Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Intersect ENT, Inc. | d474421dex991.htm |
| 8-K - FORM 8-K - Intersect ENT, Inc. | d474421d8k.htm |
Exhibit 99.2

The role of corticosteroid-eluting stents for the management of recurrent nasal polyposis Robert C. Kern MD George A. Sisson Professor of Otolaryngology Chairman, Department of Otolaryngology-Head and Neck Surgery Northwestern University Feinberg School of Medicine Chicago, Illinois

Disclosures Received research grants from NIH (Core PI) and Intersect ENT (Co-PI) Received consulting fees from 480 Biomedical, Genentech, Sanofi, NUIG and GSK The steroid-releasing implant discussed in this presentation is not approved by the US FDA and has not received CE Mark. The implant is limited by the US law to investigational use only and is not for sale in any geography. Intersect ENT provided funding, administrative support, and materials to complete this study. Sites were compensated for study-related labor and expenses in enrolling patients and acquiring data.
Chronic Rhinosinusitis with Nasal Polyps The multi-factorial pathophysiology of CRSwNP prevents long-term effectiveness of any single treatment option Medical management includes systemic and intranasal corticosteroids (INCS) Surgical treatment relieves blockage and improves ventilation o 40% recurrence rates of nasal polyps in 1.5 yr1 o Recurrent polyps positive predictor for revision surgery2 1DeConde et al Laryngoscope 2016;127:550-5 2Orlandi et al IFAR 2016;6S:S22-209

Investigational In-Office Therapy Designed for Nasal Polyps in CRS Patients An investigational corticosteroid-eluting, bioabsorbable sinus implant Designed to deliver 1350 mcg of mometasone furoate directly to the ethmoid sinus mucosa over approximately 90 days In-office placement under topical anesthesia The safety and efficacy evaluated in 4 studies (2 RCTs, 1 Pilot, 1 PK), totaling 400 patients The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.
The RESOLVE II Study Randomized, sham-controlled, double-blind, phase 3, pivotal trial To evaluate the safety and efficacy of the investigational sinus implant in adults with CRSwNP, who were indicated for repeat surgery to treat moderate-to-severe, medically-refractory symptoms of nasal obstruction/congestion and recurrent NPs in both ethmoid sinuses The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.

Patients CRSwNP Prior ESS, including bilateral total ethmoidectomy Indicated for revision ESS Persistent moderate-to-severe symptoms of nasal obstruction/congestion score ³ 2 (scale 0 to 3) on 5 out of 7 days despite ongoing INCS and recent course of high-dose steroids Post-nasal discharge, facial pain/pressure, or decreased sense of smell Bilateral ethmoid sinus polyps grade ³ 2 (scale 0 to 4 on each side) by an independent reviewer The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.
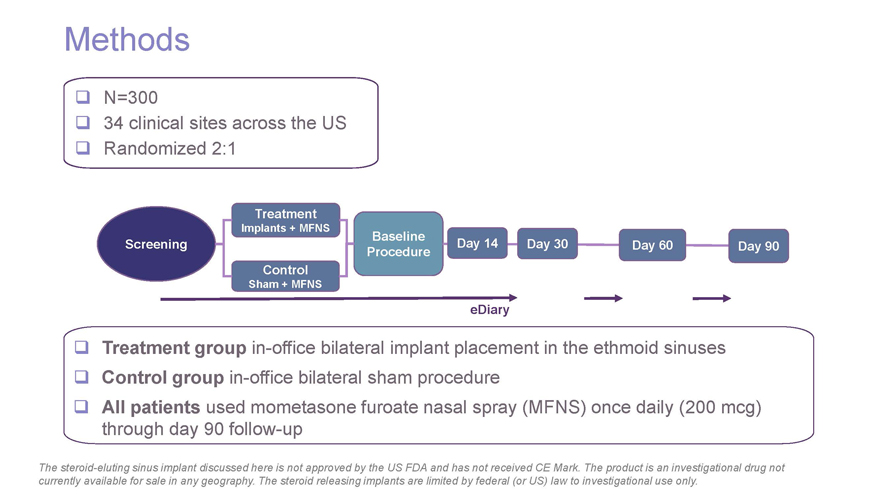
Methods N=300 34 clinical sites across the US Randomized 2:1 Treatment Implants + MFNS Baseline Screening Day 14 Day 30 Day 60 Day 90 Procedure Control Sham + MFNS eDiary Treatment group in-office bilateral implant placement in the ethmoid sinuses Control group in-office bilateral sham procedure All patients used mometasone furoate nasal spray (MFNS) once daily (200 mcg) through day 90 follow-up The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.
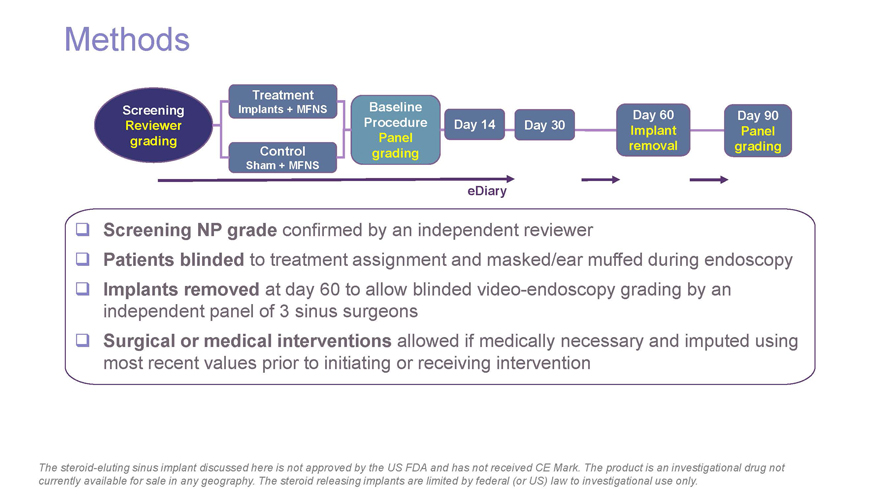
Methods Treatment Screening Implants + MFNS Baseline Day 60 Day 90 Reviewer Procedure Day 14 Day 30 Implant Panel grading Panel Control removal grading Sham + MFNS grading eDiary Screening NP grade confirmed by an independent reviewer Patients blinded to treatment assignment and masked/ear muffed during endoscopy Implants removed at day 60 to allow blinded video-endoscopy grading by an independent panel of 3 sinus surgeons Surgical or medical interventions allowed if medically necessary and imputed using most recent values prior to initiating or receiving intervention The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.

Assessment Patient-reported outcomes Endoscopic examination Adverse events The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.
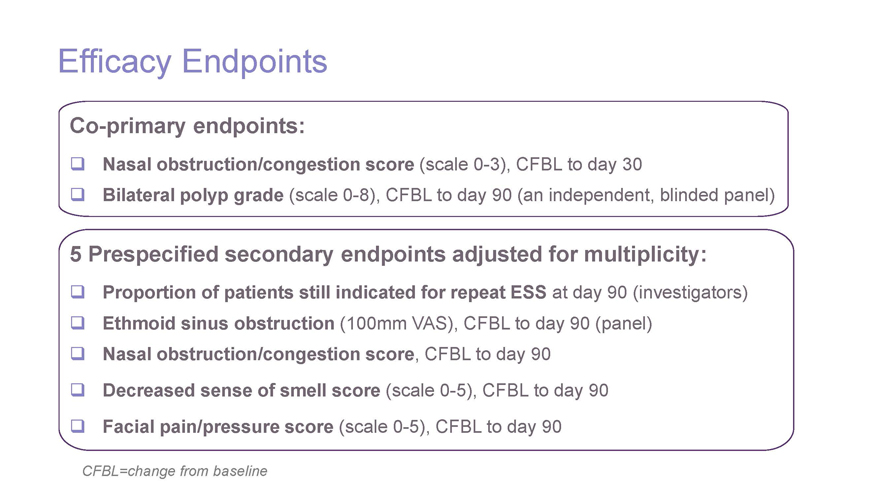
Efficacy Endpoints Co-primary endpoints: Nasal obstruction/congestion score (scale 0-3), CFBL to day 30 Bilateral polyp grade (scale 0-8), CFBL to day 90 (an independent, blinded panel) 5 Prespecified secondary endpoints adjusted for multiplicity: Proportion of patients still indicated for repeat ESS at day 90 (investigators) Ethmoid sinus obstruction (100mm VAS), CFBL to day 90 (panel) Nasal obstruction/congestion score, CFBL to day 90 Decreased sense of smell score (scale 0-5), CFBL to day 90 Facial pain/pressure score (scale 0-5), CFBL to day 90 CFBL=change from baseline
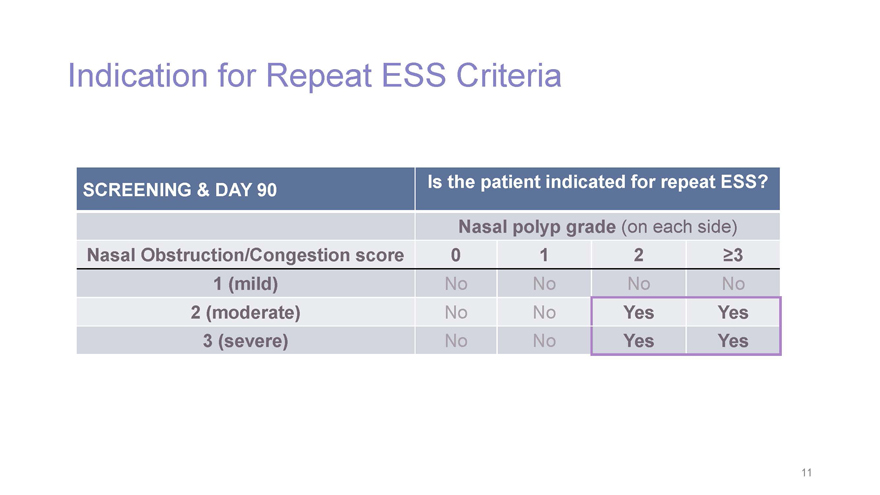
Indication for Repeat ESS Criteria SCREENING & DAY 90 Is the patient indicated for repeat ESS? Nasal polyp grade (on each side) Nasal Obstruction/Congestion score 0 1 2 ³3 1 (mild) NoNo No No 2 (moderate) NoNo Yes Yes 3 (severe) NoNo Yes Yes 11
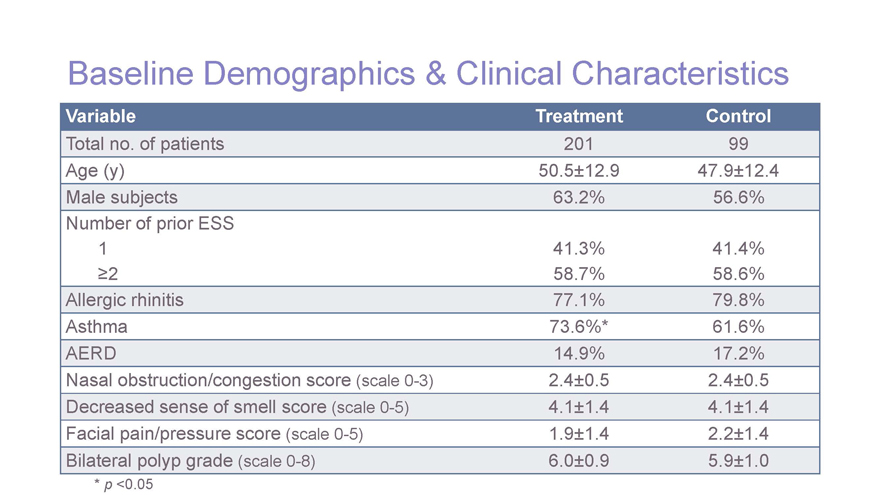
Baseline Demographics & Clinical Characteristics Variable Treatment Control Total no. of patients 20199 Age (y)50.5±12.947.9±12.4 Male subjects63.2%56.6% Number of prior ESS 141.3%41.4% ³258.7%58.6% Allergic rhinitis77.1%79.8% Asthma73.6%*61.6% AERD14.9%17.2% Nasal obstruction/congestion score (scale 0-3)2.4±0.52.4±0.5 Decreased sense of smell score (scale 0-5)4.1±1.44.1±1.4 Facial pain/pressure score (scale 0-5)1.9±1.42.2±1.4 Bilateral polyp grade (scale 0-8)6.0±0.95.9±1.0 * p <0.05
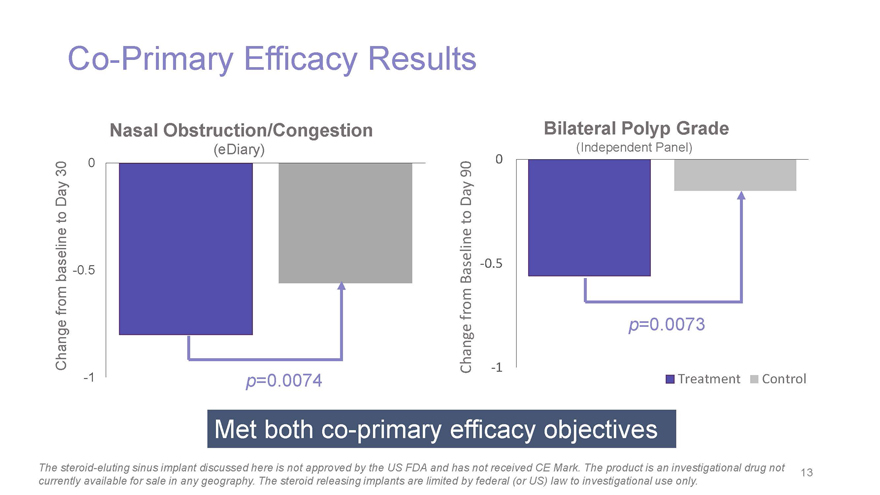
Co-Primary Efficacy Results Nasal Obstruction/Congestion Bilateral Polyp Grade (eDiary) (Independent Panel) 0 0 30 90 Day Day to to baseline -0.5 Baseline -0.5 from Change from p=0.0073 Change -1 -1 p=0.0074 Treatment Control Met both co-primary efficacy objectives The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not 13 currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.
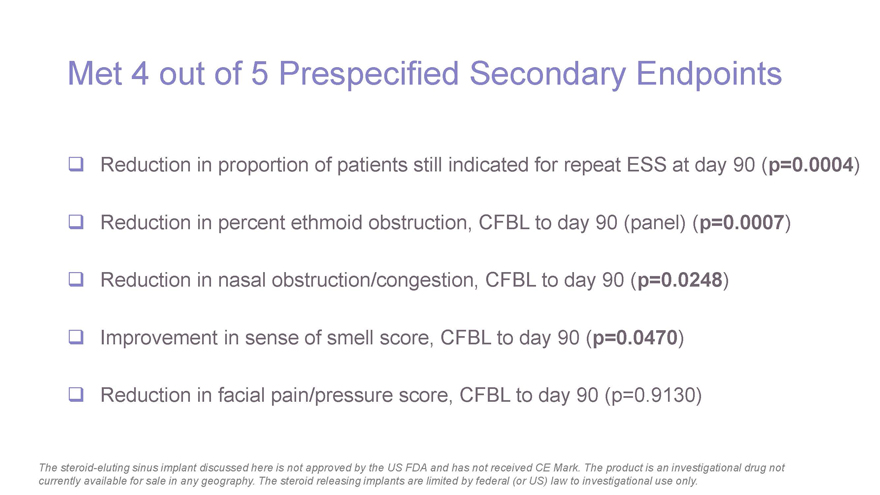
Met 4 out of 5 Prespecified Secondary Endpoints Reduction in proportion of patients still indicated for repeat ESS at day 90 (p=0.0004) Reduction in percent ethmoid obstruction, CFBL to day 90 (panel) (p=0.0007) Reduction in nasal obstruction/congestion, CFBL to day 90 (p=0.0248) Improvement in sense of smell score, CFBL to day 90 (p=0.0470) Reduction in facial pain/pressure score, CFBL to day 90 (p=0.9130) The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.
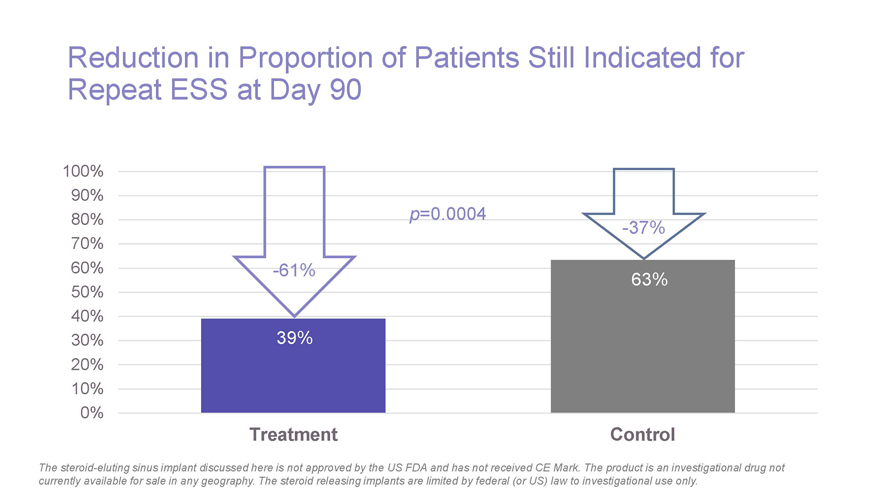
Reduction in Proportion of Patients Still Indicated for Repeat ESS at Day 90 100% 90% .0004 80% p=0 -37% 70% 60% -61% 63% 50% 40% 30% 39% 20% 10% 0% Treatment Control The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.
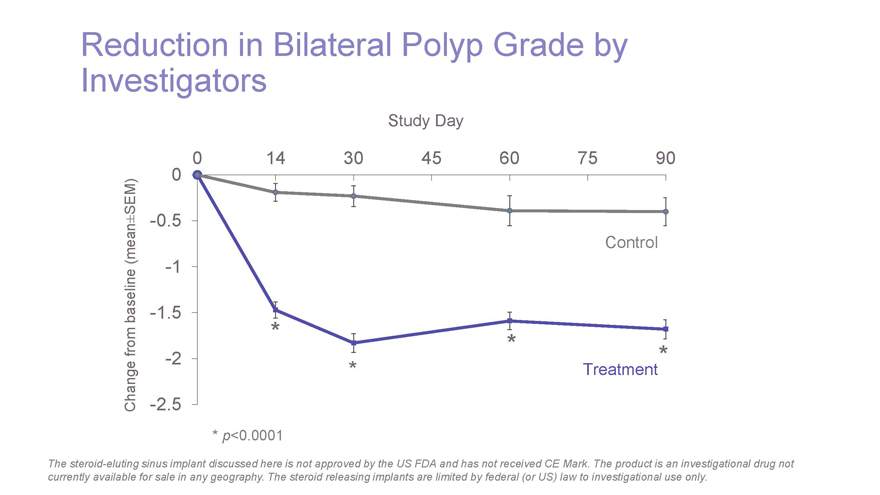
Reduction in Bilateral Polyp Grade by Investigators Study Day 0 14 30 45 60 75 90 SEM) 0 ± -0.5 (mean Control -1 baseline -1.5 * from * -2 * Treatment* Change -2.5 * p-value < 0.05 * p<0.0001 The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.
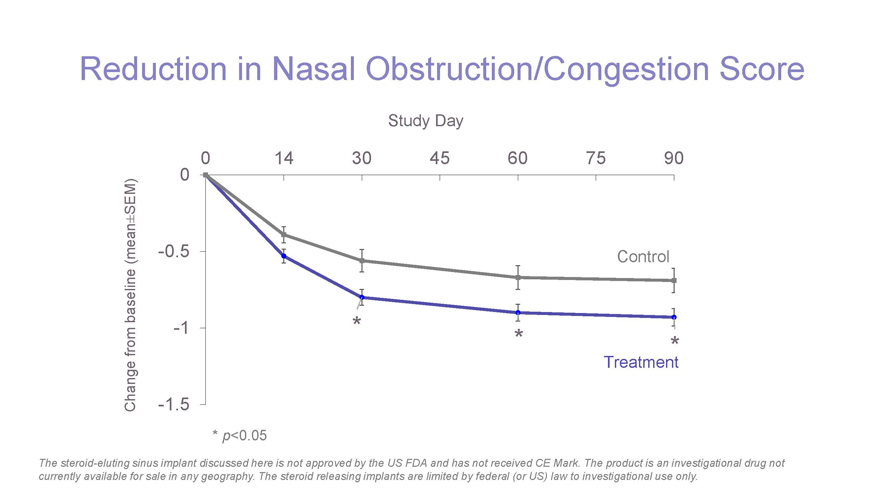
Reduction in Nasal Obstruction/Congestion Score Study Day 0 14 30 45 60 75 90 SEM) 0 ± (mean -0.5 Control baseline -1 * * from * Treatment Change -1.5 * p-value < 0.05 * p<0.05 The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.
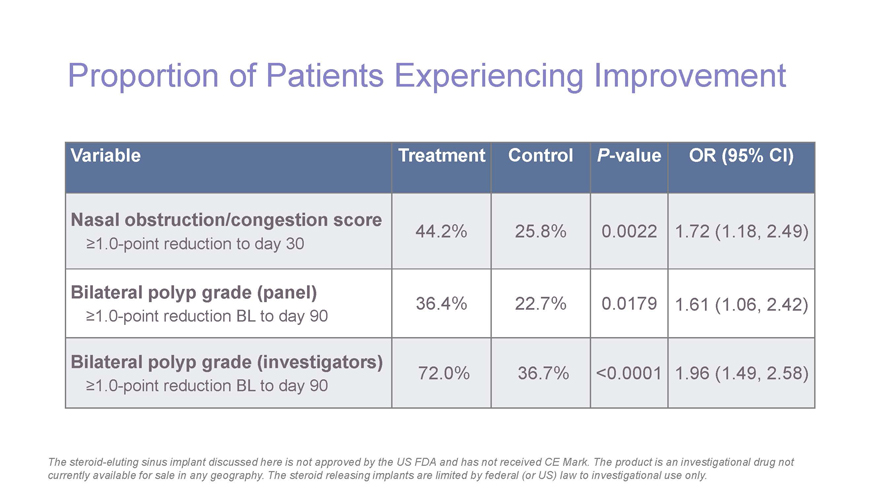
Proportion of Patients Experiencing Improvement Variable Treatment Control P-value OR (95% CI) Nasal obstruction/congestion score 44.2% 25.8% 0.0022 1.72 (1.18, 2.49) ³1.0-point reduction to day 30 Bilateral polyp grade (panel) 36.4% 22.7% 0.0179 1.61 (1.06, 2.42) ³1.0-point reduction BL to day 90 Bilateral polyp grade (investigators) 72.0% 36.7% <0.0001 1.96 (1.49, 2.58) ³1.0-point reduction BL to day 90 The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.
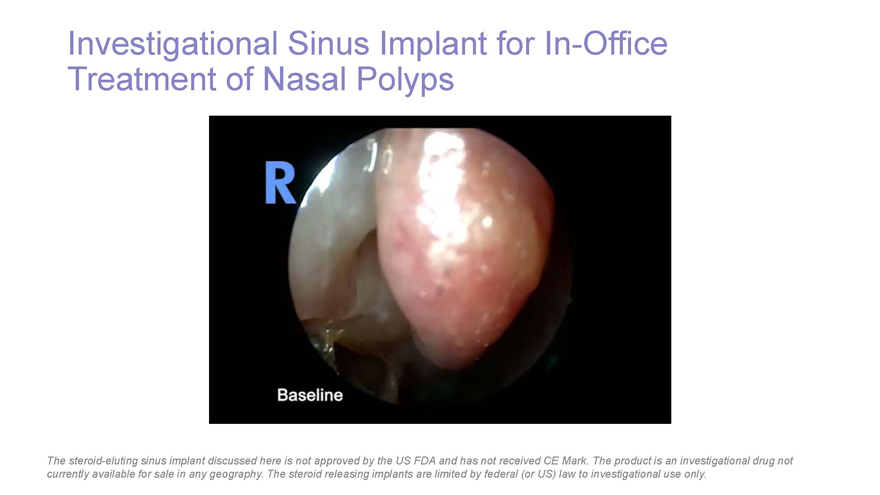
Investigational Sinus Implant for In-Office Treatment of Nasal Polyps The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.

Safety 4 Serious adverse events in 3 patients (2 treatment and 1 control), but only 1 (0.5%) implant-related (epistaxis) Similar incidence of adverse events between treatment and control groups (45.3% vs. 37.4%) The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.
Conclusion The RESOLVE II study following in-office placement of the corticosteroid-releasing implants in patients with recurrent nasal polyps met both co-primary objectives: o Reduction in nasal obstruction/congestion and o Reduction in bilateral polyp grade The study also showed a reduction in the need for revision surgery. The steroid-eluting sinus implant discussed here is not approved by the US FDA and has not received CE Mark. The product is an investigational drug not currently available for sale in any geography. The steroid releasing implants are limited by federal (or US) law to investigational use only.
