Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Synthetic Biologics, Inc. | v474743_8k.htm |
Exhibit 99.1

September 07, 2017 Investor Presentation

Forward - Looking Statements This presentation includes forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 , as amended, on Synthetic Biologics’ current expectations and projections about future events . In some cases forward - looking statements can be identified by terminology such as "may," "should," "potential," "continue," "expects," "anticipates," "intends," "plans," "believes,“ "estimates,” “indicates,” and similar expressions . These statements are based upon management’s current beliefs, expectations and assumptions and are subject to a number of risks and uncertainties, many of which are difficult to predict and include statements regarding our timeline for our SYN - 004 (ribaxamase) and SYN - 010 clinical trials and reporting of data, the size of the market, benefits to be derived from use of SYN - 004 (ribaxamase) and SYN - 010 , our anticipated patent portfolio, and our execution of our growth strategy . The forward - looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those set forth or implied by any forward - looking statements . Important factors that could cause actual results to differ materially from those reflected in Synthetic Biologics’ forward - looking statements include, among others, our product candidates demonstrating safety and effectiveness, as well as results that are consistent with prior results, our ability to initiate clinical trials and if initiated, our ability to complete them on time and achieve the desired results and benefits, our clinical trials continuing enrollment as expected, our ability to obtain regulatory approval for our commercialization of product candidates or to comply with ongoing regulatory requirements, regulatory limitations relating to our ability to promote or commercialize our product candidates for the specific indications, acceptance of our product candidates in the marketplace and the successful development, marketing or sale of our products, developments by competitors that render our products obsolete or non - competitive, our ability to maintain our license agreements, the continued maintenance and growth of our patent estate, our ability to become or remain profitable, our ability to establish and maintain collaborations, our ability to obtain or maintain the capital or grants necessary to fund our research and development activities, a loss of any of our key scientists or management personnel, and other factors described in Synthetic Biologics’ annual report on Form 10 - K for the year ended December 31 , 2016 , subsequent quarterly reports on Form 10 - Qs and any other filings we make with the SEC . The information in this presentation is provided only as of the date presented, and Synthetic Biologics undertakes no obligation to update any forward - looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law . 2

“Pioneering elegant solutions that leverage the microbiome to improve global health” Our Mission 3

Our Strategy Core Future Developing simple therapeutics that have the potential to influence the microbiome to protect the health of patients A robust pipeline of new programs designed to improve global health Growth Advancing innovative microbiome drugs with billion - dollar market potential that are poised for pivotal trials 4

Solving Critical Problems 5

The Gut Microbiome: A Community of Microbes The microbiome affects our health 4,5,6 • Immune system • Metabolism • Obesity • Inflammatory diseases • Central nervous system disorders gut bacterial species have now been characterized 3 Containing bacteria, archaea, protists, fungi and viruses in the digestive tract > 1,000 ~ 100 trillion microbial cells in each person (primarily bacteria in the gut) 1 2 5 to when the microbiome resembles that of an adult in terms of composition and diversity 2 years of age 6

Antibiotics Cause Dysbiosis to the Gut Microbiome A healthy microbiome protects the body from infection Antibiotics wipe out good and bad bacteria Resistant bacteria (bad bacteria) multiply and take over Body primed for infection resistant bacteria spreads to others Dysbiosis — disruption to the balance of beneficial and harmful bacteria Image Credit: CDC 7 7
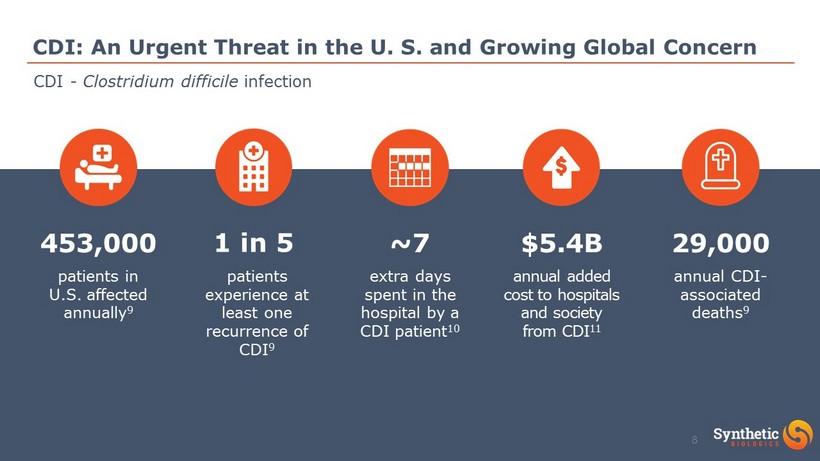
CDI: An Urgent Threat in the U. S. and Growing Global Concern patients experience at least one recurrence of CDI 9 patients in U.S. affected annually 9 453,000 1 in 5 extra days spent in the hospital by a CDI patient 10 ~7 annual added cost to hospitals and society from CDI 11 $5.4B annual CDI - associated deaths 9 29,000 CDI - Clostridium difficile infection 8

Antimicrobial Resistance is a Serious Global Threat AMR (antimicrobial resistance): the ability of a microbe to resist the effects of medication (previously) used to treat them Can cause antibiotics to become ineffective Sometimes referred to as super bugs infections persist and increase risk of spreading Source: WHO 8 9

Other patients are exposed to CDI & resistant bacteria John seeks medical attention and could receive more antibiotics John spreads resistant bacteria in his community Patients spread CDI & resistant bacteria in their community How Does AMR Spread? Patients go home John receives antibiotics which cause dysbiosis Image Credit: CDC 12 CDI & AMR pass from person to person in the community How Do CDI and AMR Spread?

Antimicrobial Resistance: Public Policy Tailwind • AMR is a serious threat — world leaders are taking action • Government recognizes need to expedite drug review timelines • Recruiting leaders in industry and academia to develop action plans • Synthetic Biologics is pioneering microbiome science: Declaration on Combatting Antibiotic Resistance – 2016 HHS • Synthetic Biologics awarded CDC contract to evaluate ability of ribaxamase to prevent AMR (CDC BAA – 2016) • Breakthrough Therapy Designation from the FDA for ribaxamase for the prevention of CDI 11

Section Title Innovating Great Solutions

Building a Deep Pipeline Focused on the Microbiome Therapeutic Area Product Candidate Preclinical Phase 1 Phase 2 Phase 3 Phase 4 Prevention of CDI and AMR [Degrade IV β - lactam Antibiotics] ribaxamase SYN - 004 Prevention of CDI and AMR [Degrade IV Carbapenem Antibiotics] Oral enzyme SYN - 006 Prevention of CDI and AMR [Degrade Oral β - lactam Antibiotics] Oral enzyme SYN - 007 Treatment of IBS - C Oral modified - release lovastatin lactone SYN - 010 C Preservation of gut microbiome barrier/Treat Inflammation Oral intestinal alkaline phosphatase (IAP) SYN - 020 M Treatment/Prevention of Pertussis (whooping cough) Monoclonal antibodies SYN - 005 I,T PKU Biotherapeutic SYN - 200 I C - License and collaboration with Cedars - Sinai Medical Center I - License and collaboration with Intrexon Corporat ion collaboration T - License and collaboration with The University of Texas at Austin M - Scientific collaboration with Massachusetts Gen eral Hospital 13

SYN - 004 (ribaxamase)

Building a Deep Pipeline Focused on the Microbiome Therapeutic Area Product Candidate Preclinical Phase 1 Phase 2 Phase 3 Phase 4 Prevention of CDI and AMR [Degrade IV β - lactam Antibiotics] ribaxamase SYN - 004 Prevention of CDI and AMR [Degrade IV Carbapenem Antibiotics] Oral enzyme SYN - 006 Prevention of CDI and AMR [Degrade Oral β - lactam Antibiotics] Oral enzyme SYN - 007 Treatment of IBS - C Oral modified - release lovastatin lactone SYN - 010 C Preservation of gut microbiome barrier/Treat Inflammation Oral intestinal alkaline phosphatase (IAP) SYN - 020 M Treatment/Prevention of Pertussis (whooping cough) Monoclonal antibodies SYN - 005 I,T PKU Biotherapeutic SYN - 200 I C - License and collaboration with Cedars - Sinai Medical Center I - License and collaboration with Intrexon Corporat ion collaboration T - License and collaboration with The University of Texas at Austin M - Scientific collaboration with Massachusetts Gen eral Hospital 15

• Oral 75mg capsule (custom designed oral β - lactamase) • Designed to protect the natural balance of microbes in the gut microbiome during antibiotic use • Did not interfere with the efficacy of IV antibiotics in Phase 2 studies What is Ribaxamase? 16

How Does Ribaxamase Work? • be co - administered with certain IV antibiotics • b reak down excess IV antibiotic excreted into the GI tract • h elp prevent CDI, pathogen overgrowth and AMR Designed to: 17

Delivering Clinical Outcomes: Ribaxamase Phase 2b Data • Achieved primary endpoint of significant (P=0.045) reduction of CDI • 71.4% relative risk reduction in CDI rates vs placebo • Significant (p=0.002) reduction in new VRE colonization vs placebo • Significant reduction of ceftriaxone - mediated loss of microbial diversity Data demonstrate a statistically significant reduction in Clostridium difficile infection (CDI) and in new colonization* by vancomycin resistant enterococci (VRE) *New colonization was defined as a negative screening sample followed by a positive sample at 72 hours or 4 weeks. 18

Prevented Ceftriaxone(CRO) - mediated loss of α - diversity and enhanced microbiome recovery Ribaxamase Protected Microbial Diversity Note: Shannon Index and Chao1 represent α - diversity, a measure of the microbial community composition within a sample Size of each ball is relative to the standard error of the sample group 7.2 7.3 7.4 7.5 7.6 7.7 7.8 7.9 8 8.1 8.2 1400 1600 1800 2000 2200 2400 2600 Shannon Diversity Chao1 Diversity 7.2 7.3 7.4 7.5 7.6 7.7 7.8 7.9 8 8.1 8.2 1400 1600 1800 2000 2200 2400 2600 Shannon Diversity Chao1 Diversity Placebo Ribaxamase T0 T1 T1 CRO Treatment Recovery CRO Treatment T0 Recovery T2 T2 Compared to T0, patients receiving ribaxamase demonstrated significantly better maintenance and recovery of microbial diversity at T1 and T2 versus Placebo T0: Baseline T1: 72 Hours T2: 4 Weeks 19

Goal of Impacting Global Health ~500k cases of CDI per year 13 2M illnesses and 23k deaths caused by AMR 13 US, Canada & Mexico 49% increase in antibiotic use in NZ between 2006 - 2014 18 25% of Australian CDI cases come from community settings 16 Australia & New Zealand Japan High use of Cephalosporin - class antibiotics 19 China, Korea & India 1 child dies every 9 min from an infection caused by antibiotic - resistant bacteria in India 17 EU ~185k cases of CDI per year 15 25k people die as a result of AMR per year 15 World Wide Total Antibiotic usage has reached ~75B standard doses 14 Brazil 68% increase in antibiotic use 2000 - 2010 16 Russia South Africa High annual incidence of CDI, 8.7 cases per 10k admissions 16 Committed to AMR Strategy & Action Plan 20 Spread of CDI is an emerging clinical challenge 20 Maximize reach to save lives, improve global health, reduce burden 20

A Potential Transformation in Anti - Infective Standard of Care The dominoes begin to fall… Preventing C. difficile infection Inhibiting the emergence of AMR Preserving use of existing antibiotics Avoiding downstream problems • Prevention of secondary infections • Shortened hospital stays • Reduced readmission, transmission, mortality and costs Protecting the microbiome from dysbiosis 21

Valuing Ribaxamase Value Story The total economic cost of antibiotic resistance to the U.S. is estimated to be $20 billion in excess direct healthcare costs, with additional costs to society for lost productivity as high as $35 billion a year (2008 dollars) 13 C. difficile costs up to up to $5.4 billion/year in excess health care and community costs 11 US Cost Burden Customer Value Pricing Research Health Economics Disease Burden Standard of Care R&D Investment Stakeholder Benefit 22

Patient Access is Priority Improved patient access delivers value to patients and stakeholders Define and Communicate Value Price Effectively Unite Focus of Stakeholders in Healthcare System • Establish common goals with KPI’s • Map the patient journey • Understand payer, PBM, and provider pathways • Price for maximum value and patient access • Taking note from other industries • Dynamic approach to pricing Improve Internal Capabilities for Patient Access • Starting early • Cross - functional team responsibility • Integrating into core business processes • Defining value for patients, caregivers and society • Early alignment from stakeholders on value • Improve value measurement KPI: Key Performance Indicator PBM: Pharmacy Benefit Manager 23

SYN - 010

Building a Deep Pipeline Focused on the Microbiome Therapeutic Area Product Candidate Preclinical Phase 1 Phase 2 Phase 3 Phase 4 Prevention of CDI and AMR [Degrade IV β - lactam Antibiotics] ribaxamase SYN - 004 Prevention of CDI and AMR [Degrade IV Carbapenem Antibiotics] Oral enzyme SYN - 006 Prevention of CDI and AMR [Degrade Oral β - lactam Antibiotics] Oral enzyme SYN - 007 Treatment of IBS - C Oral modified - release lovastatin lactone SYN - 010 C Preservation of gut microbiome barrier/Treat Inflammation Oral intestinal alkaline phosphatase (IAP) SYN - 020 M Treatment/Prevention of Pertussis (whooping cough) Monoclonal antibodies SYN - 005 I,T PKU Biotherapeutic SYN - 200 I C - License and collaboration with Cedars - Sinai Medical Center I - License and collaboration with Intrexon Corporat ion collaboration T - License and collaboration with The University of Texas at Austin M - Scientific collaboration with Massachusetts Gen eral Hospital 25

Pain, Constipation and Bloating in the Gut • The archaeon, Methanobrevibacter smithii ( M. smithii ), produces methane in the human intestine during digestion • Clinical evidence has established that elevated intestinal methane production is a cause of constipation in Irritable Bowel Syndrome with Constipation (IBS - C) and Chronic Idiopathic Constipation (CIC) 21 • Reducing excess methane production can relieve constipation and associated symptoms, including pain and bloating 21 26

IBS - C and CIC: Large Economic Burden and Huge Opportunity adults in U.S. suffer from IBS - C 23 people suffer from chronic constipation 22 1 in 7 16M satisfaction of current treatment across the globe 24 average additional expense per person, per year with IBS - C 25 $3.9k current drugs treat the underlying cause < 8% 0 27

What is SYN - 010? Modified - release formulation of lovastatin lactone optimal for reducing methane - production by certain gut microorganisms ( M. smithii ) 28
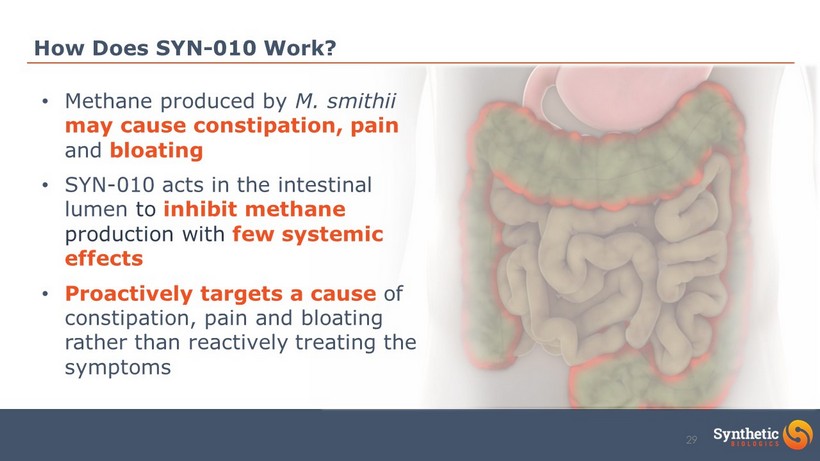
How Does SYN - 010 Work? • Methane produced by M. smithii may cause constipation, pain and bloating • SYN - 010 acts in the intestinal lumen to inhibit methane production with few systemic effects • Proactively targets a cause of constipation, pain and bloating rather than reactively treating the symptoms 29

SYN - 010 had no SAEs, few TEAEs and no incidences of drug - related diarrhea reported in clinical trials SYN - 010 was not microbicidal indicating minimal disruption to the gut microbiome 29 SYN - 010 showed compelling improvements in CSBM frequency, abdominal pain and bloating SYN - 010 Appears to Improve Symptoms Minimizes Improves Well Tolerated Based on Phase 2 Clinical Results (data previously presented) 30 SAE: Serious Adverse Event TEAE: Treatment Emergent Adverse Event 30

SYN - 010: Competitive Overview Product Candidate SYN - 010 Linzess Amitiza Trulance OTC Laxatives Company Synthetic Allergan Takeda Synergy Various Phase/Status Planned pivotal 2b/3 Marketed Marketed Marketed Marketed Designed to treat underlying cause of IBS - C Designed to treat symptoms w/o causing severe diarrhea Designed to treat symptoms Designed to relieve constipation Designed to relieve abdominal pain & bloating Designed to cause more regular bowel movements 31

Goal of Enhancing Global Health Maximize reach to save lives, improve global health, reduce burden 71M addressable patients US, Canada & Mexico 4M addressable patients Australia & New Zealand Japan 18M addressable patients China, Korea & India 406M addressable patients EU 105M addressable patients World Wide ~1/7 people suffer from CIC ~5% suffer from IBS - C Brazil 30M addressable patients Russia South Africa 8M addressable patients 20.5M addressable patients Linzess Sales Amitiza Sales *Source: UN Population Report, IMS Health Analytics Link 26 32

IBS and Constipation Markets are Growing More than 2x growth in 4 years! US IBS and Constipation Market 1 • Digital and DTC campaigns are enhancing awareness of both IBS and gut health • New entrants are growing the market , not cannibalizing it 27 • Only 26% of patients are “very satisfied” with current FDA approved prescription medication 28 $2.5B Market Growth Drivers 33

Synthetic Biologics Building Capabilities

Raymond Stapleton, PhD, SVP Manufacturing Merck & Co., Inc. Jeffrey Riley CEO Pfizer, Nichols Institute (Quest), SmithKline Beecham, QIC Steven Shallcross CFO Vanda Pharmaceuticals, Inc., Empire Petroleum Partners, LLC, Innocoll AG Joseph Sliman, MD, MPH, CMO Vanda Pharmaceuticals, Inc., MedImmune, Inc., DynPort Vaccine Michael Kaleko, MD, PhD, SVP R&D Genetic Therapy, Inc. (Novartis), Advanced Vision Therapies Deb Mathews, PharmD, VP Medical Affairs Bayer Healthcare Pharmaceuticals, Novartis Jaimee Martinez, Marketing Hewlett - Packard Vincent Perrone, MBA, Corporate Communication New York Stock Exchange Executive Leadership Team 35

Building Capabilities Financial Stewardship Clinical/ Regulatory and Med Affairs Manufacturing Innovation R&D Capabilities Marketing and Commercial • Developing innovative solutions and platforms • Manufacturing process development, tech transfer and scale up • Completed two phase 2 clinical trials • Oral formulation of large and small molecules for specialized dose delivery throughout the human gut • Building regulatory expertise HIGHLIGHTS: 36

2023 SYN - 010: Patented Methods of Treatment (Broadest Indication) SYN - 010: Patented and Pending Methods of Treating Constipation with SYN - 010 ribaxamase : Patented Composition of Matter & Uses ribaxamase : Patented other β - lactamase Composition of Matter & Uses ribaxamase : Pending Patent Applications • Methods of Manufacture • Clinical Dosing & Formulation • Methods of Treatment, etc. 2035/36 2034 2031 2035 Expires* 2035/36 SYN - 010: Pending Patent Applications • Formulations, Clinical Dosing • Methods of Use in Specific Patient Populations 2035 Synthetic Biologics Strong Patent Position 110+ U.S. & Foreign Granted Patents, 85+ U.S. & Foreign Pending Patents *Dates do not account for possible patent term extensions 37
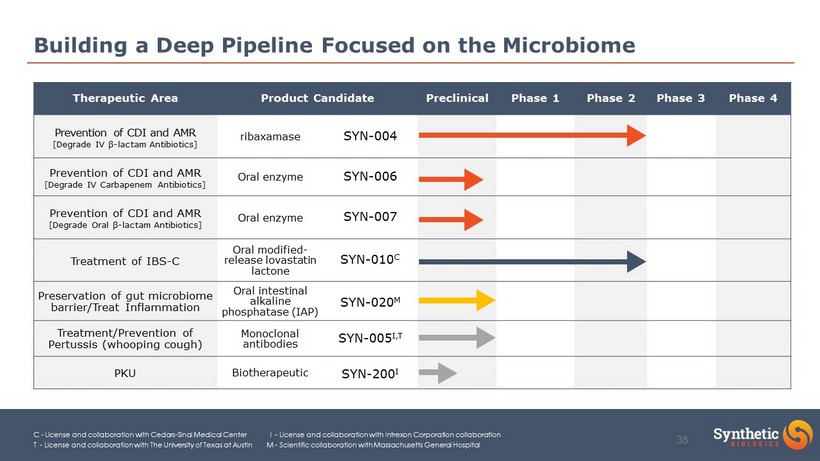
Building a Deep Pipeline Focused on the Microbiome C - License and collaboration with Cedars - Sinai Medical Center I - License and collaboration with Intrexon Corporat ion collaboration T - License and collaboration with The University of Texas at Austin M - Scientific collaboration with Massachusetts Gen eral Hospital Therapeutic Area Product Candidate Preclinical Phase 1 Phase 2 Phase 3 Phase 4 Prevention of CDI and AMR [Degrade IV β - lactam Antibiotics] ribaxamase SYN - 004 Prevention of CDI and AMR [Degrade IV Carbapenem Antibiotics] Oral enzyme SYN - 006 Prevention of CDI and AMR [Degrade Oral β - lactam Antibiotics] Oral enzyme SYN - 007 Treatment of IBS - C Oral modified - release lovastatin lactone SYN - 010 C Preservation of gut microbiome barrier/Treat Inflammation Oral intestinal alkaline phosphatase (IAP) SYN - 020 M Treatment/Prevention of Pertussis (whooping cough) Monoclonal antibodies SYN - 005 I,T PKU Biotherapeutic SYN - 200 I 38

Thank You

1. Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the Human Microbiome. Nutrition reviews . 2012;70(Suppl 1):S38 - S44. doi:10.1111/j.1753 - 4887.2012.00493.x. 2. Rodríguez JM, Murphy K, Stanton C, et al. The composition of the gut microbiota throughout life, with an emphasis on early li fe. Microbial Ecology in Health and Disease . 2015;26:10.3402/mehd.v26.26050. doi:10.3402/mehd.v26.26050. 3. Lloyd - Price J., Abu - Ali G., & Huttenhower, C. (2016). The healthy human microbiome. Genome Medicine. 8:51. https://doi.org/10 .1186/s13073 - 016 - 0307 - y. 4. Institute of Medicine (US) Food Forum. The Human Microbiome, Diet, and Health: Workshop Summary. Washington (DC): National Ac ademies Press (US); 2013. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK109559/ doi: 10.17226/13522. 5. Mulle JG, Sharp WG, Cubells JF. The Gut Microbiome: A New Frontier in Autism Research. Current psychiatry reports . 2013;15(2):337. doi:10.1007/s11920 - 012 - 0337 - 0. 6. Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden m eta bolic organ. Therapeutic Advances in Gastroenterology . 2013;6(4):295 - 308. doi:10.1177/1756283X13482996. 7. The Centers for Disease Control and Prevention. The Microbiome and Innovations to Slow Antibiotic Resistance. (Last update d M ay 10, 2017). Retrieved from https://www.cdc.gov/drugresistance/solutions - initiative/microbiome - innovations.html#microbiome (Last accessed August 2017). 8. World Health Organization (WHO). Antimicrobial resistance fact sheet. Updated September 2016. Retrieved from http://www. who .int/mediacentre/factsheets/fs194/en/ (last accessed September 2017). 9. Lessa, F.C., Winsto., & McDonald, L.C; (2015). Emerging Infections Program C. difficile Surveillance Team. Burden of Clostrid ium difficile infection in the United States. New England Journal of Medicine. Retrieved from http://www.nejm.org/doi/full/10.1056/NEJMc1505190#t=article (Last accessed August 20 17). 10. Kleef, E van et al. “Excess length of stay and mortality due to Clostridium difficile infection: a multi - state modelling app roach.” The Journal of hospital infection 88 4 (2014): 213 - 7. DOI: 10.1016/j.jhin.2014.08.008 11. Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infectious Diseases . 2016;16:303. doi:10.1186/s12879 - 016 - 1610 - 3. 12. Centers for Disease Control and Prevention (CDC). About Antimicrobial Resistance. Page last updated April 6, 2017. Retri eve d from https://www.cdc.gov/drugresistance/about.htm l (Last accessed August 2017). Sources 40

Sources (continued) 13. Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States, 2013. (Page last up dat ed April 10, 2017) Retrieved from https://www.cdc.gov/drugresistance/threat - report - 2013/index.html (Last accessed September 2017) 14. IMS Health Analytics Link. (Last accessed August 2017). Filename: IMS Health Analytics Link - All Antibiotics Market Volume WW.pdf. 15. ENVI 2013 - 04 - 10 European Report on Patient Safety, 3 Clostridium difficile infection in Europe. A CDI Europe Report. 16. Gelband, Hellen, Molly Miller - Petrie, Suraj Pant, Sumanth Gandra, Jordan Levinson,Devra Barter, Andrea White, and Ramanan La xminarayan. The State of the World's Antibiotics 2015. Report. 2015. Retrieved from http://cddep.org/publications/state_worlds_antibiotics_ 2015/ Last accessed August 2017 17. Laxminarayan et al. Antibiotic resistance - the need for global solutions. Lancet Infectious Diseases Volume 13, Issue 12, 1 057 - 1098 18. Surveillance report: Antibiotic Consumption in New Zealand, 2006 - 2015. Prepared by Institute of Environmental Science and Research Limited for the Ministry of Health. Client Report No: FW15031. May 2016. Retrieved from https://surv.esr.cri.nz/PDF_surveillance/AntibioticConsumption/2014/Anti bio tic_Consumption_Report_Final.pdf 19. Otake, Tomoko. New Guidelines tackle misuse of antibiotics amid rise of superbugs. The Japan Times. March 8, 2017. Ret rie ved from: https://www.japantimes.co.jp/news/2017/03/08/national/science - health/new - guidelines - tackle - misuse - antibiotics - amid - rise - superbug s/#.WaRCf1GGNPY 20. Sukhina, Marina et al. Clostridium difficile associated infection in Russia: epidemiology, virulence, pathophysiological as pects. State Scientific Centre for Coloproctology; Microbiology and immunology. ECCMID Vienna, Austria. April 25, 2017. P2025. 21. Triantafyllou K, Chang C, Pimentel M. Methanogens, Methane and Gastrointestinal Motility. Journal of Neurogastroenterology and Motility . 2014;20(1):31 - 40. doi:10.5056/jnm.2014.20.1.31. 22. Nicole C. Suares , MBChB 1 and Alexander C. Ford , MBChB, MD, MRCP. Prevalence of, and Risk Factors for, Chronic Idiopath ic Constipation in the Community: Systematic Review and Meta - analysis. The American Journal of Gastroenterology 106, 1582 - 1592 (September 2011) doi:10.1028/ajg.20 11.164 23. Quigley EMM, Fried M, Gwee KA et al (2015) Irritable Bowel Syndrome: A Global Perspective. World Gastroenterology Organization, 555 East Wells Street, Suite 1100, Milwaukee, WI 53202 - 3823. Retrieved from http://www.worldgastroenterology.org/guidelines/global - guidelines/irritable - bowel - syndrome - ibs/irritable - bowel - syndrome - ibs - english (Last accessed: August 2017.) 41

Sources (continued 2) 24. IBS Patients: Their Illness Experience and Unmet Needs – US Study – International Foundation for Functional Gastrointestina l Disorders (IFFGD) (Last accessed: August 2017) 25. Doshi JA, Cai Q, Buono JL, et al. Economic burden of irritable bowel syndrome with constipation: a retrospective analysis of health care costs in a commercially insured population. J Manag Care Spec Pharm. 2014;20(4):382 - 90. Retrieved from http://www.jmcp.org/doi/abs/10.18553/jmcp.2014.20.4.382. 26. IMS Health Analytics Link: Query Builder. Accessed, August 2017. Filename: SYN - 010 Molecule Sales Data by Country.pdf 27. IMS Health Analytics Link custom report. (Last accessed, August 2017). Population estimates: United Nations Population Div ision. Department of Economic and Social Affairs. World Population Prospects: The 2015 Revision. (July 2015.) 28. American Gastroenterological Association. Irritable Bowel Syndrome in America. A Survey Report conducted by AGA. (Decembe r 2 015). (Last accessed 03/19/2017). 29. Morales W et al. (2015) Lovastatin improves stool form in Methanobrevibacter smithii colonized rats with constipation. Ga str oenterology 148: S - 779 - 80 30. Gottlieb K et al. (2015) [Su1210] SYN - 010, a proprietary modified - release formulation of lovastatin lactone, lowered breath methane and improved stool frequency in patients with IBS - C: results of a multi - center randomized double - blind placebo - controlled Phase 2a trial. Gastroenterology 150: S - 496 42

Appendix
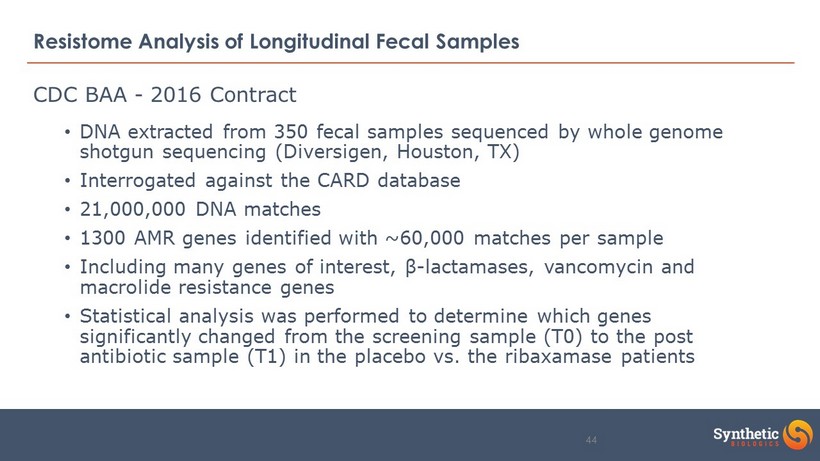
Resistome Analysis of Longitudinal Fecal Samples • DNA extracted from 350 fecal samples sequenced by whole genome shotgun sequencing (Diversigen, Houston, TX) • Interrogated against the CARD database • 21,000,000 DNA matches • 1300 AMR genes identified with ~60,000 matches per sample • Including many genes of interest, β - lactamases, vancomycin and macrolide resistance genes • Statistical analysis was performed to determine which genes significantly changed from the screening sample (T0) to the post antibiotic sample (T1) in the placebo vs. the ribaxamase patients CDC BAA - 2016 Contract 44

Analysis of the Change in Relative Abundance of AMR Genes Collection point T0 to T1, Placebo vs. Ribaxamase - treated patients Placebo Ribaxamase Decreased Increased T0 T1 Vancomycin resistance genes Β - lactamase genes Tet and erm resistance genes LefSe Analysis 45

Comparison of Ribaxamase vs. Placebo - Treated Gene of Interest P value gb|AY082011|0-699|ARO:3002923|vanRD 0.00533347 gb|EU999036|688-1390|ARO:3002934|vanSD 0.01298036 gb|AY769934|27-993|ARO:3003096|CfxA5 0.015348147 gb|U38243|149-1115|ARO:3003001|CfxA 0.020131869 gb|AY769933|0-966|ARO:3003005|CfxA4 0.021717324 gb|AF118110|71-1037|ARO:3003002|CfxA2 0.024848669 gb|AF472622|52-1018|ARO:3003003|CfxA3 0.029625208 gb|AY082011|5900-6932|ARO:3000005|vanD 0.084866514 gb|GQ343019|132-1023|ARO:3002999|CblA-1 0.175826761 gb|GQ342996|797-1793|ARO:3003097|CfxA6 0.453301435 gb|DQ212986|7030-7795|ARO:3003069|vanXYG 0.583249332 gb|DQ212986|5137-5983|ARO:3002965|vanWG 0.637510487 gb|NC_023287|65291-66509|ARO:3000615|mefA 0.692877942 gb|DQ212986|7787-9926|ARO:3002972|vanTG 0.737817419 gb|DQ212986|5984-7034|ARO:3002909|vanG 0.762362859 gb|NC_012469|1800927-1802391|ARO:3000616|mel 0.801144237 gb|U05883|555-1458|ARO:3003559|cepA 0.825294268 Change in relative abundance from T0 to T1 in genes of interest Cfx β - lactamase Vancomycin R 46
