Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Corvus Pharmaceuticals, Inc. | exh_991.htm |
| 8-K - FORM 8-K - Corvus Pharmaceuticals, Inc. | f8k_040417.htm |
Exhibit 99.2

CPI - 444, an oral adenosine A2A receptor ( A2AR ) antagonist, demonstrates clinical activity in patients with advanced solid tumors Leisha A. Emens 1 , John Powderly 2 , Lawrence Fong 3 , Joshua Brody 4 , Patrick Forde 1 , Matthew Hellmann 5 , Brett Hughes 6 , Shivaani Kummar 7 , Sherene Loi 8 Jason Luke 9 , Daruka Mahadevan 10 , Ben Markman 11 , Ian McCaffery 12 , Richard Miller 12 , and Ginna Laport 12 1 Johns Hopkins University, Baltimore MD ; 2 Carolina BioOncology Institute, Charlotte NC ; 3 University of California, San Francisco, San Francisco CA ; 4 Mount Sinai School of Medicine, New York NY ; 5 Memorial Sloan Kettering Cancer Center, New York NY ; 6 Royal Brisbane and Women’s Hospital, Herston, Australia 7 Stanford University School of Medicine, Palo Alto CA ; 8 Peter MacCallum Cancer Center, Melbourne, Australia ; 9 University of Chicago, Chicago IL ; 10 University of Arizona, Tucson AZ ; 11 Monash Health, Victoria, Australia ; 12 Corvus Pharmaceuticals, Burlingame CA 1

This presentation contains forward - looking statements, including statements related to the potential safety and efficacy of CPI - 444, both as a sin gle agent and in combination with anti - PD - 1 and anti - PD - L1 , the utility of biomarker data collected and the suitability of the dosing regimen selected for the Company’s Phase 1/ 1b clinical trial of CPI - 444. All statements other than statements of historical fact contained in this press release are forward - l ooking statements. These statements often include words such as “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate,” “seek,” “will,” “may” or similar expre ssi ons. Forward - looking statements are subject to a number of risks and uncertainties, many of which involve factors or circumstances that are beyond the Company’s con trol. The Company’s actual results could differ materially from those stated or implied in forward - looking statements due to a number of factors, including but not limited to, risks detailed in the Company’s Annual Report on Form 10 - K for the year ended December 31, 2016, filed with the Securities and Exchange Commission on March 10, 2017, as well as other documents that may be filed by the Company from time to time with the Securities and Exchange Commission. In particular , t he following factors, among others, could cause results to differ materially from those expressed or implied by such forward - looking statements: the Company ’s ability to utilize biomarker data, select a suitable dosing regimen and demonstrate evidence of efficacy and safety for CPI - 444 during its Phase 1/ 1b clinical trial; the accuracy of the Company’s estimates relating to its ability to initiate and/or complete clinical trials; the results of early clinical trials may not b e p redictive of future results. Although the Company believes that the expectations reflected in the forward - looking statements are reasonable, it cannot guarantee that the events a nd circumstances reflected in the forward - looking statements will be achieved or occur, and the timing of events and circumstances and actual results could differ materially from those projected in the forward - looking statements. Accordingly, you should not place undue reliance on these forward - looking statements. All such state ments speak only as of the date made, and the Company undertakes no obligation to update or revise publicly any forward - looking statements, whether as a result of new information, future events or otherwise. Additional information may be available in press releases or other public announcements and public filings made a fte r the date of this presentation. This presentation concerns products that have not yet been approved for marketing by the U.S. Food and Drug Administration (“ FDA ”). No representation is made as to their safety or effectiveness for the purposes of which they are being investigated. 2 Forward Looking Statements

Disclosure Information 3 3 I have the following financial relationships to disclose: Consultant for : Vaccinex , Celgene, Bristol Meyers Squibb, AstraZeneca, Amgen, Syndax , Molecuvax , eTHeRNA , Peregrine, Bayer Grant/Research support from : Genentech/Roche, EMD Serono , Maxcyte , Merck, AstraZeneca, Aduro , Corvus I will discuss the following off - label use and/or investigational use: CPI - 444 alone and combined with atezolizumab for advanced solid cancers. Study funding provided by Corvus Pharmaceuticals. Roche Genentech provided atezolizumab and support for biomarker analyses AACR Annual Meeting 2017: Leisha A. Emens

Adenosine Signaling Suppresses Immunity in the Tumor Microenvironment 4 • PD - 1/PD - L1 antibodies are effective immunotherapies with response rates ~20 - 30% • Novel agents that enhance response or overcome resistance to immunotherapy are a high priority • The adenosine pathway is a potential new immunotherapy target

CPI - 444: A Novel Inhibitor of the A2AR Pathway • Pharmaceutical Properties – Molecular weight = 407Da – A2AR Ki= 3.5 nM – > 55 - fold selective over A1R, >400 - fold A2BR and A3R – Oral bioavailability >50 % – Plasma half life: ~ 10 - 14 hours • Single agent activity in multiple preclinical models* – Synergy with anti - PD - (L)1 and anti - CTLA - 4 antibodies and other checkpoint inhibitors • Well - tolerated in early trials with healthy volunteers and ADHD patients • This is the first evaluation of safety and clinical activity of an A2AR antagonist in patients with cancer CPI - 444 *See Abstract #5598 N N N N N NH 2 O N O O 5

A Phase 1/1b , Open - Label, Multicenter, Repeat - Dose, Dose - Selection Study of CPI - 444 as a Single Agent and in Combination with Atezolizumab ( a tezo ) in Patients with Selected Incurable Cancers Primary Objectives – Evaluate the safety of CPI - 444 alone and with atezo – Identify a recommended dose and schedule for CPI - 444 alone and with atezo • S afety, PK and PD data* – Measure the clinical activity of CPI - 444 alone and with a tezo • ORR, CBR and DOR* 6 *PK – pharmacokinetics; PD = pharmacodynamics; ORR = overall response rate; CBR - clinical benefit rate; DOR = duration of respon se

Trial Design: Step 1 Dose Selection (Accrual completed) 7 Select Single Agent Dose Select Combination Dose** 100 mg CPI - 444 BID days 1 - 14/28 days* 100 mg CPI - 444 BID 28 days continuous* 200 mg CPI - 444 daily days 1 - 14/28 days* 50 mg CPI - 444 BID d1 - 14/28 days with 840 mg atezo every 2 weeks* 100 mg CPI - 444 BID 14 or 28 days with 840 mg atezo every 2 weeks* DLT evaluation DLT evaluation Eligibility • Selected incurable cancers: NSCLC , Melanoma, RCC, TNBC, Others (UBC, CRPC, CRC - MSI+, SCCHN) • 1 to 5 lines of prior therapy • Stable, treated brain metastases allowed • Resistant/refractory (R/R) to prior anti PD - 1/PDL - 1 allowed • PD - L1, CD73, A2aR expression not required for enrollment *1 cycle=28 days **See Abstract #5593

Trial Design: Step 2 Cohort Expansion by Disease (Accrual ongoing) 8 *Others: CRPC, CRC - MSI, UBC, SCCHN Randomize Single Agent Arm Expansion Combination Arm Expansion NSCLC N=14 MEL N=14 RCC N=14 TNBC N=14 Others* N=14 NSCLC N=14 MEL N=14 RCC N=14 TNBC N=14 Others * N=14 Potential expansion to 26 and 48 patients
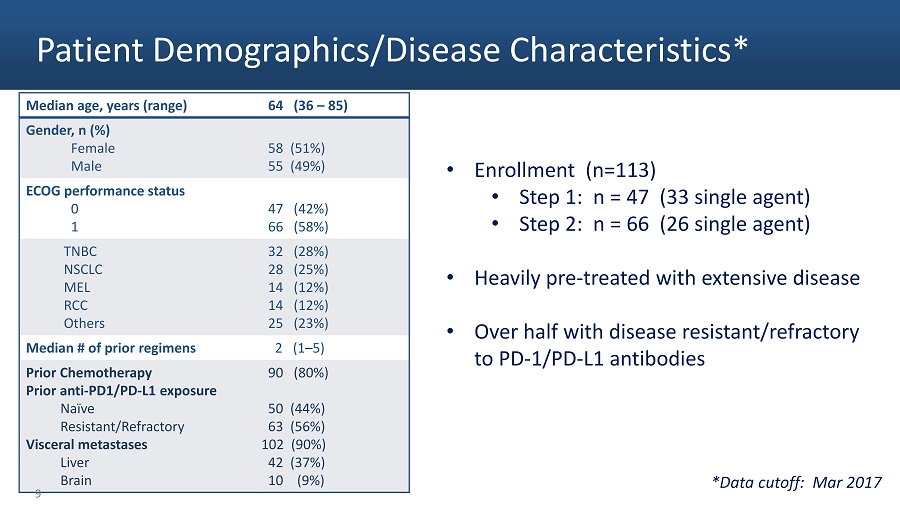
Patient Demographics/Disease Characteristics* Median age, years (range) 64 (36 – 85) Gender, n (%) Female Male 58 (51%) 55 (49%) ECOG performance status 0 1 47 (42%) 66 (58%) TNBC NSCLC MEL RCC Others 32 (28%) 28 (25%) 14 (12%) 14 (12%) 25 (23%) Median # of prior regimens 2 (1 – 5) Prior Chemotherapy Prior anti - PD1/PD - L1 exposure Naïve Resistant/Refractory Visceral metastases Liver Brain 90 (80%) 50 (44%) 63 (56%) 102 (90%) 42 (37%) 10 (9%) • Enrollment (n=113) • Step 1: n = 47 (33 single agent) • Step 2: n = 66 (26 single agent) • Heavily pre - treated with extensive disease • Over half with disease resistant/refractory to PD - 1/PD - L1 antibodies *Data cutoff: Mar 2017 9

Treatment - Related Adverse Events (AE) 10 • Median duration of treatment: 9 weeks (range: up to 40+) • 56% of patients experienced a treatment - related AE (any grade) • No grade 3/4 AEs with single agent CPI - 444 • Immune - related AEs seen only with combination of CPI - 444 and atezo (n=1 for each): - Pancreatitis (Gr 2) - Autoimmune hemolytic anemia (Gr 3) - Meningoencephalitis/thrombocytopenia (Gr 4) Adverse Events > 5% Frequency (Gr 1/2) Single Agent Combo Nausea 14% 13% Pruritus 10% 9% Fatigue 5% 7% Abdominal Pain 5% --- Rash 5% --- Diarrhea 5% --- Pyrexia 5% 7% Decreased Appetite 5% 7% Chills 5% ---

Overall Patient Outcomes Disease Control Rate (CR, PR, SD) in Evaluable Patients 11 CPI - 444 (n=52) CPI - 444/ Atezolizumab (n=44) All Subjects (n=96) All subjects 20 (38%) 17 (39%) 37 (38%) Prior PD - 1/PD - L1 Experience Naïve Resistant/Refractory 13/29 (45%) 7/23 (30%) 5/18 (28%) 12/26 (46%) 18/47 (38%) 19/49 (39%) Disease Histology - NSCLC - MEL - RCC - TNBC - Others 4/14 (29%) 2/5 (40%) 3/5 (60%) 7/17 (41%) 4/11 (36%) 5/10 (50%) 2/6 (33%) 5/5 (100%) 3/14 (21%) 2/9 (22%) 9/24 (38%) 4/11 (36%) 8/10 (80%) 10/31 (32%) 6/20 (30%) • Median follow up time for DCR: 16 weeks (range, 4 - 44 weeks) • 23/37 of PR and SD patients remain on study

Clinical Activity: Overall Patient Population* 12 • SDs and PRs observed with CPI - 444 alone and in combination with atezo % Change from Baseline *Patients with disease evaluable by CT, n= 70 Treatment : Single Agent Combination

Clinical Activity: By Disease Type 13 • Tumor regression observed in RCC, NSCLC, TNBC, SCCHN and CRC % Change from Baseline OTHER TNBC MEL NSCLC RCC

Clinical Activity by Prior PD - (L)1 Experience 14 • CPI - 444 has activity in patients resistant/refractory to PD - 1 blockade • 2 PRs and 7 minor regressions in PD - 1 resistant/refractory patients • 1 PR and 4 minor regressions in PD - 1 naïve patients % Change from Baseline Resistant/Refractory Naive

Duration of Treatment 15 OTHER TNBC MEL NSCLC RCC Single Agent CPI - 444 CPI - 444 Combined with Atezo

Tumor Regression in Nivolumab Refractory Lung Cancer Single Agent CPI - 444 Pre - treatment 2 months of treatment 16 • 2 prior chemotherapy regimens • Refractory to nivolumab • Started single agent CPI - 444

Regression in Nivolumab Resistant Lung Cancer Combination CPI - 444/Atezolizumab Pre - treatment 2 months on treatment 17 • 1 prior chemotherapy • Responded to nivolumab, then progressed • Started CPI - 444 + atezo

Tumor Regression in Nivolumab Refractory Renal Cancer Single Agent CPI - 444 Pre - treatment 3 months of treatment 18 • Five prior regimens including TKIs and mTOR inhibitor • Tumor progression on nivolumab • Started CPI - 444

19 Serial Biopsies of Liver Metastasis from PD - 1 Refractory RCC Patient Treated with Single Agent CPI - 444 18 Pre - treatment Post treatment (2 months) Inflammatory Infiltrate in Tumor = 1% Inflammatory Infiltrate in Tissue = 20% CD8 + in tumor = 14% CD8 + in tissue > 70 %; no tumor cells detectable Inflammation and CD8 + T Cell Infiltration After Progression on PD - 1 Therapy Increased with Single Agent CPI - 444 Therapy H&E H&E IHC IHC See Abstract #5593

Conclusions • CPI - 444 is well tolerated as a single agent and in combination with atezo – Most common Grade 1/2 toxicities: nausea, fatigue, pruritus – irAEs of hemolytic anemia (Gr3), meningoencephalitis (Gr4), and pancreatitis (Gr2) seen with combination therapy • Selected dose of CPI - 444 is 100 mg bid continuous • Observed clinical activity: – As single agent and in combination with atezo in multiple tumor types in advanced cancer patients – In patients refractory/resistant to PD - 1/PD - L1 blockade – 23/37 patients with PR/SD remain on study median 16 weeks • I ncreased inflammation and CD8 + T cells in biopsy observed in an anti PD(L) - 1 - experienced patients responding to single agent CPI - 444 20

Acknowledgements • The patients and their families • Participating Centers: Carolina BioOncology Institute, Columbia University Medical Center, Cross Cancer Institute, Emory University, Indiana University, Johns Hopkins University, Juravinski Cancer Centre, Karmanos Cancer Center, Mary Crowley Cancer Research Centers, Medical College of Wisconsin, Memorial Sloan Kettering Cancer Center, Monash Health, Mount Sinai, Royal Brisbane and Women’s Hospital, START, University of California at San Francisco Medical Center, University of Arizona Medical Center, University of Chicago Medical Center, University of Colorado Cancer Center, University of Pittsburgh, University of Washington, Washington University at Saint Louis • C olleagues at Corvus • Colleagues at Roche Genentech 21
