Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Blueprint Medicines Corp | ex-99d1.htm |
| EX-99.3 - EX-99.3 - Blueprint Medicines Corp | ex-99d3.htm |
| 8-K - 8-K - Blueprint Medicines Corp | f8-k.htm |
Exhibit 99.2
|
|
GIST: imatinib and beyond Clinical activity of BLU-285 in advanced gastrointestinal stromal tumor (GIST) Michael Heinrich1, Robin Jones2, Margaret von Mehren3, Patrick Schoffski4, Sebastian Bauer5, Olivier Mir6, Philippe Cassier7, Ferry Eskens8, Hongliang Shi9, Terri Alvarez-Diez9, Oleg Schmidt-Kittler9, Mary Ellen Healy9, Beni Wolf9, Suzanne George10 1Oregon Health & Sciences University, Oregon, USA; 2Royal Marsden Hospital/Institute of Cancer Research, London, UK; 3Fox Chase Cancer Center, Pennsylvania, USA; 4Leuven Cancer Institute, Leuven, Belgium; 5University of Essen, Essen, Germany; 6Institut Gustave Roussy, Paris, France; 7Centre Leon Berard, Lyon, France; 8 Erasmus MC Cancer Institute, Rotterdam, Netherlands; 9Blueprint Medicines Corporation, Massachusetts, USA; 10Dana-Farber Cancer Institute, Massachusetts, USA Abstract no: 11011 Presented by: Dr. Michael Heinrich |
|
|
Disclosures BLU-285 is an investigational agent currently in development by Blueprint Medicines Corporation (Blueprint Medicines) Dr. Michael Heinrich is an investigator for Blueprint Medicines’ ongoing Phase 1 studies in unresectable gastrointestinal stromal tumor Dr. Michael Heinrich has the following disclosures: Consultant: Blueprint Medicines, Novartis, MolecularMD Equity interest: MolecularMD Research funding: Blueprint Medicines, Deciphera, Ariad Expert testimony: Novartis Patents: four patents on diagnosis and treatment of PDGFR-mutant GIST 2 |
|
|
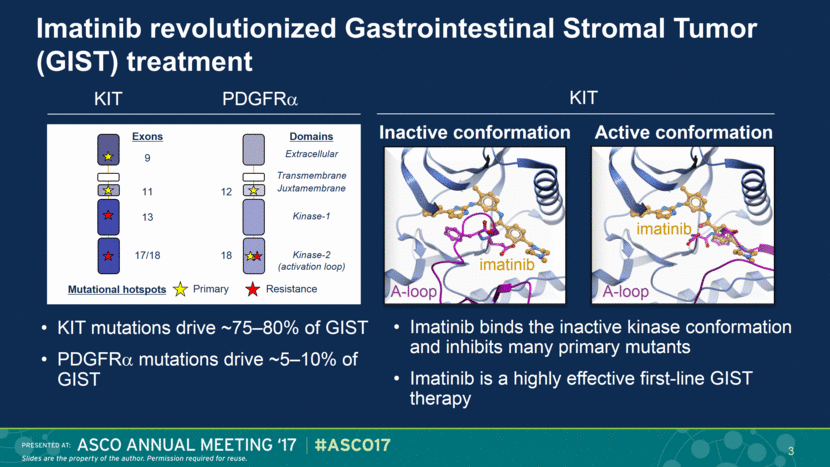
Imatinib revolutionized Gastrointestinal Stromal Tumor (GIST) treatment Imatinib binds the inactive kinase conformation and inhibits many primary mutants Imatinib is a highly effective first-line GIST therapy A-loop imatinib A-loop imatinib Inactive conformation Active conformation KIT KIT mutations drive ~75–80% of GIST PDGFR mutations drive ~5–10% of GIST KIT PDGFR Extracellular Juxtamembrane Kinase-1 Kinase-2 (activation loop) Transmembrane Exons 12 18 9 11 17/18 13 Domains Primary Resistance Mutational hotspots 3 |
|
|
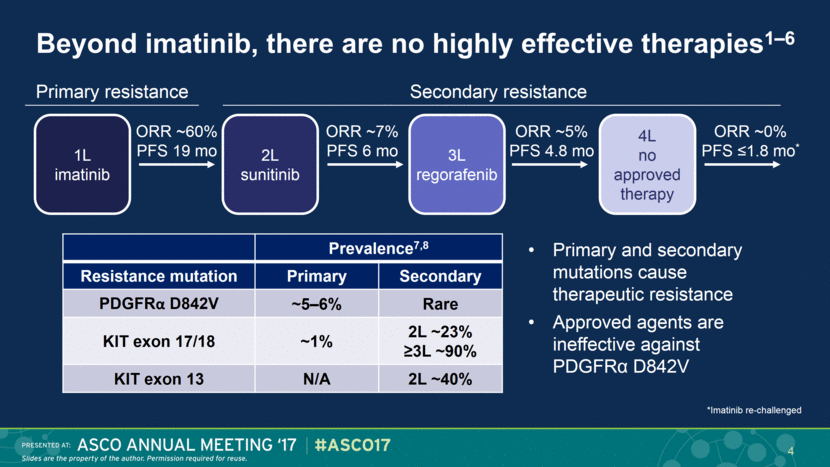
Beyond imatinib, there are no highly effective therapies1–6 Primary resistance Secondary resistance 3L regorafenib 1L imatinib 2L sunitinib ORR ~60% PFS 19 mo ORR ~7% PFS 6 mo 4L no approved therapy ORR ~5% PFS 4.8 mo ORR ~0% PFS ≤1.8 mo* 4 Prevalence7,8 Resistance mutation Primary Secondary PDGFRα D842V ~5–6% Rare KIT exon 17/18 ~1% 2L ~23% ≥3L ~90% KIT exon 13 N/A 2L ~40% Primary and secondary mutations cause therapeutic resistance Approved agents are ineffective against PDGFRα D842V *Imatinib re-challenged |
|
|
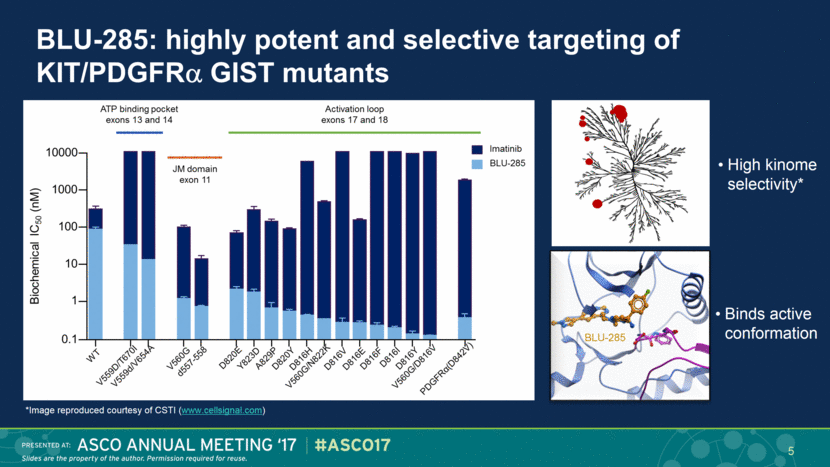
BLU-285: highly potent and selective targeting of KIT/PDGFR GIST mutants 5 High kinome selectivity* Binds active conformation BLU-285 *Image reproduced courtesy of CSTI (www.cellsignal.com) |
|
|
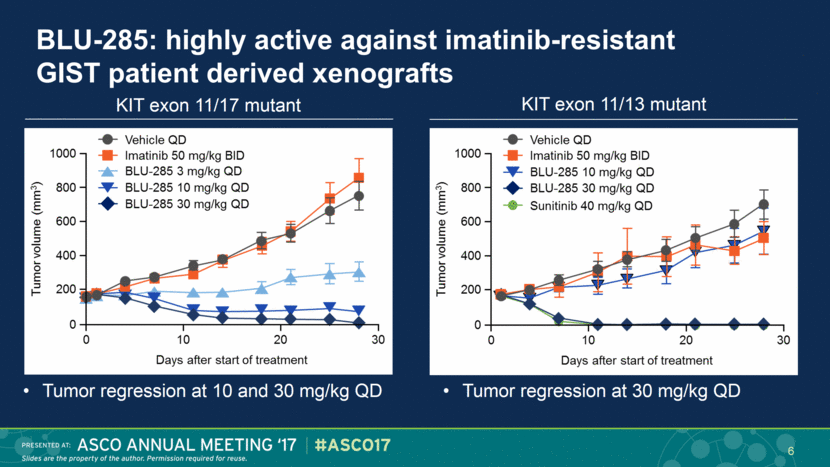
KIT exon 11/17 mutant KIT exon 11/13 mutant BLU-285: highly active against imatinib-resistant GIST patient derived xenografts Tumor regression at 10 and 30 mg/kg QD Tumor regression at 30 mg/kg QD 6 |
|
|
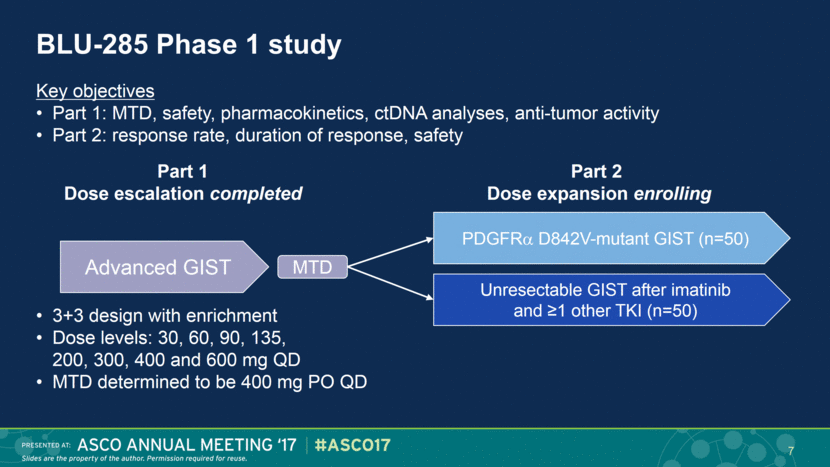
BLU-285 Phase 1 study Advanced GIST MTD Part 2 Dose expansion enrolling Unresectable GIST after imatinib and ≥1 other TKI (n=50) PDGFR D842V-mutant GIST (n=50) Part 1 Dose escalation completed Key objectives Part 1: MTD, safety, pharmacokinetics, ctDNA analyses, anti-tumor activity Part 2: response rate, duration of response, safety 3+3 design with enrichment Dose levels: 30, 60, 90, 135, 200, 300, 400 and 600 mg QD MTD determined to be 400 mg PO QD 7 |
|
|
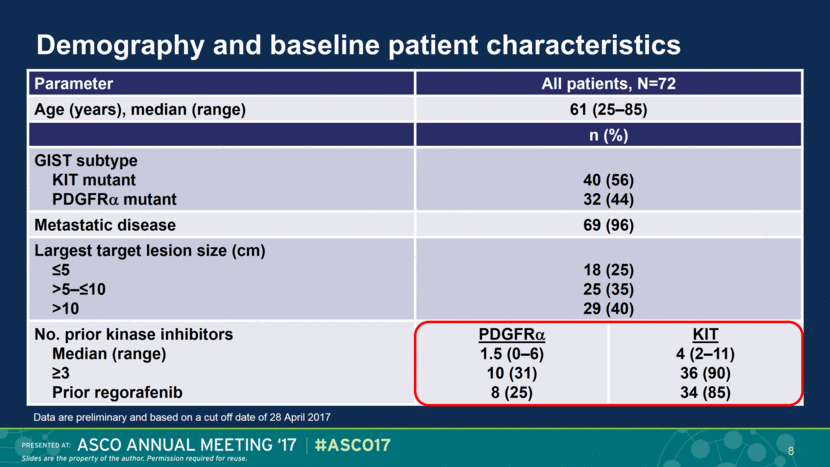
Demography and baseline patient characteristics Parameter All patients, N=72 Age (years), median (range) 61 (25–85) n (%) GIST subtype KIT mutant PDGFR mutant 40 (56) 32 (44) Metastatic disease 69 (96) Largest target lesion size (cm) ≤5 >5–≤10 >10 18 (25) 25 (35) 29 (40) No. prior kinase inhibitors Median (range) ≥3 Prior regorafenib PDGFR 1.5 (0–6) 10 (31) 8 (25) KIT 4 (2–11) 36 (90) 34 (85) Data are preliminary and based on a cut off date of 28 April 2017 8 |
|
|
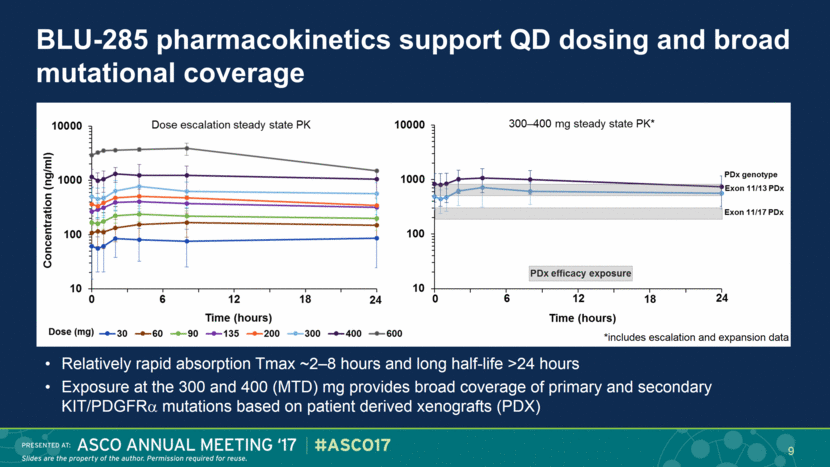
BLU-285 pharmacokinetics support QD dosing and broad mutational coverage Relatively rapid absorption Tmax ~2–8 hours and long half-life >24 hours Exposure at the 300 and 400 (MTD) mg provides broad coverage of primary and secondary KIT/PDGFR mutations based on patient derived xenografts (PDX) 9 *includes escalation and expansion data |
|
|
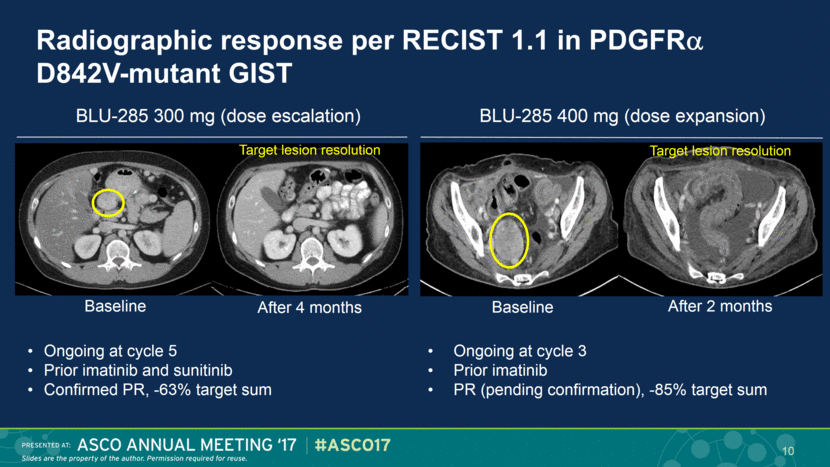
Radiographic response per RECIST 1.1 in PDGFR D842V-mutant GIST Ongoing at cycle 5 Prior imatinib and sunitinib Confirmed PR, -63% target sum Ongoing at cycle 3 Prior imatinib PR (pending confirmation), -85% target sum Target lesion resolution Baseline After 4 months BLU-285 300 mg (dose escalation) BLU-285 400 mg (dose expansion) 10 Baseline After 2 months Target lesion resolution |
|
|
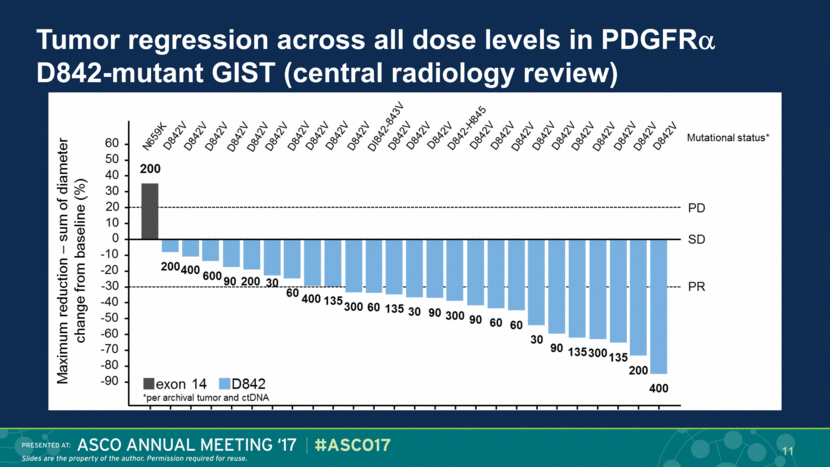
Tumor regression across all dose levels in PDGFR D842-mutant GIST (central radiology review) 11 |
|
|
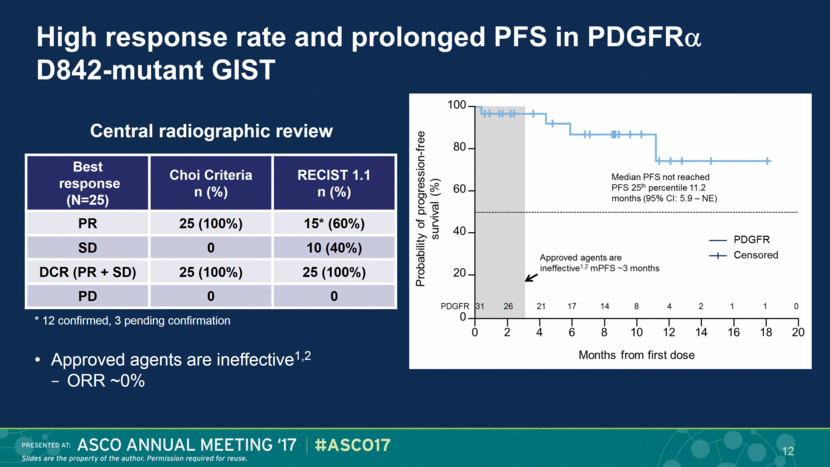
High response rate and prolonged PFS in PDGFR D842-mutant GIST Best response (N=25) Choi Criteria n (%) RECIST 1.1 n (%) PR 25 (100%) 15* (60%) SD 0 10 (40%) DCR (PR + SD) 25 (100%) 25 (100%) PD 0 0 Central radiographic review Approved agents are ineffective1,2 ORR ~0% * 12 confirmed, 3 pending confirmation 12 |
|
|
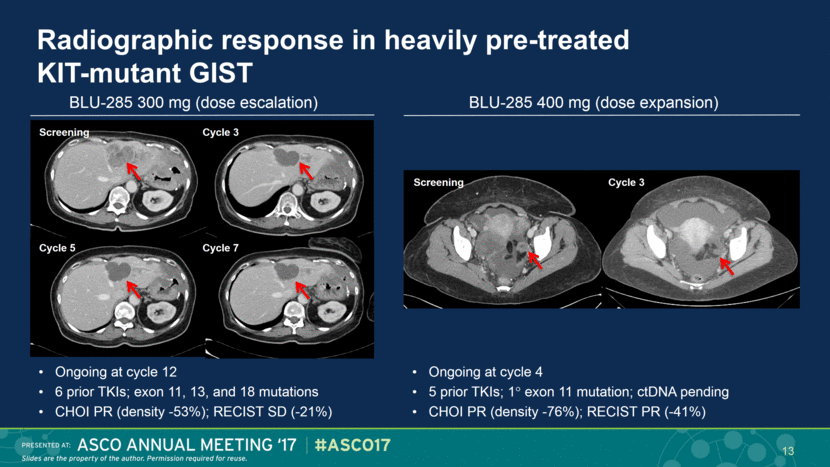
Radiographic response in heavily pre-treated KIT-mutant GIST Ongoing at cycle 12 6 prior TKIs; exon 11, 13, and 18 mutations CHOI PR (density -53%); RECIST SD (-21%) Screening Cycle 3 Cycle 5 Cycle 7 Ongoing at cycle 4 5 prior TKIs; 1 exon 11 mutation; ctDNA pending CHOI PR (density -76%); RECIST PR (-41%) BLU-285 300 mg (dose escalation) BLU-285 400 mg (dose expansion) 13 Screening Cycle 3 |
|
|
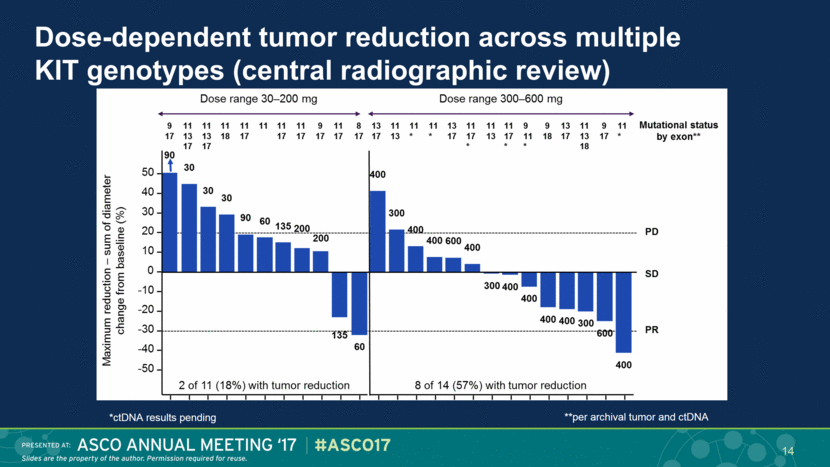
Dose-dependent tumor reduction across multiple KIT genotypes (central radiographic review) 14 *ctDNA results pending **per archival tumor and ctDNA |
|
|
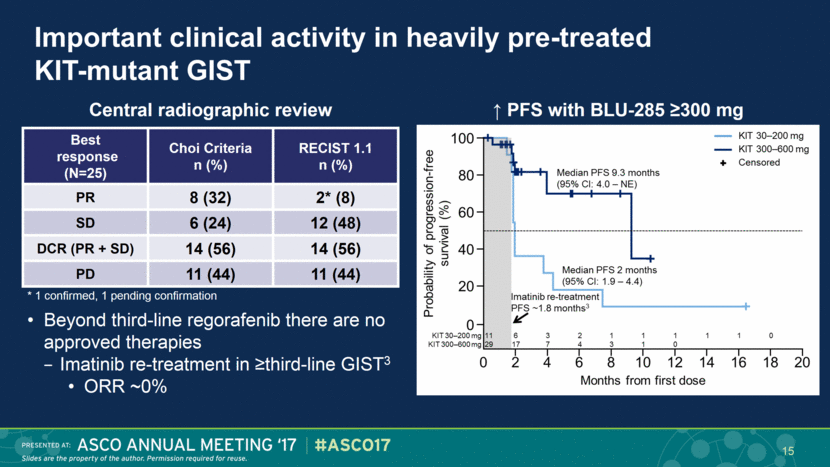
Important clinical activity in heavily pre-treated KIT-mutant GIST Beyond third-line regorafenib there are no approved therapies Imatinib re-treatment in ≥third-line GIST3 ORR ~0% Central radiographic review * 1 confirmed, 1 pending confirmation ↑ PFS with BLU-285 ≥300 mg Best response (N=25) Choi Criteria n (%) RECIST 1.1 n (%) PR 8 (32) 2* (8) SD 6 (24) 12 (48) DCR (PR + SD) 14 (56) 14 (56) PD 11 (44) 11 (44) 15 |
|
|
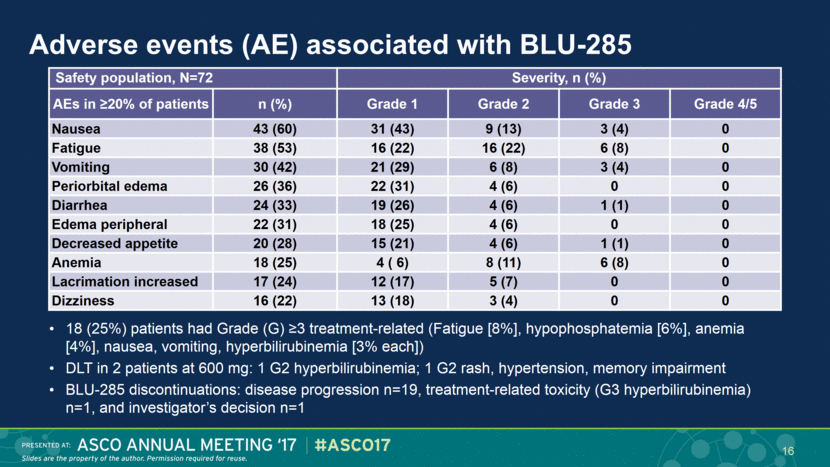
Adverse events (AE) associated with BLU-285 18 (25%) patients had Grade (G) ≥3 treatment-related (Fatigue [8%], hypophosphatemia [6%], anemia [4%], nausea, vomiting, hyperbilirubinemia [3% each]) DLT in 2 patients at 600 mg: 1 G2 hyperbilirubinemia; 1 G2 rash, hypertension, memory impairment BLU-285 discontinuations: disease progression n=19, treatment-related toxicity (G3 hyperbilirubinemia) n=1, and investigator’s decision n=1 Safety population, N=72 Severity, n (%) AEs in ≥20% of patients n (%) Grade 1 Grade 2 Grade 3 Grade 4/5 Nausea 43 (60) 31 (43) 9 (13) 3 (4) 0 Fatigue 38 (53) 16 (22) 16 (22) 6 (8) 0 Vomiting 30 (42) 21 (29) 6 (8) 3 (4) 0 Periorbital edema 26 (36) 22 (31) 4 (6) 0 0 Diarrhea 24 (33) 19 (26) 4 (6) 1 (1) 0 Edema peripheral 22 (31) 18 (25) 4 (6) 0 0 Decreased appetite 20 (28) 15 (21) 4 (6) 1 (1) 0 Anemia 18 (25) 4 ( 6) 8 (11) 6 (8) 0 Lacrimation increased 17 (24) 12 (17) 5 (7) 0 0 Dizziness 16 (22) 13 (18) 3 (4) 0 0 16 |
|
|
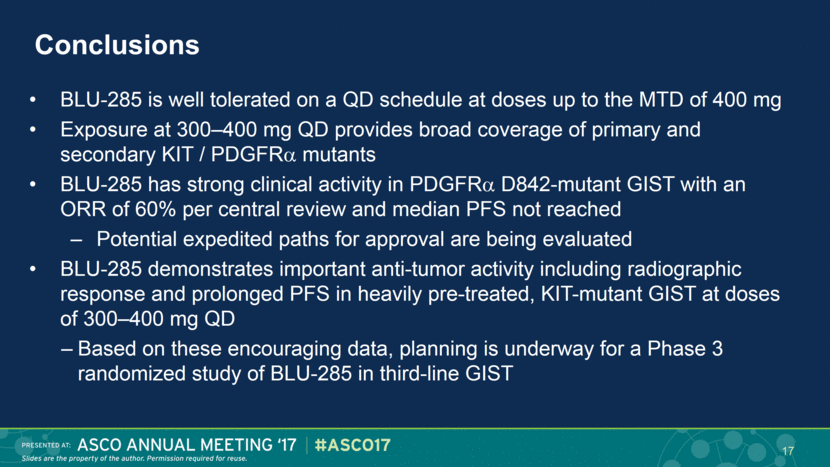
Conclusions BLU-285 is well tolerated on a QD schedule at doses up to the MTD of 400 mg Exposure at 300–400 mg QD provides broad coverage of primary and secondary KIT / PDGFR mutants BLU-285 has strong clinical activity in PDGFR D842-mutant GIST with an ORR of 60% per central review and median PFS not reached Potential expedited paths for approval are being evaluated BLU-285 demonstrates important anti-tumor activity including radiographic response and prolonged PFS in heavily pre-treated, KIT-mutant GIST at doses of 300–400 mg QD Based on these encouraging data, planning is underway for a Phase 3 randomized study of BLU-285 in third-line GIST 17 |
|
|
Acknowledgments We thank the participating patients, their families, all study co-investigators, and research coordinators at the following institutions: Oregon Health & Sciences University Royal Marsden Hospital/Institute for Cancer Research Leuven Cancer Institute University of Essen Fox Chase Cancer Center Erasmus MC Cancer Institute Centre Leon Berard Institut Gustave Roussy Dana-Farber Cancer Institute We also thank Sarah Jackson, PhD, of iMed Comms, an Ashfield company, who provided editorial writing support funded by Blueprint Medicines 18 |
|
|
References Cassier et al. Clin Cancer Res. 2012;18(16):4458–64 Yoo et al. Cancer Res Treat. 2016;48(2):546–52 Kang et al. Lancet Oncol. 2013;14(12):1175–82 National Comprehensive Cancer Network. Gastrointestinal Stromal Tumors. 2016 Demetri et al. Lancet. 2006;368:1329 Demetri et al. Lancet. 2013;381:295-302 Corless et al. J Clin Oncol. 2005;23:5357 Barnett and Heinrich. Am Soc Clin Onc Ed Book. 2012;663 19 |