Attached files
| file | filename |
|---|---|
| 8-K/A - 8-K/A - LUMINEX CORP | form8-ka102017.htm |

October 21, 2017
Luminex Corporate
Update

© Copyright 2017 Luminex Corporation 2
Safe Harbor Statement
Certain statements made during the course of this presentation may not be purely historical and consequently may be forward looking statements within the
meaning of the Private Securities Litigation Reform Act of 1995, including but not limited to statements made regarding: our Licensed Technologies Group model
and the ability of our licensees and installed base to drive future growth; the ability of our technology to enhance productivity and efficiency; our financial position
and long-term revenue growth; our ability to integrate our recent acquisition of Nanosphere Inc.; our molecular diagnostic business model, the markets we are
targeting, market segmentation, expected growth of such markets, and the ability of our products to address those markets; sales of our products, their technical
capabilities, and the anticipated market size and acceptance, demand and regulatory environment and approvals therefor; our direct sales efforts; our system
placements; our system and assay product pipeline and anticipated timelines for regulatory approvals and market releases, including for ARIES® and VERIGENE®
instrumentation and assays; market opportunity for ARIES® and VERIGENE ®; functionality and benefits of ARIES® and VERIGENE ® and competitive position;
reimbursement trends; our ability to drive growth through investment in R&D and next generation systems and focus on operating leverage and managing
operating costs; our long term financial targets; our key steps and strategies for growth; our strategic outlook and growth plan for our business for 2017 and
beyond; operational trends, including those related to sales of systems, assays, consumables, and royalty revenues; competitive threats and products offered by
other companies; 2017 revenue guidance; our business outlook, financial targets and projections about revenues, cash flow, system shipments, expenses and
market conditions, and their anticipated impact on Luminex for 2017 and beyond; and, any statements of the plans, strategies and objectives of management for
future operations.
These forward looking statements speak only as of the date hereof and are based on our current beliefs and expectations and are subject to known or unknown
risks and uncertainties some of which are beyond our control that could cause actual results or plans to differ materially and adversely from those anticipated in the
forward looking statements. Factors that could cause or contribute to such differences are detailed in our annual, quarterly, or other filings with the Securities and
Exchange Commission. We undertake no obligation to update these forward looking statements.
Also, certain non-GAAP financial measures as defined by SEC Regulation G, may be covered in this presentation. To the extent that any non-GAAP financial
measures are covered, a presentation of and reconciliation to the most directly comparable GAAP financial measures will be included in this presentation may be
available on our website at www.luminexcorp.com in accordance with Regulation G.

© Copyright 2017 Luminex Corporation 3
Today's Agenda
Introductory
Comments
Licensed
Technologies
Group
General Trends
Molecular
Diagnostics
Group
How We Are
Winning
Finance &
Corporate
Development
Supporting
Growth
Q&A
Concluding
Comments
Homi Shamir
President and CEO
Todd Bennett
SVP, Global Sales and
Customer Operations
Reid Sadler
VP, Sales & Marketing,
Americas MDx
Harriss Currie
SVP, Finance and CFO
Homi Shamir
President and CEO

Homi Shamir
President and CEO
Introductory Comments

© Copyright 2017 Luminex Corporation 5
A Track Record of Success
2014 2015 2016
Total Annual Revenue Growth Accelerating
+13%
$227M
$238M
$271M
$300M - $310M
2017E
Consistency:
11 Straight Quarters
Meeting or Exceeding Street
Revenue Expectations

© Copyright 2017 Luminex Corporation 6
Executive Summary
STRATEGIC GOALS
• Continue to drive double digit total revenue growth, excluding LabCorp
• Continued focus on profitable activities (high ROI) in both LTG and MDx
• Leverage strong balance sheet to pursue expansion opportunities
GROWTH DRIVERS
• Licensed Technologies Group: stable, mid-single digit revenue growth with high GM
• MDx infectious disease testing: double-digit revenue growth driven by high growth
S2A molecular testing platforms
• MDx well positioned for future growth with broad breadth of testing solutions and
pricing strategies (e.g. Flex) as well as VERIGENE® II and menu
LICENSED
TECHNOLOGIES
GROUP
MOLECULAR
DIAGNOSTICS
GROUP

Todd Bennett
SVP, Global Sales and Customer Operations
Licensed Technologies Group (LTG)
General Trends

© Copyright 2017 Luminex Corporation 8
Licensed Technologies Group: Summary
1H17 LTG-Related Revenue, by Product:
+5%
in
1H17
~40%
Total:
$74.5M Other
12%
% of Total 1H17 LTG-Related Revenue, by Market:
Royalty
System
Consumable
LTG:
~50%
of our
business
~40%
~20%
Transplant
Diagnostics
Immuno-dx
Protein
Research
…Luminex
bead-based
platforms are an
open technology
and the applications
are extremely broad.
There are uses in all
fields from
academia to the
clinic…”
– Director,
Academic Core Facility
37%
29%
22%
LMNX IP Portfolio*:
568
Granted
Patents
160
Patents
Pending
* Worldwide

© Copyright 2017 Luminex Corporation 9
LTG: Multiplexing Systems Overview
0
5,000
10,000
15,000
2008 2010 2012 2014 2016
0
500
1,000
1,500
2,000
2,500
'01 / '02 '03 / '04 '05 / '06 '07 / '08 '09 / '10 '11 / '12 '13 / '14 '15 / '16
Total Cumulative Systems Shipped to Date
Multiplexing System Shipments (2-Year Totals)

© Copyright 2017 Luminex Corporation 10
LTG: High Margin Revenue Trends
End-User Sales (Partner Reported)*
$0
$50
$100
$150
$200
$250
$300
'01/'02 '03/'04 '05/'06 '07/'08 '09/'10 '11/'12 '13/'14 '15/'16
LMNX LTG Revenue: Total*
$0
$200
$400
$600
$800
$1,000
$1,200
'01/'02 '03/'04 '05/'06 '07/'08 '09/'10 '11/'12 '13/'14 '15/'16
$0
$20
$40
$60
$80
$100
'01/'02 '03/'04 '05/'06 '07/'08 '09/'10 '11/'12 '13/'14 '15/'16
* $ in Millions, 2-Year Totals
$0
$20
$40
$60
$80
$100
$120
'01/'02 '03/'04 '05/'06 '07/'08 '09/'10 '11/'12 '13/'14 '15/'16
LMNX LTG Revenue: Consumables* LMNX LTG Revenue: Royalties*

© Copyright 2017 Luminex Corporation 11
LTG: Success Story
Large European Blood Bank and Testing Network
The Customer Why Luminex? The Result
• Network of 15 centers
• Focus on collection,
testing, preparation and
distribution of blood
products to some 1,900
health care facilities
• Dependability of the xMAP®
platform
• Unique approach to kit
supply utilizing two of our
Partners
• 15 LX200™ sold
• 9 Luminex service
contracts
• Valued at ~$2.5M in total
revenue over 5 year term

© Copyright 2017 Luminex Corporation 12
LTG: Growth Opportunities
• 70+ partners investing in our technology; long-term
contractual partnerships; addressing large markets
•Stable growth; highly profitable
• Large and growing system base (14,500+)
•New system (improved sensitivity and automation)
7

Reid Sadler
VP, Sales & Marketing Americas MDx
Molecular Diagnostics Group
How We are Winning

© Copyright 2017 Luminex Corporation 14
Molecular Diagnostics (MDx): Summary
Molecular
Diagnostics:
~50%
of our
business
1H17 MDx Assay Revenue, by Category:
Genetic
19%
Infectious
Disease
81%
Total:
$74M
100+
Highly Experienced
MDx Sales &
Support Professionals
+42%
in
1H17

© Copyright 2017 Luminex Corporation 15
MDx: The Right Solution for Customers Needs
SYSTEM THROUGHPUT
M
U
L
T
IP
L
E
X
IN
G
C
A
P
A
B
IL
IT
IE
S
MAGPIX®
Launched 2010
Luminex® 200™
Launched 2005
ARIES®
Launched 2015
VERIGENE®
Acquired 2016
Automated Sample to Answer
Non-Automated
VERIGENE® II
Coming Soon

© Copyright 2017 Luminex Corporation 16
MDx: Targeted & Syndromic Testing Solutions
HSV 1&2 Flu/RSV GBS C. Difficile Norovirus GAS MRSA Respiratory Panel GI Panel BCID Panel
Targeted Syndromic
Luminex is the only manufacturer providing Targeted & Syndromic solutions

© Copyright 2017 Luminex Corporation 17
MDx: Key Recent Wins
Sizeable agreements closed over the past 6 months
Large Hospital (BF) BC,EP,RP $1M
Large Hospital (BF) EP $500K
IDN Hospital Grp (BF) BC, EP $500K
IDN Hospital Grp EP $500K
Large Hospital (BF) BC,RP $400K
Large Hospital (Cult.) BC, EP RP $400K
Large Hospital (Cult.) EP $275K
Reference Lab (BF) Bordetella $500K
IDN Hospital Grp (MB) HSV, ASRs $400K
Pathology Lab GBS $250K
Reference Lab (LDT) Bordetella $200K
Large Hospital (CPD) GBS, HSV $200K
Large Hospital (DS) FLU $200K
Pathology Lab WH ASRs $150K
Reference Lab (BF) RPP $700K
IDN Core Lab (BD) NxTAG® WH $600K
Reference Lab (BF) RPP,GPP $500K
VA Hospital (BF) RPP $400K
Large Hospital
(GMK)
RPP $400K
IDN Core Lab (GMK) RPP,CF $350K
VERIGENE® ARIES® NxTAG® & xTAG®
Closed Deal Competitive Takeaway
* Dollar amounts per year

© Copyright 2017 Luminex Corporation 18
MDx: 2017 Success Story
Commercial Lab in California
The Lab The Deal The Result
Offering confidential lab-
based drug tests as well
as:
• Upper Respiratory
Testing
• GI Pathogen Testing
• UDT Testing
• Oral Fluid Testing
• Multi-year agreement
including:
‒ xTAG® GPP
‒ NxTAG® RPP
‒ ARIES® Bordetella
‒ ARIES® Group A Strep
• Valued at $4M (over term)
• 3 ARIES® & 1 MAGPIX®
• Displacing BioFire
• Great example of Total
Portfolio Selling
(syndromic panel and
targeted testing)

© Copyright 2017 Luminex Corporation 19
MDx: Customer Sales and Support
Selling Sample to
Answer and Non-
Automated MDx Portfolio
Jan
45+ 60
100+
Highly Experienced
MDx Sales &
Support Professionals
Sales Force Sales Support
MDx
Sales Force
Integrated &
Trained
Focused on Microbiology
& Molecular Clinical
Laboratories
Targeting Hospital &
Reference Lab Market
Prefer this slide (or consolidated slide earlier) –
confirm 75 FTEs vs. 100+ in previously public slide

© Copyright 2017 Luminex Corporation 20
MDx: Overview of S2A Active Customers
VERIGENE® platform continues
strong traction.
Blood Culture panels outpace
market growth. Enteric and RP Flex
panels gaining rapid traction.
ARIES® traction due to menu
expansion and anchor assays that
can drive placements.
C. Diff and MRSA to be main
drivers in 2018.
$95,000
Average Annual Revenue per Active Customer*
VERIGENE® ARIES®
$40,000
Average Annual Revenue per Active Customer*
Active Customer = Purchases in rolling 12 month period
400+
Active
Customers
* Figures approximate

© Copyright 2017 Luminex Corporation 21
MDx: The Importance of Our Flex Testing Strategy
Single Center Experience with RP Flex*
Annual Cost Savings of MDx Respiratory Testing
Targets
Competitor’s
Full MDx Panel
Luminex
RP Flex
Overall Cost
Savings
Flu A/B + RSV $215,760 $129,920 $85,840
Respiratory Virus
Panel
$181,350 $171,600 $9,750
Total $397,110 $301,520
RP Flex
Generated
Annual
Savings of:
>$95K
Source: Dr. Yvette McCarter, College of Medicine – Jacksonville, University of Florida, Department of Pathology and Laboratory Medicine
NOTE: Single center experiences may vary
How does Flex testing work?
• Each Flex cartridge contains a
broad panel of viral, bacterial
and/or parasitic targets.
• Any combination of tiered
targets can be selected for an
individual sample at the time of
test ordering.
• Additional results not initially
reported after test completion
can be reflexed instantly at an
extra cost without running an
additional test.
© Copyright 2017 Luminex Corporation 22
Americas Corporate Accounts – Team Success
MDx: Group Purchasing Organization Execution
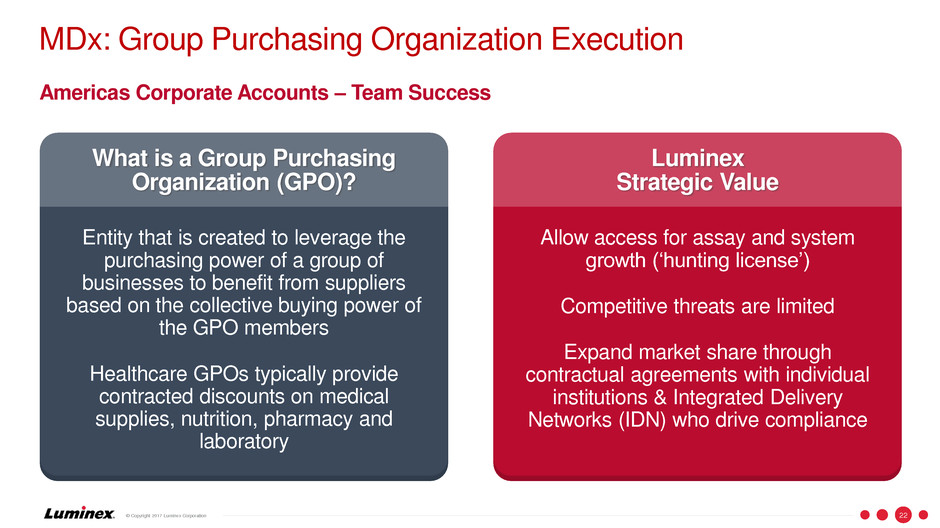
What is a Group Purchasing
Organization (GPO)?
Entity that is created to leverage the
purchasing power of a group of
businesses to benefit from suppliers
based on the collective buying power of
the GPO members
Healthcare GPOs typically provide
contracted discounts on medical
supplies, nutrition, pharmacy and
laboratory
Luminex
Strategic Value
Allow access for assay and system
growth (‘hunting license’)
Competitive threats are limited
Expand market share through
contractual agreements with individual
institutions & Integrated Delivery
Networks (IDN) who drive compliance
© Copyright 2017 Luminex Corporation 23
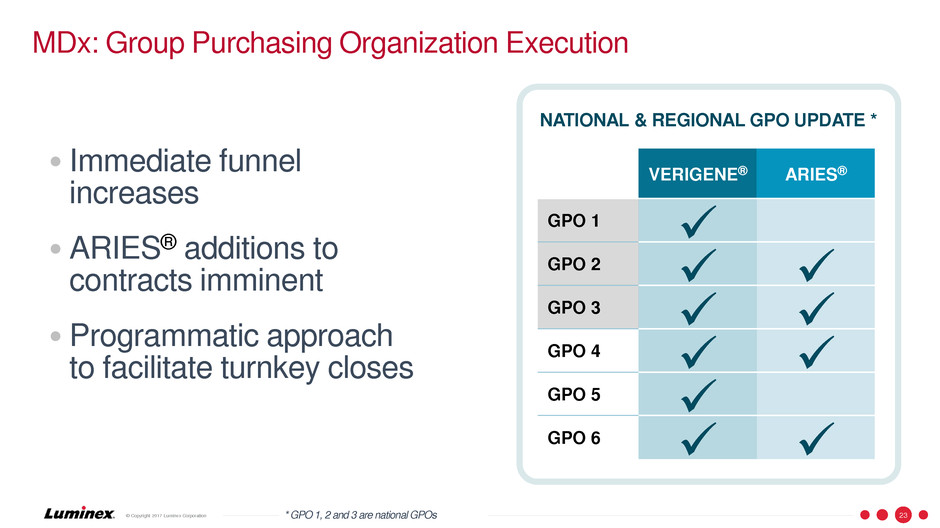
MDx: Group Purchasing Organization Execution
• Immediate funnel
increases
•ARIES® additions to
contracts imminent
•Programmatic approach
to facilitate turnkey closes
VERIGENE® ARIES®
GPO 1
GPO 2
GPO 3
GPO 4
GPO 5
GPO 6
NATIONAL & REGIONAL GPO UPDATE *
* GPO 1, 2 and 3 are national GPOs

© Copyright 2017 Luminex Corporation 24
MDx: Growth Opportunities
• Total S2A revenue – accelerated growth from over $45M in
2017E and approaching ~$100M by end of 2019E, driven by;
– Continued strong adoption of VERIGENE® I
– Expanded menu on ARIES®
– Recent expansion of GPO coverage of S2A platforms
– Initial launch of VERIGENE® II with multiple assays
• In uncertain reimbursement landscape,
Luminex is well positioned with a broad
product offering and differentiated
pricing strategies

© Copyright 2017 Luminex Corporation 25
MDx: Update on VERIGENE® II
Progress Report
• VERIGENE® II system now robust and ready
for clinical trials. Failure rates consistently
below 5%.
• Plan on commencing multiple clinical trials
with system in 2018 (enteric, respiratory,
blood culture ID) for launch in 2019
• With on-going success of VERIGENE® I,
commercialization strategy for VERIGENE® II
favors launching with multiple assays.
• Monitoring reimbursement landscape – well
positioned with Flex pricing strategy

Financial and Corporate Development
Supporting Growth
Harriss Currie
SVP, Finance and CFO

© Copyright 2017 Luminex Corporation 27
2H16 1Q17
MDx Revenue,
Automated *
2H16 1Q17
Financial Overview: Well Positioned for Future Growth
• Nanosphere deal accretive in 2Q17 -
ahead of target
• VERIGENE® gram-positive blood
culture (BC-GP) and gram-negative
blood culture (BC-GN) molecular
assays are First to Market in Japan
• Automated Sample to Answer
revenue to be more than $45M in
2017 and approaching $100M by
end of 2019
• LTG-related revenue to grow 3 - 5%
in 2017
1H17 revenue grew
5% over 1H16,
reaching $74 million;
High Margin Items
accounted for 78% of
1H17 revenue
MDx Revenue,
Non-Automated
Financial Strength LTG - Related Revenue
* Pro-forma
$57M
+6%
$22M
+55%
$103M
2Q17 Cash and
Investments
$20M
1H17 Cash
Flow from
Operations
1H17 1H17 2H16 2H16

Thank You
