Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Corvus Pharmaceuticals, Inc. | exh_991.htm |
| 8-K - FORM 8-K - Corvus Pharmaceuticals, Inc. | f8k_060517.htm |
Exhibit 99.2

Abstract #: 3004 Safety and clinical activity of adenosine A2a receptor (A2aR) antagonist, CPI - 444, in anti - PD1/PDL1 treatment - refractory renal cell (RCC) and non - small cell lung cancer (NSCLC) patients Presenter: Lawrence Fong, M.D . Leader, Cancer Immunotherapy Program Co - Director , Parker Institute of Cancer Immunotherapy @ UCSF University of California, San Francisco

This presentation contains forward - looking statements, including statements related to the potential safety and efficacy of CPI - 444, both as a single agent and in combination with anti - PD - 1 and anti - PD - L1, and the Company’s ability to develop and advance product candidates int o and successfully complete clinical trials, including the Company’s Phase 1/1b clinical trial of CPI - 444. All statements other than statements of historical fact contained in this press release are forward - looking statements. These statements often include words such as “believe,” “expect,” “anticipate ,” “intend,” “plan,” “estimate,” “seek,” “will,” “may” or similar expressions. Forward - looking statements are subject to a number of risks and uncert ainties, many of which involve factors or circumstances that are beyond the Company’s control. The Company’s actual results could differ materially fro m those stated or implied in forward - looking statements due to a number of factors, including but not limited to, risks detailed in the Company’s Quarterly Report on Form 10 - Q for the three months ended March 31, 2017, filed with the Securities and Exchange Commission on May 4, 2017, as well a s other documents that may be filed by the Company from time to time with the Securities and Exchange Commission. In particular, the fol lowing factors, among others, could cause results to differ materially from those expressed or implied by such forward - looking statements: the C ompany’s ability to demonstrate evidence of efficacy and safety for CPI - 444 during its Phase 1/ 1b clinical trial; the results of early clinical trials may not be predictive of future results; the unpredictability of the regulatory process; and regulatory developments in the United States and foreign cou ntries. Although the Company believes that the expectations reflected in the forward - looking statements are reasonable, it cannot guarantee that the events and circumstances reflected in the forward - looking statements will be achieved or occur, and the timing of events and circumstances and actual results could differ materially from those projected in the forward - looking statements. Accordingly, you should not place undue reliance on these forward - looking statements. All such statements speak only as of the date made, and the Company undertakes no obligation to update or re vise publicly any forward - looking statements, whether as a result of new information, future events or otherwise. Additional information may be a vailable in press releases or other public announcements and public filings made after the date of this presentation. This presentation concerns products that have not yet been approved for marketing by the U.S. Food and Drug Administration (“ FDA ”). No representation is made as to their safety or effectiveness for the purposes of which they are being investigated. 2 Forward Looking Statements Forward Looking Statements

Safety and clinical activity of adenosine A2a receptor (A2aR) antagonist, CPI - 444 in anti - PD(L)1 treatment - refractory renal cell (RCC) and non - small cell lung cancer (NSCLC) patients Lawrence Fong, Patrick Forde , John Powderly II, Jonathan Goldman , John Nemunaitis , Jason Luke , Matthew Hellmann , Shivaani Kummar, Robert Doebele, Daruka Mahadevan, Shirish Gadgeel , Brett Hughes , Ben Markman, Matthew Riese, Joshua Brody , Leisha Emens, Ian McCaffery, Richard Miller and Ginna Laport University of California, San Francisco, San Francisco, CA; Johns Hopkins Kimmel Cancer Center and Bloomberg - Kimmel Institute for Cancer Immunotherapy, Baltimore, MD; Carolina BioOncology Institute, Huntersville, NC; David Geffen School of Medicine at UCL A, Los Angeles, CA; Mary Crowley Cancer Research Centers, Dallas, TX; University of Chicago, Chicago, IL; Memorial Sloan Kettering Cancer Center, New York, NY; Stanford University School of Medicine, Stanford, CA; University of Colorado Anschutz Medical Campus, A uro ra, CO; The University of Arizona, Phoenix, AZ; Karmanos Cancer Institute/Wayne State University, Detroit, MI; Royal Brisbane Hospital, Chapel Hill, Australia; Monash Health and Monash University, Melbourne, Australia; Med Coll of Wisconsin, Milwaukee, WI; Icahn School of Medicine at Mount Sinai, New York, NY; The Johns Hopkins University, Baltimore, MD; Corvus Pharmaceuticals, Burlingame, CA Ph I CPI - 444 Trial 2 2

Background • Anti - PD - (L)1 antibodies are approved for treatment of RCC and NSCLC but a small proportion of patients benefit. • No approved agents overcome resistance to anti - PD - (L)1 with few reporting benefit in PD - 1 resistant/ refractory setting. • Converting tumors devoid of T cell infiltration (“cold tumors”) into T cell inflamed tumors (“hot tumors”) could improve response to immunotherapies. • Adenosine is a mediator of immunosuppression within the tumor microenvironment. • CPI - 444 is an oral small molecule antagonist of the adenosine A2A receptor ( A2AR) ( Emens , AACR 2017). Ph I CPI - 444 Trial 4 4 (Sharma et al. Cell, 2017) 2

Adenosine Suppresses Immunity and is a Potential Mechanism of Resistance to PD - (L)1 Therapy CD73 expression in baseline tumor biopsies from the CPI - 444 phase 1 trial Adenosine in the tumor microenvironment Tumors can generate adenosine in response to anti - PD - (L)1 (Beavis et al, Can Immunol Res 2015) Adenosine A2AR ATP AMP CD73 Tumor T Cell CD39 T Cell PD1 PDL - 1 Anti - PD - (L)1 N=17 10 CD73 Expression (mRNA) p=0.001 Anti - PD - 1 Naive 100 1,000 10,000 Anti - PD - 1 Resist/Refract N=19 Ph I CPI - 444 Trial 5 5 T cell inhibition 3
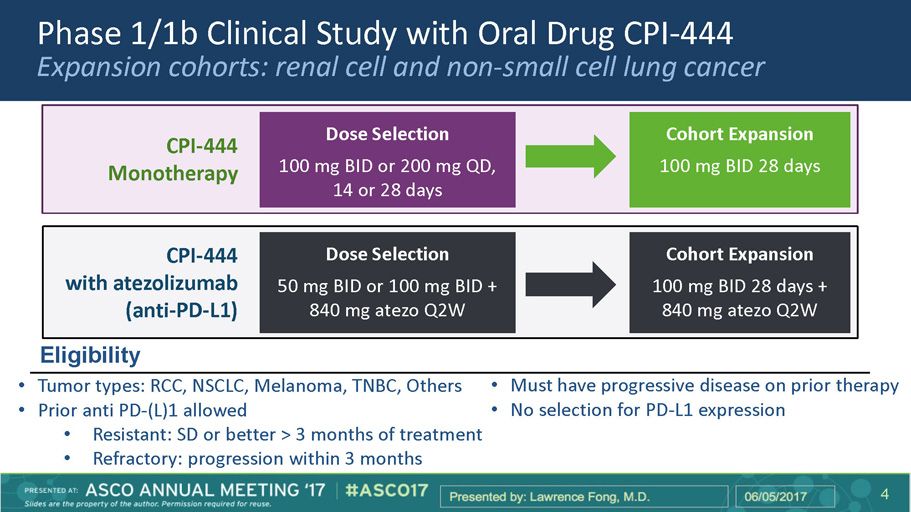
Phase 1/1b Clinical Study with Oral Drug CPI - 444 Expansion cohorts: renal cell and non - small cell lung cancer CPI - 444 Monotherapy Dose Selection 100 mg BID or 200 mg QD, 14 or 28 days Cohort Expansion 100 mg BID 28 days CPI - 444 with atezolizumab (anti - PD - L1) Dose Selection 50 mg BID or 100 mg BID + 840 mg atezo Q2W Cohort Expansion 100 mg BID 28 days + 840 mg atezo Q2W • Tumor types: RCC, NSCLC, Melanoma, TNBC, Others • Prior anti PD - (L)1 allowed • Resistant: SD or better > 3 months of treatment • Refractory: progression within 3 months • Must have progressive disease on prior therapy • No selection for PD - L1 expression Eligibility 6 6 4

CPI - 444 Blocks A2A Receptor Signaling 50mg BID 100mg BID 200mg QD pCREB cAMP A2AR Adenosine CPI - 444 CD4+ T cell Ph I CPI - 444 Trial 0 5000 10000 15000 0 50 100 150 CPI-444 (ng/mL) A 2 A R P a t h w a y A c t i v i t y ( % R e l a t i v e t o B a s e l i n e ) Analysis of pCREB by flow cytometr y Adenosine (NECA) stimulation ex vivo 7 7 CPI - 444 Plasma CPI - 444 (ng/ml) 5

Patient Characteristics Non - Small Cell Lung Cancer (N=45) Renal Cell Cancer (N=30) Prior anti - PD - (L)1 exposure Naïve Resistant/Refractory 8 (18%) 37 (82%) 8 (27%) 22 (73%) PD - L1 Negative* 54% 95% Median time since IO agent , months (range) 2.8 (0.6 – 24) 1.7 (1 – 71) Histology 28 (62%) Non squamous 17 (38%) Squamous 28 (93%) Clear cell 2 (7%) Papillary Median age, years (range) No. of patients single agent / No. of patients combination Median number prior therapies 70 (41 - 85) 22/23 2 (1 - 5) 65 (44 - 76) 14/16 3 (1 - 5) *Archive samples d ata available on 19 RCC and 28 NSCLC patients based on FDA - approved test 8 8 6

Treatment - Related Adverse Events Grade > 3 AEs: • Single a gent: none • Combination CPI - 444 + atezolizumab • One patient with Gr 3 immune related hepatitis, pneumonitis, mucositis and dermatitis Adverse Events (Gr1/2) > 5% Frequency (n=75) Single Agent (%) Combination (%) Fatigue 11 15 Nausea 6 8 Pruritus 8 5 Constipation 6 --- Dizziness 6 --- Hypertension 6 --- Pyrexia 6 --- Rash --- 5 AST increased --- 5 ALT increased --- 5 9 9 7
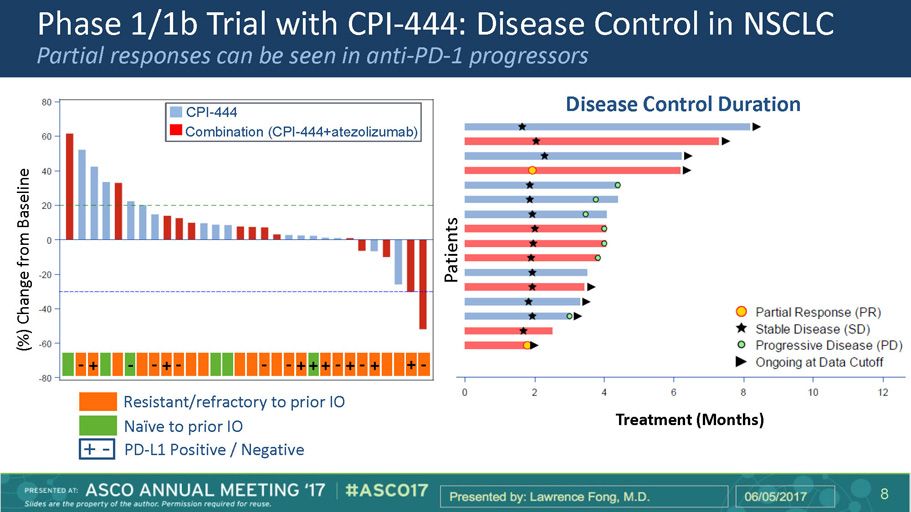
Phase 1/1b Trial with CPI - 444: Disease Control in NSCLC Partial responses can be seen in anti - PD - 1 progressors 10 (%) Change from Baseline Patients CPI - 444 Combination (CPI - 444+atezolizumab) Disease Control Duration Treatment (Months) Resistant/refractory to prior IO Naïve to prior IO PD - L1 Positive / Negative - + - + - - + - - - + + + - + - + + - 8

Phase 1/1b Trial with CPI - 444: Disease Control in Renal Cell Cancer Partial responses can be seen in an anti - PD - 1 progressing and naïve patients Resistant/refractory to prior IO Naïve to prior IO Treatment (Months) - - - - - - - - - - - - - - PD - L1 Positive / Negative + - CPI - 444 Combination (CPI - 444+atezolizumab) (%) Change from Baseline Patients Disease Control Duration 9

Tumor Growth Kinetics in “ Stable ” RCC Patients Days 12 12 -100 100 200 300 -80 -60 -40 -20 20 101001 SLD R e l a t i v e T a r g e t L e s i o n ( s ) S i z e Days -400 -200 200 400 -30 -20 -10 10 20 103101 SLD R e l a t i v e T a r g e t L e s i o n ( s ) S i z e Days -600 -400 -200 200 -30 -20 -10 10 20 100410 SLD R e l a t i v e T a r g e t L e s i o n ( s ) S i z e Days -100 100 200 -30 -20 -10 10 20 103210 SLD R e l a t i v e T a r g e t L e s i o n ( s ) S i z e Days -100 100 200 300 400 -30 -20 -10 10 20 101501 SLD R e l a t i v e T a r g e t L e s i o n ( s ) S i z e Days CPI - 444 Single Agent CPI - 444 in Combination with Atezolizumab Anti - PD - 1 Naïve Nivolumab Refractory Pembrolizumab Resistant Nivolumab Refractory Anti - PD - 1 Naïve Anti - PD - 1 Naïve Prior to CPI - 444 On CPI - 444 Percent Change in Target Lesion(s) -150 -100 -50 50 100 150 -40 -20 20 10
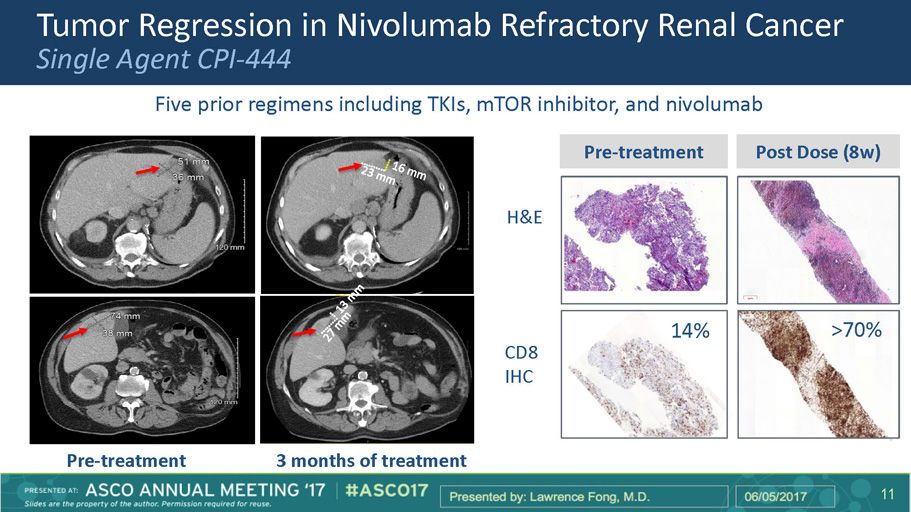
Tumor Regression in Nivolumab Refractory Renal Cancer Single Agent CPI - 444 Pre - treatment 3 months of treatment Five prior regimens including TKIs, mTOR inhibitor, and nivolumab Pre - treatment Post Dose (8w) 14% >70% H&E CD8 IHC Ph I CPI - 444 Trial 13 13 11

CPI - 444 Induces CD8 T Cell Infiltration and Th1 Gene Expression in Tumor Tissues 14 14 Log Fold Change Gene Expression Post vs pre biopsies (N=14) Immune Gene Expression (paired biopsies) CD8 T Cell Change (IHC) NSCLC RCC Median 95% CI Pre - dose NSCLC Post - dose RCC Post - dose Pre - dose C D 8 0.1 1 10 C D 8 R a t i o ( P o s t / P r e b i o p s y ) SD PR -2 -1 0 1 2 1 2 3 4 5 Log Fold Change Gene Expression - l o g P v a l u e CXCL10 GZMB PD-L1 OPG CXCL11 CXCL16 GZMA CCL17 TEFF_SIG PD-L2 ICAM1 NFATC4 IL2RA EOTAXIN CCL13 CD44 IL7R CXCL9 CD8A 12

CPI - 444 Expands New T Cell Clones in Blood and Tumor Treatment induces expansion of identical T cell clones in blood and tumor Post treatment Pre - treatment Blood Tumor Biopsy 0.01 0.1 1 10 0.01 0.1 1 10 Screen Biopsy P o s t d o s e B i o p s y 15 15 • RCC patient with PR on single - agent CPI - 444 • T cell receptor (TCR) sequencing of blood and tumor biopsies pre - and post - treatment • T cell clonotypes can be matched between blood and tumor Pre - treatment Post treatment 13

Phase 1/1b Trial with CPI - 444: Summary • CPI - 444 is well - tolerated as a monotherapy and in combination with atezolizumab • CPI - 444 has clinical activity alone and in combination with atezolizumab • Anti - tumor activity seen in: – Patients who have progressed on prior anti - PD - (L)1 – Patients with PD - L1 negative tumors • CPI - 444 can induce CD 8 T cell infiltration and expression of T cell activation genes within the tumor microenvironment • CPI - 444 induces new T cell clonotypes in the blood, which are capable of migrating to tumors • Accrual of patients into the expansion cohorts for NSCLC and RCC is ongoing 16 16 14

Acknowledgements • The patients and their families • Participating Centers: British Columbia Cancer Agency, Carolina BioOncology Institute, Cleveland Clinic, Columbia University Medical Center, Cross Cancer Institute, Emory University, Georgetown University, Indiana University, Johns Hopkins University, Juravinski Cancer Centre, Karmanos Cancer Center, Mary Crowley Cancer Research Centers, Massachusetts General Hospital, Medical College of Wisconsin, Memorial Sloan Kettering Cancer Center, Monash Health, Mount Sinai School of Medicine, Ohio State University, Ottawa Hospital Cancer Centre, Peter McCallum Cancer Center, Royal Brisbane and Women’s Hospital, Rush University, Stanford University, University of California at Los Angeles Medical Center, University of California at San Francisco Medical Center, University of Arizona Medical Center, University of Chicago Medical Center, University of Colorado Cancer Center, University of Nebraska, University of Pittsburgh, University of Washington, UT Southwestern, Washington University at Saint Louis, Yale University • C olleagues at Corvus • Colleagues at Roche Genentech Ph I CPI - 444 Trial 17 15
