Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Blueprint Medicines Corp | ex-99d1.htm |
| EX-99.2 - EX-99.2 - Blueprint Medicines Corp | ex-99d2.htm |
| 8-K - 8-K - Blueprint Medicines Corp | f8-k.htm |
Exhibit 99.3
|
|
2017 ASCO Annual Meeting Monday, June 5, 2017 Advances in GIST |
|
|
Forward-looking statements 2 This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. In this presentation, forward-looking statements include, without limitation, statements about plans and timelines for the clinical development of BLU-285, BLU-554 and BLU-667 and the ability of Blueprint Medicines Corporation (the “Company”) to implement those clinical development plans; the potential benefits of the Company’s current and future drug candidates in treating patients; plans and timelines for regulatory submissions, filings or discussions; plans and timelines for the development and commercialization of companion diagnostics for the Company’s current or future drug candidates; plans and timelines for current or future discovery programs; plans and timelines for future collaborations, if any, with strategic partners; the future financial performance of the Company; expectations regarding potential milestones in 2017; and the Company’s strategy, business plans and focus. The Company has based these forward-looking statements on management’s current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks, uncertainties and other important factors, many of which are beyond the Company’s control and may cause actual results, performance or achievements to differ materially from those expressed or implied by any forward-looking statements. These risks and uncertainties include, without limitation, risks and uncertainties related to the delay of any current or future clinical trials or the development of the Company’s drug candidates, including BLU-285, BLU-554 and BLU-667; the Company's advancement of multiple early-stage efforts; the Company’s ability to successfully demonstrate the efficacy and safety of its drug candidates; the preclinical and clinical results for the Company’s drug candidates, which may not support further development of such drug candidates; actions or decisions of regulatory agencies or authorities, which may affect the initiation, timing and progress of current or future clinical trials; the Company’s ability to obtain, maintain and enforce patent and other intellectual property protection for any drug candidates it is developing; the Company’s ability to develop and commercialize companion diagnostics for its current and future drug candidates, including a companion diagnostic for BLU-554 with Vantaa Medical Systems, Inc. and a companion diagnostic for BLU-285 with QIAGEN Manchester Limited; and the success of the Company’s rare genetic disease collaboration with Alexion Pharma Holding and its cancer immunotherapy collaboration with F. Hoffmann-La Roche Ltd and Hoffmann-La Roche Inc. These and other risks and uncertainties are described in greater detail under “Risk Factors” in the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2017, as filed with the Securities and Exchange Commission (“SEC”) on May 3, 2017, and any other filings the Company may make with the SEC in the future. The Company cannot guarantee future results, outcomes, levels of activity, performance, developments, or achievements, and there can be no assurance that the Company’s expectations, intentions, anticipations, beliefs, or projections will result or be achieved or accomplished. The forward-looking statements in this presentation are made only as of the date hereof, and except as required by law, the Company undertakes no obligation to update any forward-looking statements contained in this presentation as a result of new information, future events or otherwise. This presentation also contains estimates, projections and other statistical data made by independent parties and by the Company relating to market size and growth and other data about the Company’s industry. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of the Company’s future performance and the future performance of the markets in which the Company operates are necessarily subject to a high degree of uncertainty and risk. |
|
|
Agenda Welcome Jeff Albers, Chief Executive Officer, Blueprint Medicines Overview of BLU-285 in GIST Andy Boral, MD, Chief Medical Officer, Blueprint Medicines Phase 1 clinical trial results Michael Heinrich, MD, Professor, Oregon Health and Science University Proposed registration path Andy Boral, MD, Chief Medical Officer, Blueprint Medicines Question and answer session Michael C. Heinrich, MD, Professor, Oregon Health and Science University Jeff Albers, Chief Executive Officer, Blueprint Medicines Andy Boral, MD, Chief Medical Officer, Blueprint Medicines Closing remarks Jeff Albers, Chief Executive Officer, Blueprint Medicines 3 |
|
|
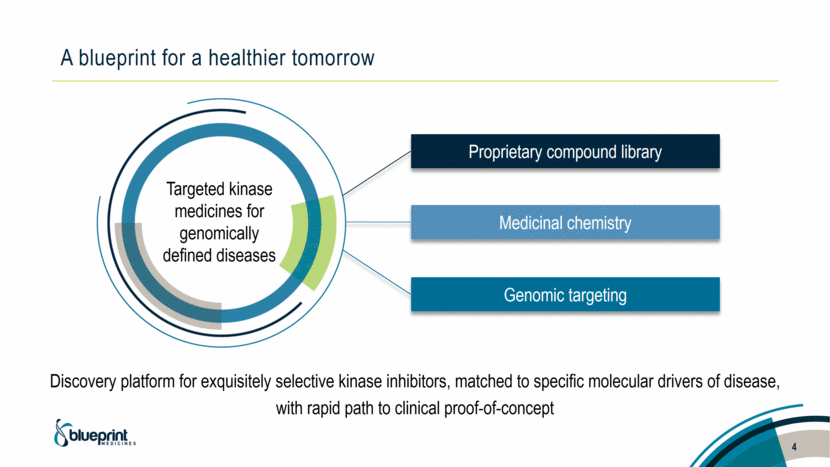
Medicinal chemistry Proprietary compound library Genomic targeting A blueprint for a healthier tomorrow 4 Targeted kinase medicines for genomically defined diseases Discovery platform for exquisitely selective kinase inhibitors, matched to specific molecular drivers of disease, with rapid path to clinical proof-of-concept |
|
|
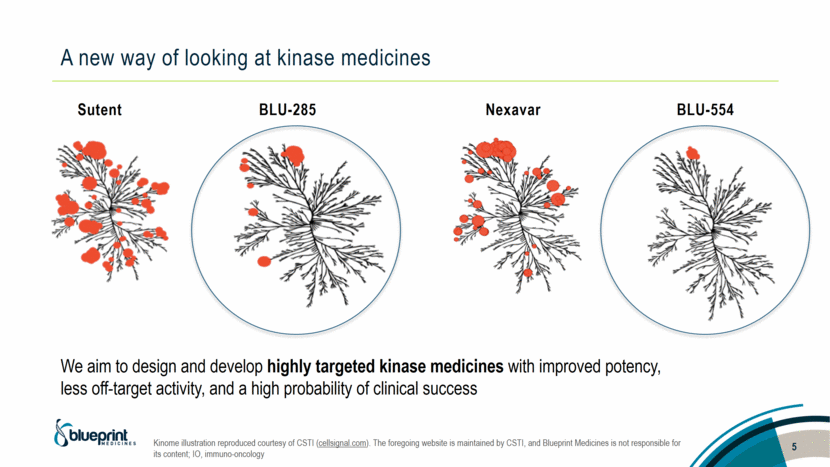
A new way of looking at kinase medicines Kinome illustration reproduced courtesy of CSTI (cellsignal.com). The foregoing website is maintained by CSTI, and Blueprint Medicines is not responsible for its content; IO, immuno-oncology 5 BLU-554 Nexavar Sutent BLU-285 We aim to design and develop highly targeted kinase medicines with improved potency, less off-target activity, and a high probability of clinical success |
|
|
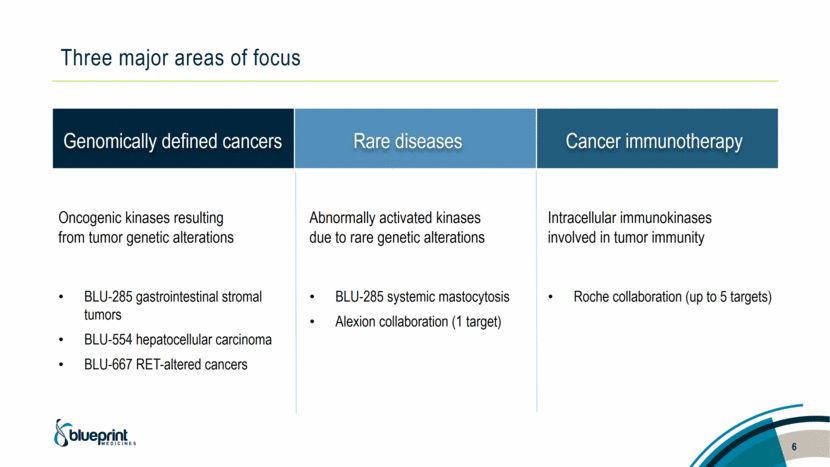
Three major areas of focus 6 Genomically defined cancers Rare diseases Cancer immunotherapy Oncogenic kinases resulting from tumor genetic alterations BLU-285 gastrointestinal stromal tumors BLU-554 hepatocellular carcinoma BLU-667 RET-altered cancers Abnormally activated kinases due to rare genetic alterations BLU-285 systemic mastocytosis Alexion collaboration (1 target) Intracellular immunokinases involved in tumor immunity Roche collaboration (up to 5 targets) |
|
|
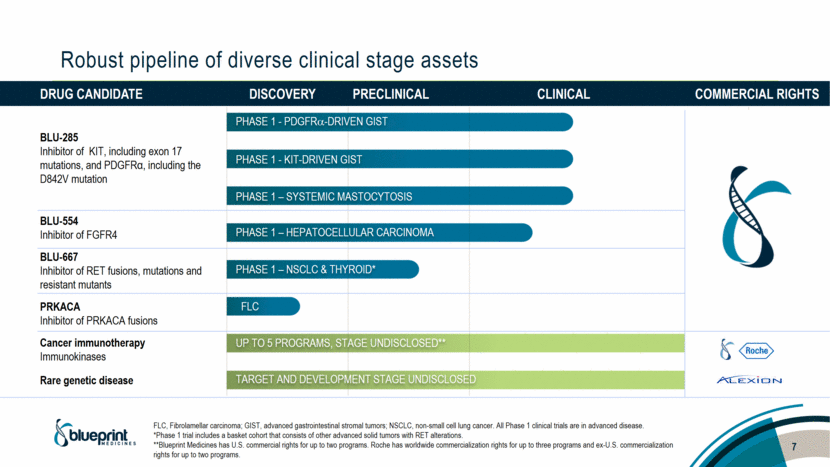
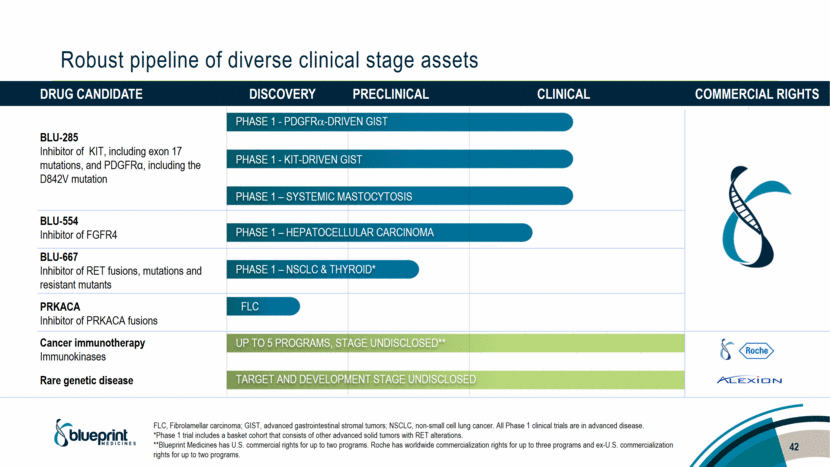
Drug candidate Discovery Preclinical Clinical Commercial rights BLU-285 Inhibitor of KIT, including exon 17 mutations, and PDGFRα, including the D842V mutation BLU-554 Inhibitor of FGFR4 BLU-667 Inhibitor of RET fusions, mutations and resistant mutants PRKACA Inhibitor of PRKACA fusions Cancer immunotherapy Immunokinases Rare genetic disease Robust pipeline of diverse clinical stage assets PHASE 1 - PDGFR-DRIVEN GIST PHASE 1 - KIT-DRIVEN GIST PHASE 1 – SYSTEMIC MASTOCYTOSIS PHASE 1 – HEPATOCELLULAR CARCINOMA PHASE 1 – NSCLC & THYROID* FLC Up to 5 programs, STAGE UNDISCLOSED** Target and development stage undisclosed FLC, Fibrolamellar carcinoma; GIST, advanced gastrointestinal stromal tumors; NSCLC, non-small cell lung cancer. All Phase 1 clinical trials are in advanced disease. *Phase 1 trial includes a basket cohort that consists of other advanced solid tumors with RET alterations. **Blueprint Medicines has U.S. commercial rights for up to two programs. Roche has worldwide commercialization rights for up to three programs and ex-U.S. commercialization rights for up to two programs. 7 |
|
|
BLU-285 Drug Discovery Overview Andy Boral, M.D. Chief Medical Officer |
|
|
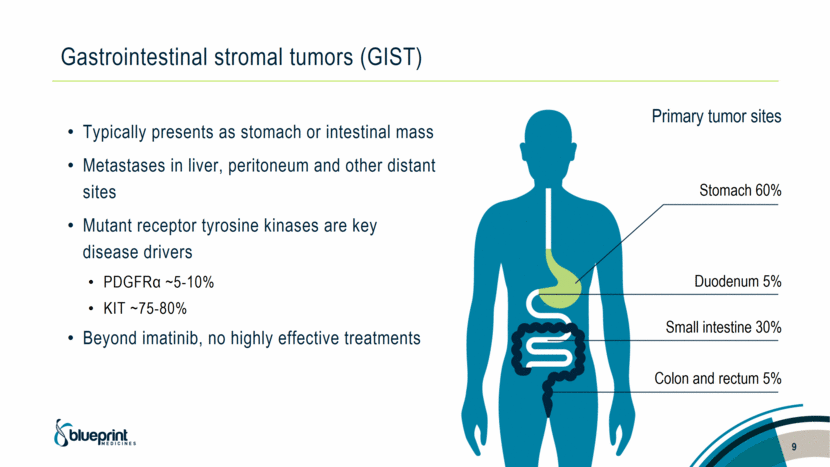
Gastrointestinal stromal tumors (GIST) Colon and rectum 5% Small intestine 30% Stomach 60% Duodenum 5% Primary tumor sites 9 Typically presents as stomach or intestinal mass Metastases in liver, peritoneum and other distant sites Mutant receptor tyrosine kinases are key disease drivers PDGFRα ~5-10% KIT ~75-80% Beyond imatinib, no highly effective treatments |
|
|
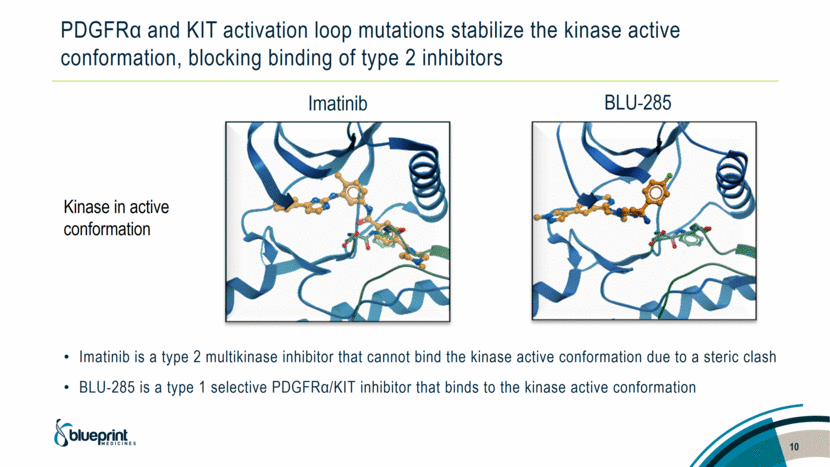
PDGFRα and KIT activation loop mutations stabilize the kinase active conformation, blocking binding of type 2 inhibitors Imatinib is a type 2 multikinase inhibitor that cannot bind the kinase active conformation due to a steric clash BLU-285 is a type 1 selective PDGFRα/KIT inhibitor that binds to the kinase active conformation BLU-285 Kinase in active conformation 10 Imatinib |
|
|
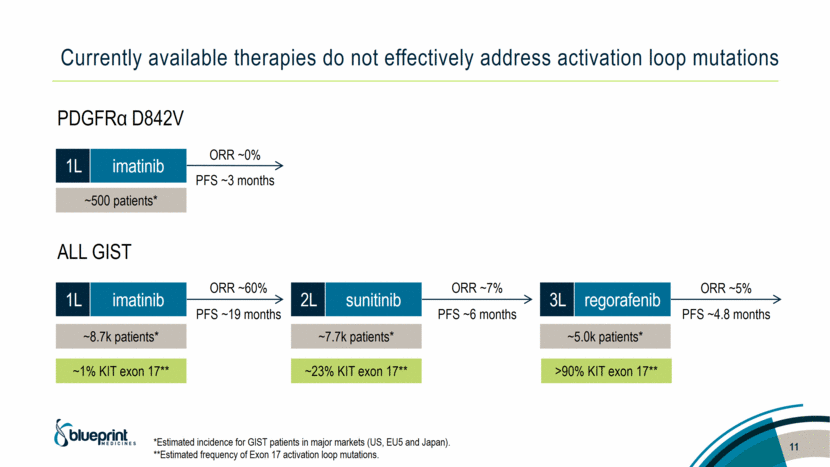
Currently available therapies do not effectively address activation loop mutations *Estimated incidence for GIST patients in major markets (US, EU5 and Japan). **Estimated frequency of Exon 17 activation loop mutations. 11 PDGFRα D842V ALL GIST 1L imatinib PFS ~3 months ORR ~0% ~500 patients* 1L imatinib 2L sunitinib ORR ~60% PFS ~19 months ORR ~7% PFS ~6 months ORR ~5% PFS ~4.8 months 3L regorafenib ~8.7k patients* ~7.7k patients* ~5.0k patients* ~1% KIT exon 17** ~23% KIT exon 17** >90% KIT exon 17** |
|
|
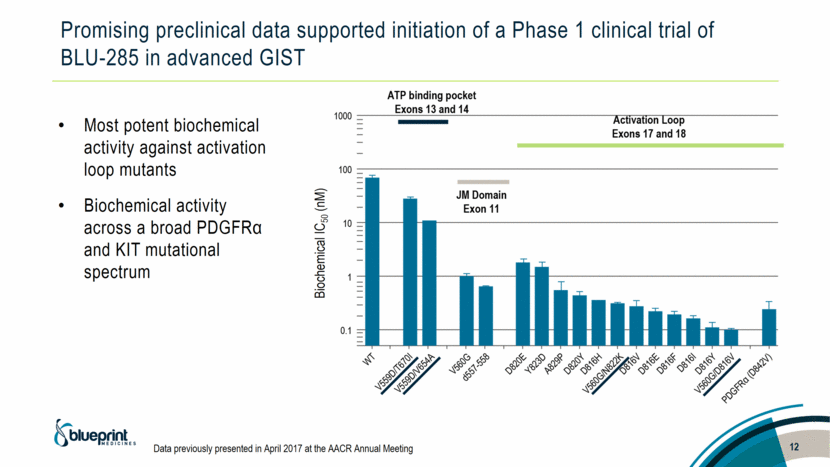
Promising preclinical data supported initiation of a Phase 1 clinical trial of BLU-285 in advanced GIST Most potent biochemical activity against activation loop mutants Biochemical activity across a broad PDGFRα and KIT mutational spectrum 12 Biochemical IC50 (nM) Data previously presented in April 2017 at the AACR Annual Meeting |
|
|
Updated Phase 1 clinical trial results Michael C. Heinrich, M.D. Oregon Health & Sciences University (OHSU) |
|
|
GIST: imatinib and beyond Clinical activity of BLU-285 in advanced gastrointestinal stromal tumor (GIST) Michael Heinrich1, Robin Jones2, Margaret von Mehren3, Patrick Schoffski4, Sebastian Bauer5, Olivier Mir6, Philippe Cassier7, Ferry Eskens8, Hongliang Shi9, Terri Alvarez-Diez9, Oleg Schmidt-Kittler9, Mary Ellen Healy9, Beni Wolf9, Suzanne George10 1Oregon Health & Sciences University, Oregon, USA; 2Royal Marsden Hospital/Institute of Cancer Research, London, UK; 3Fox Chase Cancer Center, Pennsylvania, USA; 4Leuven Cancer Institute, Leuven, Belgium; 5University of Essen, Essen, Germany; 6Institut Gustave Roussy, Paris, France; 7Centre Leon Berard, Lyon, France; 8 Erasmus MC Cancer Institute, Rotterdam, Netherlands; 9Blueprint Medicines Corporation, Massachusetts, USA; 10Dana-Farber Cancer Institute, Massachusetts, USA Abstract no: 11011 Presented by: Dr. Michael Heinrich |
|
|
Disclosures BLU-285 is an investigational agent currently in development by Blueprint Medicines Corporation (Blueprint Medicines) Dr. Michael Heinrich is an investigator for Blueprint Medicines’ ongoing Phase 1 studies in unresectable gastrointestinal stromal tumor Dr. Michael Heinrich has the following disclosures: Consultant: Blueprint Medicines, Novartis, MolecularMD Equity interest: MolecularMD Research funding: Blueprint Medicines, Deciphera, Ariad Expert testimony: Novartis Patents: four patents on diagnosis and treatment of PDGFR-mutant GIST 15 |
|
|
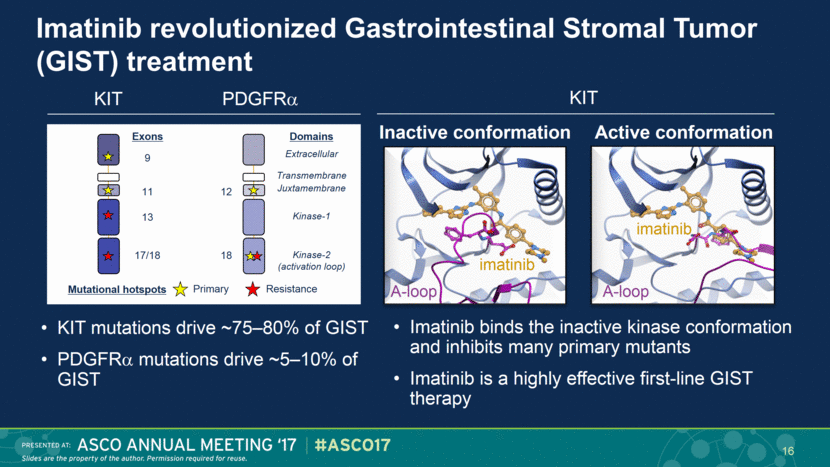
Imatinib revolutionized Gastrointestinal Stromal Tumor (GIST) treatment Imatinib binds the inactive kinase conformation and inhibits many primary mutants Imatinib is a highly effective first-line GIST therapy A-loop imatinib A-loop imatinib Inactive conformation Active conformation KIT KIT mutations drive ~75–80% of GIST PDGFR mutations drive ~5–10% of GIST KIT PDGFR Extracellular Juxtamembrane Kinase-1 Kinase-2 (activation loop) Transmembrane Exons 12 18 9 11 17/18 13 Domains Primary Resistance Mutational hotspots 16 |
|
|
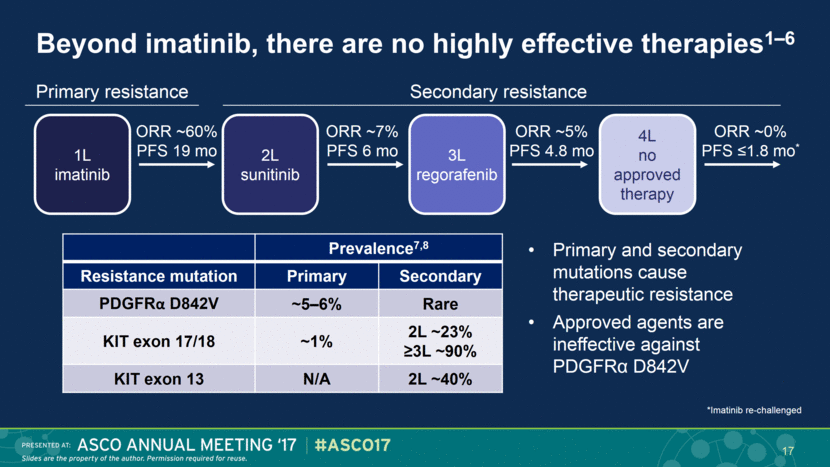
Beyond imatinib, there are no highly effective therapies1–6 Primary resistance Secondary resistance 3L regorafenib 1L imatinib 2L sunitinib ORR ~60% PFS 19 mo ORR ~7% PFS 6 mo 4L no approved therapy ORR ~5% PFS 4.8 mo ORR ~0% PFS ≤1.8 mo* 17 Prevalence7,8 Resistance mutation Primary Secondary PDGFRα D842V ~5–6% Rare KIT exon 17/18 ~1% 2L ~23% ≥3L ~90% KIT exon 13 N/A 2L ~40% Primary and secondary mutations cause therapeutic resistance Approved agents are ineffective against PDGFRα D842V *Imatinib re-challenged |
|
|
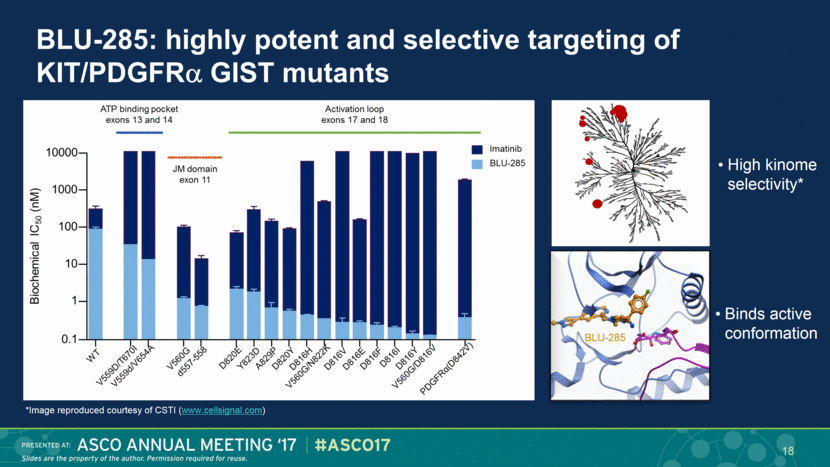
BLU-285: highly potent and selective targeting of KIT/PDGFR GIST mutants 18 High kinome selectivity* Binds active conformation BLU-285 *Image reproduced courtesy of CSTI (www.cellsignal.com) |
|
|
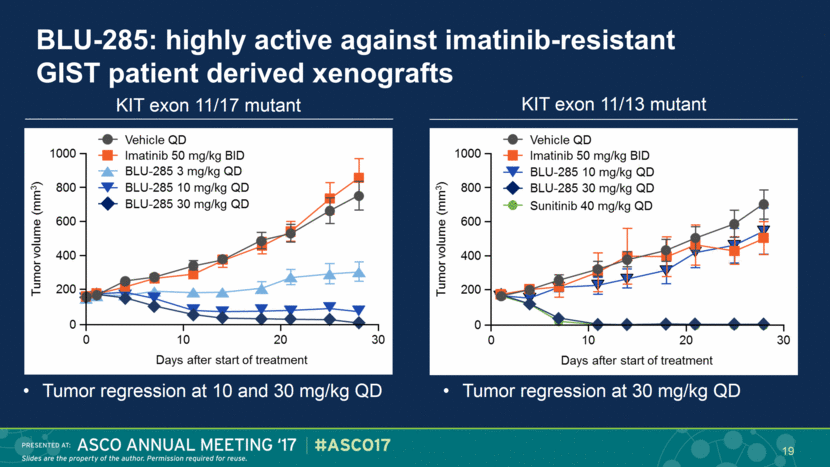
KIT exon 11/17 mutant KIT exon 11/13 mutant BLU-285: highly active against imatinib-resistant GIST patient derived xenografts Tumor regression at 10 and 30 mg/kg QD Tumor regression at 30 mg/kg QD 19 |
|
|
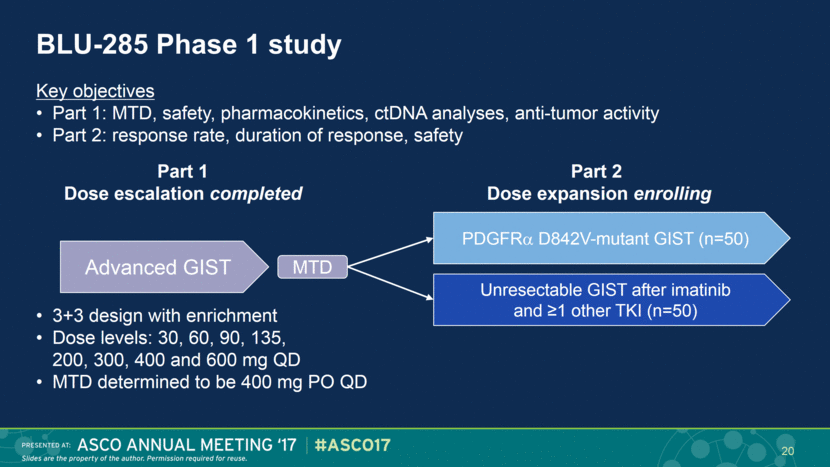
BLU-285 Phase 1 study Advanced GIST MTD Part 2 Dose expansion enrolling Unresectable GIST after imatinib and ≥1 other TKI (n=50) PDGFR D842V-mutant GIST (n=50) Part 1 Dose escalation completed Key objectives Part 1: MTD, safety, pharmacokinetics, ctDNA analyses, anti-tumor activity Part 2: response rate, duration of response, safety 3+3 design with enrichment Dose levels: 30, 60, 90, 135, 200, 300, 400 and 600 mg QD MTD determined to be 400 mg PO QD 20 |
|
|
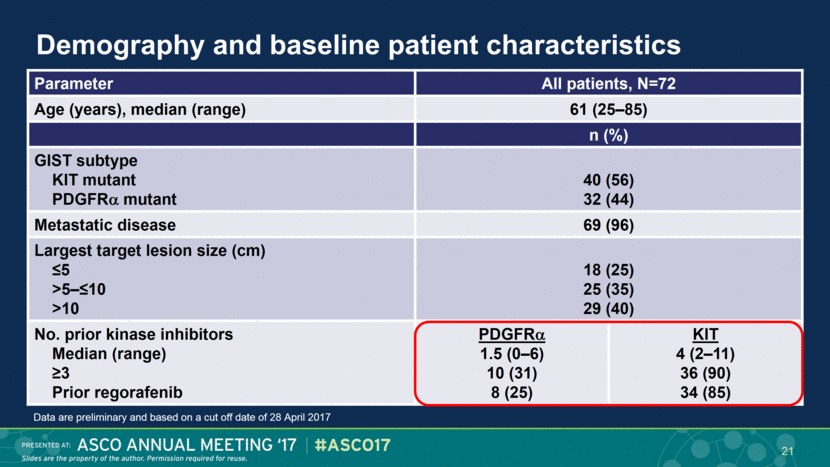
Demography and baseline patient characteristics Parameter All patients, N=72 Age (years), median (range) 61 (25–85) n (%) GIST subtype KIT mutant PDGFR mutant 40 (56) 32 (44) Metastatic disease 69 (96) Largest target lesion size (cm) ≤5 >5–≤10 >10 18 (25) 25 (35) 29 (40) No. prior kinase inhibitors Median (range) ≥3 Prior regorafenib PDGFR 1.5 (0–6) 10 (31) 8 (25) KIT 4 (2–11) 36 (90) 34 (85) Data are preliminary and based on a cut off date of 28 April 2017 21 |
|
|
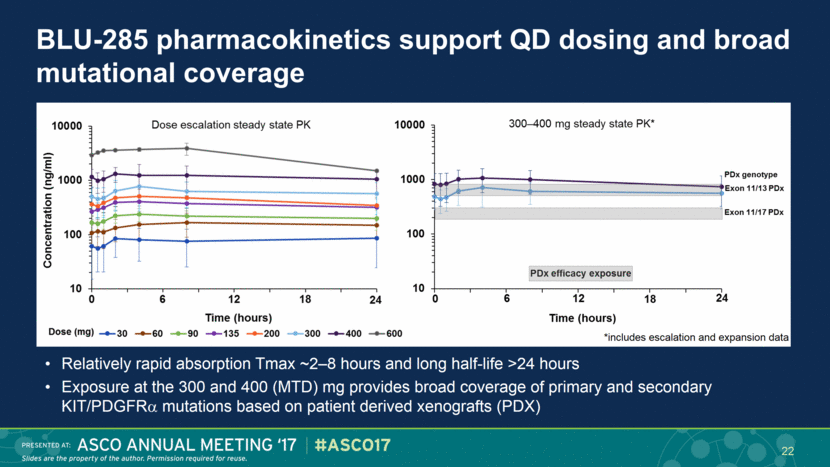
BLU-285 pharmacokinetics support QD dosing and broad mutational coverage Relatively rapid absorption Tmax ~2–8 hours and long half-life >24 hours Exposure at the 300 and 400 (MTD) mg provides broad coverage of primary and secondary KIT/PDGFR mutations based on patient derived xenografts (PDX) 22 *includes escalation and expansion data |
|
|
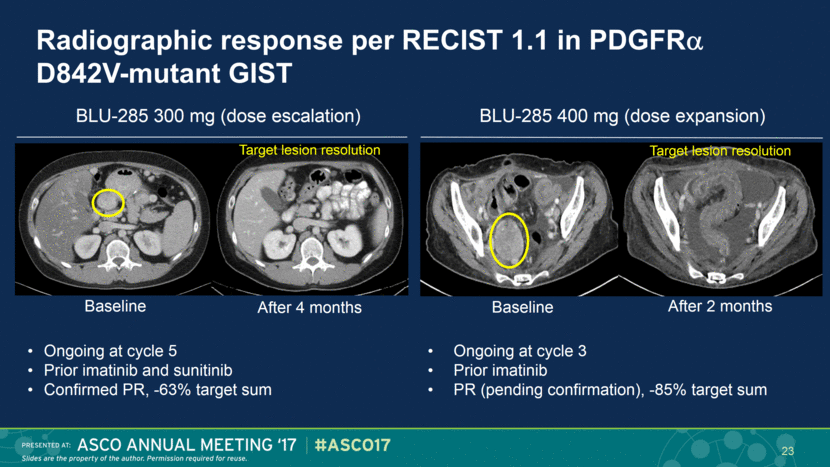
Radiographic response per RECIST 1.1 in PDGFR D842V-mutant GIST Ongoing at cycle 5 Prior imatinib and sunitinib Confirmed PR, -63% target sum Ongoing at cycle 3 Prior imatinib PR (pending confirmation), -85% target sum Target lesion resolution Baseline After 4 months BLU-285 300 mg (dose escalation) BLU-285 400 mg (dose expansion) 23 Baseline After 2 months Target lesion resolution |
|
|
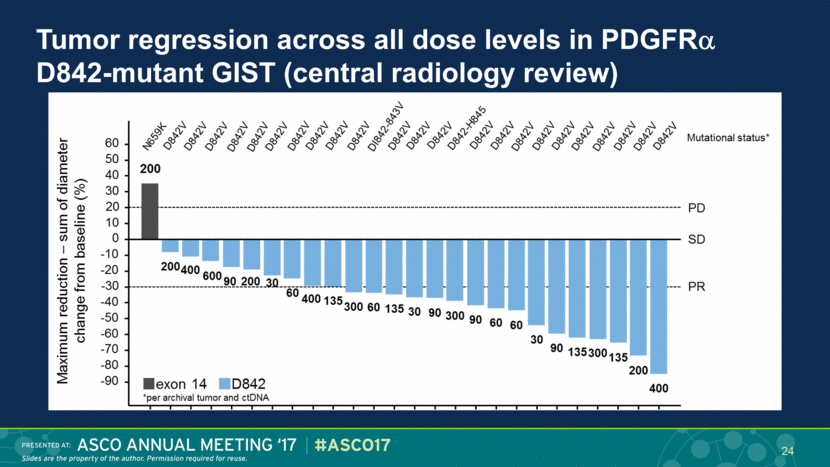
Tumor regression across all dose levels in PDGFR D842-mutant GIST (central radiology review) 24 |
|
|
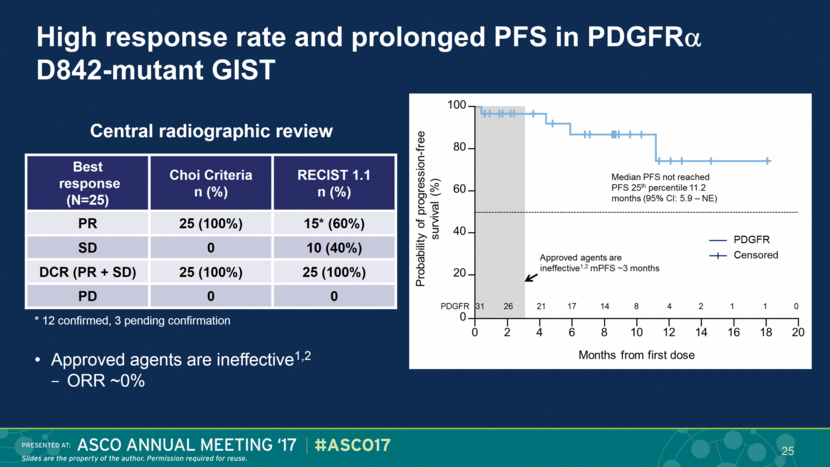
High response rate and prolonged PFS in PDGFR D842-mutant GIST Best response (N=25) Choi Criteria n (%) RECIST 1.1 n (%) PR 25 (100%) 15* (60%) SD 0 10 (40%) DCR (PR + SD) 25 (100%) 25 (100%) PD 0 0 Central radiographic review Approved agents are ineffective1,2 ORR ~0% * 12 confirmed, 3 pending confirmation 25 |
|
|
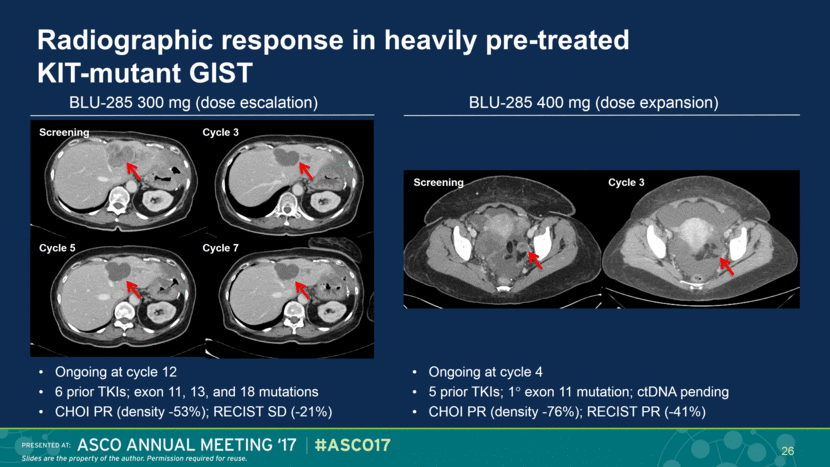
Radiographic response in heavily pre-treated KIT-mutant GIST Ongoing at cycle 12 6 prior TKIs; exon 11, 13, and 18 mutations CHOI PR (density -53%); RECIST SD (-21%) Screening Cycle 3 Cycle 5 Cycle 7 Ongoing at cycle 4 5 prior TKIs; 1 exon 11 mutation; ctDNA pending CHOI PR (density -76%); RECIST PR (-41%) BLU-285 300 mg (dose escalation) BLU-285 400 mg (dose expansion) 26 Screening Cycle 3 |
|
|
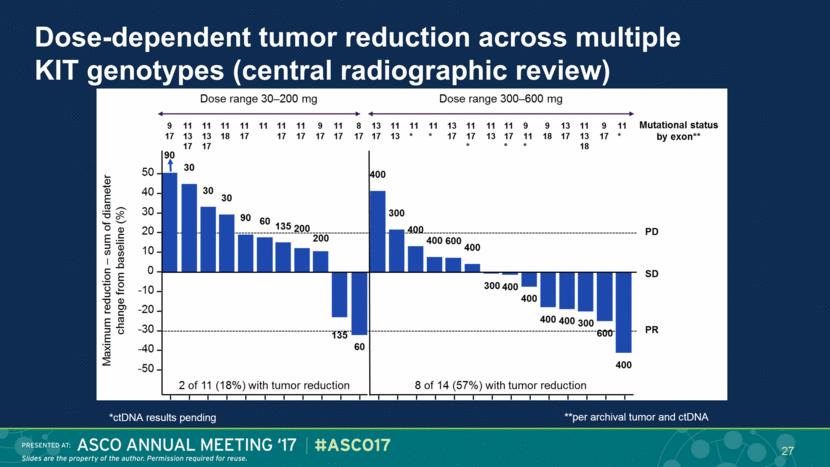
Dose-dependent tumor reduction across multiple KIT genotypes (central radiographic review) 27 *ctDNA results pending **per archival tumor and ctDNA |
|
|
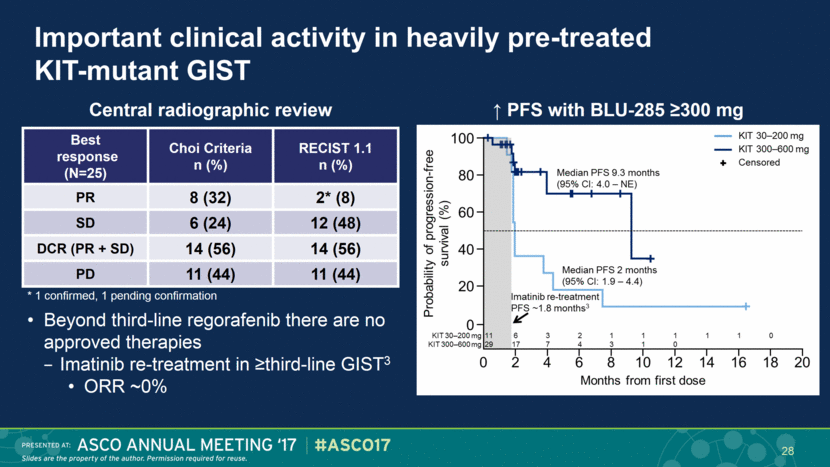
Important clinical activity in heavily pre-treated KIT-mutant GIST Beyond third-line regorafenib there are no approved therapies Imatinib re-treatment in ≥third-line GIST3 ORR ~0% Central radiographic review * 1 confirmed, 1 pending confirmation ↑ PFS with BLU-285 ≥300 mg Best response (N=25) Choi Criteria n (%) RECIST 1.1 n (%) PR 8 (32) 2* (8) SD 6 (24) 12 (48) DCR (PR + SD) 14 (56) 14 (56) PD 11 (44) 11 (44) 28 |
|
|
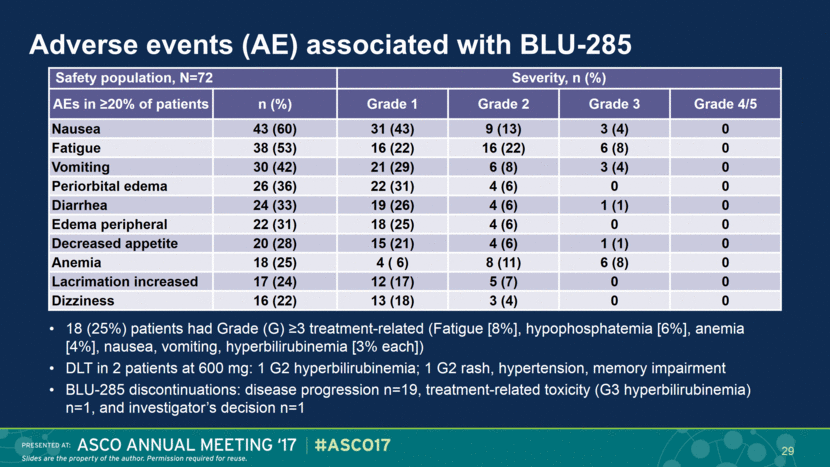
Adverse events (AE) associated with BLU-285 18 (25%) patients had Grade (G) ≥3 treatment-related (Fatigue [8%], hypophosphatemia [6%], anemia [4%], nausea, vomiting, hyperbilirubinemia [3% each]) DLT in 2 patients at 600 mg: 1 G2 hyperbilirubinemia; 1 G2 rash, hypertension, memory impairment BLU-285 discontinuations: disease progression n=19, treatment-related toxicity (G3 hyperbilirubinemia) n=1, and investigator’s decision n=1 Safety population, N=72 Severity, n (%) AEs in ≥20% of patients n (%) Grade 1 Grade 2 Grade 3 Grade 4/5 Nausea 43 (60) 31 (43) 9 (13) 3 (4) 0 Fatigue 38 (53) 16 (22) 16 (22) 6 (8) 0 Vomiting 30 (42) 21 (29) 6 (8) 3 (4) 0 Periorbital edema 26 (36) 22 (31) 4 (6) 0 0 Diarrhea 24 (33) 19 (26) 4 (6) 1 (1) 0 Edema peripheral 22 (31) 18 (25) 4 (6) 0 0 Decreased appetite 20 (28) 15 (21) 4 (6) 1 (1) 0 Anemia 18 (25) 4 ( 6) 8 (11) 6 (8) 0 Lacrimation increased 17 (24) 12 (17) 5 (7) 0 0 Dizziness 16 (22) 13 (18) 3 (4) 0 0 29 |
|
|
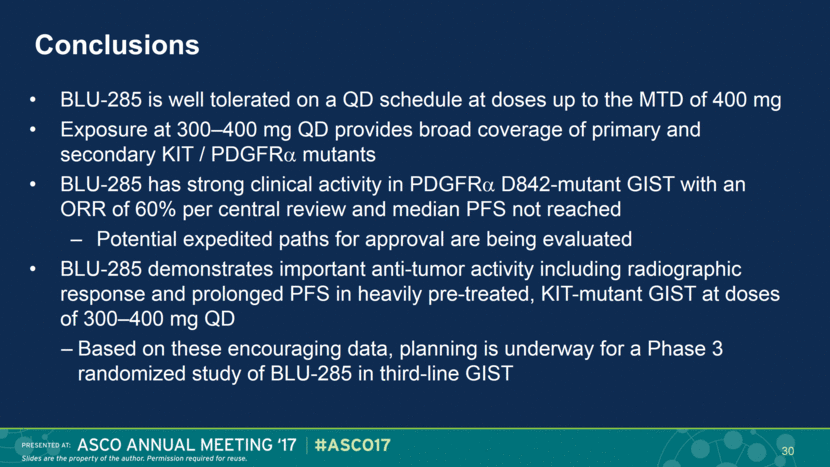
Conclusions BLU-285 is well tolerated on a QD schedule at doses up to the MTD of 400 mg Exposure at 300–400 mg QD provides broad coverage of primary and secondary KIT / PDGFR mutants BLU-285 has strong clinical activity in PDGFR D842-mutant GIST with an ORR of 60% per central review and median PFS not reached Potential expedited paths for approval are being evaluated BLU-285 demonstrates important anti-tumor activity including radiographic response and prolonged PFS in heavily pre-treated, KIT-mutant GIST at doses of 300–400 mg QD Based on these encouraging data, planning is underway for a Phase 3 randomized study of BLU-285 in third-line GIST 30 |
|
|
Acknowledgments We thank the participating patients, their families, all study co-investigators, and research coordinators at the following institutions: Oregon Health & Sciences University Royal Marsden Hospital/Institute for Cancer Research Leuven Cancer Institute University of Essen Fox Chase Cancer Center Erasmus MC Cancer Institute Centre Leon Berard Institut Gustave Roussy Dana-Farber Cancer Institute We also thank Sarah Jackson, PhD, of iMed Comms, an Ashfield company, who provided editorial writing support funded by Blueprint Medicines 31 |
|
|
References Cassier et al. Clin Cancer Res. 2012;18(16):4458–64 Yoo et al. Cancer Res Treat. 2016;48(2):546–52 Kang et al. Lancet Oncol. 2013;14(12):1175–82 National Comprehensive Cancer Network. Gastrointestinal Stromal Tumors. 2016 Demetri et al. Lancet. 2006;368:1329 Demetri et al. Lancet. 2013;381:295-302 Corless et al. J Clin Oncol. 2005;23:5357 Barnett and Heinrich. Am Soc Clin Onc Ed Book. 2012;663 32 |
|
|
Proposed registration path Andy Boral, M.D. Chief Medical Officer |
|
|
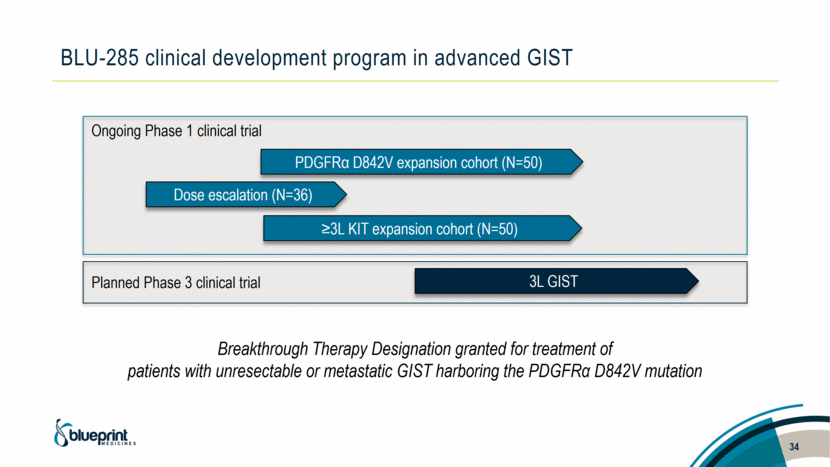
BLU-285 clinical development program in advanced GIST 34 Dose escalation (N=36) PDGFRα D842V expansion cohort (N=50) ≥3L KIT expansion cohort (N=50) Ongoing Phase 1 clinical trial 3L GIST Breakthrough Therapy Designation granted for treatment of patients with unresectable or metastatic GIST harboring the PDGFRα D842V mutation Planned Phase 3 clinical trial |
|
|
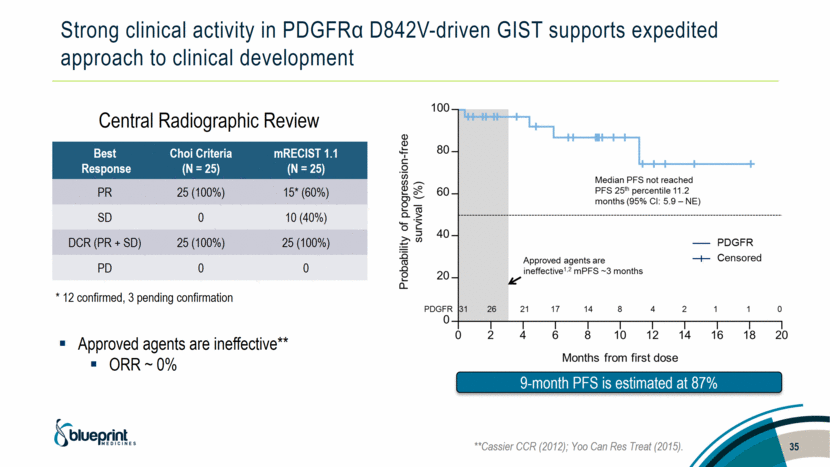
Strong clinical activity in PDGFRα D842V-driven GIST supports expedited approach to clinical development Best Response Choi Criteria (N = 25) mRECIST 1.1 (N = 25) PR 25 (100%) 15* (60%) SD 0 10 (40%) DCR (PR + SD) 25 (100%) 25 (100%) PD 0 0 Central Radiographic Review Approved agents are ineffective** ORR ~ 0% * 12 confirmed, 3 pending confirmation **Cassier CCR (2012); Yoo Can Res Treat (2015). 35 9-month PFS is estimated at 87% |
|
|
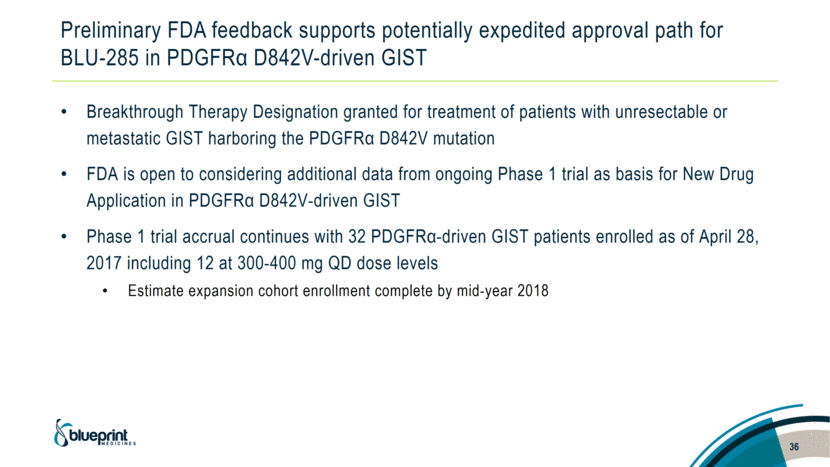
Preliminary FDA feedback supports potentially expedited approval path for BLU-285 in PDGFRα D842V-driven GIST Breakthrough Therapy Designation granted for treatment of patients with unresectable or metastatic GIST harboring the PDGFRα D842V mutation FDA is open to considering additional data from ongoing Phase 1 trial as basis for New Drug Application in PDGFRα D842V-driven GIST Phase 1 trial accrual continues with 32 PDGFRα-driven GIST patients enrolled as of April 28, 2017 including 12 at 300-400 mg QD dose levels Estimate expansion cohort enrollment complete by mid-year 2018 36 |
|
|
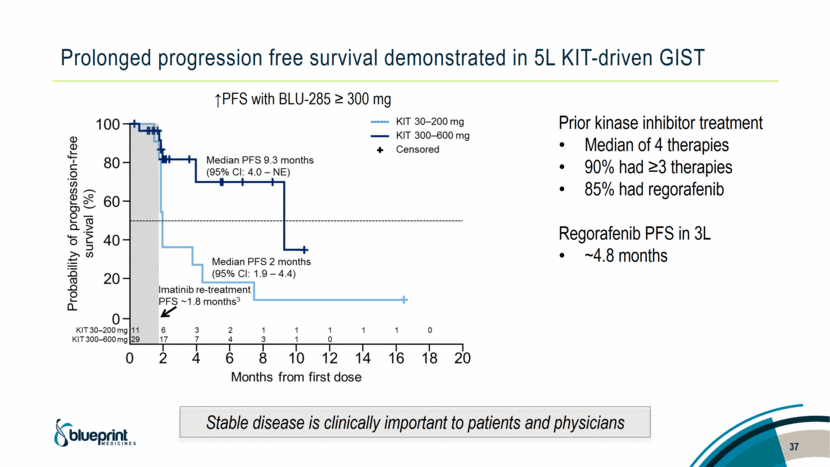
Prolonged progression free survival demonstrated in 5L KIT-driven GIST ↑PFS with BLU-285 ≥ 300 mg Prior kinase inhibitor treatment Median of 4 therapies 90% had ≥3 therapies 85% had regorafenib Regorafenib PFS in 3L ~4.8 months 37 Stable disease is clinically important to patients and physicians |
|
|
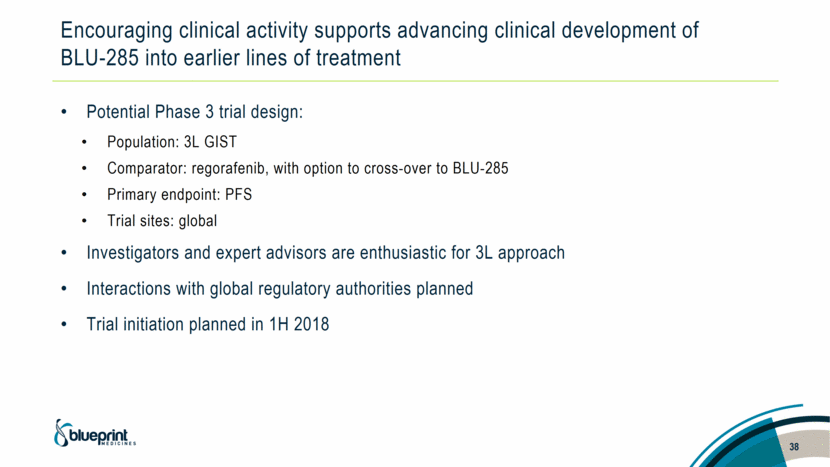
Encouraging clinical activity supports advancing clinical development of BLU-285 into earlier lines of treatment Potential Phase 3 trial design: Population: 3L GIST Comparator: regorafenib, with option to cross-over to BLU-285 Primary endpoint: PFS Trial sites: global Investigators and expert advisors are enthusiastic for 3L approach Interactions with global regulatory authorities planned Trial initiation planned in 1H 2018 38 |
|
|
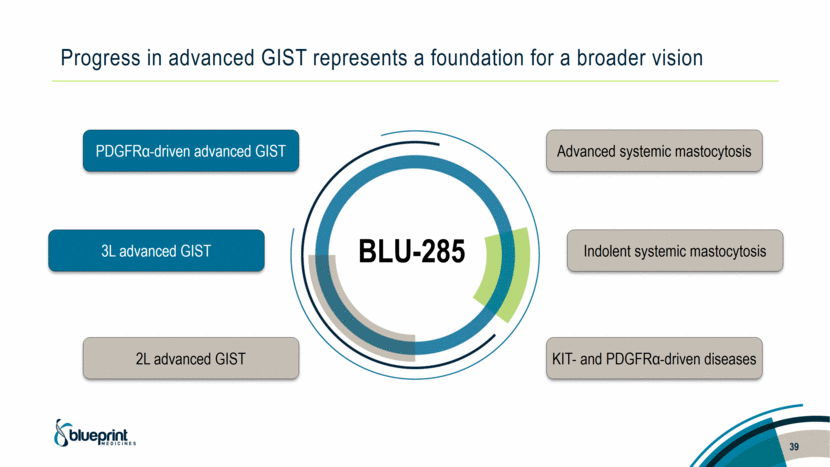
Progress in advanced GIST represents a foundation for a broader vision 39 BLU-285 PDGFRα-driven advanced GIST 3L advanced GIST 2L advanced GIST Advanced systemic mastocytosis Indolent systemic mastocytosis KIT- and PDGFRα-driven diseases |
|
|
Question & Answer Session |
|
|
Closing Remarks Jeff Albers Chief Executive Officer 41 |
|
|
Drug candidate Discovery Preclinical Clinical Commercial rights BLU-285 Inhibitor of KIT, including exon 17 mutations, and PDGFRα, including the D842V mutation BLU-554 Inhibitor of FGFR4 BLU-667 Inhibitor of RET fusions, mutations and resistant mutants PRKACA Inhibitor of PRKACA fusions Cancer immunotherapy Immunokinases Rare genetic disease Robust pipeline of diverse clinical stage assets PHASE 1 - PDGFR-DRIVEN GIST PHASE 1 - KIT-DRIVEN GIST PHASE 1 – SYSTEMIC MASTOCYTOSIS PHASE 1 – HEPATOCELLULAR CARCINOMA PHASE 1 – NSCLC & THYROID* FLC Up to 5 programs, STAGE UNDISCLOSED** Target and development stage undisclosed FLC, Fibrolamellar carcinoma; GIST, advanced gastrointestinal stromal tumors; NSCLC, non-small cell lung cancer. All Phase 1 clinical trials are in advanced disease. *Phase 1 trial includes a basket cohort that consists of other advanced solid tumors with RET alterations. **Blueprint Medicines has U.S. commercial rights for up to two programs. Roche has worldwide commercialization rights for up to three programs and ex-U.S. commercialization rights for up to two programs. 42 |
|
|
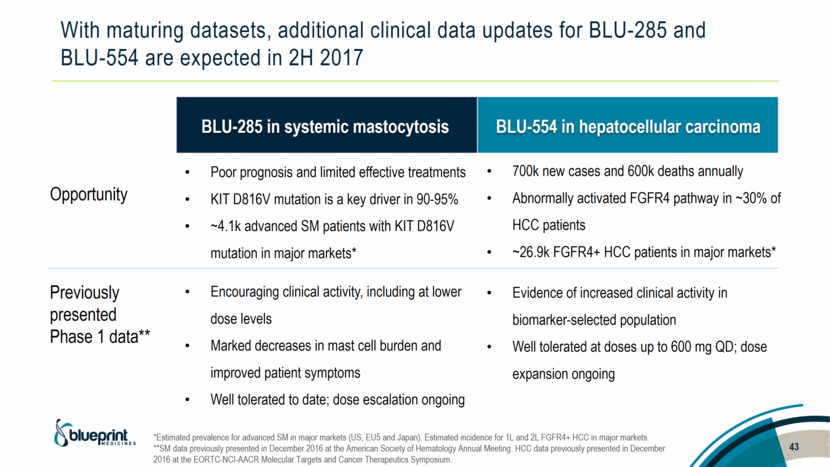
With maturing datasets, additional clinical data updates for BLU-285 and BLU-554 are expected in 2H 2017 Opportunity Previously presented Phase 1 data** BLU-285 in systemic mastocytosis BLU-554 in hepatocellular carcinoma Poor prognosis and limited effective treatments KIT D816V mutation is a key driver in 90-95% ~4.1k advanced SM patients with KIT D816V mutation in major markets* Encouraging clinical activity, including at lower dose levels Marked decreases in mast cell burden and improved patient symptoms Well tolerated to date; dose escalation ongoing 700k new cases and 600k deaths annually Abnormally activated FGFR4 pathway in ~30% of HCC patients ~26.9k FGFR4+ HCC patients in major markets* Evidence of increased clinical activity in biomarker-selected population Well tolerated at doses up to 600 mg QD; dose expansion ongoing 43 *Estimated prevalence for advanced SM in major markets (US, EU5 and Japan). Estimated incidence for 1L and 2L FGFR4+ HCC in major markets. **SM data previously presented in December 2016 at the American Society of Hematology Annual Meeting. HCC data previously presented in December 2016 at the EORTC-NCI-AACR Molecular Targets and Cancer Therapeutics Symposium. |
|
|
Thank you |