Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Endo International plc | d360004d8k.htm |

Endo International plc UBS Healthcare Conference May 23, 2017 ©2017 Endo Pharmaceuticals Inc. All rights reserved. Exhibit 99.1

Forward Looking Statements; Non-GAAP Financial Measures This presentation contains forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Canadian securities legislation. Statements including words such as “believes,” “expects,” “anticipates,” “intends,” “estimates,” “plan,” “will,” “may,” “look forward,” “intend,” “guidance,” “future projects” or similar expressions are forward looking statements. Because these statements reflect our current views, expectations and beliefs concerning future events, these forward looking statements involve risks and uncertainties. Although Endo believes that these forward looking statements and information are based upon reasonable assumptions and expectations, readers should not place undue reliance on them, or any other forward looking statements or information in this presentation. Investors should note that many factors, as more fully described in the documents filed by Endo with securities regulators in the United States and Canada including under the caption “Risk Factors” in Endo’s Form 10-K, Form 10-Q and Form 8-K filings, as applicable, with the Securities and Exchange Commission and with securities regulators in Canada on System for Electronic Document Analysis and Retrieval (“SEDAR”) and as otherwise enumerated herein or therein, could affect Endo’s future financial results and could cause Endo’s actual results to differ materially from those expressed in any forward looking statements. The forward looking statements in this presentation are qualified by these risk factors. Endo assumes no obligation to publicly update any forward looking statements, whether as a result of new information, future developments or otherwise, except as may be required under applicable securities law. This presentation may refer to non-GAAP financial measures, including, among others, adjusted diluted EPS and adjusted EBITDA, that are not prepared in accordance with accounting principles generally accepted in the United States and that may be different from non-GAAP financial measures used by other companies. Investors are encouraged to review Endo’s current report on Form 8-K furnished to the SEC for Endo’s reasons for including those non-GAAP financial measures in this presentation. Investors are also encouraged to review the reconciliation of the non-GAAP financial measures used in the Presentation to their most directly comparable GAAP financial measures as included in the appendix of the Presentation and in Exhibit 99.1 of Form 8-K filed with the U.S. Securities and Exchange Commission on May 9, 2017. However, the Company does not provide reconciliations of projected non-GAAP financial measures to GAAP financial measures, nor does it provide comparable projected GAAP financial measures for such projected non-GAAP financial measures, except for projected adjusted diluted EPS. The Company is unable to provide such reconciliations without unreasonable efforts due to the inherent difficulty in forecasting and quantifying certain amounts that are necessary for such reconciliations, including adjustments that could be made for asset impairments, contingent consideration adjustments, legal settlements, loss on extinguishment of debt, adjustments to inventory and other charges reflected in the reconciliation of historic numbers, the amount of which could be significant. ©2017 Endo Pharmaceuticals Inc. All rights reserved.
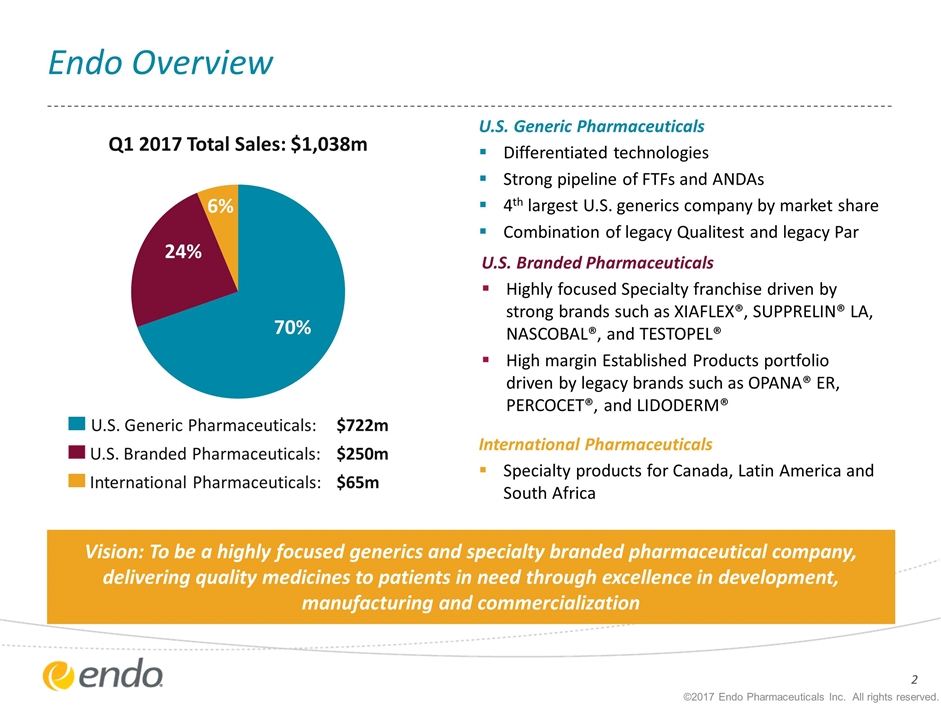
Endo Overview ©2017 Endo Pharmaceuticals Inc. All rights reserved. Q1 2017 Total Sales: $1,038m U.S. Generic Pharmaceuticals: U.S. Branded Pharmaceuticals: International Pharmaceuticals: $250m $722m $65m U.S. Generic Pharmaceuticals Differentiated technologies Strong pipeline of FTFs and ANDAs 4th largest U.S. generics company by market share Combination of legacy Qualitest and legacy Par U.S. Branded Pharmaceuticals Highly focused Specialty franchise driven by strong brands such as XIAFLEX®, SUPPRELIN® LA, NASCOBAL®, and TESTOPEL® High margin Established Products portfolio driven by legacy brands such as OPANA® ER, PERCOCET®, and LIDODERM® International Pharmaceuticals Specialty products for Canada, Latin America and South Africa Vision: To be a highly focused generics and specialty branded pharmaceutical company, delivering quality medicines to patients in need through excellence in development, manufacturing and commercialization
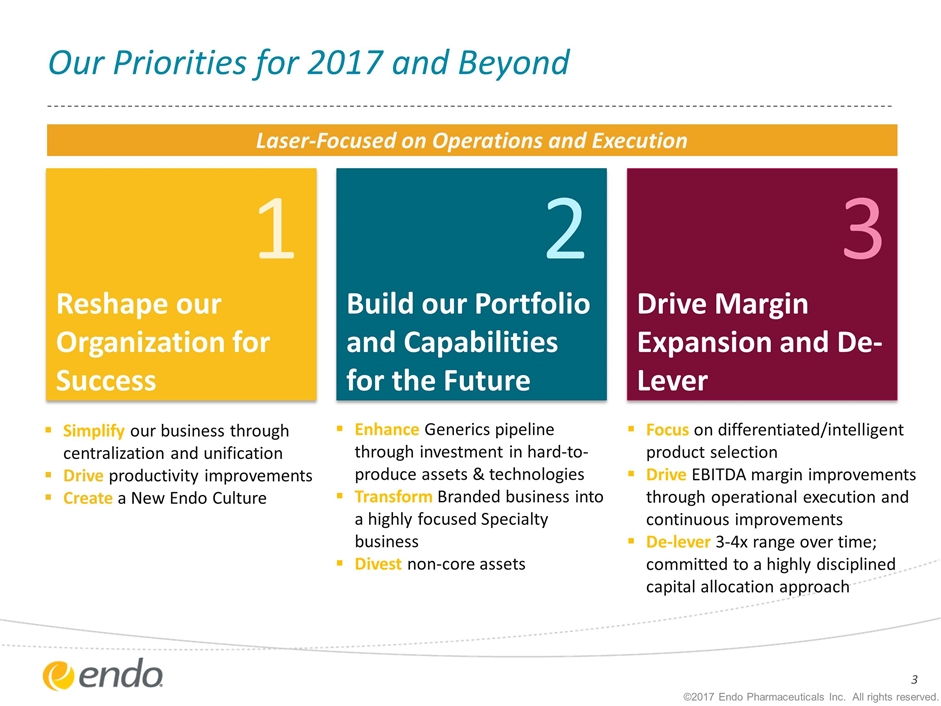
1 Reshape our Organization for Success Focus on differentiated/intelligent product selection Drive EBITDA margin improvements through operational execution and continuous improvements De-lever 3-4x range over time; committed to a highly disciplined capital allocation approach 3 Drive Margin Expansion and De-Lever Enhance Generics pipeline through investment in hard-to-produce assets & technologies Transform Branded business into a highly focused Specialty business Divest non-core assets Simplify our business through centralization and unification Drive productivity improvements Create a New Endo Culture ©2017 Endo Pharmaceuticals Inc. All rights reserved. Our Priorities for 2017 and Beyond 2 Build our Portfolio and Capabilities for the Future Laser-Focused on Operations and Execution
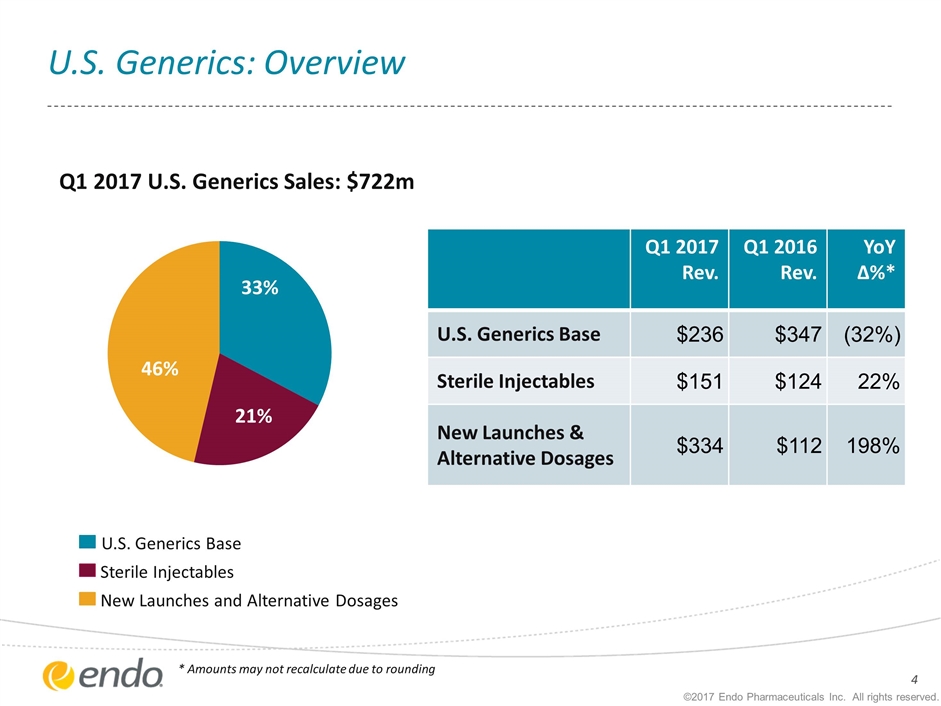
U.S. Generics: Overview ©2017 Endo Pharmaceuticals Inc. All rights reserved. Q1 2017 Rev. Q1 2016 Rev. YoY ∆%* U.S. Generics Base $236 $347 (32%) Sterile Injectables $151 $124 22% New Launches & Alternative Dosages $334 $112 198% Q1 2017 U.S. Generics Sales: $722m U.S. Generics Base Sterile Injectables New Launches and Alternative Dosages * Amounts may not recalculate due to rounding
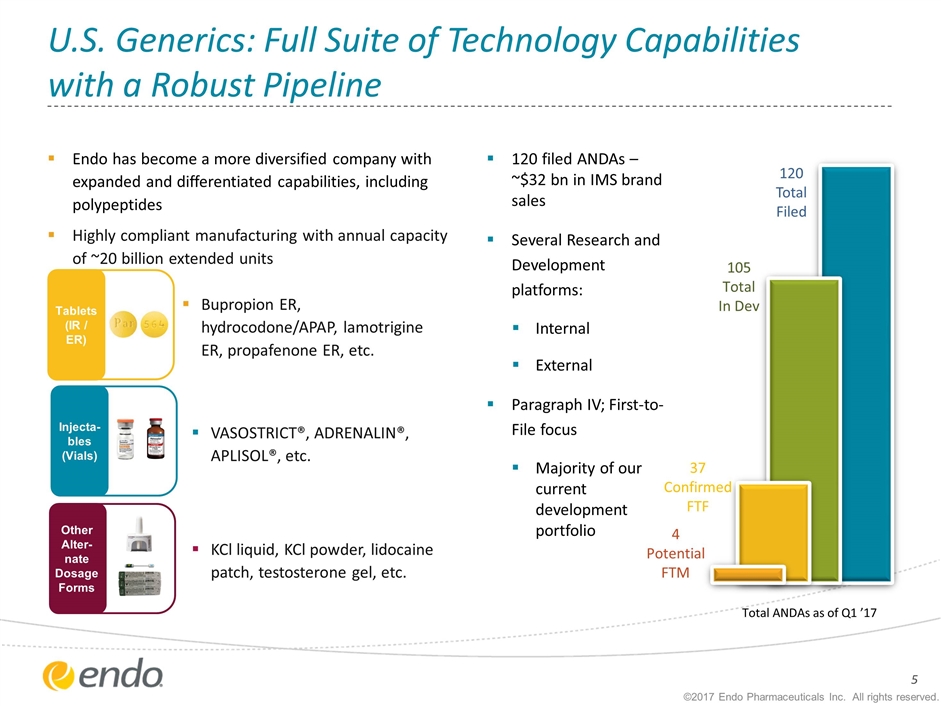
U.S. Generics: Full Suite of Technology Capabilities with a Robust Pipeline Endo has become a more diversified company with expanded and differentiated capabilities, including polypeptides Highly compliant manufacturing with annual capacity of ~20 billion extended units Tablets (IR / ER) Bupropion ER, hydrocodone/APAP, lamotrigine ER, propafenone ER, etc. KCl liquid, KCl powder, lidocaine patch, testosterone gel, etc. VASOSTRICT®, ADRENALIN®, APLISOL®, etc. ©2017 Endo Pharmaceuticals Inc. All rights reserved. Injecta-bles (Vials) Other Alter-nate Dosage Forms 37 Confirmed FTF 105 Total In Dev Total ANDAs as of Q1 ’17 120 Total Filed 4 Potential FTM 120 filed ANDAs – ~$32 bn in IMS brand sales Several Research and Development platforms: Internal External Paragraph IV; First-to-File focus Majority of our current development portfolio
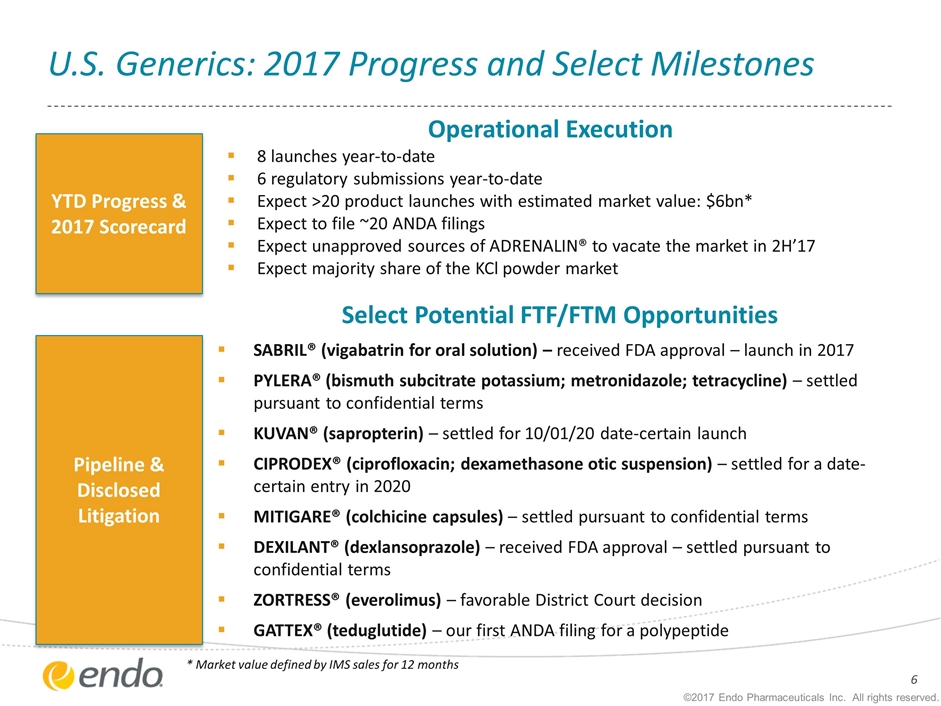
U.S. Generics: 2017 Progress and Select Milestones ©2017 Endo Pharmaceuticals Inc. All rights reserved. * Market value defined by IMS sales for 12 months Select Potential FTF/FTM Opportunities SABRIL® (vigabatrin for oral solution) – received FDA approval – launch in 2017 PYLERA® (bismuth subcitrate potassium; metronidazole; tetracycline) – settled pursuant to confidential terms KUVAN® (sapropterin) – settled for 10/01/20 date-certain launch CIPRODEX® (ciprofloxacin; dexamethasone otic suspension) – settled for a date-certain entry in 2020 MITIGARE® (colchicine capsules) – settled pursuant to confidential terms DEXILANT® (dexlansoprazole) – received FDA approval – settled pursuant to confidential terms ZORTRESS® (everolimus) – favorable District Court decision GATTEX® (teduglutide) – our first ANDA filing for a polypeptide YTD Progress & 2017 Scorecard Pipeline & Disclosed Litigation Operational Execution 8 launches year-to-date 6 regulatory submissions year-to-date Expect >20 product launches with estimated market value: $6bn* Expect to file ~20 ANDA filings Expect unapproved sources of ADRENALIN® to vacate the market in 2H’17 Expect majority share of the KCl powder market
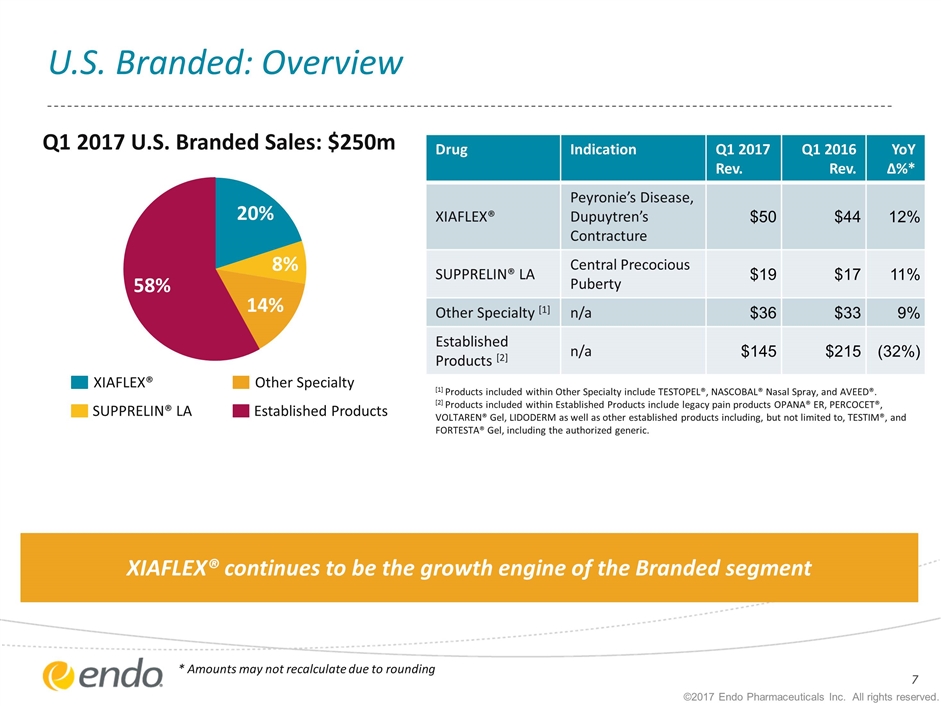
U.S. Branded: Overview [1] Products included within Other Specialty include TESTOPEL®, NASCOBAL® Nasal Spray, and AVEED®. [2] Products included within Established Products include legacy pain products OPANA® ER, PERCOCET®, VOLTAREN® Gel, LIDODERM as well as other established products including, but not limited to, TESTIM®, and FORTESTA® Gel, including the authorized generic. ©2017 Endo Pharmaceuticals Inc. All rights reserved. Drug Indication Q1 2017 Rev. Q1 2016 Rev. YoY ∆%* XIAFLEX® Peyronie’s Disease, Dupuytren’s Contracture $50 $44 12% SUPPRELIN® LA Central Precocious Puberty $19 $17 11% Other Specialty [1] n/a $36 $33 9% Established Products [2] n/a $145 $215 (32%) XIAFLEX® SUPPRELIN® LA Other Specialty Established Products Q1 2017 U.S. Branded Sales: $250m * Amounts may not recalculate due to rounding XIAFLEX® continues to be the growth engine of the Branded segment
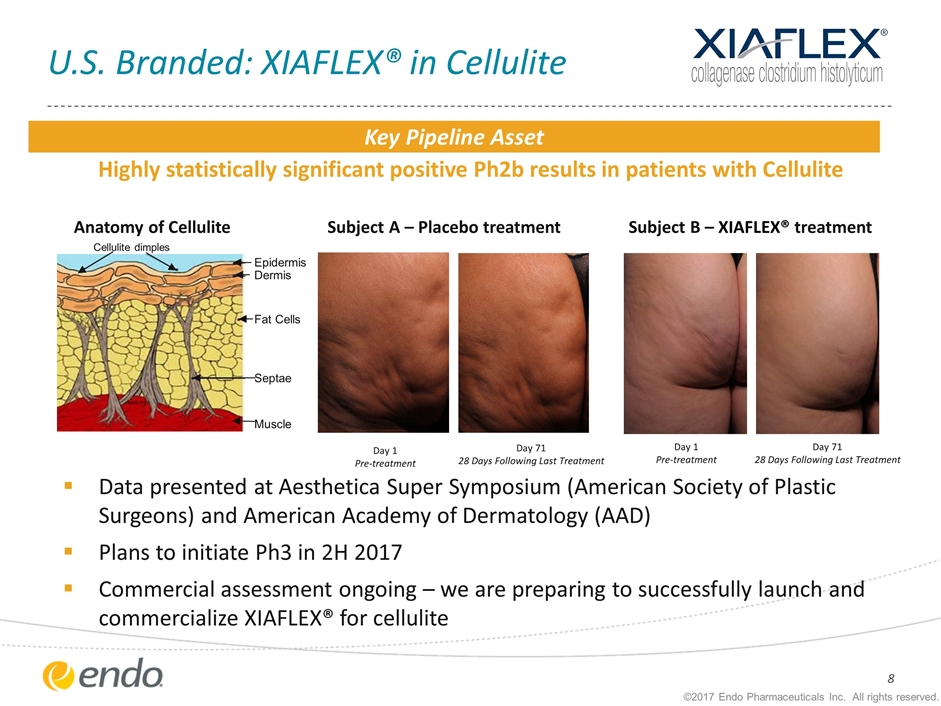
U.S. Branded: XIAFLEX® in Cellulite ©2017 Endo Pharmaceuticals Inc. All rights reserved. Data presented at Aesthetica Super Symposium (American Society of Plastic Surgeons) and American Academy of Dermatology (AAD) Plans to initiate Ph3 in 2H 2017 Commercial assessment ongoing – we are preparing to successfully launch and commercialize XIAFLEX® for cellulite Highly statistically significant positive Ph2b results in patients with Cellulite Day 1 Pre-treatment Day 71 28 Days Following Last Treatment Day 1 Pre-treatment Subject A – Placebo treatment Subject B – XIAFLEX® treatment Day 71 28 Days Following Last Treatment Key Pipeline Asset Anatomy of Cellulite Muscle Cellulite dimples Dermis Fat Cells Septae Epidermis
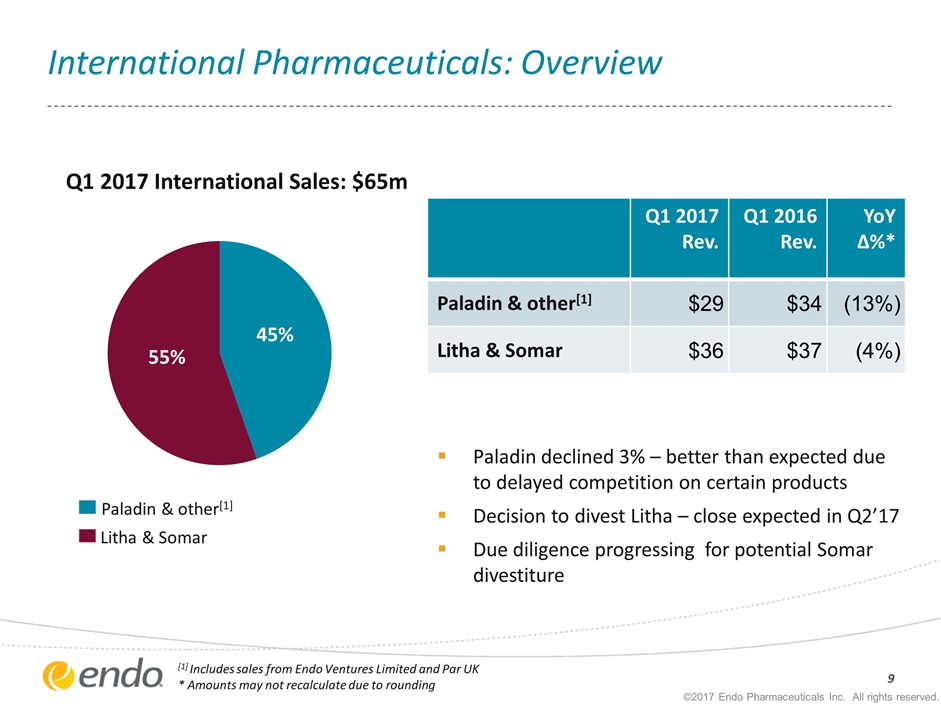
International Pharmaceuticals: Overview ©2017 Endo Pharmaceuticals Inc. All rights reserved. Q1 2017 Rev. Q1 2016 Rev. YoY ∆%* Paladin & other[1] $29 $34 (13%) Litha & Somar $36 $37 (4%) Q1 2017 International Sales: $65m Paladin & other[1] Litha & Somar [1] Includes sales from Endo Ventures Limited and Par UK * Amounts may not recalculate due to rounding Paladin declined 3% – better than expected due to delayed competition on certain products Decision to divest Litha – close expected in Q2’17 Due diligence progressing for potential Somar divestiture
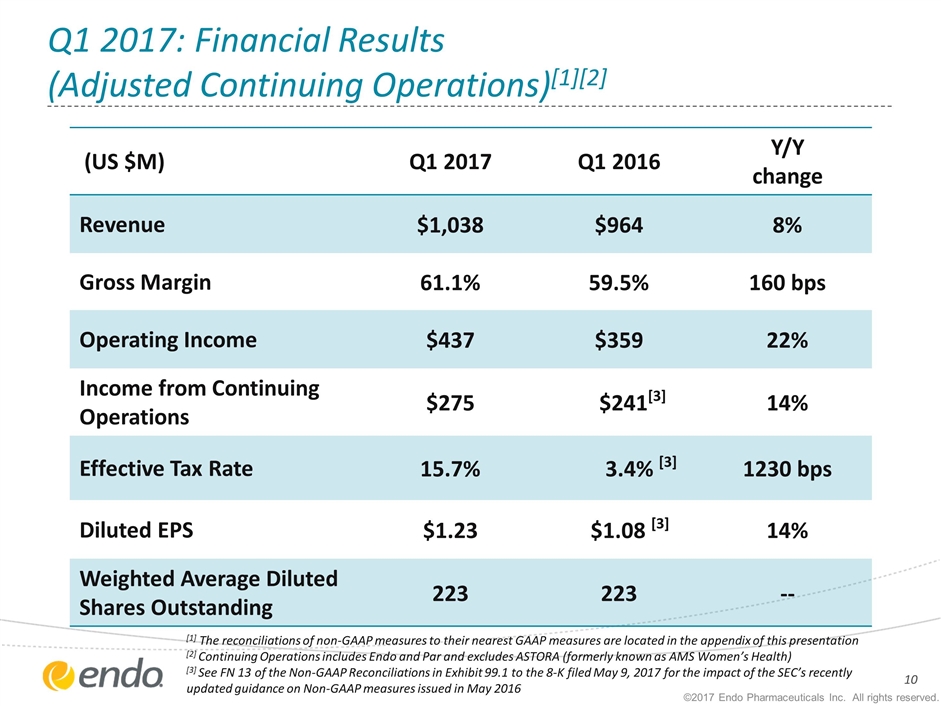
Q1 2017: Financial Results (Adjusted Continuing Operations)[1][2] ©2017 Endo Pharmaceuticals Inc. All rights reserved. (US $M) Q1 2017 Q1 2016 Y/Y change Revenue $1,038 $964 8% Gross Margin 61.1% 59.5% 160 bps Operating Income $437 $359 22% Income from Continuing Operations $275 $241[3] 14% Effective Tax Rate 15.7% 3.4% [3] 1230 bps Diluted EPS $1.23 $1.08 [3] 14% Weighted Average Diluted Shares Outstanding 223 223 -- [1] The reconciliations of non-GAAP measures to their nearest GAAP measures are located in the appendix of this presentation [2] Continuing Operations includes Endo and Par and excludes ASTORA (formerly known as AMS Women’s Health) [3] See FN 13 of the Non-GAAP Reconciliations in Exhibit 99.1 to the 8-K filed May 9, 2017 for the impact of the SEC’s recently updated guidance on Non-GAAP measures issued in May 2016
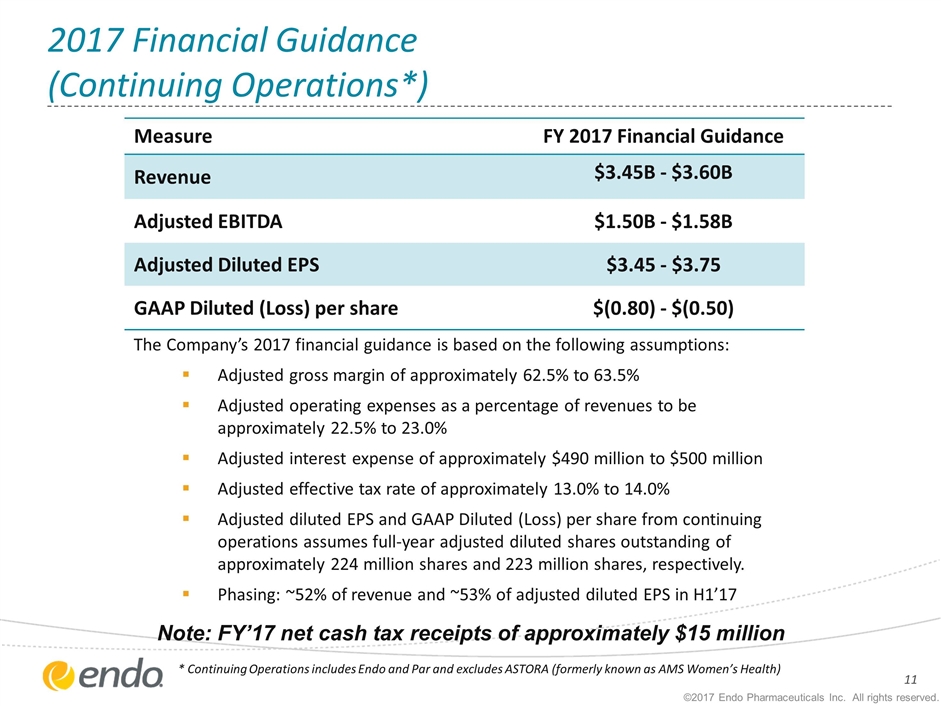
2017 Financial Guidance (Continuing Operations*) ©2017 Endo Pharmaceuticals Inc. All rights reserved. * Continuing Operations includes Endo and Par and excludes ASTORA (formerly known as AMS Women’s Health) Measure FY 2017 Financial Guidance Revenue $3.45B - $3.60B Adjusted EBITDA $1.50B - $1.58B Adjusted Diluted EPS $3.45 - $3.75 GAAP Diluted (Loss) per share $(0.80) - $(0.50) Note: FY’17 net cash tax receipts of approximately $15 million The Company’s 2017 financial guidance is based on the following assumptions: Adjusted gross margin of approximately 62.5% to 63.5% Adjusted operating expenses as a percentage of revenues to be approximately 22.5% to 23.0% Adjusted interest expense of approximately $490 million to $500 million Adjusted effective tax rate of approximately 13.0% to 14.0% Adjusted diluted EPS and GAAP Diluted (Loss) per share from continuing operations assumes full-year adjusted diluted shares outstanding of approximately 224 million shares and 223 million shares, respectively. Phasing: ~52% of revenue and ~53% of adjusted diluted EPS in H1’17
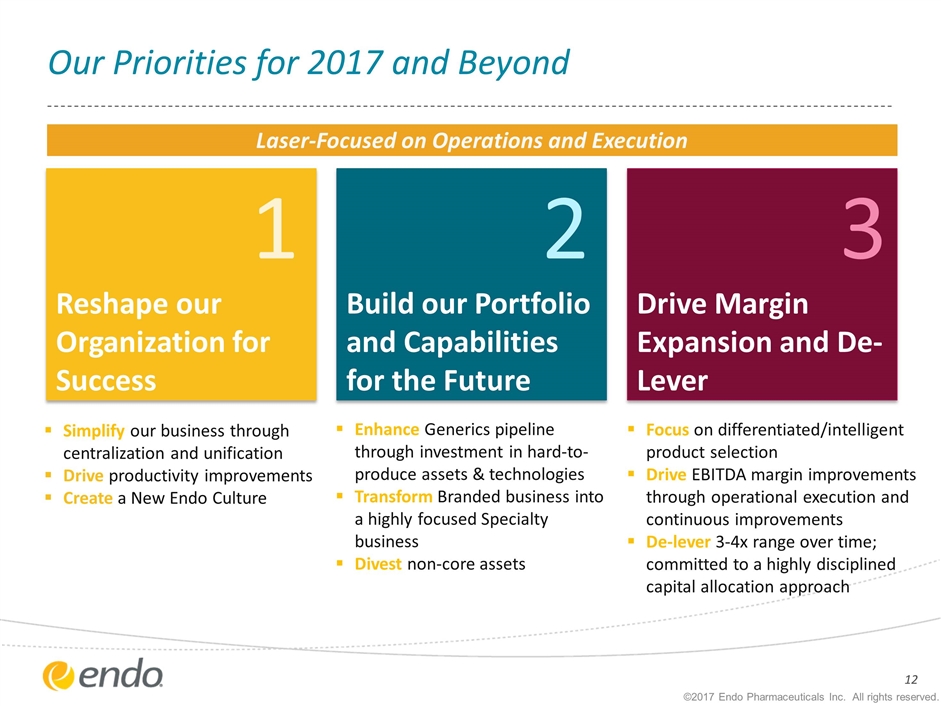
1 Reshape our Organization for Success Focus on differentiated/intelligent product selection Drive EBITDA margin improvements through operational execution and continuous improvements De-lever 3-4x range over time; committed to a highly disciplined capital allocation approach 3 Drive Margin Expansion and De-Lever Enhance Generics pipeline through investment in hard-to-produce assets & technologies Transform Branded business into a highly focused Specialty business Divest non-core assets Simplify our business through centralization and unification Drive productivity improvements Create a New Endo Culture ©2017 Endo Pharmaceuticals Inc. All rights reserved. Our Priorities for 2017 and Beyond 2 Build our Portfolio and Capabilities for the Future Laser-Focused on Operations and Execution

Endo International plc UBS Healthcare Conference May 23, 2017 ©2017 Endo Pharmaceuticals Inc. All rights reserved.

Appendix ©2017 Endo Pharmaceuticals Inc. All rights reserved.
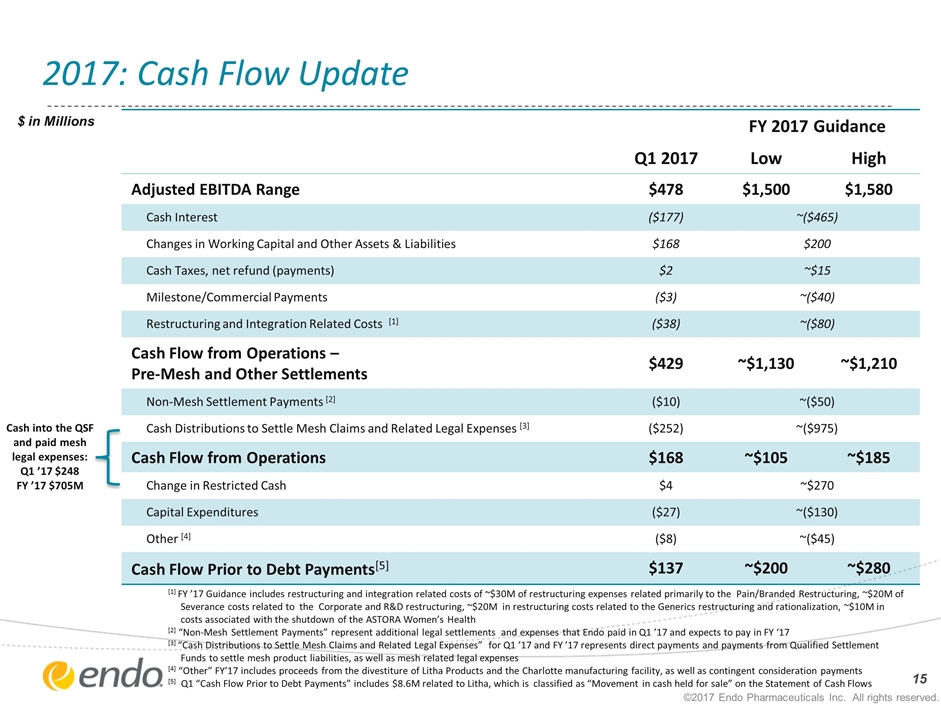
2017: Cash Flow Update [1] FY ’17 Guidance includes restructuring and integration related costs of ~$30M of restructuring expenses related primarily to the Pain/Branded Restructuring, ~$20M of Severance costs related to the Corporate and R&D restructuring, ~$20M in restructuring costs related to the Generics restructuring and rationalization, ~$10M in costs associated with the shutdown of the ASTORA Women’s Health [2] “Non-Mesh Settlement Payments” represent additional legal settlements and expenses that Endo paid in Q1 ’17 and expects to pay in FY ‘17 [3] “Cash Distributions to Settle Mesh Claims and Related Legal Expenses” for Q1 ’17 and FY ’17 represents direct payments and payments from Qualified Settlement Funds to settle mesh product liabilities, as well as mesh related legal expenses [4] “Other” FY’17 includes proceeds from the divestiture of Litha Products and the Charlotte manufacturing facility, as well as contingent consideration payments [5] Q1 “Cash Flow Prior to Debt Payments” includes $8.6M related to Litha, which is classified as “Movement in cash held for sale” on the Statement of Cash Flows $ in Millions FY 2017 Guidance Q1 2017 Low High Adjusted EBITDA Range $478 $1,500 $1,580 Cash Interest ($177) ~($465) Changes in Working Capital and Other Assets & Liabilities $168 $200 Cash Taxes, net refund (payments) $2 ~$15 Milestone/Commercial Payments ($3) ~($40) Restructuring and Integration Related Costs [1] ($38) ~($80) Cash Flow from Operations – Pre-Mesh and Other Settlements $429 ~$1,130 ~$1,210 Non-Mesh Settlement Payments [2] ($10) ~($50) Cash Distributions to Settle Mesh Claims and Related Legal Expenses [3] ($252) ~($975) Cash Flow from Operations $168 ~$105 ~$185 Change in Restricted Cash $4 ~$270 Capital Expenditures ($27) ~($130) Other [4] ($8) ~($45) Cash Flow Prior to Debt Payments[5] $137 ~$200 ~$280 ©2017 Endo Pharmaceuticals Inc. All rights reserved. Cash into the QSF and paid mesh legal expenses: Q1 ’17 $248 FY ’17 $705M
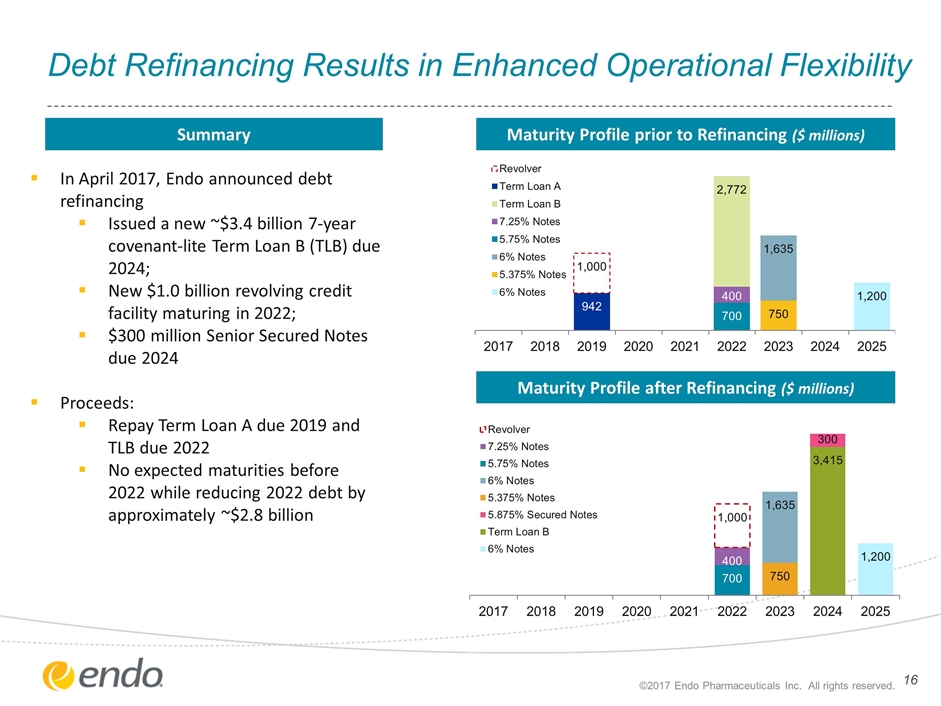
Debt Refinancing Results in Enhanced Operational Flexibility ©2017 Endo Pharmaceuticals Inc. All rights reserved. Maturity Profile prior to Refinancing ($ millions) Maturity Profile after Refinancing ($ millions) Summary In April 2017, Endo announced debt refinancing Issued a new ~$3.4 billion 7-year covenant-lite Term Loan B (TLB) due 2024; New $1.0 billion revolving credit facility maturing in 2022; $300 million Senior Secured Notes due 2024 Proceeds: Repay Term Loan A due 2019 and TLB due 2022 No expected maturities before 2022 while reducing 2022 debt by approximately ~$2.8 billion
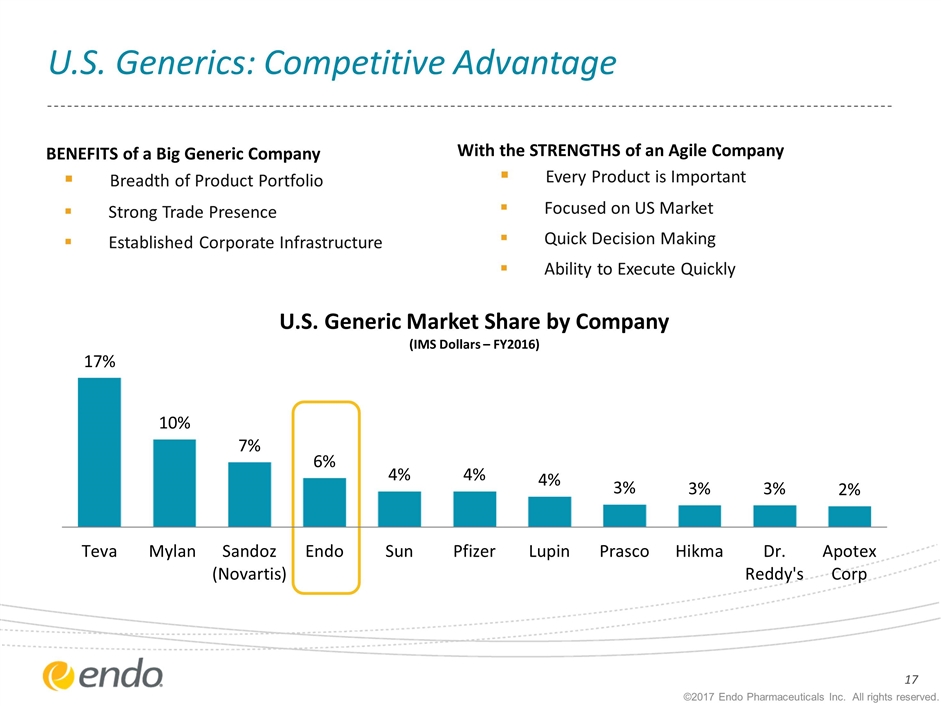
U.S. Generics: Competitive Advantage BENEFITS of a Big Generic Company Breadth of Product Portfolio Strong Trade Presence Established Corporate Infrastructure With the STRENGTHS of an Agile Company Every Product is Important Focused on US Market Quick Decision Making Ability to Execute Quickly ©2017 Endo Pharmaceuticals Inc. All rights reserved.
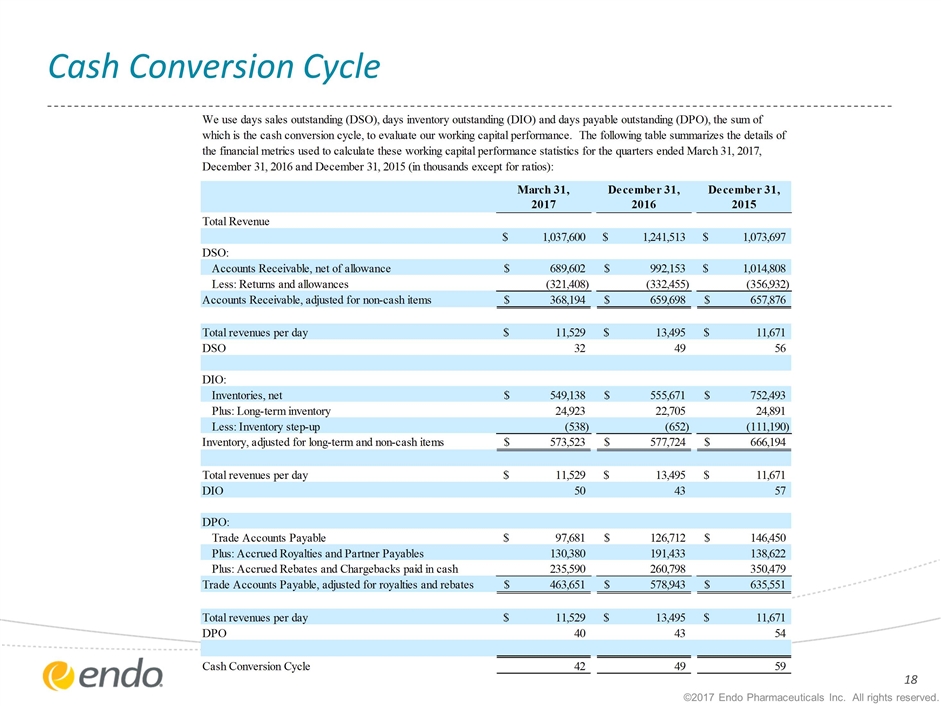
Cash Conversion Cycle ©2017 Endo Pharmaceuticals Inc. All rights reserved.
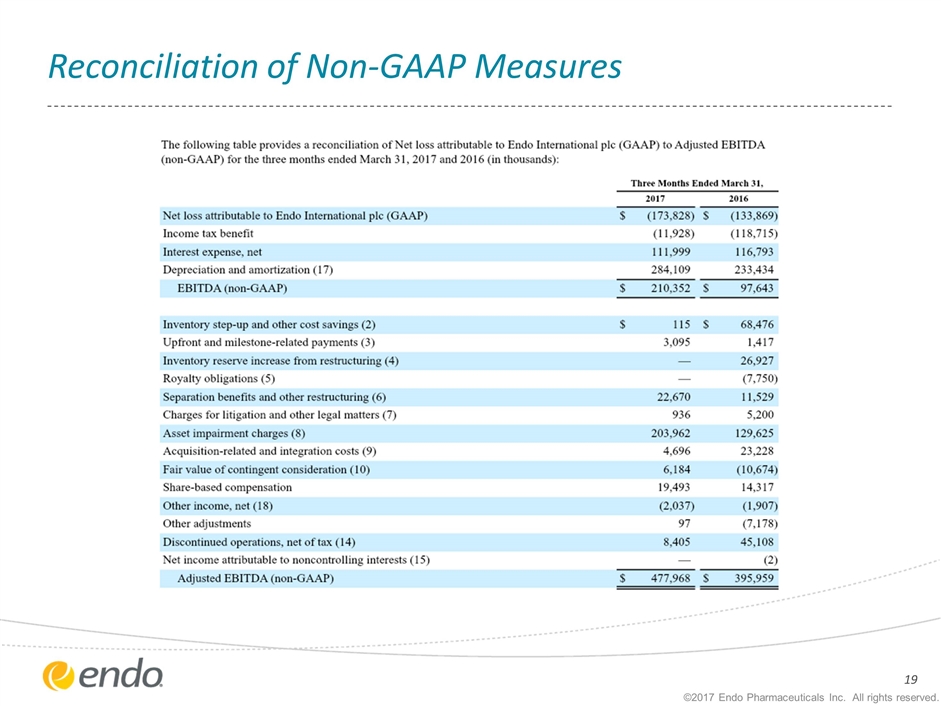
Reconciliation of Non-GAAP Measures ©2017 Endo Pharmaceuticals Inc. All rights reserved.
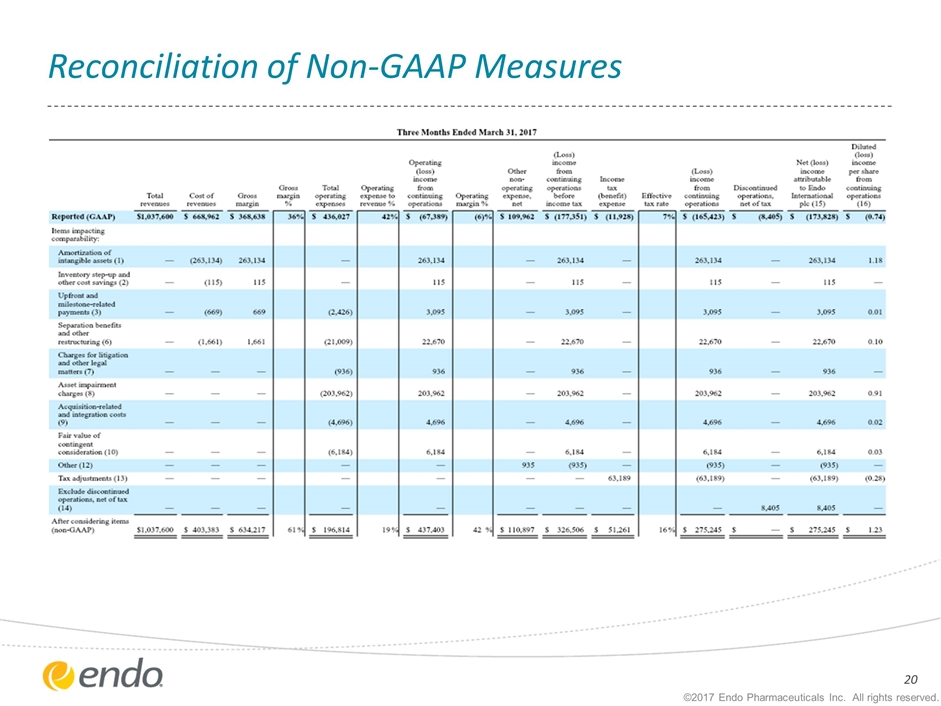
Reconciliation of Non-GAAP Measures ©2017 Endo Pharmaceuticals Inc. All rights reserved.
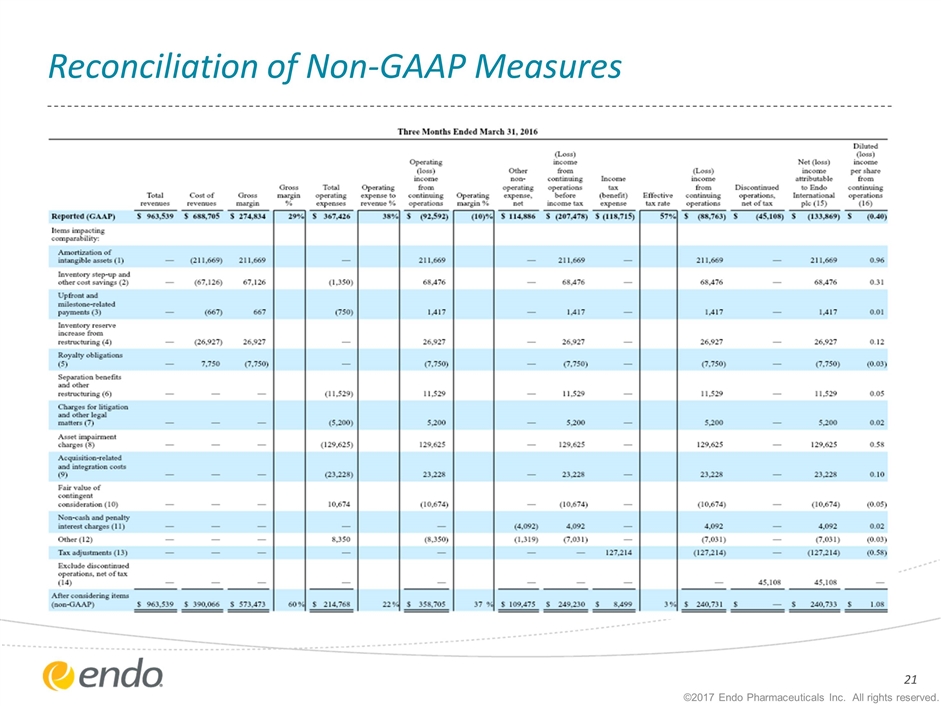
Reconciliation of Non-GAAP Measures ©2017 Endo Pharmaceuticals Inc. All rights reserved.
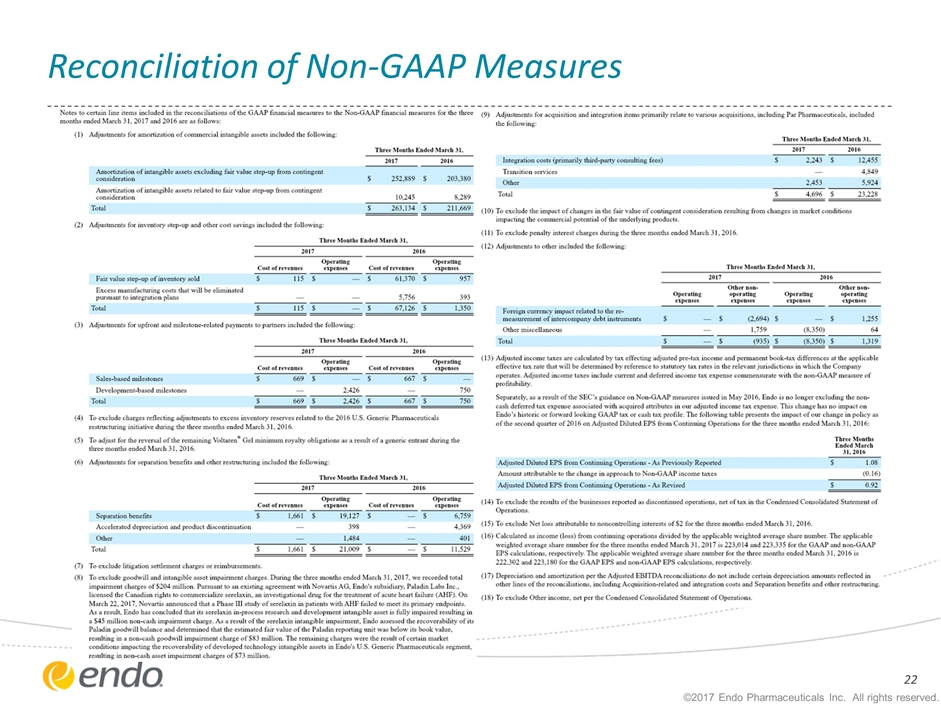
Reconciliation of Non-GAAP Measures ©2017 Endo Pharmaceuticals Inc. All rights reserved.
