Attached files
| file | filename |
|---|---|
| EX-99.5 - RespireRx Pharmaceuticals Inc. | ex99-5.htm |
| EX-99.4 - RespireRx Pharmaceuticals Inc. | ex99-4.htm |
| EX-99.2 - RespireRx Pharmaceuticals Inc. | ex99-2.htm |
| EX-99.1 - RespireRx Pharmaceuticals Inc. | ex99-1.htm |
| 8-K - RespireRx Pharmaceuticals Inc. | form8-k.htm |

Ampakines®
Development Summary – July, 2017
RespireRx Overview
The primary mission of RespireRx Pharmaceuticals is to develop innovative and revolutionary treatments to combat respiratory diseases caused by disruption of neuronal signaling. We are addressing respiratory conditions that affect millions of people, but for which there are few treatment options and no drug therapies, including sleep apneas - both Obstructive (OSA), and Central (CSA), opioid induced apnea and overdose, and disordered breathing from spinal cord injury (SCI) and neural dysfunction.
RespireRx is developing a pipeline of new drug products based on our broad patent portfolios for two drug platforms, including dronabinol (D-9THC), and the Ampakines, which positively modulate AMPA-type glutamate receptors to promote neuronal function.
Strategic Focus: Ampakine® Platform
RespireRx is developing a class of proprietary compounds known as ampakines, a term used to designate their actions as positive allosteric modulators of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor. Ampakines are small molecule compounds that enhance the excitatory actions of the neurotransmitter, glutamate at the AMPA receptor complex, which mediates most excitatory transmission in the central nervous system (CNS). These drugs do not have agonistic or antagonistic properties but instead modulate the receptor rate constants for transmitter binding, channel opening, and desensitization (see Figure 1).
Figure 1. AMPA Receptors Mediate Synaptic Transmission in the Brain
► Glutamate is the major excitatory neurotransmitter in the CNS
► Fast excitatory transmission is mediated by AMPA-type glutamate receptors
► Ampakines are positive, allosteric modulators of the AMPA-type glutamate receptor
► Ampakines prolong and strengthen synaptic transmission
► Ampakines work to enhance neuronal signaling
|
 |
Through an extensive translational research effort from the cellular level through Phase 2 clinical trials, the company has developed a family of ampakines, including CX717, CX1739 and CX1942 that have clinical application in the treatment of CNS-driven respiratory disorders, neurobehavioral disorders, spinal cord injury, neurological diseases, and orphan indications. In particular, we are addressing CNS-driven respiratory disorders that affect millions of people, but for which there are few treatment options and no drug therapies, including opioid induced respiratory disorders, such as apnea (transient cessation of breathing) or hypopnea (transient reduction in breathing). When these symptoms become severe, as in opioid overdose, they are the primary cause of opioid lethality. In addition, we are developing our ampakines for the treatment of disordered breathing and motor impairment resulting from spinal cord injury (SCI).
| 2 |
Additionally, the company is pursuing promising clinical development programs in neurobehavioral and cognitive disorders, with translational and clinical research programs focused on attention deficit hyperactive disorder (ADHD) and Fragile X Autism. This executive summary outlines the translational and clinical development programs for the ampakines in four therapeutic areas with unmet medical needs and large market opportunities:
| I. | Opioid Induced Respiratory Disorders | |
| II. | Spinal Cord Injury | |
| III. | Neuropsychiatric Disorders: Attention Deficit Hyperactivity Disorder (ADHD) | |
| IV. | Orphan Indications |
Ampakine® Clinical Development Program Summary (see Table 1)
RespireRx is committed to advancing the ampakines through the clinical and regulatory path to approval and commercialization. The opioid epidemic that claimed over 70,000 lives in our country last year demands that new solutions for opioid induced apnea be developed to ensure the public health. To this end, the company plans to conduct a series of clinical trials with CX1739, CX717, and CX1942 in the prevention, treatment, and management of opioid induced apnea.
RespireRx will continue to work with our collaborators to advance the ampakines CX717 and CX1739 for the treatment of patients with spinal cord injury (SCI). Early preclinical research suggests that ampakines may improve not only respiratory, but also motor function in SCI patients, especially in conjunction with acute intermittent hypoxia (AIH).
Additionally, RespireRx plans to continue clinical development of CX717 for the treatment of ADHD. There is a clear and significant medical need for new, non-stimulating ADHD drugs. With a positive Phase 2A study in ADHD completed, and with anticipated clearance from FDA following the demonstration that the previous safety concerns were unwarranted, CX717 is positioned to move into Phase 2B.
RespireRx will continue the development of orphan disease research, which is currently being supported through non-dilutive, NIH funding. The researchers hope to complete the preclinical research in the first half of 2018, paving the way for clinical trials at the end of 2018.
| 3 |
Table 1.
| Compound | Indication | Status | Start Date* | Completion* | ||||
| CX1739 | Opioid-induced Apnea | Phase 2A | 2Q2016 | x 4Q2016 | ||||
| Opioid Induced Apnea | Phase 2B | 4Q2017 | 3Q2018 | |||||
| CX717/CX1739 | Spinal Cord Injury | Phase 2A | 1Q2018 | 3Q2018 | ||||
| CX717/CX1739 | Orphan Diseases: Autism, Pompé |
Pre-Clinical to Clinical | 2Q2017 | Ongoing | ||||
| CX717 | ADHD | Phase 2B | Being planned | |||||
| CX1942 | Injectable for Opioid Overdose Rescue in Combination with Naloxone (NIDA Contract) | Pre-clinical to Clinical | 4Q2017 | Ongoing |
*Pending Funding
History of Ampakines®:
A Translational Research Approach from Chemical Structure to Molecular Function and Clinical Utility
Molecular Target – AMPA Glutamate Receptors on Brainstem Neurons
Glutamate, the major excitatory neurotransmitter in the nervous system, is ubiquitously present in over 50% of nervous system tissue. Glutamate receptors can be divided into two groups: 1. Ionotropic glutamate receptors (iGluRs), transmembrane proteins which form ion channel pores that, when glutamate binds to the receptor, open and allow the passage of cations, causing neuronal depolarization and subsequent biochemical signaling cascades; and 2. Metabotropic glutamate receptors (mGluRs) that directly activate a signaling cascade involving G proteins. iGluRs tend to be quicker in relaying information, but mGluRs are associated with a more prolonged stimulus. The three ionotropic glutamate receptors are defined by the classic pharmacological agonists used to activate these receptors: AMPA, NMDA and kainate.
| 4 |
When released from presynaptic neurons, glutamate attaches to and rapidly dissociates from its binding site on the AMPA receptor, producing a brief neuronal depolarization that rapidly deactivates as part of the neuronal signaling process. The depolarization produced by AMPA receptor activation also removes a voltage sensitive Mg2+ ion block from the NMDA receptor allowing it to be activated by glutamate. Glutamate binding to AMPA receptor further activates downstream signal pathways involving growth factors and G-proteins, which in turn activate kinase cascades, all of which lead to synaptic plasticity.
Translational research efforts have focused on a family of brainstem neurons in the pre-Bötzinger’s complex (pre-BötC) of the brainstem, which regulates respiratory drive. These pre-BötC neurons express a variety of neurotransmitter receptors, including AMPA glutamate and µ-opioid receptors. Using in situ brain slice preparations, local application of opioids reduces the electrical activity of these cells and their concomitant respiratory motor neurons. AMPA receptor activation increases respiratory motor neuron activity and AMPA receptor blockade reduces respiratory motor neuron activity. For these reasons, the study of respiratory rhythm and drive represents an ideal translational platform to study drugs that act at AMPA receptors. Target site engagement and proof of principle studies can be conducted from in vitro cellular, in situ organ and in vivo whole animal levels leading up to the human level.
Ampakines
Since the elucidation of the molecular structure of the AMPA glutamate receptor and the discovery of two structurally distinct classes of compounds that could selectively potentiate the currents mediated by glutamate stimulation of the AMPA receptors, considerable attention has focused on the possible pharmacotherapeutic uses for these compounds. The term ampakine was coined by RespireRx scientists to describe any drug that enhanced the actions of glutamate at its AMPA receptor by positive allosteric modulation. The first ampakine to be discovered was aniracetam, followed by the benzothiazides, diazoxides and cyclothiazides. These early efforts led to directed chemical synthetic efforts by a number of groups, but primarily RespireRx (then called Cortex Pharmaceuticals) and Lilly Research Labs. Lilly scientists developed a novel series of highly active biarylpropylsulfonamides, based on their ability to act at the cyclothiazide binding site. RespireRx scientists developed several novel families of compounds, initially using aniracetam as a starting point (see Figure 2).
These early ampakines were active in a variety of animal models that had been developed to predict pharmacotherapeutic benefits. Unfortunately, because these early ampakines were not designed for their actions as ampakines, they suffered from various problems that limited their use as drugs. While the thiazides were very potent and efficacious, their therapeutic ratio was limited by the production of undesirable side effects, such as convulsions, a problem also faced by their descendant compounds. On the other hand, aniracetam was limited in its therapeutic development because of its low potency and rapid metabolism, a problem also displayed in its early descendant compounds. It should be emphasized, however, that neither aniracetam nor the thiadiazides had specifically been developed for AMPA receptors but, despite their limited therapeutic use, these early ampakines became important tools for exploring molecular mechanisms of action.
| 5 |
Figure 2
The Lego School of Drug Design
High Impact Ampakines

Low Impact Ampakines

Early in the development of ampakines, RespireRx chemists synthesized two novel benzoylpiperidine ampakines (CX516 and CX546) that, despite their structural similarities, appeared to have significantly different pharmacological properties (see Figure 2). This discovery led to the identification of two classes of ampakines with markedly different pharmacological profiles. Like benzothiazides and biarylpropylsulfonamides, CX546 (termed a high impact compound) bound to the cyclothiazide binding site of the AMPA receptor, prolonged time to deactivation, inhibited desensitization in excised hippocampal patches, and prolonged synaptically evoked response duration using whole-cell recordings from CA1 pyramidal neurons of hippocampal slices. In contrast, CX516 (termed a low impact compound) did not bind to the cyclothiazide binding site, primarily increased amplitude much more than it prolonged deactivation, inhibited desensitization in excised hippocampal patches, and increased rather than prolonged synaptically evoked responses. Based on these findings, RespireRx developed a kinetic receptor model in which CX546 (high impact) mainly slows channel closing, while CX516 (low impact) preferentially accelerates channel opening. Highlighting the differences between these two compounds are the observations that CX546 produced epileptiform-like discharges in hippocampal slices, while CX516 did not, thereby differentiating the high impact versus low impact ampakines by their propensity to produce convulsions, or seizure potential.
Subsequent studies demonstrated that, despite differences in their molecular mechanisms of action, these two compounds displayed very similar behavioral effects in certain animal models of cognition, depression, ADHD and other central nervous system disorders. The positive behavioral effects of CX516, in the absence of the convulsant effects produced by CX546, raised the possibility that it might be possible to differentiate the therapeutic effects of the ampakines from their undesirable seizure potential. For this reason, and despite its relatively short half-life, CX516 was employed in a few clinical trials to evaluate the compound’s memory enhancing potential. When administered to healthy young male volunteers, a relatively low dose of CX516 significantly improved delayed retention of visual material, ability to identify difficult odors and learning of a visuospatial maze. While results from later studies were equivocal, some of these differences were believed to be due to the low potency of CX516 as well as the drug’s short half-life.
While additional high impact ampakines such as CX614 (see Figure 2) were synthesized, the poor side effect profiles of high impact ampakines led to the termination of their research programs at both RespireRx and Lilly. The continuation of the low impact ampakine program at RespireRx subsequently led to the discovery and patenting of several new classes of chemical structures, exemplified by CX717 and CX1739 (see Figure 2). These compounds retained the beneficial properties of the early low impact ampakines, while proving to be more potent with considerably better pharmacokinetic profiles.
| 6 |
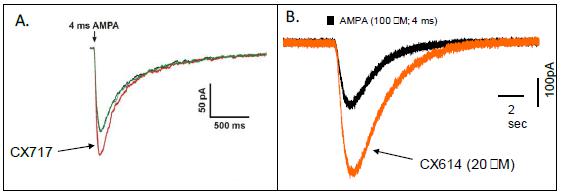
The differences between the high and low impact ampakines can best be appreciated in the next series of figures. In Figure 3A, brief application of AMPA to an individual neuron produces a fast current that is rapidly deactivated. The co-application of CX717, a low impact ampakine, enhances the amplitude of the AMPA mediated current, but does not affect the deactivation. In Figure 3B, the co-application of CX614, a high impact ampakine, enhances the amplitude of the AMPA mediated current, but also prolongs the current by slowing the deactivation.
Figure 3. Effects of Ampakines on AMPA Receptor Mediated Currents in Individual Neurons
This distinction between low and high impact ampakines is also seen after prolonged application of AMPA. As shown in Figure 4A and B, the initial depolarization produced by AMPA undergoes rapid and almost complete desensitization, despite the continued application of AMPA. We believe that this desensitization process is an adaptive, regulatory mechanism to prevent over-stimulation of the neurons. CX614 produces a dose-related, complete inhibition of desensitization, while CX717 produces only minimal effects. We believe that interference with basic, adaptive, regulatory mechanisms that neurons use to prevent hyper-excitability is responsible for the convulsive properties of the high impact ampakines.
Figure 4. Effects of Ampakines® on AMPA Receptor Desensitization in Individual Neurons

| 7 |
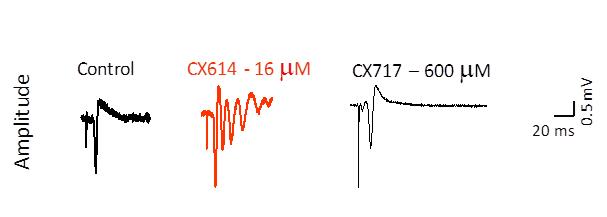
In order to test this hypothesis, extracellular field potentials from CA1 pyramidal cells were recorded as population spikes with glass micropipettes after single pulse electrical stimulation of the Schaffer-commissural fiber afferents. As can be seen in Figure 5, under control conditions, single pulses produced single population spikes. In the presence of CX614, multiple spikes were recorded, reminiscent of epileptiform-like activity. These effects were not produced by CX717 or any other low impact compounds that have been tested in this procedure. Additionally, unlike high impact ampakines and even after administration of high doses, no low impact ampakines have been observed to produce in animals either overt convulsions or increased sensitivity to known convulsant agents. Likewise, low impact ampakines are safe and well tolerated in humans.
Figure 5. Assessment of Ampakine® Seizure Potential
In addition to these translational studies, CX717 and CX1739, our lead clinical compounds, were tested and shown to be active in a variety of animal models designed to predict pharmacotherapeutic activity and have undergone animal toxicological testing sufficient to support 180 and 28 days of clinical administration, respectively. To date, two hundred and eighty-one (281) human subjects have been administered CX717 in 7 Phase 1 and 2 clinical trials, with no deaths, serious adverse events, or dose-limiting adverse events encountered. In two Phase 2A studies, single dose administration of CX717 antagonized the respiratory depressant effects of alfentanil, a potent opioid, without altering its analgesic effects (see below). It also demonstrated positive effects in a 3-week Phase 2 study in patients with adult ADHD (see below).
Likewise, to date, 127 human subjects have received at least one dose of CX1739, including single doses up to 1200 mg and multiple daily doses of up to 1200 mg/day for 7 days for the assessment of safety, tolerability, and pharmacokinetic and pharmacodynamic parameters. No deaths, serious adverse events, or dose-limiting adverse events have been associated with the administration of CX1739. In a recently completed Phase 2A clinical trial, CX1739 antagonized the respiratory depressant effects of remifentanil in a model of chronic opioid consumption, without altering its analgesic properties (see below).
In addition, RespireRx is developing CX1942, a soluble pro-drug, as a low impact ampakine. CX1942 is in the preclinical research stage, where it has demonstrated the ability to selectively antagonize opioid induced respiratory depression (OIRD), as well as positive results in certain animal models predictive of other pharmacotherapeutic effects.
Ampakine® Commercialization Plan:
I. Combating the Opioid Epidemic with Translational Approaches to Life-Saving Solutions
Demographics
Across the United States people are suffering from what has been called “The Opioid Epidemic”, with tens of thousands of people dying every year from overdose. This epidemic is not restricted to people consuming illegal street drugs. In fact, more than half of the deaths result from the legal, prescription use of opioids, and 80% of heroin users report that they used prescription drugs prior to heroin. The cause of these fatalities is the respiratory depressant effects of the opioids, or opioid induced apnea.
| 8 |
Opioid analgesics are now the most commonly prescribed class of medications in the United States, where chronic pain is estimated to affect around 68 million people each year. In 2014 alone, U.S. retail pharmacies dispensed 245 million prescriptions for opioid pain relievers. While the opiate epidemic has been widely highlighted in the medical and popular press, the extent of the crisis is still underappreciated. To put the problem in perspective, one-third of all adults, and nearly 40% of older Americans experience chronic pain, with an estimated 10 to 11 million people in the U.S. being prescribed chronic opioid analgesics for non-cancer pain.
Trends in opioid prescribing, which have seen a four-fold increase in the last decade, have serious consequences to the health of the nation. A potentially life-threatening side effect of opioid therapy is apnea, the primary cause of death in opioid overdose. In 2016, more persons died from drug overdoses in the United States than during any previous year on record, culminating with an estimated 70,000 deaths with 61% of drug overdose deaths attributable to prescription opioids. The rate of emergency department visits for opioid overdose quadrupled from 1993 to 2010 and now, every day, over 1,000 people are treated in emergency departments for adverse effects of prescription opioids. Increased risk of overdose was observed among patients receiving medically prescribed opioids at higher dosage levels; most opioid overdoses were medically serious, and 12% were fatal. The Department of Health and Human Services (HHS) has deemed prescription-opioid overdose deaths an epidemic and prompted multiple federal, state, and local actions.
Among patients on chronic opioid therapy for at least 6 months, the prevalence of apnea and hypopnea has been diagnosed in 50% - 75% of patients screened. Initially, these symptoms usually appear during sleep. The National Institutes of Health (NIH) and the National Institute of Drug Addiction (NIDA) have listed apnea and hypopnea, including central sleep apnea, as a significant risk factor for opioid addiction and overdose. Unfortunately, the vast majority of chronic opioid users are never screened for apnea, so the risk, therefore, may be underestimated. Opioid induced apnea can cause significant declines in health-related quality of life and increased health care costs. Undiagnosed apnea leads to increased risk of health complications, work-place errors and traffic accidents, leading to significant economic costs estimated to be between $1,950 and $3,899, per patient, per year. Based on the epidemiology of CSA and opioid prescribing trends, however, there are at least 3 million, and perhaps as many as 6 million people on chronic opioid therapy who are at increased risk of opioid induced apnea and overdose.
While naloxone, in its various forms, has proven to be of immense value in the acute, emergency setting to rescue people from opioid overdose, it cannot be used chronically as a prophylactic because naloxone negates the desired pain-relieving effects at the same time that it antagonizes the respiratory depressant effects of opioids. Various groups are attempting to develop novel analgesics that are devoid of the respiratory depressant effects of the opioids, but ultimate success is uncertain. Attempts to interfere with the addictive properties of the opioids so as to minimize their illegal use are equally uncertain and ignore the fact that the majority of deaths occur among patients who are taking legal prescription opioids for chronic pain. Until these alternative approaches meet with success, there exists an enormous unmet need to save lives now by providing some sort of prophylactic agent that will reduce the lethality without diminishing the therapeutic actions of the opioids.
Pre-Clinical Translational Research Program
RespireRx has focused its translational research program to develop treatments for the prevention of opioid overdose. In collaboration with Dr. John Greer from the University of Alberta, who conducted the initial groundbreaking work, RespireRx has conducted an elegant series of translational research experiments ranging from single cell electrophysiological recordings, to in vitro brain-slice methodology, through animal models of behavior and physiology, and ultimately leading to Phase 2 human clinical trials.
Pre-clinical experiments in animal models of respiration have demonstrated that activation of opioid receptors decreases the activity of pre-BötC neurons, leading to hypopnea (a slowing of breathing), apnea (a cessation of breathing) and, at high opioid doses, death. In animals treated with ampakines, however, activation of the AMPA glutamate receptors on the pre-BötC cells selectively counteracts the respiratory depressant effects of the opioids and protects from lethality without altering the analgesic properties of the opioids.
| 9 |
Using plethysmographic recordings from rats, administration of fentanyl produces a rapid cessation of breathing, followed by lethality (see Figure 6A). CX717, administered either after (Figure 6B) or before (Figure 6C) fentanyl, dramatically reduced the respiratory depression and lethality. Dose dependent antagonism of opioid induced respiratory depression was obtained from all three low impact ampakines being developed - CX717, CX1739 and CX1942 (see Figure 7).
Figure 6. Ampakine Protects From Opioid Induced Respiratory Depression and Lethality*

*Tracings taken from plethysmographic recordings of rats. Each deflection reflects a breath.
Figure 7. Dose Response Antagonism of Opioid Induced Respiratory Depression

| 10 |
In addition, opioid induced analgesia was measured in a rat hot plate procedure in which latency to remove paws from the painful thermal stimulus was determined. If no response was made by 20 seconds, the trial was terminated and latency was recorded as >20 sec. As can be seen in Table 1, fentanyl dramatically increased latency times, an effect that was not altered by administration of an ampakine at a dose that completely antagonized the respiratory depression. As an active control, naloxone produced its well known antagonism of the opioid induced analgesia.
Table 1. Opioid Induced Analgesia in the Rat Hot Plate Procedure

Clinical Translational Research Program
The results of our preclinical research studies have been replicated in three separate Phase 2A human clinical trials with two ampakines, CX717 and CX1739, confirming the translational mechanism and target site engagement and demonstrating proof of principle that ampakines can be used in humans for the prevention of opioid induced apnea.
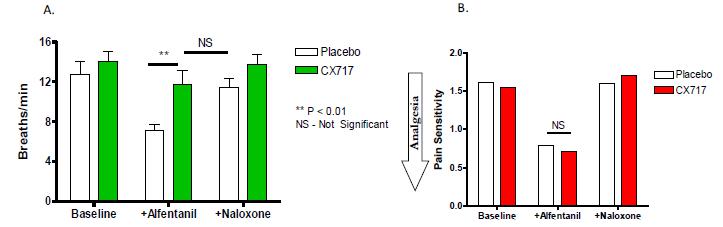
For example, in a previously reported study, oral administration of CX717 prior to intravenous infusion of alfentanil produced no effect on basal respiration, but completely antagonized the respiratory depression produced by alfentanil. This antagonism was comparable to that produced by naloxone, an opioid receptor antagonist (see Figure 8A). Alfentanil reduced the perceived pain produced by electrical stimulation to the finger, an effect which was antagonized by naloxone, but not by CX717 (Figure 8B).
Figure 8. CX717 Maintains the Analgesic Properties of Opioids While Antagonizing Respiratory Depression
| 11 |
RespireRx recently completed a Phase 2A clinical trial that evaluated the ability of CX1739 to overcome the respiratory depression induced by the powerful, yet short-acting opioid, remifentanil in two models of opioid use: 1.) REMI-Bolus evaluated respiratory parameters in an opioid overdose model, using a bolus of remifentanil (1 mcg/kg) to achieve significant respiratory depression; 2.) REMI-Infusion evaluated respiratory, pain, and pupilometry parameters using an infusion of remifentanil at a steady state blood concentration of 2 ng/ml to achieve approximately 40 - 50% respiratory depression as a model of sub-acute, post-surgical intravenous opioid treatment as well as chronic oral opioid treatment for chronic pain. The results of the REMI-Infusion analysis demonstrated that, compared to placebo, an acute dose of CX1739 (300 mg or 900 mg) significantly reduced OIRD under steady-state opioid concentrations (see Figure 9). The results of the REMI-Bolus analysis demonstrated that CX1739 was unable to prevent the rapid respiratory depression that occurs after intravenous, bolus injection of remifentanil. The remifentanil effects on analgesia, pupilometry and bispectral index were not altered by CX1739. Administration of CX1739 at an acute dose up to 900 mg was safe and comparable to placebo.
Figure 9. CX717 Maintains the Analgesic Properties of Opioids While Antagonizing Respiratory Depression

In conjunction with NIDA, we are planning a clinical strategy to develop ampakines as a prophylactic treatment to prevent opioid overdose and a formulation strategy to develop combination therapies with ampakines and naloxone that offer enhanced respiratory safety advantages as a long-term rescue medication with extended duration of action.
Ampakine® Commercialization Plan:
II. Ampakines® for Improving Recovery from Spinal Cord Injury (SCI)
An estimated 17,000 new cases of spinal cord injury occur each year in the United States, most a result of automobile accidents. Currently, there are roughly 282,000 people living with spinal cord injuries, and many of these patients have impaired respiratory motor function, a major cause of morbidity and mortality following spinal cord injury. In fact, diseases of the respiratory system (particularly pneumonia) are the leading cause of death in people with SCI. Ampakines have potential utility in the treatment and management of SCI to enhance respiration and other motors functions, and improve the quality of life for SCI patients.
Spinal cord injury can profoundly impair respiratory motor function and neural plasticity leading to significant morbidity and mortality in human accident victims. Plasticity is a fundamental property of the nervous system that enables continuous alteration of neural pathways and synapses in response to experience or injury. One frequently studied model of respiratory plasticity is long-term facilitation of phrenic motor output (pLTF). A large body of literature exists regarding the ability of ampakines to stimulate neural plasticity, possibly due to an enhanced synthesis and secretion of various growth factors.
| 12 |
Recently, studies of acute intermittent hypoxia (AIH) in patients with spinal cord injury demonstrate that neural plasticity can be induced to restore respiratory function and reduce the need for mechanical ventilation. This approach, known as long-term facilitation (LTF), is based on physiological mechanisms associated with the ability of spinal circuitry to learn how previous repeated hypoxic bouts affect breathing by adjusting synaptic strength between respiratory neurons. Because AIH induces spinal plasticity, the potential exists to harness repetitive AIH as a means of inducing functional recovery in conditions causing respiratory insufficiency, such as cervical spinal injury. Because repetitive AIH induces phenotypic plasticity in respiratory motor neurons, it may restore respiratory motor function in patients with incomplete spinal injury. AIH has also been reported to improve other motor functions as well.
RespireRx has been working with Dr. David Fuller, at the University of Florida, to evaluate the use of ampakines with and without AIH for the treatment of compromised motor function in SCI. Eight weeks following C2 spinal hemisection in mice, intravenous injections of CX717 (15mg/kg) increases phrenic nerve amplitude bilaterally with no impact on burst frequency (see Figure 10). The effect on the hemisected side is greater than that measured on the intact side, with the recovery approximating that seen on the intact side prior to administration of ampakine.
Figure 10. CX717 Promotes Phrenic Nerve Function in SCI

In addition, recordings of medullary inspiratory output from the hypoglossal nerve of anesthetized mice show that, in sham treated animals, AIH induced LTF as evidenced by approximately 200% increases in mean recording amplitude (see Figure 11 A & B) with no changes in frequency (see Figure 11C). CX717 produced a considerable potentiation of the response to AHI, with mean recording amplitudes increasing 2.5 times greater than those recorded with AHI alone and for considerably longer periods of time.(see Figure 11 A & B)
| 13 |
Figure 11. CX717 Increases the Magnitude of Respiratory Long Term Facilitation

These animal models of respiration following spinal cord injury support proof of concept for a new treatment paradigm using ampakines in conjunction with AIH to promote respiratory and other motor functions in patients with SCI. RespireRx is continuing it collaborative preclinical research with Dr. Fuller while it is planning a clinical trial program focused on developing ampakines with and without AIH for the restoration of respiratory and other motor functions in patients with SCI. The company is working with our clinical advisory panel and with researchers at the University of Miami, the Miami Project and the Detroit VA to finalize a Phase II clinical trial protocol.
Ampakine® Commercialization Plan:
III. Ampakines® for the Treatment of Attention Deficit Hyperactivity Disorder (ADHD)
ADHD is one of the most common neurobehavioral disorders, with 6.1% of all American children taking medication for treatment. ADHD is estimated to affect 7.8% of U.S. children aged 4 to 17, according to the U.S. Centers for Disease Control and Prevention or approximately 4.5 million children. The principal characteristics of ADHD are inattention, hyperactivity and impulsivity. In up to 65% of children affected by this disorder, symptoms will persist into adulthood, with estimates of approximately 9.9 million adults in the United States having ADHD. ADHD can negatively impair many aspects of daily life, including home, school, work and interpersonal relationships.
Currently available treatments for ADHD include stimulant and non-stimulant agents targeting the monoaminergic receptor systems in the brain. However, these receptors are not restricted to the brain and are widely found throughout the body. Thus, while these agents can be effective in ameliorating ADHD symptoms, they also can produce adverse cardiovascular effects, such as increased heart rate and blood pressure. Existing treatments also affect eating habits and can reduce weight gain and growth in children, and have been associated with suicidal ideation in adolescents and adults. In addition, approved stimulant treatments, such as amphetamine, are DEA classified as controlled substances and present logistical issues for distribution and protection from diversion.
Various investigators have generated data supporting the concept that alterations in AMPA receptor function might underly the production of some of the symptoms of ADHD. In rodent and primate models of cognition, ampakines have been demonstrated to reduce inattention and impulsivity, two of the cardinal symptoms of ADHD. Furthermore, ampakines do not stimulate spontaneous locomotor activity in either mice or rats, unlike the stimulants presently used for the treatment of ADHD, nor do they increase the stimulation produced by amphetamine or cocaine.
| 14 |
These preclinical considerations prompted us to conduct, in adults with ADHD, a Phase 2, randomized, double-blind, two period crossover study comparing the efficacy and safety of two separate doses of CX717 (200 mg BID or 800 mg BID) with placebo. Subjects were orally administered either placebo, low dose (200 mg BID) or high dose (800 mg BID) for three weeks, followed by a two week washout and then an additional three weeks of dosing using a randomized crossover design. The primary outcome measure was the ADHD-RS total score, which evaluates the severity of ADHD symptoms, as well as the ADHD-RS hyperactivity and inattentiveness subscales, which were secondary efficacy variables.
In a repeated measures analysis, a significant treatment effect on ADHD-RS was observed with high dose CX717 compared to placebo (p< 0.003). The differences between high dose CX717 and placebo were seen as early as Week 1 of treatment and continued throughout the remainder of the study. No meaningful differences in the ADHD RS total scores were observed between low dose CX717 and placebo. In general, results for both the ADHD-RS hyperactivity and inattentiveness subscales paralleled the results of the total score. Using a repeated measures analysis, both hyperactivity (p<0.02) and inattentiveness (p<0.03) symptoms improved significantly with the high dose compared to placebo.
Based on these clinical results, we believe that CX717 might represent a breakthrough opportunity to develop a non-stimulating therapeutic for ADHD. We are planning to continue this program with a Phase 2B clinical trial in patients with adult ADHD.
Ampakine® Commercialization Plan:
IV. Ampakines® for the Treatment of Autism Spectrum Disorders and Fragile X Syndrome
More than 3.5 million Americans live with an Autism Spectrum Disorder (ASD), a complex neurodevelopmental disorder with a rising global prevalence of over 1%. Fragile X Syndrome (FXS) is the most common identifiable single-gene cause of autism, responsible for approximately 5% of cases. Individuals with FXS and ASD exhibit a range of abnormal behaviors comprising hyperactivity and attention problems, executive function deficits, hyper-reactivity to stimuli, anxiety and mood instability. The prevalence rate of ASD has risen from 1 in 150 children in 2000 to 1 in 68 children in 2010, with current estimates indicating a significant rise in ASD diagnosis to over 2% or 1 in 45 children, placing a significant emotional and economic burden on families and educational systems.
Since “autistic disturbances” were first identified in children in 1943, extensive research efforts have attempted to identify the genetic, molecular, environmental, and clinical causes of ASD, but until recently the underlying etiology of the disorder remained elusive. Today, there are no medications that can treat ASD or its core symptoms, and only two FDA approved anti-psychotic drugs, aripiprazole and risperidone, are approved for the treatment of irritability associated with ASD.
Thanks to wide ranging translational research efforts, FXS and ASD are currently recognized as disorders of the synapse with alterations in different forms of synaptic communication and neuronal network connectivity. Focusing on the proteins and subunits of the AMPA receptor complex, autism researchers at the University of San Diego (UCSD) have proposed that AMPA receptor malfunction and disrupted glutamate signal transmission may play an etiologic role in the behavioral, emotional and neurocognitive phenotypes that remain the standard for ASD diagnosis. For example, Stargazin, also known as CACNG2 (Ca2+ channel g2 subunit) is one of four closely related proteins recently categorized as transmembrane AMPA receptor regulating proteins (TARPs).
Researchers at the University of California San Diego (UCSD) have been studying genetic mutations in the AMPA receptor complex that lead to cognitive and functional deficiencies along the autism spectrum. They work with patients and their families to conduct detailed genetic analyses in order to better understand the underlying mechanisms of autism. In one case, they have been working with a teenage patient who has an autism diagnosis, with a phenotype that is characterized by subtle Tourette-like behaviors, extreme aggression, and verbal & physical outbursts with disordered thought. Despite the behaviors, his language is normal. Using next generation sequencing and genome editing technologies, the researchers identified a Stargazin mutation (deletion of exon 2 of CACNG2) and introduced the aberrant sequence into C57bL6 mice using CRISPR (Clustered Regulatory Interspaced Short Palindromic Repeats). The heterozygous allele has a dominant negative effect on the trafficking of post-synaptic AMPA receptors and produces behaviors that are consistent with a glutamatergic deficit, including reduced anxiety-like behavior, reduced grooming, and reduced pre-pulse inhibition, similar to what has been observed in the teenage patient.
| 15 |
With funding from the National Institutes of Health, RespireRx is working with UCSD to explore the use of ampakines for the amelioration of the cognitive deficits associated with the AMPA receptor gene mutations. Preliminary results indicate that the ampakines might reduce inattention and impulsivity measured in rodent behavioral models and, depending on outside funding, we anticipate beginning a small clinical trial late this year.
Ampakine® Commercialization Plan:
V. Ampakines® for the Treatment of Orphan Diseases
Orphan diseases represent significant market opportunities for ampakines. Orphan drugs provide market exclusivity of 7 Years in the US and 10 years in the EU. Further, the US offers reduced research and development costs with a 50% tax credit, and FDA user fees are waived for products with revenues less than $50 million.
Preclinical research on ampakines has demonstrated that CX717 and CX1739 can improve respiratory function in animal models of certain orphan disorders, such as Pompé Disease and perinatal respiratory distress. The company is actively working with experts and noted centers of excellence for the treatment of these rare disorders to bring new therapies to the clinic.
RespireRx is working with researchers at the University of Florida, who have demonstrated the ability of ampakines to treat respiratory insufficiency in a mouse model of Pompé Disease. Additionally, we have supported the research at the University of Alberta that demonstrated the potential for ampakines to treat apnea of prematurity. Lastly, RespireRx has teamed with researchers at UCSD on a personalized, translational medicine project to facilitate a treatment for a boy with Fragile X Autism (see above). These research and development partnerships with key opinion leaders at leading academic institutions demonstrate the potential for developing the ampakines for the treatment of rare pediatric diseases.
The U.S. FDA has established a priority review program to encourage treatments for rare pediatric diseases (RPDs). Pompé Disease, Apnea of Prematurity, and Fragile X Autism each meet the criteria for a rare pediatric disease. The priority review program confers regulatory review benefits on the developers of drugs for RPDs, but as importantly, the Agency provides an added incentive with a priority review voucher, to whit: The Secretary shall award a priority review voucher to the sponsor of a rare pediatric disease product application upon approval by the Secretary of such rare pediatric disease product application.
The PRD Voucher, sometimes referred to as the “Golden Ticket” bestows accelerated regulatory review and approval times that can provide six months or more of market exclusivity and increased sales opportunities. The real beauty of the voucher is that it can be sold to an unrelated third party: The sponsor of a rare pediatric disease product application that receives a priority review voucher under this section may transfer (including by sale) the entitlement to such voucher. There is no limit on the number of times a priority review voucher may be transferred before such voucher is used. To date, six vouchers have been awarded and four have been sold, the latest to AbbVie for $350 Million.
Pompé Disease results from a mutation in the acid alpha-glucosidase gene leading to lysosomal glycogen accumulation. Respiratory insufficiency is common and the current FDA-approved treatment, enzyme replacement, has limited effectiveness. Ampakines stimulate respiratory neuromotor output and ventilation in a mutant mouse model of Pompé and may have potential as an adjunctive therapy in Pompé disease. In this mutant mouse model, ampakines robustly increase phrenic and hypoglossal inspiratory bursting and reduced respiratory cycle variability in anesthetized mutant mice, and increased inspiratory tidal volume in unanesthetized mutant mice.
| 16 |
Apnea of prematurity is defined as cessation of breathing by a premature infant that lasts for more than 15 seconds and is accompanied by low blood oxygen levels (hypoxemia) and lowered heart rate (bradycardia). Apnea of prematurity, which can be obstructive, central, and mixed, occurs to varying degrees in more than 85% of infants who are born at less than 34 weeks of gestation. Ampakines have been shown to enhance weak endogenous respiratory drive and reduce the incidence of apneas in perinatal rats. This includes alleviation of respiratory depression and apneas induced by hypoxia. Using in vitro brainstem–spinal cord preparations, the laboratory of Dr. John Greer at the University of Alberta has demonstrated that ampakines markedly increase respiratory drive and reduce the incidence of apnea using in vitro and in vivo perinatal rat models under normoxic and hypoxic conditions. Additionally, in vivo plethysmographic recordings from postnatal Day 0 rats demonstrated that ampakines increase the frequency and regularity of ventilation, reduce apneas and protect against hypoxia-induced respiratory depression. These data indicate a potential supplementary pharmacological therapy to methylxanthenes for countering apnea of prematurity in the clinic.
Clinical Advisory Panel
To augment the executive team, RespireRx has assembled a world-renowned group of experts in the fields of sleep medicine, anesthesia, pain management, neurology, and psychiatry to act as a clinical advisory panel in helping guide our strategic clinical development effort.
Intellectual Property
RespireRx owns patents claiming the composition of matter for various ampakines, including CX1739, CX1942 and related compounds, and owns and has licensed exclusive rights to patents and patent applications claiming the use of ampakines, including CX717, CX1739 and CX1942, for the treatment of certain breathing disorders and neuropsychiatric disorders, including ADHD, impaired cognition and depression.
Special Note Regarding Forward-Looking Statements
Certain statements included or incorporated by reference in this Executive Summary, including information as to the future financial or operating performance of the Company and its drug development programs, constitute forward-looking statements. The words “believe,” “expect,” “anticipate,” “contemplate,” “target,” “plan,” “intend,” “continue,” “budget,” “estimate,” “may,” “schedule” and similar expressions identify forward-looking statements. Forward-looking statements include, among other things, statements regarding future plans, targets, estimates and assumptions. Forward-looking statements are necessarily based upon a number of estimates and assumptions that, while considered reasonable by the Company, are inherently subject to significant business, economic and competitive uncertainties and contingencies. Many factors could cause the Company’s actual results to differ materially from those expressed or implied in any forward-looking statements made by, or on behalf of, the Company. Due to these various risks and uncertainties, actual events may differ materially from current expectations. Investors are cautioned that forward-looking statements are not guarantees of future performance and, accordingly, investors are cautioned not to put undue reliance on forward-looking statements due to the inherent uncertainty therein. Forward-looking statements are made as of the date of this news release and the Company disclaims any intent or obligation to update publicly such forward-looking statements, whether as a result of new information, future events or results or otherwise.
Contacts
Jeff Margolis
Chief Financial Officer and Senior VP Finance
jmargolis@respirerx.com
(917) 834-7206
| 17 |
