Attached files
| file | filename |
|---|---|
| EX-32 - PetLife Pharmaceuticals, Inc. | ex32.htm |
| EX-31 - PetLife Pharmaceuticals, Inc. | ex31.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| [X] | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended August 31, 2017
| [ ] | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number: 000-52445

PETLIFE PHARMACEUTICALS, INC,
(Name of registrant as specified in its charter)
| Nevada | 33-1133537 | |
| (State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization) | Identification No.) |
38 West Main Street Hancock, MD |
21750 | |
| (Address of principal executive offices) | (Zip Code) |
(844) 473-8543
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $0.001 par value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. [ ] Yes [X] No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. [ ] Yes [X] No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the issuer was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. [X] Yes [ ] No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). [X] Yes [ ] No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer [ ] | Accelerated filer [ ] |
| Non-accelerated filer [ ] (Do not check if a smaller reporting company) | Smaller reporting company [X] |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). [ ] Yes [X] No
On December 31, 2016, the last business day of the registrant’s most recently completed second quarter, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was $4,595,945, based upon the closing price on that date of the common stock of the registrant on the OTC Bulletin Board system of $0.13. For purposes of this response, the registrant has assumed that its directors, executive officers and beneficial owners of 5% or more of its Common Stock are deemed affiliates of the registrant.
As of December 14, 2017, the registrant had 73,966,195 shares of its common stock, $0.001 par value, issued, issuable, and outstanding.
TABLE OF CONTENTS
| 2 |
The document (including information incorporated herein by reference) contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, which involve a degree of risk and uncertainty due to various factors affecting PetLife Pharmaceuticals, Inc.
Forward-Looking Statements
This Annual Report on Form 10-K contains forward-looking statements, including, without limitation, in the sections captioned “Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Result of Operations,” and elsewhere. Any and all statements contained in this Current Report that are not statements of historical fact may be deemed forward-looking statements. Terms such as “may,” “might,” “would,” “should,” “could,” “project,” “estimate,” “pro-forma,” “predict,” “potential,” “strategy,” “anticipate,” “attempt,” “develop,” “plan,” “help,” “believe,” “continue,” “intend,” “expect,” “future,” and terms of similar import (including the negative of any of the foregoing) may be intended to identify forward-looking statements. However, not all forward-looking statements may contain one or more of these identifying terms. Forward-looking statements in this Annual Report may include, without limitation, statements regarding (i) the plans and objectives of management for future operations, (ii) a projection of income (including income/loss), earnings (including earnings/loss) per share, capital expenditures, dividends, capital structure or other financial items, (iii) our future financial performance, including any such statement contained in a discussion and analysis of financial condition by management or in the results of operations included pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”), (iv) our beliefs regarding potential clinical and other health benefits of our PetLife products, and (v) the assumptions underlying or relating to any statement described in points (i), (ii), (iii) or (iv) above.
The forward-looking statements are not meant to predict or guarantee actual results, performance, events or circumstances and may not be realized because they are based upon our current projections, plans, objectives, beliefs, expectations, estimates and assumptions and are subject to a number of risks and uncertainties and other influences, many of which we have no control over. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of these risks and uncertainties. Factors that may influence or contribute to the inaccuracy of the forward-looking statements or cause actual results to differ materially from expected or desired results may include, without limitation, our inability to obtain adequate financing, the significant length of time and resources associated with the development of our products and related insufficient cash flows and resulting illiquidity, our inability to expand our business, significant government regulation of our business and the healthcare industry, the results of clinical studies or trials, lack of product diversification, volatility in the price of our raw materials, existing or increased competition, results of arbitration and litigation, stock volatility and illiquidity, and our failure to implement our business plans or strategies. A description of some of the risks and uncertainties that could cause our actual results to differ materially from those described by the forward-looking statements in this Annual Report appears in the section captioned “Risk Factors” and elsewhere in this Annual Report.
Readers are cautioned not to place undue reliance on forward-looking statements because of the risks and uncertainties related to them and to the risk factors. We disclaim any obligation to update the forward-looking statements contained in this Annual Report to reflect any new information or future events or circumstances or otherwise.
Readers should read this Annual Report in conjunction with the discussion under the caption “Risk Factors,” our financial statements and the related notes thereto in this Annual Report, and other documents which we may file from time to time with the SEC.
All references in this Form 10-K that refer to the “Company,” “PetLife Pharmaceuticals, Inc.,” “Registrant,” “we,” “us,” or “our” are to PetLife Pharmaceuticals, Inc., a Nevada formed corporation.
| 3 |

Historical Company Information
PetLife Pharmaceuticals, Inc. (“PetLife”) were incorporated on April 5, 2002 under the laws of the State of Nevada as “Aztek Ventures Inc.” Effective November 13, 2007, we filed a Certificate of Amendment to our Articles of Incorporation to change our name from “Aztek Ventures Inc.” to “Genesis Uranium Corp.” Effective April 21, 2008, we amended our Articles of Incorporation to change our name from “Genesis Uranium Corp.” to “Vault Technology Inc.” to reflect the change in our business focus beyond solely that of uranium exploration. Effective July 10, 2009, we filed a Certificate of Amendment to our Articles of Incorporation to change our name from “Vault Technology, Inc.” to “Modern Renewable Technologies, Inc.” (“Modern”). On May 27, 2011, Modern, merged with Eco Ventures Group, Inc., and the name of the Company was changed to Eco Ventures Group, Inc. On July 18, 2013, the Company declared a 15-for-1 reverse stock split for all of its common and preferred stock. On June 26, 2014, Eco Ventures Group, Inc. entered into an Agreement and Plan of Merger with its subsidiary, PetLife Pharmaceuticals, Inc., a Nevada Corporation, with PetLife Pharmaceuticals, Inc. being the surviving entity. As part of that merger, the name of the Company was changed to PetLife Pharmaceuticals, Inc. and each 20 shares of our common stock were exchanged for one share in the surviving company. Effective August 12, 2014 we completed the closing of the Share Exchange Agreement and the acquisition of PetLife and changed our name to PetLife Pharmaceuticals, Inc. Effective July 19, 2016, we agreed to complete a subsidiary merger in which we effectively complete a 1 for 5 reduction in our outstanding shares. All references herein to the number of shares outstanding and per-share amounts have been retroactively restated to reflect both the reverse stock split on July 18, 2013, the exchange ratio in the merger with PetLife Pharmaceuticals, Inc., and the reduction in September 2016.
Business of PetLife Pharmaceuticals, Inc.
Summary
We have developed and are launching a new generation of high potency veterinary cancer medications and nutraceuticals.
PetLife’s main product, Vitalzul™ inhibits blood vessels formation in solid tumors. Chlorotoxin blocks trans-membrane fluxes of chlorine and regulates the adjusting of cell growth, cell division, metastasis and induces apoptosis (dose dependent) that leads to tumor cells death. The regulating of activity of the potassium (K+) 3-4 kDa, sodium (N+) 6-8 kDa and chloride (CL+) voltgate ionic channels of the tumor cells leads to growth arrest and causes cell death (apoptosis); additionally, calcium dependent potassium channels inhibit growth of other cancer cells.
The National Cancer Institute reported that in the United States alone, nearly 6 million dogs and 6.5 million cats are diagnosed with cancer annually with dogs being 35 times and cats 40 times more likely to suffer from cancer than humans. Sixty percent of dogs and cats over six years of age will be diagnosed with cancer.
| 4 |
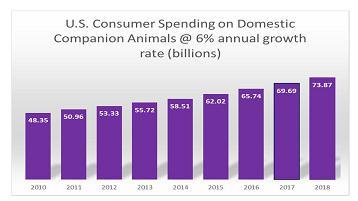
In the United States over 92 million households own a dog or cat, representing 83.3 million dogs and 95.6 million cats. Consumer spending on domestic companion animals in the United States alone is expected to reach over $60.28 billion in 2015, with over $13 billion being spent on over the counter medications. Demand for domestic pet health products is expected to grow approximately 4 - 6% annually through 2018
PetLife plans to apply to the FDA in the first quarter of 2018 to begin a New Animal Drug Application for prescription strength version of VitalzulTM. Although there can be no assurance the FDA will work this quickly, the Company anticipates FDA approval of its prescription strength products in the second half of 2019, and intends to roll out those products shortly thereafter.
The Company has a developed a sophisticated, multi-channel marketing strategy, to be implemented in the second half of 2017, that includes direct sales, retail, veterinarian vertical market, affiliate sales and infomercials for its nutraceutical formulation.
As part of our mission to bring health and well-being to our companion animals, PetLife has acquired “Healthy Life Pets “a natural based pet food company founded by renowned veterinarian, Dr. Geoffrey Broderick. The Company plans to market a new brand “Dr. Geoff’s by PetLife” as a premium product and will seek to provide evidence of the benefit of the food on cancer prevention and general pet health.
Corporate Overview
PetLife Pharmaceuticals, Inc. is a registered U.S. Veterinary Pharmaceutical company whose mission is to bring its scientifically proven, potentiated bioactive medication and nutraceuticals — “Vitalzul™” — to the world of veterinary oncology. The Company specializes in the research, development, sales and support of advanced drugs and nutraceuticals for pet cancer and autoimmune related diseases such as arthritis.
Since the histology (cellular biology) of human, dogs and cats is quite similar at the molecular level, it is notable that PetLife, upon the completion of our veterinary trials, PetLife can continue to develop Vitalzul™, for human use or out license the technology as deemed appropriate by management and our medical team.
Business Strategy
| ● | Initially, enter the market with an oral nutraceutical version of Vitalzul™ with a focus on the oncology needs of dogs and cats. | |
| ● | Raise capital through traditional means, including through this private placement of securities, additional private placements, public offerings and/or bank financings. | |
| ● | Apply for FDA approval of the Caribbean Blue Scorpion animal pharmaceutical drug, VitalzulTM for cancer treatment, a prescription strength oral pharmaceutical as well as a concentrated intravenous and injectable version for direct administration to a tumor. | |
| ● | Concurrently develop edible dog and cat treats that are infused with Vitalzul™’s active ingredients that deliver preventative benefits for both dogs and cats. |
FDA Process, Cost and Time Line
The Company plan outlines the necessary studies and regulatory actions required to move Vitalzul TM thought the Food and Drug Administration (“FDA”) and Center for Veterinary Medicine (“CVM”) approval process.
The lead for our FDA approval of the pharmaceutical version of VitalzulTM is Dr. Vivek Ramana, our Chief Medical Officer, who has greater than 20 years of experience in the pharmaceutical industry and has worked on more than 25 drugs that have been approved by the FDA. Dr. Ramana has more than four years’ experience working with Scorpion venom and has successfully secured approval of a scorpion-based Chlorotoxin Oncology drug for humans in India. His combined professional experience and personal knowledge of Vitalzul™ gives PetLife great confidence in his ability to manage the process, adhere to the timelines and maintain the project budget.
Dr. Ramana has provided PetLife with a time line of approximately 24 months to successfully complete the required clinical studies and register the pharmaceutical version of Vitalzul TM with the FDA/CVM. We will also push to fast track the drug and can potentially have it approved as a compassionate use drug for pets in as little as nine months.
Prior to our discussions with the FDA, the Company, under the direction of Dr. Ramana, has completed a preliminary dosing and toxicology study. The third study required prior to our FDA discussion is a cell line study, which should be completed within the next several months.
During the initial stage of our proposed FDA trial, the drug will be analyzed to establish its structure, stability and toxicity. This stage will establish the ability to produce a stable, replicable drug product supply for the subsequent clinical studies in animals. This stage is estimated to take 3-9 months to complete at a cost of approximately $700,000.
The second stage is pre-clinical development. In this stage, InnoVision Therapeutics will perform the studies necessary to establish the safety of the product. These studies include toxicology and dosing studies as well as establish the pharmokinetics and pharmodynamics of the drug. Essentially, we want to learn what the drug does to the body and what the body does to the drug. This stage is estimated to take between 9 and 24 months. Many of these studies can be performed during the initial stage of the project if funding is available. The estimated cost of this stage is approximately $2,200,000.
In the third stage will include standard FDA phase I, II and III studies on dogs. These studies will establish safety and efficacy of the drug in a canine model and allow for the determination of appropriate dosing. Some of these studies can be run concurrently with earlier stages. The estimated to take between 12-24 months at accost of $3,600,000.
| 5 |
Based on the information provided in our Development Plan, assuming immediate full funding allowing concurrent studies, we expect to complete our FDA submission within 24 months.
The FDA/CVM registration is only necessary for the pharmaceutical version products PetLife wishes to sell globally. The nutraceutical version, pet food, treats and other products do require FDA/CVM approval under the Nutraceutical Division. Having this stamp of approval and completing these necessary tests will allow PetLife to make the necessary claims on the product label and in its advertising programs. This will move PetLife from a company with lots of anecdotal evidence and a few clinical trials to one with certified studies and evidence meeting all the necessary requirements by the FDA/CVM.
The Company’s Vitalzul TM Product

PetLife will initially develop dedicated cancer preventative products, such as animal treats. These products are designed to prolong pets’ lives when taken consistently. The Company anticipates this will drive sales for PetLife as clients adopt these types of products as part of their pet’s normal daily diets. Part of PetLife’s sales and marketing framework is to not only sell the products to animal’s post cancer diagnosis, but to educate owners on cancer and autoimmune prevention. PetLife’s preventative animal treats, for example, are an excellent way for pet owners to promote lasting health and viability for their companions.
PetLife anticipates developing the following products for the cancer preventative products:
| ● | Vitalzul™ Preventative – a natural pet treat preventative | |
| ● | Vitalzul™ Tabs – a natural daily pet preventative tablet | |
| ● | Vitalzul™ Pet Foods – natural preventative foods |
VitalzulTM has demonstrated an effect on several different cancer types. With inexpensive modifications, PetLife may be able to create cancer specific products that may increase sales. The following are the top veterinary cancer types that PetLife intends to target:
| ● | Lymphoma or Lymph sarcoma | |
| ● | Hemangiosarcoma | |
| ● | Osteosarcoma | |
| ● | Mast Cell Tumor | |
| ● | Melanoma | |
| ● | Squamous Cell Carcinoma | |
| ● | Mammary Carcinoma | |
| ● | Apocrine Gland Carcinoma (Anal Sac) | |
| ● | Transitional Cell Carcinoma | |
| ● | Soft Tissue Sarcoma | |
| ● | Lymphoma | |
| ● | Squamous carcinoma | |
| ● | Fibrosarcoma | |
| ● | Lung tumors | |
| ● | Brain tumors | |
| ● | Nasal tumors | |
| ● | Liver tumors |
PetLife plans to design three products for differentiated intake protocols for home and veterinary usage.
| ● | Oral / Rectal - Home oral and rectal administration of the VitalzulTM liquid makes the animal easy to treat. The solution is colorless and odorless and can be administered through a droplet or enema. This is an effective tool which clients can use in the convenience of their home. |
| 6 |
| ● | Injectable/Intravenous - The injectable/intravenous version can be used by veterinarians to directly inject the product into the tumor for effective, targeted high dose treatments. This will increase sales with vets who will have an effective in clinic, targeted product will require clients to bring their pets into the vet clinic for treatment. |
PetLife, through the work of its analytical scientists, has identified a diversity of diseases that it believes will respond positively from treatments with the Vitalzul™ nutraceuticals and drugs. The diseases below illustrate those areas of potential expansion beyond the current focus on veterinary oncology related illnesses. The expansion of the application of PetLife’s products and technical knowledge into other health related areas will require the addition of skilled research scientists and pharmacological experts. PetLife will expand its market reach and increase its revenue by treating other diseases.
PetLife intends to also develop products for pets in the following areas:
| ● | Arthritis: Arthritis (or osteoarthritis) is a slowly progressive, degenerative disease of the joints for which there is no cure. However, Vitalzul TM may be able to help to prevent, delay, and manage arthritis in dogs or cats. Arthritis affects 1 in 5 dogs over the age of 7 and over 90% of geriatric cats have arthritis; currently 12 million cats have arthritis. | |
| ● | Hepatitis: As with humans, Hepatitis in dogs and cats affects the liver. This contagious disease includes symptoms such as fever, vomiting and diarrhea accompanied by abdominal pain. Furthermore, Hepatitis may lead to kidney damage. Approximately, 12% of dogs and 40% of cats get Hepatitis. |
| ● | Lyme disease: Lyme disease is a dangerous bacterial disease that can cause irreversible damage to a companion animal’s health. About 5% of dogs contract Lyme disease while the statistics for cats is nominal. |
Global Market for Vitalzul TM and the Company’s Products
The global veterinary pharmaceutical industry is composed of various veterinary health products including biological, veterinary pharmaceuticals and medicated food additives. Over the past few years, the range of animal health products has diversified, currently encompassing metabolic drugs, reproductive aids, anti-infectives, feed additives, imaging diagnostics, vaccines, parasite control, and topical solutions. Veterinary products have emerged for treating chronic diseases such as cancer, osteoarthritis and cardiovascular disease. Pet owners, more than ever before, are spending a larger proportion of their income to take care of their pets’ health.
In the future, the global market for pet health products is expected to grow by an average of 4 - 6% annually and become more specialized. The major drivers will be: (1) the continued strengthening of the bond between owners and their animal companions, (2) increasing companion animal owner awareness and willingness to pay for care, (3) increasing occurrence of cancer in dogs and cats than people while only a fraction are treated because of prohibitive costs, (4) offering an FDA drug at competitive prices, and potentially more effective, as an incentive for more pet owners to treat their pets and extend their duration and quality of life. Companion animals have come to play an important part in the lives of many people. Unsurprisingly the bond between companion animals and people continues to strengthen in parallel to the market for products that contribute significantly to the health and well-being of these animals, such as the products offered by PetLife.
The current focus of the Company’s market is veterinary oncology, a branch of veterinary medicine whose emphasis is dealing with cancer diagnosis and treatment in animals, especially companion animals. Over the years, the number of animals dying of cancer has increased. For example, approximately 45% of dogs aged ten years and above die of cancer. Part of PetLife’s product expansion will include an injectable version that will focus on decreasing and eliminating tumors that are accessible to direct injection
PetLife realizes the parallels between animals and their owners regarding certain conditions, such as poor diet, and its consequences. Overall, the companion animal segment is considered to be the health segment that parallels the human pharmaceutical sector. Many of the innovations in human medicine, at least in terms of new medications, are subsequently adapted and tailored to suit companion animals, like Vitalzul™.
The global animal health market is consolidated with the top ten players controlling the majority share of the market. For many years the largest players in the companion animal market have been Merial and Zoetis. However, the market has seen considerable consolidation mainly through acquisitions and mergers. Proposed mergers and acquisitions are monitored very carefully by governments around the world (such as the Directorate General for Economic and Financial Affairs in the European Union and the United States Federal Trade Commission) to protect the consumer from reduced competition, price increases, and reduced innovation.
Medication and treatments for veterinary cancer are limited and largely ineffective - mainly aimed at temporarily delaying disease progression rather than effective treatments. The veterinary health market for companion animals has grown slowly but steadily by 4% - 6% annually while companion animal expenditures has grown to more than 6.9% compound annual growth rate.
While the U.S. represents the biggest regional market for animal medication, Asia-Pacific region represents the fastest growing market for pet medication with annual dollar sales in the region growing at an annual average of 10.5%. This can be attributed to factors such as increasing income, improvement in per capita consumption per animal, improved living standards, as well as increased pet populations in various regions all over the world. About half of Brazilian households have a dog, more than any other country, according to data tracker Euromonitor, and pet food sales there rose 10 percent to $5.6 billion last year, trailing only the U.S. and U.K. in the $71 billion worldwide market.
| 7 |
Despite remaining challenges in the overall economy, the pet industry has remained unshaken and has been less affected by the recent global recession than other retail sectors. Over the past years, the United States and the United Kingdom have been the leaders in the pet industry. However, today there are a number of other countries that are coming out as players in the global pet industry. The trend in world pet industry has increased tremendously, which is a clear indication of the growing market for veterinary oncology services.
Market in the United States
The veterinary oncology market in the United States is expected to grow mainly due to an increase in the pet population in the United States. People’s attitude towards companion animals has changed, as most pet owners now treat their pets as family members. In a report by Global Industry Analysts, Animal Medication: A Global Strategic Business Report, the authors indicate that animal care often mirrors the trends in human health care. Diagnosis and disease monitoring in veterinary medicine, particularly for companion animals, has also followed trends in human medicine. Diagnostic imaging techniques, such as ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI), have become commonplace and many veterinary practices have in-house analyzers for clinical chemistry and hematology, as well as rapid patient-side tests, e.g., for infectious agents.
The National Cancer Institute reported that nearly six million dogs in the United States alone are diagnosed with cancer annually, with dogs being 35 times more likely to suffer from cancer than humans. Over six years of age, sixty percent of America’s 83.3 million dogs and 95.6 million cats will be diagnosed with cancer. Unfortunately, only an estimated 10% of those diagnosed receive treatment. PetLife understands that 90% of dogs and cats with cancer go untreated because of unrealistically high costs associated with treatments as well as the dramatic suffering typical of treating pets with toxic, conventional chemotherapy
Marketing Plan
PetLife will deliver its nutraceutical and prescription strength medicines to the enormous population of untreated animals by making the product affordable and convenient in both the home and veterinary setting. PetLife will also capitalize on the increased willingness of pet owners to spend more on their animals’ preventative health, and of veterinarians to meet that demand as key drivers of this market. PetLife will also dedicate their marketing efforts to include educating the public as to the risk their companion animal has at developing cancer. The Company will emphasize the importance on pet owners being proactive and taking the necessary steps to protect their animal. The Company will offer VitalzulTM as the preventative solution.
Currently, apart from surgery which carries its own risks for older pets, the preponderance of treatment for dogs and cats with cancer is simply the same, drastically expensive (most pets do not have medical insurance), toxic chemotherapy drugs developed for humans — scaled down in dosage by the pet’s weight — with many of the same side effects such as nausea, diarrhea, lost appetite, cachexia (“wasting” loss of muscle and fat tissues) headache, hair loss, liver and kidney toxicity and susceptibility to opportune infections.
Direct Sales
PetLife has lined up key team members to execute a traditional, multichannel marketing campaign. This campaign, composed of the traditional five “P” marketing mix will include:
| ● | Product – PetLife will capitalize on the proprietary nature of their primary product offering, Vitalzul™. PetLife will sell Vitalzul™ to veterinarians using multiple respected veterinarian centers of influence to promote the product. PetLife will also sell directly to pet owners through online and brick and mortar establishments; this will be achieved using a well-connected sales force to market to both distributors and direct to retail outlets. | |
| ● | Physical attributes – a newly redesigned bottle has been approved. | |
| ● | Place or distribution – the Company will configure its corporate structure to accommodate the most efficient global supply chain, production and distribution through regional sales centers. PetLife representatives will manage the supply chain, local final production (as required) and distribution. |
| 8 |
| ● | Promotion – direct to vet, direct to consumer, direct to wholesaler; there will also be trade shows, direct vet outreach and online webinars. PetLife will also implement an aggressive online marketing campaign to drive sales in the United States and key international markets using: |
| (i) | DOSEP Campaign: DOSEP (Direct Organic Search Engine Optimization) is a groundbreaking online awareness campaign and technology that will push PetLife’s web presence to the top of Google organic search results for targeted search keywords determined by PetLife management. | |
| (ii) | Multi-Touch Facebook Campaign: The retained Marketing Company will execute a targeted multi-touch Facebook advertising campaign that will generate major social awareness (and resulting online sales) of Vet Oncology products. The marketing messages will appear directly in the Facebook newsfeeds of specific demographic groups who additionally have expressed personal or professional interest in cancer treatment, based on research and targeting of the specific interests contained in their Facebook profiles. | |
| (iii) | Scientifically-Optimized Banner Ad Campaign: The Marketing Company will conduct research for optimal placement, and perform continuous split testing of ads to quickly determine “winners” and then further refine campaigns to determine the optimal combination of performance metrics to justify increasing ad budget spend for top-performing banner campaigns. |
| ● | Media – the Company will engage a top-notch boutique public relations firm to generate media coverage for its products in general news, family, pet and veterinarian publications, e-zines, videos and the blogosphere to communicate Vitalzul™ features and benefits to consumers. |
The online strategic marketing campaigns will repeatedly “local-launch” PetLife into specific metro markets while concurrently building PetLife’s overall brand and marketing presence globally. Repeating the online marketing campaign in each additional targeted metro market will not only localize and establish the PetLife’s brand and products, each event will also increase PetLife’s local and nationwide Google search rankings via DOSEP, Multi-Touch Facebook and Scientifically-Optimized Banner Ads, producing both short- and long-term online sales growth, and firmly establish PetLife as a market leader.
Direct Response: Infomercials
This year an estimated $150 billion dollars in product sales will be generated by infomercials. Specifically, over $1.5 billion will be spent on pet-related purchases through infomercials. Research has shown that retail sales driven by infomercials all range from two to 15 times the infomercial sales. More and more major brands are integrating infomercials into their marketing mix. Infomercials share the product story and benefits with millions of additional prospects at a cost per lead or cost per order that usually matches or beats other direct marketing channels such as direct mail or print ads.
PetLife anticipates the infomercial will:
| ● | Reach millions of new customers and present a complete product story. | |
| ● | Enhance, support, and fill in where other advertising avenues, particularly spot television, leaves off. | |
| ● | Generate qualified leads. | |
| ● | Reduce advertising costs per order and avoid print and mailing costs. | |
| ● | Rapidly and cost-effectively introduce a new product or revive an under-marketed but potentially profitable product. |
PetLife intends to capitalize on the lucrative channels of distribution available to an infomercial marketer. Only one out of every 100 consumers watching an infomercial actively calls an 800 number and purchases the product immediately. Therefore, the retail channel provides a means for the other 99 individuals - who may have been primed by the infomercial - to buy the product.
One-step infomercials are commonly utilized for products previously unable to get retail shelf space in order to gain retailers attention and establish instant distribution. For example, PetLife can inform a retailer that more than a million consumers ordered their product directly from their television ads and thousands of others called for more information. These kinds of figures are meant to grab the attention of brick and mortar retailers, who then purchase and showcase infomercial products realizing that “as seen on TV” are big sellers at retail.
Competition
For most of the products the Company offers there are a number of competitors, several of which are publicly-traded where they not only manufacture and produce their own products but also have established distribution and sales networks and participate in large group purchasing organizations within the medical industry. As mature companies, they also have extensive legacy systems and expensive administrative and sales commission cost structures. In addition, there are independent distributorships of pet medications primarily focused on limited geographic markets and products located across the United States.
| 9 |
Key among the Company’s competitors are:
| Paccal Vet: Paccal Vet®-CA1 has been granted conditional approval by the FDA for the treatment of: non-resectable stage III, IV or V mammary carcinoma in dogs that have not received previous chemotherapy, or radiotherapy and resectable and non-resectable squamous cell carcinoma in dogs that have not received previous chemotherapy, or radiotherapy. | ||
| Palladia: The only drug fully approved by the FDA for the treatment of cancer in canines is Palladia. Palladia is a prescription-only therapy is used to treat grade II or III recurrent cutaneous mast cell tumors with or without regional lymph node involvement. It works by blocking the activity of key receptors important in the development of blood vessels that supply tumors, as well as receptors vital for tumor survival | ||
| Zoetis, Inc.: the world’s largest producer medicine and vaccinations for pets and livestock. Zoetis is engaged in the discovery, development, manufacture and commercialization of animal health medicines and vaccines, with a focus on both livestock and companion animals. It offers a diversified product portfolio, including vaccines, parasiticides, anti-infectives, medicated feed additives and other pharmaceuticals, for both livestock and companion animal customers. | ||
| Merial, Inc.: Sanofi Pasteur is a worldwide leader in the vaccine industry. Its net sales amounted to €3,716 million in 2013, with leading vaccines in five areas: pediatric vaccines, influenza vaccines, adult and adolescent booster vaccines, meningitis vaccines, and travel and endemic vaccines. The company’s Animal Health activity is carried out through Merial, one of the world’s leading animal healthcare companies, dedicated to the research, development, manufacture and delivery of innovative pharmaceuticals and vaccines used by veterinarians, farmers and pet owners and providing a comprehensive line of products to enhance the health, well-being and performance of a wide range of production and companion animals. | ||
| Virbac SA: Virbac SA develops, manufactures, and sells vaccines and medicines to prevent and treat pathologies for companion and food-producing animals in France, the rest of Europe, North America, Latin America, Africa, the Middle East, Asia, and the Pacific region. The company’s products are comprised of a multitude of products in the animal health sector. | ||
Dechra Pharmaceuticals PLC: Dechra Pharmaceuticals PLC is engaged in the development, manufacture, distribution, sale, and marketing of veterinary pharmaceuticals worldwide. It markets and sells licensed branded pharmaceuticals and specialist pet foods to the veterinary professionals in Europe, as well as manufactures products for third party customers. The company also markets and sells a range of endocrine, ophthalmic, dermatological, and equine products in North America. In addition, it develops and licenses its branded veterinary product portfolio of novel and generic pharmaceuticals, and specialist pet diets.
Preveceutical, Inc.: Preveceutical and partner, Uniquest, are currently screening peptides isolated from Caribbean Blue Scorpion venom across some of the most aggressive diseases where there exists unmet clinical need, such as cancer. |
In addition to drug companies, PetLife competes with animal supplements companies. That segment is fragmented, and is dominated by small firms. However, it is witnessing tremendous growth due to the following trends;
● Pet owners view supplements as natural ways to promote health and wellness
● Veterinarians have come to view supplements as reasonable alternatives to animal drugs and are more willing to recommend them as adjuvant or even replacement therapy
● Well formulated supplements with functional ingredients that target specific physiological—and psychological—needs of today’s pets are enticing a whole population of pet parents committed to optimizing the quality of their animals’ lives
● The marketing of recognizable label claims and ingredients found in human diets, such as chicken fillets or fresh salmon, glucosamine, omega 3 fatty acids, probiotics and antioxidants attract shoppers who want to provide the “best” for their pets.
Intellectual Property
PetLife has developed its own patent-pending formulation of Chlorotoxin (the primary active of Blue Scorpion venom) and other naturally occurring plant-based nutrients with known cancer fighting properties. Additionally, PetLife has acquired the formulations of Healthy Life Pets, which is the basis of our “Dr. Geoff’s by PetLife” food line.
Regulatory Issues
There are worldwide, national, state and local rules, regulations and statutes that may impact the Company’s ability to fully implement our strategic plan. The sale of animal health products is governed by the laws and regulations specific to each country in which we sell our products. To maintain compliance with these regulatory requirements, we have established processes, systems and dedicated resources with involvement from product concept to launch and maintenance in the market. In the majority of our markets, the relevant animal health authority is separate from those governing human medicinal products.
| 10 |
United States
The regulatory body that is responsible for the regulation of animal health pharmaceuticals in the United States is the Center for Veterinary Medicine (CVM), housed within the United States Food and Drug Administration (FDA). All manufacturers of animal health pharmaceuticals must show their products to be safe, effective and produced by a consistent method of manufacture as defined under the Federal Food, Drug and Cosmetic Act. Post-approval monitoring of products is required by law, with reports being provided to the CVM’s Surveillance and Compliance group. Reports of product quality defects, adverse events or unexpected results are produced in accordance with the law. Additionally, we are required to submit all new information for a product, regardless of the source.
The regulatory body in the United States for veterinary vaccines is the United States Department of Agriculture (USDA). The USDA’s Center for Veterinary Biologics is responsible for the regulation of animal health vaccines, including immunotherapeutics. All manufacturers of animal health biologicals must show their products to be pure, safe, effective and produced by a consistent method of manufacture as defined under the Virus Serum Toxin Act. Post-approval monitoring of products is required. Reports of product quality defects, adverse events or unexpected results are produced in accordance with the agency requirements.
Outside of the United States
Country-specific regulatory laws have provisions that include requirements for certain labeling, safety, efficacy and manufacturers’ quality control procedures (to assure the consistency of the products), as well as company records and reports. With the exception of the European Union, most other countries’ regulatory agencies will generally refer to the FDA, USDA, EU and other international animal health entities, including the World Organization for Animal Health, Codex Alimentarius, in establishing standards and regulations for veterinary pharmaceuticals and vaccines.
Employees
Currently, we have one employee, which is an executive officer. We have good relationships with our employees and do not anticipate issues relative to our employees.
Properties
We presently lease our principal executive offices, located at 38 West Main Street, Hancock, MD 20715. We believe that our present business property is adequate and suitable to meet our needs.
The PetLife Scorpion Ranch is located in Haiti just a few hours from the capital of Port Au Prince. The ranch will also be the location of our expanded laboratory for the collection and initial processing of scorpion venom. The Company has yet to lease the ranch which is independently owned by unrelated parties.
Legal Proceedings
We are not currently a party in any legal proceeding or governmental regulatory proceeding nor are we currently aware of any pending or potential legal proceeding or governmental regulatory proceeding proposed to be initiated against us.
Our business and an investment in our securities are subject to a variety of risks. The following risk factors describe some of the most significant events, facts or circumstances that could have a material adverse effect upon our business, financial condition, results of operations, ability to implement our business plan and the market price for our securities. Many of these events are outside of our control. If any of these risks actually occurs, our business, financial condition or results of operations may be materially adversely affected. In such case, the trading price of our common stock could decline and investors in our common stock could lose all or part of their investment.
Risks Related to our Business
We were formed on December 12, 2012 and have a limited operating history and accordingly may not be able to effectively operate our business.
We are still in the early stages of company development and accordingly, there is only a limited basis upon which to evaluate our prospects for achieving our intended business objectives. There can be no assurance that we will ever achieve positive cash flow or profitability, or that if either is achieved, that it will be at the levels estimated by management.
We have not yet generated any revenue to date. Our failure to generate significant revenues would seriously harm our business. Even if we are able to access capital, we anticipate that we will experience operating losses and incur significant and increasing losses in the future due to growth, expansion, development and marketing. In the event that we are able to raise adequate capital, we expect to significantly increase our sales and marketing and general and administrative expenses. As a result of these additional expenses, we would need to generate substantial revenues to become profitable. We expect to incur significant operating losses for at least the next several years.
| 11 |
Our independent auditors have expressed substantial doubt about our ability to continue as a going concern, which may hinder our ability to obtain future financing.
The report of our independent auditors on our consolidated financial statements for the year ended August 31, 2017 included an explanatory paragraph indicating that there is substantial doubt about our ability to continue as a going concern. Our auditors’ doubts are based on our incurring significant losses from operations and our working capital deficit position. Our ability to continue as a going concern will be determined by our ability to obtain additional funding in the short term to enable us to realize the commercialization of our planned business operations. Our consolidated financial statements do not include any adjustments that might result from the outcome of this uncertain.
Our Vitalzul ™ product has not been approved by the FDA.
U.S. pet owners may be hesitant to consider Vitalzul™ for treating cancer without FDA approval. Alternative cancer treatments are not well-accepted in the U.S. The Company will need to educate and change the mindset of those who normally would not seek medicinal alternatives for themselves or for their pets for us to be successful. Obtaining approval for our products will be a lengthy and very costly process, the success of which cannot be assured.
Our brand is not well known in the United States.
Marketing Vitalzul™ products will require the Company to establish branding reputation of credibility. U.S. pet owners and veterinarians must become familiar with the products. Due to lack of brand recognition, significant advertising budgets will be required to market and promote products in the U.S. Additional costs are required for a team of experts to seek approval of the PetLife products in foreign markets. The brand initially may not be able to position itself on price, within the natural remedies markets. While the product is less costly than chemotherapy treatments, it may still not be reasonably priced for entry in some markets.
We will require approval for the products in numerous countries.
If Vitalzul™ is not approved in the countries selected to market and promote the products, PetLife will need to consider secondary markets, which may have limited sales potential. In addition, many chemotherapy drugs used by veterinarians today are used as “off label” treatments with no FDA approval for veterinary use, regardless of successful use in treating humans. Veterinarians who have had success with these drugs may be hesitant to try something new or more natural in treating pet cancers.
We are highly dependent on the continued availability of our scorpion farm facilities and would be harmed if they were unavailable for any prolonged period of time.
Any failure in the physical infrastructure of our scorpion ranch facilities or services could lead to significant costs and disruptions that could reduce our revenues and harm our business reputation and financial results. We are highly reliant on our Haiti facilities. Any natural or man-made event that impacts our ability to utilize these facilities could have a significant impact on our operating results, reputation and ability to continue operations. Our ability to rebuild facilities would take a considerable amount of time and expense and may cause a significant disruption in service to our customers. The Company has the ability to store venom offsite which will help mitigate this risk.
We are not currently profitable and we will need to raise additional funds in the future; however, additional funds may not be available on acceptable terms, or at all.
We have substantial operating expenses associated with the sales and marketing of our products. The sales and marketing expenses are anticipated to be funded from operating cash flow and from potential financing transactions. There can be no assurance that we will have sufficient access to liquidity or cash flow to meet our operating expenses and other obligations. If we do not increase our revenue or reduce our expenses, we will need to raise additional capital, which would result in dilution to our stockholders, or seek additional loans. The incurrence of indebtedness would result in increased debt service obligations and could require us to agree to operating and financial covenants that would restrict our operations. Financing may not be available in amounts or on terms acceptable to us, if at all. Any failure by us to raise additional funds on terms favorable to us, or at all, could result in our inability to pay our expenses as they come due, limit our ability to expand our business operations, and harm our overall business prospects.
We may not be able to raise capital or, if we can, it may not be on favorable terms. We may seek to raise additional capital through public or private equity financings, partnerships, joint ventures, dispositions of assets, debt financings or restructurings, bank borrowings or other sources. To obtain additional funding, we may need to enter into arrangements that require us to relinquish rights to certain technologies, products and/or potential markets. If adequate funds are not otherwise available, we would be forced to curtail operations significantly, including reducing our sales and marketing expenses which could negatively impact product sales and we could even be forced to cease operations, liquidate our assets and possibly even seek bankruptcy protection.
We operate in a highly regulated environment, and any legal or regulatory action could be time-consuming and costly.
If we fail to comply with all applicable laws, standards and regulations, action by the FDA or other regulatory agencies could result in significant restrictions, including restrictions on the marketing or use of our products or the withdrawal of products from the market. Any such restrictions or withdrawals could materially affect our business and operations. In addition, governmental authorities could impose fines, seize our inventory of products, or force us to recall any product already in the market if we fail to comply with governmental regulations.
| 12 |
Competitive products exist and more will be developed, and we may not be able to successfully compete because we are smaller and have fewer financial resources.
Our business is in a very competitive and evolving field. Rapid new developments in this field have occurred over the past few years, and are expected to continue to occur. Other companies already have competing products available or may develop products to compete with ours. Many of these products have short regulatory timeframes and our competitors, many with more substantial development resources, may be able to develop competing products that are equal to or better than ours. This may make our products obsolete or undesirable by comparison and reduce our revenue. Our success will depend, in large part, on our ability to maintain a competitive position concerning our intellectual property, and to develop new technologies and new applications for our technologies. Many of our competitors have substantially greater financial and technical resources, as well as greater production and marketing capabilities, and our ability to compete remains uncertain.
We will need to continue to innovate and develop new products to be desirable to our customers.
The markets for our products and services are characterized by rapid technological change, frequent new introductions, changes in customers’ demands and evolving industry standards. Accordingly, we will need to continue to innovate and develop additional products. These efforts can be costly, subject to long development and regulatory delays and may not result in products approved for sale. These costs may hurt operating results and may require additional capital. If additional capital is not available, we may be forced to curtail development activities. In addition, any failure on our behalf to react to changing market conditions could create an opportunity for other market participants to capture a critical share of the market within a short period of time.
Our success will depend on our ability to engage and retain qualified technical personnel who are difficult to attract.
Our success will depend on our ability to attract and retain qualified technical personnel to assist in research and development, testing, product implementation, low-scale production and technical support. The demand for such personnel is high and the supply of qualified technical personnel is limited. A significant increase in the wages paid by competing employers could result in a reduction of our technical work force and increases in the wage rates that we must pay or both. If either of these events were to occur, our cost structure could increase, and our growth potential could be impaired.
Loss of key members of our management who we need to succeed could adversely affect our business.
We are highly dependent on the services of key members of our management team, and the loss of any of their services could have an adverse effect on our future operations. We do not currently maintain key-man life insurance policies insuring the life of any member of our management team.
We will be required to invest in facilities and equipment on a continuing basis, which will put pressure on us to finance these investments.
We have invested, and intend to continue to invest, in facilities and state-of-the-art equipment in order to increase, expand or update our capabilities and facilities. Changes in technology or sales growth beyond currently established production capabilities, which we anticipate, will require further investment. However, there can be no assurance that we will generate sufficient funds from operations to maintain our existing facilities and equipment or to finance any required capital investments or that other sources of funding will be available. Additionally, there can be no guarantee that any future expansion will not negatively affect earnings.
Future revenue will depend on our ability to increase sales.
We intend to sell our products through numerous means, including direct sales by our employees, through infomercials and through a multi-level marketing program. We have and will continue to incur increased sales and marketing expenses in building and expanding our sales force, and there can be no assurance that we will generate increased sales as a result of this effort.
Our operating results will be harmed if we are unable to effectively manage and sustain our future growth.
We might not be able to manage our future growth efficiently or profitably. Our business is unproven on a large scale and actual revenue and operating margins, or revenue and margin growth, may be less than expected. If we are unable to scale our production capabilities efficiently, we may fail to achieve expected operating margins, which would have a material and adverse effect on our operating results. Growth may also stress our ability to adequately manage our operations, quality of products, safety and regulatory compliance. In order to grow, we may be required to obtain additional financing, which may increase our indebtedness or result in dilution to our stockholders. Further, there can be no assurance that we would be able to obtain any additional financing.
Our success depends on our ability to avoid infringing on the intellectual property rights of third parties which could expose us to litigation or commercially unfavorable licensing arrangements.
Our commercial success depends in part on our ability to avoid infringing patents and proprietary rights of third parties. Third parties may accuse us of employing their proprietary technology in our products, or in the materials or processes used to research or develop our products, without authorization. Any legal action against us claiming damages and/or seeking to stop our commercial activities relating to the affected products, materials and processes could, in addition to subjecting us to potential liability for damages, require us to obtain a license to continue to utilize the affected materials or processes or to manufacture or market the affected products. We cannot predict whether we would prevail in any of these actions or whether any license required under any of these patents would be made available on commercially reasonable terms, if at all. If we are unable to obtain such a license, we may be unable to continue to utilize the affected materials or processes or manufacture or market the affected products or we may be obligated by a court to pay substantial royalties and/or other damages to the patent holder. Even if we are able to obtain such a license, the terms of such a license could substantially reduce the commercial value of the affected product or products and impair our prospects for profitability. Accordingly, we cannot predict whether or to what extent the commercial value of the affected product or products or our prospects for profitability may be harmed as a result of any of the liabilities discussed above. Furthermore, infringement and other intellectual property claims, with or without merit, can be expensive and time-consuming to litigate and can divert management’s attention from our core business. We may be unable to obtain and enforce intellectual property rights to adequately protect our products and related intellectual property.
| 13 |
Our business will become subject to continuing regulatory compliance by the FDA and other authorities which is costly and could result in delays in the commercialization of our products.
Upon completion of the FDA process for our approved drug Vitalzul™ product, we will become subject to extensive regulation by the FDA and potentially other federal governmental agencies and, in some jurisdictions, by state and foreign governmental authorities. These regulations govern the introduction of new pharmaceuticals even for pets, the observance of certain standards with respect to the design, manufacture, testing, labeling, promotion and sales of the pharmaceuticals, the maintenance of certain records, the ability to track devices, the reporting of potential problems, and other matters.
Future revenue will depend on our ability to develop new sales channels and there can be no assurance that these efforts will result in significant revenues.
We are heavily dependent on developing sales channels for our products but there can be no assurance that these channels can be developed or that we will continue to be successful in selling our products. We are engaging in a major initiative to build and further expand our direct sales force, as well as develop infomercial and multi-level marketing sales. This effort will have significant costs that will be incurred prior to the generation of revenue sufficient to cover these costs. The costs incurred for these efforts may impact our operating results and there can be no assurance of their effectiveness. Many of our competitors have well-developed sales channels and it may be difficult for us to break through these competitors to take market share. If we are unable to develop these sales channels, we may not be able to grow revenue or maintain our current level of revenue generation.
Risks Related to our Stock
There may be fluctuations in our operating results, which will impact our stock price.
Significant annual and quarterly fluctuations in our results of operations may be caused by, among other factors, our volume of revenues, the timing of new product or service announcements, releases by us and our competitors in the marketplace of new products or services, seasonality and general economic conditions. There can be no assurance that the level of revenues achieved by us in any particular fiscal period will not be significantly lower than in other comparable fiscal periods. Our expense levels are based, in part, on our expectations as to future revenues. As a result, if future revenues are below expectations, net income or loss may be disproportionately affected by a reduction in revenues, as any corresponding reduction in expenses may not be proportionate to the reduction in revenues.
Because we became public through a reverse merger, we may not be able to attract the attention of major brokerage firms or certain investors.
There are coverage risks associated with our becoming public through a reverse merger, including, among other things, security analysts of major brokerage firms may not provide coverage of us since there is no incentive to brokerage firms to recommend the purchase of our common stock. In addition, we may not attract the attention of major brokerage firms and certain investors due to the possibility of a low stock price. We cannot assure you that brokerage firms would want to conduct any public offerings on our behalf in the future.
The market price of our common stock is extremely volatile, which may affect our ability to raise capital in the future and may subject the value of your investment to sudden decreases.
The market price for securities of biotechnology companies, including ours, historically has been highly volatile, and the market from time to time has experienced significant price and volume fluctuations that are unrelated to the operating performance of such companies. Fluctuations in the trading price or liquidity of our common stock may harm the value of your investment in our common stock.
Our stockholders may experience significant dilution if future equity offerings are used to fund operations or acquire complementary businesses.
If our future operations or acquisitions are financed through the issuance of equity securities, our stockholders could experience significant dilution. In addition, securities issued in connection with future financing activities or potential acquisitions may have rights and preferences senior to the rights and preferences of our common stock.
We do not anticipate paying dividends in the foreseeable future; you should not buy our stock if you expect dividends.
We currently intend to retain our future earnings to support operations and to finance expansion and, therefore, we do not anticipate paying any cash dividends on our common stock in the foreseeable future.
| 14 |
Our current management can exert significant influence over us and make decisions that are not in the best interests of all stockholders.
As of August 31, 2017, our executive officers and directors beneficially owned as a group approximately 32.6% of our outstanding shares of common stock. These officers continue to own a controlling interest in the stock. As a result, these stockholders will be able to assert significant influence over all matters requiring stockholder approval, including the election and removal of directors and any change in control. In particular, this concentration of ownership of our outstanding shares of common stock could have the effect of delaying or preventing a change in control, or otherwise discouraging or preventing a potential acquirer from attempting to obtain control. This, in turn, could have a negative effect on the market price of our common stock. It could also prevent our stockholders from realizing a premium over the market prices for their shares of common stock. Moreover, the interests of the owners of this concentration of ownership may not always coincide with our interests or the interests of other stockholders and, accordingly, could cause us to enter into transactions or agreements that we would not otherwise consider.
Our common stock is considered “penny stock” and may be difficult to sell.
The SEC has adopted Rule 3a51-1, which establishes the definition of a “penny stock” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. The market price of our common stock is less than $5.00 per share and therefore may be designated as a “penny stock” according to SEC rules. For any transaction involving a penny stock, unless exempt, Rule 15g-9 requires:
● that a broker or dealer approve a person’s account for transactions in penny stocks; and
● that the broker or dealer receives from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must:
● obtain financial information and investment experience objectives of the person; and
● make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks. The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form:
● sets forth the basis on which the broker or dealer made the suitability determination; and
● that the broker or dealer received a signed, written agreement from the investor prior to the transaction.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of our Common Stock and cause a decline in the market value of our stock. In addition, since the Common Stock is currently traded on the OTC Bulletin Board, investors may find it difficult to obtain accurate quotations of the Common Stock and may experience a lack of buyers to purchase such stock or a lack of market makers to support the stock price.
We could issue “blank check” preferred stock without stockholder approval with the effect of diluting then current stockholder interests and impairing their voting rights, and provisions in our charter documents and under Nevada law could discourage a takeover that stockholders may consider favorable.
Our certificate of incorporation provides for the authorization to issue up to 50,000,000 shares of “blank check” preferred stock with designations, rights and preferences as may be determined from time to time by our board of directors. Our board of directors is empowered, without stockholder approval, to issue one or more series of preferred stock with dividend, liquidation, conversion, voting or other rights which could dilute the interest of, or impair the voting power of, our common stockholders. The issuance of a series of preferred stock could be used as a method of discouraging, delaying or preventing a change in control. For example, it would be possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of our company.
Item 1B. Unresolved Staff Comments
Not applicable
| 15 |
Properties
We presently lease our principal executive offices, located at 38 West Main Street, Hancock, MD 21750. We believe that our present business property is adequate and suitable to meet our needs.
The PetLife Scorpion Ranch is located in Haiti just a few hours from the capital of Port Au Prince. The ranch will also be the location of our expanded laboratory for the collection and initial processing of scorpion venom. The Company has yet to lease the ranch which is independently owned by unrelated parties.
We are not currently a party in any legal proceeding or governmental regulatory proceeding nor are we currently aware of any pending or potential legal proceeding or governmental regulatory proceeding proposed to be initiated against us.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market for Common Equity
Marketing Information
The Company’s common stock is quoted on OTC Markets Group, Inc.’s OTCQB under the symbol “PTLF.” As of August 31, 2017, the Company’s common stock was held by 327 shareholders of record, which does not include shareholders whose shares are held in street or nominee name.
Below is a table indicating the range of high and low closing price information for the common stock as reported by the OTC Markets Group for the periods listed. These prices do not necessarily reflect actual transactions.
| For the Years Ended August 31, | ||||||||||||||||
| 2017 | 2016 | |||||||||||||||
| High | Low | High | Low | |||||||||||||
| First Quarter | $ | 0.99 | $ | 0.45 | $ | 0.35 | $ | 0.075 | ||||||||
| Second Quarter | $ | 0.55 | $ | 0.159 | $ | 0.253 | $ | 0.03 | ||||||||
| Third Quarter | $ | 0.5055 | $ | 0.19 | $ | 0.3111 | $ | 0.02 | ||||||||
| Fourth Quarter | $ | 0.2896 | $ | 0.125 | $ | 1.00 | $ | 0.0202 | ||||||||
Source: OTC Markets
Transfer Agent
Our current transfer agent and registrar for our common stock is Empire Stock Transfer, 1859 Whitney Mesa Drive, Henderson, NV 89014.
Dividend Distributions
We have not paid any cash dividends on our common stock and have no present intention of paying any dividends on the shares of our common stock for the foreseeable future. Our current policy is to retain earnings, if any, for use in our operations and in the development of our business. Our future dividend policy may be modified from time to time by our board of directors.
Securities authorized for issuance under equity compensation plans
The Company established a stock option plan on June 9, 2016. The Board of Directors approved the 2016 Stock Option Plan which reserved 20,000,000 shares of common stock.
Penny Stock
Our common stock is considered “penny stock” under the rules the Securities and Exchange Commission (the “SEC”) under the Securities Exchange Act of 1934. The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or quoted on the NASDAQ Stock Market System, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or quotation system. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock, to deliver a standardized risk disclosure document prepared by the Commission, that:
| 16 |
| ● | contains a description of the nature and level of risks in the market for penny stocks in both public offerings and secondary trading; |
| ● | contains a description of the broker’s or dealer’s duties to the customer and of the rights and remedies available to the customer with respect to a violation to such duties or other requirements of Securities’ laws; contains a brief, clear, narrative description of a dealer market, including bid and ask prices for penny stocks and the significance of the spread between the bid and ask price; |
| ● | contains a toll-free telephone number for inquiries on disciplinary actions; |
| ● | defines significant terms in the disclosure document or in the conduct of trading in penny stocks; and |
| ● | contains such other information and is in such form, including language, type, size and format, as the Commission shall require by rule or regulation. |
The broker-dealer also must provide, prior to effecting any transaction in a penny stock, the customer with:
| ● | bid and offer quotations for the penny stock; |
| ● | the compensation of the broker-dealer and its salesperson in the transaction; |
| ● | the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the marker for such stock; and |
| ● | monthly account statements showing the market value of each penny stock held in the customer’s account. |
In addition, the penny stock rules that require that prior to a transaction in a penny stock not otherwise exempt from those rules; the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written acknowledgement of the receipt of a risk disclosure statement, a written agreement to transactions involving penny stocks, and a signed and dated copy of a written suitably statement.
These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our stock.
Related Stockholder Matters
None.
Purchase of Equity Securities
None.
Item 6. Selected Financial Data.
As the Company is a “smaller reporting company,” this item is inapplicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Rule 175 of the Securities Act of 1933, as amended, and Rule 3b-6 of the Securities Act of 1934, as amended, that involve substantial risks and uncertainties. These forward-looking statements are not historical facts, but rather are based on current expectations, estimates and projections about our industry, our beliefs and our assumptions. Words such as “anticipate,” “expects,” “intends,” “plans,” “believes,” “seeks” and “estimates” and variations of these words and similar expressions are intended to identify forward-looking statements. These statements are not guarantees of future performance and are subject to risks, uncertainties and other factors, some of which are beyond our control and difficult to predict and could cause actual results to differ materially from those expressed or forecasted in the forward-looking statements. You should not place undue reliance on these forward-looking statements, which apply only as of the date of this Form 10-K. Investors should carefully consider all of such risks before making an investment decision with respect to the Company’s stock. The following discussion and analysis should be read in conjunction with our consolidated financial statements and summary of selected financial data for PetLife Pharmaceuticals, Inc. Such discussion represents only the best present assessment from our Management.
DESCRIPTION OF COMPANY
PetLife is launching a new generation of non-toxic veterinary cancer medications and nutraceuticals, based on the venom of the Blue Scorpion. This treatment is based on the same patented formula and production processes used in the human formulation. PetLife is developing, packaging, and marketing a new product line, “Vitalzul for Pets™.” The Company’s mission is to bring several non-invasive treatments to market with the goal of improving the quality of life in our companion animals. The anticipated registration of a U.S. Food and Drug Administration (“FDA”) approved drug, “Vitalzul For Pets™,” for the treatment of cancer in animals, as well as the introduction of nutraceuticals, food, and pet treats, are all in the works today. This product has not been approved by the FDA or the Center for Veterinary Medicine for the treatment, cure or prevention of cancer or any other disease in animals. Currently, Vitalzul™ is a nutraceutical product and falls under the category of a Dietary Supplement.
| 17 |
PetLife’s primary goal is to bring its products to the world of veterinary oncology, with the ultimate goal of extending the life of pets with cancer and improving their quality of life. In the process of achieving these objectives, PetLife will transition into a world renowned, professionally respected veterinary pharmaceutical company that will create new industry standards as well as being profitable and innovative.
We have had limited operations and have been issued a “going concern” opinion by our auditor.
The following Management Discussion and Analysis should be read in conjunction with the consolidated financial statements and accompanying notes included in this Form 10-K.
Merger Agreements
On July 20, 2016, PetLife Pharmaceuticals, Inc. entered into an Agreement and Plan of Merger with its wholly-owned subsidiary, PetLife Merger Subsidiary, Inc., a Nevada Corporation, with PetLife Merger Subsidiary, Inc. being the surviving entity. As part of that merger, the name of the PetLife Merger Subsidiary was changed back to PetLife Pharmaceuticals, Inc. The purpose of the subsidiary merger was to effectuate a 1 for 5 reverse exchange of PetLife’s common stock pursuant to the terms of the merger. The combined entities continue on public markets pursuant to Rule 12g-3 of the Securities Exchange Act of 1934, as amended.
COMPARISON OF THE YEAR ENDED AUGUST 31, 2017 TO THE YEAR ENDED AUGUST 31, 2016
Results of Operations
Revenue
For the years ended August 31, 2017 and 2016, the Company had no revenues.
Operating expenses
For the year ended August 31, 2017, we incurred $22,655,379 in operating expenses as compared to $3,687,040 in operating expenses we incurred for the year ended August 31, 2016, primarily due to increased stock-based compensation from $736,374 to $21,310,740. General and administrative expenses decreased from $2,950,666 to $1,272,554 primarily for the accrual of stock payable for services of $2,315,000 during the year ended August 31, 2016.
Net loss
We incurred a net loss of $23,256,850 for the year ended August 31, 2017 as compared to the net loss we incurred for the year ended August 31, 2016 of $3,687,040. The increase in net loss is attributable principally to increased stock-based compensation.
Liquidity and Capital Resources
General. At August 31, 2017, we had cash and cash equivalents of $54,254. We have historically met our cash needs through a combination of cash flows from operating activities and proceeds from private placements of our securities and loans. Our cash requirements are generally for selling, general and administrative activities. We believe that our cash balance is not sufficient to finance our cash requirements for expected operational activities, capital improvements, and partial repayment of debt through the next 12 months.
Our operating activities used cash of $318,918 for the year ended August 31, 2017, and we used cash in operations of $151,839 during the same period in 2016. The principal elements of cash flow used by operations for the year ended August 31, 2017 are due to the net loss of $23,256,850 offset by stock compensation of $21,524,979, loss on settlement of $211,600 and the increase in accounts payable and accrued expenses of $451,076 and the common stock issued for debt, inducement of conversion of debt and accrued expenses of $658,450.
Cash used in investing activities during the year ended August 31, 2017, was $0 compared to $0 during the same period in 2016.
Cash generated in our financing activities was $372,000 for the year ended August 31, 2017, compared to cash generated of $153,011 during the comparable period in 2016. The Company received proceeds from the issuance of convertible debt of $220,500. The Company received $150,000 in proceeds from the issuance of a note payable.
As of August 31, 2017, current liabilities exceeded current assets by $1,381,450. Current assets increased from $1,172 at August 31, 2016 to $661,810 at August 31, 2017, whereas current liabilities decreased from $3,236,032 at August 31, 2016, to $1,436,454 at August 31, 2017.
| For the years ended | ||||||||
| August 31, | ||||||||
| 2017 | 2016 | |||||||
| Cash used in operating activities | $ | (318,918 | ) | $ | (151,839 | ) | ||
| Cash used in investing activities | - | - | ||||||
| Cash provided by financing activities | 372,000 | 153,011 | ||||||
| Net changes to cash | $ | 53,082 | $ | 1,172 | ||||
| 18 |
Going Concern
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company had no sales and net losses of $23,256,850 for the year ended August 31, 2017, compared to no sales and net losses of $3,687,040 for the year ended August 31, 2016. The Company had a working capital, stockholders’ deficit, and accumulated deficit of $(1,381,450) and $30,388,180, respectively, at August 31, 2017. These factors raise substantial doubt about the ability of the Company to continue as a going concern for a reasonable period of time. The Company is highly dependent on its ability to continue to obtain investment capital from future funding opportunities to fund the current and planned operating levels. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern. The Company’s continuation as a going concern is dependent upon its ability to bring in income generating activities and its ability to continue receiving investment capital from future funding opportunities. No assurance can be given that the Company will be successful in these efforts.
Critical Accounting Policies
Use of Estimates. The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates in the accompanying consolidated financial statements include the amortization period for intangible assets, valuation and impairment valuation of intangible assets, depreciable lives of the web site and property and equipment, valuation of warrant and beneficial conversion feature debt discounts, valuation of share-based payments and the valuation allowance on deferred tax assets.
Changes in Accounting Principles. No significant changes in accounting principles were adopted during fiscal 2017 and 2016.
Derivatives. The Company evaluates its convertible debt, options, warrants or other contracts to determine if those contracts or embedded components of those contracts qualify as derivatives to be separately accounted for. The result of this accounting treatment is that under certain circumstances the fair value of the derivative is marked-to-market each balance sheet date and recorded as a liability. In the event that the fair value is recorded as a liability, the change in fair value is recorded in the statement of operations as other income or expense. Upon conversion or exercise of a derivative instrument, the instrument is marked to fair value at the conversion date and then that fair value is reclassified to equity. Equity instruments that are initially classified as equity that become subject to reclassification under this accounting standard are reclassified to liability at the fair value of the instrument on the reclassification date.
Impairment of Long-Lived Assets. The Company accounts for long-lived assets in accordance with the provisions of Statement of Financial Accounting Standards ASC 360-10, “Accounting for the Impairment or Disposal of Long-Lived Assets”. This statement requires that long-lived assets and certain identifiable intangibles be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
Fair Value of Financial Instruments and Fair Value Measurements. The Company measures their financial assets and liabilities in accordance with generally accepted accounting principles. For certain of our financial instruments, including cash, accounts payable, accrued expenses escrow liability and short-term loans the carrying amounts approximate fair value due to their short maturities.
We have adopted accounting guidance for financial and non-financial assets and liabilities. The adoption did not have a material impact on our results of operations, financial position or liquidity. This standard defines fair value, provides guidance for measuring fair value and requires certain disclosures. This standard does not require any new fair value measurements, but rather applies to all other accounting pronouncements that require or permit fair value measurements. This guidance does not apply to measurements related to share-based payments. This guidance discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flow), and the cost approach (cost to replace the service capacity of an asset or replacement cost). The guidance utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: Inputs other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active.
Level 3: Unobservable inputs in which little or no market data exists, therefore developed using estimates and assumptions developed by us, which reflect those that a market participant would use.
| 19 |
Revenue Recognition. The Company recognizes revenue for our services in accordance with ASC 605-10, “Revenue Recognition in Financial Statements.” Under these guidelines, revenue is recognized on transactions when all of the following exist: persuasive evidence of an arrangement did exist, delivery of service has occurred, the sales price to the buyer is fixed or determinable and collectability is reasonably assured. The Company has five primary revenue operating classes as follows:
| ● | Consulting services. | |
| ● | Advertising services. | |
| ● | Branding, marketing and selling products for companies. | |
| ● | Educational seminars. | |
| ● | Selling branded products. |
Stock-Based Compensation. The Company accounts for stock-based instruments issued to employees in accordance with ASC Topic 718. ASC Topic 718 requires companies to recognize in the statement of operations the grant-date fair value of stock options and other equity based compensation issued to employees. The Company accounts for non-employee share-based awards in accordance with ASC Topic 505-50. The value of the portion of an award that is ultimately expected to vest is recognized as an expense over the requisite service periods using the straight-line attribution method. The Company estimates the fair value of each stock option at the grant date by using the Black-Scholes option-pricing model. The Company estimates the fair value of each stock option at the grant date by using the Black-Scholes option-pricing model.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
As the Company is a “smaller reporting company,” this item is inapplicable.
| 20 |
Item 8. Consolidated Financial Statements and Supplementary Data.
PetLife Pharmaceuticals, Inc.
Table of Contents
| 21 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of
PetLife Pharmaceuticals, Inc.
Hancock, MD
We have audited the accompanying consolidated balance sheets of PetLife Pharmaceuticals, Inc. (the “Company”) as of August 31, 2017 and 2016, and the related consolidated statements of operations, stockholders’ deficit, and cash flows for each of the years in the two-year period ended 2017. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits include consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting.
Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of PetLife Pharmaceuticals, Inc., as of August 31, 2017 and 2016, and the results of its consolidated operations, stockholders’ deficit, and its cash flows for each of the years in the two-year period ended 2017, in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 3 to the consolidated financial statements, the Company’s absence of significant revenues, recurring losses from operations, and its need for additional financing in order to fund its projected loss in 2018 raise substantial doubt about its ability to continue as a going concern. The 2017 consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/S/ LBB & ASSOCIATES LTD., LLP
LBB & Associates Ltd., LLP
Houston, Texas
December 14, 2017
| F-1 |
PETLIFE PHARMACEUTICALS, INC.
and Subsidiary
August 31,
| 2017 | 2016 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash | $ | 54,254 | $ | 1,172 | ||||
| Prepaid | 750 | - | ||||||
| Total current assets | 55,004 | 1,172 | ||||||
| Total assets | $ | 55,004 | $ | 1,172 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current liabilities | ||||||||
| Convertible notes payable, net of discounts | $ | 57,318 | $ | - | ||||
| Note payable | 312,000 | - | ||||||
| Notes payable to related parties | 336,358 | 153,011 | ||||||
| Due to shareholder | 10,000 | 10,000 | ||||||
| Loan from officer | 11,500 | - | ||||||
| Accounts payable | 307,478 | 374,047 | ||||||
| Accounts payable to related parties | 70,000 | 383,974 | ||||||
| Accrued expenses | 223,897 | - | ||||||
| Accrued expenses to related parties | 5,526 | - | ||||||
| Derivative liabilities | 102,377 | - | ||||||
| Stock payable | - | 2,315,000 | ||||||
| Total current liabilities | 1,436,454 | 3,236,032 | ||||||
| Total liabilities | 1,436,454 | 3,236,032 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ deficit | ||||||||
| Preferred stock, $0.001 par value, 50,000,000 shares authorized, 3,250,000 and 0 shares issued and outstanding at August 31, 2017 and 2016, respectively | 3,250 | - | ||||||
| Common stock, $0.001 par value, 750,000,000 shares authorized, 73,842,320 and 8,399,422 shares issued, issuable, and outstanding at August 31, 2017 and 2016, respectively | 73,842 | 8,400 | ||||||
| Additional paid-in capital | 28,929,638 | 3,888,070 | ||||||
| Accumulated deficit | (30,388,180 | ) | (7,131,330 | ) | ||||
| Total stockholders’ deficit | (1,381,450 | ) | (3,234,860 | ) | ||||
| Total liabilities and stockholders’ deficit | $ | 55,004 | $ | 1,172 | ||||
See accompanying notes to consolidated financial statements.
| F-2 |
PETLIFE PHARMACEUTICALS, INC.
and Subsidiary
Consolidated Statements of Operations
For the Years Ended August 31,
| 2017 | 2016 | |||||||
| Revenue, net | $ | - | $ | - | ||||
| Operating expenses | ||||||||
| General and administrative | 1,272,554 | 2,950,666 | ||||||
| Impairment | 270,000 | - | ||||||
| Stock-based compensation | 21,112,825 | 736,374 | ||||||
| Operating loss | (22,655,379 | ) | (3,687,040 | ) | ||||
| Other income (expense) | ||||||||
| Interest expense | (383,729 | ) | - | |||||
| Loss on settlement of debt | (211,600 | ) | - | |||||
| Other expense | (6,142 | ) | - | |||||
| Net loss | $ | (23,256,850 | ) | $ | (3,687,040 | ) | ||
| Net loss per share - basic and diluted | $ | (0.33 | ) | $ | (0.40 | ) | ||
| Weighted average number of shares outstanding - basic and diluted | 68,805,018 | 9,124,246 | ||||||
See accompanying notes to consolidated financial statements.
| F-3 |
PETLIFE PHARMACEUTICALS, INC.
and Subsidiary
Consolidated Statement of Shareholders’ Deficit
August 31, 2017
| Common Stock | Additional | |||||||||||||||||||||||||||||||||||
| Preferred Stock | Issuable | Common Stock | Paid In | Accumulated | ||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Capital | Deficit | Total | ||||||||||||||||||||||||||||
| Balance at August 31, 2015 | - | $ | - | - | $ | - | 11,706,431 | $ | 11,707 | $ | 3,067,456 | $ | (3,444,290 | ) | $ | (365,127 | ) | |||||||||||||||||||
| Shares returned to treasury | - | - | - | - | (3,538,249 | ) | (3,538 | ) | 3,538 | - | - | |||||||||||||||||||||||||
| Issuance of options for common stock | - | - | - | - | - | - | 736,374 | - | 736,374 | |||||||||||||||||||||||||||
| Issuance of common stock for debt | - | - | - | - | 231,240 | 231 | 80,702 | - | 80,933 | |||||||||||||||||||||||||||
| Net loss for the period ended August 31, 2016 | - | - | - | - | - | - | - | (3,687,040 | ) | (3,687,040 | ) | |||||||||||||||||||||||||
| Balance at August 31, 2016 | - | $ | - | - | $ | - | 8,399,422 | $ | 8,400 | $ | 3,888,070 | $ | (7,131,330 | ) | $ | (3,234,860 | ) | |||||||||||||||||||
| Common stock for services | - | - | 16,468,308 | 16,468 | 42,450,590 | 42,451 | 23,508,517 | - | 23,567,435 | |||||||||||||||||||||||||||
| Common stock issued in connection with debt | - | - | 250,000 | 250 | - | - | 149,750 | - | 150,000 | |||||||||||||||||||||||||||
| Notes payable converted into common stock | - | - | 2,899,000 | 2,899 | - | - | 352,101 | - | 355,000 | |||||||||||||||||||||||||||
| Conversion of common stock into preferred stock | 3,250,000 | 3,250 | - | - | (3,250,000 | ) | (3,250 | ) | 121,875 | - | 121,875 | |||||||||||||||||||||||||
| Common stock for assets | - | - | 1,000,000 | 1,000 | 500,000 | 500 | 268,500 | - | 270,000 | |||||||||||||||||||||||||||
| Common stock for debt | - | - | 5,000,000 | 5,000 | - | - | 620,000 | - | 625,000 | |||||||||||||||||||||||||||
| Common stock for financing fees | - | - | 125,000 | 125 | - | - | 20,825 | - | 20,950 | |||||||||||||||||||||||||||
| Net loss for the period ended August 31, 2017 | - | - | - | - | - | - | - | (23,256,850 | ) | (23,256,850 | ) | |||||||||||||||||||||||||
| Balance at August 31, 2017 | 3,250,000 | $ | 3,250 | 25,742,308 | $ | 25,742 | 48,100,012 | $ | 48,100 | $ | 28,929,638 | $ | (30,388,180 | ) | $ | (1,381,450 | ) | |||||||||||||||||||
See accompanying notes to consolidated financial statements.
| F-4 |
PETLIFE PHARMACEUTICALS, INC.
and Subsidiary
Consolidated Statements of Cash Flows
For the Years Ended August 31,
| 2017 | 2016 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (23,256,850 | ) | $ | (3,687,040 | ) | ||
| Adjustments to reconcile net loss to net cash used in operations: | ||||||||
| Impairment | 270,000 | 600 | ||||||
| Amortization of debt discount | 8,652 | - | ||||||
| Bad debt | - | 4,800 | ||||||
| Common stock issued for inducement of conversion of debt | 162,500 | - | ||||||
| Common stock for notes payable | 170,950 | - | ||||||
| Common stock issued for inducement of accrued expenses | 325,000 | - | ||||||
| Stock compensation | 21,344,979 | 736,374 | ||||||
| Loss on settlement | 211,600 | - | ||||||
| Change in derivative liability | (8,627 | ) | - | |||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses | 10,250 | - | ||||||
| Accounts payable and accrued expenses | 451,076 | 56,120 | ||||||
| Accounts payable and accrued expenses to related parties | (8,448 | ) | 422,307 | |||||
| Stock payable | - | 2,315,000 | ||||||
| Net cash used in operating activities | (318,918 | ) | (151,839 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Proceeds from convertible note payable | 220,500 | - | ||||||
| Proceeds from loan from officer | 36,330 | 153,011 | ||||||
| Payments on loan from officer | (24,830 | ) | - | |||||
| Proceeds from notes payable | 150,000 | - | ||||||
| Payments on note payable | (10,000 | ) | - | |||||
| Net cash provided by financing activities | 372,000 | 153,011 | ||||||
| Net increase in cash | 53,082 | 1,172 | ||||||
| Cash at beginning of period | 1,172 | - | ||||||
| Cash at end of period | $ | 54,254 | $ | 1,172 | ||||
| Supplemental disclosure of cash flow information: | ||||||||
| Cash paid for interest | $ | - | $ | - | ||||
| Cash paid for taxes | $ | - | $ | - | ||||
| Non-cash investing and financing activities: | ||||||||
| Common stock returned to treasury and cancelled | $ | - | $ | 21,583 | ||||
| Expenses paid directly by related party | $ | 183,346 | $ | - | ||||
| Common stock for accounts payable | $ | 300,000 | $ | 80,933 | ||||
| Common stock returned to treasury and cancelled | $ | - | $ | 3,538 | ||||
| Conversion of notes payable into common stock | $ | 192,500 | $ | - | ||||
| Common stock issued for purchase of asset | $ | 270,000 | $ | - | ||||
| Common stock converted into preferred stock | $ | 3,250,000 | $ | - | ||||
See accompanying notes to consolidated financial statements.
| F-5 |
PETLIFE PHARMACEUTICALS, INC.
and Subsidiary
Notes to Consolidated Financial Statements
August 31, 2017
NOTE 1 – NATURE OF OPERATIONS AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Organization
PetLife Pharmaceuticals, Inc. (the “Company,” “we,” “our,” “PetLife”) was incorporated in the State of Nevada on April 5, 2002 as Aztek Ventures Inc. Effective November 13, 2007, we filed a Certificate of Amendment to our Articles of Incorporation to change our name to Genesis Uranium Corp. Effective April 21, 2008, we amended our Articles of Incorporation to change our name to Vault Technology, Inc. to reflect the change in our business focus beyond solely that of uranium exploration. Effective July 10, 2009, we filed a Certificate of Amendment to our Articles of Incorporation to change our name to “Modern Renewable Technologies, Inc. (“Modern”). On May 27, 2011, Modern, merged with Eco Ventures Group, Inc., and the name of the Company was changed to Eco Ventures Group, Inc. On July 15, 2013, the Company entered into an Agreement and Plan of Merger with Clear TV Ventures, Inc. Under the terms of the merger, Clear TV became the surviving corporation. On June 26, 2014, Eco Ventures Group, Inc. entered into an Agreement and Plan of Merger with its subsidiary, PetLife Pharmaceuticals, Inc., a Nevada corporation, with PetLife Pharmaceuticals, Inc. being the surviving entity. As part of that merger, the name of the Company was changed to PetLife Pharmaceuticals, Inc. and each 15 shares of our common stock were exchanged for one share in the surviving company. Effective August 12, 2014, we completed the closing of the Share Exchange Agreement and the acquisition of PetLife and changed our name to PetLife Pharmaceuticals, Inc. On July 20, 2016, PetLife Pharmaceuticals, Inc. entered into an Agreement and Plan of Merger with its wholly-owned subsidiary, PetLife Merger Subsidiary, Inc., a Nevada corporation, with PetLife Merger Subsidiary, Inc. being the surviving entity. As part of that merger, the name of the PetLife Merger Subsidiary was changed back to PetLife Pharmaceuticals, Inc. The purpose of the subsidiary merger was to effectuate a 1 for 5 reverse exchange of PetLife’s common stock pursuant to the terms of the merger. The combined entities continue on public markets pursuant to Rule 12g-3 of the Securities Exchange Act of 1934, as amended.
On April 19, 2017, the Company organized in the State of Maryland a wholly-owned subsidiary, Dr. Geoff’s by PetLife, Inc. (“Dr. Geoff’s by PetLife”), to operate the Company’s pet food division. See Note 2.
Nature of Operations
PetLife is a registered U.S. Veterinary Pharmaceutical company whose mission is to bring its scientifically proven medication and nutraceuticals, Vitalzul™, to the world of veterinary oncology. The Company specializes in the research, development, sales and support of advanced drugs and nutraceuticals for pet cancer and autoimmune related diseases such as arthritis. The Company will also introduce a line of natural pet food and other complementary products.
Basis of Presentation
The Company prepares its consolidated financial statements in conformity with generally accepted accounting principles in the United States of America.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates in the accompanying financial statements include the amortization period for intangible assets, valuation and impairment valuation of intangible assets, allowance for accounts receivable, depreciable lives of the web site and fixed assets, and valuation of share-based payments.
Principles of Consolidation
The consolidated financial statements include the accounts of PetLife and its wholly-owned subsidiary, Dr. Geoff’s by PetLife. All significant inter-company balances and transactions have been eliminated in consolidation.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less when purchased to be cash equivalents.
Property, Equipment and Depreciation
Property and equipment is recorded at cost. Depreciation is computed using the straight-line method based on the estimated useful lives of the related assets of three years for computer equipment, five years for office furniture and fixtures, and the lesser of the lease term or the useful life of the leased equipment. Leasehold improvements, if any, would be amortized over the lesser of the lease term or the useful life of the improvements. Expenditures for maintenance and repairs along with fixed assets below our capitalization threshold are expensed as incurred.
| F-6 |
Intangible Assets
On May 10, 2017, the Company acquired certain intangible assets from Healthy Life Pets, LLC (“Healthy Life Pets”), which included the intellectual property of Healthy Life Pets’ product line, including the trademarked brand, “Dr. Geoff’s Real Pet Food.” The purchase price of the assets was 1,500,000 shares of common stock of the Company, 500,000 of which were issued at execution, with the remaining 1,000,000 shares of common stock vesting over two years. The Company has valued the assets at the value of the 1,500,000 shares of common stock issued, or $270,000 of which $252,085 are intangible assets. The Company has yet to commercially use the intellectual property as of the date of this report. The Company impaired the assets acquired during the year ended August 31, 2017.
Accounting for Derivatives
The Company evaluates its convertible debt, options, warrants or other contracts to determine if those contracts or embedded components of those contracts qualify as derivatives to be separately accounted for. The result of this accounting treatment is that under certain circumstances the fair value of the derivative is marked-to-market each balance sheet date and recorded as a liability. In the event that the fair value is recorded as a liability, the change in fair value is recorded in the statement of operations as other income or expense. Upon conversion or exercise of a derivative instrument, the instrument is marked to fair value at the conversion date and then that fair value is reclassified to equity. Equity instruments that are initially classified as equity that become subject to reclassification under this accounting standard are reclassified to liability at the fair value of the instrument on the reclassification date.
Impairment of Long-Lived Assets
The Company accounts for long-lived assets in accordance with the provisions of Statement of Financial Accounting Standards ASC 360-10, “Accounting for the Impairment or Disposal of Long-Lived Assets.” This statement requires that long-lived assets and certain identifiable intangibles be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
Fair Value of Financial Instruments
The Company measures its financial assets and liabilities in accordance with generally accepted accounting principles. For certain of our financial instruments, including cash, accounts payable, accrued expenses, and short-term loans the carrying amounts approximate fair value due to their short maturities.
We follow accounting guidance for financial and non-financial assets and liabilities. This standard defines fair value, provides guidance for measuring fair value and requires certain disclosures. This standard does not require any new fair value measurements, but rather applies to all other accounting pronouncements that require or permit fair value measurements. This guidance does not apply to measurements related to share-based payments. This guidance discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flow), and the cost approach (cost to replace the service capacity of an asset or replacement cost). The guidance utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: Inputs other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active.
Level 3: Unobservable inputs in which little or no market data exists, therefore developed using estimates and assumptions developed by us, which reflect those that a market participant would use.
Revenue Recognition
The Company recognizes revenue for our services in accordance with ASC 605-10, “Revenue Recognition in Financial Statements.” Under these guidelines, revenue is recognized on transactions when all of the following exist: persuasive evidence of an arrangement did exist, delivery of service has occurred, the sales price to the buyer is fixed or determinable and collectability is reasonably assured. The Company has no revenue streams at this time.
Stock-Based Compensation
The Company accounts for stock-based instruments issued to employees in accordance with ASC Topic 718. ASC Topic 718 requires companies to recognize in the statement of operations the grant-date fair value of stock options and other equity based compensation issued to employees. The Company accounts for non-employee share-based awards in accordance with ASC Topic 505-50. The value of the portion of an award that is ultimately expected to vest is recognized as an expense over the requisite service periods using the straight-line attribution method. The Company estimates the fair value of each stock option at the grant date by using the Black-Scholes option-pricing model. The Company estimates the fair value of each stock option at the grant date by using the Black-Scholes option-pricing model.
| F-7 |
Income Taxes
The Company adopted the provisions of ASC 740-10, “Accounting for Uncertain Income Tax Positions.” When tax returns are filed, it is highly certain that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. In accordance with the guidance of ASC 740-10, the benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above should be reflected as a liability for unrecognized tax benefits in the accompanying balance sheets along with any associated interest and penalties that would be payable to the taxing authorities upon examination. The Company believes its tax positions are all highly certain of being upheld upon examination. As such, the Company has not recorded a liability for unrecognized tax benefits. As of August 31, 2017, tax years 2012 - 2016 remain open for IRS audit. The Company has received no notice of audit from the IRS for any of the open tax years.
Company adopted ASC 740-10, “Definition of Settlement in FASB Interpretation No. 48” (“ASC 740-10”), which was issued on May 2, 2007. ASC 740-10 amends FIN 48 to provide guidance on how an entity should determine whether a tax position is effectively settled for the purpose of recognizing previously unrecognized tax benefits. The term “effectively settled” replaces the term “ultimately settled” when used to describe recognition, and the terms “settlement” or “settled” replace the terms “ultimate settlement” or “ultimately settled” when used to describe measurement of a tax position under ASC 740-10. ASC 740-10 clarifies that a tax position can be effectively settled upon the completion of an examination by a taxing authority without being legally extinguished. For tax positions considered effectively settled, an entity would recognize the full amount of tax benefit, even if the tax position is not considered more likely than not to be sustained based solely on the basis of its technical merits and the statute of limitations remains open. The adoption of ASC 740-10 did not have an impact on the accompanying financial statements.
Net Earnings (Loss) Per Share
In accordance with ASC 260-10, “Earnings Per Share,” basic net earnings (loss) per common share is computed by dividing the net earnings (loss) for the period by the weighted average number of common shares outstanding during the period. Diluted earnings (loss) per share are computed using the weighted average number of common stock shares outstanding during the period.
Reclassifications
For comparability, certain prior period amounts have been reclassified, where appropriate, to conform to the financial statement presentation used in 2017. The reclassifications have no impact on net loss.
Effect of Recent Accounting Pronouncements
The Company reviews new accounting standards and updates as issued. No new standards or updates had any material effect on these unaudited financial statements. The accounting pronouncements and updates issued subsequent to the date of these audited financial statements that were considered significant by management were evaluated for the potential effect on these audited financial statements. Management does not believe any of the subsequent pronouncements will have a material effect on these audited financial statements as presented and does not anticipate the need for any future restatement of these audited financial statements because of the retro-active application of any accounting pronouncements issued subsequent to August 31, 2017 through the date these audited financial statements were issued.
In February 2016, the Financial Accounting Standards Board (“FASB”) issued an ASU on lease accounting. The ASU requires the lease rights and obligations arising from lease contracts, including existing and new arrangements, to be recognized as assets and liabilities on the balance sheet. The ASU is effective for reporting periods beginning after December 15, 2018 with early adoption permitted. While the Company is still evaluating the ASU, the Company does not expect the adoption of the ASU to have a material effect on the Company’s financial condition due to the recognition of the lease rights and obligations as assets and liabilities. The Company does not expect the ASU to have a material effect on the Company’s results of operations, and the ASU will have no effect on cash flows.
NOTE 2 – DEFINITIVE AGREEMENTS
On May 10, 2017, Dr. Geoff’s by PetLife, a subsidiary of PetLife, entered into an asset purchase agreement and a supply agreement with Healthy Life Pets, LLC (“Healthy Life Pets”) to acquire certain assets, including the intellectual property of Healthy Life Pets’ product line, including the trademarked brand, “Dr. Geoff’s Real Pet Food,” and to purchase product for a period of time from Healthy Life Pets. The purchase price of the assets was 1,500,000 shares of common stock of the Company, 500,000 of which were issued at execution, with the remaining 1,000,000 shares of common stock vesting over two years. All shares of Company common stock to be issued pursuant to these agreements are subject to restrictions on resale pursuant to a leak out agreement. In connection with the agreement, the Company recorded $17,915 of inventory and $252,085 of intangible assets for the issuance of common stock. The inventory and intangible assets were impaired during the year ended August 31, 2017.
| F-8 |
NOTE 3 – GOING CONCERN
The Company has a net loss for the year ended August 31, 2017 of $23,655,379 and negative working capital as of August 31, 2017 of $1,381,450, and has used cash in operations of $318,918 for the year ended August 31, 2017. In addition, as of August 31, 2017, the Company had a stockholders’ deficit and accumulated deficit of $1,381,450 and $30,388,180, respectively. These conditions raise substantial doubt about the Company’s ability to continue as a going concern.
The accompanying consolidated financial statements have been prepared in conformity with U.S. GAAP, which contemplate continuation of the Company as a going concern and the realization of assets and satisfaction of liabilities in the normal course of business. The ability of the Company to continue its operations is dependent on the execution of management’s plans, which include the raising of capital through the debt and/or equity markets, until such time that funds provided by operations are sufficient to fund working capital requirements. If the Company were not to continue as a going concern, it would likely not be able to realize its assets at values comparable to the carrying value or the fair value estimates reflected in the balances set out in the preparation of the consolidated financial statements.
There can be no assurances that the Company will be successful in generating additional cash from the equity/debt markets or other sources to be used for operations. The consolidated financial statements do not include any adjustments relating to the recoverability of assets and classification of assets and liabilities that might be necessary. Based on the Company’s current resources, the Company will not be able to continue to operate without additional immediate funding. Should the Company not be successful in obtaining the necessary financing to fund its operations, the Company would need to curtail certain or all operational activities and/or contemplate the sale of its assets, if necessary.
NOTE 4 – NOTES PAYABLE
The Company has notes payable as of August 31, 2017 and 2016 are as follows:
| Notes payable and | August 31, 2017 | August 31, 2016 | ||||||||||||||||||||||||||
| convertible notes payable | Debt | Debt | ||||||||||||||||||||||||||
| Principal | Discount | OID | Principal | Principal | Discount | Principal | ||||||||||||||||||||||
| Shelton Davis (1) | $ | 25,000 | $ | - | $ | - | $ | 25,000 | $ | - | $ | - | $ | - | ||||||||||||||
| Steven Sass (1) | 25,000 | - | - | 25,000 | - | - | - | |||||||||||||||||||||
| Alkmini Anastasiadou | 312,000 | - | - | 312,000 | - | - | - | |||||||||||||||||||||
| Luke Hoppel (1) | 75,000 | (59,697 | ) | (18,331 | ) | (3,028 | ) | - | - | - | ||||||||||||||||||
| PowerUp (1) | 53,000 | (42,654 | ) | - | 10,346 | - | - | - | ||||||||||||||||||||
| $ | 490,000 | $ | (102,351 | ) | $ | (18,331 | ) | $ | 369,318 | $ | - | $ | - | $ | - | |||||||||||||
(1) Convertible
On July 27, 2016, the Company executed a convertible promissory note with Shelton Avery Davis, as part of a private offering, for $25,000. The note has a maturity date of July 27, 2017 and bears interest of 10% which accrues. The note converts into common stock at $0.25 per share. As of August 31, 2017, $2,500 of interest has been accrued. On July 27, 2017, the note was extended to July 27, 2018.
On September 30, 2016, the Company executed a convertible promissory note with William Bodenheimer III (“Bodenheimer”), as part of a private offering, for $30,000. The note has a maturity date of September 30, 2017 and bears interest of 10% which accrues. The note converts into common stock at $0.25 per share. On July 22, 2017, Bodenheimer and the Company agreed to convert the principal and accrued interest into 266,000 shares of common stock. The common shares have not been issued as of August 31, 2017.
On October 4, 2016, the Company executed a convertible promissory note with Peter Sherman (“Sherman”), as part of a private offering, for $12,500. The note has a maturity date of October 4, 2017 and bears interest of 10% which accrues. The note converts into common stock at $0.25 per share. On July 22, 2016, Sherman and the Company agreed to convert the principal and accrued interest into 133,000 shares of common stock. The common shares have not been issued as of August 31, 2017.
On December 8, 2016, the Company executed a convertible promissory note with Steven Sass, as part of a private offering, for $25,000. The note has a maturity date of December 8, 2017 and bears interest of 10% which accrues. The note converts into common stock $0.25 per share. As of August 31, 2017, $1,829 of interest has been accrued.
On July 18, 2017, the Company executed a convertible promissory note with Luke Hoppel for $75,000. The note has a maturity date of July 18, 2018 and bears interest of 8% which accrues. As of August 31, 2017, $740 of interest has been accrued. The note is convertible at 60% of the average of the three lowest daily traded prices during the 25 consecutive trading days immediately preceding the applicable conversion date.
On August 28, 2017, the Company executed a convertible promissory note with PowerUp for $53,000. The note has a maturity date of May 30, 2018 and bears interest of 12% which accrues. As of August 31, 2017, $70 of interest has been accrued. The note is convertible at 58% of the average of the three lowest daily traded prices during the 10 consecutive trading days immediately preceding the applicable conversion date.
Note payable
On May 15, 2017, the Company entered into a settlement agreement with Arthur G. Mikaelian, et al (see Note 7 for all parties, the “Settlement Agreement”). Mikaelian had a financial obligation to Alkmini Anastasiadou (“Anastasiadou”), which was unrelated to the Company. As part of the Settlement Agreement, the Company would assume the debt of Mikaelian to Anastasiadou, as settlement between them, of $322,000, in a promissory note (the “Anastasiadou Note”). The terms of the Anastasiadou Note are 3% interest, with installment payments of $5,000 monthly beginning June 1, 2017 with payment in full by December 31, 2017. As of August 31, 2017, the balance of the note was $312,000 and the accrued interest was $2,858. In connection with the settlement, the Company issued $322,000 in notes payable, reversed accounts payable of $110,400 and recorded a loss of $211,600.
| F-9 |
The Company has notes payable to related parties, net of discounts, as of August 31, 2017 and August 31, 2016, as follows:
| August 31, 2017 | August 31, 2016 | |||||||||||||||||||||||||||
| Notes payable to related parties, net of discounts | Principal | Debt Discount | OID | Principal | Principal | Debt Discount | Principal | |||||||||||||||||||||
| Ralph Salvagno | $ | 153,011 | $ | - | $ | - | $ | 153,011 | $ | 153,011 | $ | - | $ | 153,011 | ||||||||||||||
| Ralph Salvagno | 59,852 | - | - | 59,852 | - | - | - | |||||||||||||||||||||
| Ralph Salvagno | 24,830 | - | - | 24,830 | - | - | - | |||||||||||||||||||||
| Ralph Salvagno | 98,664 | - | - | 98,664 | - | - | - | |||||||||||||||||||||
| $ | 336,357 | $ | - | $ | - | $ | 336,357 | $ | 153,011 | $ | - | $ | 153,011 | |||||||||||||||
On August 31, 2016, the Company executed a promissory note with Ralph Salvagno (“Salvagno”), the Company’s CEO and Director, for $153,011. The note is due on demand and bears interest at 2% per annum which accrues. As of August 31, 2017, $3,060 of interest has been accrued. See Note 5.
On October 25, 2016, the Company executed a promissory note with Dreadnought 1906, Inc., which is controlled by Vyvyan Campbell (“Campbell”), the Company’s Director, for $150,000. The note matures on November 1, 2017 and bears interest at 10% per annum which accrues. As an incentive for the issuance of the note, 250,000 shares of common stock were issued and recorded as a debt discount. The shares were valued at $150,000 for the debt discount. On August 26, 2017, Campbell and the Company agreed on a conversion for the principal and accrued interest to be converted into 2,500,000 shares of common stock. See Note 5. The common shares have not been issued as of August 31, 2017.
On January 14, 2017, the Company executed a promissory note with Salvagno for $59,852. The note converted various payables to Salvagno. The note is due on demand and bears interest at 4% per annum which accrues. As of August 31, 2017, $1,509 of interest has been accrued. See Note 5.
On March 18, 2017, the Company executed a promissory note with Salvagno for $24,830. The note converted various payables to Salvagno. The note is due on demand and bears interest at 4% per annum which accrues. As of August 31, 2017, $454 of interest has been accrued. See Note 5.
On May 31, 2017, the Company executed a promissory note with Salvagno for $98,664. The note converted various payables to Salvagno. The note is due on demand and bears interest at 4% per annum which accrues. As of August 31, 2017, $503 of interest has been accrued. See Note 5.
NOTE 5 – RELATED PARTY TRANSACTIONS
During the year ended August 31, 2015, we received $10,000 from a shareholder advance which is unsecured, non-interest bearing, and due on demand. As of August 31, 2017, $10,000 remains outstanding.
As of August 31, 2017, the Company has a loan from an officer of $11,500. The loan is unsecured, non-interest bearing, and due on demand.
As of August 31, 2017, and 2016, there are related party accounts payable and accrued expenses of $75,526 and $383,974, respectively, related to payments, consulting services and accrued interest.
On August 31, 2016, the Company executed a promissory note with Salvagno, the Company’s CEO and Director, for $153,011. The note is due on demand and bears interest at 2% per annum which accrues. As of August 31, 2017, $3,060 of interest has been accrued. See Note 4.
On October 25, 2016, the Company executed a promissory note with Dreadnought 1906, Inc., which is controlled by Vyvyan Campbell (“Campbell”), the Company’s Director, for $150,000. The note matures on November 1, 2017 and bears interest at 10% per annum which accrues. As an incentive for the issuance of the note, 250,000 shares of common stock were issued and recorded as a debt discount. The shares were valued at $150,000 for the debt discount. On August 26, 2017, Campbell and the Company agreed on a conversion for the principal and accrued interest to be converted into 2,500,000 shares of common stock. See Note 4. The common shares have not been issued as of August 31, 2017.
On January 14, 2017, the Company executed a promissory note with Salvagno for $59,852. The note converted various payables to Salvagno. The note is due on demand and bears interest at 4% per annum and accrues monthly. As of August 31, 2017, $1,509 of interest has been accrued. See Note 4.
On March 18, 2017, the Company executed a promissory note with Salvagno for $24,830. The note converted various payables to Salvagno. The note is due on demand and bears interest at 4% per annum which accrues. As of August 31, 2017, $454 of interest has been accrued. See Note 4.
On May 31, 2017, the Company executed a promissory note with Salvagno for $98,664. The note converted various payables to Salvagno. The note is due on demand and bears interest at 4% per annum which accrues. As of August 31, 2017, $503 of interest has been accrued. See Note 4.
| F-10 |
During the year ended August 31, 2017, the Company has issued or is contractually obligated to issue, 40,000 shares of common stock for board advisory services valued at $13,000 of which $13,000 has been recorded as stock-based compensation.
In February 2017, the Company entered into a consulting agreement for a spokesman for the PetLife pet foods division. The agreement is through January 2020. The Company issued 1,000,000 shares of restricted common stock which 27,778 shares of common stock vest every month. The shares of common stock were valued at $160,000 and $31,111 was recorded as stock-based compensation for the year ended August 31, 2017. The Company will record the remaining $128,889 over the service period or through January 2020.
During the year ended August 31, 2017, the Company exchanged 3,250,000 common shares owned by officers and directors for 3,250,000 Series A Preferred shares and valued the transaction at $121,875. The Series A Preferred Stock have voting rights of 1,000 votes for each share of Series A Preferred Stock.
NOTE 6 - STOCKHOLDERS’ DEFICIT
Preferred Stock
The Company is authorized to issue up to 50,000,000 shares of preferred stock, par value $0.001 per share. Each share of preferred stock is convertible into common stock on a one-for-one basis. As of August 31, 2017, there are 3,250,000 shares of preferred stock issuable, and outstanding.
During the year ended August 31, 2017, the Company exchanged 3,250,000 common shares owned by officers and directors for 3,250,000 Series A Preferred shares and valued the transaction at $121,875. The Series A Preferred Stock have voting rights of 1,000 votes for each share of Series A Preferred Stock.
The Corporation will pay dividends or distributions equal to the amount of dividends or distributions per share of Common Stock if and when declared.
In the event of any liquidation, dissolution or winding up of the Corporation, either voluntary or involuntary, the holders of the Corporation's Preferred Stock entitled, by reason of their ownership of Preferred Stock, receive the preferential amount, prior and in preference to any distribution of any of the assets of the Corporation to the holders of Series A Stock and Common Stock.
Common Stock
The Company was authorized to issue up to 750,000,000 shares of common stock, par value $0.001 per share. Each outstanding share of common stock entitles the holder to one vote per share on all matters submitted to a stockholder vote. All shares of common stock are non-assessable and non-cumulative, with no pre-emptive rights.
On May 10, 2017, the Company issued 500,000 shares of common stock and will issue a 1,000,000 shares of common stock for the acquisition of certain assets from Healthy Life Pets, LLC. The shares were valued at $270,000. See Note 2.
During the year ended August 31, 2017, the Company has issued or is contractually obligated to issue, 58,918,898 common shares for services valued at $24,310,040, of which $23,703,234 has been recorded as stock-based compensation. The Company will amortize $606,806 over the remaining service period.
During the year ended August 31, 2017, the Company issued 250,000 common shares to a shareholder for debt valued at $150,000. The Company recorded the $150,000 in interest expense during the year ended August 31, 2017.
During the year ended August 31, 2017, the Company issued 2,500,000 common shares to a shareholder for the conversion of debt valued at $150,000. The Company recorded $162,500 in interest expense for the inducement during the year ended August 31, 2017.
During the year ended August 31, 2017, the Company issued 399,000 common shares to a shareholder for the conversion of debt valued at $42,500.
During the year ended August 31, 2017, the Company issued 5,000,000 common shares to a shareholder for the settlement of accrued expenses of $300,000. The Company valued the common stock at $625,000 and recorded $325,000 in stock compensation for the settlement during the year ended August 31, 2017.
During the year ended August 31, 2017, the Company issued 125,000 common shares valued at $20,950 to a shareholder for debt of $75,000. The Company recorded the $20,950 in interest expense during the year ended August 31, 2017.
Employment Agreements
Included in the above paragraph, in January 2017, the Company entered into an employment agreement with our Vice-President of marketing. The agreement is through February 2019. The Company will pay $6,300 per month and 1,500,000 shares of restricted common stock which 100,000 vest immediately and 350,000 common shares vest every six months. The common shares were valued at $240,000 and $56,000 was recorded as stock compensation for the year ended August 31, 2017. The Company terminated the agreement effective August 31, 2017. The Company is obligated to issue 350,000 common shares in connection with the agreement.
Included in the above paragraph, in January 2017, the Company entered into an employment agreement with the President of the pet foods division. The agreement is through December 2018. The Company will pay $12,500 per month and 3,000,000 shares of restricted common stock which 350,000 common shares vest every three months. The common shares were valued at $600,000 and $150,000 was recorded as stock compensation for the year ended August 31, 2017. The Company will record the remaining $450,000 over the service period or through December 2018. The Company is obligated to issue 150,000 common shares in connection with the agreement.
| F-11 |
The Company terminated all employment agreements as of August 31, 2017. The Company, upon sufficient additional funding, will renegotiate employment agreements accordingly.
Stock Option Plan
On June 9, 2016, the Board of Directors approved the 2016 Stock Option Plan which reserved 20,000,000 shares of common stock.
The Company has granted options to an employee and to a consultant. Options activity for the year ended August 31, 2017 is as follows:
| Weighted | ||||||||||||||||
| Weighted | Average | |||||||||||||||
| Average | Remaining | Aggregate | ||||||||||||||
| Number | Exercise | Contractual | Intrinsic | |||||||||||||
| of Options | Price | Terms | Value | |||||||||||||
| Outstanding at August 31, 2016 | 4,000,000 | $ | 0.25 | |||||||||||||
| Granted | - | $ | - | |||||||||||||
| Exercised | - | $ | - | |||||||||||||
| Forfeited | - | $ | - | |||||||||||||
| Expired | - | $ | - | |||||||||||||
| Outstanding at August 31, 2017 | 4,000,000 | $ | 0.25 | 1.77 | $ | 369,200 | ||||||||||
| Exercisable at August 31, 2017 | 4,000,000 | $ | 0.25 | |||||||||||||
NOTE 7 – COMMITMENTS AND CONTINGENCIES
Legal Matters
From time to time, we may be involved in litigation relating to claims arising out of our operations in the normal course of business. As of the date of this report, there were no pending or threatened lawsuits. The Company entered into a settlement agreement as follows:
On May 15, 2017, the Company entered into a settlement agreement with Arthur G. Mikaelian (a/k/a Arthur Grant Mikaelian, Artur Mikaelian, collectively, “Mikaelian”), the Irrevocable de Fidecomison Confianza General De Familiar Artur Mikaelian (the “Mikaelian Trust”), Artur Mikaelian, Jr., Tigran Mikaelian, Grant Mikaelian, Armani Minasyan, Medolife Corp. (“Medolife”), and Alkmini Anastasiadou (“Anastasiadou”). Mikaelian, a former officer of the Company, the other parties, and the Company finalized a settlement to sever all ties between the Company and all other parties (the “Settlement Agreement”). Mikaelian had a financial obligation to Anastasiadou, which was unrelated to the Company. The Settlement Agreement provided the following: a) full release between all parties, b) all common stock of the Company held by all parties, excluding Anastasiadou (as they did not hold any common stock of the Company) would be returned to the Company as treasury stock (the “Treasury Stock”), and c) the Company would assume the debt of Mikaelian to Anastasiadou, as settlement between them, of $322,000, in a promissory note (the “Anastasiadou Note”). The Treasury Stock would be held in escrow until the Anastasiadou Note is paid in full by the Company, which then would be retired by the Company. The Treasury Stock is 4,794,000 shares which, as of August 31, 2017, constitutes 7.25% of the outstanding common stock of the Company. The terms of the Anastasiadou Note are 3% interest, with installment payments of $5,000 monthly beginning June 1, 2017 with payment in full by December 31, 2017. See Note 4.
Lease Commitment
On February 1, 2016, the Company entered into a lease space effective June 1, 2016 through May 30, 2018. Lease payments of $750 per month payable in advance on the first day of each month for total lease payments of $18,000.
Future minimum lease payments are as follows:
| 2018 | $ | 6,750 | ||
| 2019 | - | |||
| 2020 | - | |||
| 2021 | - | |||
| 2022 | - | |||
| Future | - | |||
| Total | $ | 6,750 |
Rent expense for the years ended August 31, 2017 and 2016 was $9,000 and $23,825, respectively.
| F-12 |
NOTE 8 – CONCENTRATIONS
Concentration of Credit Risk
Financial instruments, which potentially subject the Company to a concentration of credit risk, consist principally of temporary cash investments.
The Company places its temporary cash investments with financial institutions insured by the Federal Deposit Insurance Corporation (“FDIC”) for the United States. No amounts exceeded federally insured limits as of August 31, 2017. There have been no losses in these accounts through August 31, 2017.
Concentration of Intellectual Property
The Company owns or has filed for the trademarks “Dr. Geoff’s Real Pet Food” as filed with the United States Patent and Trademark Office.
NOTE 9 – INCOME TAX
The Company follows ASC 740-10-50 “Accounting for Income Taxes.” Deferred income taxes reflect the net effect of (a) temporary difference between carrying amounts of assets and liabilities for financial purposes and the amounts used for income tax reporting purposes, and (b) net operating loss carry-forwards. No net provision for refundable Federal income tax has been made in the accompanying statement of loss because no recoverable taxes were paid previously. Similarly, no deferred tax asset attributable to the net operating loss carry-forward has been recognized, as it is not deemed likely to be realized.
The provision for refundable federal income tax consists of the following for the years ending:
| For the Years Ended | ||||||||
| August 31, | ||||||||
| 2017 | 2016 | |||||||
| Tax expense (benefit) at the statutory rate | ||||||||
| Federal | $ | (7,907,000 | ) | $ | (1,254,000 | ) | ||
| Change in valuation allowance | 7,907,000 | 1,254,000 | ||||||
| Total | $ | - | $ | - | ||||
The cumulative tax effect at the expected rate of 34% of significant items comprising our net deferred tax amount is as follows:
| August 31, | ||||||||
| 2017 | 2016 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforward | $ | 10,332,000 | $ | 2,425,000 | ||||
| Less: Deferred tax asset valuation allowance | (10,332,000 | ) | (2,425,000 | ) | ||||
| Total net deferred taxes | $ | - | $ | - | ||||
At August 31, 2017, the Company had an unused net operating loss carry-forward approximating $10,332,000 that is available to offset future taxable income; the loss carry-forward will start to expire in 2037.
NOTE 13 – DERIVATIVES
Embedded Conversion Option Derivatives
Due to the conversion terms of certain promissory notes, the embedded conversion options met the criteria to be bifurcated and presented as derivative liabilities. The Company calculated the estimated fair values of the liabilities for embedded conversion option derivative instruments at the original note inception date and as of August 31, 2017 using the Black-Scholes option pricing model using the share prices of the Company’s stock on the dates of valuation and using the following ranges for volatility, expected term and the risk-free interest rate at each respective valuation date, no dividend has been assumed for any of the periods:
| F-13 |
Note Inception Date | August 31, 2017 | |||||||
| Volatility | 313% - 350 | % | 291 | % | ||||
| Expected Term | 0.75 – 0.88 years | 0.33 - 0.88 years | ||||||
| Risk Free Interest Rate | 1.07% - 1.19 | % | 1.23 | % | ||||
The following reflects the initial fair value on the note inception date and changes in fair value through August 31, 2017:
| Note inception date fair value allocated to debt discount | $ | 111,004 | ||
| Change in fair value in 2017 | (8,627 | ) | ||
| Embedded conversion option derivative liability fair value on August 31, 2017 | $ | 102,377 |
NOTE 14 – SUBSEQUENT EVENTS
The Company has evaluated subsequent events through the date the financial statements were issued and filed with the Securities and Exchange Commission. The Company has determined that there are no other such events that warrant disclosure or recognition in the financial statements, except as stated herein.
On November 6, 2017, the Company entered into a convertible promissory note for $135,000 with Auctus Fund, LLC (“Auctus”). The note matures on August 6, 2018 and bears interest of 12%. The conversion feature provides for a discount of 40%. The Company has to issue 123,875 shares of common stock to Auctus which were an inducement for the financing.
| F-14 |
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Management’s Annual Report on Internal Control Over Financial Reporting
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in rule 13a-15(f) and 15d-15(f) under the Exchange Act. Internal control over financial reporting is a process designed by, or under the supervision of the CEO to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with generally accepted accounting principles. Internal controls over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records which in reasonable detail accurately and fairly reflect the transactions and disposition of the Company’s assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made in accordance with authorizations of management and directors of the issuer; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the Company’s consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency or a combination of deficiencies in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
In evaluating the effectiveness of the Company’s internal control over financial reporting as of August 31, 2017, management used the criteria established in the Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based on the criteria established by COSO, management (with the participation of the CEO and the CFO) identified the following material weaknesses in the Company’s internal control over financial reporting as of August 31, 2017, which arose from the limited number of number of staff at the Company and the inability to achieve proper segregation of duties:
| ○ | The Company lacked effective controls for ensuring the accuracy of reporting over significant account balances, including the review, approval, and documentation of related transactions and account reconciliations and other complex accounting procedures. |
| ○ | The Company lacked effective controls because their directors are not independent. |
As a result of these material weaknesses, management concluded that the Company did not maintain effective internal control over financial reporting as of August 31, 2017, based on the criteria established in Internal Control – Integrated Framework (2013) issued by COSO.
This Annual Report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to rules of the Security and Exchange Commission that permit us to provide only management’s report in this Annual Report.
Changes in Internal Control over Financial Reporting
Except as set forth above, there were no changes in our internal control over financial reporting that occurred during the period covered by this report that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on the Effectiveness of Controls
The Company’s management, including the CEO and CFO, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of the control system must reflect that there are resource constraints and that the benefits must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of controls effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
| 21 |
Item 9B. Other Information.
None.
Item 10. Directors, Executive Officers and Corporate Governance Identify Directors and Executive Officers
Executive Officers and Directors
The following table sets forth information with respect to persons who are serving as directors and officers of the Company. Each director holds office until the next annual meeting of shareholders or until his successor has been elected and qualified.
| Name | Age | Position | ||
| Ralph T. Salvagno, MD | 62 | Chairman, Chief Executive Officer and Chief Financial Officer | ||
Dr. Vivek Ramana Vyvyan Campbell |
58 74 |
Chief Medical Officer Director |
Biographies of Directors and Officers
The following is a brief account of the education and business experience during at least the past five years of each director, executive officer and key employee of our company, indicating the person’s principal occupation during that period, and the name and principal business of the organization in which such occupation and employment were carried out.
Ralph T. Salvagno, MD. – Chief Executive Officer, Chief Financial Officer and Chairman
Dr. Salvagno is Chairman of the Board and CEO of PetLife Pharmaceuticals Inc. and an Orthopaedic Surgeon by training. From his early childhood he has always had family pets. From purebred Golden Retrievers to stray mixed breeds, dogs were always a part of his life. More recently, his affinity has been for felines.
A Bachelor of Science Honors graduate of the University of Maryland, Dr. Salvagno received his M.D. degree from the University of Maryland Medical School and completed an Orthopaedic Surgery residency at Case Western Reserve University/ University Hospitals of Cleveland. Since 1987 he was in private practice and was the founding partner and president of the Center for Joint Surgery and Sports Medicine in Hagerstown, Md.
He is Board Certified in Orthopaedic Surgery and a fellow of the American Academy of Orthopaedic Surgeons.
His professional memberships include the American Medical Association, the American Academy of Hip and Knee Surgeons, the American Association for Physician Leadership and the International Society for Stem Cell Research. He has presented new technology for arthritis treatment at recent national meetings.
Dr. Salvagno has previously served on the Board of the Washington County Arthritis Foundation and served for 10 years on the Board of Meritus Enterprises, the for-profit arm of Meritus Medical Center. Dr. Salvagno currently serves as Chief of Staff at Meritus Medical Center in Hagerstown, Maryland.
Dr. Vivek Ramana – Chief Medical Officer
Prior to joining PetLife, Dr. Ramana served as Senior Safety Medical Officer/Senior Medical Director for Ambit Biosciences. In 2008 - 2009, Dr. Ramana served as Senior Medical Director/Therapeutic Area Lead for US Astellas. From 2006 to 2008 he was Chief Medical Officer and President for AdPharma. From 2003 to 2006, he served as Vice President, Medical Services at Ovation Pharmaceuticals. During the early part of Dr. Ramana’s career, from 1984 to 2003, Dr. Ramana served as Medical Director of R&D for Pharmaceutical Clinical Research, Inc., which involved joint ventures with BMS, Pfizer, Schering Plough, and Astra Zeneca.
Dr. Ramana received his medical degree from the University of Belgrade, Serbia and specialized in Radiation Oncology and Clinical Pathology. Post Graduate studies in Molecular Immunology, Genetics and Molecular Biology from University of New Haven, CT; Post-Doctoral Studies in Clinical Pharmacology, Drug Research & Development and Regulation from TUFTS University in Boston.
Vyvyan Campbell - Director
Vyvyan Campbell’s diverse career in television and film spans over 30 years and several continents. Vyvyan has achieved an outstanding reputation as a producer in lifestyle and reality television. She is the President and Executive Producer of JV Productions, based in Toronto Canada.
| 22 |
Indemnification of Directors and Officers
Nevada Corporation Law allows for the indemnification of officers, directors, and any corporate agents in terms sufficiently broad to indemnify such persons under certain circumstances for liabilities, including reimbursement for expenses, incurred arising under the 1933 Act. The Bylaws of the Company provide that the Company will indemnify its directors and officers to the fullest extent authorized or permitted by law and such right to indemnification will continue as to a person who has ceased to be a director or officer of the Company and will inure to the benefit of his or her heirs, executors and Consultants; provided, however, that, except for proceedings to enforce rights to indemnification, the Company will not be obligated to indemnify any director or officer in connection with a proceeding (or part thereof) initiated by such person unless such proceeding (or part thereof) was authorized by the Board of Directors. The right to indemnification conferred will include the right to be paid by the Company the expenses (including attorney’s fees) incurred in defending any such proceeding in advance of its final disposition.
The Company may, to the extent authorized from time to time by the Board of Directors, provide rights to indemnification and to the advancement of expenses to employees and agents of the Company similar to those conferred to directors and officers of the Company. The rights to indemnification and to the advancement of expenses are subject to the requirements of the 1940 Act to the extent applicable.
Furthermore, the Company may maintain insurance, at its expense, to protect itself and any director, officer, employee or agent of the Company or another company against any expense, liability or loss, whether or not the Company would have the power to indemnify such person against such expense, liability or loss under the Nevada General Corporation Law.
Director Compensation
During the fiscal year ended August 31, 2017 and 2016, we had one independent director. Directors that were employees were not paid any fees for their role as director.
Involvement on Certain Material Legal Proceedings During the Last Five Years
No director, officer, significant employee or consultant has been convicted in a criminal proceeding, exclusive of traffic violations.
No bankruptcy petitions have been filed by or against any business or property of any director, officer, significant employee or consultant of the Company nor has any bankruptcy petition been filed against a partnership or business association where these persons were general partners or executive officers.
No director, officer, significant employee or consultant has been permanently or temporarily enjoined, barred, suspended or otherwise limited from involvement in any type of business, securities or banking activities.
No director, officer or significant employee has been convicted of violating a federal or state securities or commodities law.
Directors’ and Officers’ Liability Insurance
The Company has directors’ and officers’ liability insurance insuring our directors and officers against liability for acts or omissions in their capacities as directors or officers.
Code of Ethics
We intend to adopt a code of ethics that applies to our officers, directors and employees, including our principal executive officer and principal accounting officer, but have not done so to date due to our relatively small size. We intend to adopt a written code of ethics in the near future.
Corporate Governance & Board Independence
Our Board of Directors consists of three directors and has not established a Nominating or Governance Committees as standing committees. The Board does not have an executive committee or any committees performing a similar function. We are not currently listed on a national securities exchange or in an inter-dealer quotation system that has requirements that a majority of the board of directors be independent.
Due to our lack of operations and size, and since we are not currently listed on a national securities exchange, we are not subject to any listing requirements mandating the establishment of any particular committees; all functions of a nominating/governance committee were performed by our whole board of directors. Our board of directors intends to appoint such persons and form such committees as are required to meet the corporate governance requirements imposed by the national securities exchanges as necessary. Our board of directors does not believe that it is necessary to have such committees at the early stage of the company’s development, and our board of directors believes that the functions of such committees can be adequately performed by the members of our board of directors.
We believe that members of our board of directors are capable of analyzing and evaluating our consolidated financial statements and understanding internal controls and procedures for financial reporting. We believe that retaining an independent director who would qualify as an “audit committee financial expert” would be overly costly and burdensome and is not warranted in our circumstances given the early stages of our development and the fact that we have not generated any material revenues to date.
| 23 |
Board Leadership Structure and the Board’s Role in Risk Oversight.
The Board of Directors is led by the Chairman who is also the Chief Executive Officer. Although our sole officer is also one of our directors, the Board believes that the most effective leadership structure at this time is not to separate the roles of Chairman and Chief Executive Officer. A combined structure provides the Company with a single leader who represents the company to our stockholders, regulators, business partners and other stakeholders, among other reasons set forth below.
| ● | This structure creates efficiency in the preparation of the meeting agendas and related Board materials as the Company’s Chief Executive Officer works directly with those individuals preparing the necessary Board materials and is more connected to the overall daily operations of the Company. Agendas are also prepared with the permitted input of the full Board of Directors allowing for any concerns or risks of any individual director to be discussed as deemed appropriate. The Board believes that the Company has benefited from this structure, and Mr. Perry’s continuation in the combined role of the Chairman and Chief Executive Officer is in the best interest of the stockholders. |
| ● | The Company believes that the combined structure is necessary and allows for efficient and effective oversight, given the Company’s relatively small size, its corporate strategy and focus. |
The Board of Directors does not have a specific role in risk oversight of the Company. The Chairman, President and Chief Executive Officer and other executive officers and employees of the Company provide the Board of Directors with information regarding the Company’s risks.
Item 11. Executive Compensation
The table below sets forth, for our last two fiscal years, the compensation earned by Dr. Ralph Salvagno, as our Chief Executive Officer and Chief Financial Officer, Dr. Vivek Ramana, our Chief Medical Officer, and Vyvyan Campbell, our director.
| Deferred | Option and | All Other | ||||||||||||||||||||||||||||||
| Name and | Compen- | Stock | Warrant | Compen- | ||||||||||||||||||||||||||||
| Principal Position | Salary | sation | Bonus | Awards | Awards | sation | Total | |||||||||||||||||||||||||
| Dr. Ralph Salvagno (1) | 2017 | $ | 240,000 | $ | - | $ | - | $ | 112,000 | $ | - | $ | - | $ | 352,000 | |||||||||||||||||
| Chief Executive Officer, Chief Financial Officer and Director | 2016 | $ | - | $ | - | $ | - | $ | 840,000 | $ | 204,849 | $ | - | $ | 1,044,849 | |||||||||||||||||
| Dr. Vivek Ramana (2) | 2017 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||||||
| Chief Medical Officer | 2016 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||||||
(1) Dr. Salvagno was appointed as CEO, CFO and Director on June 9, 2016.
(2) Dr. Ramana was appointed as CMO on June 30, 2016.
| Director | Fees
Earned or Paid in Cash | Stock Awards | Warrant Awards (1) | Non-Equity Incentive Plan Compensation | Change
in Pension Value and Nonqualified Deferred Compensation Earnings | All
Other Compensation | Total | |||||||||||||||||||||||
| Vyvyan Campbell (1) | ||||||||||||||||||||||||||||||
| 2017 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||||
| 2016 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - |
(1) Ms. Campbell was appointed as Director on September 6, 2016.
Executive Employment Agreements
The Company had several employment agreements which, as of August 31, 2017, were terminated for the purpose of restructuring certain contracts in the future to accommodate the working capital of the Company.
| 24 |
Equity Compensation Plans
We have no current equity compensation plans.
Outstanding Equity Awards at Fiscal Year-end
There are 4,000,000 stock option equity awards outstanding for our Executive officers for the years ended August 31, 2017 and August 31, 2016, respectively.
Stock Option Exercised
There were no stock options exercised on common shares during the years ended August 31, 2016 and August 31, 2015.
Expense Reimbursement
We will reimburse our officers and directors for reasonable expenses incurred during the course of their performance.
Retirement Plans and Benefits.
None.
Standard Director Compensation Arrangement
We do not have a standard compensation arrangement for directors.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth information regarding the beneficial ownership of the Company’s common stock (and preferred stock) as of June 30, 2017, for (i) each person or entity who, to our knowledge, beneficially owns more than 5% of our common stock; (ii) each executive officer and named officer; (iii) each director; and (iv) all of our officers and directors as a group. Unless otherwise indicated in the footnotes to the following table, each of the stockholders named in the table has sole voting and investment power with respect to the shares of our common stock beneficially owned. Except as otherwise indicated, the address of each of the stockholders listed below is: c/o 3571 E. Sunset Road, Suite 420, Las Vegas, Nevada 89120.
| Amount & | ||||||||||
| Nature | ||||||||||
| Name of Beneficial | of Beneficial | Percent of | ||||||||
| Title of Class | Owners | Ownership (1) | Class (2) | |||||||
| Common stock, $0.001 par value | Dr. Ralph Salvagno (3) | 24,450,000 | 31.0 | % | ||||||
| Common stock, $0.001 par value | Dr. Vivek Ramana (4) | 1,000,000 | 1.3 | % | ||||||
| Common stock, $0.001 par value | Vyvyan Campbell (5) | 240,000 | 0.3 | % | ||||||
| Series A preferred stock, $0.001 par value | Dr. Ralph Salvagno (3) | 1,250,000 | 38.5 | % | ||||||
| Series A preferred stock, $0.001 par value | Dr. Vivek Ramana (4) | 1,000,000 | 30.8 | % | ||||||
| Series A preferred stock, $0.001 par value | Vyvyan Campbell (5) | 1,000,000 | 30.8 | % | ||||||
| Common stock, $0.001 par value | All officers and directors as a Group | 25,690,000 | 32.6 | % | ||||||
| Series A preferred stock, $0.001 par value | All officers and directors as a Group | 3,250,000 | 100 | % | ||||||
(1) Beneficial Ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. Each of the beneficial owners listed above has direct ownership of and sole voting power and investment power to the shares of the Company’s common stock.
(2) As of August 31, 2016, a total of 78,775,982 shares of the Company’s common stock are considered to be outstanding (includes issuable) pursuant to SEC Rule 13d-3(d)(1). For each Beneficial Owner listed, any options exercisable within 60 days have been also included for purposes of calculating their percent of class.
(3) Director and officer.
(4) Officer.
(5) Director.
| 25 |
Item 13. Certain Relationships and Related Transactions, and Director Independence
Transactions with Related Persons
Director Independence
We currently do not have any independent directors, as the term “independent” is defined in Section 803A of the NYSE Amex LLC Company Guide. Since the Over the Counter Pink (“OTCPK”) does not have rules regarding director independence, the Board makes its determination as to director independence based on the definition of “independence” as defined under the rules of the New York Stock Exchange (“NYSE”) and American Stock Exchange (“Amex”).
Item 14. Principal Accountant Fees and Services
The following table sets forth the fees billed by our principal independent accountants, LBB & Associates Ltd., LLP (“LBB”) for 2017 and 2016.
| Years Ended August 31, | ||||||||
| Category | 2017 | 2016 | ||||||
| Audit Fees | $ | 43,000 | $ | 18,400 | ||||
| Audit Related Fees | – | – | ||||||
| Tax Fees | – | – | ||||||
| All Other Fees | – | – | ||||||
| Total | $ | 43,000 | $ | 18,400 | ||||
Audit fees. Consists of fees billed for the audit of our annual consolidated financial statements and review of our interim financial information and services that are normally provided by the accountant in connection with year-end and quarter-end statutory and regulatory filings or engagements.
Audit-related fees. Consists of fees billed for services relating to review of other regulatory filings including registration statements, periodic reports and audit related consulting.
Tax fees. Consists of professional services rendered by our principal accountant for tax compliance, tax advice and tax planning.
Other fees. Other services provided by our accountants.
Item 15. Exhibits and Financial Statement Schedules
See the Exhibit Index following the signature page of this Registration Statement, which Exhibit Index is incorporated herein by reference.
(1) Filed herewith
Financial Statement Schedules
None.
| 26 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| /s/ Ralph Salvagno | December 14, 2017 | |
Ralph Salvagno, Principal Executive Officer and Principal Accounting Officer |
Date |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| /s/ Ralph Salvagno | December 14, 2017 | |
| Ralph Salvagno, Director | Date |
| /s/ Vyvyan Campbell | December 14, 2017 | |
| Vyvyan Campbell, Director | Date |
| 27 |
