Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Integrity Applications, Inc. | zk1720717.htm |
Exhibit 99.1

GlucoTrackA First In Class Non-Invasive GlucoseMonitoring Platform Digital Health SolutionSupporting healthy behaviors by providingindividual context to glucose data The Integrity Applications Growth Story Attractive Industry Dynamics Glucose monitoring undergoing rapid disruptive change

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Statements contained in this presentation that are not statements of historical fact may be deemed to be forward-looking statements. Without limiting the generality of the foregoing, words such as “expect”, “plan” and “will” are intended to identify forward-looking statements. Readers are cautioned that certain important factors may affect Integrity Applications’ actual results and could cause such results to differ materially from any forward-looking statements that may be made in this news release. Factors that may affect Integrity Applications’ results include, but are not limited to, the ability of Integrity Applications to raise additional capital to finance its operations (whether through public or private equity offerings, debt financings, strategic collaborations or otherwise); risks relating to the receipt (and timing) of regulatory approvals (including FDA approval); risks relating to enrollment of patients in, and the conduct of, clinical trials; risks relating to its current and future distribution agreements; risks relating to its ability to hire and retain qualified personnel, including sales and distribution personnel; and the additional risk factors described in Integrity Applications’ filings with the U.S. Securities and Exchange Commission (SEC), including its Annual Report on Form 10-K for the year ended December 31, 2016 as filed with the SEC on March 31, 2017.

Unique product , solid clinical dataFirst -in-class non-invasive glucose monitorUnique “triple-sensor” technology combined with proprietary algorithm provides unmatched accuracyApproved for use in T2 and pre-diabetes patients in Europe and AsiaMultiple clinical trials in over 1000 patients Attractive High Growth MarketDiabetes disease burden is high and growing in every country, fueled by obesity and unhealthy lifestyle"eHealth solutions for Diabetes" is already a $1BN market opportunity in 2017, growing at 30% annuallyAdvancements in design, technology and mass adoption of mobile devices enables consumer driven diabetes care to be feasible.User penetration expected to double every five years. Clear plan for transformationBuilt world class management team with proven track recordCommercialize products in most attractive marketsOverhaul commercial strategy Renovate, modernize and expand product offeringAdditional pillars of growth over and above the existing commercial productLeverage eHealth opportunity to turbocharge growthFocus on US approval Executive Summary
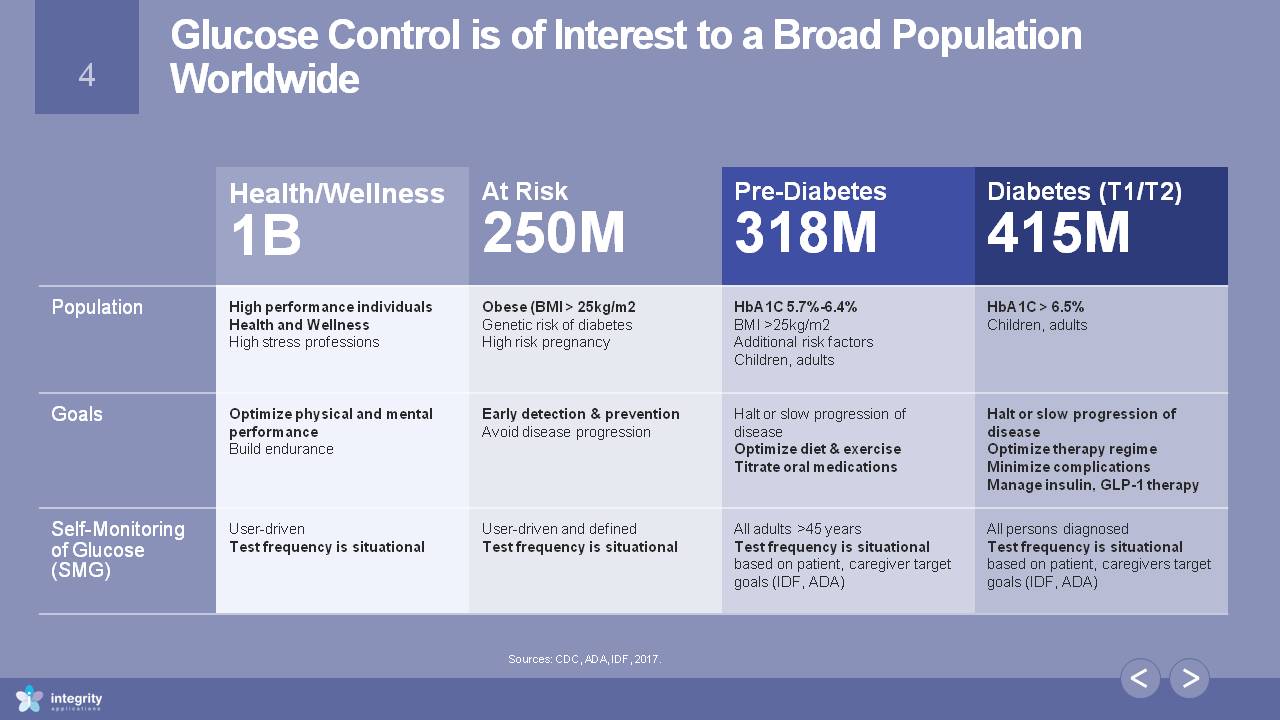
Glucose Control is of Interest to a Broad Population Worldwide Health/Wellness1B At Risk250M Pre-Diabetes318M Diabetes (T1/T2)415M Population High performance individualsHealth and WellnessHigh stress professions Obese (BMI > 25kg/m2Genetic risk of diabetesHigh risk pregnancy HbA1C 5.7%-6.4%BMI >25kg/m2Additional risk factorsChildren, adults HbA1C > 6.5%Children, adults Goals Optimize physical and mental performanceBuild endurance Early detection & preventionAvoid disease progression Halt or slow progression of diseaseOptimize diet & exerciseTitrate oral medications Halt or slow progression of diseaseOptimize therapy regime Minimize complicationsManage insulin, GLP-1 therapy Self-Monitoring of Glucose (SMG) User-driven Test frequency is situational User-driven and definedTest frequency is situational All adults >45 years Test frequency is situational based on patient, caregiver target goals (IDF, ADA) All persons diagnosedTest frequency is situational based on patient, caregivers target goals (IDF, ADA) Sources: CDC, ADA, IDF, 2017.

Glucose Measurement is Undergoing a Major Transformation Commercial Monitor only R&D Stage of Development IntegratedSolution Digital HealthProductSolution Google/Novartis GlucoWise (Mediwise) Cnoga Medical Symphony (Echo Tx) Noviosense Medella Health Light Touch Medical GlucoVista Sano GlucoTrack K’Track (PKVitality) Libre (Abbott) = interstitialfluid sensing GlucoTrack 2.0 Dexcom G6
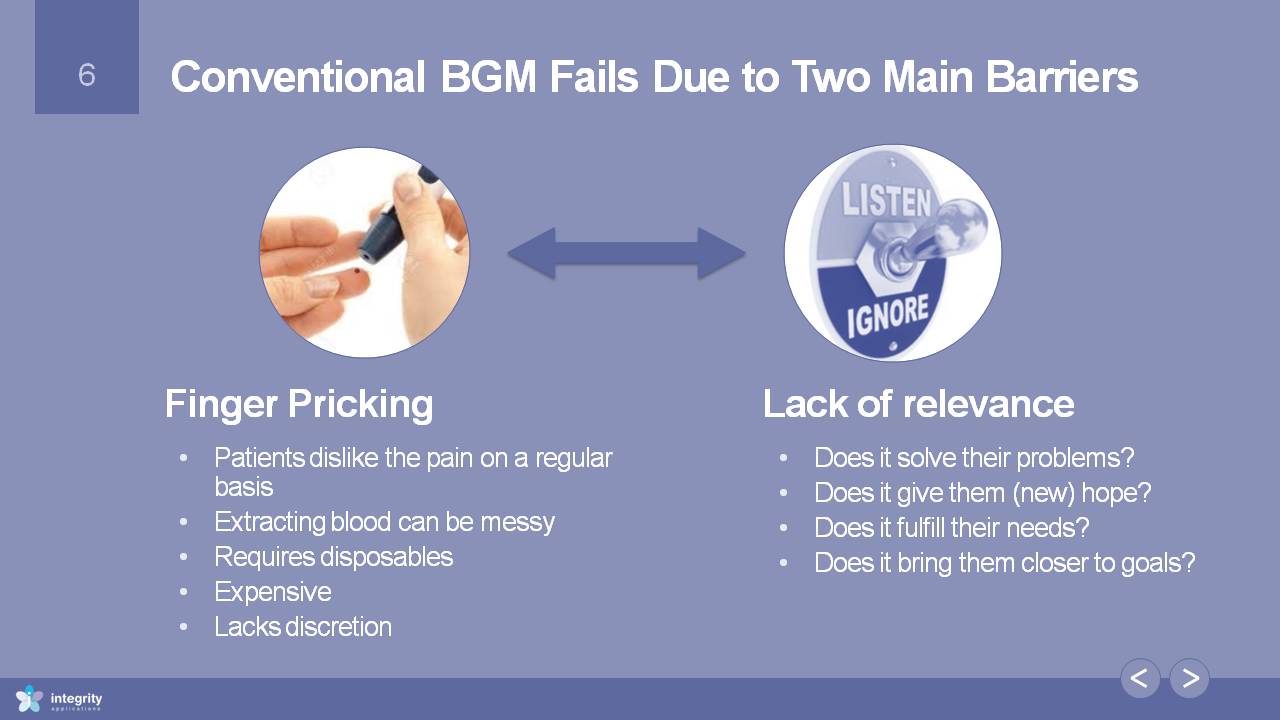
Conventional BGM Fails Due to Two Main Barriers Finger Pricking Patients dislike the pain on a regular basisExtracting blood can be messyRequires disposablesExpensiveLacks discretion Lack of relevance Does it solve their problems?Does it give them (new) hope?Does it fulfill their needs?Does it bring them closer to goals?

Eliminates daily finger pricksSimple calibration with ear clip replacement once every six months Cost-effectiveUnlimited testingLess than cost of conventional glucose monitoring Verified algorithm for estimated HBA1CTrend data allows individual monitoring over time GlucoTrackA Unique Non-Invasive GlucoseMonitoring Solution Pocket-sized Simple to useReading in <1 minUnlimited measurements Discreet use in publicPatented ear clip sensor comfortable to useNo pain Accurate, rapid reading in less than one minute

Ultrasonic Sound velocity through tissue varies with glucose concentration Electromagnetic Tissue impedance changes with glucose concentration. thermal Heat transfer characteristics of tissue vary with glucose concentration. GlucoTrack’s Novel Triple-Sensor Technology The three independent readings are combined and analyzed using a proprietary algorithm.

Accuracy Equivalent to Gold Standard Female: Age: 59; BMI: 29.6; T2DM duration: 1 mo.; No Medications Male; Age: 64; BMI: 22.3; T2DM duration: 24 mos.; Medications: Acrose, Glucophage Sources: Pfutzner Science & Health Institute, Germany and Soroka University Medical Center, Israel, November 2016.
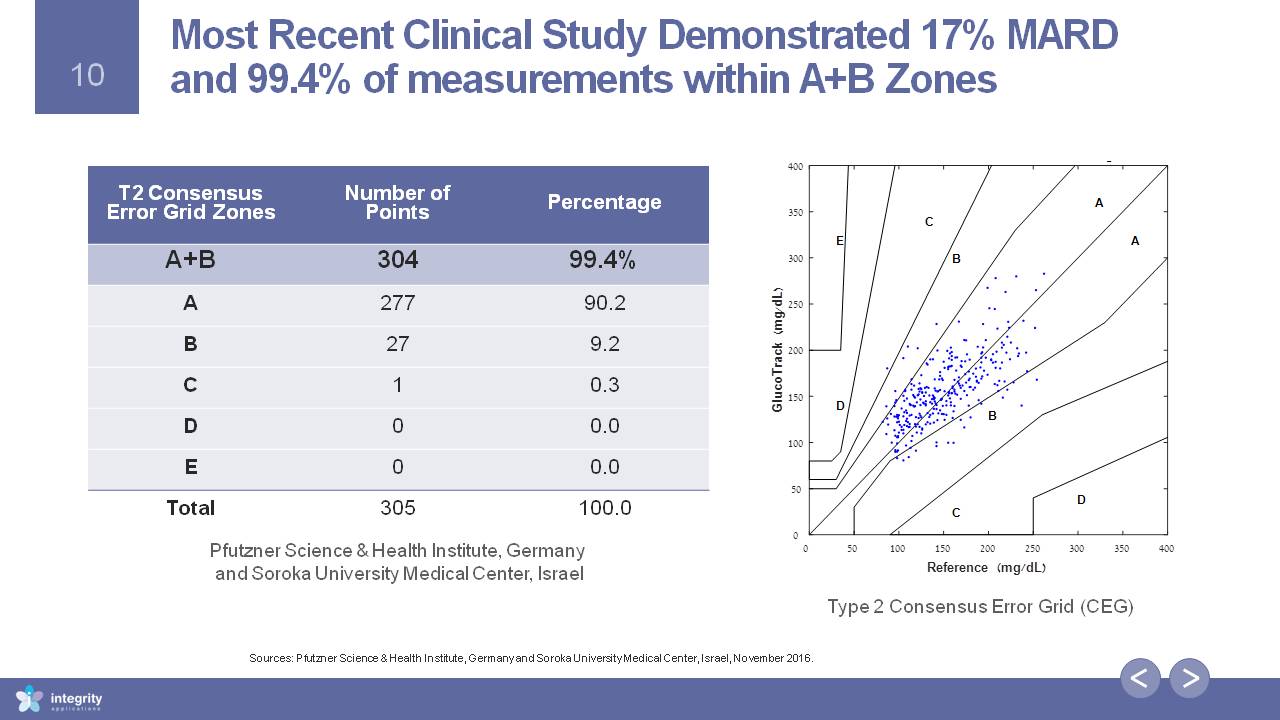
T2 Consensus Error Grid Zones Number of Points Percentage A+B 304 99.4% A 277 90.2 B 27 9.2 C 1 0.3 D 0 0.0 E 0 0.0 Total 305 100.0 Most Recent Clinical Study Demonstrated 17% MARD and 99.4% of measurements within A+B Zones Type 2 Consensus Error Grid (CEG) Sources: Pfutzner Science & Health Institute, Germany and Soroka University Medical Center, Israel, November 2016. Pfutzner Science & Health Institute, Germany and Soroka University Medical Center, Israel

Glucotrack can Combine Non-invasive Measurement with Relevant and Actionable Information. . . …Empowering Patients to Take Control of their Health
Two Strategic Paths, Multi-Billion Global Markets Diabetes Regulated Product HealthConsumer Product

DIGITAL HEALTH SOLUTIONS GlucoTrack app and integration with digital health solutions and health monitoring devices Accuracy & HUMAN FACTORS Reduce calibration, smaller handset, algorithm modifications based on real world use MINIATURIZATION & SCALE Wireless earclip, manufacturing transition to partner Wistron (global electronics manufacturer) BROADEN TARGET MARKET Expand clinical utility in T2, T1 (e.g., hypo and hyperglycemic alerts)Consumer health glucose product for non-diabetics Product Strategy
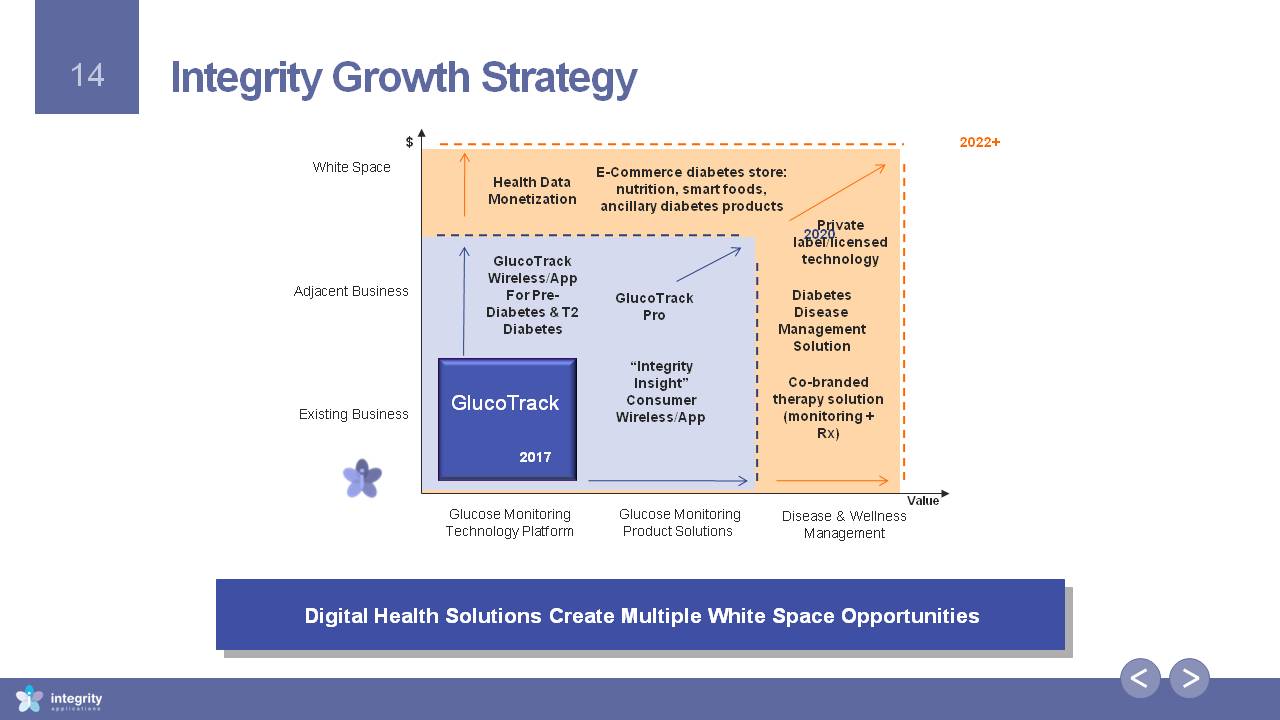
Integrity Growth Strategy Digital Health Solutions Create Multiple White Space Opportunities Glucose MonitoringTechnology Platform Glucose MonitoringProduct Solutions Existing Business Adjacent Business White Space Value $ Disease & WellnessManagement 2017 2020 2022+ GlucoTrack Pro Private label/licensed technology DiabetesDisease Management Solution E-Commerce diabetes store: nutrition, smart foods, ancillary diabetes products Health DataMonetization Co-branded therapy solution (monitoring + Rx) “Integrity Insight” ConsumerWireless/App GlucoTrack Wireless/AppFor Pre-Diabetes & T2 Diabetes GlucoTrack

FutureGlucoTrack Wireless Wireless ear clip App tailored to maximize healthy patient behaviors

Transforming Integrity Management & Talent Structure & Process Commercial Mindset Product Portfolio Distribution Partners US Focus Integrity in 2016 Where will we be in 2018? Significant Talent GapLack of commercial experience World class managementTrack record in commercialization & diabetes Lack of process No formal business planning Established process, structure and controls Not commercially orientedLack of focus on priority markets Clear commercial strategyStrong commercial team Single product orientationMissed opportunities in adjunct markets Development of product pipelineExpansion into digital health Inadequate selection of partnersUnder-management of partnerships Highly experienced & competent partnersCollaborative partnering model US market ignored in favor of othersLack of US management US approval process advancing rapidlyEstablish US headquarters & upgrade talent

Commercialization Commencement of phased product launch in EuropeImproved distributor selectionTransformed sales modelReset pricing and reimbursementBuild patient and HCP advocacyExpand clinical evidenceImplement Digital Health Applications

Proven and Scalable Manufacturing Process Contract manufacturing partners:AY Electronics (Israel)A few thousand systems manufactured to dateWistron CorporationTaiwan, Industrial Design & Medical manufacturing arm of Acer ComputersCapable of high volume manufacturingResearch servicesTaiwan FDA (TFDA) Good Manufacturing Practice (GMP) audit successfully completed
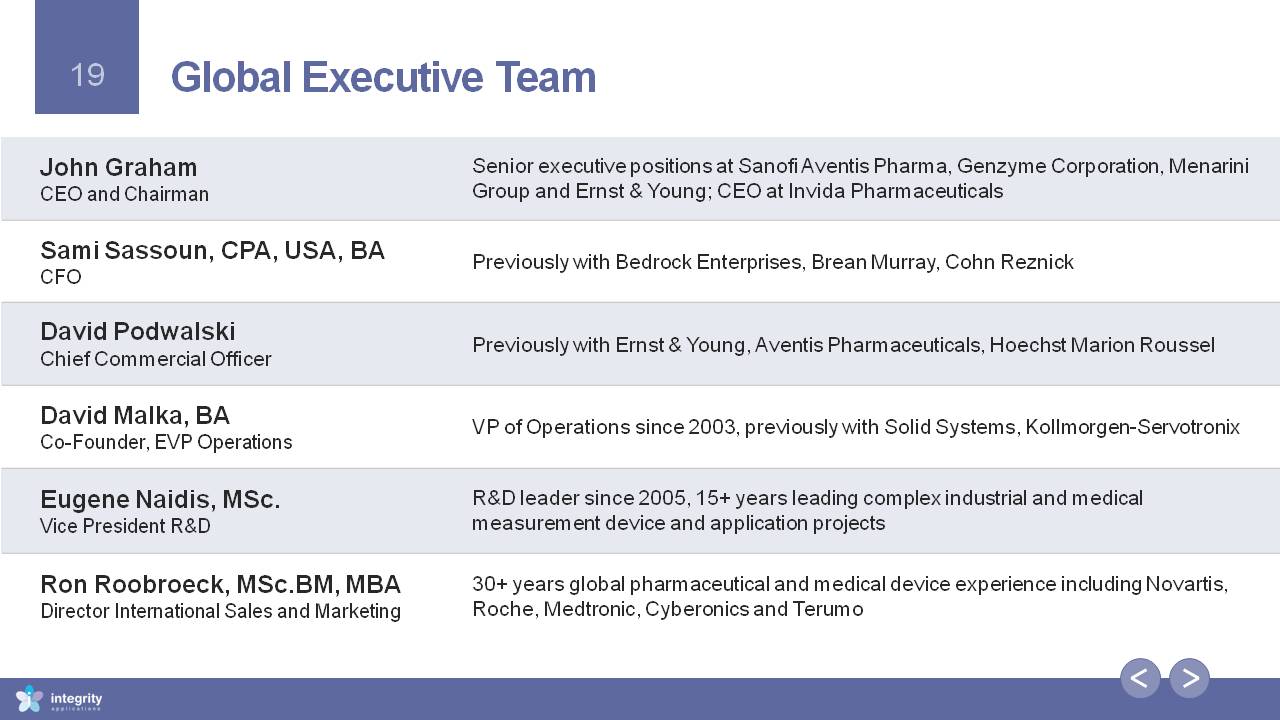
Global Executive Team John GrahamCEO and Chairman Senior executive positions at Sanofi Aventis Pharma, Genzyme Corporation, Menarini Group and Ernst & Young; CEO at Invida Pharmaceuticals Sami Sassoun, CPA, USA, BACFO Previously with Bedrock Enterprises, Brean Murray, Cohn Reznick David PodwalskiChief Commercial Officer Previously with Ernst & Young, Aventis Pharmaceuticals, Hoechst Marion Roussel David Malka, BACo-Founder, EVP Operations VP of Operations since 2003, previously with Solid Systems, Kollmorgen-Servotronix Eugene Naidis, MSc.Vice President R&D R&D leader since 2005, 15+ years leading complex industrial and medical measurement device and application projects Ron Roobroeck, MSc.BM, MBADirector International Sales and Marketing 30+ years global pharmaceutical and medical device experience including Novartis, Roche, Medtronic, Cyberonics and Terumo

Advisors and Directors Angela Strand, Vice-ChairmanFounder, senior executive of Nohm; Founder of Strand Strategy; Founder, CEO of Cliffdive Records. Former executive with multiple biotech and digital health companies in diabetes and metabolic disease. Dr. Robert FischellInventor of more than 200 issued patents and founder of multiple companies that have been licensed or acquired by more than 14 organizations. Inventor of the first implanted insulin pump (acquired by MDT). Mike HauckExecutive Director of the Getz Group; Various executive level and director positions with multiple logistics, healthcare, and technology companies. Former CEO of Walsh International (NASDAQ listed, sold to IMS Health). Revan R. Schwartz Esq.Attorney in private practice. Previously Executive Vice President and General Counsel for Andrew Garrett, Inc. Les SeffFounder, COO of AIMPaaS; Founder, President of Matthew B. Management. Built and ran the NASDAQ trading department at Fidelity. Mr. Avner GalCo-founder, Chairman Emeritus and Senior Consultant Prof. Jan BolinderProfessor of Clinical Diabetes Research, Department of Medicine, Huddinge, SwedenProf. Katherine BarnardHealth Psychologist, Bournemouth University, Faculty of Health and Social Science Dr. Barry GinsbergDiabetes Consultant DMTCProf. Dr. Michael HeiseUniversity of Applied Science of South Westphalia Prof. Dr. Lutz Heinemann (Chairman)CEO, Science & Co.Prof. Irl B. HirschUniversity of Washington School of Medicine Scientific Advisors Independent Directors

A 21st Century Company for 21st Century Patients Positioned to leverage patients’ increasing willingness to self-manage their healthAbility to have an impact on cost of diabetesInnovative technology enables a new digital health approach to diabetesPotential to improve compliance and play a role in disease prevention Financial Information (July17)(OTCQB: IGAP) Shares outstanding: Common and PFD 12.3MMGlobal HQ: Wilmington, DER&D: Ashdod, IsraelEmployees: 30

Recap of the Story Attractive industry dynamicsUnique product with strong clinical dataTransformation underway with near-term tangible resultsCurrently at an inflection point in commercializationUnder-managed and under-invested in the past with upside potentialStrong technology with significant opportunity in portfolio development
