Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - NEXEON MEDSYSTEMS INC | ex23_1.htm |
As filed with the Securities and Exchange Commission on April 28, 2017
Registration No. 333-____________
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
NEXEON MEDSYSTEMS INC
(Exact name of registrant as specified in its charter)
|
Nevada
|
|
3841
|
|
81-0756622
|
|
(State or other jurisdiction
|
|
(Primary Standard Industrial
|
|
(I.R.S. Employer
|
|
of incorporation or organization)
|
|
Classification Code Number)
|
|
Identification Number)
|
1708 Jaggie Fox Way
Lexington, Kentucky
Telephone: (844) 919-9990
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
William Rosellini
Chief Executive Officer
Nexeon MedSystems Inc
1708 Jaggie Fox Way
Lexington, Kentucky
Telephone: (844) 919-9990
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With a copy to:
Harvey Kesner, Esq.
Sichenzia Ross Ference Kesner LLP
61 Broadway, 32nd Floor
New York, NY 10006
(212) 930-9700
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: ☑
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐
|
Accelerated filer ☐
|
|
Non-accelerated filer ☐ (Do not check if a smaller reporting company)
|
Smaller reporting company ☑
|
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of
Securities to be Registered
|
Amount to be
Registered(1)
|
Proposed
Maximum
Offering Price
per Share(2)
|
Proposed
Maximum
Aggregate
Offering Price
|
Amount of
Registration Fee
|
||||||||||||
|
|
||||||||||||||||
|
Common Stock, $0.001 par value
per share
|
6,052,240
|
$
|
1.00
|
$
|
6,052,240
|
$
|
701.45
|
|||||||||
+
| (1) |
Pursuant to Rule 416 under the Securities Act of 1933, as amended, the shares being registered hereunder include such indeterminate number of shares of common stock, as may be issuable with respect to the shares being registered hereunder as a result of stock splits, stock dividends or similar transactions.
|
| (2) |
Estimated solely for purposes of calculating the registration fee pursuant to Rule 457(c) under the Securities Act of 1933, as amended.
|
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell and is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
|
PRELIMINARY PROSPECTUS
|
SUBJECT TO COMPLETION
|
DATED APRIL 28, 2017
|

6,052,240 Shares of Common Stock
We are registering an aggregate of 6,052,240 shares of common stock, $0.001 par value per share (the “Common Stock”) (collectively, the “Resale Shares”) of Nexeon MedSystems Inc (referred to herein as “we”, “us”, “our”, “Nexeon”, “Registrant”, or the “Company”) for resale by certain of our shareholders identified in this prospectus (the “Selling Shareholders”). Please see “Selling Shareholders” beginning at page 43.
The Selling Shareholders may sell some or all of their shares at a fixed price of $1.00 per share until our shares are quoted on the OTCQX for anticipated aggregate net proceeds of approximately $6,052,240 and thereafter at prevailing market prices or privately negotiated prices. The offering price bears no relationship to our assets, book value, earnings or any other customary investment criteria. We will not receive any proceeds from the sale of the Resale Shares by the Selling Shareholders. We will bear all costs relating to the registration of the Resale Shares.
Our Common Stock is not currently listed for trading on any exchange. It is our intention to seek quotation on the OTCQX. The Company and its sponsoring broker dealer have filed form 211 with FINRA to obtain the Company’s trading symbol and are waiting for the issuance; provided, however, there can be no assurance that our Common Stock will be approved for trading on the OTCQX Marketplace or any other trading exchange.
The Resale Shares may be sold by the Selling Shareholders to or through underwriters or dealers, directly to purchasers or through agents designated from time to time. For additional information regarding the methods of sale you should refer to the section entitled “Plan of Distribution” in this Prospectus.
Our business and an investment in our securities involve a high degree of risk. See “Risk Factors” beginning on page 5 of this prospectus for a discussion of information that you should consider before investing in our securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2017
|
|
Page
|
|
|
|
|
3
|
|
|
5
|
|
|
12
|
|
|
13
|
|
|
13
|
|
|
13
|
|
|
13
|
|
|
14
|
|
|
19
|
|
|
34
|
|
|
42
|
|
|
44
|
|
|
45
|
|
|
46
|
|
|
50
|
|
|
51
|
|
|
52
|
|
|
53
|
You should rely only on the information contained in this prospectus or in any free writing prospectus that we may specifically authorize to be delivered or made available to you. We have not authorized anyone to provide you with any information other than that contained in this prospectus or in any free writing prospectus we may authorize to be delivered or made available to you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus may only be used where it is legal to offer and sell our securities. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of our securities. Our business, financial condition, results of operations and prospects may have changed since that date. We are not making an offer of these securities in any jurisdiction where the offer is not permitted.
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before investing in our Common Stock, you should carefully read this entire prospectus, including our financial statements and the related notes and the information set forth under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in each case included elsewhere in this prospectus.
Unless the context otherwise requires, references to “we,” “our,” “us,” “Nexeon” or the “Company” in this prospectus mean Nexeon MedSystems Inc., a Nevada corporation, on a consolidated basis with its wholly-owned subsidiaries, as applicable.
COMPANY BACKGROUND
We were incorporated in the State of Nevada on December 7, 2015. Our principal corporate office is located at 1708 Jaggie Fox Way, Lexington, Kentucky 40511 and our phone number is (844) 919-9990. Our internet address is www.nexeonmed.com.
We are a bioelectronics company developing active medical devices for the treatment of chronic medical conditions. The solutions we are developing are a unique blend of traditional device technologies such as electronics, software, mechanical engineering, and material science, as well as pharmaceuticals, protein chemistry, and cell biology.
On February 16, 2016, Nexeon MedSystems, Inc., a Delaware corporation (“NXDE”) merged with and into the Company (the “Merger”) with the Company surviving the Merger as the surviving corporation. As a result of the Merger, (i) 100% of NXDE’s issued and outstanding shares of preferred stock were converted into an aggregate of 1,659,943 shares of the Company’s Common Stock and (ii) all shares of NXDE’s common stock, options and deferred compensation units were cancelled. In addition, pursuant to the terms of the Merger, the Company will pay Nexeon Shareholder Royalty Group LLC, a West Virginia limited liability company formed in connection with the Merger, a 3% royalty on net product sales derived by the Company, its affiliates and licensees in connection with the commercialization of patents and intellectual property acquired by the Company pursuant to the Merger. Former NXDE shareholders are members of Nexeon Shareholder Royalty Group LLC.
The Nexeon Neurostimulation System
The Nexeon Neurostimulation System (the “NNS”) was developed to address the shortcomings of present day neuromodulators. The NNS is an implantable neurostimulation and recording platform, and is one of the very few implantable stimulation and recording devices that has received regulatory approval (Conformité Européenne, or “CE” conformity) for human use in connection with use as a deep brain stimulation system in the treatment of certain movement disorders. The Multi-Purpose Implant Controller is the engine of the NNS system. This custom-made computer chip utilizes features from cochlear implants and we believe addresses the shortcomings of present day neuromodulators. The flexibility of in-house manufacturing and the unique ability to reprogram the operation of the NNS allows anticipation of future needs and increasing knowledge of deep brain stimulation therapy for a broad spectrum of neural diseases.
On January 10, 2017, we entered into an exclusive, irrevocable agreement with Rosellini Scientific LLC (“Rosellini Scientific”) pursuant to which we will acquire 100% of the issued and outstanding capital stock of Nexeon Medsystems Belgium, SPRL (“NMB”), an entity controlled by William Rosellini, our Chief Executive Officer. In conjunction with the acquisition of NMB, we will be continuing the development of the NNS that was initially developed by NMB. As part of the continued development, we intend to extend capabilities and target new diseases and disorders.
Micro-Perforated Catheter Balloon
We have developed, Micro-Perforated Catheter Balloon, a novel balloon material that utilizes carbon nanotubes and polymer. The carbon nanotubes help maintain structural integrity of the Micro-Perforated Catheter Balloon during inflation and drug delivery while allowing a more compliant polymer, such as nylon, to be used. While current drug-coated balloons provide only passive drug delivery, management believes that Micro-Perforated Catheter Balloon brings a much-needed, innovative improvement to intravascular drug delivery by enabling controllable pulsed flow delivery of anti-restenotic drugs to the diseased site. If successful, our approach will reduce the need for follow up procedures by efficiently delivering anti-restenotic drugs, intravascularly, to direct the therapy immediately into the lesion. The foregoing balloon material is protected by approximately 36 patents which we acquired in the Merger, and we anticipate that federal and state funding will enable us to develop our solution and proceed with a U.S. Food & Drug Administration (“FDA”) 510(k) approval. We expect to develop a suite of passive and electrically-active drug eluting balloons that will be owned by our wholly-owned subsidiary, Pulsus Medical LLC, a Kentucky limited liability company and wholly-owned subsidiary of the Company (“Pulsus”).
Siemens Intellectual Property
Effective as of December 15, 2016, we acquired a non-exclusive license to a portfolio of 86 patents that originated from Siemens AG, a global technology powerhouse and one of the world’s preeminent engineering companies. The intellectual property relates to Internet-of-Things (“IOT”) technology as described by a system of interrelated computing devices, mechanical and digital machines, objects, animals, and/or people that have unique identifiers and a subsequent ability to transfer data over a network without requiring human-to-human or human-to-computer interaction. This technology can be utilized in a wide variety of medical device applications, most notably in hospitals, nursing facilities, or patients’ homes.
Summary of the Offering
|
Resale Shares
|
6,052,240 shares of Common Stock (collectively, the “Resale Shares”)
|
|
|
Offering Price
|
$1.00 per share until a market develops and thereafter at market prices or privately negotiated prices.
|
|
|
Common Stock Outstanding
Before and After this Offering
|
(1) 22,663,621 and (2) 22,663,621
|
|
|
Use of Proceeds
|
We will not receive any proceeds from the sale of the Resale Shares by the Selling Shareholders.
|
|
|
Market for our Common
Stock
|
There is currently no market for our securities. Our Common Stock is not currently listed for trading on any exchange. It is our intention to seek quotation on the OTCQX.The Company and its sponsoring broker dealer have filed form 211 with FINRA to obtain the Company’s trading symbol and are waiting for the issuance; provided, however, there can be no assurance that our Common Stock will be approved for trading on the OTCQX or any other trading exchange.
|
|
|
|
|
|
|
Risk Factors
|
See “Risk Factors” beginning on page 5 of this prospectus and the other information included in this prospectus for a discussion of factors you should carefully consider before investing in our securities.
|
| (1) |
The number of outstanding shares before the offering is based upon 22,663,621 shares outstanding as of April 27, 2017, and excludes:
|
| · |
757,997 shares of our Common Stock issuable upon exercise of outstanding vested options at a weighted average exercise price of $1.00 per share as of April 27, 2017 and 2,024,003 shares of our Common Stock issuable upon exercise of outstanding unvested options at a weighted average exercise price of $1.00 per share as of April 27, 2017; and
|
| · |
2,458,762 shares of our Common Stock issuable upon exercise of outstanding warrants at a weighted average exercise price of $2.00 per share as of April 27, 2017.
|
| (2) |
The number of outstanding shares after the offering includes the Resale Shares already issued and outstanding.
|
Any investment in our Common Stock involves a high degree of risk. Investors should carefully consider the risks described below and all of the information contained in this prospectus before deciding whether to purchase our Common Stock. Our business, financial condition and results of operations could be materially adversely affected by these risks if any of them actually occur. This prospectus also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks we face as described below and elsewhere in this prospectus.
RISKS RELATED TO OUR BUSINESS AND INDUSTRY
We have only a limited history upon which an evaluation of our prospects and future performance can be made and have no history of profitable operations. Since we have a limited operating history, it may be difficult for potential investors to evaluate our business.
We were incorporated in the State in Nevada on December 7, 2015, and as a result, we have only a limited history upon which an evaluation of our prospects and future performance can be made and have no history of profitable operations. Due to our lack of operating history, our proposed operations are subject to all business risks associated with new enterprises. The likelihood of our success must be considered in light of the problems, expenses, difficulties, complications, and delays frequently encountered in connection with the startup of a business and operation in a competitive and regulated industry. We may sustain losses in the future as we implement our business plan. There can be no assurance that we will ever generate revenues or operate profitably. Investors should evaluate an investment in us in light of the uncertainties encountered by developing companies in a competitive environment. Our business is dependent upon the implementation of our business plan. We may not be successful in implementing such plan and cannot guarantee that, if implemented, we will ultimately be able to attain profitability.
We will need to obtain additional financing to fund our operations.
We will need additional capital in the future to continue to execute our business plan. Therefore, we will be dependent upon additional capital in the form of either debt or equity to continue our operations and commercialize our products. At the present time, we do not have arrangements to raise all of the needed additional capital and we will need to identify potential investors and negotiate appropriate arrangements with them. We may not be able to arrange enough investment within the time the investment is required or that if it is arranged, that it will be on favorable terms. If we cannot get the needed capital, we may not be able to become profitable and may have to curtail or cease our operations.
We have a history of losses, and we anticipate that we will continue to incur losses in the future. Our auditors have included in their audit report an explanatory paragraph as to substantial doubt as to our ability to continue as a going concern.
We have experienced net losses since our inception. As of December 31, 2016, our net loss was $1,591,423 compared to a net loss of $915 for the period between December 7, 2015 (inception) and December 31, 2015. As of December 31, 2016, we had an accumulated deficit of $1,592,338. Our auditors have included in their audit report a “going concern” explanatory paragraph as to substantial doubt as to our ability to continue as a going concern that assumes the realization of our assets and the satisfaction of our liabilities and commitments in the normal course of business. We anticipate continuing to incur substantial additional losses over at least the next several years due to, among other factors, expenses related to the following: investor and public relations, SEC compliance efforts, anticipated research and development activities and the general and administrative expenses associated with each of these activities. We may never achieve profitability, and even if we do, we may not be able to sustain being profitable.
Our management and our independent registered public accounting firm have not identified any material weaknesses in our internal control over financial reporting. However, if we are unable to maintain certain internal controls, we may not be able to produce timely and accurate financial statements, and our independent registered public accounting firm could conclude that our internal control over financial reporting is not effective, which could adversely impact investor confidence and our stock price.
In connection with the audit of our financial statements for the year ended December 31, 2016, our management and our independent registered public accounting firm have not identified any material weaknesses in our internal control over financial reporting. However, due to the size of our current operation and existing personnel, errors may occur if we are not able to maintain certain internal controls over financial reporting. Our management is responsible for maintaining, implementing and testing our internal controls over financial reporting. These efforts are intended to maintain an effective control environment but may not prevent significant deficiencies from occurring. If we are unable to maintain such internal controls, we may not be able to produce timely and accurate financial statements, and our independent registered public accounting firm could conclude that our internal control over financial reporting is not effective, which could adversely impact investor confidence and our stock price.
We may be unable to complete or integrate intellectual property acquisitions effectively, which may adversely affect our growth, profitability, and results of operations.
The Company expects future acquisitions of intellectual property to play a significant role in the Company’s product development growth. As of the date hereof, we have made two such acquisition; however, we cannot be certain that we will be able to continue to identify attractive acquisition opportunities, obtain financing for acquisitions on satisfactory terms if needed, or successfully acquire identified targets. Additionally, we may not be successful in integrating acquired intellectual property into our existing operations and realizing anticipated synergies. Competition for acquisition opportunities in the industry in which we operate may increase our costs of acquisitions or causing us to refrain from engaging in acquisitions. These and other factors relating to our acquisition of intellectual property could negatively and adversely impact our growth, profitability, and results of operations.
The Company may experience delays or unforeseen issues during the requisite studies and trials required prior to regulatory approval of our medical devices.
Although there are no foreseeable risks with respect to obtaining an Investigational Device Exemption (“IDE”) following biocompatibility and active animal safety testing, even minor issues with the testing and application process can delay the IDE, which will in turn delay the clinical studies and increase the costs to complete the testing. For our FDA premarket approval (“PMA”), we will hire a regulatory consultant to facilitate all interactions with the FDA and ensure that we make the most time and capital efficient steps towards regulatory approval.
The Company may not be able to compete effectively with larger companies in the medical device space with greater resources and market recognition.
Our primary competitor, Medtronic, a provider of medical devices has been involved in the manufacturing and sale of deep brain stimulation devices for several years. In addition, Boston Scientific and Abbott (formerly St. Jude Medical) have a CE mark and PMA approval to market and sell their neurostimulation implant devices in Europe and in the U.S. These companies may have substantially greater financial, research and development, manufacturing, marketing and sales experience, and resources than us. As a result, our competitors may be more successful than us in developing their products, obtaining regulatory approvals, and marketing their products to consumers. We cannot assure investors that we will be able to compete effectively against current and future competitors.
The Company’s neurostimulation device may not be approved for reimbursement which could adversely affect the adoption of our device.
Even if the device and treatment are successful and approved for use, reimbursement may not be forthcoming, which could lead to slower than anticipated adoption of this technology. Additionally, new drugs or devices may also affect market acceptance. These risks are mitigated by the current reimbursement standards, which we believe our system will be reimbursed under. Additionally, the Company’s management team is experienced in device reimbursement, in the U.S. and in Germany, as well as throughout the European Union (“EU”). William Rosellini, our Chief Executive Officer, is a published author in the area of Medicare and Medicaid reimbursement. Previously, he has utilized reimbursement consultants to map existing reimbursement codes in order to develop proper data collection and clinical trial design strategies to shorten the path to eventual Medicare reimbursement. Although the Company’s management team is experienced in device reimbursement there can be no guarantee that the Company’s neurostimulation device will be approved for reimbursement, which could adversely affect the adoption of our device.
Laws and regulations that could affect the industry in which we operate may be enacted, which could result in a delay or cessation of our research and development activities or the imposition of additional costs that could hinder our ability to achieve and maintain profitable operations.
Current laws and regulations with respect to our industry and additional laws and regulations which may be enacted in the future could impose new and/or unexpected operational considerations or constraints upon us. Complying with existing laws or regulations may require significant time and resource allocation for medical device manufacturers, including us. Additionally, changing or new legislation may force us to redesign one or more of our products. In such an event, our proprietary neurostimulation device may have to be altered or modified as to ensure that it is in compliance with all applicable laws and regulations. Such alterations or modifications would cause us to incur substantial research and development costs. Moreover, if we cannot modify or alter our neurostimulation device’s design or functionality, then our device could be rendered obsolete, which would substantially reduce our future profitability and harm our business. Additionally, since we intend to operate both domestically and in the European Union, we must remain cognizant of the legislative and regulatory landscape in both regions. Compliance with these regulations, when applicable, increases the research and development and production costs and could make our proposed products and services less attractive to potential customers.
International operations, if conducted by us in the future, would subject us to a number of risks, including unfavorable political, regulatory, labor, and tax conditions.
We may sell medical devices to customers located outside the United States in the future. International operations are subject to the legal, political, regulatory, and social requirements and economic conditions in the jurisdictions in which they are conducted. Risks inherent to international operations and sales, include, but are not limited to, the following:
| · |
exposure to violations of the Foreign Corrupt Practices Act of 1977, as amended;
|
| · |
difficulty in enforcing agreements, judgments, and arbitration awards in foreign legal systems;
|
| · |
impediments to the flow of foreign exchange capital payments and receipts due to exchange controls instituted by certain foreign governments and the fact that the local currencies of these countries are not freely convertible;
|
| · |
inability to obtain maintain, or enforce our intellectual property rights;
|
| · |
changes in general economic and political conditions in foreign countries;
|
| · |
changes in foreign government regulations and technical standards, including additional regulation of medical devices, which may reduce or eliminate our ability to sell or license in certain markets;
|
| · |
requirements or preferences of foreign nations for domestic technologies, which could reduce demand for our technologies;
|
| · |
trade barriers such as export requirements, tariffs, taxes, and other restrictions and expenses, which could increase the prices of our technologies and make us less competitive; and
|
| · |
longer payment cycles typically associated with international sales and potential difficulties in collecting accounts receivable, which may reduce the future profitability of foreign sales or licensing.
|
Conducting business in foreign jurisdictions would require us to respond to rapid changes in market conditions in these countries. The Company believes that its overall success as a global business would depend on its ability to succeed in different legal, regulatory, economic, social, and political situations and conditions. We may not be able to develop and implement effective policies and strategies in each foreign jurisdiction where we may do business in the future.
The Company depends on its key management personnel for its future success.
The Company’s success depends largely on the skills of its key management and technical personnel. The loss of one or more of its key management and technical personnel may materially and adversely affect business and results of operations. The Company does not maintain key person insurance for any of its employees. The Company cannot guarantee that it will be able to replace any of its key management personnel in the event that their services become unavailable.
We only have a limited number of employees to manage and operate our business.
As of April 27, 2017, we had a total of 10 full-time employees and 1 part-time employees. We cannot assure you that we will be able to retain adequate staffing levels to run our operations and/or to accomplish all of the objectives that we otherwise would seek to accomplish.
Failure to raise the necessary capital could restrict the Company’s growth, limit its development of new products and services and hinder its ability to compete.
The Company needs to raise funds in order to achieve its business objectives. Failure to raise these funds may:
| · |
Restrict its growth;
|
| · |
Limit its development of new products and services; and
|
| · |
Hinder its ability to compete.
|
Any of these aforementioned consequences would have a materially adverse effect on the Company’s business, operations, and financial position.
We may be subject to product liability claims, and may not have sufficient product liability insurance to cover any such claims, which may expose us to substantial liabilities.
We may be exposed to product liability claims from consumers of our products. It is possible that any product liability insurance coverage we obtain will be insufficient to protect us from future claims. Further, we may not be able to obtain or maintain insurance on acceptable terms or that such insurance would be sufficient to cover any potential product liability claim or recall. Failure to obtain or maintain sufficient insurance coverage could have a material adverse effect on our business, prospects, and results of operations if claims are made that exceed our coverage.
Our revenues will depend upon adequate reimbursement from public and private insurers and health systems.
Our success will depend on the extent to which reimbursement for the costs of its treatments will be available from third party payers, such as public and private insurers and health systems. Government and other third party payers attempt to contain healthcare costs by limiting both coverage and the level of reimbursement of new treatments. Therefore, significant uncertainty usually exists as to the reimbursement status of new healthcare treatments. If we are not successful in obtaining adequate reimbursement for our treatment from these third party payers, the market’s acceptance of our treatment could be adversely affected. Inadequate reimbursement levels also likely would create downward price pressure on our treatment. Even if we succeed in obtaining widespread reimbursement for our treatment, future changes in reimbursement policies could have a negative impact on our business, financial condition and results of operations.
To be commercially successful, we must convince physicians that our devices are safe and effective alternatives to existing medical devices and that our devices should be used.
We believe physicians will only adopt our devices if they determine, based on experience, clinical data and published peer reviewed journal articles, that the use of our devices is a favorable alternative to conventional devices/methods. Physicians may be slow to change their practices for the following reasons, among others:
| · |
Lack of evidence supporting additional patient benefits and our devices over conventional devices/methods;
|
| · |
Perceived liability risks generally associated with the use of new devices; and
|
| · |
Limited availability of reimbursement from third party payers
|
In addition, we believe that recommendations for and support of our devices by influential physicians are essential for market acceptance and adoption. If we do not receive this support or are unable to demonstrate favorable long-term clinical data, physicians and hospitals may not use our devices, which would significantly reduce our ability to achieve revenue and would prevent us from sustaining profitability.
We are an “emerging growth company,” and the reduced disclosure requirements applicable to emerging growth companies may make our Common Stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups (“JOBS”) Act, and may remain an emerging growth company for up to five years. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
| · |
being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure;
|
| · |
not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting;
|
| · |
not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements;
|
| · |
reduced disclosure obligations regarding executive compensation; and
|
| · |
exemptions from the requirements of holding a non-binding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
|
We cannot predict whether investors will find our Common Stock less attractive if we rely on these exemptions. If some investors find our Common Stock less attractive as a result, there may be a less active trading market for our Common Stock and our stock price may be more volatile.
RISKS RELATED TO OUR INTELLECTUAL PROPERTY
Variability in intellectual property laws may adversely affect our intellectual property position.
Intellectual property laws, and patent laws and regulations in particular, have been subject to significant variability either through administrative or legislative changes to such laws or regulations or changes or differences in judicial interpretation, and it is expected that such variability will continue to occur. Additionally, intellectual property laws and regulations differ among countries. Variations in the patent laws and regulations or in interpretations of patent laws and regulations in the United States and other countries may diminish the value of our intellectual property and may change the impact of third-party intellectual property on us. Accordingly, we cannot predict the scope of patents that may be granted to us, the extent to which we will be able to enforce our patents against third parties, or the extent to which third parties may be able to enforce their patents against us.
We may need to negotiate modifications or extensions of existing intellectual property agreements.
We license intellectual property from third parties. We may in the future need to extend, modify, or otherwise negotiate changes to such licenses. There can be no assurance that the third parties with whom we have (or in the future may have) agreements will agree to such modifications, or that if they do, that they will do so on terms favorable to us.
We, or the third parties whom we license intellectual property from, may become involved in legal proceedings to protect or enforce our intellectual property rights, which could be expensive and time-consuming.
Competitors or others may infringe upon our intellectual property rights. To counteract infringement or unauthorized use, we or third parties whom we license intellectual property from may be required to file patent infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that certain of our intellectual property is not valid or is unenforceable, or the court may refuse to stop the other party from using the technology at issue on the grounds that our intellectual property rights do not cover such technology.
An adverse determination of any litigation or defense proceedings could put our intellectual property at risk of being invalidated or interpreted narrowly, and could put outstanding intellectual property applications at risk of not being granted.
Interference proceedings brought by the United States Patent and Trademark Office may be necessary to determine the priority of inventions with respect to our intellectual property applications. Litigation or interference proceedings may fail and, even if successful, may result in substantial costs, diversion of resources, and distraction of our management. We, or our licensors, may not be able to prevent misappropriation of our proprietary rights, particularly in countries where the laws may not protect such rights as comprehensively as in the United States.
Furthermore, due to the substantial amount of discovery associated with intellectual property litigation, there is a risk that some of our, or our licensors’, confidential information could be compromised by disclosure. In addition, during the course of this litigation, there could be public announcement of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, then it could have a substantial adverse effect on the price of our Common Stock. We, or our licensors, may not prevail in any litigation or interference proceeding in which we may be involved in the future. Even if we, or our licensors prevail, such legal proceedings would likely be expensive and time-consuming.
RISKS RELATED TO OUR COMMON STOCK
Our shareholders may not be able to resell their stock due to a lack of public trading market.
There is presently no public trading market for our Common Stock. Although the Company and its sponsoring broker dealer have filed form 211 with FINRA to obtain the Company’s trading symbol and are waiting for the issuance, there can be no assurance that our shares of Common Stock will be quoted in a public trading market. Until there is an established trading market, holders of our Common Stock may find it difficult to sell their stock or to obtain accurate quotations for the price of the Common Stock. If a market for our Common Stock does develop, our stock price may be volatile.
Even if a market develops for our shares, our shares may be thinly traded with wide share price fluctuations, low share process and minimal liquidity.
If a market for our shares develops, the share price may be volatile with wide fluctuations in response to several factors, including:
| · |
potential investors’ anticipated feeling regarding our results of operations;
|
| · |
increased competition;
|
| · |
our ability or inability to generate future revenues; and
|
| · |
market perception of the future of development of the products and services we offer.
|
In addition, if our shares are quoted on the OTC Markets or another trading platform or exchange for which we qualify, our share price may be affected by factors that are unrelated or disproportionate to our operating performance. Our share price might be affected by general economic, political, and market conditions such as recessions, interest rates or international currency fluctuations. In addition, even if our stock is approved for quotation by a market maker through the OTC Markets or on another trading platform or exchange, stocks traded over this quotation system are usually thinly traded, highly volatile and not followed by analysts. These factors, which are not under our control, may have a material effect on our share price.
Because we can issue additional shares of Common Stock, purchasers of our Common Stock will suffer immediate dilution, and may experience further dilution in the future.
We are authorized to issue up to 75,000,000 shares of Common Stock. As of April 27, 2017, 22,663,621 shares of Common Stock are issued and outstanding. Our Board of Directors has the authority to mandate the issuance of additional shares of Common Stock without the consent of any of our shareholders. Consequently, our shareholders may experience further dilution of their ownership of the Company in the future, which could have an adverse effect on the trading market for our Common Stock.
We have not paid cash dividends in the past and do not expect to pay dividends in the future. Any return on investment may be limited to the value of our Common Stock.
We have never paid cash dividends on our Common Stock and do not anticipate doing so in the foreseeable future. The payment of dividends on our Common Stock will depend on earnings, financial condition and other business and economic factors affecting us at such time as our Board of Director may consider relevant. If we do not pay dividends, our Common Stock may be less valuable because a return on your investment will only occur if our stock price appreciates.
Our Common Stock is deemed a “penny stock,” which would make it more difficult for our investors to sell their shares.
Our Common Stock is subject to the “penny stock” rules adopted under Section 15(g) of the Securities Exchange Act of 1934, as amended (“Exchange Act”). The penny stock rules generally apply to companies whose Common Stock is not listed on the NASDAQ Stock Market or other national securities exchange and trades at less than $4.00 per share, other than companies that have had average revenue of at least $6,000,000 for the last three years or that have tangible net worth of at least $5,000,000 ($2,000,000 if the company has been operating for three or more years). These rules require, among other things, that brokers who trade penny stock to persons other than “established customers” complete certain documentation, make suitability inquiries of investors and provide investors with certain information concerning trading in the security, including a risk disclosure document and quote information under certain circumstances. Many brokers have decided not to trade penny stocks because of the requirements of the penny stock rules and, as a result, the number of broker-dealers willing to act as market makers in such securities is limited. If we remain subject to the penny stock rules for any significant period, it could have an adverse effect on the market, if any, for our securities. If our securities are subject to the penny stock rules, investors will find it more difficult to dispose of our securities.
Our stock price may be volatile; you may not be able to resell your shares at or above your purchase price.
The market prices for securities of companies similar to ours have been highly volatile, with price and volume fluctuations, and may continue to be highly volatile in the future. Although there is presently no public trading market for our Common Stock, if a market for our shares develops, the following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our common stock, some of which are beyond our control:
| · |
announcements of technological innovations or new commercial products by our competitors or us
|
| · |
our issuance of equity or debt securities, or disclosure or announcements relating thereto;
|
| · |
developments concerning proprietary rights, including patents;
|
| · |
regulatory developments in the United States and foreign countries;
|
| · |
litigation;
|
| · |
economic and other external factors or other disaster or crisis; or
|
| · |
period-to-period fluctuations in our financial results.
|
We may not be able to achieve secondary trading of our stock in certain states because our Common Stock is not nationally traded, which could subject our shareholders to significant restrictions and costs.
Our Common Stock is not eligible for trading on The NASDAQ Capital Market or on a national securities exchange. Therefore, our Common Stock is subject to the securities laws of the various states and jurisdictions of the United States in addition to federal securities law. While we may register our Common Stock or qualify for exemptions for our Common Stock in one of more states, if we fail to do so the investors in those states where we have not taken such steps may not be allowed to purchase our stock or those who presently hold our stock may not be able to resell their shares without substantial effort and expense. These restrictions and potential costs could be significant burdens on our shareholders.
This prospectus contains forward-looking statements. Such forward-looking statements include those that express plans, anticipation, intent, contingency, goals, targets or future development and/or otherwise are not statements of historical fact. These forward-looking statements are based on our current expectations and projections about future events and they are subject to risks and uncertainties known and unknown that could cause actual results and developments to differ materially from those expressed or implied in such statements.
In some cases, you can identify forward-looking statements by terminology, such as “expects”, “anticipates”, “intends”, “estimates”, “plans”, “potential”, “possible”, “probable”, “believes”, “seeks”, “may”, “will”, “should”, “could” or the negative of such terms or other similar expressions. Accordingly, these statements involve estimates, assumptions and uncertainties that could cause actual results to differ materially from those expressed in them. Any forward-looking statements are qualified in their entirety by reference to the factors discussed throughout this prospectus.
You should read this prospectus and the documents that we reference herein and therein and have filed as exhibits to the registration statement, of which this prospectus is part, completely and with the understanding that our actual future results may be materially different from what we expect. You should assume that the information appearing in this prospectus is accurate as of the date on the front cover of this prospectus only. Because the risk factors referred to above could cause actual results or outcomes to differ materially from those expressed in any forward-looking statements made by us or on our behalf, you should not place undue reliance on any forward-looking statements. These risks and uncertainties, along with others, are described above under the heading “Risk Factors” beginning on page 5 of this prospectus. Further, any forward-looking statement speaks only as of the date on which it is made, and we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. We qualify all of the information presented in this prospectus, and particularly our forward-looking statements, by these cautionary statements.
This prospectus also includes estimates of market size and industry data that we obtained from industry publications and surveys and internal company sources. The industry publications and surveys used by management to determine market size and industry data contained in this prospectus have been obtained from sources believed to be reliable.
The Company will not receive any proceeds from this offering. The Selling Shareholders will receive all of the net proceeds from the offering of their shares of Common Stock.
There is currently no public or other market for our Common Stock, and we cannot guarantee that any such market will develop in the foreseeable future. It is our intention to seek quotation on OTCQX. The Company and its sponsoring broker dealer have filed form 211 with FINRA to obtain the Company’s trading symbol and are waiting for the issuance; provided, however, there can be no assurances that our Common Stock will be approved for trading on the OTCQX or any other trading exchange.
As of April 27, 2017, there were 154 shareholders of record of our Common Stock.
Since inception we have not declared or paid any cash dividends and do not intend to declare any such dividends in the foreseeable future. We currently intend to retain any future earnings for use in the operation of our business. Our ability to pay dividends is subject to limitations imposed by Nevada law. Under Nevada law, dividends may be paid to the extent that a corporation’s assets exceed its liabilities and it is able to pay its debts as they become due in the usual course of business. Any future determination to pay dividends will be at the discretion of our Board of Directors.
We may, from time to time, issue certain equity awards pursuant to our 2016 Omnibus Incentive Plan (the "2016 Plan''). The 2016 Plan was adopted by our Board of Directors on January 2, 2016 and was subsequently approved by our shareholders on January 2, 2016. As of December 31, 2016, incentive stock options to purchase an aggregate of 1,178,000 shares of Common Stock and non-qualified options to purchase an aggregate of 1,154,000 shares of the Company's Common Stock were issued under the 2016 Plan, all with an exercise price of $1.00 per share. Of such issuances, 138,000 options vested immediately upon grant and the remaining vest in varying amounts ranging from 100,000 annually to monthly increments ranging from 3,333 to 17,000 based on individual stock option agreements. Each option has a three-year term commencing on each date of vesting.
The 2016 Plan will be administered by a committee of two or more non-employee independent directors designated by the Board. The committee shall perform the requisite duties with respect to awards granted. The committee currently determines to whom awards are made, the timing of any such awards, the type of securities, and number of shares covered by each award, as well as the terms, conditions, performance criteria, restrictions and other provisions of awards. The committee has the authority to cancel or suspend awards, accelerate the vesting or extend the exercise period of any awards made pursuant to the 2016 Plan.
Shares Available under the 2016 Plan
The maximum shares available for issuance under the 2016 Plan are 5,000,000, subject to adjustment as set forth in the 2016 Plan. Any shares subject to an award that expires and that are cancelled, forfeited or settled for cash shall become available under the 2016 Plan. The committee can issue awards comprised of restricted stock, stock options, stock appreciation rights, stock units and other awards, as set forth in the 2016 Plan.
The following table sets forth, as of December 31, 2016, (A) the number of securities to be issued upon the exercise of outstanding options, warrants and rights issued under our equity compensation plans, (B) the weighted-average exercise price of such options, warrants and rights, and (C) the number of securities remaining available for future issuance under our equity compensation plans (excluding those securities set forth in Item (A)).
|
Plan Category
|
Number of securities
to be issued upon exercise of outstanding options, warrants and rights (A) |
Weighted average
price of outstanding options, warrants and rights (B) |
Number of securities
remaining available for future issuance under equity compensation plans (excluding (A)) (C) |
|||||||||
|
|
||||||||||||
|
Equity compensation plans approved by
security holders:
|
||||||||||||
|
2016 Plan
|
2,332,000
|
$
|
1.00
|
2,668,000
|
||||||||
|
|
||||||||||||
|
Equity compensation plans not approved by
security holders:
|
||||||||||||
|
Stock options issued outside the plans
|
—
|
—
|
—
|
|||||||||
|
|
||||||||||||
|
Total
|
2,332,000
|
$
|
1.00
|
2,668,000
|
||||||||
The following discussion and analysis of financial condition and results of operations should be read together with our financial statements and accompanying notes appearing elsewhere in this Prospectus. This Management’s Discussion and Analysis contains forward-looking statements that involve risks and uncertainties. Please see “Forward-Looking Statements” set forth in the beginning of this Prospectus, and see “Risk Factors” beginning on page 5 for a discussion of certain risk factors applicable to our business, financial condition, and results of operations. Operating results are not necessarily indicative of results that may occur in future periods.
Overview of Business
Nexeon MedSystems Inc was incorporated on December 7, 2015 in the State of Nevada. We are a development stage enterprise focusing on the development and commercialization of physician-driven medical device innovations for the treatment of cardiovascular disease. The Company’s consolidated operations include operations of the following wholly-owned subsidiaries: Nexeon Medsystems Europe, SARL (“Nexeon Europe”), Nexeon Medsystems Puerto Rico Operating Company Corporation (“NXPROC”) and Pulsus. Nexeon Europe is the holding company for NXPROC and will be the holding company for NMB upon acquisition of NMB. NXPROC is focused on research and development, software development and data analysis for the implantable neurotechnology device, NNS. Pulsus will conduct research and development on the Company’s cardiovascular disease technology (the Micro-Perforated Catheter Balloon).
Our operations to date have been limited to organizing and staffing the Company, limited research and development activities, our Merger with NXDE, which included acquisition of certain intellectual property and organization of a private placement offering.
As of December 31, 2016, we had an accumulated deficit of $1,592,338. Our net loss was $1,591,423 for the year ended December 31, 2016, compared with a net loss of $915 for the period between December 7, 2015 (inception) and December 31, 2015.
We expect that we will continue to incur significant expenses and increasing operating losses in connection with our ongoing activities, particularly as we continue to invest in research and development and initiate clinical trials required to receive regulatory approval for our medical devices in both the United States and European Union. Additionally, if and when we initiate a launch of one or more of our products, we expect to incur substantial commercialization expenses related to the manufacture and distribution, as well as sales and marketing, of these products. In addition, the Company is subject to additional costs associated with operating as a public company. Accordingly, we may need to obtain additional funding to continue operations. Such financing may not be available to us on acceptable terms, or at all. In the event we require additional capital and are unable to secure such funding, we could be forced to delay, reduce, or eliminate our research and development activities, as well as any future commercialization of our products.
Results of Operations - Fiscal year ended December 31, 2016 compared to fiscal year ended December 31, 2015.
Our results of operations reflect the year ended December 31, 2016 and the period from December 7, 2015 (inception) through December 31, 2015.
Revenue
To date, we have not generated any revenues. Our ability to generate revenues will depend heavily on the successful completion of the requisite clinical trials and studies necessary to achieve approval to begin marketing our contemplated medical devices from the relevant regulatory authorities in the United States and European Union.
Research & Development Activities
Research and Development (“R&D”) expenses consist of the costs associated with our research and discovery efforts related to the design and development of our proposed medical devices. Primarily, R&D expenses are expected to include, but may not be limited to:
| · |
Facilities, laboratory supplies, equipment and related expenses;
|
| · |
Employee-related expenses, which among other things includes salaries, benefits, travel, and stock-based compensation;
|
| · |
External R&D activities incurred under arrangements with third-parties such as contract research organizations, manufacturing organizations, consultants, and possibly a scientific advisory board; and
|
| · |
License fees and other costs associated with securing and protecting intellectual property.
|
For the year ending December 31, 2016 and the period ending December 31, 2015, the Company’s R&D expenses were $266,586 and $0, respectively, primarily reflecting fees for consultants, materials and supplies.
It is expected that our R&D activities and related expenses will increase significantly in the future as we increase the scope and rate of such efforts and begin more expensive development activities, including clinical trials and similar studies as required by the relevant regulatory authorities in our targeted jurisdictions (i.e. the United States and European Union).
The successful completion of the requisite clinical trials and studies to bring our contemplated medical devices to the U.S. and E.U. markets is highly uncertain. We cannot reasonably estimate or know the nature, timing, and estimated costs of the related efforts, nor can we make any assurances as to the period, if any, in which material net cash inflows from sales of our medical devices may commence. This uncertainty is due to the numerous risks and variables associated with developing and marketing medical devices, including, but not limited to:
| · |
The scope and degree of progress associated with our research and discovery efforts, as well as related development activities;
|
| · |
The extent of expenses incurred in conjunction with the foregoing activities;
|
| · |
The safety and efficacy of our medical device as compared to traditional treatment modalities;
|
| · |
Our ability to articulate successfully the benefits of our medical device to medical professionals who will be responsible for introducing patients to our product;
|
| · |
The results of anticipated clinical studies and trials as required by the relevant regulatory approval processes;
|
| · |
The terms and timing of potential regulatory approvals, if any; and
|
| · |
The expense of securing licenses to relevant intellectual property and/or the expense of filing, prosecuting, defending, and enforcing patent claims and other intellectual property rights that we either own outright or have licensed.
|
Unfavorable developments with respect to any of the foregoing could mean significant changes in the cost and timing associated with our ability to bring our medical device to market and begin generating revenues.
General & Administrative Expenses
General and administrative expenses generally consist of salaries and similar costs associated with employees, including stock-based compensation expense. This category of expenses may also include facility costs and professional fees related to (i) legal and patent services; (ii) capital formation; (iii) investor and public relations services; and (iv) consulting and accounting services.
For the year ending December 31, 2016 and the period ending December 31, 2015, the Company’s general and administrative expenses were $582,867 and $915, respectively.
It is expected that our general and administrative expenses will increase in the future as we expand our R&D activities in pursuit of regulatory approval for our contemplated medical device. Such a rise in expenses could result from:
| · |
Increased number of employees;
|
| · |
Expanded infrastructure;
|
| · |
Higher legal and compliance costs;
|
| · |
Increased complexity of our financial statements that could precipitate a rise in our bookkeeping and accounting costs;
|
| · |
Higher insurance premiums; or
|
| · |
Increased need for investor and public relation services.
|
Depreciation and Amortization
Depreciation and amortization expenses consist of amortization of acquired intangibles and depreciation for office equipment and furniture and fixtures. Equipment is depreciated using the straight-line method over the estimated useful lives of the assets. During the year ended December 31, 2016, the Company acquired $986 in office equipment. During the year ended December 31, 2016, the Company acquired $6,120,000 in patents pertaining to the cardiovascular disease technology acquired pursuant to the merger with NXDE and $3,190,000 in patent licenses for the underlying patents referred to as the Siemens Intellectual Property. The amortization period for each of the individual patents depends on the legal terms for patents in the countries in which they are granted. In most countries, including the United States, the patent term is generally 20 years from the earliest claimed filing date of a non-provisional patent application in the applicable country. The patents and patent licenses are amortized using the straight-line method over the remaining time until expiration. The majority of these patents and patents underlying the license will expire between 2020 and 2036.
For the year ended December 31, 2016 and the period ending December 31, 2015, the Company's depreciation expenses were $99 and $0, respectively. For the year ended December 31, 2016 and the period ending December 31, 2015, the Company's amortization expenses were $562,714 and $0, respectively.
Liquidity and Capital Resources
Sources of Liquidity
To date, the Company has not generated any revenues and has financed operations through a private placement of our Common Stock and from loans from the Company's largest shareholder, Rosellini Scientific. On December 2, 2016, the Company closed a private placement (the “December Private Placement”) pursuant to which it received $2,860,946 in net cash proceeds from the issuance of 2,840,946 units. The purchase price for each unit was $1.00 per unit and each unit consisted of one share of Common Stock and one warrant to purchase one additional share of Common Stock. The warrants have an exercise price of $2.00 per share and expire 36 months from the date of issuance of the warrants.
In addition, the Company also issued 347,336 shares of Common Stock for certain legal, corporate structuring and research and development consulting services rendered by third-party consultants. These shares were valued at $347,336. During the next 12 months, the Company may elect to issue additional debt or equity capital either by private placement or a registered offering. There can be no assurance that the Company will be successful in completing any new debt and/or equity financing or receive assignments of grants. In the event that the Company is unable to secure needed financing or is unable to secure such financing on terms it finds favorable, the Company may be forced to delay, limit, or terminate product development and/or future product commercialization.
As of December 31, 2016, we had cash on hand of $2,039,907 and a working capital surplus of $2,004,347. Based upon our budgeted burn rate, we currently have operating capital for approximately five months. The Company has historically relied on equity or debt financings to finance its ongoing operations.
During 2016, Rosellini Scientific, an affiliate of the Company, transferred approximately $751,000 of federal research grants applicable to Pulsus’ products to the Company. These awards commence in the first quarter of 2017. Pulsus has applied for Matching Funds from the Kentucky SBIR Matching Funds Program funded by the Cabinet for Economic Development (“CED”), Office of Entrepreneurship. The Kentucky Science and Technology Corporation administers the Kentucky SBIR Matching Funds Program under a contract with the CED. If the Kentucky Matching Funds applications are approved, the state of Kentucky will award up to $150,000 associated with the National Institute of Health (“NIH”)/ Small Business Innovative Research Program (“SBIR”) Phase I Grant and up to $500,000 associated with the NIH/SBIR Phase II Grant. The Company and Pulsus have several additional NIH/SBIR Grant applications pending.
NMB currently has €1,496,000 (approximately US$1,615,000) in remaining funds from previously awarded grants and €1,203,000 (approximately US$1,299,000) in pending grant applications from the La Region Wallone in Belgium. Subjection to the competition of the acquisition of NMN, the NMB grant revenues would be beneficial to the Company.
From December 7, 2015 (inception) until December 31, 2015, the Company did not generate any revenues and did not raise any funds through equity or debt financings.
Material changes in the financial condition of the Company during the year ended December 31, 2016 are as follows:
| · |
The contribution on January 2, 2016 of various securities with a fair market value of $322,360 for 15,000,000 shares of the Company’s Common Stock.
|
| · |
On February 16, 2016, the Company entered into a Merger Agreement with NXDE pursuant to which the Company issued aggregate of 1,659,943 shares of the Company’s Common Stock in exchange for 100% of NXDE’s issued and outstanding shares of preferred stock and the assumption of $1,614,513 of NXDE’s liabilities. As part of the Merger Agreement, $821,482 of assumed stockholder loans and accrued interest was converted into 821,482 shares of the Company’s Common Stock and $202,825 of assumed accrued interest was cancelled. In subsequent transactions, the Company converted $466,082 of assumed stockholder loans and accrued interest into 466,082 shares of the Company’s Common Stock.
|
| · |
On December 15, 2016, pursuant to the terms of the License Agreement, William Rosellini sold, assigned and transferred any and all of his right, title and interest in and to the License owned by him related to the Siemens Intellectual Property to the Company. As consideration for the transfer of the Siemens Intellectual Property and the license related thereto, the Company paid to Mr. Rosellini the sum of $140,000 in cash and will issue to Mr. Rosellini 3,050,000 shares of the Company’s Common Stock valued at $3,050,000. The Company has not yet issued the shares of Common Stock to Mr. Rosellini.
|
Future Financing; Continued Operations
Until such time, if ever, as we can generate substantial revenues, we expect to finance our cash needs through a combination of future debt and equity financing, as well as expected non-dilutive research grant awards. Besides certain grant awards, as described above, the Company does not have any committed external source of funds. To the extent that the Company secures additional capital through the sale of convertible debt or equity securities, the ownership interest of our shareholders may be diluted, and the terms of any such securities we issue may include liquidation or other preferences that adversely affect the rights of common shareholders. In cases where the Company secures certain debt financing, if any such is available, we may become subject to certain covenants limiting or restricting our ability to take certain actions, such as incurring additional debt, making capital expenditures, or declaring dividends. In the event the Company is unable to secure needed financing, or is unable to secure such financing on terms we find favorable, we may be forced to delay, limit, or terminate product development and/or future commercialization of the same.
Off-Balance Sheet Arrangements
During the fiscal year ended December 31, 2016, we did not engage in any off-balance sheet arrangements as set forth in Item 303(a)(4) of the Regulation S-K. We have no off-balance sheet arrangements, including arrangements that would affect our liquidity, capital resources, market risk support and credit risk support, or other benefits.
Critical Accounting Policies
Our management’s discussion and analysis of the Company’s financial condition and results of operations is based on our consolidated financial statements, which were prepared in conformity with U.S. generally accepted accounting principles. The preparation of our consolidated financial statements requires us to establish accounting policies and make estimates and assumptions that affect our reported amounts of assets and liabilities at the date of the consolidated financial statements. These consolidated financial statements include some estimates and assumptions that are based on informed judgments and estimates of management. We evaluate our policies and estimates on an on-going basis and discuss the development, selection and disclosure of critical accounting policies with the Board of Directors. Predicting future events is inherently an imprecise activity and as such requires the use of judgment. Our consolidated financial statements may differ based upon different estimates and assumptions.
The Company’s significant accounting policies are described in more detail in the notes to our consolidated financial statements, which are included in the Company’s Annual Report on Form 10-K as of and for the year ended December 31, 2016. which we believe sets forth the most critical accounting policies to aid you in fully understanding and evaluating our financial condition and results of operations.
Management does not believe that any recently issued, but not yet effective accounting pronouncements, if adopted, would have a material effect on the accompanying consolidated financial statements.
Business Overview
Nexeon is a bioelectronics company developing active medical devices for the treatment of chronic medical conditions. The solutions we are developing are a unique blend of traditional device technologies such as electronics, software, mechanical engineering, and material science, as well as pharmaceuticals, protein chemistry, and cell biology.
The Company has developed a novel balloon material that utilizes carbon nanotubes and polymer. This technology, in addition to patents related to interventional cardiology products being developed by the Company, are protected with nearly 36 patent matters. Non-dilutive federal and state funding will enable the Company to develop the solution and proceed with an FDA 510(k) approval. If successful, our approach will reduce the need for follow up procedures by efficiently delivering anti-restenotic drugs, intravascularly, to direct the therapy immediately into the lesion. We expect to develop a suite of passive and electrically-active drug eluting balloons that will be owned by our wholly owned subsidiary, Pulsus.
In addition, the Company is developing and commercializing NNS, an implantable neurostimulation and recording platform. The platform is one of the very few implantable stimulation and recording devices that has received regulatory approval (CE conformity) for human use.
Key Transactions:
| · |
On February 16, 2016, NXDE merged with and into the Company, with the Company surviving the Merger as the surviving corporation. As a result of the Merger, the Company converted 100% of NXDE’s issued and outstanding common and preferred stock into an aggregate of 1,659,943 shares of the Company’s Common Stock and all shares of NXDE’s common stock, options and deferred compensation units were cancelled. In addition pursuant to the terms of the Merger, the Company will pay Nexeon Shareholder Royalty Group LLC, a West Virginia limited liability company formed in connection with the Merger, a three percent (3%) royalty of net product sales received by the Company, its affiliates and licensees derived in connection with the commercialization of patents or other intellectual property acquired by the Company pursuant to the Merger. The patents acquired pursuant to the Merger and other intellectual property will be spun-out into Pulsus, and will serve as the basis for our balloon catheter product, which will be the first product we shall attempt to develop to commercialization.
|
| · |
The Company acquired a non-exclusive license, subject to a 6% royalty fee, to a portfolio of 86 patents that originated from Siemens AG, a global technology company. With this license, the Company controls nearly 125 patents and patent applications in the bioelectronics space. The intellectual property relates to IOT technology as described by a system of interrelated computing devices, mechanical and digital machines, objects, animals, and/or people that have unique identifiers and a subsequent ability to transfer data over a network without requiring human-to-human or human-to-computer interaction. This technology, with further development, can be utilized in a wide variety of medical device applications, most notably in hospitals, nursing facilities, or patients’ homes.
|
| · |
On January 10, 2017, the Company has entered into an exclusive, irrevocable agreement with Rosellini Scientific pursuant to which the Company will acquire 100% of the issued and outstanding shares of NMB, an entity controlled by William Rosellini, our Chief Executive Officer.
|
Neurological Disease Markets
In conjunction with the acquisition of NMB, Nexeon will be developing implantable neurotechnology to be applied for the relief of chronic diseases and disorders such as using deep brain stimulation (“DBS”). Central to Nexeon’s future products is, NNS, an implantable neurostimulation and recording platform. The platform is one of the very few implantable stimulation and recording devices that has previously received regulatory approval in Europe (CE conformity) for human use.
The complete system is designed, developed, and validated according to International Organization for Standardization (“ISO”) 14708-3 and ISO 10993 standards. The programmer software and all embedded software in the Implantable Pulse Generator, Remote Control and Battery Charger are compliant with the International Electrontechnical Commission (“IEC”) 62304 standard.
Implantable Pulse Generator: The central component of the NNS is a thin, implantable pulse generator (“IPG”) with soft contours that is designed for patient comfort and aesthetics. The IPG provides two connectors for electrode leads with up to eight contacts each. The leads are stretchable, though unbreakable, allowing for normal patient movement. The heart of the IPG is a custom chip designed to deliver current in multiple contacts independently and simultaneously, making it suitable for selective electrical stimulation. The chip also incorporates a recording system to measure local field and evoked potentials, or to monitor stimulation and the system’s integrity.
Remote Control and Battery Charger: The patient’s Remote Control (“RC”) allows the patient to start and stop the stimulation and to adjust the stimulation level within the limits given by the physician. A color screen provides feedback on the stimulation in the IPG (on or off, level) and on the IPG battery level using icons without text, making the system language independent. It warns the patient when the battery must be charged. To charge the IPG battery, the Battery Charger (“BC”) is placed over the implanted IPG using a shoulder harness, and the RC is connected to the BC. The RC continuously monitors charging voltage, current and temperature in the IPG and optimizes the inductive power generated by the BC for efficient charging. At the same time, the RC battery is charged too. The RC and IPG communicate with each other over a wireless radio microphone link (“MICS”). MICS wireless technology is suitable for remote monitoring of patients to collect user and usage information. A 3G/4G-enabled device can interrogate the IPG over the MICS link and stream the anonymous data to a central database.
Physician Programmer: A physician programmer allows the clinician to set IPG parameters for each patient and to check the integrity of the IPG and electrode leads. The programmer has a database that maintains individual patient records with all programmed parameters and recorded data.
Industry & Target Market
Deep Brain Stimulation Market Overview
DBS is the application of electrical pulses to specific target structures inside the brain. DBS has been used to treat more than 125,000 patients worldwide (http://www.medtronic.co.uk/your-health/parkinsons-disease/about-dbs/dbs-faq/index.htm), predominantly for neurological diseases with motor symptoms such as Parkinson’s disease.
Programming of the correct electrode-contact and optimizing the pulse setting is an inefficient approach. The neurologist has no feedback other than the clinical output for interpreting a patient’s state. We believe this trial-and-error method often results in sub-optimal parameters and is time consuming. The time commitment for the nurse, clinician and patient can exceed 40 hours in the first year of treatment.
Funding & Development
NMB currently has €1,496,000 (approximately U.S. $1,615,000) in remaining funds from previously awarded grants related to its implantable neurotechnology, NNS, from the La Region Wallone in Belgium. The NMB grant revenues would be beneficial to the Company, subject to completing the acquisition of NMB.
Cardiovascular Disease Technology Business Summary
The Company is also developing an intravascular drug delivery system comprised of an inflatable Micro-Perforated Catheter Balloon. This balloon will be comprised of carbon nanotubes embedded within the host nylon polymer for increased mechanical strength. The technology has demonstrated only a small amount of carbon nanotubes (<5% by weight) is needed within the polymer to provide the necessary mechanical reinforcement to withstand high pressure (>20 Atmospheres, “ATM”). While high-pressure (>20 ATM) balloons are currently available, they are made from stiffer polymers such as polyethylene terephthalate (“PET”) and are not perforated. Therefore, existing high-pressure balloon technology without perforations are not available. The carbon nanotubes help maintain structural integrity of the Micro-Perforated Catheter Balloon during inflation and drug delivery while allowing a more compliant polymer, such as nylon, to be used. Anti-restenotic drugs, such as paclitaxel, can then be delivered using pulsed flow. The drug is introduced through the proximal shaft of the catheter balloon. The drug and carrier medium then fills up the catheter balloon and is released through the pores in the catheter balloon. The drug then exits the catheter balloon and is forced into the surrounding neointima for maximal therapeutic effect.
When compared to drug-coated catheter balloons and drug-eluting stents, management believes the Company’s Micro-Perforated Catheter Balloon is expected to provide the following benefits:
| · |
No distal outflow occlusion due to no excipient needed;
|
| · |
Minimal downstream loss of drug;
|
| · |
Predictable depth and load of drug delivery;
|
| · |
Adjustable drug dosage based on clinical variables; and
|
| · |
Ability to use wider range of drugs, including non-lipophilic drugs.
|
Innovation
Current drug-coated balloons provide only passive drug delivery. In management’s opinion, the Company’s Micro-Perforated Catheter Balloon brings a much-needed, innovative improvement to intravascular drug delivery by enabling controllable pulsed flow delivery of anti-restenotic drugs to the diseased site. The specific innovations that are being developed include, but are not limited to:
| · |
Carbon nanotube-nylon composite materials and associated processing procedures to manufacture mechanically robust, micro-perforated catheter balloons; and
|
| · |
An intravascular method that can deliver lipophilic and hydrophilic drugs.
|
Advantages of our Approach
Our drug delivery approach using the Micro-Perforated Catheter Balloon reduces the need for follow up procedures by efficiently delivering anti-restenotic drugs, intravascularly, to direct the therapy immediately into the lesion. Currently, repeat angioplasty procedures are required to continually counter restenosis. The need for compliant materials to perform intravascular drug delivery within the arteriovenous (“AV”) graft site (AV graft is when a synthetic tube connects an artery to avein) eliminates the use of stents for this application. While drug-coated catheter balloons could potentially be used to treat blockages in proximity to the AV graft, the high flow rate associated with such grafts has the potential to wash out the drug.
Commercial Applications
The targeted clinical application for the Micro-Perforated Catheter Balloon is as an intervention for hemodialysis graft failures, particularly in delivering paclitaxel or similar anti-restenotic drugs. Pulsed-flow intravascular drug delivery can also potentially be applied to bodily sites where drug-eluting stents and drug-coated balloons are being used, including the superficial femoral artery in the thigh and the popliteal artery in the knee (Pastromas et al. 2014). Management believes the Micro-Perforated Catheter Balloon has the ability to deliver almost any type of drug, and that the Company’s approach not only has the potential to treat many blockages within the peripheral vasculature but may also transcend to other diseases such as cancer. It is management’s belief drugs that are difficult or impossible to take orally or drugs that lose efficacy when taken by standard intravenous delivery could potentially be used much more effectively with the Company’s intravascular delivery system. Management believes, this approach may not only extend or broaden the useful life of existing drugs, but it may also revive interest in abandoned drugs.
Production Plan
Production of the Micro-Perforated Catheter Balloon will require contract manufacturing organizations (“CMOs”) that implement Good Manufacturing Practices (“GMP”) pursuant to FDA requirements. The balloon manufacturing partner, Interface Catheter Solutions, is well-versed in GMP procedures and it is management’s belief the manufacturing and testing procedures used in this effort will be readily scalable and GMP-compliant. Currently, Interface Catheter Solutions is a component vendor and no contract exists. We plan to use FDA-registered, GMP-compliant testing facilities at Nelson Laboratories to validate the biological safety of our materials. It is management’s belief these processes can be readily transferable to a strategic partner who could eventually market our Micro-Perforated Catheter Balloon.
Funding & Development
During 2016, Rosellini Scientific, pursuant to the contribution agreement entered into with the Company, transferred approximately $751,000 of federal research grants applicable to Pulsus’ Micro-Perforated Catheter Balloon to the Company. Pulsus has applied to receive matching funds from the Kentucky Small Business Innovation Research (“SBIR”) Matching Funds Program funded by the Cabinet for Economic Development (“CED”), Office of Entrepreneurship. The Kentucky Science and Technology Corporation (KSTC) administers the Kentucky SBIR Matching Funds Program under a contract with the CED. If the Kentucky Matching Funds’ applications are approved, the state of Kentucky will award up to $150,000 associated with the NIH/SBIR Phase I Grant and up to $500,000 associated with the National Institute of Health (“NIH”)/SBIR Phase II grant. The Company and Pulsus have several additional NIH/SBIR grant applications pending. In addition, NMB currently has a pending grant application in the amount of €1,203,000 (approximately U.S. $1,299,000) from the La Region Wallone in Belgium. The NMB grant revenues would be beneficial to the Company, subject to completing the acquisition of NMB.
Investments in research and development related to intravascular drug delivery system totaled $266,586 for the twelve months ended December 31, 2016.
As of April 27, 2017, we own 36 U.S. and foreign patents and patent applications reflecting the intellectual property underlying the intravascular drug delivery system technology platform. The term of our individual patents depends on the legal terms for patents in the countries in which they are granted. In most countries, including the United States, the patent term is generally 20 years from the earliest claimed filing date of a non-provisional patent application in the applicable country. The majority of our patents will expire between 2020 and 2029.
Regulatory Plan
The Company intends to file for a CE Mark in the European Union at the end of 2017 for NNS for the relief of Parkinson’s disease using DBS. Nexeon intends to perfect its Technical File (including implementing its quality system, manufacturing and packaging procedures, labeling, sterilization, packaging, and performing any remaining testing, verification, and/or validation activities). It is also Nexeon’s expectation that, while the EU designated Notified Body (“NB”) for a DBS medical device may want Nexeon to conduct the clinical study that it reviewed at the outset of this Clinical Evaluation Review (“CER”) process, the NB still will require only that this study be ongoing and progressing by the time that review of the Technical File is completed. In fact, while there have been some recent increases in the stringency of the relevant EU regulatory requirements, human clinical trial data is not always necessary when pursuing product approval via a CE Mark. Ultimately, then, this CER process is expected to take somewhere between three to six months, with issuance of a CE Mark early in the first quarter of 2018.
The Company has had a number of discussions with FDA and intends to begin a pivotal study for DBS in the U.S. in 2018. Bench experiments and preclinical animal testing requirements are already in progress and management expects that such experiments will be concluded in 2017 with the completion of an active safety in porcine. Some of the biocompatibility testing may be replaced by published literature of the material being used. Preclinical animal studies to demonstrate efficacy and functioning of the device may be, but are not always, a regulatory requirement.
The tests could include preclinical animal testing for cytotoxicity, sensitization, genotoxicity, and implantation biocompatibility. Additional testing that may be applicable includes irritation or intracutaneous reactivity, acute system toxicity, and sub-chronic toxicity (sub-acute toxicity). Supplementary evaluation testing that could be required includes testing for chronic toxicity and carcinogenicity. In lieu of some of these testing requirements, the regulatory submissions may be able to include published toxicity data on the lead material and/or provide justification or risk assessment for not conducting the appropriate tests.
The Company also intends to complete preclinical testing for the drug eluting balloon program in 2017 and expects to submit a clinical evaluation review request to the notified bodies in order to gain more information concerning the regulatory pathway of the program. This will be done in conjunction with a pre-IDE meeting with the FDA. The SBIR funding supports all preclinical work and ISO 13485 testing needed to complete preclinical safety analysis.
Sales Approach
Our strategy for sales and distribution will vary depending on product line and region. We expect to utilize a targeted sales approach for the DBS product, NNS, while achieving U.S. PMA from the FDA. We expect to hire a small, experienced sales force, all with prior experience of training 50 or more clinicians on active implantable devices. Each member of the sales force will be expected to have previous sales of at least $1M annually and a wealth of contacts in their regional area.
Territories in the U.S. and EU not targeted by our sales force will be covered using a distributor to reduce upfront costs and training. This approach will likely be altered if we form a strategic partnership with an existing medical device company prior to initial sales.
Intellectual Property
Intellectual Property Protection
Nexeon possesses a strong patent portfolio for each of its products under development ensuring the ability to operate, which is critical to successful commercialization of the Company’s platforms. In addition, maintaining a focus in the internet of medical things allows Nexeon to utilize issued and pending patents for maximum commercial benefit and growth. Since inception, Nexeon has actively pursued patent coverage of the proprietary systems and methods of delivering therapy. The patents have been deliberately written to provide broad and specific protection.
Issued and pending U.S. and foreign method and technology patents protect the Company’s intellectual property (“IP”) (Table 1).
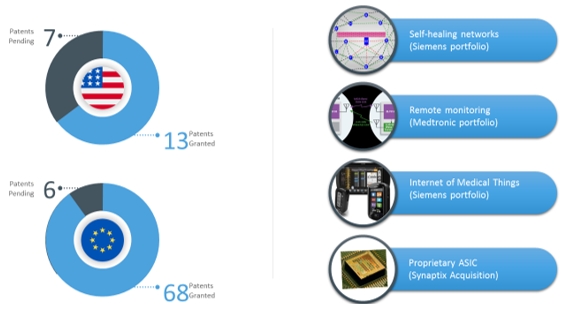
Table 1: IP Portfolio with Patents Issued
Nexeon’s strategy to create temporal barriers to others and to protect proprietary positions is to continue to acquire and file for U.S. and foreign patent applications related to its technology, inventions, and improvements to enhance the Company’s business and competitive advantages. Continuing to rigorously analyze competitive IP applications and their prosecution history will ensure that this freedom to operate position remains viable. As development of new products and prosecution of pending patent applications progresses, we will continue to strategically file additional applications, including continuations, continuations-in-part, and divisional applications, to protect the new developments and those already being prosecuted.
In addition to seeking patent protection, the Company utilizes other available intellectual property rights to protect its developments. For example, Nexeon utilizes copyrighted software, manuals, and reports. Nexeon also maintains a number of trade secrets that are essential to its business. Nexeon has implemented procedures to maintain such secrecy required of such trade secrets. Finally, Nexeon has filed for and obtained several trademark registrations related to the branding of its products. This multifaceted approach provides Nexeon with the maximum protection available for its developments.
Patents Acquired by the Company pursuant to the Merger
Pursuant to the terms of the Merger, the Company acquired a 100% ownership interest in certain domestic and international patents as described below, some of which relate to a micro-perforated balloon catheter system for use in the treatment of restenosis associated with hemodialysis. The Company intends to initially focus on the development and commercialization of the micro-perforated balloon catheter system and intends to transfer the intellectual property acquired pursuant to the Merger to Pulsus. It is expected that Pulsus will then enter into sub-license agreements as well as development and supply agreements in order to enhance the technology and expedite the development process, although we cannot guarantee that sublicensining will occur. In addition to developing the medical device, the Company may acquire patents or companies which are complimentary to the intellectual property acquired pursuant to the Merger with an intent to develop and commercialize various medical technologies.
|
NANOTUBE-REINFORCED BALLOONS FOR DELIVERING PATENTS
|
|
|
US 8187221
|
Therapeutic agents within or beyond the wall of blood vessels, and methods of making and using same
|
|
JP 5481479
|
Therapeutic agents within or beyond the wall of blood vessels, and methods of making and using same
|
|
EP 9771434.9
|
Therapeutic agents within or beyond the wall of blood vessels, and methods of making and using same
|
|
CN 200980134724.2
|
Therapeutic agents within or beyond the wall of blood vessels, and methods of making and using same
|
|
IRIS FILTER-SHEATH CATHETER SYSTEM FOR EMBOLISM PROTECTION PATENTS
|
|
|
US 8728113
|
Interventional catheter for retrograde use having embolic protection capability and methods of use
|
|
US 8257384
|
Interventional catheter for retrograde use having embolic protection capability and methods of use
|
|
US 7837702
|
Interventional catheter for retrograde use having embolic protection capability and methods of use
|
|
EP 6847981.5
|
Interventional catheter for retrograde use having embolic protection capability and methods of use
|
|
APPARATUS AND METHODS FOR RENAL STENTING PATENTS
|
|
|
US 8702744
|
Apparatus and methods for renal stenting
|
|
EP 6770151.6
|
Apparatus and methods for renal stenting
|
|
AU 2006244070
|
Apparatus and methods for renal stenting
|
|
JP 4990887
|
Apparatus and methods for renal stenting
|
|
METHODS AND APPARATUS FOR TREATING AN INJURED NERVE PATHWAY PATENTS
|
|
|
US 7842304
|
Methods and apparatus for treating an injured nerve pathway
|
|
US 8603512
|
Methods and apparatus for treating an injured nerve pathway
|
|
SYSTEMS AND METHODS FOR ATRAUMATIC IMPLANTATION OF BIO-ACTIVE AGENTS PATENTS
|
|
|
US 7632262
|
Systems and methods for atraumatic implantation of bio-active agents
|
|
US 8377032
|
Systems and methods for atraumatic implantation of bio-active agents
|
|
EP 9736537.3
|
Systems and methods for atraumatic implantation of bio-active agents
|
|
EP 5773060.8
|
Systems and methods for atraumatic implantation of bio-active agents
|
|
EP 11150671.3
|
Systems and methods for atraumatic implantation of bio-active agents
|
|
AU 2005274775
|
Systems and methods for atraumatic implantation of bio-active agents
|
|
CN 2573844
|
Systems and methods for atraumatic implantation of bio-active agents
|
|
JP 4774403
|
Systems and methods for atraumatic implantation of bio-active agents
|
|
APPARATUS AND METHODS FOR TREATING TISSUE USING PASSIVE INJECTION SYSTEMS PATENTS
|
|
|
US 7338471
|
Apparatus and methods for treating tissue using passive injection systems
|
|
US 7862551
|
Apparatus and methods for treating tissue using passive injection systems
|
|
AU 2005274774
|
Apparatus and methods for treating tissue using passive injection systems
|
|
JP 4934029
|
Apparatus and methods for treating tissue using passive injection systems
|
|
GB & FR 1773440
|
Apparatus and methods for treating tissue using passive injection systems
|
|
DE 602005030269.7
|
Apparatus and methods for treating tissue using passive injection systems
|
|
CA 2574089
|
Apparatus and methods for treating tissue using passive injection systems
|
|
IMPLANTABLE DEVICE FOR TREATING DISEASE STATES AND METHODS OF USING SAME PATENTS
|
|
|
US 7651696
|
Implantable device for treating disease states and methods of using same
|
|
METHODS AND APPARATUS FOR TREATING INFARCTED REGIONS OF TISSUE FOLLOWING PATENTS
|
|
|
US 7819856
|
Acute myocardial infarction
|
|
US 8062283
|
Acute myocardial infarction
|
|
APPARATUS FOR THE DELIVERY OF DRUGS OR GENE THERAPY PATENTS
|
|
|
US 7648495
|
Into a patient's vasculature and methods of use
|
|
INVENTORY SPARING CATHETER SYSTEM
|
|
|
CA 2739841
|
Inventory sparing catheter system
|
|
CN 200980149617.7
|
Inventory sparing catheter system
|
Patent License Agreement (Siemens Patents)
On September 29, 2016, William Rosellini, our Chief Executive Officer and director and a majority shareholder of the Company, entered into a patent license agreement (the “License Agreement”) with Magnus IP GmbH, German corporation (“Magnus”). Pursuant to the terms of the License Agreement, Magnus granted to Mr. Rosellini, and his affiliates, a non-exclusive, non-transferable, non-assignable without the right to sublicense worldwide license to a portfolio of 86 patents, referred to herein as the “Siemens Patents”.
The intellectual property relates to IOT technology as described by a system of interrelated computing devices, mechanical and digital machines, objects, animals, and/or people that have unique identifiers and a subsequent ability to transfer data over a network without requiring human-to-human or human-to-computer interaction. This technology can be utilized in a wide variety of medical device applications, most notably in hospitals, nursing facilities, or patients’ homes.
Patent License Asset Purchase Agreement
On December 15, 2016, Mr. Rosellini sold, assigned and transferred any and all of his right, title and interest in and to the license owned by him related to the Siemens Patents to the Company pursuant to a Patent License Asset Purchase Agreement (the “Purchase Agreement”). As consideration for the transfer of the Siemens Patents and the license related thereto, the Company paid to Mr. Rosellini the sum of $140,000 in cash and will issue to Mr. Rosellini 3,050,000 shares of the Company’s restricted Common Stock valued at $3,050,000. The Company has not yet issued Mr. Rosellini the shares of restricted Common Stock.
Siemens Patents
The following is a list of the Siemens patents the Company acquired the right to use pursuant to the Purchase Agreement.
|
MAGNUS IP GmbH
|
|||||
|
Asset List
|
|||||
|
Country
|
Patent #
|
Issued Date
|
Apln. #
|
Apln. Date
|
Pub. #
|
|
AUSTRIA
|
AT521160
|
08/17/11
|
5109169.2
|
10/04/05
|
EP1646185
|
|
AUSTRIA
|
AT530961
|
10/26/11
|
3735054.3
|
01/28/03
|
EP1470457
|
|
AUSTRIA
|
AT615998
|
06/05/13
|
5108954.8
|
09/28/05
|
EP1643324
|
|
AUSTRIA
|
AT334459
|
07/26/06
|
4100499.5
|
02/10/04
|
EP1494191
|
|
BELGIUM
|
BE1494191
|
07/26/06
|
4100499.5
|
02/10/04
|
EP1494191
|
|
BRASIL
|
PI0710612
|
|
BR0710612
|
03/30/07
|
|
|
BRASIL
|
|
|
PI0821881-1
|
12/16/08
|
|
|
CANADA
|
2647652
|
11/20/12
|
2647652.0
|
03/30/07
|
2647652
|
|
CANADA
|
2662014
|
07/08/14
|
2662014.0
|
08/29/07
|
2662014
|
|
CANADA
|
2711225
|
01/21/14
|
2711225.0
|
12/16/08
|
2711225
|
|
CANADA
|
2836941
|
05/17/16
|
2836941.0
|
08/29/07
|
2836941
|
|
CHINA
|
|
|
200780032280.2
|
08/29/07
|
CN101512976
|
|
CHINA
|
CN101422019
|
09/10/14
|
200780012887.4
|
03/30/07
|
101422019
|
|
CHINA
|
CN101971568
|
10/30/13
|
200880123790.5
|
12/16/08
|
101971568
|
|
CHINA
|
|
|
201410392441.0
|
03/30/07
|
CN104243248
|
|
EPO
|
EP1470456
|
12/30/09
|
3707570.2
|
01/28/03
|
EP1470456
|
|
EPO
|
EP1470457
|
10/26/11
|
3735054.3
|
01/28/03
|
EP1470457
|
|
EPO
|
EP1494191
|
07/26/06
|
4100499.5
|
02/10/04
|
EP1494191
|
|
EPO
|
EP1626532
|
03/19/14
|
5012617.6
|
06/13/05
|
EP1626532
|
|
EPO
|
EP1643324
|
06/05/13
|
5108954.8
|
09/28/05
|
EP1643324
|
|
EPO
|
EP1646185
|
08/17/11
|
5109169.2
|
10/04/05
|
EP1646185
|
|
EPO
|
EP2225854
|
02/12/14
|
8869509.3
|
12/16/08
|
EP2225854
|
|
EPO
|
|
|
7101252.0
|
10/04/05
|
EP1783959
|
|
EPO
|
|
|
7774303.7
|
03/30/07
|
EP2005713
|
|
EPO
|
|
|
13172203.5
|
06/13/05
|
EP2642696A3
|
|
EPO
|
|
|
13172204.3
|
06/13/05
|
EP2642697A3
|
|
FINLAND
|
FI1470456
|
12/30/09
|
3707570.2
|
01/28/03
|
EP1470456
|
|
MAGNUS IP GmbH
|
|||||
|
Asset List
|
|||||
|
Country
|
Patent #
|
Issued Date
|
Apln. #
|
Apln. Date
|
Pub. #
|
|
FRANCE
|
FR1470456
|
12/30/09
|
3707570.2
|
01/28/03
|
EP1470456
|
|
FRANCE
|
FR1470457
|
10/26/11
|
3735054.3
|
01/28/03
|
EP1470457
|
|
FRANCE
|
FR1626532
|
03/19/14
|
5012617.6
|
06/13/05
|
EP1626532
|
|
FRANCE
|
FR1643324
|
06/05/13
|
5108954.8
|
09/28/05
|
EP1643324
|
|
FRANCE
|
FR1646185
|
08/17/11
|
5109169.2
|
10/04/05
|
EP1646185
|
|
FRANCE
|
FR2225854
|
02/12/14
|
8869509.3
|
12/16/08
|
EP2225854
|
|
FRANCE
|
FR1494191
|
07/26/06
|
4100499.5
|
02/10/04
|
EP1494191
|
|
GERMANY
|
|
|
10317962.3
|
04/17/03
|
|
|
GERMANY
|
DE602005029532.1
|
08/17/11
|
5109169.2
|
10/04/05
|
EP1646185
|
|
GERMANY
|
DE602005039869.4
|
06/05/13
|
5108954.8
|
09/28/05
|
EP1643324
|
|
GERMANY
|
DE602005042990.5
|
03/19/14
|
5012617.6
|
06/13/05
|
EP1626532
|
|
GERMANY
|
DE602008030307.1
|
02/12/14
|
8869509.3
|
12/16/08
|
EP2225854
|
|
GERMANY
|
DE60330750.7
|
12/30/09
|
3707570.2
|
01/28/03
|
EP1470456
|
|
GERMANY
|
DE60338908.2
|
10/26/11
|
3735054.3
|
01/28/03
|
EP1470457
|
|
GERMANY
|
DE502004001017.2
|
07/26/06
|
4100499.5
|
02/10/04
|
EP1494191
|
|
GERMANY
|
|
|
112007001804.0
|
08/29/07
|
|
|
GREECE
|
GR1494191
|
07/26/06
|
4100499.5
|
02/10/04
|
EP1494191
|
|
IRELAND
|
IE1494191
|
07/26/06
|
4100499.5
|
02/10/04
|
EP1494191
|
|
IRELAND
|
1470456
|
12/30/09
|
3707570.2
|
01/28/03
|
1470456
|
|
ITALY
|
IT1470457
|
10/26/11
|
3735054.3
|
01/28/03
|
EP1470457
|
|
ITALY
|
IT1626532
|
03/19/14
|
5012617.6
|
06/13/05
|
EP1626532
|
|
ITALY
|
IT1643324
|
06/05/13
|
5108954.8
|
09/28/05
|
EP1643324
|
|
ITALY
|
IT1646185
|
08/17/11
|
5109169.2
|
10/04/05
|
EP1646185
|
|
ITALY
|
IT2225854
|
02/12/14
|
8869509.3
|
12/16/08
|
EP2225854
|
|
ITALY
|
IT1494191
|
07/26/06
|
4100499.5
|
02/10/04
|
EP1494191
|
|
MEXICO
|
MX313282
|
09/12/13
|
MX/a/2010/007211
|
12/16/08
|
|
|
MEXICO
|
MX322526
|
08/01/14
|
MX/a/2013/010239
|
12/16/08
|
|
|
NETHERLANDS
|
NL1470456
|
12/30/09
|
3707570.2
|
01/28/03
|
EP1470456
|
|
NETHERLANDS
|
NL1470457
|
10/26/11
|
3735054.3
|
01/28/03
|
EP1470457
|
|
NETHERLANDS
|
NL1626532
|
03/19/14
|
5012617.6
|
06/13/05
|
EP1626532
|
|
NETHERLANDS
|
NL1643324
|
06/05/13
|
5108954.8
|
09/28/05
|
EP1643324
|
|
NETHERLANDS
|
NL1646185
|
08/17/11
|
5109169.2
|
10/04/05
|
EP1646185
|
|
NETHERLANDS
|
NL1494191
|
07/26/06
|
4100499.5
|
02/10/04
|
EP1494191
|
|
SOUTH KOREA
|
100989017
|
10/20/10
|
1020087024880.0
|
03/30/07
|
1.02008E+12
|
|
SOUTH KOREA
|
101272384
|
06/07/13
|
1020107014107.0
|
06/25/10
|
1.0201E+12
|
|
SOUTH KOREA
|
KR101202914B1
|
11/20/12
|
1020107014718.0
|
12/16/08
|
1.0201E+12
|
|
SPAIN
|
ES2270271
|
07/26/06
|
4100499.5
|
02/10/04
|
EP1494191
|
|
SWEDEN
|
SE1470456
|
12/30/09
|
3707570.2
|
01/28/03
|
EP1470456
|
|
SWEDEN
|
SE1470457
|
10/26/11
|
3735054.3
|
01/28/03
|
EP1470457
|
|
SWEDEN
|
SE1626532
|
03/19/14
|
5012617.6
|
06/13/05
|
EP1626532
|
|
SWEDEN
|
SE1643324
|
06/05/13
|
5108954.8
|
09/28/05
|
EP1643324
|
|
SWEDEN
|
SE1646185
|
08/17/11
|
5109169.2
|
10/04/05
|
EP1646185
|
|
SWEDEN
|
SE2225854
|
02/12/14
|
8869509.3
|
12/16/08
|
EP2225854
|
|
SWEDEN
|
SE1494191
|
07/26/06
|
4100499.5
|
02/10/04
|
EP1494191
|
|
SWITZERLAND
|
CH1470456
|
12/30/09
|
3707570.2
|
01/28/03
|
EP1470456
|
|
SWITZERLAND
|
CH1470457
|
10/26/11
|
3735054.3
|
01/28/03
|
EP1470457
|
|
SWITZERLAND
|
CH1626532
|
03/19/14
|
5012617.6
|
06/13/05
|
EP1626532
|
|
SWITZERLAND
|
CH1643324
|
06/05/13
|
5108954.8
|
09/28/05
|
EP1643324
|
|
SWITZERLAND
|
CH1646185
|
08/17/11
|
5109169.2
|
10/04/05
|
EP1646185
|
|
SWITZERLAND
|
CH2225854
|
02/12/14
|
8869509.3
|
12/16/08
|
EP2225854
|
|
SWITZERLAND
|
CH1494191
|
07/26/06
|
4100499.5
|
02/10/04
|
EP1494191
|
|
UNITED KINGDOM
|
GB1470456
|
12/30/09
|
3707570.2
|
01/28/03
|
EP1470456
|
|
MAGNUS IP GmbH
|
|||||
|
Asset List
|
|||||
|
Country
|
Patent #
|
Issued Date
|
Apln. #
|
Apln. Date
|
Pub. #
|
|
UNITED KINGDOM
|
GB1470457
|
10/26/11
|
3735054.3
|
01/28/03
|
EP1470457
|
|
UNITED KINGDOM
|
GB1626532
|
03/19/14
|
5012617.6
|
06/13/05
|
EP1626532
|
|
UNITED KINGDOM
|
GB1643324
|
06/05/13
|
5108954.8
|
09/28/05
|
EP1643324
|
|
UNITED KINGDOM
|
GB1646185
|
08/17/11
|
5109169.2
|
10/04/05
|
EP1646185
|
|
UNITED KINGDOM
|
GB2225854
|
02/12/14
|
8869509.3
|
12/16/08
|
EP2225854
|
|
UNITED KINGDOM
|
GB1494191
|
07/26/06
|
4100499.5
|
02/10/04
|
EP1494191
|
|
US
|
|
|
60/352,452
|
|
|
|
US
|
8131399
|
|
10/353,142
|
|
|
|
US
|
8538589
|
|
13/366,095
|
|
|
|
US
|
|
|
10/353,110
|
|
|
|
US
|
|
|
10/672,527
|
|
|
|
US
|
7860495
|
|
10/915,034
|
|
|
|
US
|
|
|
12/629,548
|
|
|
|
US
|
8200273
|
|
12/953,244
|
|
|
|
US
|
7139239
|
|
10/958,770
|
|
|
|
US
|
7437596
|
|
11/538,654
|
|
|
|
US
|
7664573
|
|
10/952,705
|
|
|
|
US
|
7746887
|
|
11/402,743
|
|
|
|
US
|
|
|
60/823,788
|
|
|
|
US
|
9030315
|
|
11/846,218
|
|
|
|
US
|
|
|
60/823,909
|
|
|
|
US
|
|
|
60/823,912
|
|
|
|
US
|
8264371
|
|
11/969,111
|
|
|
|
US
|
|
|
12/124,452
|
|
|
|
US
|
|
|
61/037,739
|
|
|
|
US
|
8224282
|
|
12/406,799
|
|
|
|
US
|
8350691
|
|
12/269,136
|
|
|
|
US
|
7363036
|
|
10/824,800
|
|
|
|
WO/PCT
|
|
|
PCT/US2003/002556
|
01/28/03
|
WO/2003/064933
|
|
WO/PCT
|
|
|
PCT/US2003/002559
|
01/28/03
|
WO/2003/065136
|
|
WO/PCT
|
|
|
PCT/US2007/007984
|
03/30/07
|
WO/2007/123738
|
|
WO/PCT
|
|
|
PCT/US2007/077107
|
08/29/07
|
WO/2008/027964
|
|
WO/PCT
|
|
|
PCT/US2008/013746
|
12/16/08
|
WO/2009/088429
|
Company Obligations Pursuant to the License Agreement
Pursuant to the terms of the Purchase Agreement and the License Agreement, the Company assumed the following obligations:
Royalties
The Company shall pay Magnus royalties equal to 6% of the gross amount invoiced by the Company for sales of the licensed products, less, without duplication, the sum of the following: (a) reasonable discounts, rebates, allowances or price adjustments actually granted; (b) sales, value added, use, consumption, excise and/or similar taxes actually incurred; and (c) amounts refunded, credited or allowed on returns for, or rejections of, licensed products of all licensed products sold, licensed, leased or otherwise disposed by or on behalf of Magnus.
Term of the License Agreement
The term of the License Agreement commenced on September 29, 2016 and shall continue until the expiration of the last-to-expire of the licensed patents.
If Mr. Rosellini materially breaches the License Agreement and does not cure such breach, to the extent capable of being cured, within 21 days after receipt of written notice from Magnus, then the License Agreement may be terminated upon written notice to that effect from Magnus at any time after the expiration of 21 days following receipt by Mr. Rosellini of such notice. If the License Agreement is terminated, the Company will no longer have rights to the Siemens Patents.
Fair Market Value of Patents; Board Approval
The Company engaged a third-party valuation firm to provide a fair market value of the Siemens Patents acquired pursuant to the Purchase Agreement and related License Agreement in accordance with ASC 820-10, Fair Value Measurements. Management and the Company’s Board of Directors have relied on the fair market value provided by the third-party valuation firm in approving the Purchase Agreement transactions. The Board of Directors approved the Purchase Agreement and related transactions on December 15, 2016 in accordance with the Company’s Code of Business Conduct and Ethics. Management believes the primary value of the transaction is reflected in the patent portfolio acquired.
The Augmented Market Approach (“AMA”) was used to determine the fair market value of the Siemens Patents. AMA was developed from actual data of patent market transactions. Essentially, the valuation computed from AMA would indicate the fair market value of patents if they were sold in today's medical patent market. The estimated fair value for the Siemens Patents was $3,190,000.
For the twelve months ended December 31, 2016, the Company had not initiated any research and development activities for the technology underlying the IOT technology license.
The patents underlying the license reflects 106 U.S. and foreign patents and patent applications for the IOT technology. The license term for each patent reflects the expiration dates of the underlying patents. The term of the individual patents depends on the legal terms for patents in the countries in which they are granted. In most countries, including the United States, the patent term is generally 20 years from the earliest claimed filing date of a non-provisional patent application in the applicable country. The majority of these patents will expire between 2022 and 2036.
Other Investments
The Company currently owns minority holdings in the following two entities:
|
1)
|
approximately a 10% equity interest in Nuviant Medical Inc (“Nuviant”), the largest shareholder of which is Rosellini Scientific; and
|
|
2)
|
267 shares of common stock, representing a less than 1% equity interest in MicroTransponder, Inc.
|
The Company has recorded impairment of $173,500 on its investment in Nuviant due to a significant change to Nuviant’s financial condition.
Government Regulations
The medical device industry is regulated extensively by governmental authorities, principally the FDA and corresponding state and foreign regulatory agencies and authorities, such as the EU legislative bodies and the European Economic Area (“EEA”) Member State Competent Authorities. The FDA, EEA and other U.S. and foreign governmental agencies and authorities regulate and oversee, among other things, with respect to medical devices:
| · |
design, development and manufacturing;
|
| · |
testing, labeling, content and language of instructions for use and storage;
|
| · |
clinical trials;
|
| · |
product safety;
|
| · |
marketing, sales and distribution;
|
| · |
pre-market regulatory clearance and approval;
|
| · |
conformity assessment procedures;
|
| · |
record-keeping procedures;
|
| · |
advertising and promotion;
|
| · |
recalls and other field safety corrective actions;
|
| · |
post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury;
|
| · |
post-market studies; and
|
| · |
product import and export.
|
The laws and regulations to which we are subject are complex and have become more stringent over time. Legislative or regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales.
Our failure to comply with U.S. federal and state regulations, EEA or other foreign regulations applicable in the countries where we operate could lead to the issuance of warning letters or untitled letters, the imposition of injunctions, suspensions or loss of regulatory clearance or approvals, product recalls, termination of distribution, product seizures or civil penalties. In the most extreme cases, criminal sanctions or closure of our manufacturing facilities are possible. If any of these risks materialize, our business would be adversely affected.
Foreign Regulatory Approval
Outside of the United States, our ability to market our product candidates will be contingent also upon our receiving marketing authorizations from the appropriate foreign regulatory authorities, whether or not FDA approval has been obtained. The foreign regulatory approval process in most industrialized countries generally encompasses risks similar to those we will encounter in the FDA approval process. The requirements governing conduct of clinical trials and marketing authorizations, and the time required to obtain requisite approvals, may vary widely from country to country and differ from those required for FDA approval.
In the European Union, we are required to affix the CE mark to certain of our products in order to sell the products in member countries of the European Union pursuant to the European Medical Device Directive (Council Directive 93/42/EEC). The CE mark is an international symbol that represents adherence to certain essential principles of safety and effectiveness mandated in the European Medical Device Directive, which are referred to as the “essential requirements”. Once affixed, the CE mark enables a product to be sold within the EEA which is composed of the 28 member states of the EU and Norway, Iceland and Liechtenstein as well as other countries that accept products with the CE mark.
To demonstrate compliance with the “essential requirements”, we must undergo a conformity assessment procedure which varies according to the type of medical device and its classification. Except for low risk medical devices (Class I with no measuring function and which are not sterile) where the manufacturer can issue a Declaration of Conformity based on a self-assessment of the conformity of its products with the “essential requirements” of the Medical Devices Directive, a conformity assessment procedure requires the intervention of an organization accredited by a member state of the EEA to conduct conformity assessments, or a NB. Depending on the relevant conformity assessment procedure, the NB would typically audit and examine the technical file and the quality system for the manufacture, design and final inspection of our devices. The NB issues a CE Certificate of conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the “essential requirements”. This Certificate of Conformity entitles the manufacturer to affix the CE mark to its medical devices after having prepared and signed a related Declaration of Conformity.
If we modify our devices, we may need to apply for permission to affix the CE mark to our modified product. Additionally, we may need to apply for a CE mark for any new products that we may develop in the future. Certain products regulated as medical devices according to one or more U-EEC Directives are subject to vigilance requirements for reporting of adverse events.
We will be subject to additional regulations in other countries in which we market, sell and import our products, including Canada. We or our distributors must receive all necessary approvals or clearance prior to marketing and/or importing our products in such markets.
The ISO promulgates internationally recognized standards, including those for the requirements of quality systems. To support ISO certifications, surveillance audits are conducted by a notified body yearly and recertification audits every three years that assess continued compliance with the relevant ISO standards.
Other Regulatory Requirements
After approval, drug products are subject to extensive continuing regulation by the FDA, which include company obligations to (i) manufacture products in accordance with GMP, (ii) maintain and provide to the FDA updated safety and efficacy information, (iii) report adverse experiences with the product, (iv) keep certain records and submit periodic reports, (v) obtain FDA approval of certain manufacturing or labeling changes and (vi) comply with FDA promotion and advertising requirements and restrictions. Failure to meet these obligations can result in various adverse consequences, both voluntary and FDA-imposed, including, but not limited to, product recalls, withdrawal of approval, restrictions on marketing, and the imposition of civil fines and criminal penalties against the NDA holder. In addition, later discovery of previously unknown safety or efficacy issues may result in restrictions on the product, manufacturer or NDA holder.
We and any manufacturers of our products are required to comply with applicable FDA manufacturing requirements contained in the FDA’s GMP regulations. GMP regulations require, among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation. The manufacturing facilities for our products must meet GMP requirements to the satisfaction of the FDA pursuant to a pre-approval inspection before we can such facilities to manufacture our products. We and any third-party manufacturers are also subject to periodic inspections of facilities by the FDA and other authorities, including procedures and operations used in the testing and manufacture of our products to assess our compliance with applicable regulations.
With respect to post-market product advertising and promotion, the FDA imposes a number of complex regulations on entities that advertise and promote pharmaceuticals, which include, among others, standards for direct-to-consumer advertising, promoting drugs for uses or in patient populations that are not described in the drug’s approved labeling (known as “off-label use”), industry-sponsored scientific and educational activities, and promotional activities involving the internet. Failure to comply with FDA requirements can have negative consequences, including adverse publicity, enforcement letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties. Although physicians may prescribe legally available drugs for off-label uses, manufacturers may not market or promote such off-label uses.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
Adverse event reporting and submission of periodic reports is required following FDA approval of an NDA. The FDA also may require post-marketing testing, known as Phase 4 testing, risk minimization action plans and surveillance to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product.
Outside of the United States, our ability to market a product is contingent upon receiving marketing authorization from the appropriate regulatory authorities. The requirements governing marketing authorization, pricing and reimbursement vary widely from jurisdiction to jurisdiction. At present, foreign marketing authorizations are applied for at a national level, although within the European Union registration procedures are available to companies wishing to market a product in more than one European Union member state.
We are also subject to various environmental, health and safety regulations including those governing laboratory procedures and the handling, use, storage, treatment, and disposal of hazardous materials. From time to time, and in the future, our operations may involve the use of hazardous materials.
Competition
The neuromodulation industry is characterized by rapidly evolving technology and intense competition. Some of our current and potential competitors include Medtronic, Boston Scientific, Abbott (formerly St. Jude Medical), PINS Medical, Aleva Neurotherapeutics, and Nevro Company, many of which have financial, technical, and marketing resources significantly greater than our resources. In addition, there are a significant number of companies, working on evolving technologies that may supplant our technology or make it obsolete. Academic institutions, government agencies, and other public and private research organizations are also conducting research activities and seeking patent protection and may commercialize products on their own or through joint ventures. In addition, we are aware of certain development projects for alternative therapies to prevent or treat certain diseases targeted by us. The existence of these potential products, or other products or treatments of which we are not aware, or products or treatments that may be developed in the future, may adversely affect the marketability of our products or product candidates.
Research and Development
For the year ending December 31, 2016 and the period ending December 31, 2015, the Company’s research and development expenses were $266,586 and $0, respectively, primarily reflecting consultants and materials and supplies. It is expected that the Company’s research and development expenses activities and related expenses will increase significantly in the future as we increase the scope and rate of such efforts and begin more expensive development activities, including clinical trials and similar studies as required by the relevant regulatory authorities in our targeted jurisdictions (i.e. the United States and European Union).
Employees
As of April 27, 2017, we have 10 full-time employees and three consultants. None of our employees or consultants are represented by a labor union or covered by a collective bargaining agreement. We have employment agreements with all full time employees. We consider our relationship with our employees to be very good.
Properties
The Company does not currently own any property. The Company leases approximately 400 sq. ft. of office space in Lexington, Kentucky for $5,700 per year.
Legal Proceedings
We are not currently subject to any legal proceedings, and to the best of our knowledge, no such proceeding is threatened, the results of which would have a material impact on our properties, results of operation, or financial condition, nor to the best of our knowledge, are any of our officers or directors involved in any legal proceedings in which we are an adverse party.
Available Information
The Company is a Nevada corporation with its principal executive office located at 1708 Jaggie Fox Way, Lexington, Kentucky 40511. The Company’s telephone number is 844-919-9990. The Company’s website address is www.nexeonmed.com. Our website contains links to download, free of charge, our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Unless expressly noted, none of the information on our website is part of this Registration Statement.
EXECUTIVE OFFICERS
The following table sets forth information regarding the Company’s executive officers and key personnel.
Executive Officers:
|
Name
|
Age
|
Position(s)
|
|
|
|
|
|
William Rosellini
|
37
|
Chief Executive Officer and Director
|
|
Brian Blischak
|
52
|
President and Chief Commercial Officer
|
|
Mark C. Bates
|
56
|
Chief Innovation Officer and Director
|
|
Christopher R. Miller
|
48
|
Chief Financial Officer
|
|
Ronald Conquest
|
72
|
Executive Vice President of Finance and Director
|
|
Kent J. George
|
57
|
Director
|
|
Michael Neitzel
|
38
|
Director
|
The following is a brief summary of the background of each of our executive officers and directors.
William Rosellini
William Rosellini has served as Chief Executive Officer and as a director of the Company since inception on December 7, 2015. He is a 15-year veteran of the neurotechnology space and has expertise in accelerating the development of emerging technologies with minimal at-risk capital. Since 2005, Mr. Rosellini has served as Chairman of Rosellini Scientific, which develops medical rehabilitation devices to support patients post-procedure. He previously founded and led Lexington Technology Group, LLC, a database company commercializing a database solution with an exit to ("DSS" NYSE) Sarif Biomedical LLC, a stereotactic microsurgery company exit to ("MARA" NSDQ) and Telemend Medical, a clinical engineering services company exit to ("SPRV"OTC). Mr. Rosellini founded Microtransponder in May 2005 and served as CEO from January 2006 until July 2012. From March 2013 until September 2016, Mr. Rosellini served as a member of the board of directors of Marathon Patent Group. Other than the foregoing, Mr. Rosellini does not currently, and has not for the last five years, served as a member of the board of directors for any other public companies. Mr. Rosellini received separate Masters Degrees in the fields of Regulatory Science and Regulatory Affairs (University of Southern California), Computational Biology (New Jersey Institute of Technology), Neuroscience (University of Texas at Dallas), and Accounting (University of Texas at Dallas). Additionally, Mr. Rosellini obtained an MBA from the University of Texas at Dallas and a Juris Doctor from Hofstra University School of Law.
We believe that Mr. Rosellini is qualified to serve on our Board of Directors due to his role in founding the Company and in the development of its business strategy, as well as his experience developing medical devices.
Brian Blischak
Brian Blischak has served as the Company’s President and Chief Commercial Officer since December 1, 2016. Mr. Blischak has over 20 years of experience in the medical device field and 16 years of experience in neuromodulation serving in senior management and executive roles for ImThera Medical, Inc. (“ImThera”) and Abbott (formerly St. Jude Medical). Prior to his appointment as the Company’s President and Chief Commercial Officer, Mr. Blischak served as Senior Vice President of Sales and Marketing at ImThera, a developer of an implantable neurostimulation system for obstructive sleep apnea, from December 2011 to August 2015. Prior to that, from April 2008 to November 2011 Mr. Blischak worked in the St. Jude Medical Neuromodulation Division, where he led the commercialization and launch of two major deep brain stimulation product families consisting of over 20 products in more than 15 countries, and the patient recruitment efforts for two pivotal Investigational Device Exemption (“IDE”) studies for Parkinson's disease and essential tremor and two IDE feasibility studies for major depressive disorder . Mr. Blischak earned an MBA and MS in Biomedical Engineering from The University of Texas at Austin, Texas.
Mark C. Bates
Dr. Mark C. Bates, MD, DSc. (hon), FACC, FSCAI, FSVM is the co-founder and Chief Innovation Officer of the Company and has been on the Board of Directors since his appointment in February 2016. In addition, Dr. Bates was the founder of the company we acquired, NXDE, where he was also the Board Chairman and Chief Executive Officer from November 2004 through the date of merger of NXDE with the Company. Dr. Bates was responsible for leading NXDE through several important milestones including: manufacturing facility quality systems development and ISO certification, managing products through clinical trials, spinout and merger of the stent portfolio with CeloNova BioSciences in 2011, sale of an inventory saving system to BioSensors International in 2013, and the sale of a cell therapy regenerative medicine portfolio to Cook Medical in 2014. Since 2011 he has been on the Board of CeloNova BisoSciences, which recently sold their embolization portfolio to Boston Scientific. Since 2001 he has been the co-director of a CAMC Vascular Center of Excellence in Charleston, West Virginia and has more than 20 years of interventional cardiology and endovascular surgery experience during which time he has performed or supervised thousands of coronary and peripheral procedures. In addition to the Company’s, Dr. Bates has been named as inventor or co-inventor on over 100 U.S. and foreign patents in the medical field, though not all of these patents have led to commercialization. Over the past five years Dr. Bates has been involved in teaching with a part-time faculty position as Professor of Medicine and Surgery at West Virginia University School of Medicine in Charleston, West Virginia. Dr. Bates does not currently, and has not for the last five years, served as a member of the Board of Directors for any other public companies. Dr. Bates obtained a Bachelor of Science in Chemistry from West Virginia State University and a Doctor of Medicine from West Virginia University Medical School. Dr. Bates does not currently, and has not for the last five years, served as a member of any other Board of Directors for any public company.
Dr. Bates is qualified to serve on our Board of Directors as a result of his past business endeavors over the last three decades.
Christopher R. Miller
Mr. Miller served as Interim Chief Financial Officer of the Company from January 1, 2016 until December 1, 2016 when he was appointed as the Chief Financial Officer. Since 2002, Mr. Miller has been providing financial and business development consulting and interim CFO services, with a focus on early-stage companies. From October 2006 to June 2008, Mr. Miller provided public company valuation, financial modeling and due diligence services to Doherty & Company, LLC, a Los Angeles based broker-dealer specializing in venture capital, private equity funding, mergers and acquisitions advisory and valuations for early-stage companies. From June 2009 to June 2010, Mr. Miller served as a member of the Board of Directors of WindGen Energy, Inc. Mr. Miller obtained a Bachelor of Science degree in Finance from Arizona State University. Other than the foregoing, Mr. Miller does not currently, and has not for the last five years, served as a member of the Board of Directors for any public companies.
Ronald Conquest
Mr. Conquest served as the Chief Operating Officer and a director of the Company since inception on December 7, 2015. On October 16, 2016, Mr. Conquest was appointed to the office of Executive Vice President of Finance and remains as a director of the Company. Since November 2009, Mr. Conquest has served as Chairman and CEO of WindGen Energy, Inc. Mr. Conquest’s executive experience includes Chairman of the Board and CEO-level corporate management along with domestic and international corporate finance. From January 1979 to December 1983 Mr. Conquest served as Chairman and President for RH Energy, Inc., from January 1984 to December 1988 Mr. Conquest served as Chairman and CEO for American Medical Support, Inc., and from January 1989 to February 1999 Mr. Conquest served as Managing Director of Warwick Clarendon Investors and the Conquest Group. From March 1999 to October 2001 Mr. Conquest served as Chairman and CEO for FTM Media, Inc., and from 2005 to 2009 he served as President and Manager for GEM Distribution Systems, LLC. Mr. Conquest has served as a director of Nuviant since its inception 2014, and since September of 2015 Mr. Conquest has served as Chief Operating Officer of Rosellini Scientific. Other than the foregoing, Mr. Conquest does not currently, and has not for the last five years, served as a member of the Board of Directors for any public companies.
Mr. Conquest is qualified to serve on our Board of Directors because, as a result of his past business endeavors over the last four decades, we believe Mr. Conquest has gained significant insight into the challenges that entrepreneurs face in today’s competitive business environment and could help the Company navigate many of the challenges facing an early-stage company.
Kent J. George
Mr. George has served as a Director of the Company since January 1, 2017. Mr. George been associated with Robinson & McElwee PLLC, a mid-Atlantic corporate law firm, since 1987. From January 1999 through December 2014 Mr. George served as Managing Member of Robinson & McElwee, PLLC, and has served as the Chief Executive Officer since January 2014. With over three decades of experience in commercial transactions, representing public and private companies, Mr. George has been recognized for his work in real estate by Chambers USA since 2014 and is AV peer-rated by Martindale-Hubbell. Mr. George’s practice focuses upon business transactions, including mergers and acquisitions, business litigation, arbitration and dispute resolutions and real estate transactions (retail, industrial, resort, lodging, and other commercial development projects). Other than the foregoing, Mr. George does not currently, and has not for the last five years, served as a member of the Board of Directors for any public companies. Mr. George holds a B.A. from Swarthmore College, a J.D. from the University of Chicago and a B.A. and M.A. (Law) from Oxford University.
We believe that Mr. George’s legal and business experience qualifies him to serve on our Board of Directors. Mr. George’s experience and institutional knowledge in this regard is relevant to the business of and is strategically beneficial to the Company. Further, in the course of his business experience, he has developed and displayed significant senior leadership, deal-making, negotiation, problem solving and interpersonal skills which are relevant to the operation of the Company.
Michael Neitzel
Mr. Neitzel has served as a Director of the Company since January 1, 2017. Since April 2017, Mr. Neitzel has served as the Director of Land Acquisition for Cambridge Homes, and since January 2014 Mr. Neitzel has served as Managing Partner of DartPoints Holdings, LLC (“DartPoints”), a data center construction and management firm, where he leveraged his 15 years of experience in real estate acquisition, design, and construction management to the delivery and operation of the data centers. Prior to DartPoints, from August 2009 until January 2014, Mr. Neitzel served as the Land Acquisition and Land Development Manager at Gehan Homes and has held management positions for both public and private, large-scale building companies where his responsibilities included deal flow sourcing, acquisition, and development and delivery of subdivisions throughout Texas. Other than the foregoing, Mr. Neitzel does not currently, and has not for the last five years, served as a member of the Board of Directors for any public companies. Mr. Neitzel holds a B.A. in business administration from the University of Kansas and an MBA in finance from Southern Methodist University.
We believe that Mr. Neitzel is qualified to serve on our Board of Directors based on his education, business and financial experience.
Independence of the Board of Directors
As of December 31, 2016, our Board of Directors was composed of three members, none of whom qualified as “independent directors” as defined by the rules of The NASDAQ Stock Market. As of January 1, 2017, Kent J. George and Michael Neitzel were appointed to the Board of Directors. Each meets the standard of an independent director as defined by the rules of The NASDAQ Stock Market.
Arrangements between Officers and Directors
To our knowledge, there is no arrangement or understanding between any of our officers and any other person, including directors, pursuant to which the officer was selected to serve as an officer.
Family Relationships
As of December 31, 2016, there are no family relationships of any kind among our executive officers, directors or persons nominated or chosen by us to become executive officers or directors.
Legal Proceedings
We are not aware of any of our directors or officers being involved in any legal proceedings in the past ten years relating to any matters in bankruptcy, insolvency, criminal proceedings (other than traffic and other minor offenses) or being subject to any of the items set forth under Item 401(f) of Regulation S-K.
EXECUTIVE COMPENSATION
The tables below summarize all compensation awarded to, earned by, or paid to our executive officers for all services rendered in all capacities to us for the fiscal period(s) indicated.
Summary Executive Compensation
|
|
|
Nonequity
|
Nonqualified
|
|||||||||||||||||||||||||||||||
|
|
|
incentive
|
deferred
|
|||||||||||||||||||||||||||||||
|
|
|
Stock
|
Option
|
plan
|
compensation
|
All other
|
||||||||||||||||||||||||||||
|
Name and
|
|
Salary
|
Bonus
|
Awards
|
Awards
|
compensation
|
earnings
|
compensation
|
Total
|
|||||||||||||||||||||||||
|
Position
|
Year
|
($)
|
($)
|
($)
|
($)
|
($)
|
($)
|
($)
|
($)
|
|||||||||||||||||||||||||
|
(a)
|
(b)
|
(c)
|
(d)
|
(e)
|
(f)
|
(g)
|
(h)
|
(i)
|
(j)
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||
|
William Rosellini
|
2016
|
$
|
—
|
$
|
10,000
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
10,000
|
|||||||||||||||||
|
Chief Executive
Officer
|
2015
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
|||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||
|
Brian Blischak
|
2016
|
$
|
20,833
|
$
|
—
|
$
|
—
|
$
|
356,343
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
386,176
|
|||||||||||||||||
|
President & Chief
Commercial Officer
|
2015
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
|||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||
|
Mark C. Bates
|
2016
|
$
|
9,900
|
$
|
—
|
$
|
—
|
$
|
47,056
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
56,956
|
|||||||||||||||||
|
Chief Innovation
Officer
|
2015
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
|||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||
|
Ronald Conquest
|
2016
|
$
|
81,500
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
6,500
|
$
|
88,000
|
|||||||||||||||||
|
Executive Vice
President of Finance
|
2015
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
|||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||
|
Elizabeth Rosellini
|
2016
|
$
|
20,000
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
20,000
|
|||||||||||||||||
|
Vice President of
Clinical Affairs
(Resigned effective
December 31, 2016)
|
2015
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
|||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||
|
Christopher R. Miller
|
2016
|
$
|
21,577
|
$
|
—
|
$
|
252
|
$
|
66,234
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
88,063
|
|||||||||||||||||
|
Chief Financial Officer
|
2015
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
|||||||||||||||||
Summary Director Compensation
The following table provides information with respect to the total compensation granted to our directors for services as a director as of December 31, 2016.
|
Name
|
Year
|
Fees Earned or
Paid inCash ($)
|
Stock
Awards ($) |
Option
Awards ($) |
Non-Equity
Incentive Plan Compensation ($)
|
Non-Qualified
Deferred Compensation Earnings ($)
|
All Other
Compensation ($) |
Total
($)
|
||||||||||||||||||||||
|
William Rosellini
|
2016
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
|||||||||||||||
|
Mark C. Bates
|
2016
|
—
|
—
|
—
|
—
|
—
|
—
|
—
|
||||||||||||||||||||||
|
Ron Conquest
|
2016
|
—
|
—
|
—
|
—
|
—
|
—
|
—
|
||||||||||||||||||||||
On January 1, 2016, the Company entered into an employment agreement with Ronald Conquest. On October 14, 2016, the Company entered in an executive services agreement with Ronald Conquest which replaced the employment agreement dated January 1, 2016.
On May 1, 2016, the Company entered into a director services agreement with Dr. Mark Bates. On September 1, 2016, the Company entered into a subsequent director services agreement with Dr. Mark Bates which replaced the agreement dated May 1, 2016.
2016 Omnibus Incentive Plan
We may, from time to time, issue certain equity awards pursuant to our 2016 Plan. The 2016 Plan was adopted by our Board of Directors on January 2, 2016 and was subsequently approved by our shareholders on January 2, 2016. As of December 31, 2016, incentive stock options to purchase an aggregate of 1,178,000 shares of Common Stock and non-qualified options to purchase an aggregate of 1,154,000 shares of the Company's common stock were issued under the 2016 Plan, all with an exercise price of $1.00 per share. Of such issuances, 138,000 options vested immediately upon grant and the remaining vest in varying amounts ranging from 100,000 annually to monthly increments ranging from 3,333 to 17,000 based on individual stock option agreements. Each option has a three-year term commencing on each date of vesting.
The 2016 Plan will be administered by a committee of two or more non-employee directors designated by the Board once outside directors have been elected to the Board. In the interim, the Board shall perform the requisite duties of the committee with respect to awards granted. The Committee currently determines to whom awards are made, the timing of any such awards, the type of securities, and number of shares covered by each award, as well as the terms, conditions, performance criteria, restrictions and other provisions of awards. The committee has the authority to cancel or suspend awards, accelerate the vesting or extend the exercise period of any awards made pursuant to the 2016 Plan.
Shares Available under the 2016 Plan
The maximum shares available for issuance under the 2016 Plan are 5,000,000, subject to adjustment as set forth in the 2016 Plan. Any shares subject to an award that expires and that are cancelled, forfeited or settled for cash shall become available under the 2016 Plan. The committee can issue awards comprised of restricted stock, stock options, stock appreciation rights, stock units and other awards, as set forth in the 2016 Plan.
Transferability
Except as otherwise provided in the 2016 Plan, (i) during the lifetime of a participant, only the participant or the participant’s guardian or legal representative may exercise an option or stock appreciation right, or receive payment with respect to any other award and (ii) no award may be sold, assigned, transferred, exchanged or encumbered, voluntarily or involuntarily, other than by will or the laws of descent and distribution.
Change in Control
In the event of a merger, the surviving or successor entity (or its parent) may continue, assume or replace outstanding awards as of the date of the relevant transaction and such awards or replacements therefore shall remain outstanding and be governed by their respective terms. Such awards or replacements can be executed in part on the condition that the contractual obligations represented by the award are expressly assumed by the surviving or successor entity (or its parent) with appropriate adjustments to the number and type of securities subject to the award and the exercise price thereof so as to preserve the intrinsic value of the award existing at the time of the relevant transaction. Alternatively, the surviving or successor entity (or its parent) could issue to a participant a comparable equity-based award that preserves the intrinsic value of the original award existing at the time of the relevant transaction and contains terms and conditions that are substantially similar to those of the award.
If and to the extent that outstanding awards under the 2016 Plan are not continued, assumed or replaced in connection with a merger or relevant corporate transaction, then all outstanding awards shall become fully vested and exercisable for such period of time prior to the effective date of the relevant transaction as is deemed fair and equitable by the committee and shall terminate at the effective date of said transaction.
Amendment & Termination
The 2016 Plan shall remain in effect until all shares subject to it are distributed, all awards have expired or terminated, or the tenth anniversary of the 2016 Plan’s effective date; however, our Board of Directors may terminate, suspend or amend the 2016 Plan at any time. The Company shall submit any amendment to the 2016 Plan to its shareholders for approval only to the extent required by applicable laws or regulations or the rules of any securities exchange on which the Company’s shares may then be listed. No termination, suspension, or amendment of the 2016 Plan may materially impair the rights of any participant under a previously granted award without the participant’s consent, unless such action is necessary to comply with applicable law or stock exchange rules.
Employment Agreements
William Rosellini
The Company currently does not have a formal employment agreement with William Rosellini in his executive capacity as Chief Executive Officer.
Pursuant to the contribution agreement between the Company and Rosellini Scientific, any compensation paid by the Company for Mr. Rosellini’s services to the Company will be paid directly to Rosellini Scientific. Mr. Rosellini is the Chairman of Rosellini Scientific.
Brian Blischak
Effective December 1, 2016, the Company executed an employment agreement with Brian Blischak (the “Blischak Employment Agreement”) in his capacity of President and Chief Commercial Officer. The term of the Blischak Employment Agreement is four years and may automatically be extended for one additional year. Upon expiration of the term of the Blischak Employment Agreement, Mr. Blischak shall remain an “at will” employee of the Company, but shall still be subject to and bound by the terms of the Blischak Employment Agreement. The Blischak Employment Agreement provides that Mr. Blischak will have a minimum annual base salary of $250,000. The base salary shall not include any benefits made available to Mr. Blischak or any contributions or payments made on his behalf pursuant to any employee benefit plan or program of the Company, including any health, disability or life insurance plan or program, 401(k) plan, cash bonus plan, stock incentive plan, retirement plan or similar plan or program of any nature.
Benefit Programs. Mr. Blischak shall be eligible to participate in various Company benefit programs, as they become available, pursuant to the terms of the Company’s applicable benefit plans and policies available to other similarly situated employees of the Company. In addition, the Company shall establish and maintain a Health Reimbursement Account (Section 105 Plan) (“HRA”) for the benefit of Mr. Blischak and his immediate family. During the term of the Blischak Employment Agreement and until Company makes available a health insurance benefit that Mr. Blischak deems superior to this arrangement, the Company shall contribute $1,350 per month to Mr. Blischak’s HRA account. Mr. Blischak will draw upon this account to pay for health insurance premiums, deductibles, co-payments and any other health care expenses permitted by the HRA Plan (“Health Costs”), and unused funds shall roll forward until thirty days after the his employment terminates for any reason, at which time any remaining funds would revert to the Company. The amount of Company’s contribution to the HRA shall be reviewed and increased effective January 1 of each year of the Blischak Employment Agreement to reflect changes in Health Costs and the cost of health insurance available to Mr. Blischak and his immediate family.
Bonus. The Blischak Employment Agreement provides that in addition to the annual base salary described above, Mr. Blischak shall also be eligible for an annual performance-based bonus of 30% of his annual base salary, to be earned by satisfactorily meeting criteria established by the Company’s Chief Executive Officer and approved by the Compensation Committee of the Board of Directors prior to March 1 each year. Mr. Blischak will receive the full 30% bonus amount if such criteria are satisfactorily met. In the event that Mr. Blischak’s performance exceeds this standard, he may be considered for a bonus in an amount greater than 30%. In the event that his performance falls short of this standard, he may receive less than the full bonus percentage. A minimum of 70% of the annual bonus compensation shall be paid in cash, and the balance shall be paid in unrestricted shares of Common Stock, or such other mutually agreeable consideration. During the term of the Blischak Employment Agreement, the yearly annual bonus shall be paid within sixty days of the calendar year end.
Termination.
Termination for Cause. In the event that Mr. Blischak’s employment is terminated by the Company for Cause (as defined in the Blischak Employment Agreement), Mr. Blischak shall be entitled to all accrued compensation, including vested stock and stock options, up through the date of termination but shall not be entitled to additional severance payments.
Termination without Cause or Change in Control. In the event that Mr. Blischak’s employment is terminated by the Company without Cause or as a result of a Change in Control (as defined in the Blischak Employment Agreement), Mr. Blischak will receive severance compensation pursuant to the following formulas:
(i) In the event of a termination without Cause, or a Change in Control occurs, occurring prior to the first, second, or third year anniversary of the Blischak Employment Agreement, Mr. Blischak shall receive a lump sum severance amount equal to 4/12th, 5/12th, or 6/12th, respectively, of the sum of (A) his highest annual base salary in effect at any time during the term of the Blischak Employment Agreement or his annual base salary in effect immediately prior to the termination or Change in Control, whichever is the larger amount, plus (B) the amount of the bonus or incentive compensation targeted for payment to him for the fiscal year during which the termination or the Change in Control occurs.
(ii) In the event of a termination without Cause, or a Change in Control occurs, occurring at any time after the third-year anniversary of the Blischak Employment Agreement, Mr. Blischak will receive a lump sum severance amount equal to 6/12th of the sum of (A) his highest annual base salary in effect at any time during the term of the Blischak Employment Agreement or his annual base salary in effect immediately prior to the termination or Change in Control, whichever is the larger amount, plus (B) the amount of the bonus or incentive compensation targeted for payment to him for the fiscal year during which the termination or the Change in Control occurs.
Termination for Good Reason. In the event that Mr. Blischak terminates his employment with the Company for Good Reason (as defined in the Blischak Employment Agreement), he will receive a severance payment equal to 3/12thof the sum of (A) his highest annual base salary in effect at any time during the term of the Blischak Employment Agreement or his annual base salary in effect immediately prior to the termination, whichever is the larger amount, plus (B) the amount of the bonus or incentive compensation targeted for payment to him for the fiscal year during which the termination occurs.
Release of Claims. As a condition to receiving any severance, Mr. Blischak must execute a full general release satisfactory to the Company, releasing all claims, known or unknown that Mr. Blischak may have against the Company arising out of or in any way related to his employment or termination of employment with Company prior to receipt of the severance payment. If Company fails to provide Mr. Blischak with a signed, full general release within seven days of the termination date, then Company shall waive this requirement. Any severance and other amounts due shall be paid in full within seven days of the termination date, and execution or waiver of the full general release as applicable.
Stock Options. Upon execution of the Blischak Employment Agreement, Mr. Blischak was granted an initial grant of non-transferable stock options to purchase up to 1,150,000 shares of the Company’s Common Stock, consisting of 500,000 incentive stock options (“Blischak ISO”), and 650,000 non-qualified stock options (“Blischak NQSO”). With respect to the Blischak ISO options, 100,000 Blischak ISO options vested on December 1, 2016, and additional lots of 100,000 Blischak ISO options shall vest on January 2, 2017, 2018, 2019 and 2020. With respect to the Blischak NQSO options, 38,000 Blischak NQSO options vested on December 1, 2016, and 17,000 Blischak NQSO options vested on January 1, 2017. An additional 17,000 Blischak NQSO options shall vest on the first day of each month thereafter until all Blischak NQSO options are fully vested on December 1, 2019. The exercise price of all options is $1.00 per share and the options shall expire in eight years from the grant date. All options shall vest immediately upon a termination without Cause, Change in Control, or Termination for Good Reason as set forth above.
Dr. Mark C. Bates
The Company currently does not have a formal employment agreement with Dr. Mark Bates in his executive capacity as Chief Innovation Officer.
On May 1, 2016, the Company executed a director services agreement with Dr. Bates. Pursuant to this agreement, Dr. Bates was granted 252,000 nonqualified stock options. The options vest in monthly increments of 7,000, with a three-year term for each option beginning upon each date of vesting. On September 1, 2016, the Company entered into a subsequent director services agreement with Dr. Bates which included a fee of $3,000 paid quarterly to serve as a director and a fee of $9,000 paid quarterly for research and development consulting services in addition to the 252,000 nonqualified stock options grant received pursuant to the agreement dated March 1, 2016.
Ronald Conquest
On January 1, 2016, the Company executed an employment agreement with Ronald Conquest (the “Conquest Employment Agreement”) in the capacity of Chief Operating Officer and director. Pursuant to this agreement Mr. Conquest initially received an annual salary of $100,000, and commencing on October 1, 2016, Mr. Conquest received an annual salary of $150,000. Commencing October 1, 2017, Mr. Conquest will receive an annual salary of $175,000. The term of the Conquest Employment Agreement is two years.
In the event Mr. Conquest is terminated without Cause (as defined in the Conquest Employment Agreement), the Company will be required to pay Mr. Conquest’s then existing salary and benefits for a period equal to 50% of the remaining term of the Conquest Employment Agreement. In the event the Company terminates Mr. Conquest’s employment as a result of a Change in Control, the Company will be responsible to pay his then existing salary and benefits for the remaining term of the agreement.
The Company also reimburses Mr. Conquest and his spouse for his health insurance and all out of pocket expenses.
Christopher R. Miller
Effective December 1, 2016, the Company entered into an executive employment contract with Christopher R. Miller (the “Miller Employment Contract”). The term of the Miller Employment Contract i three years. The Miller Employment Contract shall automatically renew for an additional one year term. The Miller Employment Contract provides that Mr. Miller will receive an annual base salary of (i) $125,000 per year from December 1, 2016 through December 31, 2017, (ii) $150,000 per year from January 1, 2018 through December 31, 2018 and (iii) $175,000 per year from January 1, 2019 through December 31, 2019.
Benefit Programs. Mr. Miller shall be eligible to participate in various Company benefit programs, as they become available, pursuant to the terms of the Company’s applicable benefit plans and policies available to other similarly situated employees of the Company.
Bonus. In addition to the annual base salary described above, Mr. Miller shall also be eligible for an annual performance-based bonus of 20% of his annual base salary, to be earned by satisfactorily meeting criteria established by the Company’s Chief Executive Officer and approved by the Compensation Committee of the Board of Directors prior to March 1 each year. Mr. Miller will receive the full 20% bonus amount if such criteria are satisfactorily met. In the event that Mr. Miller’s performance exceeds this standard, he may be considered for a bonus in an amount greater than 20%. In the event that his performance falls short of this standard, he may receive less than the full bonus percentage. A minimum of 70% of the annual bonus compensation shall be paid in cash, and the balance shall be paid in unrestricted shares of Common Stock of the Company, or such other mutually agreeable consideration. During the term of the Miller Employment Contract, the yearly annual bonus shall be paid within sixty days of the calendar year end. In the event the Miller Employment Contract is terminated by the Company or Mr. Miller terminates his employment under the Miller Employment Contract, Mr. Miller will earn the base salary prorated to the date of termination. The prorated base salary will be based on a thirty day calendar month.
Stock Options. Upon execution of the Miller Employment Contract, Mr. Miller was granted stock options to purchase up to 306,000 shares of the Company’s restricted Common Stock pursuant to the Company’s 2016 Plan. The option vest at the rate of 8,500 shares per month for a period of thirty-six months and is exercisable at a price of $1.00 per share. Any portion of the option that has not vested as of the date of the end of the term of the Miller Employment Contract shall be subject to forfeiture.
Stock Award. In January 2016, the Company issued to Mr. Miller 252,000 shares of the Company’s restricted Common Stock for prior and then ongoing consulting services. On the effective date of the Miller Employment Contract, 148,000 shares had vested and the, balance, or 104,000 shares will vest at the rate of 8,000 shares per month for a period of thirteen months.
Termination.
Death or Disability. In the event that Mr. Miller’s employment terminates due to his death, Mr. Miller’s estate shall receive severance benefits equivalent to 30 days’ of Mr. Miller’s then base salary. In the event that Mr. Miller’s employment terminates due to Disability (as defined in the Miller Employment Contract), Mr. Miller will receive severance benefits equal to 90 days’ of his then base salary.
Termination for Cause. In the event that Mr. Miller’s employment is terminated by the Company for Cause (as defined in the Miller Employment Contract), Mr. Miller shall be entitled to all accrued compensation, including vested stock options, up through the date of termination but shall not be entitled to additional severance payments.
Termination Without Cause. In the event that Mr. Miller’s employment is terminated by the Company without Cause, Mr. Miller will receive the base salary then in effect, prorated to the date of termination. In addition, Mr. Miller will receive a severance payment equivalent to 90 days’ of the base salary then in effect.
Termination for Good Reason. In the event that Mr. Miller terminates his employment with the Company for Good Reason (as defined in the Miller Employment Contract), he will receive a severance payment equivalent to 90 days’ of base salary then in effect
Release of Claims. As a condition to receiving any severance, Mr. Miller must execute a full general release satisfactory to the Company, releasing all claims, known or unknown that Mr. Miller may have against the Company arising out of or in any way related to his employment or termination of employment with Company prior to receipt of the severance package.
Outstanding Equity Awards at Fiscal Year End
As of December 31, 2016, the Company had no outstanding equity awards made to our executives.
The following table sets forth information regarding each unexercised options held by each of the Company’s named executive officers as of December 31, 2016:
|
Name
|
Number of Securities
Underlying Unexercised Options Exercisable |
Number of Securities
Underlying Unexercised Options Unexercisable |
Option Exercise
Price |
Option Expiration
Date
|
|||||||||||
|
|
|||||||||||||||
|
William Rosellini
|
—
|
—
|
$
|
—
|
—
|
||||||||||
|
Brian Blischak
|
138,000
|
1,012,000
|
$
|
1.00
|
12/01/2027
|
||||||||||
|
Mark C. Bates
|
56,000
|
196,000
|
$
|
1.00
|
04/01/2022
|
||||||||||
|
Ronald Conquest
|
—
|
—
|
$
|
—
|
—
|
||||||||||
|
Christopher R. Miller
|
—
|
306,000
|
$
|
1.00
|
12/01/2022
|
||||||||||
____________
| (1) |
All option grants reflected in the table above were granted pursuant to the Company’s 2016 Plan.
|
The following table sets forth, as of April 27, 2017, certain information regarding beneficial ownership of our capital stock according to the information supplied to us, that were beneficially owned by (i) each person known by the Company to be the beneficial owner of more than 5% of each class of the Company’s outstanding voting stock, (ii) each director, (iii) each named executive officer identified in the Summary Compensation Table, and (iv) all named executive officers and directors as a group.
Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In general, a person is deemed to be the beneficial owner of (i) any shares of the Company’s Common Stock over which such person has sole or shared voting power or investment power, plus (ii) any shares which such person has the right to acquire beneficial ownership of within 60 days of the above date, whether through the exercise of options, warrants or otherwise. Applicable percentages are based on 22,663,621 shares of Common Stock outstanding on the date above, adjusted as required by rules promulgated by the SEC.
Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o Nexeon MedSystems Inc, 1708 Jaggie Fox Way, Lexington, Kentucky 40511.
|
|
Shares Beneficially
Owned Prior to Offering
|
|||||||
|
Name of Beneficial Owner
|
Number
|
Percent
|
||||||
|
|
||||||||
|
Beneficial Shareholders ( Greater than 5%):
|
||||||||
|
Rosellini Scientific, LLC
|
12,543,807
|
(1)
|
55.35
|
%
|
||||
|
Michael Rosellini
|
1,523,890
|
(2)
|
6.72
|
%
|
||||
|
Elizabeth Rosellini
|
1,800,000
|
7.94
|
%
|
|||||
|
|
||||||||
|
Directors and Executive Officers:
|
||||||||
|
William Rosellini
|
15,593,807
|
(3)
|
60.64
|
%
|
||||
|
Brian Blischak
|
340,000
|
(4)
|
1.48
|
%
|
||||
|
Mark C. Bates
|
917,112
|
(5)
|
4.03
|
%
|
||||
|
Ronald Conquest
|
500,000
|
2.21
|
%
|
|||||
|
Kent J. George
|
54,899
|
(6)
|
*
|
%
|
||||
|
Michael Neitzel
|
597,500
|
(7)
|
2.63
|
%
|
||||
|
Christopher R. Miller
|
303,000
|
(8)
|
1.33
|
%
|
||||
|
All Directors and Officers as a Group (8 persons)
|
18,306,318
|
69.78
|
%
|
|||||
____________
| * |
Less than 1%
|
| (1) |
Rosellini Scientific LLC is located at 3824 Cedar Springs Rd #419, Dallas, Texas. Mr. Rosellini is the sole Member and Manager of Rosellini Scientific and in such capacity has voting and dispositive power over the securities held by such entity.
|
| (2) |
Represents (i) 821,890 shares of Common Stock held by Michael Rosellini, and (ii) 702,000 shares of Common Stock held by IRA Resources, FBO Randy Michael Rosellini, ROTH IRA (“IRA”). Nrose LLC (“Nrose”) owns 58,000 shares of Common Stock. Michael Rosellini’s spouse, Nancy Rosellini, is the sole member and manager of Nrose and in such capacity has voting and dispositive power over the securities held by such entity. Michael Rosellini has disclaimed beneficial ownership of the securities held by NRose. Michael Rosellini is the trustee of his Roth IRA and in such capacity has voting and dispositive power over the securities held by such entity.
|
| (3) |
Represents (i) 3,050,000 shares of Common Stock issuable to Mr. Rosellini and (ii) 12,543,807 shares of Common Stock owned by Rosellini Scientific. The Company has not yet issued the 3,050,000 shares of restricted common stock issuable to Mr. Rosellini pursuant to the License Agreement, but they are considered as being issued for beneficial ownership calculation purposes. Mr. Rosellini is the sole Member and Manager of Rosellini Scientific and in such capacity has voting and dispositive power over the securities held by such entity.
|
| (4) |
Represents (i) an incentive stock option to purchase up to 200,000 shares of Common Stock which represents the vested portion (including shares vesting within 60 days) of an incentive stock option to purchase up to 500,000 shares of Common Stock pursuant to the 2016 Plan and (ii) a non-qualified stock option to purchase up to 140,000 shares of Common Stock which represents the vested portion (including shares vesting within 60 days) of a non-qualified stock option to purchase up to 650,000 shares of Common Stock.
|
| (5) |
Represents (i) 819,112 shares of Common Stock and (ii) and 98,000 shares of Common Stock which represents the vested portion (including shares vesting within 60 days) of a non-qualified option to purchase up to 252,000 shares of Common Stock.
|
| (6) |
Represents (i) 12,500 shares of Common Stock which represents the vested portion (including shares vesting within 60 days) of option to purchase up to 12,500 shares of Common Stock, (ii) 5,817 shares of Common Stock held by George Brothers Investment Partnership (“George Brothers”), (iii) warrants to purchase up to 5,817 shares of Common Stock held by George Brothers and (iv) 30,765 shares of Common Stock held by Paragon Investment Group (“Paragon”). Mr. George is the Managing Partner of George Brothers and the Manager of Paragon and in such capacities has voting and dispositive power over the securities held by such entities.
|
| (7) |
Represents (i) 12,500 shares of Common Stock which represents the vested portion (including shares vesting within 60 days) of option to purchase up to 12,500 shares of Common Stock, and (ii) 585,000 shares of Common Stock held by Yorkville MGB Investments, LLC (“Yorkville”). Mr. Neitzel is the Manager of Yorkville and in such capacity has voting and dispositive power over the securities held by such entity.
|
| (8) |
Represents (i) a grant of 252,000 shares of Common Stock and (ii) 51,000 shares of Common Stock which represents the vested portion (including shares vesting within 60 days) of an option to purchase up to 306,000 shares of Common Stock issued pursuant to the 2016 Plan.
|
The following reflects certain relationships and related transactions as of December 31, 2016:
On January 2, 2016, the Company entered into a contribution agreement with Rosellini Scientific, a company controlled by our Chief Executive Officer, William Rosellini and its wholly-owned subsidiary, Belltower Associates, LLC (collectively, Rosellini Scientific and Belltower Associates, LLC are hereinafter referred to as “RSBA”). Pursuant to the contribution agreement, the Company issued 13,200,000 shares of Common Stock in return for, among other consideration:
| · |
RSBA’s assignment (subject to regulatory transfer approval) to the Company of Phase II, should it be granted, of the federal NIH/SBIR awarded Grant #1R44HL129870-01;
|
| · |
1,675,000 shares of common stock of Nuviant;
|
| · |
167 shares of common stock of MicroTransponder, Inc., a Delaware corporation; and
|
| · |
175 shares of common stock of Emeritus Clinical Solutions, Inc., a Delaware corporation (“Emeritus”).
|
On January 2, 2016, the Company issued 1,800,000 shares of Common Stock to our former Vice President of Clinical Affairs, Dr. Elizabeth Rosellini, the sister of William Rosellini, our Chief Executive Officer in exchange for 214 shares of common stock of Emeritus and 60,000 shares of common stock of Nuviant. In addition, Dr. Rosellini was paid cash compensation of $20,000 during the year ended December 31, 2016. Dr. Rosellini resigned as Vice President of Clinical Affairs on December 31, 2016.
In October 2016, the Company paid NBM $124,870 for research and development services with respect to the Company’s intravascular drug delivery system technology platform. NMB is controlled by William Rosellini, our Chief Executive Officer.
On October 28, 2016, Michael Rosellini entered into a contribution agreement with the Company pursuant to which he acquired 617,000 shares of Common Stock and three year warrants to purchase up to 617,000 shares of Common Stock. The exercise price of the warrants is $2.00 per share. In addition, IRA Resources, FBO Randy Michael Rosellini, ROTH IRA was issued 600,000 shares of Common Stock and three year warrants to purchase up to 600,000 shares of Common Stock at an exercise price of $2.00 per share. Michael Rosellini is the trustee of IRA and in such capacity has voting and dispositive power over the securities held by such entity. Michael Rosellini is the father of William Rosellini, our Chief Executive Officer.
On December 15, 2016, pursuant to the terms of the Asset Purchase Agreement, William Rosellini sold, assigned and transferred any and all of his right, title and interest in and to the License owned by him related to the Siemens Intellectual Property to the Company in consideration for $140,000 in cash and the issuance of 3,050,000 shares of Common Stock. The Company has not yet issued the shares of Common Stock to Mr. Rosellini.
During the year ended December 31, 2016, Rosellini Scientific, the largest shareholder in the Company, loaned $145,475 to the Company. The non-interest bearing has no set terms of repayment. As of December 31, 2016, the loan was repaid in full in cash. William Rosellini, our Chief Executive Officer, is the Chairman of Rosellini Scientific.
The following description summarizes the material terms and provisions of our capital stock.
General
Our authorized capital stock consists of 75,000,000 shares of common stock with a par value of $0.001. As of April 27, 2017, there were 22,687,471 shares of Common Stock outstanding. We have not authorized any shares of preferred stock.
Common Stock
Each share of our Common Stock is entitled to one vote at all meetings of our shareholders. Our shareholders are not permitted to cumulate votes in the election of directors. All shares of our Common Stock are equal to each other with respect to liquidation rights and dividend rights. In the event of our liquidation, dissolution or winding up, holders of our Common Stock will be entitled to receive, on a pro-rata basis, all of our assets remaining after satisfaction of all liabilities and preferences of outstanding preferred stock, if any. Except as otherwise required by Nevada law, holders of shares of our Common Stock do not have cumulative voting rights, which means that the holders of more than 50% of the outstanding shares, voting for the election of directors, can elect all of the directors to be elected, if they so choose, and in such event, the holders of the remaining shares will not be able to elect any of our directors.
Warrants
As of April 27, 2017, we had 636,762 warrants outstanding, all of which are currently exercisable, with a weighted average price of 2.00 per share.
Stock Options Under Equity Plans
As of April 27, 2017, there were approximately 5,000,000 shares of Common Stock reserved for issuance under our stock option and equity plans. Of this number, approximately 2,782,000 shares are reserved for issuance upon exercise of outstanding options that were previously granted under our equity plans, and 2,218,000 shares may be granted in the future under our equity plans.
Anti-Takeover Provisions
In the future, we may become subject to Nevada’s control share law. A corporation is subject to Nevada’s control share law if it has more than 200 shareholders, at least 100 of whom are shareholders of record and residents of Nevada/ conduct business in Nevada or through an affiliated corporation. The law focuses on the acquisition of a “controlling interest”, which means the ownership of outstanding voting shares sufficient, but for the control share law, to enable the acquiring person to exercise the following proportions of the voting power of the corporation in the election of directors: (i) one-fifth or more but less than one-third, (ii) one-third or more but less than a majority, or (iii) a majority or more. The ability to exercise such voting power may be direct or indirect, as well as individual or in association with others.
The effect of the control share law is that the acquiring entity, and those acting in association with it, obtains only such voting rights in the control shares as are conferred by a resolution of the shareholders of the corporation, approved at a special or annual meeting of shareholders. The control share law contemplates that voting rights will be considered only once by the other shareholders. Thus, there is no authority to strip voting rights from the control shares of an acquiring entity once those rights have been approved. If the shareholders do not grant voting rights to the control shares acquired by an acquiring entity, then those shares do not become permanent non-voting shares. The acquiring entity is free to sell its shares to others.
If the buyers of those shares themselves do not acquire a controlling interest, then the control share law does not govern their shares. If control shares are accorded full voting rights and the acquiring entity has acquired control shares with a majority or more of the voting power, then any shareholders of record, other than an acquiring entity, who has not voted in favor of approval of voting rights is entitled to demand fair value for such shareholder’s shares. Nevada’s control share law may have the effect of discouraging takeovers of the Company.
In addition to the control share law, Nevada has a business combination law that prohibits certain business combinations between Nevada corporations and “interested shareholders” for three years after the “interested shareholders” first becomes an “interested shareholders,” unless the corporation’s board of directors approves the combination in advance.
For purposes of Nevada law, an “interested shareholders” is any person who is: (i) the beneficial owner, directly or indirectly, of 10% or more of the voting power of the outstanding voting shares of the corporation, or (ii) an affiliate or associate of the corporation and at any time within the three previous years was the beneficial owner, directly or indirectly, of 10% or more of the voting power of the then outstanding shares of the corporation. The definition of the term “business combination” is sufficiently broad to cover virtually any kind of transaction that would allow a potential acquirer to use the corporation’s assets to finance the acquisition or otherwise to benefit its own interests rather than the interests of the corporation and its other shareholders.
The effect of Nevada’s business combination law is to discourage parties potentially interested in taking control of our Company from doing so if it cannot obtain the approval of our Board of Directors.
Listing
Our Common stock is not currently listed for trading on any exchange or market.
Transfer Agent and Registrar
The transfer agent and registrar for our Common Stock is Action Stock Transfer Corp. Its address is 2469 E. Fort Union Blvd, Suite 214, Salt Lake City, Utah 84121 and its telephone number is (801) 274-1088.
We are registering an aggregate of 6,052,240 Resale Shares for resale by the Selling Shareholders listed in the table below. All expenses incurred with respect to the registration of the Common Stock will be paid by us, but we will not be obligated to pay any underwriting fees, discounts, commissions or other expenses incurred by the Selling Shareholders in connection with the sale of such shares.
The Selling Shareholders may sell some or all of their Resale Shares at a fixed price of $1.00 per share until our shares are quoted on the OTCQX for anticipated aggregate net proceeds of approximately $6,052,240 and thereafter at prevailing market prices or privately negotiated prices. The offering price bears no relationship to our assets, book value, earnings or any other customary investment criteria. We will not receive any proceeds from the sale of the Resale Shares by the Selling Shareholders.
The Selling Shareholders may also resell all or a portion of their securities in reliance upon Rule 144 under the Securities Act provided that they meet the criteria and conform to the requirements of that rule or by any other available means.
The Selling Shareholders named below may from time to time offer and sell pursuant to this prospectus up to 6,052,240 Resale Shares.
The following table sets forth:
| · |
the name of the Selling Shareholder;
|
| · |
the number and percent of shares of our Common Stock that the Selling Shareholders beneficially owned prior to the offering for resale of the shares under this prospectus;
|
| · |
the number of shares of our Common Stock that may be offered for resale for the account of the Selling Shareholders under this prospectus; and
|
| · |
the number and percent of shares of our Common Stock to be beneficially owned by the Selling Shareholders after the offering of the Resale Shares (assuming all of the offered Resale Shares are sold by the Selling Shareholders).
|
The number of shares in the column “Number of Shares Being Offered” represents all of the shares that each Selling Shareholder may offer under this prospectus. We do not know how long the Selling Shareholders will hold the shares before selling them or how many shares they will sell, and we currently have no agreements, arrangements or understandings with any of the Selling Shareholders regarding the sale of any of the Resale Shares.
This table is prepared solely based on information supplied to us by the Selling Shareholders, any Schedules 13D or 13G and Forms 3 and 4, and other public documents filed with the SEC. The applicable percentages of beneficial ownership are based on an aggregate of 22,663,621 shares of our Common Stock issued and outstanding on April 27, 2017. Unless otherwise indicated, no Selling Shareholder is a broker dealer or affiliated with a broker dealer. Unless otherwise indicated, the address of each Selling Shareholder is c/o Nexeon MedSystems Inc, 1708 Jaggie Fox Way, Lexington, Kentucky 40511.
|
Name of Selling Shareholder
|
|
|
Common
Stock Beneficially Owned Prior to the Offering (1) |
|
|
|
Common
Stock Covered in Prospectus |
|
|
|
Common
Stock Beneficially Owned Upon Completion of this Offering (1)(2) |
|
|
|
Percentage of
Common Stock Owned Upon Completion of this Offering (1) (3) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mark C. Bates
|
|
|
917,112
|
|
|
|
432,900
|
|
|
|
484,212
|
|
|
|
2.13
|
%
|
|
Atlas Ventures LLC (4)
|
|
|
50,000
|
|
|
|
25,000
|
|
|
|
25,000
|
|
|
|
*
|
|
|
Ballard Investments Inc. (5)
|
|
|
651,993
|
|
|
|
76,752
|
|
|
|
575,241
|
|
|
|
2.54
|
%
|
|
Ronald Edward Duke/SEPP-IRA (6)
|
|
|
100,000
|
|
|
|
50,000
|
|
|
|
50,000
|
|
|
|
*
|
|
|
George Brothers, Investment Partnership (7)
|
|
|
11,634
|
|
|
|
5,817
|
|
|
|
5,817
|
|
|
|
*
|
|
|
Andrew Jordan
|
|
|
73,222
|
|
|
|
22,627
|
|
|
|
50,595
|
|
|
|
*
|
|
|
Mitchell N. Rashid
|
|
|
17,228
|
|
|
|
5,817
|
|
|
|
11,411
|
|
|
|
*
|
|
|
Hou Invests LLC (8)
|
|
|
60,000
|
|
|
|
30,000
|
|
|
|
30,000
|
|
|
|
*
|
|
|
ISM Holdings LLC (9)
|
|
|
100,000
|
|
|
|
50,000
|
|
|
|
50,000
|
|
|
|
*
|
|
|
Adam August
|
|
|
29,250
|
|
|
|
29,250
|
|
|
|
0
|
|
|
|
*
|
|
|
Thomas August
|
|
|
58,500
|
|
|
|
58,500
|
|
|
|
0
|
|
|
|
*
|
|
|
Liberty Trust Company Ltd. CUST FBO Stacie
Hyatt IRA (10)
|
|
|
50,000
|
|
|
|
25,000
|
|
|
|
25,000
|
|
|
|
*
|
|
|
Jordan Foster
|
|
|
29,250
|
|
|
|
29,250
|
|
|
|
0
|
|
|
|
*
|
|
|
George Alonistiotis
|
|
|
29,250
|
|
|
|
29,250
|
|
|
|
0
|
|
|
|
*
|
|
|
Amy Rosellini
|
|
|
10,000
|
|
|
|
5,000
|
|
|
|
5,000
|
|
|
|
*
|
|
|
Nicholas Pipikios
|
|
|
50,000
|
|
|
|
25,000
|
|
|
|
25,000
|
|
|
|
*
|
|
|
Michael J. Bacsik
|
|
|
89,442
|
|
|
|
89,442
|
|
|
|
0
|
|
|
|
*
|
|
|
Brenda J. Long
|
|
|
11,700
|
|
|
|
11,700
|
|
|
|
0
|
|
|
|
*
|
|
|
Harvey W. Wiggins Jr.
|
|
|
29,250
|
|
|
|
29,250
|
|
|
|
0
|
|
|
|
*
|
|
|
LXT Strategic Advisors Ltd. (11)
|
|
|
20,000
|
|
|
|
10,000
|
|
|
|
10,000
|
|
|
|
*
|
|
|
Michael Rosellini & Michael Rosellini DDS Roth
IRA (12)
|
|
|
1,523,890
|
|
|
|
802,000
|
|
|
|
721,890
|
|
|
|
3.19
|
%
|
|
William Ward Jr and Donna Sue Ward
|
|
|
23,400
|
|
|
|
23,400
|
|
|
|
0
|
|
|
|
*
|
|
|
Andreas Spiegl
|
|
|
35,100
|
|
|
|
35,100
|
|
|
|
0
|
|
|
|
*
|
|
|
Midway Pasquale Holdings LP (13)
|
|
|
40,950
|
|
|
|
40,950
|
|
|
|
0
|
|
|
|
*
|
|
|
Mountain West IRA Inc. FBO David Spann IRA
(14)
|
|
|
29,250
|
|
|
|
29,250
|
|
|
|
0
|
|
|
|
*
|
|
|
NRose LLC (15)
|
|
|
58,500
|
|
|
|
58,500
|
|
|
|
0
|
|
|
|
*
|
|
|
RBC Trust Co (Delaware) Ltd. TTEE FBO Briana
N. Ballard Irrev. Trust (16)
|
|
|
143,961
|
|
|
|
20,714
|
|
|
|
123,247
|
|
|
|
*
|
|
|
RBC Trust Co (Delaware) Ltd. TTEE FBO Derek
M. Ballard Irrev. Trust (17)
|
|
|
182,732
|
|
|
|
26,348
|
|
|
|
156,384
|
|
|
|
*
|
|
|
RBC Trust Co (Delaware) Ltd. TTEE FBO Ralph
L. Ballard IV Irrev. Trust (18)
|
|
|
182,732
|
|
|
|
26,348
|
|
|
|
156,384
|
|
|
|
*
|
|
|
Kevin M. Sobczyk
|
|
|
10,000
|
|
|
|
5,000
|
|
|
|
5,000
|
|
|
|
*
|
|
|
Anne Marie Bierman
|
|
|
10,000
|
|
|
|
5,000
|
|
|
|
5,000
|
|
|
|
*
|
|
|
Spellbound Partners Ltd. (19)
|
|
|
58,500
|
|
|
|
58,500
|
|
|
|
0
|
|
|
|
|
Name of Selling Shareholder
|
Common
Stock Beneficially Owned Prior to the Offering (1) |
Common
Stock Covered in Prospectus |
Common
Stock Beneficially Owned Upon Completion of this Offering (1)(2) |
Percentage
of Common Stock Owned Upon Completion of this Offering (1) (3) |
||||||||||||
|
|
||||||||||||||||
|
Thomas Russell
|
500,000
|
250,000
|
250,000
|
1.09
|
%
|
|||||||||||
|
Yorkville MGB Investments LLC (20)
|
597,500
|
585,000
|
12,500
|
*
|
||||||||||||
|
Dimitrios Sikaras
|
20,000
|
10,000
|
10,000
|
*
|
||||||||||||
|
Gina Vanessa Palavicini
|
20,000
|
10,000
|
10,000
|
*
|
||||||||||||
|
Giovanni Palavicini
|
40,000
|
20,000
|
20,000
|
*
|
||||||||||||
|
Ryan Malady
|
29,250
|
29,250
|
0
|
*
|
||||||||||||
|
Harold Levine
|
15,000
|
7,500
|
7,500
|
*
|
||||||||||||
|
Sarah Levine
|
15,000
|
7,500
|
7,500
|
*
|
||||||||||||
|
Todd Upchurch
|
58,500
|
58,500
|
0
|
*
|
||||||||||||
|
Discovery Consulting, LLC (21)
|
25,000
|
25,000
|
0
|
*
|
||||||||||||
|
Erin Von Rosenberg
|
5,000
|
2,500
|
2,500
|
*
|
||||||||||||
|
Joshua E. Duke
|
30,000
|
15,000
|
15,000
|
*
|
||||||||||||
|
Ronald Edward Duke
|
100,000
|
50,000
|
50,000
|
*
|
||||||||||||
|
Weidema van Tol, GmbH (22)
|
146,500
|
146,500
|
0
|
*
|
||||||||||||
|
Ronald Conquest
|
500,000
|
500,000
|
0
|
*
|
||||||||||||
|
Elizabeth S. Rosellini
|
1,800,000
|
800,000
|
1,000,000
|
4.41
|
%
|
|||||||||||
|
George Hamilton III
|
60,000
|
60,000
|
0
|
*
|
||||||||||||
|
An Bobbaers
|
80,938
|
80,938
|
0
|
*
|
||||||||||||
|
Austin Robert Duke
|
427,815
|
215,317
|
212,498
|
*
|
||||||||||||
|
Health Wildcatters Fund, LLC (23)
|
200,000
|
200,000
|
0
|
*
|
||||||||||||
|
Montgomery Coscia Greilich, LLP (24)
|
39,000
|
39,000
|
0
|
*
|
||||||||||||
|
Granicus IP, LLC (25)
|
158,500
|
158,500
|
0
|
*
|
||||||||||||
|
Christopher R. Miller
|
303,000
|
252,000
|
51,000
|
*
|
||||||||||||
|
Christopher R. Davis
|
45,570
|
45,570
|
0
|
*
|
||||||||||||
|
Acorn Management Partners (26)
|
125,000
|
125,000
|
0
|
*
|
||||||||||||
|
Sichenzia Ross Ference Kesner, LLP (27)
|
12,500
|
12,500
|
0
|
*
|
||||||||||||
|
Atidtek LLC (28)
|
175,000
|
175,000
|
0
|
*
|
||||||||||||
|
Total
|
10,215,919
|
6,052,240
|
4,163,679
|
18.34
|
%
|
|||||||||||
______________
| * |
Represents less than 1%.
|
| (1) |
Under applicable SEC rules, a person is deemed to beneficially own securities which the person has the right to acquire within 60 days through the exercise of any option or warrant or through the conversion of a convertible security. Also under applicable SEC rules, a person is deemed to be the “beneficial owner” of a security with regard to which the person directly or indirectly, has or shares (a) voting power, which includes the power to vote or direct the voting of the security, or (b) investment power, which includes the power to dispose, or direct the disposition, of the security, in each case, irrespective of the person’s economic interest in the security. Each listed Selling Shareholder has the sole investment and voting power with respect to all shares of Common Stock shown as beneficially owned by such Selling Shareholder, except as otherwise indicated in the footnotes to the table.
|
| (2) |
Represents the amount of shares that will be held by the Selling Shareholders after completion of this offering based on the assumptions that (a) all shares registered for sale by the registration statement of which this prospectus is part will be sold and (b) that no other shares of our Common Stock beneficially owned by the Selling Shareholders are acquired or are sold prior to completion of this offering by the Selling Shareholders.
|
| (3) |
In determining the percent of Common Stock beneficially owned by a Selling Shareholder following the offering, (a) the numerator is the number of shares of Common Stock beneficially owned by such Selling Shareholder (including shares that he has the right to acquire within 60 days of April 27, 2017), and (b) the denominator is 22,663,621 shares outstanding after offering based upon 22,663,621 shares of Common Stock outstanding on April 27, 2017 and (ii) the number of shares of Common Stock which such Selling Shareholder has the right to acquire within 60 days of April 27, 2017 after the offering.
|
| (4) |
Mitchel Gervais as Manager of Atlas Ventures, LLC has voting and dispositive power over the shares held by such entity.
|
| (5) |
Ralph L. Ballard and Debra K Ballard as the officers and directors of Ballard Investments Inc. have voting and dispositive power over the shares held by such entity.
|
| (6) |
Ronald Edward Duke as Trustee of Ronald Edward Duke SEPP/IRA has voting and dispositive power over the shares held by such entity.
|
| (7) |
Kent George as Managing Partner of George Brothers Investment Partnership has voting and dispositive power over the shares held by such entity.
|
| (8) |
Austin Hancock as Manager of Hou Invests, LLC has voting and dispositive power over the shares held by such entity.
|
| (9) |
Arvin Zeinali as Manager of ISM Holdings LLC has voting and dispositive power over the shares held by such entity.
|
| (10) |
Glenn Martin as Trustee of Liberty Trust Company, Ltd. FBO Stacie Hyatt has voting and dispositive power over the shares held by such entity.
|
| (11) |
Jason Mann as the sole officer and director of LXT Strategic Advisors Ltd has voting and dispositive power over the shares held by such entity.
|
| (12) |
Michael Rosellini as Trustee of Michael Rosellini DDS Defined Benefit Pension Plan has voting and dispositive power over the shares held by such entity.
|
| (13) |
Jill and James Ombrello as Managers of Midway Pasquale Holdings LP have voting and dispositive power over the shares held by such entity.
|
| (14) |
David Spann as Trustee of Mountain West IRA FRO David Spann IRA has voting and dispositive power over the shares held by such entity.
|
| (15) |
Nancy Rosellini as sole member and manager of NRose LLC has voting and dispositive power over the shares held by such entity.
|
| (16) |
Kevin Fretz as Trust Officer of RBC Trust Co. (Delware) Ltd. TIEE FBO Briana N. Ballard Irrevocable Trust has voting and dispositive power over the shares held by such entity.
|
| (17) |
Kevin Fretz as Trust Officer of RBC Trust Co. (Delware) Ltd. TIEE FBO Derek M. Ballard Irrevocable Trust has voting and dispositive power over the shares held by such entity.
|
| (18) |
Kevin Fretz as Trust Officer of RBC Trust Co. (Delware) Ltd. TIEE FBO Ralph L. Ballard IV Irrevocable Trust has voting and dispositive power over the shares held by such entity.
|
| (19) |
Richard J. Cree as Manager of Spellbound Partners, Ltd has voting and dispositive power over the shares held by such entity.
|
| (20) |
Michael Neitzel as Manager of Yorkville MGB Investments, LLC has voting and dispositive power over the shares held by such entity.
|
| (21) |
Eric Geoff White as the sole Member and Manager of Discovery Consulting, LLC has voting and dispositive power over the shares held by such entity.
|
| (22) |
Frank Weidema and Pieter van Tol as the Co-Managing Partners of Weidema van Tol, GmbH have voting and dispositive power over the shares held by such entity.
|
| (23) |
Dr. Hubert Zajick and J.R. Garcia as the Co-Managers of Health Wildcatters Fund, LLC has voting and dispositive power over the shares held by such entity.
|
| (24) |
Tom Montgomery as the Managing Partner of Montgomery Coscia Greilich, LLP has voting and dispositive power over the shares held by such entity
|
| (25) |
Eric Spangneberg as the sole Member and Manager of Granicus IP, LLC has voting and dispositive power over the shares held by such entity
|
| (26) |
John R. Exley III as General Partner of Acorn Management Partners has voting and dispositive power over the shares held by such entity.
|
| (27) | Harvey Kesner as Partner of Sichenzia Ross Ference Kesner, LLP has voting and dispositive power over the shares held by such entity. |
| (28) |
Alejandro Covalin as sole Member and Manager of Atidtek LLC has voting and dispositive power over the shares held by such entity.
|
Each Selling Shareholder of the Common Stock and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of Common Stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These sales may be at fixed or negotiated prices; provided, however, until such time that our shares are quoted on the OTCQX or any other trading exchange, the Selling Shareholders may sell some or all of their Resale Shares at a fixed price of $1.00 per share.
A Selling Shareholder may use any one or more of the following methods when selling shares:
| · |
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
| · |
block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
|
| · |
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
| · |
an exchange distribution in accordance with the rules of the applicable exchange;
|
| · |
privately negotiated transactions;
|
| · |
settlement of short sales;
|
| · |
in transactions through broker-dealers that agree with the Selling Shareholders to sell a specified number of such Common stock at a stipulated price per security;
|
| · |
through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
|
| · |
a combination of any such methods of sale; or
|
| · |
any other method permitted pursuant to applicable law.
|
The Selling Shareholders may also sell shares under Rule 144 under the Securities Act, if available, rather than under this prospectus.
Broker-dealers engaged by the Selling Shareholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Shareholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2440; and in the case of a principal transaction a markup or markdown in compliance with FINRA IM-2440.
In connection with the sale of the Common Stock or interests therein, the Selling Shareholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the Common Stock in the course of hedging the positions they assume. The Selling Shareholders may also sell shares of the Common Stock short and deliver these securities to close out their short positions, or loan or pledge the Common Stock to broker-dealers that in turn may sell these securities. The Selling Shareholders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Shareholders and any broker-dealers or agents that are involved in selling the shares may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each Selling Shareholder has informed us that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the Common Stock.
We are required to pay certain fees and expenses incurred by us incident to the registration of the shares. We have agreed to indemnify the Selling Shareholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We agreed to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Shareholders without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for the Company to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar effect, or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The Resale Shares will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the Resale Shares covered hereby may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the Resale Shares may not simultaneously engage in market making activities with respect to the Common Stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Selling Shareholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of shares of the Common Stock by the Selling Shareholders or any other person. We will make copies of this prospectus available to the Selling Shareholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
The validity of the securities being offered by this prospectus been passed upon for us by Sichenzia Ross Ference Kesner LLP, New York, New York and the related consolidated statements of operations, changes in stockholders’ equity and cash flows for the year ended December 31, 2016 and for the period from December 7, 2015 (inception) to December 31, 2015 have been audited by Paritz & Company, P.A., , independent registered public accounting firm, as stated in their report which is incorporated herein. Such financial statements have been incorporated herein in reliance on the report of such firm given upon their authority as experts in accounting and auditing.
We are a reporting company and file annual, quarterly and special reports, and other information with the SEC. Copies of the reports and other information may be read and copied at the SEC’s Public Reference Room at 100 F Street N.E., Washington, D.C. 20549. You can request copies of such documents by writing to the SEC and paying a fee for the copying cost. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains a web site at http://www.sec.gov that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC.
This prospectus is part of a registration statement on Form S-1 that we filed with the SEC. Certain information in the registration statement has been omitted from this prospectus in accordance with the rules and regulations of the SEC. We have also filed exhibits and schedules with the registration statement that are excluded from this prospectus. For further information you may:
· read a copy of the registration statement, including the exhibits and schedules, without charge at the SEC’s Public Reference Room;
· obtain a copy from the SEC upon payment of the fees prescribed by the SEC.
NEXEON MEDSYSTEMS INC
|
|
|
Page
|
|
|
|
|
|
|
F-1
|
|
|
|
|
|
|
|
F-2
|
|
|
|
|
|
|
|
F-3
|
|
|
|
|
|
|
|
F-4
|
|
|
|
|
|
|
|
F-5
|
|
|
|
|
|
|
|
F-6
|
To the Board of Directors and Stockholders’
Nexeon MedSystems, Inc.
We have audited the accompanying consolidated balance sheets of Nexeon MedSystems, Inc.(the “Company”) and its subsidiaries as of December 31, 2016 and 2015 and the related consolidated statements of operations, changes in stockholders’ equity and cash flows for the year ended December 31, 2016 and for the period from December 7, 2015 (inception) to December 31, 2015. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company and its subsidiaries as of December 31, 2016 and 2015, and the results of their operations and their cash flows for the years ended December 31, 2016 and for the period from December 7, 2015 (inception) to December 31, 2015 in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. As discussed in Note 3 the Company has not generated any revenues since inception and has an accumulated a deficit of $1,592,338 at December 31, 2016. The Company currently has not completed its efforts to establish a source of revenues sufficient to cover operating costs over an extended period of time. These factors, among others, raise substantial doubt about the ability of the Company to continue as a going concern. Management plans are also discussed in Note 3.The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/S/Paritz & Company, P.A.
Hackensack, New Jersey
March 29, 2017
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
|
|
December 31, 2016
|
December 31, 2015
|
||||||
|
Assets
|
||||||||
|
|
||||||||
|
Current Assets
|
||||||||
|
Cash and cash equivalents
|
$
|
2,039,907
|
$
|
—
|
||||
|
Other current assets
|
127,597
|
—
|
||||||
|
Total Current Assets
|
$
|
2,167,504
|
—
|
|||||
|
|
||||||||
|
Property and equipment, net
|
888
|
—
|
||||||
|
Investments
|
148,860
|
—
|
||||||
|
Patents and license, net of accumulated amortization of $562,714
|
8,747,286
|
—
|
||||||
|
Total Assets
|
$
|
11,064,538
|
$
|
—
|
||||
|
|
||||||||
|
Liabilities and Stockholders’ Equity
|
||||||||
|
|
||||||||
|
Current Liabilities
|
||||||||
|
Accounts payable
|
112,302
|
—
|
||||||
|
Accrued liabilities
|
33,434
|
—
|
||||||
|
Due to related party
|
415
|
415
|
||||||
|
Credit facility
|
14,813
|
—
|
||||||
|
Accrued interest payable – stockholder
|
2,193
|
—
|
||||||
|
Total Current Liabilities
|
163,157
|
415
|
||||||
|
|
||||||||
|
Notes payable stockholders - long term
|
10,000
|
—
|
||||||
|
Total Liabilities
|
$
|
173,157
|
415
|
|||||
|
|
||||||||
|
Stockholders' Equity
|
||||||||
|
Common Stock - 75,000,000 shares authorized, $.001 par value;
21,711,953 and 500,000 issued and outstanding at December 31, 2016
and December 31, 2015, respectively
|
21,712
|
500
|
||||||
|
Additional paid-in capital
|
9,392,007
|
—
|
||||||
|
Equity instruments to be issued
|
3,070,000
|
—
|
||||||
|
Accumulated deficit
|
(1,592,338
|
)
|
(915
|
)
|
||||
|
Total Stockholders' Equity
|
10,891,381
|
(415
|
)
|
|||||
|
|
||||||||
|
Total Liabilities and Stockholders' Equity
|
$
|
11,064,538
|
$
|
—
|
||||
The accompanying notes are an integral part of the consolidated financial statements.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
|
|
For the year
Ended December 31, 2016 |
From Inception,
December 7, 2015 to December 31, 2015 |
||||||
|
|
||||||||
|
Revenues
|
$
|
—
|
$
|
—
|
||||
|
|
||||||||
|
Depreciation and amortization
|
562,813
|
—
|
||||||
|
General and administrative expenses
|
582,867
|
915
|
||||||
|
Research and development expenses
|
266,586
|
—
|
||||||
|
|
||||||||
|
Loss from operations
|
(1,412,266
|
)
|
(915
|
)
|
||||
|
|
||||||||
|
Other (Expense)
|
||||||||
|
Loss on impairment of investment
|
(173,500
|
)
|
—
|
|||||
|
Interest expense – stockholders
|
(2,098
|
)
|
—
|
|||||
|
Interest expense – other
|
(3,559
|
)
|
—
|
|||||
|
|
||||||||
|
Loss before provision (benefit) for taxes
|
$
|
(1,591,423
|
)
|
$
|
(915
|
)
|
||
|
Provision (benefit) for taxes
|
—
|
—
|
||||||
|
|
||||||||
|
Net loss
|
$
|
(1,591,423
|
)
|
$
|
(915
|
)
|
||
|
|
||||||||
|
BASIC AND DILUTED PER SHARE DATA:
|
||||||||
|
Net Loss per common share, basic and diluted
|
$
|
(0.08
|
)
|
$
|
(0.00
|
)
|
||
|
Weighted average common shares outstanding, basic and diluted
|
19,044,803
|
500,000
|
||||||
The accompanying notes are an integral part of the consolidated financial statements.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
|
|
Additional
|
Equity
|
||||||||||||||||||||||
|
|
Common Stock
|
Paid-In
|
Instruments
|
Accumulated
|
||||||||||||||||||||
|
|
Shares
|
Amount
|
Capital
|
to be Issued
|
Deficit
|
Total
|
||||||||||||||||||
|
|
||||||||||||||||||||||||
|
Balances at December 7, 2015
(Inception)
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
|||||||||||||
|
Issuance of common stock to founder
|
500,000
|
500
|
500
|
|||||||||||||||||||||
|
Net loss for the period from inception through December
31, 2015
|
(915
|
)
|
(915
|
)
|
||||||||||||||||||||
|
Balances at December 31, 2015
|
500,000
|
500
|
—
|
—
|
(915
|
)
|
(415
|
)
|
||||||||||||||||
|
Common stock issued for services
|
423,500
|
423
|
171,329
|
171,752
|
||||||||||||||||||||
|
Common stock issued for acquisition
|
16,659,943
|
16,660
|
4,811,186
|
4,827,846
|
||||||||||||||||||||
|
Common stock to be issued for patent license
|
3,050,000
|
3,050,000
|
||||||||||||||||||||||
|
Common stock issued for conversion of notes payable and
accrued interest
|
1,287,564
|
1,288
|
1,389,335
|
1,390,623
|
||||||||||||||||||||
|
Common stock issued in private placement for cash
|
2,840,946
|
2,841
|
2,675,283
|
2,678,124
|
||||||||||||||||||||
|
Common stock to be issued in private placement for cash
|
20,000
|
20,000
|
||||||||||||||||||||||
|
Options expense
|
82,284
|
82,284
|
||||||||||||||||||||||
|
Warrants issued for conversion of notes payable and
accrued interest
|
162,823
|
162,823
|
||||||||||||||||||||||
|
Warrants issued in private placement for cash
|
99,767
|
99,767
|
||||||||||||||||||||||
|
Net loss for the twelve months ended December 31, 2016
|
(1,591,423
|
)
|
(1,591,423
|
)
|
||||||||||||||||||||
|
Balances at December 31, 2016
|
21,711,953
|
$
|
21,712
|
$
|
9,392,007
|
$
|
3,070,000
|
$
|
(1,592,338
|
)
|
$
|
10,891,381
|
||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
|
|
For the year Ended
December 31, 2016
|
From Inception,
December 7, 2015 to
December 31, 2015
|
||||||
|
|
||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||
|
Net Loss
|
$
|
(1,591,423
|
)
|
$
|
(915
|
)
|
||
|
Adjustment to reconcile net loss to net cash used in operating
activities:
|
||||||||
|
Depreciation and amortization
|
562,813
|
—
|
||||||
|
Stock-based compensation
|
254,036
|
—
|
||||||
|
Loss on impairment of investment
|
173,500
|
—
|
||||||
|
Settlement of stockholder note accrued interest
|
(690
|
)
|
—
|
|||||
|
Change in operating assets and liabilities:
|
||||||||
|
Other current asset
|
(127,597
|
)
|
—
|
|||||
|
Accounts payable
|
40,454
|
—
|
||||||
|
Accrued liabilities
|
20,448
|
—
|
||||||
|
Accrued interest
|
898
|
—
|
||||||
|
Net cash used in operating activities
|
(667,561
|
)
|
(915
|
)
|
||||
|
|
||||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||
|
Acquisition of property and equipment
|
(986
|
)
|
—
|
|||||
|
Acquisition of intellectual property
|
(140,000
|
)
|
—
|
|||||
|
Net cash used in investing activities
|
(140,986
|
)
|
—
|
|||||
|
|
||||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||
|
Proceeds from issuance of common stock
|
2,860,946
|
500
|
||||||
|
Proceeds of loan from related party
|
—
|
415
|
||||||
|
Repayment of shareholder note
|
(10,000
|
)
|
—
|
|||||
|
Repayment of revolving credit facility
|
(2,492
|
)
|
—
|
|||||
|
Net cash provided by financing activities
|
2,848,454
|
915
|
||||||
|
|
||||||||
|
Net increase in cash and cash equivalents
|
2,039,907
|
—
|
||||||
|
Cash and cash equivalents at beginning of period
|
—
|
—
|
||||||
|
Cash and cash equivalents at end of period
|
2,039,907
|
—
|
||||||
|
|
||||||||
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW
INFORMATION:
|
||||||||
|
Cash paid during period for interest
|
3,559
|
—
|
||||||
|
Cash paid during period for taxes
|
—
|
—
|
||||||
|
|
||||||||
|
SUPPLEMENTAL DISCLOSURE OF NON-CASH ACTIVITIES:
|
||||||||
|
Common stock issued for acquisition
|
4,505,486
|
—
|
||||||
|
Equity instruments to be issued for acquisition for patent license
|
3,050,000
|
—
|
||||||
|
Common stock issued for conversion of shareholder notes and
accrued interest
|
1,490,390
|
—
|
||||||
|
Common stock issued for investments
|
322,360
|
—
|
||||||
The accompanying notes are an integral part of the consolidated financial statements.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTE 1 – BUSINESS – NATURE OF ORGANIZATION
Nexeon MedSystems Inc (the “Company” or “ Nexeon”) was incorporated in the State of Nevada on December 7, 2015. We are a development stage enterprise focusing on the development and commercialization of physician-driven, medical device innovations. The Company’s primary purposes are to develop and commercialize the drug-eluting balloon technology acquired in the acquisition of Nexeon MedSystems, Inc., a private Delaware corporation (“NXDE”) and to commercialize the implantable neurotechnology and recording platform to be applied for the relief of chronic diseases and disorders, upon completing the acquisition of Nexeon Medsystems Belgium, SPRL (“NMB”), as well as acquire medical device manufacturing capability.
During 2016, the Company formed the following wholly owned subsidiaries: Nexeon Medsystems Europe, SARL (“Nexeon Europe”), Nexeon Medsystems Puerto Rico Operating Company Corporation (“NXPROC”), and Pulsus Medical LLC. Nexeon Europe is the holding company for NXPROC and NMB, subject to the acquisition. NXPROC is focused on research and development, software development and data analysis for the implantable neurotechnology. Pulsus Medical, LLC will conduct research and development on the cardiovascular disease technology.
Acquisitions
On February 16, 2016, NXDE entered into an Agreement and Plan of Merger (“Merger Agreement”) with the Company, pursuant to which NXDE was acquired by the Company, with the Company continuing as the surviving entity. The transaction is being accounted for as a business combination. The primary reason for the acquisition of NXDE was to acquire unique intellectual property owned by NXDE and technical knowledge retained by NXDE and to further develop these technologies for potential commercialization. The foregoing transaction is referred to herein as the “Merger.” The effective date of the Merger was February 16, 2016 and 100% of the equity voting interest was acquired.
In exchange for 100% of the issued and outstanding preferred stock of NXDE, immediately prior to the closing of the Merger, the Company issued 1,659,943 shares of common stock to the preferred stockholders of NXDE. As a result of the Merger, the stockholders of the NXDE acquired 9.67% of the Company's issued and outstanding common stock as of the effective date of the Merger.
In addition, the Merger Agreement provides for the conversion of debt of NXDE under the provisions of the Private Placement of the Company. See Note 10 - Equity for details on the Private Placement.
The Merger Agreement further provides for the payment of a royalty to a limited liability company to be formed by the former preferred shareholders of NXDE (the “Royalty”). The Royalty payment is equal to 3% of net product sales made by the Company that directly result from the patent portfolio of NXDE and has a term coinciding with the term of the Company’s patent portfolio.
Pursuant to the Merger Agreement, the Company exchanged $645,000 in NXDE stockholder loans and $176,482 in accrued interest related to those loans for 821,482 Units in the Private Placement, each Unit consisting of one share of restricted common stock and one common stock purchase warrant. $202,825 in accrued interest related to those loans was cancelled. See Note 10 - Equity for additional information regarding the Private Placement.
The Company considered the provisions of ASC 845 Nonmonetary transaction whereby assets acquired in exchange for another nonmonetary asset is the fair value of the assets surrendered or received, whichever is more clearly evident. Due to the lack of trading activity of the Company’s common stock surrendered in the acquisition, the Company has determined that the fair value of the assets acquired, less liabilities assumed of NXDE is a more clearly evident value of the consideration transferred in the acquisition.
The aggregate fair market value of the consideration issued $4,505,486, was determined based on the fair market value of the assets received in the acquisition less the liabilities assumed through the acquisition. 1,659,943 shares of the Company's par value $.001 common stock were transferred for all the issued and outstanding preferred stock of NXDE.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BUSINESS – NATURE OF ORGANIZATION (Continued)
The following table shows the allocation of the purchase price of the identified assets acquired and liabilities assumed.
|
Cash and cash equivalents
|
$
|
—
|
||
|
Patents, net
|
6,120,000
|
|||
|
Total identifiable assets
|
6,120,000
|
|||
|
Accounts payable
|
(89,152
|
)
|
||
|
Accrued liabilities
|
(12,986
|
)
|
||
|
Accrued interest payable – stockholders
|
(542,376
|
)
|
||
|
Notes payable – stockholders
|
(970,000
|
)
|
||
|
Total liabilities assumed
|
(1,614,514
|
)
|
||
|
|
||||
|
Total Purchase Price
|
$
|
4,505,486
|
Patent License Asset Purchase Agreement
On September 29, 2016, William Rosellini (“Licensee”), the Chief Executive Officer, a Director and a majority shareholder of the Company, entered into a patent license agreement (the “License Agreement”) with Magnus IP GmbH, incorporated and existing under the laws of Germany (“Magnus” or “Licensor”). Pursuant to the terms of the License Agreement, Magnus granted to Mr. Rosellini, and his “Affiliates” (an entity that Mr. Rosellini has ownership, directly or indirectly, of 50% or more of the voting equity of such entity), a non-exclusive, non-transferable, non-assignable without the right to sublicense worldwide license under the Licensed Patents (described below), to make, have made, use, import, export, distribute, sell, offer for sale, develop and advertise Licensed Products.
On December 15, 2016, pursuant to the terms of the License Agreement, Mr. Rosellini sold, assigned and transferred any and all of his right, title and interest in and to the License owned by him related to the Siemens Patents to the Company pursuant to the Patent License Asset Purchase Agreement (the “Purchase Agreement”). As consideration for the transfer of the Siemens Patents and the License related thereto, the Company paid to Mr. Rosellini the sum of $140,000 in cash and will issue to Mr. Rosellini 3,050,000 shares of the Company’s restricted common stock valued at $3,050,000. The Company has not yet issued the shares of restricted common stock.
NOTE 2 –SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates
The preparation of the Company’s consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting periods. Management makes these estimates using the best information available at the time the estimates are made; however, actual results could differ materially from these estimates.
Cash and Cash Equivalents
The Company considers those short-term, highly liquid investments with maturities of three months or less as cash and cash equivalents. At times, cash in banks may be in excess of the FDIC limits. The Company currently has no cash equivalents.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 –SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Principals of Consolidation
The consolidated financial statements include the accounts of the Company, and its wholly-owned subsidiaries. All material inter-company accounts, transactions, and profits have been eliminated in consolidation.
Investments in Non-Consolidated Subsidiaries
Investments in non-consolidated entities are accounted for using the equity method or cost basis depending upon the level of ownership and/or the Company's ability to exercise significant influence over the operating and financial policies of the investee. When the equity method is used, investments are recorded at original cost and adjusted periodically to recognize the Company's proportionate share of the investees' net income or losses after the date of investment. When net losses from an investment accounted for under the equity method exceed its carrying amount, the investment balance is reduced to zero and additional losses are not provided for. The Company resumes accounting for the investment under the equity method if the entity subsequently reports net income and the Company's share of that net income exceeds the share of net losses not recognized during the period the equity method was suspended. Investments are written down only when there is clear evidence that a decline in value that is other than temporary has occurred. The Company accounts for its investments in Nuviant Medical Inc. and MicroTransponder, Inc. under the cost method due to the lack of significant influence (see Note 10 - Equity).
Long-lived Assets
Long-lived assets such as property, equipment and identifiable intangibles are reviewed for impairment whenever facts and circumstances indicate that the carrying value may not be recoverable. When required impairment losses on assets to be held and used are recognized based on the fair value of the asset. The fair value is determined based on estimates of future cash flows, market value of similar assets, if available, or independent appraisals, if required. If the carrying amount of the long-lived asset is not recoverable from its undiscounted cash flows, an impairment loss is recognized for the difference between the carrying amount and fair value of the asset. When fair values are not available, the Company estimates fair value using the expected future cash flows discounted at a rate commensurate with the risk associated with the recovery of the assets. The Company recognized impairment of $173,500 on one of its investments in non-consolidated entities.
Common Stock Purchase Warrants and Other Derivative Financial Instruments
The Company classifies as equity any contracts that require physical settlement or net-share settlement or provide us a choice of net-cash settlement or settlement in our own shares (physical settlement or net-share settlement) provided that such contracts are indexed to our own stock as defined in ASC 815-40 "Contracts in Entity's Own Equity." We classify as assets or liabilities any contracts that require net-cash settlement (including a requirement to net cash settle the contract if an event occurs and if that event is outside our control) or give the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement). We assess classification of our common stock purchase warrants at each reporting date to determine whether a change in classification between assets and liabilities is required.
Property and Equipment
Property and equipment are stated at cost. Equipment is depreciated using the straight-line method over the estimated useful lives of the assets. Leasehold improvements are amortized based upon the lesser of the term of the lease or the useful life of the asset and such expense is included in depreciation expense. Repair and maintenance costs are expensed as incurred.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 –SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Research and Development
The Company expenses all research and development costs as incurred. Research and development expenses include, but are not limited to, product development, clinical and regulatory expenses, payroll and other personnel expenses, materials, supplies, and consulting costs.
Stock-Based Compensation
ASC 718 requires companies to measure all stock compensation awards using a fair value method and recognize the related compensation cost in its financial statements. Beginning with the Company’s quarterly period that began on January 1, 2016, the Company adopted the provisions of FASB ASC 718 and expenses the fair value of employee stock options and similar awards in the financial statements. The Company accounts for share based payments in accordance with ASC 718, Compensation - Stock Compensation, which requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statements based on the grant date fair value of the award. In accordance with ASC 718-10-30-9, Measurement Objective – Fair Value at Grant Date, the Company estimates the fair value of the award using the Black-Scholes option pricing model for valuation of the share-based payments. The Company believes this model provides the best estimate of fair value due to its ability to incorporate inputs that change over time, such as volatility and interest rates, and to allow for actual exercise behavior of option holders. The simplified method is used to determine compensation expense since historical option exercise experience is limited relative to the number of options issued. The compensation cost is recognized ratably using the straight-line method over the expected vesting period.
The Company accounts for stock-based compensation to other than employees in accordance with FASB ASC 505-50. Equity instruments issued to other than employees are valued at the earlier of a commitment date or upon completion of the services, based on the fair value of the equity instruments and is recognized as expense over the service period.
The Company accounts for stock-based compensation to other than employees in accordance with FASB ASC 505-50. Equity instruments issued to other than employees are valued at the earlier of a commitment date or upon completion of the services, based on the fair value of the equity instruments and is recognized as expense over the service period.
The Company recognized stock-based compensation aggregating $82,284 and $0 for common stock options issued to Company personnel, directors and consultants during the fiscal years ended December 31, 2016 and 2015, respectively. Also during the fiscal years ended December 31, 2016 and 2015, the Company paid stock-based compensation consisting of common stock issued to non-employees aggregating $171,500 and $0, respectively and to affiliates aggregating $252 and $0 respectively.
Net Income (Loss) Per Share
The Company calculates net income (loss) per share as required by Accounting Standards Codification subtopic 260-10, Earnings per Share (ASC 260-10”). Basic earnings (loss) per share is calculated by dividing net income (loss) by the weighted average number of common shares outstanding for the period. Diluted earnings per share is calculated by dividing net income (loss) by the weighted average number of common shares and dilutive common stock equivalents outstanding. During the periods when they are anti-dilutive, common stock equivalents, if any, are not considered in the computation. For the fiscal years ended December 31, 2016 and 2015, the impact of outstanding stock equivalents has not been included as they would be anti-dilutive. During the years ended December 31, 2016 and 2015, 2,332,000 and 0 options and 4,128,510 and 0 warrants were excluded.
Comprehensive Income (Loss)
FASC Topic No. 220, “Comprehensive Income,” establishes standards for reporting and display of comprehensive income and its components in a full set of general-purpose financial statements. As at December 31, 2016 and 2015, the Company had no items of other comprehensive income.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 –SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Derivative Financial Instruments
The Company does not use derivative instruments to hedge exposures to cash flow or, market risks. The Company reviews the terms of convertible debt, equity instruments and other financing arrangements to determine whether there are embedded derivative instruments, including embedded conversion options that are required to be bifurcated and accounted for separately as a derivative financial instrument. Also, in connection with the issuance of financing instruments, the Company may issue freestanding options or warrants that may, depending on their terms, be accounted for as derivative instrument liabilities, rather than as equity. The Company may also issue options or warrants to non-employees in connection with consulting or other services.
Derivative financial instruments are initially measured at their fair value. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported as charges or credits to income. For warrant-based derivative financial instruments, the Company uses the Black-Scholes Option Pricing Model to value the derivative instruments. To the extent that the initial fair values of the freestanding and/or bifurcated derivative instrument liabilities exceed the total proceeds received, an immediate charge to income is recognized, in order to initially record the derivative instrument liabilities at their fair value.
The discount from the face value of the convertible debt or equity instruments resulting from allocating some or all of the proceeds to the derivative instruments, together with the stated interest on the instrument, is amortized over the life of the instrument through periodic charges to income, using the effective interest method.
The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is reassessed at the end of each reporting period. If reclassification is required, the fair value of the derivative instrument, as of the determination date, is reclassified. Any previous charges or credits to income for changes in the fair value of the derivative instrument are not reversed. Derivative instrument liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement of the derivative instrument could be required within twelve months of the balance sheet date.
Development Stage Company
The Company is considered to be in the development stage as defined in ASC 915 “Development Stage Entities.” The Company is devoting substantially all of its efforts to the development of its business plans. The Company has adopted Accounting Standards Update No. 2014-10, Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements; and does not present or disclose inception-to-date information and other remaining disclosure requirements of Topic 915.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes in accordance with ASC Topic 740, “Income Taxes.” Under this method, income tax expense is recognized for the amount of: (i) taxes payable or refundable for the current year and (ii) deferred tax consequences of temporary differences resulting from matters that have been recognized in an entity’s financial statements or tax returns. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is provided to reduce the deferred tax assets reported if based on the weight of the available positive and negative evidence, it is more likely than not some portion or all of the deferred tax assets will not be realized.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 –SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
ASC Topic 740.10.30 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740.10.40 provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. The Company has no material uncertain tax positions for any of the reporting periods presented.
All tax positions are first analyzed to determine if the weight of available evidence indicates that it is more likely than not that the position will be sustained under audit, including resolution of any related appeals or litigation processes. After the initial analysis, the tax benefit is measured as the largest amount that is more than 50% likely of being realized upon ultimate settlement.
If the Company is required to pay interest on the underpayment of income taxes, the Company recognizes interest expense in the first period the interest becomes due according to the provisions of the relevant tax law.
If the Company is subject to payment of penalties, the Company recognizes an expense for the amount of the statutory penalty in the period when the position is taken on the income tax return. If the penalty was not recognized in the period when the position was initially taken, the expense is recognized in the period when the Company changes its judgment about meeting minimum statutory thresholds related to the initial position taken.
Fair Value Measurements
The Company adopted the provisions of ASC Topic 820, “Fair Value Measurements and Disclosures,” which defines fair value as used in numerous accounting pronouncements, establishes a framework for measuring fair value and expands disclosure of fair value measurements.
The estimated fair value of certain financial instruments, including cash and cash equivalents are carried at historical cost basis, which approximates their fair values because of the short-term nature of these instruments.
ASC 820 defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 describes three levels of inputs that may be used to measure fair value:
Level 1 — quoted prices in active markets for identical assets or liabilities
Level 2 — quoted prices for similar assets and liabilities in active markets or inputs that are observable
Level 3 — inputs that are unobservable (for example cash flow modeling inputs based on assumptions)
The Company currently has no assets or liabilities valued at fair value on a recurring basis.
Recently Issued Accounting Pronouncements
Management does not believe that any recently issued, but not yet effective accounting pronouncements, if adopted, would have a material effect on the accompanying financial statements.
Other recent accounting pronouncements issued by the FASB (including its Emerging Issues Task Force), the AICPA, and the SEC during the current reporting period did not, or are not believed by management to have a material impact on the Company’s present or future consolidated financial statements.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Acquired Intangibles
Acquired intangibles include patents and patent licenses acquired by the Company, which are recorded at fair value, assigned an estimated useful life, and are amortized on a straight-line basis over their estimated useful lives ranging from 3 to19 years . The Company periodically evaluates whether current facts or circumstances indicate that the carrying values of its acquired intangibles may not be recoverable. If such circumstances are determined to exist, an estimate of the undiscounted future cash flows of these assets, or appropriate asset groupings, is compared to the carrying value to determine whether an impairment exists. If the asset is determined to be impaired, the loss is measured based on the difference between the carrying value of the intangible asset and its fair value, which is determined based on the net present value of estimated future cash flows. See Note 4 and Note 10.
NOTE 3 – GOING CONCERN
The accompanying financial statements have been prepared in conformity with generally accepted accounting principles, which contemplate continuation of the Company as a going concern. However, the Company has not generated any revenues since inception and has an accumulated a deficit of $1,592,338 at December 31, 2016. The Company currently has not completed its efforts to establish a source of revenues sufficient to cover operating costs over an extended period of time. These factors among others raise substantial doubt about the Company’s ability to continue as a going concern.
Management expects to seek potential business opportunities for merger or acquisition of existing companies. Management is not currently limiting their search for merger or acquisition candidates to any industry or locations. Management, while not especially experienced in matters relating to public company management, will rely upon their own efforts and, to a much lesser extent, the efforts of the Company’s stockholders, in accomplishing the business purposes of the Company.
NOTE 4 – RELATED PARTY TRANSACTIONS
During the year ended December 31, 2015, the Company had the following transactions with related parties:
On December 15, 2015, the Company issued 500,000 shares of its common stock to its Chief Operating Officer for the sum of $500 at the par value of $0.001. 212,000 shares of the 500,000 shares became vested upon issue and the remaining 288,000 shares shall vest over a 36 month period at the rate of 8,000 shares per month. Vesting began on January 1, 2016.
The Company's Executive Vice President of Finance loaned $415 to the Company. The loan is non-interest bearing with no set terms of repayment. The loan is outstanding as of December 31, 2016.
During the year ended December 31, 2016, the Company had the following transactions with related parties:
On January 1, 2016, the Company issued 252,000 shares of common stock to Christopher Miller, with a par value of $0.001, for certain accounting and budget-related services rendered as well as serving as Interim CFO until such time as a permanent CFO was hired. In addition, Mr. Miller was paid $11,600 in cash compensation in the year ended December 31, 2016 prior to entering into an Executive Employment Agreement with the Company effective December 1, 2016. Pursuant to the Executive Employment Contract dated December 1, 2016 between Christopher Miller and the Company to serve as the permanent Chief Financial Officer, 148,000 shares were vested, with the remaining 104,000 shares vesting at a rate 8,000 shares per month for thirteen months.
Effective December 1, 2016, Christopher R. Miller, our Chief Financial Officer, pursuant to the 2016 Omnibus Incentive Plan was granted 306,000 three-year incentive stock options to purchase 306,000 shares of common stock. The options vest in monthly increments of 8,500 shares, with the three-year term for each option beginning upon each date of vesting. As of March 28, 2017, 25,500 options were vested but none exercised. The exercise price of all options is $1.00 per share.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 4 – RELATED PARTY TRANSACTIONS (Continued)
On January 2, 2016, the Company entered into a Contribution Agreement with Rosellini Scientific, LLC – a company controlled by our CEO, William Rosellini – and its wholly owned subsidiary Belltower Associates, LLC (collectively, Rosellini Scientific, LLC and Belltower Associates, LLC are hereinafter referred to as “RS”). Under this agreement, the Company issued 13,200,000 shares of its common stock in return for, among other consideration:
| i. |
RS’s agreement to an assignment (subject to regulatory transfer approval) to the Company of Phase II, should it be granted of the Federal NIH/SBIR awarded Grant #1R44HL129870-01;
|
| ii. |
1,675,000 shares of common stock of Nuviant Medical, Inc., a Nevada corporation;
|
| iii. |
167 shares of common stock of MicroTransponder, Inc., a Delaware corporation; and
|
| iv. |
175 shares of common stock of Emeritus Clinical Solutions, Inc., a Delaware corporation.
|
These transactions were valued based on the value of the contributed assets as the Company’s shares had no ascertainable value as of the date of issuance of the shares. This was in accordance with ASC 845 Non-monetary transactions whereby non-monetary assets acquired in exchange for another non-monetary asset is the fair value of the asset surrendered or received, whichever is more clearly evident. In this case the value of the contributed assets were more ascertainable than the value of the shares issued.
Prior to the contribution William Rosellini was not a related party of the Company, but became a related party on January 2, 2016 through the issuance of the 13,200,000 shares and a controlling interest in the Company.
On January 2, 2016, the Company issued 1,800,000 shares of its common stock to its then Vice President of Clinical Affairs, Dr. Elizabeth Rosellini DDS (the sister of our CEO), in return for 214 shares of common stock of Emeritus Clinical Solutions, Inc., a Delaware corporation, and 60,000 shares of common stock of Nuviant Medical, Inc., a Nevada corporation. In addition, Dr. Rosellini was paid cash compensation of $20,000 during the year ended December 31, 2016. Effective December 31, 2016, Dr. Rosellini resigned as an officer of the Company.
On December 15, 2016, pursuant to the terms of the License Agreement, Mr. Rosellini sold, assigned and transferred any and all of his right, title and interest in and to the License owned by him related to the Siemens Patents to the Company pursuant to the Patent License Asset Purchase Agreement (the “Purchase Agreement”). As consideration for the transfer of the Siemens Patents and the License related thereto, the Company paid to Mr. Rosellini the sum of $140,000 in cash and will issue to Mr. Rosellini 3,050,000 shares of the Company’s restricted common stock valued at $3,050,000. The Company has not yet issued the shares of restricted common stock.
In October 2016, the Company paid $124,870 to Nexeon Medsystems Belgium, SPRL (“NMB”), formerly Rosellini Scientific Benelux, SPRL for research and development services for the Company’s the intravascular drug delivery system technology platform. NMB is a company controlled by our CEO, William Rosellini.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 4 – RELATED PARTY TRANSACTIONS (Continued)
During the year ended December 31, 2016, Rosellini Scientific, LLC, the largest shareholder in the Company, loaned $145,475 to the Company. The loan was non-interest bearing with no set terms of repayment. As of December 31, 2016, the loan was repaid in full in cash.
Mr. Rosellini, the CEO of the Company, is the sole Member and Manager of Rosellini Scientific, LLC.
On April 1, 2016, pursuant to the 2016 Omnibus Incentive Plan, the Company issued non-qualified options to purchase 252,000 shares of common stock, with an exercise price of $1.00 per share, with a term of three years to each of the following: Dr. Mark C. Bates MD, Chief Innovation Officer; Dr. Elizabeth Rosellini DDS, Vice President of Clinical Affairs; and Sheneka Rains, appointed Chief Clinical Engineering Officer, for services rendered to the Company since inception as well as ongoing services to be provided from time to time. The options vest in monthly increments of 7,000, with a three-year term for each option beginning upon each date of vesting. Effective December 31, 2016, Dr. Rosellini resigned as an officer of the Company and the 252,000 options were cancelled.
As part of the Merger Agreement with NXDE, Dr. Mark Bates, M.D, the Company’s Chief Innovation Officer, and Director, received a total of 386,212 shares of the Company’s Common Stock upon conversion of the NXDE preferred shares, and converted $370,000 of debt owed to him by NXDE into 370,000 shares of our common stock and warrants to purchase 370,000 additional shares of common stock at a strike price of $2.00 per share and with a term of 36 months. In addition, Dr. Bates contributed $202,825 of accrued interest on his debt, which has been reflected as additional paid-in capital to the Company during the first quarter of 2016. Dr. Bates, beginning September 1, 2016, will receive a fee of $3,000 paid quarterly to serve as a Director and a fee of $9,000 paid quarterly for research and development consulting services.
On June 1, 2016, pursuant to the 2016 Omnibus Incentive Plan, the Company issued to Dr. Melanie McWade PhD, Vice President of Emerging Therapies, non-qualified stock options to purchase 252,000 shares of common stock, with an exercise price of $1.00 per share, with a term of three years. The options vest in monthly increments of 7,000 with a three-year term for each option beginning upon each date of vesting.
On October 28, 2016, Dr. Michael Rosellini acquired 1,217,000 shares of Common Stock of the Company and 1,217,000 warrants to acquire another 1,217,000 shares of Common Stock of the Company as follows: (i) 617,000 shares of Common Stock and 617,000 warrants to purchase 617,000 shares of Common Stock at a strike price of $2.00 per share with a term of 36 months are held by Dr. Rosellini individually and (ii) 600,000 shares of Common Stock and 600,000 warrants to purchase 600,000 shares of Common Stock at a strike price of $2.00 per share with a term of 36 months are held by the IRA Resources, FBO Randy Michael Rosellini, ROTH IRA. Dr. Rosellini has the sole power to vote and dispose of the shares held by the Roth IRA. Alll of the shares of Common Stock and warrants were purchased from the Company pursuant to the Company’s private placement which closed on December 2, 2016. The warrants are currently exercisable and expire on October 28, 2019. Dr. Rosellini is the father of William Rosellini our Chief Executive Officer.
On August 21, 2016, NRose, LLC entered into a contribution agreement with the Company to acquire 50,000 shares of Common Stock and 50,000 warrants to purchase 50,000 shares of Common Stock at a strike price of $2.00 per share with a term of 36 months. Nancy Rosellini has the sole power to vote and dispose of the shares/warrants held by the NRose, LLC. The shares of Common Stock and the warrants were purchased from the Company pursuant to the Company’s private placement which closed on December 2, 2016. The warrants are currently exercisable and expire on August, 21, 2019. Nancy Rosellini is the mother of William Rosellini, our Chief Executive Officer.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 4 – RELATED PARTY TRANSACTIONS (Continued)
Effective December 1, 2016, Brian Blischak, our President and Chief Commercial Officer, pursuant to the 2016 Omnibus Incentive Plan was granted an initial grant of 1,150,000 non-transferable stock options to purchase shares of the Company’s Common Stock, consisting of 500,000 incentive stock options (“ISO”), and 650,000 non-qualified stock options (“NQSO”). With respect to the ISO options, 100,000 ISO options vested on the Effective Date, and 100,000 ISO options vested on January 1, 2017. Additional lots of 100,000 ISO options each shall vest on January 1, 2018, 2019 and 2020. With respect to the NQSO options, 38,000 NQSO options vested on the Effective Date, and 17,000 NQSO options vested on January 1, 2017. An additional 17,000 options shall vest on the first day of each month thereafter until all NQSO options are fully vested on December 1, 2019. As of March 28, 2017, a total of 289,000 ISO and NQSO options were vested but none exercised. The exercise price of all options is $1.00 per share and the options shall expire in eight years from the date of vesting.
NOTE 5 – INVESTMENTS
As of December 31, 2016 investments consists of the following, all of which are being accounted for under the cost method. See Note 2 – Summary of Significant Accounting Policies.
|
Name of Investment
|
Shares
|
Book Value
|
||||||
|
|
||||||||
|
Nuviant Medical Inc.
|
1,735,000
|
$
|
173,500
|
|||||
|
MicroTransponder Inc
|
167
|
69,472
|
||||||
|
Emeritus Clinical Solutions, Inc.
|
389
|
79,388
|
||||||
|
Total Investments
|
322,360
|
|||||||
|
|
||||||||
|
Less Impairment
|
(173,500
|
)
|
||||||
|
|
||||||||
|
Net Investments
|
$
|
148,860
|
||||||
The Company has recorded impairment of $173,500 on its investment in Nuviant Medical Inc., due to a significant change to Nuviant Medical Inc.’s financial condition.
NOTE 6 – NOTES PAYABLE
During the year ended December 31, 2015, the Company did not have any notes payable.
The Company's Executive Vice President of Finance loaned $415 to the Company. The loan is non-interest bearing with no set terms of repayment. The loan is outstanding as of December 31, 2016.
During the year ended December 31, 2016, the Company incurred the following notes payable:
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6 – NOTES PAYABLE (Continued)
During the 12 months ended December 31, 2016, the Company held notes payable with two stockholders in the amount of $10,000 each, which bear interest at the rate of 12% per annum and mature March 31, 2018. One note payable with a stockholder in the amount of $10,000 was paid in full along with $1,200 in accrued interest during the year ended December 31, 2016.
The Company has a revolving credit card with BB&T Financial with an outstanding balance of $14,633 as of December 31, 2016 with a credit limit of $60,000 and a current APR of 25.4%. And a revolving credit card with Comerica Bank with an outstanding balance of $180 as of December 31, 2016 with a credit limit of $11,000 with a current APR of 0%.
NOTE 7 – COMMITMENTS AND CONTINGENCIES
The Company is subject to a patent royalty agreement that requires 3% of Net Product Sales received from commercialization of the patents or other intellectual property acquired in the Merger with NXDE. To be paid to NXDE, LLC. No sales have been generated from any of the acquired patents or intellectual property.
The Company acquired a non-exclusive license to a portfolio of 86 patents and is subject to a 6% royalty to Magnus IP GmbH of the Net Sales of all licensed products sold, licensed, leased or otherwise disposed pursuant to the license. No sales have been generated from the licensed intellectual property.
NOTE 8 – INCOME TAXES
The reconciliation of income tax benefit at the U.S. statutory rate of 34% for the years ended December 31, 2016 and 2015 to the Company’s effective tax rate is as follows:
|
Years Ended
|
||||||||
|
|
December 31,
2016
|
December 31,
2015
|
||||||
|
|
||||||||
|
Statutory federal income tax rate
|
-34
|
%
|
-34
|
%
|
||||
|
State income tax, net of federal benefits
|
-6
|
%
|
-6
|
%
|
||||
|
Valuation allowance
|
40
|
%
|
40
|
%
|
||||
|
Income tax provision (benefit)
|
0
|
%
|
0
|
%
|
||||
The benefit for income tax is summarized as follows:
|
Years Ended
|
||||||||
|
December 31,
2016
|
December31,
2015
|
|||||||
|
Federal
|
||||||||
|
Current
|
$
|
541,000
|
$
|
300
|
||||
|
Deferred
|
—
|
—
|
||||||
|
State
|
||||||||
|
Current
|
95,000
|
50
|
||||||
|
Deferred
|
—
|
—
|
||||||
|
Change in valuation allowance
|
(636,000
|
)
|
(350
|
)
|
||||
|
Income tax provision (benefit)
|
$
|
—
|
$
|
—
|
||||
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 8 – INCOME TAXES (Continued)
The tax effects of temporary differences that give rise to the Company’s net deferred tax liability as of December 31, 2015 and 2014 are as follows:
|
Years Ended
|
||||||||
|
|
December 31,
2016
|
December 31,
2015
|
||||||
|
|
||||||||
|
Deferred tax asset
|
||||||||
|
Net operating loss carryforwards – tax effect
|
$
|
636,350
|
$
|
350
|
||||
|
Valuation allowance
|
(636,350
|
)
|
(350
|
)
|
||||
|
Deferred tax asset, net of allowance
|
$
|
—
|
$
|
—
|
||||
As of December 31, 2016 and 2015, the Company had $1,592,338 of Federal and State net operating loss carryovers (“NOLs”) which begin to expire in 2035. Utilization of the NOLs may be subject to limitation under the Internal Revenue Code Section 382 should there be a greater than 50% ownership change as determined under regulations.
In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. Based on the assessment, management has established a full valuation allowance against the entire deferred tax asset relating to NOLs for every period because it is more likely than not that all of the deferred tax asset will not be realized.
The Company files U.S. Federal and Kentucky tax returns that are subject to audit by tax authorities beginning with the year ended December 31, 2015. The Company’s policy is to classify assessments, if any, for tax and related interest and penalties as tax expense.
NOTE 9 – 2016 OMNIBUS INCENTIVE PLAN
The Company may, from time to time, issue certain equity awards pursuant to our 2016 Omnibus Incentive Plan (the "Plan''). The Plan was adopted by our Board of Directors on January 2, 2016 and was subsequently approved by our shareholders. As of December 31, 2016, options to purchase a total of 2,332,000 shares of the Company's common stock were issued under the Plan, all with an exercise price of $1.00 per share. 138,000 vested immediately upon grant and the remaining vest in varying amounts ranging from 100,000 annually to monthly increments ranging from 3,333 to 17,000 based on individual stock option agreements. Each option has a three-year term starting on each date of vesting.
The Plan will be administered by a Committee of two or more non-employee Directors designated by the Board once outside Directors have been elected to the Board. In the interim the Board shall perform the requisite duties of the Committee with respect to awards made. The Committee currently determines to whom awards are made, the timing of any such awards, the type of securities, and number of shares covered by each award, as well as the terms, conditions, performance criteria, restrictions and other provisions of awards. The Committee has the authority to cancel or suspend awards, accelerate the vesting or extending the exercise period of any awards made pursuant to the Plan.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 9 – 2016 OMNIBUS INCENTIVE PLAN (Continued)
Shares Available under the 2016 Omnibus Incentive Plan
The maximum shares available for issuance under the Plan are 5,000,000, subject to adjustment as set forth in the Plan. Any shares subject to an award that expires, is cancelled or forfeited or is settled for cash shall, to the extent of such cancelation, forfeiture, expiration or cash settlement, again become available for awards under the Plan. The Committee can issue awards comprised of restricted stock, stock options, stock appreciation rights, stock units and other awards, as set forth in the Plan.
Transferability
Except as otherwise provided in the Plan, (i) during the lifetime of a Participant, only the Participant or the Participant’s guardian or legal representative may exercise an option or stock appreciation right, or receive payment with respect to any other award and (ii) no award may be sold, assigned, transferred, exchanged or encumbered, voluntarily or involuntarily, other than by will or the laws of descent and distribution.
Change in Control
In the event of a merger, the surviving or successor entity (or its parent) may continue, assume or replace outstanding awards as of the date of the relevant transaction and such awards or replacements therefore shall remain outstanding and be governed by their respective terms. Such awards or replacements can be executed in part on the condition that the contractual obligations represented by the award are expressly assumed by the surviving or successor entity (or its parent) with appropriate adjustments to the number and type of securities subject to the award and the exercise price thereof so as to preserve the intrinsic value of the award existing at the time of the relevant transaction. Alternatively, the surviving or successor entity (or its parent) could issue to a Participant a comparable equity-based award that preserves the intrinsic value of the original award existing at the time of the relevant transaction and contains terms and conditions that are substantially similar to those of the award.
If and to the extent that outstanding awards under the Plan are not continued, assumed or replaced in connection with a merger or relevant corporate transaction, then all outstanding awards shall become fully vested and exercisable for such period of time prior to the effective date of the relevant transaction as is deemed fair and equitable by the Committee and shall terminate at the effective date of said transaction.
NOTE 10 –EQUITY
Common and Preferred Stock
The Company’s Articles of Incorporation authorize 75,000,000 shares of Common Stock, $0.001 par value, and 25,000,000 shares of Preferred Stock, $0.001 par value. The Articles of Incorporation were amended on February 22, 2016 to cancel the authorization of Preferred Stock.
Common Stock Issuances
On December 15, 2015, the Company issued 500,000 shares of its common stock to its Chief Operating Officer for the sum of $500 at the par value of $0.001. 212,000 shares of the 500,000 shares became vested upon issue and the remaining 288,000 shares shall vest over a 36 month period at the rate of 8,000 shares per month. Vesting began on January 1, 2016. No other shares were issued during the year ended December 31, 2015.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 – EQUITY (Continued)
During the year ended December 31, 2016, the Company:
| (i) |
Issued to its Interim Chief Financial Officer 252,000 shares of restricted Common Stock of the Company for certain accounting and budget-related services rendered as well as serving as Interim CFO until such time as a permanent CFO was hired. As of December 1, 2016 148,000 shares were vested and the remaining 104,000 shares vest at the rate of 8,000 shares per month over a term of 13 months from December 1, 2016. The shares were valued at $252.
|
| (ii) |
Issued to Rosellini Scientific, LLC 13,200,000 shares of Common Stock in exchange for 1,675,000 shares of common stock in Nuviant Medical Inc., a Nevada corporation, 175 shares of common stock in Emeritus Clinical Solutions, Inc., a Delaware corporation, 167 shares of common stock in MicroTransponder, Inc., a Delaware corporation, the assignment of one Federal NIH Grant in the amount of $218,377 and one State of Kentucky Matching Funds Grant in the amount of $150,000. Mr. Rosellini, the CEO of the Company, is the sole Member and Manager of Rosellini Scientific, LLC. The shares had a fair value of $272,686 on the date of issuance and were valued based on the value of the contributed assets as the Company’s shares had no ascertainable value as of the date of issuance of the shares. This was in accordance with ASC 845, Nonmonetary Transactions, whereby nonmonetary assets acquired in exchange for another nonmonetary asset is the fair value of the asset surrendered or received, whichever is more clearly evident. In this case the value of the contributed assets was more ascertainable than the value of the shares issued.
|
On December 15, 2016, pursuant to the terms of the License Agreement, Mr. Rosellini sold, assigned and transferred any and all of his right, title and interest in and to the License owned by him related to the Siemens Patents to the Company pursuant to the Patent License Asset Purchase Agreement (the “Purchase Agreement”). As consideration for the transfer of the Siemens Patents and the License related thereto, the Company paid to Mr. Rosellini the sum of $140,000 in cash and will issue to Mr. Rosellini 3,050,000 shares of the Company’s restricted common stock valued at $3,050,000. The Company has not yet issued the shares of restricted common stock.
| (iii) |
Issued 1,800,000 shares of restricted common stock to its Vice President of Clinical Affairs in return for 214 shares of common stock of Emeritus Clinical Solutions, Inc., a Delaware corporation, and 60,000 shares of common stock of Nuviant Medical, Inc., a Nevada corporation. The shares had a fair value of $49,673 on the date of issuance and were valued based on the value of the contributed assets as the Company’s shares had no ascertainable value as of the date of issuance of the shares. This was in accordance with ASC 845, Nonmonetary Transactions, whereby nonmonetary assets acquired in exchange for another nonmonetary asset is the fair value of the asset surrendered or received, whichever is more clearly evident. In this case the value of the contributed assets was more ascertainable than the value of the shares issued.
|
| (iv) |
Issued 1,659,943 shares of common stock to the stockholders of NXDE, pursuant to the terms of the Merger Agreement (see Note 1 Business – Acquisition), in exchange for 10,222,137 shares of Series A Preferred Stock and 832,034 shares of Series B Preferred Stock, or 100% of the issued and outstanding preferred stock of NXDE. The shares had a fair value of $4,505,486 on the date of issuance.
|
| (v) |
Pursuant to the Merger Agreement and subsequent to the Merger, the Company exchanged $950,000 in NXDE stockholder loans and $337,564 in accrued interest related to those loans for 1,287,564 Units in the Private Placement at $1.00 per Unit, each Unit consisting of one share of restricted common stock and one warrant to purchase one additional share of restricted common stock. The warrants have an exercise price of $2.00 per share and expire 36 months from the date of issue. For information regarding the Private Placement, see Note 10 - Equity – Warrants, below. The shares of Common Stock issued as part of the Units had a fair value of $1,287,564 on the date of issuance.
|
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 – EQUITY (Continued)
| (vi) |
As of December 31, 2016, $2,860,946 in Units were issued for cash in the Private Placement, resulting in 2,840,946 shares of restricted Common Stock being issued and 20,000 shares not yet issued.
|
| (vii) |
As of December 31, 2016, 146,500 shares of restricted Common Stock of the Company have been issued for certain legal services rendered to third-party consultants. The shares were valued at $146,500.
|
| (viii) |
As of December 31, 2016, 25,000 shares of restricted Common Stock of the Company have been issued for certain research and development consulting services rendered to third-party consultants. The shares were valued at $25,000.
|
Option Grants
The Company may, from time to time, issue certain equity awards pursuant to our 2016 Omnibus Incentive Plan (the “2016 Plan”). The 2016 Plan was adopted by our Board of Directors on January 2, 2016 and was subsequently approved by our shareholders on January 2, 2016. The Company reserved 5,000,000 shares of common stock for issuance pursuant option grants under the 2016 Plan. During the twelve months ended December 31, 2016, the Company issued stock options to purchase a total of 2,332,000 shares of the Company’s common stock under the 2016 Plan, all with an exercise price of $1.00 per share, as follows:
| (i) |
Granted to the Company’s Chief Innovation Officer 252,000 three-year nonqualified options to purchase 252,000 shares of common stock, with an exercise price of $1.00 per share. The options vest in monthly increments of 7,000 shares, with the three-year term for each option beginning upon each date of vesting. The fair value of the options was determined to be $47,056 using the Black-Scholes Option Pricing Model.
|
| (ii) |
Granted to the Company’s Vice President Clinical Affairs 252,000 three-year nonqualified options to purchase 252,000 shares of common stock, with an exercise price of $1.00 per share. The options vest in monthly increments of 7,000 shares, with the three-year term for each option beginning upon each date of vesting. The fair value of the options was determined to be $47,056 using the Black-Scholes Option Pricing Model. The Vice President of Clinical Affairs resigned effective December 31, 2016 and all options granted to such officer were cancelled.
|
| (iii) |
Granted to Chief Clinical Engineering Officer 252,000 three-year nonqualified options to purchase 252,000 shares of common stock, with an exercise price of $1.00 per share. The options vest in monthly increments of 7,000 shares, with the three-year term for each option beginning upon each date of vesting. The fair value of the options was determined to be $47,056 using the Black-Scholes Option Pricing Model.
|
| (iv) |
Granted to the Vice President of Emerging Therapies 252,000 three-year nonqualified options to purchase 252,000 shares of common stock, with an exercise price of $1.00 per share. The options vest in monthly increments of 7,000 shares, with the three-year term for each option beginning upon each date of vesting. The fair value of the options was determined to be $42,113 using the Black-Scholes Option Pricing Model.
|
| (v) |
Granted to the Chief Financial Officer 306,000 three-year incentive stock options to purchase 306,000 shares of common stock, with an exercise price of $1.00 per share. The options vest in monthly increments of 8,500 shares, with the three-year term for each option beginning upon each date of vesting. The fair value of the options was determined to be $66,233 using the Black-Scholes Option Pricing Model.
|
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 – EQUITY (Continued)
| (vi) |
Granted 1,150,000 stock options to purchase shares of the Company’s Common Stock, consisting of 500,000 incentive stock options (“ISO”), and 650,000 non-qualified stock options (“NQSO”). With respect to the ISO options, 100,000 ISO options vested on the Effective Date, and 100,000 ISO options vested on January 1, 2017. Additional lots of 100,000 ISO options each shall vest on January 1, 2018, 2019 and 2020. With respect to the NQSO options, 38,000 NQSO options vested on the Effective Date, and 17,000 NQSO options vested on January 1, 2017. An additional 17,000 options shall vest on the first day of each month thereafter until all NQSO options are fully vested on December 1, 2019. The exercise price of all options is $1.00 per share and the options shall expire in eight years from the date of vesting. The fair value of the options was determined to be $365,343 using the Black-Scholes Option Pricing Model.
|
| (vii) |
Granted to the Director of Clinical Research 120,000 three-year incentive stock options to purchase 120,000 shares of common stock, with an exercise price of $1.00 per share. The options vest in monthly increments of 3,333 shares, with the three-year term for each option beginning upon each date of vesting. The fair value of the options was determined to be $25,974 using the Black-Scholes Option Pricing Model.
|
The options were valued at $593,775 using the Black-Scholes option pricing model with the following assumptions:
|
Risk-free interest rate
|
1.68%
|
||
|
Expected life
|
6.73 years
|
||
|
Expected dividends
|
0.00%
|
||
|
Expected volatility
|
42.88.%
|
||
|
Fair value of Company's common stock
|
$
|
1.00
|
|
Aggregate options expense recognized for the year ended December 31, 2016 was $82,284.
As of December 31, 2016, there were 2,668,000 shares available for grant under the 2016 Plan.
Warrants
On February 1, 2016, the Company initiated a private placement for the sale of up to 5,500,000 units (the “Units”) at $1.00 per Unit (the “Private Placement”). Each Unit consists of one share of restricted common stock and one warrant to purchase one additional share of restricted common stock. The Private Placement was closed on December 2, 2016. The warrants have an exercise price of $2.00 per share and expire 36 months from the date of issue. The warrants have limited transferability to an affiliate of the holder only, cannot be sold as a warrant and do not contain cashless exercise provisions. The warrants do not include any terms or contracts to issue additional shares and have no liquidation preferences or participation rights.
During the year ended December 31, 2016, the Company issued a total of 4,128,510 warrants to purchase shares of the Company’s common stock pursuant to the Private Placement, as follows:
| (i) |
Pursuant to the Merger Agreement, the Company exchanged $645,000 in NXDE stockholder loans and $176,482 in accrued interest related to those loans for 821,482 Units in the Private Placement at $1.00 per Unit, each Unit consisting of one share of restricted common stock and one warrant to purchase one additional share of restricted common stock, resulting in 821,482 warrants being issued.
|
| (ii) |
Subsequent to the Merger the Company exchanged $305,000 in NXDE stockholder loans and $161,082 in accrued interest related to those loans for 466,082 Units in the Private Placement at $1.00 per Unit, each Unit consisting of one share of restricted common stock and one warrant to purchase one additional share of restricted common stock, resulting in 466,082 warrants being issued.
|
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 – EQUITY (Continued)
| (iii) |
As of December 31, 2016, $2,860,946 in Units have been subscribed in the Private Placement associated with cash subscriptions, resulting in 2,840,946 warrants being issued and 20,000 warrants not yet issued.
|
As of December 31, 2016, no warrants have been exercised.
As of December 31, 2016, a total of 4,128,510 shares of Common Stock of the Company have been reserved for issuance upon exercise of the warrants.
Stock option activity, both within and outside the Plan, and warrant activity for the year ended December 31, 2016, are as follows:
|
|
Stock Options
|
Stock Warrants
|
||||||||||||||
|
|
Weighted
|
Weighted
|
||||||||||||||
|
|
Average
|
Exercise
|
||||||||||||||
|
|
Shares
|
Price
|
Shares
|
Price
|
||||||||||||
|
|
||||||||||||||||
|
Outstanding at January 2, 2016
|
—
|
$
|
—
|
—
|
$
|
—
|
||||||||||
|
Granted
|
2,584,000
|
1.00
|
4,128,510
|
2.00
|
||||||||||||
|
Canceled
|
252,000
|
1.00
|
—
|
—
|
||||||||||||
|
Expired
|
—
|
—
|
—
|
—
|
||||||||||||
|
Exercised
|
—
|
—
|
—
|
—
|
||||||||||||
|
Outstanding at December 31, 2016
|
2,332,000
|
$
|
1.00
|
4,128,510
|
$
|
2.00
|
||||||||||
|
Exercisable at December 31, 2016
|
292,000
|
$
|
1.00
|
4,128,510
|
$
|
2.00
|
||||||||||
The range of exercise prices and remaining weighted average life of the options outstanding at December 31, 2016 were $1.00 to $1.00 and 3.88 to 9.45 years, respectively. The aggregate intrinsic value of the outstanding options at December 31, 2016 was $593,775.
The range of exercise prices and remaining weighted average life of the warrants outstanding at December 31, 2016 were $2.00 to $2.00 and 2.11 to 2.86 years, respectively. The aggregate intrinsic value of the outstanding warrants at December 31, 2016 was $262,590.
NOTE 11 – SUBSEQUENT EVENTS
The Company has evaluated subsequent events occurring through the date that the financial statements were issued.
Subsequent to December 31, 2016, 175,836 shares of restricted Common Stock of the Company have been issued for certain legal and corporate consulting services rendered to third-party consultants. These shares were valued at $175,836.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 11 – SUBSEQUENT EVENTS (Continued)
January 1, 2017 Appointment of Director of Emerging Therapy
On January 1, 2017, the Board of Directors of the Company, appointed Emily Hamilton, MD to serve as the Director of Emerging Therapy of the Company.
Dr. Hamilton was the President of Rosellini Scientific LLC from 2010 to 2012 where she provided leadership to position the company at the forefront of the biomedical engineering industry. At Rosellini Scientific she helped to develop a strategic plan to advance the company's scientific mission and objectives and to promote revenue, profitability and growth as an organization. She also oversaw the company operations to ensure production efficiency, quality, service, and cost-effective management of clinical and pre-clinical research. Dr. Hamilton graduated from Oklahoma State University with a degree in Physiology. She then attended the University of Texas Medical School at Houston ('06) and went on to complete an Anesthesiology Residency. She is board certified in Anesthesiology in Texas and for the last seven years has been affiliated with Baylor Scott & White Medical Center Plano, Medical City Dallas Hospital, Texas Health Presbyterian Hospital and Texas Health Presbyterian Hospital Dallas.
Dr. Hamilton is the wife of our CEO, William Rosellini.
January 6, 2017 Stock Exchange Agreement
On January 6, 2017, (the "Effective Date), The Company and Rosellini Scientific, LLC, a Texas limited liability company ("RS"), entered into a Stock Exchange Agreement (the “Agreement”). Subject to the terms and conditions set forth the Agreement, on the Effective Date, RS sold, transferred, and assigned to Nexeon all of its right, title and interest in and to 100 shares of common stock of MicroTransponder Inc., a Delaware corporation (the "MTI Shares") in exchange for 389 shares of common stock of Emeritus Clinical Solutions, Inc. (formerly Telemend, Inc.), a Texas corporation, owned by Nexeon and Nexeon sold, transferred and assigned to RS 389 shares of common stock of Emeritus Clinical Solutions, Inc. (the “Emeritus Shares”) in exchange for the 100 MTI Shares (the MTI Shares and Emeritus Shares are collective referred to as the “Exchange Shares”).
January 10, 2017 Acquisition Agreement
On January 10, 2017, Rosellini Scientific, LLC, (“RS”) and Nexeon Medsystems Europe, S.a.r.l., a Luxembourg private limited liability company (hereinafter referred to as “Nexeon Europe”), which is a wholly-owned subsidiary Company, and in the presence of Nexeon Medsystems Belgium, SPRL, a company incorporated under the laws of Belgium, (hereinafter referred to as “NMB”), entered into an Acquisition Agreement. RS is the sole shareholder of NMB owning 107,154 shares (the “Shares”).
Pursuant to the Acquisition Agreement, RS is granting to Nexeon Europe the exclusive and irrevocable right to purchase the Shares upon the terms and conditions set forth in the Acquisition Agreement (the “Right to Purchase”). The consideration for the Right to Purchase is US $1,000 (the “Acquisition Price”). Nexeon Europe shall have the right to exercise the Right to Purchase commencing from the date of the Acquisition Agreement and terminating on December 31, 2017 (the “Acquisition Period”). In the event Nexeon Europe exercises the Right to Purchase, the Agreement shall be automatically deemed converted into and considered a share transfer agreement for the purchase of the Shares and the Acquisition Price shall be considered the Purchase Price of the Shares and shall be deemed to have been satisfied by Nexeon Europe to RS as of the date of the Acquisition Agreement. If Nexeon Europe elects not to exercise the Right to Purchase on or before December 31, 2017, then the Acquisition Agreement shall become null and void and of no further force and effect.
Pursuant to the terms of the Acquisition Agreement, closing of the transaction is conditioned upon the delivery to Nexeon Europe of a two year audit for years ending December 31, 2015 and 2016 of NMB, to be completed by April 15, 2017. RS shall be solely responsible and liable for any and all fees, costs and expenses associated with such audit.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 11 – SUBSEQUENT EVENTS (Continued)
Description of Nexeon Medsystems Belgium, SPRL
Nexeon Medsystems Belgium, SPRL, formerly known as Rosellini Scientific Benelux, is wholly-owned subsidiary of RS and is a medical device manufacturing company. NMB was originally formed in 2013 and is located in Liege, Belgium. NMB has previously received a number of subsidies from the government of the Walloon region in Belgium to develop active implantable medical devices. In addition, NMB has acquired assets related to an implantable neurostimulation device system, the Synapse™, for use in the treatment of neurological diseases. The Synapse™ was previously issued a CE Mark for use in the treatment of certain movement disorders associated with Parkinson's disease. It also is being manufactured for a number of commercial partners, including Galvani Bioelectronics and John Hopkin's University, for use in their various research projects.
Description of Nexeon Europe
Nexeon Europe is a wholly owned subsidiary of the Company formed on October 28, 2016. The Company and Nexeon Europe are part of the Nexeon group of companies (the “Group”) that is currently being restructured in order to achieve a more efficient and cost-effective Group structure.
Loan Agreement and Promissory Note
In connection with the Acquisition Agreement described above and based on the contemplation that Nexeon Europe shall acquire all of the shares of NMB, Nexeon Europe (Lender) and NMB (Borrower) entered into a Loan Agreement and related Promissory Note pursuant to which Nexeon Europe agrees to make a loan to NMB in the aggregate principal amount of EUR 1,000,000 (One Million Euros) (the “Loan”). The Loan shall mature on the first Business Day falling one (1) year from the date of the Loan Agreement (the “Maturity Date”). The Maturity Date shall be extended and the term of the Loan Agreement automatically renewed for successive thirty (30) day periods unless the NMB notifies the Nexeon Europe within ten (10) days of the then upcoming Maturity Date that it intends to repay the full amount or the then outstanding amount of the Loan prior to the upcoming Maturity Date. NMB may repay, either partially or entirely, the Loan and/or accrued interest thereon at any time prior to the Maturity Date without penalty, upon giving at least two (2) Business Days’ prior written notice to Nexeon Europe. NMB and Nexeon Europe may also agree to settle the Loan through the inter-company account netting procedure or to capitalize as an investment in the subsidiary.
The Loan shall bear interest at the rate of 5% per annum. Accrued interest on the unpaid principal amount of the Loan shall be payable on the Maturity Date. Accrued interest on any partial repayment of the Loan shall be payable on the earlier of the date of repayment or the first business day of the month following partial repayment.
As of March 28, 2017, $592,059 has been loaned to NMB pursuant to the terms of the Loan Agreement and Promissory Note.
Security Agreement
As collateral security for the payment in full when due (whether at stated maturity, by acceleration or otherwise) of the Loan, NMB pledged and granted to Nexeon Europe a security interest in all of NMB’s right, title and interest in, to and under all of its properties, including but not limited to personal and real property, in each case whether tangible or intangible.
NEXEON MEDSYSTEMS INC
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 11 – SUBSEQUENT EVENTS (Continued)
Services Agreements for Common Stock
On December 9, 2016, Nexeon Medsystems Puerto Rico, a wholly owned subsidiary of the Company, entered into a services agreement with Adaptive Business Solutions, LLC to provided corporate structuring consulting services in exchange for $60,000 in restricted shares of the Company’s common stock. No stock was issued at the time the agreement was entered into in 2016. In January 2017, 38,336 shares of the Company’s common stock were issued in exchange for services provided.
On February 14, 2017, the Company entered into a services agreement with ACORN Management Partners, LLC to provide strategic business outreach and strategic relations services in exchange for a monthly fee of $7,500 and $125,000 in restricted shares of the Company’s common stock. The Company has made cash payments of $7,500 and issued 125,000 shares of the Company’s commons stock in February 2017. The initial term of the agreement is six months.
On February 23, 2017, the Company entered into a services agreement with Sichenzia Ross Ference Kesner, LLP to provide drafting and filing of a registration statement on Form S-1for a flat rate of $40,000. The fee shall be paid $20,000 in cash and $20,000 in restricted shares of the Company’s common stock. The Company has issued 12,500 shares of the Company’s commons stock and made cash payments of $12,500 in February 2017.

6,052,240 Shares of Common Stock
PROSPECTUS
______________, 2017
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth all expenses to be paid by the Registrant, other than estimated underwriting discounts and commissions. All amounts shown are estimates except for the SEC registration fee:
|
SEC registration fee
|
$
|
701.45
|
||
|
Legal fees and expenses
|
40,000
|
|||
|
Accounting fees and expenses
|
*
|
|||
|
Transfer agent and registrar fees
|
*
|
|||
|
Printing and engraving expenses
|
*
|
|||
|
Miscellaneous fees and expenses
|
*
|
|||
|
Total
|
$
|
*
|
_________________
* To be filed by amendment.
Item 14. Indemnification of Directors and Officers
Our Officers and Directors are indemnified as provided by the Nevada Revised Statutes (“NRS”) and our Bylaws. Under the NRS, unless modified by a corporation's Articles of Incorporation, a director is not liable to a corporation, its stockholders, or creditors for damages unless the director's action or failure constituted a breach of fiduciary duty and such breach involved intentional misconduct, fraud, or a knowing violation of law. Our Bylaws provide that we will indemnify our directors and officers to the fullest extent permissible under Nevada law if such person acted in good faith and in a manner which such person reasonably believed to be in, or not opposed to, the best interests of the Company and, with respect to any criminal action, had no reasonable cause to believe such conduct was unlawful.
The Company may purchase and maintain directors’ and officers’ liability insurance or make other financial arrangements on behalf of any individual entitled to indemnity. Our Bylaws also provide that we will advance all expenses incurred to any person entitled to indemnity upon receipt of an undertaking by, or on behalf of, such person to repay said amounts should it be ultimately determined that the person was not entitled to indemnification.
Item 15. Recent Sales of Unregistered Securities
Set forth below is an enumeration of all securities issued by the Company since December 7, 2015 (inception) that have not been registered under the Securities Act.
As a result of the Merger, 100% of NXDE’s issued and outstanding shares of preferred stock were converted into an aggregate of 1,659,943 shares of the Company’s Common Stock.
As part of the Merger, Dr. Mark Bates, our Chief Innovation Officer and director, received an aggregate of 386,212 shares of the Company’s Common Stock upon conversion of the NXDE preferred shares, and converted $370,000 of debt owed to him by NXDE into 370,000 shares of the Company’s Common Stock and warrants to purchase up to 370,000 shares of Common Stock at an exercise price of $2.00 per share. The issuance of these securities was deemed to be exempt from the registration requirements of the Securities Act of 1933, as amended (the “Securities Act”), by virtue of Section 4(2) thereof and/or Rule 506 of Regulation D thereunder, as a transaction by an issuer not involving a public offering.
As part of the Merger, Mr. Ralph Ballard, co-founder and director of NXDE, received an aggregate of 123,759 shares of the Company’s Common Stock upon conversion of the NXDE preferred shares as follows: 1,398 shares of Common Stock were issued to Mr. Ballard, 7,691 shares of Common Stock were issued to a Custodial IRA FBO Ralph Ballard and 114,670 shares of Common Stock were issued to Ballard Investments. In addition, Mr. Ballard converted $451,482 of debt owed to him by NXDE into 451,482 shares of the Company’s Common Stock and warrants to purchase up to 451,482 shares of Common Stock at an exercise price of $2.00 per share with a term of 36 months, which shares and warrants were issued to Ballard Investments. In addition, three trusts representing three of Mr. Ballard’s children converted an aggregate of $431,821 of debt into 431,821 shares of the Company’s Common Stock and warrants to purchase up to 431,821 shares of Common Stock at an exercise price of $2.00 per share with a term of 36 months, divided between and issued to the three trusts. Mr. Ballard disclaims any beneficial ownership in the Common Stock and warrants issued to the three trusts. The issuance of these securities was deemed to be exempt from the registration requirements of the Securities Act by virtue of Section 4(2) thereof and/or Rule 506 of Regulation D thereunder, as a transaction by an issuer not involving a public offering.
Subsequent to the Merger, three additional NXDE shareholders converted an aggregate of $34,261 in debt for 34,261 shares of the Company’s Common Stock and warrants to purchase up to 34,261 shares of Common Stock at an exercise price of $2.00 per share with a term of 36 months. The issuance of these securities was deemed to be exempt from the registration requirements of the Securities Act by virtue of Section 4(2) thereof and/or Rule 506 of Regulation D thereunder, as a transaction by an issuer not involving a public offering.
Subsequent to the Merger, Rosellini Scientific exchanged a $175,000 promissory note with a term of five years bearing an annual interest rate of 8%, payable to Rosellini Scientific by Emeritus in exchange for 175,000 shares of the Company’s Common Stock and three year warrants to purchase up to 175,000 shares of Common Stock with an exercise price of $2.00 per share. The issuance of these securities was deemed to be exempt from the registration requirements of the Securities by virtue of Section 4(2) thereof and/or Rule 506 of Regulation D thereunder, as a transaction by an issuer not involving a public offering. This transaction was rescinded as of June 30, 2016 and the shares were returned.
On December 15, 2015, the Company issued 500,000 shares of its Common Stock to Ronald Conquest, the Executive Vice President of Finance and a director for the sum of $500. Of the 500,000 shares, 212,000 vested upon issuance and the balance shall vest over a 36 month period commencing on January 1, 2016. The issuance of these securities was deemed to be exempt from the registration requirements of the Securities Act by virtue of Section 4(2) thereof and/or Rule 506 of Regulation D thereunder, as a transaction by an issuer not involving a public offering.
On January 1, 2016, the Company issued 252,000 shares of Common Stock to Christopher Miller, the Company’s Chief Financial Officer, for certain accounting and budget-related services rendered as well as serving as Interim CFO until such time as a permanent CFO was hired. The shares vest over a 36 month period. The issuance of these securities was deemed to be exempt from the registration requirements of the Securities Act by virtue of Section 4(2) thereof, as a transaction by an issuer not involving a public offering.
On January 2, 2016, the Company issued 1,800,000 shares of Common Stock to its then Vice President of Clinical Affairs, Dr. Elizabeth Rosellini in exchange for 214 shares of common stock of Emeritus and 60,000 shares of common stock of Nuviant. The issuance of these securities was deemed to be exempt from the registration requirements of the Securities Act, by virtue of Section 4(2) thereof and/or Rule 506 of Regulation D thereunder, as a transaction by an issuer not involving a public offering.
On January 2, 2016, the Company entered into a contribution agreement with Rosellini Scientific, a company controlled by our Chief Executive Officer, William Rosellini and its wholly-owned subsidiary, Belltower Associates, LLC. Pursuant to the contribution agreement, the Company issued 13,200,000 shares of Common Stock in return for, among other consideration:
| · |
RSBA’s assignment (subject to regulatory transfer approval) to the Company of Phase II, should it be granted, of the federal NIH/SBIR awarded Grant #1R44HL129870-01;
|
| · |
1,675,000 shares of common stock of Nuviant;
|
| · |
167 shares of common stock of MicroTransponder, Inc., a Delaware corporation; and
|
| · |
175 shares of common stock of Emeritus.
|
The issuance of these securities was deemed to be exempt from the registration requirements of the Securities Act by virtue of Section 4(2) thereof and/or Rule 506 of Regulation D thereunder, as a transaction by an issuer not involving a public offering.
Between March 2016 and November 21, 2016, the Company issued 2,311,946 shares of Common Stock and three year warrants to purchase up to 2,311,946 shares of Common Stock with an exercise price of $2.00 per share for proceeds of $2,311,946. The issuance of these securities was deemed to be exempt from the registration requirements of the Securities Act by virtue of Section 4(2) thereof and/or Rule 506 of Regulation D thereunder, as a transaction by an issuer not involving a public offering.
On December 2, 2016, pursuant to the December Private Placement, the Company received $2,860,946 in net cash proceeds from the issuance of 2,840,946 units. Each Unit consisted of one share of Common Stock and one warrant to purchase one additional share of Common Stock. The issuance of these securities was deemed to be exempt from the registration requirements of the Securities Act by virtue of Section 4(2) thereof and/or Rule 506 of Regulation D thereunder, as a transaction by an issuer not involving a public offering.
On March 21, 2017 the Company offered to current warrant holders who participated in the December Private Placement, the opportunity to convert their warrants into Common Stock of the Company. The offer terms included the exercise of 17 warrants for 17 shares of the Company’s Common Stock at an exercise price of $0.01 per share for every 100 warrants owned. The remaining 83 warrants per 100 warrants owned would be cancelled. The offer was on an all-or-nothing basis to convert all warrants held by each warrant holder.
As of April 27, 2017 593,598 warrants have been exercised for an aggregate of 593,598 shares of the Company’s Common Stock and 2,898,151 warrants were cancelled in connection with the warrant conversion offering. The issuance of these securities was deemed to be exempt from the registration requirements of the Securities Act, by virtue of Section 4(2) thereof, as a transaction by an issuer not involving a public offering.
As of April 27, 2017, the Company issued an aggregate of 529,570 shares of Common Stock for certain legal, corporate structuring and research and development consulting services rendered by third-party consultants. The foregoing shares were valued at $529,570. The issuance of these securities was deemed to be exempt from the registration requirements of the Securities Act, by virtue of Section 4(2) thereof, as a transaction by an issuer not involving a public offering.
Item 16. Exhibits and Financial Statement Schedules
| (a) |
Exhibits.
|
|
Exhibit
Number
|
|
Description
|
|
|
|
|
|
3.1
|
|
Articles of Incorporation as filed with the Nevada Secretary of State (incorporated by reference to Exhibit 3.01 of the Company’s Form 10 Registration Statement filed on July 6, 2016)
|
|
3.2
|
|
Certificate of Amendment to the Articles of Incorporation filed with the Nevada Secretary of State (incorporated by reference to Exhibit 3.02 of the Company’s Form 10 Registration Statement filed on July 6, 2016)
|
|
3.3
|
|
Articles of Merger filed with the Nevada Secretary of State (incorporated by reference to Exhibit 3.03 of the Company’s Form 10 Registration Statement filed on July 6, 2016)
|
|
3.4
|
|
Certificate of Merger filed with the Delaware Secretary of State (incorporated by reference to Exhibit 3.04 of the Company’s Form 10 Registration Statement filed on July 6, 2016)
|
|
3.5
|
|
By-laws (incorporated by reference to Exhibit 3.05 of the Company’s Form 10 Registration Statement filed on July 6, 2016)
|
|
4.1
|
|
2016 Omnibus Incentive Plan (incorporated by reference to Exhibit 4.01 of Amendment No. 1 to the Company’s Form 10 Registration Statement filed on August 16, 2016)
|
|
4.2
|
|
2016 Omnibus Incentive Plan - Form of Stock Option Award Agreement (incorporated by reference to Exhibit 4.02 of the Company’s Form 10 Registration Statement filed on July 6, 2016)
|
|
5.1**
|
|
Opinion of Sichenzia Ross Ference Kesner LLP, as to the legality of securities being registered
|
|
10.1
|
|
Agreement and Plan of Merger dated February 8, 2016 between the Company and Nexeon MedSystems, Inc., a Delaware corporation (incorporated by reference to Exhibit 10.01 of Amendment No. 2 to the Company’s Form 10 Registration Statement filed on September 9, 2016)
|
|
10.2
|
|
Contribution Agreement between the Company and Rosellini Scientific, LLC dated January 2, 2016 (incorporated by reference to Exhibit 10.02 of the Company’s Form 10 Registration Statement filed on July 6, 2016)
|
|
10.3
|
|
Contribution Agreement between the Company and Elizabeth Rosellini dated January 2, 2016 (incorporated by reference to Exhibit 10.03 of the Company’s Form 10 Registration Statement filed on July 6, 2016)
|
|
10.4
|
|
Executive Services Agreement between the Company and Ronald Conquest dated January 1, 2016 (incorporated by reference to Exhibit 10.04 of the Company’s Form 10 Registration Statement filed on July 6, 2016)
|
|
10.5
|
|
Director Services Agreement between the Company and Dr. Mark Bates dated May 1, 2016 (incorporated by reference to Exhibit 10.05 of the Company’s Form 10 Registration Statement filed on July 6, 2016)
|
|
10.6
|
|
Form of Director Indemnification Agreement (incorporated by reference to Exhibit 10.07 of the Company’s Form 10 Registration Statement filed on July 6, 2016)
|
|
10.7
|
|
Patent License Asset Purchase Agreement between the Company and William Rosellini dated December 15, 2016 (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed on December 20, 2016)
|
|
10.8
|
Employment Agreement between the Company and Brian Blischak dated December 20, 2016 and Stock Option Award Agreement (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed on December 29, 2016)
|
|
|
10.9
|
Executive Employment Contract between the Company and Christopher R. Miller dated December 21, 2016 and Stock Option Award Agreement (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed on December 29, 2016)
|
|
Exhibit
Number
|
|
Description
|
|
|
|
|
|
10.10
|
|
Acquisition Agreement between Rosellini Scientific, LLC and Nexeon Medsystems Europe, S.a.r.l. (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC on January 17, 2017)
|
|
10.11
|
|
Loan Agreement between Nexeon Medsystems Europe, S.a.r.l. and Nexeon Medsystems Belgium, SPRL (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed with the SEC on January 17, 2017)
|
|
10.12
|
|
Promissory Note of Nexeon Medsystems Belgium, SPRL (incorporated by reference to Exhibit 10.3 of the Company’s Current Report on Form 8-K filed with the SEC on January 17, 2017)
|
|
10.13
|
|
Security Agreement between Nexeon Medsystems Europe, S.a.r.l. and Nexeon Medsystems Belgium, SPRL (incorporated by reference to Exhibit 10.4 of the Company’s Current Report on Form 8-K filed with the SEC on January 17, 2017)
|
|
10.14
|
|
Stock Exchange Agreement between the Company and Rosellini Scientific, LLC (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed on January 19, 2017)
|
|
10.15
|
|
Executive Employment Contract between the Company and Emily Hamilton dated January 1, 2017 (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed on February 28, 2017)
|
|
10.16
|
|
Director Services Agreement between the Company and Kent J. George dated January 1, 2017 (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed on February 28, 2017)
|
|
10.17
|
|
Director Services Agreement between the Company and Michael Neitzel dated January 1, 2017 (incorporated by reference to Exhibit 10.3 of the Company’s Current Report on Form 8-K filed on February 28, 2017)
|
|
10.18
|
|
Professional Relations and Consulting Agreement between the Company and Acorn Management Partners, L.L.C. dated February 14, 2017 (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed on March 10, 2017)
|
|
14.1
|
|
Code of Business Conduct and Ethics (incorporated by reference to Exhibit 14.01 of the Company’s Form 10 Registration Statement filed on July 6, 2016)
|
|
22.1
|
|
Subsidiaries (incorporated by reference to Exhibit 21.1 of the Company’s Annual Report on Form 10-K filed on March 29, 2017)
|
|
23.1*
|
|
Consent of Paritz & Company, P.A.
|
|
23.2**
|
|
Consent of Sichenzia Ross Ference Kesner LLP (included as part of Exhibit 5.1)
|
|
24.1
|
|
Power of Attorney (included on the signature page of this Form S-1)
|
__________________
| * |
Filed herewith
|
| ** |
To be filed by amendment
|
Item 17. Undertakings
(a) The undersigned registrant hereby undertakes:
| (1) |
To file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement:
|
| (i) |
To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
|
| (ii) |
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
|
| (iii) |
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
|
Provided, however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) of this section do not apply if the registration statement is on Form S–3 (§239.13 of this chapter) or Form F–3 (§239.33 of this chapter) and the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) (§230.424(b) of this chapter) that is part of the registration statement.
| (2) |
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
|
| (3) |
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
|
| (4) |
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
|
| (i) |
If the registrant is relying on Rule 430B (§230.430B of this chapter):
|
| (A) |
Each prospectus filed by the registrant pursuant to Rule 424(b)(3) (§230.424(b)(3) of this chapter) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
|
| (B) |
Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) (§230.424(b)(2), (b)(5), or (b)(7) of this chapter) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) (§230.415(a)(1)(i), (vii), or (x) of this chapter) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date; or
|
| (ii) |
If the registrant is subject to Rule 430C (§230.430C of this chapter), each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A (§230.430A of this chapter), shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
|
| (5) |
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities:
|
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
| (i) |
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§230.424 of this chapter);
|
| (ii) |
(ii)Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
|
| (iii) |
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
|
| (iv) |
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
|
(b) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Lexington, State of Kentucky, on this 28th day of April, 2017.
|
|
NEXEON MEDSYSTEMS INC
|
|
|
|
|
|
|
|
By:
|
/s/ William Rosellini
|
|
|
|
William Rosellini
|
|
|
|
Chief Executive Officer
(Principal Executive Officer)
|
|
|
By:
|
/s/ Christopher R. Miller
|
|
|
|
Christopher R. Miller
|
|
|
|
Chief Financial Officer
(Principal Financial and Accounting Officer)
|
We, the undersigned officers and directors of Nexeon MedSystems Inc. hereby severally constitute and appoint William Rosellini and Christopher R. Miller, our true and lawful attorney-in-fact and agents, with full power of substitution and resubstitution for him and in his name, place and stead, and in any and all capacities, to sign for us and in our names in the capacities indicated below any and all amendments (including post-effective amendments) to this registration statement (or any other registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933, as amended), and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as full to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities held on the dates indicated.
|
Name
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ William Rosellini
|
|
Chief Executive Officer and Director
|
|
April 28, 2017
|
|
William Rosellini
|
|
(Principal Executive Officer)
|
|
|
|
|
|
|
|
|
|
/s/ Christopher R. Miller
|
|
Chief Financial Officer
|
|
April 28, 2017
|
|
Christopher R. Miller
|
|
(Principal Financial and Accounting Officer)
|
|
|
|
|
|
|
|
|
|
/s/ Brian Blischak
|
|
President and Chief Commercial Officer
|
|
April 28, 2017
|
|
Brian Blischak
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Mark C. Bates
|
|
Chief Innovation Officer and Director
|
|
April 28, 2017
|
|
Mark C. Bates
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Ronald Conquest
|
|
Executive Vice President of Finance and Director
|
|
April 28, 2017
|
|
Ronald Conquest
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Kent J. George
|
|
Director
|
|
April 28, 2017
|
|
Kent J. George
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Michael Neitzel
|
|
Director
|
|
April 28, 2017
|
|
Michael Neitzel
|
|
|
|
S-1
