Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - MEDICINES CO /DE | a17-8530_1ex99d1.htm |
| 8-K - 8-K - MEDICINES CO /DE | a17-8530_18k.htm |
Exhibit 99.2
ORION-1 Primary efficacy & safety outcomes LDL-C reduction from 6 to 9 months following single injections of inclisiran, a novel siRNA compound or second Kausik K Ray, Ulf Landmesser, Lawrence A Leiter, David Kallend, Peter Wijngaard, R Scott Wright, and John JP Kastelein On behalf of the ORION-1 investigators 1

Background Major progress is being made in ASCVD PCSK9 inhibition is now a validated target for reducing LDL-C and ASCVD1 PCSK9 mAb therapy requires 12-26 injections per year statins2 Adherence data with PCSK9 mAbs show no substantial improvement over Poor adherence and LDL-C variability are associated with poor outcomes3 These limitations are most relevant in high risk patients with high LDL-C 1. 2. 3. Sabatine M et al NEJM 2017 Hines D et al ACC 2017 abstract #1203-313 Bangalore S et al JACC 2015;65:1539-48 2
Rationale and objective Inclisiran: a novel agent to address unmet needs Harnessing RNAi offers an alternative treatment for PCSK9 and LDL-C1 Inclisiran, a synthetic siRNA molecule, inhibits PCSK9 synthesis in the liver2 In Phase I, 300 mg inclisiran lowered LDL-C 50-60% for 84 days (n=69)3 • • Objective of ORION-1 • Evaluate optimal dosing regimens in patients with elevated LDL-C and high CV risk 1. 2. 3. Wittrup A & Lieberman J Nature Rev Gen 2015;16: 543-52 Fitzgerald K et al. Lancet 2013;9911:60-8 Fitzgerald K et al. N Engl J Med 2017;376 (1):41-51 3
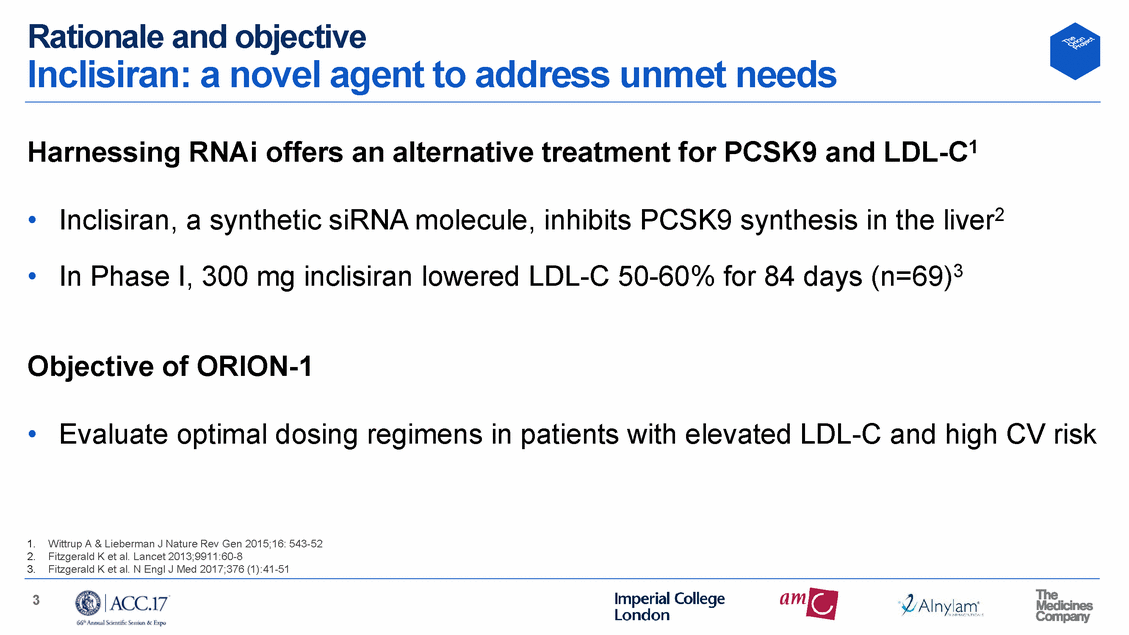
Methods Trial design N=61 Day 360 Extended follow-up Day 360 Extended follow-up 4 Screening (Day -14 to Day -1) Randomization (Day 1, n=501) One dose starting regimen Randomized (n=501) Two dose starting regimen Placebo N=65 200 mg N=60 300 mg N=61 500 mg N=65 Treated (n=497) Placebo N=62 100 mg N=61 200 mg N=62 300 mg Day 1 Day 14 Day 30 Day 90 Day 180 Day 210 Study drug given 1st follow-up visit Monthly follow-up visits Primary evaluation End of study visit Day 1 Day 14 Day 30 Day 90 Day 180 Day 210 Study drug given 1st follow-up visit Monthly follow-up visits Study drug given Primary evaluation End of study visit Completed (n=483)
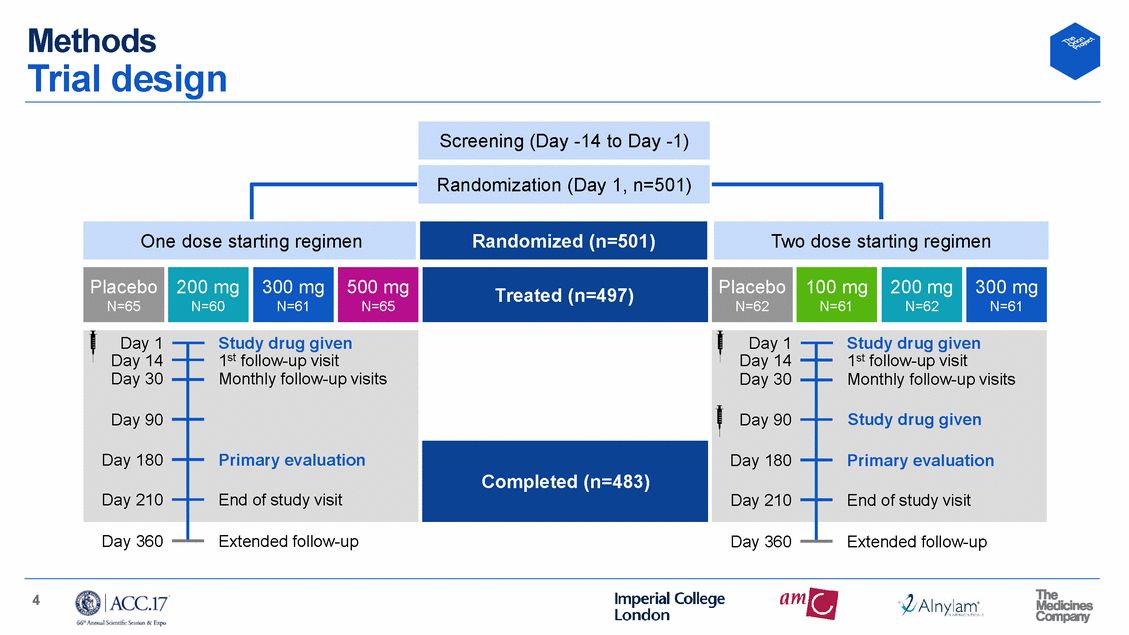
Patients High-risk CV patients, balanced by randomization One dose starting regimen Two dose starting regimen Placebo N=65 Inclisiran N=186 Placebo N=62 Inclisiran N=184 Age 62 63 63 64 Mean years Prior ASCVD 69.2 67.9 74.2 68.3 % LDL-C 128.5 125.9 125.2 133.0 Mean mg/dL Apo-B 102.4 103.2 104.6 107.7 Mean mg/dL PCSK9 404.7 428.7 431.3 416.2 Mean ng/mL 5 Lipoprotein(a)Median nmol/L27.034.050.540.0 Non-HDL-CMean mg/dL157.8156.5157.1165.6 Statin Rx%70.374.477.070.2 Male sex%64.667.753.266.3
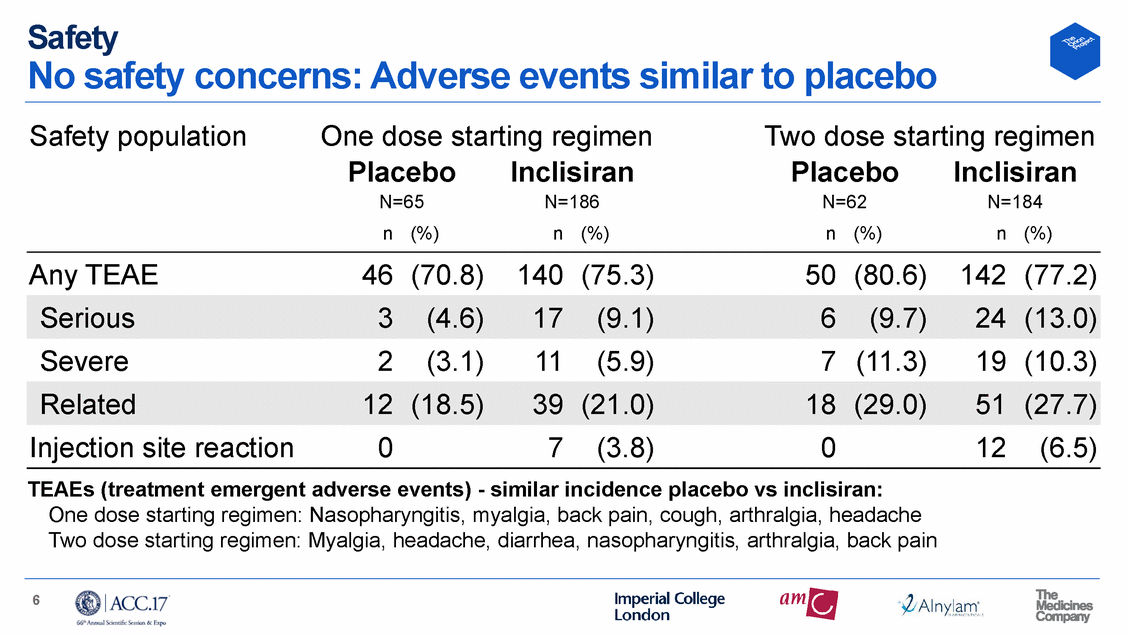
Safety No safety concerns: Adverse events similar to placebo Safety population One dose starting regimen Two dose starting regimen Placebo N=65 Inclisiran N=186 Placebo N=62 Inclisiran N=184 n (%) n (%) n (%) n (%) Any TEAE 46 (70.8) 140 (75.3) 50 (80.6) 142 (77.2) Severe 2 (3.1) 11 (5.9) 7 (11.3) 19 (10.3) Injection site reaction 0 7 (3.8) 0 12 (6.5) TEAEs (treatment emergent One dose starting regimen: adverse events) - similar incidence placebo vs inclisiran: Nasopharyngitis, myalgia, back pain, cough, arthralgia, headache Two dose starting regimen: Myalgia, headache, diarrhea, nasopharyngitis, arthralgia, back pain 6 Related12(18.5)39(21.0)18(29.0)51(27.7) Serious3(4.6)17(9.1)6(9.7)24(13.0)
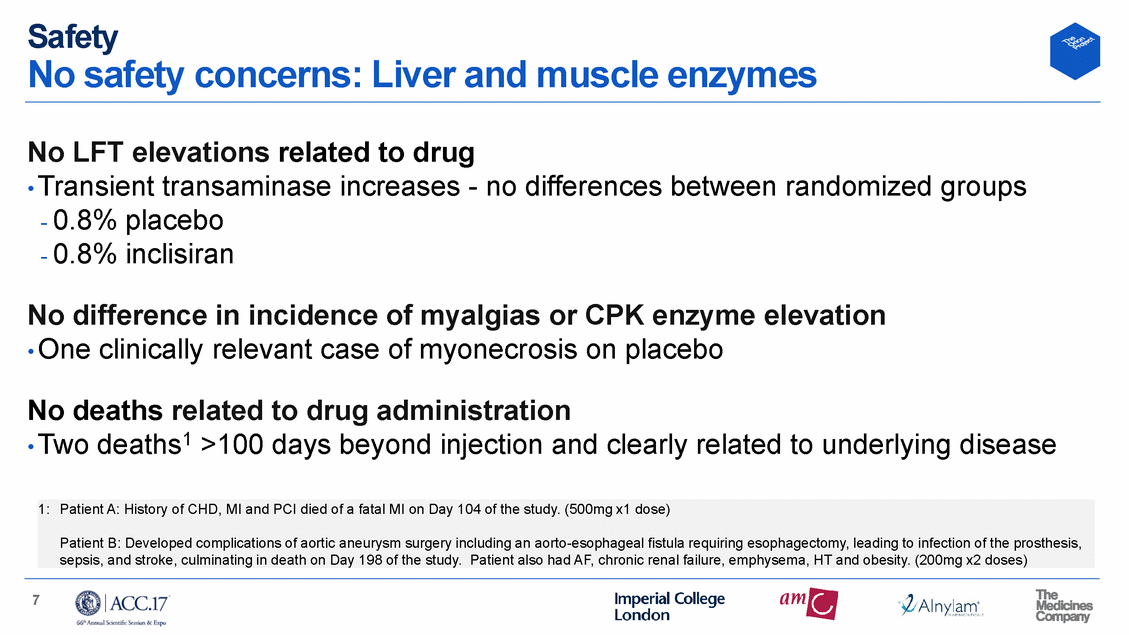
Safety No safety concerns: Liver and muscle enzymes No LFT elevations related to drug • Transient transaminase increases - no differences between randomized - 0.8% placebo - 0.8% inclisiran groups No difference in incidence of myalgias or CPK enzyme elevation • One clinically relevant case of myonecrosis on placebo No deaths related to drug administration • Two deaths1 >100 days beyond injection and clearly related to underlying disease 7 1: Patient A: History of CHD, MI and PCI died of a fatal MI on Day 104 of the study. (500mg x1 dose) Patient B: Developed complications of aortic aneurysm surgery including an aorto-esophageal fistula requiring esophagectomy, leading to infection of the prosthesis, sepsis, and stroke, culminating in death on Day 198 of the study. Patient also had AF, chronic renal failure, emphysema, HT and obesity. (200mg x2 doses)
Safety No safety concerns: Other relevant parameters No No No No thrombocytopenia neuropathy immunogenicity (no anti-drug antibodies) pro-inflammatory symptoms or elevated markers 8
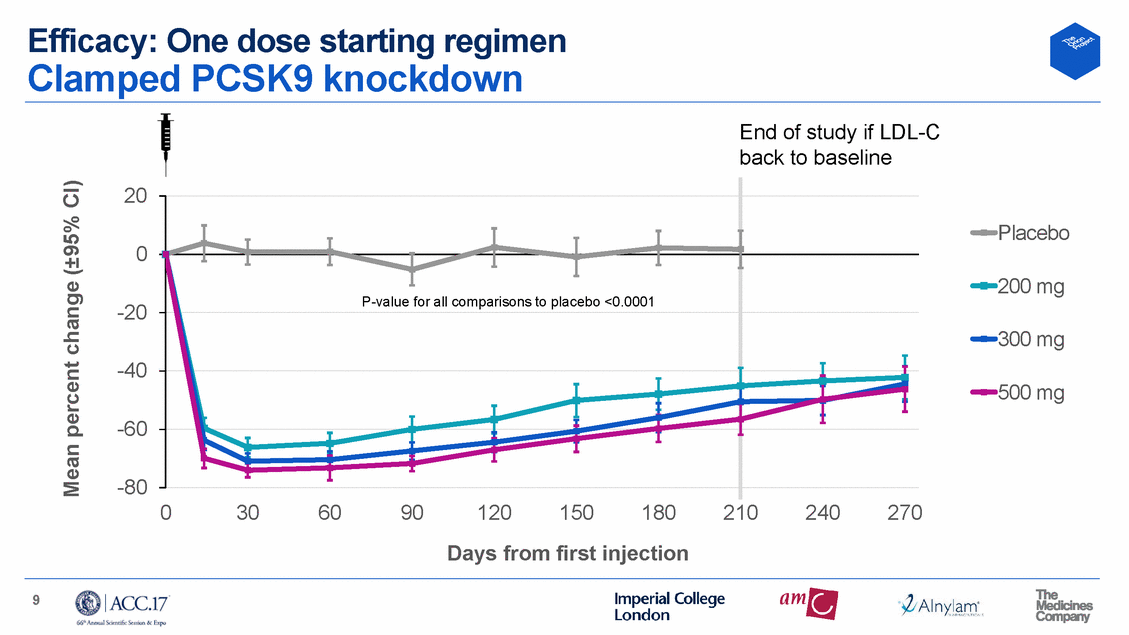
Efficacy: One dose starting regimen Clamped PCSK9 knockdown End of study if LDL-C back to baseline 20 Placebo 0 200 mg -20 300 mg -40 500 mg -60 -80 0 30 60 90 120 150 180 210 240 270 Days from first injection 9 Mean percent change (±95% CI) P-value for all comparisons to placebo <0.0001
Efficacy: Two dose starting regimen Clamped PCSK9 knockdown End of study if LDL-C back to baseline 20 Placebo 0 100 mg -20 200 mg -40 300 mg -60 -80 0 30 60 90 120 150 180 210 240 270 Days from first injection 10 Mean percent change (±95% CI) P-value for all comparisons to placebo <0.0001
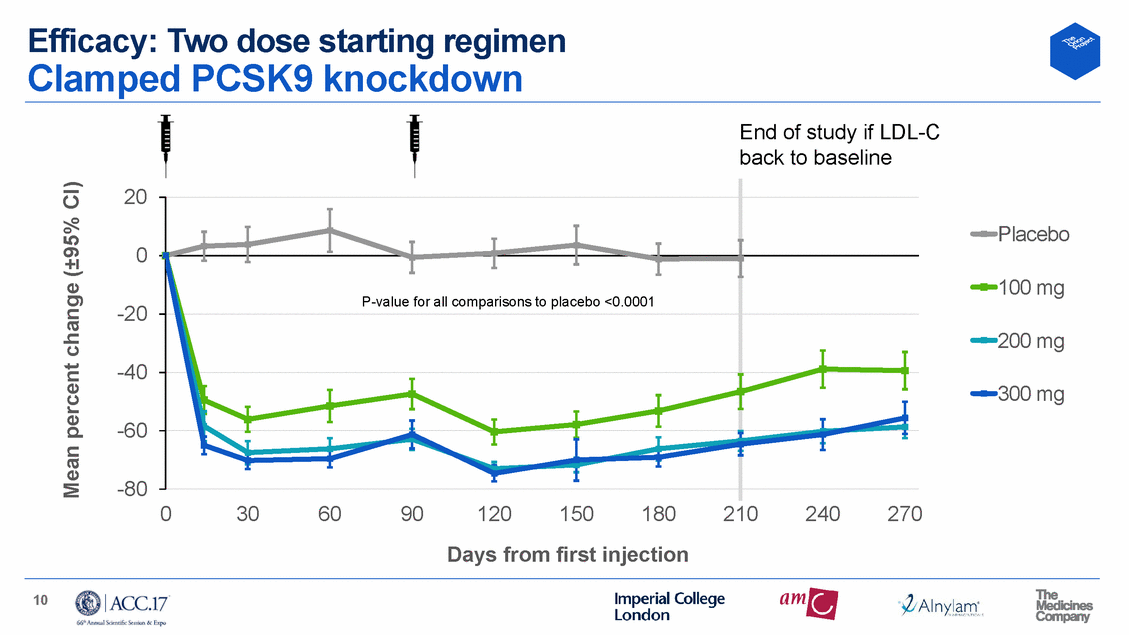
Efficacy: One dose starting regimen Robust, sustained LDL-C reductions – 300 mg optimal 300 mg 300 mg 9 months time adjusted 50.9% reduction 38.4% reduction mean 41% reduction 10 0 -10 -20 -30 -40 -50 -60 Placebo 200 mg 300 mg 500 mg 0 30 60 90 120 150 180 210 240 270 Days from first injection 11 Mean percent change (±95% CI) P-value for all comparisons to placebo <0.0001
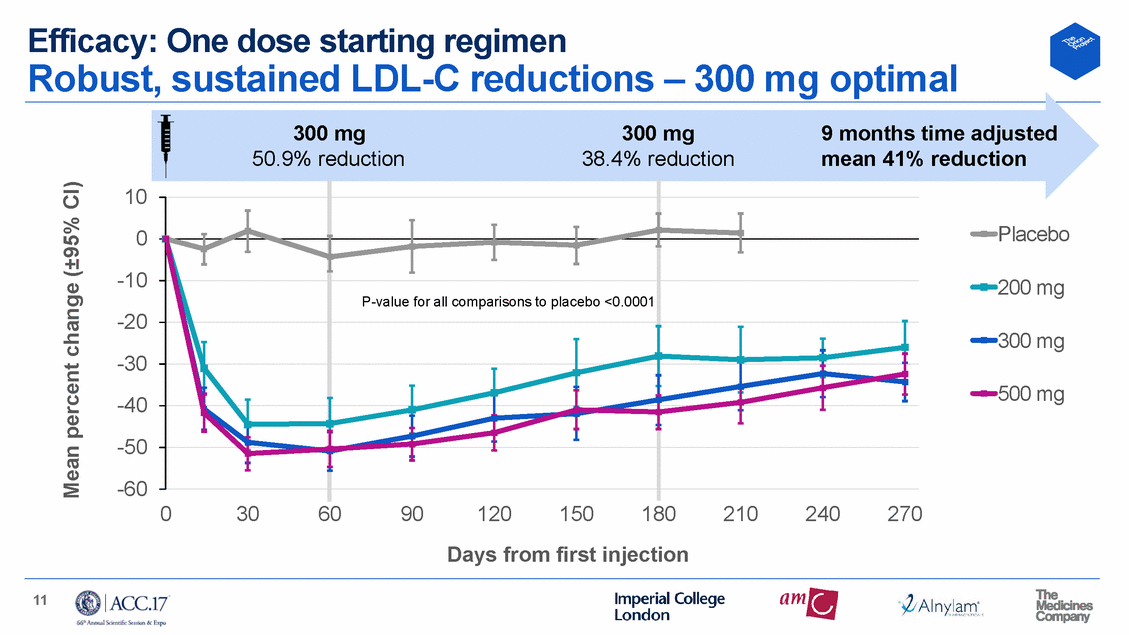
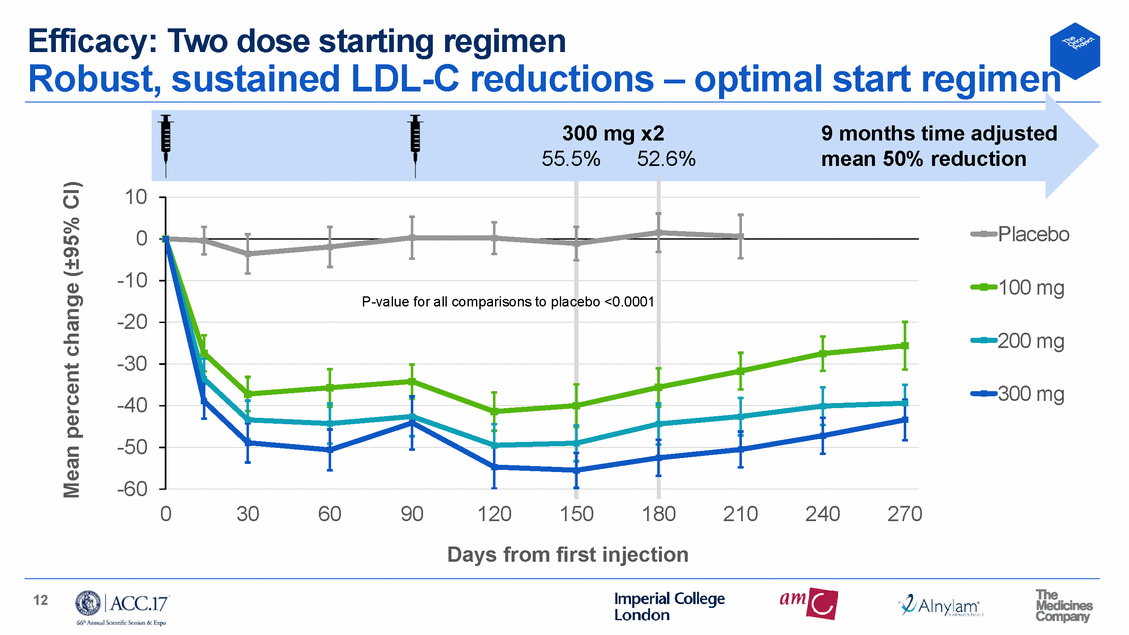
Efficacy: Two dose starting regimen Robust, sustained LDL-C reductions – optimal start regimen 300 mg x2 9 months time adjusted 55.5% 52.6% mean 50% reduction 10 0 -10 -20 -30 -40 -50 -60 Placebo 100 mg 200 mg 300 mg 0 30 60 90 120 150 180 210 240 270 Days from first injection 12 Mean percent change (±95% CI) P-value for all comparisons to plac ebo <0.0001
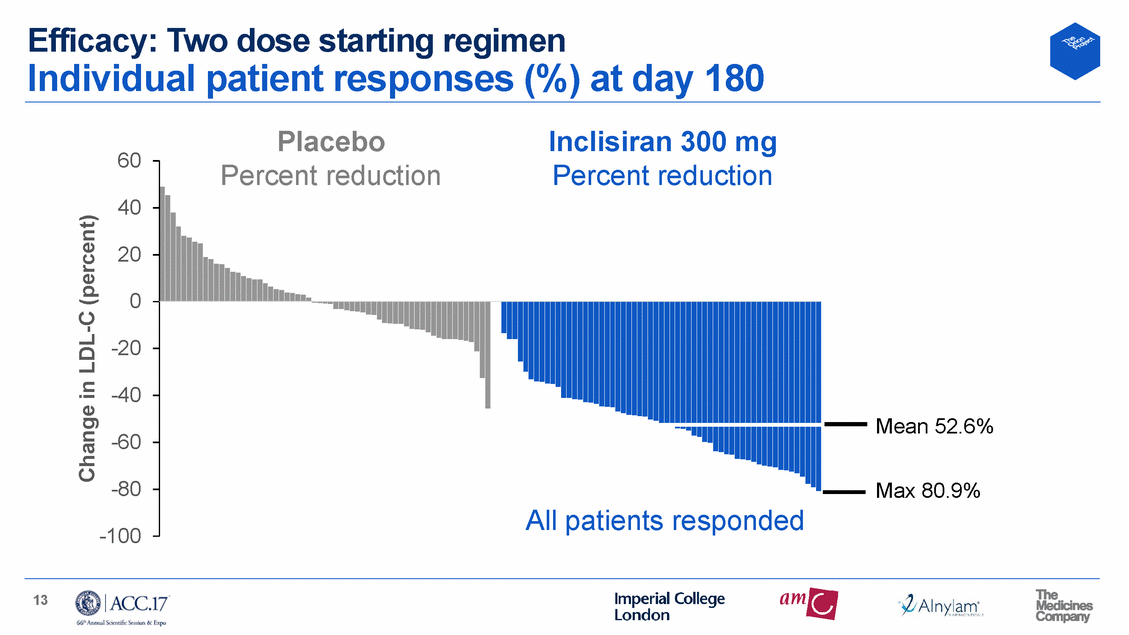
Efficacy: Two dose starting regimen Individual patient responses (%) at day 180 Placebo Inclisiran 300 mg 60 rcent reduction Percent reduction 40 20 0 -20 -40 Mean 52.6% -60 -80 Max 80.9% -100 13 Change in LDL-C (percent) Pe All patients responded
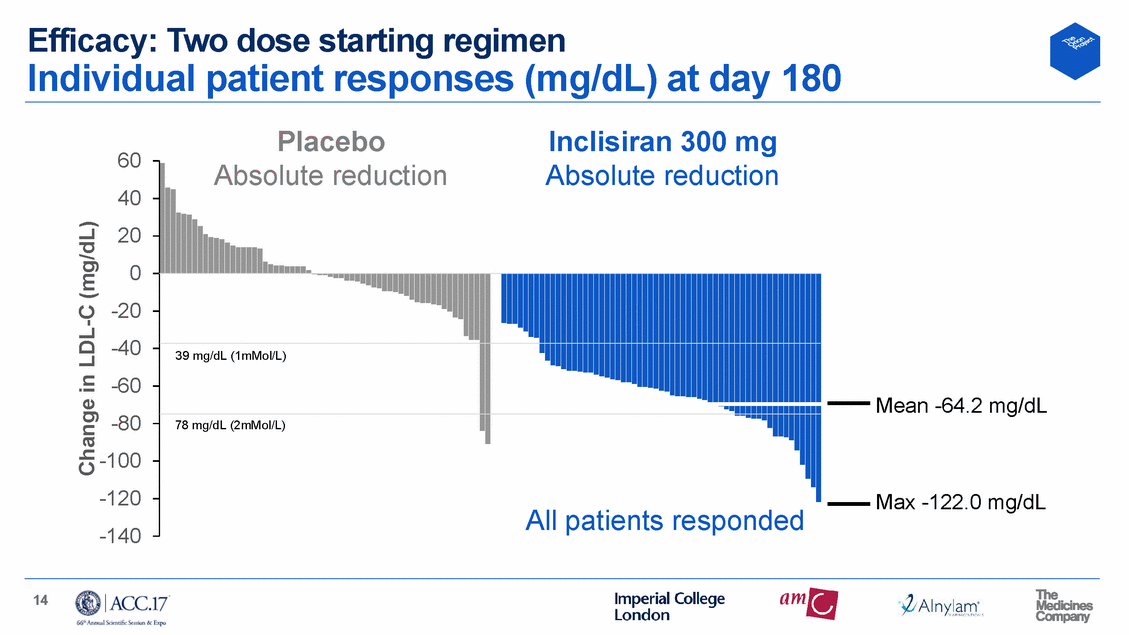
Efficacy: Two dose starting regimen Individual patient responses (mg/dL) at day 180 Placebo Inclisiran 300 mg 60 40 20 0 -20 -40 -60 -80 -100 -120 -140 ute reduction Absolute reduction Mean -64.2 mg/dL Max -122.0 mg/dL 14 Change in LDL-C (mg/dL) Absol 39 mg/dL (1mMol/L) 78 mg/dL (2mMol/L) All patients responded
Conclusions Two 300 mg starting dose regimen for inclisiran selected No safety concerns Optimal dosage 300 mg given twice as starting regimen then Q6 monthly • • All patients responded with significant LDL-C lowering At 6 months, mean LDL-C of 52.6% (64 mg/dL), and up to 81% (122 mg/dL) Unique attributes of inclisiran address multiple unmet needs • • • • LDL-C variability within individuals is practically Injection burden reduced substantially Sustained effect between infrequent injections Opportunity to improve patient adherence eliminated 15
Implications Inclisiran will move into phase III trials In an ORION-1-like population, inclisiran 300 mg delivers sustained LDL-C lowering of 60-65 mg/dL In a CVOT, this is likely to confer substantial reductions in MACE ORION-4 will study CV outcomes with inclisiran in high risk primary secondary prevention patients with average LDL-C ~130 mg/dL and 16
Online publication New England Journal of Medicine, 17th March 2017 T he NEW ENGLAND JOURNAL of MEDICINE II o_R_I_GI_N_A_L_A_R_TI_c_LE Inclisiran in Patients at High Cardiovascular Risl< with Elevated LDL Cholesterol Kausik K. Ray, M.D., Ulf Landmesser, M.D., Lawrence A. Leiter, M.D., David Kallend, M.D., Robert Dufour, M.D., Mahir Karakas, M.D., Tim Hall, M.D., Roland P.T. Troquay, M.D., Traci Turner, M.D., Frank L.J. Visseren, M.D., PeterWijngaard, Ph.D., R. Scott Wright, M.D., andJohnJ.P. Kastelein, M.D., Ph.D. The ImperialCollege London 17 @I ACC.lt Medicines Company

