Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - ReShape Lifesciences Inc. | a17-18401_2ex99d3.htm |
| EX-99.1 - EX-99.1 - ReShape Lifesciences Inc. | a17-18401_2ex99d1.htm |
| 8-K - 8-K - ReShape Lifesciences Inc. | a17-18401_28k.htm |
Exhibit 99.2
Our Company
We are a medical device company focused on the development and commercialization of technology to treat obesity and metabolic diseases.
The vBloc®System, our initial product, is a U.S. Food and Drug Administration (FDA)-approved pacemaker-like device that delivers vBloc® Neurometabolic Therapy (vBloc Therapy) to help patients feel full and eat less by intermittently blocking hunger signals on the vagus nerve. Our therapy limits the expansion of the stomach, helps control hunger sensations between meals, reduces the frequency and intensity of stomach contractions and produces a feeling of early and prolonged fullness. We believe the vBloc System offers obese patients a minimally-invasive treatment that can result in significant, durable and sustained weight loss. We believe that our vBloc System allows bariatric surgeons to offer a new option to obese patients who are concerned about the risks and complications associated with currently available anatomy-altering, restrictive or malabsorptive surgical procedures.
We have a limited operating history and only recently received FDA approval to sell the vBloc System in the United States. In addition, we have regulatory approval to sell the vBloc System in the European Economic Area and other countries that recognize the European CE Mark and do not have any other source of revenue. We were incorporated in Minnesota on December 19, 2002 and later reincorporated in Delaware on July 22, 2004. We have devoted substantially all of our resources to the development and commercialization of the vBloc System, which was formerly known as the Maestro or vBloc Rechargeable System.
On May 22, 2017, we acquired the Gastric Vest System™ through our acquisition of BarioSurg. The Gastric Vest System is an investigational, minimally invasive, laparoscopically implanted medical device being studied for weight loss in morbidly obese patients. The device wraps around the stomach, emulating the effect of conventional weight-loss surgery, and is intended to enable gastric volume reduction without permanently changing patient anatomy. The acquisition was completed under the terms of a merger agreement pursuant to which BarioSurg became a wholly-owned subsidiary of our company. The aggregate merger consideration we paid for all of the outstanding shares of capital stock and outstanding options of BarioSurg was: (i) 1.38 million shares of our common stock, (ii) 1.0 million shares of our newly created conditional convertible preferred stock, which shares will convert into 5.0 million shares of our common stock subject to and contingent upon the post-closing approval of our stockholders in accordance with the NASDAQ Stock Market Rules, and (iii) $2 million in cash.
We received FDA approval on January 14, 2015 for vBloc Therapy, delivered via the vBloc System, for the treatment of adult patients with obesity who have a Body Mass Index (BMI) of at least 40 to 45 kg/m2, or a BMI of at least 35 to 39.9 kg/m2 with a related health condition such as high blood pressure or high cholesterol levels, and who have tried to lose weight in a supervised weight management program and failed within the past five years. In 2015 we began a controlled commercial launch at select surgical centers in the United States and had our first commercial sales. During 2015, we initiated a controlled expansion of our commercial operations and started the process of building a sales force. In January 2016, we hired new executives to oversee this expansion. Our direct sales force is supported by field clinical engineers who provide training, technical and other support services to our customers. Throughout 2016, our sales force called directly on key opinion leaders and bariatric surgeons at commercially-driven surgical centers that met our certification criteria. Additionally, in 2016, through a distribution agreement with Academy Medical, LLC, U.S. Department of Veterans Affairs (VA) medical facilities now offer the vBloc System as a treatment option for veterans at little to no cost to veterans in accordance with their veteran healthcare benefits. We plan to build on these efforts in 2017 with self-pay and veteran focused direct-to-patient marketing, key opinion leader and center-specific partnering, and a multi-faceted reimbursement strategy. Our vBloc Therapy is a covered benefit for over 21 million U.S. veterans. The VA estimates that 78% of U.S. veterans are overweight or obese and nearly 25% of VA patients have diabetes. To date, we have relied on, and anticipate that we will continue to rely on, third-party manufacturers and suppliers for the production of the vBloc System.
In 2016, we sold 62 units for $787,000 in revenue, and in 2015 we sold 24 units for $292,000 in revenue. We have incurred and expect to continue to incur significant sales and marketing expenses prior to recording sufficient revenue to offset these expenses. Additionally, our selling, general and administrative expenses have
increased since we commenced commercial operations, and we expect that they will continue to increase as we continue to build the infrastructure necessary to support our expanding commercial sales, operate as a public company and develop our intellectual property portfolio. For these reasons, we expect to continue to incur operating losses for the next several years. We have financed our operations to date principally through the sale of equity securities, debt financing and interest earned on cash investments.
Our goal for the vBloc System remains broad coverage and reimbursement for vBloc Therapy. We believe that the most significant barrier to adoption for patients who want vBloc Therapy has been cost and lack of payer coverage. In June 2017, we launched our vBloc Now program. The vBloc Now program provides qualified patients battling obesity the opportunity to receive vBloc Therapy, including the device, procedure, and vBloc Achieve follow up program, at an affordable price in exchange for sharing detailed health data with EnteroMedics. The program is available for a limited time, will reduce patient total out-of-pocket costs, and compete with leading covered bariatric surgery procedures as well as other low-cost weight loss devices
In addition, the vBloc Now program provides us with additional commercial data concerning vBloc Therapy in order to enhance our case with third-party payers that the vBloc System can have a clinically meaningful level of effectiveness in reducing the incidence of diabetes and other comorbidities in certain patients. While we do not expect to recognize any revenues in conjunction with the vBloc Now program, the Company anticipates that vBloc Now program expenses will be offset by a reduction in marketing and advertising expenses and will not increase the Company’s overall operating expenses.
Data from our ReCharge trial was used to support the premarket approval (PMA) application for the vBloc System, submitted to the FDA in June 2013. The ReCharge trial was a randomized, double-blind, sham-controlled, multicenter pivotal clinical trial testing the effectiveness and safety of vBloc Therapy utilizing our vBloc System. All patients in the trial received an implanted device and were randomized in a 2:1 allocation to treatment or sham control groups. The sham control group received a non-functional device during the trial period. All patients were expected to participate in a standard weight management counseling program. The primary endpoints of efficacy and safety were evaluated at 12 months. As announced, the ReCharge trial met its primary safety endpoint with a 3.7% serious adverse event rate. The safety profile at 12 months was further supported by positive cardiovascular signals including a 5.5 mmHg drop in systolic blood pressure, a 2.8 mmHg drop in diastolic blood pressure and a 3.6 bpm drop in average heart rate.
Additionally, the trial demonstrated in the intent to treat (ITT) population (n=239) a clinically meaningful and statistically significant excess weight loss (EWL) of 24.4% (approximately 10% total body weight loss (TBL)) for vBloc Therapy-treated patients, with 52.5% of patients achieving at least 20% EWL, although it did not meet its co-primary efficacy endpoints due to higher than expected weight loss levels in the sham control group. In the per protocol population, the trial demonstrated an EWL of 26.3% for vBloc Therapy-treated patients, with 56.8% of patients achieving at least 20% EWL. We subsequently announced that vBloc Therapy-treated patients were maintaining their weight loss at 18 months and 24 months with an EWL of 23.5% and 21.1%, respectively. The trial’s positive safety profile also continued throughout this reported time period.
In the ReCharge trial, two-thirds of vBloc Therapy-treated patients achieved at least 5% TBL at 12 months. According to the Centers for Disease Control and Prevention (CDC), 5% TBL can have significant health benefits on obesity related risk factors, or comorbidities, including reduction in blood pressure, improvements in Type 2 diabetes and reductions in triglycerides and cholesterol. Further analysis of our data at 12 months showed a meaningful impact on these comorbidities as noted in the below table showing the improvements seen at 10% TBL, the average weight loss in vBloc Therapy-treated patients.
|
Risk Factor |
|
10% TBL |
|
|
Systolic BP (mmHg) |
|
(9 |
) |
|
Diastolic BP (mmHg) |
|
(6 |
) |
|
Heart Rate (bpm) |
|
(6 |
) |
|
Total Cholesterol (mg/dL) |
|
(15 |
) |
|
LDL (mg/dL) |
|
(9 |
) |
|
Triglycerides (mg/dL) |
|
(41 |
) |
|
HDL (mg/dL) |
|
3 |
|
|
Waist Circumference (inches) |
|
(7 |
) |
|
HbA1c (%) |
|
(0.5 |
) |
We obtained European CE Mark approval for our vBloc System in 2011 for the treatment of obesity. The CE Mark approval for our vBloc System was expanded in 2014 to also include use for the management of Type 2 diabetes in obese patients. Additionally, the final vBloc System components were previously listed on the Australian Register of Therapeutic Goods by the Therapeutic Goods Administration. The costs and resources required to successfully commercialize the vBloc System internationally are currently beyond our capability. Accordingly, we will continue to devote our near-term efforts toward mounting a successful system launch in the United States. We intend to explore select international markets to commercialize the vBloc System as our resources permit, using direct, dealer and distributor sales models as the targeted market best dictates.
To date, we have not observed any mortality related to the vBloc System or any unanticipated adverse device effects in our human clinical trials. We have also not observed any long-term problematic clinical side effects in any patients. In addition, data from our VBLOC-DM2 ENABLE trial outside the United States demonstrate that vBloc Therapy may hold promise in improving obesity-related comorbidities such as diabetes and hypertension. We are conducting, or plan to conduct, further studies in each of these comorbidities to assess vBloc Therapy’s potential in addressing multiple indications.
Our Market
The Obesity and Metabolic Disease Epidemic
Obesity is a disease that has been increasing at an alarming rate with significant medical repercussions and associated economic costs. Since 1980, the worldwide obesity rate has more than doubled, with about 13% of the world’s adult population now being obese. The World Health Organization (WHO) currently estimates that as many as 600 million people worldwide are obese and more than 1.9 billion adults are overweight. Being overweight or obese is also the fifth leading risk for global deaths, with approximately 3.4 million adults dying each year as a result.
According to the WHO, there are over 70 progressive obesity-related diseases and disorders associated with obesity, which are also known as comorbidities, including Type 2 diabetes, hypertension, infertility and certain cancers. Worldwide, 44% of the diabetes burden, 23% of the heart disease burden and between 7% and 41% of certain cancer burdens are attributable to overweight and obesity.
We believe that this epidemic will continue to grow worldwide given dietary trends in developed nations that favor highly processed sugars, larger meals and fattier foods, as well as increasingly sedentary lifestyles. Despite the growing obesity rate, increasing public interest in the obesity epidemic and significant medical repercussions and economic costs associated with obesity, there continues to be a significant unmet need for effective treatments.
The United States Market
Obesity has been identified by the U.S. Surgeon General as the fastest growing cause of disease and death in the United States, and according to a 2014 McKinsey Report is the leading cause of preventable death in the U.S. Currently, the Center for Disease Control (the CDC) estimates that 35.7% of U.S. adults (or approximately 73 million people) are obese, having a BMI of 30 or higher. BMI is calculated by dividing a person’s weight in kilograms by the square of their height in meters. It is estimated that if obesity rates stay consistent, 51% of the U.S. population will be obese by 2030. According to data from the U.S. Department of Health and Human Services, almost 80% of adults with a BMI above 30 have a comorbidity, and almost 40% have two or more of these comorbidities. According to The Obesity Society and the CDC, obesity is associated with many significant weight-related comorbidities including Type 2 diabetes, high blood-pressure, sleep apnea, certain cancers, high cholesterol, coronary artery disease, osteoarthritis and stroke. According to the American Cancer Society, 572,000 Americans die of cancer each year, about one-third of which are linked to excess body weight, poor nutrition and/or physical inactivity. Over 75% of hypertension cases are directly linked to obesity, and approximately two-thirds of U.S.
adults with Type 2 diabetes are overweight or have obesity. Currently, medical costs associated with obesity in the U.S. are estimated to be up to $210 billion per year and nearly 21% of medical costs in the U.S. can be attributed to obesity. An estimated approximately $1.5 billion was spent in 2015 alone on bariatric surgical procedures to treat obesity. Researchers estimate that if obesity trends continue, obesity related medical costs could rise by another $44-$66 billion each year in the U.S. by 2030. The medical costs paid by third-party payers for people who are obese were $2,741 per year, or 42% higher than those of people who are normal weight and the average cost to employers is $6,627 to $8,067 per year per obese employee (BMI of 35 to 40 and higher).
Current Treatment Options and Their Limitations
We believe existing options for the treatment of obesity have seen limited adoption to date due to patient concerns and potential side effects including morbidity. The principal treatment alternatives available today for obesity include:
· Behavioral modification. Behavioral modification, which includes diet and exercise, is an important component in the treatment of obesity; however, most obese patients find it difficult to achieve and maintain significant weight loss with a regimen of diet and exercise alone.
· Pharmaceutical therapy. Pharmaceutical therapies often represent a first option in the treatment of obese patients but carry significant safety risks and may present troublesome side effects and compliance issues.
· Bariatric surgery. In more severe cases of obesity, patients may pursue more aggressive surgical treatment options such as gastric balloon, gastric banding, sleeve gastrectomy and gastric bypass. These procedures promote weight loss by surgically restricting the stomach’s capacity and outlet size. While largely effective, these procedures generally result in major lifestyle changes, including dietary restrictions and food intolerances, and they may present substantial side effects and carry short- and long-term safety and side effect risks that have limited their adoption.
Market Opportunity
Given the limitations of behavioral modification, pharmaceutical therapy and traditional bariatric surgical approaches, we believe there is a substantial need for a patient-friendly, safer, effective and durable solution that:
· preserves normal anatomy;
· is “non-punitive” in that it supports continued ingestion and digestion of foods and micronutrients such as vitamins and minerals found in a typical, healthy diet while allowing the user to modify his or her eating behavior appropriately without inducing punitive physical restrictions that physically force a limitation of food intake;
· enables non-invasive adjustability while reducing the need for frequent clinic visits;
· minimizes undesirable side-effects;
· minimizes the risks of re-operations, malnutrition and mortality; and
· reduces the natural hunger drive of patients.
Our Products
The vBloc System
The vBloc System, our initial product, uses vBloc Therapy to block the gastrointestinal effects of the vagus nerve using high-frequency, low-energy electrical impulses to intermittently interrupt naturally occurring neural
impulses on the vagus nerve between the brain and the digestive system. Our therapy controls hunger sensations between meals, limits the expansion of the stomach and reduces the frequency and intensity of stomach contractions, leading to earlier fullness. The resulting physiologic effects of vBloc Therapy produce a feeling of early and prolonged fullness following smaller meal portions. By intermittently blocking the vagus nerve and allowing it to return to full function between therapeutic episodes, our therapy limits the body’s natural tendency to circumvent the therapy, which can result in long-term weight loss.
Benefits. We have designed our vBloc System to address a significant market opportunity that we believe exists for a patient-friendly, safe, effective, less-invasive and durable therapy that is intended to address the underlying causes of hunger and obesity. Our vBloc System offers each of the following benefits, which we believe could lead to the adoption of vBloc Therapy as the surgical therapy of choice for obesity and its comorbidities:
· Preserves Normal Anatomy. The vBloc System is designed to deliver therapy that blocks the neural signals that influence a patient’s hunger and sense of fullness without altering digestive system anatomy. Accordingly, patients should experience fewer and less severe side effects compared to treatments that incorporate anatomical alterations.
· Allows Continued Ingestion and Digestion of Foods Found in a Typical, Healthy Diet. Because our therapy leaves the digestive anatomy unaltered, patients are able to maintain a more consistent nutritional balance compared to conventional surgical approaches, thus allowing them to effect positive changes in their eating behavior in a non-forced and potentially more consistent way.
· May be Implanted on an Outpatient Basis and Adjusted Non-Invasively. The vBloc System is designed to be laparoscopically implanted within a 60-90 minute procedure, allowing patients to leave the hospital or clinic on the same day. The implantable system is designed to be turned off and left in place for patients who reach their target weight. When desired, the follow-up physician can simply and non-invasively turn the therapy back on. Alternatively, the implantable system can be removed in a laparoscopic procedure.
· Offers Favorable Safety Profile. We have designed our clinical trials to demonstrate the safety of the vBloc System. In our clinical trials to date, including the ReCharge trial, we have not observed any mortality related to our device or any unanticipated adverse device effects. We have also not observed any long-term problematic clinical side effects in any patients, including in those patients who have been using vBloc Therapy for more than one year.
· Targets Multiple Factors that Contribute to Hunger and Obesity. We designed vBloc Therapy to target the digestive, metabolic and information transmission functions of the vagus nerve and to affect the perception of hunger and fullness, which together contribute to obesity and its metabolic consequences.
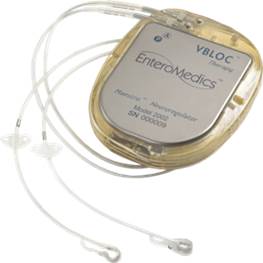
The vBloc System, Implantation Procedure and Usage.
The vBloc System. Our vBloc System delivers vBloc Therapy via two small electrodes that are laparoscopically implanted and placed in contact with the trunks of the vagus nerve just above the junction between the esophagus and the stomach, near the diaphragm.
|
|
|
The major components of the vBloc System include:
· Neuroregulator. The neuroregulator, a pacemaker-like device, is an implanted device that controls the delivery of vBloc Therapy to the vagus nerve. It is surgically implanted just below, and parallel to, the skin, typically on the side of the body over the ribs.
· Lead System. Proprietary leads are powered by the neuroregulator and deliver electrical pulses to the vagus nerve via the electrodes. The leads and electrodes are similar to those used in traditional cardiac rhythm management products.
· Mobile Charger. The mobile charger is an electronic device worn by the patient externally while recharging the device. It connects to the transmit coil and provides information on the battery status of the neuroregulator and the mobile charger.
· Transmit Coil. The transmit coil is positioned for short periods of time on top of the skin over the implanted neuroregulator to deliver radiofrequency battery charging and therapy programming information across the skin into the device.
· Clinician Programmer. The clinician programmer connects to the mobile charger to enable clinicians to customize therapy settings as necessary and retrieve reports stored in system components. The reports include patient use and system performance information used to manage therapy. The clinician programmer incorporates our proprietary software and is operated with a commercially available laptop computer.
Implantation Procedure. The vBloc System is implanted by a laproscopically trained surgeon using a procedure that is typically performed within 60-90 minutes. During the procedure, the surgeon laparoscopically implants the electrodes in contact with the vagal nerve trunks and then connects the lead wires to the neuroregulator, which is subcutaneously implanted. The implantation procedure and usage of the vBloc System carry some risks, such as the risks generally associated with laparoscopic procedures as well as the possibility of device malfunction. Adverse events related to the therapy, device or procedure may include, but are not limited to: transient pain at the implant site, heartburn, constipation, nausea, depression, diarrhea, infection, organ or nerve damage, surgical explant or revision, device movement, device malfunction and allergic reaction to the implant.
Usage of the vBloc System. The physician activates the vBloc System after implantation. vBloc Therapy is then delivered intermittently through the neuroregulator each day as scheduled (recommended during the patient’s
waking hours when food is consumed) through the neuroregulator. The scheduled delivery of the intermittent pulses blocking the vagus nerve is customized for each patient’s weight loss and overall treatment objectives.
The physician is able to download reports to monitor patient use and system performance information. This information is particularly useful to physicians to ensure that patients are properly using the system. Although usage of our vBloc System generally proceeds without complications, as part of the therapy or intentional weight loss, patients in our clinical trials have observed side-effects such as transient pain at the implant site, heartburn, bloating, dysphagia, eructation, cramps, diarrhea, nausea, constipation, and excessive feelings of fullness, especially after meals. In addition, patient noncompliance with properly charging the vBloc System may render vBloc Therapy less effective in achieving long-term loss.
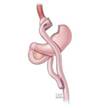
The Gastric Vest System
The Gastric Vest System, which we acquired in May 2017 in connection with our acquisition of BarioSurg, is an investigational, minimally invasive, laparoscopically implanted medical device being studied for weight loss in morbidly obese patients with a BMI of at least 35. This device is designed to restrict the intake of food and provide the feeling of fullness without cutting or permanently removing portions of the stomach, or bypassing, any portion of the gastrointestinal tract. The implantation of the device mimics a traditional weight-loss surgery without permanently altering the anatomy and does not appear to require vitamin supplementation.
In a small pilot study conducted outside the U.S., at 12 months, Gastric Vest patients demonstrated a mean percent excess weight loss (%EWL) of 85%, an average drop in HbA1c (Hemoglobin A1c) of 2.1 points, and an average waist circumference reduction of 38 centimeters, or approximately 15 inches.
Benefits. The Gastric Vest System, if approved for sale, would allow us to offer an additional weight loss solution that emulates the effect of conventional weight loss surgery through a procedure that is minimally invasive. The Gastric Vest System potentially offers the following benefits:
· Minimizes Changes to Normal Anatomy. The Gastric Vest System emulates the effects of conventional weight-loss surgery without stapling, cutting or removing any portion of the stomach.
· Minimally Invasive Procedure. Unlike conventional weight loss surgery, which typically is performed in a hospital setting under general anesthesia and requires a hospital stay of up to four days, the Gastric Vest System is inserted laparoscopically in an outpatient procedure.
Removable/Reversible. The Gastric Vest System is designed to be removed laparoscopically, permitting the removal of the device at a later time, if that is desired.
· Allows Continued Ingestion and Digestion of Foods Found in a Typical, Healthy Diet. Because the Gastric Vest System also leaves the digestive anatomy largely unaltered, patients are able to maintain a more consistent nutritional balance compared to conventional surgical approaches, thus allowing them to effect positive changes in their eating behavior in a non-forced and potentially more consistent way.
System. The Gastric Vest System is a thin, implantable-grade silicon device that wraps around the stomach, as shown below. Following stomach plication, the device wraps around the stomach, emulating the effect of conventional weight-loss surgery, and is intended to enable gastric volume reduction without permanently changing patient anatomy. By decreasing the cross-sectional area of the stomach, food travels faster through the stomach, resulting in faster gastric emptying. The smaller amount of food in the stomach coupled with restriction is intended to stimulate the stretch receptors along the stomach, which send signals to the brain that the patient should stop eating.
|
|
|
Alternative Weight Loss Solutions
If we are able to commercialize the Gastric Vest System, we believe we will be able to offer three distinct approaches as weight loss solutions for obesity and its related comorbidities: the vBloc System; the Gastric Vest System; and a combination of both the vBloc System and the Gastric Vest System.
Our Commercialization Strategy
We started the process of building a sales force and a controlled expansion of our operations and hired three new executives in January 2016 to oversee this expansion. The direct sales force is supported by field technical managers who provide training, technical and other support services to our customers. Throughout 2015 and 2016 our sales force called directly on key opinion leaders and bariatric surgeons at commercially-driven bariatric centers of excellence that met our certification criteria. Additionally, in 2016, through a distribution agreement with Academy Medical, VA medical facilities now offer the vBloc System as a treatment option to veteran healthcare benefits. We intend to continue to build on these efforts in 2017 through self-pay patient and veteran focused direct-to-patient marketing and key opinion leader and center specific partnering.
Account management and patient registration processes used during the clinical trial are being transitioned to a commercial registration structure. Centers responsible for implanting our product will be expanded and trained to perform patient selection, implant the vBloc System and manage appropriate follow-up procedures.
Our sales representatives are supported by field clinical experts who are responsible for training, technical support, and other support services at various implant centers. Our sales representatives implement consumer marketing programs and provide surgical centers and implanting surgeons with educational patient materials.
We market directly to patients but sell the vBloc System to select surgical centers throughout the United States that have patients that would like to use the vBloc System to treat obesity and its comorbidities. The surgical centers then sell our product to the patients and implant and administer vBloc Therapy. In 2015 and 2016, almost all the patients that purchased the vBloc System paid for the therapy themselves and did not receive reimbursement from an insurance provider, and we expect that most of our sales will come from self-pay patients and veterans in 2017. Additionally, through our distribution agreement with Academy Medical, VA medical facilities now offer the vBloc System as a treatment option for veterans at little to no cost to veterans in accordance with their veteran healthcare benefits.
We are working to obtain coverage for our product from the U.S. Centers for Medicare and Medicaid Services (CMS), Medicare Administrative Contractors (MACs), major insurance carriers, local coverage entities and self-insured plans, including Integrated Delivery Networks (IDNs). We received coverage from one significant IDN in the northeast, Winthrop University Hospital, in 2016 and are in active discussions with other IDNs throughout the country.
Identify Appropriate Coding, Obtain Coverage and Payment for the vBloc System. While payers are not our direct customers, their coverage and reimbursement policies influence patient and physician selection of obesity treatment. We are employing a focused campaign to obtain payer support for vBloc Therapy. We are seeking specific and appropriate coding, coverage and payment for our vBloc System from private insurers and CMS. We plan to establish a market price for the vBloc System in the United States that is competitive with other available weight loss surgical procedures and comparable to other active implantable devices such as implantable cardioverter defibrillators, neurostimulation devices for chronic pain and depression, and cochlear implant systems.
CMS issued a national coverage determination for several specific types of bariatric surgery in 2006, which we view as positive potential precedent and guidance factors that CMS might use in deciding to cover our therapy. Although Medicare policies are often emulated or adopted by other third-party payers, other governmental and private insurance coverage currently varies by carrier and geographic location. We are actively working with major insurance carriers, local coverage entities and self-insured plans, as well as CMS, on obtaining coverage for procedures using our product. Initial coverage for vBloc will likely occur in self-contained healthcare systems that operate as IDNs, as these systems are able to evaluate risk-benefit ratios in a closed environment. For example, in the first quarter of 2016, we announced that the Winthrop Hospital System in New York, a significant IDN in the northeast, would cover our therapy for their employees. Other similar arrangements are in active discussion.
Drive the Adoption and Endorsement of vBloc Therapy Through Obesity Therapy Experts and Patient Ambassadors. Our Clinical Development strategy is to collaborate closely with regulatory bodies, obesity therapy experts and others involved in the obesity management process, patients and their advocates and scientific experts. We have established credible and open relationships with obesity therapy experts and have identified vBloc Therapy patient ambassadors and we believe these individuals will be important in promoting patient awareness and gaining widespread adoption of the vBloc System.
Expand and Protect Our Intellectual Property Position. We believe that our issued patents and our patent applications encompass a broad platform of neuromodulation therapies, including vagal blocking and combination therapy focused on obesity, diabetes, hypertension and other gastrointestinal disorders. We intend to continue to pursue further intellectual property protection through U.S. and foreign patent applications.
Leverage our vBloc Technology for Other Disease States. We intend to continue to conduct research and development for other potential applications for our vBloc Therapy and believe we have a broad technology platform that will support the development of additional clinical applications and therapies for other metabolic and gastrointestinal disorders in addition to obesity.
Concentrate Our Resources on the U.S. Market. We intend to devote our near-term efforts toward mounting a successful system launch in the United States. We intend to explore select international markets to commercialize the vBloc System as our resources permit, using direct, dealer and distributor sales models as the targeted market best dictates. Specifically, we are currently evaluating Canada as a market due to its relatively low barrier to entry and an established cash-pay bariatric patient market.
Our Clinical Experience
We have conducted a series of clinical trials to date, which have shown that vBloc Therapy offers physicians a programmable method to selectively and reversibly block the vagus nerve resulting in clinically and statistically significant EWL.
We have not observed any mortality related to our device or any unanticipated adverse device effects in any of our completed or ongoing studies. Reported events include those associated with laparoscopic surgery or any implantable electronic device. The effects of vBloc Therapy include changes in appetite, and, in some patients, effects that may be expected with decreased intra-abdominal vagus nerve activity, such as temporary abdominal discomfort and short episodes of belching, bloating, cramping or nausea.
Findings from our clinical trials have resulted in publication in numerous peer-reviewed journals including The Journal of the American Medical Association, Journal of Obesity, Obesity Surgery, Surgery for Obesity and
Related Diseases, Journal of Diabetes and Obesity, Surgery and Journal of Neural Engineering, and data have been presented at several scientific sessions including the American Society for Metabolic and Bariatric Surgery, International Federation for Surgery of Obesity and Metabolic Disorders, the Obesity Surgery Society of Australia & New Zealand and The Obesity Society.
Below is a more detailed description of our ongoing clinical studies:
ReCharge Trial
In October 2010, we received an unconditional Investigational Device Exemption (IDE) Supplement approval from the FDA to conduct a randomized, double-blind, sham-controlled, multicenter pivotal clinical trial, called the ReCharge trial, testing the effectiveness and safety of vBloc Therapy utilizing our second generation vBloc System. Enrollment and implantation in the ReCharge trial was completed in December 2011 in 239 randomized patients (233 implanted) at 10 centers. All patients in the trial received an implanted device and were randomized in a 2:1 allocation to treatment or control groups. The control group received a non-functional device during the trial period. All patients were expected to participate in a standard weight management counseling program. The primary endpoints of efficacy and safety were evaluated at 12 months. The ReCharge trial met its primary safety endpoint with a 3.7% serious adverse event rate, significantly lower than the threshold of 15% (p<0.0001). The safety profile at 12 months was further supported by positive cardiovascular signals including a 5.5 mmHg drop in systolic blood pressure, a 2.8 mmHg drop in diastolic blood pressure and a 3.6 bpm drop in average heart rate.
Although the trial did not meet its predefined co-primary efficacy endpoints, it did demonstrate in the ITT population (n=239) a clinically meaningful and statistically significant EWL of 24.4% (approximately 10% TBL) for vBloc Therapy-treated patients, with 52.5% of patients achieving at least 20% EWL. In the per protocol population, the trial demonstrated an EWL of 26.3% for vBloc Therapy-treated patients, with 56.8% of patients achieving at least 20% EWL. As a result of the positive safety and efficacy profile of vBloc Therapy, we used the data from the ReCharge trial to support a PMA application for the vBloc System, which was submitted to the FDA in June 2013 and was accepted for review and filing in July 2013. An Advisory Panel meeting was held on June 17, 2014 to review our PMA application for approval of the vBloc System. The Advisory Panel voted 8 to 1 “in favor” that the vBloc System is safe when used as designed and voted 4 to 5 “against” on the issue of a reasonable assurance of efficacy. The final vote, on whether the relative benefits outweighed the relative risk, was 6 to 2 “in favor,” with 1 abstention. We received FDA approval on January 14, 2015 for vBloc Therapy, delivered via the vBloc System, for the treatment of adult patients with obesity who have a BMI of at least 40 to 45 kg/m2, or a BMI of at least 35 to 39.9 kg/m2 with a related health condition such as high blood pressure or high cholesterol levels, and who have tried to lose weight in a supervised weight management program and failed within the past five years.
Further analysis of the 12 month data show that in the primary analysis (ITT) population (n=239), vBloc Therapy-treated patients achieved a 24.4% average EWL (approximately 10% TBL) compared to 15.9% for sham control patients. This 8.5% difference demonstrated statistical superiority over sham control (p=0.002), but not super-superiority at the pre-specified 10% margin (p=0.705). In total, 52.5% of vBloc Therapy-treated patients had 20% or more EWL compared to 32.5% in the control group (p=0.004), and 38.3% of vBloc Therapy-treated patients had 25% or more EWL compared to 23.4% in the sham control group (p=0.02). While the respective co-primary endpoint targets of 55% and 45% were not met, the endpoint targets were within the 95% confidence intervals for the observed rates and therefore the observed rates were not significantly lower than these pre-specified rates. These efficacy data demonstrate vBloc Therapy’s positive effect on weight loss.
In the per protocol group, which included only those patients who received therapy per the trial design (n=211), the vBloc Therapy-treated patients had a 26.3% average EWL (approximately 10% TBL) compared to 17.3% for the sham control group (p=0.003). In total, 56.8% of vBloc Therapy-treated patients achieved at least 20% EWL, which was above the predefined threshold of 55% compared to 35.4% in the sham control group (p=0.004). 41.8% of vBloc Therapy-treated patients also achieved at least 25% EWL in this population, which is slightly less than the predefined threshold of 45%, compared to 26.2% in the sham control group (p=0.03).
Additionally, two-thirds of vBloc Therapy-treated patients achieved at least 5% TBL at 12 months. According to the CDC, 5% TBL can have significant health benefits on obesity related risk factors, or comorbidities,
including reduction in blood pressure, improvements in Type 2 diabetes and reductions in triglycerides and cholesterol. Further analysis of our data at 12 months showed a meaningful impact on these comorbidities as noted in the below table showing the improvements seen at 10% TBL, the average weight loss in vBloc Therapy-treated patients.
|
Risk Factor |
|
10% TBL |
|
|
Systolic BP (mmHg) |
|
(9 |
) |
|
Diastolic BP (mmHg) |
|
(6 |
) |
|
Heart Rate (bpm) |
|
(6 |
) |
|
Total Cholesterol (mg/dL) |
|
(15 |
) |
|
LDL (mg/dL) |
|
(9 |
) |
|
Triglycerides (mg/dL) |
|
(41 |
) |
|
HDL (mg/dL) |
|
3 |
|
|
Waist Circumference (inches) |
|
(7 |
) |
|
HbA1c (%) |
|
(0.5 |
) |
Approximately 93% of patients reached the 12 month assessment in the trial, consistent with a rigorously executed trial. vBloc Therapy-treated patients maintained their weight loss at 18 months and 24 months with an EWL of 23.5% and 21.1%, respectively. The trial’s positive safety profile also continued throughout this reported time period.
VBLOC-DM2 ENABLE Trial
Enrollment of the VBLOC-DM2 ENABLE trial began in 2008. The VBLOC-DM2 ENABLE trial is designed to evaluate the efficacy and safety of vBloc Therapy on obese subjects as well as its effect on glucose regulation in approximately 30 patients who are using the vBloc System. The trial is an international, open-label, prospective, multi-center study. At each designated trial endpoint the efficacy of vBloc Therapy is evaluated by measuring average percentage EWL, HbA1c (blood sugar), FPG (fasting plasma glucose), blood pressure, calorie intake, appetite and other endpoints at one week, one month, three, six, 12 and 18 months and longer. The following results were reported at 12 month intervals.
· Percent EWL (from implant, Company updated interim data):
|
Visit (post-device activation) |
|
% EWL |
|
N |
|
|
12 Months |
|
(24.5 |
) |
26 |
|
|
24 Months |
|
(22.7 |
) |
22 |
|
|
36 Months |
|
(24.3 |
) |
18 |
|
· HbA1c change in percentage points (Baseline HbA1c = 7.8 + 0.2%) (Company updated interim data):
|
|
|
% |
|
|
|
|
Visit (post-device activation) |
|
change |
|
N |
|
|
12 Months |
|
(1 |
) |
26 |
|
|
24 Months |
|
(0.5 |
) |
24 |
|
|
36 Months |
|
(0.6 |
) |
17 |
|
· Fasting Plasma Glucose change (Baseline 151.4 + 6.5 mg/dl average) (Company updated interim data):
|
|
|
Glucose |
|
|
|
|
|
|
change |
|
|
|
|
Visit (post-device activation) |
|
(mg/dl) |
|
N |
|
|
12 Months |
|
(27.6 |
) |
25 |
|
|
24 Months |
|
(20.3 |
) |
24 |
|
|
36 Months |
|
(24 |
) |
17 |
|
· Change in mean arterial pressure (MAP) in hypertensive patients (baseline 99.5 mmHg) (Company updated interim data):
|
|
|
MAP |
|
|
|
|
|
|
change |
|
|
|
|
Visit (post-device activation) |
|
(mmHg) |
|
N |
|
|
12 Months |
|
(7.8 |
) |
14 |
|
|
24 Months |
|
(7.5 |
) |
12 |
|
|
36 Months |
|
(7.3 |
) |
10 |
|
To date, no deaths related to our device or unanticipated adverse device effects have been reported during the VBLOC-DM2 ENABLE trial and the safety profile is similar to that seen in the other vBloc trials.
Caloric Intake Sub-study: A sub-study, conducted as part of the VBLOC-DM2 ENABLE trial, evaluated 12-month satiety and calorie intake in 10 patients with Type 2 diabetes mellitus enrolled in the trial. Follow-up measures among patients enrolled in the sub-study included EWL, 7-day diet records assessed by a nutritionist, calorie calculations and visual analogue scale (VAS) questions to assess satiety by 7-day or 24-hour recall at the following time periods: baseline, 4 and 12 weeks and 6 and 12 months post device initiation. A validated program, Food Works™, was used to determine calorie and nutrition content. Results include:
· Mean EWL for the sub-study was 33+5% (p<0.001) at 12 months;
· Calorie intake decreased by 45% (p<0.001), 48% (p<0.001), 38% (p<0.001) and 30% (p=0.02), at 4 and 12 weeks, 6 months and 12 months, respectively, from a baseline of 2,062 kcal/day; and
· VAS recall data, using a repeated measures analysis, documented fullness at the beginning of meals (p=0.005), less food consumption (p=0.02) and less hunger at the beginning of meal (p=0.03) corroborating the reduction in caloric intake.
ReNew Trial
The ReNew Trial is a Post Approval Study required by the FDA as a condition of approval. ReNew is a five-year, single arm, multi-center trial to evaluate the long-term safety and efficacy of the Maestro Rechargeable System in treating obesity in 200 patients at 10 to 15 sites. We expect enrollment for the ReNew trial to begin in the second half of 2017.
Kaiser Diabetes Trial
On April 26, 2017, we entered into a Clinical Trial Agreement with Southern California Permanente Medical Group (“Southern”), a division of Kaiser Permanente, with an effective date of June 1, 2017. Under the agreement, we are sponsoring an investigator-initiated study with Southern to study vBloc Therapy as a treatment for Type 2 diabetic patients with obesity. As sponsor of the study, we are obligated to pay Southern approximately $3.4 million over three years to fund the study. We expect enrollment to begin in the fourth quarter of 2017.
All clinical data generated during the study will be disclosed to us and may be used for any purpose stated in the informed consent form or otherwise in compliance with applicable law. We will have the right to publish, present or use any final results arising out of the study.
Gastric Vest System ENDURE Trial
The Gastric Vest System was studied internationally in the ENDURE trial, which was designed to evaluate the safety and efficacy of the Gastric Vest System. Of the 17 patients enrolled, 14 have completed their 12-month follow-up visit. Results from these 14 patients show that the Gastric Vest System demonstrated a mean excess weight loss (%EWL) of 85.5% compared to approximately 75% and 65% for gastric bypass and sleeve gastrectomy. The patients also experienced an average HgA1c decrease of 2.1%, and an average waist circumference reduction of
38 cm, or 15 inches. The Gastric Vest System will continue to be studied in upcoming trials in the US and internationally.
Our Research and Development
Current R&D Focus
We have an experienced research and development team, including clinical, regulatory affairs and quality assurance, comprised of scientists, electrical engineers, software engineers and mechanical engineers with significant clinical knowledge and expertise. Our research and development efforts are focused in the following major areas:
· supporting the current vBloc System;
· testing and developing the Gastric Vest System;
· developing the next-generation vBloc System;
· identifying the effect of vagal blocking on nerve and organ function; and
· investigating the vBloc and Gastric Vest platform for the treatment of gastrointestinal disorders and comorbidities in addition to obesity.
We have spent a significant portion of our capital resources on research and development. Our research and development expenses were $5.1 million in 2016, $8.1 million in 2015 and $11.0 million in 2014. Having obtained FDA approval in January 2015, our main focus has been on commercialization efforts, resulting in decreases in spending on research and development in each of 2015 and 2016 compared to 2014, when we were still working through the FDA approval process.
Other Diseases and Disorders
We believe that our vBloc Therapy and Gastric Vest System may have the potential, if validated through appropriate clinical studies, to treat a number of additional gastrointestinal disorders or comorbidities frequently associated with obesity, including the following:
· Type 2 Diabetes. Type 2 diabetes is an escalating global health epidemic often related to obesity that affects nearly 200 million people worldwide, 50 million in the United States alone. Those with diabetes are susceptible to cardiovascular morbidity and mortality, and up to two out of three people with diabetes have high blood pressure. We believe that vBloc Therapy has significant potential in treating metabolic syndrome (diabetes with high blood pressure). We have launched an international feasibility trial, VBLOC-DM2 ENABLE, to further explore the efficacy of vBloc Therapy in this patient population and have reported preliminary findings in the “Our Clinical Experience” section above. vBloc Therapy for patients with Type 2 diabetes will continue to be studied primarily in our Kaiser Diabeties Trial.
· Hypertension. Blood pressure normally rises and falls throughout the day. When it consistently stays too high for too long, it is called hypertension. Globally, nearly one billion people have high blood pressure (hypertension); of these, two-thirds are in developing countries. About one in three American adults has high blood pressure or hypertension. Hypertension is one of the most important causes of premature death worldwide and the problem is growing; in 2025, an estimated 1.56 billion adults will be living with hypertension. Hypertension kills nearly 8 million people every year worldwide. We believe that vBloc Therapy may improve mean systolic and diastolic blood pressure in hypertensive patients. We completed a subgroup analysis of patients from an earlier clinical trial and have included an evaluation of the blood pressure effects of vBloc Therapy in our international feasibility trial,
VBLOC-DM2 ENABLE, to further explore the efficacy of vBloc Therapy in this patient population and have reported preliminary findings in the “Our Clinical Experience” section above.
· Pancreatitis. Primary and recurrent cases of acute pancreatitis are estimated to number from 150,000 to 200,000 annually, resulting in approximately 80,000 hospital admissions each year in the United States. In animal studies, we have shown that vBloc Therapy suppresses pancreatic exocrine secretion, suggesting its potential efficacy in treating pancreatitis.
· Other Gastrointestinal Disorders. We believe that vBloc Therapy may have potential in a number of other gastrointestinal disorders, including irritable bowel syndrome and inflammatory bowel disease.
None of the above conditions were included in our PMA application that was approved by the FDA on January 14, 2015, nor are they approved for sale internationally. Additional approvals will be required to market the vBloc System or Gastric Vest System for these indications in the United States or internationally.
Medical Advisors
In addition to our collaboration with Mayo Clinic, we also have medical advisors who provide strategic guidance to our development programs, consult with us on clinical investigational plans and individual study protocols, and advise on clinical investigational site selection. Members of our medical advisory group also:
· serve on our Data Safety Monitoring Board and Clinical Events Committee;
· provide consultation on professional meeting presentations and journal manuscript submissions; and
· develop and participate in clinical site training programs, including study surgical technique training and study subject follow-up training.
Our Competition
The market for obesity treatments is competitive, subject to technological change and significantly affected by new product development. Our primary competition in the obesity treatment market is currently from surgical obesity procedures and from various devices used to implement neurostimulation and gastric stimulation systems. We believe we are the first company having neuroblocking therapy for the treatment of obesity. There are currently no other FDA-approved neuromodulation or neuroblocking therapies for the treatment of obesity, but in the future we expect other new stimulation systems and neurotechnology devices to come on the market.
|
|
|
|
|
|
Balloon |
Band |
Sleeve |
Bypass |
Our vBloc System competes, and we expect that our Gastric Vest System will compete, with surgical obesity procedures, including gastric bypass, gastric balloon, gastric banding, sleeve gastrectomy and the endoscopic sleeve. These current surgical procedures are performed in less than 1% of all eligible obese patients today. Current manufacturers of approved gastric balloon and banding products include Apollo Endosurgery Inc. (Lap-Band,ORBERA Intragastric Balloon System, and OverStitch Endoscopic Suturing System), ReShape Medical, Inc. (ReShape Integrated Dual Balloon System), Obalon Therapeutics, Inc. (Obalon Balloon System) and Johnson & Johnson (Realize Adjustable Gastric Band).
In June 2016, Aspire Bariatrics, Inc. received FDA approval on the Aspire Assist® System, an endoscopic alternative to weight loss surgery for people with moderate to severe obesity. We are also aware that GI Dynamics, Inc. has received approvals in various international countries to sell its EndoBarrier Gastrointestinal Liner.
We also compete against the manufacturers of pharmaceuticals that are directed at treating obesity and the 99% of obese patients eligible for surgery that are not willing to pursue a surgical option . We are aware of a number of drugs that are approved for long-term treatment of obesity in the United States: Orlistat, marketed by Roche as Xenical and GlaxoSmithKline as Alli, Belviq marketed by Arena Pharmaceuticals, Inc., Qsymia, marketed by VIVUS, Inc. and Contrave, marketed by Orexigen Therapeutics, Inc.
In addition to competition from surgical obesity procedures, we compete with several private early-stage companies developing neurostimulation devices for application to the gastric region and related nerves for the treatment of obesity. These companies may prove to be significant competitors, particularly through collaborative arrangements with large and established companies. They also compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and subject registration for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business.
In addition, there are many larger potential competitors experimenting in the field of neurostimulation to treat various diseases and disorders. For example, Medtronic, Inc., which develops deep brain stimulators and spinal cord stimulators, acquired TransNeuronix, which sought to treat obesity by stimulating the smooth muscle of the stomach wall and nearby tissue. St. Jude Medical, Inc., through its acquisition of Advanced Neuromodulation Systems, is developing spinal cord stimulators. LivaNova PLC is developing vagus nerve stimulators to modulate epileptic seizures and other neurological disorders. Boston Scientific Corporation, through its Advanced Bionics division, is developing neurostimulation devices such as spinal cord stimulators and cochlear implants. Ethicon-Endo Surgery acquired LivaNova PLC’s patents and patent applications pertaining to vagus nerve stimulation for the treatment of obesity and two related comorbidities, diabetes and hypertension, in overweight patients.
We believe that the principal competitive factors in our market include:
· acceptance by healthcare professionals, patients and payers;
· published rates of safety and efficacy;
· reliability and high quality performance;
· effectiveness at controlling comorbidities such as diabetes and hypertension;
· invasiveness and the inherent reversibility of the procedure or device;
· cost and average selling price of products and relative rates of reimbursement;
· effective marketing, education, sales and distribution;
· regulatory and reimbursement expertise;
· technological leadership and superiority; and
· speed of product innovation and time to market.
Many of our competitors are either publicly-traded or are divisions of publicly-traded companies, and they enjoy several competitive advantages over us, including:
· significantly greater name recognition;
· established relations with healthcare professionals, customers and third-party payers;
· established distribution networks;
· greater experience in research and development, manufacturing, preclinical testing, clinical trials, obtaining regulatory approvals, obtaining reimbursement and marketing approved products; and
· greater financial and human resources.
As a result, we cannot assure you that we will be able to compete effectively against these companies or their products.
Our Intellectual Property
Our success will depend in part on our ability to obtain and defend patent protection for our products and processes, to preserve our trade secrets and to operate without infringing or violating the proprietary rights of third parties. We own numerous U.S. and foreign patents, and have numerous patent applications pending, most of which pertain to treating gastrointestinal disorders and we believe provide us with broad intellectual property protection covering electrically-induced vagal blocking and methods for treating obesity. Assuming timely payment of maintenance fees as they become due, many of these patents will expire in 2023. Our acquisition of the Gastric Vest System included four U.S. patents, one pending U.S. patent application, four foreign patents, and five pending foreign patent applications. The patents we acquired related to the Gastric Vest System will expire between 2028 and 2034. We have also received or applied for patents in Europe, Australia, China, India and Japan. These applications primarily pertain to our vagal blocking technology and its application to obesity as well as other gastrointestinal disorders. The applications that we acquired related to the Gastric Vest System primarily pertain to methods of gastric restriction for treating obesity.
We also register the trademarks and trade names through which we conduct our business. To date, in the United States we have registered trademarks for vBloc®, ENTEROMEDICS® and MAESTRO®, each registered with the United States Patent and Trademark Office, and trademark applications for vBloc POWER TO CHOOSE and vBloc POWER TO CHOOSE AND DESIGN. In addition, some or all of the marks vBloc, ENTEROMEDICS, MAESTRO, MAESTRO SYSTEM ORCHESTRATING OBESITY SOLUTIONS, vBloc POWER TO CHOOSE and vBloc POWER TO CHOOSE AND DESIGN are the subject of either a trademark registration or application for registration in Australia, Brazil, China, the European Community, India, Kuwait, Mexico, Saudi Arabia, Switzerland and the United Arab Emirates. While the Company believes that it has common law trademark rights to GASTRIC VEST, it has not applied for the registration of this mark.
In addition to our patents, we rely on confidentiality and proprietary information agreements to protect our trade secrets and proprietary knowledge. These confidentiality and proprietary information agreements generally provide that all confidential information developed or made known to individuals by us during the course of their relationship with us is to be kept confidential and not disclosed to third parties, except in specific circumstances. The agreements also provide for ownership of inventions conceived during the course of such agreements. If our proprietary information is shared or our confidentiality agreements are breached, we may not have adequate remedies, or our trade secrets may otherwise become known to or independently developed by competitors.
Our Manufacturers and Suppliers
We have designed and developed all of the elements of our vBloc System, except for the clinician programmer hardware, which uses a commercially available laptop computer. To date, all of the materials and components of the system are procured from qualified suppliers and contract manufacturers in accordance with our proprietary specifications. We use third parties to manufacture our vBloc System to minimize our capital investment, help control costs and take advantage of the expertise these third parties have in the large-scale production of medical devices. We do not currently plan to manufacture our vBloc System ourselves. All of our key
manufacturers and suppliers have experience working with commercial implantable device systems, are ISO certified and are regularly audited by us. Our key manufacturers and suppliers have a demonstrated record of compliance with international regulatory requirements.
Since we received FDA approval for the vBloc System on January 14, 2015, and commenced commercialization of the vBloc System in the United States, we have increased our production volume slowly in connection with the controlled commercial launch of the vBloc System in the United States. Given that we rely primarily on third-party manufacturers and suppliers for the production of our products, our ability to increase production going forward will depend upon the experience, certification levels and large scale production capabilities of our suppliers and manufacturers. Qualified suppliers and contract manufacturers have been and will continue to be selected to supply products on a commercial scale according to our proprietary specifications. We have modestly increased our inventory levels to support commercial forecasts as we expand our implanting centers and intend to continue to increase our inventory levels as we determine necessary. Our FDA approval process required us to name and obtain approval for the suppliers of key components of our vBloc System.
Many of our parts are custom designed and as a result, we may not be able to quickly qualify and establish additional or replacement suppliers for the components of our vBloc System. Any new approvals of vendors required by the FDA or other regulatory agencies in other international markets for our vBloc System as a result of the need to qualify or obtain alternate vendors for any of our components would delay our ability to sell and market the vBloc System and could have a material adverse effect on our business.
We believe that our current manufacturing and supply arrangements will be adequate to continue our controlled commercial launch and our ongoing and planned clinical trials. In order to produce the vBloc System in the quantities we anticipate to meet future market demand, we will need our manufacturers and suppliers to increase, or scale up, manufacturing production and supply arrangements by a significant factor over the current level of production. There are technical challenges to scaling up manufacturing capacity and developing commercial-scale manufacturing facilities that may require the investment of substantial additional funds by our manufacturers and suppliers and hiring and retaining additional management and technical personnel who have the necessary experience. If our manufacturers or suppliers are unable to do so, we may not be able to meet the requirements to expand the launch of the product in the United States or launch the product internationally or to meet future demand, if at all. We may also represent only a small portion of our suppliers’ or manufacturers’ business and if they become capacity constrained they may choose to allocate their available resources to other customers that represent a larger portion of their business. We currently anticipate that we will continue to rely on third-party manufacturers and suppliers for the production of the vBloc System as we expand our commercial launch. If we are unable to obtain a sufficient supply of our product, our revenue, business and financial prospects would be adversely affected.
Government Regulations
United States
Our vBloc System and our proposed Gastric Vest System are regulated by the FDA as medical devices under the Federal Food, Drug, and Cosmetic Act (FFDCA) and the regulations promulgated under the FFDCA. Pursuant to the FFDCA, the FDA regulates the research, design, testing, manufacture, safety, labeling, storage, record keeping, advertising, sales and distribution, post-market adverse event reporting, production and advertising and promotion of medical devices in the United States. Noncompliance with applicable requirements can result in warning letters, fines, injunctions, civil penalties, recall or seizure of products, total or partial suspension of production, failure of the government to grant premarket approval for devices and criminal prosecution.
Medical devices in the United States are classified into one of three classes, Class I, II or III, on the basis of the amount of risk and the controls deemed by the FDA to be necessary to reasonably ensure their safety and effectiveness. Class I, low risk, devices are subject to general controls (e.g., labeling and adherence to good manufacturing practices). Class II, intermediate risk, devices are subject to general controls and to special controls (e.g., performance standards, and premarket notification). Generally, Class III devices are those which must receive premarket approval by the FDA to ensure their safety and effectiveness (e.g., life-sustaining, life-supporting and implantable devices, or new devices which have not been found substantially equivalent to legally marketed devices), and require clinical testing to ensure safety and effectiveness and FDA approval prior to marketing and
distribution. The FDA also has the authority to require clinical testing of Class II devices. In both the United States and certain international markets, there have been a number of legislative and regulatory initiatives and changes, such as the Modernization Act, which could and have altered the healthcare system in ways that could impact our ability to sell our medical devices profitably.
The FFDCA provides two basic review procedures for medical devices. Certain products may qualify for a submission authorized by Section 510(k) of the FFDCA, where the manufacturer submits to the FDA a premarket notification of the manufacturer’s intention to commence marketing the product. The manufacturer must, among other things, establish that the product to be marketed is substantially equivalent to another legally marketed product. Marketing may commence when the FDA issues a letter finding substantial equivalence. If a medical device does not qualify for the 510(k) procedure, the manufacturer must file a premarket approval (PMA) application with the FDA. This procedure requires more extensive pre-filing clinical and preclinical testing than the 510(k) procedure and involves a significantly longer FDA review process.
Premarket Approval
Our vBloc System is an implanted device that required PMA from the FDA to market in the United States. The FDA approved the vBloc System on January 14, 2015 with post-approval conditions intended to ensure the safety and effectiveness of the device. Failure to comply with the conditions of approval can result in material adverse enforcement action, including the loss or withdrawal of the approval. Even after approval of the PMA, new PMAs or supplemental PMAs will be required for significant modifications to the manufacturing process, labeling, use and design of a device that is approved through the premarket approval process. Premarket approval supplements often require submission of the same type of information as a PMA except that the supplement is limited to information needed to support any changes from the device covered by the original PMA. In addition, holders of an approved PMA are required to submit annual reports to the FDA that include relevant information on the continued use of the device.
The Gastric Vest System will likely be considered a Class III Long Term Implantable (LTI) product by the FDA requiring the premarket approval (PMA) path. A PMA is required to establish the safety and effectiveness of the device and a key component of a PMA submission is the pivotal clinical trial data, as discussed in more detail below. A pivotal trial for the Gastric Vest System will likely include 200 to 250 implanted patients monitored up to three years. Other implantable devices for the treatment of obesity relied on 12 month endpoints for the PMA submission with annual follow-up visits up to five years and we expect the pivotal trial for the Gastric Vest System to be similar. A US pivotal trial requires FDA Investigational Device Exemption (IDE) submission and approval. We expect to submit our IDE application to the FDA in the first quarter of 2018 and expect the first U.S. PMA implants of the Gastric Vest System to take place in the second quarter of 2018. Our goal is to obtain PMA approval by the end of 2020. We intend to initiate a CE Mark trial in the European Union of 50 to 100 patients with a six-month weight loss and safety endpoint with a minimum 12-month follow up. We expect the first EU implants will start in the first quarter of 2018. Our goal is to obtain CE mark approval by the second quarter of 2019.
Clinical Trials
A clinical trial is almost always required to support a PMA. Clinical trials for a “significant risk” device such as ours require submission to the FDA of an application for an IDE for clinical studies to be conducted within the United States. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. Clinical trials for a significant risk device in the United States may begin once the IDE application is approved by the FDA and by the Institutional Review Boards (IRBs) overseeing the clinical trial at the various investigational sites.
Clinical trials require extensive recordkeeping and detailed reporting requirements. Our clinical trials must be conducted under the oversight of an IRB for each participating clinical trial site and in accordance with applicable regulations and policies including, but not limited to, the FDA’s good clinical practice requirements. We, the trial Data Safety Monitoring Board, the FDA or the IRB for each site at which a clinical trial is being performed may suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits.
Pervasive and Continuing U.S. Regulation
Numerous regulatory requirements apply. These include:
· Quality System Regulation, which requires manufacturers to follow design, testing, control, documentation, complaint handling and other quality assurance procedures during the design and manufacturing processes;
· regulations which govern product labels and labeling, prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling and promotional activities;
· medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur;
· notices of correction or removal and recall regulations;
· periodic reporting of progress related to clinical trials, post approval studies required as conditions of PMA approval and relevant changes to information contained within the PMA approval; and
· reporting of transfers of value and payments to physicians and teaching hospitals.
Advertising and promotion of medical devices are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. Recently, some promotional activities for FDA-regulated products have resulted in enforcement actions brought under healthcare reimbursement laws and consumer protection statutes. In addition, under the federal Lanham Act, competitors and others can initiate litigation relating to advertising claims.
Compliance with regulatory requirements is enforced through periodic facility inspections by the FDA, which may be unannounced. Because we rely on contract manufacturing sites and service providers, these additional sites are also subject to these FDA inspections. Failure to comply with applicable regulatory requirements can result in enforcement action, which may include any of the following sanctions:
· warning letters or untitled letters;
· fines, injunction and civil penalties;
· recall or seizure of our products;
· customer notification, or orders for repair, replacement or refund;
· operating restrictions, partial suspension or total shutdown of production or clinical trials;
· refusing our request for premarket approval of new products;
· withdrawing premarket approvals that are already granted; and
· criminal prosecution.
International
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA approval, and the requirements may differ. The primary regulatory environment
in Europe is that of the European Economic Community (EEC), which consists of 28 European Union (EU) member states encompassing nearly all the major countries in Europe. Additional countries that are not part of the EU, but are part of the European Economic Area (EEA), and other countries, such as Switzerland, have voluntarily adopted laws and regulations that mirror those of the EEC with respect to medical devices. The EEC has adopted Directive 90/385/EEC as amended by 2007/47/EC for active implantable medical devices and numerous standards that govern and harmonize the national laws and standards regulating the design, manufacture, clinical trials, labeling and adverse event reporting for medical devices that are marketed in member states. Medical devices that comply with the requirements of the national law of the member state in which their Notified Body is located will be entitled to bear CE marking, indicating that the device conforms to applicable regulatory requirements, and, accordingly, can be commercially marketed within the EEA and other countries that recognize this mark for regulatory purposes.
We obtained European CE Mark approval for our vBloc System in 2011 for the treatment of obesity. The CE Mark approval for our vBloc System was expanded in 2014 to also include use for the management of Type 2 diabetes in obese patients. The method of assessing conformity with applicable regulatory requirements varies depending on the class of the device, but for our vBloc System (which is considered an Active Implantable Medical Device (AIMD) in Australia and the EEA, and falls into Class III within the United States), the method involved a combination of self-assessment and issuance of declaration of conformity by the manufacturer of the safety and performance of the device, and a third-party assessment by a Notified Body of the design of the device and of our quality system. A Notified Body is a private commercial entity that is designated by the national government of a member state as being competent to make independent judgments about whether a product complies with applicable regulatory requirements. The assessment included, among other things, a clinical evaluation of the conformity of the device with applicable regulatory requirements. We use DEKRA Certification B.V. (formerly known as KEMA Quality) in the Netherlands as the Notified Body for our CE marking approval process.
Continued compliance with CE marking requirements is enforced through periodic facility inspections by the Notified Body, which may be unannounced. Because we rely on contract manufacturing sites and service providers, these additional sites may also be subject to these Notified Body inspections.
Patient Privacy Laws
United States and various international laws have been evolving to protect the confidentiality of certain patient health information, including patient medical records. These laws restrict the use and disclosure of certain patient health information. Enforcement actions, including financial penalties, related to patient privacy issues are globally increasing. The management of patient data may have an impact on certain clinical research activities and product design considerations.
Employees
As of June 30, 2017, we had a total of 41 employees. All of these employees are located in the United States.
From time to time we also employ independent contractors, consultants and temporary employees to support our operations. None of our employees are subject to collective bargaining agreements. We have never experienced a work stoppage and believe that our relations with our employees are good.
Executive Officers
The following table sets forth information regarding our executive officers, including their ages, as of June 30, 2017:
|
Name |
|
Age |
|
Position |
|
Dan W. Gladney |
|
64 |
|
President and Chief Executive Officer |
|
Scott P. Youngstrom |
|
57 |
|
Chief Financial Officer and Chief Compliance Officer |
|
Rajesh Nihalani, M.D. |
|
47 |
|
Chief Technology Officer |
|
Naqeeb (Nick) A. Ansari |
|
56 |
|
Senior Vice President of Sales |
|
Peter M. DeLange |
|
48 |
|
Senior Vice President of Operations and Business Development |
|
Paul F. Hickey |
|
52 |
|
Senior Vice President of Marketing and Reimbursement |
Dan W. Gladney has served as our President and Chief Executive Officer since November 16, 2015 and as Chairman of our board of directors since October 14, 2016. Mr. Gladney joined the Company on November 2, 2015 as President-Elect and a member of the board of directors. Prior to joining us, Mr. Gladney served as Chairman and Chief Executive Officer of Lanx, Inc., a medical device company focused on developing and commercializing innovative devices for spinal surgery. Prior to his time at Lanx, Inc., Mr. Gladney was a Healthcare Operating Partner at Norwest Equity Partners (NEP) from 2008 until 2010, where he was responsible for strategic planning, business growth and corporate governance for NEP portfolio companies and executing new investment opportunities for the firm. Prior to joining NEP, Mr. Gladney served as President and Chief Executive Officer of several medical device companies, including Heart Leaflet Technologies and Acist Medical Systems, both of which were acquired by The Bracco Group. He also served as Chairman, Chief Executive Officer and President of Compex Technologies, a publicly traded orthopedic and health and wellness electro therapy company, from 2002 until 2006. Mr. Gladney currently serves on the board of directors of Aria CV, Inc. and has been a member of a number of other private and public company boards. After the sale of Lanx, he acted as a private investor and small business consultant.
Scott P. Youngstrom has served as our Chief Financial Officer and Chief Compliance Officer since October 3, 2016. Mr. Youngstrom has over 25 years of strategic financial and operational experience in a variety of medical device companies, most recently having served as Chief Financial Officer and Vice President, Finance at Galil Medical, a leading developer of cryotherapy technology. Prior to Galil Medical, from 2009-2014, Mr. Youngstrom served as Vice President, Chief Operating Officer, and Chief Financial Officer at DGIMED Ortho, Inc., a developer of orthopedic medical devices. Mr. Youngstrom has previously served as Chief Financial Officer and Vice President, Finance with Anulex Technologies, Enpath Medical, Compex Technologies, Acist Medical Systems, and Cardiotronics.
Rajesh Nihalani, M.D. has served as our Chief Technology Officer since May 22, 2017, the date we completed our acquisition of BarioSurg, Inc. From August 2008 to May 2017, Dr. Nihalani served as the Chief Executive Officer of BarioSurg, Inc., a privately held medical device company that developed a minimally invasive and reversible device to treat obesity and glycemic control. Dr. Nihalani holds a medical degree from the University of Nagpur, India.
Naqeeb (Nick) A. Ansari has served as our Senior Vice President of Sales since January 6, 2016. Mr. Ansari has over 20 years of experience in the medical device industry, having held various senior sales positions at Stryker Corporation, DePuy, Medtronic, Inc., Lanx, Inc. and Globus Medical Inc. Prior to joining the Company he spent two years as the owner of an independent distributor solely selling Biomet products. Prior to this, he served as Senior Vice President of Sales at Lanx, Inc. from 2010 to 2013.
Peter M. DeLange has served as our Senior Vice President of Operations and Business Development since January 18, 2016. Mr. DeLange has spent the last 11 years as the owner and President of Devicix, LLC a medical device engineering development company that was sold in 2015. At Devicix, he contracted with large medical device companies and worked closely with individual surgeons to develop new technologies. Since 2011, Mr. DeLange has also served as a Co-Founder and Board Member of FocusStart LLC, an early stage technology development company utilizing a capital efficient business model to advance medical technology. Prior to Devicix, he held software engineer and product development positions at numerous companies including Acist Medical Systems, Nellcor Puritan Bennett, Emerson EMC and Quester Technology.
Paul F. Hickey has served as our Senior Vice President of Marketing and Reimbursement since January 18, 2016. Mr. Hickey has over 15 years of experience as a medical device executive, most recently having served as Chief Executive Officer of Pantheon Spinal, a small spine implant start-up company based in Austin, Texas, since 2014. Prior to Pantheon, he spent three years as Senior Vice President, Global Commercialization at Lanx, Inc., which was acquired by Biomet Spine in 2013, where he oversaw marketing, clinical reimbursement and R&D. Mr. Hickey also spent 17 years at Zimmer-Spine where he held numerous marketing and developments positions, most recently as Vice President, Global R&D and Emerging Technology from 2004-2008.
Our Corporate Information
We were incorporated in Minnesota in December 2002 as two separate legal entities, Alpha Medical, Inc. and Beta Medical, Inc., both of which were owned 100% by a common stockholder. In October 2003, the two entities were combined and we changed our name to EnteroMedics Inc. In 2004 we reincorporated in Delaware. We file reports and other information with the Securities and Exchange Commission (SEC) including annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and proxy or information statements. Those reports and statements as well as all amendments to those documents filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (1) are available at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, DC 20549, (2) may be obtained by sending an electronic message to the SEC at publicinfo@sec.gov or by sending a fax to the SEC at 1-202-777-1027, (3) are available at the SEC’s internet site (http://www.sec.gov), which contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC and (4) are available free of charge through our website as soon as reasonably practicable after electronic filing with, or furnishing to, the SEC. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
Our principal executive offices are located at 2800 Patton Road, St. Paul, Minnesota 55113, and our telephone number is (651) 634-3003. Our website address is www.enteromedics.com. The information on, or that may be accessed through, our website is not incorporated by reference into this Current Report on Form 8-K and should not be considered a part of this Current Report on Form 8-K.