Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SYNERGY PHARMACEUTICALS, INC. | a17-8950_18k.htm |
Exhibit 99.1
OPPENHEIMER HEALTHCARE CONFERENCE Marino Garcia EVP, Chief Strategy Officer March 21, 2017

SAFE HARBOR STATEMENT 2 This presentation and any statements made for and during any presentation or meeting contain forward-looking statements related to Synergy Pharmaceuticals Inc. under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995 and are subject to risks and uncertainties that could cause actual results to differ materially from those projected. These statements may be identified by the use of forward-looking words such as "anticipate," "planned," "believe," "forecast," "estimated," "expected," and "intend," among others. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, the development, launch, introduction and commercial potential of TRULANCE™; growth and opportunity, including peak sales and the potential demand for TRULANCE, as well as its potential impact on applicable markets; market size; substantial competition; our ability to continue as a going concern; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payer reimbursement; dependence upon third parties; our financial performance and results, including the risk that we are unable to manage our operating expenses or cash use for operations, or are unable to commercialize our products, within the guided ranges or otherwise as expected; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. There are no guarantees that future clinical trials discussed in this presentation will be completed or successful or that any product will receive regulatory approval for any indication or prove to be commercially successful. Investors should read the risk factors set forth in our most recent periodic reports filed with the Securities and Exchange Commission, including our Form 10-K for the year ended December 31, 2016. While the list of factors presented here is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Forward-looking statements included herein are made as of the date hereof, and we do not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances except as required by law. The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation. Oppenheimer Healthcare Conference I March 21, 2017
TRULANCE IS NOW AVAILABLE IN THE U.S. FOR THE TREATMENT OF CIC IN ADULTS 3 Only product thought to replicate the pH-sensitive activity of naturally occurring uroguanylin Highly experienced sales force trained and now fully deployed – 100% focused on Trulance On-track to submit supplemental NDA (sNDA) in IBS-C this month Oppenheimer Healthcare Conference I March 21, 2017
TRULANCE AND SYNERGY ARE WELL POSITIONED 4 ~ 33M U.S. Adults with CIC Significant unmet need; multiple drivers for future growth Strong Product Profile Based on clinical data, the label and market research/customer insights Strong. Focused. Efficient. Launch brand / launch mode, targeting most productive prescribers /influencers Highly Experienced Team Significant GI experience and several product launches RIGHT MARKET RIGHT PRODUCT RIGHT STRATEGY RIGHT TEAM Oppenheimer Healthcare Conference I March 21, 2017
TRULANCE commercial opportunity 5

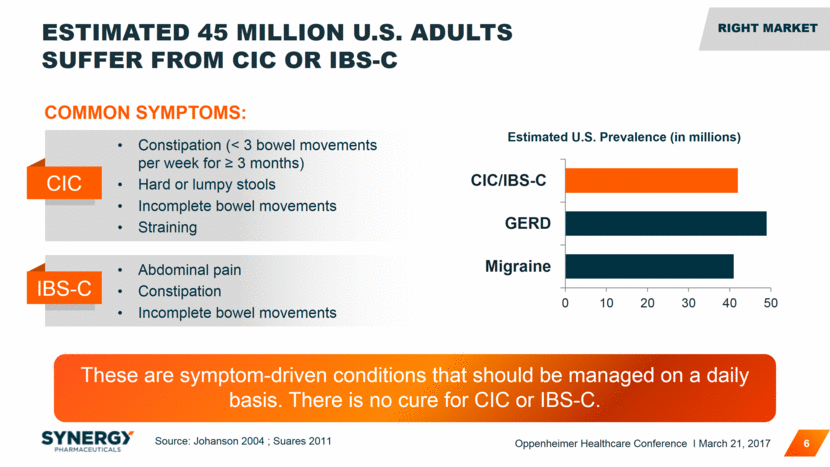
ESTIMATED 45 MILLION U.S. ADULTS SUFFER FROM CIC OR IBS-C 6 Source: Johanson 2004 ; Suares 2011 Estimated U.S. Prevalence (in millions) COMMON SYMPTOMS: Constipation (< 3 bowel movements per week for > 3 months) Hard or lumpy stools Incomplete bowel movements Straining CIC Abdominal pain Constipation Incomplete bowel movements IBS-C RIGHT MARKET These are symptom-driven conditions that should be managed on a daily basis. There is no cure for CIC or IBS-C. Oppenheimer Healthcare Conference I March 21, 2017 0 10 20 30 40 50 Migraine GERD CIC/IBS-C
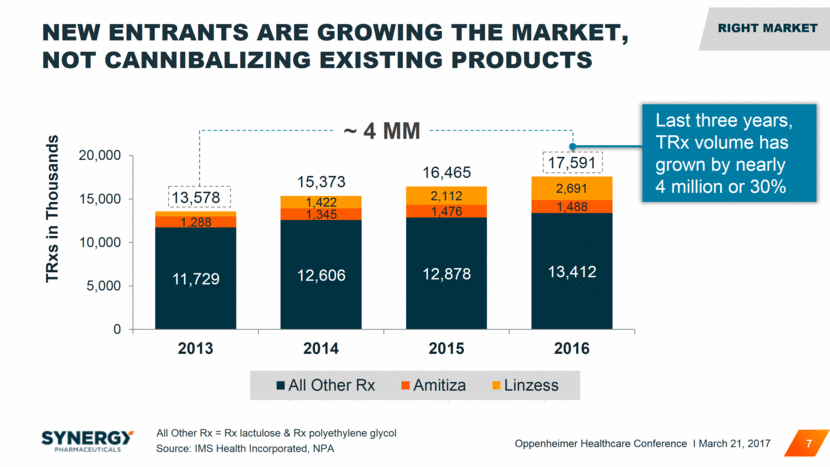
NEW ENTRANTS ARE GROWING THE MARKET, NOT CANNIBALIZING EXISTING PRODUCTS 7 ~ 4 MM Source: IMS Health Incorporated, NPA All Other Rx = Rx lactulose & Rx polyethylene glycol Last three years, TRx volume has grown by nearly 4 million or 30% RIGHT MARKET RIGHT MARKET Oppenheimer Healthcare Conference I March 21, 2017 11,729 12,606 12,878 13,412 1,288 1,345 1,476 1,488 1,422 2,112 2,691 13,578 15,373 16,465 17,591 0 5,000 10,000 15,000 20,000 2013 2014 2015 2016 TRxs in Thousands All Other Rx Amitiza Linzess
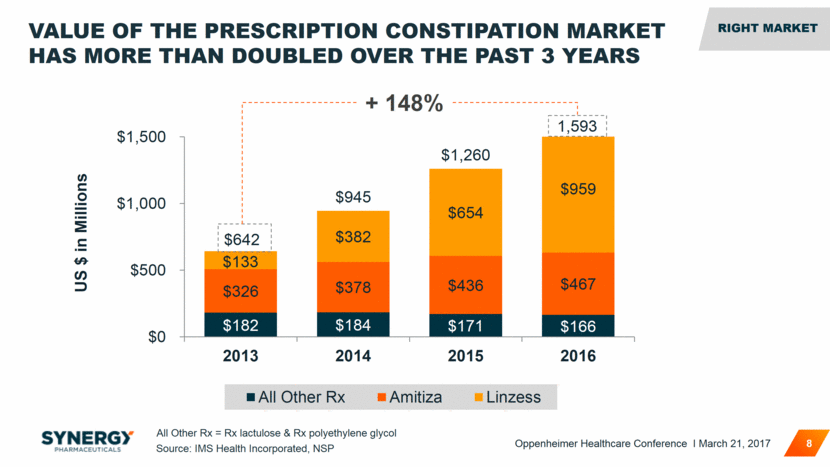
VALUE OF THE PRESCRIPTION CONSTIPATION MARKET HAS MORE THAN DOUBLED OVER THE PAST 3 YEARS 8 + 148% Source: IMS Health Incorporated, NSP All Other Rx = Rx lactulose & Rx polyethylene glycol 1,593 $133 RIGHT MARKET RIGHT MARKET Oppenheimer Healthcare Conference I March 21, 2017 $182 $184 $171 $166 $326 $378 $436 $467 $382 $654 $959 $642 $945 $1,260 $0 $500 $1,000 $1,500 2013 2014 2015 2016 US $ in Millions All Other Rx Amitiza Linzess
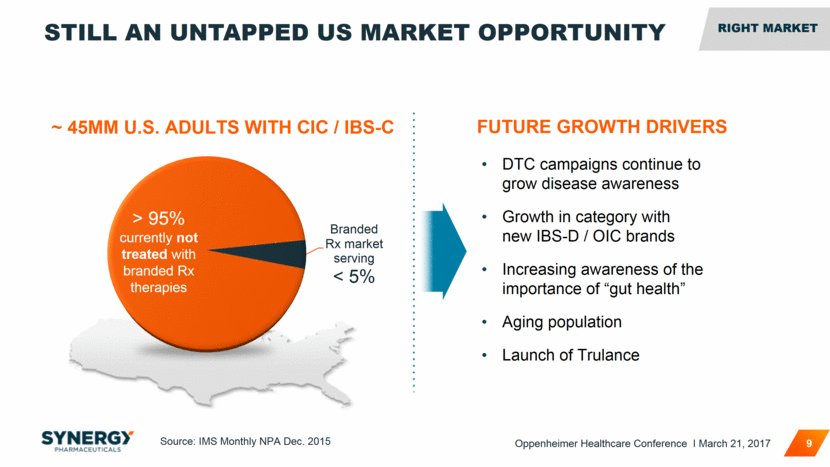
Source: IMS Monthly NPA Dec. 2015 STILL AN UNTAPPED US MARKET OPPORTUNITY 9 FUTURE GROWTH DRIVERS DTC campaigns continue to grow disease awareness Growth in category with new IBS-D / OIC brands Increasing awareness of the importance of “gut health” Aging population Launch of Trulance ~ 45MM U.S. ADULTS WITH CIC / IBS-C > 95% currently not treated with branded Rx therapies RIGHT MARKET RIGHT MARKET Oppenheimer Healthcare Conference I March 21, 2017 Branded Rx market serving < 5%
TRULANCE commercial launch 10

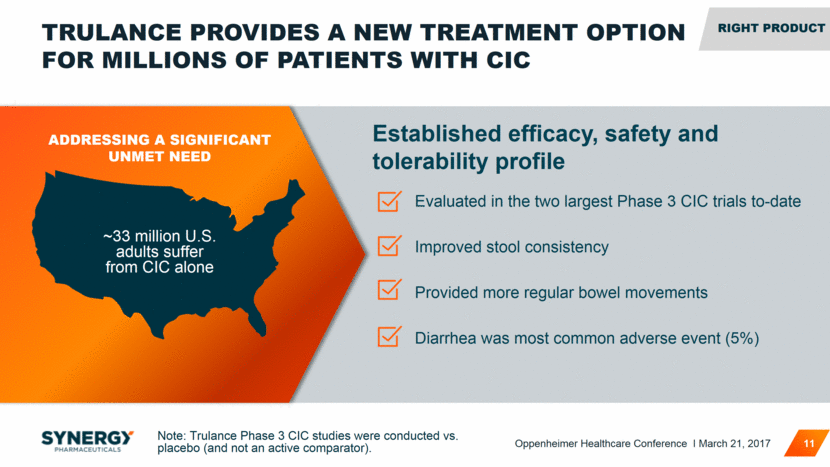
TRULANCE PROVIDES A NEW TREATMENT OPTION FOR MILLIONS OF PATIENTS WITH CIC 11 Evaluated in the two largest Phase 3 CIC trials to-date Improved stool consistency Provided more regular bowel movements Diarrhea was most common adverse event (5%) Established efficacy, safety and tolerability profile ~33 million U.S. adults suffer from CIC alone ADDRESSING A SIGNIFICANT UNMET NEED RIGHT Product Note: Trulance Phase 3 CIC studies were conducted vs. placebo (and not an active comparator). Oppenheimer Healthcare Conference I March 21, 2017
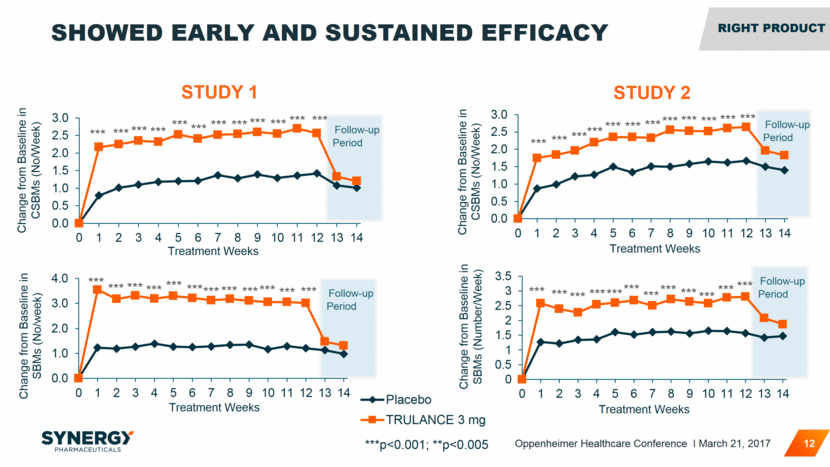
Follow-up Period Follow-up Period Follow-up Period *** Change from Baseline in CSBMs (No/Week) *** *** *** *** *** *** *** *** *** *** *** Follow-up Period *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** SHOWED EARLY AND SUSTAINED EFFICACY 12 STUDY 1 STUDY 2 ***p<0.001; **p<0.005 Change from Baseline in SBMs (Number/Week) *** *** *** *** *** *** *** *** *** *** *** *** RIGHT Product Oppenheimer Healthcare Conference I March 21, 2017 0.0 0.5 1.0 1.5 2.0 2.5 3.0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Change from Baseline in CSBMs ( No/Week ) Treatment Weeks 0.0 0.5 1.0 1.5 2.0 2.5 3.0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Treatment Weeks
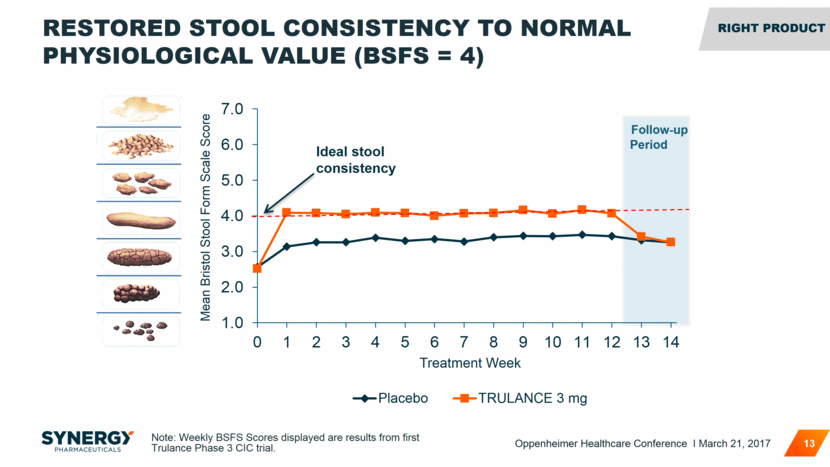
Follow-up Period RESTORED STOOL CONSISTENCY TO NORMAL PHYSIOLOGICAL VALUE (BSFS = 4) 13 Mean Bristol Stool Form Scale Score Ideal stool consistency Note: Weekly BSFS Scores displayed are results from first Trulance Phase 3 CIC trial. RIGHT Product Oppenheimer Healthcare Conference I March 21, 2017 1.0 2.0 3.0 4.0 5.0 6.0 7.0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Treatment Week Placebo TRULANCE 3 mg
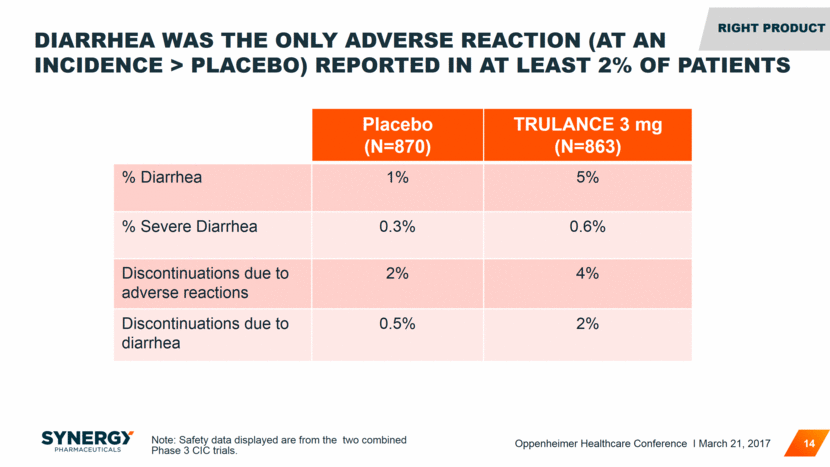
DIARRHEA WAS THE ONLY ADVERSE REACTION (AT AN INCIDENCE > PLACEBO) REPORTED IN AT LEAST 2% OF PATIENTS Placebo (N=870) TRULANCE 3 mg (N=863) % Diarrhea 1% 5% % Severe Diarrhea 0.3% 0.6% Discontinuations due to adverse reactions 2% 4% Discontinuations due to diarrhea 0.5% 2% 14 RIGHT Product Note: Safety data displayed are from the two combined Phase 3 CIC trials. Oppenheimer Healthcare Conference I March 21, 2017
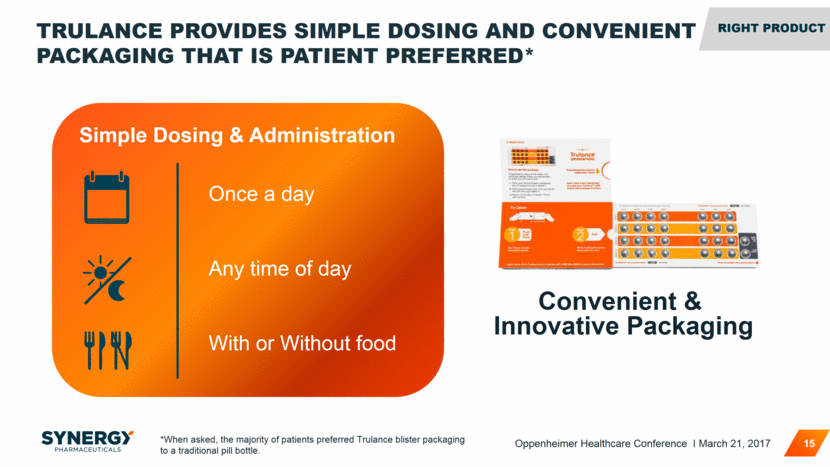
TRULANCE PROVIDES SIMPLE DOSING AND CONVENIENT PACKAGING THAT IS PATIENT PREFERRED* 15 Convenient & Innovative Packaging Simple Dosing & Administration *When asked, the majority of patients preferred Trulance blister packaging to a traditional pill bottle. Once a day Any time of day With or Without food RIGHT PROFILE RIGHT Product Oppenheimer Healthcare Conference I March 21, 2017
STRONG CREATIVE CAMPAIGN BASED ON EXTENSIVE MARKET RESEARCH AND CUSTOMER INSIGHTS 16 RIGHT STRATEGY Oppenheimer Healthcare Conference I March 21, 2017

WELL POSITIONED TO ENTER A CLEARLY DEFINED MARKET Most productive prescribers and influencers (~27,000) gastroenterologists primary care physicians 17 Experienced Sales Force Congress and Conventions Promotional Peer to Peer Programs Non-Personal Promotion Trulance samples RIGHT STRATEGY Oppenheimer Healthcare Conference I March 21, 2017
OUR COMMITMENT TO PROVIDE MARKET ACCESS 18 RIGHT Strategy Oppenheimer Healthcare Conference I March 21, 2017
EXPERIENCED FIELD FORCE EXECUTING ON A STRONG AND FOCUSED LAUNCH STRATEGY 19 Synergy Regional Business Directors average over 11 years of management experience with over 10 years in relevant GI fields Synergy Regional GI Account Specialists average 13 years of pharmaceutical experience and 8.5 years of GI experience National and Regional Market Access team members have been in the field introducing Synergy to payers since January 2016 Market Access team has met with all key commercial and public payers, representing ~ 230 million lives Utilizing flexible and innovative hybrid sales model to reach key prescribers and influencers Sales representatives average 11.5 years of pharmaceutical experience and nearly 6 years in GI, with over 90% coming from peer GI and PCP companies Sales representatives are fully dedicated to Trulance RIGHT TEAM Synergy Sales Leaders Market Access Team Hybrid Sales Model Oppenheimer Healthcare Conference I March 21, 2017
TRULANCE ibs-c CLINICAL program 20

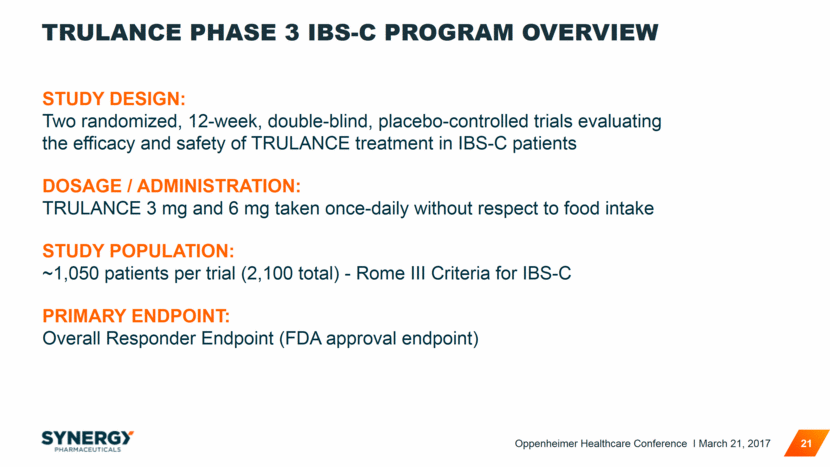
TRULANCE PHASE 3 IBS-C PROGRAM OVERVIEW 21 STUDY DESIGN: Two randomized, 12-week, double-blind, placebo-controlled trials evaluating the efficacy and safety of TRULANCE treatment in IBS-C patients DOSAGE / ADMINISTRATION: TRULANCE 3 mg and 6 mg taken once-daily without respect to food intake STUDY POPULATION: ~1,050 patients per trial (2,100 total) - Rome III Criteria for IBS-C PRIMARY ENDPOINT: Overall Responder Endpoint (FDA approval endpoint) Oppenheimer Healthcare Conference I March 21, 2017
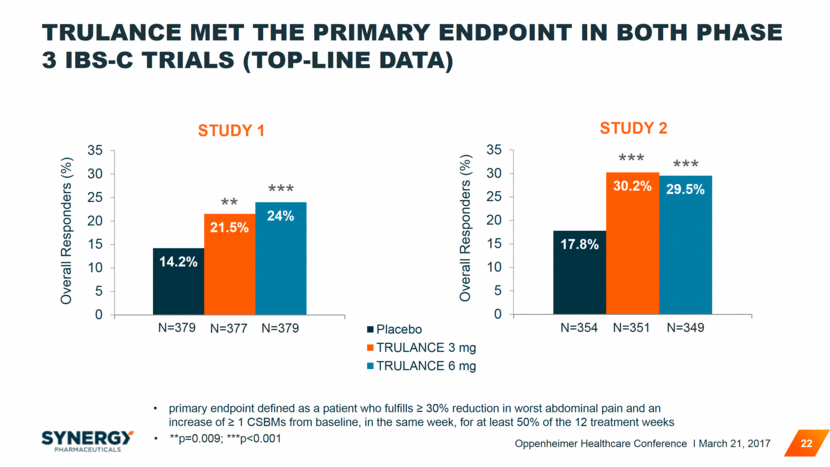
TRULANCE MET THE PRIMARY ENDPOINT IN BOTH PHASE 3 IBS-C TRIALS (TOP-LINE DATA) 22 STUDY 1 **p=0.009; ***p<0.001 ** *** STUDY 2 *** *** primary endpoint defined as a patient who fulfills > 30% reduction in worst abdominal pain and an increase of > 1 CSBMs from baseline, in the same week, for at least 50% of the 12 treatment weeks N=354 N=351 N=349 N=379 N=377 N=379 Oppenheimer Healthcare Conference I March 21, 2017 17.8% 30.2% 29.5% 0 5 10 15 20 25 30 35 Overall Responders (%)
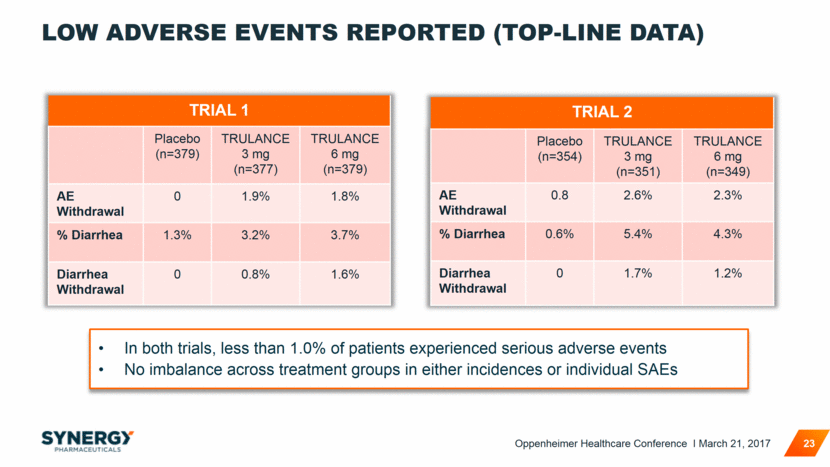
LOW ADVERSE EVENTS REPORTED (TOP-LINE DATA) TRIAL 1 Placebo (n=379) TRULANCE 3 mg (n=377) TRULANCE 6 mg (n=379) AE Withdrawal 0 1.9% 1.8% % Diarrhea 1.3% 3.2% 3.7% Diarrhea Withdrawal 0 0.8% 1.6% 23 TRIAL 2 Placebo (n=354) TRULANCE 3 mg (n=351) TRULANCE 6 mg (n=349) AE Withdrawal 0.8 2.6% 2.3% % Diarrhea 0.6% 5.4% 4.3% Diarrhea Withdrawal 0 1.7% 1.2% In both trials, less than 1.0% of patients experienced serious adverse events No imbalance across treatment groups in either incidences or individual SAEs Oppenheimer Healthcare Conference I March 21, 2017
CORPORATE SUMMARY

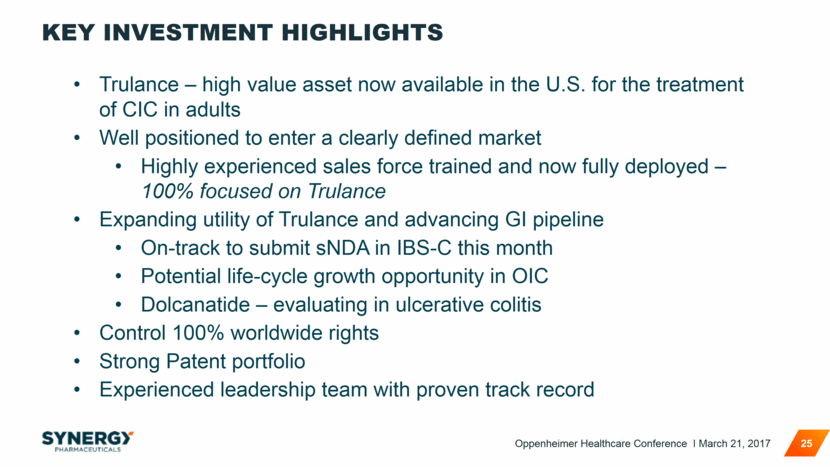
KEY INVESTMENT HIGHLIGHTS 25 Trulance – high value asset now available in the U.S. for the treatment of CIC in adults Well positioned to enter a clearly defined market Highly experienced sales force trained and now fully deployed – 100% focused on Trulance Expanding utility of Trulance and advancing GI pipeline On-track to submit sNDA in IBS-C this month Potential life-cycle growth opportunity in OIC Dolcanatide – evaluating in ulcerative colitis Control 100% worldwide rights Strong Patent portfolio Experienced leadership team with proven track record Oppenheimer Healthcare Conference I March 21, 2017
AT SYNERGY, we’re challenging the status quo in gastrointestinal (GI) medicine to develop new treatments that offer the potential to change people’s lives. IGNITING CHANGE FOR NEW SOLUTIONS

