Attached files
| file | filename |
|---|---|
| 10-Q - CIDARA THERAPEUTICS, INC. FORM 10-Q - Cidara Therapeutics, Inc. | cdtx10-q2017x03.htm |
| EX-32.2 - CERTIFICATION OF CFO PURSUANT TO SARBANES-OXLEY ACT OF 2002 - Cidara Therapeutics, Inc. | exhibit322-q12017.htm |
| EX-32.1 - CERTIFICATION OF CEO PURSUANT TO SARBANES-OXLEY ACT OF 2002 - Cidara Therapeutics, Inc. | exhibit321-q12017.htm |
| EX-31.2 - CERTIFICATION OF CFO PURSUANT TO SECURITIES EXCHANGE ACT OF 1934 - Cidara Therapeutics, Inc. | exhibit312-q12017.htm |
| EX-31.1 - CERTIFICATION OF CEO PURSUANT TO SECURITIES EXCHANGE ACT OF 1934 - Cidara Therapeutics, Inc. | exhibit311-q12017.htm |
| EX-10.2 - FORM OF RESTRICTED STOCK UNIT AWARD GRANT NOTICE - Cidara Therapeutics, Inc. | cidara-restrictedstockunit.htm |

Exhibit 10.1
***Text Omitted and Filed Separately
with the Securities and Exchange Commission.
Confidential Treatment Requested
Under 17 C.F.R. Sections 200.80(b)(4)
and 240.24b-2
Cost Reimbursement Research Subaward Agreement
Pass-Through Entity (PIE) Trustees of Boston University Subrecipient: Cidara Therapeutics, Inc.
PTE Principal Investigator (PI): Levin Outterson Subrecipient Principal Investigator: James Balkovec
PTE Federal Award No: 1 IDSEP160030-01-02
PTE non-Federal Award No:
FAIN: IDSEP160030 Federal Awarding Agency: HHS/ASPR
non-Federal Sponsor: The Wellcome Trust
Federal Award Issue Date:
07/27/2016, 09/16/2016, 1/12/2017
Total Amount of Federal Award to
PTE: $30,000,000.00
Total Amount of non-Federal Award
to PTE: $18,660,000.00
CFDA No. 93 360 CFDA Title
see Attachment 2a
Project Title: A Novel, Bifunctional Immunotherapy Effective against Multidrug-Resistant Gram-Negative (MDRGN) Infections
Subaward Period of Performance
Start 04/01/2017 End 04/30/2018
Amount Funded this Action:
Total: $ 3,853,576.00
Federal Award: $2,312,146.00
non-Federal Award
$1,541,430.00
Subaward No
4500002324
Estimated Project Period (if incrementally funded)
Start 04/01/2017 End: 04/30/2018
Is this award R&D
Yes or No
Check all that apply: Subject to FFATA (Attachment 3B) Cost Sharing (Attachment 5)
Terms and Conditions
1) PTE hereby awards a cost reimbursable subaward, as described above, to Subrecipient. The statement of work and budget for this subaward are (check one)
as specified in Subrecipient’s proposal dated or as shown in Attachment 5. In this performance of subaward work, Subrecipient shall be an
independent entity and not an employee or agent of PTE.
2) PTE shall reimburse Subrecipient not more often than monthly for allowable costs. All invoices shall be submitted using Subrecipient’s standard invoice, but at a
minimum shall include current and cumulative costs (including cost sharing), subaward number, and certification, as required in 2 CFR 200.415(a). See
Attachment 2/Special Terms and Conditions #21 for required certification. Invoices that do not reference PTE Subaward number shall be returned to
Subrecipient. Invoice and questions concerning invoice receipt or payments should be directed to the appropriate party’s Financial Contact, as shown i
Attachment 3. All costs and financials must be expressed in U.S. dollars using an exchange rate applicable at the time the invoice is submitted and including
documentation of conversion rate used. All payments will be in U.S. dollars. Documentation with applicable translation in English to support claimed expenses
must be submitted with all invoices. Supporting documentation includes, but is not limited to, payroll distribution records, time sheets, time and effort reports for
subject personnel, general ledger support, vouchers, expense reports, requisitions and receipts for non-personnel expenditures and contracts issued for services.
All invoices and reports shall be submitted in English.
3) A final statement of cumulative costs incurred, including cost sharing, marked “FINAL” moat he submitted to FTE’s Financial Contact, as shown in
Attachment 3A. NOT LATER THAN 45 days after subaward end date. The final statement of costs shall constitute Subrecipient’s final financial report.
4) All payments shall be considered provisional and subject to adjustment within the total estimated cost in the event such adjustment is necessary as a result of an
adverse audit finding against the Subrecipient. PTE reserves the right to reject an invoice, in accordance with 2 CFR 203 305.
5) Matters concerning the technical performance of this subaward should be directed to the appropriate party’s Principal Investigator as shown in Attachments 3A
and 3B. Technical reports are required as shown in Attachment 4.
6) Matters concerning the request or negotiation of any changes in the terms, conditions, or amounts cited in this Subaward Agreement and airy changes requiring
prior approval, should he directed to the appropriate party’s Administrative Contact, as shown in Attachments 3A and 3B. Any such changes made to this
Subaward Agreement require the written approval of each party’s Authorized Official, as shown in Attachments 3A and 3B.
7) Substantive changes made to this Subaward Agreement require the written approval of each party’s Authorized Official as shown in Attachments 3A and 3B.
The PTE may issue non-substantive changes to the Period of Performance (check one) Bilaterally, or Unilaterally
8) Each party shall be responsible for its negligent acts or omissions and the negligent acts or omissions of its employees, officers or directors, to the extent allowed
by law.
9) PTE may terminate this subaward with thirty days written notice to the Subrecipient’s Administrative Contact, as shown in Attachment 3B. If Federal Awarding
Agency or non-Federal Sponsor terminate PTE award, PTE will terminate this subaward. PTE shall pay Subrecipient for termination costs as allowable under
Subpart 31.2 of the Federal Acquisition Regulations (48 CFR Subpart 31 2) applicable to commercial organizations.
10) No-cost extensions require the approval of the PTE. Any requests for a no-cost extension should be addressed to and received by the Administrative Contact, as
shown in Attachments 3A, not less than 30 days prior to the desired effective date of the requested change.
11) The Subaward Agreement is subject to the terms and conditions of the PTE Award (Attachment 2A) and other special terms and conditions, as identified in
Attachments 2 and 6, as applicable.
12) By signing this Subaward Agreement, Subrecipient makes the certifications and assurances shown in Attachments 1 and 2. This Subaward Agreement is not
final, binding or enforceable until countersigned below by PTE.
By and Authorized Official of PTE
/s/ Dolores Markey 3/30/2017
Dolores Markey Date
Director, Special Projects, Policy and Process
By and Authorized Official of Subrecipient
/s/ Matthew Onaitis March 27, 2017
Matthew Onaitis Date

Attachment 1
Research Subaward Agreement
Certifications and Assurances
By signing the Subaward Agreement, the Authorized Official of Subrecipient certifies, to the best of his/her knowledge and belief,
that:
Certification Regarding Lobbying
1) No Federal appropriated funds have been paid or will be paid, by or on behalf of the Subrecipient, to any person for
influencing or attempting to influence an officer or employee of any agency, a Member of Congress, an officer or employee of
Congress, or an employee of a Member of Congress in connection with the awarding of any Federal contract, the making of any
Federal grant, the making of any Federal loan, the entering into of any cooperative agreement, and the extension, continuation,
renewal, amendment, or modification of any Federal contract, grant, loan, or cooperative agreement.
2) If any funds other than Federal appropriated funds have been paid or will be paid to any person for influencing or intending
to influence an officer or employee of any agency, a Member of Congress, an officer or employee of Congress, or an employee of a
Member of Congress in connection with this Federal contract, grant, loan, or cooperative agreement, the Subrecipient shall complete
and submit Standard Form -LLL, “Disclosure Form to Report Lobbying,” to the PTE. The form may be found at
https://www.gsa.gov/portal/forms/download/116430
3) The Subrecipient shall require that the language of this certification be included in the award documents for all subawards
at all tiers (including subcontracts, subgrants, and contracts under grants, loans, and cooperative agreements) and that all subrecipients
shall certify and disclose accordingly.
This certification is a material representation of fact upon which reliance was placed when this transaction was made or entered into.
Submission of this certification is a prerequisite for making or entering into this transaction imposed by section 1352, title 31, U. S.
Code. Any person who fails to file the required certification shall be subject to a civil penalty of not less than $10,000 and not more
than $100,000 for each such failure.
Debarment, Suspension, and Other Responsibility Matters
Subrecipient certifies by signing this Subaward Agreement that neither it nor its principals are presently debarred, suspended,
proposed for debarment, declared ineligible or voluntarily excluded from participation in this transaction by any federal department or
agency. Subrecipients that are foreign governments or governmental entities, public international organizations, or foreign-owner or -
controlled (in whole or in part) entities are not subject to the debarment or suspension certification requirement or to debarment or
suspension under 2 CFR 376. All other foreign organizations and international organizations are subject to these requirements.
Audit and Access to Records
Subrecipient certifies by signing this Subaward Agreement that it complies with the Uniform Administrative Requirements (2 CFR
Part 200), will provide notice of the completion of required audits and any adverse findings which impact this subaward as required
by parts 200.501- 200.521, and HHS implementation 45 CFR parts 75.501-75.521, and will provide access to records as required by
parts 200.336, 200.337, and 200.201, and HHS implementation 45 CFR parts 75.364, 75 .365, and 75.50 1, as applicable.
In accordance with 45 CFR §75.50 1, subrecipients that are commercial organizations (including for-profit hospitals) have two
options regarding audits:
(1) A financial related audit (as defined in the Government Auditing Standards, GPO Stock #020-000-00-265-4) of a particular
award in accordance with Government Auditing Standards, in those cases where the recipient receives awards under only
one HHS program; or, if awards are received under multiple HHS programs, a financial related audit of all HHS awards in
accordance with Government Auditing Standards; or
(2) An audit that meets the requirements contained in this subpart.
Commercial organizations that receive annual HHS awards totaling less than $750,000 are exempt from requirements for a non-
Federal audit for that year, but records must be available for review by appropriate officials of Federal agencies.
PTE (or a third party designated by PTE) reserves the right to inspect, upon PTE’s reasonable advance notice and during normal
business hours, Subrecipient’s physical facilities, all aspects of the Statement of Work undertaken under this Subagreement, and all
books, records. and documents of any kind pertaining to the Subagreement. Subrecipient agrees to provide copies of any records,
receipts, accounts or other documentation to PTE in a timely fashion as reasonably requested by PTE.

Subrecipient will keep all usual and proper records and books of accounts in accordance with Generally Accepted Accounting
Principles (GAAP) relating to performance of the Statement of Work for a minimum period of three (3) years after completion of
closeout of the Subaward Agreement and after the final Report has been submitted to PTE and approved. During this period, PTE or
an authorized representative shall have the right to audit, at its own expense, all financial books, accounts, and records of funds
received and costs and commitments incurred under this Subaward Agreement. If any audit reveals a material discrepancy or error in
reporting, Subrecipient will repay the unallowable cost(s). Subrecipient expressly acknowledges its understanding that its activities
pursuant to this Subaward Agreement and all financial books, records, and accounts pertaining thereto may be subject to audit by the
Sponsor, and Subrecipient agrees to cooperate fully in the performance of any such audit.

Attachment 2
Subaward Agreement
Prime Award Terms and Conditions
HHS
Agency-Specific Certifications/Assurances
In addition to all applicable public policy requirement included in Part I and Part II of the HHS Grants Policy Statement, by signing
this Subaward Agreement, the authorized official of Subrecipient assures compliance with the following, as applicable:
1. Research Misconduct. The research misconduct requirements included in “Public Policy Requirements,” HHS Grants Policy
Statement Revised 1/1/07, http://www.hhs.gov/grants/grants/grants-policies-regulations/, Part II-13.
2. Animal Welfare. U.S. Federal and home country requirements. The animal welfare requirements contained in “Public Policy
Requirements,” HHS Grants Policy Statement Revised 1/1/07, http://www.hhs.gov/grants/grants/grants-policies-regulations/,
Part II-12, including the requirement to file a written Animal Welfare Assurance with the Office of Laboratory Animal
Welfare (OLAW) as detailed at https://grants.nih.gov/grants/olaw/sampledoc/foreign.htm, and PTE Award (Attachment 2a,
p13-14). See also Attachment 6, section 503 “Additional Research Standards.”
3. Human Subjects. U.S. Federal and home country requirements. The human subjects requirements contained in “Public
Policy Requirements,” HHS Grants Policy Statement Revised 1/1/07, http ://www.hhs.gov/grants/grants/grants-policies-
regulations/, Part II-9), including the requirement for an assurance (Section 4.1.15.1), as detailed at
http://www.hhs.gov/ohrp/register-irbs-and-obtain-fwas/fwas/index.html, and PTE Award (Attachment 2a, p22-23). See also
Attachment 6, section 503 “Additional Research Standards.”
4. Research Involving Recombinant DNA and Human Gene Transfer Research. The requirements for recombinant DNA and
Human Gene Transfer Research included in “Public Policy Requirements,” HHS Grants Policy Statement Rev 1/1/07,
http://www.hhs.gov/grants/grants/grants-policies-reeulations/, Part II-15, which are under the current NIH Guidelines for
Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines),
http://osp.od.nih.gov/officebiotechnology-activities/biosafety/nih-guidelines.
5. Inclusion of Women, Minorities and Children in Clinical Research. The requirements contained in PTE Award
(Attachment 2a, pgs 13, 28-29, 33) and HHS Grants Policy Statement Rev 1/1/07, http://www.hhs.gov/grants/grants/grants-
policies-regulations/, Part I-18, I-19, Part II-9, II-10.
General terms and conditions (as of the effective date of this Subaward Agreement):
1. Conditions on activities and restrictions on expenditure of federal funds in appropriations acts are applicable to this Subaward
Agreement to the extent those restrictions are pertinent http://grants/nih.gov/grants/policy/appropriations_info.htm.
2. 2 CFR 200, Uniform Administrative Requirements, Cost Principles, and Audit Requirements for Federal Awards (Uniform
Guidance), http://www.ecfr.gov/cgi-bin/text-idx?tpl=/ecfrbrowse/Title02/2cfr200_main_02.tpl
3. 45 CFR Part 75, Uniform Administrative Requirements, Cost Principles, and Audit Requirements for HHS Award (HHS
Implementation of Uniform Guidance), http://www.ecfr.gov/cgi-bin/text-idx?node=pt45.1.75
4. The HHS Grants Policy Statement, including addenda in effect as of the beginning date of the period of performance. See HHS
website, http://www.hhs.gov/grants/grants/get-ready-for-grants-management/index.html, for agency-specific grant
management guidance. The following is excepted from the HHS Grants Policy Statement:
a. The right to initiate an automatic one-time extension of the end date is replaced by the need to obtain prior written
approval from the PTE.
b. Any prior approvals are to be sought from the PTE and not the Federal Awarding Agency.
5. Equipment title and accountability: For-profit subrecipients of HHS grants are non-exempt and subject to the requirements of
45 CFR 75.320, as well as the conditions set forth in 45 CFR 75.316 6 – 75.323 (Property Standards), and 45 CFR 75.327 –
75.335 (Procurement Standards).
6. Treatment of Program Income: Additive Other, Pass-through Entity specify alternative from HHS Agreement
7. Travel shall be in accordance with Federal Travel Regulation (http://www.gsa.gov/federaltravelregulation) and HHS Grants
Policy Statement Parts II-42, II-43 (Cost Considerations, Allowable Costs and Activities), and the Fly America Act (49 USC
40118) and Open Skies Agreements as detailed at http://www.gsa.gov/portal/content/103191.

PHS-Specific Requirements Promoting Objectivity in Research Applicable to Subrecipients (42 CFR
Par 50 Subpart F)
a) 42 CFR Part 50. 604 requires that institutions conducting PHS-funded research “Maintain an up-to-date, written, enforced
policy on financial conflicts of interest.” Further, “If the Institution carries out the PHS-funded research through a subrecipient
(e.g., subcontractors or consortium members), the Institution (awardee Institution) must take reasonable steps to ensure that
any subrecipient Investigator complies with this subpart by incorporating as part of a written agreement with the subrecipient
terms that establish whether the financial conflicts of interest policy of the awardee Institution or that of the subrecipient will
apply to the subrecipient’s Investigators.”
Subrecipient must designate herein whether the financial conflicts of interest policy of x Pass-through Entity
Institution, or ____________ Subrecipient Institution (check one) will apply. If applying its own financial conflicts of interest
policy, by execution of this Subaward Agreement, Subrecipient Institution certifies that its policy complies with 42 CFR
Part 50.
b) Subrecipient shall report any financial conflict of interest to Pass-through Entity’s Administrative Representative, as
designated on Attachment 3A. Any financial conflicts of interest identified shall subsequently be reported to HHS. Such report
shall be made before expenditure of funds authorized in this Subaward Agreement and within 45 days of any subsequently
identified financial conflict of interest.
Special terms and conditions:
1. Automatic Carry Forward: [ ] Yes [ x ] No
(If No, Carry Forward requests must be sent to Pass-through Entity’s Administrative contact, as shown in Attachment 3.)
2. In accordance with 48 CFR 3.908 Pilot Program for Enhancement of Contractor Employee Protections, Subrecipient is hereby
notified that they are required to:
a. Inform their employees working on any Federal award that they are subject to the whistleblower rights and remedies
of the pilot program;
b. Inform their employees in writing of employee whistleblower protections under 41 U.S.C §4712 in the predominant
native language of the workforce; and
c. Contractors and grantees will include such requirements in any agreement made with a subcontractor or subgrantee.
3. Rebudgeting:
a. Rebudgeting of any direct cost budget line ≥10% requires PTE prior approval.
b. Addition of any new contracted service ≥%50,000 not included in the approved budget that represents a <10%
change in the direct cost category (contracted services) requires contemporaneous notification to PTE.
c. Addition of any new contracted service ≥$50,000 not included in the approved budget that represents a ≥10% change
in the direct cost category (contracted services) requires PTE prior approval per 3.a above.
4. Trafficking in Persons - Required flow-down in full text:
a. Provisions applicable to a recipient that is a private entity.
1. You as the recipient, your employees, subrecipients under this award, and subrecipients’ employees may not –
i. Engage in severe forms of trafficking in persons during the period of time that the award is in effect;
ii. Procure a commercial sex act during the period of time that the award is in effect; or
iii. Use forced labor in the performance of the award or subawards under the award.
See Att 2a (PTE NOA), General Terms and Conditions #16 for the complete clause on Trafficking in Persons.
5. Publications:
See Att 2a/General Terms and Conditions #18 for required acknowledgment and disclaimer. See also Attachment 6,
“Publication and Open Access.”
Abstracts or manuscripts from any CARB-X partner involving CARB-X data must be coordinated with PTE PI at the
beginning of the writing process. Subrecipient will provide the PTE with advance copies of all manuscripts related to the
Project when they are submitted or re-submitted for publication. PI will have no role in the preparation, editing or approval of
the manuscript.

6. Press Releases: See also Attachment 6, “Publication and Open Access.” Any press release directly relating to CARB-X will be
coordinated in advance with the PTE PI. Subrecipient will provide the PTE PI with advance copies of all press releases related
to the Project at least five (5) days before release.
7. Funding Restrictions: All salaries paid under the Subaward Agreement (including cost-sharing) are capped at the rate of
Executive Level II. Pre-award costs are not allowed. See Att 2a, CARB-X Terms and Conditions/Other Terms for complete
list. Facilities and Administrative Costs (F&A) are unallowable for any foreign Subrecipient.
8. Copyrights
Subrecipient x grants / shall grant (check one) to PTE an irrevocable, royalty-free, non-transferable, non-exclusive right
and license to use, reproduce, make derivative works, display, and perform publicly any copyrights or copyrighted material
(including any computer software and its documentation and/or databases) first developed and delivered under this Subaward
Agreement solely for the purpose of and only to the extent required to meet PTE’s obligations to the Federal Government
under its Prime Award. Per 45 CFR 75 .322(b), the subrecipient may copyright any work that is subject to copyright and was
developed, or for which ownership was acquired, under a Federal award. HHS/ASPR reserves a royalty-free, non-exclusive
and irrevocable right to reproduce, publish, or otherwise use the work for Federal purposes, and to authorize others to do so.
9. Data Rights
Subrecipient grants to PTE the right to use data created in the performance of this Subaward Agreement solely for the purpose
of and only to the extent required to meet PTE’s obligations to the Federal Government under its Prime Award. Per 45 CFR
75.322(d), the Federal Government has the right to: (1) obtain, reproduce, publish, or otherwise use the data produced under a
Federal award; and (2) authorize others to receive, reproduce, publish, or otherwise use such data for Federal purposes.
10. Cost Principles
Subrecipient must operate the Subaward Agreement under the Federal Cost Principles set forth in Subpart 31.2 of the Federal
Acquisition Regulations (48 CFR Subpart 31.2) applicable to commercial organizations, found at
https://www.acquisition.gov/far/html/Subpart%2031_2 html.
11. Intellectual Property
See Att 2a (PTE NOA), CARB-X Terms & Conditions/Other Terms, “Intellectual Property.”
12. Lower-Tier Subawards
Subrecipient may not issue any Subawards under this Subaward Agreement without the express prior written consent of PTE.
The requirement for prior approval does not include contracted services.
13. Other Research-Related Activities
This Subaward Agreement does not include research involving human embryonic stem cell research and cloning or research on
transplantation of human fetal tissue.
14. CARB-X Special Terms & Conditions
This Subaward Agreement is subject to the provisions set forth in the CARB-X Special Terms and Conditions, incorporated
herein as Attachment 6.
15. Fringe Benefits
If Subrecipient does not have federally negotiated rate agreement including fringe benefits rates, invoices must reflect actual
fringe benefits costs.
16. Disputes (this clause applicable to foreign subrecipients only)
The parties shall attempt to resolve disputes through good faith negotiations. Any dispute arising under or related to this
Subaward Agreement shall be resolved to the maximum possible extent through informal dispute resolution. Unresolved issues
shall be arbitrated in accordance with the International Arbitration Rules of the American Arbitration Association.
17. Governing Language
In the event that a translation of this Subaward Agreement is prepared and signed by the parties, this English language version
shall be the official version and shall govern if there is a conflict between this English language version and the translation. All
disputes under this Subaward Agreement shall be resolved and conducted, regardless of the means or authority, in the English
language.
18. Governing Law

This Agreement shall be governed, construed and enforced for all purposes in accordance with the laws of The Commonwealth
of Massachusetts, without regard to such laws governing choice of law. Subrecipient acknowledges that PTE is subject to the
laws of the United States and PTE will not be obligated to take any action that is violative of such laws.
19. Export Control
It is understood that PTE is subject to United States laws and regulations controlling the export of technical data, computer
software, laboratory prototypes and other commodities, and that its obligations hereunder are contingent on compliance with
applicable U.S. export laws and regulations (including the International Traffic in Arms Regulations (ITAR) and Export
Administration Regulations (EAR) in activities under this Subaward Agreement. In the event that Subrecipient intends to
provide any technical information, computer software, laboratory prototypes. or other items controlled under the applicable
U.S. export control laws, the Subrecipient shall first notify PTE of its intent to provide such export-controlled items or
information and shall not transfer the export-controlled items or information until PTE’s Authorized Representative agrees in
writing to accept. Prior to the transfer of any export-controlled items or information (excluding items or information
designated as EAR99 under the EAR), recipient shall conspicuously designate such items or information as “Export
Controlled” and identify the applicable export control category under the United States Munitions List (ITAR) or ECCN under
the Commerce Control List (EAR). The transfer of any such items may require a license or authorization from the cognizant
agency of the United States Government, and/or may require written assurances by the receiving party that it shall not re-
export such items to certain foreign destinations and/or to certain recipients without prior approval of the cognizant
government agency, and/or may require the involved individuals and entities comply with certain conditions. PTE cannot
guarantee that such licenses will be granted.
20. Anti-terrorist Compliance
Subrecipient hereby agrees that all funds, including subawards to subrecipients, will be used in compliance with all applicable
United States anti-terrorist financing and asset control laws, regulations, rules and executive orders.
21. Required Certification for Invoices (see face page Article 2)
“By signing this report. I certify to the best of my knowledge and belief that the report is true, complete, and accurate, and
the expenditures, disbursements and cash receipts are for the purposes and objectives set forth in the terms and conditions
of the Federal award. I am aware that any false, fictitious, or fraudulent information, or the omission of any material fact,
may subject me to criminal, civil or administrative penalties for fraud, false statements, false claims or otherwise. (U.S.
Code Title 18, Section 1001 and Title 31, Sections 3729-3730 and 3801-3812).”

Attachment 2a
Subaward Agreement
PTE Federal Award from BARDA (HHS/ASPR)

Att 2a, Page 1 of 44
Attachment 2a
1. DATE ISSUED
MM/DD/YYYY
07/27/2016
2. CFDA NO.
93.360
3. ASSISTANCE TYPE
Cooperative Agreement
DEPARTMENT OF HEALTH AND HUMAN SERVICES
ASSISTANT SECRETARY FOR PREPAREDNESS & RESPONSE
ASPR Acquisition Management Contracts and Grants
200 C Street, SW
Washington, DC 20024
NOTICE OF AWARD
AUTHORIZATION (Legislation/Regulations)
Pub. L. 109-148 119 Stat. 2680, 2786 (2005)
1a. SUPERSEDES AWARD NOTICE dated
except that any additions or restrictions previously imposed remain
in effect unless specifically rescinded
4. GRANT NO.
1 IDSEP160030-01-00
Formerly
5. ACTION TYPE
New
6. PROJECT PERIOD MM/DD/YY
From 08/01/2016
MM/DD/YY
Through 07/31/2021
7. BUDGET PERIOD MM/DD/YY
From 08/01/2016
MM/DD/YY
Through 07/31/2017
8. TITLE OF PROJECT (OR PROGRAM)
CARB-X
9a. GRANTEE NAME AND ADDRESS
Trustees of Boston University
881 Commonwealth Ave
Boston Univ School of Law
Boston, MA 02215-1390
9b. GRANTEE PROJECT DIRECTOR
Prof. Michael Kevin Outterson
881 Commonwealth Avenue
Boston, MA 02215-1300
Phone: 617-935-6517
10a. GRANTEE AUTHORIZING OFFICIAL
Mrs. Diane Baldwin
881 Commonwealth Avenue
Boston, MA 02215-1300
Phone: 617-638-4600
10b. FEDERAL PROJECT OFFICER
Tyler Merkeley
200 Independence Ave., S.W.
Room 638-G
Washington, DC 20201
Phone: 202-679-9539
ALL AMOUNTS ARE SHOWN IN USD
11. APPROVED BUDGET (Excludes Direct Assistance) 12. AWARD COMPUTATION
I Financial Assitance from the Federal Awarding Agency Only
II Total project costs including grant funds and all other financial participation
a. Amount of Federal Financial Assistance (from item 11m) 30,000,000.00
a. Salaries and Wages ................... 327,261.00 b. Less Unobligated Balance From Prior Budget Periods 0.00
b. Fringe Benefits ................... 86,860.00 c. Less Cumulative Prior Award(s) This Budget Period 0.00
c. Total Personnel Costs .................... 414,121.00 d. AMOUNT OF FINANCIAL ASSISTANCE THIS ACTION 30,000,000.00
d. Equipment ........................................... 0.00 13. Total Federal Funds Awarded to Date for Project Period 30,000,000.00
e. Supplies ........................................... 5,000.00 14. RECOMMENDED FUTURE SUPPORT
(Subject to the availability of funds and satisfactory progress of the pojrect):
f. Travel ........................................... 51,004.00
g. Construction ........................................... 0.00 YEAR TOTAL DIRECT COSTS YEAR TOTAL DIRECT COSTS
h. Other ........................................... 158,600.00 a. 2 55,000,000.00 d. 5 55,000,000.00
i. Contractual ........................................... 69,734,943.00 b. 3 55,000,000.00 e. 6
j. TOTAL DIRECT COSTS 70,363,668.00 c. 4 55,000,000.00 f. 7
k. INDIRECT COSTS 776,403.00 15. PROGRAM INCOME SHALL BE USED IN ACCORD OF THE FOLLOWING
ALTERNATIVES
a. DEDUCTION
b. ADDITIONAL COSTS
c. MATCHING
d. OTHER RESEARCH (Add / Deduct Option)
e. OTHER (See REMARKS)
l. TOTAL APPROVED BUDGET 71,140,071.00
m. Federal Share 30,000,000.00 16. THIS AWARD IS BASED ON AN APPLICATION SUBMITTED TO, AND AS APPROVED BY, THE FEDERAL AWARDING AGENCY ON THE ABOVE TITLED PROJECT AND IS SUBJECT TO THE TERMS AND CONDITIONS INCORPORATED EITHER
DIRETLY OR BY REFERENCE IN THE FOLLOWING:
a. The grant program legislation
b. The grant program regulations.
c. This award notice including terms and conditions, if any, noted below under REMARKS
d. Federal administrative requirements, cost principles and audit requirements applicable to this grant
In the event there are conflicting or otherwise inconsistent policies applicable to the grant, the above order of precedence shall
prevail. Acceptance of the grant terms and conditions is acknowledged by the grantee when funds are drawn or otherwise
obtained from the grant payment system.
n. Non-Federal Share 41,140,071.00
REMARKS (Other Terms and Conditions Attached – Yes No)
GRANTS MANAGEMENT OFFICIAL Virginia Simmons, Chief Grants Management Officer
17. OBJ CLASS 41.22 18a. VENDOR CODE 1042103547C9 18b. EIN 042103547 19. DUNS 049435266 20. CONG. DIST. 07
FY-ACCOUNT NO. DOCUMENT NO. ADMINISTRATIVE CODE AMT ACTION FIN ASST APPROPRIATION
21. a. 6-1992016 b. IDESEP0030A c. HEP-ID d. $30,000,000.00 e. 75-1617-0140
22. a. b. c. d. e.
23. a. b. c. d. e.

Att 2a, Page 2 of 44
1. DATE ISSUED
MM/DD/YYYY
09/16/2016
2. CFDA NO.
93.360
3. ASSISTANCE TYPE
Cooperative Agreement
DEPARTMENT OF HEALTH AND HUMAN SERVICES
ASSISTANT SECRETARY FOR PREPAREDNESS & RESPONSE
ASPR Acquisition Management Contracts and Grants
200 C Street, SW
Washington, DC 20024
NOTICE OF AWARD
AUTHORIZATION (Legislation/Regulations)
Pub. L. 109-148 119 Stat. 2680, 2786 (2005)
1a. SUPERSEDES AWARD NOTICE dated 07/28/2016
except that any additions or restrictions previously imposed remain
in effect unless specifically rescinded
4. GRANT NO.
6 IDSEP160030-01-01
Formerly
5. ACTION TYPE
Post Award
Amendment
6. PROJECT PERIOD MM/DD/YY
From 08/01/2016
MM/DD/YY
Through 07/31/2021
7. BUDGET PERIOD MM/DD/YY
From 08/01/2016
MM/DD/YY
Through 07/31/2017
8. TITLE OF PROJECT (OR PROGRAM)
CARB-X
9a. GRANTEE NAME AND ADDRESS
Trustees of Boston University
881 Commonwealth Ave
Boston Univ School of Law
Boston, MA 02215-1390
9b. GRANTEE PROJECT DIRECTOR
Prof. Michael Kevin Outterson
881 Commonwealth Avenue
Boston, MA 02215-1300
Phone: 617-935-6517
10a. GRANTEE AUTHORIZING OFFICIAL
Mrs. Diane Baldwin
881 Commonwealth Avenue
Boston, MA 02215-1300
Phone: 617-638-4600
10b. FEDERAL PROJECT OFFICER
Tyler Merkeley
200 Independence Ave., S.W.
Room 638-G
Washington, DC 20201
Phone: 202-679-9539
ALL AMOUNTS ARE SHOWN IN USD
11. APPROVED BUDGET (Excludes Direct Assistance) 12. AWARD COMPUTATION
I Financial Assitance from the Federal Awarding Agency Only
II Total project costs including grant funds and all other financial participation
a. Amount of Federal Financial Assistance (from item 11m) 30,000,000.00
a. Salaries and Wages ................... 327,261.00 b. Less Unobligated Balance From Prior Budget Periods 0.00
b. Fringe Benefits ................... 86,860.00 c. Less Cumulative Prior Award(s) This Budget Period 0.00
c. Total Personnel Costs .................... 414,121.00 d. AMOUNT OF FINANCIAL ASSISTANCE THIS ACTION 30,000,000.00
d. Equipment ........................................... 0.00 13. Total Federal Funds Awarded to Date for Project Period 30,000,000.00
e. Supplies ........................................... 5,000.00 14. RECOMMENDED FUTURE SUPPORT
(Subject to the availability of funds and satisfactory progress of the pojrect):
f. Travel ........................................... 51,004.00
g. Construction ........................................... 0.00 YEAR TOTAL DIRECT COSTS YEAR TOTAL DIRECT COSTS
h. Other ........................................... 158,600.00 a. 2 55,000,000.00 d. 5 55,000,000.00
i. Contractual ........................................... 69,734,943.00 b. 3 55,000,000.00 e. 6
j. TOTAL DIRECT COSTS 70,363,668.00 c. 4 55,000,000.00 f. 7
k. INDIRECT COSTS 776,403.00 15. PROGRAM INCOME SHALL BE USED IN ACCORD OF THE FOLLOWING
ALTERNATIVES
a. DEDUCTION
b. ADDITIONAL COSTS
c. MATCHING
d. OTHER RESEARCH (Add / Deduct Option)
e. OTHER (See REMARKS)
l. TOTAL APPROVED BUDGET 71,140,071.00
m. Federal Share 30,000,000.00 16. THIS AWARD IS BASED ON AN APPLICATION SUBMITTED TO, AND AS APPROVED BY, THE FEDERAL AWARDING AGENCY ON THE ABOVE TITLED PROJECT AND IS SUBJECT TO THE TERMS AND CONDITIONS INCORPORATED EITHER
DIRETLY OR BY REFERENCE IN THE FOLLOWING:
a. The grant program legislation
b. The grant program regulations.
c. This award notice including terms and conditions, if any, noted below under REMARKS
d. Federal administrative requirements, cost principles and audit requirements applicable to this grant
In the event there are conflicting or otherwise inconsistent policies applicable to the grant, the above order of precedence shall
prevail. Acceptance of the grant terms and conditions is acknowledged by the grantee when funds are drawn or otherwise
obtained from the grant payment system.
n. Non-Federal Share 41,140,071.00
REMARKS (Other Terms and Conditions Attached – Yes No)
This amendment reflects changes to the administrative and programmatic terms and conditions.
GRANTS MANAGEMENT OFFICIAL Virginia Simmons, Chief Grants Management Officer
17. OBJ CLASS 41.15 18a. VENDOR CODE 1042103547C9 18b. EIN 042103547 19. DUNS 049435266 20. CONG. DIST. 07
FY-ACCOUNT NO. DOCUMENT NO. ADMINISTRATIVE CODE AMT ACTION FIN ASST APPROPRIATION
21. a. 6-1992016 b. IDESEP0030A c. HEP-ID d. $0.00 e. 75-1617-0140
22. a. b. c. d. e.
23. a. b. c. d. e.

Att 2a, Page 3 of 44
1. DATE ISSUED
MM/DD/YYYY
01/12/2017
2. CFDA NO.
93.360
3. ASSISTANCE TYPE
Cooperative Agreement
DEPARTMENT OF HEALTH AND HUMAN SERVICES
ASSISTANT SECRETARY FOR PREPAREDNESS & RESPONSE
ASPR Acquisition Management Contracts and Grants
200 C Street, SW
Washington, DC 20024
NOTICE OF AWARD
AUTHORIZATION (Legislation/Regulations)
Pub. L. 109-148 119 Stat. 2680, 2786 (2005)
1a. SUPERSEDES AWARD NOTICE dated 09/16/2016
except that any additions or restrictions previously imposed remain
in effect unless specifically rescinded
4. GRANT NO.
6 IDSEP160030-01-02
Formerly
5. ACTION TYPE
Post Award
Amendment
6. PROJECT PERIOD MM/DD/YY
From 08/01/2016
MM/DD/YY
Through 07/31/2021
7. BUDGET PERIOD MM/DD/YY
From 08/01/2016
MM/DD/YY
Through 07/31/2017
8. TITLE OF PROJECT (OR PROGRAM)
CARB-X
9a. GRANTEE NAME AND ADDRESS
Trustees of Boston University
881 Commonwealth Ave
Boston Univ School of Law
Boston, MA 02215-1390
9b. GRANTEE PROJECT DIRECTOR
Prof. Michael Kevin Outterson
881 Commonwealth Avenue
Boston, MA 02215-1300
Phone: 617-935-6517
10a. GRANTEE AUTHORIZING OFFICIAL
Mrs. Diane Baldwin
881 Commonwealth Avenue
Boston, MA 02215-1300
Phone: 617-638-4600
10b. FEDERAL PROJECT OFFICER
Tyler Merkeley
200 Independence Ave., S.W.
Room 638-G
Washington, DC 20201
Phone: 202-679-9539
ALL AMOUNTS ARE SHOWN IN USD
11. APPROVED BUDGET (Excludes Direct Assistance) 12. AWARD COMPUTATION
I Financial Assitance from the Federal Awarding Agency Only
II Total project costs including grant funds and all other financial participation
a. Amount of Federal Financial Assistance (from item 11m) 30,000,000.00
a. Salaries and Wages ................... 463,195.00 b. Less Unobligated Balance From Prior Budget Periods 0.00
b. Fringe Benefits ................... 116,058.00 c. Less Cumulative Prior Award(s) This Budget Period 0.00
c. Total Personnel Costs .................... 579,253.00 d. AMOUNT OF FINANCIAL ASSISTANCE THIS ACTION 30,000,000.00
d. Equipment ........................................... 0.00 13. Total Federal Funds Awarded to Date for Project Period 30,000,000.00
e. Supplies ........................................... 9,000.00 14. RECOMMENDED FUTURE SUPPORT
(Subject to the availability of funds and satisfactory progress of the pojrect):
f. Travel ........................................... 75,764.00
g. Construction ........................................... 0.00 YEAR TOTAL DIRECT COSTS YEAR TOTAL DIRECT COSTS
h. Other ........................................... 396,800.00 a. 2 55,000,000.00 d. 5 55,000,000.00
i. Contractual ........................................... 65,693,536.00 b. 3 55,000,000.00 e. 6
j. TOTAL DIRECT COSTS 66,754,353.00 c. 4 55,000,000.00 f. 7
k. INDIRECT COSTS 958,352.00 15. PROGRAM INCOME SHALL BE USED IN ACCORD OF THE FOLLOWING
ALTERNATIVES
a. DEDUCTION
b. ADDITIONAL COSTS
c. MATCHING
d. OTHER RESEARCH (Add / Deduct Option)
e. OTHER (See REMARKS)
l. TOTAL APPROVED BUDGET 67,712,705.00
m. Federal Share 30,000,000.00 16. THIS AWARD IS BASED ON AN APPLICATION SUBMITTED TO, AND AS APPROVED BY, THE FEDERAL AWARDING AGENCY ON THE ABOVE TITLED PROJECT AND IS SUBJECT TO THE TERMS AND CONDITIONS INCORPORATED EITHER
DIRETLY OR BY REFERENCE IN THE FOLLOWING:
a. The grant program legislation
b. The grant program regulations.
c. This award notice including terms and conditions, if any, noted below under REMARKS
d. Federal administrative requirements, cost principles and audit requirements applicable to this grant
In the event there are conflicting or otherwise inconsistent policies applicable to the grant, the above order of precedence shall
prevail. Acceptance of the grant terms and conditions is acknowledged by the grantee when funds are drawn or otherwise
obtained from the grant payment system.
n. Non-Federal Share 37,712,705.00
REMARKS (Other Terms and Conditions Attached – Yes No)
This amendment provides a revised budget. All previous terms and conditions remain the same.
GRANTS MANAGEMENT OFFICIAL Virginia Simmons, Chief Grants Management Officer
17. OBJ CLASS 41.15 18a. VENDOR CODE 1042103547C9 18b. EIN 042103547 19. DUNS 049435266 20. CONG. DIST. 07
FY-ACCOUNT NO. DOCUMENT NO. ADMINISTRATIVE CODE AMT ACTION FIN ASST APPROPRIATION
21. a. 6-1992016 b. IDESEP0030A c. HEP-ID d. $0.00 e. 75-1617-0140
22. a. b. c. d. e.
23. a. b. c. d. e.
Att 2a, Page 4 of 44
NOTICE OF AWARD (Continuation Sheet)
PAGE 2 of 2 DATE ISSUED
01/12/2017
GRANT NO. 6 IDSEP160030-01-02
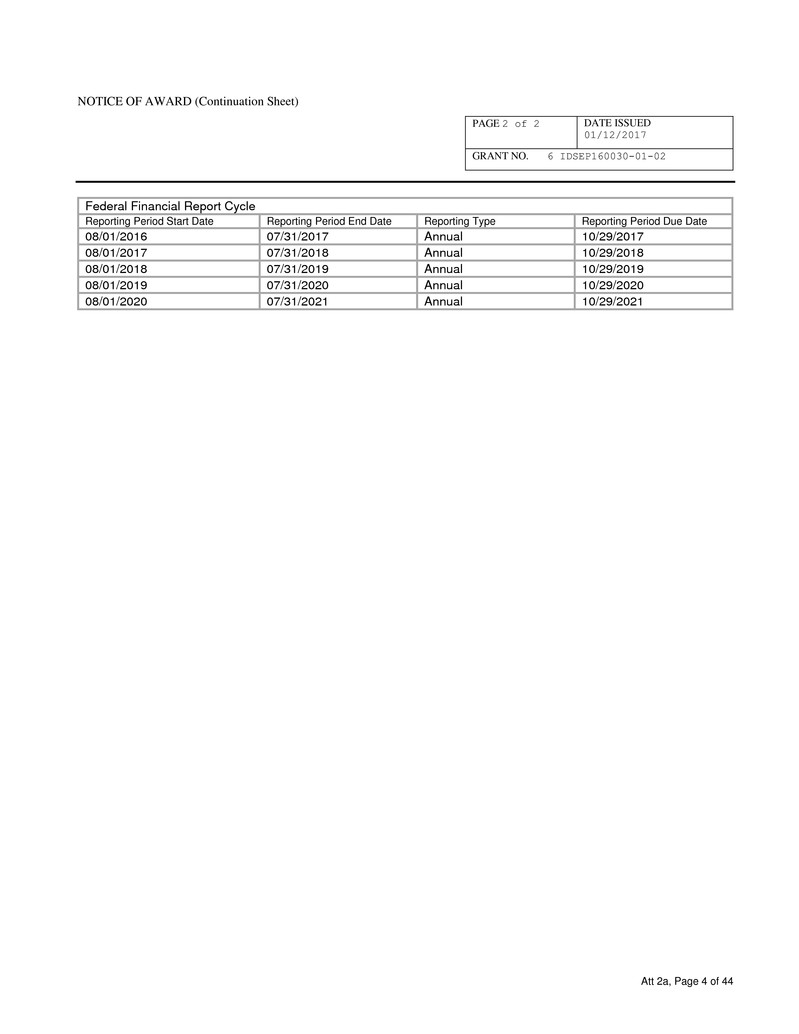
Federal Financial Report Cycle
Reporting Period Start Date Reporting Period End Date Reporting Type Reporting Period Due Date
08/01/2016 07/31/2017 Annual 10/29/2017
08/01/2017 07/31/2018 Annual 10/29/2018
08/01/2018 07/31/2019 Annual 10/29/2019
08/01/2019 07/31/2020 Annual 10/29/2020
08/01/2020 07/31/2021 Annual 10/29/2021

Att 2a, Page 5 of 44
AWARD ATTACHMENTS
Trustees of Boston University 6 IDSEP160030-01-02
1. CARB-X Terms and Conditions

***Confidential Treatment Requested
PROGRAM OVERVIEW
Authority
This cooperative agreement is issued under the authority of Section 301 of the Public Health Service
(PHS) Act (42 U.S.C. 241), “Research and Investigations”; Section 319L of the PHS Act (42 U.S.C.
247d-7e), “Biomedical Advanced Research and Development Authority”; 14Section 405 of the PHS
Act (42 U.S.C. 284), “Authority of the Directors of the National Research Institutes”; Section 446 of the
PHS Act (42 U.S.C. 285f), “National Institute of Allergy and Infectious Diseases, Purpose of Institute.”
By receiving funds under this award, the recipient assures that it will carry out the project/program as
authorized and will comply with the terms and conditions and other requirements of this award.
Project Objective:
The current pipeline of candidate antimicrobial products is insufficient to counter the threat of
antimicrobial resistance1. A novel collaborative model is needed to spur innovation and investment
towards new antimicrobial products to repopulate the early development pipeline. In 2014, the United
States Government released the National Strategy for Combating Antimicrobial Resistant Bacteria. A
component of the National Strategy is to establish a Biopharmaceutical Accelerator2 for Combating
Antibiotic-Resistant Bacteria [Accelerator] to fund Research and Development (R&D) activities to help
progress candidate products from the proof-of-concept stage through pre-clinical development.
Candidates that graduate from the Accelerator will be better positioned for R&D investment and clinical
development. There are various Accelerator3 models in the marketplace. The Biomedical Advanced
Research and Development Authority (BARDA) and the National Institute of Allergy and Infectious
Diseases (NIAID) designed the Accelerator to will be a non-equity accelerator4 that provides non-
dilutive
1 Antimicrobial Resistance: A microbial internal mechanism(s) that confers resistance or ineffectiveness to an
antimicrobial product(s).
2 The 2014 National Strategy for Combating Antimicrobial Resistant Bacteria discusses a "biopharmaceutical incubator."
Following market research and discussion with key stakeholders the program was renamed the biopharmaceutical
accelerator as referred to in this document.
3 The use of the word "Accelerator" in this FOA implies an entity that provides unique and highly flexible combination of
technical support, business development processes, infrastructure, and people designed to nurture new and small
businesses grow through the difficult and vulnerable early stages of development. The term implies capabilities typical
of both Accelerators and Incubators. Both types of existing organizations may have the ability to augment their existing
capabilities and acquire remaining capabilities discussed in this FOA to respond to this announcement.
4 An accelerator that does not take an equity position in the companies it supports through its partnership with NIAID and
BARDA.

Att 2a, Page 7 of 44
funding5 to product developers for R&D activities and enables the product developers to retain full
ownership and control of their company. The initiative will be focusing only on antibacterial products.
BARDA and NIAID stimulate public good through establishment of a public-private partnership with the
Trustees of Boston University. Under this notice of award, Trustees of Boston University will assemble
an international collaboration bringing together four separate life science accelerators to form a global
antibacterial innovation initiative to accelerate the pace of preclinical product development antibacterial
products to combat resistant bacteria. The partnership unknown as Combatting Antibiotic-Resistant
Bacteria Biopharmaceutical Accelerator (CARB-X) will be led by the Trustees of Boston University, a
non-profit research university and a member in the Association of American Universities. The Principal
Investigator is Professor Kevin Outterson, Boston University School of Law. Trustees of Boston
University and brings together four accelerators, Wellcome Trust and AMRC (U.K.), MassBio and the
California Life Sciences Institute (CLSI). CARB-X will focus on rejuvenating the antibacterial pipeline
by providing nondilutive financing, Research and Development (R&D) support, and business
mentoring services to innovative product developers worldwide to accelerate the pace of preclinical
antibacterial product development to combat resistant bacteria. Product developers retain full
ownership and control of their company. These services are provided through several Accelerators
(described below in Section 2).
BARDA will provide direct funding and NIAID will provide in-kind services (e.g. preclinical services,
technical expertise) to CARB-X [the cooperative agreement recipient]. CARB-X will manage a portfolio
of investments of early stage antimicrobial product6 candidates. Antimicrobial products may enter the
CARB-X at proof of concept, optimization, pre-clinical development stages or after IND filing. CARB-X
will assist/expedite the development of these products through IND filing such that they are poised for
clinical development outside the accelerator. The CARB-X will fund products across a broad
technology landscape (e.g. bacterial vaccines, therapeutics, devices and diagnostics) to align with
BARDA and NIAID’s strategic goals.
CARB-X will focus on:
Funding development of antimicrobial products to further enhance the pipeline,
Offering a suite of capabilities to rapidly shuttle successful product candidates through
early development,
Provide business and drug development guidance,
Decrease the risks and barriers that impact further R&D investment by pharmaceutical
companies, private investors and government partners.
5 Non-dilutive funding is financing that does not require the sale of your company's shares, and hence does not cause
dilution of the existing shareholders.
6 Antimicrobial Products: A product that supports the treatment, prevention, or detection of a microbial infection across
various technology landscapes including but not limited to therapies, preventions, vaccines, diagnostics, devices, and
other innovations.

Att 2a, Page 8 of 44
CARB-X has the opportunity to leverage four existing life science accelerators, a consortium of
academic, biotechnology, and pharmaceutical companies to fund R&D activities to help progress
antimicrobial candidate products and aid these developers in overcoming business hurdles that
smaller companies and academic start-ups currently face. By leveraging an industry Accelerator
model, CARB-X will rapidly fund product candidates, support technical milestone driven management,
and efficient down selection of less promising candidates. The focus of CARB-X will be the support of
activities that incentivize development in the field and rapidly assess whether a proof-of-concept or
prototype technology will lead to an antimicrobial product candidate. Since there are various
Accelerator models it is important to define the specific Accelerator model issued under the award of
this cooperative agreement. CARB-X will be a non-equity non-government entity that provides non-
dilutive funding to product developers for R&D activities and enables the product developers to retain
full ownership and control of their company. CARB-X or any of its Alliance or Partner accelerators may
not take an equity position in any companies the entity funds supported by this cooperative agreement.
Accelerators ICARB-X] Capabilities
Under this award, CARB-X must at maintain the below qualifications, experience and capability and/or
have the ability to provide the following:
Ability to provide functions typical of a Life Science Accelerator
CARB-X will leverage four existing life science accelerators, a consortium of academic,
biotechnology, and pharmaceutical companies to fund R&D activities to help progress
antimicrobial candidate products and aid these developers in overcoming business
hurdles that smaller companies and academic start-ups currently face.
Ability to manage an extensive portfolio (15-20) antimicrobial product candidates.
An accelerator’s ability to provide Directed Research:
Conduct research and development on new therapeutics and vaccines, first-in class
drugs, and new combination therapies for the treatment or prevention of resistant
bacterial infections.
Develop non-traditional therapeutics and innovative strategies to treat or prevent resistant
bacterial infections. Examples could include: targeting bacterial virulence factors,
resistance genes, using phage or phage derived lysins, development of monoclonal or
polyclonal antibodies, developing products that restore or preserve beneficial bacteria in
humans (e.g. probiotics, prebiotics, or synthetic [sense and respond] microbiota), and
new natural products and synthetic scaffolds with antibiotic activity.
Conduct research and development on new diagnostics, including tests that rapidly
distinguish between viral and bacterial pathogens and tests that detect presence of
specific antibiotic resistance determinants such that targeted antimicrobials can be used
to reduce misuse of antibiotics. These diagnostics can then be implemented in a wide
range of settings.

Att 2a, Page 9 of 44
An accelerator’s ability to provide Internal Capabilities;
Provide industry standard services and capabilities commensurate with the stage of
candidate development.
Leverage a scientific advisory board comprised of subject matter experts in antimicrobial
product development, diagnostics for antibiotic resistance bacteria, and vaccines against
bacterial pathogens.
Provide scientific and technical expertise in the area of medicinal chemistry, preclinical
testing, pharmacokinetics, toxicology, and manufacturing (e.g. for IND enabling studies
and clinical trial material).
Business consulting services that function to aid early stage entrepreneurs in establishing
a business plan/model and position the company for external opportunities. The
accelerator should provide business, financial, strategic consulting services while also
linking entrepreneurs with key stakeholders developing products and technologies to
combat antimicrobial resistance. These services should help accelerate innovation and
mitigate corporate and business development risks inherent to startup companies.
A physical and virtual environment that is programmed to increase collaboration and spur
antimicrobial product innovation by leveraging the best practices from incubators,
accelerators, technical hubs, co-working sites.
Access to translational partners for advanced development including venture capital (VC)
firms, other product development partnerships, large pharmaceutical companies, etc.
Incentivizing strategies to bring in new researchers and candidates to work with VC and
Pharma.
Mechanisms to incentivize innovation and identify early potential research through
workshops, hosted challenges, tech days, seminars, etc.
An accelerator’s ability to provide External Capabilities:
A robust and extensive R&D network of subcontractors to support preclinical studies,
toxicology studies, pharmacokinetic studies, ADME studies, and animal efficacy and
challenge studies.
A robust and extensive R&D network of subcontractors to conduct medicinal chemistry for
lead optimization, small scale chemical synthesis, cGMP chemical manufacturing, cGMP
fermentation/production, biologic and vaccine manufacturing.
A robust R&D network of subcontractors that are capable of diagnostic development and
engineering, including characterization and feasibility demonstration of diagnostic targets,
test system development and critical design requirements, reagent development, non-
GLP prototype development, and pilot scale manufacturing.
A robust and extensive R&D network of subcontractors capable of developing analytical
methods and assays suitable to support process control.
Regulatory support to assist in the preparation of regulatory submissions [e.g.
Investigational New Drug (IND) or Investigational Device Exemption (IDE) applications].

Att 2a, Page 10 of 44
Access to innovators and industry partners and the ability to leverage partnerships and
resources to successfully advance promising products for follow-on R&D investment,
including business capital and USG funding.
Portfolio Priorities
The overall scope of this project for cooperative agreement is to stimulate public good by providing
funding to accelerate antimicrobial product development. On an annual basis, BARDA and NIAID will
outline the gaps to the CARB-X that the agencies seek to address with their funding and provide
recommendations towards focus areas and potential technology types that may fulfill those gaps.
BARDA and NIAID will not identify specific candidates but, rather, strategic priorities.
The priorities may adjust from year to year. BARDA and NIAID provide the Year 1 priorities below for
planning and consideration.
Year 1 Portfolio Priorities:
Antimicrobial products to target gram negative infections on the “Urgent” and “Serious” list
as defined in the CDC report [CDC Antibiotic Resistance Threats in the US, 2013 ]
Non-traditional products to target and prevent antimicrobial infections on the “Urgent” and
“Serious” list as defined in the CDC report [CDC Antibiotic Resistance Threats in the US,
2013 ]
Budget and Cost Sharing:
As part of the FOA, BARDA encouraged Applicants to propose innovative ways to leverage federal
funding to attract additional capabilities/incentives to bolster the Accelerator’s portfolio and success.
This may include identification of external opportunities or leveraging funding at the state or local level
(e.g. public, private, etc.) BARDA envisioned that every $1 of BARDA funding would be matched by up
to $2 of non-dilutive funding contributed by Alliance members of the Accelerator.7
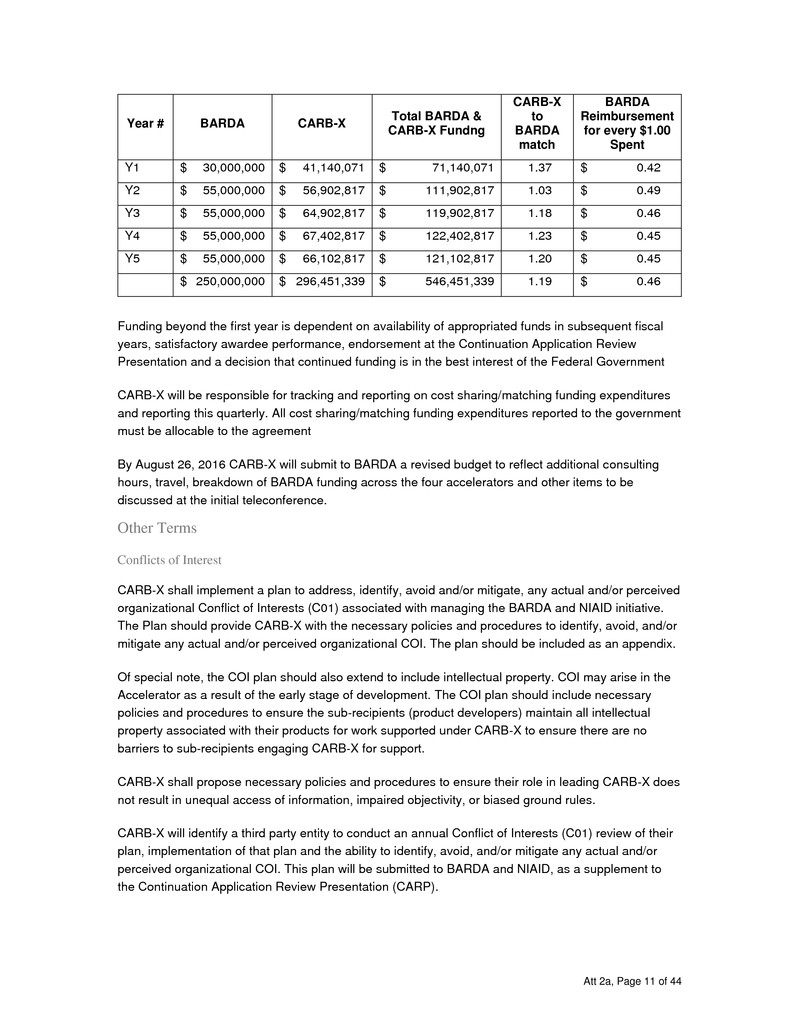
In response to the FOA, CARB-X proposed a $295 Million cost share match as part of their proposal
over the 5 years of the program. Year #1 match is proposed at $41M by CARB-X which is a 1.44
leveraged investment. In Year #1 for every $1.00 spent by CARB-X to stimulate antibacterial product
development, BARDA will reimburse $0.42. All CARB-X submissions for reimbursement will align to
the costs share outline in the below table and leverage the exchange rate on day of submission by the
CARB-X or dates invoice was submitted to CARB-X by a partner or alliance accelerator.
7 In-kind support may be used for match. In-kind support includes the value of goods and services donated to the
operation of the Accelerator, including but not limited to office space, volunteer secretarial services, pro bono
accounting services, and other volunteer services to support the organization's/entity's work. All matches must follow
the federal cost principles Applicants cannot submit match that would not be an allowable purchase with ASPR funds. A
match level over the ideal amount will not result in a higher peer review score. All proposed match is an obligation on
the part of the applicant. The HHS Grants Policy Statement provides information on allowable costs, volunteer rates,
and conflict of interest issues. This document is http://www.hhs.gov/asfriogapa/aboutog/hhsgps107.pdf Federal funds,
including those passed through a state or local government cannot be used toward the required match.
Att 2a, Page 11 of 44
Year # BARDA CARB-X
Total BARDA &
CARB-X Fundng
CARB-X
to
BARDA
match
BARDA
Reimbursement
for every $1.00
Spent
Y1 $ 30,000,000 $ 41,140,071 $ 71,140,071 1.37 $ 0.42
Y2 $ 55,000,000 $ 56,902,817 $ 111,902,817 1.03 $ 0.49
Y3 $ 55,000,000 $ 64,902,817 $ 119,902,817 1.18 $ 0.46
Y4 $ 55,000,000 $ 67,402,817 $ 122,402,817 1.23 $ 0.45
Y5 $ 55,000,000 $ 66,102,817 $ 121,102,817 1.20 $ 0.45
$ 250,000,000 $ 296,451,339 $ 546,451,339 1.19 $ 0.46
Funding beyond the first year is dependent on availability of appropriated funds in subsequent fiscal
years, satisfactory awardee performance, endorsement at the Continuation Application Review
Presentation and a decision that continued funding is in the best interest of the Federal Government
CARB-X will be responsible for tracking and reporting on cost sharing/matching funding expenditures
and reporting this quarterly. All cost sharing/matching funding expenditures reported to the government
must be allocable to the agreement
By August 26, 2016 CARB-X will submit to BARDA a revised budget to reflect additional consulting
hours, travel, breakdown of BARDA funding across the four accelerators and other items to be
discussed at the initial teleconference.
Other Terms
Conflicts of Interest
CARB-X shall implement a plan to address, identify, avoid and/or mitigate, any actual and/or perceived
organizational Conflict of Interests (C01) associated with managing the BARDA and NIAID initiative.
The Plan should provide CARB-X with the necessary policies and procedures to identify, avoid, and/or
mitigate any actual and/or perceived organizational COI. The plan should be included as an appendix.
Of special note, the COI plan should also extend to include intellectual property. COI may arise in the
Accelerator as a result of the early stage of development. The COI plan should include necessary
policies and procedures to ensure the sub-recipients (product developers) maintain all intellectual
property associated with their products for work supported under CARB-X to ensure there are no
barriers to sub-recipients engaging CARB-X for support.
CARB-X shall propose necessary policies and procedures to ensure their role in leading CARB-X does
not result in unequal access of information, impaired objectivity, or biased ground rules.
CARB-X will identify a third party entity to conduct an annual Conflict of Interests (C01) review of their
plan, implementation of that plan and the ability to identify, avoid, and/or mitigate any actual and/or
perceived organizational COI. This plan will be submitted to BARDA and NIAID, as a supplement to
the Continuation Application Review Presentation (CARP).

Att 2a, Page 12 of 44
Intergovernmental Review
This funding opportunity announcement is not subject to the requirements of Executive Order 12372,
“Intergovernmental Review of Federal Programs.”
Funding Restrictions
The following activities are not fundable:
Cost of money is not allowed even if it’s in your negotiated rate agreement
All salaries are capped at the rate of Executive Level II.
Construction is not allowed.
To carry out any program of distributing sterile needles or syringes for the hypodermic
injection of any illegal drug.
To advocate or promote gun control.
Funds cannot be used to lobby.
Pre-award costs are not allowed.
Lobbying Restrictions: http://www.hhs.gov/grants/grants/grants-policies-
regulations/lobbying-restrictions.html
In addition to the restrictions listed above the following funding restriction apply to all foreign
entities applying under this application:
Continuation of existing projects without expansion or new and innovative approaches
Alteration and Renovation (A&R). Major A&R or construction costs are unallowable under
foreign grants and domestic grants with foreign components. (unless specifically
authorized in legislation)
Customs and import duties. These costs, which include consular fees, customs surtax,
value-added taxes, and other related charges, are unallowable under foreign grants and
domestic grants with foreign components.
Indirect costs. Indirect costs will not be reimbursed, With the exception of the American
University of Beirut, which is not considered a foreign organization, and the World Health
Organization. Research patient care costs. Research patient care costs are allowable
only in exceptional circumstances as determined by the OPDIV.
Document Review
Upon request, the CARB-X shall provide BARDA sufficient opportunity to review key documents for
programs supported under the portfolio associated with sub-recipients. These documents may include
but not limited to study protocols, reports, and FDA submissions and correspondences.
Sharing of agreement reports within United States Government (USG)
In an effort to build a robust medical countermeasure pipeline through increased collaboration, BARDA
and NIAID may share technical reports with Government entities responsible for Medical
Countermeasure Development. This provision applies to all reports and data developed during
performance including reports and data paid for by the CARB-X under the matching and cost sharing
arrangements.

Att 2a, Page 13 of 44
Funding beyond the first year is dependent on availability of appropriated funds in subsequent fiscal
years, satisfactory awardee performance, endorsement at the Continuation Application Review
Presentation and a decision that continued funding is in the best interest of the Federal Government
Inclusion of Women, Minorities, and Children
When the proposed project involves clinical research, the CARB-X will evaluate the proposed plans for
inclusion of minorities and members of both genders, as well as the inclusion of children.
Intellectual Property
It is ASPR policy that the results and accomplishments of the activities that it funds should bemade
available to the public. PD/Pls and grantee organizations are expected to make the results and
accomplishments of their activities available to the research community and to the public at large. If the
outcomes of the research result in inventions, the provisions of the Bayh-Dole 5 Act of 1980, as
implemented in 37 CFR Part 401, apply. The guidelines listed in the HHS Grants Policy Statement and
applicable regulations regarding Rights in Data, Access to Research Data, Patents and Inventions,
Royalties and Licensing Fees, etc. apply to this award.
Inventions:
Acceptance of grant funds obligates recipients to comply with the standard patent rights clause in 37
CFR 401.
Publications:
Publications, journal articles, etc. produced under a grant support project must bear an
acknowledgment and disclaimer, as appropriate, for example:
This publication (journal article, etc.) was supported by the Cooperative Agreement
Number above from Biomedical Advanced Research and Development Authority
(BARDA). Its contents are solely the responsibility of the authors and do not necessarily
represent the official views of The Assistant Secretary for Preparedness and Response.
Vertebrate Animals
If applicable, this award is subject to the following special condition: Under governing policy, Federal
funds administered by the Public Health Service (PHS) shall not be expended for research involving
live vertebrate animals without prior approval by the Office of Laboratory Animal Welfare (OLAW) of an
assurance to comply with the PHS Policy on Human Care and Use of Laboratory Animals. This
restriction applies to all performance sites (e.g., collaborating institutions, subcontractors, subgrantees)
without OLAW-approved assurances, whether domestic or foreign.
Only activities that do not involve live vertebrate animals may be conducted at any performance site
until OLAW has approved an Animal Welfare Assurance of Compliance for that site. The Assurance
documents must be submitted to OLAW not less than 60 days prior to the involvement of animals in
the research. In addition, the grantee must provide ASPR with a copy of the approved Assurance. No
funds may be drawn down from the payment system and no obligations may be made against federal
funds for any research involving live vertebrate animals at any site that does not have an OLAW-
approved Assurance.

Att 2a, Page 14 of 44
Failure to submit the assurance to OLAW within the required timeframe or to otherwise comply with the
above requirements can result in suspension and/or termination of this award, withholding of support,
audit disallowances, and/or other appropriate action.
Biohazards
CARB-X should propose a plan for how CARB-X will assess whether materials or procedures
proposed are potentially hazardous to research personnel and/or the environment, and if needed,
determine whether adequate protection is proposed. Post award, BARDA and NIAID will require
CARB-X to complete this assessment.
Cooperative Agreement Award and Substantial Involvement
The Federal Grant and Cooperative Agreement Act of 1977, 31 U.S.C. 6305, defines the cooperative
agreement as similar to a grant in that a thing of value is transferred to a recipient to carry out a public
purpose. However, a cooperative agreement is used whenever substantial federal involvement with
the recipient during performance is anticipated. The difference between grants and cooperative
agreements is the degree of federal programmatic involvement rather than the type of administrative
requirements imposed.
The administrative and funding mechanism used for this program will be the cooperative agreement for
which substantial NIAID and BARDA programmatic involvement with awardees is anticipated during
the performance period. This award is subject to the awardee(s) and collaborative requirements and
responsibilities set forth in the Cooperative Agreement outlined in the program announcement under
this funding opportunity and are hereby incorporated by reference as terms and conditions of this
award.
Example of Substantial involvement:
Provide funding to the CARB-X and/or in-kind services to product developers supported
by the CARB-X,
Share pertinent information related to the performance of CARB-X between agencies on
a regular basis.
NIAID and BARDA staff will participate in all technical meetings at the portfolio level with
the CARB-X,
Identify and release strategic priorities to the CARB-X on an annual basis that identify
focus areas and potential technology types to address strategic gaps
Serve as members of the Joint Oversight Committee (JOC) for the CARB-X. The JOC will
provide strategic guidance and direction to the CARB-X. The JOC will mutually
interrogate risks and progress of assets, concur on potential new assets and confer on
allocation of funding across assets in the CARB-X portfolio. The JOC will also jointly
evaluate achievement of Portfolio Progress Milestones.
BARDA and MAID Activities:
BARDA and NIAID staff collaborator(s) and/or designee(s) activities for this program are as follows:
Participate in orientation and/or summary update meetings with CARB-X on expectations,
regulations and key management requirements, as well as reporting requirements,
formats and contents.

Att 2a, Page 15 of 44
Participate in every two week teleconferences, Face to Face Technical Quarterly Review
meetings, as discussed in “M anagem ent o f the Agreem ent” section of this FOA.
Serve as members of the Joint Oversight Committee (JOC) for the CARB-X. The JOC will
provide strategic guidance and direction to CARB-X. The JOC will mutually interrogate
risks and progress of assets, concur on potential new assets and confer on allocation of
funding across assets in the CARB-X portfolio. The JOC will also jointly evaluate
achievement of Portfolio Progress Milestones.
Identify and release strategic priorities to the CARB-X on an annual basis that identify
focus areas and potential technology types to address strategic gaps
Participate in the development, review and approval of the CARB-X’s quarterly work plan,
detailed budget, and monitoring and evaluation plan.
Provide awardee with technical assistance and consultation in identification of appropriate
resources outside of both the CARB-X and HHS to support CARB-X activities.
Coordinate activities and synergies with the CARB-X for this FOA with other BARDA &
NIAID stakeholders.
Work cooperatively with the CARB-X to assure that all necessary information and
progress resulting from this agreement is provided to BARDA in a format that will allow
the Department of Health and Human Services to assess the continuing benefits and
communicate the successes of the agreement to the general public.
BARDA Responsibilities
BARDA will be responsible for the review and approval of program activities including, but not limited
to, the proposed use of funds and activities to meet the terms and conditions of the award. BARDA will
also approve timelines and review progress of the program as well as monitor reporting requirements.
Progress evaluation will include the development of timelines and milestones and the oversight of
proposed activities through quarterly reports and on-site joint visits with principal investigator / program
managers and others in the United States Government (USG) as needed.
Accelerator’s Responsibilities
CARB-X has the primary responsibility for defining objectives and approaches for planning,
conducting, identifying, selecting, fostering, advancing R&D product development and ultimately
transitioning the products for follow-on clinical development. The CARB-X and/or sub-recipients
[product developers] will retain custody of and primary rights to any data developed under this award,
subject to US Government rights of access consistent with U.S. law and current Department of Health
and Human Services (HHS) and Public Health Service (PHS) regulations and policies.
CARB-X will be responsible for coordinating activities approved under the award. CARB-X will be
responsible for developing achievable program plans. CARB-X will also be responsible for tracking that
all activities and processes, follow terms and conditions of the agreement, and satisfactorily adhere to
budget and Monitoring and Evaluation (M&E) reporting plans. CARB-X will compile program results
from sub-recipient (contract and sub-awards, if any) affiliates into consolidated quarterly and annual
reports.
CARB-X will be responsible for planning and participating collaboratively with BARDA and NIAID under
this agreement. Through participation in every two week teleconferences, Face to Face Technical
Quarterly Review meetings, and JOC meetings as outlined in the “ Management of t he Agreement”
section of, CARB-X will work with BARDA and NIAID in order to make progress toward its goal of
executing CARB-X annual work plans.

Att 2a, Page 16 of 44
The following is a summary of the annual program requirements for CARB-X:
Submit required technical reports, progress reports and program and financial data.
Ensure that technical data from both the awardee and any possible “sub-recipients”
necessary to evaluate the progress of the program are available to BARDA.
Have in place fiscal and programmatic systems to document accountability and
performance.
Project Meetings
Every Two Week Teleconferences. A conference call between BARDA, NIAID and the
CARB-X shall occur every two weeks or as directed by the BARDA program officer.
During this call, the CARB-X will discuss the activities during the reporting period, any
problems that have arisen and the activities planned for the ensuing reporting period. The
principal investigator may choose to include other key personnel on the conference call to
give detailed updates on specific projects or the program officer may make this request.
CARB-X will maintain a table of expected activities, an actions log an identified risk log as
well as a decisions log as a means of managing and conducting these teleconferences.
These meeting will review data as well as status and budgetary items.
Kick-off and Quarterly Meetings. CARB-X and the Government shall participate in
Project Meetings to coordinate the performance of the Agreement. These meetings may
include face-to- face meetings with BARDA/NIAID/AMCG at CARB-X site. Such meetings
may include, but are not limited to, meetings of the CARB-X to discuss technical
approach, operational capability and initial portfolio that CARB-X proposes to manage.
Site visits to the CARB-X and subcontractor’s facilities, and meetings with the CARB-X
and HHS officials to discuss the technical, regulatory, and ethical aspects of the program.
These meetings will also formulate and agree on the activities for the subsequent three
months. In order to facilitate review of agreement activities, it is expected that CARB-X
will provide data, reports, and presentations to groups of outside experts (subject to
appropriate agreements to protect CARB-X’s and sub-recipients confidential or
proprietary data) and USG personnel as requested by the program officer.
Continuation Application Review Presentation (CARP): On an annual base prior to
the allocation of additional continuation funding, the Government will invite the CARB-X to
give a presentation at a CARP meeting attended by BARDA, NIAID, AMCG, and select,
invited interagency representatives. CARB-X will present progress of their portfolio under
the Agreement over the corresponding period of time. CARB-X will discuss successes
and challenges of the programs and portfolio and present their action plan for the coming
year to align with the issued BARDA and NIAID portfolio priorities. The CARP will
evaluate progress against portfolio progress milestones and make a recommendation on
the action plan, budget allocation and whether the agreement should continue and/or
receive additional funding.
Table 3.1 Technical and Portfolio Review Meeting Schedule
Meeting Frequency Method Members Action
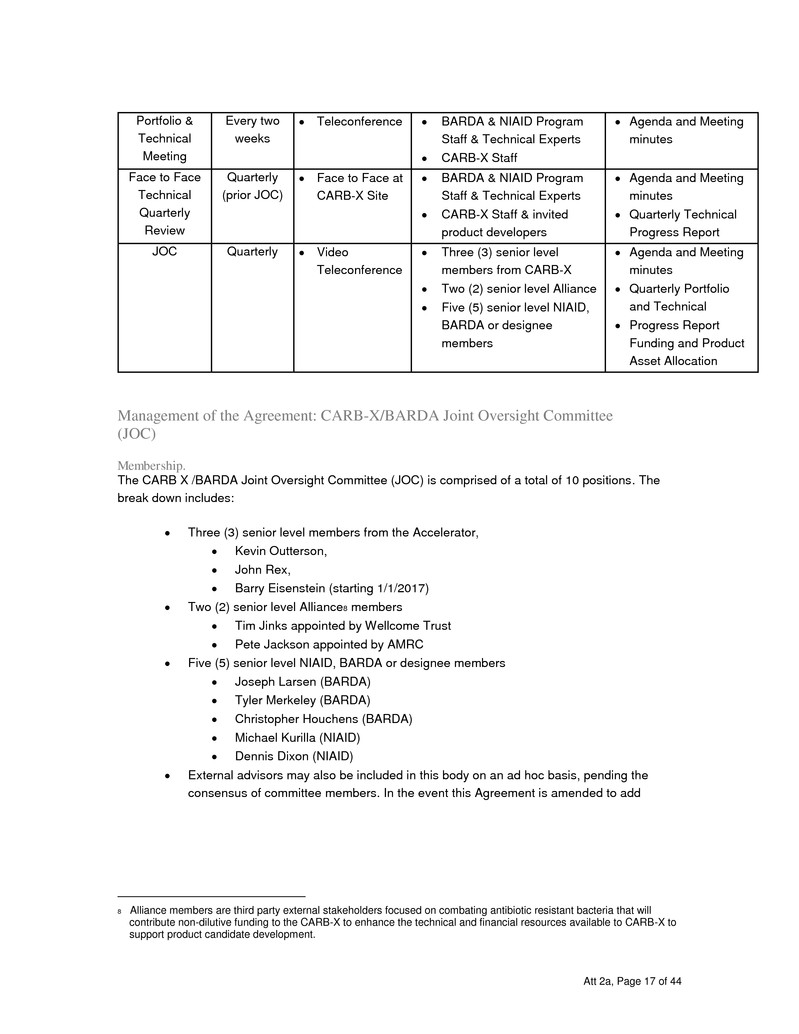
Att 2a, Page 17 of 44
Portfolio &
Technical
Meeting
Every two
weeks
Teleconference BARDA & NIAID Program
Staff & Technical Experts
CARB-X Staff
Agenda and Meeting
minutes
Face to Face
Technical
Quarterly
Review
Quarterly
(prior JOC)
Face to Face at
CARB-X Site
BARDA & NIAID Program
Staff & Technical Experts
CARB-X Staff & invited
product developers
Agenda and Meeting
minutes
Quarterly Technical
Progress Report
JOC Quarterly Video
Teleconference
Three (3) senior level
members from CARB-X
Two (2) senior level Alliance
Five (5) senior level NIAID,
BARDA or designee
members
Agenda and Meeting
minutes
Quarterly Portfolio
and Technical
Progress Report
Funding and Product
Asset Allocation
Management of the Agreement: CARB-X/BARDA Joint Oversight Committee
(JOC)
Membership.
The CARB X /BARDA Joint Oversight Committee (JOC) is comprised of a total of 10 positions. The
break down includes:
Three (3) senior level members from the Accelerator,
Kevin Outterson,
John Rex,
Barry Eisenstein (starting 1/1/2017)
Two (2) senior level Alliance8 members
Tim Jinks appointed by Wellcome Trust
Pete Jackson appointed by AMRC
Five (5) senior level NIAID, BARDA or designee members
Joseph Larsen (BARDA)
Tyler Merkeley (BARDA)
Christopher Houchens (BARDA)
Michael Kurilla (NIAID)
Dennis Dixon (NIAID)
External advisors may also be included in this body on an ad hoc basis, pending the
consensus of committee members. In the event this Agreement is amended to add
8 Alliance members are third party external stakeholders focused on combating antibiotic resistant bacteria that will
contribute non-dilutive funding to the CARB-X to enhance the technical and financial resources available to CARB-X to
support product candidate development.

Att 2a, Page 18 of 44
additional parties, representatives of such parties will also be represented on the JOC as
appropriate.
JOC’s Responsibility:
The responsibility of the CARB-X /BARDA Joint Oversight Committee is to mutually evaluate risks and
progress of assets covered under this Agreement, concur on potential new assets into the CARB-X
and confer on allocation of R&D funding across activities covered under the Agreement. This
Committee will also jointly evaluate achievements of Portfolio Progress Milestones
Frequency and Process:
The CARB-X/BARDA Joint Oversight Committee will meet every quarter or as needed to review
progress. The Committee will recommend the strategy to be covered under this Agreement during the
subsequent funding period, as well as how Government funding will be allocated across these
activities. CARB-X will be responsible for performing all technical and business due diligence on
potential new assets prior to submitting their recommendation to the JOC for funding. This may include
evaluating, submitting, to the relevant governance board(s) for endorsement prior to a
recommendation to the JOC. After concurrence on the product strategic alignment to the priorities, in a
majority manner by the JOC, the CARB-X will incorporate the new asset into the portfolio. This enables
the CARB-X/Recipient to issue a funding opportunity to the Sub-recipient. The JOC process, funding
selection is further discussed in the CARB-X Charter
The CARB-X’s internal stage gate process9 will first evaluate any Sub-recipients request for
supplemental funding to support follow-on R&D development. Prior to issuing additional funding,
beyond the funding approved in consultation with the JOC, the CARB-X will recommend to the JOC
additional funding at a JOC meeting. After concurrence on the product in a majority manner by the
JOC, and approval by BARDA, CARB-X can issue an additional funding opportunity to the Sub-
recipient.
Metrics
NIAID and BARDA have developed Key Performance Parameters (KPP) to define the metrics for the
Accelerator. Meeting all threshold objectives is required to ensure a successful program. The six KPPs
summarized in Table 1 below are deemed essential for the successful implementation of the
Accelerator.
Table: CARB-X Key Performance Parameters
KPP Top-Level Metrics
Threshold
(Minimally Acceptable
Performance)
Objective
(Optimal or Ideal Level
of Performance)
Ability to populate a
diverse R&D
CARB-X possesses the
ability to aggressively
target, incentivize and
CARB-X is partially
populated with an array of
at least 15 R&D candidate
CARB-X is fully populated
with a diverse array of 20
R&D candidate
9 A stage gate process in product development consists of a set of information gathering phases, which are divided into
stages of product development which have a Go/No decision point between each stage
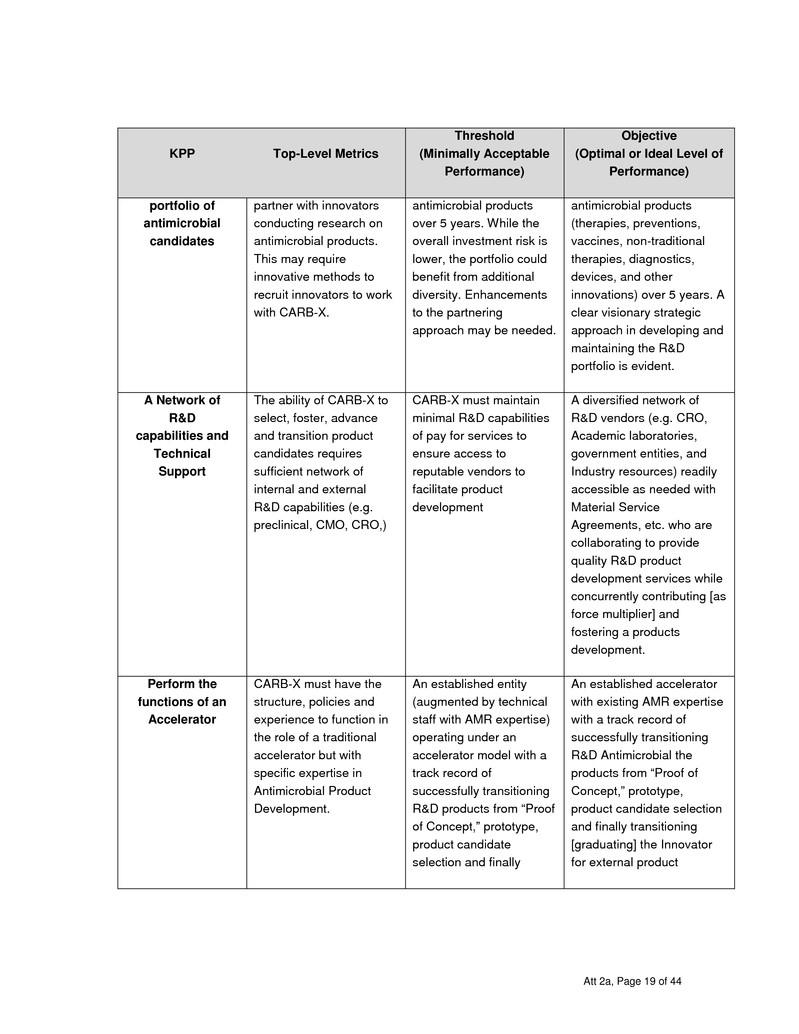
Att 2a, Page 19 of 44
KPP Top-Level Metrics
Threshold
(Minimally Acceptable
Performance)
Objective
(Optimal or Ideal Level of
Performance)
portfolio of
antimicrobial
candidates
partner with innovators
conducting research on
antimicrobial products.
This may require
innovative methods to
recruit innovators to work
with CARB-X.
antimicrobial products
over 5 years. While the
overall investment risk is
lower, the portfolio could
benefit from additional
diversity. Enhancements
to the partnering
approach may be needed.
antimicrobial products
(therapies, preventions,
vaccines, non-traditional
therapies, diagnostics,
devices, and other
innovations) over 5 years. A
clear visionary strategic
approach in developing and
maintaining the R&D
portfolio is evident.
A Network of
R&D
capabilities and
Technical
Support
The ability of CARB-X to
select, foster, advance
and transition product
candidates requires
sufficient network of
internal and external
R&D capabilities (e.g.
preclinical, CMO, CRO,)
CARB-X must maintain
minimal R&D capabilities
of pay for services to
ensure access to
reputable vendors to
facilitate product
development
A diversified network of
R&D vendors (e.g. CRO,
Academic laboratories,
government entities, and
Industry resources) readily
accessible as needed with
Material Service
Agreements, etc. who are
collaborating to provide
quality R&D product
development services while
concurrently contributing [as
force multiplier] and
fostering a products
development.
Perform the
functions of an
Accelerator
CARB-X must have the
structure, policies and
experience to function in
the role of a traditional
accelerator but with
specific expertise in
Antimicrobial Product
Development.
An established entity
(augmented by technical
staff with AMR expertise)
operating under an
accelerator model with a
track record of
successfully transitioning
R&D products from “Proof
of Concept,” prototype,
product candidate
selection and finally
An established accelerator
with existing AMR expertise
with a track record of
successfully transitioning
R&D Antimicrobial the
products from “Proof of
Concept,” prototype,
product candidate selection
and finally transitioning
[graduating] the Innovator
for external product

Att 2a, Page 20 of 44
KPP Top-Level Metrics
Threshold
(Minimally Acceptable
Performance)
Objective
(Optimal or Ideal Level of
Performance)
transitioning [graduating]
the Innovator for external
product development.
development.
Ability to progress
R&D candidate
antimicrobial
products in
preclinical
development
The ability of CARB-X to
identify 15-20 R&D
antimicrobial product
targets over 5 years,
engage these innovators,
support proof of concept,
prototype and transition
these products out of the
CARB-X for follow-on
investment.
CARB-X provides the
minimal R&D capabilities
to achieve two IND
candidates within the 5
year timeframe of the
program and has
previously demonstrated
development of a product
to an IND stage.
CARB-X possesses the
R&D capabilities and has
previously demonstrated
development of a product to
an IND stage, which will
allow it to exceed the
required two IND
candidates’ goal within the 5
year timeframe of the
program.
Supporting the
Business Needs of
Innovators
The ability of CARB-X to
provide wrap-around
support capabilities and
services (e.g. technical,
strategic, business,
access to stakeholders.)
to ensure innovators have
access to the necessary
resources to foster
success.
CARB-X to provide
strategic, regulatory and
business development
support services to
innovators after selection
through graduation. /
CARB-X will provide
introductions to follow-on
support and/or R&D
transition partner prior to
graduation from CARB-X.
CARB-X to provide
strategic, regulatory and
business development and
support services to
innovators after selection
through graduation. / The
Innovator will secure follow-
on support and/or R&D
transition partner prior to
graduation from CARB-X.
Accelerator Portfolio Metrics:
On a quarterly basis BARDA and NIAID will conduct a performance evaluation of CARB-X. CARB-X
will be measured on several different parameters. First, the ability of CARB-X to attract and partner
with innovators will be measured. CARB-X will also be responsible for tracking the progress and
success of each project within the four Accelerators. This data will be helpful in assessing how the
CARB-X is progressing technically with the R&D candidate products, but also how well they are
operating (i.e. within cost and schedule parameters). The effectiveness of the strategic, regulatory and
business development support services of the CARB-X alliance and partner accelerators will also be
regularly assessed. This may include a review of external services provided through the CARB-X for
the Innovators to determine if the services are helpful in maturing the business and product
development strategy.

Att 2a, Page 21 of 44
Additional performance benchmarks are related to the overall progression of the portfolio. CARB-X will
be evaluated on its ability to identify at least 15 antimicrobial product targets over 5 years, engage
these innovators, support proof of concept, prototype and transition these products out of the
accelerator for follow-on investment. In the first year of the program, a minimum of five antimicrobials
should be identified and supported by CARB-X. Longer term benchmarks are related to the
establishment of a robust and diverse pipeline of antimicrobial products.
Each project within CARB-X will be required to propose specific performance benchmarks that will be
set on a project-by-project basis. Commonly used R&D portfolio management systems will be applied
to the CARB-X portfolio of projects/companies.
Contacts:
The Grants Management Specialist, Nancy Brown, reached at Nancy.Brown@hhs.gov, is responsible
for the negotiation, award and administration of this project and for interpretation of grants
administration policies and provisions.
The Program Officer at BARDA is Tyler Merkeley, at 202-260-0315 and Tyler.Merkeley@hhs.gov is
responsible for the programmatic and technical aspects of this project.

Att 2a, Page 22 of 44
Attachment A: Research and Related Project Information
Awardee will provide the following information to the related questions when a clinical study or
Vertebrate Animals studies are identified within CARB-X
If activities involving human subjects are planned at any time during the proposed project at any
performance site, check yes. Check yes even if the proposed project is exempt from Regulations for
the Protection of Human Subjects. If activities involving human subjects are not planned at any time
during the proposed project at any performance site, select no.
Applications proposing human subjects research may be required to submit additional information,
forms, or attachments with the application, in accordance with policies covering human
subjects research.
1. Are Human Subjects Involved? YES NO
1.a. If YES to Human Subjects
Is the Project Exempt from Federal Regulations? YES NO
Yes: If the project is exempt from Federal regulations, check Yes.
No: If the project is not exempt from Federal regulations, check No.
If yes, Select the appropriate exemption number from 1, 2, 3, 4, 5, 6.
If human subject activities are exempt from Federal regulations, provide the exemption numbers
corresponding to one or more of the exemption categories. The six categories of research that qualify
for exemption from coverage by the regulations are defined in the Common Rule for the Protection of
Human Subjects. These regulations can be found at
http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html.
OHPR guidance states that appropriate use of Exemptions described in 45 CFR 46 should be
determined by an authority independent from the investigators
(http://answers.hhs.gov/ohrp/categories/1564). Institutions often designate their IRB to make
this determination. Since IRB approval is not required at the time of application, the
exemptions designated often represent the opinion of the PD/PI, and the justification provided
for the exemption by the PD/PI is evaluated during peer review. Human subjects research
should be designated as exempt if the proposed research meets the criteria for one or more of
the six exemptions.
If no, is the IRB review Pending? YES NO
Enter the latest Institutional Review Board (IRB) approval date. Leave blank if Pending.
Applicants should check “Yes” to the question “Is the IRB review Pending?” even if the IRB
review/approval process has not yet begun at the time of submission. Also note that an IRB
Approval Date is not required at the time of submission. This may be requested later in the
pre-award cycle. If IRB is still pending at time of award then affected components of the award
will be restricted. Failure to obtain IRB approval within the agreed upon time frame may result
in the termination of an award.

Att 2a, Page 23 of 44
Human Subject Assurance Number
Enter the approved Federal Wide Assurance (FWA) that the applicant has on file with the Office for
Human Research Protections, if available. If the applicant has a FWA number, enter the 8-digit
number.
Insert “None” if the applicant organization does not have an approved assurance on file with OHRP. In
this case, the applicant organization, by the signature in item 21 on the SF424 declaring that it
will comply with 45 CFR part 46 and proceed to obtain a human subjects assurances (see
http://www.hhs.gov/ohrp). Do not insert the human subjects assurance number of any
collaborating institution in the space provided.
2. Are Vertebrate Animals Used? YES NO
If activities involving vertebrate animals are planned at any time during the proposed project at any
performance site, check yes. If no, skip the rest of block 2.
Note that the generation of custom antibodies constitutes an activity involving vertebrate animals.
2.a. If YES to Vertebrate Animals
Is the Institutional Animal Care and Use Committee (IACUC) review Pending?
YES NO
IACUC Approval Date:
Enter the latest IACUC approval date (if available). Leave blank if Pending.
Animal Welfare Assurance Number:
Enter the Federally approved assurance number, if available.
To determine if your organization holds an Animal Welfare Assurance, see
http://grants.nih.gov/grants/olaw/olaw.htm#assur. Applicants should check “Yes” to the
question “Is the IACUC review Pending?” even if the IACUC review/approval process has not
yet begun at the time of submission. Also note that an IACUC Approval Date is not required at
the time of submission. However, the approval date and other data may be requested later in
the pre-award cycle. If the applicant organization does not have an approved Animal Welfare
Assurance on file with the Office of Laboratory Animal Welfare (OLAW), NIH, enter “None” in
the Animal Welfare Assurance Number field. Do not enter the Animal Welfare Assurance
number of any collaborating institution. By inserting “None” at the time of submission, the
applicant organization is essentially declaring that it will comply with the PHS Policy on
Humane Care and Use of Laboratory Animals by submitting an Animal Welfare Assurance
and verification of IACUC approval when requested to do so by OLAW. If IACC approval is
still pending at time of award then affected components of the award will be restricted. Failure
to obtain IACC approval within the agreed upon time frame may result in the termination of an
award.
3. Is proprietary/privileged information included in the application? YES NO
Patentable ideas, trade secrets, privileged or confidential commercial or financial information,
disclosure of which may harm the applicant, should be included in applications only when such
information is necessary to convey an understanding of the proposed project. If the application
includes such information, check yes and clearly mark each line or paragraph on the pages containing
the proprietary/privileged information with a legend similar to: “The following contains
proprietary/privileged information that (name of applicant) requests not be released to persons outside
the Government, except for purposes of review and evaluation. “

Att 2a, Page 24 of 44
4. Environmental Questions
Most research grants are not expected to individually or cumulatively have a significant effect on the
environment, and there are several categorical exclusions allowing most applicants to answer
‘No’ to this question unless a specific FOA indicates that the National Environmental Policy
Act (NEPA) applies. However, if an applicant expects that the proposed project will have an
actual or potential impact on the environment, or if any part of the proposed research and/or
project includes one or more of the following categorical exclusions listed below, the line
marked “Yes” should be checked and an explanation provided in field 4.b.
1. The potential environmental impacts of the proposed research may be of greater
scope or size than other actions included within a category.
2. The proposed research threatens to violate a Federal, State, or local law established
for the protection of the environment or for public health and safety.
3. Potential effects of the proposed research are unique or highly uncertain.
4. Use of especially hazardous substances or processes is proposed for which
adequate and accepted controls and safeguards are unknown or not available.
5. The proposed research may overload existing waste treatment plants due to new
loads (volume, chemicals, toxicity, additional hazardous wasted, etc.)
6. The proposed research may have a possible impact on endangered or threatened
species.
7. The proposed research may introduce new sources of hazardous/toxic wastes or
require storage of wastes pending new technology for safe disposal.
8. The proposed research may introduce new sources of radiation or radioactive
materials.
9. Substantial and reasonable controversy exists about the environmental effects of the
proposed research.
4.a. Does this project have an actual or potential impact on the environment?
YES NO
4.b. If yes, please explain
Explanation of the actual or potential impact on the environment.
4.c. If this project has an actual or potential impact on the environment, has an exemption
been authorized or an Environmental Assessment (EA) or an Environmental Impact Statement
(EIS) been performed? YES NO
4.d. If yes, please explain
Enter additional details about the EA or EIS. If desired, you can provide the information in a separate
file, and attach by clicking Add Attachments located to the right of Step 11- Other Attachments.
5. Is the research performance site designated, or eligible to be designated, as a historic place? YES
NO
If any research performance site is designated, or eligible to be designated, as a historic place, if Yes,
check the Yes and then provide an explanation. Otherwise, check the No.

Att 2a, Page 25 of 44
5.a. If yes, please explain:
If you checked the Yes box indicating any performance site is designated, or eligible to be designated,
as a historic place, provide the explanation here.
6. Does this project involve activities outside of the United States or partnerships with International
Collaborators? YES NO
Indicate whether this project involves activities outside of the United States or partnerships with
international collaborators. Check yes or no..
Applicants to PHS agencies must check “Yes” if the applicant organization is a foreign institution or if
the project includes a foreign component.
6.a. If yes, identify countries
Enter the countries with which international cooperative activities are involved.

***Confidential Treatment Requested
Attachment B: Reporting Table
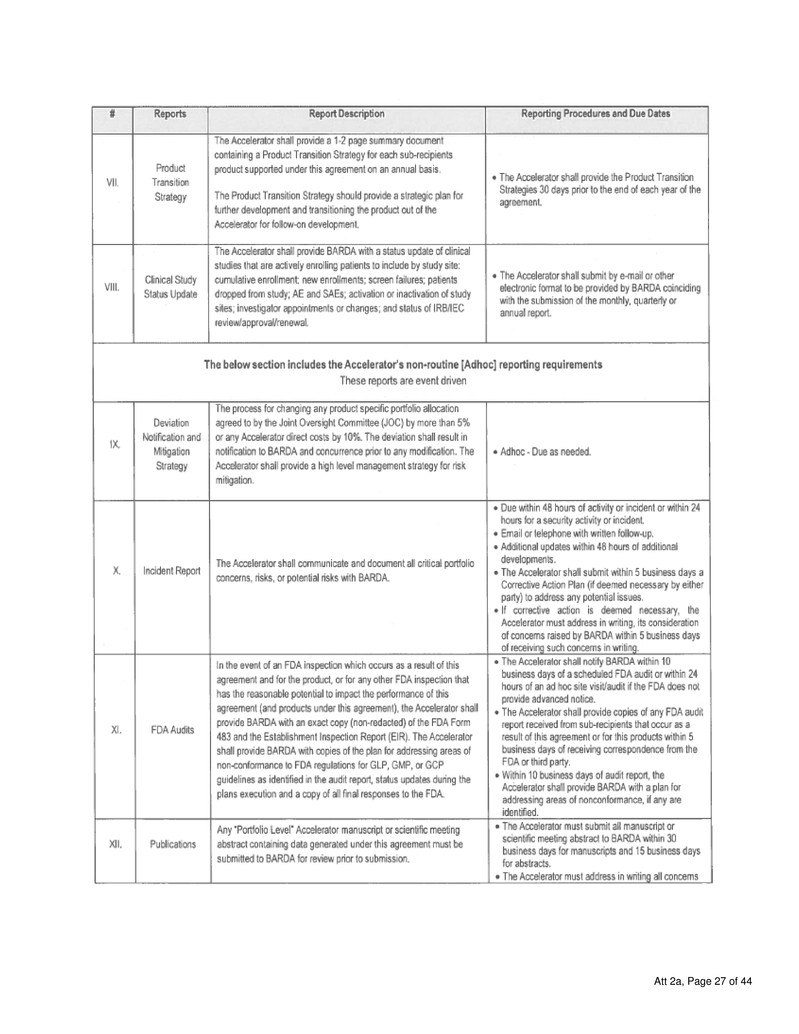
Att 2a, Page 27 of 44
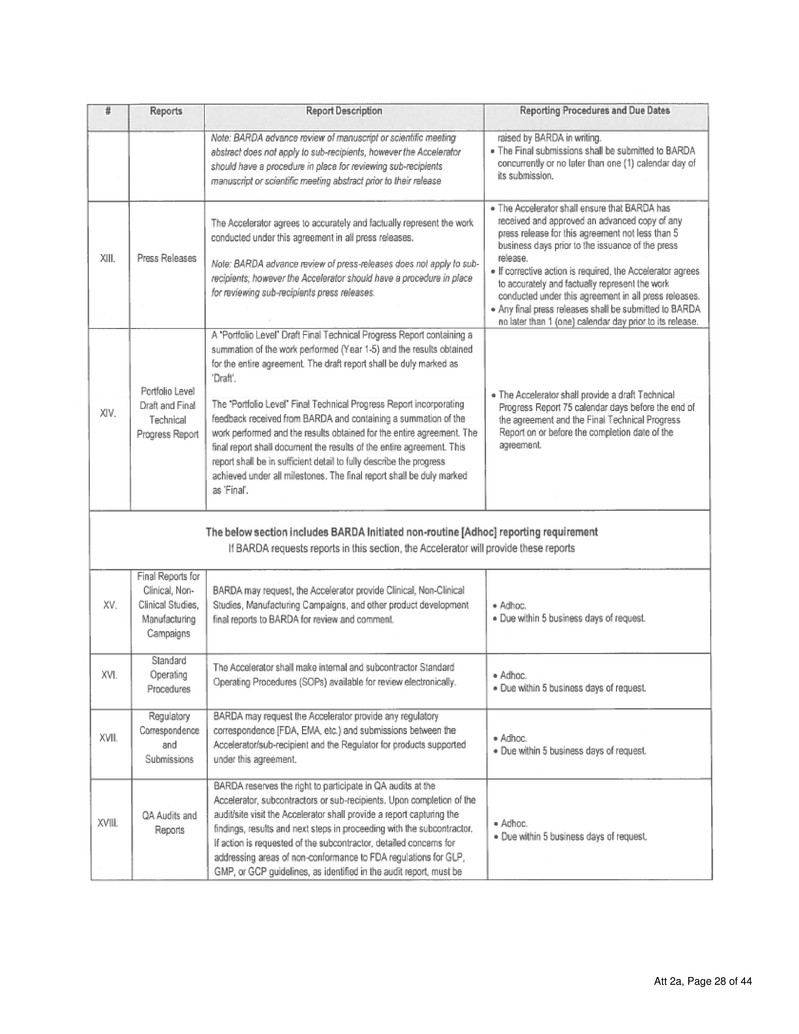
Att 2a, Page 28 of 44
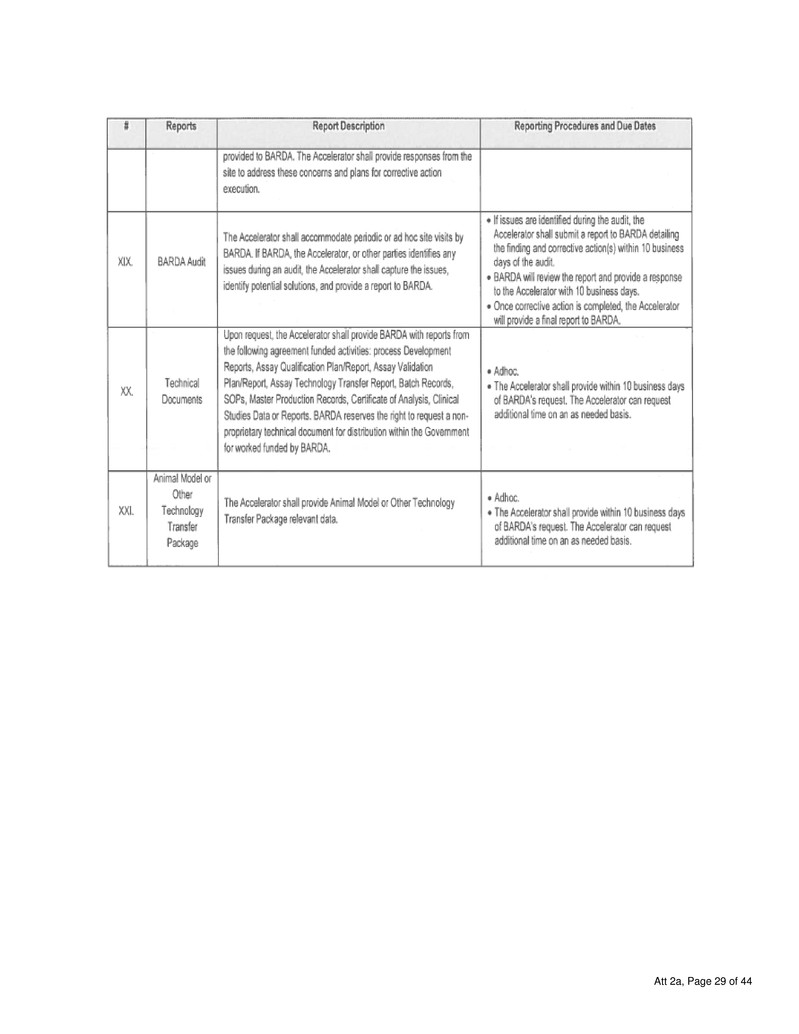
Att 2a, Page 29 of 44
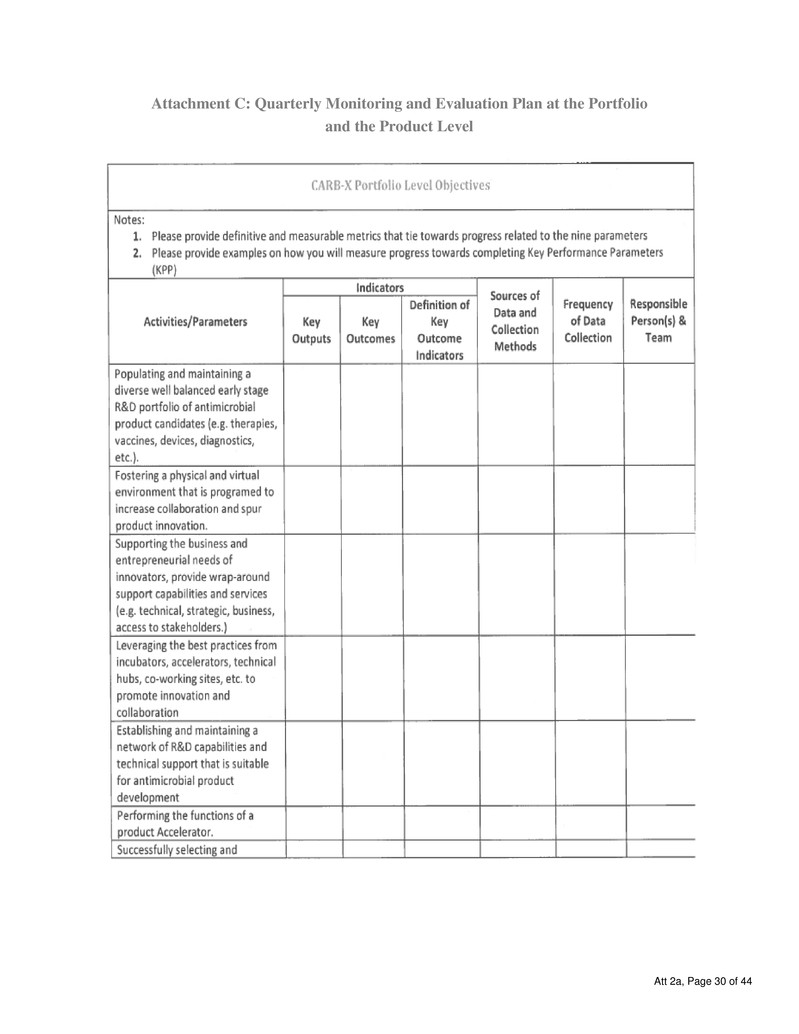
Att 2a, Page 30 of 44
Attachment C: Quarterly Monitoring and Evaluation Plan at the Portfolio
and the Product Level
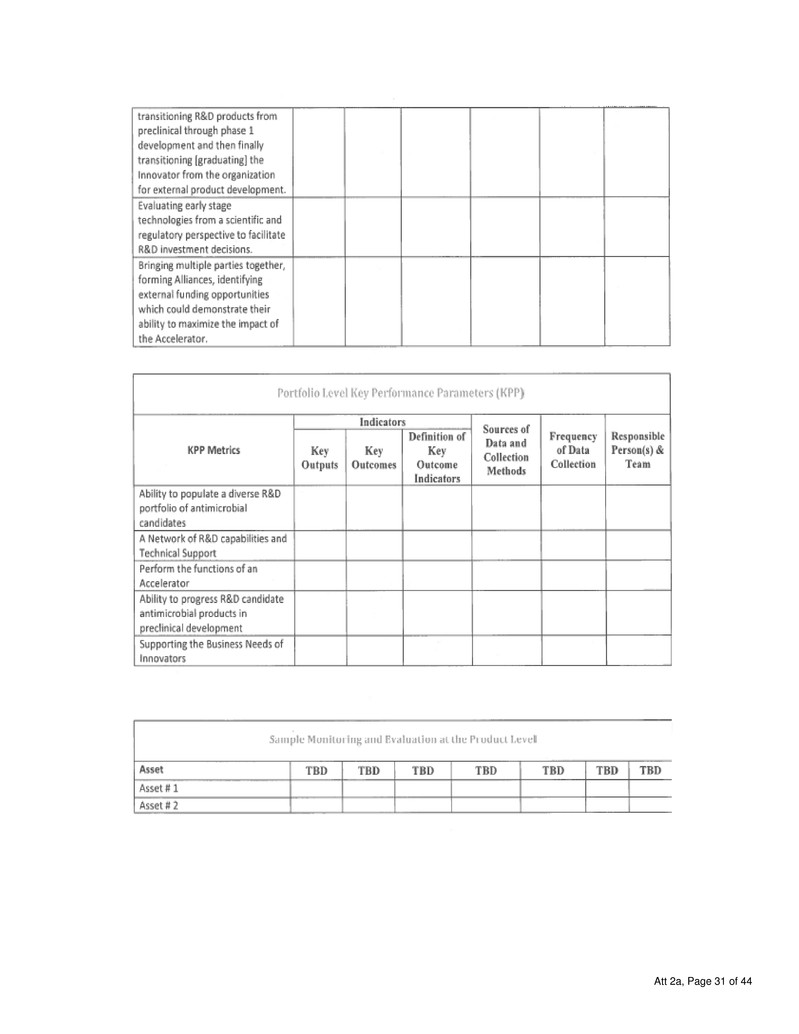
Att 2a, Page 31 of 44

***Confidential Treatment Requested
Standard Terms and Conditions of award:
This cooperative agreement is issued under the authority of Section 301 of the Public Health
Service (PHS) Act (42 U.S.C. 241), “Research and Investigations”; Section 319L of the PHS Act
(42 U.S.C. 247d-7e), “Biomedical Advanced Research and Development Authority”; Section 405
of the PHS Act (42 U.S.C. 284), “Authority of the Directors of the National Research Institutes”;
Section 446 of the PHS Act (42 U.S.C. 2850, “National Institute of Allergy and Infectious
Diseases, Purpose of Institute.” By receiving funds under this award, the recipient assures that it
will carry out the project/program as authorized and will comply with the terms and conditions
and other requirements of this award.
This grant is subject to the applicable requirements of the Uniform Administrative Requirements
for Awards and Subawards to Institutions of Higher Education, Hospitals, Other Nonprofit
Organizations, and Commercial Organizations under Title 45 Code of Federal Regulations, Part
75.
Any applicable statutory or regulatory requirements, including 45 CFR Part 75 and 2 CFR Part
200, directly apply to this award apart from any coverage in the HHS GPS
The terms and conditions of this Notice of Award and other requirements have the following
order of precedence if there is any conflict in what they require: (1) Public Health Service Act,
Section 311 (42 U.S.C. 243).(2) terms and conditions of the award (3) CFR Part 75; (4) HHS
Grants Policy Statement.
Requests that require prior approval from the awarding office must be submitted in writing to the
Grants Management Specialist via GrantSolutions. Only responses signed by the Grants
Management Specialist or Grants Management Officer are to be considered valid. Grantees who
take action on the basis of responses from other ASPR officials do so at their own risk. Such
responses will not be considered binding by or upon ASPR.
Subaward Equal Treatment
The recipient must comply with 45 CFR 75, including the provision that no State or local
government recipient nor any intermediate organization with the same duties as a governmental
entity shall, in the selection of service providers, discriminate for or against an organization’s
religious character or affiliation.

Att 2a, Page 33 of 44
Public Policy Requirements
All public policy requirements included in “Public Policy Requirements” in Part I and Part II
(pages 11-2 throughll-24) of the HHS GPS apply as appropriate. See FOA under which this
award was issued for more information.
Title VI in Division G of the “Consolidated Appropriation Act, 2015”
Consistent with the Consolidated and Further Continuing Appropriations Act, 2015 (Public Law
113-235), Division G, Title VI, Sec. 603, the grantee agrees to comply with existing and future
guidance from the Secretary regarding control of the spread of the Ebola virus.
GENERAL TERMS AND CONDITIONS:
1) As required by the Federal Funding Accountability and Transparency Act of 2006, this
new award is subject to the subaward and executive compensation reporting requirement of 2
CFR Part 170. Although the full text of this regulation is attached, you may access the language
online at https://www.fsrs.gov/.
Reporting Subawards and Executive Compensation
a. Reporting of first-tier subawards.
1. Applicability. Unless you are exempt as provided in paragraph d. of this award term, you must
report each action that obligates $25,000 or more in Federal funds that does not include Recovery
funds (as defined in section 1512(a)(2) of the American Recovery and Reinvestment Act of 2009,
Pub. L. 111-5) for a subaward to an entity (see definitions in paragraph e. of this award term).
2. Where and when to report.
i. You must report each obligating action described in paragraph a.1. of this award term to
http://www.fsrs.gov.
ii. For subaward information, report no later than the end of the month following the month in
which the obligation was made. (For example, if the obligation was made on November 7, 2010,
the obligation must be reported by no later than December 31, 2010.)

Att 2a, Page 34 of 44
3. What to report. You must report the information about each obligating action that the
submission instructions posted at http://www-Ssrs.gov specify
b. Reporting Total Compensation of Recipient Executives.
1. Applicability and what to report. You must report total compensation for each of your five
most highly compensated executives for the preceding completed fiscal year, if
i. the total Federal funding authorized to date under this award is $25,000 or more;
ii. in the preceding fiscal year, you received
(A) 0 percent or more of your annual gross revenues from Federal procurement contracts (and
subcontracts) and Federal financial assistance subject to the Transparency Act, as defined at 2
CFR 170.320 (and subawards); and
(B) 25,000,000 or more in annual gross revenues from Federal procurement contracts (and
subcontracts) and Federal financial assistance subject to the Transparency Act, as defined at 2
CFR 170.320 (and subawards); and
iii. The public does not have access to information about the compensation of the executives
through periodic reports filed under section 13(a) or 15(d) of the Securities Exchange Act of 1934
(15 U.S.C. 78m(a), 78o(d)) or section 6104 of the Internal Revenue Code of 1986. (To determine
if the public has access to the compensation information, see the U.S. Security and Exchange
Commission total compensation filings at http://www.sec.gov/answers/execomp.htm.)
2. Where and when to report. You must report executive total compensation described in
paragraph b.1. of this award term:
i. As part of your registration profile, you must access the System for Award Management
(SAM) at: https://ww•.sam.gov/portal/public/SAM/ .
ii. By the end of the month following the month in which this award is made, and annually
thereafter.
c. Reporting of Total Compensation of Subrecipient Executives.
1. Applicability and what to report. Unless you are exempt as provided in paragraph d. of this
award term, for each first-tier subrecipient under this award, you shall report the names and total
compensation of each of the subrecipient’s five most highly compensated executives for the
subrecipient’s preceding completed fiscal year, if
i. in the subrecipient’s preceding fiscal year, the subrecipient received
(A) 0 percent or more of its annual gross revenues from Federal procurement contracts (and
subcontracts) and Federal financial assistance subject to the Transparency Act, as defined at 2
CFR 170.320 (and subawards); and
(B) 25,000,000 or more in annual gross revenues from Federal procurement contracts (and
subcontracts), and Federal financial assistance subject to the Transparency Act (and subawards);
and
ii. The public does not have access to information about the compensation of the executives
through 3

Att 2a, Page 35 of 44
periodic reports filed under section 13(a) or 15(d) of the Securities Exchange Act of 1934 (15
U.S.C. 78m(a), 78o(d)) or section 6104 of the Internal Revenue Code of 1986. (To determine if
the public has access to the compensation information, see the U.S. Security and Exchange
Commission total compensation filings at http://www.sec.gov/answers/execomp.httri.)
2. Where and when to report. You must report subrecipient executive total compensation
described in paragraph c.1. of this award term:
i. To the recipient.
ii. By the end of the month following the month during which you make the subaward. For
example, if a subaward is obligated on any date during the month of October of a given year (i.e.,
between October 1 and 31), you must report any required compensation information of the
subrecipient by November 30 of that year.
d. Exemptions
If, in the previous tax year, you had gross income, from all sources, under $300,000. you are
exempt from the requirements to report:
i. Subawards, and
ii. The total compensation of the five most highly compensated executives of any
subrecipient.
e. Definitions. For purposes of this award term:
1. Entity means all of the following, as defined in 2 CFR part 25:
i. A Governmental organization, which is a State, local government, or Indian tribe;
ii. A foreign public entity;
iii. A domestic or foreign nonprofit organization;
iv. A domestic or foreign for-profit organization;
v. A Federal agency, but only as a subrecipient under an award or subaward to a non-
Federal entity.
2. Executive means officers, managing partners, or any other employees in management
positions.
3. Subaward:
i. This term means a legal instrument to provide support for the performance of any portion of
the substantive project or program for which you received this award and that you as the recipient
award to an eligible subrecipient.
ii. The term does not include your procurement of property and services needed to carry out the
project or program (for further explanation, see Sec. 11.210 of the attachment to OMB Circular
A¬133. “Audits of States, Local Governments, and Non-Profit Organizations”).
iii. A subaward may be provided through any legal agreement, including an agreement that you
or a 4

Att 2a, Page 36 of 44
subrecipient considers a contract.
4. Subrecipient means an entity that:
i. Receives a subaward from you (the recipient) under this award; and
ii. Is accountable to you for the use of the Federal funds provided by the subaward.
5. Total compensation means the cash and noncash dollar value earned by the executive during
the recipient’s or subrecipient’s preceding fiscal year and includes the following (for more
information see 17 CFR 229.402(c)(2)):
i. Salary and bonus.
ii. Awards of stock, stock options, and stock appreciation rights. Use the dollar amount
recognized for financial statement reporting purposes with respect to the fiscal year in accordance
with the Statement of Financial Accounting Standards No. 123 (Revised 2004) (FAS 123R),
Shared Based Payments.
iii. Earnings for services under non-equity incentive plans. This does not include group life,
health, hospitalization or medical reimbursement plans that do not discriminate in favor of
executives, and are available generally to all salaried employees.
iv. Change in pension value. This is the change in present value of defined benefit and actuarial
pension plans.
v. Above-market earnings on deferred compensation which is not tax-qualified.
vi. Other compensation, if the aggregate value of all such other compensation (e.g. severance,
termination payments, value of life insurance paid on behalf of the employee, perquisites or
property) for the executive exceeds $10,000.
2) Indirect Cost Rates: §200.414/§75.414(f) In addition to the procedures outlined in the
appendices in paragraph (e) of this section, any non-Federal entity that has never received a
negotiated indirect cost rate, except for those non-Federal entities described in Appendix VII to
Part 200/Appendix VII to part 75 —States and Local Government and Indian Tribe Indirect Cost
Proposals. paragraph D.1.b, may elect to charge a de minimis rate of 10% of modified total direct
costs (MTDC) which may be used indefinitely. As described in §200.403/§75.403 Factors
affecting allowability of costs, costs must be consistently charged as either indirect or direct costs,
but may not be double charged or inconsistently charged as both. If chosen, this methodology
once elected must be used consistently for all Federal awards until such time as a non-Federal
entity chooses to negotiate for a rate, which the non-Federal entity may apply to do at any time.
(g) Any non-Federal entity that has a current federally negotiated indirect cost rate may apply for
a one-time extension of the rates in that agreement for a period of up to four years. This extension
will be subject to the review and approval of the cognizant agency for indirect costs. If an
extension is granted the non-Federal entity may not request a rate review until the extension
period ends. At the end of the 4-year extension, the non-Federal entity must re-apply to negotiate
a rate. Subsequent one-time extensions (up to four years) are permitted if a renegotiation is
completed between each extension request.
3) Mandatory disclosures.

Att 2a, Page 37 of 44
The non-Federal entity or applicant for a Federal award must disclose, in a timely manner, in
writing to the Federal awarding agency or pass-through entity all violations of Federal criminal
law involving fraud, bribery, or gratuity violations potentially affecting the Federal award. Failure
to make required disclosures can result in any of the remedies described in §200.338 Remedies
for noncompliance, including suspension or debarment. (See also 2 CFR part 180 and 31 U.S.C.
3321).
4) English Language
All Federal financial assistance announcements and Federal award information must be in the
English language. Applications must be submitted in the English language and must be in the
terms of U.S. dollars. If the Federal awarding agency receives applications in another currency,
the Federal awarding agency will evaluate the application by converting the foreign currency to
United States currency using the date specified for receipt of the application.
Non-Federal entities may translate the Federal award and other documents into another language.
In the event of inconsistency between any terms and conditions of the Federal award and any
translation into another language, the English language meaning will control. Where a significant
portion of the non-Federal entity’s employees who are working on the Federal award are not
fluent in English, the non-Federal entity must provide the Federal award in English and the
language(s) with which employees are more familiar.
5) As the recipient organization, you acknowledge acceptance of the grant terms and conditions
by drawing down or otherwise obtaining funds from the Payment Management System. In doing
so, your organization must ensure that you exercise prudent stewardship over Federal funds and
that all costs are allowable, allocable and reasonable.
6) This grant is subject to the terms and conditions as stated in Section III (Terms and
Conditions) of the NoA. Refer to the “order of precedence” that explains the laws and regulations
that govern the award.
7) Legal and Financial Responsibility-The recipient organization is legally and financially
responsible for all aspects of this grant, including funds provided to sub-recipients.
8) Executive Level II Salary Cap
For FY 2016, the Consolidated Appropriations Act, 2015 (Public Law 113-76) signed into law on
January 10, 2016, restricts the amount of direct salary to Executive Level II of the Federal
Executive Pay scale. The Executive Level II salary is $185,100 annually. Funds made available
by this award shall not be used by the grantee or subrecipient to pay the salary and bonuses of an
individual, either as direct costs or indirect costs, at a rate in excess of current Executive Level II
compensation requirements.
9) Gun Control
None of the funds made available through this award may be used, in whole or in part, to
advocate or promote gun control.

Att 2a, Page 38 of 44
10) Pornography
None of the funds made available through this award may be used to maintain or establish a
computer network unless
such network blocks the viewing, downloading, and exchanging of pornography.
11) Lobby Restrictions
The grantee must comply with 45 CFR Part 93. None of the funds made available through this
award shall be used to pay any person for influencing or attempting to influence an officer or
employee of any agency, a Member of Congress, an officer or employee of Congress, or an
employee of a Member of Congress in connection with any of the following covered Federal
actions: the awarding of any Federal contract, grant or cooperative agreement, the making of any
Federal loan, and the extension, continuation, renewal, amendment, or modification of any
Federal contract, grant, loan, or cooperative agreement. Influencing or attempting to influence
means making, with the intent to influence, any
communication to or appearance before an officer or employee of any agency, a Member of
Congress, an officer or employee of Congress, or an employee of a Member of congress in
connection with any covered action.
12) Sterile Needle Distribution
No funds made available through this award shall be used to carry out any program of distributing
sterile needles or syringes for the hypodermic injection of any illegal drug.
13) Accounting Records and Disclosure - Awardees and sub-recipients must maintain
records which adequately identify the source and application of funds provided for financially
assisted activities. These records must contain information pertaining to grant or subgrant awards
and authorizations,

Att 2a, Page 39 of 44
obligations, unobligated balances, assets, liabilities, outlays or expenditures, and income. The
awardee, and all its sub-recipients, should expect that A, or its designee, may conduct a financial
compliance audit and on-site program review of grants with significant amounts of Federal
funding.
14) Procurement
When procuring equipment, the recipient must comply with the procurement standards at 45 CFR
Part 75.329 Procurement procedures, which requires the performance and documentation of some
form of cost or price analysis with every procurement action.
15) DUNS Number Annual Update
The DUNS number recipients use on their application must be registered and active in the System
for Award Management (SAM) which can be accessed at https://www.sam.gov. Recipients must
update their SAM information at least every 12 months to maintain an active account.
16) Trafficking In Persons
a. Provisions applicable to a recipient that is a private entity.
1. You as the recipient, your employees, subrecipients under this award, and subrecipients’
employees may not
i. Engage in severe forms of trafficking in persons during the period of time that the award
is in effect;
ii. Procure a commercial sex act during the period of time that the award is in effect; or
iii. Use forced labor in the performance of the award or subawards under the award.
2. We as the Federal awarding agency may unilaterally terminate this award, without penalty, if
you or a subrecipient that is a private entity —
i. Is determined to have violated a prohibition in paragraph a.1 of this award term; or
ii. Has an employee who is determined by the agency official authorized to terminate the
award to have violated a prohibition in paragraph a.1 of this award term through conduct that is
either—
A. Associated with performance under this award; or
B. Imputed to you or the subrecipient using the standards and due process for imputing the
conduct of an individual to an organization that are provided in 2 CFR part 180, “OMB
Guidelines to Agencies on Governmentwide Debarmentand Suspension (Nonprocurement),” as
implemented by our agency at 2 CFR part 376.
b. Provision applicable to a recipient other than a private entity. We as the Federal awarding
agency may unilaterally terminate this award, without penalty, if a subrecipient that is a private
entity
1. Is determined to have violated an applicable prohibition in paragraph a.1 of this award
term; or
2. Has an employee who is determined by the agency official authorized to terminate the
award to have violated an applicable prohibition in paragraph a.1 of this award term through
conduct that is either
i. Associated with performance under this award; or
ii. Imputed to the subrecipient using the standards and due process for imputing the conduct
of an individual to an organization that are provided in 2 CFR part 180, “OMB Guidelines to

Att 2a, Page 40 of 44
Agencies on Government wide Debarment and Suspension (Non-procurement),” as implemented
by our agency at 2 CFR part 376
c. Provisions applicable to any recipient.
1. You must inform us immediately of any information you receive from any source
alleging a violation of a prohibition in paragraph a.1 of this award term
2. Our right to terminate unilaterally that is described in paragraph a.2 or b of this section: i.
Implements section 106(g) of the Trafficking Victims Protection Act of 2000 (TVPA), as
amended (22 U.S.C. 7104(g)), and ii. Is in addition to all other remedies for noncompliance that
are available to us under this award.
3. You must include the requirements of paragraph a.1 of this award term in any subaward
you make to a private entity.
17) Reducing Text Messaging While Driving
In accordance with Executive Order 13513, Federal Leadership On Reducing Text Messaging
While Driving, dated October 1, 2009, contractors, subcontractors, and recipients and
subrecipients are encouraged “to adopt and enforce policies that ban text messaging while driving
company-owned or -rented vehicles or GOV, or while driving POV when on official Government
business or when performing any work for or on behalf of the Government. Agencies should also
encourage Federal contractors, subcontractors, and grant recipients and subrecipients as described
in this section to conduct initiatives of the type described in section 3(a) of this order.”
18) Publications: All grantee publications, including: research publications press releases
other publications or documents about research that is funded by ASPR must include the
following two statements: A specific acknowledgment of ASPR grant support,
such as:
“Research reported in this [publication/press release] was supported by [name of the program
office(s), or other ASPR offices] the Department of Health and Human Services Office of the
Assistant Secretary for Preparedness and Response under award number [specific ASPR grant
number(s)].” A disclaimer that says:
“The content is solely the responsibility of the authors and does not necessarily represent the
official views of the Department of Health and Human Services Office of the Assistant Secretary
for Preparedness and Response.”
19) Federal Information Security Management Act (FISMA):
If applicable, all information systems, electronic or hard copy which contain federal data need to
be protected from unauthorized access. This also applies to information associated with ASPR
grants. Congress and the OMB have instituted laws, policies and directives that govern the
creation and implementation of federal information security practices that pertain specifically to
grants and contracts. The current regulations are pursuant to the Federal Information Security
Management Act (FISMA), Title III of the E-Government Act of 2002 Pub. L. No. 107-347.
20) Health and Safety Regulations and Guidelines
Grantees are responsible for meeting applicable Federal, State, and local health and safety
standards and for establishing and implementing necessary measures to minimize their
employees’ risk of injury or illness in activities related to ASPR grants. In addition to applicable

Att 2a, Page 41 of 44
Federal, State, and local laws and regulations, the following regulations must be followed when
developing and implementing health and safety operating procedures and practices for both
personnel and facilities:
29 CFR 1910.1030, Blood borne pathogens; 29 CFR 1910.1450. Occupational exposure to
hazardous chemicals in laboratories; and other applicable occupational health and safety
standards issued by the Occupational Health and Safety Administration (OSHA) and included in
29 CFR 1910. These regulations are available at
http://www.osha.gov/p1s/oshaweb/owastand.displav_standard_group?p_toc_level=l&p_j.)art_nu
mber=1910.
Nuclear Regulatory Commission Standards and Regulations, pursuant to the Energy
Reorganization Act of 1974 (42 U.S.C. 5801 et seq.). Copies may be obtained from the U.S.
Nuclear Regulatory Commission, Washington, DC 20555-0001. The following guidelines are
recommended for use in developing and implementing health and safety operating procedures and
practices for both personnel and facilities:
Biosafety in Microbiological and Biomedical Laboratories, CDC and NIH, HHS. This
publication is available at http://www.cdc.wv/OD/ohs/biosftv/bmb15/BMBL 5th_Edition.pdf.
Prudent Practices for Safety in Laboratories (1995), National Research Council, National
Academy Press, 500 Fifth Street, NW, Lockbox 285, Washington, DC 20055 (ISBN 0-309-
05229-7). This publication can be obtained by telephoning 800-624-8373. It also is available at
http://www.nap.eclu/catalog/4911.html. Grantee organizations are not required to submit
documented assurance of their compliance with or implementation of these regulations and
guidelines. However, if requested by ASPR, grantees should be able to provide evidence that
applicable Federal, State, and local health and safety standards have been considered and have
been put into practice.
Reporting Requirements:
1) Federal Financial Report (FFR) — Awardees are required to electronically submit a semi-
annual (due months from date of award w/ 30 day grace period) and annual (due 90 days after the
end of budget period) Federal Financial Report (Standard Form 425) via grantsolutions.
2) Progress Reporting: Awardees are required to electronically submit monthly and annual
program progress reports via GrantSolutions. As part of the progress report. financial information
will be reported both per major category of expense, and by objectives. Grantees will include sub-
recipient monitoring activities that were completed during each quarter.
3) Cash Transaction Reporting: Recipients must report cash transaction data using the Federal
Financial Report (FFR), SF-425. Recipients will utilize the SF-425 lines 10.a through 10.c to

Att 2a, Page 42 of 44
report cash transaction data to the Division of Payment Management. The FFR SF-425 (lines 10.a
through 10.c) is due to the Payment Management System 30 days after the end of each calendar
quarter. The FFR SF-425 electronic submission and dates for the new quarters will be announced
through the Payment Management/SmartLink Payment System’s bulletin board. Funds will be
frozen if the report is not
filed on or before the due date.
4) Subaward and Executive Compensation Reporting: Awardees must ensure that they have
the necessary processes and systems in place to comply with the sub-award and executive total
compensation reporting requirements established under OMB guidance at 2 CFR Part 170, unless
they qualify for an exception from the requirements, should they be selected for funding. CFDA
number is to be included on all Subawards, including contracts and consultant agreements, so
ASPR staff may track compliance.
5) Tangible Property Report: Awardees will be required to submit an annual (after each 12
month period) Tangible Property Report (SF 428). Final SF 428 reports are due 90 days after the
end of the project period.
6) Audit requirements for Federal award recipients are detailed at
http://www.whitehouse.govisites/default/files/omb/assets/a133_revised_2007.pdf.
Specifically, non-Federal entities that expend a total of $750,000 or more in Federal awards,
during each Fiscal Year, are required to have an audit completed in accordance with OMB
Circular A-133. The Circular defines Federal awards as Federal financial assistance (grants) and
Federal cost-reimbursement (contracts) received both directly from a Federal awarding agency as
well as indirectly from a pass-through entity and requires entities submit, to the Federal Audit
Clearinghouse (FAC), a completed Data Collection Form (SF-SAC) along with the Audit Report,
within the earlier of 30 days after receipt of the report or 9 months after the fiscal year end.
The Data Collection Forms and Audit Reports MUST be submitted to the FAC electronically at
http://harvester.census.gov/fac/collect/ddeindex.html . For questions and information concerning
the submission process, please visit http://harvester.census.gov/sac/ or call the FAC 1-800-
253¬0696.
7) Reporting of Matters Related to Recipient Integrity and Performance
1. General Reporting Requirement
If the total value of your currently active grants, cooperative agreements, and procurement
contracts from all Federal awarding agencies exceeds $10,000,000 for any period of time during
the period of performance of this Federal award, then you as the recipient during that period of
time must maintain the currency of information reported to the System for Award Management
(SAM) that is made available in the designated integrity and performance system (currently the
Federal Awardee Performance and Integrity Information System (FAPIIS)) about civil, criminal,
or administrative proceedings described in paragraph 2 of this award term and condition. This is a
statutory requirement under section 872 of Public Law 110-417, as amended (41 U.S.C. 2313).
As required by section 3010 of Public Law 111-212, all information posted in the designated

Att 2a, Page 43 of 44
integrity and performance system on or after April 15, 2011, except past performance reviews
required for Federal procurement contracts, will be publicly available.
2. Proceedings About Which You Must Report
Submit the information required about each proceeding that:
a. Is in connection with the award or performance of a grant, cooperative agreement, or
procurement contract from the Federal Government;
b. Reached its final disposition during the most recent five year period; and
c. If one of the following:
(1) A criminal proceeding that resulted in a conviction, as defined in paragraph 5 of this award
term and condition;
(2) A civil proceeding that resulted in a finding of fault and liability and payment of a monetary
fine, penalty, reimbursement, restitution, or damages of $5,000 or more;
(3) An administrative proceeding, as defined in paragraph 5 of this award term and condition,
that resulted in a finding of fault and liability and your payment of either a monetary fine or
penalty of $5,000 or more or reimbursement, restitution, or damages in excess of $100,000; or (4)
Any other criminal, civil, or administrative proceeding if:
(i) It could have led to an outcome described in paragraph 2.c.(1), (2), or (3) of this award term
and condition;
(ii) It had a different disposition arrived at by consent or compromise with an acknowledgement
of fault on your part; and (iii) The requirement in this award term and condition to disclose
information about the proceeding does not conflict with applicable laws and regulations.
3. Reporting Procedures
Enter in the SAM Entity Management area the information that SAM requires about each
proceeding described in paragraph 2 of this award term and condition. You do not need to submit
the information a second time under assistance awards that you received if you already provided
the information through SAM because you were required to do so under Federal procurement
contracts that you were awarded.
4. Reporting Frequency
During any period of time when you are subject to this requirement in paragraph 1 of this award
term and condition, you must report proceedings information through SAM for the most recent
five year period, either to report new information about any proceeding(s) that you have not
reported previously or affirm that there is no new information to report. Recipients that have
Federal contract, grant, and cooperative agreement awards with a cumulative total value greater
than $10,000,000 must disclose semiannually any information about the criminal, civil, and
administrative
proceedings.
5. Definitions
For purposes of this award term and condition:
a. Administrative proceeding means a nonjudicial process that is adjudicatory in nature in order to
make a determination of fault or liability (e.g., Securities and Exchange Commission
Administrative proceedings. Civilian Board of Contract Appeals

Att 2a, Page 44 of 44
proceedings, and Armed Services Board of Contract Appeals proceedings). This includes
proceedings at the Federal and State level but only in connection with performance of a Federal
contract or grant. It does not include audits, site visits, corrective plans, or inspection of
deliverables.
b. Conviction, for purposes of this award term and condition, means a judgment or conviction of
a criminal offense by any court of competent jurisdiction, whether entered upon a verdict or a
plea, and includes a conviction entered upon a plea of nolo contendere.
c. Total value of currently active grants, cooperative agreements, and procurement contracts
includes—
(1) Only the Federal share of the funding under any Federal award with a recipient cost share or
match; and
(2) The value of all expected funding increments under a Federal award and options, even if not
yet exercised
Failure to comply with the above stated terms and conditions may result in suspension,
classification as High Risk status, termination of this award or denial of funding in the
future.
All previous terms and conditions remain in effect until specifically approved and removed
by the Grants Management Officer.
All responses to special terms and conditions of award and postaward requests must be
electronically mailed to the ASPR Grants Management Specialist and to the Program Official as
identified on your Notice of Award.
CLOSEOUT
All required closeout documents are due within 90 days of the end of the project period. At a
minimum those documents will include the final progress report, final financial reports. final
equipment and supply reports and invention reports. ASPR will convey final instructions.
Remarks:
Payment under this award will be made available through the HHS Departmental Payment
Management System (PMS). PMS provides instructions for making withdrawals of Federal
funds. Inquiries regarding payments should be directed to Program Support Center/Division of
Payment Management (PSC/DPM), DHHS; Post Office Box 6021; Rockville, MD 20852; 1¬877-
614-5533; PMSSupport@psc.gov
Contacts:
The Grants Management Specialist Nancy.Brown@hhs.gov is responsible for the negotiation,
award and administration of this project and for interpretation of grants administration policies
and provisions.
The Project Officer, Tyler.Merkeley@hhs.gov is responsible for the programmatic and technical
aspects.

***Confidential Treatment Requested
Research Subaward
Attachment 3A
Subaward No.
Pass-Through Entity Contacts
[…***…]
***Confidential Treatment Requested
Attachment 3B
Research Subaward Agreement
Subrecipient Contacts
[…***…]
Subaward Number:

***Confidential Treatment Requested
Attachment 3B Page 2
Research Subaward Agreement
Highest Compensated Officers
[…***…]
Subaward Number:

Attachment 4
Research Subaward Agreement
Reporting Requirements
Subrecipient agrees to the following:
A final technical/progress report will be submitted to PTE PI within 30 days after the end of the period of performance.
Monthly technical/progress reports will be submitted to PTE PI within 7 days of the end of each month. Report should
include programmatic and financial (sponsored and cost-share) components as detailed in PTE Prime Award Attachment
B/Reporting Table (Subaward Attachment 2a, p26), as applicable to Subrecipient. See Attachment 6, Article III for additional
information to be included on a quarterly basis in the monthly report. Negative reports are required. Monthly progress reports
are not required for the periods when the Annual Report is due.
Annual technical/progress reports will submitted to PTE PI within 7 days after the end of each reporting period. Report
should include programmatic and financial (sponsored and cost-share) components as detailed in PTE Prime Award
Attachment B/Reporting Table (Subaward Attachment 2a, p26), as applicable to Subrecipient.
In accordance with 37 CFR 401.14, Subrecipient agrees to notify PTE’s Administrative Contact within 30 days after
Subrecipient’s inventor discloses invention(s) in writing to Subrecipient’s personnel responsible for patent matters. The
Subrecipient will submit a final invention report using Awarding Agency specific forms to the PTE PI within 30 days of the
end of the period of performance to that it may be included with the PTE’s final invention report to the Awarding Agency. A
negative report is required.
Annual property inventory reports will be submitted to PTE’s Administrative contact within 7 days after the end of each 12-
month period. A final report is due 30 days after the project end date. The Subrecipient will submit property inventory reports
using Tangible Property Report (SF 428).
Subrecipient shall provide PTE PI with a status update of clinical studies that are actively enrolling patients to include by
study site: cumulative enrollment; new enrollments; screen failures; patients dropped from study; AE and SAEs; activation or
inactivation of study sites; investigator appointments or changes; and status of IRB/IEC review/approval/renewal.
Subrecipient shall include such status updates with the submission of the monthly programmatic report.
Other Special Reporting Requirements: It is anticipated that Subrecipient invoices and monthly financial reports will be
submitted through the CARB-X Digital Resources system. See also Att 4a (Adhoc Reporting Requirements).
All reports shall be in English. All information provided to PTE under this Subaward Agreement may be shared with
members of the CARB-X Joint Oversight Committee, which includes representatives from the Wellcome Trust, BARDA,
and NIAID for non-proprietary purposes relating to oversight under this Subaward Agreement.

Attachment 4a
Research Subaward Agreement
Ad hoc Reporting Requirements
The below section includes non-routine (Ad hoc) reporting requirements. These reports are event driven and are due contingent upon
such events occurring.
1. Incident Report
Subrecipient shall communicate and document all critical program concerns, risks, or potential risks with PTE.
○ Due within 24 hours of activity or incident or within 12 hours for a security activity or incident.
○ Email or telephone with written follow-up.
○ Additional updates within 24 hours of additional developments.
○ Subrecipient shall submit within 3 business days a Corrective Action Plan (if deemed necessary by either party) to
address any potential issues.
○ If corrective action is deemed necessary, Subrecipient must address in writing, its consideration of concerns raised by
PTE within 3 business days of receiving such concerns in writing.
2. FDA or EMA Audits
In the event of an FDA or EMA inspection which occurs that relates to products under this Subaward Agreement, or for any other
FDA or EMA inspection that has the reasonable potential to impact the performance of this Subaward Agreement, Subrecipient
shall provide PTE with an exact copy (non-redacted) of the FDA Form 483 and the Establishment Inspection Report (EIR) (or the
corresponding forms from the EMA). Subrecipient shall provide PTE with copies of the plan for addressing areas of non-
conformance to FDA or EMA regulations for GLP, GMP, or GCP guidelines as identified in the audit report, status updates
during the plan’s execution and a copy of all final responses to the FDA or EMA.
○ Subrecipient shall notify PTE within 5 business days of a scheduled FDA or EMA audit or within 12 hours of an ad hoc
site visit/audit if the FDA or EMA does not provide advanced notice.
○ Subrecipient shall provide copies of any FDA or EMA audit report received from sub-recipients that occur as a result of
this agreement or for products funded hereunder within three (3) business days of receiving correspondence from the
FDA or EMA.
○ Within five (5) business days of audit report, Subrecipient shall provide PTE with a plan for addressing areas of
nonconformance, if any are identified.
○ For the purposes of this Attachment 4a, “EMA” includes all constituent national drug regulatory authorities.
The below section includes PTE Initiated non-routine [Ad hoc] reporting requirements. These reports are due three (3) business days
after request.
1. Final Reports for Clinical, Non-Clinical Studies, Manufacturing Campaigns
PTE may request that Subrecipient provide Clinical, Non-Clinical Studies, Manufacturing Campaigns, and other product
development final reports to PTE for review and comment.
2. Standard Operating Procedures
PTE may request that Subrecipient shall make internal and subcontractor Standard Operating Procedures (SOPs) available for
review electronically.
3. Regulatory Correspondence and Submissions
PTE may request that Subrecipient provide any regulatory correspondence (FDA, EMA, etc.) for products supported under this
agreement.
4. QA Audits and Reports
PTE reserves the right to participate in QA audits at the Subrecipient. Upon completion of the audit/site visit the Subrecipient
shall provide a report capturing the findings, results and next steps in proceeding. If action is requested of the Subrecipient,
detailed concerns for addressing areas of non-conformance to FDA or EMA regulations for GLP, GMP, or GCP guidelines, as
identified in the audit report, must be provided to PTE. The Subrecipient shall provide responses from the site to address these
concerns and plans for corrective action execution.
5. Technical Documents

PTE may request that Subrecipient provide PTE with reports from the following agreement funded activities: Process
Development Reports, Assay Qualification Plan/Report, Assay Validation Plan/Report, Assay Technology Transfer Report, Batch
Records, SOPs, Master Production Records, Certificate of Analysis, Clinical Studies Data or Reports, Toxicology Reports or any
other reasonably requested Technical Documents.
6. Animal Model or Other Technology Transfer Package
PTE may request that Subrecipient provide Animal Model or Other Technology Transfer Package relevant data.
7. Post-completion Reporting
Subrecipient shall report to the PTE during the 24-month period following the completion of the Subaward Agreement any
significant funding, regulatory events, or major transactions involving the Project, including new equity funding, outlicensing or
collaboration agreements, regulatory approvals, and litigation involving the Project, as well as the Ad Hoc reporting specified
above as reasonably requested by the PTE. Subrecipient shall also annually report to the PTE the Subrecipients Stewardship and
Access Plan (as defined in Attachment 6, Article V) after the termination of the Subaward Agreement.

***Confidential Treatment Requested
Attachment 5
Cost Reimbursement Research Subaward Agreement
Statement of Work, Cost Sharing, Indirect & Budget
[…***…]
Subaward Number:

***Confidential Treatment Requested
[…***…]

***Confidential Treatment Requested
[…***…]
***Confidential Treatment Requested
Project Milestones
GO/NO GO PROJECT MILESTONES
Mstn
#
GO/NO GO Contract Milestone Go Criteria No Go Criteria Technical Report SOW/WBS # Stage
[…***…]

***Confidential Treatment Requested
[…***…]
***Confidential Treatment Requested
CIDARA THERAPEUTICS
Risk Mitigation
RISK MITIGATION MATRIX (RMM)
Prior to Risk Mitigation Strategy Post Risk Mitigation Strategy
WBS Risks Probability
of
Occurrence
Risk to
Project
(Severity)
Risk to
Cost
Risk to
Schedule
Risk to Tech
Performance
Risk Mitigation
Effort
Probability
of
Occurrence
Risk to
Project
(Severity)
Risk to
Cost
Risk to
Schedule
Risk to Tech
Performance
[…***…]
7
Confidential

Attachment 6
Research SubawardAgreement
CARB-X Special Terms and Conditions
Article I. The Project
Subrecipient has applied for funding from the PTE in support of the project described above and led by the
principal investigator named above, which aims to conduct research and development (R&D) to reduce the
threat to human health from antimicrobial resistance (the “Project”). The Subrecipient shall furnish or
arrange for the provision of all the necessary services, qualified personnel, material, equipment, and
facilities as needed to perform the Project to completion.
Section 1.01 The Project will begin with an initial phase of work, the Base Stage, and may include one or
more Option Stage(s).
Section 1.02 Work performed during the Base Stage and during each exercised Option Stage each constitute
independent, non-severable, discrete work segments that cannot be subdivided for separate performance and
are each necessary for the Project. Each non-severable, work segment constitutes a discrete requirement,
which shall contain multiple R&D activities that, when reviewed in total, shall result in a defined end
product.
Section 1.03 Statement of Work.
(a) The Statement of Work in Attachment 5 of this Subaward describes the Budget and Milestones for this
Subaward Agreement.
(b) This Subaward Agreement requires the Subrecipient to provide the PTE certain information regarding
monitoring and reporting contained in this Attachment 6 and in Attachments 4 and 5 (“Deliverables”).
(c) The PTE is providing Subrecipient with funding for the Project. The Subrecipient’s success in
completing the required Project tasks under each Stage (Base Stage and any exercised Option Stage)
must be demonstrated to the satisfaction of the PTE through completion of the Statement of Work and
Deliverables for that Stage.

(d) This Statement of Work in Attachment 5 may be extended, modified or terminated only as provided for
in this Subaward.
Article II. Option Stages
Section 2.01 No Automatic Option Stages.
(a) Unless an Option Stage is exercised in writing by the PTE as described herein, the Project consists only
of the Base Stage.
Section 2.02 Option Stages
(a) The PTE will evaluate Milestones and Deliverables at the completion of the Base Stage, and at each
subsequent exercised Option Stage.
(b) The PTE has no obligation to exercise any Option Stage and has no obligation to pay for any work under
any Option Stage, unless:
(i) all of the specified Milestones and Deliverables are fully satisfied by the intended dates, to the
satisfaction of the PTE;
(ii) the CARB-X Joint Oversight Committee, in its sole discretion, chooses to fund the Option Stage;
and
(iii)the Subrecipient and the PTE agree on a Statement of Work to be incorporated as Attachment 5 for
the Option Stage, evidenced in a fully-executed, new Subaward Agreement.
(c) Notice of intent to exercise an Option Stage shall be provided in the following manner:
(i) The PTE shall give written notice to the Subrecipient, signed by the PTE PI, of intent to exercise an
Option Stage;
(ii) The PTE will endeavor to give the Subrecipient provisional notice at least thirty (30) days before the
Subaward Agreement expires; and
(iii)The Subrecipient has ten (10) business days to provide written notice to the PTE to accept or decline
the Option. Nonacceptance is presumed if the Subrecipient fails to respond in writing within the ten
(10) business days.

Article III. Monitoring
Section 3.01 The Subrecipient’s progress in furtherance of Milestones and Deliverables will be monitored:
(a) By the Subrecipient’s Scientific Advisory Board (the “SAB”), with progress incorporated on a quarterly
basis in the monthly written reports by the Subrecipient as detailed in Attachment 4 (Reporting)
submitted to PTE;
(b) In the course of regularly scheduled meetings with the company support team provided by the PTE (the
“Company Support Team”);
(c) In monthly reports provided by the Subrecipient on the CARB-X Digital Resources as detailed in
Attachment 4 (Reporting), or as otherwise requested by the PTE;
(d) Subrecipient ad hoc reports reasonably requested by the PTE or as detailed in Attachment 4a; and
(e) A written Subrecipient report provided to the PTE when the Subrecipient considers it has completed a
Milestone.
Section 3.02 Subrecipient SAB.
(a) The Subrecipient shall establish (or shall demonstrate that it has already established) a SAB. The
Subrecipient will inform the PTE of SAB membership, including changes to the SAB membership.
(b) If the PTE reasonably believes that the Milestones and Deliverables are not being met, the PTE may
designate an independent expert advisor with relevant experience to attend SAB meetings related to this
Project.
Article IV. Subrecipient Representations and Warranties
Section 4.01 The Subrecipient makes the following representations and warranties to the best of its
knowledge:
(a) The Subrecipient has all requisite power and authority to execute, deliver, and perform this Subaward
Agreement and to deliver the Project.
(b) The Subrecipient has obtained or will obtain all third-party approvals and consents required for the
Subrecipient to execute, deliver, and perform this Subaward Agreement where failure to obtain such

approvals and consents would have a material adverse effect on Subrecipient’s ability to perform its
obligations under the Subaward Agreement.
(c) The execution and performance of this Subaward Agreement by the Subrecipient does not and will not
violate or conflict with, as applicable, the Subrecipient’s charter documents, contract(s) or intellectual
property agreements to which Subrecipient is a party, which violation or conflict would have a material
adverse effect on Subrecipient’s ability to perform its obligations under the Subaward Agreement.
(d) Subrecipient will perform the Subaward Agreement and the Project in compliance with all applicable
laws.
(e) All written statements made by the Subrecipient to the PTE during the application process, including
Expressions of Interest, Short Form and Long Form Applications, Presentations to the CARB-X
Advisory Board, and written responses to the Funding Award - Due Diligence Form, and all other
communications relating to this Subaward Agreement, are true and correct as of the time each such
statement was made.
(f) For each representation and warranty above, the statements are made: (a) as of the date of this Subaward
Agreement; (b) as of the date any Option Stage is exercised; and (c) as of the date the Subrecipient
provides any Deliverable to the PTE.
Article V. Additional Subrecipient Obligations
Section 5.01 Access, Not Excess.
(a) The purpose of CARB-X is to protect human health from threats related to antibiotic resistant bacteria.
CARB-X supports R&D efforts through this Subaward to improve the pipeline of products to protect
human health. These efforts must be sustainable, transparent and enforceable in order to protect human
health from antibiotic resistant bacteria. Over the long term, new products and technologies must be
sustainably managed and used to promote “Access, Not Excess,” including:

(i) Thoughtful and effective stewardship of new antibacterial products to prevent inappropriate use and
therefore premature resistance, in line with the Global Action Plan on Antimicrobial Resistance
developed by the World Health Organization;
(ii) Reasonable plans for appropriate access to and stewardship of developed antibiotic products and
technologies , especially in low- and middle-income countries; and
(iii) Avoidance of misaligned commercial incentives, which go against the above-stated goals.
(b) Therefore, the Subrecipient agrees that any products or technologies resulting from the Project (the
“Product”) will be manufactured, marketed, and sold in compliance with (at minimum) the principles the
Industry Roadmap for Progress on Combatting Antimicrobial Resistance - September 2016;
(c) Wellcome Trust Reporting Requirement. As the last Deliverable at the end of each Stage, the
Subrecipient shall create a plan reasonably describing how it intends to meet the above stewardship and
access obligations for the Product, (the “Stewardship and Access Plan”). The Stewardship and Access
Plan shall include:
(i) identifying obstacles and constraints to access, pricing and stewardship delivery;
(ii) identifying strategy to ensure access, pricing and stewardship delivery (e.g. proposed reliable
production with sufficient capacity, supply systems and pricing policies);
(iii) exploitation strategy for IP Rights;
(iv) global registration strategy;
(v) strategy for monitoring effectiveness of access, pricing and stewardship delivery and proposed
metrics to measure success; and
(vi) target pricing policies with a view to ensuring equitable access.
(d) The Subrecipient shall update the Stewardship and Access Plan and provide it to the PTE when the
Product enters Phase III trials, files a New Drug Application, or is approved by the FDA or EMA. After
marketing approval, the Stewardship and Access Plan shall be updated every other year. The
Subrecipient shall use best reasonable efforts to comply with its Plan at all times.

(e) The Stewardship and Access Plan will be a non-confidential document and will be publicly posted on
the PTE website.
(f) Obligations Follow the Product
(i) If ownership of the Subrecipient’s IP Rights resulting from the Project changes, whether through
sale, transfer, license, assignment or otherwise, the Subrecipient will require the above Stewardship
and Access Plan commitments to follow the Product and be incorporated into any such sale, transfer,
license, assignment or otherwise to the new company (the “Acquirer”). Prompt notice will be
provided by the Subrecipient to the PTE of any such event. If the Acquirer accepts obligations under
this Section 5.01, the Subrecipient is discharged from further obligations from this Section 5.01.
(ii) If the Subrecipient fails to provide the Stewardship and Access Plan as provided in Section 5.01, the
PTE can demand the same in writing. If the Subrecipient fails to provide such plan within sixty (60)
days of such written demand, then Subrecipient will be deemed to be in breach of this Section 5.01
and without limiting any other claims, damages or remedies, shall repay to the PTE, as stipulated
damages, the sum of $10,000 and the Subrecipient will not be deemed to be in good standing with
CARB-X.
(iii)The obligations of this Section 5.01 survive the termination of this Subaward Agreement and shall
continue in force until the expiration of the last period of patent or exclusivity periods in the United
States or the European Union for any IP Rights related to the Project.
Section 5.02 Open Science.
(a) CARB-X supports the unrestricted access to the published research resulting from the Project and the
public dissemination of the results of any clinical trial, including positive and negative results, which
may include clinical trial data.
(b) Therefore, the Subrecipient will endeavor, to the greatest extent possible, consistent with timely filing
of patents, to publish results from the Project (whether positive or negative), so that the results of this

Project are placed in the peer-reviewed literature as soon as practical, consistent with the protection of
the Subrecipient’s patents.
(c) CARB-X Awardees will make available any publications of research funded by CARB-X through
PubMed Central (PMC) and Europe PubMed Central as soon as possible and no later than six months
from the date of final publication (in accordance with Wellcome’s Open Access policy:
https://wellcome.ac.uk/funding/managing-grant/open-access-policy).
(d) The Subrecipient will provide the PTE with advance copies of all manuscripts related to the Project
when they are submitted or re-submitted for publication. The PTE will have no role in the preparation,
editing or approval of the manuscript.
Section 5.03 Additional Research Standards.
Subject in all cases to item 2 in the Agency Specific Certifications & Assurances included in Attachment
2 (describing minimum US government mandated standards for research with animals):
(a) Where the Subrecipient is undertaking research using non-human primates, cats, dogs or horses, the
Subrecipient must also comply with the Wellcome Trust policies on research involving animals and the
use of animals in medical and veterinary research.
(b) Where the Subrecipient is undertaking research involving human participants, the Subrecipient is
required to have the relevant regulatory and ethical approvals and appropriate governance mechanism in
place before such research begins and comply with item 3 in the Agency Specific Certifications &
Assurances included in Attachment 2 (describing US government regulations on human subjects
research). It is the responsibility of the Subrecipient (not the PTE) to ensure that appropriate
compensation arrangements (including insurance or indemnity cover, where available) are in place to
cover research participants or their dependents against injuries or damage caused as a result of their
participation in research, in accordance with local law and best practices. The PTE will not fund the
costs of such insurance or indemnity cover, and will not be liable for any such compensation.
(c) Where a healthcare intervention is being examined as part of research, the standard of healthcare
provided to a control group member must be at least equivalent to the best local, currently available and

affordable standard of care. The Subrecipient’s research protocol shall include proposals for any
necessary post-research health monitoring related to a volunteer’s participation.
Article VI. Intellectual Property
Section 6.01 Subrecipient Use of the Powered by CARB-X Logo.
(a) For the limited purposes of your participation in CARB-X relating to the Project, your use of the
following logo:
shall be permitted for the Term, subject to:
(i) your full performance of the terms and conditions of your Subaward Agreement with the PTE,
(ii) The PTE’s determination, in its sole discretion, that use of the term is either no longer accurate or
appropriate, in any way misrepresents Subrecipient participation, or for any reason reflects
negatively on the PTE or the PTE’s other partners, collaborators, sponsors or grantees, or
(iii)discontinuation of the trademark at the order or request of BARDA, NIAID or the Wellcome Trust.
(b) Subrecipient use of the term “Powered by CARB-X” shall be subject to CARB-X Brand Guidelines, as
set forth in Attachment 6a.
(c) Any other use of the CARB-X name, its servicemarks or trademarks, or any of its other distinguishable
marks, whether registered or not, shall be limited to those granted by the express, written permission of
the PTE. Those to whom such permission is granted must agree that the PTE shall remain the final
arbiter of the use of the mark.
Section 6.02 PTE Use of Subrecipient Logo.
The Subrecipient hereby grants the PTE and the institutions represented on the CARB-X Joint Oversight
Committee permission and the right to use the Subrecipient logo artwork for presentations, the PTE internal
and external websites, and other reasonable promotional and reporting uses relating to the Project.
Section 6.03 Other IP Rights.

(a) In addition to the reporting requirements detailed in Attachment 4 of this Subaward, the Subrecipient
shall provide written notice to PTE of any material:
(i) filing or re-filing, prosecution, defense or enforcement activities including any litigation or
threatened litigation of any IP Rights during the Term; and
(ii) completed transactions between the Company and third parties to sublicense, transfer, or otherwise
exploit the IP Rights.
(b) Subrecipient shall use its best commercial efforts to include provisions in its contracts with its
subcontractors performing service(s) requiring the subcontractors to assign to the Subrecipient the entire
right, title, and interest throughout the world to any inventions arising out of the services performed that
are specifically related to the asset under development in the Project.
Section 6.04 License to Wellcome Trust of Unexploited Intellectual Property
(a) Subject in all cases to the provisions of the PTE NOA, applicable Federal laws and regulations
governing patents and inventions, including government-wide regulations at 37 CFR part 401 (per 45
CFR 75.322(c)) and, in particular, and without limitation, to U.S. Government march-in rights set forth
in 37 CFR Sect. 401.6 and 401.14(j), and further subject to Section 6.04(e), if any IP Rights remain
unexploited or not further developed by the Subrecipient and/or its licensees in any country as of the
date that is five (5) years following the end date of the Project, the Wellcome Trust Limited as trustee of
the Wellcome Trust (“Wellcome”) shall have the option in its sole discretion by giving written notice to
the Subrecipient, to take responsibility on behalf of the Subrecipient for the commercialization and
exploitation of such IP Rights in that country, which includes discretion to make any and all decisions
regarding the negotiation, acceptance, and conclusion of terms for any agreement regarding the
commercial development and exploitation of such unexploited IP Rights (including development and
exploitation by way of license, sale, assignment, materials transfer or other transfer of rights, as well as
any transaction which involves placing such unexploited IP Rights into a separate corporate vehicle) in
such country.

(b) If Wellcome exercises its right to exploit on behalf of the Subrecipient under Section 6.04(a), the
Subrecipient will license or assign the IP Rights to Wellcome or its nominee and provide Wellcome with
access to any associated data, documents, materials, regulatory approvals or information as required for
Wellcome to exploit such rights.
(c) If Wellcome exercises its right to exploit on behalf of the Subrecipient under Section 6.04(a), the
Subrecipient agrees that it shall pass to Wellcome promptly any or all exploitation opportunities in the
applicable country that it becomes aware of from time to time in connection with the IP Rights. The
Subrecipient further undertakes that it shall not engage in any activities that could reasonably lead to the
loss of an exploitation opportunity in the applicable country without the prior written consent of
Wellcome.
(d) If Wellcome exercises its right to exploit under Section 6.04(a), the Trust shall share revenues and
equity holdings received in respect of exploitation of the IP Rights at a 50:50 ratio between Wellcome
and the Subrecipient after reimbursement of costs that Wellcome directly incurs in exploiting the Project
IP Rights.
(e) Notwithstanding anything to the contrary set forth in this Section 6.04, in the event that the Subrecipient
licenses a third party to exploit the IP Rights (whether alone or together with other IP Rights of the
Subrecipient) in any country, then Wellcome shall have no rights under this Section 6.04 with respect to
such IP Rights in such country, provided that either: (a) under a written agreement with the Subrecipient,
such licensee is required to use diligent efforts to exploit the licensed IP Rights in such country, and
such written agreement provides for a reversion to the Subrecipient of the IP Rights in such country if
the licensee materially breaches this diligence obligation; or (b) Wellcome has approved such license in
writing.
(f) For purposes of this Section 6.04 only, Wellcome is a third party beneficiary of this Subaward
Agreement, able to enforce the right to exploit in any country any unexploited IP Rights derived from
the Project as described herein.

(g) The rights and obligations of this Section 6.04 survive the termination of this Subaward Agreement and
shall continue in force until ten (10) years following the end date of this Subaward Agreement.
Article VII. Budget.
Section 7.01 Cost Share Principles.
(a) The Subrecipient will meet their full amount of cost share obligation as stated in Attachment 5 (the
“Cost Share Obligation”).
(b) The Cost Share Obligation must meet the requirements described in this Subaward Agreement.
(c) The Subrecipient shall use best efforts to reach an agreement with NIAID so that the Subrecipient
utilizes an amount of NIAID Preclinical Services (“PCS”) valued at no less than 15% of the budget
amount stated in Attachment 5 for the Base Stage (the “NIAID PCS Target Amount”).
Section 7.02 Budget Reconciliation.
(a) At the end of the Base Stage and at the end of any exercised Option Stage(s), the Subrecipient and PTE
will perform a budget reconciliation to determine:
(i) Whether the Subrecipient fully met their Cost Share Obligation; and
(ii) Whether the Subrecipient fully met their NIAID PCS Target Amount.
(b) If the Subrecipient does not meet the Cost Share Obligation in full, the PTE may withhold payment or
request a refund to recover the unmet Cost Share Obligation.
Article VIII. Definitions
All other capitalized terms are defined as described in the text of this Subrecipient Agreement.
Section 8.01 Base Stage means the initial scope of work contracted for in this Subaward Agreement as
detailed in Subaward Attachment 5 and further described in Article I of this Attachment 6.
Section 8.02 CARB-X Funders means HHS/ASPR/BARDA, the Wellcome Trust, and HHS/NIH/NIAID.
Section 8.03 IP Rights means all legally protectable patents, know-how, and trade secret rights that cover the
making, use, or sale of the specific asset under development in the Project.

Section 8.04 Milestones means a reference point that marks a specified task and is used to monitor the
Project’s progress.
Section 8.05 Option Stage means additional, optional, segment of work that builds upon Base Stage or
previously exercised Option Stage as described in Article II of this Attachment 6.
Section 8.06 Subaward Agreement means this Cost Reimbursement Research Subaward Agreement.

Attachment 6a
Research Subaward Agreement
Powered by CARB-X Logo and Brand Guidelines
We encourage the use of the Powered by CARB-X Logo in all communications that serve to
raise the profile of CARB-X in a positive manner and to raise awareness about its mission. Any
use of the Powered by CARB-X Logo must be in accordance with the Sub-Award Agreement
between the Company and Boston University.
The following are guidelines to assist in the graphic design of communications materials in
which you would like to use the Powered by CARB-X Logo.
1. Display: In order to preserve the integrity of the Powered by CARB-X Logos, it is
important that no other logos, type or other graphic elements infringe on its space. The
minimum “clear space” around the Logo in all uses is equivalent to ½ the height of the
Logo.
2. Background Color: The Powered by CARB-X Logo should always be used in color for
online use, fully visible and displayed on a white background. The Powered by
CARB-X Logo should be used in color for print applications unless the color version is
not practical, in which case the Powered by CARB-X Logo can be reproduced in solid
black on a white background. For all other uses, such as in videos and digital
communications, please consult with your CARB-X liaison for guidance prior to
distribution or publication.
3. The Powered by CARB-X Logo should be displayed in high resolution as appropriate
to the medium.
4. Do not use the Powered by CARB-X Logo in a manner that might create confusion as
to the CARB-X brand or imply that CARB-X is the source of your products or services.
5. The Powered by CARB-X Logo must not be used as your own product names, service
names, trademarks, logos, company names, domain names, website title, application
icon, favicon, or the like.
