Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - BIOGEN INC. | lte2.htm |
| 8-K - 8-K - BIOGEN INC. | december920168-k.htm |

Interim analysis presented at CTAD 2016
Aducanumab Titration Dosing Regimen:
12-Month Interim Analysis from PRIME,
a Randomized, Double-Blind, Placebo-Controlled
Phase 1b Study in Patients With Prodromal or
Mild Alzheimer’s Disease
Vissia Viglietta,1 John O’Gorman,1 Leslie Williams,1 Tianle Chen,1
Ahmed Enayetallah,1 Ping Chiao,1 Christoph Hock,2 Roger M. Nitsch,2
Samantha Budd Haeberlein,1 Alfred Sandrock1
1Biogen, Cambridge, MA, USA; 2Neurimmune, Schlieren-Zurich, and University of Zurich, Switzerland

Interim analysis presented at CTAD 2016
Disclosures
This study is funded by Biogena
VV, JO, LW, TC, AE, PC, SBH, and AS are employees and
shareholders of Biogen
CH and RMN are employees and shareholders of Neurimmune
aMedical writing support for this presentation was provided by Erin Bekes, PhD, of Complete Medical Communications and funded by Biogen.

Interim analysis presented at CTAD 2016
Introduction
Aducanumab is a human monoclonal antibody selective for aggregated
forms of Aβ, including soluble oligomers and insoluble fibrils
PRIME is an ongoing Phase 1b study assessing the safety, tolerability, PK
and PD of aducanumab in patients with prodromal or mild Alzheimer’s
disease
Results from a 12-month interim analysis from fixed-dose cohorts have been
previously published1
Here we present 12-month interim data for both fixed-dose and titrated
aducanumab in PRIME
1. Sevigny et al. Nature 2016;537:50-56
PD, pharmacodynamics; PK, pharmacokinetics

Interim analysis presented at CTAD 2016
1 mg/kg
3 mg/kg
Placebo
Titration (ApoE ε4 carriers)
Placebo (ApoE ε4 carriers)
6 mg/kg
Placebo
PRIME Study Design:
Placebo-Controlled and LTE Periods
Randomization: 3:1 active: placebo within cohorts, fixed-dose cohorts stratified by ApoE ε4 status
Planned sample size: 188 patients
Titration cohort of ApoE ε4 carriers added after enrollment into fixed-dose arms was complete (planned sample size: 21
aducanumab: 7 placebo)
CDR–SB, Clinical Dementia Rating‒Sum of Boxes; LTE, long-term extension; MMSE, Mini-Mental State Examination; PET, positron emission
tomography
10 mg/kg
Placebo
36-Month
LTE
Prodromal or mild
AD
MMSE ≥20
Stable
concomitant
medications
Positive amyloid
PET scan
Population
Staggered Parallel-Group Design
Primary endpoint:
safety and
tolerability
Secondary
endpoints: serum
PK,
immunogenicity,
change in amyloid
PET (Week 26)
Exploratory
endpoints included
CDR–SB, MMSE,
change in amyloid
PET (Week 54)
Endpoints
12-Month
Placebo-Controlled Period

Interim analysis presented at CTAD 2016
Titration Dosing Regimen
in the 12-Month Placebo-Controlled Period
3 mg/kg 6 mg/kg 10 mg/kg
Study
week: 0 4 8 12 16 20 24 28 32 36 40 44 48 52
Dose
(mg/kg):
Placebo
1 1 3 3 3 3 6 6 6 6 6 10 10 10
1 mg/kg
By Week 52
average expected
dose
= 5.3 mg/kg
By Week 24
average expected
dose
= 2.9 mg/kg

Interim analysis presented at CTAD 2016
Patient Disposition at 12 Months
AE, adverse event
Analysis of data from all cohorts up to Week 54
Randomized
Dosed
Discontinued treatment
AE
Lost to follow-up
Disease progression
Consent withdrawn
Investigator decision
Death
Other
197
randomized
(by cohort)
1 mg/kg
31
Pooled
placebo
48
3 mg/kg
33
6 mg/kg
30
10 mg/kg
32
31 48 32 30 32
7
3
0
0
2
0
0
2
10
3
0
0
0
1
1
5
6
2
1
0
1
0
0
2
5
3
0
1
1
0
0
0
12
10
0
0
1
1
0
0
Titration
23
23
4
2
0
0
0
0
0
2

Interim analysis presented at CTAD 2016
Baseline Disease Characteristics
AD, Alzheimer’s disease; SD, standard deviation; SUVR, standardized uptake value ratio
aCholinesterase inhibitors and/or memantine.
Aducanumab
Placebo
(n=48)
1 mg/kg
(n=31)
3 mg/kg
(n=32)
6 mg/kg
(n=30)
10 mg/kg
(n=32)
Titration
(n=23)
Age in years, mean ± SD 73.3 ± 6.8 72.6 ± 7.8 70.5 ± 8.2 73.3 ± 9.3 73.7 ± 8.3 73.1 ± 7.8
ApoE ε4, n (%)
Carriers
Non-carriers
34 (71)
14 (29)
19 (61)
12 (39)
21 (66)
11 (34)
21 (70)
9 (30)
20 (63)
12 (38)
23 (100)
0
Clinical stage, n (%)
Prodromal
Mild
22 (46)
26 (54)
10 (32)
21 (68)
14 (44)
18 (56)
12 (40)
18 (60)
13 (41)
19 (59)
13 (57)
10 (43)
MMSE, mean ± SD 24.7 ± 3.6 23.6 ± 3.3 23.2 ± 4.2 24.4 ± 2.9 24.8 ± 3.1 24.7 ± 3.0
CDR Global Score, n (%)
0.5
1
40 (83)
8 (17)
22 (71)
9 (29)
22 (69)
10 (31)
25 (83)
5 (17)
24 (75)
8 (25)
18 (78)
5 (22)
CDR-SB, mean ± SD 2.69 ± 1.54 3.40 ± 1.76 3.50 ± 2.06 3.32 ± 1.54 3.14 ± 1.71 3.24 ± 1.84
PET SUVR, mean composite 1.435 1.441 1.464 1.429 1.441 1.325
AD medications used,a n (%) 30 (63) 21 (68) 28 (88) 20 (67) 17 (53) 12 (52)

Interim analysis presented at CTAD 2016
PET AMYLOID IMAGING

Interim analysis presented at CTAD 2016
Aducanumab Reduces Amyloid Plaques
Nominal p values: * P<0.05; **P<0.01; ***P<0.001 vs placebo.
1. Ostrowitzki et al. Arch Neurol 2012
Analyses based on observed data. ANCOVA for change from baseline with factors of treatment, laboratory ApoE ε4 status (carrier and non-carrier), and baseline composite
SUVR. PD analysis population is defined as all randomized patients who received at least 1 dose of study medication and had at least 1 post-baseline assessment of the
parameter. ANCOVA, analysis of covariance; SE, standard error
Adu
c
anu
m
a
b

Interim analysis presented at CTAD 2016
CLINICAL ENDPOINTS
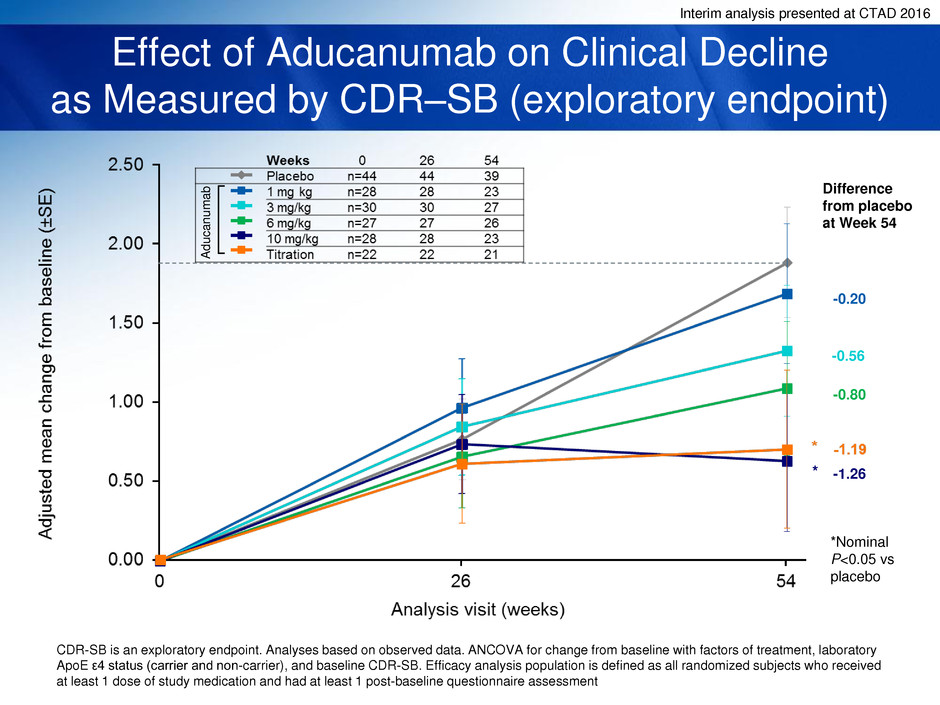
Interim analysis presented at CTAD 2016
Effect of Aducanumab on Clinical Decline
as Measured by CDR–SB (exploratory endpoint)
CDR-SB is an exploratory endpoint. Analyses based on observed data. ANCOVA for change from baseline with factors of treatment, laboratory
ApoE ε4 status (carrier and non-carrier), and baseline CDR-SB. Efficacy analysis population is defined as all randomized subjects who received
at least 1 dose of study medication and had at least 1 post-baseline questionnaire assessment
Aducanu
m
a
b
*Nominal
P<0.05 vs
placebo
Difference
from placebo
at Week 54
-0.20
-0.56
-1.26
-0.80
*

Interim analysis presented at CTAD 2016
Effect of Aducanumab on Clinical Decline
as Measured by MMSE (exploratory endpoint)
MMSE is an exploratory endpoint. Analyses based on observed data. ANCOVA for change from baseline with factors of treatment, laboratory ApoE ε4 status
(carrier and non-carrier), and baseline MMSE. Efficacy analysis population is defined as all randomized patients who received at least 1 dose of study
medication and had at least 1 post-baseline questionnaire assessment.
*Nominal
P<0.05 vs
placebo
Difference
from placebo
at Week 52
1.91
1.70
0.25
0.47
*

Interim analysis presented at CTAD 2016
SAFETY AND
TOLERABILITY

Interim analysis presented at CTAD 2016
No New Safety Signals Identified in Titration Cohort
During 12-Month Placebo-Controlled Period
No new safety signals were identified in the titration cohort
As previously presented for the fixed-dose cohorts:
- The most common AE/SAE was ARIA
- Other AEs/SAEs were consistent with the patient population
• Three deaths; none considered treatment-related; two in placebo and one in 10 mg/kg arm
(two occurred after study discontinuation)
- No significant changes in chemistry, hematology, urinalysis, ECGs, or vital signs
ARIA, amyloid-related imaging abnormalities; ECG, electrocardiogram; SAE, serious adverse event
Aducanumab
Placebo
(N=48)
1 mg/kg
(N=31)
3 mg/kg
(N=32)
6 mg/kg
(N=30)
10 mg/kg
(N=32)
Titration
(N=23)
Number with an AE (%) 47 (98) 28 (90) 27 (84) 28 (93) 29 (91) 21 (91)
Number with an SAE (%) 16 (33) 4 (13) 4 (13) 4 (13) 12 (38) 5 (22)
Number discontinuing
treatment due to AE (%)
4 (8) 3 (10) 2 (6) 3 (10) 10 (31) 2 (9)

Interim analysis presented at CTAD 2016
Dose Titration Slightly Attenuated Incidence of
ARIA-E Versus Higher Fixed Doses
Incidence of ARIA based on MRI.
ARIA-E, ARIA‒vasogenic edema; ARIA-H, ARIA‒microhemorrhages, macrohemorrhages, or superficial siderosis; MRI, magnetic resonance
imaging
Placebo
Aducanumab
1 mg/kg 3 mg/kg 6 mg/kg 10 mg/kg Titration
Patients with at least 1
post-baseline MRI
46 31 32 30 32 23
ARIA-E,a n (%) 0/46 1/31 (3) 2/32 (6) 11/30 (37) 13/32 (41) 8/23 (35)
aARIA-E with or without ARIA-H.
ApoE ε4 carrier 0/32 1/19 (5) 1/21 (5) 9/21 (43) 11/20 (55) 8/23 (35)
ApoE ε4 non-carrier 0/14 0/12 1/11 (9) 2/9 (22) 2/12 (17) --
Isolated ARIA-H, n (%) 3/46 (7) 2/31 (6) 3/32 (9) 0/30 2/32 (6) 0/23
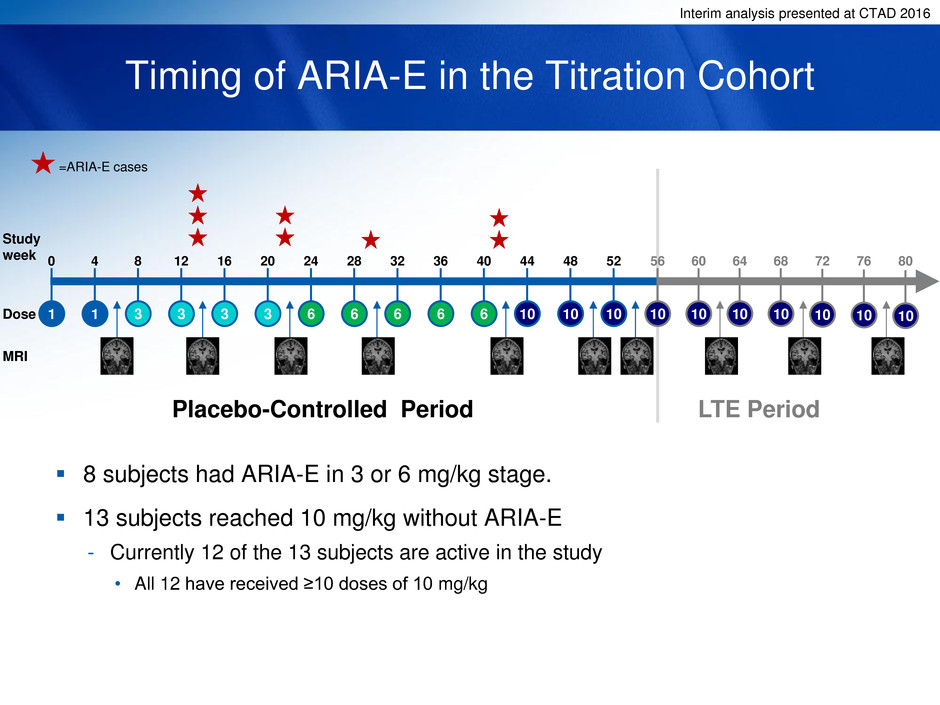
Interim analysis presented at CTAD 2016
Timing of ARIA-E in the Titration Cohort
8 subjects had ARIA-E in 3 or 6 mg/kg stage.
13 subjects reached 10 mg/kg without ARIA-E
- Currently 12 of the 13 subjects are active in the study
• All 12 have received ≥10 doses of 10 mg/kg
1 1 3 3 3 3 6 6 6 6 6 10 10 10
Study
week 0 4 8 12 16 20 24 28 32 36 40 44 48 52
Dose
Placebo-Controlled Period LTE Period
10
56
10
60
10
64
10
68
10
72
10
76
10
80
MRI
=ARIA-E cases

Interim analysis presented at CTAD 2016
Most Titration Patients with ARIA-E
Continued Treatment
Among ApoE ε4 carriers with ARIA-E,
4/11 (36%) in the 10 mg/kg group continued treatment
7/9 (78%) in the 6 mg/kg group continued treatment
6/8 (75%) in the titration group continued treatment
Aducanumab
1 mg/kg 3 mg/kg 6 mg/kg 10 mg/kg Titration
ApoE ε4 carriers with at least 1
post-baseline MRI
19 21 21 20 23
ARIA-E, n (%) 1 (5) 1 (5) 9 (43) 11 (55) 8 (35)
Continued treatment, n (%) 0 1 (5) 7 (33) 4 (20) 6 (26)
Same dose 0 0 1 0 0
Reduced dose 0 1 6 4 6
Discontinued treatment, n (%) 1 (5) 0 2 (10) 7 (35) 2 (9)

Interim analysis presented at CTAD 2016
ARIA-E Characteristics in the Titration Cohort
Majority of cases occurred within the first 5 months of
treatment
75% of events were asymptomatic
2 patients (25%) had mild symptoms that resolved
MRI findings typically resolved within 412 weeks
Interim analysis presented at CTAD 2016
Summary
Both titration and fixed doses of aducanumab significantly reduced amyloid plaque
burden following 12 months of treatment versus placebo
Clinical effects with titrated aducanumab were generally consistent with findings in the
fixed-dose cohorts
- Slowing of decline as measured by the CDR–SB and MMSE was observed in the
titration and fixed-dose cohorts
Titration up to 10 mg/kg may reduce incidence of ARIA-E compared with higher fixed
dosing based on the ApoE ε4 cohort studied
PRIME results support the study design of the ENGAGE and EMERGE Phase 3 trials,
which are investigating the clinical efficacy and safety of aducanumab in patients with
early AD
24-month data with fixed doses of aducanumab from PRIME will also be presented at
CTAD 2016 (Fri Dec 9, 9:15 AM)

Interim analysis presented at CTAD 2016
Acknowledgments
We thank all the patients and their family
members participating in the aducanumab
studies, as well as the investigators and their staff
conducting these studies.
