Attached files
| file | filename |
|---|---|
| EX-99.3 - EXHIBIT 99-3 - Tonix Pharmaceuticals Holding Corp. | s104910_ex99-03.htm |
| 8-K - 8-K - Tonix Pharmaceuticals Holding Corp. | s104910_8k.htm |

© 2016 Tonix Pharmaceuticals Holding Corp December 2016 Version P0043 12 - 8 - 16 Investor Presentation

© 2016 Tonix Pharmaceuticals Holding Corp 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, substantial competition ; our need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties ; and risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by the Company on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2015 , as filed with the Securities and Exchange Commission (the “SEC”) on March 3 , 2016 , and future periodic reports filed with the SEC on or after the date hereof . All of the Company's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .

© 2016 Tonix Pharmaceuticals Holding Corp 3 Posttraumatic Stress Disorder (PTSD) program On May 19, 2016, we reported encouraging topline data for TNX - 102 SL* 5.6 mg in a military - related PTSD trial (“AtEase”) • PTSD was our “second” clinical program using our proprietary formulation TNX - 102 SL • Prior to the AtEase trial, no other investigational new drug or approved therapy had demonstrated efficacy in military PTSD in a large adequate well - controlled study On August 29, 2016, we reported U.S. Food and Drug Administration (FDA) acceptance of the PTSD Phase 3 clinical program at the End - of - Phase 2 meeting • However, advancing TNX - 102 SL for PTSD was not in our budget * TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an Investigational New Drug and has not been approved for any indication

© 2016 Tonix Pharmaceuticals Holding Corp 4 Tonix (TNXP): Value proposition On September 6, 2016 our lead Phase 3 program TNX - 102 SL for fibromyalgia narrowly missed its primary endpoint in the first Phase 3 study (“AFFIRM”) • Received strong negative investor market response • Reassuring safety profile and activity of TNX - 102 SL at 2.8 mg for improvement in sleep quality in fibromyalgia sets stage for new clinical direction in PTSD We simultaneously announced that we discontinued our fibromyalgia program and we are focusing our resources to PTSD • High value Phase 3 clinical asset not well known to the market • Encouraging evidence of safety and efficacy of TNX - 102 SL was demonstrated in Phase 2 AtEase trial • Regulatory clarity for PTSD based on FDA acceptance of PTSD Phase 3 clinical program at End - of - Phase 2 meeting

© 2016 Tonix Pharmaceuticals Holding Corp 5 Pivot to PTSD: Rationale • Unmet medical need • PTSD is a serious condition and the prevalence is increasing, especially combat - related • Military - related PTSD is not satisfactorily treated by existing FDA approved therapies • Two selective serotonin reuptake inhibitors (SSRIs) are approved for PTSD, but have not shown efficacy in military - related PTSD • Endpoint • TNX - 102 SL 5.6 mg had significant effects on the FDA - recognized primary endpoint, the Clinician Administered PTSD Scale, “CAPS - 5” • Potential development and commercialization p artners • Several companies have U.S. psychiatry - focused specialty sales forces • Department of Defense (DoD) is interested in military - related PTSD and has the potential to support basic science and clinical development • Important target population • U.S. veterans are in great need of a medicine that works for this indication • TNX - 102 SL for PTSD has the potential for “Breakthrough Therapy Designation”

© 2016 Tonix Pharmaceuticals Holding Corp 6 Tonix Pharmaceuticals Posttraumatic Stress Disorder (PTSD) Program Phase 3 Ready Program Targeting An Attractive Market TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) • A unique, innovative pr oduct designed for bedtime administration • Targeting a chronic and serious psychiatric disorder: PTSD x Therapeutic dose identified in Phase 2 study x Phase 3 clinical and product registration plan accepted by the FDA 1 Target ing commencement of Phase 3 study in military - related PTSD in 1Q 2017 PTSD • High prevalence worldwide and receiving greater attention • Not well served - high off - label usage 2 with unproven or contraindicated treatments 3 • Potential opportunity to displace current therapies and expand market © 2016 Tonix Pharmaceuticals Holding Corp 1. August 2016 FDA End - of - Phase 2 Meeting Minutes 2. Bernardy et al., J Clin Psychiatry, 2012; 73: 297 3. VA/DoD Clinical Practice Guideline for the Management of Post - Traumatic Stress, Version 2, 2010

© 2016 Tonix Pharmaceuticals Holding Corp 7 What is PTSD? A chronic response to traumatic event(s) • A majority of people will experience a traumatic event at some point in their lifetime 1 • 20% of women and 8% of men in the U.S. who experience significant trauma develop PTSD 1 • Lifetime prevalence: 6.8% 2 (~ 17 million adults in the U.S.) • Persistent - >1/3 fail to recover, even after several years following the trauma 2 • Twelve month prevalence: U.S. 3.5% 3 (~ 8.6 million adults) EU 2.3% 4 (~10 million adults) Most common forms of trauma 1 • Witnessing someone being badly injured or killed • Natural disaster • Life - threatening accident • Sexual or physical assault 1. Kessler et al, Arch Gen Psychiatry 1995;52:1048. 2. Kessler et al., Arch Gen Psychiatry. 2005;, 62:593 3. Kessler et al., Arch Gen Psychiatry. 2005 ; 62: 617 ; Prevalence rate of 3.5% applied to U.S. Census estimate of 247 million U.S. adult ( > 18) population in 2015 (www.census.gov/quickfacts/table/PST045215/00) 4. The European Union Market Potential for a New PTSD Drug. Prepared for Tonix Pharmaceuticals by Procela Consultants Ltd Septe mbe r 2016.

© 2016 Tonix Pharmaceuticals Holding Corp 8 What Are the Symptoms of PTSD? Symptoms of PTSD fall into four clusters: 1. Intrusion ( aversive memories, nightmares, flashbacks ) 2. Avoidance ( avoiding persons, places or situations ) 3. Mood/cognitions ( memory block, emotional numbing, detachment from others ) 4. Hyperarousal ( anxiety, agitation & sleep disturbance ) Symptoms assessed for diagnosis, severity and treatment effect • Clinician Administered PTSD Scale ("CAPS - 5") • Recognized as the standard for rating PTSD severity in clinical trials • Takes into account all four symptom clusters

© 2016 Tonix Pharmaceuticals Holding Corp 9 What Are the Consequences of PTSD? Consequences: • Impaired daily function and substantial interference with work and social interactions • Reckless or destructive behavior • Increased health care utilization and greater medical morbidity PTSD as a risk f actor for: • Depression • Alcohol or substance abuse • Absenteeism/unemployment • Homelessness • Violent acts • Suicidal thoughts and suicide Unmet Needs : • Effective therapy for populations not well served by current treatment (males, military trauma) • Alternative therapy for the majority of patients unresponsive or intolerant to current treatments • Drug therapy compatible and complementary with behavioral therapy
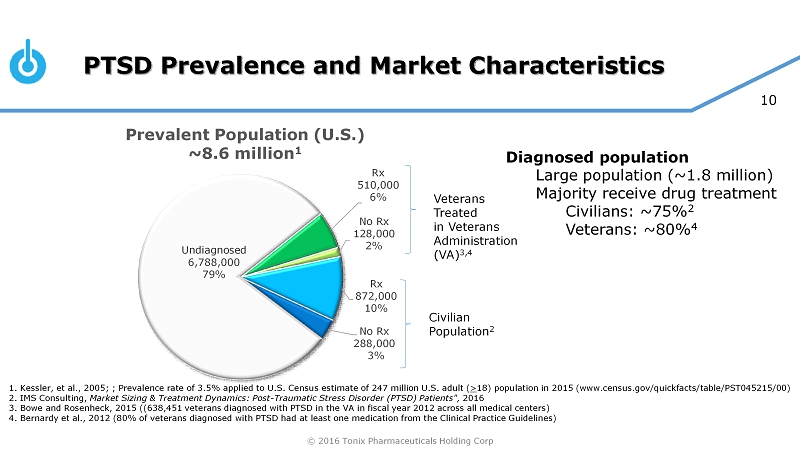
© 2016 Tonix Pharmaceuticals Holding Corp 10 PTSD Prevalence and Market Characteristics Undiagnosed 6,788,000 79% Rx 510,000 6% No Rx 128,000 2% Rx 872,000 10% No Rx 288,000 3% 1. Kessler, et al., 2005; ; Prevalence rate of 3.5% applied to U.S. Census estimate of 247 million U.S. adult ( > 18) population in 2015 (www.census.gov/quickfacts/table/PST045215/00) 2. IMS Consulting, Market Sizing & Treatment Dynamics: Post - Traumatic Stress Disorder (PTSD) Patients", 2016 3. Bowe and Rosenheck, 2015 ((638,451 veterans diagnosed with PTSD in the VA in fiscal year 2012 across all medical centers) 4. Bernardy et al., 2012 (80% of veterans diagnosed with PTSD had at least one medication from the Clinical Practice Guidelin es) Veterans Treated in Veterans Administration (VA) 3,4 Civilian Population 2 Diagnosed population Large population (~1.8 million) Majority receive drug treatment Civilians: ~75% 2 Veterans: ~80% 4 Prevalent Population (U.S.) ~8.6 million 1

© 2016 Tonix Pharmaceuticals Holding Corp 11 What Drug Classes are Used to Treat PTSD? * TRx = Total prescriptions 1. VA/DoD Clinical Practice Guideline for the Management of Post - Traumatic Stress, Version 2, 2010 2. IMS Consulting, Market Sizing & Treatment Dynamics: Post - Traumatic Stress Disorder (PTSD) Patients", 2016 Benzodiazepines 2.84 M TRx SSRIs 2.84 M TRx Anticonvulsants/Mood Stabilizers Atypical Antipsychotics Other Antidepressants Serotonin and norepinephrine reuptake inhibitors Non - benzodiazpepine Hypnotics Adrenergic Agents Tricyclic Antidepressants All Others Estimated PTSD Market Volume (Civilian Population Only) ~14.1 million TRx* 2 Market highly fragmented, with benzodiazepines being widely prescribed (but not indicated 1 ) • Multiple medications per patient (or “Polypharmacy”) is the norm • Approximately 55% of patients receive a benzodiazepine, and 53% receive an selective serotonin reuptake inhibitor (SSRI) • SSRIs are the only FDA approved drug class

© 2016 Tonix Pharmaceuticals Holding Corp 12 Relevance of Sleep Disturbances for PTSD Sleep disturbances : • Core symptom of PTSD (component of intrusion and hyperarousal symptom clusters) • Believed to play roles in the pathophysiology of PTSD om Sleep As a Core Symptom P athophysiology Pharmacological Action 2 o Clinical Endpoint Therapeutic Benefit 1 o Clinical Endpoint PTSD • Nightmares • Hyperarousal Stress ≈ Hyperarousal ≈ Sleep Disturbances Hyperarousal and hypervigilance interfere with sleep and emotional memory processing during sleep Reduced hyperarousal Reduced PTSD symptoms and disability

© 2016 Tonix Pharmaceuticals Holding Corp 13 • CBP is more selective for high affinity sites believed to have a role in sleep quality 2 • 5 - HT 2A • a 1 - adrenergic • histamine H 1 • CBP undergoes extensive first - pass hepatic metabolism when administered orally • Major metabolite, norcyclobenzaprine (nCBP) • Long half - life (~72 hours) • Less selective for target receptors ( 5 - HT 2A, a 1 - adrenergic, histamine H 1 ) • More selective for norepinephrine transporter TNX - 102 SL: Innovative and Unique By Design Active Pharmaceutical Ingredient (API) is cyclobenzaprine (CBP), is structurally related to tricyclic antidepressants (TCAs) • TCAs and their metabolites differ widely in their receptor binding and pharmacokinetic profiles 1 TNX - 102 SL: Innovative sublingual formulation of CBP • Rapid systemic exposure • Increased bioavailability • Avoids first - pass metabolism • Lowers exposure to long - lived active major metabolite, nCBP Different from other tricyclics Different from oral CBP formulation 1. Rudorfer and Potter, Cellular and Molecular Neurobiology, 1999 19:373 2. Daugherty et al., Abstract 728, Society of Biological Psychiatry 70th Annual Scientific Convention, May 14 - 16, 2015,Toronto, Ontario, Canada

© 2016 Tonix Pharmaceuticals Holding Corp 14 Why Initially Target Military - Related PTSD? 1. Friedman et al., J Clin Psychiatry 2007; 68:711, 2. Zoloft Package Insert, August, 2014, 3. Paxil Package Insert, June, 2014, 4. Fava et al., Psychother Psychosom 84:72 - 81, 2015 Military - related PTSD not well - served by existing FDA - approved therapies • No clear treatment response observed in U.S. military population Sertraline: failed to show efficacy in a large multicenter trial in U.S. military (placebo numerically better) 1 Paroxetine: no large trials conducted with predominantly military trauma • Inconsistent treatment response observed in males Sertraline: FDA - conducted post - hoc analysis concluded no effect for male civilian subgroup 2 Paroxetine: no sex - related difference in treatment outcomes 3 • Important tolerability issues with SSRIs in this population Sexual dysfunction 2,3 Insomnia 2,3 SSRI withdrawal syndrome 4 © 2016 Tonix Pharmaceuticals Holding Corp
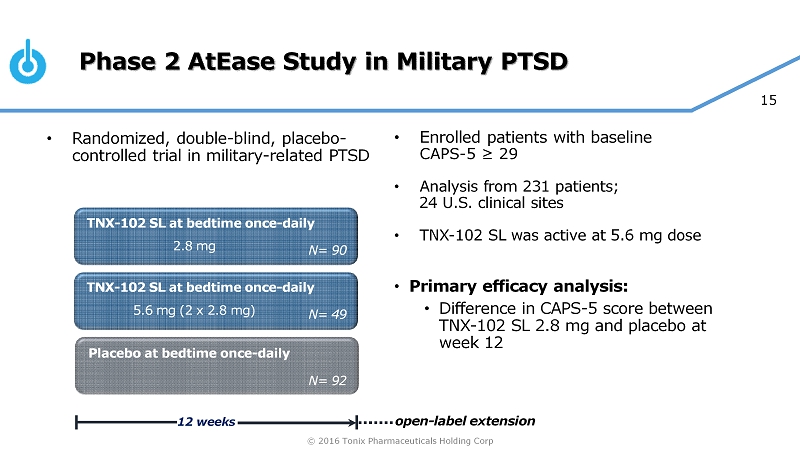
© 2016 Tonix Pharmaceuticals Holding Corp 15 Phase 2 AtEase Study in Military PTSD • Enrolled patients with baseline CAPS - 5 ≥ 29 • Analysis from 231 patients; 24 U.S. clinical sites • TNX - 102 SL was active at 5.6 mg dose • Primary efficacy analysis: • Difference in CAPS - 5 score between TNX - 102 SL 2.8 mg and placebo at week 12 TNX - 102 SL at bedtime once - daily Placebo at bedtime once - daily 12 weeks N= 90 TNX - 102 SL at bedtime once - daily N= 92 N= 49 2.8 mg 5.6 mg (2 x 2.8 mg) open - label extension • Randomized, double - blind, placebo - controlled trial in military - related PTSD

© 2016 Tonix Pharmaceuticals Holding Corp 16 AtEase Study Demographics and Characteristics 93% of the randomized patients were male 98% had trauma during military service Deployed an average of 2.3 times Mean time since index trauma was 7 years Race and ethnicity generally consistent with U.S. military distribution Current Major Depressive Disorder 14% by MINI 7.0 Similar baseline CAPS - 5 scores and MADRS scores across treatment arms Variable Placebo N=92 TNX - 102 SL 2.8 mg N=90 TNX - 102 SL 5.6 mg N=49 Overall N=231 Baseline CAPS - 5 Scores (SD) 39.5 (7.7) 39.5 (8.0) 39.3 (8.1) 39.5 (7.85) Baseline MADRS Scores (SD) 17.3 (6.5) 17.6 (5.2) 16.1 (5.5) 17.1 (5.83) CAPS - 5, Clinician Administered PTSD Scale for DSM - 5 MADRS, Montgomery - Åsberg Depression Rating Scale MINI 7.0, Mini - International Neuropsychiatric Interview, version 7 SD, standard deviation
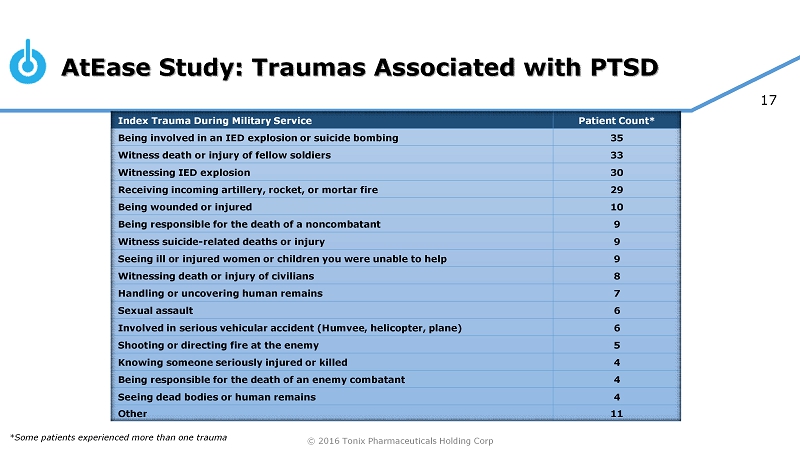
© 2016 Tonix Pharmaceuticals Holding Corp 17 AtEase Study: Traumas Associated with PTSD Index Trauma During Military Service Patient Count* Being involved in an IED explosion or suicide bombing 35 Witness death or injury of fellow soldiers 33 Witnessing IED explosion 30 Receiving incoming artillery, rocket, or mortar fire 29 Being wounded or injured 10 Being responsible for the death of a noncombatant 9 Witness suicide - related deaths or injury 9 Seeing ill or injured women or children you were unable to help 9 Witnessing death or injury of civilians 8 Handling or uncovering human remains 7 Sexual assault 6 Involved in serious vehicular accident (Humvee, helicopter, plane) 6 Shooting or directing fire at the enemy 5 Knowing someone seriously injured or killed 4 Being responsible for the death of an enemy combatant 4 Seeing dead bodies or human remains 4 Other 11 *Some patients experienced more than one trauma

© 2016 Tonix Pharmaceuticals Holding Corp 18 AtEase Study – Summary of Primary and Secondary Analyses (week 12)
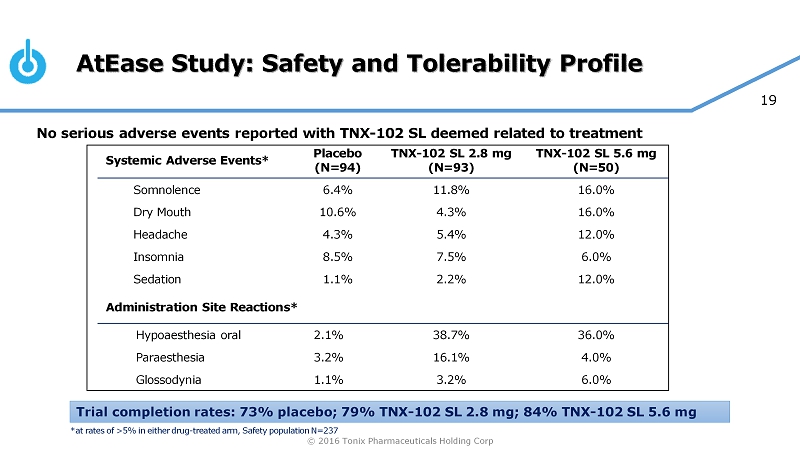
© 2016 Tonix Pharmaceuticals Holding Corp 19 AtEase Study: Safety and Tolerability Profile No serious adverse events reported with TNX - 102 SL deemed related to treatment Systemic Adverse Events* Placebo (N=94) TNX - 102 SL 2.8 mg (N=93) TNX - 102 SL 5.6 mg (N=50) Somnolence 6.4% 11.8% 16.0% Dry Mouth 10.6% 4.3% 16.0% Headache 4.3% 5.4% 12.0% Insomnia 8.5% 7.5% 6.0% Sedation 1.1% 2.2% 12.0% Administration Site Reactions* Hypoaesthesia oral 2.1% 38.7% 36.0% Paraesthesia 3.2% 16.1% 4.0% Glossodynia 1.1% 3.2% 6.0% Trial completion rates: 73% placebo; 79% TNX - 102 SL 2.8 mg; 84% TNX - 102 SL 5.6 mg *at rates of >5% in either drug - treated arm, Safety population N=237

© 2016 Tonix Pharmaceuticals Holding Corp 20 0 0.1 0.2 0.3 0.4 0.5 0.6 Effect Size CAPS - 5 ≥ 29 0 0.1 0.2 0.3 0.4 0.5 0.6 Effect Size CAPS - 5 ≥33 AtEase: Retrospective Analysis for TNX - 102 SL 5.6 mg * MMRM, mixed - effects model repeated measures, p<0.05 Prior pharmacotherapy trials in PTSD used earlier versions of CAPS (e . g . CAPS - 2 ) • Different scoring range from CAPS - 5 • Most frequently used a score of ≥ 50 for entry (similar to CAPS - 5 ≥ 33 1 ) • FDA has accepted this higher entry criteria (CAPS - 5 ≥ 33 ) for Phase 3 program Compared CAPS - 5 Severity Entry Criteria ≥ 29 versus ≥ 33 on Effect Size for AtEase • Retrospective analysis showed more robust effect with high entry criteria * * * * 1 Sullivan, Gregory, et al. Poster session presented at: Military Health System Research Symposium (MHSRS) 2016 Annual Meeting; 20 16 Aug 15 - 18; Kissimmee, FL. URL: http://bit.ly/2bFo4mx

© 2016 Tonix Pharmaceuticals Holding Corp 21 Retrospective Analysis of CAPS - 5 in patients with entry CAPS - 5 ≥ 33 1 -30.0 -25.0 -20.0 -15.0 -10.0 -5.0 0.0 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 LS Mean (SE) of CAPS - 5 Total Score Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg 6.8 point difference in CAPS - 5 change from baseline between placebo and TNX - 102 SL 5.6 mg Effect Size = 0.53 * * * * * * p<0.025, comparing 5.6 mg group with placebo, MMRM * p=0.029, comparing 2.8 mg group with placebo, MMRM 1 Primary analysis was TNX - 102 SL 2.8 mg with entry CAPS - 5 ≥ 29

© 2016 Tonix Pharmaceuticals Holding Corp 22 Week 12 outcome measures for TNX - 102 SL 5.6 mg vs. placebo in military - related PTSD for CAPS - 5 ≥ 29 and CAPS - 5 ≥ 33 at baseline PBO N=92, 5.6mg N=49; CAPS - 5 ≥ 29 PBO N=77, 5.6mg N=38; CAPS - 5 ≥ 33 Outcome Measure ES 1 p - value 2 ES 1 p - value 2 CAPS - 5 Total score 0.36 0.053 0.53 *0.013 Cluster B (intrusion) 0.26 0.161 0.46 *0.026 Cluster C (avoidance) 0.04 0.963 0.12 0.522 Cluster D (mood/cognition) 0.35 0.062 0.39 0.065 Cluster E (arousal and reactivity) 0.35 *0.048 0.52 *0.012 E6 (Sleep item) 0.51 *0.010 0.51 *0.013 E2 (Reckless/Self Destruct) 0.15 0.140 0.30 *0.012 CGI – I (responders) 2.11 *0.041 2.29 *0.042 SDS Total Score 0.33 0.079 0.35 0.093 Work/School item 0.34 0.050 0.41 *0.040 Social/Leisure item 0.38 *0.031 0.35 0.116 Family Life/Home Responsibilities item 0.12 0.524 0.15 0.455 1Cohen’s d for CAPS - 5 and Sheehan Disability Scale outcome measures; odds ratio from logistic regression for CGI - I. 2CAPS - 5 and Sheehan Disability Scale outcome measures: p - values from mixed - effects model repeated measures statistical test on the mean change from baseline difference between TNX - 102 SL 5.6 mg and placebo; CGI - I: p - values from a repeated measure logistic regression (Responde r defined as a patient who was rated as “1” very much improved, or “2” much improved at week 12). *denotes statistical significance difference with p<0 .05, not adjusted for multiple comparisons; Abbreviations: 5.6 mg=TNX - 102 SL 5.6 mg; CAPS - 5=Clinician - Administered PTSD Scale for DSM - 5; ES=Effect Siz e; N=number of patients; PBO=Placebo; SDS=Sheehan Disability Scale.
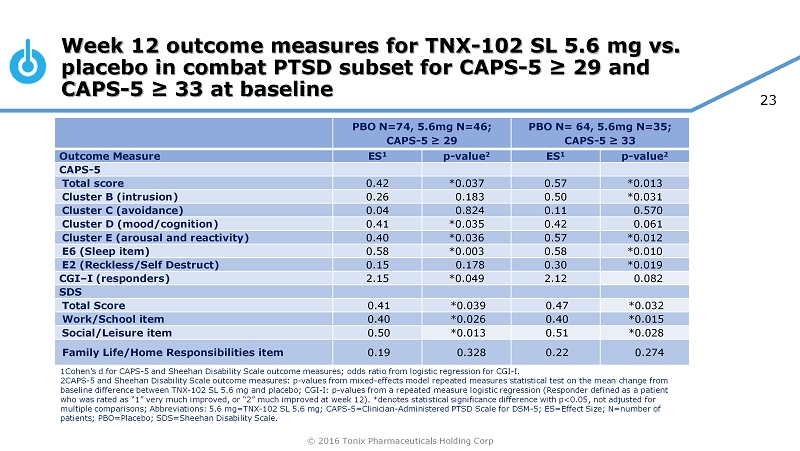
© 2016 Tonix Pharmaceuticals Holding Corp 23 Week 12 outcome measures for TNX - 102 SL 5.6 mg vs. placebo in combat PTSD subset for CAPS - 5 ≥ 29 and CAPS - 5 ≥ 33 at baseline PBO N=74, 5.6mg N=46; CAPS - 5 ≥ 29 PBO N= 64, 5.6mg N=35; CAPS - 5 ≥ 33 Outcome Measure ES 1 p - value 2 ES 1 p - value 2 CAPS - 5 Total score 0.42 *0.037 0.57 *0.013 Cluster B (intrusion) 0.26 0.183 0.50 *0.031 Cluster C (avoidance) 0.04 0.824 0.11 0.570 Cluster D (mood/cognition) 0.41 *0.035 0.42 0.061 Cluster E (arousal and reactivity) 0.40 *0.036 0.57 *0.012 E6 (Sleep item) 0.58 *0.003 0.58 *0.010 E2 (Reckless/Self Destruct) 0.15 0.178 0.30 *0.019 CGI – I (responders) 2.15 *0.049 2.12 0.082 SDS Total Score 0.41 *0.039 0.47 *0.032 Work/School item 0.40 *0.026 0.40 *0.015 Social/Leisure item 0.50 *0.013 0.51 *0.028 Family Life/Home Responsibilities item 0.19 0.328 0.22 0.274 1Cohen’s d for CAPS - 5 and Sheehan Disability Scale outcome measures; odds ratio from logistic regression for CGI - I. 2CAPS - 5 and Sheehan Disability Scale outcome measures: p - values from mixed - effects model repeated measures statistical test on t he mean change from baseline difference between TNX - 102 SL 5.6 mg and placebo; CGI - I: p - values from a repeated measure logistic regression (Responde r defined as a patient who was rated as “1” very much improved, or “2” much improved at week 12). *denotes statistical significance difference with p<0 .05, not adjusted for multiple comparisons; Abbreviations: 5.6 mg=TNX - 102 SL 5.6 mg; CAPS - 5=Clinician - Administered PTSD Scale for DSM - 5; ES=Effect Siz e; N=number of patients; PBO=Placebo; SDS=Sheehan Disability Scale.

© 2016 Tonix Pharmaceuticals Holding Corp 24 Analysis Using CAPS - 5 ≥ 33 As Entry Criteria Reckless or Self - Destructive Behavior Item on CAPS - 5 0 0.5 1 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 Reckless or Self - Destructive Mean Score Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg * * p=0.012, TNX - 102 SL 5.6 mg v. placebo, mixed model repeated measures Reckless or self - destructive behavior can include dangerous driving, high - risk thrill - seeking, excessive alcohol or drug use, injurious behaviors to self or others, or suicidal behaviors.

© 2016 Tonix Pharmaceuticals Holding Corp 25 First large multi - center trial demonstrating efficacy of an Investigational New Drug (IND) in military - related PTSD x TNX - 102 SL therapeutic dose (5.6 mg) identified x Symptom reduction (CAPS - 5) x Functional improvement (Sheehan Disability Scale domains) x Clinical global impression of improvement (CGI - I) Effects on sleep and hyperarousal x Consistent with mechanistic hypothesis Well - tolerated; side effects include: x Systemic: somnolence, dry mouth, headache and sedation x Local administration site reactions: transient tongue numbness Phase 2 AtEase Study Conclusions Comprehensive AtEase study results from scientific presentations available at: http://www.tonixpharma.com/research - development/scientific - presentations

© 2016 Tonix Pharmaceuticals Holding Corp 26 Hypothesized mechanism of action in P TSD Attenuation of Sleep Disruption by TNX - 102 SL Leads to Recovery Recovery (over weeks) Diagrams adapted from Pace - Schott et al. Biol Mood Anxiety Disord 2015;5:3

© 2016 Tonix Pharmaceuticals Holding Corp 27 Planned Phase 3 Program in PTSD General Study Characteristics: • Randomized, double - blind, placebo - controlled, entrance CAPS - 5 ≥ 33 • One to two planned unblinded interim analyses (IAs) • First IA (N ~180) for efficacy stop or sample size adjustment • Potential to enroll 550 patients • Approximately 30 U.S. clinical sites Primary Efficacy Endpoint: • Mean change from baseline in total CAPS - 5 at Week 12 compared between TNX - 102 SL 5.6 mg and placebo Placebo once - daily at bedtime 12 weeks TNX - 102 SL once - daily at bedtime N ~ 275 (90*) N ~ 275 (90*) 5.6 mg First IA topline data anticipated 2H 2017 To confirm AtEase findings in military - related PTSD: • Larger adaptive design study • Targeting start in 1Q 2017 * First interim analysis

© 2016 Tonix Pharmaceuticals Holding Corp 28 Commercialization Options Tonix is exploring a variety of options to commercialize TNX - 102 SL, including commercializing on our own or partnering all or some indications in specific regions of the world. Commercial Considerations: • Primary physician audience is well defined: psychiatrists (~30,000 in U.S.) • Small specialty sales force sufficient for coverage • Primary market research with psychiatrists indicate strong interest in new therapeutic options

© 2016 Tonix Pharmaceuticals Holding Corp 29 Intellectual Property Portfolio of international patents filed under the Patent Cooperation Treaty (PCT) C omposition - of - matter (eutectic) • Patents filed • Protection expected to 2034 Pharmacokinetics (PK) • Patents filed • Protection expected to 2033 Method - of - use • PTSD: patents filed TNX - 102 SL

© 2016 Tonix Pharmaceuticals Holding Corp 30 Financial overview NASDAQ: TNXP Cash, cash equivalents, and marketable securities reported at September 30, 2016 $26.7 million Net proceeds from underwritten offering in 4Q16 $4.6 million Shares outstanding (December 1, 2016) 39.2 million

© 2016 Tonix Pharmaceuticals Holding Corp 31 Management Team Seth Lederman, MD President & CEO Jessica Morris EVP, Operations Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer

© 2016 Tonix Pharmaceuticals Holding Corp 32 Board of Directors Seth Lederman, MD Chairman Ernest Mario, PhD ALZA, Glaxo, Reliant Pharma John Rhodes NYSERDA, NRDC, Booz Allen Hamilton Samuel Saks, MD Jazz Pharma, ALZA, Johnson & Johnson Charles Mather BTIG, Janney, Jefferies, Cowen, Smith Barney Stuart Davidson Labrador Ventures, Alkermes , Combion Patrick Grace Apollo Philanthropy, WR Grace, Chemed Donald Landry, MD, PhD Chair of Medicine, Columbia University

© 2016 Tonix Pharmaceuticals Holding Corp 33 Milestones – recent and upcoming TNX - 102 SL – Posttraumatic Stress Disorder □ December 2015 Entered into Collaborative Research and Development Agreement (CRADA) with the United States Army Medical Materiel Development Activity (USAMMDA) □ May 2016 Report results from AtEase study □ August 2016 End - of - Phase 2 meeting with FDA - Proposed Phase 3 clinical and NDA plan accepted - Breakthrough Therapy Designation Request can be submitted for review □ 1Q 2017 Target commencement of Phase 3 study in military - related PTSD □ 2H 2017 Anticipated t opline data from interim analysis of Phase 3 study in ~180 military - related PTSD patients x x x

© 2016 Tonix Pharmaceuticals Holding Corp 34 Summary Strong position for value growth with Phase 3 trial in a major medical indication: PTSD • Phase 3 asset not previously well - known to the investor marketplace Funded through 1 st interim analysis (180 patients) of Phase 3 PTSD trial expected to initiate in 1Q2017 • Topline data from 1 st interim analysis expected to be available 2H2017

© 2016 Tonix Pharmaceuticals Holding Corp Thank you ! NASDAQ: TNXP
