Attached files
| file | filename |
|---|---|
| EX-99.5 - RespireRx Pharmaceuticals Inc. | ex99-5.htm |
| EX-99.4 - RespireRx Pharmaceuticals Inc. | ex99-4.htm |
| EX-99.3 - RespireRx Pharmaceuticals Inc. | ex99-3.htm |
| EX-99.1 - RespireRx Pharmaceuticals Inc. | ex99-1.htm |
| 8-K - RespireRx Pharmaceuticals Inc. | form8-k.htm |

Dronabinol for the Treatment of Obstructive Sleep Apnea (OSA)
OSA, the most common form of sleep-related breathing disorders, is a serious respiratory disorder that involves repetitive cessation of breathing during sleep. It is estimated that up to 30 million people in the United States suffer from OSA, which has been linked to increased risk for hypertension, heart failure, depression, and diabetes. According to the American Academy of Sleep Medicine, the annual economic cost of OSA in the U.S. was $162 Billion in 2015. The current standard of care for OSA, continuous positive airway pressure (CPAP), involves a mechanical device that has poor patient compliance, adherence, and satisfaction. Despite these shortcomings, the sleep apnea devices market size is projected to exceed $8.7 billion by 2023 (Global Market Insights, 2017). There are no approved drug treatments for OSAS, and 80% of patients (23.5 million) are undiagnosed.
Dronabinol Dronabinol (D9-THC) is a generic, orally active cannabinoid that that has been shown to act at CB1 & CB2 receptors on neurons to stabilize respiratory patterns and augment upper airway muscles. Two Phase 2 clinical trials have been completed and both have demonstrated significant reductions in sleep apnea produced by dronabinol. The results of the first, a Phase 2a study conducted by the company, have been published in two peer-reviewed journal articles, and the results from the recently completed Phase 2b study of dronabinol in OSA are in press. The PACE Trial was fully-funded by NIH through a $5 million grant.
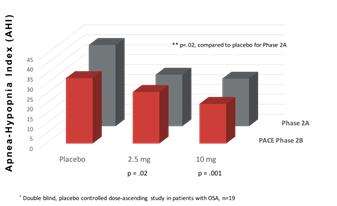
Phase 2b Clinical Trial Results The results of the PACE Trial show that 2.5 and 10-mg doses of dronabinol produce statistically significant improvement in the primary endpoints, including improvement in the Apnea-Hypopnea Index (p=.001), sleepiness, as measured by the Epworth Scale (p<.02), and patient satisfaction (p=.04). The figure below illustrates how the PACE Trial replicated the statistically significant primary endpoint results of the Phase 2a study.
PACE Trial Summary: Efficacy in the Treatment of OSA - AHI Scores
Through an extensive series of translational research projects from the cellular level through proof-of-concept clinical trials, research has demonstrated that dronabinol is an effective drug therapy for the widespread and underappreciated disease, OSA.
|
BACKGROUND RespireRx is developing a pipeline of new drug products based on our broad patent portfolios for two drug platforms: the cannabinoids, including dronabinol (D9-THC), and the ampakines, which allosterically and positively modulate AMPA-type glutamate receptors to promote neuronal function.
We are a leader in the development of drugs for respiratory disorders caused by disruptions of neural signaling, with a focus on sleep apneas, opioid-induced respiratory depression, and other CNS-driven breathing disorders. Additionally, we are developing new drugs and formulations that address the widespread problem of opioid overdose.
RespireRx has an extensive patent portfolio, with intellectual property for composition of matter and use across multiple indications.
OBSTRUCTIVE SLEEP APNEA MARKET SIZE: Prevalence: ~ 30 MM Diagnosed: 5.9 MM Undiagnosed: 23.5 MM Device Market: $7.5 B Economic Impact: $162 B
INTELLECTUAL PROPERTY Extensive IP Portfolio Patent Extensions Market Exclusivity Patents Pending to 2037
MANAGEMENT TEAM James Manuso,PhD, CEO Vice Chairman Arnold Lippa, PhD, CSO Exec Chairman Jeff Margolis, CFO, Senior VP Finance Richard Purcell, Senior VP R&D
OFFICE LOCATION 126 Valley Rd. Glen Rock, NJ 07452
Market OTC: RSPI
Contact: Jeff Margolis jmargolis@respirerx.com (917) 834-7206 |
Special Note Regarding Forward-Looking Statements Follows
Special Note Regarding Forward-Looking Statements: Certain statements included or incorporated by reference in this Platform Summary, including information as to the future financial or operating performance of the Company and its drug development programs, constitute forward-looking statements. The words “believe,” “expect,” “anticipate,” “contemplate,” “target,” “plan,” “intend,” “continue,” “budget,” “estimate,” “may,” “schedule” and similar expressions identify forward-looking statements. Forward-looking statements include, among other things, statements regarding future plans, targets, estimates and assumptions. Forward-looking statements are necessarily based upon a number of estimates and assumptions that, while considered reasonable by the Company, are inherently subject to significant business, economic and competitive uncertainties and contingencies. Many factors could cause the Company’s actual results to differ materially from those expressed or implied in any forward-looking statements made by, or on behalf of, the Company. Due to these various risks and uncertainties, actual events may differ materially from current expectations. Investors are cautioned that forward-looking statements are not guarantees of future performance and, accordingly, investors are cautioned not to put undue reliance on forward-looking statements due to the inherent uncertainty therein. Forward-looking statements are made as of the date of this news release and the Company disclaims any intent or obligation to update publicly such forward-looking statements, whether as a result of new information, future events or results or otherwise.