Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AveXis, Inc. | a17-1660_18k.htm |
Exhibit 99.1
COMPANY OVERVIEW January 2017

2 Forward-Looking Statements This presentation contains forward-looking statements, including statements about: the timing, progress and results of preclinical studies and clinical trials for AVXS-101, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, our manufacturing strategy and developments, key regulatory and development milestones and our research and development programs. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Disclaimers

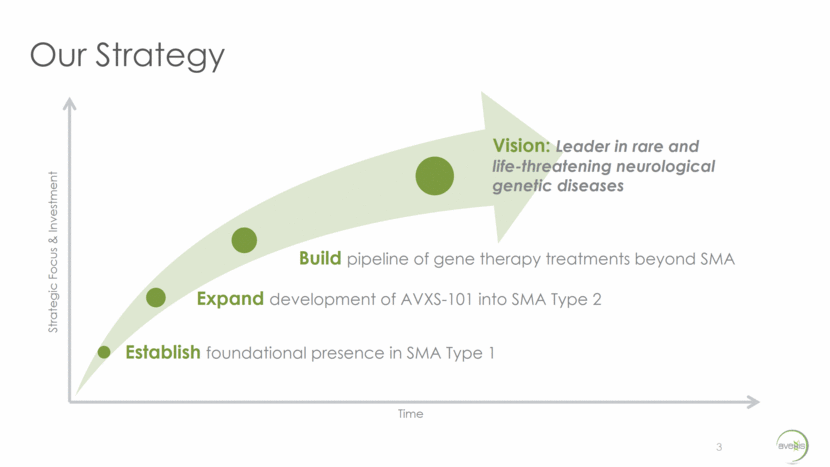
Our Strategy 3 Build pipeline of gene therapy treatments beyond SMA Expand development of AVXS-101 into SMA Type 2 Establish foundational presence in SMA Type 1 Vision: Leader in rare and life-threatening neurological genetic diseases Strategic Focus & Investment Time
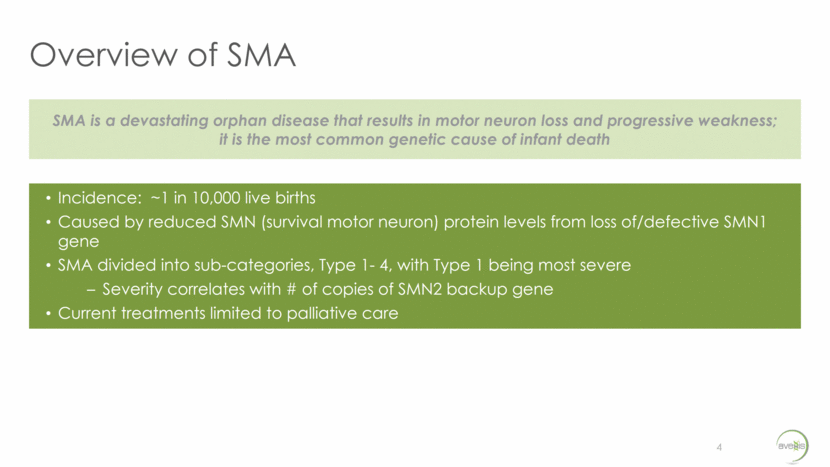
Overview of SMA 4 SMA is a devastating orphan disease that results in motor neuron loss and progressive weakness; it is the most common genetic cause of infant death Incidence: ~1 in 10,000 live births Caused by reduced SMN (survival motor neuron) protein levels from loss of/defective SMN1 gene SMA divided into sub-categories, Type 1- 4, with Type 1 being most severe Severity correlates with # of copies of SMN2 backup gene Current treatments limited to palliative care
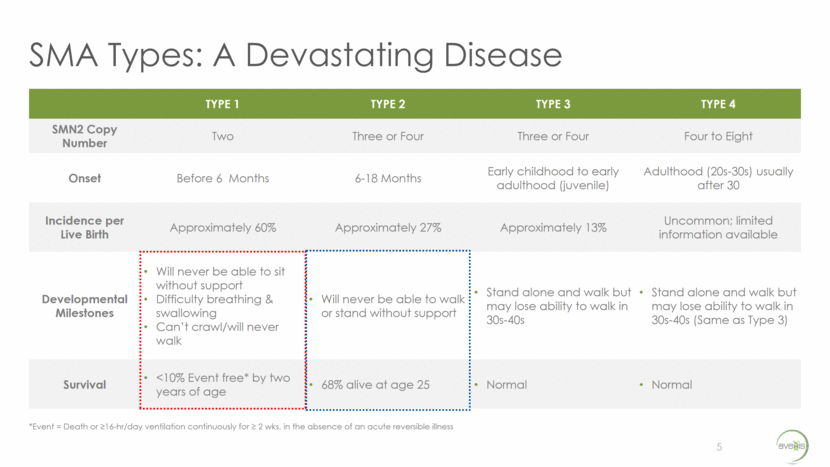
SMA Types: A Devastating Disease 5 TYPE 1 TYPE 2 TYPE 3 TYPE 4 SMN2 Copy Number Two Three or Four Three or Four Four to Eight Onset Before 6 Months 6-18 Months Early childhood to early adulthood (juvenile) Adulthood (20s-30s) usually after 30 Incidence per Live Birth Approximately 60% Approximately 27% Approximately 13% Uncommon; limited information available Developmental Milestones Will never be able to sit without support Difficulty breathing & swallowing Can’t crawl/will never walk Will never be able to walk or stand without support Stand alone and walk but may lose ability to walk in 30s-40s Stand alone and walk but may lose ability to walk in 30s-40s (Same as Type 3) Survival <10% Event free* by two years of age 68% alive at age 25 Normal Normal *Event = Death or >16-hr/day ventilation continuously for > 2 wks, in the absence of an acute reversible illness
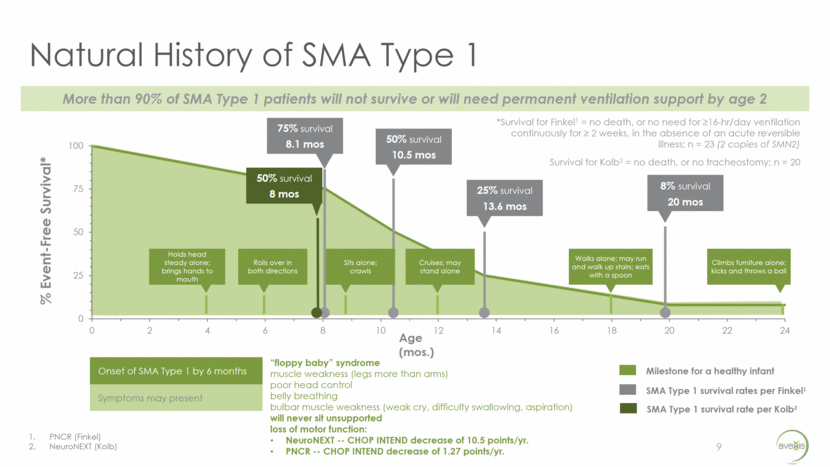
SMA Market Opportunity 6 The SMA patient population is well-defined in the U.S. and has similar incidence rates in Europe 1 in 50 (~6 million Americans) are genetic carriers ~10,000 SMA patients in the U.S. All SMA has same root cause – deletion or point mutation of SMN1 gene SMA in the U.S. Incidence Approximate % Prevalence Approximate % Type 1 58% 5% Type 2 29% 50% Type 3 13% 45%
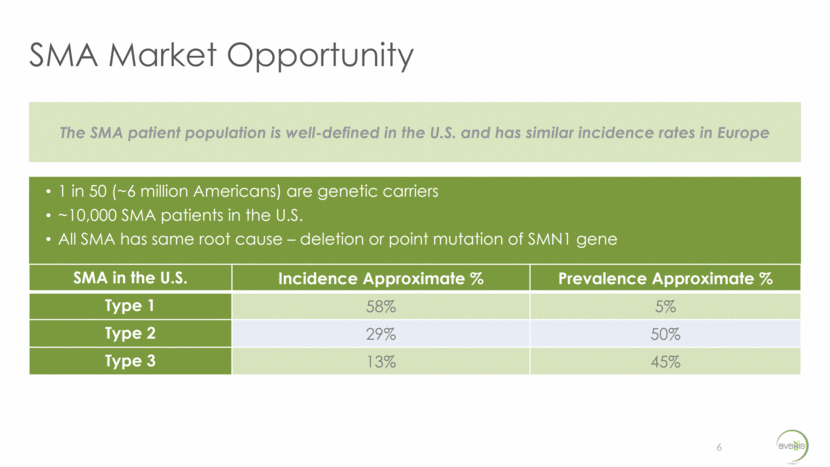
AVXS-101 Targets the Primary SMN Gene 7 NORMAL INDIVIDUAL SMA-AFFLICTED INDIVIDUAL SMA-AFFLICTED INDIVIDUAL TREATED WITH AVXS-101 SMN Genes SMN Protein SMN1 Primary SMN2 Back up SMN Genes SMN Protein SMN1 SMN2 Back up SMN Genes SMN Protein SMN1 SMN2 Back up AVXS-101 Primary Functional SMN Protein Not-functional SMN Protein
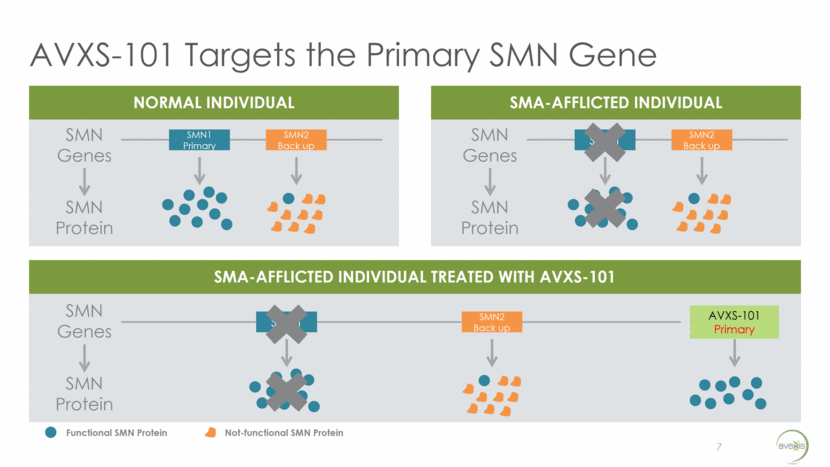
Our Solution: AVXS-101 An Innovative Treatment Approach for SMA 8 scAAV ITR Continuous Promoter Human SMN Transgene scAAV ITR KEY COMPONENTS PURPOSE Recombinant AAV9 Capsid Shell Ability to deliver across the blood brain barrier (BBB) and into the spinal cord - Avoids the need for intrathecal delivery when treating infants Non-replicating virus does not modify the existing DNA of the patient. scAAV ITR (Self-complementary DNA technology) Enables rapid onset of effect which is key in a quickly deteriorating population Continuous Promoter Activates the transgene to allow for continuous and sustained SMN expression Human SMN Transgene Full copy of a stable, functioning SMN gene that is introduced into the cell’s nucleus Recombinant AAV9 Capsid Shell Rendering adapted from DiMattia et al. Structural Insight into the Unique Properties of Adeno-Associated Virus Serotype 9. J. Virol. June 2012. Gene therapy is the right approach for SMA: Monogenic mutation that drives the pathology
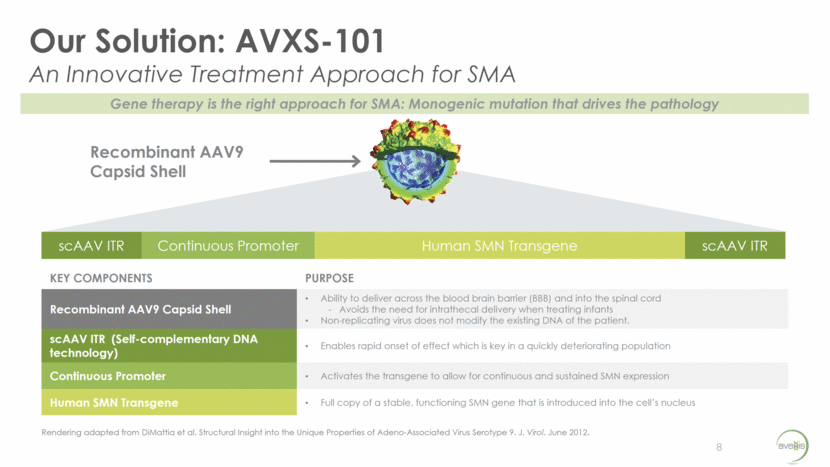
Natural History of SMA Type 1 “floppy baby” syndrome muscle weakness (legs more than arms) poor head control belly breathing bulbar muscle weakness (weak cry, difficulty swallowing, aspiration) will never sit unsupported loss of motor function: NeuroNEXT -- CHOP INTEND decrease of 10.5 points/yr. PNCR -- CHOP INTEND decrease of 1.27 points/yr. % Event-Free Survival* Age (mos.) PNCR (Finkel) NeuroNEXT (Kolb) *Survival for Finkel1 = no death, or no need for >16-hr/day ventilation continuously for > 2 weeks, in the absence of an acute reversible illness; n = 23 (2 copies of SMN2) Survival for Kolb2 = no death, or no tracheostomy; n = 20 75% survival 8.1 mos 50% survival 10.5 mos 25% survival 13.6 mos 8% survival 20 mos Holds head steady alone; brings hands to mouth Rolls over in both directions Sits alone; crawls Cruises; may stand alone Walks alone; may run and walk up stairs; eats with a spoon Climbs furniture alone; kicks and throws a ball Onset of SMA Type 1 by 6 months Symptoms may present More than 90% of SMA Type 1 patients will not survive or will need permanent ventilation support by age 2 9 Milestone for a healthy infant SMA Type 1 survival rates per Finkel1 50% survival 8 mos SMA Type 1 survival rate per Kolb2 0 25 50 75 100 0 2 4 6 8 10 12 14 16 18 20 22 24
Children with SMA Type 1 Do Not Reach Any Major Motor Milestones Retrospective Study from large multi-center datasets (US and EU) Patients (n=33) have genetically confirmed homozygous deletion of exon 7 in SMN1 gene; categorized according to Dubowitz’s decimal classification (confirmation of SMN1 status and clinical observations) Study visits at baseline, every 2-3 months until the age of 12 months, and every 6 months thereafter, when possible Hammersmith Infant Neurological Examination (HINE) used to assess intermediate steps leading to full achievement of milestones DESIGN Prolongation of survival with supportive care does not impact achievement of motor milestones in SMA Type 1 infants SMA Type 1 infants with symptom onset <6 months: Will not reach any major motor milestones, such as sitting, crawling, standing, and walking Any early intermediate milestones in 1B patients will be quickly lost The highest milestone achieved is seen in the child’s first visit followed by a rapid decline Any improvement or achievement of milestones not usually achieved in a child with SMA Type 1 in a drug intervention trial can be attributed to the drug and not due to survival or enhanced standard of care CONCLUSIONS Developmental Milestones in Type 1 Spinal Muscular Atrophy De Sanctis et al. Neuromuscular Disorders, Nov-2016 10
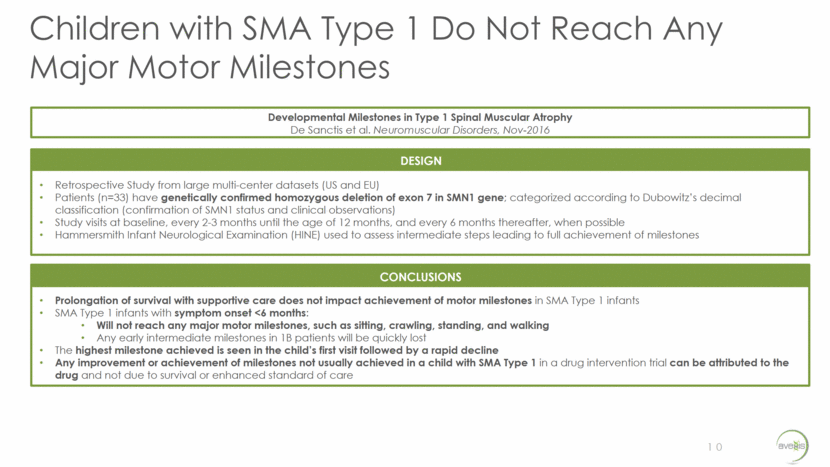
Phase 1 Trial Design: SMA Type 1 11 TRIAL OVERVIEW Route of Administration One-time intravenous infusion through peripheral limb vein Prednisolone 1 mg/kg 1 day Pre-GT Trial Design Open-label, dose-escalation Principal Investigator Jerry R. Mendell, M.D. TRIAL OVERVIEW Study Site OBJECTIVES Primary Safety and Tolerability Secondary Time from birth until death or time to >16-hour ventilation continuously for >2 weeks in the absence of an acute reversible illness or perioperatively Video confirmed achievement of ability to sit unassisted Additional CHOP INTEND Bayley Motor Scales of Infant/Toddler development – Gross Motor Exploratory Ability Captured Through Interactive Video Evaluation-mini (ACTIVE-mini) Comp Motor Action Pot (CMAP) Motor Unit Number (MUNE) Electrical Impedance Myography (EIM) OBJECTIVES OBJECTIVES Inclusion 9 months of age / 6 months of age¹ and younger at day of vector infusion with SMA Type 1 as defined by the following features: Bi-allelic SMN1 gene mutations (deletion or point mutations) 2 copies of SMN2 Onset of disease at birth to 6 months of age Hypotonia by clinical evaluation with delay in motor skills, poor head control, round shoulder posture and hypermobility of joints Exclusion Active viral infection (includes HIV or serology positive for hepatitis B or C) Use of invasive ventilatory support (tracheotomy with positive pressure)* or pulse oximetry <95% saturation Patients with Anti-AAV9 antibody titers >1:50 as determined by ELISA binding immunoassay Abnormal laboratory values considered to be clinically significant Patients with the c.859G>C mutation in SMN2 exon 7 (predicted mild phenotype)2 Key Enrollment Criteria Clinicaltrials.gov Identifier = NCT02122952 ¹ Inclusion criteria was 9 months of age and younger for the first nine patients. 6 months of age and younger for the last six patients. 2 Exclusion criteria related to c.859G>C was confirmed for all patients. *Patients may be put on non-invasive ventilatory support (BiPAP) for <16 hours/day at discretion of their physician or study staff.
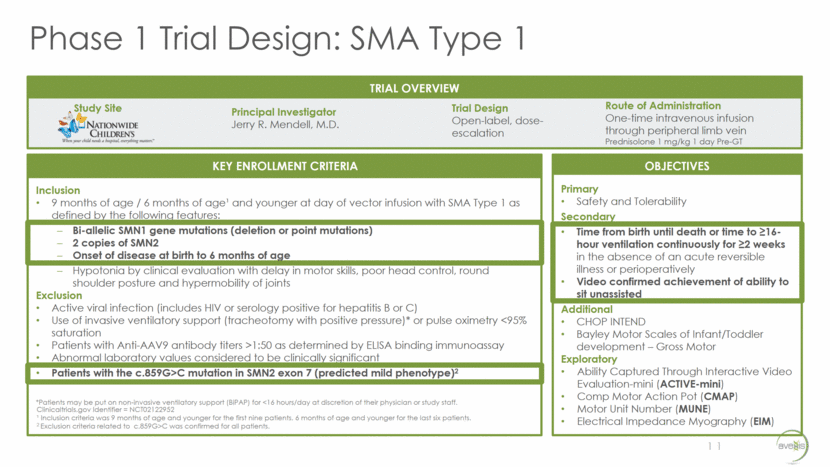
* A month is defined as 30 days Cohort 1 6.7E13 vg/kg Cohort 2 2.0E14 vg/kg PNCR (Finkel) 2014 Natural History Study: 75% event-free 50% event-free 25% event-free 8% event-free 12 SURVIVAL DATA 9/9 reached 20 mo. event-free (8% PNCR) 12/12 reached 13.6 mo. event-free (25% PNCR) 15/15 reached 10.5 mo. event-free (50% PNCR) 15/15 reached 8.1 mo. event-free (75% PNCR) Age at Last Follow-up Cohort 1*: 30.8 months (median) 30.3 months (mean) Cohort 2: 17.3 months (median) 17.9 months (mean) *reflects E.01’s age at Last Trial Visit, E.02’s age at Pulmonary Event Day of Gene Transfer Last Trial Visit – Age Fixed Pulmonary Event – Age Fixed Event-Free Survival Data – September 15, 2016 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Age (months*)
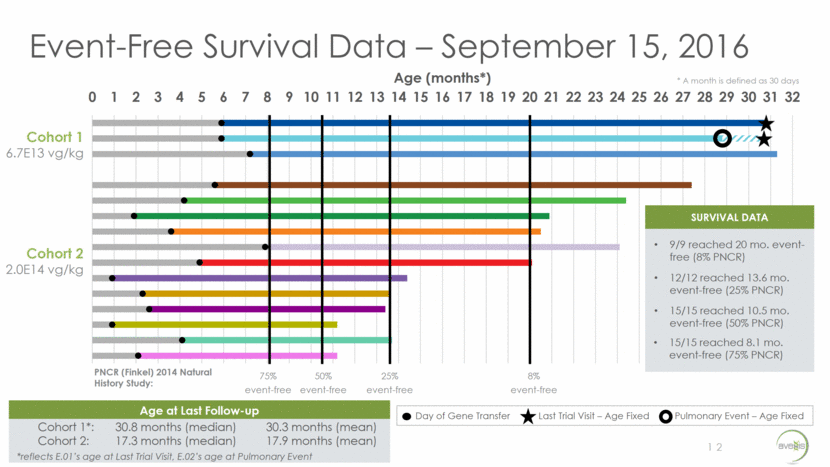
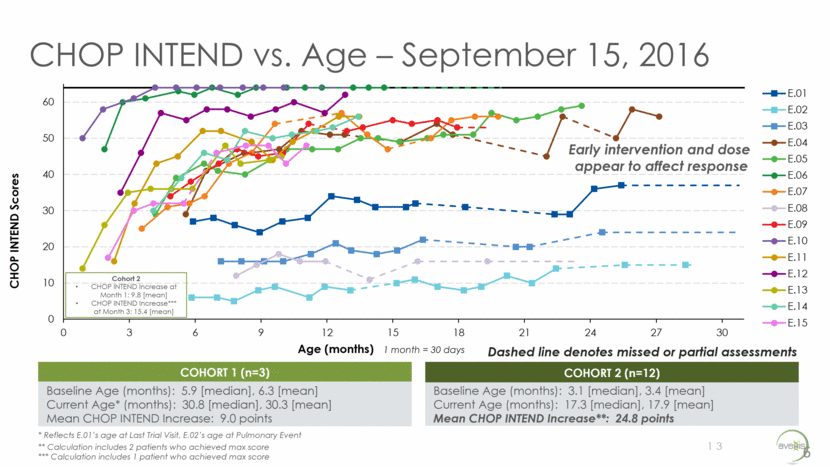
CHOP INTEND vs. Age – September 15, 2016 Early intervention and dose appear to affect response 6 13 COHORT 1 (n=3) Baseline Age (months): 5.9 [median], 6.3 [mean] Current Age* (months): 30.8 [median], 30.3 [mean] Mean CHOP INTEND Increase: 9.0 points COHORT 2 (n=12) Baseline Age (months): 3.1 [median], 3.4 [mean] Current Age (months): 17.3 [median], 17.9 [mean] Mean CHOP INTEND Increase**: 24.8 points * Reflects E.01’s age at Last Trial Visit, E.02’s age at Pulmonary Event ** Calculation includes 2 patients who achieved max score *** Calculation includes 1 patient who achieved max score Dashed line denotes missed or partial assessments Cohort 2 CHOP INTEND Increase at Month 1: 9.8 [mean] CHOP INTEND Increase*** at Month 3: 15.4 [mean] 0 10 20 30 40 50 60 0 3 6 9 12 15 18 21 24 27 30 CHOP INTEND Scores Age (months) 1 month = 30 days E.01 E.02 E.03 E.04 E.05 E.06 E.07 E.08 E.09 E.10 E.11 E.12 E.13 E.14 E.15
Safety Data – September 15, 2016 SAFETY AND TOLERABILITY OBSERVATIONS No new treatment-related SAEs or AEs observed As previously reported, a total of 5 treatment-related AEs in 4 patients have been observed to date Treatment-related SAEs and AEs were clinically asymptomatic elevated liver function enzymes (LFEs) resolved with prednisolone treatment* 2 were SAEs experienced by 2 patients (SAEs defined on the basis of laboratory tests showing elevated LFEs) 3 were AEs experienced by 2 patients A total 118 AEs (34 SAEs and 84 non-serious AEs) have been reported AVXS-101 appears to have a favorable safety profile and appears to be generally well-tolerated in patients studied to date 14 *No drug-induced liver injury (DILI) as defined by Hy’s Law

Children with SMA Type 1 Never Sit Unassisted 15 Disease Characteristics Disease onset <6 months Hypotonia and weakness Bulbar muscle weakness Difficulty breathing and swallowing Inexorable progression to nutritional failure Inexorable progression to respiratory failure Developmental Milestone Prognosis Progressive decline in motor function soon after birth Rapid loss of any early milestones (e.g. head control, hands to mouth) Will never be able to sit unassisted Will never be able to roll Will never be able to crawl, stand, or walk The Natural History of SMA Type 1 is marked by the inability to achieve or maintain developmental milestones
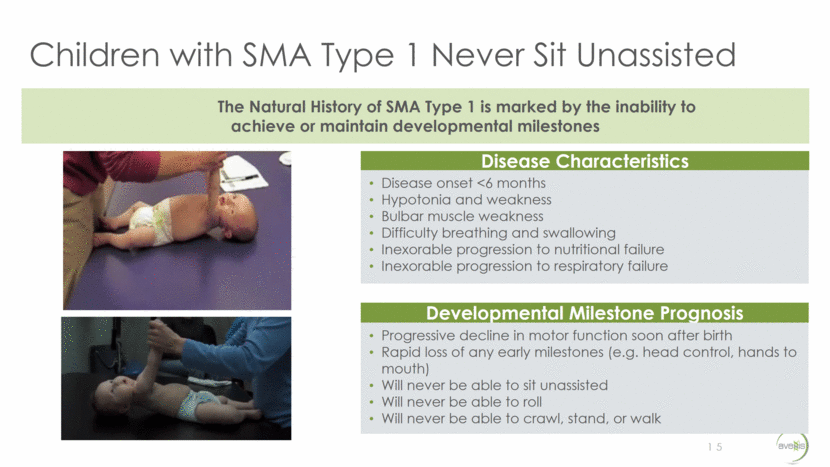
Motor Milestone Achievement in Proposed Therapeutic Dose (Interim Data) 16 Cohort 2 2.0e14 vg/kg Age at GT (mos) Motor Milestone Achievement Brings hand to mouth Head control Rolls over (partial) Rolls over (complete) Sitting with assistance Sitting unassisted E.04 6 E.05 4 E.06 2 E.07 4 E.08 8 E.09 5 E.10 1 E.11 2 E.12 3 E.13 1 E.14 4 E.15 2 a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a E.11 sitting unassisted and standing with support, E.10 walking independently, E.12 standing with support, and E.15 feeding self were confirmed with video evidence after September 15, 2016. 7 patients are feeding themselves 5 patients are speaking (1 bilingual) 2 patients are crawling 4 patients are standing with support 2 patients are standing alone 2 patients are walking independently
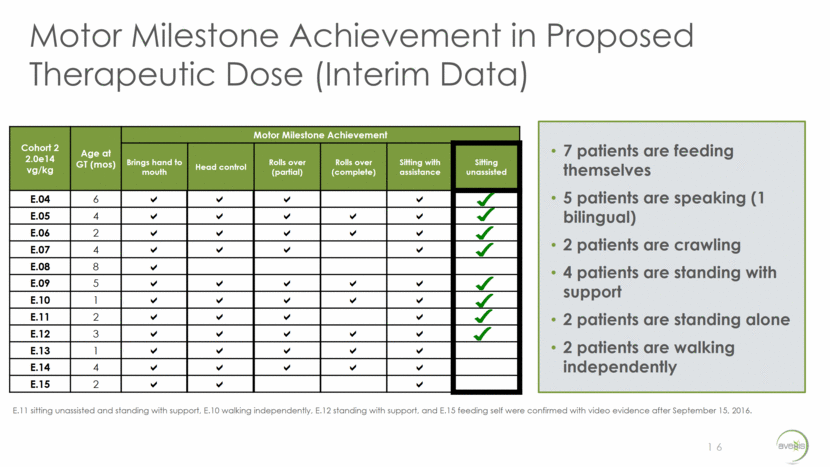
AVXS-101 Pivotal Trial in SMA Type 1 Update Based on Ongoing Dialogue and FDA Type B Meeting Minutes Held 9/30/16 17 Study Overview To reflect single-arm design with a natural history comparator Expected to enroll approximately 20 patients Endpoints to include safety, achievement of motor milestones (e.g. ability to sit unassisted), event-free survival Additional trial design details to be provided at time of initiation Acknowledged company’s rationale for single-arm trial Indicated preference for co-primary endpoints consisting of developmental milestones and a clinically meaningful measure of survival Strongly recommended end-of-Phase 1 meeting to evaluate adequacy of data from ongoing study to support future product development, including discussion of whether these data may provide the substantial evidence needed to support a marketing application FDA feedback in late December 2016 on protocol submission resulted in continued alignment on single-arm design and endpoints FDA Feedback Next Steps Complete ongoing Phase 1 trial and continue collaborative discussions with FDA regarding most expeditious pathway for approval Planned trial start in H1’17
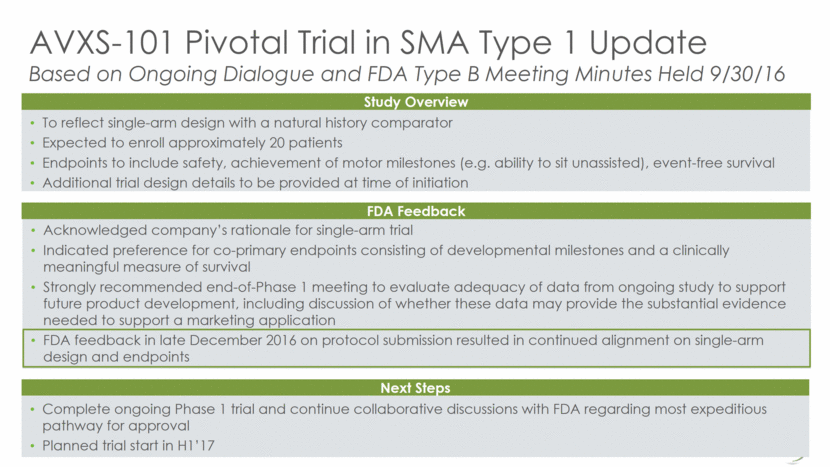
SMA Type 2 Strategy Potentially streamlines development of Type 2 clinical data set Eliminates the need for a comparability analysis later in development Simplifies the development pathway by using one, consistent product; FDA in general prefers use of a consistent product process in program development, if at all possible Potentially accelerates the timeframe for gathering clinical data that may be used as part of a data set to support a future SMA Type 2 indication Plan to initiate Type 2 study in Q2 2017, assuming a positive outcome from the planned Type B manufacturing meeting This strategy does not impact the development program for Type 1 Optimal approach to use scalable GMP-derived product in clinical evaluation of AVXS-101 in SMA Type 2 18
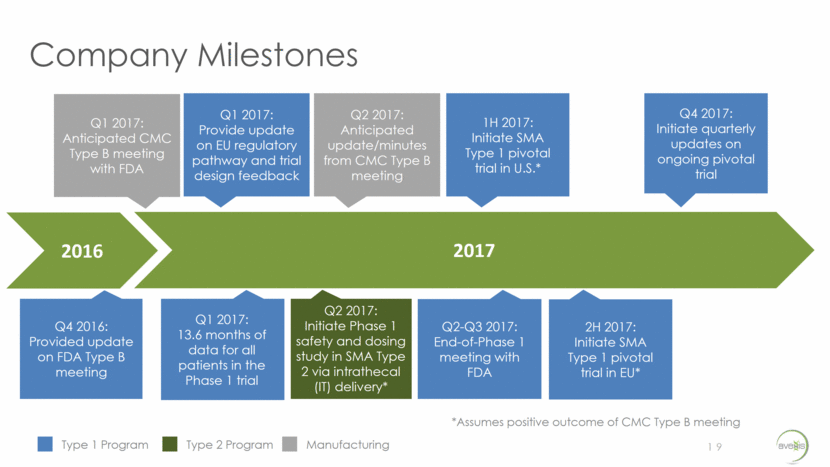
Company Milestones 19 Type 1 Program Type 2 Program 2016 2017 Manufacturing Q4 2017: Initiate quarterly updates on ongoing pivotal trial 1H 2017: Initiate SMA Type 1 pivotal trial in U.S.* Q1 2017: Anticipated CMC Type B meeting with FDA Q1 2017: Provide update on EU regulatory pathway and trial design feedback Q2 2017: Anticipated update/minutes from CMC Type B meeting Q1 2017: 13.6 months of data for all patients in the Phase 1 trial Q2 2017: Initiate Phase 1 safety and dosing study in SMA Type 2 via intrathecal (IT) delivery* Q2-Q3 2017: End-of-Phase 1 meeting with FDA Q4 2016: Provided update on FDA Type B meeting 2H 2017: Initiate SMA Type 1 pivotal trial in EU* *Assumes positive outcome of CMC Type B meeting
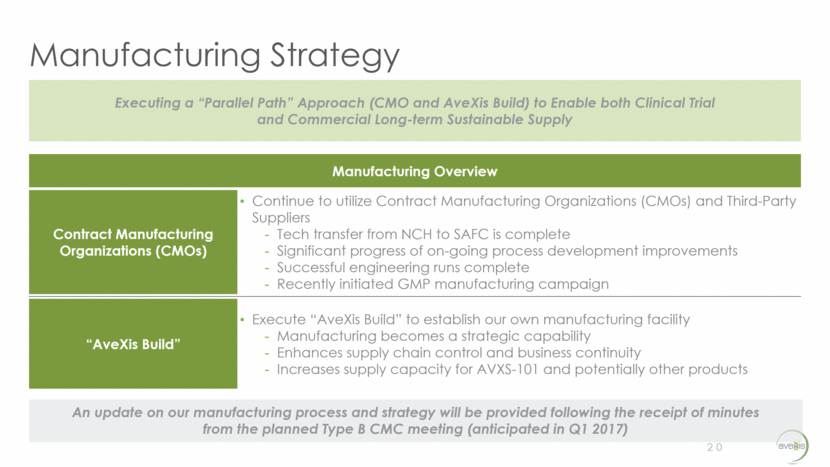
Manufacturing Strategy 20 Executing a “Parallel Path” Approach (CMO and AveXis Build) to Enable both Clinical Trial and Commercial Long-term Sustainable Supply Manufacturing Overview Contract Manufacturing Organizations (CMOs) Continue to utilize Contract Manufacturing Organizations (CMOs) and Third-Party Suppliers Tech transfer from NCH to SAFC is complete Significant progress of on-going process development improvements Successful engineering runs complete Recently initiated GMP manufacturing campaign “AveXis Build” Execute “AveXis Build” to establish our own manufacturing facility Manufacturing becomes a strategic capability Enhances supply chain control and business continuity Increases supply capacity for AVXS-101 and potentially other products An update on our manufacturing process and strategy will be provided following the receipt of minutes from the planned Type B CMC meeting (anticipated in Q1 2017)
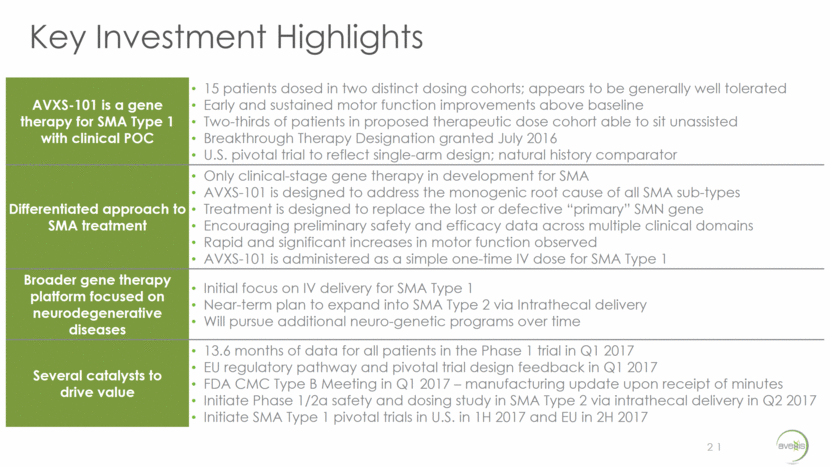
Key Investment Highlights 21 21 AVXS-101 is a gene therapy for SMA Type 1 with clinical POC 15 patients dosed in two distinct dosing cohorts; appears to be generally well tolerated Early and sustained motor function improvements above baseline Two-thirds of patients in proposed therapeutic dose cohort able to sit unassisted Breakthrough Therapy Designation granted July 2016 U.S. pivotal trial to reflect single-arm design; natural history comparator Differentiated approach to SMA treatment Only clinical-stage gene therapy in development for SMA AVXS-101 is designed to address the monogenic root cause of all SMA sub-types Treatment is designed to replace the lost or defective “primary” SMN gene Encouraging preliminary safety and efficacy data across multiple clinical domains Rapid and significant increases in motor function observed AVXS-101 is administered as a simple one-time IV dose for SMA Type 1 Broader gene therapy platform focused on neurodegenerative diseases Initial focus on IV delivery for SMA Type 1 Near-term plan to expand into SMA Type 2 via Intrathecal delivery Will pursue additional neuro-genetic programs over time Several catalysts to drive value 13.6 months of data for all patients in the Phase 1 trial in Q1 2017 EU regulatory pathway and pivotal trial design feedback in Q1 2017 FDA CMC Type B Meeting in Q1 2017 – manufacturing update upon receipt of minutes Initiate Phase 1/2a safety and dosing study in SMA Type 2 via intrathecal delivery in Q2 2017 Initiate SMA Type 1 pivotal trials in U.S. in 1H 2017 and EU in 2H 2017
Thank You