Attached files
| file | filename |
|---|---|
| 8-K - 8-K - KUBOTA PHARMACEUTICAL HOLDINGS CO LTD | kph20170221-8xkanalystmeet.htm |

Kubota Pharmaceutical Holdings is
committed to translating innovation
into a diverse portfolio of drugs and
devices to preserve and restore
vision for millions of people
worldwide.
Kubota Pharmaceutical Holdings
FY2016 Analyst Meeting
Tokyo
February 22, 2017

Cautionary Statement Regarding
Forward Looking Statements
2
This presentation contains forward-looking statements concerning our product development, our ability to successfully commercialize our product candidates, our
technology, our competitors, our intellectual property, our financial condition and operating results and our plans for research and development programs and the
timing thereof that involve risks, uncertainties and assumptions. Such forward-looking statements typically can be identified by the use of words such as “expect,”
“estimate,” “anticipate,” “forecast,” “intend,” “project,” “target,” “plan,” “believe” and similar terms and expressions. These statements are based on the current
estimates and assumptions of the management of Kubota Pharmaceutical Holdings Co., Ltd. as of the date of this presentation and are subject to uncertainty and risks
in circumstances, including, but not limited to the risk that our product candidates will not demonstrate the expected benefits and will not achieve regulatory approval
or be successfully commercialized, the risk of delays in our ongoing or expected clinical trials, the risk that new developments in the intensely competitive ophthalmic
pharmaceutical and device markets require changes in our clinical trial plans or limit the potential benefits of our product candidates, the accuracy of our estimates of
the size and characteristics of the markets that may be addressed by our product candidates, the risk that our pre-clinical development efforts may not yield additional
product candidates, and other risks and uncertainties inherent in the process of discovering and developing therapeutics and devices that demonstrate safety and
efficacy. Given these uncertainties, you should not place undue reliance upon these forward-looking statements. Such forward-looking statements are subject to risks,
uncertainties, assumptions and other factors that may cause our actual results to be materially different from those reflected in such forward-looking statements.
Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, those set forth in
our reports on file with the Tokyo Securities Exchange and the United States Securities and Exchange Commission. The Company does not undertake any obligation to
release publicly any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated
events. All statements contained in this presentation are made only as of the date of this presentation.
“Acucela,” the Acucela logo and “Kubota” are among the registered trademarks or trademarks of Kubota Pharmaceutical Holdings in various jurisdictions.
Kubota Pharmaceutical Holdings Co., Ltd. [4596]

Company Overview
An Ophthalmology-Focused, Science-Driven Company
3
Partnership
• Partnership with YouHealth and University of
California, San Diego to address cataracts and
presbyopia with lanosterol
• Partnership with The University of Manchester to
address retinal degenerative disease with gene
therapy
• Partnership with the University of Southern California
and EyeMedics to address retinal neovascular
disease using biomimetic technology
Financials
• Cash, short-term and long-term investments as of
12/31/16 was JPY16.5 billion to invest in ongoing
programs, business development and internal
research and development
People and Strategy
• Executive leadership with experience in health care
management, life science administration &
technology
• Broad-skilled employee base in R&D and operations
with broad industry relationships
• Strategic plan to develop an innovative portfolio of
ophthalmology products
• Quick Win – Fast Fail
Internal Research
• We believe an investment chasm exists for
exploratory projects for unmet medical needs in the
US, creating an opportunity to bridge the chasm
between early and late stage development by
focusing on innovative ophthalmic technologies
• Emixustat, a visual cycle modulator with novel
mechanism of action for retinal diseases with unmet
medical need
• PBOS - Patient Based Ophthalmology Suite
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
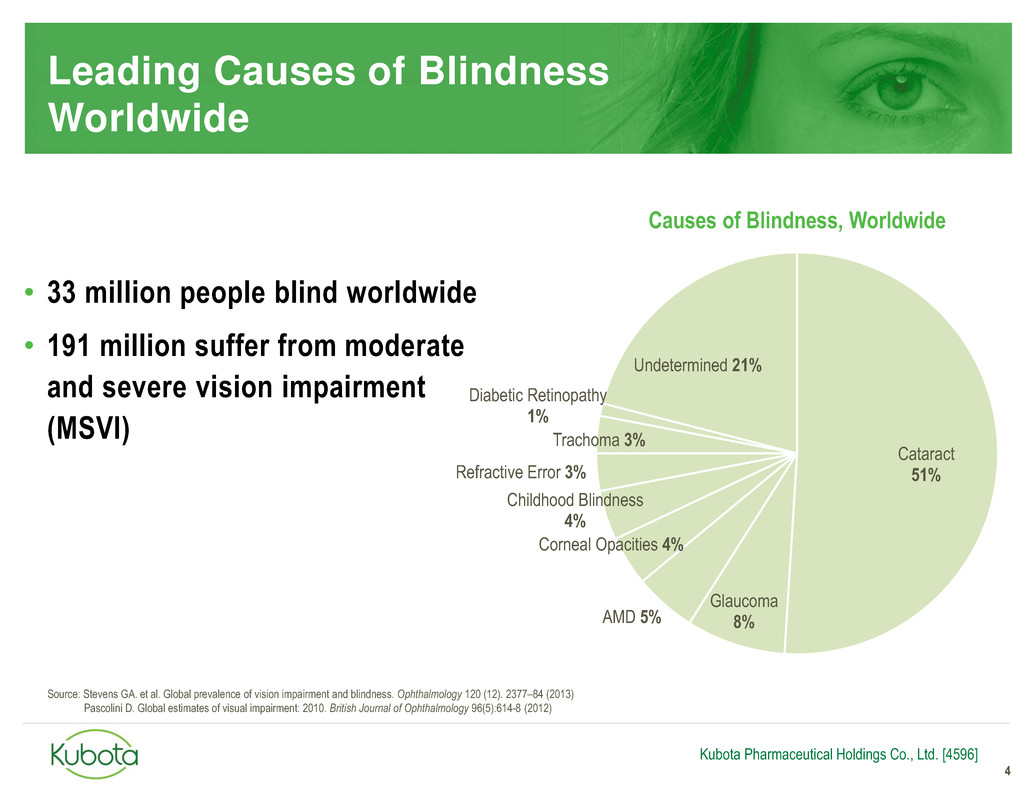
Cataract
51%
Glaucoma
8% AMD 5%
Corneal Opacities 4%
Childhood Blindness
4%
Refractive Error 3%
Trachoma 3%
Diabetic Retinopathy
1%
Undetermined 21%
Leading Causes of Blindness
Worldwide
4
• 33 million people blind worldwide
• 191 million suffer from moderate
and severe vision impairment
(MSVI)
Source: Stevens GA. et al. Global prevalence of vision impairment and blindness. Ophthalmology 120 (12). 2377–84 (2013)
Pascolini D. Global estimates of visual impairment: 2010. British Journal of Ophthalmology 96(5):614-8 (2012)
Causes of Blindness, Worldwide
Kubota Pharmaceutical Holdings Co., Ltd. [4596]

Leading Causes of Blindness
in Japan, US and Europe
5
Glaucom
a
21%
16%
8%
34%
10%
Source:
1) 厚生労働省 難治性疾患等克服研究事業「網膜脈絡膜・視神経萎縮症に関する調査研究 平成25(2013)年度」報告書 (A 2013 Report by Ministry of Health, Labour and Welfare)
2) Nathan C. et al. Causes and Prevalence of Visual Impairment Among Adults in the United States. Arch Ophthalmol122 (2004)
3) Kocur I, Resnikoff S. Visual Impairment and blindness in Europe and their prevention. British Journal of Ophthalmology 86, 716-722 (2002)
AMD 26%
Glaucom
a 20%
DR 9%
Others
45%
Retinitis
Pigmentosa 11%
AMD
Retinochoroidal
Atrophy
Others
DR AMD
54%
Catara
ct 9%
Glauco
ma 6%
DR 5%
Other
25%
Japan1 Unites States2 Europe3
Kubota Pharmaceutical Holdings Co., Ltd. [4596]

Global revenue forecast to reach $17.5B in 2020
CAGR 6.7% from 2017 – 2020
6
Sources:
Visiongain, Macular Degeneration (AMD) and Other Retinal Diseases: World Drug Industry and Market 2015-2025。
Market Scope, 2015 Comprehensive Report on the Global IOL Market。
Retinal Diseases Market Forecast: Global Market
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
(US$ in millions)
1,716 1,986 2,246 2,492
6,220 6,424
6,676 6,767
429
818
1,036
1,285 2,359
2,453
2,520
2,570
3,651
3,864
4,092
4,358
2017 2018 2019 2020
DR Wet AMD Dry AMD Other Retinal Dieases IOL
14,375
17,472
15,545
16,570
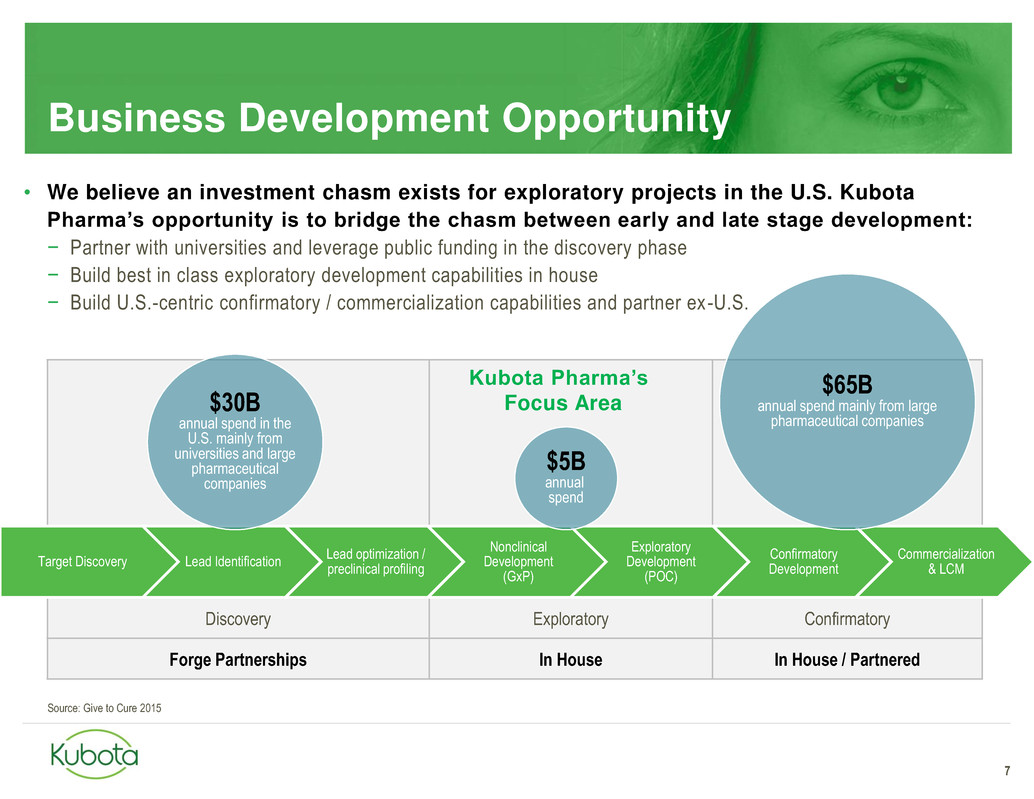
Discovery Exploratory Confirmatory
Forge Partnerships In House In House / Partnered
Business Development Opportunity
7
• We believe an investment chasm exists for exploratory projects in the U.S. Kubota
Pharma’s opportunity is to bridge the chasm between early and late stage development:
− Partner with universities and leverage public funding in the discovery phase
− Build best in class exploratory development capabilities in house
− Build U.S.-centric confirmatory / commercialization capabilities and partner ex-U.S.
Target Discovery Lead Identification
Lead optimization /
preclinical profiling
Nonclinical
Development
(GxP)
Exploratory
Development
(POC)
Confirmatory
Development
Commercialization
& LCM
Source: Give to Cure 2015
$30B
annual spend in the
U.S. mainly from
universities and large
pharmaceutical
companies
$5B
annual
spend
$65B
annual spend mainly from large
pharmaceutical companies
Kubota Pharma’s
Focus Area
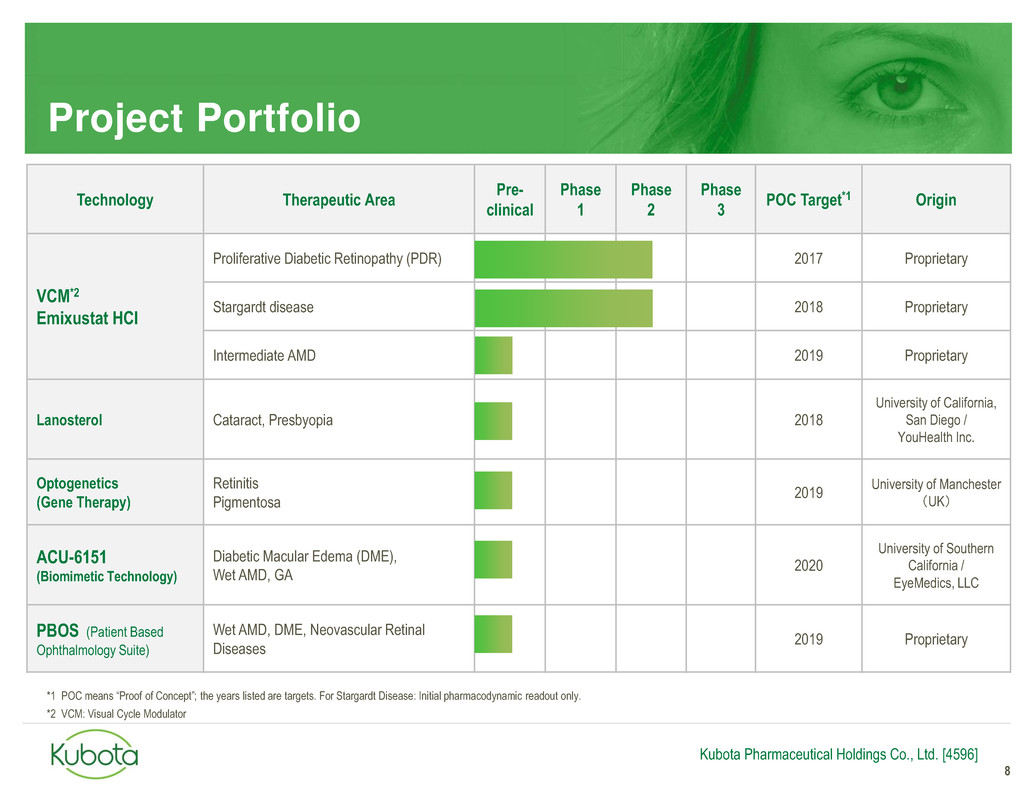
Project Portfolio
8
Technology Therapeutic Area
Pre-
clinical
Phase
1
Phase
2
Phase
3
POC Target*1 Origin
VCM*2
Emixustat HCI
Proliferative Diabetic Retinopathy (PDR) 2017 Proprietary
Stargardt disease 2018 Proprietary
Intermediate AMD 2019 Proprietary
Lanosterol Cataract, Presbyopia 2018
University of California,
San Diego /
YouHealth Inc.
Optogenetics
(Gene Therapy)
Retinitis
Pigmentosa
2019
University of Manchester
(UK)
ACU-6151
(Biomimetic Technology)
Diabetic Macular Edema (DME),
Wet AMD, GA
2020
University of Southern
California /
EyeMedics, LLC
PBOS (Patient Based
Ophthalmology Suite)
Wet AMD, DME, Neovascular Retinal
Diseases
2019 Proprietary
*1 POC means “Proof of Concept”; the years listed are targets. For Stargardt Disease: Initial pharmacodynamic readout only.
*2 VCM: Visual Cycle Modulator
Kubota Pharmaceutical Holdings Co., Ltd. [4596]

Emixustat Hydrochloride
for Diabetic Retinopathy
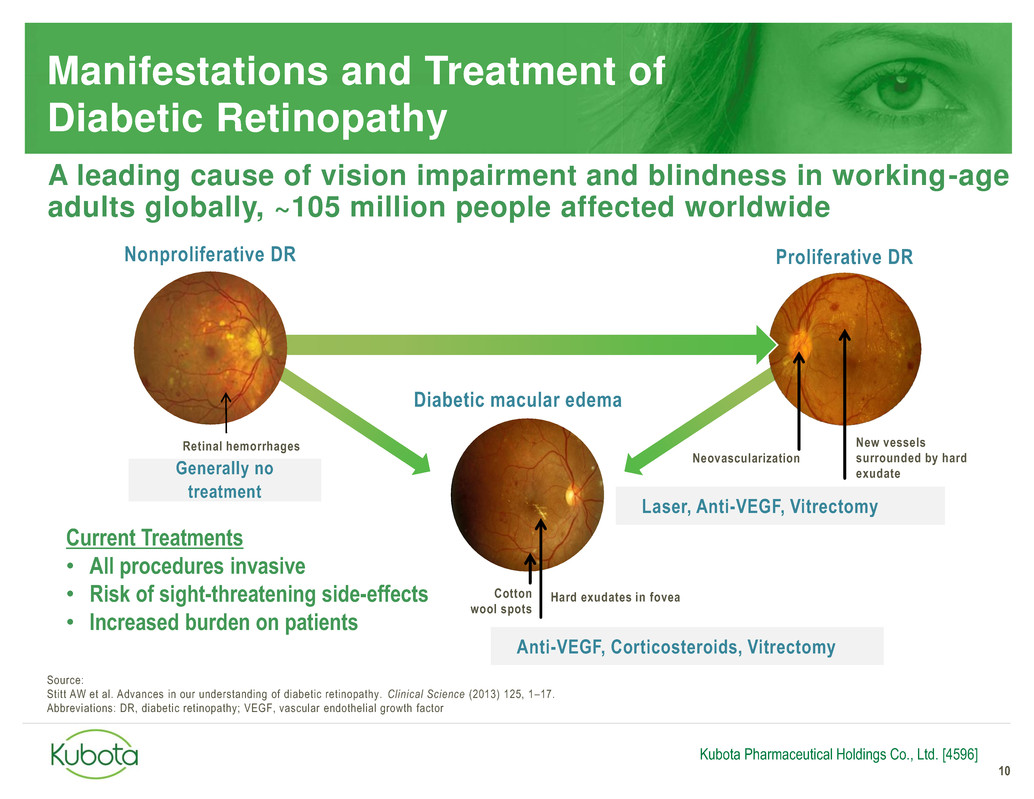
Generally no
treatment
Anti-VEGF, Corticosteroids, Vitrectomy
Manifestations and Treatment of
Diabetic Retinopathy
10
Diabetic macular edema
Hard exudates in fovea Cotton
wool spots
Neovascularization
Proliferative DR
New vessels
surrounded by hard
exudate
Nonproliferative DR
Retinal hemorrhages
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
Current Treatments
• All procedures invasive
• Risk of sight-threatening side-effects
• Increased burden on patients
Laser, Anti-VEGF, Vitrectomy
A leading cause of vision impairment and blindness in working-age
adults globally, ~105 million people affected worldwide
Source:
Stitt AW et al. Advances in our understanding of diabetic retinopathy. Clinical Science (2013) 125, 1–17.
Abbreviations: DR, diabetic retinopathy; VEGF, vascular endothelial growth factor

Emixustat: a Potential Paradigm Shift
in the Treatment of Diabetic Retinopathy
11
Mimic constitutive
phototransduction
Reduction in
neovascularization
Oral administration
Potential to transform
current therapeutic
landscape of invasive
treatment modalities
To benefit more patients
• Initial focus on PDR, the
most serious form of DR
• Evaluate potential in slowing NPDR
progression to PDR
and also DME
Highly
O2-consuming
dark current
Emixustat
Totally new approach to
diabetic retinopathy
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
Abbreviations: DME, diabetic macular edema; DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PDR, proli ferative diabetic retinopathy

Phase 2 Study of Emixustat in Proliferative
Diabetic Retinopathy (PDR) is Underway
12
• Multicenter (up to 6 clinical sites in the US)randomized, double-blinded, placebo-
controlled
• Up to 20 patients diagnosed with PDR will take emixustat (up to 40 mg, step up
titration) or placebo once daily for 85 days
• Evaluations
− Change in biomarkers of diabetic retinopathy disease
− Effects on retinal hemorrhage, neovascularization, and vision
2016 2017 2018 2019
Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2
Phase 2(1)(2)
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
POC (Proof of Concept) Target 2017
Source:
1) ClinicalTrials.gov identifier NCT02753400. Available at: https://clinicaltrials.gov/ct2/show/NCT02753400?term=emixustat&ra nk=1. Accessed February 14, 2017.
2) Acucela press release. May 8, 2016. Available at: http://acucela.com/Read -About-Us/Press-Releases/160508_PDR.

Forecast for the Global Diabetic Retinopathy(1)
Market
13
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
1,716
1,986
2,246
2,492
2017 2018 2019 2020
Source:
Visiongain, Macular Degeneration (AMD) and Other Retinal Diseases: World Drug Industry and Market 2015-2025。
1) Including Proliferative diabetic retinopathy and diabetic macular edema
CAGR 13.3%
(US$ in millions)

Emixustat Hydrochloride
for Stargardt Disease

Stargardt Disease: a Genetic Disorder
Stargardt disease is a genetically inherited disorder of the
retina
15
• Stargardt is a serious unmet medical need, with fewer than 150,000
people estimated to be patients in the US, Japan and Europe,
combined.
• There are many symptoms a person with Stargardt may experience
including spots in the vision, color vision deficits, distortion,
blurriness, and loss of central vision in both eyes.
• Mutations in a gene called ABCA4 are the most common cause of
Stargardt disease.
• Kubota Pharma receives Orphan Drug Designation from the FDA for
the treatment of Stargardt disease in January 2017
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
Source: National Eye Institute. https://nei.nih.gov/health/stargardt/star_facts. Retrieved July 2, 2016

Stargardt Disease: Proposed Disease
Progression and Emixustat Mechanism of Action
16
Photoreceptor Cell Death
Death of RPE
RPE Dysfunction
Accumulation of lipofuscin (lipid, proteins, vitamin A toxins) leading to free
radical production and activation of complement system
Defective gene: ABCA4
Blocks the
accumulation of
lipofuscin and
vitamin A toxins
Emixustat
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
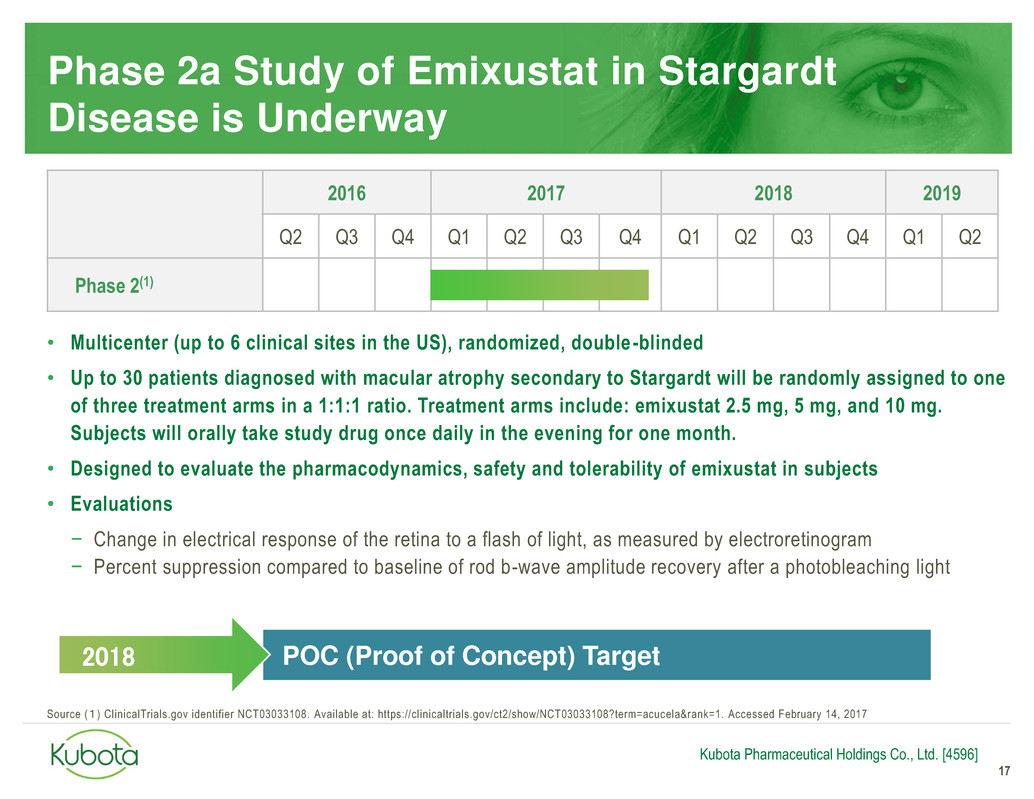
Phase 2a Study of Emixustat in Stargardt
Disease is Underway
17
• Multicenter (up to 6 clinical sites in the US), randomized, double -blinded
• Up to 30 patients diagnosed with macular atrophy secondary to Stargardt will be randomly assigned to one
of three treatment arms in a 1:1:1 ratio. Treatment arms include: emixustat 2.5 mg, 5 mg, and 10 mg.
Subjects will orally take study drug once daily in the evening for one month.
• Designed to evaluate the pharmacodynamics, safety and tolerability of emixustat in subjects
• Evaluations
− Change in electrical response of the retina to a flash of light, as measured by electroretinogram
− Percent suppression compared to baseline of rod b-wave amplitude recovery after a photobleaching light
2016 2017 2018 2019
Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2
Phase 2(1)
Source (1) ClinicalTrials.gov identifier NCT03033108. Available at: https://clinicaltrials.gov/ct2/show/NCT03033108?term=acucela&rank=1. Accessed February 14, 2017
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
POC (Proof of Concept) Target 2018

Lanosterol for Cataracts
and Presbyopia

Protein Aggregation in Crystalline
Lens of the Eye Leads to Cataract
19
Clear Vision
Highly ordered
proteins
Impact on Vision
Cloudy Vision
Denatured
proteins
Low protein order
/ protein
aggregation
Denatured proteins aggregate and obstruct light, causing cloudy vision
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
Source: Zhao L, Chen XJ, Zhu J, et al. Lanosterol reverses protein aggregation in cataracts. Nature 523 (7562). 607-611 (2015)
National Eye Institute. https://nei.nih.gov/health/cataract/cataract_facts. Retrieved July 2, 2016

Lanosterol:
New Treatment Potential
20
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
Currently, there is no non-surgical treatment available for
cataracts
1. Multiple incisions are made to access the lens
2. High-frequency ultrasound breaks up the lens into small pieces, then removed with
suction (phacoemulsification)
3. A clear intraocular lens is inserted in the same location the natural lens occupied
4. The incisions are closed and a protective shield is placed over the eye (in some
procedures)
Lanosterol
• Non-invasive, pharmacological treatment
• Dissolves protein aggregates and establishes order
• Naturally occurring compound in the human body
• Has the potential to reverse lens opacification
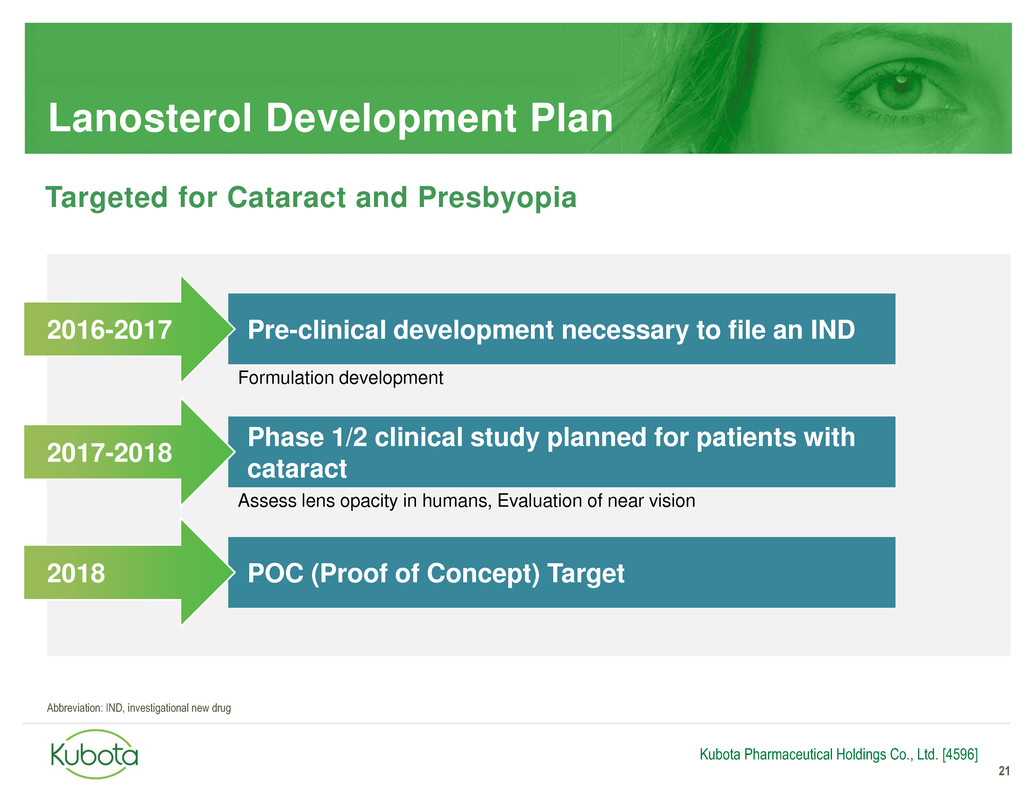
Lanosterol Development Plan
21
Pre-clinical development necessary to file an IND
Phase 1/2 clinical study planned for patients with
cataract
2016-2017
2017-2018
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
Assess lens opacity in humans, Evaluation of near vision
Formulation development
Targeted for Cataract and Presbyopia
POC (Proof of Concept) Target 2018
Abbreviation: IND, investigational new drug

Forecast for the Global IOL Market
22
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
3,651
3,864
4,092
4,358
2017 2018 2019 2020
Source:
Market Scope, 2015 Comprehensive Report on the Global IOL Market
CAGR 6.1%
(US$ in millions)

Optogenetics for
Retinitis Pigmentosa

Retinitis Pigmentosa is a Blinding Disease
24
• Affects roughly 1 in 4,000 people, both in the United States and worldwide (NEI)1
• Approximately 1.5 million people worldwide are affected by the disease2
• Earliest and most frequent symptom is night blindness, decreased vision or tunnel vision 3
• Progressive loss of vision in childhood leads generally to blindness by age 404
• The cause of RP is complex, with over 100 different gene mutations identified5
NORMAL VISION DECREASED VISION TUNNEL VISION BLINDNESS
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
Source:
1) Genetics Home Reference, Retinitis Pigmentosa. https://ghr.nlm.nih.gov/condition/retinitis-pigmentosa. Retrieved Nov 7, 2016
2) Vaidya P, Vaidaya A. Retinitis Pigmentosa: Disease Encumbrance in the Eurozone. Int J Ophthalmol Clin Res. 2:030 (2015)
3) MedilinePlus, Retinitis Pigmentosa. https://medlineplus.gov/ency/article/001029.htm. Retrieved Nov 7, 2016
4) American Academy of Ophthalmology. Retinitis Pigmentosa Causes. http://www.aao.org/eye-health/diseases/retinitis-pigmentosa-cause, Retrieved July 2, 2016
5) National Human Genome Research Institute. Leaning About Retinitis Pigmentosa. https://www.genome.gov/13514348/. Retrieved Nov 7, 2016
Abbreviations: NEI, National Eye Institute; RP, retinitis pigmentosa
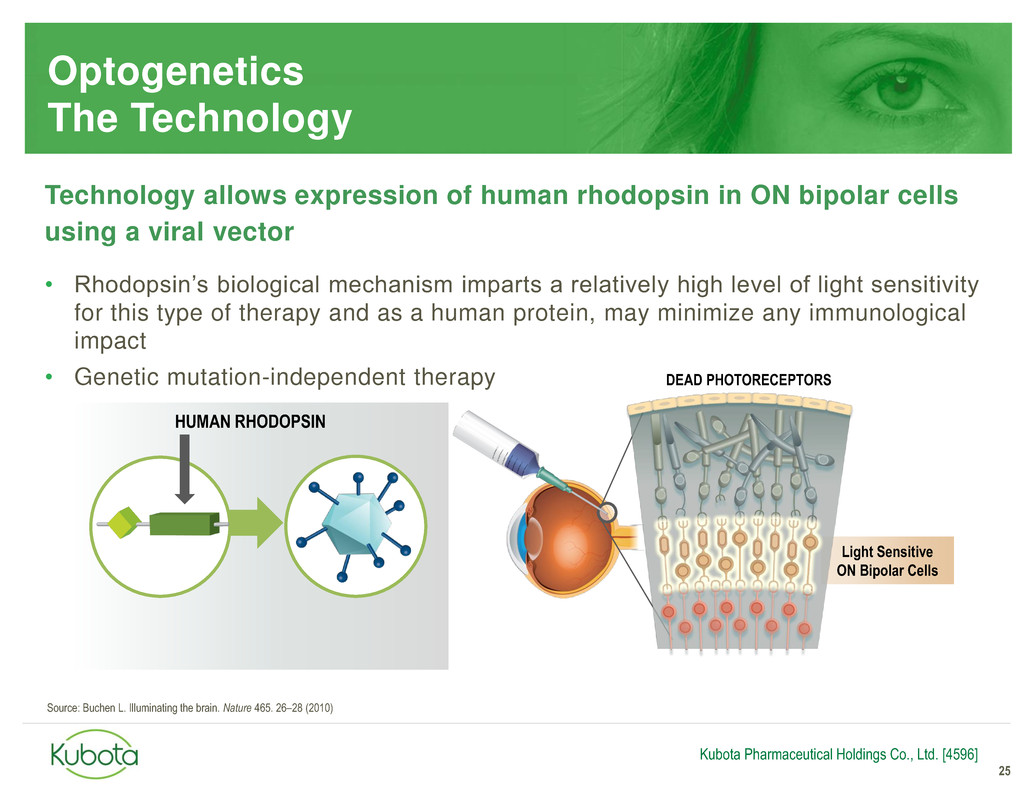
Optogenetics
The Technology
Technology allows expression of human rhodopsin in ON bipolar cells
using a viral vector
• Rhodopsin’s biological mechanism imparts a relatively high level of light sensitivity
for this type of therapy and as a human protein, may minimize any immunological
impact
• Genetic mutation-independent therapy
25
HUMAN RHODOPSIN
Light Sensitive
ON Bipolar Cells
DEAD PHOTORECEPTORS
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
Source: Buchen L. Illuminating the brain. Nature 465. 26–28 (2010)
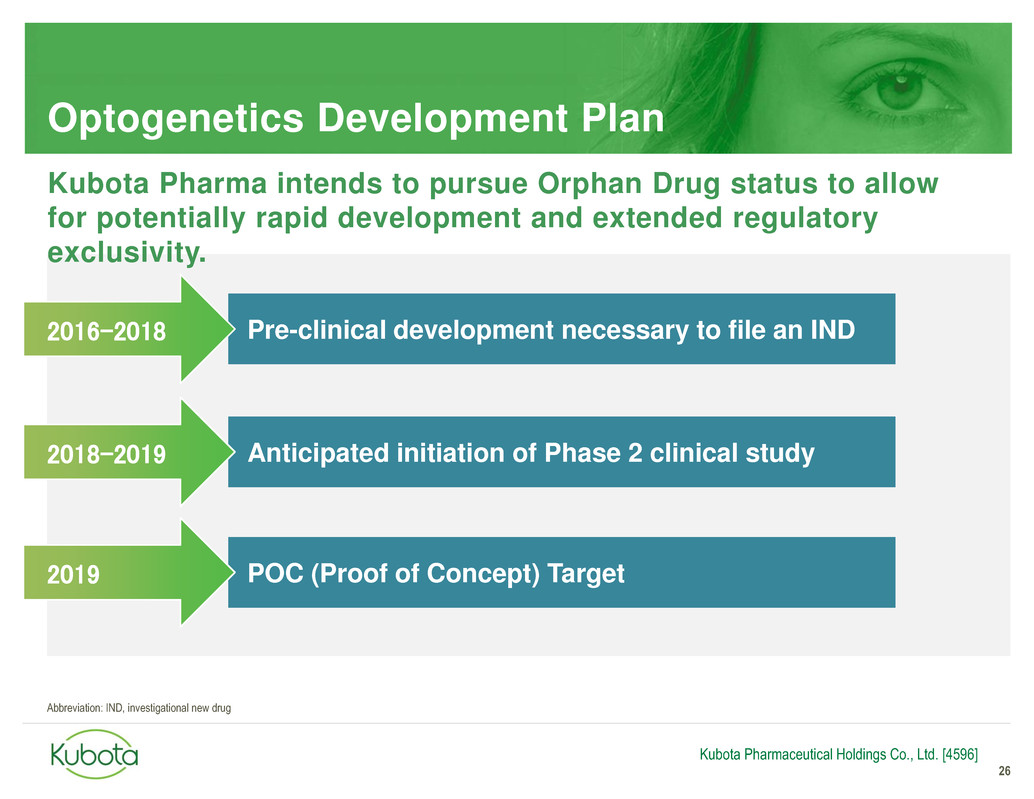
Optogenetics Development Plan
26
Pre-clinical development necessary to file an IND
Anticipated initiation of Phase 2 clinical study
2016-2018
2018-2019
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
Kubota Pharma intends to pursue Orphan Drug status to allow
for potentially rapid development and extended regulatory
exclusivity.
POC (Proof of Concept) Target 2019
Abbreviation: IND, investigational new drug

ACU-6151
Biomimetic Technology

Retinal Vascular Abnormalities are a Core Pathology in
Multiple Sight-threatening Ocular Diseases
28
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
Reduced visual
acuity
Loss of vision
Potentially
irreversible
blindness
Vascular leakage
Proliferation of new abnormal blood
vessels at back of eye
Persistent inflammation
Disrupted ocular architecture
Proliferative
diabetic
retinopathy
Retinal vein
occlusion*
Retinopathy
of prematurity
Diabetic patients Older people
Premature babies
Vascular Abnormalities
Diabetic retinopathies
Diabetic
macular
edema
Wet AMD*
*Predominantly but not exclusively in older people
Wet AMD: wet age-related macular degeneration

Biomimetic Technology
Potential small molecule pharmacologic treatment for retinal
neovascular disease
29
• The proprietary technology modulates endogenous factors released during the
inflammatory process at the early pathogenic stages of diabetic macular edema
(DME), age-related macular degeneration (wet AMD and GA associated with dry
AMD) and other retinal neovascular conditions.
− Demonstrated the ability to inhibit vascular endothelial growth factor (VEGF) induced
vascular leakage comparable to anti-VEGF therapy, and without loss of native
microvasculature
− Vessels appeared to be better preserved than with the anti -VEGF treatment
suggesting less occlusion
• Evaluate the potential of these proprietary molecules to be dosed less frequently
and exert therapeutic effects over a longer period of time than current anti -VEGF
biologic drugs used as standard of care.
− Potential of this novel therapeutic approach to provide better outcomes and an
improved delivery paradigm, administered either intravitreally or orally, for patients
suffering from a variety of retinal neovascular diseases
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
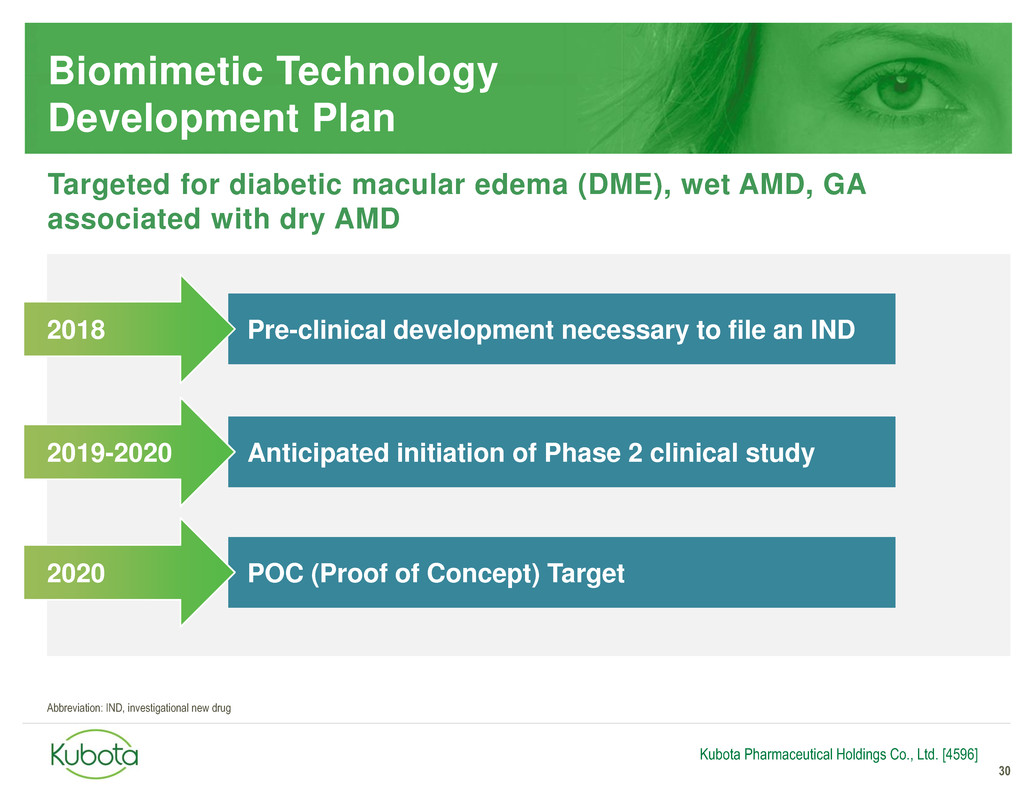
Biomimetic Technology
Development Plan
30
Pre-clinical development necessary to file an IND
Anticipated initiation of Phase 2 clinical study
2018
2019-2020
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
Targeted for diabetic macular edema (DME), wet AMD, GA
associated with dry AMD
POC (Proof of Concept) Target 2020
Abbreviation: IND, investigational new drug

Source:
Visiongain, Macular Degeneration (AMD) and Other Retinal Diseases: World Drug Industry and Market 2015-2025
Forecast for the Global Wet AMD Market
31
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
6,220 6,424
6,676 6,767
2017 2018 2019 2020
CAGR 2.9%
(US$ in millions)

PBOS
Patient Based Ophthalmology Suite

PBOS
Patient Based Ophthalmology Suite
At home, patient self-use retinal monitoring device
33
• Increase in demand for mobile Health applications (mHealth) in home care and
remote medical care field
• In its first iteration, the PBOS aims to improve treatment outcome in patients
diagnosed and treated for wet AMD, DME and other neovascular retinal diseases
• The PBOS is being designed to detect nascent disease progression and support
patient re-treatment prior to irreversible vision loss due to disease progression.
• Key Component of the PBOS
− Micromini handheld device equipped with optical coherence tomography
− Network connectivity and cloud based technologies
− Alert the patients and their physicians of disease progression and re-treatment needs
− Patient administered device used to capture changes in retinal anatomy
− Improve distribution of routine and emergency health services and provide diagnostic
services
Kubota Pharmaceutical Holdings Co., Ltd. [4596]

PBOS:Micromini Handheld Device Equipped with
Optical Coherence Tomography Development Plan
34
Invention and prototyping
Anticipated initiation of pre-clinical and clinical
studies
2017-2018
2019
Kubota Pharmaceutical Holdings Co., Ltd. [4596]
Targeted for wet AMD, DME, and other neovascular retinal
disease
POC (Proof of Concept) Target 2019
Abbreviation: IND, investigational new drug

FY2016 Financial Results

Overview of FY2016: P/L
JPY in thousands
December
31, 2015
December
31, 2016
Reasons for change
Revenues from collaboration 2,902,721 870,198
The decrease in revenue from collaborations was primarily due to billing fewer full-time
employees as a result of the completion of the Emixustat clinical trial during the year and wind-
down activities related to Emixustat following the termination of our collaboration with Otsuka.
Wind-down activities related to Emixustat were completed in December 2016.
Expenses
R&D 2,730,128 2,370,363
Emixustat 2,540,027 1,324,661
Lower expenditures as the Phase 2b/3 clinical trial came to completion in May 2016 and
expenses associated with wind-down activities.
Internal research 189,962 1,044,622
Higher expenditures in connection with the Company’s Strategic Plan to in-license new molecules
and compounds and self-fund related research activities.
OPA 6566 139 1,080 Expenses associated with wind-down activities.
General and administrative 3,375,512 2,620,904
Decrease primarily due to lower: (i) stock based compensation expense of ¥396mn; (ii) charges
related to the one-time May 2015 special meeting of the shareholders and related transaction
costs of ¥271mn; (iii) charges related to bonus and retention payments of ¥151mn; (iv)
severance costs of ¥139mn.
The decrease was partially offset by increase in legal expenses and charges related to the
Redomicile Transaction of ¥372mn.
Income (loss) from operations -3,202,919 -4,121,069
Net income (loss) -3,076,641 -3,952,549
Note: Figures of the former Acucela Inc. are used for FY 2015 for the sake of convenience of our shareholders.
36
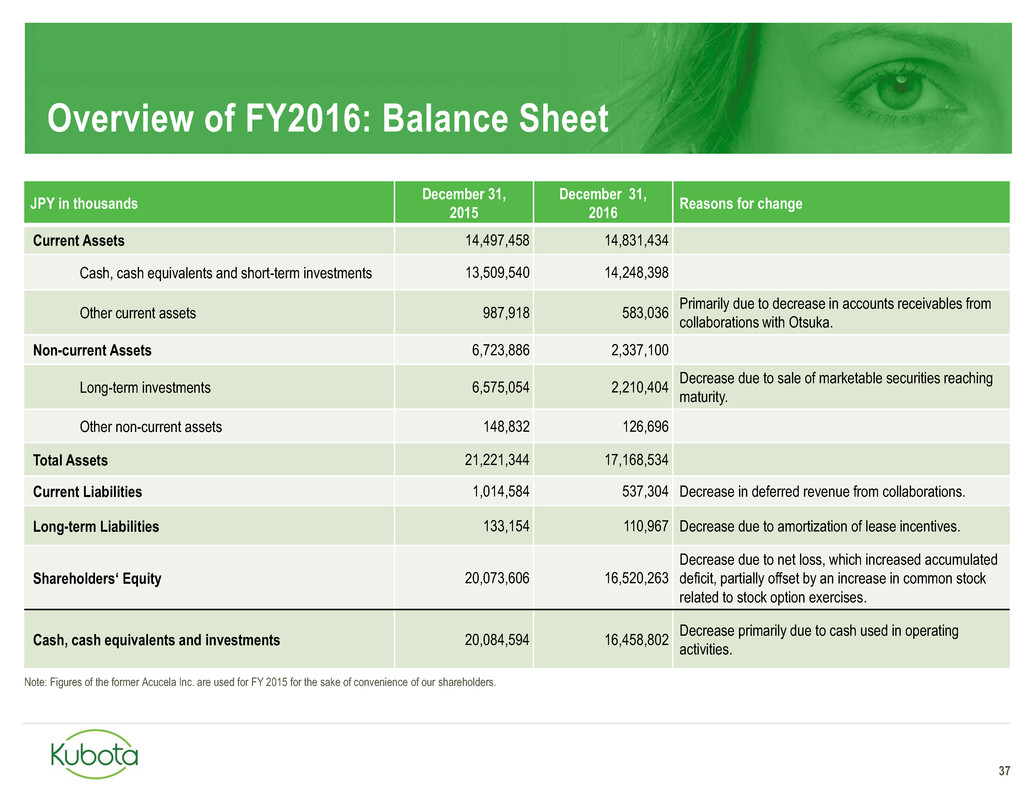
Overview of FY2016: Balance Sheet
37
JPY in thousands
December 31,
2015
December 31,
2016
Reasons for change
Current Assets 14,497,458 14,831,434
Cash, cash equivalents and short-term investments 13,509,540 14,248,398
Other current assets 987,918 583,036
Primarily due to decrease in accounts receivables from
collaborations with Otsuka.
Non-current Assets 6,723,886 2,337,100
Long-term investments 6,575,054 2,210,404
Decrease due to sale of marketable securities reaching
maturity.
Other non-current assets 148,832 126,696
Total Assets 21,221,344 17,168,534
Current Liabilities 1,014,584 537,304 Decrease in deferred revenue from collaborations.
Long-term Liabilities 133,154 110,967 Decrease due to amortization of lease incentives.
Shareholders‘ Equity 20,073,606 16,520,263
Decrease due to net loss, which increased accumulated
deficit, partially offset by an increase in common stock
related to stock option exercises.
Cash, cash equivalents and investments 20,084,594 16,458,802
Decrease primarily due to cash used in operating
activities.
Note: Figures of the former Acucela Inc. are used for FY 2015 for the sake of convenience of our shareholders.

Financial Outlook*
38
• Outlook for Revenue from collaborations
− The Company is pursuing various partnering efforts and is expecting to generate
revenue in the future through collaborations with strategic partners.
• Outlook for loss from operations
− Total research and development expenses are expected to increase as the Company
pursues development of its product candidates in multiple indications and executes
in-licensing transactions resulting in potential upfront and milestone payments.
− The Company is conducting a comprehensive evaluation of its cost structure.
* Forward-looking financial information and estimates contained in this presentation were previously disclosed by the Company in the Company’s Kessan Tanshin dated February 14, 2017 and/or in the Company’s Form 8-K
filed with the SEC on February 14, 2017. Such forward-looking financial information and estimates speak only as of the dates of initial disclosure and this presentation is neither updating nor confirming the previously provided
forward-looking financial information, guidance and estimates.
Kubota Pharmaceutical Holdings Co., Ltd. [4596]

848 844
(23)
(3,203)
(4,121) (4,290)
(6,000)
(4,000)
(2,000)
0
2,000
FY12 FY13 FY14 FY15 FY16 FY17 Est.
Operating Profit (Loss)
Financial Overview*
39
5,672 6,519
23,756
21,221
17,169
0
5,000
10,000
15,000
20,000
25,000
FY12 FY13 FY14 FY15 FY16
Total Assets
3,088 3,754
22,236
20,074
16,520
0
5,000
10,000
15,000
20,000
25,000
FY12 FY13 FY14 FY15 FY16
Total Shareholder's Equity
5,599
6,386
4,269
2,903
870
0
0
2,000
4,000
6,000
8,000
FY12 FY13 FY14 FY15 FY16 FY17 Est.
Revenues from Collaboration
(JPY in millions)
* Forward-looking financial information and estimates contained in this presentation were previously disclosed by the Company in the Company’s Kessan Tanshin dated February 14, 2017 and/or in the Company’s Form 8-K
filed with the SEC on February 14, 2017. Such forward-looking financial information and estimates speak only as of the dates of initial disclosure and this presentation is neither updating nor confirming the previously provided
forward-looking financial information, guidance and estimates.
Figures of the former Acucela Inc. are used for FY2015 and earlier for the sake of convenience of our shareholders.
Kubota Pharmaceutical Holdings Co., Ltd. [4596]

Appendix

Management Team
Globally experienced management team
in the ophthalmology industry
41
Lukas Scheibler, PhD
EVP R&D, Acucela Inc.
Alcon
Novartis Pharmaceuticals
John Gebhart
Chief Financial Officer
Qliance Medical Management Consultant to Remote
Medical International, Ventripoint, and others
Ted Danse, MBA
Chief Business Officer, Acucela Inc.
Neurotech Pharmaceuticals, Inc.
ISTA Pharmaceuticals Inc., Allergan, Inc.,
Coopervision, Bausch & Lomb , Schering-Plough
Ryo Kubota, MD, PhD
Chairman, President and CEO
Kubota Pharmaceutical Holdings Co., Ltd. [4596]

Board of Directors
42
Directors Background
Ryo Kubota, MD PhD Chairman, President and Chief Executive Officer and also founder of Acucela Inc.
Shintaro Asako
Chief Executive Officer and Chief Financial Officer — DeNA Corp
Previously: Chief Financial Officer — MediciNova, Inc.
Shiro Mita, PhD
President and Chief Executive Officer — M’s Science Corporation
Previously: Executive Director of Drug Discovery, Director — Santen Pharmaceuticals Co., Ltd
Eisaku Nakamura
Corporate Auditor — Koinobori Associates Inc.
Previously: Director and General Manager — Bio Sight Capital Co., Ltd , Chief Executive Officer and
President — Berevno Corporation, Board of Directors — CanBas Corporation and Activus Pharma Co. Ltd.
Robert Takeuchi
President — RT Consulting, Inc.
Previously: President — SOFTBANK Finance, America Corporation, Director of International Equity Sales —
Credit Suisse First Boston, Board of Directors SBI Investment Co., Ltd. and Quark Pharmaceuticals, Inc.
Kubota Pharmaceutical Holdings Co., Ltd. [4596]

Recent Events
43
Date Highlights
Mar 17 ‘16 Acucela Secures Option to Exclusively License Novel Cataract Treatment
April 5 ‘16 Acucela Licenses Gene Therapy from The University of Manchester for Retinal Degenerative Disease
May 25 ’16 Announcement of Top-Line Results from Phase 2b/3 Clinical Trial of Emixustat
S.E.A.T.T.L.E. study failed to demonstrate a reduction in GA lesion growth rate
Jun 13 ’16 Announcement on the Termination of Emixustat Agreement and OPA-6566 Agreement with Otsuka Pharmaceutical, and
Postponement of Annual Shareholder Meeting
Oct 18 ‘16 Acucela Issues Notice of Resolutions of the Annual Meeting of Shareholders
Dec 6 ‘16 Listing of Kubota Pharmaceutical Holdings Co., Ltd. and Forecast for Fiscal Year End 2016
Dec 13 ‘16 Acucela Secures Option to Exclusively Acquire Novel Retinal Technology
Dec 26 ‘16 Kubota Pharmaceutical Holdings Signs Letter of Intent to Form a Joint Venture with SBI Holdings
Jan 5 ‘16 Acucela Receives Orphan Drug Designation from the FDA for the Treatment of Stargardt Disease
Jan 26 ‘17 Acucela Initiates Phase 2a Study of Emixustat Hydrochloride Addressing Patients with Stargardt Disease
Kubota Pharmaceutical Holdings Co., Ltd. [4596]

Kubota Pharmaceutical Holdings is
committed to translating innovation
into a diverse portfolio of drugs and
devices to preserve and restore
vision for millions of people
worldwide.
