Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Nabriva Therapeutics plc | a17-14242_38k.htm |
Exhibit 99.1
A Transformational Year for Nabriva June 19, 2017 2017 Analyst & Investor Day

This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements about our future expectations, plans and prospects, including but not limited to statements about, our planned redomicile from Austria to Ireland, the development of our product candidates, such as plans for the design, conduct and timelines of Phase 3 clinical trials of lefamulin for CABP, the clinical utility of lefamulin for CABP and our plans for filing of regulatory approvals and efforts to bring lefamulin to market, the development of lefamulin for additional indications, the development of additional formulations of lefamulin, plans to pursue research and development of other product candidates, and other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties inherent in the initiation and conduct of clinical trials, availability and timing of data from clinical trials, whether results of early clinical trials or trials in different disease indications will be indicative of the results of ongoing or future trials, uncertainties associated with regulatory review of clinical trials and applications for marketing approvals, the availability or commercial potential of product candidates, the sufficiency of cash resources, whether research programs will result in product candidates that are advanced into future clinical trials, and need for additional financing and such other important factors as are set forth under the caption "Risk Factors" in the annual and quarterly reports we file with the United States Securities and Exchange Commission. In addition, the forward-looking statements included in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. Please note that any opinions, estimates or forecasts regarding the company or otherwise made by the key opinion leaders are theirs alone and may not represent the opinions, estimates or forecasts of the company. Such opinions, estimates or forecasts should not be relied upon as representing our views, and we specifically disclaim any obligation to update them, except as required by law. Safe Harbor and Disclaimer

Agenda Welcome & Introduction David Garrett, Vice President, Corporate Controller & Head of Investor Relations Building A Fully Integrated Anti-Infectives Company Colin Broom, M.D., Chief Executive Officer Lefamulin Clinical Program Overview Elyse Seltzer, M.D., Chief Medical Officer Current Treatment Approach to CABP in Adults: What are the Medical Needs? Richard Wunderink, M.D. Hospital Initiated Therapy for CABP: Perspectives from the Front-Line Alpesh Amin, M.D., MBA, MACP, SFHM, FACC Break Clinical Conundrums in the Management of CABP Andrew Shorr, M.D., MPH Treatment of Patients with CABP: Defining the Medical Need Thomas Lodise, Pharm.D., Ph.D. Preparing for U.S. Commercialization Will Sargent, Vice President, Commercial Strategy Q&A Nabriva Management & External Experts Milestones / Catalysts & Closing Colin Broom, M.D., Chief Executive Officer Lunch

Building a Fully Integrated Anti-Infectives Company Colin Broom, M.D. Chief Executive Officer

2017- A Transformational Year for Nabriva Building a Fully Integrated Anti-Infectives Company Expert team advancing pleuromutilins, a new class of antibiotics, for systemic (IV and oral) use in humans Investing in pre-commercial activities to maximize US commercial potential and evaluating ex-US partner(s) Cash expected to fund operations into 2Q18 Lefamulin in two international, pivotal Phase 3 trials for moderate-to-severe CABP with top-line data expected in September 2017 and in 1Q18
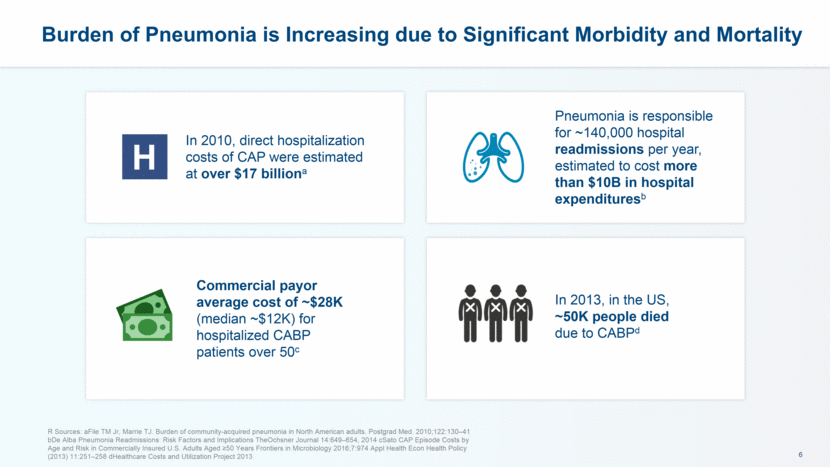
Burden of Pneumonia is Increasing due to Significant Morbidity and Mortality R Sources: aFile TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130–41 bDe Alba Pneumonia Readmissions: Risk Factors and Implications TheOchsner Journal 14:649–654, 2014 cSato CAP Episode Costs by Age and Risk in Commercially Insured U.S. Adults Aged >50 Years Frontiers in Microbiology 2016;7:974 Appl Health Econ Health Policy (2013) 11:251–258 dHealthcare Costs and Utilization Project 2013 In 2010, direct hospitalization costs of CAP were estimated at over $17 billiona H Pneumonia is responsible for ~140,000 hospital readmissions per year, estimated to cost more than $10B in hospital expendituresb In 2013, in the US, ~50K people died due to CABPd Commercial payor average cost of ~$28K (median ~$12K) for hospitalized CABP patients over 50c
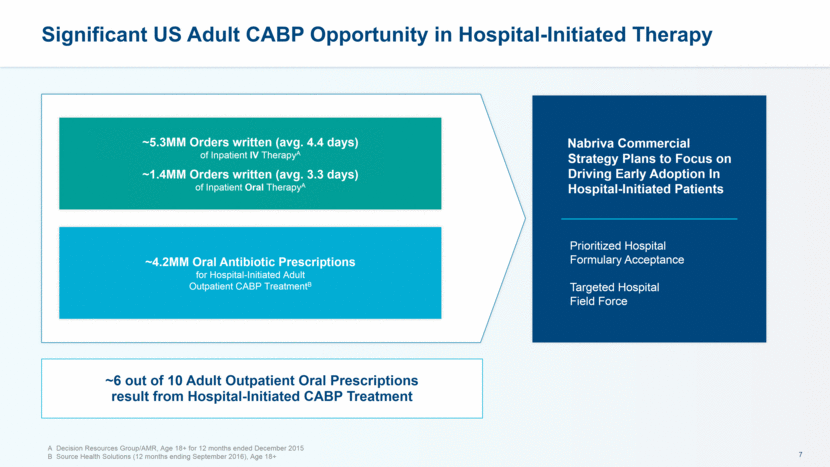
Significant US Adult CABP Opportunity in Hospital-Initiated Therapy A Decision Resources Group/AMR, Age 18+ for 12 months ended December 2015 B Source Health Solutions (12 months ending September 2016), Age 18+ ~4.2MM Oral Antibiotic Prescriptions for Hospital-Initiated Adult Outpatient CABP TreatmentB Nabriva Commercial Strategy Plans to Focus on Driving Early Adoption In Hospital-Initiated Patients Prioritized Hospital Formulary Acceptance Targeted Hospital Field Force ~5.3MM Orders written (avg. 4.4 days) of Inpatient IV TherapyA ~1.4MM Orders written (avg. 3.3 days) of Inpatient Oral TherapyA ~6 out of 10 Adult Outpatient Oral Prescriptions result from Hospital-Initiated CABP Treatment
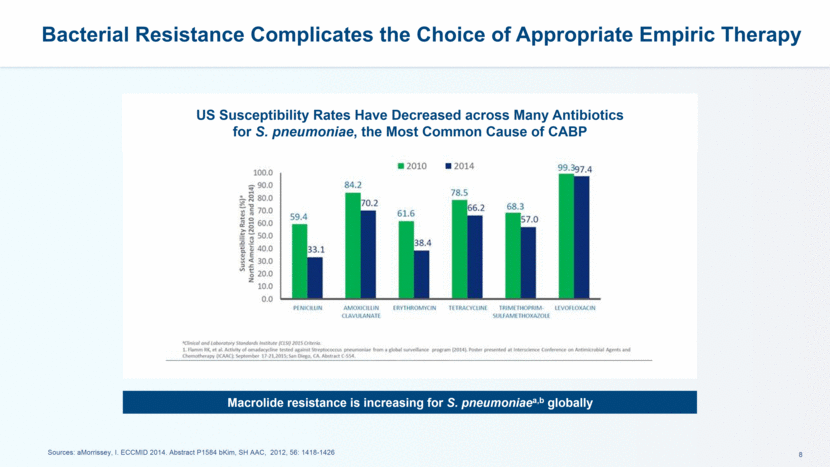
Bacterial Resistance Complicates the Choice of Appropriate Empiric Therapy Sources: aMorrissey, I. ECCMID 2014. Abstract P1584 bKim, SH AAC, 2012, 56: 1418-1426 US Susceptibility Rates Have Decreased across Many Antibiotics for S. pneumoniae, the Most Common Cause of CABP Macrolide resistance is increasing for S. pneumoniaea,b globally
Antibiotic Stewardship Should Increasingly Drive Antibiotic Choices Sources: a Policy Statement on Antimicrobial Stewardship by SHEA, IDSA, and Pediatric Infectious Diseases Society (PIDS)nfect Control Hosp Epidemiol 2012;33(4):322-327 Achieve the best clinical outcome, while lessening or mitigating adverse events and limiting selective pressures Effective January 1, 2017, Joint Commission & CMS require that all hospitals have Antibiotic Stewardship Committees (ASC) Decrease excessive costs due to suboptimal antibiotic use Realize a fiduciary responsibility across the continuum of care IDSA/SHEA Antibiotic Stewardship guidelines for antibiotic use updated in 2016: Reduce the use of high-risk antibiotics associated with a high risk of C. Diff infections Increase use of oral antibiotics as a strategy to improve outcomes or decrease costs Reduce antibiotic therapy to the shortest effective duration ASC Goalsa
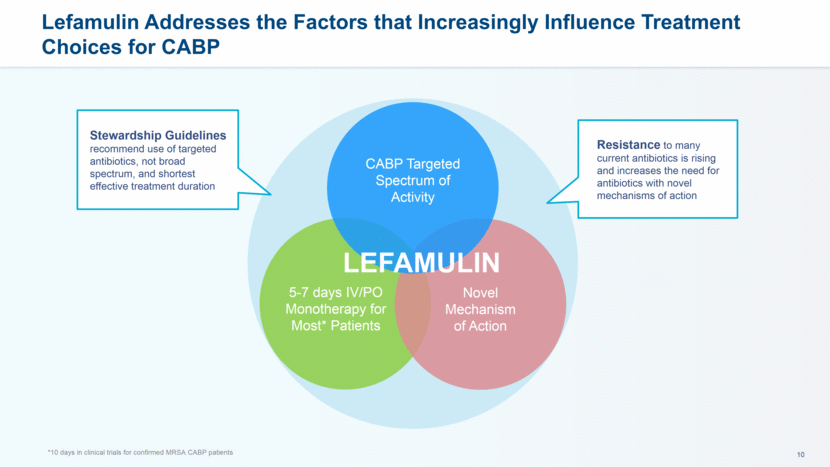
Lefamulin Addresses the Factors that Increasingly Influence Treatment Choices for CABP *10 days in clinical trials for confirmed MRSA CABP patients 5-7 days IV/PO Monotherapy for Most* Patients Novel Mechanism of Action CABP Targeted Spectrum of Activity Stewardship Guidelines recommend use of targeted antibiotics, not broad spectrum, and shortest effective treatment duration Resistance to many current antibiotics is rising and increases the need for antibiotics with novel mechanisms of action LEFAMULIN
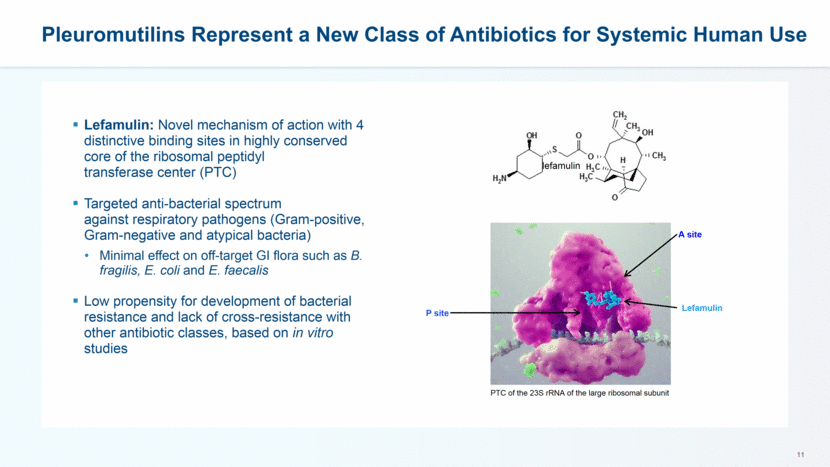
Pleuromutilins Represent a New Class of Antibiotics for Systemic Human Use Lefamulin A site P site PTC of the 23S rRNA of the large ribosomal subunit Lefamulin: Novel mechanism of action with 4 distinctive binding sites in highly conserved core of the ribosomal peptidyl transferase center (PTC) Targeted anti-bacterial spectrum against respiratory pathogens (Gram-positive, Gram-negative and atypical bacteria) Minimal effect on off-target GI flora such as B. fragilis, E. coli and E. faecalis Low propensity for development of bacterial resistance and lack of cross-resistance with other antibiotic classes, based on in vitro studies lefamulin
Lefamulin Clinical Program Overview Elyse Seltzer, M.D. Chief Medical Officer

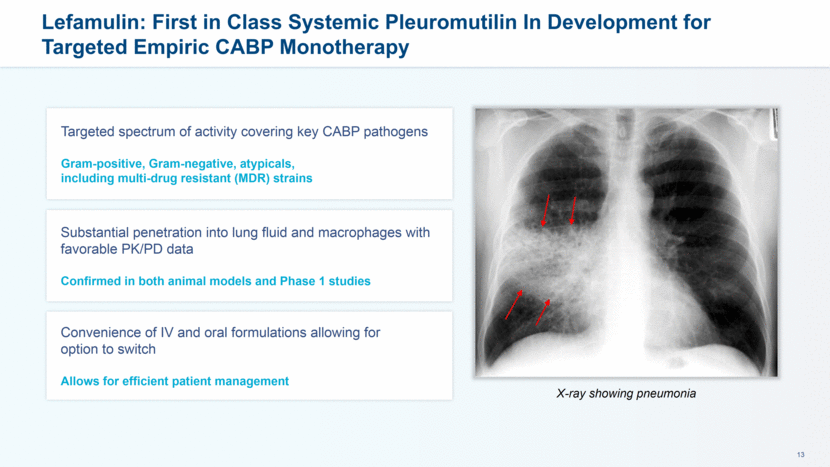
Lefamulin: First in Class Systemic Pleuromutilin In Development for Targeted Empiric CABP Monotherapy Targeted spectrum of activity covering key CABP pathogens Gram-positive, Gram-negative, atypicals, including multi-drug resistant (MDR) strains Substantial penetration into lung fluid and macrophages with favorable PK/PD data Confirmed in both animal models and Phase 1 studies Convenience of IV and oral formulations allowing for option to switch Allows for efficient patient management X-ray showing pneumonia
Lefamulin Addresses the Factors that Increasingly Influence Treatment Choices for CABP *10 days in clinical trials for confirmed MRSA CABP patients 5-7 days IV/PO Monotherapy for Most* Patients Novel Mechanism of Action CABP Targeted Spectrum of Activity Stewardship Guidelines recommend use of targeted antibiotics, not broad spectrum, and shortest effective treatment duration Resistance to many current antibiotics is rising and increases the need for antibiotics with novel mechanisms of action LEFAMULIN
Phase 3 CABP Program Supported by Robust Preclinical and Clinical Data Lefamulin Evaluated in over 400 Patients and Healthy Subjects in Phase 1/2 Clinical Trials Lack of target organ toxicity in preclinical studies CABP Animal Studies PK/PD and Microbiology Phase 2 ABSSSI Proof of Concept Trial PK-PD modeling simulations suggest that the food effect seen may not be clinically relevant Lack of target organ toxicity in preclinical studies Achieved clinical response rates compared to iv vancomycin Generally well tolerated
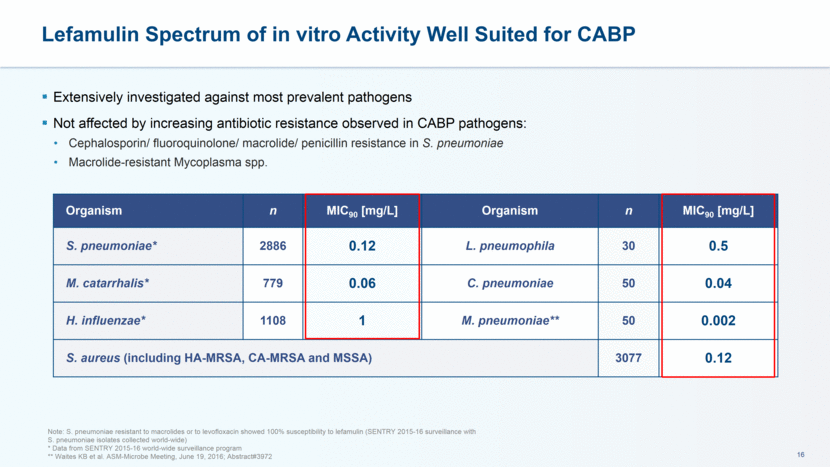
Lefamulin Spectrum of in vitro Activity Well Suited for CABP Extensively investigated against most prevalent pathogens Not affected by increasing antibiotic resistance observed in CABP pathogens: Cephalosporin/ fluoroquinolone/ macrolide/ penicillin resistance in S. pneumoniae Macrolide-resistant Mycoplasma spp. Note: S. pneumoniae resistant to macrolides or to levofloxacin showed 100% susceptibility to lefamulin (SENTRY 2015-16 surveillance with S. pneumoniae isolates collected world-wide) * Data from SENTRY 2015-16 world-wide surveillance program ** Waites KB et al. ASM-Microbe Meeting, June 19, 2016; Abstract#3972 Organism n MIC90 [mg/L] Organism n MIC90 [mg/L] S. pneumoniae* 2886 0.12 L. pneumophila 30 0.5 M. catarrhalis* 779 0.06 C. pneumoniae 50 0.04 H. influenzae* 1108 1 M. pneumoniae** 50 0.002 S. aureus (including HA-MRSA, CA-MRSA and MSSA) 3077 0.12
Lefamulin Clinical Development Plan Supports Differentiation in CABP Phase 3 CABP registration program initiated in the fall of 2015 Lefamulin Evaluation of Adults with Pneumonia (LEAP 1) trial (IV to oral): moderate-to-severe CABP (Pneumonia Outcomes Research Team (PORT) III, IV and V) February 2017- Confirmation of ~550 patients sample size April 2017 – Completed enrollment Data anticipated in September 2017 Second trial (LEAP 2 – oral): moderate CABP (PORT II, III and IV) initiated April 2016 ~160 sites enrolling patients Anticipate completing enrollment in 4Q 2017 Data anticipated in 1Q 2018 Hepatic / Renal Impairment Additional Phase 1 studies LEAP 2: CABP Oral CP3, global, ~740 pts. LEAP 1: CABP IV to Oral CP3, global, ~550 pts. 2016 2018 2017 Q1 Q4 Q4 Q2 Q3 Q4 Non-Clinical Activities Blue = Clinical Programs Q3 Q2 Q1 Q3 Q2 Q1 NDA/MAA submission target in 2018
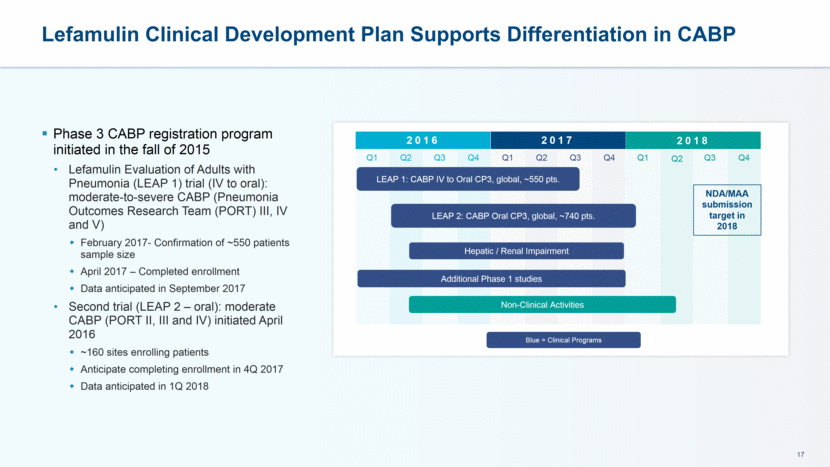
LEAP Phase 3 Trial Designs Cover Multiple Treatment Settings for CABP Double-blind, randomized, active comparator >100 multinational centers Stratification according to: Geographic region (US vs. ex-US) Prior short-acting antibiotic therapy for CABP vs. none PORT Class ~740 adult patients with PORT Risk Class II-IV (moderate) 1:1 randomization: lefamulin vs. moxifloxacin 5 days lefamulin / 7 days moxifloxacin PORT Risk Class II vs. III and IV >50% of patients will have a PORT risk class of III or IV LEAP 2 (Oral) Trial LEAP 1 (IV to Oral) Trial ~550 adult patients with PORT Risk Class >III (moderate to severe) 1:1 randomization: lefamulin vs. moxifloxacin ± linezolid Rx for 7 days (10 days for MRSA) PORT Risk Class III vs. IV and V >25% of patients will have a PORT risk class of IV or V Special Protocol Assessment with FDA
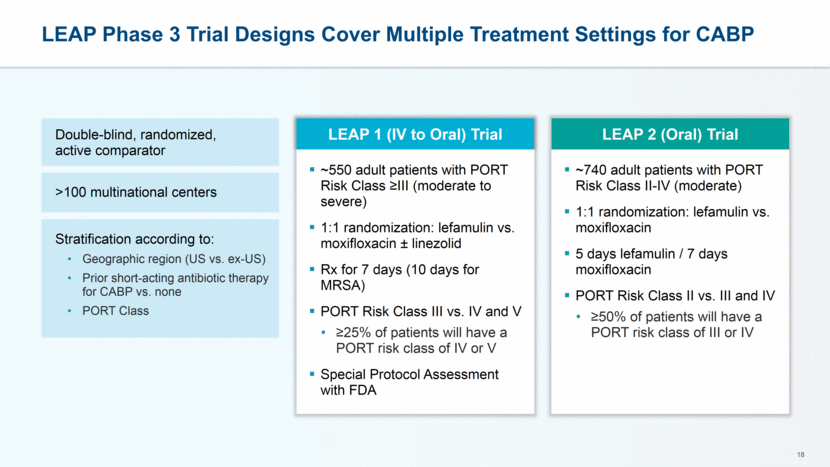
LEAP 1 Study Overview Lefamulin (± Placebo) Moxifloxacin (± Linezolid) Follow Up Study Drug Administration Enrollment: Must Dose Within 24 h Informed Consent & Baseline Assessments Randomization Early Clinical Response Assessment (96 +/- 24 hrs After 1st Dose) End of Treatment Within 2 Days After Last Dose Test of Cure (TOC) 5-10 Days After Last Dose Late Follow Up 30 +/-3 Days After 1st Dose >3 days of IV therapy Change to PO based on predefined signs of improvement and investigator discretion
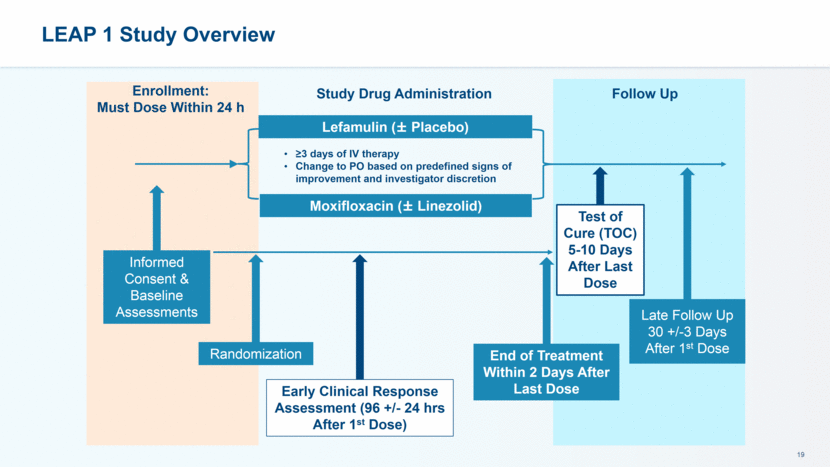
LEAP 1 and 2: Powering and Primary Objectives FDA Endpoint: Early Clinical Response (ECR) 72-120 hours after first dose of study drug Intent to Treat (ITT) Population LEAP 1 non-inferiority margin 12.5%, >90% power LEAP 2 non-inferiority margin 10%, 90% power EMA endpoint: Investigator Assessment of Clinical Response (IACR) at Test of Cure 5-10 days after the last dose of study drug Modified-ITT (mITT) and Clinically Evaluable Populations LEAP 1 non-inferiority margin 10%, 80% power LEAP 2 non-inferiority margin 10%, >90% power
LEAP 1 and 2: Additional Endpoints for Analysis Early Clinical Response (FDA primary endpoint) in additional populations Early Clinical Response plus improvement in vital signs - patient reported symptoms with objective signs of disease Investigator’s Assessment of Clinical Response (EMA endpoint) in additional populations By-Pathogen Response for FDA and EMA primary endpoints Safety and tolerability of lefamulin versus comparator 28 day all-cause mortality
Preparing for U.S. Commercialization Will Sargent Vice President, Commercial Strategy

What You Will Hear Today Patient treatment patterns Antibiotic prescription trends Hospital treatment data Physician market research Review CABP market highlights Nabriva’s anticipated Go To Market (GTM) strategy
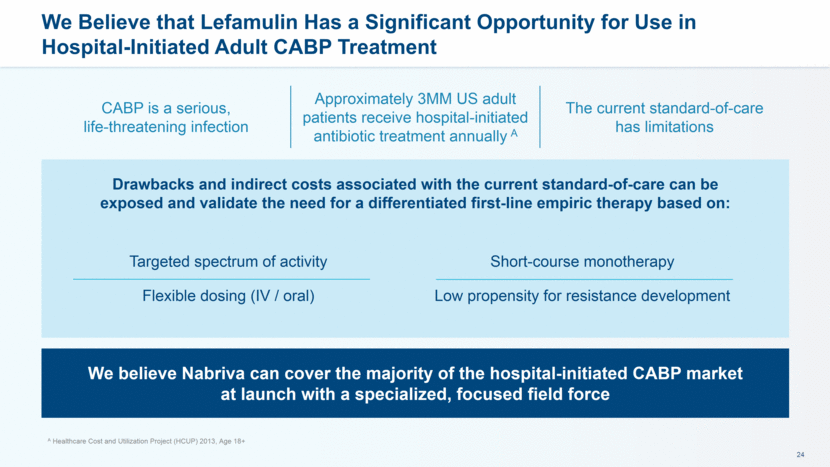
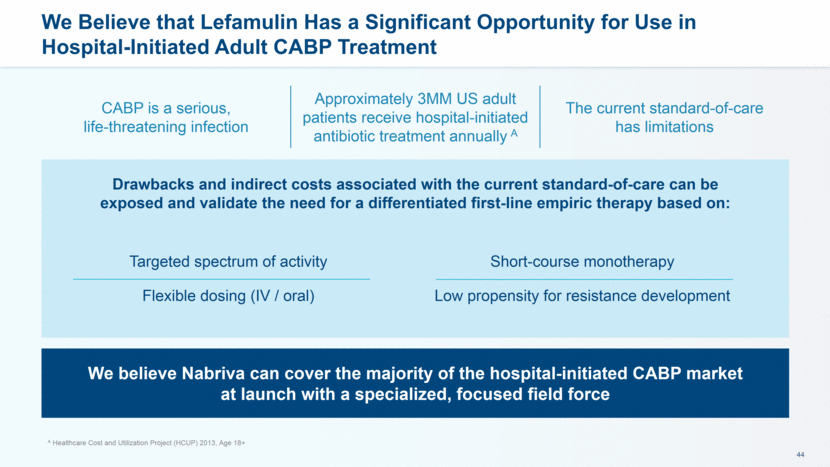
CABP is a serious, life-threatening infection Approximately 3MM US adult patients receive hospital-initiated antibiotic treatment annually A The current standard-of-care has limitations We Believe that Lefamulin Has a Significant Opportunity for Use in Hospital-Initiated Adult CABP Treatment Drawbacks and indirect costs associated with the current standard-of-care can be exposed and validate the need for a differentiated first-line empiric therapy based on: We believe Nabriva can cover the majority of the hospital-initiated CABP market at launch with a specialized, focused field force Targeted spectrum of activity Flexible dosing (IV / oral) Short-course monotherapy Low propensity for resistance development A Healthcare Cost and Utilization Project (HCUP) 2013, Age 18+
Adult CABP Patient Treatment Patterns
A Company estimate B CDC: National Ambulatory Medical Care Survey (NAMCS) and the National Hospital Ambulatory Medical Care Survey (NHAMCS) (2007), All ages estimate percent that are Age 18+ using HCUP ED discharge age split (65% Age 18+) C Healthcare Cost and Utilization Project (HCUP) 2013, Age 18+ # of Adult Patients In-Patient Community B Primary care offices (PCP) or community clinics, or urgent care ED / Out-patient Other Nursing home, home health, etc. ~2.4MMC ~.9MM 1 diagnosis, ~1.6MM 2 diagnosis ~0.7MMC ~.5MM 1 diagnosis, ~.2MM 2 diagnosis ~1.7MMB N/A >5MM Adult Patients go to a site of care for CABP treatment annually A Hospital ~3.1MMC Adult CABP Treatment Journey Aligns with Hospital-Initiated Opportunity Allows for a focused commercial strategy
Adult CABP Prescription Trends
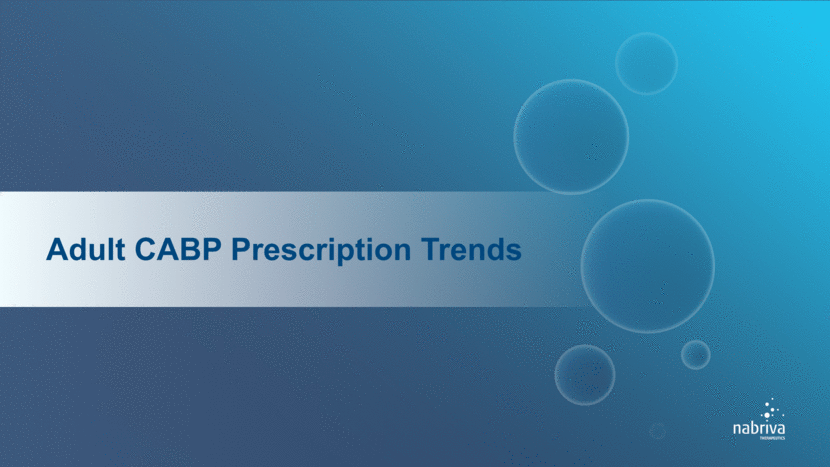
Based on AMR 2010-2015 prescription data Hospital Inpatient Market Dominated by Macrolides, Fluoroquinolones and IV Cephalosporins No current treatment option is ideally-suited for adult CABP population
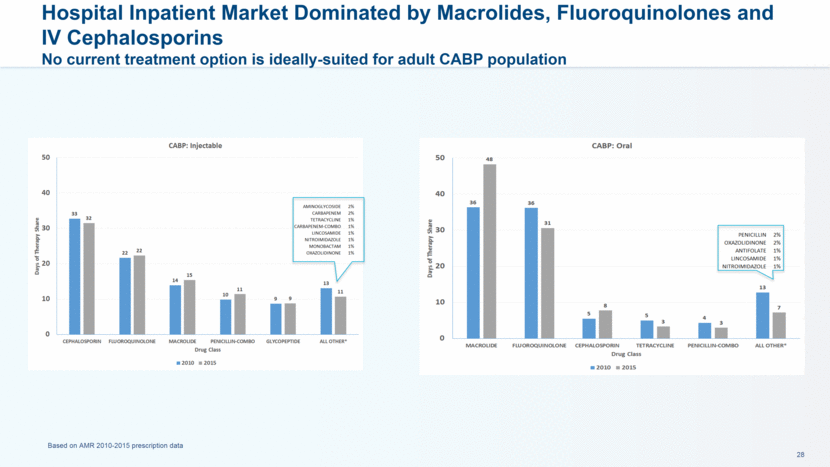
Retail Outpatient Market Dominated by Fluoroquinolones and Macrolides No ideal oral therapy exists to treat adult CABP outpatients Based on 6-12-17 data provided by Source Health Solutions (12 months ending September 2016), Age 18+ Hospital Initiated, Retail Dispensed Adult Oral TRX share
Adult CABP Hospital Treatment Data
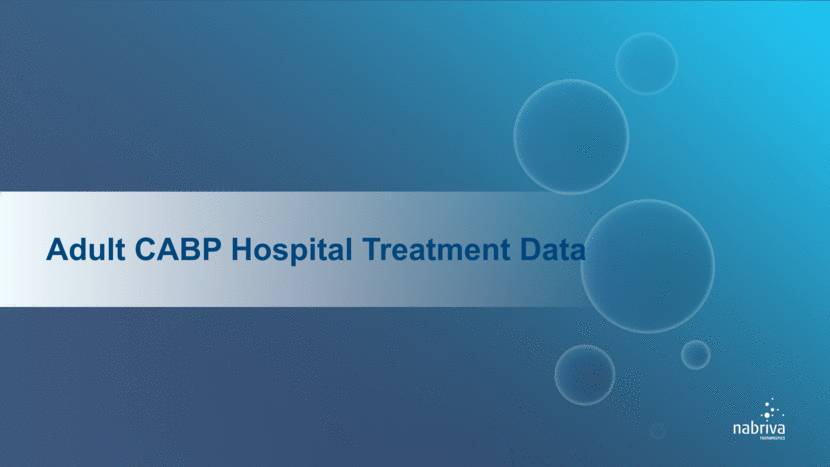
Challenges with Current Hospital Initiated CABP Treatment Paradigm A Length of stay is primary focus of hospitalists, but not necessarily other specialties 90% Approximate number of CABP patients treated empirically in the Emergency Department B CABP is perceived to be one of the easier infections to treat in the hospital due to several “good enough” low-cost generic options, but adverse outcomes aren’t always viewed as “antibiotic costs” associated with the course of therapy. On average, generic antibiotics generate negative margins for hospitals treating CABP patients C Both antimicrobial stewardship sub-committee and Pharmacy & Therapeutics (P&T) committee review new antibiotics A Company sponsored market research, Medical Marketing Economics, July 2016, (N=122) B Company sponsored market research, MEDACorp Market Research, July 2016 (N=152) C Company sponsored market research, Avalere 2016 Analysis of CABP 2015 MedPAR data
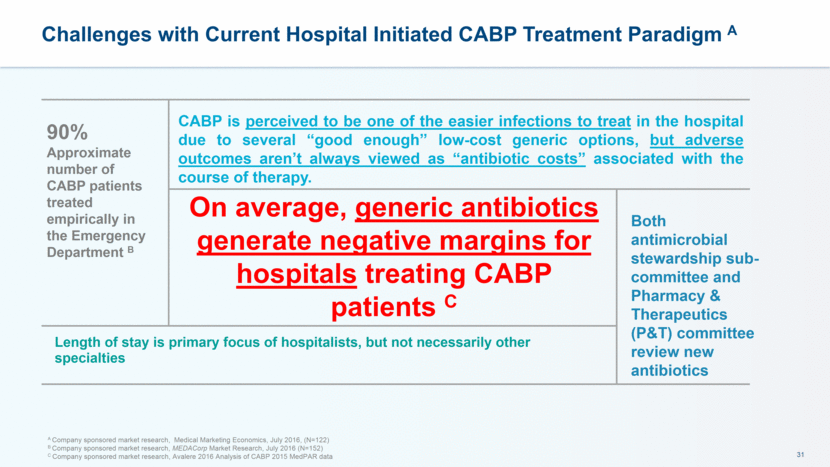
Estimated 1/3 of All CABP Patients Need Second- or Third-line Therapy, which Can Increase Length and Cost of Hospital Stay A A Company sponsored market research, Medical Marketing Economics, July 2016, (N=122) Percent of CABP patients who receive first-, second- and third line therapy for CABP Non-ICU setting ICU setting Community setting % of patients % of patients % of patients Overall % of patients
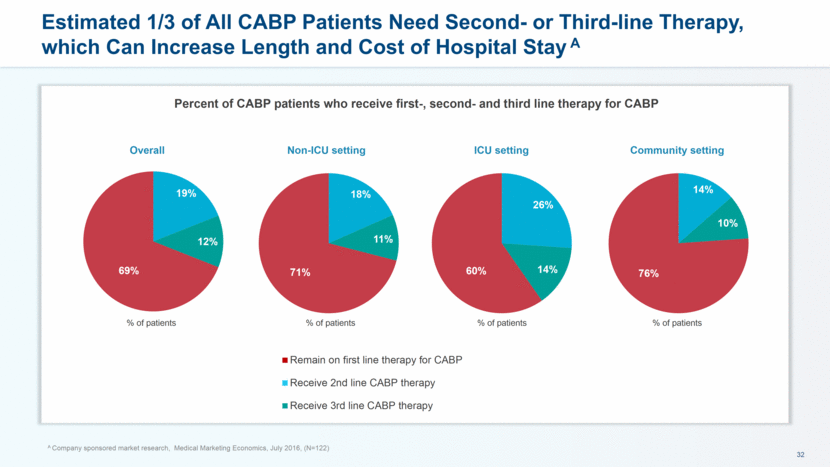
Most Hospitals Lose Money when Treating Adult Medicare CABP Inpatients Treatment scenario Number of cases Average length of stay Average CABP patient cost Average CABP patient payment Average margin Primary Dx CABP 354,423 5.2 days ~$9,600 ~$8,901 -7.5% Secondary Dx CABP 752,985 8.2 days ~$21,000 ~$17,000 -17.3% A Company sponsored market research, Avalere 2016 Analysis of CABP 2015 MedPAR data Reality challenges perception that the existing treatment paradigm is “good enough” with regard to patient outcomes and cost effectiveness Outcomes and budget impact models can help hospitals understand the true costs of the current standard of care for antibiotics These data set the stage for conversations to demonstrate potential value of new antibiotics
Physician Market Research

Lefamulin Addresses the Factors that Increasingly Influence Treatment Choices for CABP *10 days in clinical trials for confirmed MRSA CABP patients 5-7 days IV/PO Monotherapy for Most* Patients Novel Mechanism of Action CABP Targeted Spectrum of Activity Stewardship Guidelines recommend use of targeted antibiotics, not broad spectrum, and shortest effective treatment duration Resistance to many current antibiotics is rising and increases the need for antibiotics with novel mechanisms of action LEFAMULIN
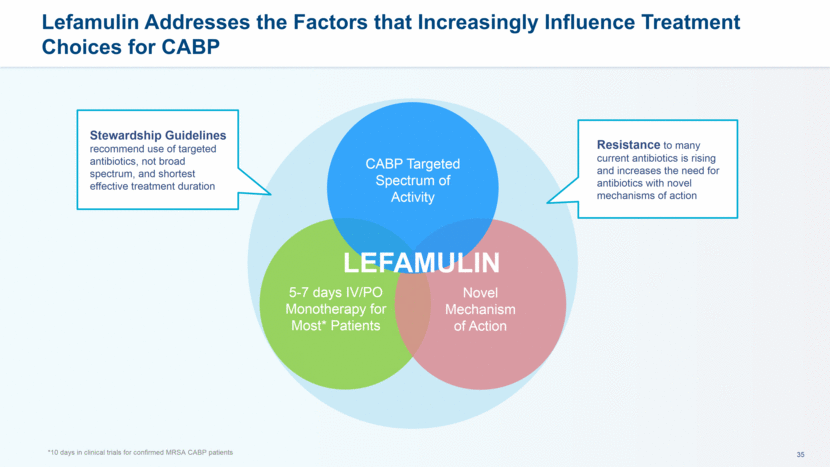
Current CABP Treatment Paradigm for Empiric Use is not Fully Aligned with Stewardship Principles Empiric Broad Spectrum Antibiotics CABP Narrow Spectrum Antibiotics If pathogen is determined S. aureus S. pneumo CABP Gut flora UTI/ gram neg.
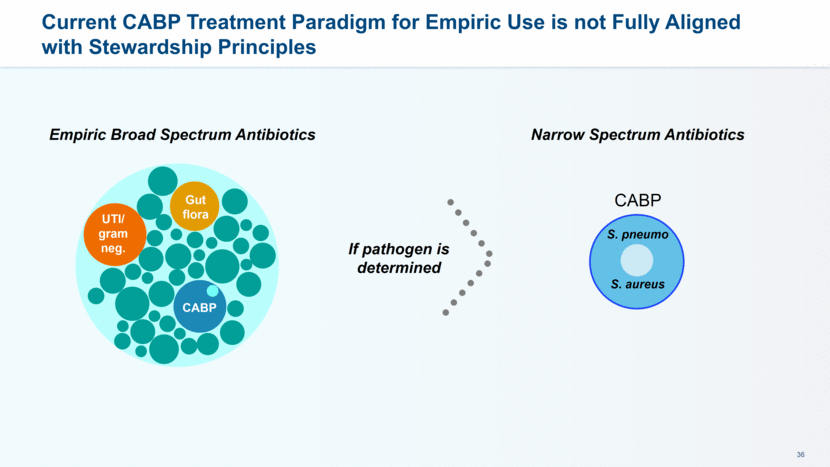
A Targeted Antibiotic for Empiric Treatment of CABP Aligns with Stewardship Principles Empiric Targeted Spectrum Antibiotic Save Broader Spectrum Antibiotics for Second- and Third-line Use If patient isn’t cured MRSA M. Cat DRSP H. Flu L. Pne C. Pne M. Pne CABP Gut flora UTI/ gram neg.
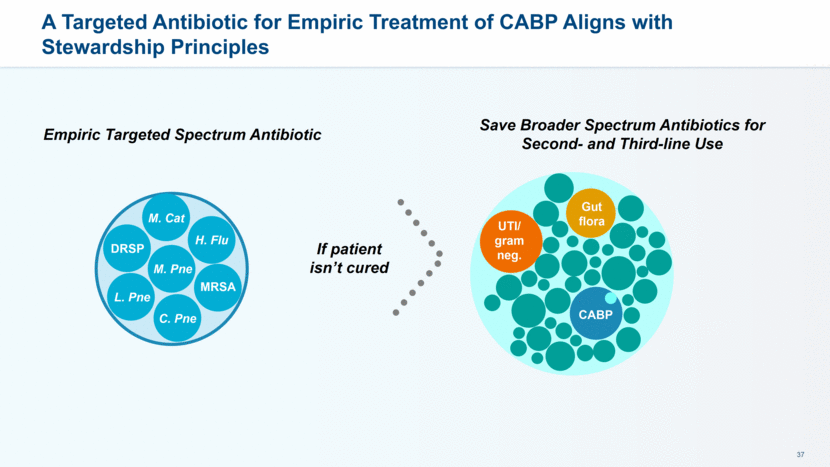
Physicians Believe Targeted Coverage of the Most Common Pathogens that Cause CABP Will Have Greater Impact than Broad Spectrum Coverage A Infectious Disease Critical Care Hospitalist Emergency Room General Medicine 4.4 4.5 4.4 4.6 4.8 Targeted coverage of most common CABP pathogens 4.0 3.9 4.1 3.6 4.0 Atypical coverage 3.1 3.7 3.7 3.5 4.0 Broad pathogen coverage 3.0 3.1 3.4 2.8 3.5 Multi Drug Resistant S. Pneumonia coverage 2.9 3.4 3.7 2.6 3.5 Methicillin Sensitive S Aureus coverage 2.9 3.6 3.7 3.3 3.4 Methicillin Resistant S Aureus coverage 2.6 3.3 3.2 2.9 3.1 Pseudomonas coverage 2.3 2.5 3.3 2.5 3.2 Macrolide-resistant mycoplasma coverage How do the following features of an antibiotic pathogen coverage impact on your choice of antibiotic for CABP? Please rate each on a scale of 1-5 where 5 is high-impact and 1=no impact at all A Company sponsored market research, MEDACorp. July 2016 (N=152)
Nabriva’s Market Research Focuses on Understanding the Multiple Factors Affecting CABP Treatment Choice Physician Choice of Antibiotic Patient Profile Comorbidities and safety HCP Perceptions Guidelines and Protocols Stewardship Outcomes
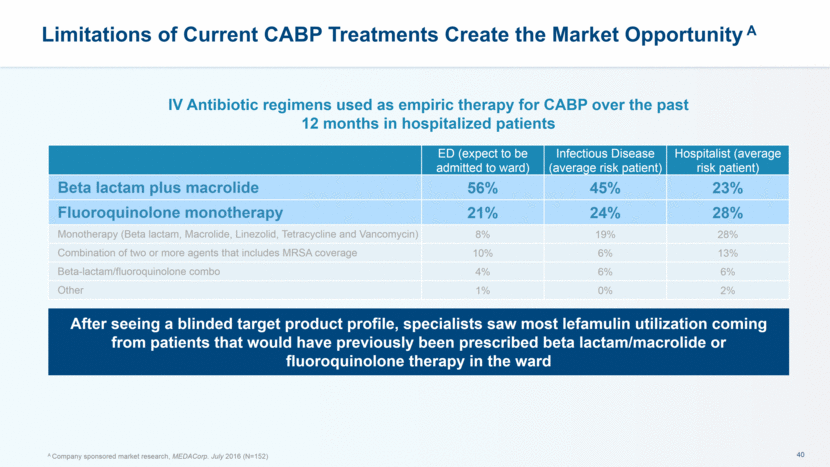
After seeing a blinded target product profile, specialists saw most lefamulin utilization coming from patients that would have previously been prescribed beta lactam/macrolide or fluoroquinolone therapy in the ward Limitations of Current CABP Treatments Create the Market Opportunity A ED (expect to be admitted to ward) Infectious Disease (average risk patient) Hospitalist (average risk patient) Beta lactam plus macrolide 56% 45% 23% Fluoroquinolone monotherapy 21% 24% 28% Monotherapy (Beta lactam, Macrolide, Linezolid, Tetracycline and Vancomycin) 8% 19% 28% Combination of two or more agents that includes MRSA coverage 10% 6% 13% Beta-lactam/fluoroquinolone combo 4% 6% 6% Other 1% 0% 2% IV Antibiotic regimens used as empiric therapy for CABP over the past 12 months in hospitalized patients A Company sponsored market research, MEDACorp. July 2016 (N=152)
Potential Nabriva GTM Strategy

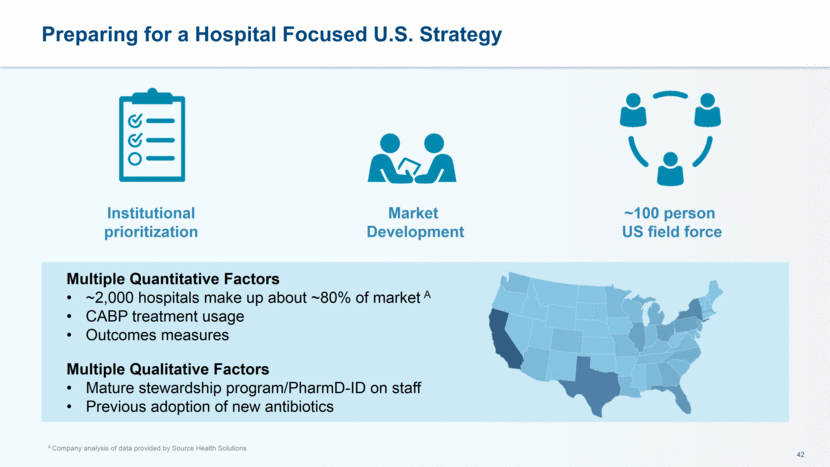
Multiple Quantitative Factors ~2,000 hospitals make up about ~80% of market A CABP treatment usage Outcomes measures Multiple Qualitative Factors Mature stewardship program/PharmD-ID on staff Previous adoption of new antibiotics Preparing for a Hospital Focused U.S. Strategy A Company analysis of data provided by Source Health Solutions Institutional prioritization Market Development ~100 person US field force
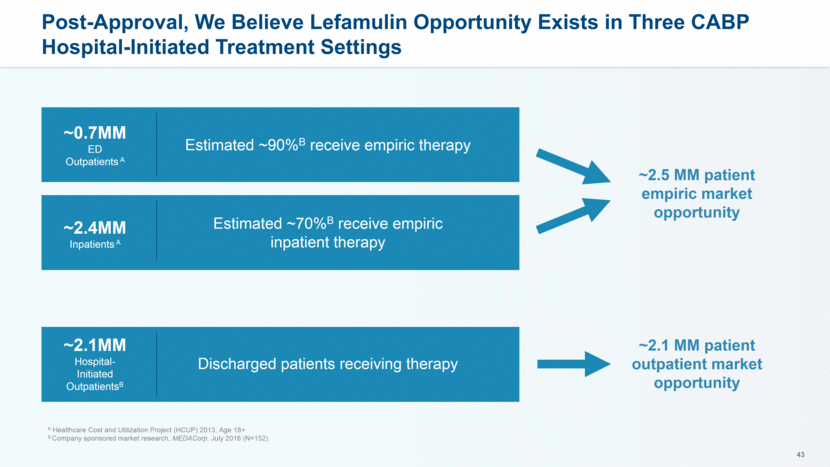
Post-Approval, We Believe Lefamulin Opportunity Exists in Three CABP Hospital-Initiated Treatment Settings A Healthcare Cost and Utilization Project (HCUP) 2013, Age 18+ B Company sponsored market research, MEDACorp. July 2016 (N=152) ~2.1MM Hospital-Initiated OutpatientsB Discharged patients receiving therapy ~2.1 MM patient outpatient market opportunity ~0.7MM ED Outpatients A ~2.4MM Inpatients A Estimated ~70%B receive empiric inpatient therapy Estimated ~90%B receive empiric therapy ~2.5 MM patient empiric market opportunity
CABP is a serious, life-threatening infection Approximately 3MM US adult patients receive hospital-initiated antibiotic treatment annually A The current standard-of-care has limitations We Believe that Lefamulin Has a Significant Opportunity for Use in Hospital-Initiated Adult CABP Treatment Drawbacks and indirect costs associated with the current standard-of-care can be exposed and validate the need for a differentiated first-line empiric therapy based on: We believe Nabriva can cover the majority of the hospital-initiated CABP market at launch with a specialized, focused field force Targeted spectrum of activity Flexible dosing (IV / oral) Short-course monotherapy Low propensity for resistance development A Healthcare Cost and Utilization Project (HCUP) 2013, Age 18+
Q&A 2017 Analyst & Investor Day

A Transformational Year for Nabriva Colin Broom, M.D. Chief Executive Officer

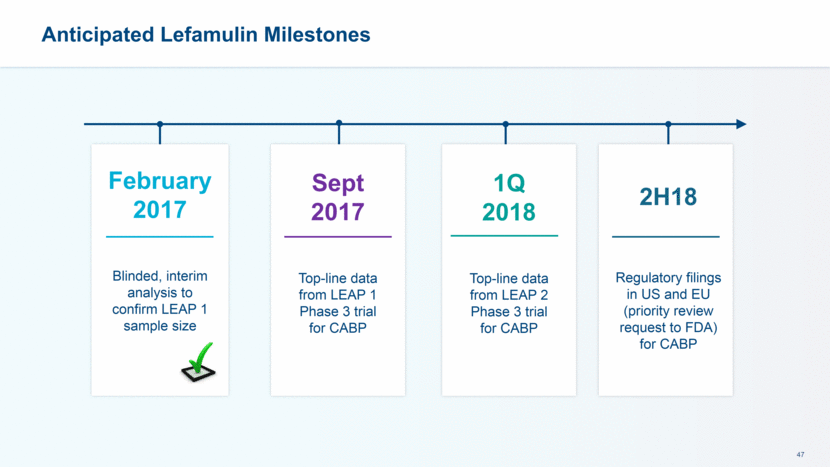
Anticipated Lefamulin Milestones February 2017 Blinded, interim analysis to confirm LEAP 1 sample size 1Q 2018 Top-line data from LEAP 2 Phase 3 trial for CABP 2H18 Regulatory filings in US and EU (priority review request to FDA) for CABP Sept 2017 Top-line data from LEAP 1 Phase 3 trial for CABP
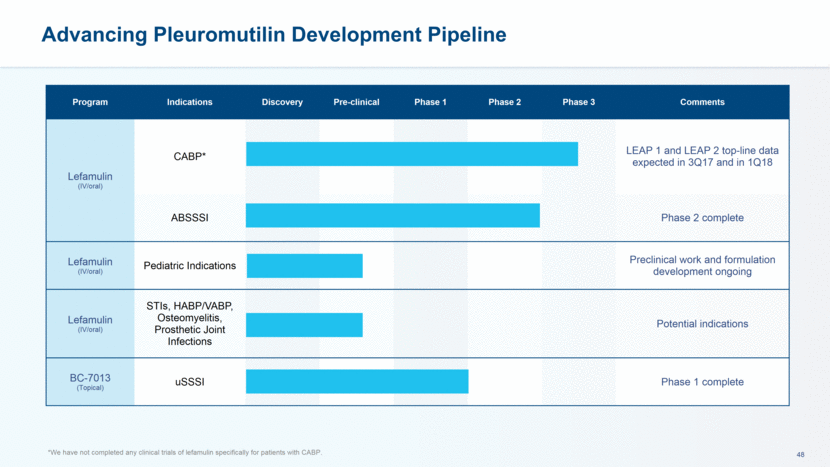
Advancing Pleuromutilin Development Pipeline *We have not completed any clinical trials of lefamulin specifically for patients with CABP. Program Indications Discovery Pre-clinical Phase 1 Phase 2 Phase 3 Comments Lefamulin (IV/oral) CABP* LEAP 1 and LEAP 2 top-line data expected in 3Q17 and in 1Q18 ABSSSI Phase 2 complete Lefamulin (IV/oral) Pediatric Indications Preclinical work and formulation development ongoing Lefamulin (IV/oral) STIs, HABP/VABP, Osteomyelitis, Prosthetic Joint Infections Potential indications BC-7013 (Topical) uSSSI Phase 1 complete
Nabriva: Positioned for Long-Term Growth & Success CABP represents a significant medical need Rising rates of bacterial resistance to commonly used antibiotic therapies Utility of available treatment options becoming more limited Lefamulin is well-positioned to address limitations of current antibiotics for the treatment of adult patients with CABP Well-designed Phase 3 clinical development program consistent with regulatory guidance Potential to be first new class of antibiotics for CABP approved since 2000 Nabriva has the right team in place to successfully execute its strategy Focused on capital efficiency Developing pipeline of follow-on indications for lefamulin