Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Cardiff Oncology, Inc. | a17-27641_18k.htm |
Transforming Oncology With Precision Cancer Therapeutics Company Overview november, 2017 Company Overview January 2017

Forward-Looking Statements Certain statements in this presentation are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of words such as "anticipate," "believe," "forecast," "estimated" and "intend" or other similar terms or expressions that concern Trovagene's expectations, strategy, plans or intentions. These forward-looking statements are based on Trovagene's current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. While the list of factors presented in the 10-K is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Forward-looking statements included herein are made as of the date hereof, and Trovagene does not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances.
Company Overview Precision Cancer Therapeutics Clinical stage biotechnology company developing treatments for hematologic and solid tumor malignancies Core DNA/RNA Technology Expertise Proprietary technology in tumor genomics and CLIA/CAP-accredited laboratory for drug development programs Lead Therapeutic Candidate – PCM-075 Polo-like Kinase 1 (PLK1) Inhibitor NASDAQ: TROV Headquarters: San Diego, CA Shares (07/31/17): 37.3M outstanding
Lead Drug Candidate PCM-075 Investment Highlights Progressing Pipeline Broad clinical applications in hematologic and solid tumor malignancies Hematologic: Acute Myeloid Leukemia, Non-Hodgkin Lymphoma Solid Tumors: Castrate-Resistant Prostate cancer CRPC), Adrenocortical Carcinoma (ACC), Small Cell Lung Cancer (SCLC), Triple-Negative Breast Cancer (TNBC) Technology Platform Proprietary biomarker technology to optimize drug development NPM1 for diagnosis and monitoring therapy response in AML Partnerships with pharma and research institutions to purchase NextCollect™ urine collection and DNA preservation device Significant Market Opportunities FDA approved IND and protocol for Trovagene to conduct a Phase 1b/2 trial with PCM-075 in Acute Myeloid Leukemia (AML) Completed Phase 1 trial in solid tumor cancers published in Investigational New Drugs Phase 2 protocol in metastatic Castration-Resistant Prostate Cancer in development Highly-selective, oral, Polo-like Kinase 1 (PLK1) Inhibitor Licensed exclusive global development and commercialization rights from Nerviano Medical Sciences (NMS), S.r.l. Orphan Drug Designation granted by the FDA for the treatment of Acute Myeloid Leukemia (AML)
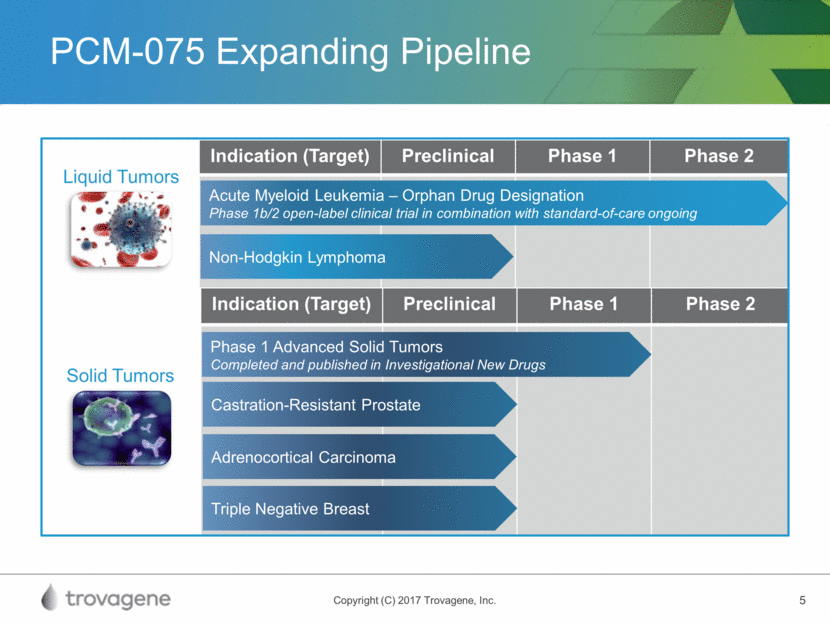
PCM-075 Expanding Pipeline Indication (Target) Preclinical Phase 1 Phase 2 Non-Hodgkin Lymphoma Acute Myeloid Leukemia – Orphan Drug Designation Phase 1b/2 open-label clinical trial in combination with standard-of-care ongoing Indication (Target) Preclinical Phase 1 Phase 2 Phase 1 Advanced Solid Tumors Completed and published in Investigational New Drugs Castration-Resistant Prostate Adrenocortical Carcinoma Triple Negative Breast Liquid Tumors Solid Tumors
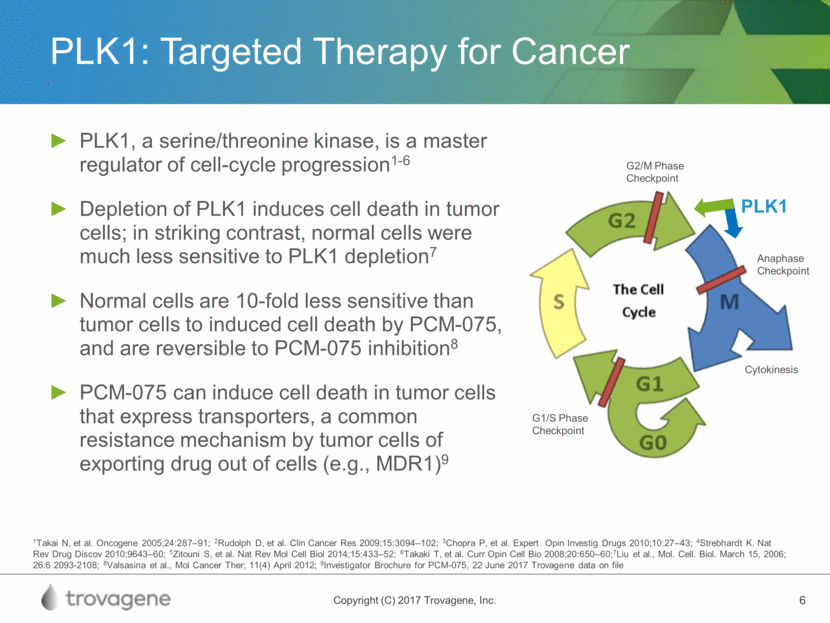
PLK1: Targeted Therapy for Cancer PLK1, a serine/threonine kinase, is a master regulator of cell-cycle progression1-6 Depletion of PLK1 induces cell death in tumor cells; in striking contrast, normal cells were much less sensitive to PLK1 depletion7 Normal cells are 10-fold less sensitive than tumor cells to induced cell death by PCM-075, and are reversible to PCM-075 inhibition8 PCM-075 can induce cell death in tumor cells that express transporters, a common resistance mechanism by tumor cells of exporting drug out of cells (e.g., MDR1)9 1Takai N, et al. Oncogene 2005;24:287–91; 2Rudolph D, et al. Clin Cancer Res 2009;15:3094–102; 3Chopra P, et al. Expert Opin Investig Drugs 2010;10:27–43; 4Strebhardt K. Nat Rev Drug Discov 2010;9643–60; 5Zitouni S, et al. Nat Rev Mol Cell Biol 2014;15:433–52; 6Takaki T, et al. Curr Opin Cell Bio 2008;20:650–60;7Liu et al., Mol. Cell. Biol. March 15, 2006; 26:6 2093-2108; 8Valsasina et al., Mol Cancer Ther; 11(4) April 2012; 9Investigator Brochure for PCM-075, 22 June 2017 Trovagene data on file PLK1 Anaphase Checkpoint Cytokinesis G1/S Phase Checkpoint G2/M Phase Checkpoint
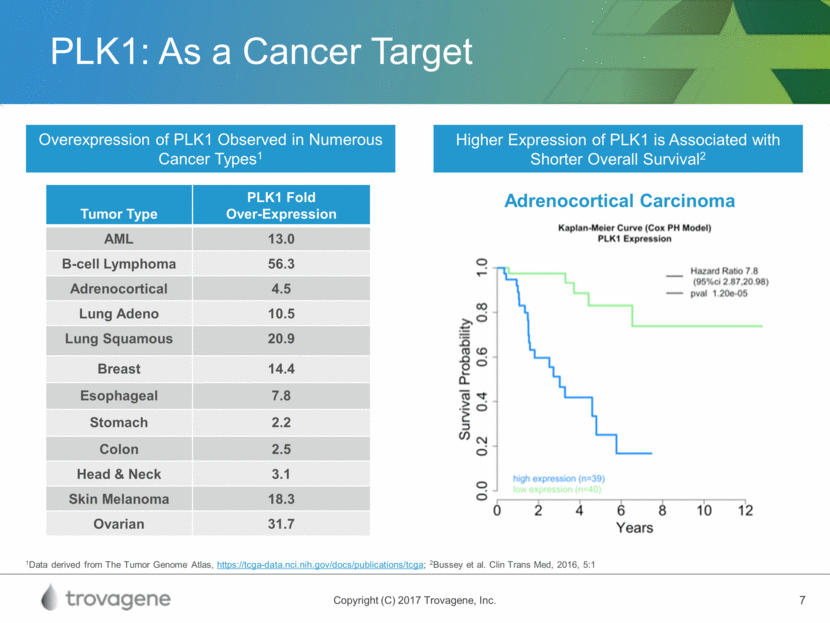
PLK1: As a Cancer Target Higher Expression of PLK1 is Associated with Shorter Overall Survival2 Overexpression of PLK1 Observed in Numerous Cancer Types1 Adrenocortical Carcinoma 1Data derived from The Tumor Genome Atlas, https://tcga-data.nci.nih.gov/docs/publications/tcga; 2Bussey et al. Clin Trans Med, 2016, 5:1 Tumor Type PLK1 Fold Over-Expression AML 13.0 B-cell Lymphoma 56.3 Adrenocortical 4.5 Lung Adeno 10.5 Lung Squamous 20.9 Breast 14.4 Esophageal 7.8 Stomach 2.2 Colon 2.5 Head & Neck 3.1 Skin Melanoma 18.3 Ovarian 31.7
PCM-075 Attributes Highly Selective Short Half-Life Acceptable Safety Profile Broad Applicability Orally Available
PCM-075 Biochemical Profile Selective, adenosine triphosphate (ATP) competitive PLK1 inhibitor Selectivity driven by polar interaction with the carboxyl side chain of Glutamate 140 position of PLK1 Tested against over 260 kinases and PLK1 was the only active target with IC50 of 2nM All other kinase hits at IC50’s of ~500nM to 10uM and above Polo-like kinase 1 (PLK1) inhibitor PLK Member PCM-075 IC50 (nM)1 PLK1 2 PLK2 > 10,000 PLK3 > 10,000 1Data on File, Trovagene, Inc.
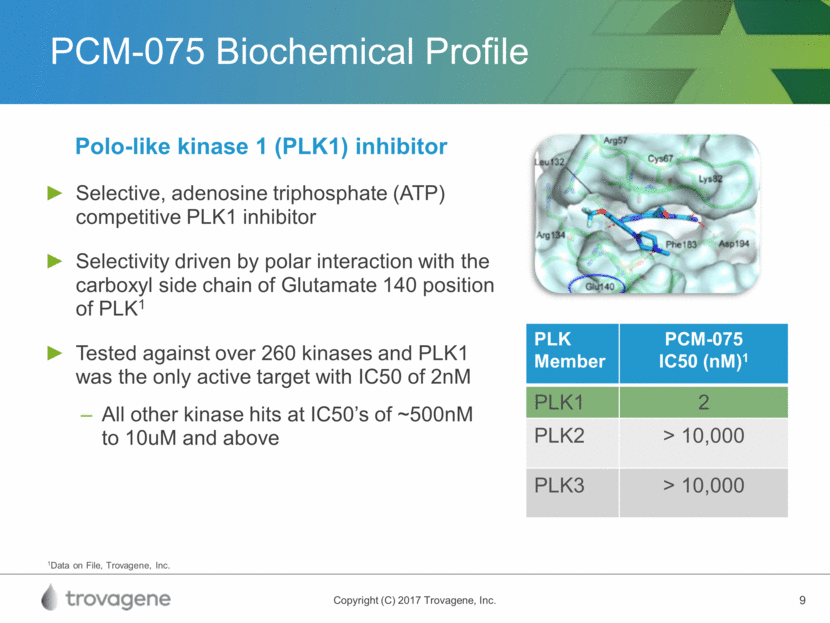
PCM-075 Mechanism of Action Induces a cellular phenotype of G2/M, leading to cell death Clear evidence of in-vitro pathway modulation in AML Mitotic inhibitor demonstrating anti-proliferative activity* AML-NS8 Patient-Derived Cells Treated with 200 nM PCM-075 for 24 Hrs1 * In numerous human cell lines; 1Casolaro et al (2013) PLOS One PCM-075 PLK1 blocking
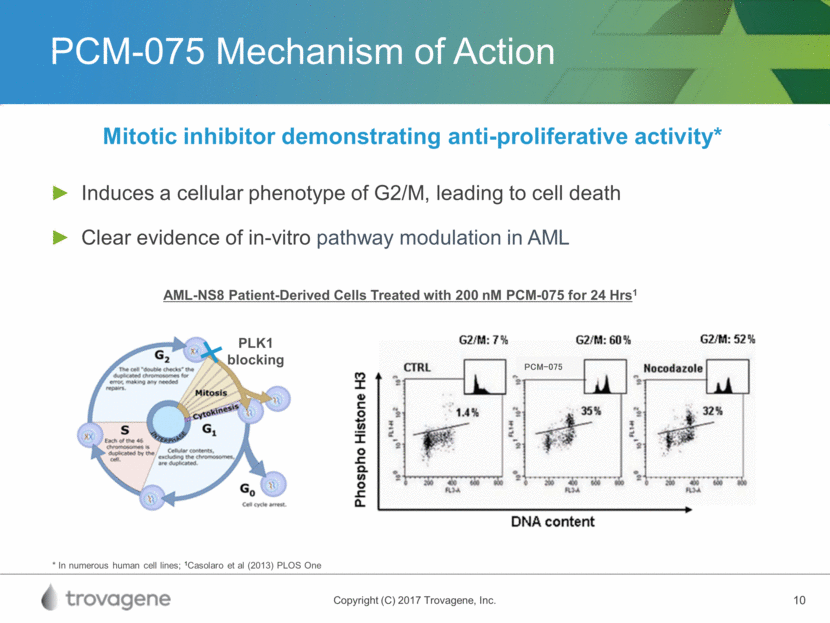
PCM-075 In-Vivo Activity Response observed in various xenograft models as a single agent and in combination1 Compound Median Survival Time (days) %TGI Placebo 28 Cytarabine 38 128.6 PCM-075 62 221.4 In Vivo Disseminated Leukemia Model (AML-NS8 Cells) Treatment with PCM-075 Started 20 Days Post-Inoculation 60 mg/kg BID (Days 1 and 2, 3 Cycles) 1Casolaro et al (2013) PLOS One Vehicle PCM-075 60 mg/kg BID Cytarabine 75 mg/kg Vehicle PCM-075 60 mg/kg BID Cytarabine 75 mg/kg
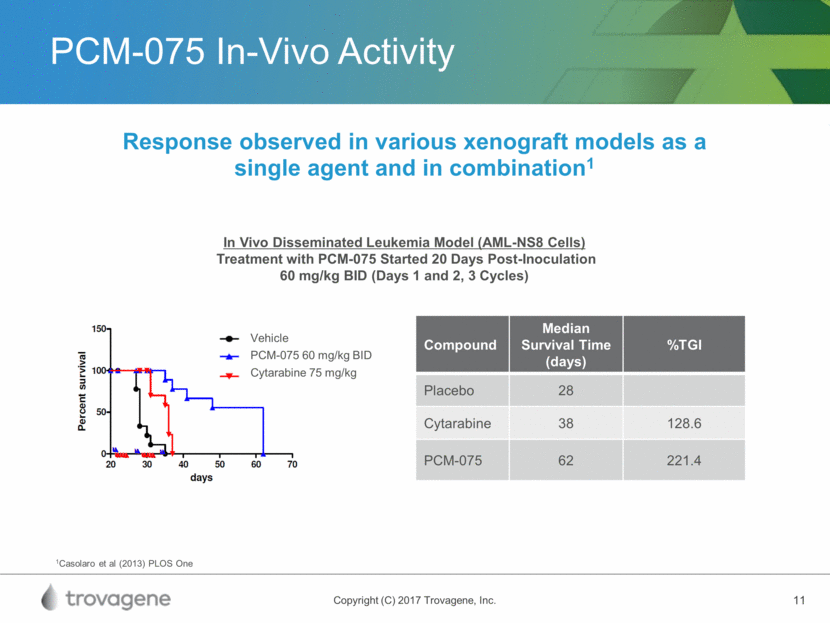
Phase 1 Study Completed in Solid Tumors* Phase 1 Study Design 19 of 21 patients enrolled administered PCM-075 orally, once daily for 5 consecutive days, every 3 weeks PCM-075 Study Results Included: colorectal, pancreatic, lung, sarcomas, hepatocellular, ampullary, prostate, ovarian, skin Open-label dose escalation trial in patients with solid tumor malignancies Established safety of PCM-075 and identified a recommended Phase 2 dose of 24 mg/m2/day 16 of the 19 patients (84.2%) treated with PCM-075, were evaluable for efficacy, with stable disease at any dose observed in 5 (31.2%) of the patients Reversible, on-target thrombocytopenia and neutropenia, consistent with the expected mechanism of action, were the primary adverse events No GI disorders, mucositis, or alopecia was observed, confirming that bone marrow cells are the most sensitive to PCM-075 inhibition with the applied dosing schedule Phase 1 dose escalation study in patients with advanced or metastatic solid tumors * Phase 1 Dose-Escalation Study of NMS-1286937, an Orally Available Polo-Like Kinase 1 Inhibitor,.
Acute Myeloid Leukemia Market Opportunity1 AML: aggressive hematologic malignancy Incidence: 20,000* new cases and 10,400 deaths annually in the U.S. Prognosis: 5 year survival rate is 25% Treatment options vary based on patient condition / age, but can include: Chemotherapy Radiation Stem cell transplant Genetically diverse landscape: AML is known to be genetically diverse and PLK1 selectivity presents opportunity to work across patient sub-populations *Orphan Drug Designation granted by the FDA September 28, 2017; 1National Cancer Institute SEER 2016
PLK Inhibition in AML Pan-PLK inhibitor demonstrated increased response rates, improvements in event-free survival, and overall survival benefit in combination with low-dose cytarabine (LDAC)1 We believe that the adverse events observed in Phase 3 study were due to drug accumulation and pan-PLK inhibitor activity Opportunity for a more selective PLK inhibitor with less drug accumulation paired with a precision medicine diagnostic Clinical activity published on PLK inhibitors Compound Selectivity Half-Life (hrs) Dosing Clinical Activity in AML Volasertib pan-PLK 135 iv 25-30% CR+CRi (+LDAC)* PCM-075 PLK1 24 oral P1b/2 Trial *higher incidence of severe adverse events with volasertib plus LDAC in phase 3 study. 1Dohner et al. Randomized, Phase 2 Trial of Cytarabine with or without Volasertib in AML Patients Not Suitable for Induction Therapy. 2Phase 3 POLO-AML-2 Study of Volasertib – June 11, 2016 Press Release
PCM-075 Phase 1b/2 AML Clinical Trial PCM-075 in Combination with Standard-of-Care (SOC) in Subjects with Acute Myeloid Leukemia (AML) Principal Investigator – Dr. Jorge Cortes, MD Anderson Open-label trial to evaluate safety and anti-leukemic activity Phase 1b: dose escalation in combination with SOC to assess safety, tolerability, dose and scheduling, and determine recommended dose for Phase 2 continuation trial Phase 2: administration of recommended clinical dose in combination with SOC to further assess safety, tolerability and evaluate anti-leukemic activity Patient Selection and Dosing Schedule Phase 1b: Up to 42 patients with relapsed refractory disease up to 3 prior regimens Starting dose of 12 mg/m2, administered orally, in combination with SOC, for 5 consecutive days on Day 1 – Day 5, every 28-days in 3-patient cohorts Dose escalation in 50% increments, in subsequent cohorts, to achieve the maximum tolerated dose (MTD), or recommended Phase 2 dose Phase 2: Up to 32 patients newly diagnosed, treatment naïve, and ineligible for, or have refused, standard intensive induction therapy or have relapsed or refractory disease to 1 prior regimen Recommended Phase 2 dose and scheduling based on Phase 1b results
PCM-075 Phase 1b/2 AML Clinical Trial Primary Endpoints Safety: characterization of does-limiting toxicities (DLTs), adverse events (AEs) by type, incidence, severity, seriousness and relationship to treatment effects on vital signs and laboratory parameters Efficacy: Rate of complete response (CR + CRi) in Phase 2, defined as morphologic leukemia-free state (MLF) plus: For CR: Patient is independent of transfusions Absolute neutrophil count (ANC) of >1000/mm3 Platelets of >100,000/mm3 For CRi: Meets all criteria for CR except for either neutropenia (ANC <1000/mm3) or thrombocytopenia (<100,000/mm3) Exploratory Endpoints Pharmacodynamic and diagnostic correlative biomarker evaluation Evaluation of the rate of complete response (CR + CRi) PCM-075 in Combination with Standard-of-Care (SOC) in Subjects with Acute Myeloid Leukemia (AML) Principal Investigator – Dr. Jorge Cortes, MD Anderson
Phase 1b/2 Biomarker Assessment1 Biomarkers will be measured and correlated with pharmacokinetic drug levels to assess: Inhibition of PLK1 enzymatic activity by assessing inhibition of TCTP phosphorylation Blockage of cell division (G2/M arrest) Tumor DNA biomarkers Blood sample (baseline) AML Patient + PCM-075 Blood sample (post PCM-075) Pre- and Post-Dose Blood Sampling 1TruSight Myeloid Sequencing Panel; Illumina
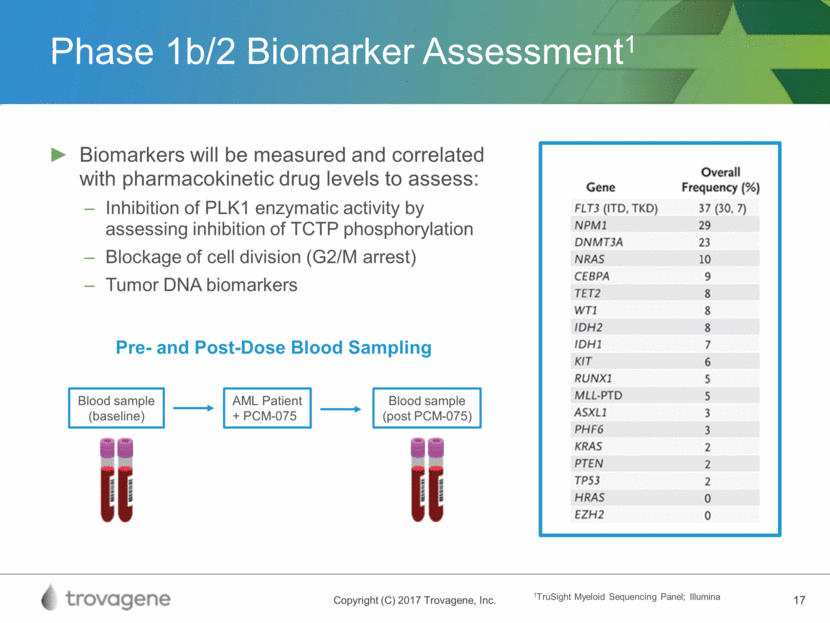
PLK1 Enzymatic Activity Biomarker for PCM-075 inhibition1 PCM-075 (µM): 0 .01 .032 .1 .32 1 3 10 pTCTP Total TCTP 1Trovagene, data on file PLK1 activity: as measured by pTCTP (Translationally Controlled Tumor Protein) which is activated by PLK1 1Trovagene Data on file Vehicle (DMSO) PCM-075 Positive Control (Nocodazole) Blockage of Cell Division by PCM-075 G2/M Arrest by Analysis of DNA Content PLK1 Enzymatic Activity Inhibited by PCM-075 PLK1 activity: as measured by G2/M arrest of cell division
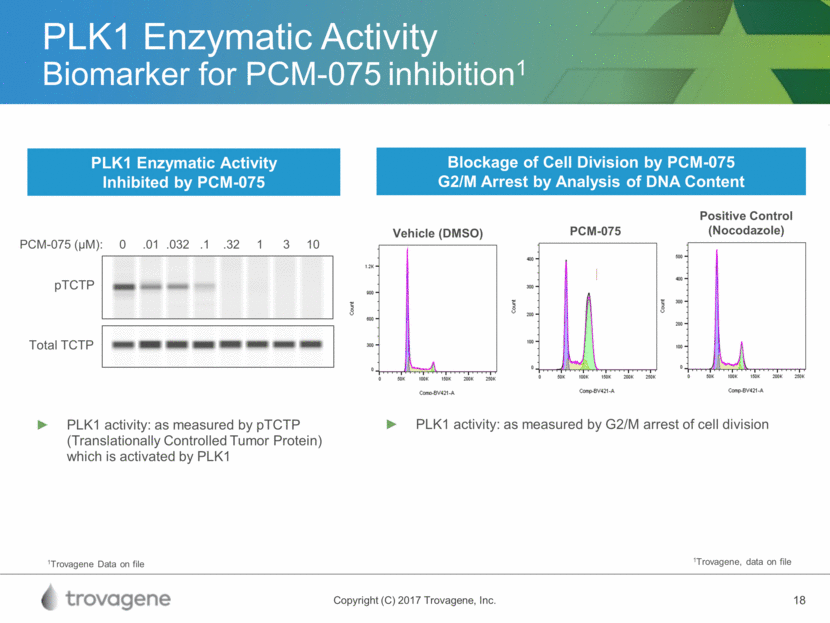
Molecular Profiling and Patient Response AML Genomic Subgroup Frequency of Patients Most Frequently Mutated Genes (%) DNA Panel RNA Panel NPM1 mutation 27% NPM1(100), DNMT3A(54), FLT3(39), NRAS(19), TET2(16), PTPN11(15) Mutated chromatin, RNA-splicing genes, or both 18% RUNX1(39), MLLPTD(25), SRSF2(22), DNMT3A(20), ASXL1(17), STAG2(16), NRAS(16),TET2(15),FLT3ITD(15) TP53mutations, chromosomal aneuploidy, or both 13% Complex karyotope(68), -5/5q(47), -7/7q(44), TP53(44), -17/17p(31), +8/8q(16) inv(16)(p13.1q22) or t(16;16)(p13.1;q22);CBFB-MYH11 5% inv(16) (100), NRAS(53), +8/8q(16), KIT(15), FLT3TKD(15) biallelic CEBPA mutations 4% CEBPAbiallelic(100), NRAS(30), WT1(21), GATA2(20) t(15;17)(q22;q12); PML-RARA 4% t(15;17) (100), FLT3 ITD(35), WT1(17) t(8;21)(q22;q22); RUNX1-RUNX1T1 4% t(8;21) (100), KIT(38), -Y(33), -9q(18) MLL fusion genes; t(x;11)(x;q23) 3% t(x;11q23) (100), NRAS(23) inv(3)(q21q26.2) or t(3;3)(q21;q26.2); GATA2,MECOM(EVI1) 1% inv(3) (100), -7(85), KRAS(30), NRAS(30), PTPN11(30), ETV6(15), PHF6(15), SF3B1(15) IDH2R172 mutations and no other class-defining lesions 1% IDH2R172(100), DNMT3A(67), +8/8q(17) t(6;9)(p23;q34); DEK-NUP214 1% t(6;9) (100), FLT3ITD(80), KRAS(20) Papaemmanuil et al. Genomic classification and prognosis in acute myeloid leukemia. NEJM 2016;374:2209-2221.
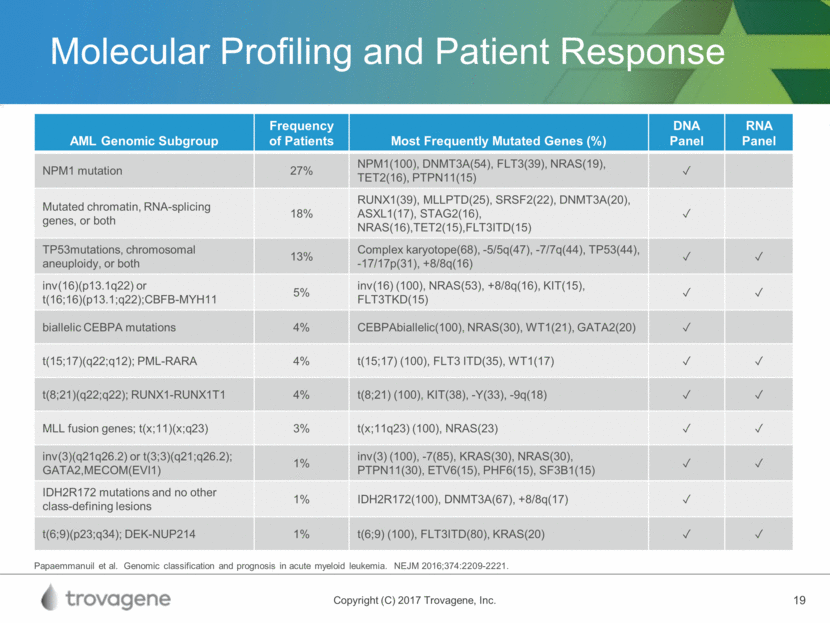
Combination Therapy Opportunities High PLK1 expression is associated with the most aggressive forms of hematologic malignancies and solid tumor cancers PCM-075 may be synergistic and enhance the efficacy of current and future standard-of-care therapies 1Alphabetical order. 2Preclinical data on file with PCM-075 and these combined therapeutics Potentially Synergistic Drugs1,2 Bortezomib Cisplatin Cytarabine Doxorubicin Gemcitabine HDAC Inhibitors Pacitaxel Quizartinib (FLT3) Associated Cancers2 Liquid Tumors: Acute Myeloid Leukemia Acute Lymphocytic Leukemia Non-Hodgkin Leukemia Multiple Myeloma Solid Tumors Castration-Resistant Prostate Adrenocortical Carcinoma Triple Negative Breast Sarcomas Small Cell Lung
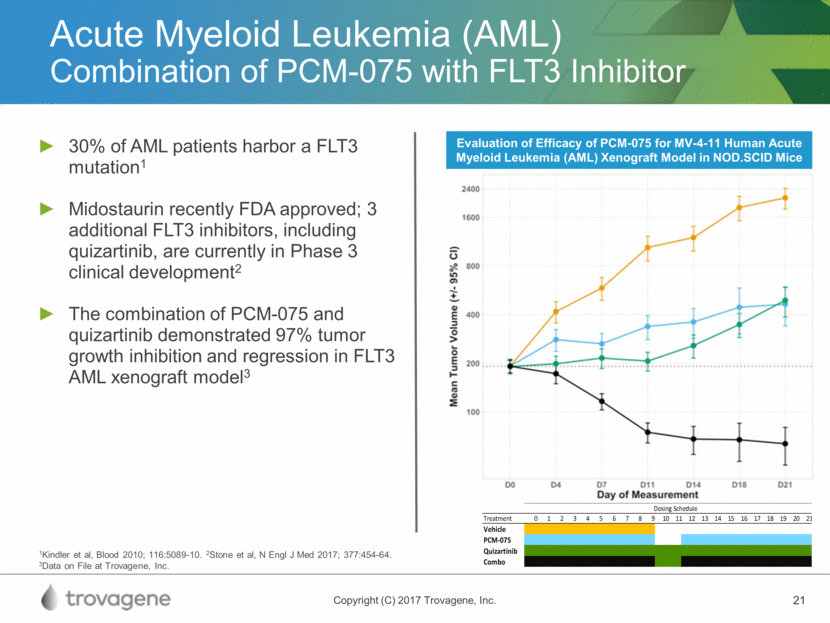
Acute Myeloid Leukemia (AML) Combination of PCM-075 with FLT3 Inhibitor Evaluation of Efficacy of PCM-075 for MV-4-11 Human Acute Myeloid Leukemia (AML) Xenograft Model in NOD.SCID Mice 1Kindler et al, Blood 2010; 116:5089-10. 2Stone et al, N Engl J Med 2017; 377:454-64. 3Data on File at Trovagene, Inc. 30% of AML patients harbor a FLT3 mutation1 Midostaurin recently FDA approved; 3 additional FLT3 inhibitors, including quizartinib, are currently in Phase 3 clinical development2 The combination of PCM-075 and quizartinib demonstrated 97% tumor growth inhibition and regression in FLT3 AML xenograft model3 T r e a t m e n t 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 2 1 V e h i c l e P C M - 0 7 5 Q u i z a r t i n i b C o m b o D o s i n g S c h e d u l e
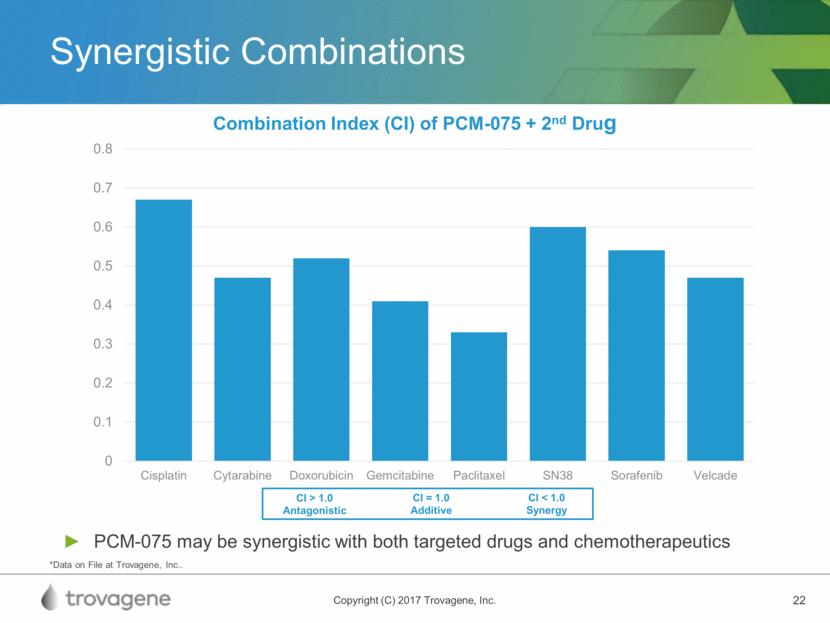
Synergistic Combinations PCM-075 may be synergistic with both targeted drugs and chemotherapeutics CI > 1.0 Antagonistic CI < 1.0 Synergy CI = 1.0 Additive *Data on File at Trovagene, Inc..
Metastatic Castration-Resistant Prostate Cancer (mCRPC) Market Opportunity 25,000 men progress to metastatic prostate cancer resistant to standard androgen-deprivation therapy, anually1 Five-year survival rate of 37%2 Risk of metastases increases as the disease progresses; most common metastases are adrenal gland, bone, and lung3 Treatments Abiraterone (Zytiga® – Johnson & Johnson) and prednisone Enzalutamide (Xtandi® – Astellas/Pfizer) Docetaxel (Docefrez, Taxotere) and prednisone Ongoing need to increase duration of response for mCRPC patients Patients develop resistance to abiraterone and enzalutamide (within 9-15 months)4 and do not respond well to subsequent therapies Further testing suggested to determine whether PCM-075 in combination with abiraterone would prolong response to anti-androgen therapy 12017 Annual Report on Prostate Disease – Harvard Health Publications; 2GlobalData. Prostate Cancer—Global Drug Forecast and Market Analysis to 2023. Apr, 2015; 3 National Cancer Institute Metastatic cancer. Mar, 2013. Available at: http://www.cancer.gov/about-cancer/what-is-cancer/metastatic-fact-sheet; 4GAntonarakis, Emmannel – Current Understanding of Resistance to Abiraterone and Enzalutamide in Advanced Prostate Cancer; Clinical Advances in Hematology & Oncology – May 2016 – Volume 14, Issue 5
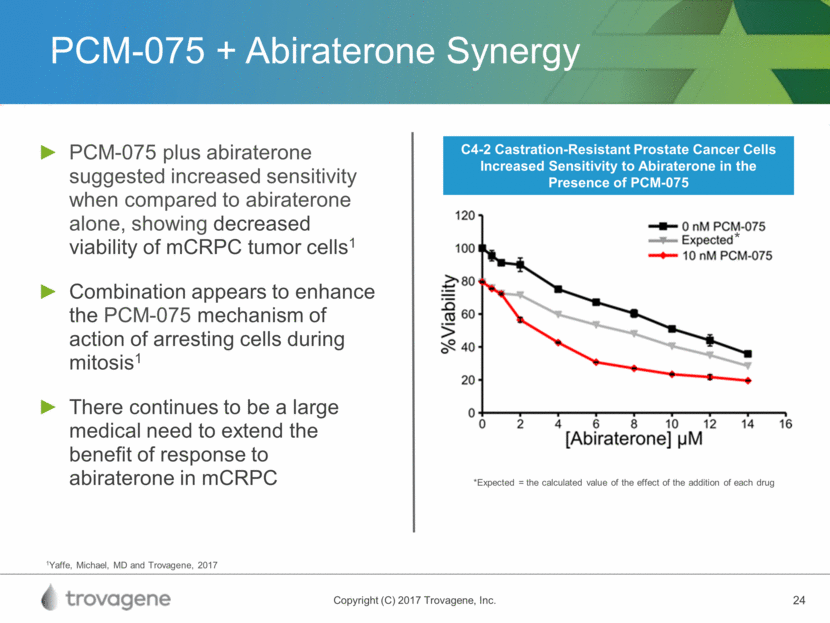
PCM-075 + Abiraterone Synergy PCM-075 plus abiraterone suggested increased sensitivity when compared to abiraterone alone, showing decreased viability of mCRPC tumor cells1 Combination appears to enhance the PCM-075 mechanism of action of arresting cells during mitosis1 There continues to be a large medical need to extend the benefit of response to abiraterone in mCRPC 1Yaffe, Michael, MD and Trovagene, 2017 C4-2 Castration-Resistant Prostate Cancer Cells Increased Sensitivity to Abiraterone in the Presence of PCM-075 *Expected = the calculated value of the effect of the addition of each drug *
mCRPC Path Forward Active IND for PCM-075 in solid tumors Recommended Phase 2 (RP2) study dose of 24 mg/m2 Dosing Schedule: 21 day cycle, 5 days on and 16 days off Engaging key opinion leaders in metastatic castration resistant prostate cancer (mCRPC) to develop Phase 2 clinical trial protocol Combination PCM-075 and abiraterone (oral dosing of four 250mg once daily plus prednisone 5mg twice daily) Patient eligibility: progressing on abiraterone as observed by rising Prostate Specific Antigen (PSA) levels Initial study structure: Open label with ~40 patients Measure PSA levels to evaluate efficacy response; progression-free survival and stable disease
Established Drug Class PCM-075 Value Proposition Demonstrated Safety PLK1-selective inhibitor in clinical development and Orphan Drug Designation for the treatment of acute myeloid leukemia (AML) Demonstrated synergy across multiple chemo and targeted therapies Clinical Activity Drug class demonstrated combined 25-30% response in Phase 2 studies in Acute Myeloid Leukemia (AML) when used in conjunction with standard therapy Phase 1b/2 clinical trial ongoing Highly-Selective Completed Phase 1 trial showing potential anti-tumor activity, acceptable safety profile and providing recommended dosage for additional studies Preclinical and clinical data supporting PLK1 as a potential cancer drug target
PCM-075 Value Inflection Points Acute Myeloid Leukemia Phase 1b/2 Trial Initiate patient enrollment and dose escalation in Q4’17 Data release of Phase 1b results in Q2’18 with subsequent Phase 2 trial initiated (pending number of dose escalation cohorts studied) Publication of pharmacokinetic and pharmacodynamic data from open-label trial throughout 2018 Data release of Phase 2 results Q4’18 – Q2’19 Clinical Development in Other Cancers Announce second clinical indication with FDA protocol submission in Q1’18 (castration-resistant prostate cancer) Commercialization Precision Diagnostics royalties and business development Precision Therapeutics business development Presentations and publications
Thank you for more information, please email: ir@trovagene.com

Appendix Slides

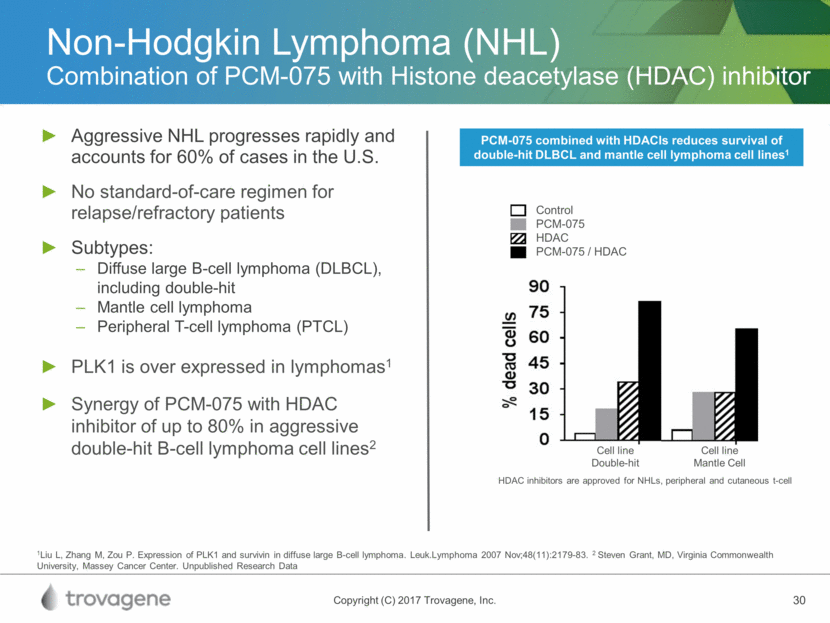
Non-Hodgkin Lymphoma (NHL) Combination of PCM-075 with Histone deacetylase (HDAC) inhibitor Aggressive NHL progresses rapidly and accounts for 60% of cases in the U.S. No standard-of-care regimen for relapse/refractory patients Subtypes: Diffuse large B-cell lymphoma (DLBCL), including double-hit Mantle cell lymphoma Peripheral T-cell lymphoma (PTCL) PLK1 is over expressed in lymphomas1 Synergy of PCM-075 with HDAC inhibitor of up to 80% in aggressive double-hit B-cell lymphoma cell lines2 1Liu L, Zhang M, Zou P. Expression of PLK1 and survivin in diffuse large B-cell lymphoma. Leuk.Lymphoma 2007 Nov;48(11):2179-83. 2 Steven Grant, MD, Virginia Commonwealth University, Massey Cancer Center. Unpublished Research Data PCM-075 combined with HDACIs reduces survival of double-hit DLBCL and mantle cell lymphoma cell lines1 Cell line Double-hit Cell line Mantle Cell Control PCM-075 HDAC PCM-075 / HDAC HDAC inhibitors are approved for NHLs, peripheral and cutaneous t-cell
Adrenocortical Carcinoma (ACC) Market Opportunity Adrenocortical carcinoma (ACC) is a rare cancer with significant unmet clinical needs Incidence: 0.7 to 2 cases per 1 million population per year1,2 Prognosis: 5-year survival in patients with metastatic disease is <15%3-7 40-70% of patients present with advanced or metastatic disease Patients with resectable tumors have 5-year survival of 40% Treatment is mitotane, a highly toxic adrenolytic chemotherapy, approved in 1960 and remains the only approved therapy There is no treatment standard for advanced disease8,9 1Golden et al., J Clin Endocrinol Metab 2009;94:1853-78; 2Kebebew et al., World J Surg 2006;30:872-8; 3Icard et al, World J Surg 2001;25:891-7; 4Abiven et al., J Clin Endocrinol Metab 2006;91:2650-5; 5Assié G et al., J Clin Endocrinol Metab 2007;92:148-54; 6Fassnacht et al., Cancer 2009;115:243-50; 7Lughezzani et al., Eur J Cancer 2010;46:713-9; 8Fay et al., Crit Rev Oncol Hematol. 2014; 92: 123–132; 9Fassnacht et al, NEJM, 2012; 366:2189-2197 Malignant cells form in the outer layer of the adrenal gland
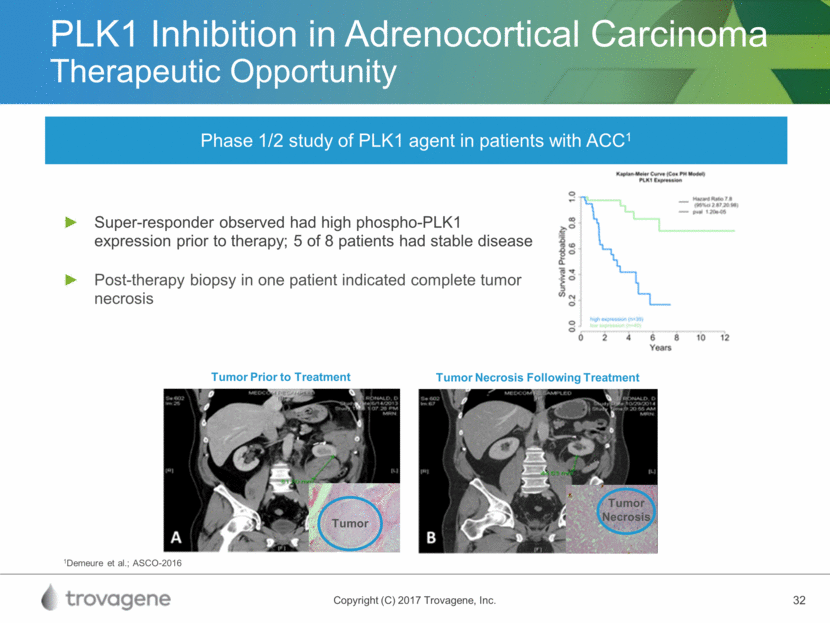
PLK1 Inhibition in Adrenocortical Carcinoma Therapeutic Opportunity 1Demeure et al.; ASCO-2016 Tumor Prior to Treatment Tumor Necrosis Following Treatment Super-responder observed had high phospho-PLK1 expression prior to therapy; 5 of 8 patients had stable disease Post-therapy biopsy in one patient indicated complete tumor necrosis Phase 1/2 study of PLK1 agent in patients with ACC1 Tumor Tumor Necrosis