Attached files
| file | filename |
|---|---|
| 8-K - 8-K - CAPRICOR THERAPEUTICS, INC. | v476343_8k.htm |
Exhibit 99.1

NASDAQ: CAPR October 2017 Capricor Therapeutics

Forward - Looking Statements 2 Statements in this presentation regarding the efficacy, safety, and intended utilization of Capricor's product candidates; the initiation, conduct, size, timing and results of discovery efforts and clinical trials; the pace of enrollment of clinical trials; plans regarding regulatory filings, future research and clinical trials; regulatory developments involving products, including the ability to obtain regulatory approvals or otherwise bring products to market; plans regarding current and future collaborative activities and the ownership of commercial rights; scope, duration, validity and enforceability of intellectual property rights; future royalty streams, expectations with respect to the expected use of proceeds from the recently completed offerings and the anticipated effects of the offerings, and any other statements about Capricor's management team's future expectations, beliefs, goals, plans or prospects constitute forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements that are not statements of historical fact (including statements containing the words "believes," "plans," "could," "anticipates," "expects," "estimates," "should," "target," "will," "would" and similar expressions) should also be considered to be forward - looking statements. There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward - looking statements. More information about these and other risks that may impact Capricor's business is set forth in Capricor's Annual Report on Form 10 - K for the year ended December 31, 2016 as filed with the Securities and Exchange Commission on March 16, 2017, in its Registration Statement on Form S - 3, as filed with the Securities and Exchange Commission on September 28, 2015, together with the prospectus included therein and prospectus supplements thereto, and in its Quarterly Report on Form 10 - Q for the quarter ended June 30, 2017, as filed with the Securities and Exchange Commission on August 14, 2017. All forward - looking statements in this presentation are based on information available to Capricor as of the date hereof, and Capricor assumes no obligation to update these forward - looking statements.

Investment Highlights ‒ Pipeline focused on rare pediatric disorders for which current options are inadequate ‒ Clinical proof - of - concept demonstrated in lead indication ▪ Potential registration trial planned to initiate in 1Q18 * ▪ $1B+ U.S. sales opportunity ▪ Capricor holds worldwide IP rights ‒ Scalable, cost - efficient manufacturing process in development ‒ Disruptive technology platform that may address several challenging diseases ‒ Significant ownership by insiders and strategic investor 3 * Subject to regulatory approval

Capricor’s Product Pipeline 4 Candidate Indication Development Phase Status Preclinical Clinical Market CAP - 1002 (allogeneic CDCs) Duchenne Muscular Dystrophy ▪ Improvement in skeletal and cardiac muscle function shown in randomized clinical trial in advanced DMD ▪ Plan to initiate potential registration trial in 1Q18 * ▪ Orphan Drug and Rare Pediatric Disease Designations; RMAT eligible CAP - 2003 (CDC - e xosomes) Hypoplastic Left Heart Syndrome ▪ Plan to submit IND in 2018 ▪ Awarded NIH grant of up to $4.2 M Inflammatory Disorders ▪ Exploring potential indications * Subject to regulatory approval. CDCs = cardiosphere - derived cells

Evolution of Capricor’s Science and Clinical Development 5

Capricor’s Technology 6 ‒ Capricor’s core technology = cardiosphere - derived cells (CDCs) ▪ Unique population of cells which include cardiac progenitor cells with a distinct surface marker profile ▪ Subject of over 100 peer - reviewed scientific publications ▪ CAP - 1002 (allogeneic CDCs) has been evaluated in several clinical trials ‒ CDCs shown to exert diverse bioactivities ▪ Regenerative ▪ Anti - fibrotic ▪ Anti - apoptotic ‒ CDCs effect their actions at a distance ▪ Secrete extracellular vesicles (CDC - exosomes) that contain a variety of signalling molecules ▪ Do not act by “ stemness ” – do not engraft into host tissue ▪ Angiogenic ▪ Anti - inflammatory ▪ Immunomodulatory

CAP - 1002 Exerts its Actions via Extracellular Vesicles (EVs) 7 — Exosomes are nano - scale extracellular vesicles released by most cells ▪ Charged with a variety of biomolecules, including RNAs and proteins ▪ Emerging as major players in cell - to - cell communication cardiosphere - derived cells Skeletal muscle: Exercise tolerance Contractile force Energy generation Cell formation epigenetic modulation of protein expression CDC exosomes

CAP - 1002 for Duchenne Muscular Dystrophy 8 ‒ CAP - 1002’s initial market opportunity is for the treatment of DMD ▪ Rare pediatric disorder – WW incidence ~1 / 3,600 male births ▪ Progressive muscle weakness with eventual loss of function starting in early childhood – Affects skeletal, cardiac, and respiratory muscles ▪ Loss of ambulation typically by early teens; death typically before age 30 ▪ DMD poorly met by current therapeutic options ‒ Plan to initiate potential U.S. registration trial in the first quarter of 2018* ▪ Clinical proof - of - concept has been demonstrated for CAP - 1002 in DMD ▪ Excellent safety record per cumulative clinical experience in ~140 human subjects ▪ Being developed as a 30 minute intravenous infusion to be given every 90 days * Subject to regulatory approval.

Progressive Muscle Wasting Leads to Weakness, Loss of Motor Function and Early Death 9
Treatment and Management Options are Limited 10 Glucocorticosteroids ▪ Anti - inflammatory ▪ Slow muscle degeneration ▪ Delay progression ▪ Onerous, permanent side effects Exondys 51 ( eteplirsen ) ▪ “Exon - skipping drug” approved in 2016 ▪ Dystrophin expression via read - through ▪ Addresses only 13% of DMD population ▪ Functional improvement yet to be rigorously shown Occupational Therapy Medication Surgery Assistive Devices Physical Therapy

Lack of Dystrophin Predisposes Muscle to Damage 11 • X - linked genetic disorder • Nearly all affected are male • Dystrophin is a structural protein in muscle • Acts both as a cushion and a kind of glue • Without dystrophin, muscles are unable to function properly, suffer progressive damage and eventually die
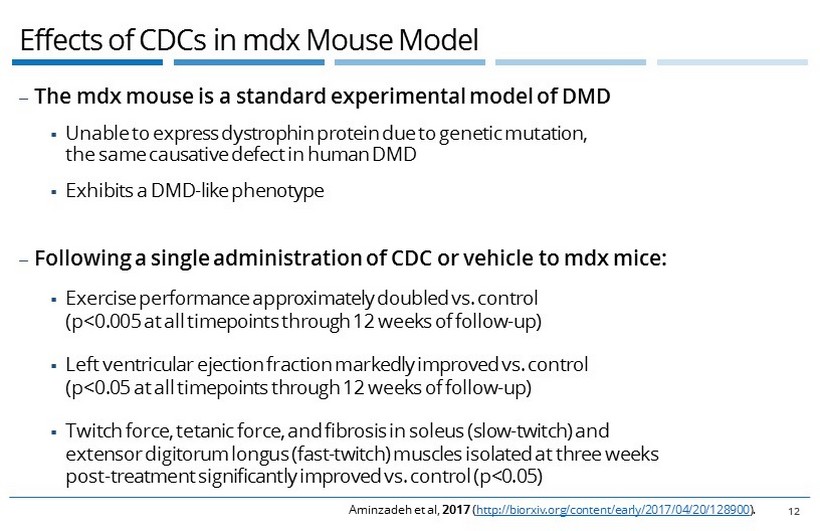
Effects of CDCs in mdx Mouse Model 12 ‒ The mdx mouse is a standard experimental model of DMD ▪ Unable to express dystrophin protein due to genetic mutation, the same causative defect in human DMD ▪ Exhibits a DMD - like phenotype ‒ Following a single administration of CDC or vehicle to mdx mice: ▪ Exercise performance approximately doubled vs. control (p<0.005 at all timepoints through 12 weeks of follow - up) ▪ Left ventricular ejection fraction markedly improved vs. control (p<0.05 at all timepoints through 12 weeks of follow - up) ▪ Twitch force, tetanic force, and fibrosis in soleus (slow - twitch) and extensor digitorum longus (fast - twitch) muscles isolated at three weeks post - treatment significantly improved vs. control (p<0.05) Aminzadeh et al, 2017 ( http://biorxiv.org/content/early/2017/04/20/128900 ).

Physiological Effects of CDC in mdx Model 13 Oxidative Stress Fibrosis Inflammation Mitochondrial Integrity Muscle Cell Generation phospho - Akt Nrf2 (cytoplasmic) Nrf2 (nuclear) HO - 1 catalase SOD - 2 GCLC cat. sub. phospho - IkB p65 (nuclear) MCP1 CD68+ macrophages CD3+ T cells collagen I collagen III m itochondrial DNA copy number level of respiratory chain subunits RESTORED mitochondrial ultrastructure NORMALIZED deficient respiratory capacity of isolated mitochondria Ki67 + cardiomyocytes Aurora B cardiomyocytes NF - kB Adapted from Aminzadeh et al, 2017 ( http://biorxiv.org/content/early/2017/04/20/128900 ).

Phase I / II HOPE - Duchenne Clinical Trial 14 CAP - 1002 (1 x 75M cells) Usual Care n=12 25 patients Ages 12 and older DMD heart disease n=13 6 — Trial population characterized by advanced disease: CAP - 1002 given by intracoronary infusion ▪ all are receiving steroids ▪ majority are non - ambulant — Most DMD clinical development to date has been conducted in less sick patients 12 top - line interim data reported in April 2017

PUL Results Indicated Skeletal Muscle Benefit 15 ‒ The Performance of the Upper Limb (PUL) test was designed specifically for use in the DMD setting ‒ Individual tasks represent real - world activities of daily living (ns) improvement Thought leaders in DMD with whom we have shared these data view them as indicating a meaningful functional improvement in those treated with CAP - 1002 (ns) (ns)

Cardiac Functional Measures Improved in CAP - 1002 Patients 16 improvement improvement Spontaneous scar reduction is not normally observed in DMD Systolic thickening is believed to be a principal mechanism of cardiac output generation in DMD Month 6 (ns) (ns) (ns) (ns)

Cardiac and Skeletal Muscle Effects Seen in the Same Patients 17 Inferior Wall Thickening + Middle - Level PUL Scar Reduction + Middle - Level PUL * Concordance of improvements support a treatment effect p - values:

Key Conclusions from Six - Month HOPE Data — Results support the potential benefit of CAP - 1002 to boys and young men with advanced Duchenne muscular dystrophy — Signal of skeletal muscle improvement (PUL) — Signal of cardiac muscle improvement (wall thickening) — Approx. three month duration of effect following a single dose — CAP - 1002 generally safe and well - tolerated 18 Sets the stage for registration program based on functional improvement
▪ Randomized, double - blind, placebo - controlled ▪ Peripheral intravenous delivery – supported by preclinical studies ▪ Repeat - dose design – potential to achieve sustained benefit ▪ Primary efficacy endpoint to be based on the PUL test at six months ▪ Principal Investigator – Craig M. McDonald, M.D. — FDA willing to accept PUL as an efficacy endpoint for registration ▪ Type B meeting held following six - month HOPE data 19 * Subject to regulatory approval. Plan to Initiate HOPE - 2 Clinical Trial of CAP - 1002 in 1Q18 *

Manufacturing 20 ‒ CAP - 1002 is manufactured from donor hearts via a proprietary process ‒ Clinical trial material currently produced at Capricor facility ‒ High - yield process in advanced development ‒ Selection of CMO in process for anticipated early - stage commercial manufacture CSps CDCs Wash Formulate Fill CAP - 1002
Commercial Outlook for CAP - 1002 in DMD 21 — DMD prevalence: U.S. ~15,000 Europe ~20,000 ▪ WW incidence ~ one per 3,600 live male births — CAP - 1002 positioned to have broad treatment potential ▪ Acts upon multiple pathways that contribute to DMD disease process ▪ Lack of mutation dependence due to downstream site of action — Opportunity for “ultra - orphan” pricing ▪ Exondys 51 carries average cost of ~USD 300,000 per year

Capricor has Assembled a World - Class DMD Advisory Board 22 Barry Byrne, M.D., Ph.D. University of Florida (USA) Michelle Eagle, Ph.D., M.Sc., MCSP Atom International Ltd (UK) Richard Finkel , M.D. Nemours Children's Hospital (USA) Pat Furlong Parent Project Muscular Dystrophy (USA) Kan Hor , M.D. Nationwide Children's Hospital (USA) John Jefferies, M.D. Cincinnati Children's Hospital Medical Center (USA) Oscar Henry Mayer, M.D. Children's Hospital of Philadelphia (USA) Craig McDonald, M.D. University of California at Davis (USA) Eugenio Mercuri , M.D., Ph.D. Catholic University of the Sacred Heart (Italy) Francesco Muntoni , M.D. University College London (UK) Ron Victor, M.D. Cedars - Sinai Medical Center (USA) Thomas Voit , M.D. University College London (UK)

Relationships with Key DMD Advocacy Organizations 23

Current Resources Expected to Fund Operations Through 2Q18 24 Capricor has received over $30 million in competitive grants and a loan award from: California Institute of Regenerative Medicine National Institutes of Health U.S. Department of Defense Cash and cash equivalents $12.3 million (as of 6/30/17) Shares outstanding 23.5 million (as of August 10 , 2017 ) Capricor reported the above cash, cash equivalents, and shares outstanding in its most recent Quarterly Report on Form 10 - Q filed with the SEC on August 14, 2017.

Recent and Upcoming Milestones 25 CAP - 1002 in Duchenne Muscular Dystrophy x April 2017: Reported positive top - line six - month results of HOPE trial x July 2017: Granted Rare Pediatric Disease Designation x July 2017: Announced results of FDA meeting x September 2017: Announced Craig McDonald, M.D. to lead HOPE - 2 clinical trial October 2017: Present six - month HOPE results at World Muscle Society Congress 4Q 2017: Submit IND for i.v. CAP - 1002 in DMD with request for RMAT Designation 1Q 2018: Plan to initiate HOPE - 2 Trial of repeat - dose, intravenous CAP - 1002 * CAP - 2003 2018: Expect to submit IND for hypoplastic left heart syndrome (HLHS) * Subject to regulatory approval.
