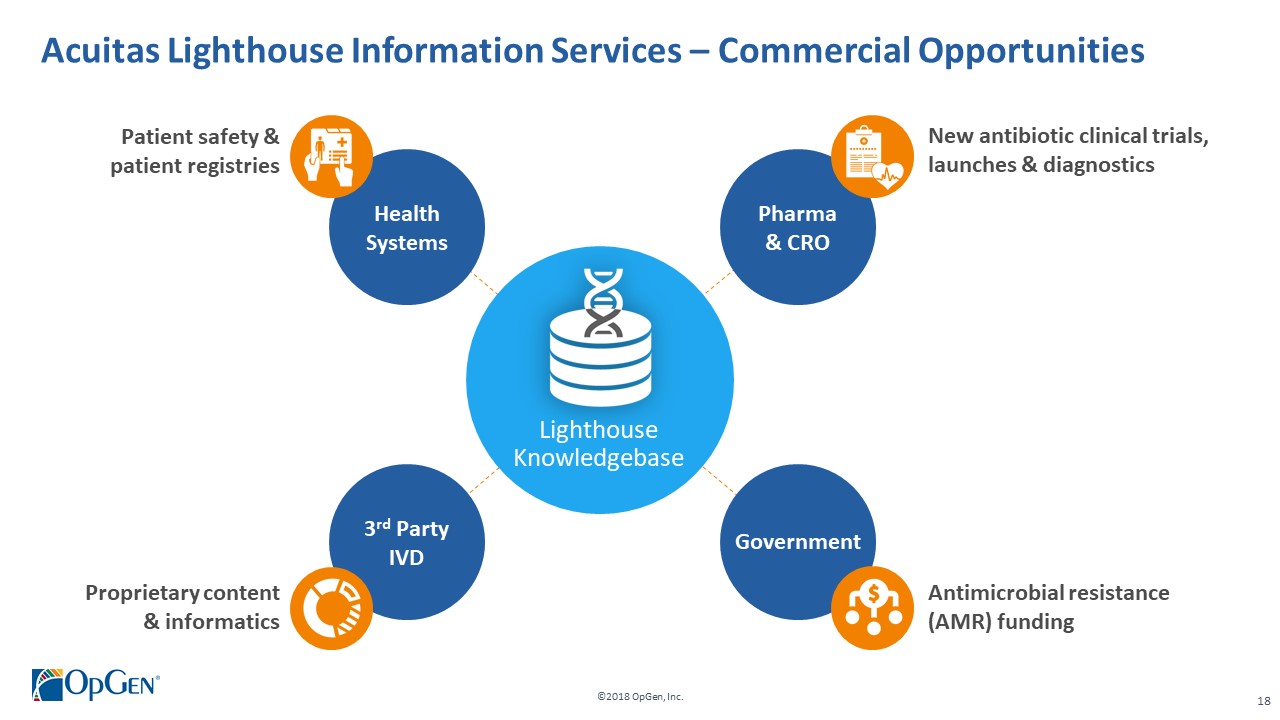
->$2 billion market in need of novel solutions to help combat the rise of multi-drug resistant infections •Uniquely positioned with disruptive technologies for helping guide precision antibiotic therapy -Acuitas® Lighthouse is a proprietary and continually evolving MDRO knowledge database with analytic tools enabling actionable clinical decision support •Validation of OpGenvalue proposition from early adopter U.S. health systems •Experienced management team with a proven track record of value creation
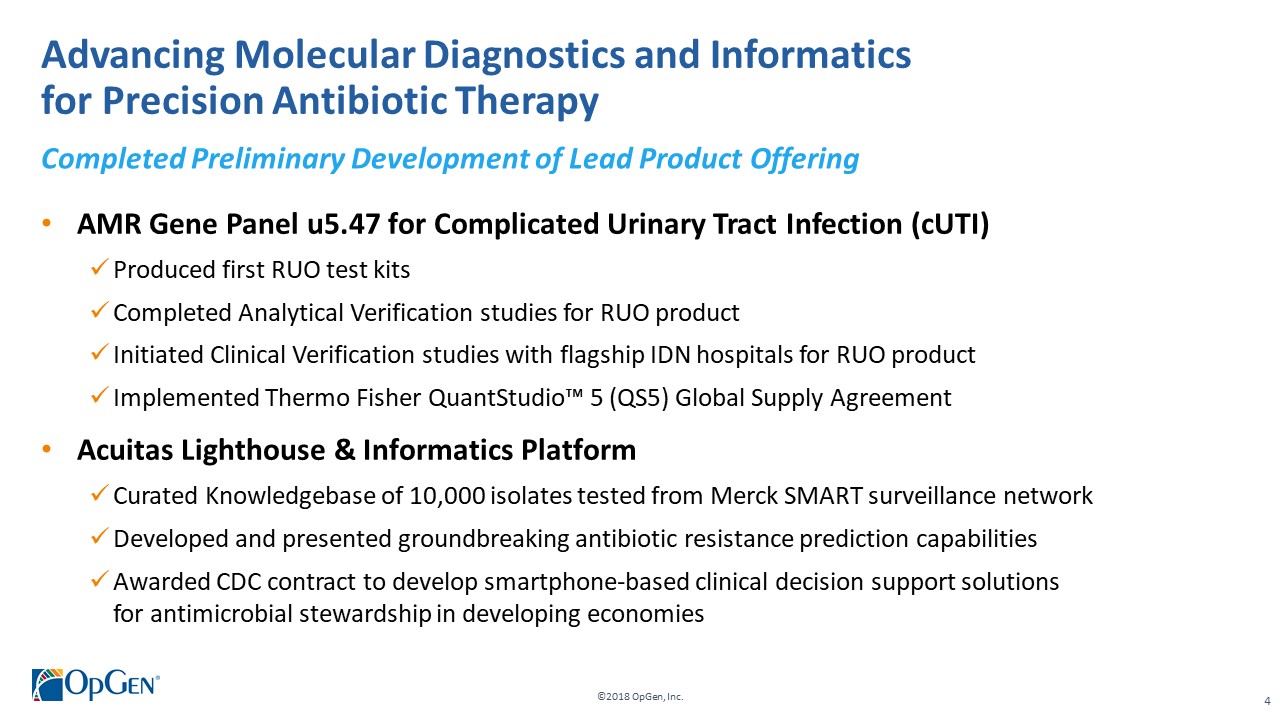
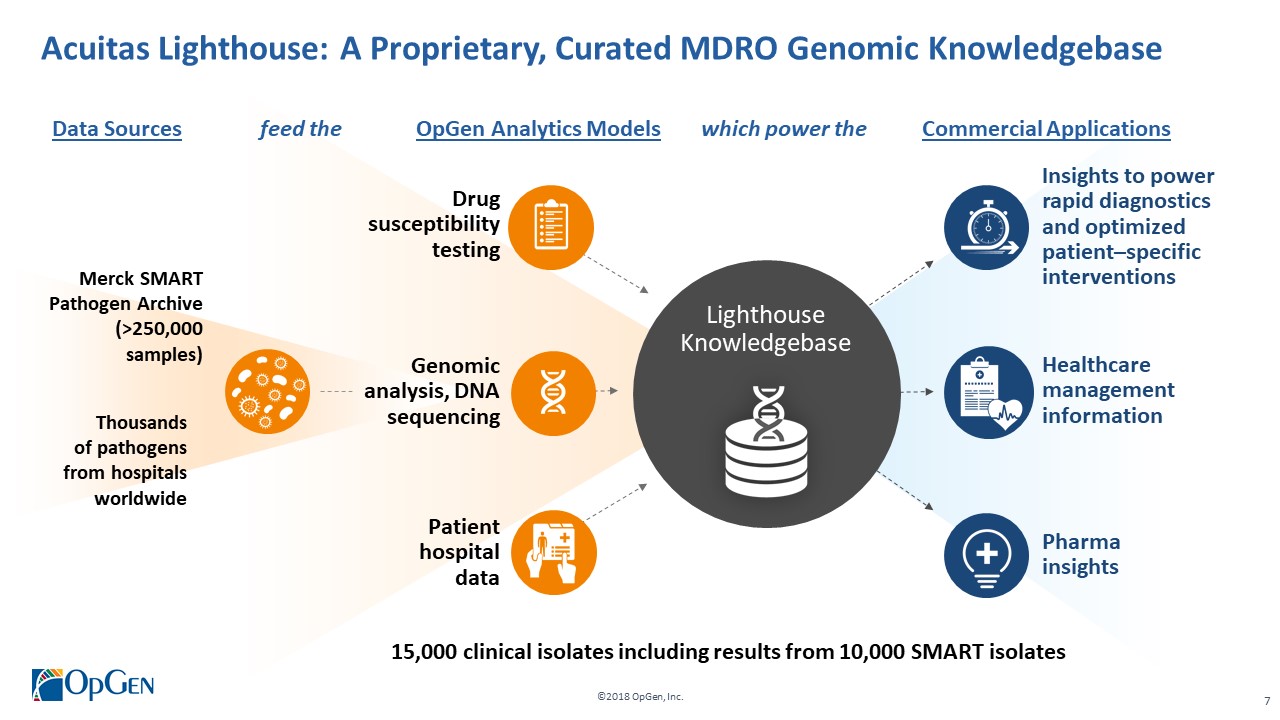
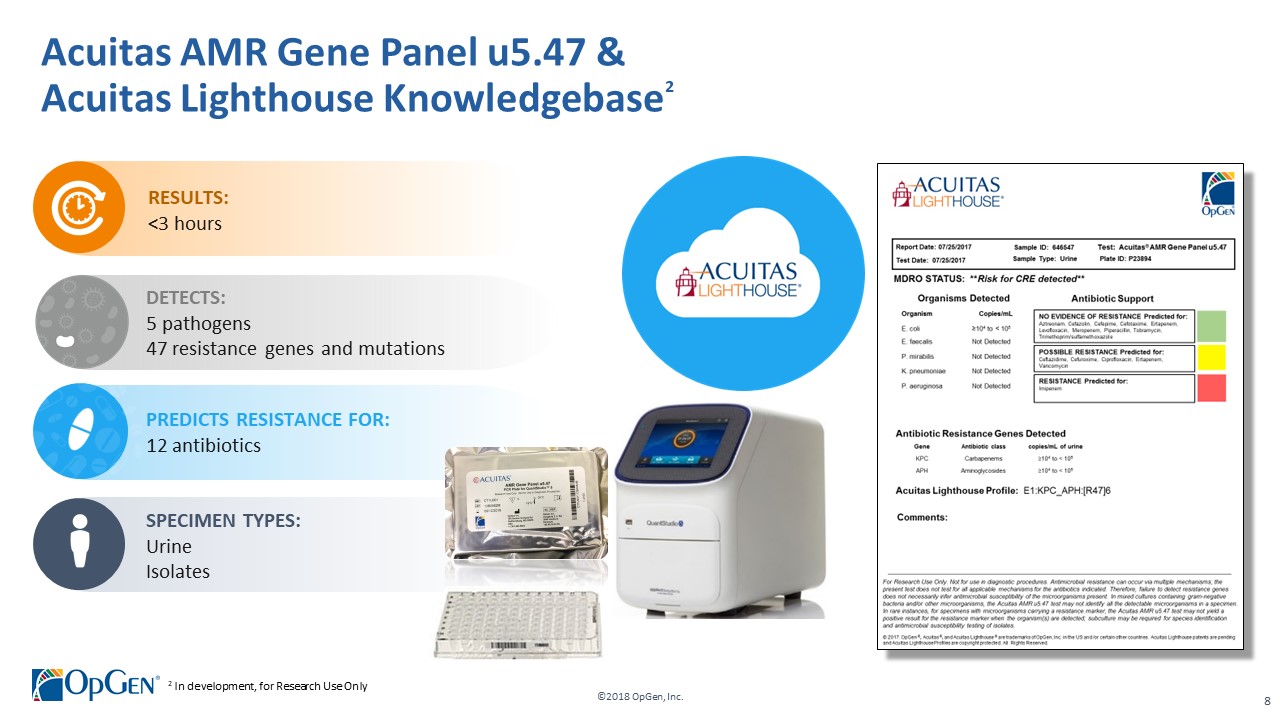
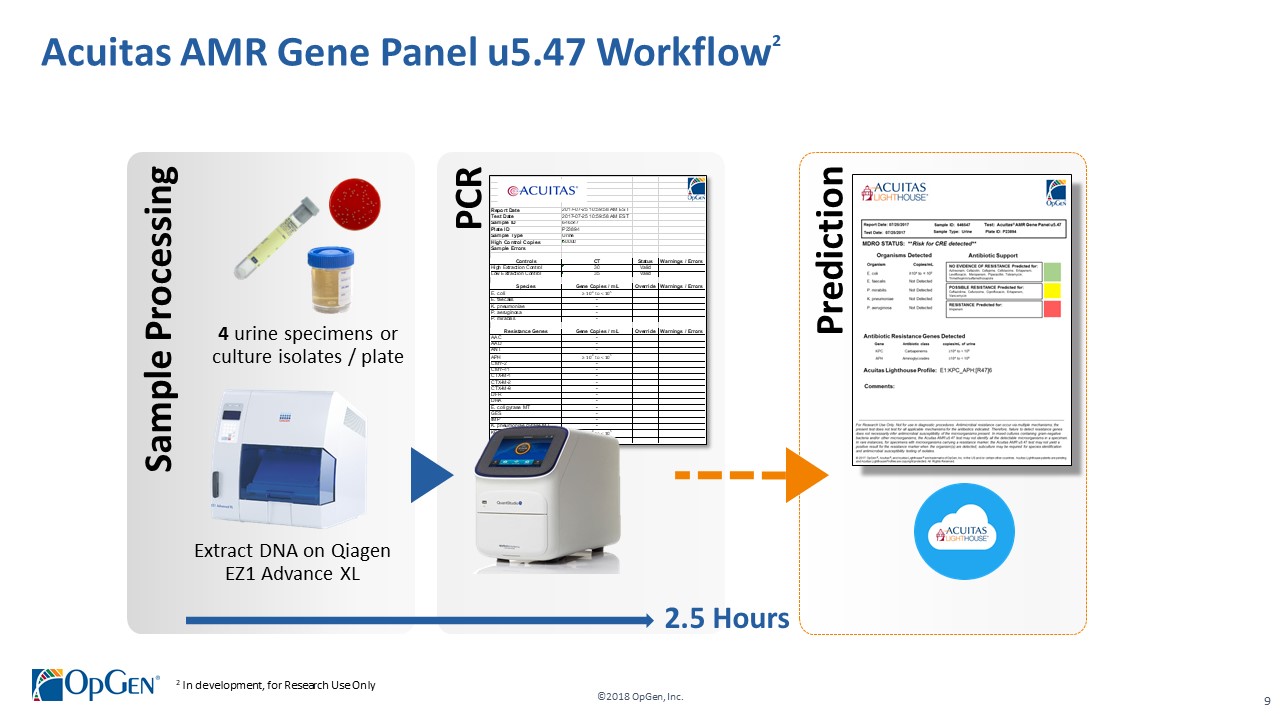
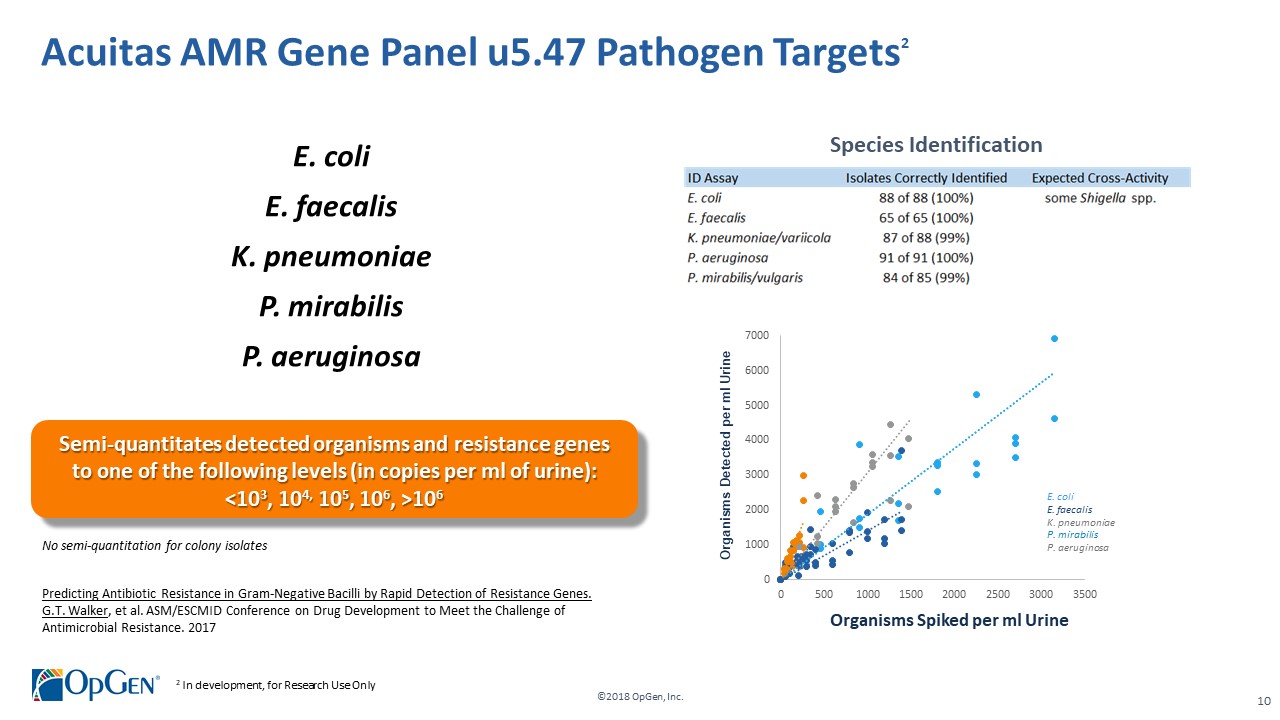
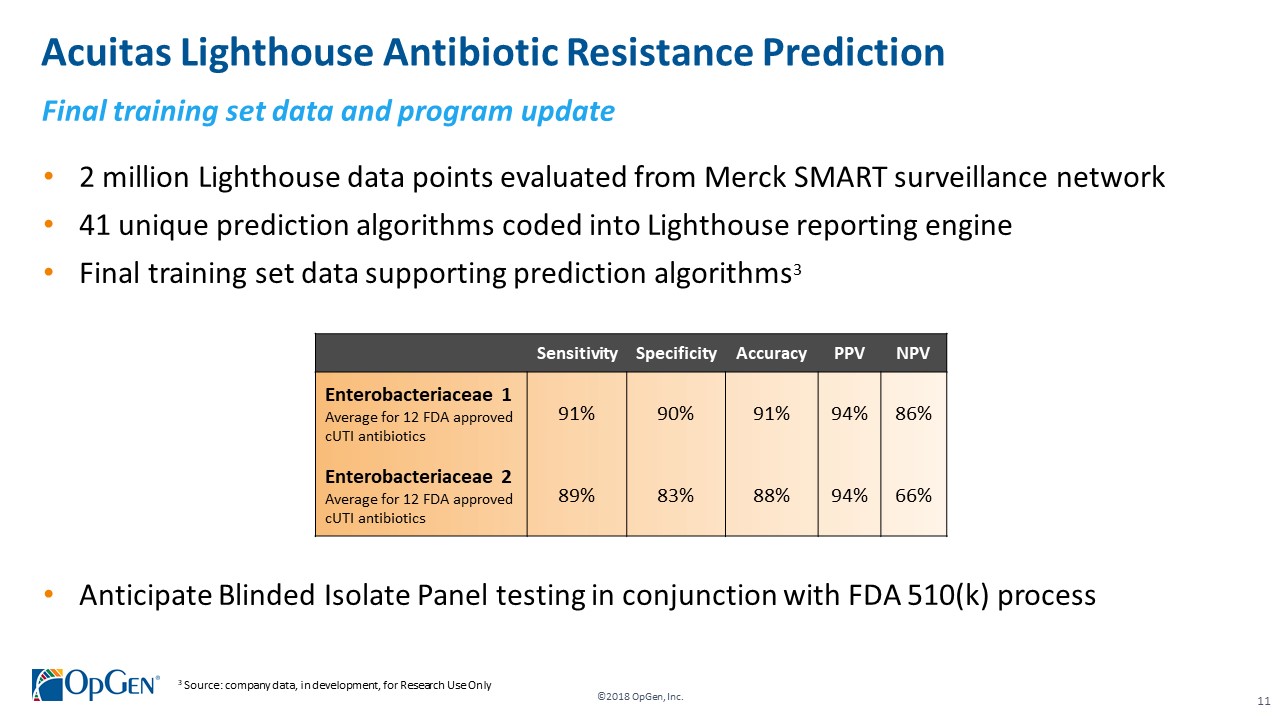
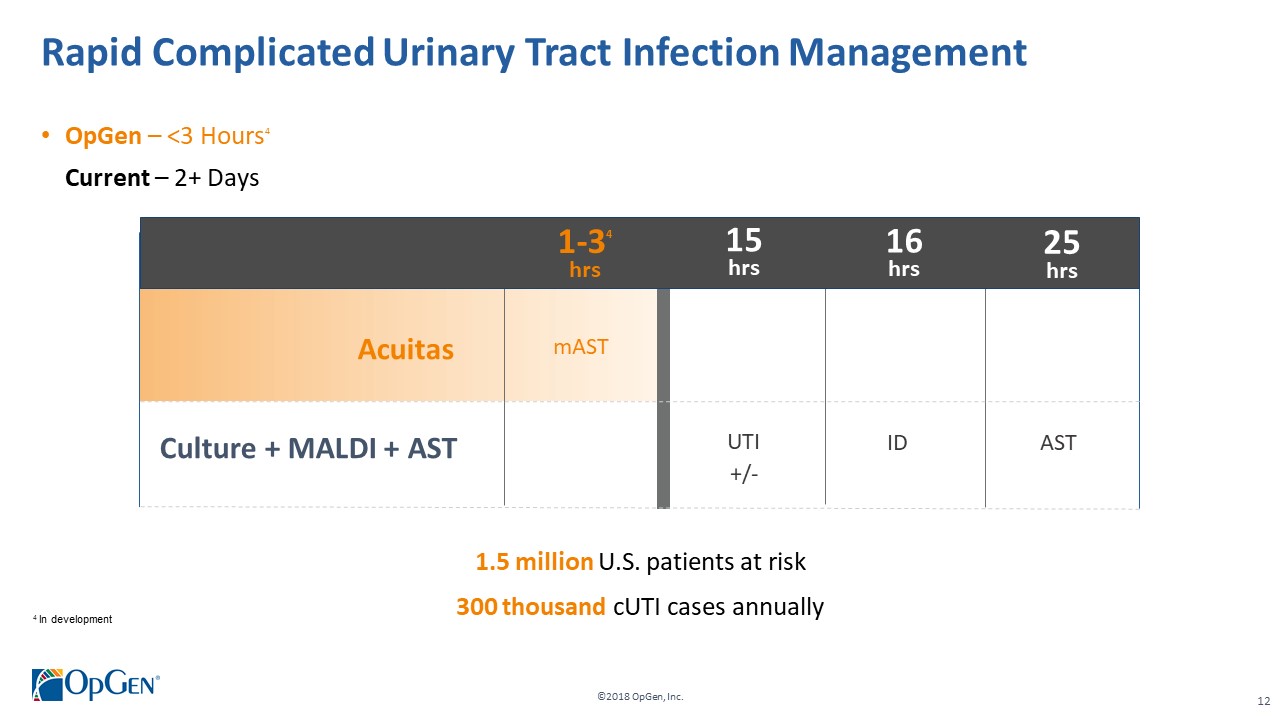
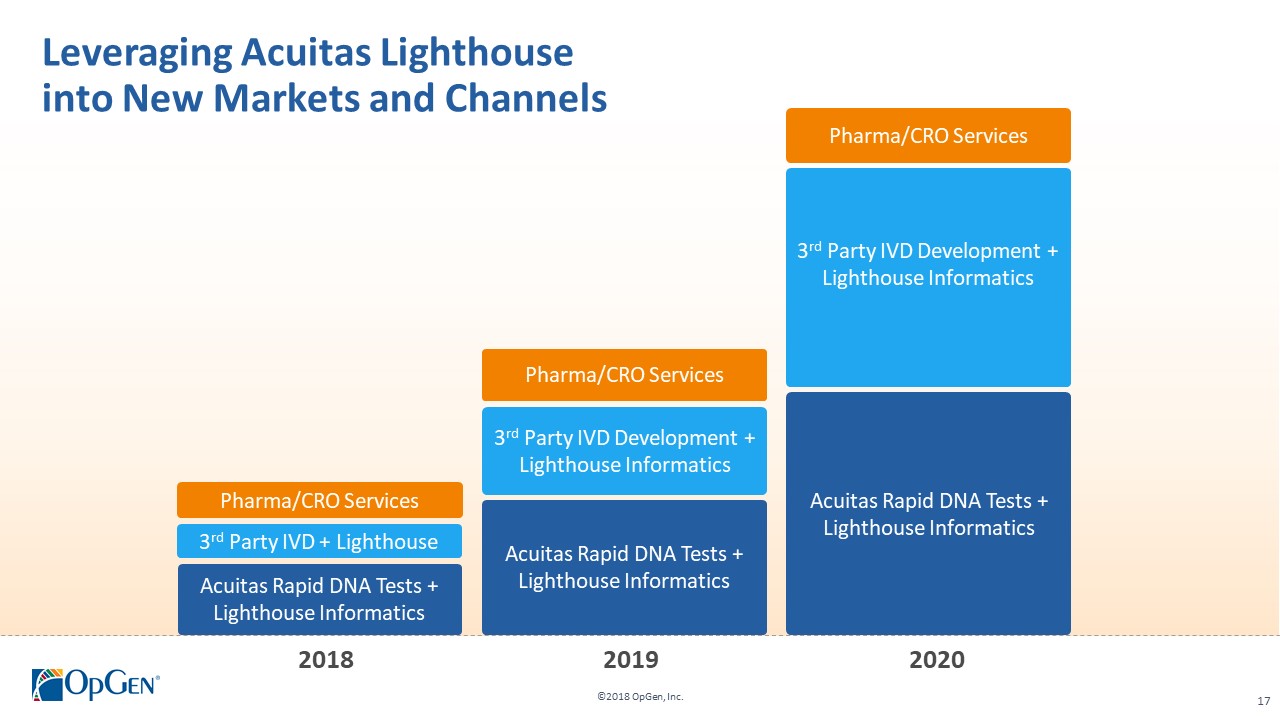
•AMR Gene Panel u5.47 for Complicated Urinary Tract Infection (cUTI) Produced first RUO test kits üCompleted Analytical Verification studies for RUO product üInitiated Clinical Verification studies with flagship IDN hospitals for RUO product üImplemented Thermo Fisher QuantStudio™ 5 (QS5) Global Supply Agreement •Acuitas Lighthouse & Informatics Platform üCurated Knowledgebase of 10,000 isolates tested from Merck SMART surveillance network üDeveloped and presented groundbreaking antibiotic resistance prediction capabilities
üAwarded CDC contract to develop smartphone-based clinical decision support solutions for antimicrobial stewardship in developing economies

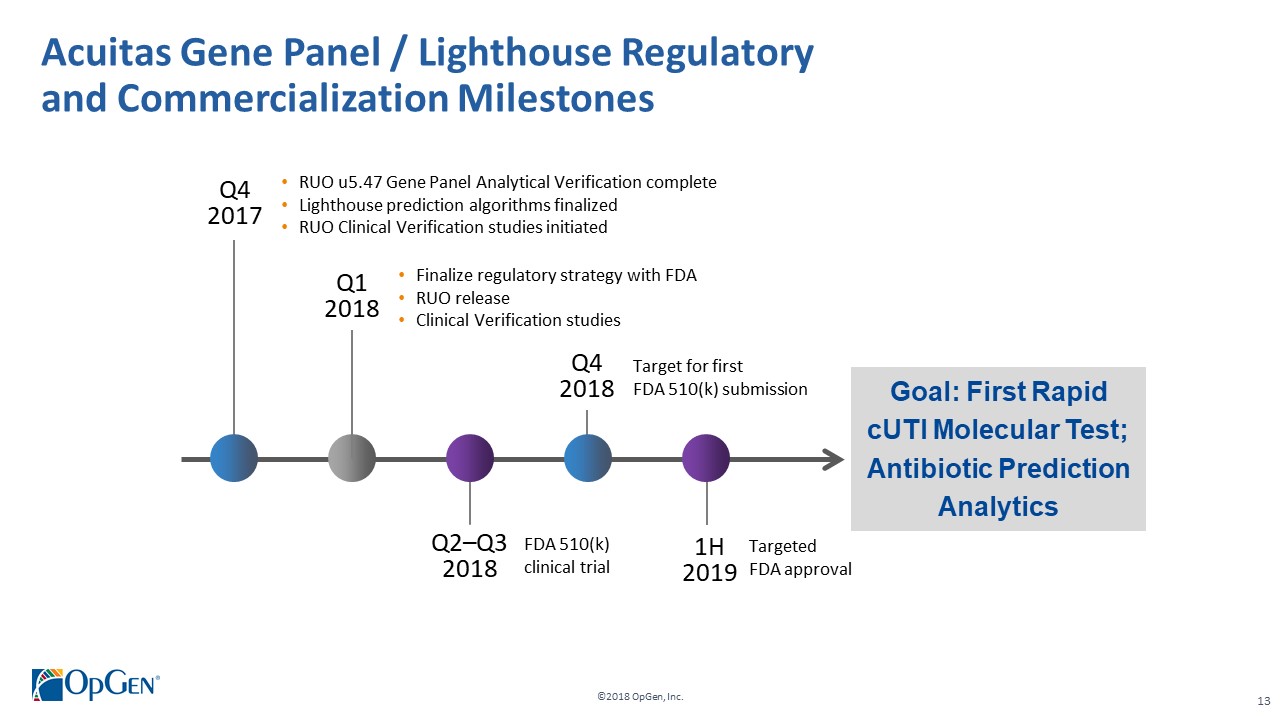
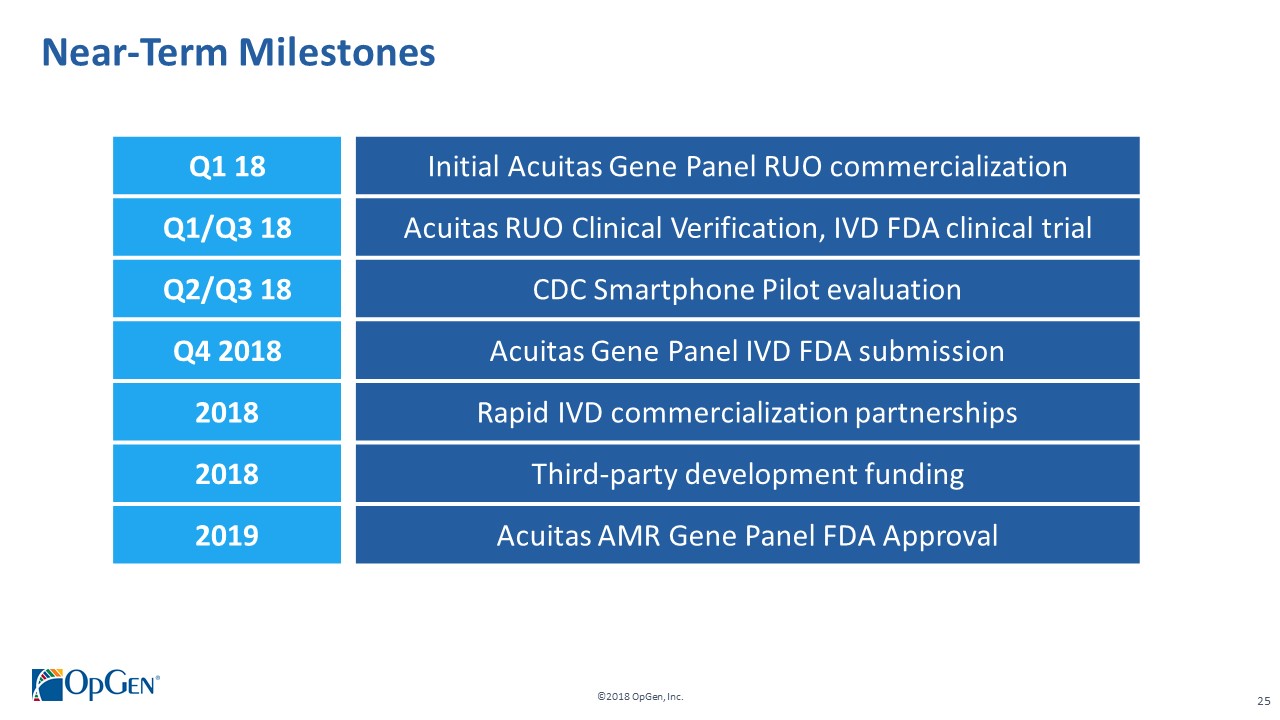
•Q1 RUO release for AMR Gene Panel u5.47 for clinical isolate testing and cUTI research studies •Completing 3rd party RUO Clinical Verification studies and FDA clinical trials for IVD u5.47 •Making FDA 510(k) submission of u5.47 Gene Panel to support full commercial launch •Adding QS5 and Qiagen EZ1 revenue generating system placements •Finalizing Lighthouse Knowledgebase Portal and prediction technology •Conducting CDC smartphone demonstration pilot in Colombia with potential expansion opportunities
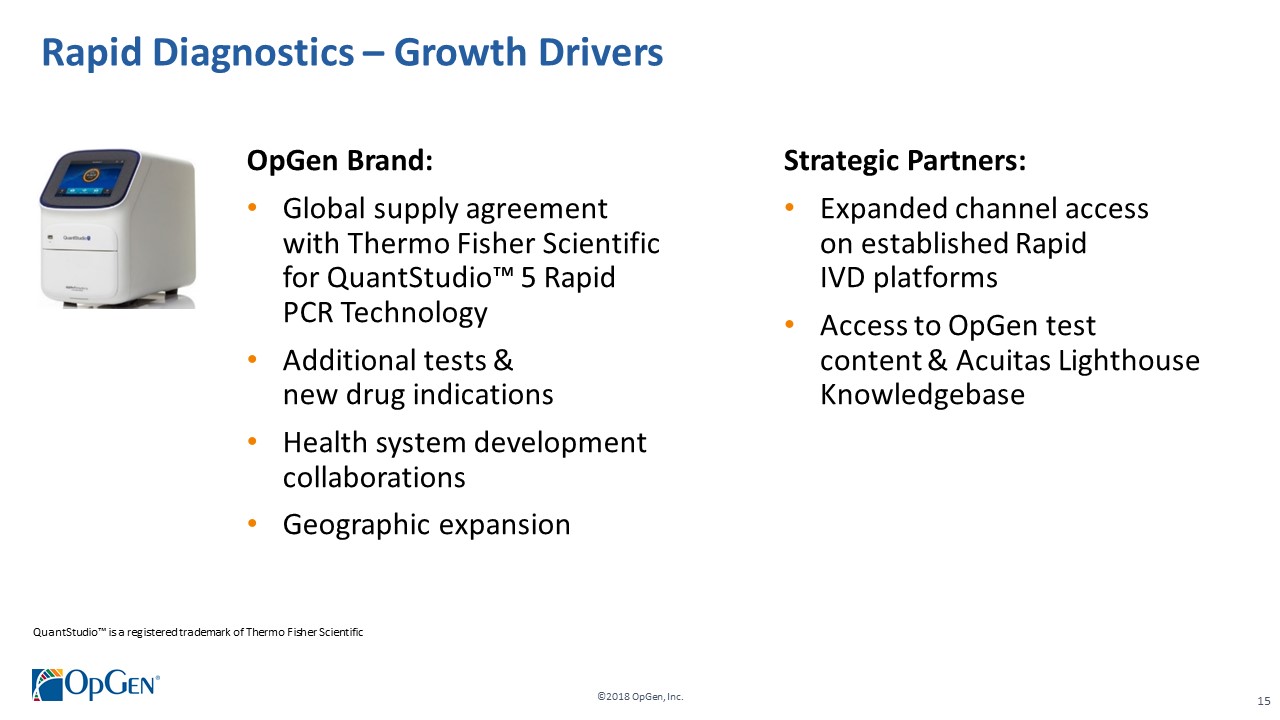
OpGen Brand: •Global supply agreement with Thermo Fisher Scientific for QuantStudio™ 5 Rapid PCR Technology •Additional tests & new drug indications •Health system development collaborations •Geographic expansion Strategic Partners: •Expanded channel access on established Rapid IVD platforms •Access to OpGen test content & Acuitas Lighthouse Knowledgebase
•Merck Global Health Investment Fund invests where Merck’s expertise can help accelerate revenue growth and enhance value creation -$10.5 million investment (May 2016, July 2015) •November 2016: Research collaboration with Merck to develop novel rapid diagnostics and informatics tools to combat antibiotic resistance -Access to Merck’s 250,000 SMART pathogen bacterial archive •December 2017: Ilύm /Opgen CDC smartphone antibiotic stewardship collaboration agreement
Revenue •$2.2 million total revenue through September 30, 2017 •$4.0 million total revenue in 2016 ̶$3.1 million from ongoing FISH business ̶$0.9 million from legacy and other Balance Sheet •Raised $8.8 million in net proceeds in July 2017 •Cash balance $4.9 million •$11.5 million ATM program (raised $8.8M to date - $2.7M remaining capacity) December 2017 Update •Filed S1 Registration Statement to raise up to $7 million of equity capital •Special meeting for reverse stock split January 2018
•OpGen is a precision medicine informatics company focused on the rapidly growing Multi-Drug Resistant Organism (MDRO) market -Collaboration and investment from Merck provides differentiated competitive advantage •Addressing a significant global challenge and market opportunity
->$2 billion market in need of novel solutions to help combat the rise of multi-drug resistant infections •Uniquely positioned with disruptive technologies for helping guide precision antibiotic therapy -AcuitasLighthouse is a proprietary and continually evolving MDRO knowledge database with analytic tools enabling actionable clinical decision support •Validation of OpGenvalue proposition from early adopter U.S. health systems •Experienced management team with a proven track record of value creation