Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - PRECIGEN, INC. | d511494d8k.htm |

ActoBio Therapeutics™, Inc. Pieter Rottiers, PhD, Chief Executive Officer January 2018 Industriepark 7C Building D, 9052 Gent Belgium Tel: +32 9 277 11 77 | E-mail: IA.info@intrexon.com Exhibit 99.1


ActoBio Therapeutics™ - Introduction A new class of microbe-based biopharmaceuticals (ActoBiotics®) for expression and local delivery of therapeutics at disease sites For oral, gastrointestinal, and autoimmune/allergic disorders that are poorly served by current therapeutics Oral or topical administration is safer and more efficacious than injectable biologicals Straightforward cGMP manufacturing process and low cost profile Strong IP portfolio Strong R&D pipeline; latest candidate is in Phase 2b Extensive portfolio of previously engineered candidates ready for clinical development across different diseases License to Intrexon Crop Protection for use of ActoBiotics® microbe-based technology in crop protection applications

ActoBio Therapeutics™ - History Scientists at Flemish Institute for Biotechnology and University of Ghent began developing platform in 1995 ActoGeniX NV, a Belgian based company, was founded in 2006 to commercialize the scientists’ research that focused on ActoBiotics® for in situ expression / secretion of proteins & peptides First team to use live GM bacteria to deliver therapeutic molecules in humans Completed one early stage investigator-driven and four ICH/GCP clinical trials ActoGeniX was acquired by Intrexon in 2015, complementing its technology suite and focus on engineering of biology to deliver efficacious, cost-effective therapeutics Since acquisition: increased efficiency of strain development and validation, expanded platform market opportunity in new disease areas, and entered several new partnerships for the development of ActoBiotics® based therapeutics ActoBio Therapeutics™ is headquartered in Belgium and operates as a subsidiary of Intrexon Corporation

ActoBio Therapeutics™ - Key Team Members PIETER ROTTIERS, PhD Chief Executive Officer, Director Entrepreneurial leader with over 15 years experience in drug discovery and development using the ActoBiotics® platform, including over 13 years of leadership and managerial experience in biopharmaceuticals Held several R&D and executive management functions at ActoGeniX and Intrexon and progressed four ActoBiotics® products from discovery research into clinical trials PhD in Biotechnology, University of Ghent, Belgium LOTHAR STEIDLER, PhD Chief Technology Officer Inventor of ActoBiotics® technology, published author in Science and Nature Biotechnology, co-founder of ActoGeniX Twenty-five years of experience of genetic engineering of L. lactis Led all molecular biology at ActoGeniX and Intrexon ActoBiotics N.V. PhD in Biotechnology, University of Ghent, Belgium LUC VAN FRAEYENHOVEN Chief Financial Officer Finance executive with more than twenty years experience in international finance functions Held various senior finance positions including venture capital investor, CFO of the Ghent Volvo plant, and CFO of Volvo Europe Commercial engineer and Master in Financial Management
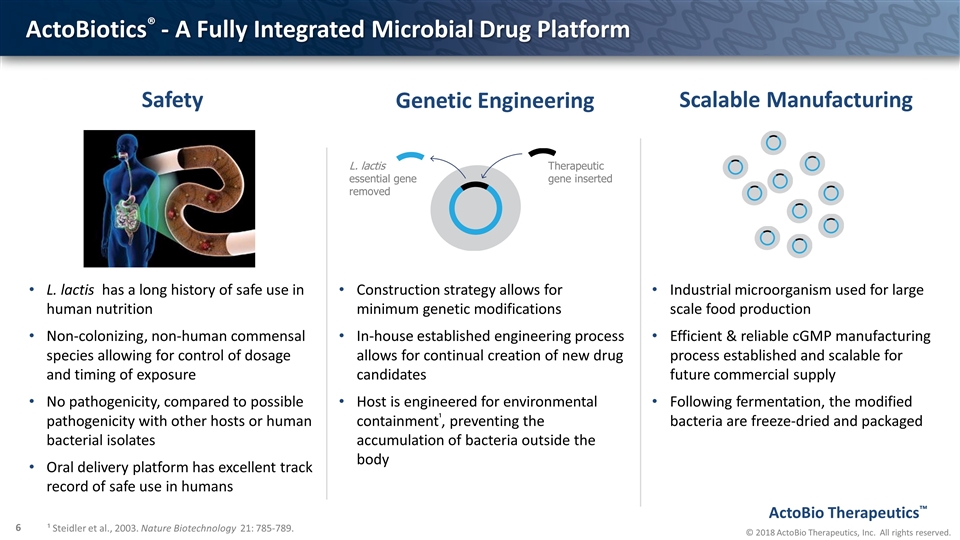
ActoBiotics® - A Fully Integrated Microbial Drug Platform L. lactis essential gene removed Therapeutic gene inserted Construction strategy allows for minimum genetic modifications In-house established engineering process allows for continual creation of new drug candidates Host is engineered for environmental containment¹, preventing the accumulation of bacteria outside the body Industrial microorganism used for large scale food production Efficient & reliable cGMP manufacturing process established and scalable for future commercial supply Following fermentation, the modified bacteria are freeze-dried and packaged Genetic Engineering Scalable Manufacturing ¹ Steidler et al., 2003. Nature Biotechnology 21: 785-789. L. lactis has a long history of safe use in human nutrition Non-colonizing, non-human commensal species allowing for control of dosage and timing of exposure No pathogenicity, compared to possible pathogenicity with other hosts or human bacterial isolates Oral delivery platform has excellent track record of safe use in humans Safety
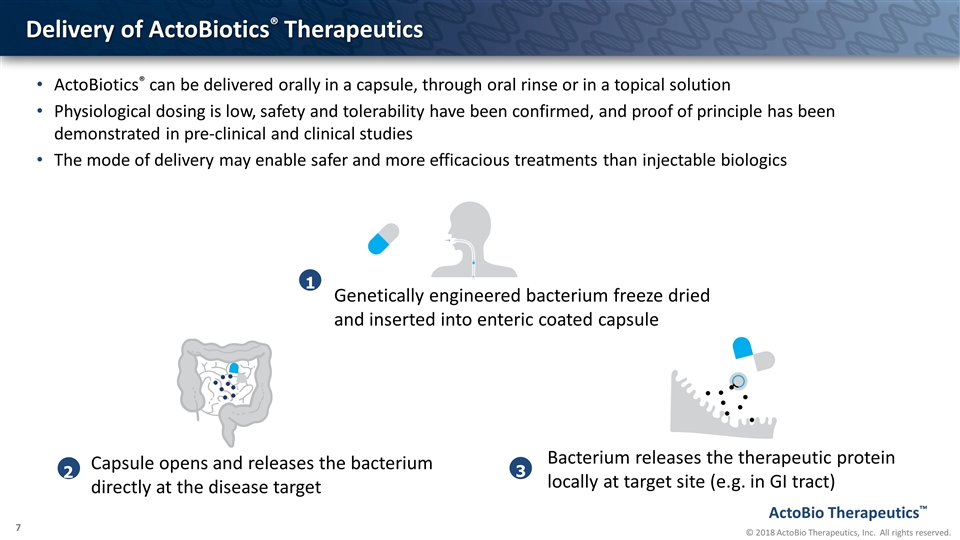
Delivery of ActoBiotics® Therapeutics 1 Genetically engineered bacterium freeze dried and inserted into enteric coated capsule 2 Capsule opens and releases the bacterium directly at the disease target 3 Bacterium releases the therapeutic protein locally at target site (e.g. in GI tract) ActoBiotics® can be delivered orally in a capsule, through oral rinse or in a topical solution Physiological dosing is low, safety and tolerability have been confirmed, and proof of principle has been demonstrated in pre-clinical and clinical studies The mode of delivery may enable safer and more efficacious treatments than injectable biologics
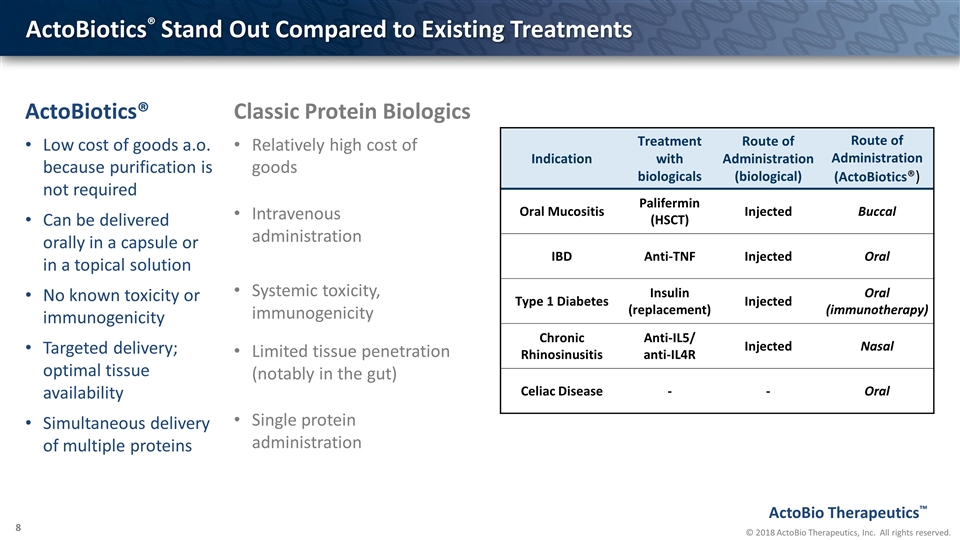
ActoBiotics® Stand Out Compared to Existing Treatments ActoBiotics® Low cost of goods a.o. because purification is not required Can be delivered orally in a capsule or in a topical solution No known toxicity or immunogenicity Targeted delivery; optimal tissue availability Simultaneous delivery of multiple proteins Classic Protein Biologics Relatively high cost of goods Intravenous administration Systemic toxicity, immunogenicity Limited tissue penetration (notably in the gut) Single protein administration Indication Treatment with biologicals Route of Administration (biological) Route of Administration (ActoBiotics®) Oral Mucositis Palifermin (HSCT) Injected Buccal IBD Anti-TNF Injected Oral Type 1 Diabetes Insulin (replacement) Injected Oral (immunotherapy) Chronic Rhinosinusitis Anti-IL5/ anti-IL4R Injected Nasal Celiac Disease - - Oral
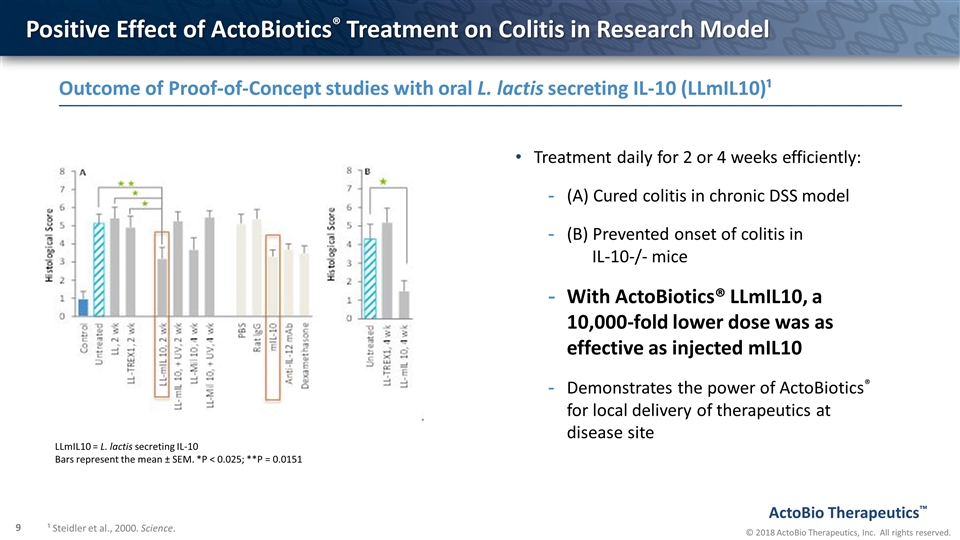
Positive Effect of ActoBiotics® Treatment on Colitis in Research Model Treatment daily for 2 or 4 weeks efficiently: (A) Cured colitis in chronic DSS model (B) Prevented onset of colitis in IL-10-/- mice With ActoBiotics® LLmIL10, a 10,000-fold lower dose was as effective as injected mIL10 Demonstrates the power of ActoBiotics® for local delivery of therapeutics at disease site LLmIL10 = L. lactis secreting IL-10 Bars represent the mean ± SEM. *P < 0.025; **P = 0.0151 Outcome of Proof-of-Concept studies with oral L. lactis secreting IL-10 (LLmIL10)¹ ¹ Steidler et al., 2000. Science.
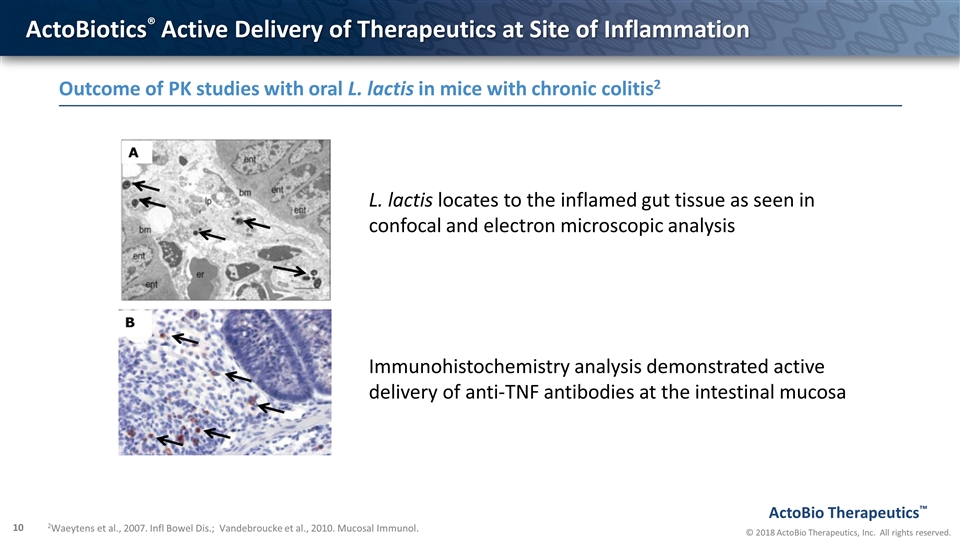
ActoBiotics® Active Delivery of Therapeutics at Site of Inflammation Outcome of PK studies with oral L. lactis in mice with chronic colitis2 2Waeytens et al., 2007. Infl Bowel Dis.; Vandebroucke et al., 2010. Mucosal Immunol. L. lactis locates to the inflamed gut tissue as seen in confocal and electron microscopic analysis Immunohistochemistry analysis demonstrated active delivery of anti-TNF antibodies at the intestinal mucosa
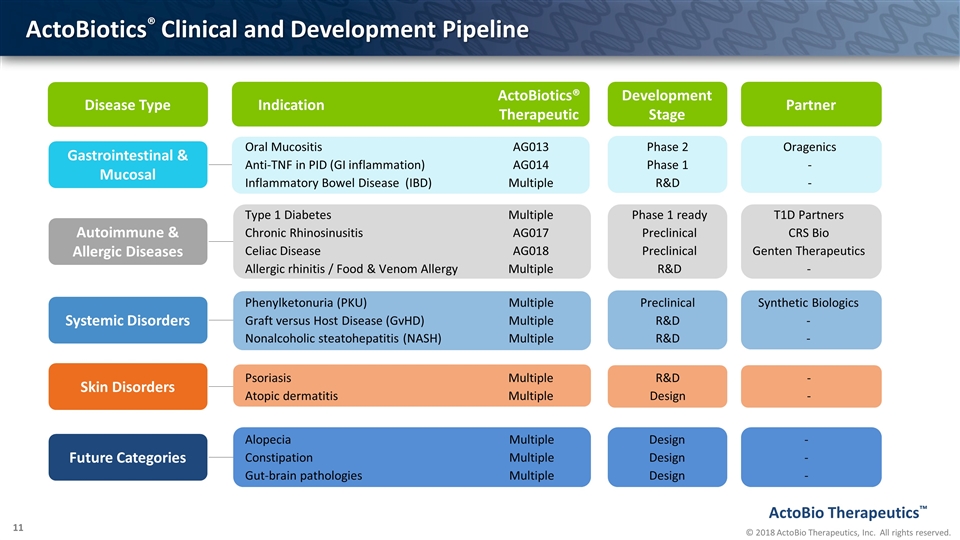
Oral Mucositis AG013 Phase 2 Oragenics Anti-TNF in PID (GI inflammation) AG014 Phase 1 - Inflammatory Bowel Disease (IBD) Multiple R&D - ActoBiotics® Clinical and Development Pipeline Gastrointestinal & Mucosal Autoimmune & Allergic Diseases Systemic Disorders Disease Type Type 1 Diabetes Multiple Phase 1 ready T1D Partners Chronic Rhinosinusitis AG017 Preclinical CRS Bio Celiac Disease AG018 Preclinical Genten Therapeutics Allergic rhinitis / Food & Venom Allergy Multiple R&D - Phenylketonuria (PKU) Multiple Preclinical Synthetic Biologics Graft versus Host Disease (GvHD) Multiple R&D - Nonalcoholic steatohepatitis (NASH) Multiple R&D - Psoriasis Multiple R&D - Atopic dermatitis Multiple Design - Alopecia Multiple Design - Constipation Multiple Design - Gut-brain pathologies Multiple Design - Indication ActoBiotics® Therapeutic Development Stage Partner Skin Disorders Future Categories

ActoBiotics® Therapeutics in Autoimmune & Allergic Diseases

Strategy Builds on Strong Research Foundation Takiishi et al. JCI 2012 Huibregtse et al. J. of Immunol. 2009
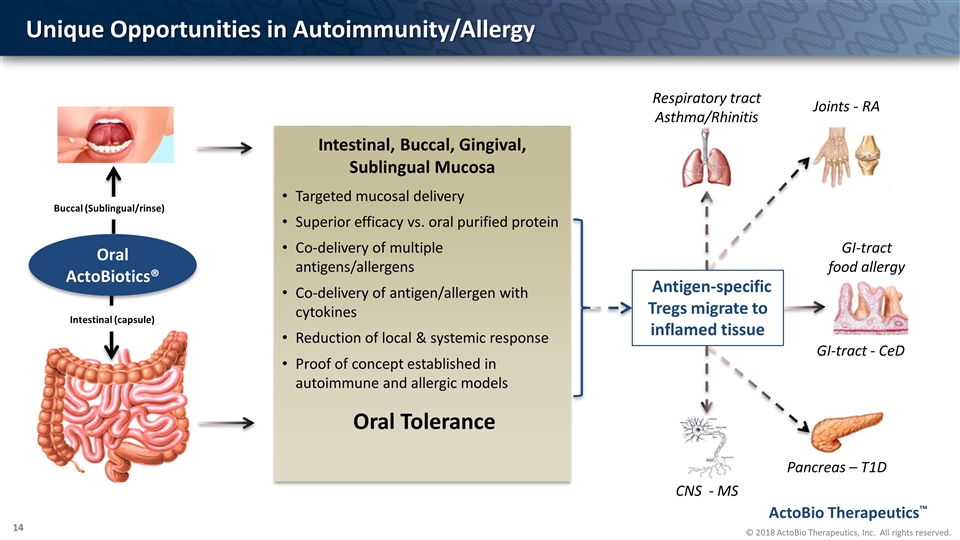
Unique Opportunities in Autoimmunity/Allergy Targeted mucosal delivery Superior efficacy vs. oral purified protein Co-delivery of multiple antigens/allergens Co-delivery of antigen/allergen with cytokines Reduction of local & systemic response Proof of concept established in autoimmune and allergic models Intestinal, Buccal, Gingival, Sublingual Mucosa Intestinal (capsule) Oral ActoBiotics® Pancreas – T1D GI-tract - CeD CNS - MS Joints - RA Respiratory tract Asthma/Rhinitis GI-tract food allergy Antigen-specific Tregs migrate to inflamed tissue Buccal (Sublingual/rinse) Oral Tolerance
Re-establish Tolerance in Autoimmunity with Oral ActoBiotics® ActoBiotics® antigen-specific immunotherapy is the first to harness all required elements – antigen, tolerance enhancing cytokine, and delivery – in a practical single therapeutic platform, ideally suited for efficient tolerance induction Goal of therapy is to re-establish long-term immunological tolerance to self-antigens in order to stabilize/reverse the autoimmune phenotype Treatment option for autoimmune diseases for which triggering autoantigens are known (e.g. T1D, CeD, RA, MS)

Case study: ActoBiotics® Therapeutics in Type 1 Diabetes (Joint Venture with Intrexon T1D Partners, LLC)
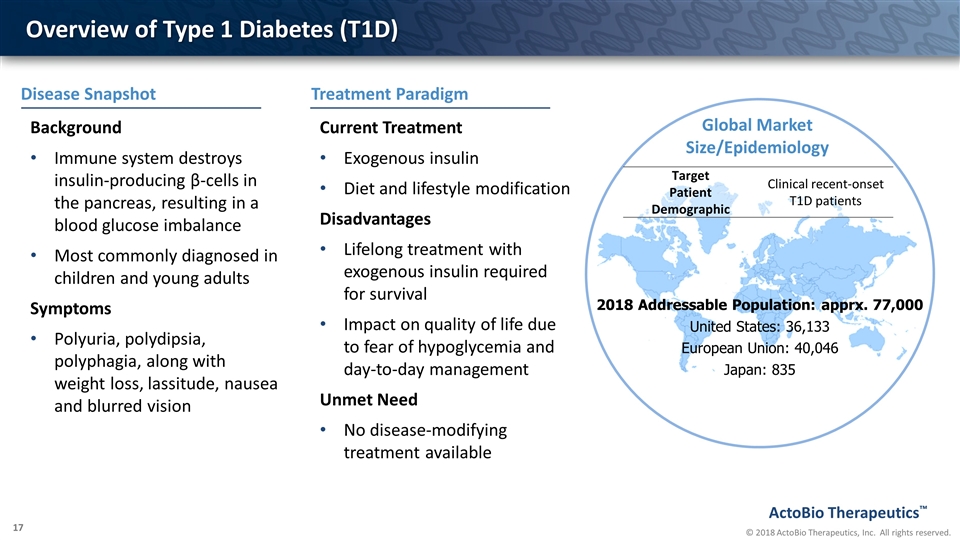
Overview of Type 1 Diabetes (T1D) Global Market Size/Epidemiology 2018 Addressable Population: apprx. 77,000 United States: 36,133 European Union: 40,046 Japan: 835 Target Patient Demographic Clinical recent-onset T1D patients Background Immune system destroys insulin-producing β-cells in the pancreas, resulting in a blood glucose imbalance Most commonly diagnosed in children and young adults Symptoms Polyuria, polydipsia, polyphagia, along with weight loss, lassitude, nausea and blurred vision Current Treatment Exogenous insulin Diet and lifestyle modification Disadvantages Lifelong treatment with exogenous insulin required for survival Impact on quality of life due to fear of hypoglycemia and day-to-day management Unmet Need No disease-modifying treatment available Treatment Paradigm Disease Snapshot
ActoBiotics® Opportunity for Disease-modifying Treatment AG019 in T1D Value Proposition Current treatments do not re-establish long-term immunological tolerance to self-antigens Need for treatment to re-establish tolerance in order to maintain functional β-cell mass Ingested ActoBiotics® therapeutics delivering self-antigens and cytokines have potential as first disease-modifying treatment that can prevent, delay or reverse T1D Combination of antigen-specific properties of ActoBiotics® with broad immunoregulatory agents Mechanism of Action Easy-to-take capsule formulation composed of L. lactis delivering the autoantigen human proinsulin (hPINS) and the tolerance-enhancing cytokine hIL-10 (AG019) Proof of concept demonstrated in mice Induction of antigen-specific Treg cells will re-establish long-lasting immunological tolerance to islet-antigens and reverse the autoimmune phenotype Clinical study scheduled for 2018
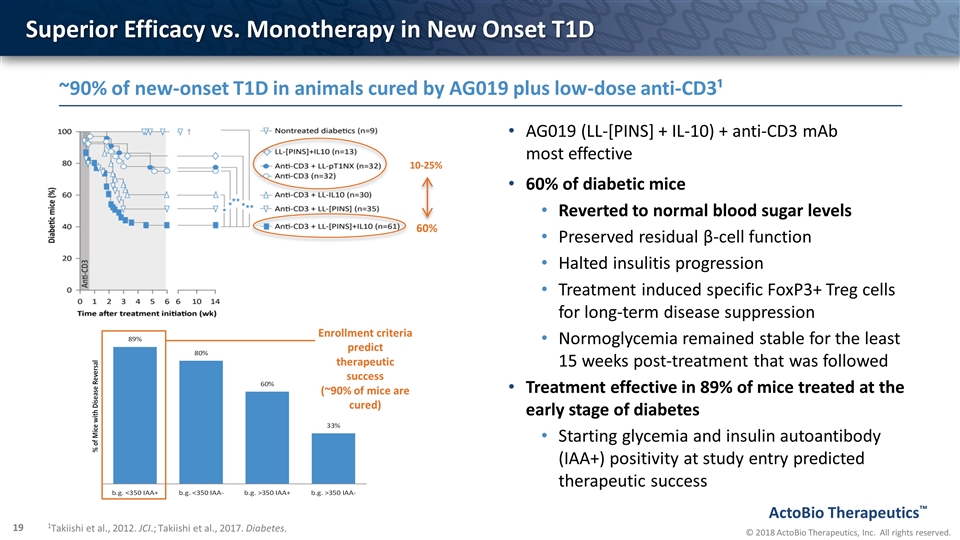
Superior Efficacy vs. Monotherapy in New Onset T1D AG019 (LL-[PINS] + IL-10) + anti-CD3 mAb most effective 60% of diabetic mice Reverted to normal blood sugar levels Preserved residual β-cell function Halted insulitis progression Treatment induced specific FoxP3+ Treg cells for long-term disease suppression Normoglycemia remained stable for the least 15 weeks post-treatment that was followed Treatment effective in 89% of mice treated at the early stage of diabetes Starting glycemia and insulin autoantibody (IAA+) positivity at study entry predicted therapeutic success 10-25% 60% ~90% of new-onset T1D in animals cured by AG019 plus low-dose anti-CD3¹ 1Takiishi et al., 2012. JCI.; Takiishi et al., 2017. Diabetes. Enrollment criteria predict therapeutic success (~90% of mice are cured)
ActoBiotics® in T1D Summary and Outlook Data Validation Proof-of-Concept and mechanism of action established in validated new-onset T1D animal model when combined with systemic low-dose anti-CD3 (2012) Progress to Date Non-clinical development plan and Phase 1b/2a clinical protocol reviewed during US FDA pre-IND meeting and Scientific-Technical Advice (EU-FAGG) (2Q’17) cGMP manufacturing process established (3Q’17) Completed PK, safety pharmacology, and RDT studies (4Q’17) Upcoming Catalysts & Strategy IND/CTA filing (1Q’18) Launch Phase Ib/IIa (2Q’18)

ActoBio Therapeutics™ Key Highlights ActoBiotics® technology is a fully integrated microbial drug delivery platform for biologicals with superior efficacy and safety potential through targeted delivery Therapeutic application across a broad set of markets with strong growth dynamics R&D engine creates new drug candidates and delivery improvements Strong IP position covering platform and specific applications Experienced leadership team with strong scientific, regulatory, and clinical experience Enables production of new, disease-modifying therapeutics through oral or topical administration
