Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SYNERGY PHARMACEUTICALS, INC. | a17-4543_18k.htm |
Exhibit 99.1
LEERINK GLOBAL HEALTHCARE CONFERENCE Marino Garcia EVP, Chief Strategy Officer February 15, 2017

SAFE HARBOR STATEMENT This presentation and any statements made for and during any presentation or meeting contain forward-looking statements related to Synergy Pharmaceuticals Inc. under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995 and are subject to risks and uncertainties that could cause actual results to differ materially from those projected. These statements may be identified by the use of forward-looking words such as "anticipate," "planned," "believe," "forecast," "estimated," "expected," and "intend," among others. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, the development, launch, introduction and commercial potential of TRULANCE™; growth and opportunity, including peak sales and the potential demand for TRULANCE, as well as its potential impact on applicable markets; market size; substantial competition; our ability to continue as a going concern; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payer reimbursement; dependence upon third parties; our financial performance and results, including the risk that we are unable to manage our operating expenses or cash use for operations, or are unable to commercialize our products, within the guided ranges or otherwise as expected; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. There are no guarantees that future clinical trials discussed in this presentation will be completed or successful or that any product will receive regulatory approval for any indication or prove to be commercially successful. Investors should read the risk factors set forth in our most recent periodic reports filed with the Securities and Exchange Commission, including our Form 10-K for the year ended December 31, 2015. While the list of factors presented here is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Forward-looking statements included herein are made as of the date hereof, and we do not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances except as required by law. The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation. Leerink Global Healthcare Conference I February 15, 2017 2
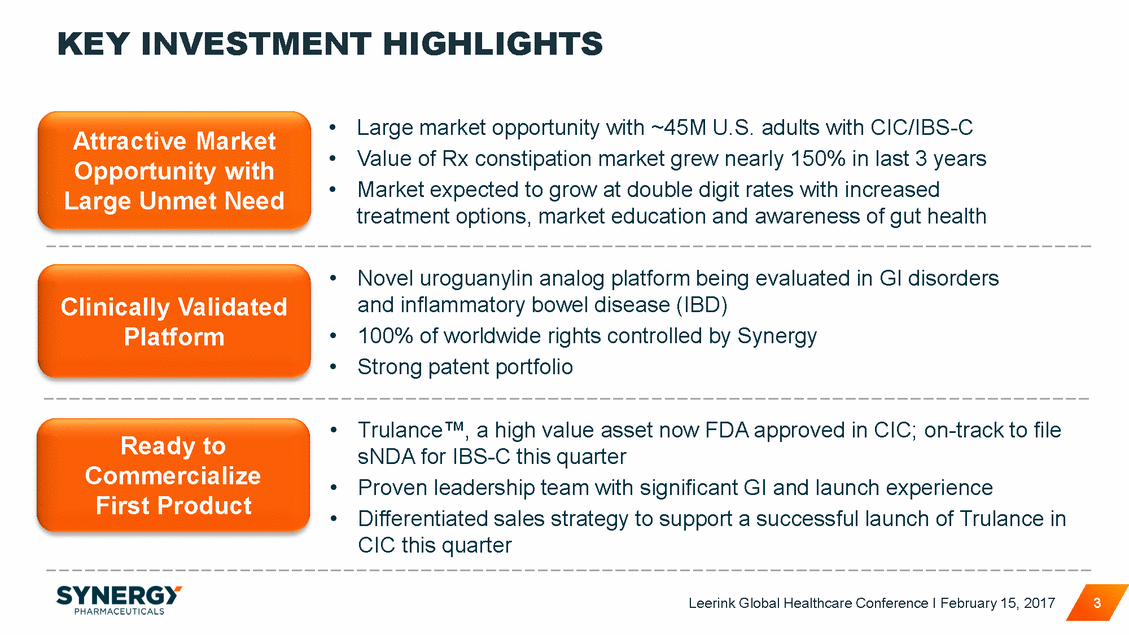
KEY INVESTMENT HIGHLIGHTS • • • Large market opportunity with ~45M U.S. adults with CIC/IBS-C Value of Rx constipation market grew nearly 150% in last 3 years Market expected to grow at double digit rates with increased treatment options, market education and awareness of gut health Attractive Market Opportunity with Large Unmet Need • Novel uroguanylin analog platform being evaluated in GI disorders and inflammatory bowel disease (IBD) 100% of worldwide rights controlled by Synergy Strong patent portfolio Clinically Validated Platform • • • Trulance™, a high value asset now FDA approved in CIC; on-track to file sNDA for IBS-C this quarter Proven leadership team with significant GI and launch experience Differentiated sales strategy to support a successful launch of Trulance in CIC this quarter Ready to Commercialize First Product • • Leerink Global Healthcare Conference I February 15, 2017 3
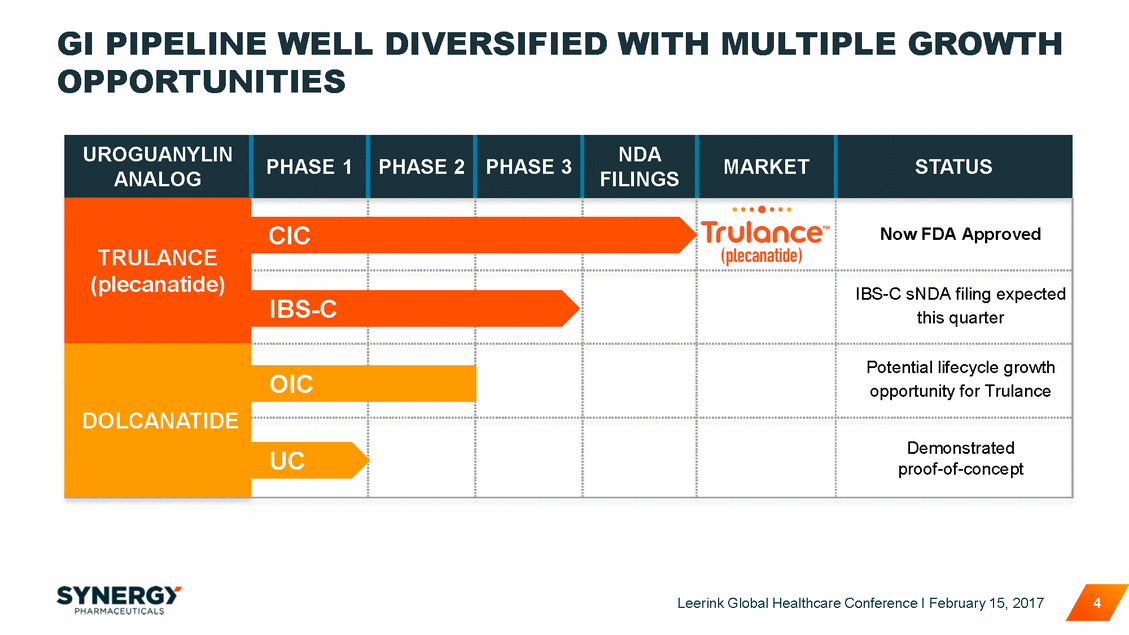
GI PIPELINE WELL OPPORTUNITIES DIVERSIFIED WITH MULTIPLE GROWTH this quarter proof-of-concept Leerink Global Healthcare Conference I February 15, 2017 4 UROGUANYLIN ANALOG PHASE 1 PHASE 2 PHASE 3 NDA FILINGS MARKET STATUS TRULANCE (plecanatide) CIC Now FDA Approved IBS-C IBS-C sNDA filing expected DOLCANATIDE Potential lifecycle growth opportunity for Trulance OIC UC Demonstrated
TRULANCE COMMERCIAL OPPORTUNITY 5

ESTIMATED 45 MILLION U.S. SUFFER FROM CIC OR IBS-C ADULTS COMMON SYMPTOMS: Estimated U.S. Prevalence (in millions) • Constipation (< 3 bowel movements per week for > 3 months) Hard or lumpy stools Incomplete bowel movements Straining CIC/IBS-C • • • GERD Migraine • • • Abdominal pain Constipation Incomplete bowel movements 0 1020 30 40 50 These are symptom-driven conditions that should be managed on a daily basis. There is no cure for CIC or IBS-C. Source: Johanson 2004; Suares 2011 Leerink Global Healthcare Conference I February 15, 2017 6 IBS-C CIC
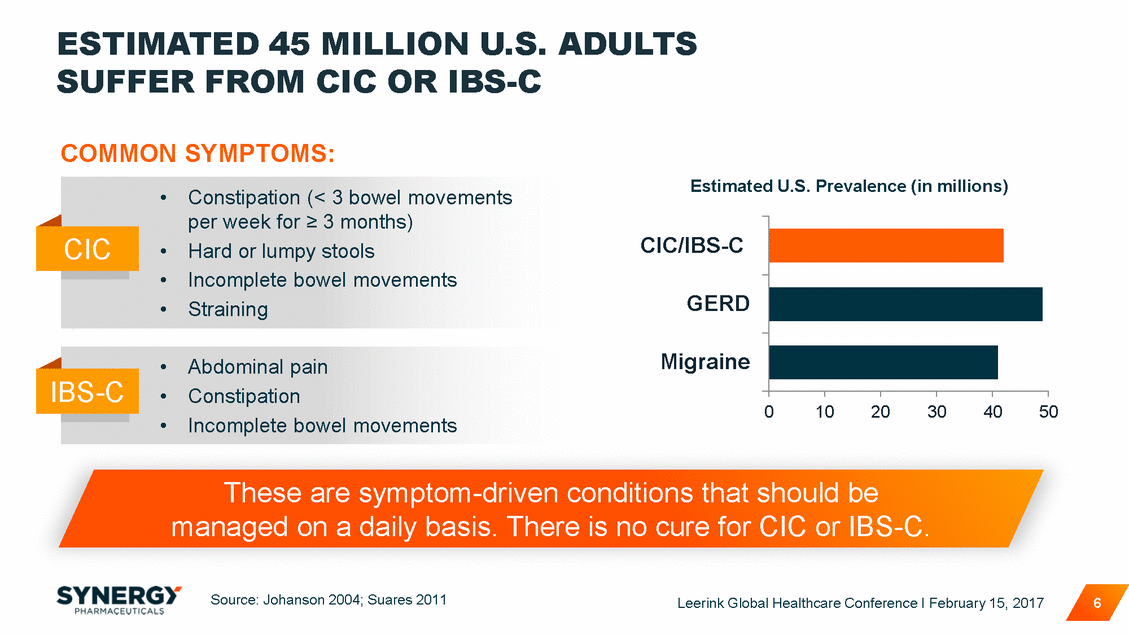
LIMITED PRESCRIPTION TREATMENT AND IBS-C AVAILABLE OPTIONS FOR CIC TYPICAL TREATMENT PROGRESSION Changes to Diet and Lifestyle (fiber, water intake, exercise) OTC Laxatives and Stool Softeners (Miralax®, Lactulose, etc.) Only 2 FDA Approved Products (Amitiza®, Linzess®) Leerink Global Healthcare Conference I February 15, 2017 7
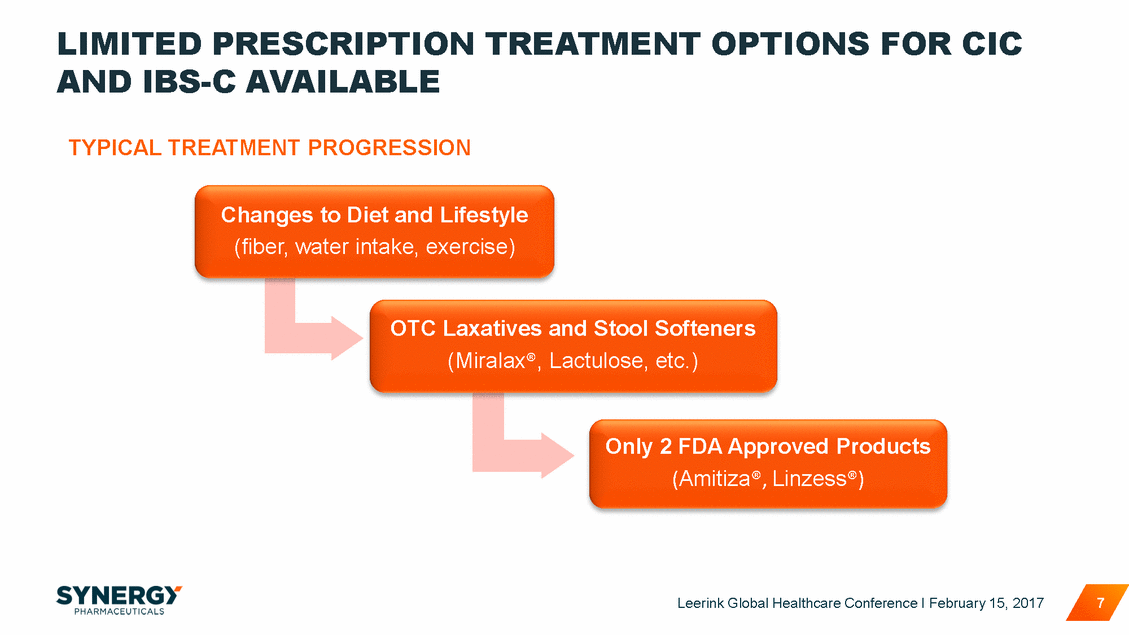
NEW ENTRANTS ARE GROWING THE MARKET, NOT CANNIBALIZING EXISTING PRODUCTS ~ 4 MM 20,000 16,465 373 15,000 10,000 5,000 0 2013 2014 2015 2016 All Other Rx = Rx lactulose & Rx polyethylene glycol Source: IMS Health Incorporated, NPA Leerink Global Healthcare Conference I February 15, 2017 8 TRxs in Thousands All Other RxAmitizaLinzess 2,691 2,112 2 1,488 1,476 1,345 1,288 13,412 12,878 12,606 11,729 15, 13,578 1,42 17,591 Last three years, TRx volume has grown by nearly 4 million or 30%
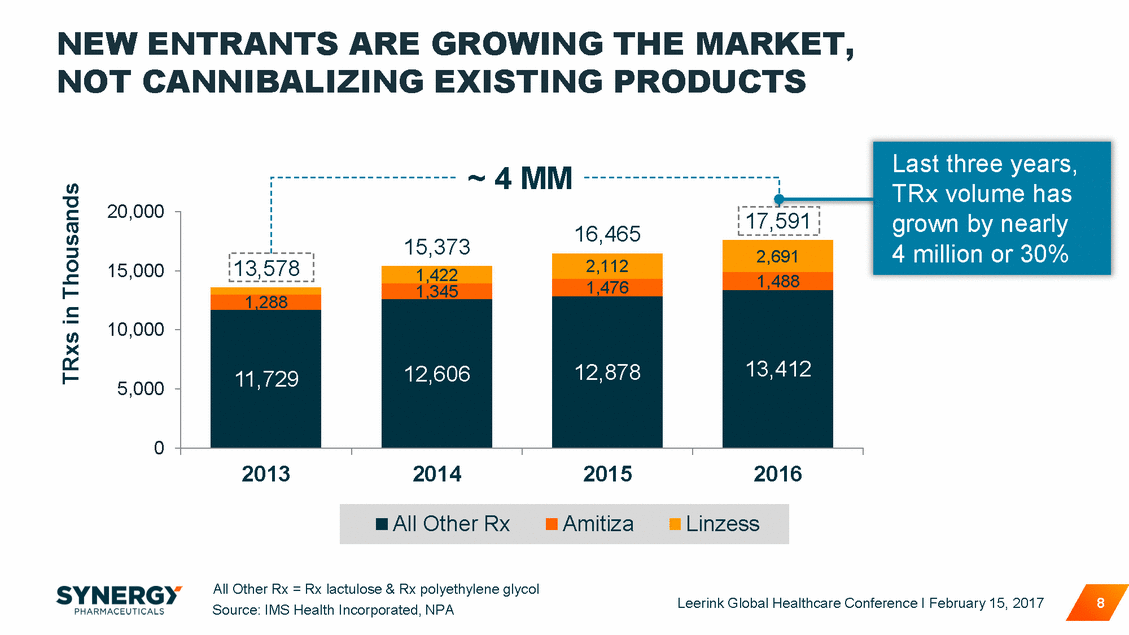
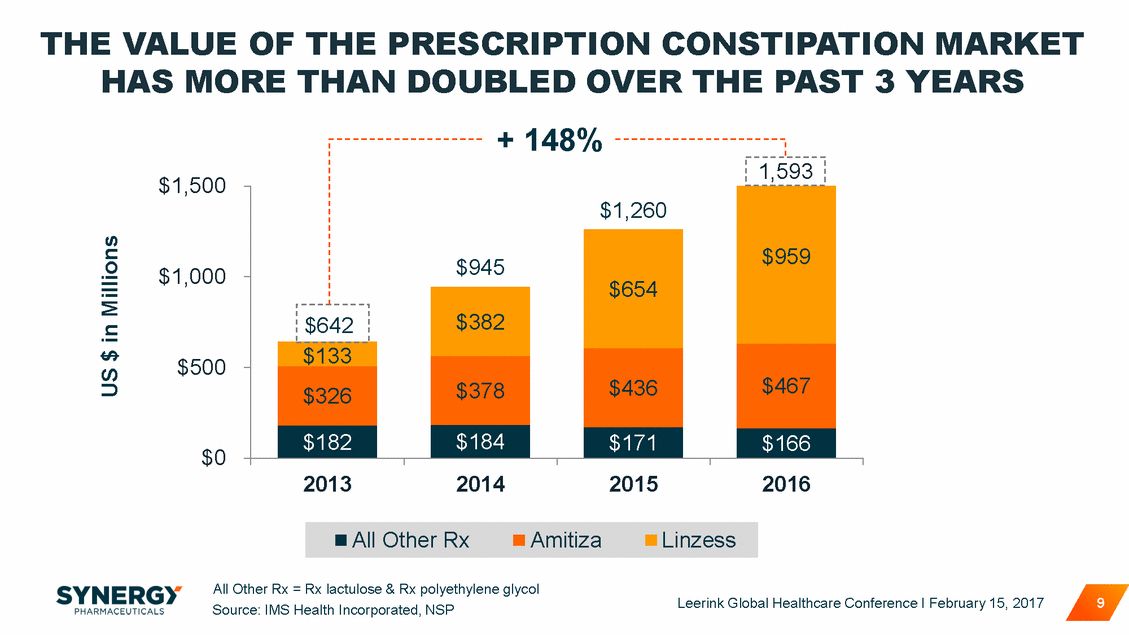
THE VALUE OF THE PRESCRIPTION CONSTIPATION MARKET HAS MORE THAN DOUBLED OVER THE PAST 3 YEARS + 148% $1,500 $1,000 $500 $0 2013 2014 2015 2016 All Other Rx = Rx lactulose & Rx polyethylene glycol Source: IMS Health Incorporated, NSP Leerink Global Healthcare Conference I February 15, 2017 9 US $ in Millions All Other RxAmitizaLinzess 1,593 $1,260 $959 $945 $654 $382 $642 $133 $467 $436 $378 $326 $166 $184 $171 $182
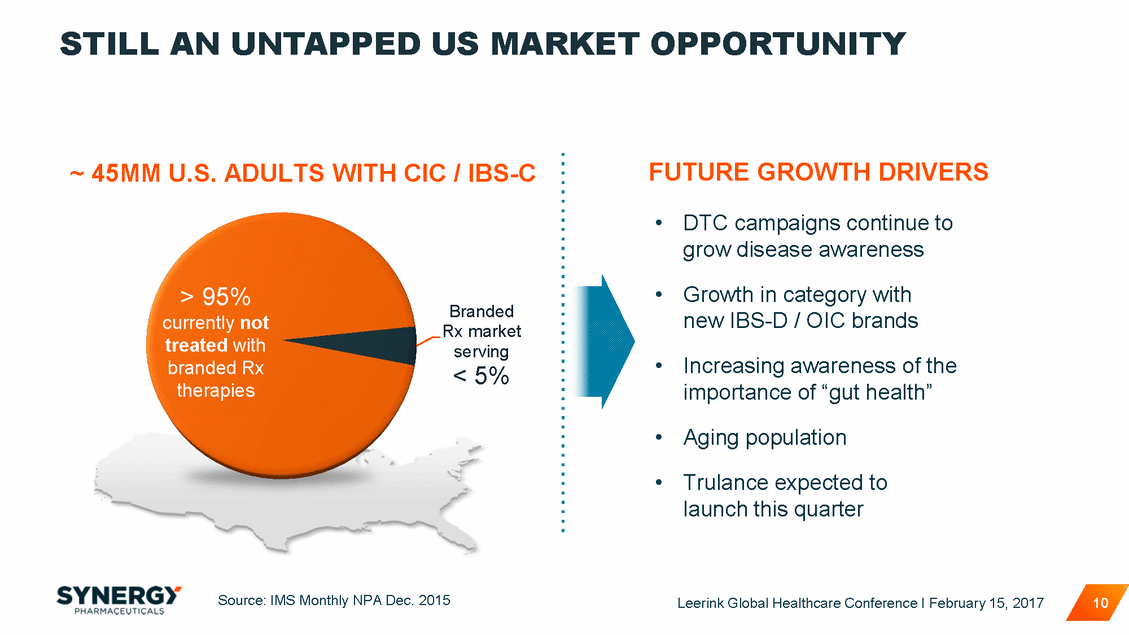
STILL AN UNTAPPED US MARKET OPPORTUNITY FUTURE GROWTH DRIVERS ~ 45MM U.S. ADULTS WITH CIC / IBS-C • DTC campaigns continue to grow disease awareness • Growth in category with new IBS-D / OIC brands > 95% currently not treated with branded Rx therapies Branded Rx market serving < 5% • Increasing awareness of the importance of “gut health” Aging population • • Trulance expected to launch this quarter Source: IMS Monthly NPA Dec. 2015 Leerink Global Healthcare Conference I February 15, 2017 10
TRULANCE COMMERCIAL & LAUNCH READINESS 11

PRODUCT QUARTER SUPPLY ON-TRACK FOR LAUNCH THIS Product Readiness Leerink Global Healthcare Conference I February 15, 2017 12 MAJOR INITIATIVES •Established robust supply chain process •Trade and sample stock ready for launch this quarter •Launching with 30-day blister pack
MARKET AND TRULANCE BRAND PREPARED FOR LAUNCH Market / Brand Readiness WAC = Wholesale Acquisition Cost Leerink Global Healthcare Conference I February 15, 2017 13 MAJOR INITIATIVES •Finalized pricing strategy --launching at WAC price of $353.48 •Completed extensive market research with more than 2,700 healthcare providers and over 5,300 patients •Conducted multiple advisory boards with national and regional GI key opinion leaders and payers •Conducted meetings with all key payers, representing ~230 million covered lives in the U.S. •Finalized Trulance core marketing strategies and launch tactics, including a compliant, value-optimizing, cost-effective promotional mix to reach broadest universe of prescribers •Initiated pre-launch multimedia and digital campaigns to drive company awareness and disease education, focusing on current unmet medical needs of patients with CIC
COMMERCIAL TEAM WITH SIGNIFICANT GI EXPERIENCE AND SEVERAL PRODUCT LAUNCHES Organizational Readiness - Commercial • • Over 25 years of experience in pharma: Shire, J&J & IMS Health Expertise in business analytics, market research, forecasting, call plan deployment & commercial IT infrastructure Significant GI/specialty and primary care experience Scott Brunetto VP, Commercial Operations • • • Over 25 years of healthcare experience: J&J & P&G Expertise in product launches, new product development, HCP & consumer marketing Significant GI/specialty and primary care experience Pam Cebulski VP, Marketing • • • Over 25 years of commercial leadership experience: Shire & AZ Expertise in general management, sales and marketing leadership, managed care strategy, and product launches Significant GI/specialty and primary care experience Marianne Jackson SVP, Sales & Market Access • Leerink Global Healthcare Conference I February 15, 2017 14
EXPERIENCED SALES AND MARKET ACCESS TEAM Organizational Readiness - Commercial • Synergy RBDs average over 11 years of management experience with over 10 years in relevant GI fields Hired from peer GI and PCP companies Regional Business Directors • • Implementing flexible and efficient hybrid sales force team to reach key prescribers and influencers at launch Hired our own Regional GI Account Specialists; averaging 13 years of pharma & 8.5 years of GI experience Partnered with Publicis Touchpoint to hire highly experienced sales reps fully dedicated to Trulance; averaging 11.5 years in pharma & 6 years of GI experience • Hybrid Sales Model • • National and Regional Market Access team members have been in the field introducing Synergy to payers since January 2016 Met with all key commercial and public payers, representing ~ 230 million lives Market Access Team • Leerink Global Healthcare Conference I February 15, 2017 15
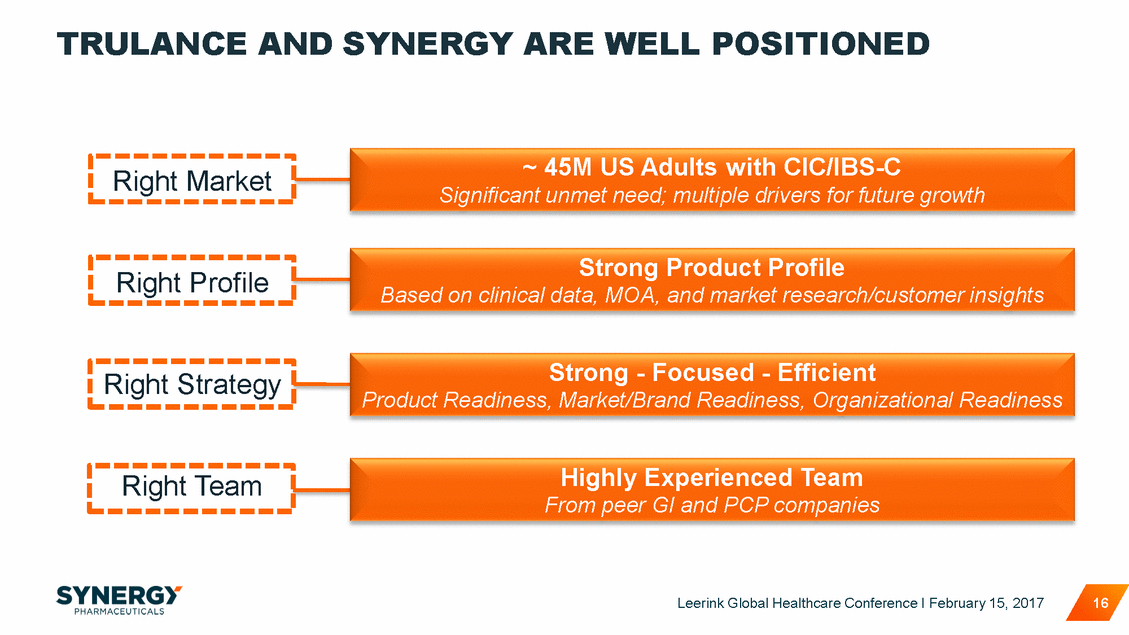
TRULANCE AND SYNERGY ARE WELL POSITIONED ~ 45M US Adults with CIC/IBS-C Significant unmet need; multiple drivers for future growth Strong Product Profile Based on clinical data, MOA, and market research/customer insights Strong - Focused - Efficient Product Readiness, Market/Brand Readiness, Organizational Readiness Highly Experienced Team From peer GI and PCP companies Leerink Global Healthcare Conference I February 15, 2017 16 Right Team Right Strategy Right Profile Right Market