Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Loxo Oncology, Inc. | a17-24012_18k.htm |
|
|
LOXO-292, a Potent, Highly Selective RET Inhibitor, in Multi-Kinase Inhibitor-Resistant RET Fusion-Positive Lung Cancer Patients with and without Brain Metastases Vamsidhar Velcheti1, Todd Bauer2, Vivek Subbiah3, Maria E. Cabanillas3, Nehal Lakhani4, Lori J. Wirth5, Geoffrey R. Oxnard6, Manisha H. Shah7, Eric J. Sherman8, Melissa Johnson2, Steven Smith9, Todd Eary9, Scott Cruickshank9, Brian B. Tuch9, Kevin Ebata9, Michele Nguyen9, Stefani Corsi-Travali9, Stephen Michael Rothenberg9, Alexander Drilon8 1Cleveland Clinic, Cleveland, OH; 2Sarah Cannon Research Institute/Tennessee Oncology, PLLC, Nashville, TN; 3The University of Texas MD Anderson Cancer Center, Houston, TX; 4South Texas Accelerated Research Therapeutics (START) Midwest, Grand Rapids, MI; 5Massachusetts General Hospital Cancer Center, Boston, MA; 6Dana Farber Cancer Institute, Boston, MA; 7The Ohio State University Comprehensive Cancer Center, Columbus, OH; 8Memorial Sloan Kettering Cancer Center, New York, NY; 9Loxo Oncology, Stamford, CT |
|
|
Dr. Vamsidhar Velcheti: advisory role/consultant for Astra Zeneca, Bristol-Myers Squibb, Merck, Novartis, Amgen, Foundation Medicine, Fulgent Genetics, Celgene, Genentech, Takeda Oncology and Clovis Oncology Disclosures |
|
|
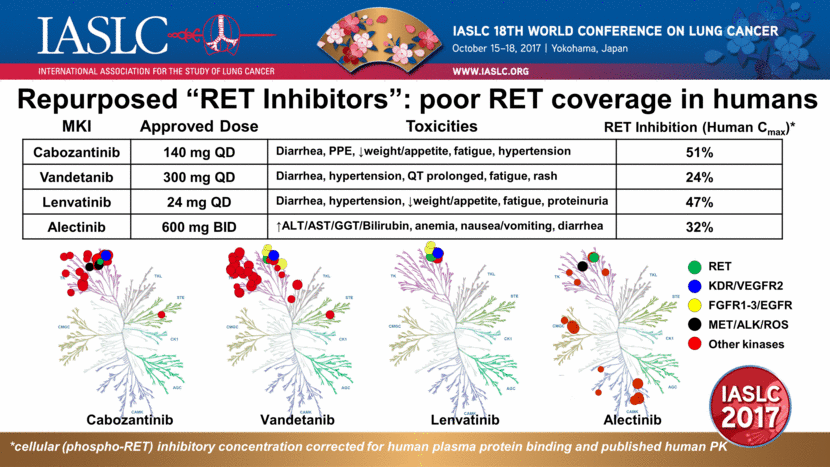
Repurposed “RET Inhibitors”: poor RET coverage in humans Cabozantinib Vandetanib Lenvatinib Alectinib *cellular (phospho-RET) inhibitory concentration corrected for human plasma protein binding and published human PK RET KDR/VEGFR2 MET/ALK/ROS Other kinases FGFR1-3/EGFR MKI Approved Dose Toxicities Cabozantinib 140 mg QD Diarrhea, PPE, weight/appetite, fatigue, hypertension 51% Vandetanib 300 mg QD Diarrhea, hypertension, QT prolonged, fatigue, rash 24% Lenvatinib 24 mg QD Diarrhea, hypertension, weight/appetite, fatigue, proteinuria 47% Alectinib 600 mg BID ALT/AST/GGT/Bilirubin, anemia, nausea/vomiting, diarrhea 32% RET Inhibition (Human Cmax)* |
|
|
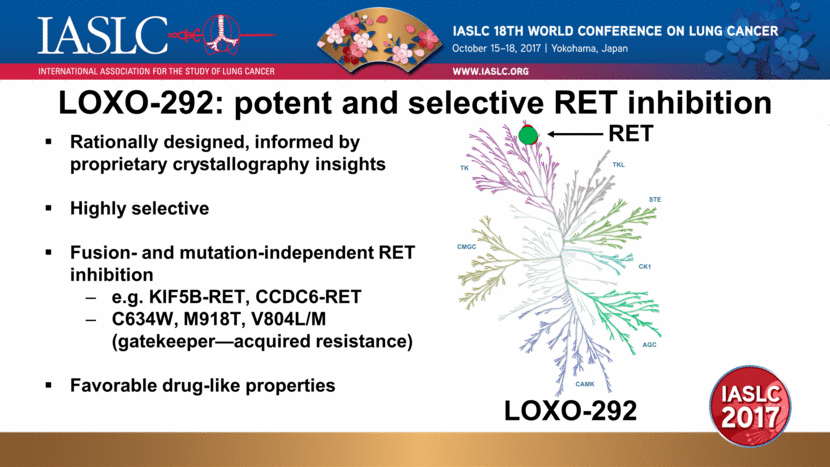
Rationally designed, informed by proprietary crystallography insights Highly selective Fusion- and mutation-independent RET inhibition e.g. KIF5B-RET, CCDC6-RET C634W, M918T, V804L/M (gatekeeper—acquired resistance) Favorable drug-like properties RET LOXO-292 LOXO-292: potent and selective RET inhibition |
|
|
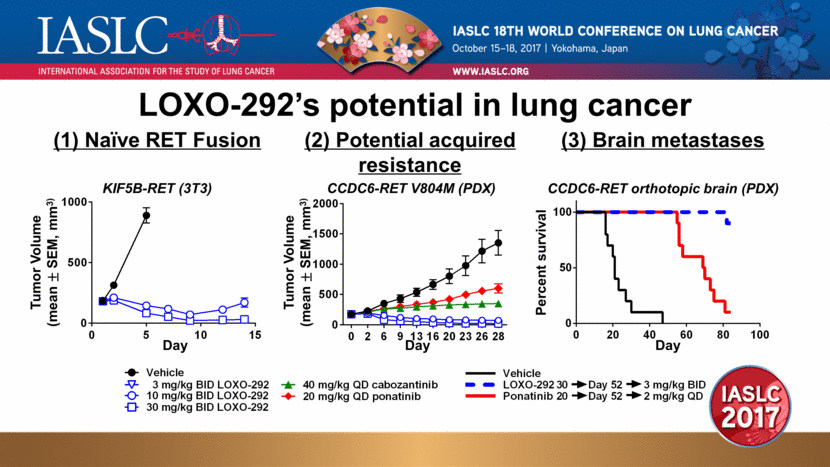
Day Tumor Volume (mean ± SEM, mm3) LOXO-292’s potential in lung cancer KIF5B-RET (3T3) CCDC6-RET V804M (PDX) (1) Naïve RET Fusion (2) Potential acquired resistance (3) Brain metastases CCDC6-RET orthotopic brain (PDX) Tumor Volume (mean ± SEM, mm3) Day Day Percent survival 0 2 6 9 13 16 20 23 26 28 0 500 1000 1500 2000 0 5 10 15 0 500 1000 0 20 40 60 80 100 0 50 100 Vehicle 3 mg/kg BID LOXO-292 10 mg/kg BID LOXO-292 30 mg/kg BID LOXO-292 40 mg/kg QD cabozantinib 20 mg/kg QD ponatinib Vehicle LOXO-292 Ponatinib Day 52 3 mg/kg BID 2 mg/kg QD Day 52 20 30 |
|
|
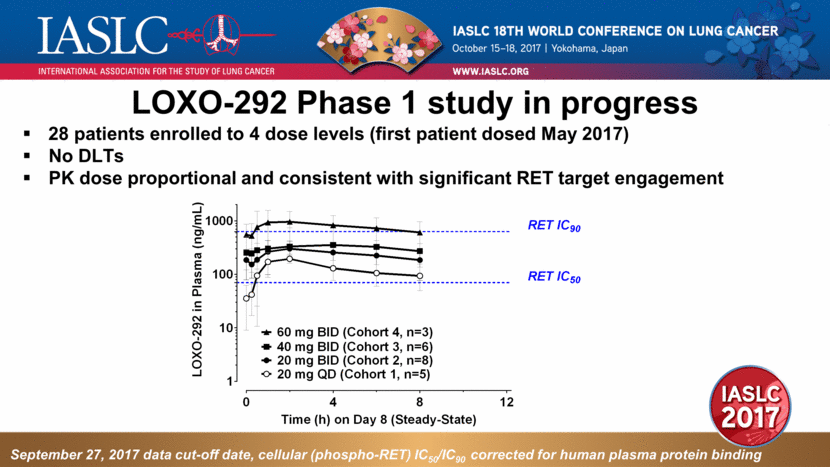
LOXO-292 Phase 1 study in progress 28 patients enrolled to 4 dose levels (first patient dosed May 2017) No DLTs PK dose proportional and consistent with significant RET target engagement September 27, 2017 data cut-off date, cellular (phospho-RET) IC50/IC90 corrected for human plasma protein binding 0 4 8 12 1 10 100 1000 Time (h) on Day 8 (Steady-State) L O X O - 2 9 2 i n P l a s m a ( n g / m L ) RET IC 50 20 mg BID (Cohort 2, n=8) 20 mg QD (Cohort 1, n=5) RET IC 90 40 mg BID (Cohort 3, n=6) 60 mg BID (Cohort 4, n=3) |
|
|
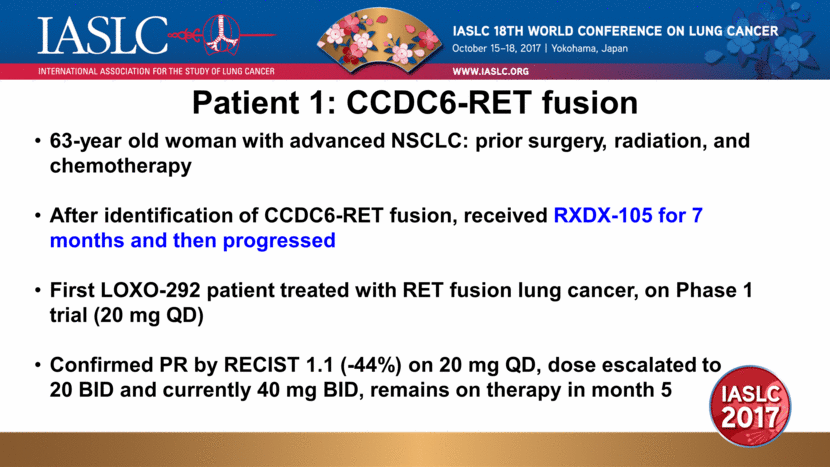
63-year old woman with advanced NSCLC: prior surgery, radiation, and chemotherapy After identification of CCDC6-RET fusion, received RXDX-105 for 7 months and then progressed First LOXO-292 patient treated with RET fusion lung cancer, on Phase 1 trial (20 mg QD) Confirmed PR by RECIST 1.1 (-44%) on 20 mg QD, dose escalated to 20 BID and currently 40 mg BID, remains on therapy in month 5 Patient 1: CCDC6-RET fusion |
|
|
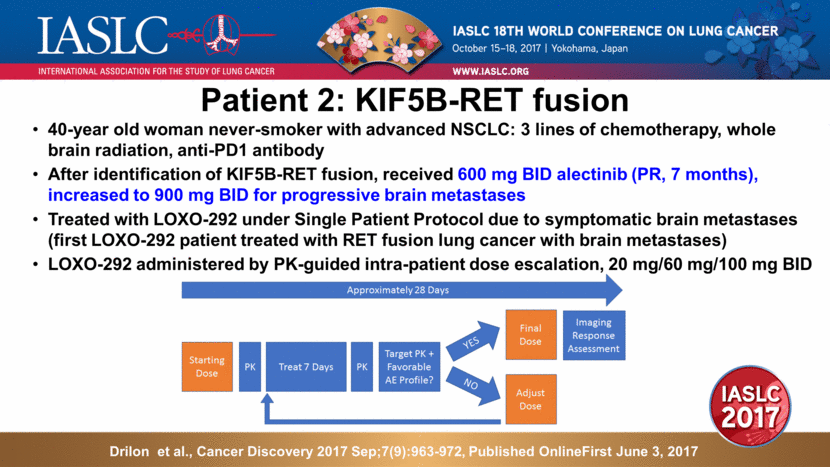
40-year old woman never-smoker with advanced NSCLC: 3 lines of chemotherapy, whole brain radiation, anti-PD1 antibody After identification of KIF5B-RET fusion, received 600 mg BID alectinib (PR, 7 months), increased to 900 mg BID for progressive brain metastases Treated with LOXO-292 under Single Patient Protocol due to symptomatic brain metastases (first LOXO-292 patient treated with RET fusion lung cancer with brain metastases) LOXO-292 administered by PK-guided intra-patient dose escalation, 20 mg/60 mg/100 mg BID Patient 2: KIF5B-RET fusion Drilon et al., Cancer Discovery 2017 Sep;7(9):963-972, Published OnlineFirst June 3, 2017 |
|
|
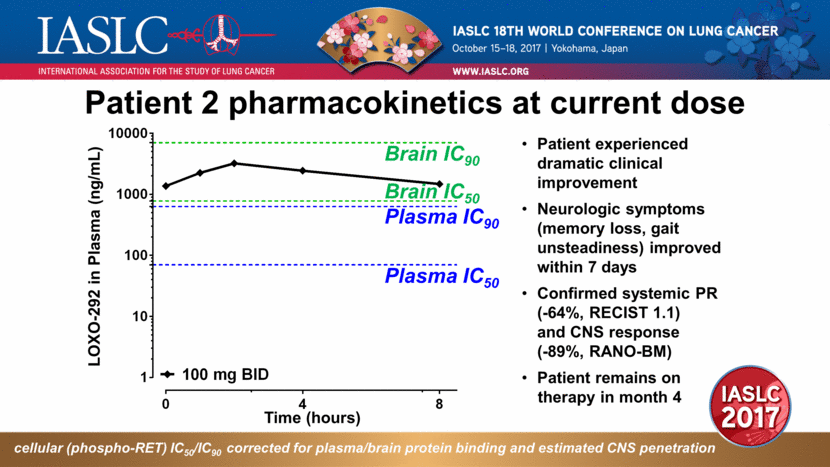
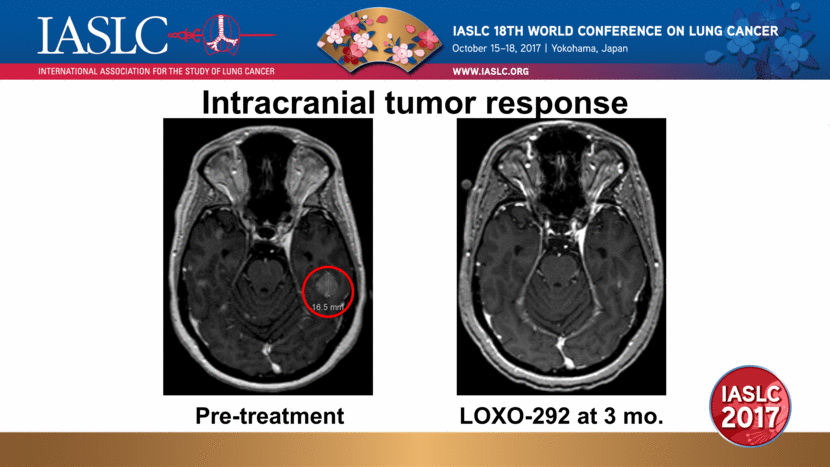
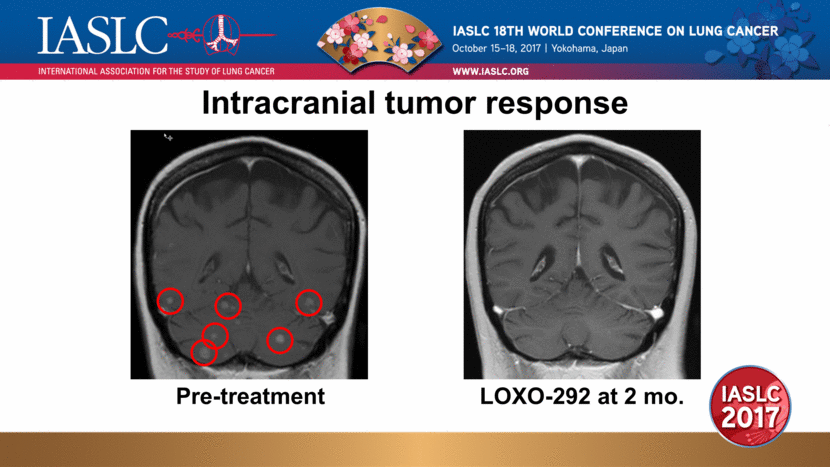
Patient 2 pharmacokinetics at current dose Patient experienced dramatic clinical improvement Neurologic symptoms (memory loss, gait unsteadiness) improved within 7 days Confirmed systemic PR (-64%, RECIST 1.1) and CNS response (-89%, RANO-BM) Patient remains on therapy in month 4 cellular (phospho-RET) IC50/IC90 corrected for plasma/brain protein binding and estimated CNS penetration LOXO-292 in Plasma (ng/mL) Time (hours) Plasma IC50 Plasma IC90 Brain IC50 Brain IC90 |
|
|
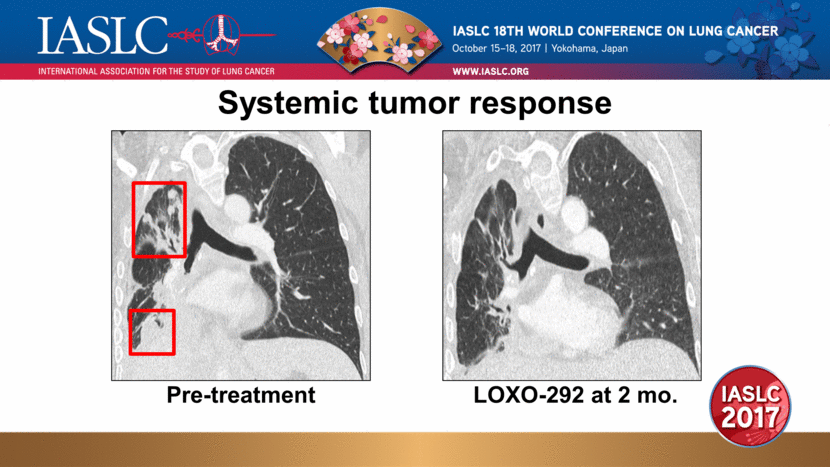
Systemic tumor response Pre-treatment LOXO-292 at 2 mo. |
|
|
Intracranial tumor response Pre-treatment LOXO-292 at 3 mo. |
|
|
Intracranial tumor response Pre-treatment LOXO-292 at 2 mo. |
|
|
Conclusions LOXO-292: RET inhibition in ongoing Phase 1 trial already greater than multikinase (MKI) inhibitors Well-tolerated with no DLTs Biologically relevant doses Proof concept anti-tumor efficacy in patients with RET-fusion NSCLC Heavily pre-treated and MKI-experienced patients With/without brain metastases Phase 1 study currently enrolling patients 855-RET-4-292 (855-738-4292) clinicaltrials@loxooncology.com |
|
|
Acknowledgements LOXO-292 patients, families and caregivers LOXO-292 Phase 1 investigators and research staff Alturas Analytics Array BioPharma Loxo Oncology |