Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - ASSEMBLY BIOSCIENCES, INC. | v475710_ex99-1.htm |
| 8-K - FORM 8-K - ASSEMBLY BIOSCIENCES, INC. | v475710_8k.htm |
Exhibit 99.2

Targeting HBV Core Protein to Achieve Higher Cure Rates Richard Colonno Targeting HBV New Drug Development for HBV & Other Infectious Diseases September 25, 2017 Boston, MA

Cautionary Note Regarding Forward - looking Statements Some of the information in this presentation contains forward - looking statements regarding future events, including statements about the clinical and therapeutic potential of Assembly’s development programs and drug candidates. Certain forward - looking statements may be identified by reference to a future period or periods or by use of forward - looking terminology such as “may,” “expected,” “should” or "predictive." Assembly intends such forward - looking statements to be covered by the safe harbor provisions contained in Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Actual results or developments may differ materially from those projected or implied in these forward - looking statements. More information about the risks and uncertainties faced by Assembly are more fully detailed under the heading "Risk Factors" in Assembly’s Annual Report on Form 10 - K for the year ended December 31, 2016, and Quarterly Report on Form 10 - Q for the quarter ending June 30, 2017 filed with the Securities and Exchange Commission. Except as required by law, Assembly assumes no obligation to update publicly any forward - looking statements, whether as a result of new information, future events or otherwise. 2

Significant Need Remains for Curative Therapies □ Number of chronically - infected HBV patients exceeds the number of patients infected with HCV (~170M ) plus HIV (~37M) combined □ The majority are undiagnosed – often asymptomatic for years □ Chronic HBV infection results in chronic inflammation and progressive liver damage, potentially leading to liver cirrhosis, HCC and death (~1M deaths/year) HBV Carrier Rate High (HBsAg prevalence >8%) Intermediate (HBsAg prevalence 2% - 8%) Low (HBsAg prevalence <2%) China Prevalence: ~90 million Global Prevalence: >300 million U.S. Prevalence: ~1.5 million EU Prevalence: ~20 million Jidong Jia. HBV HCV epidem & therapy 2013 http ://www.who.int/hepatitis/en/ 3

Current HBV Therapies □ Currently Approved ─ Nucleoside Analogs: Entecavir, Lamivudine, Telbivudine ─ Nucleotide Analogs: Tenofovir, Adefovir , Tenofovir Alafenamide ─ Interferons (IFN and peg - IFN ) □ Entecavir and Tenofovir ─ Safe , highly effective therapies and the current drugs of choice ─ Target the viral polymerase, inhibiting reverse transcription of negative - strand DNA from pgRNA and positive - strand HBV DNA synthesis to generate rcDNA ─ Highly effective reduction and maintenance of HBV DNA at undetectable levels in virtually all treatment - naïve patients ─ HBV DNA undetectability maintained for prolonged periods (years ) ─ One pill, once - a - day dosing ─ Very well tolerated, with no meaningful resistance emergence over prolonged treatment periods ─ Unfortunately, cure rates are very low despite prolonged therapy 4

Deficiencies of Current Approved Therapies HBV Patients ETV 1,2 TFV 3 Peg - IFN 4,5 HBeAg Positive N = 354 N = 176 N = 271 HBV DNA Undetectable 67% 76% 25% a HBeAg Seroconversion 21% 21% 27% ALT Normalization 68% 68% 39% HBsAg Loss 2.0% 3.2% 2.9% HBeAg Negative N = 325 N = 250 N = 177 HBV DNA Undetectable 90% 93% 63% a ALT Normalization 78% 76% 38% HBsAg Loss 0.3% 0% 0.6% b a HBV DNA <400 copies/mL; b 72 weeks Table courtesy of Geoff Dusheiko 1 Chang T - T, et al . N Engl J Med 2006:354:1001 - 10 2 Lai C - L, et al. N Engl J Med 2006:354:1011 - 20 3 Marcellin P, et al. N Engl J Med 2008:359:2442 - 55 4 Lau GKK, et al. N Engl J Med 2005:352:2682 - 95 5 Marcellin P, et al. N Engl J Med 2004:351:1206 - 17 0 20 40 60 80 100 12 24 36 62.5 53.4 51.5 44 31 30 HBeAg Pos HBeAg Neg Virologic Relapse After Nuc Discontinuation Papatheodoridis G. et al, Hepatology 2016 % Patients with HBV DNA <20,000 IU/mL Months After NUC Discontinuation HBeAg Positive Patients 14 studies, 733 initially HBeAg positive Pooled HBsAg loss: 1 % HBeAg Negative Patients 17 studies, 967 HBeAg negative Pooled HBsAg loss: 1.7 % Results at 48 Weeks 5
Woodchuck Hepatitis B Virus Model □ WHBV infection at 3 days of age results in a carrier state with life - long viremia □ Chronically - infected animals mimic the HBV carrier state in man (viral pathogenesis & development of HCC) □ Infected woodchucks have a >90% chance of dying of HCC within 4 years □ Predictive model for toxicity and effectiveness of antivirals in man □ ETV potency against WHBV equivalent to HBV Long - term treatment study conducted to determine if prolonged ETV therapy could cure woodchucks? Colonno , et al. JID 2001;184:1236 - 45 Thought We Had A Chance for Cure 12 Years Ago 6
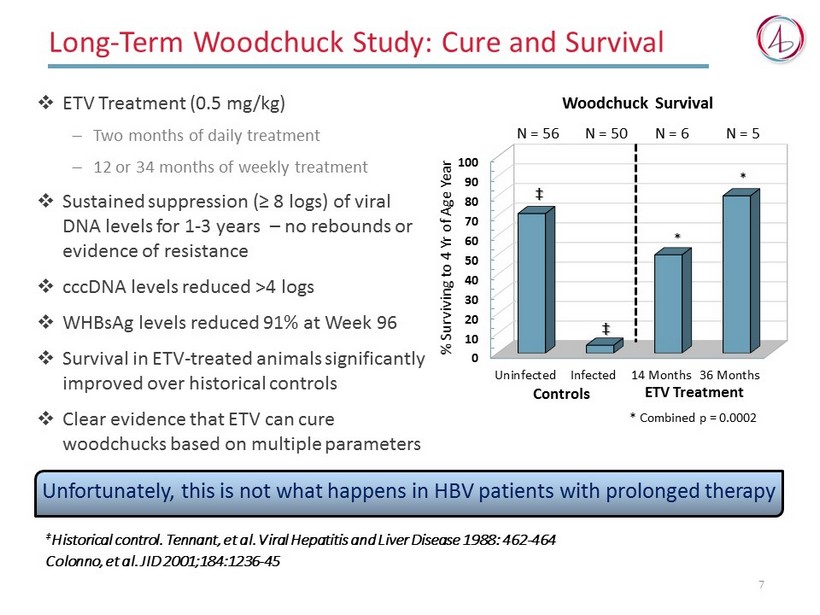
Long - Term Woodchuck Study : Cure and Survival □ ETV Treatment (0.5 mg/kg) ─ Two months of daily treatment ─ 12 or 34 months of weekly treatment □ Sustained suppression (≥ 8 logs) of viral DNA levels for 1 - 3 years – no rebounds or evidence of resistance □ cccDNA levels reduced >4 logs □ WHBsAg levels reduced 91% at Week 96 □ Survival in ETV - treated animals significantly improved over historical controls □ Clear evidence that ETV can cure woodchucks based on multiple parameters ‡ Historical control. Tennant, et al. Viral Hepatitis and Liver Disease 1988: 462 - 464 Colonno , et al. JID 2001;184:1236 - 45 0 10 20 30 40 50 60 70 80 90 100 Uninfected Infected 14 Months 36 Months * Combined p = 0.0002 % Surviving to 4 Yr of Age Year * ‡ ETV Treatment N = 56 N = 50 N = 5 N = 6 * ‡ Controls Woodchuck Survival 7 Unfortunately, this is not what happens in HBV patients with prolonged therapy

HBV Life Cycle: Complex and Poorly Understood cccDNA Maintenance cccDNA Maintenance 8

HBV Life Cycle: Failure of Nucs to Inhibit cccDNA cccDNA Maintenance cccDNA Maintenance Nucleos(t)ides 9

Aspirational Objectives for Clinical Cure in Humans □ We want what we achieved in woodchucks! □ Must cause depletion of cccDNA pools ─ Inhibit generation of new cccDNA ─ Direct silencing or elimination of existing cccDNA (more challenging) □ Decrease HBsAg levels to restore/enhance immune response □ Treatment of less than 2 years ─ Convenient dosing (QD?) and low pill burden ─ Excellent safety profile, with minimal side effects □ Sustained remission off therapy ─ Viral DNA replication remains undetectable ─ Elimination of cccDNA reservoirs □ Clinical efficacy ─ HBsAg loss and ideally, seroconversion ─ Reversal of liver damage, lack of hepatic inflammation ─ Significant reduction in the risk of future HCC development 10
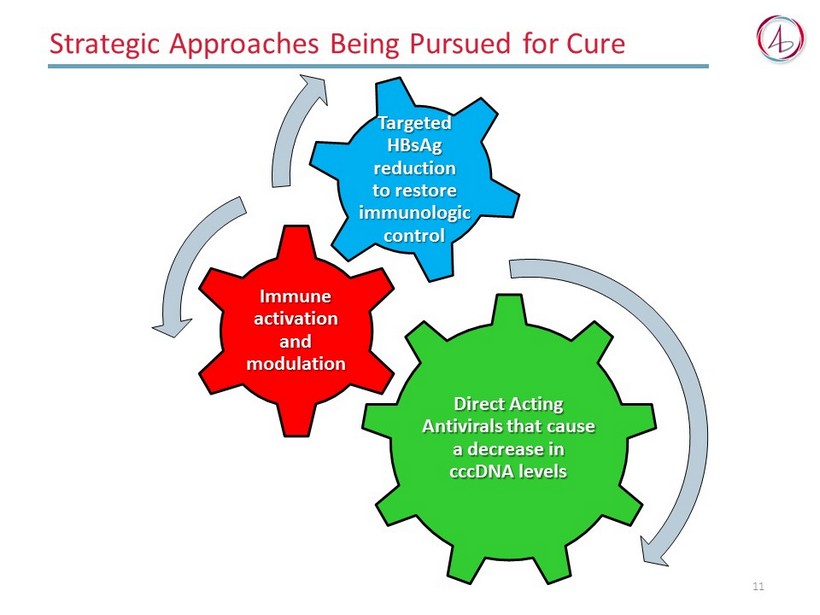
Strategic Approaches Being Pursued for Cure 11 Direct Acting Antivirals that cause a decrease in cccDNA levels Immune activation and modulation Targeted HBsAg reduction to restore immunologic control

Assembly Biosciences Focused on Targeting Core Protein to Decrease cccDNA Levels

cccDNA Maintenance ASMB Focused on Inhibitors of Core Protein CpAMs CpAMs CpAMs cccDNA Maintenance 13 • CpAM = Core protein Allosteric Modifiers

CpAMs Inhibit HBV Life Cycle at Several Steps □ CpAMs (Core protein Allosteric Modifiers) target Core protein and inhibit key functional steps required for to cccDNA generation □ Bind at dimer - dimer interface and disrupt the orderly folding of Core protein dimers into functional nucleocapsids, generating empty aberrant capsids □ Prevent encapsidation of Pol and pgRNA, a pre - requisite for RT activity and generation of rcDNA □ Prevent maturation of infectious viral particles □ Prevent trafficking of encapsidated rcDNA to nucleus for conversion to cccDNA □ Alter phosphorylation levels of Core protein, and may also lead to Core protein elimination in infected cells □ Ability to disrupt existing nucleocapsids 14 Because their distinct inhibitory mechanism(s), CpAMs and Nucs together should exhibit enhanced antiviral potency and have the potential to reduce levels of both cccDNA and HBsAg

cccDNA Maintenance Antiviral Effects of CpAMs CpAMs CpAMs cccDNA Maintenance Control + CpAM Addition of CpAMs causes formation of aberrant capsids that are larger, cracked and asymmetrical Electron Micrographs (100 nm) In Vitro Capsid Disruption Assay CpAMs 15

cccDNA Maintenance Antiviral Effects of CpAMs CpAMs CpAMs cccDNA Maintenance CpAMs 16 0 50 100 150 pgRNA DNA DMSO ETV (17x EC50) CpAM (10x EC50) % of Control CpAMs prevent encapsidation of pgRNA and subsequent synthesis of DNA in nucleocapsids Intracellular Encapsidation Study

cccDNA Maintenance Antiviral Effects of CpAMs CpAMs CpAMs cccDNA Maintenance CpAMs 17 CpAMs cause premature melting of existing nucleocapsids and prevent trafficking of rcDNA to nucleus DMSO Control ETV 167x EC 50 Capsid DNA CpAM 158x EC 50 Southern Blot of HBV DNA from HepG2 - NTCP Cells 3 h r Post Infection

cccDNA Maintenance Antiviral Effects of CpAMs CpAMs CpAMs cccDNA Maintenance CpAMs ETV >10K x EC 50 cccDNA CpAMs inhibit cccDNA generation/formation DMSO Control CpAM 8x EC 50 CpAM 38x EC 50 DMSO Control Southern Blot of HBV cccDNA From Infected HepG2 - NCTP Cells 18

Clinical POC of CpAMs as Effective HBV Antivirals □ Satisfactory safety at all dose levels, no pattern of treatment - related clinical AEs or lab abnormalities □ Oral doses of 600 mg BD in chronic HBV patients resulted in a potent antiviral response in 28 days ─ Mean 1.72 log 10 IU/mL HBV DNA reduction ─ Mean 0.86 log 10 copies/ml reduction in serum HBV RNA levels NVR 3 - 778 Phase 1b 28 - Day M onotherapy R esults in HBV - Infected P atients Lawrence Blatt, J&J R&D Day Presentation 6 - 17 19
Assembly Biosciences Establishing A Pipeline of CpAMs

Drugable Properties Will Play an Important Role □ Higher concentrations of CpAMs will be required to decrease cccDNA levels relative to inhibiting viral DNA levels □ Premium will be placed on CpAMs with favorable DMPK properties that are able to achieve and maintain higher effective concentrations in infected hepatocytes □ Convenient oral dosing (frequency and pill burden) □ Metabolic stability in hepatocytes to enable maximal sustained inhibition □ Good PK profile ─ O ral bioavailability, half - life , C max and C min ─ Rapid achievement of steady state □ Controlled and predictable liver:plasma concentrations, minimal accumulation in liver □ Limited drug - drug interactions □ Well tolerated – good safety profile with prolonged dosing 21

HBV CpAM Discovery Lead Optimization IND Enabling Phase 1a Phase 1b Phase 2 ABI - H0731 ABI - H2158 Next Generation Assembly Pipeline of CpAMs □ ABI - H0731 has completed Phase 1a evaluation, and is currently being studied in HBV patients in a 28 - day, double - blind , placebo - controlled Phase 1b study □ ABI - H2158 selected as our next generation CpAM clinical candidate and is currently undergoing IND - enabling studies for initiation of Phase 1a studies □ Plan to identify and select a third CpAM clinical candidate by year end □ All ASMB CpAMs derived from distinct proprietary chemical scaffolds unrelated to previous HAP and Novira - like molecules 22

ABI - H0731 Preclinical Overview □ Selective targeting of dimer - dimer interface of HBV Core protein, leading to i nhibition of cccDNA generation in infected cell assays □ Pangenotypic coverage of HBV genotypes (A – D) □ No significant activity noted against a panel of other viruses or in cytotoxicity assays utilizing a panel of cell types □ Additive to synergistic in combination with Nuc □ Highly favorable DMPK properties ─ High (62 - 95%) oral bioavailability observed in all animal species tested ─ T½ predictive of human QD dosing ─ Limited accumulation with repeat dosing ─ Enhanced liver concentrations relative to plasma levels, parallel half - lives ─ Highly stable, excretion predominantly as intact compound □ Clean safety profile in a panel of GLP toxicology studies, no organ toxicities identified 23

ABI - H0731 Clinical Overview Phase 1a Completed □ Single oral doses from 100 to 1,000 mg, multiple doses of 800 mg QD and 800 mg BID x 7 days evaluated □ Favorable PK profile with a half - life consistent with QD dosing □ Well absorbed, with achievement of concentrations believed to be sufficient to suppress viral replication and cccDNA generation □ No SAEs, no clinically significant AEs and no withdrawals due to AEs □ Treatment emergent AEs deemed “possibly related,” such as headache and rash, were mild and transient, and only observed at the highest doses □ No clinically significant treatment emergent laboratory abnormalities, vital sign changes or ECG findings Data to be presented at AASLD, October 2017 in Washington, DC Phase 1b In Progress □ 28 - Day monotherapy dosing in HBV patients 24

0.1 1 10 100 1000 10000 ABI - H2158 - Next Generation CpAM 25 Antiviral Activity in AD38 Induced Cells (Viral Load) Antiviral Activity in HepG2 - NTCP Infected Cells (Viral Load and HBeAg) • EC 50 (nM) 0.1 1 10 100 1000 10000 • EC 50 (nM) 0.6 281 216 22 8580 0.8 540 307 28 4950 390 HBV DNA HBV DNA HBeAg • SBA - R01 is believed to be NVR 3 - 778 or a very close analog (Pei Y. et al. , J. Med. Chem. 2017; 60, 6461− 6479) Projected

CpAMs Profiling in Primary Human Hepatocytes □ CpAMs reduced viral HBV DNA levels and known surrogate markers for cccDNA (HBeAg, HBsAg and pgRNA) □ ETV was highly effective at inhibiting HBV DNA levels, but exhibited limited effect on cccDNA surrogate markers CpAM HBV DNA EC 50 (nM) HBeAg EC 50 (nM) HBsAg EC 50 (nM) pgRNA EC 50 (nM) ETV <0.1 Est >100 Est >100 Est >100 SBA - R01 1,130 2,502 3,192 2,554 ABI - H0731 154 2,210 3,000 1,840 ABI - H2158 39 230 242 169 Cytotoxic 26 • Data to be presented at AASLD, October 2017 in Washington, DC

Profile of ASMB Clinical Candidates Virology Parameters SBA - R01 ABI - H0731 ABI - H2158 AD38 VL EC 50 (nM ) 281 170 14 HC9AT HBeAg EC 50 (nM ) 8,580 4,950 390 PHH VL EC 50 (nM) 1,130 150 23 PHH HBeAg EC 50 (nM) 2,502 2,210 230 DMPK Parameters Human Liver Microsomes (% remaining at 45 min) 100 87 91 CYP Profile (IC 50 ) All > 10 µM All >10 µM All ≥10 µM Protein Binding (%) 98 97 97 Rat PK %F (1 mg/kg) T 1/2 (hr) C max (ng/mL) Oral AUC last (hr*ng/mL ) 95 6.1 162 1,470 50 2.9 536 3,671 □ Unclear what potency/exposure levels are required to suppress cccDNA generation □ Liver concentrations of ABI - H0731 projected to be ~25x plasma concentrations □ If needed, ABI - H2158 has superior antiviral potency to ABI - H0731, while maintaining favorable DMPK properties 27

Will CpAM Treatment Result in Higher Cure Rates? Studies underway using patient samples to gain insight into both topics □ Lamivudine (LVD) and Telbivudine (LdT) treatment results in rapid emergence of resistant variants due to their low barrier to resistance □ LVD/LdT resistance maps to L180M and M204I substitutions in the Pol gene □ Used these genetic markers to monitor the turnover of cccDNA, pgRNA and viral DNA in longitudinal patient samples 28 Potential Challenges Weeks Years Long Intracellular cccDNA Half - life No Yes Significant Pools of Inactive cccDNA

cccDNA Studies – Initial Results □ All biological molecules have a half - life, including cccDNA □ Longitudinal study conducted on samples (serum and biopsies) from patients with emerging resistance to LVD and TBV □ Appearance and enrichment of resistant mutations used as a genetic marker in monitoring populations of viral DNA, pgRNA and cccDNA □ Results demonstrated rapid establishment of newly formed cccDNA pools harboring Nuc - resistant mutations □ Significant turnover of wt pgRNA molecules within months suggests that existing cccDNA may decay faster than previously predicted in the absence of any gross inflammation □ Little evidence for the existence and maintenance of substantial pools of inactive wt cccDNA in patient samples Data to be presented at AASLD, October 2017 in Washington, DC 29
CpAM Summary 30 □ M echanism - based studies demonstrated that CpAMs bind to Core protein and disrupt viral replication at multiple steps □ Importantly, and distinct from Nucs, CpAMs appear to block the generation of new cccDNA molecules! □ ASMB’s CpAM pipeline consists of candidate compounds selected and optimized from distinct and proprietary chemical series □ While the precise level of intrinsic potency needed for cccDNA inhibition in patients is yet to be established, emphasis has been placed on increasing potency while maintaining favorable DMPK properties □ Lead candidate ABI - H0731 has completed Phase 1a with favorable safety and PK properties predictive of QD dosing in patients, and is currently undergoing evaluation in chronically - infected patients (Phase 1b) □ Second generation candidate ABI - H2158 exhibits enhanced potency while retaining the favorable DMPK properties of ABI - H0731 □ Future combinations of CpAMs and Nucs should result in enhanced antiviral activity, have a high resistance barrier and most importantly, decrease cccDNA levels
Acknowledgements 31 Assembly Biosciences HBV Team Qi Huang Dawei Cai Ran Y an Yi Zhou Yuhua Zong Alex Mercier Pao - Chen Li Emily Connelly Lida Guo Lichun Li Esteban Carabajal Xuman Tang Uri Lopatin Sandy Laiw Eric Ruby Virology Clinical/Regulatory Chemistry/DMPK Leping Li Bill Turner Simon Haydar Lynn Bannen Mark Bures Roopa Rai Kelvin Chan Samson Francis Ray Kauffman Lee Arnold
