Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - VARIAN MEDICAL SYSTEMS INC | v474735_8k.htm |
Exhibit 99.1

1 | VARIAN CONFIDENTIAL – INTERNAL USE ONLY 2017 Baird Global Healthcare Conference TRANSFORMING CANCER CARE

2 | VARIAN CONFIDENTIAL – INTERNAL USE ONLY 2 THIS PRESENTATION IS INTENDED EXCLUSIVELY FOR INVESTORS. IT IS NOT INTENDED FOR USE IN SALES OR MARKETING. FORWARD LOOKING STATEMENTS Except for historical information, this presentation contains forward - looking statements within the meaning of the Private Secur ities Litigation Reform Act of 1995. Statements concerning industry outlook, including growth drivers, future trends in cancer incidence and trends in cance r t reatment needs, demand, innovation and growth opportunities; Varian Medical System, Inc.’s (“Varian” or the “company”) future orders, revenues, backl og, or earnings growth; future financial results; market acceptance of or transition to new products or technology such as our Edge™ radiosurgery system, TrueBeam ® , HyperArc ™, 360 Oncology™, HALCYON™, image - guided radiation therapy, stereotactic radiosurgery and proton therapy, and any statements using the terms “could,” “believe,” “expect,” “outlook,” “anticipate”, “vision”, “estimate”, “future”, “horizon”, “aiming”, “driving”, “target” or similar statem ent s are forward - looking statements that involve risks and uncertainties that could cause the company’s actual results to differ materially from those anticipated. Su ch risks and uncertainties include global economic conditions and changes to trends for cancer treatment regionally; the impact of changes to the Affordable Hea lth Care for America Act (including excise taxes on medical devices) and any further healthcare reforms (including changes to Medicare and Medicaid), and /or changes in third - party reimbursement levels; currency exchange rates and tax rates; demand for the company’s products; the company’s ability to deve lop , commercialize, and deploy new products; the company’s ability to meet Food and Drug Administration (FDA) and other regulatory requirements for product cle arances or to comply with FDA and other regulatory regulations or procedures; changes in the regulatory environment, including with respect to FDA requ ire ments; the company’s assessment of the goodwill associated with its particle therapy business, challenges associated with the successful commercia liz ation of the company’s particle therapy business; the effect of adverse publicity; the company’s reliance on sole or limited - source suppliers; the company’s abi lity to maintain or increase margins; the impact of competitive products and pricing; the potential loss of key distributors or key personnel; challenges to public tender awards and the loss of such awards or other orders; and the other risks listed from time to time in the company’s filings with the Securities and Ex change Commission, which by this reference are incorporated herein. The company assumes no obligation to update or revise the forward looking statements in th is presentation because of new information, future events, or otherwise. Reconciliations to GAAP financials can be found at http://investors.varian.com/financialstatements . Medical Advice Disclaimer Varian as a medical device manufacturer cannot and does not recommend specific treatment approaches. Individual treatment res ult s may vary.

3 | VARIAN CONFIDENTIAL – INTERNAL USE ONLY 2 EMPLOYEES 6,400+ FY16 REVENUES $2.6B* SOFTWARE INSTALLS 4,000+ $1.25B* SOFTWARE & SERVICE REVENUE 7,500 MEDICAL LINEAR ACCELERATORS 50+ PROTON THERAPY ROOMS * Varian FY16 Actuals, excluding Imaging Components ** YTD thru Fiscal 3 rd quarter 2017 Gross Orders, excluding North America A FOCUSED CANCER COMPANY VARIAN TODAY – A SNAPSHOT Global Leader in Radiation Therapy 50 % ** INTERNATIONAL ORDER MIX
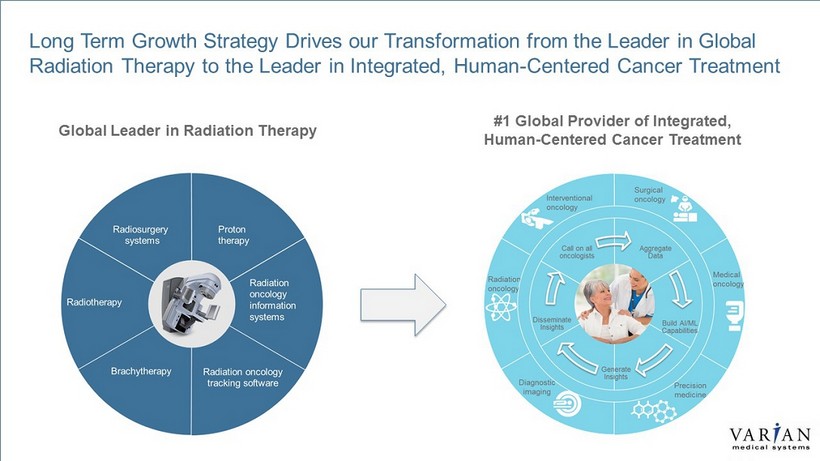
4 | VARIAN CONFIDENTIAL – INTERNAL USE ONLY Global Leader in Radiation Therapy #1 Global Provider of Integrated, Human - Centered Cancer Treatment Long Term Growth Strategy Drives our Transformation from the Leader in Global Radiation Therapy to the Leader in Integrated, Human - Centered Cancer Treatment
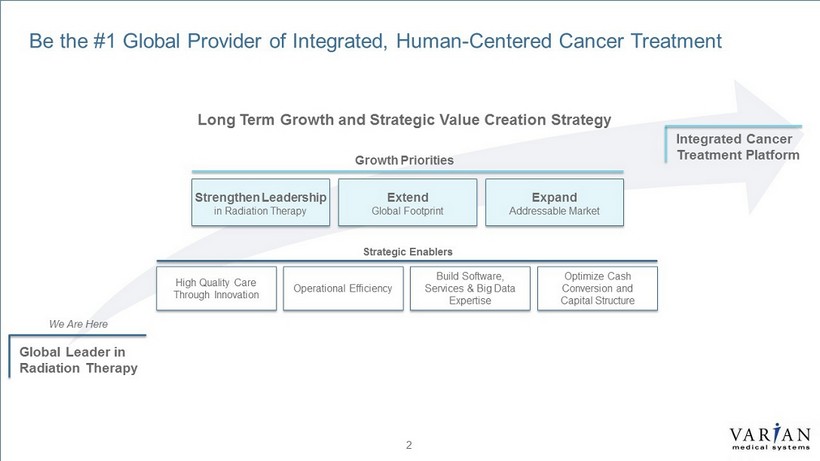
5 | VARIAN CONFIDENTIAL – INTERNAL USE ONLY 2 Be the #1 Global Provider of Integrated, Human - Centered Cancer Treatment We Are Here Global Leader in Radiation Therapy Integrated Cancer Treatment Platform Strengthen Leadership in Radiation Therapy Extend Global Footprint Expand Addressable Market Growth Priorities High Quality Care Through Innovation Operational Efficiency Build Software, Services & Big Data Expertise Optimize Cash Conversion and Capital Structure Strategic Enablers Long Term Growth and Strategic Value Creation Strategy

6 | VARIAN CONFIDENTIAL – INTERNAL USE ONLY ▪ Market leader with the strongest portfolio of product offerings ▪ Focused on extending position as leader in radiation therapy systems and software for the treatment of cancer ▪ Optimally positioned to grow in both developed and emerging markets supported by our product, software and service offerings ▪ Disciplined to drive profitable growth and improve liquidity, strengthening our financial flexibility to invest in growth opportunities In Summary

7 | VARIAN CONFIDENTIAL – INTERNAL USE ONLY VARIAN INVESTOR MEETING — MAY 10, 2017 THANK YOU
