Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - KERYX BIOPHARMACEUTICALS INC | d480884dex993.htm |
| EX-99.1 - EX-99.1 - KERYX BIOPHARMACEUTICALS INC | d480884dex991.htm |
| 8-K - FORM 8-K - KERYX BIOPHARMACEUTICALS INC | d480884d8k.htm |

U.S. FDA approval of Additional Indication for AURYXIA® (ferric citrate) TABLETS Keryx Biopharmaceuticals, Inc 11/7/2017 www.keryx.com Exhibit 99.2

Agenda Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Topic Speakers Welcome Amy Sullivan, SVP, Corporate Affairs Today’s Announcements & Importance of Auryxia® Greg Madison, President & CEO 3Q 2017 Results Scott Holmes, CFO Auryxia’s Broad Label John Neylan, M.D., Chief Medical Officer Commercial Strategy & Launch Focus Doug Jermasek, VP, Marketing & Strategy Question & Answer All

Forward-Looking Statements Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Some of the statements included in this presentation, particularly those regarding the commercialization and ongoing clinical development of Auryxia may be forward-looking statements that involve a number of risks and uncertainties. For those statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. Among the factors that could cause our actual results to differ materially are the following: our ability to successfully market Auryxia and whether we can increase adoption of Auryxia in patients with CKD on dialysis and successfully launch Auryxia for the treatment of iron deficiency anemia in patients with chronic kidney disease, not on dialysis; whether we can maintain our operating expenses to projected levels while continuing our current clinical, regulatory and commercial activities; our ability to continue to supply Auryxia to the market; the risk that increased utilization by Medicare Part D subscribers will increase our gross-to-net adjustment greater than we anticipate; and other risk factors identified from time to time in our reports filed with the Securities and Exchange Commission. Any forward looking statements set forth in this press release speak only as of the date of this press release. We do not undertake to update any of these forward-looking statements to reflect events or circumstances that occur after the date hereof. The third quarter 2017 press release and prior releases are available at http://www.keryx.com. The information found on our website is not incorporated by reference into this presentation and is included for reference purposes only.

Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Today’s Announcements Greg Madison Chief Executive Officer

Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Today’s Announcements Building a leading kidney care company

Copyright © 2017 by Keryx Biopharmaceuticals, Inc. 3Q 2017 Financial Results Scott Holmes Chief Financial Officer
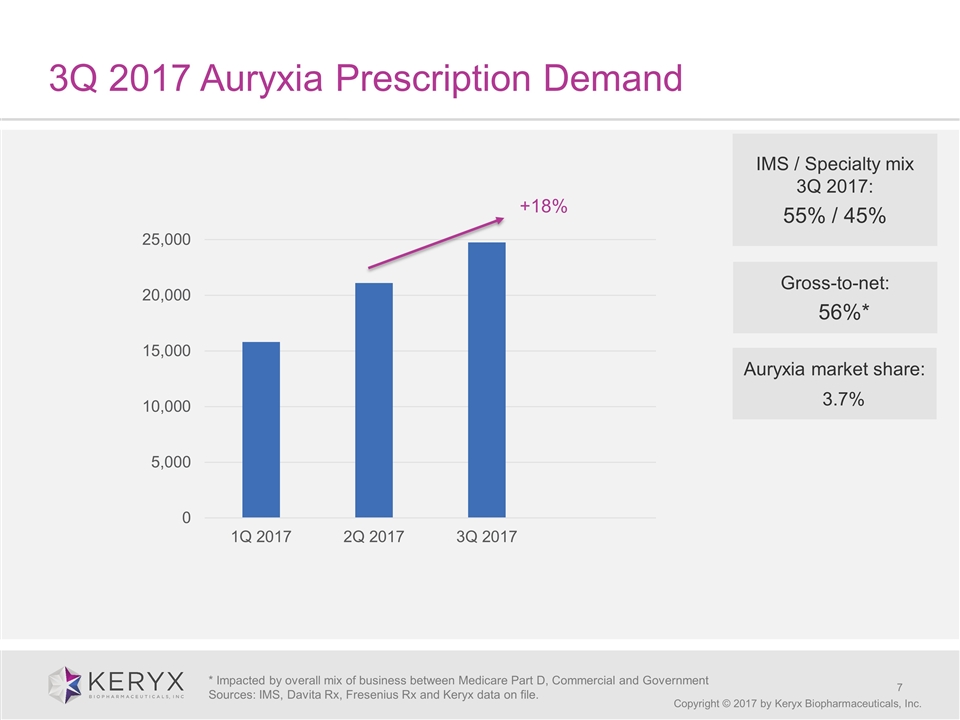
3Q 2017 Auryxia Prescription Demand Copyright © 2017 by Keryx Biopharmaceuticals, Inc. IMS / Specialty mix 3Q 2017: 55% / 45% * Impacted by overall mix of business between Medicare Part D, Commercial and Government Sources: IMS, Davita Rx, Fresenius Rx and Keryx data on file. Gross-to-net: 56%* +18% Auryxia market share: 3.7%
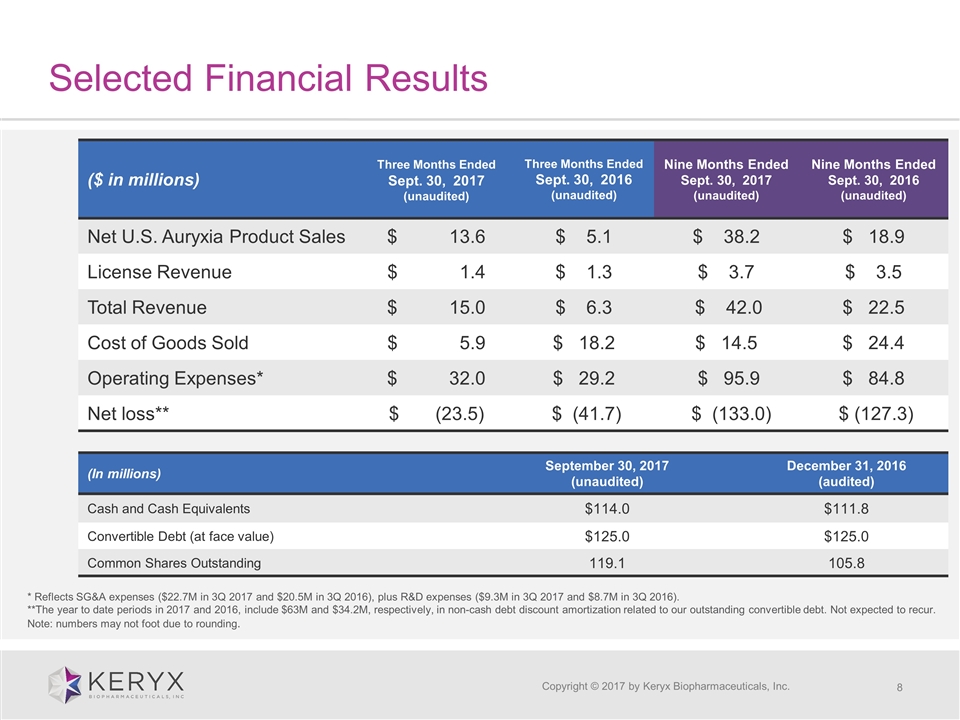
Selected Financial Results Copyright © 2017 by Keryx Biopharmaceuticals, Inc. ($ in millions) Three Months Ended Sept. 30, 2017 (unaudited) Three Months Ended Sept. 30, 2016 (unaudited) Nine Months Ended Sept. 30, 2017 (unaudited) Nine Months Ended Sept. 30, 2016 (unaudited) Net U.S. Auryxia Product Sales $ 13.6 $ 5.1 $ 38.2 $ 18.9 License Revenue $ 1.4 $ 1.3 $ 3.7 $ 3.5 Total Revenue $ 15.0 $ 6.3 $ 42.0 $ 22.5 Cost of Goods Sold $ 5.9 $ 18.2 $ 14.5 $ 24.4 Operating Expenses* $ 32.0 $ 29.2 $ 95.9 $ 84.8 Net loss** $ (23.5) $ (41.7) $ (133.0) $ (127.3) (In millions) September 30, 2017 (unaudited) December 31, 2016 (audited) Cash and Cash Equivalents $114.0 $111.8 Convertible Debt (at face value) $125.0 $125.0 Common Shares Outstanding 119.1 105.8 * Reflects SG&A expenses ($22.7M in 3Q 2017 and $20.5M in 3Q 2016), plus R&D expenses ($9.3M in 3Q 2017 and $8.7M in 3Q 2016). **The year to date periods in 2017 and 2016, include $63M and $34.2M, respectively, in non-cash debt discount amortization related to our outstanding convertible debt. Not expected to recur. Note: numbers may not foot due to rounding.

Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Importance of Auryxia® Greg Madison Chief Executive Officer

Auryxia® (ferric citrate) Tablets Copyright © 2017 by Keryx Biopharmaceuticals, Inc. New Indication: treatment of iron deficiency anemia in adults with chronic kidney disease (CKD), not on dialysis Broad label supports use of Auryxia as a first-line treatment option Auryxia’s expanded indication now gives us the opportunity to help millions of people living with iron deficiency anemia and CKD Only oral iron medicine designed, studied and approved specifically for the treatment of iron deficiency anemia in CKD patients
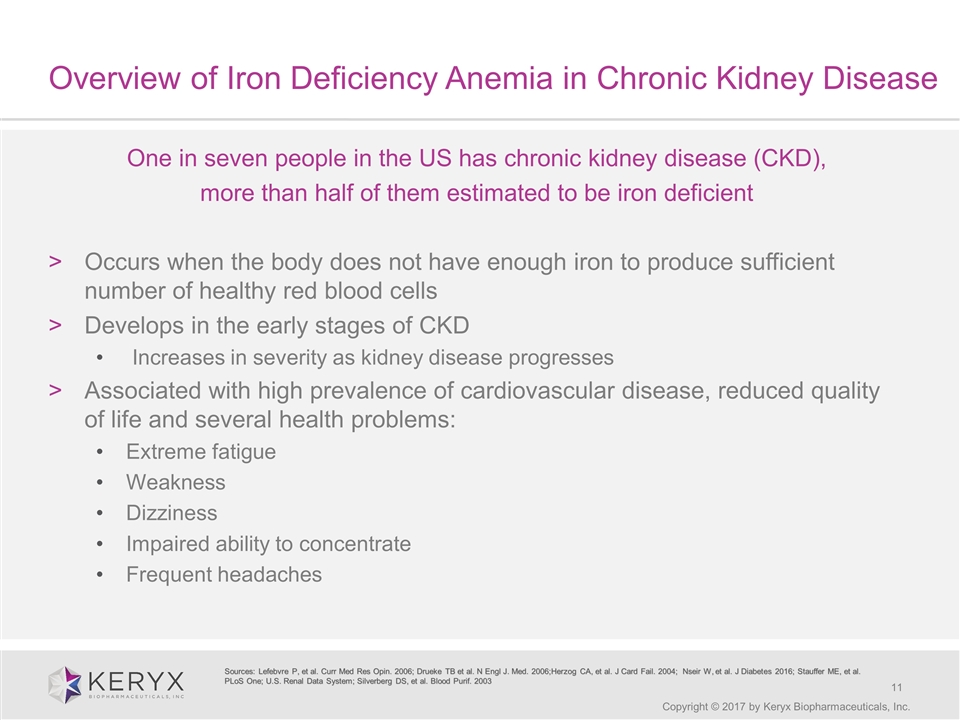
Overview of Iron Deficiency Anemia in Chronic Kidney Disease Copyright © 2017 by Keryx Biopharmaceuticals, Inc. One in seven people in the US has chronic kidney disease (CKD), more than half of them estimated to be iron deficient Occurs when the body does not have enough iron to produce sufficient number of healthy red blood cells Develops in the early stages of CKD Increases in severity as kidney disease progresses Associated with high prevalence of cardiovascular disease, reduced quality of life and several health problems: Extreme fatigue Weakness Dizziness Impaired ability to concentrate Frequent headaches Sources: Lefebvre P, et al. Curr Med Res Opin. 2006; Drueke TB et al. N Engl J. Med. 2006;Herzog CA, et al. J Card Fail. 2004; Nseir W, et al. J Diabetes 2016; Stauffer ME, et al. PLoS One; U.S. Renal Data System; Silverberg DS, et al. Blood Purif. 2003
Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Auryxia’s Profile John Neylan, M.D. Chief Medical Officer
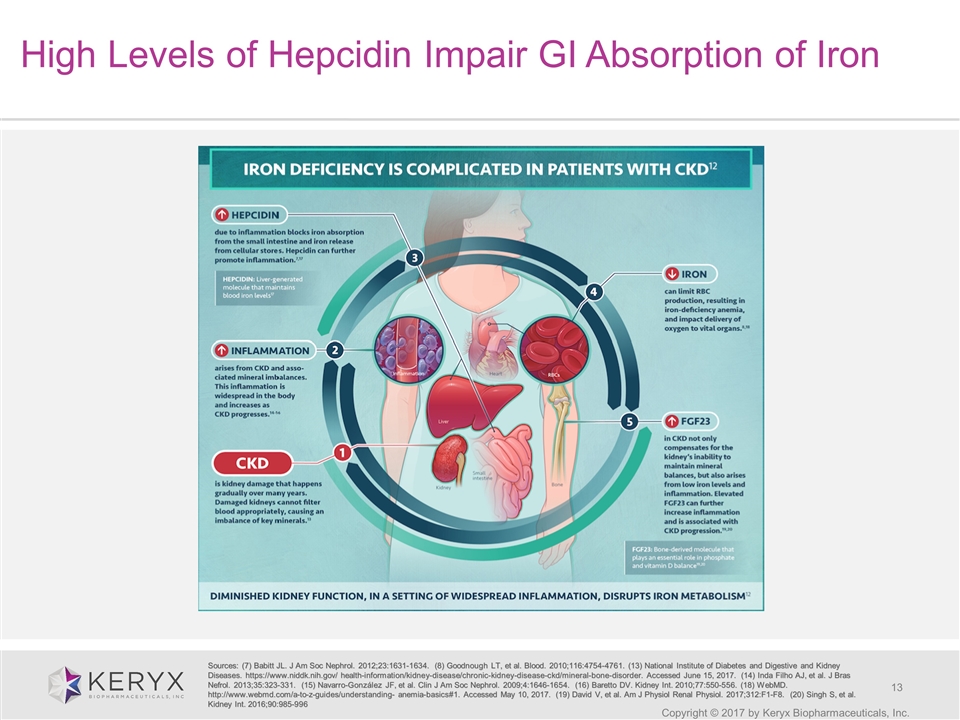
Copyright © 2017 by Keryx Biopharmaceuticals, Inc. High Levels of Hepcidin Impair GI Absorption of Iron Sources: (7) Babitt JL. J Am Soc Nephrol. 2012;23:1631-1634. (8) Goodnough LT, et al. Blood. 2010;116:4754-4761. (13) National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/ health-information/kidney-disease/chronic-kidney-disease-ckd/mineral-bone-disorder. Accessed June 15, 2017. (14) Inda Filho AJ, et al. J Bras Nefrol. 2013;35:323-331. (15) Navarro-González JF, et al. Clin J Am Soc Nephrol. 2009;4:1646-1654. (16) Baretto DV. Kidney Int. 2010;77:550-556. (18) WebMD. http://www.webmd.com/a-to-z-guides/understanding- anemia-basics#1. Accessed May 10, 2017. (19) David V, et al. Am J Physiol Renal Physiol. 2017;312:F1-F8. (20) Singh S, et al. Kidney Int. 2016;90:985-996
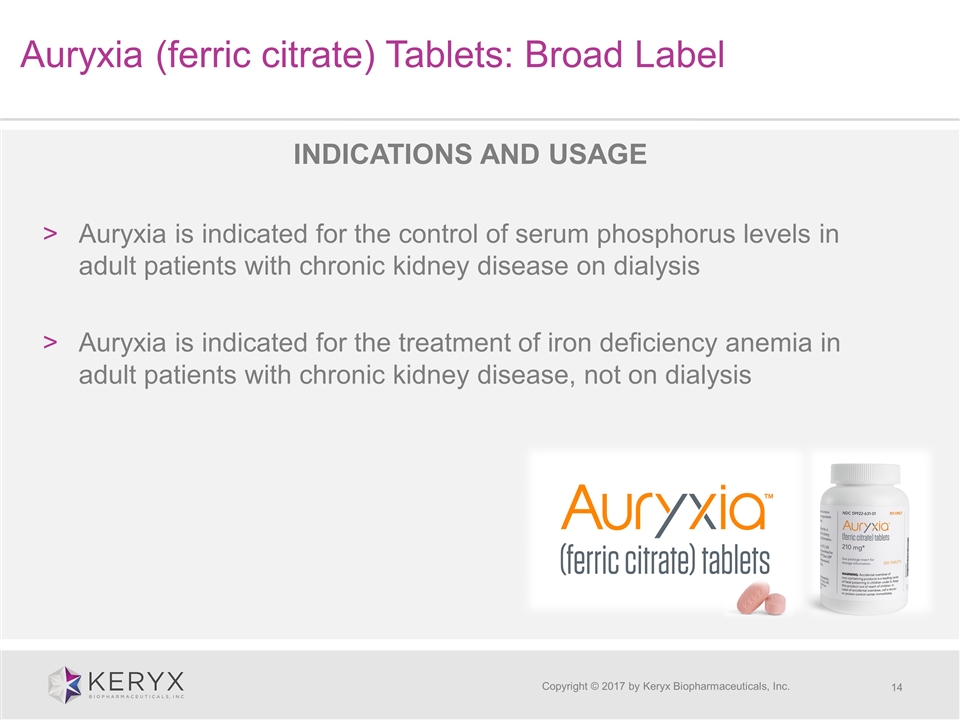
Copyright © 2017 by Keryx Biopharmaceuticals, Inc. INDICATIONS AND USAGE Auryxia is indicated for the control of serum phosphorus levels in adult patients with chronic kidney disease on dialysis Auryxia is indicated for the treatment of iron deficiency anemia in adult patients with chronic kidney disease, not on dialysis Auryxia (ferric citrate) Tablets: Broad Label
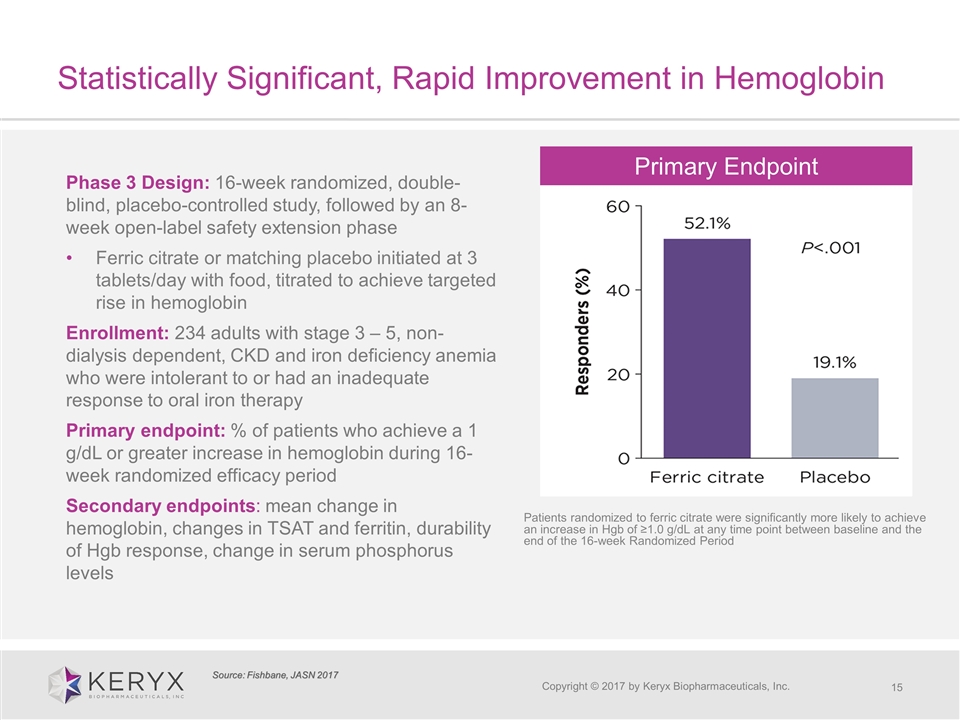
Statistically Significant, Rapid Improvement in Hemoglobin Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Phase 3 Design: 16-week randomized, double-blind, placebo-controlled study, followed by an 8-week open-label safety extension phase Ferric citrate or matching placebo initiated at 3 tablets/day with food, titrated to achieve targeted rise in hemoglobin Enrollment: 234 adults with stage 3 – 5, non-dialysis dependent, CKD and iron deficiency anemia who were intolerant to or had an inadequate response to oral iron therapy Primary endpoint: % of patients who achieve a 1 g/dL or greater increase in hemoglobin during 16-week randomized efficacy period Secondary endpoints: mean change in hemoglobin, changes in TSAT and ferritin, durability of Hgb response, change in serum phosphorus levels Source: Fishbane, JASN 2017 Patients randomized to ferric citrate were significantly more likely to achieve an increase in Hgb of ≥1.0 g/dL at any time point between baseline and the end of the 16-week Randomized Period Primary Endpoint

Auryxia® Safety Profile Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Source: Fishbane, JASN 2017 Pivotal Phase 3 study: Auryxia was well tolerated Majority of adverse events were mild to moderate Gastrointestinal adverse reactions were the most common reason for discontinuing Auryxia Incidence of hypophosphatemia reported as an adverse event in Auryxia-treated patients was rare (<1%) Full prescribing information available at www.auryxia.com

ASN 2017: Keryx Accepted Abstracts Oral: Ferric Citrate Reduces FGF23 in Patients With Non-Dialysis Dependent Chronic Kidney Disease (NDD-CKD) and Iron Deficiency Anemia (IDA) Irrespective of the Change in Serum Phosphate (P) Authors: Block GA, Pergola PE, Uhlig K, Neylan JF, Fishbane S, Chertow GM Poster: Ferric Citrate Lowered Serum Phosphate Only When Elevated in Patients With Nondialysis-Dependent (NDD) CKD and Iron Deficiency Anemia (IDA) Authors: Block GA, Pergola PE, Uhlig K, Neylan JF, Fishbane S, Chertow GM Poster: The Effect of Ferric Citrate on IV Iron, ESA utilization and laboratory parameters in Real-World Dialysis Practice Authors: Kovesdy CP, Rowan CG, Foote B, Acree LS, Meltzer LA, LeWinter R Copyright © 2017 by Keryx Biopharmaceuticals, Inc.

THANK YOU! Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Source: Fishbane, JASN 2017 To the… Patients who participated in our clinical trials and their families Physicians Nurses Study coordinators U.S. Food & Drug Administration

Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Commercial Strategy and Launch Focus Doug Jermasek VP, Marketing and Strategy

Promotional Marketing Campaign Copyright © 2017 by Keryx Biopharmaceuticals, Inc.
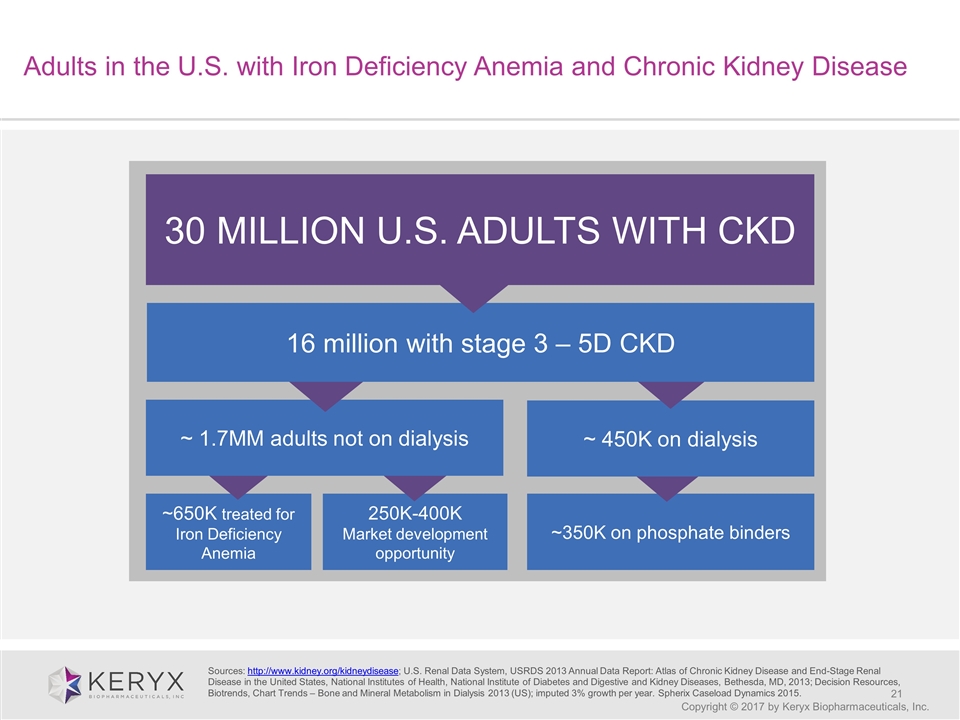
Adults in the U.S. with Iron Deficiency Anemia and Chronic Kidney Disease Copyright © 2017 by Keryx Biopharmaceuticals, Inc. 30 MILLION U.S. ADULTS WITH CKD ~650K treated for Iron Deficiency Anemia ~350K on phosphate binders ~ 450K on dialysis 250K-400K Market development opportunity ~ 1.7MM adults not on dialysis 16 million with stage 3 – 5D CKD Sources: http://www.kidney.org/kidneydisease; U.S. Renal Data System, USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013; Decision Resources, Biotrends, Chart Trends – Bone and Mineral Metabolism in Dialysis 2013 (US); imputed 3% growth per year. Spherix Caseload Dynamics 2015.

Key Launch Objectives Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Drive rapid awareness of new indication in iron deficiency anemia Differentiate Auryxia from existing treatment options Communicate favorable reimbursement access to Auryxia
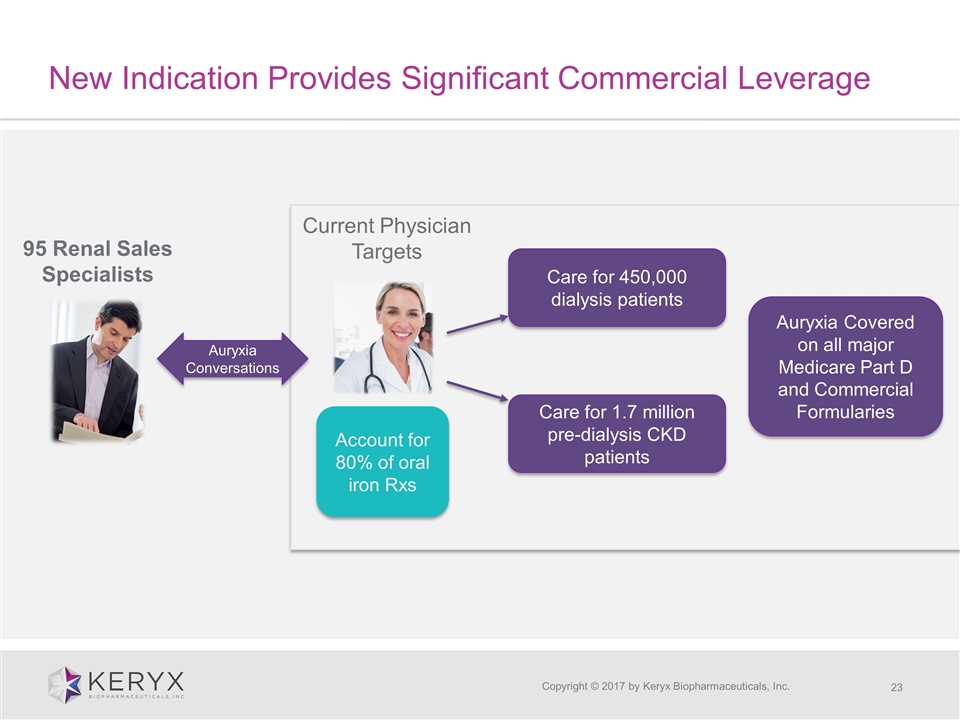
New Indication Provides Significant Commercial Leverage Copyright © 2017 by Keryx Biopharmaceuticals, Inc. 95 Renal Sales Specialists Current Physician Targets Care for 450,000 dialysis patients Care for 1.7 million pre-dialysis CKD patients Auryxia Conversations Auryxia Covered on all major Medicare Part D and Commercial Formularies Account for 80% of oral iron Rxs

Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Closing Remarks Greg Madison Chief Executive Officer Keryx Biopharmaceuticals, Inc

Key Takeaways Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Remainder of 2017 focused on: Educating physicians about Auryxia Field team training Continue to drive adoption in dialysis for Auryxia’s use as a phosphate binder Building foundation to achieve vision of multi-product kidney care company
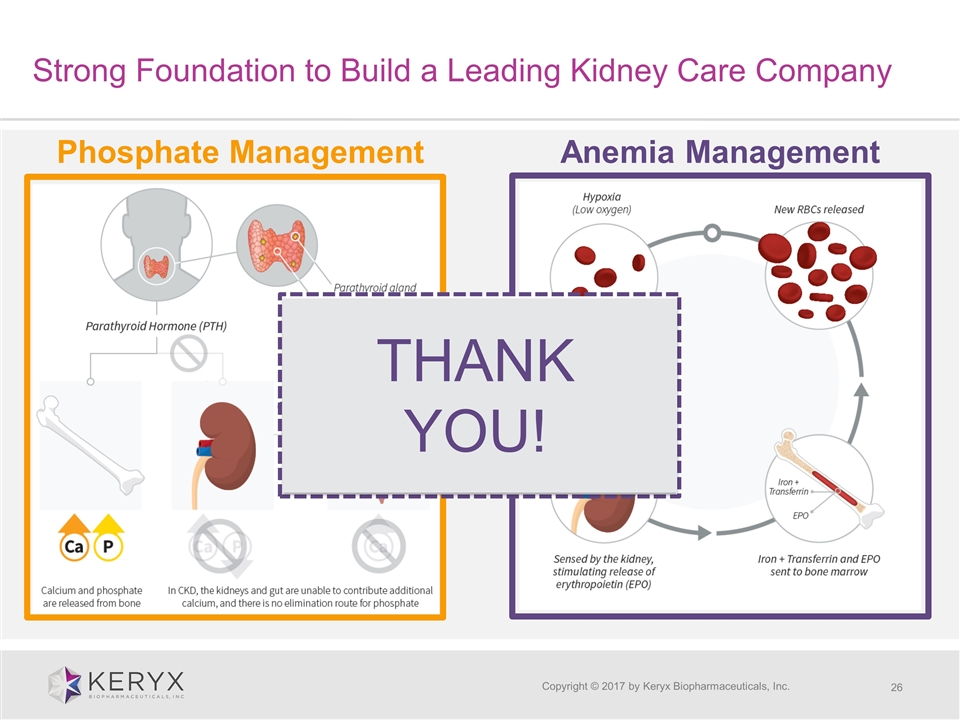
Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Phosphate Management Anemia Management Strong Foundation to Build a Leading Kidney Care Company THANK YOU!
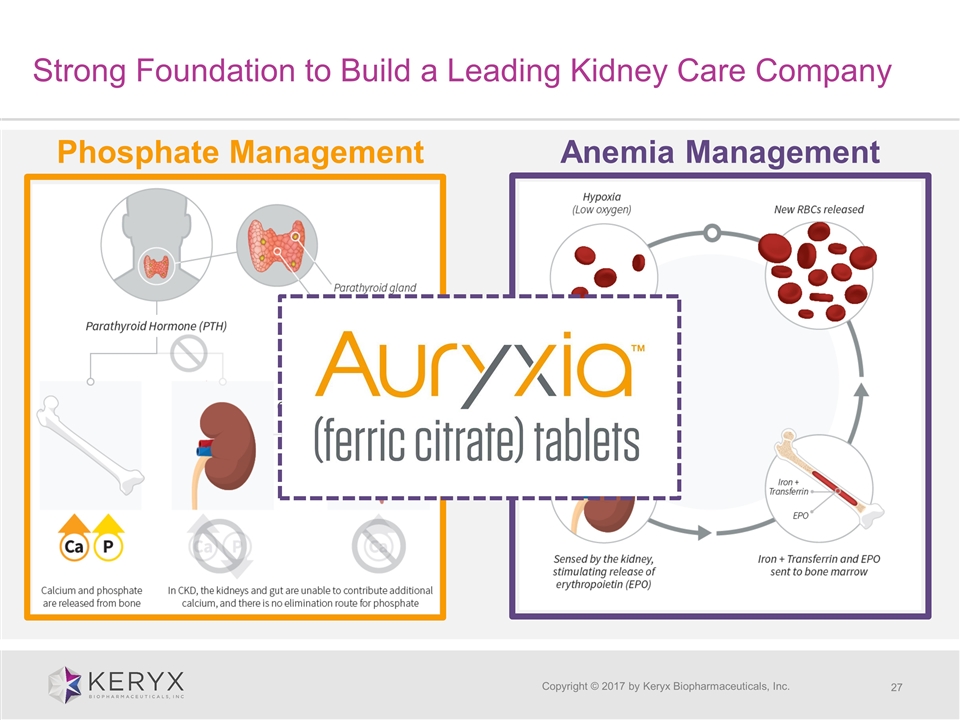
Copyright © 2017 by Keryx Biopharmaceuticals, Inc. Phosphate Management Anemia Management Strong Foundation to Build a Leading Kidney Care Company

U.S. FDA approval of Additional Indication for AURYXIA® (ferric citrate) TABLETS Keryx Biopharmaceuticals, Inc 11/7/2017 www.keryx.com
