Attached files
| file | filename |
|---|---|
| EX-32.01 - EXHIBIT 32.01 - Tonix Pharmaceuticals Holding Corp. | v432316_ex32-01.htm |
| EX-21.01 - EXHIBIT 21.01 - Tonix Pharmaceuticals Holding Corp. | v432316_ex21-01.htm |
| EX-31.01 - EXHIBIT 31.01 - Tonix Pharmaceuticals Holding Corp. | v432316_ex31-01.htm |
| EX-31.02 - EXHIBIT 31.02 - Tonix Pharmaceuticals Holding Corp. | v432316_ex31-02.htm |
| EX-23.01 - EXHIBIT 23.01 - Tonix Pharmaceuticals Holding Corp. | v432316_ex23-01.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended December 31, 2015
Commission File Number 001-36019
TONIX PHARMACEUTICALS HOLDING CORP.
(Exact name of registrant as specified in its charter)
| Nevada | 26-1434750 | |
|
(State or other jurisdiction of incorporation or organization) |
(IRS Employer Identification No.) | |
|
509 Madison Avenue, Suite 306 New York, New York |
10022 | (212) 980-9155 |
| (Address of principal executive office) | (Zip Code) | (Registrant’s telephone number, including area code) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |
| Common Stock, $0.001 par value | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined by Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer x |
| Non-accelerated filer ¨ | Smaller reporting company ¨ |
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).Yes ¨ No x
The aggregate market value of the voting common equity held by non-affiliates as of June 30, 2015, based on the closing sales price of the common stock as quoted on The NASDAQ Global Market was $107,689,550. For purposes of this computation, all officers, directors, and 5 percent beneficial owners of the registrant are deemed to be affiliates. Such determination should not be deemed an admission that such directors, officers, or 5 percent beneficial owners are, in fact, affiliates of the registrant.
As of March 2, 2016, there were 18,873,264 shares of registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant's Proxy Statement for the 2016 Annual Meeting of Stockholders are incorporated herein by reference in Part III of this Annual Report on Form 10-K to the extent stated herein. Such proxy statement will be filed with the Securities and Exchange Commission within 120 days of the registrant's fiscal year ended December 31, 2015.
TABLE OF CONTENTS
| 2 |
This Annual Report on Form 10-K (including the section regarding Management's Discussion and Analysis of Financial Condition and Results of Operations) contains forward-looking statements regarding our business, financial condition, results of operations and prospects. Words such as “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates” and similar expressions or variations of such words are intended to identify forward-looking statements, but are not deemed to represent an all-inclusive means of identifying forward-looking statements as denoted in this Annual Report on Form 10-K. Additionally, statements concerning future matters are forward-looking statements.
Although forward-looking statements in this Annual Report on Form 10-K reflect the good faith judgment of our Management, such statements can only be based on facts and factors currently known by us. Consequently, forward-looking statements are inherently subject to risks and uncertainties and actual results and outcomes may differ materially from the results and outcomes discussed in or anticipated by the forward-looking statements. Factors that could cause or contribute to such differences in results and outcomes include, without limitation, those specifically addressed under the heading “Risks Factors” below, as well as those discussed elsewhere in this Annual Report on Form 10-K. Readers are urged not to place undue reliance on these forward-looking statements, which speak only as of the date of this Annual Report on Form 10-K. We file reports with the Securities and Exchange Commission (“SEC”). You can read and copy any materials we file with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington, DC 20549. You can obtain additional information about the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet site (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, including us.
We undertake no obligation to revise or update any forward-looking statements in order to reflect any event or circumstance that may arise after the date of this Annual Report on Form 10-K. Readers are urged to carefully review and consider the various disclosures made throughout the entirety of this annual Report, which attempt to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects.
Tonix Pharmaceuticals®, Tonmya® and other trademarks and intellectual property of ours appearing in this report are our property. This report contains additional trade names and trademarks of other companies. We do not intend our use or display of other companies’ trade names or trademarks to imply an endorsement or sponsorship of us by such companies, or any relationship with any of these companies.
Tonmya is the proposed trade name for TNX-102 SL for fibromyalgia, or FM, and has been conditionally accepted by the FDA. TNX-102 SL for FM and PTSD is an investigational new drug and has not been approved for any indication.
Business Overview
Tonix Pharmaceuticals Holding Corp., together with its subsidiaries (collectively “we,” “our,” “us,” “Tonix” or the “Company”), is a clinical-stage pharmaceutical company dedicated to the invention and development of next-generation medicines. Our most advanced drug development programs are directed toward disorders affecting the central nervous system, or CNS, and include FM and post-traumatic stress disorder, or PTSD. These disorders are characterized by chronic disability, inadequate treatment options, high utilization of healthcare services, and significant economic burden. We have assembled a management team with significant industry experience to lead the development of our product candidates. We complement our management team with a network of scientific, clinical, and regulatory advisors that includes recognized experts in the fields of FM, PTSD and other central nervous system disorders.
Our lead product candidate, TNX-102 SL (cyclobenzaprine HCl sublingual tablets) is in Phase 3 clinical development as a potential treatment for FM. In addition, TNX-102 SL is in Phase 2 clinical development as a potential treatment for PTSD. Our nonclinical pipeline includes a development program for the treatment of alcohol use disorders, or AUD, as well as two biodefense development programs for protection from smallpox virus and from radiation injury. We hold worldwide development and commercialization rights to all of our product candidates.
Our clinical-stage product candidates are as follows:
TNX-102 SL
TNX-102 SL is a small, rapidly disintegrating tablet containing cyclobenzaprine, or CBP, for sublingual administration and transmucosal absorption. We are developing TNX-102 SL for two indications:
| 3 |
Fibromyalgia. Fibromyalgia is a debilitating syndrome that occurs in five to 15 million U.S. adults and is associated with a substantial negative impact on social and occupational function, including disrupted relationships with family and friends, social isolation, reduced activities of daily living and leisure activities, avoidance of physical activity, and loss of career or inability to advance in careers or education. Many patients fail to adequately respond to the medications approved for FM, or discontinue therapy due to poor tolerability. Prescription pain and sleep medications not approved for FM are frequently taken for symptomatic relief, despite the lack of evidence that such medications provide a meaningful or durable therapeutic effect.
Post-traumatic stress disorder. An estimated 8.4 million adults in the U.S. suffer from PTSD, a chronic disorder that is characterized by avoidance, emotional numbing, hyperarousal, and sleep disturbances. People with PTSD suffer significant impairment in their functioning, including occupational activities and social relations, and are at elevated risk for impulsive, violent behaviors toward others and themselves, including suicide. Many patients fail to adequately respond to the medications approved for PTSD. Antidepressants, sedative-hypnotics and antipsychotics not approved for PTSD are commonly prescribed despite generally weak evidence in support of their use.
TNX-201
TNX-201 is an oral formulation of dexisometheptene mucate that we were developing for episodic tension-type headache, or ETTH. On February 16, 2016, we announced that we discontinued development of TNX-201 because it lacked efficacy.
Our Strategy
Our objective is to develop and commercialize our product candidates. The principal components of our strategy are to:
| • | Develop TNX-102 SL for multiple central nervous system disorders. We currently are pursuing the development of TNX-102 SL for two separate indications, FM and PTSD. Our broad development strategy is designed to explore the clinical potential of TNX-102 SL in disorders that are underserved by currently available medications and represent large unmet medical needs; |
| • | Maximize the commercial potential of TNX-102 SL. We plan to commercialize TNX-102 SL for indications including FM and PTSD either on our own or through collaboration with partners. We believe TNX-102 SL can be marketed to U.S. physicians either by an internal sales force that we will build or by a contract sales organization, which we would engage. An alternative strategy would be to enter into partnership agreements with drug companies that already have significant marketing capabilities in the same, or similar, therapeutic areas. If we determine that such a strategy would be more favorable than developing our own sales capabilities, we would seek to enter into collaborations with pharmaceutical or biotechnology companies for the commercialization of TNX-102 SL; |
| • | Pursue a broad intellectual property strategy to protect our product candidates. We are pursuing a broad patent strategy for our product candidates, and we endeavor to generate new patent applications as supported by our innovations and conceptions as well as to advance their prosecution. In the case of TNX-102 SL, we own patents and patent applications protecting its composition-of-matter, certain methods of its use, its formulation, and its pharmacokinetic properties. We plan to opportunistically apply for new patents to protect TNX-102 SL and our other product candidates; |
| • | Provide value propositions to merit market demand and reimbursement for our product candidates. We are designing the development programs for our product candidates to demonstrate their value propositions to patients, prescribers, and third-party payors. In the case of TNX-102 SL, we have been engaged in market research and commercial assessment activities, the results of which we may use to inform future commercial strategy. We plan to continue these activities in tandem with our clinical development of TNX-102 SL and to conduct similar work in relation to our other product candidates as they advance in their development; and |
| • | Pursue additional indications and commercial opportunities for our product candidates. We will seek to maximize the value of TNX-102 SL, and our other product candidates by pursuing other indications and commercial opportunities for such candidates. For example, we own rights related to the development and commercialization of CBP for generalized anxiety disorder, depression, and fatigue related to disordered sleep. |
Disease and Market Overview
Our product candidates address disorders that are not well served by currently available therapies and represent large potential commercial market opportunities. Background information on the disorders and related commercial markets that may be addressed by our clinical-stage product candidates is set forth below.
| 4 |
Fibromyalgia
FM is a chronic syndrome characterized by widespread musculoskeletal pain accompanied by fatigue, sleep, memory and mood issues. The peak incidence of FM occurs between 20-50 years of age, and 80-90% of diagnosed patients are female. FM may have a substantial negative impact on social and occupational function, including disrupted relationships with family and friends, social isolation, reduced activities of daily living and leisure activities, avoidance of physical activity, and loss of career or inability to advance in career or education. According to published estimates, there are approximately five to fifteen million people suffering from FM in the U.S. (Vincent et al, Arthritis Care Res 2013;65:786-792; Lawrence et al, Arthritis Rheum 2008;58:26-35). Based on our market research, we believe that sales in the U.S. of FDA-approved medications for FM were approximately $1.2 billion in 2014, representing approximately 5.6 million prescriptions.
According to a report by Frost and Sullivan that we commissioned, despite the availability of approved medications, the majority of patients fail therapy due to either insufficient efficacy or poor tolerability, or both. Prescription pain and sleep medications are frequently prescribed off-label for symptomatic relief, despite the lack of evidence that such medications provide a meaningful or durable therapeutic benefit, and many of these medications carry significant safety risks and risk of dependence.
Post-traumatic Stress Disorder
PTSD is a chronic syndrome that may develop after a person is exposed to one or more traumatic events, such as warfare, sexual assault, serious injury, or threat of imminent death. The core symptom clusters of PTSD are avoidance, emotional numbing, hyperarousal, and intrusion, where the triggering event is commonly re-experienced by the individual through intrusive, recurrent recollections, flashbacks, and nightmares. People with PTSD suffer significant impairment in their daily functioning, including occupational activities and social relations, and are at elevated risk for impulsive violent behaviors toward others and themselves, including suicide. Of those who experience significant trauma, approximately 20% of women and 8% of men develop PTSD. According to the U.S. Department of Veterans Affairs, the prevalence rate of PTSD in the military population is higher than that among civilians. As of 2009, there were approximately 500,000 veterans receiving treatment for PTSD in the Veterans Health Administration, or VHA. Based on March 2014 VHA data, approximately 20% of military personnel involved in recent conflicts were seen at VHA facilities for potential or provisional PTSD.
The medications currently approved by the FDA for the treatment of PTSD show little evidence of a treatment effect in men, lack evidence of efficacy in those for whom the traumatic event was combat-related, and carry suicidality warnings. Sleep disturbances are central features of PTSD and are predictive of disease severity, depression, substance abuse, and suicidal ideation, yet are resistant to the approved medications and present a difficult therapeutic challenge. Current PTSD treatments include off-label use of anxiolytics, sedative-hypnotics, and antipsychotics, many of which lack reliable evidence of efficacy, and have significant safety liabilities and dependence risk.
Our Product Candidates
We currently are focused on developing a portfolio of product candidates, including one product candidate in clinical development for registration in two indications. We believe that our product candidates offer innovative therapeutic approaches and may provide significant advantages relative to current therapies. The following table summarizes our most advanced product candidates, for which we plan to complete the required clinical and nonclinical studies to support their NDA filings:
| Product Candidate | Indication | Stage of Development | Commercialization Rights | |||
| TNX-102 SL | Fibromyalgia | Phase 3 | Worldwide | |||
| TNX-102 SL | Post-traumatic stress disorder | Phase 2 | Worldwide |
TNX-102 SL
Overview
TNX-102 SL is a sublingual tablet formulation of CBP that efficiently delivers CBP across the oral mucosal membrane into the systemic circulation. We are developing TNX-102 SL for fibromyalgia and post-traumatic stress disorder. We own all rights to TNX-102 SL in all geographies, and we bear no obligations to third-parties for any future development or commercialization.
Excipients used in TNX-102 SL are approved for pharmaceutical use. Some of the excipients were specially selected to promote a local oral environment that facilitates mucosal absorption of CBP.
| 5 |
TNX-102 SL contains 2.8 mg of CBP. We selected this dose with the goal of providing a balance of efficacy, safety, and tolerability that would be acceptable as a first-line therapy and for long-term use, and in patient populations characterized by burdensome symptoms and sensitivity to medications.
TNX-102 SL is a serotonin 2A and alpha-1 adrenergic receptor antagonist as well as an inhibitor of serotonin and norepinephrine reuptake, and we refer to it as a Serotonin and Norepinephrine receptor Antagonist and Reuptake Inhibitor, or SNARI. In FM, pregabalin is believed to exert its clinical benefit primarily by blocking calcium channels, and both duloxetine and milnacipran are believed to exert their clinical benefit mainly by inhibiting the reuptake of serotonin and norepinephrine. In PTSD, both paroxetine and sertraline are believed to exert their clinical benefit primarily by blocking serotonin reuptake. As such, TNX-102 SL acts upon cellular receptors that play important roles in the treatment of FM and PTSD, including the receptors that mediate serotonin and norepinephrine reuptake. In addition, TNX-102 SL also acts upon other receptors in the central nervous system not targeted by products approved for these indications, including the serotonin 2A and alpha-1 adrenergic receptors.
CBP is the active ingredient of two products that are approved in the U.S. for the treatment of muscle spasm: FLEXERIL® (oral immediate-release tablet, 5 mg and 10 mg dosage forms) and AMRIX® (oral extended-release capsule, 15 mg and 30 mg dosage forms). FLEXERIL brand of cyclobenzaprine immediate-release tablet has been discontinued since May 2013. There are numerous generic versions of cyclobenzaprine immediate-release tablets on the market. CBP-containing products are not indicated for the treatment of FM or PTSD. CBP-containing products are approved for short term use (two to three weeks) only. Immediate-release, or IR, CBP tablets are recommended for three times per day dosing, which results in relatively stable blood levels of CBP after several days of treatment. Extended-release CBP capsules taken once a day mimic, and flatten, the pharmacokinetic profile of three times per day immediate-release CBP tablets.
We designed TNX-102 SL to be administered once-daily at bedtime and intended for long-term dosing regimen. We believe the selected dose of TNX-102 SL and its pharmacokinetic profile will enable it to achieve a desirable balance of efficacy, safety, and tolerability in FM and PTSD. Our Phase 1 comparative trials showed that, on a dose-adjusted basis, TNX-102 SL results in faster systemic absorption and significantly higher plasma levels of CBP in the first hour following sublingual administration relative to oral immediate-release CBP tablets. In clinical studies, TNX-102 SL, 2.8 mg was generally well-tolerated, with no serious adverse events reported in these studies. Some subjects experienced transient numbness of the tongue after TNX-102 SL administration.
We expect that any applications we submit to the Food and Drug Administration, or FDA, for approval of TNX-102 SL will be submitted under Section 505(b)(2) of the Federal Food, Drug, and Cosmetic Act, or FDCA, for product candidates containing an active ingredient that is similar or identical to an already approved product. In general, the development timeline for a 505(b)(2) New Drug Application, or NDA, is shorter and less expensive than an NDA developed under Section 505(b)(1), which is for new chemical entities, or NCEs, that have never been approved in the United States. Currently, we are pursuing the development of TNX-102 SL for two separate indications. These indications are fibromyalgia, for which TNX-102 SL is in Phase 3 development, and post-traumatic stress disorder, for which TNX-102 SL is in Phase 2 development. We believe that TNX-102 SL has the potential to provide clinical benefit to these and possibly other CNS indications that are underserved by currently marketed products.
TNX-102 SL – Fibromyalgia Program
We are developing TNX-102 SL for the treatment of FM under an effective investigational new drug, or IND, application. Our therapeutic approach to FM was initially supported by results from a randomized, double-blind, placebo-controlled Phase 2a clinical trial of TNX-102 immediate release capsules, or TNX-102 capsules, which we have also referred to as VLD CBP (Moldofsky et al, J Rheumatol 2011;38:2653-63). This study demonstrated significant decreases in pain and other symptoms in patients treated with TNX-102 capsules daily between dinner and bedtime for eight weeks. This study also demonstrated that treatment with TNX-102 capsules led to a significant improvement in objective measures of sleep quality, which we believe relates to the mechanism by which CBP leads to improvement of FM symptoms.
Clinical Development Plan
At an End-of-Phase 2/Pre-Phase 3 meeting with the FDA in February 2013, we discussed the design of our clinical program, including the acceptability of the pivotal study design and the proposed registration plan, to support the approval of TNX-102 SL for the management of FM. On the basis of our discussions with the FDA, we believe that positive results from two adequate, well-controlled efficacy and safety studies and long-term (six- and 12-month) safety exposure studies would provide sufficient evidence of efficacy and safety to support FDA approval of TNX-102 SL for the management of FM.
| 6 |
Phase 2b “BESTFIT” Study
In September 2013, we commenced enrollment of our BESTFIT trial, a randomized, double-blind, placebo-controlled Phase 2b clinical trial of TNX-102 SL in FM. We reported preliminary top line results from the BESTFIT trial in September 2014. In the BESTFIT trial, 205 patients with FM were randomized at 17 U.S. centers to treatment with either TNX-102 SL 2.8 mg or placebo sublingual tablets at bedtime daily for 12 weeks. The primary outcome measure of the BESTFIT trial was the mean change in week 12 average daily pain intensity from baseline on the 11-point Numeric Rating Scale, using a daily telephonic diary. In the BESTFIT trial, TNX-102 SL did not achieve statistical significance in the primary outcome measure (p=0.172). However, the trial demonstrated that TNX-102 SL had a statistically significant effect on pain as measured by a 30% responder analysis of the primary pain data (p=0.033), in which a responder is defined as a subject for whom pain intensity was reduced by at least 30% at week 12 as compared to baseline. The 30% response rate in the final analysis was 34.0% in the active treatment arm as compared to 20.6% in the control arm. The BESTFIT trial also showed statistically significant improvements with TNX-102 SL in the declared secondary analyses of the Patient Global Impression of Change (p=0.025) and the Fibromyalgia Impact Questionnaire-Revised, or FIQ-R (p=0.014). The study showed statistically significant improvement with TNX-102 SL on measures of sleep quality, including the Patient-Reported Outcomes Measurement Information System, or PROMIS, Sleep Disturbance instrument (p=0.005). In addition, statistically significant improvements with TNX-102 SL were observed on several FIQ-R items (pain, sleep quality, anxiety, stiffness, and sensitivity) as well as on the overall symptom subdomain.
TNX-102 SL was well tolerated in the BESTFIT trial. Among patients randomized to the active and control arms, 86% and 83%, respectively, completed the 12-week dosing period. The most common adverse events were local in nature, with transient tongue or mouth numbness occurring in 44% of participants on TNX-102 SL vs. 2% on placebo, and bitter taste in 8% on TNX-102 SL compared to none on placebo. These local adverse events did not appear to affect either rates of retention of study participants or their compliance with taking TNX-102 SL. Systemic adverse events were similar between TNX-102 SL and placebo. No serious adverse events were reported.
Phase 3 “AFFIRM” Study
Following our report of the results of the BESTFIT trial, we requested guidance from the FDA on our proposed use of a 30% pain responder analysis as the primary efficacy endpoint in a prospective Phase 3 registration program. In January 2015, we announced receipt of the written guidance, whereby the FDA accepted our proposal to use a 30% pain responder analysis as the primary efficacy endpoint to support the approval of TNX-102 SL for the management of FM.
In the second quarter of 2015, we commenced the AFFIRM trial, a 500 patient, randomized, double-blind trial comparing TNX-102 SL to placebo (1:1 ratio), in which study medication is administered sublingually once daily at bedtime for 12 weeks. The primary endpoint of the AFFIRM trial is the FDA-agreed upon 30% pain responder analysis. We expect to report top line results from the AFFIRM trial in the third quarter of 2016.
Long-Term Safety Exposure Study
In August 2015, we completed Study F203, a 12-month open-label extension study of TNX-102 SL in patients who had completed the BESTFIT study. The goal of Study F203 was to obtain the prerequisite long-term safety exposure data to support a New Drug Application, or NDA, filing for TNX-102 SL for the management of FM, a chronic medical condition. We believe that we have sufficient long-term exposure data from F203 to support an NDA filing for TNX-102 SL.
Bioequivalence Pharmacokinetic Study (F105)
We have completed the clinical phase of a Phase 1 bioequivalence pharmacokinetic study (F105) that compared the pharmacokinetic profiles of single-doses of TNX-102 SL, 2.8 mg tablets manufactured at two facilities: the facility used to produce study medication for our Phase 2b BESTFIT study and the facility used to produce study medication for our ongoing Phase 3 AFFIRM study and the to-be-marketed product. The study demonstrated that the TNX-102 SL 2.8 mg tablets manufactured at the two different facilities are bioequivalent.
Prospective Phase 3 “REAFFIRM” Study
In the second quarter of 2016, we plan to commence the REAFFIRM trial, a second randomized, double-blind Phase 3 clinical trial of TNX-102 SL, to support product registration. We currently expect that the size and design of the REAFFIRM trial will be similar to that of the AFFIRM trial.
Open-label Extension Study for AFFIRM and REAFFIRM
Patients who successfully complete the AFFIRM or REAFFIRM studies are or will be eligible to enroll into the three-month open label extension study, or Study F303. Study F303 is designed to capture additional safety and efficacy information after the patients completed the three month double-blind randomized treatment.
| 7 |
Prospective Multi-dose Pharmacokinetic Study
Since TNX-102 SL will be submitted as a 505(b)(2) NDA using CBP 5 mg IR tablets as our reference product, we plan to study TNX-102 SL, 2.8 mg in comparison to CBP 5 mg IR tablets in a multiple-dose pharmacokinetic study. The results of this study will provide information regarding blood levels of CBP at steady state after repeated dosing of CBP 5 mg IR tablet and TNX-102 SL. We expect the data from this study to serve as a ‘bridge’ to enable us to use the reference product labeling information to support the TNX-102 SL NDA.
Nonclinical Development
In addition to the clinical studies necessary to support the TNX-102 SL 505(b)(2) NDA filing for the management of fibromyalgia, we proposed, and the FDA has accepted, our nonclinical data package to support the NDA filing, as the NDA requires nonclinical information that is not completely available in the reference product labeling. In 2014, we completed dose-ranging studies to identify the doses for the chronic toxicity studies requested by the FDA to augment the nonclinical information in the CBP labeling which will be used to support the TNX-102 SL labeling for long-term use. In January 2015, we engaged an FDA-certified Good Laboratory Practices laboratory to conduct a six-month repeated-dose toxicology study of TNX-102 in rats and a nine-month repeated-dose toxicology study in dogs required for the NDA filing and to support Phase 3 clinical studies outside the U.S. The in-life portion of these studies has been completed and reports are being prepared. Based on the prescribing information of AMRIX and FLEXERIL (discontinued since May 2013) and the post-marketing surveillance information, there is no evidence of abuse for CBP. As a result, the FDA has advised that we will not have to assess the abuse potential of TNX-102 SL for the TNX-102 SL 505(b)(2) NDA submission.
Manufacturing
The TNX-102 SL drug product manufactured for our Phase 2b BESTFIT study was manufactured in a small-scale current Good Manufacturing Practice, or cGMP, facility that is licensed to manufacture clinical trial materials, but not equipped for large-scale commercial production. For Phase 3 trials and for the commercial product, we have engaged a commercial cGMP facility that is capable of manufacturing the registration batches to support the NDA. The product’s comparability is supported by the bioequivalence results of the single-dose pharmacokinetic study (F105).
Other NDA Requirements
We have an Agreed Initial Pediatric Study Plan, or iPSP, with the FDA. The iPSP contains (i) an agreement whereby we will submit a protocol to conduct a single dose, open-label pharmacokinetic study in adolescent patients (13-17 years of age) with fibromyalgia before our NDA filing and (ii) a partial waiver of the requirement to submit pediatric assessments of TNX-102 SL per Section 505B(a)(4)(A)(i) of the FDCA. A Final Pediatric Study Plan requirement will be determined at the time of the NDA approval.
Based our discussions with the FDA and the FDA official meeting minutes, we will not have to conduct special populations (geriatric and renal/hepatic impaired), drug-drug interaction or cardiovascular safety studies to support the NDA filing. Due to the well-established safety profile of CBP at much higher doses than we proposed for FM, the FDA has not requested a risk management plan or medication guide for this product.
Regulatory Strategy
We expect to register TNX-102 SL with the FDA through the provisions of Section 505(b)(2). This regulatory pathway may help to accelerate product development and reduce overall business risk. The 505(b)(2)-based product development plan for TNX-102 SL is designed to leverage the safety data that have been generated by other manufacturers for CBP-containing products and accepted by the FDA in support of their product registrations, in addition to the safety data we generate. TNX-102 SL contains significantly less CBP than other marketed products that contain CBP. We believe that the nonclinical safety data package from these products, together with their marketing experience, will provide an adequate safety margin to support TNX-102 SL development and marketing application for FM.
If NDA approval of TNX-102 SL is granted, in addition to the three-year marketing exclusivity provided by law, we expect this product to be protected by patents that extend through at least 2021, during which time it should not be subject to generic substitution. We plan to continue to support the TNX-102 SL program with new patent applications as we obtain data from the clinical evaluation of our new formulation in healthy human subjects and in FM patients. For example, we have recently filed patent applications on TNX-102 SL which, if issued, would be expected to provide protection from generic substitution until at least 2033.
| 8 |
TNX-102 SL – Post-traumatic Stress Disorder Program
We are developing TNX-102 SL for the management of PTSD under a separate IND, cleared by the FDA in June 2014, and we are currently conducting our AtEase trial, a Phase 2 clinical trial of TNX-102 SL in military-related PTSD. Patients who successfully complete the AtEase study are eligible to enroll into a three-month open-label extension study (P202).
Parallels between Fibromyalgia and Post-traumatic Stress Disorder
The clinical presentations of FM and PTSD share a number of similarities and clinical overlap. For example, in a survey of males with PTSD or major depression, 49% of PTSD patients met the American College of Rheumatology criteria for FM compared to 5% of major depression patients (Amital et al, J Psychosom Res 2006;61:663-669). Conversely, in a different survey of FM patients, 57% of the patients had symptoms associated with PTSD (Cohen et al, Semin Arthritis Rheum 2002;32:38-50).
As with FM, a core feature of PTSD is sleep disturbance. Sleep disturbances are believed to exacerbate daytime symptoms of PTSD, including irritability, poor concentration, and diminished interest in significant activities. The sleep disturbances of PTSD, which include nightmares and night terrors, may be more pronounced than those typically experienced by FM patients.
Development Rationale
Our rationale for developing TNX-102 SL for treatment of PTSD is supported by the following:
| • | Results from our BESTFIT study, which showed that treatment with TNX-102 SL resulted in: 1) an observed statistically significant improvement of FM, a separate but related central nervous system disorder having significant overlap with PTSD (Chrousos, 2009); 2) an observed statistically significant improvement in sleep quality in FM, which is similarly impaired in PTSD and central to the neuropathology (Germain, 2013); and 3) statistically significant improvements in anxiety and sensory sensitivity, symptoms that are of particular relevance to PTSD. |
| • | In research from peer-reviewed scientific publications, we have identified a number of compounds that are antagonists of the serotonin 2A and/or alpha-1 adrenergic receptors that have been shown to have beneficial effects in treating PTSD. Therefore, it is our belief that TNX-102 SL, a serotonin 2A and alpha-1 adrenergic receptor antagonist, will have a therapeutic effect in treating PTSD. |
Clinical Development Plan
Phase 2 “AtEase” Study
In the first quarter of 2015, we commenced the AtEase trial, a randomized, double-blind, placebo-controlled, 12-week Phase 2 trial of TNX-102 SL in approximately 240 patients with military-related PTSD. In the AtEase study, patients are randomized in a 2:1:2 ratio to TNX-102 SL 2.8 mg, TNX-102 SL 5.6 mg, or placebo, respectively, sublingually once daily at bedtime. This trial is being conducted at approximately 25 U.S. centers. In December 2015, we announced that enrollment in AtEase has completed.
The primary objective of the AtEase trial is to evaluate the efficacy of TNX-102 SL 2.8 mg as compared to placebo sublingual tablet following 12 weeks of treatment using the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). If the AtEase study achieves success in its primary outcome measure, it could serve as one of the two pivotal studies required to establish sufficient evidence of efficacy and safety to support the approval of TNX-102 SL for PTSD. We expect to report top line results from the AtEase study in the second quarter of 2016.
Manufacturing
The TNX-102 SL drug product manufactured for our Phase 2 AtEase trial was manufactured in a small-scale cGMP facility that is licensed to manufacture clinical trial materials, but not equipped for large-scale commercial production. For Phase 3 trials and for the commercial product, we have engaged a commercial cGMP facility that is capable of manufacturing the registration batches to support the NDA. The product’s comparability is supported by the bioequivalence results of single-dose pharmacokinetic study (F105). An End-of-Phase 2 Chemistry, Manufacturing and Controls Meeting has been held in February 2016 to discuss the quality data requirement for the NDA filing. As of the date of this filing, we have not received the FDA official minutes from the meeting.
| 9 |
Regulatory Strategy
We expect to register TNX-102 SL in PTSD with the FDA through the provisions of Section 505(b)(2). The FDA approvals of paroxetine and sertraline for treating PTSD established a regulatory approval pathway for symptom reduction in PTSD. Based on our communications with the FDA to date, we believe that positive results from two adequate, well-controlled efficacy and safety studies and long-term (six- and 12-month) safety exposure studies (F203 and P202) would support FDA approval of TNX-102 SL for the management of PTSD.
We plan to meet with the FDA after we complete the AtEase study to further discuss our development plan for TNX-102 SL for PTSD, including the proposed design of the registration program that would be required to support approval of an NDA for this indication. If we achieve our primary outcome measure in the AtEase study, it could qualify as one of the two studies required to support the NDA. We expect that we can use the long-term safety exposure data generated by our clinical development of TNX-102 SL in FM (F203) to supplement the long-term safety exposure data required for the PTSD NDA.
If the results from the AtEase study are positive, we plan to seek Fast Track Development and/or Breakthrough Therapy designation for TNX-102 SL in PTSD. The Breakthrough Therapy designation process is a relatively new and uncertain process for drugs intended alone or in combination with one or more other drugs to treat a serious or life threatening disease or condition, the preliminary clinical evidence of which indicates that the drug may demonstrate substantial improvement over existing therapies, in which the majority of requests for designation have been denied.
TNX-201 – Episodic Tension-type Headache Program
We were developing TNX-201 for the treatment of episodic tension-type headache under an IND cleared by the FDA in October 2014. ETTH is the most common type of headache, estimated to account for over 60% of headaches. In the second quarter of 2015, we initiated a single-dose Phase 2 proof-of-concept clinical trial to evaluate the ability of TNX-201 140 mg to improve headache pain as well as its tolerability in patients with ETTH. This trial was conducted at approximately 10 U.S. centers and randomized approximately 150 patients to receive TNX-201 140 mg or placebo capsules. This trial assessed efficacy following a dose of study medication according to a variety of measures at several time points, including the difference between the two study arms in the number of subjects who report complete relief from their headache pain at two hours. This study was designed to test the activity and tolerability of TNX-201 to support possible future efficacy and safety studies. We reported top line results from this study on February 16, 2016. Since TNX-201 failed to show any efficacy, we abandoned development of TNX-201.
Additional Product Candidates
We also have a pipeline of other product candidates, including TNX-301. TNX-301 is a fixed dose combination drug product, or CDP, containing two FDA-approved drugs, disulfiram and selegiline. We intend to develop TNX-301 CDP under Section 505(b)(2) of the FDCA as a potential treatment for AUD, and we have commenced development work on TNX-301 formulations. We held a Pre-IND Meeting with the FDA in February 2016 to discuss the clinical development of TNX-301 for AUD. As of the date of this filing, we have not received the FDA official minutes from the meeting.
In addition, we own rights to intellectual property on two biodefense technologies: one relating to the development of novel smallpox vaccines; and the other to the development of protective agents against radiation exposure. We have begun non-clinical research and development on these programs. The FDA Animal Efficacy Rule provides a mechanism for product licensure when human efficacy studies are not feasible or ethical. As a result, the licensure of these biodefense products in the U.S. may not require human efficacy studies, which we believe will reduce our development costs and risks compared to the development of other NCEs or new biologic candidates.
Competition
Our industry is highly competitive and subject to rapid and significant technological change. Our potential competitors include large pharmaceutical and biotechnology companies, specialty pharmaceutical and generic drug companies, academic institutions, government agencies and research institutions. We believe that key competitive factors that will affect the development and commercial success of our product candidates are efficacy, safety, tolerability, reliability, price and reimbursement level. Many of our potential competitors, including many of the organizations named below, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products. Accordingly, our competitors may be more successful than we may be in obtaining FDA approval for drugs and achieving widespread market acceptance. Our competitors’ drugs may be more effective, or more effectively marketed and sold, than any drug we may commercialize and may render our product candidates obsolete or non-competitive before we can recover the expenses of developing and commercializing any of our product candidates. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available. Further, the development of new treatment methods for the conditions we are targeting could render our drugs non-competitive or obsolete.
The markets for medicines to treat FM, PTSD and other CNS conditions are well developed and populated with established drugs marketed by large and small pharmaceutical, biotechnology and generic drug companies. Eli Lilly (Cymbalta), Forest Laboratories (Savella), and Pfizer (Lyrica) market FDA approved drugs for FM. Cymbalta lost its U.S. patent exclusivity in December 2013. GlaxoSmithKline (Paxil) and Pfizer (Zoloft) market FDA approved drugs for PTSD. Paxil and Zoloft lost their U.S. patent exclusivities in 2003 and 2006, respectively.
| 10 |
A number of companies are developing prescription medications for FM, including Allergan, Daiichi Sankyo, Meda, Merck, Pfizer, RiboCor and Theravance. Clinical trials in the U.S. are registered with the FDA and reported on the website www.clinicaltrials.gov. Medications that are used off-label for the treatment of FM include gabapentin; anti-depressants, such as amitriptyline, venlafaxine, and trazodone; muscle relaxants, such as cyclobenzaprine; tramadol; opioids; and benzodiazepine, as well as non-benzodiazepine sedative hypnotics.
A number of companies are developing prescription medications for PTSD, including Actavis, Bionomics, Johnson and Johnson, Lundbeck, Marinus Pharmaceuticals, Merck, Otsuka, and Pfizer. Medications that are used off-label for the treatment of PTSD include anti-depressants, such as nefazodone and trazodone; the antihistamine cyproheptadine; and certain atypical antipsychotics, such as olanzapine and risperidone.
Intellectual Property
We believe that we have an extensive patent portfolio and substantial know-how relating to TNX-102 SL and our other product candidates. Our patent portfolio, described more fully below, includes claims directed to TNX-102 SL compositions and methods of use. As of March 1, 2016, we are either the owner of record of or own the contractual right to five issued U.S. patents and 26 issued non-U.S. patents. We are actively pursuing an additional 17 U.S. patent applications, of which six are provisional and 11 are non-provisional, five international patent applications, and 70 non-U.S./non-international patent applications.
We strive to protect the proprietary technology that we believe is important to our business, including our proprietary technology platform, our product candidates, and our processes. We seek patent protection in the United States and internationally for our products, their methods of use and processes of manufacture, and any other technology to which we have rights, where available and when appropriate. We also rely on trade secrets that may be important to the development of our business.
Our success will depend on 1) the ability to obtain and maintain patent and other proprietary rights in commercially important technology, inventions and know-how related to our business, 2) the validity and enforceability of our patents, 3) the continued confidentiality of our trade secrets, and 4) our ability to operate without infringing the valid and enforceable patents and proprietary rights of third parties. We also rely on continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position.
We cannot be certain that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications we may own or license in the future, nor can we be certain that any of our existing patents or any patents we may own or license in the future will be useful in protecting our technology. For this and more comprehensive risks related to our intellectual property, please see “Risk Factors — Risks Relating to Our Intellectual Property.”
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the date of filing the first non-provisional priority application. In the United States, a patent’s term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the U.S. Patent and Trademark Office, or PTO, in granting a patent, or may be shortened if a patent is terminally disclaimed over another patent.
The term of a U.S. patent that covers an FDA-approved drug may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Hatch-Waxman Amendments permit a patent term extension of up to five years beyond the statutory 20-year term of the patent for the approved product if the active ingredient has not been previously approved in the U.S. The length of the patent term extension is related to the length of time the drug is under regulatory review. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. Similar provisions are available in Europe and some other foreign jurisdictions to extend the term of a patent that covers an approved drug. When possible, depending upon the length of clinical trials and other factors involved in the filing of a new drug application, or NDA, we expect to apply for patent term extensions for patents covering our product candidates and their methods of use.
The patent portfolios for our proprietary technology platform and our three most advanced product candidates as of March 1, 2016 are summarized below.
| 11 |
TNX-102 SL
Our patent portfolio for TNX-102 SL includes patent applications directed to compositions of matter of CBP, formulations containing CBP, and methods for treating FM and other CNS conditions utilizing these compositions and formulations. The portfolio includes issued U.S. patents, such as U.S. Patent Nos. 6,541,523, 6,395,788 and 6,358,944, and corresponding issued foreign counterpart patents or applications. U.S. Patent Nos. 6,541,523, 6,395,788 and 6,358,944 are expected to expire in 2020, unless they are eligible for patent term extensions on the basis of FDA approvals.
The unique pharmacokinetic profile of TNX-102 SL was discovered by Tonix and its development partners and is termed the “PK Technology.” The patent portfolio for TNX-102 SL relating to the PK Technology includes patent applications directed to compositions of matter of CBP, formulations containing CBP, and methods for treating FM and other CNS conditions utilizing these compositions and formulations. The PK Technology patent portfolio includes U.S. Patent Application No. 13/918,692. If U.S. and non-U.S. patents claiming priority from those applications issue, those patents would expire in 2033, excluding any patent term adjustments or extensions.
Certain eutectic compositions were discovered by development partners and are termed the “Eutectic Technology.” The patent portfolio for TNX-102 SL relating to the Eutectic Technology includes patent applications directed to eutectic compositions containing CBP, eutectic CBP formulations, methods for treating FM and other CNS conditions utilizing eutectic CBP compositions and formulations, and methods of manufacturing eutectic CBP compositions. The Eutectic Technology patent portfolio includes U.S. patent applications, such as U.S. Patent Application No. 14/214,433. If U.S. and non-U.S. patents claiming priority from those applications issue, those patents would expire in 2034 or 2035, excluding any patent term adjustments or extensions.
TNX-201 — Isometheptene Isomers
Our patent portfolio for TNX-201, relating to isometheptene isomers and termed the “Isometheptene Technology”, includes patent applications directed to purified isomers of isometheptene, pharmaceutical compositions containing that isometheptene isomer, isometheptene isomer formulations, methods for modulating headache and other CNS conditions and treating CNS conditions utilizing isometheptene isomers, and methods of manufacturing isometheptene isomers. The Isometheptene Technology patent portfolio includes U.S. Patent Application No. 14/158,735 as well as U.S. Patent Application No 14/657,885. If U.S. and non-U.S. patents claiming priority from those applications issue, those patents would expire in 2034 and 2035, respectively, excluding any patent term adjustments or extensions.
TNX-301 — Alcohol Use Disorders
Our patent portfolio for disulfiram and selegiline combinations includes patents and patent applications. It includes claims directed to disulfiram and selegiline, pharmaceutical compositions containing disulfiram and selegiline, disulfiram and selegiline formulations, methods of treating AUD, and methods of modulating alcohol abuse and dependence. It includes issued U.S. Patent Nos. 8,093,300 and 8,481,599. The patent expiring last is expected to expire in 2024, excluding any patent term extensions.
Biodefense Technology
We own the rights to develop a potential biodefense technology, which is a new vaccine candidate against smallpox. With respect to the smallpox vaccine candidate, we own U.S. non-provisional Patent Application No. 14,207,727 and related intellectual property rights. The smallpox vaccine technology relates to proprietary forms of live vaccinia vaccines which may be safer than ACAM2000, the only currently available replication competent, live vaccinia vaccine to protect against smallpox disease. We believe that this technology, after further development, may be of interest to biodefense agencies in the U.S. and other countries.
Trade Secrets
In addition to patents, we rely on trade secrets and know-how to develop and maintain our competitive position. For example, significant aspects of our proprietary technology platform are based on unpatented trade secrets and know-how. Trade secrets and know-how can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors, and commercial partners. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with a third party. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our contractors use intellectual property owned by others in their work for us, disputes may arise as to rights in related or resulting inventions and know-how.
| 12 |
Issued Patents
Our current patents owned are as follows:
Very-Low Dose Cyclobenzaprine
| Patent No. | Title | Country / Region | Expiration Date | |||
| 6,541,523 | Methods for Treating or Preventing Fibromyalgia Using Very Low Doses of Cyclobenzaprine | U.S.A | Aug. 11, 2020 | |||
| 6,395,788 | Methods and Compositions for Treating or Preventing Sleep Disturbances and Associated Illnesses Using Very Low Doses of Cyclobenzaprine | U.S.A. | Aug. 11, 2020 | |||
| 6,358,944 | Method and Compositions for Treating Generalized Anxiety Disorder | U.S.A. | Aug. 23, 2020 | |||
| 299369 | Uses Compositions for Treating or Preventing Sleep Disturbances Using Very Low Doses of Cyclobenzaprine | Austria | Aug. 11, 2020 | |||
| 1202722 | Uses Compositions for Treating or Preventing Sleep Disturbances Using Very Low Doses of Cyclobenzaprine | Belgium, France, Ireland, Luxembourg, Monaco, Portugal, Switzerland, U.K. | Aug. 11, 2020 | |||
| 60021266.1 | Uses Compositions for Treating or Preventing Sleep Disturbances Using Very Low Doses of Cyclobenzaprine | Germany | Aug. 11, 2020 | |||
| 2245944 | Uses Compositions for Treating or Preventing Sleep Disturbances Using Very Low Doses of Cyclobenzaprine | Spain | Aug. 11, 2020 | |||
| 1047691 | Uses Compositions for Treating or Preventing Sleep Disturbances Using Very Low Doses of Cyclobenzaprine | Hong Kong | Aug. 11, 2020 | |||
| 516749 | Uses Compositions for Treating or Preventing Sleep Disturbances Using Very Low Doses of Cyclobenzaprine | New Zealand | Aug. 11, 2020 |
AUD Treatment
| Patent No. | Title | Country / Region | Expiration Date | |||
| 8,093,300 | Compositions and Methods for Increasing Compliance with Therapies Using Aldehyde Dehydrogenase Inhibitors and Treating Alcoholism | U.S.A. | May 23, 2024 | |||
| 8,481,599 | Compositions and Methods for Increasing Compliance with Therapies Using Aldehyde Dehydrogenase Inhibitors and Treating Alcoholism | U.S.A. | Nov. 4, 2022 | |||
| 2002354017 | Compositions and Methods for Increasing Compliance with Therapies Using Aldehyde Dehydrogenase Inhibitors and Treating Alcoholism | Australia | Nov. 4, 2022 | |||
| 2463987 | Compositions and Methods for Increasing Compliance with Therapies Using Aldehyde Dehydrogenase Inhibitors and Treating Alcoholism | Canada | Nov. 4, 2022 | |||
| 1441708 | Compositions and Methods for Increasing Compliance with Therapies Using Aldehyde Dehydrogenase Inhibitors and Treating Alcoholism | Austria, Belgium, Denmark, France, Germany, Luxembourg, Monaco, Portugal, Switzerland, U.K. | Nov. 4, 2022 | |||
| 532583 | Compositions and Methods for Increasing Compliance with Therapies Using Aldehyde Dehydrogenase Inhibitors and Treating Alcoholism | New Zealand | Nov. 4, 2022 |
| 13 |
Pending Patent Applications
Our current pending patent applications are as follows:
Sublingual Cyclobenzaprine/Amitriptyline
| Application No. | Title | Country / Region | ||
| 13/918,692 | Compositions and Methods for Transmucosal Absorption | U.S.A. | ||
| P20130102101 | Compositions and Methods for Transmucosal Absorption | Argentina | ||
| 2013274003 | Compositions and Methods for Transmucosal Absorption | Australia | ||
| BR112014031394-6 | Compositions and Methods for Transmucosal Absorption | Brazil | ||
| Not yet assigned | Compositions and Methods for Transmucosal Absorption | Canada | ||
| Not yet assigned | Compositions and Methods for Transmucosal Absorption | China | ||
| 13804115.7 | Compositions and Methods for Transmucosal Absorption | European Patent Office | ||
| 2013/24661 | Compositions and Methods for Transmucosal Absorption | Gulf Cooperation Council | ||
| 15110186.6 | Compositions and Methods for Transmucosal Absorption | Hong Kong | ||
| P-00 2015 00202 | Compositions and Methods for Transmucosal Absorption | Indonesia | ||
| 236268 | Compositions and Methods for Transmucosal Absorption | Israel | ||
| 139/KOLNP/2015 | Compositions and Methods for Transmucosal Absorption | India | ||
| Not yet assigned | Compositions and Methods for Transmucosal Absorption | Japan | ||
| MX/a/2014/015436 | Compositions and Methods for Transmucosal Absorption | Mexico | ||
| PI 2014703784 | Compositions and Methods for Transmucosal Absorption | Malaysia | ||
| 631144 | Compositions and Methods for Transmucosal Absorption | New Zealand | ||
| 11201408318R | Compositions and Methods for Transmucosal Absorption | Singapore | ||
| 102121267 | Compositions and Methods for Transmucosal Absorption | Taiwan | ||
| 2013-000737 | Compositions and Methods for Transmucosal Absorption | Venezuela | ||
| 2015/00288 | Compositions and Methods for Transmucosal Absorption | South Africa |
PTSD Treatment
| Application No. | Title | Country / Region | ||
| 12/948,828 | Methods and Compositions for Treating Symptoms Associated with Post-Traumatic Stress Disorder Using Cyclobenzaprine | U.S.A. | ||
| 10831895.7 | Methods and Compositions for Treating Symptoms Associated with Post-Traumatic Stress Disorder Using Cyclobenzaprine | European Patent Office | ||
| 13103530.6 | Methods and Compositions for Treating Symptoms Associated with Post-Traumatic Stress Disorder Using Cyclobenzaprine | Hong Kong |
Sleep Disorder Treatment
| Application No. | Title | Country / Region | ||
| 14/477,981 | Methods and Compositions for Treating Fatigue Associated with Disordered Sleep Using Very Low Dose Cyclobenzaprine | U.S.A. |
Depression Treatment
| Application No. | Title | Country / Region | ||
| 13/412,571 | Methods and Compositions for Treating Depression Using Cyclobenzaprine | U.S.A. | ||
| 2012225548 | Methods and Compositions for Treating Depression Using Cyclobenzaprine | Australia | ||
| 2,829,200 | Methods and Compositions for Treating Depression Using Cyclobenzaprine | Canada | ||
| 12755254.5 | Methods and Compositions for Treating Depression Using Cyclobenzaprine | European Patent Office | ||
| 2013-557811 | Methods and Compositions for Treating Depression Using Cyclobenzaprine | Japan | ||
| 2016-7041 | Methods and Compositions for Treating Depression Using Cyclobenzaprine | Japan | ||
| 614725 | Methods and Compositions for Treating Depression Using Cyclobenzaprine | New Zealand | ||
| 714294 | Methods and Compositions for Treating Depression Using Cyclobenzaprine | New Zealand |
| 14 |
Cyclobenzaprine/Amitriptyline Eutectics
| Application No. | Title | Country / Region | ||
| 14/214,433 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | U.S.A. | ||
| 14/776,624 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | U.S.A. | ||
| 2014233277 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | Australia | ||
| BR112015022095-9 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | Brazil | ||
| 2,904,812 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | Canada | ||
| 201480024011.1 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | China | ||
| 14762323.5 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | Europe | ||
| P-00 2015 06570 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | Indonesia | ||
| 241353 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | Israel | ||
| 3392/KOLNP/2015 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | India | ||
| 2016-503239 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | Japan | ||
| MX/a/2015/012622 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | Mexico | ||
| PI 2015703142 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | Malaysia | ||
| 631152 | Eutectic Formulations of Cyclobenzaprine Hydrochloride | New Zealand | ||
| 515361124 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | Saudi Arabia | ||
| 11201507124X | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | Singapore | ||
| 2015/07443 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | South Africa | ||
| 103109816 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | Taiwan | ||
| 2014-000391 | Eutectic Formulations of Cyclobenzaprine Hydrochloride and Amitriptyline Hydrochloride | Venezuela | ||
| PCT/US2015/051068 | Eutectic Formulations of Cyclobenzaprine Hydrochloride | PCT |
Isometheptene Isomer
| Application No. | Title | Country / Region | ||
| 14/158,735 | Isometheptene Isomer | U.S.A. | ||
| 2014207347 | Isometheptene Isomer | Australia | ||
| BR 11 2015 017176 | Isometheptene Isomer | Brazil | ||
| 2,898,450 | Isometheptene Isomer | Canada | ||
| 02010-2015 | Isometheptene Isomer | Chile | ||
| TBA | Isometheptene Isomer | China | ||
| 14740447.9 | Isometheptene Isomer | European Patent Office | ||
| 6716/DELNP/2015 | Isometheptene Isomer | India | ||
| P00201504975 | Isometheptene Isomer | Indonesia | ||
| 239971 | Isometheptene Isomer | Israel | ||
| 2015-553872 | Isometheptene Isomer | Japan | ||
| 10-2015-7022165 | Isometheptene Isomer | Republic of Korea | ||
| PI 2015702325 | Isometheptene Isomer | Malaysia | ||
| MX/a/2015/009268 | Isometheptene Isomer | Mexico | ||
| 710347 | Isometheptene Isomer | New Zealand | ||
| 11201505558Y | Isometheptene Isomer | Singapore | ||
| 2015/05795 | Isometheptene Isomer | South Africa | ||
| 1514082.5 | Isometheptene Isomer | United Kingdom | ||
| 62/196,455 | (R)-IMH Synthesis | U.S.A. | ||
| PCT/US2015/034292 | Novel (R)-Isometheptene Compositions and Uses | PCT | ||
| 62/146,067 | Compounds for Use as Pain Therapeutics | U.S.A. | ||
| 62/251,011 | Compounds, Compositions, and their Uses as Pain Therapeutics | U.S.A. | ||
| 62/298,026 | Compounds, Compositions, and their Uses as Pain Therapeutics | U.S.A. | ||
| PCT/IB2015/00934 | Eutectic Isometheptene Mucate | PCT | ||
| 14/657,885 | Eutectic Isometheptene Mucate | U.S.A. | ||
| 62/181,012 | Imidazoline Receptor Type 1 Ligands for Use as Analgesics | U.S.A. | ||
| 62/181,030 | Imidazoline Receptor Type 1 Ligands for Use as Analgesics | U.S.A. | ||
| PCT/US2015/000154 | Compounds for Use as Pain Therapeutics | PCT | ||
| PCT/US2015/000153 | Imidazoline Receptor Type 1 Ligands for Use as Therapeutics | PCT |
| 15 |
Cocaine Addiction Treatment
| Application No. | Title | Country / Region | ||
| 13/820,338 | Treatment for Cocaine Addiction | U.S.A. | ||
| 2809966 | Treatment for Cocaine Addiction | Canada | ||
| 2011314358 | Treatment for Cocaine Addiction | Australia | ||
| 11832859.0 | Treatment for Cocaine Addiction | European Patent Office | ||
| 2013-527062 | Treatment for Cocaine Addiction | Japan | ||
| 10-2013-7008187 | Treatment for Cocaine Addiction | Republic of Korea | ||
| 13114135.2 | Treatment for Cocaine Addiction | Hong Kong |
Neurocognitive Dysfunction Treatment
| Application No. | Title | Country / Region | ||
| 12/151,200 | Method for Treating Neurocognitive Dysfunction | U.S.A. | ||
| 09743321.2 | Method for Treating Neurodegenerative Dysfunction | European Patent Office | ||
| 2723688 | Method for Treating Neurodegenerative Dysfunction | Canada |
Novel Smallpox Vaccines
| Application No. | Title | Country / Region | ||
| 14207727 | Novel Smallpox Vaccines | U.S.A. |
Trademarks and Service Marks
We seek trademark and service mark protection in the United States and outside of the United States where available and when appropriate. We are the owner of the following U.S. federally registered marks: TONIX PHARMACEUTICALS (Reg. No. 4656463, issued 12/16/2015) and TONMYA (Reg. No. 4868328, issued 12/08/2015).
We are the owner of the following marks for which applications for U.S. federal registration are currently pending: FYMRALIN (Serial No. 86/516046, filed 01/27/2015), MODALTIN (Serial No. 86/631228, filed 05/15/2015), RAPONTIS (Serial No. 86/631236, filed 05/15/2015), IMADAZIO (Serial No. 86/631242, filed 05/15/2015), PROTECTIC (Serial No. 86/636119, filed 05/20/2015) and TONIX PHARMACEUTICALS (Serial No. 86/400401, filed 09/19/2014).
Research and Development
We have approximately 15 employees dedicated to research and development. We anticipate that our research and development expenditures will increase several fold as we continue our late-stage clinical development of TNX-102 SL and advance other candidates in our pipeline. We need to raise additional capital to fund our development plans and there is no certainty that we will be successful in continuing to attract new investments. Our research and development operations are located in New York, NY, San Diego, CA, San Jose, CA, Dublin, Ireland and Montreal, Canada. We have used, and expect to continue to use, third parties to conduct our nonclinical and clinical studies.
Manufacturing
We have contracted with third-party cGMP-compliant contract manufacturing organizations, or CMOs, for the manufacture of TNX-102 SL drug substances and drug products for investigational purposes, including nonclinical and clinical testing. For TNX-102 SL, we have engaged a cGMP facility for manufacturing of to-be-marketed product for Phase 3 clinical and commercial. Our manufacturing operations are managed and controlled in Dublin, Ireland.
All of our compounds are small molecules, synthesized using industry standard processes, and our drug products are formulated using commercially available pharmaceutical grade excipients.
Government Regulation
The FDA and other federal, state, local and foreign regulatory agencies impose substantial requirements upon the clinical development, approval, labeling, manufacture, marketing and distribution of drug products. These agencies regulate, among other things, research and development activities and the testing, approval, manufacture, quality control, safety, effectiveness, labeling, storage, record keeping, advertising and promotion of our product candidates. The regulatory approval process is generally lengthy and expensive, with no guarantee of a positive result. Moreover, failure to comply with applicable FDA or other requirements may result in civil or criminal penalties, recall or seizure of products, injunctive relief including partial or total suspension of production, or withdrawal of a product from the market.
| 16 |
The FDA regulates, among other things, the research, manufacture, promotion and distribution of drugs in the United States under the FDCA and other statutes and implementing regulations. The process required by the FDA before prescription drug product candidates may be marketed in the United States generally involves the following:
| • | completion of extensive nonclinical laboratory tests, animal studies and formulation studies, all performed in accordance with the FDA’s Good Laboratory Practice regulations; |
| • | submission to the FDA of an IND, which must become effective before human clinical trials may begin; |
| • | performance of adequate and well-controlled human clinical trials in accordance with the FDA’s regulations, including Good Clinical Practices, to establish the safety and efficacy of the product candidate for each proposed indication; |
| • | submission to the FDA of an NDA for drug products, or a Biologics Drug Application, or BLA, for biologic products; |
| • | satisfactory completion of an FDA preapproval inspection of the manufacturing facilities at which the product is produced to assess compliance with cGMP regulations; and |
| • | FDA review and approval of the NDA or BLA prior to any commercial marketing, sale or shipment of the drug. |
The testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all.
Nonclinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as studies to evaluate toxicity in animals and other animal studies. The results of nonclinical tests, together with manufacturing information and analytical data, are submitted as part of an IND to the FDA. Some nonclinical testing may continue even after an IND is submitted. The IND also includes one or more protocols for the initial clinical trial or trials and an investigator’s brochure. An IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions relating to the proposed clinical trials as outlined in the IND and places the clinical trial on a clinical hold. In such cases, the IND sponsor and the FDA must resolve any outstanding concerns or questions before any clinical trials can begin. Clinical trial holds also may be imposed at any time before or during studies due to safety concerns or non-compliance with regulatory requirements. An independent institutional review board, or IRB, at each of the clinical centers proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that center. An IRB considers, among other things, whether the risks to individuals participating in the trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the consent form signed by the trial participants and must monitor the study until completed.
Clinical Trials
Clinical trials involve the administration of the product candidate to human subjects under the supervision of qualified medical investigators according to approved protocols that detail the objectives of the study, dosing procedures, subject selection and exclusion criteria, and the parameters to be used to monitor participant safety. Each protocol for a U.S. study is submitted to the FDA as part of the IND.
Human clinical trials are typically conducted in three sequential phases, but the phases may overlap, or be combined.
| • | Phase 1 clinical trials typically involve the initial introduction of the product candidate into healthy human volunteers. In Phase 1 clinical trials, the product candidate is typically tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and pharmacodynamics. |
| • | Phase 2 clinical trials are generally conducted in a limited patient population to gather evidence about the efficacy of the product candidate for specific, targeted indications; to determine dosage tolerance and optimal dosage; and to identify possible adverse effects and safety risks. Phase 2 clinical trials, in particular Phase 2b trials, can be undertaken to evaluate clinical efficacy and to test for safety in an expanded patient population at geographically dispersed clinical trial sites. |
| • | Phase 3 clinical trials are undertaken to evaluate clinical efficacy and to test for safety in an expanded patient population at geographically dispersed clinical trial sites. The size of Phase 3 clinical trials depends upon clinical and statistical considerations for the product candidate and disease, but sometimes can include several thousand patients. Phase 3 clinical trials are intended to establish the overall risk-benefit ratio of the product candidate and provide an adequate basis for product labeling. |
Post-approval clinical trials, sometimes referred to as Phase 4 clinical trials, may be conducted after initial approval. These clinical trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication, particularly for long-term safety follow-up.
| 17 |
Clinical testing must satisfy extensive FDA regulations. Reports detailing the results of the clinical trials must be submitted at least annually to the FDA and safety reports must be submitted for serious and unexpected adverse events. Success in early-stage clinical trials does not assure success in later-stage clinical trials. The FDA, an IRB or we may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk.
New Drug Applications
Assuming successful completion of the required clinical trials, the results of product development, nonclinical studies and clinical trials are submitted to the FDA as part of an NDA. An NDA also must contain extensive manufacturing information, as well as proposed labeling for the finished product. An NDA applicant must develop information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in accordance with cGMP. The manufacturing process must be capable of consistently producing quality product within specifications approved by the FDA. The manufacturer must develop methods for testing the quality, purity and potency of the final product. In addition, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product does not undergo unacceptable deterioration over its shelf life. Prior to approval, the FDA will conduct an inspection of the manufacturing facilities to assess compliance with cGMP.
The FDA reviews all NDAs submitted before it accepts them for filing. The FDA may request additional information rather than accept an NDA for filing. In this event, the NDA must be resubmitted with the additional information and is subject to review before the FDA accepts it for filing. After an application is filed, the FDA may refer the NDA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers them carefully when making decisions. The FDA may deny approval of an NDA if the applicable regulatory criteria are not satisfied. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently than we interpret the same data. The FDA may issue a complete response letter, which may require additional clinical or other data or impose other conditions that must be met in order to secure final approval of the NDA. If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. In addition, the FDA may require us to conduct Phase 4 testing which involves clinical trials designed to further assess a drug’s safety and effectiveness after NDA approval, and may require surveillance programs to monitor the safety of approved products which have been commercialized. Once issued, the FDA may withdraw product approval if ongoing regulatory requirements are not met or if safety or efficacy questions are raised after the product reaches the market.
Section 505(b) NDAs
There are two types of NDAs: the Section 505(b)(1) NDA, or full NDA, and the Section 505(b)(2) NDA. We intend to file Section 505(b)(2) NDAs for TNX-102 SL for FM and PTSD, and for certain other products, that might, if accepted by the FDA, save time and expense in the development and testing of our product candidates. We may need to file a Section 505(b)(1) NDA for certain other products in the future. A full NDA is submitted under Section 505(b)(1) of the FDCA, and must contain full reports of investigations conducted by the applicant to demonstrate the safety and effectiveness of the drug. A Section 505(b)(2) NDA may be submitted for a drug for which one or more of the investigations relied upon by the applicant was not conducted by or for the applicant and for which the applicant has no right of reference from the person by or for whom the investigations were conducted. A Section 505(b)(2) NDA may be submitted based in whole or in part on published literature or on the FDA’s finding of safety and efficacy of one or more previously approved drugs, which are known as reference drugs. Thus, the filing of a Section 505(b)(2) NDA may result in approval of a drug based on fewer clinical or nonclinical studies than would be required under a full NDA. The number and size of studies that need to be conducted by the sponsor depends on the amount and quality of data pertaining to the reference drug that are publicly available, and on the similarity of and differences between the applicant’s drug and the reference drug. In some cases, extensive, time-consuming, and costly clinical and nonclinical studies may still be required for approval of a Section 505(b)(2) NDA.
Our drug approval strategy for our new formulations of approved chemical entities is to submit Section 505(b)(2) NDAs to the FDA. As such, we plan to submit NDAs under Section 505(b)(2) for TNX-102 SL for FM and PTSD. The FDA may not agree that this product candidate is approvable for FM or PTSD as Section 505(b)(2) NDAs. If the FDA determines that Section 505(b)(2) NDAs are not appropriate and that full NDAs are required for TNX-102 SL, the time and financial resources required to obtain FDA approval for TNX-102 SL could substantially and materially increase, and TNX-102 SL might be less likely to be approved. If the FDA requires full NDAs for TNX-102 SL, or requires more extensive testing and development for some other reason, our ability to compete with alternative products that arrive on the market more quickly than our product candidates would be adversely impacted. If CBP-containing products are withdrawn from the market by the FDA for any reason, we may not be able to reference such products to support our anticipated TNX-102 SL 505(b)(2) NDA, and we may be required to follow the requirements of Section 505(b)(1).
| 18 |
Patent Protections
An applicant submitting a Section 505(b)(2) NDA must certify to the FDA with respect to the patent status of the reference drug upon which the applicant relies in support of approval of its drug. With respect to every patent listed in the FDA’s Orange Book, which is the FDA’s list of approved drug products, as claiming the reference drug or an approved method of use of the reference drug, the Section 505(b)(2) applicant must certify that: (1) there is no patent information listed in the orange book for the reference drug; (2) the listed patent has expired; (3) the listed patent has not expired, but will expire on a particular date; (4) the listed patent is invalid or will not be infringed by the manufacture, use, or sale of the product in the Section 505(b)(2) NDA; or (5) if the patent is a use patent, that the applicant does not seek approval for a use claimed by the patent. If the applicant files a certification to the effect of clause (1), (2) or (5), FDA approval of the Section 505(b)(2) NDA may be made effective immediately upon successful FDA review of the application, in the absence of marketing exclusivity delays, which are discussed below. If the applicant files a certification to the effect of clause (3), the Section 505(b)(2) NDA approval may not be made effective until the expiration of the relevant patent and the expiration of any marketing exclusivity delays.
If the Section 505(b)(2) NDA applicant provides a certification to the effect of clause (4), referred to as a paragraph IV certification, the applicant also must send notice of the certification to the patent owner and the holder of the NDA for the reference drug. The filing of a patent infringement lawsuit within 45 days of the receipt of the notification may prevent the FDA from approving the Section 505(b)(2) NDA for 30 months from the date of the receipt of the notification unless the court determines that a longer or shorter period is appropriate because either party to the action failed to reasonably cooperate in expediting the action. However, the FDA may approve the Section 505(b)(2) NDA before the 30 months have expired if a court decides that the patent is invalid or not infringed, or if a court enters a settlement order or consent decree stating the patent is invalid or not infringed.
Notwithstanding the approval of many products by the FDA pursuant to Section 505(b)(2), over the last few years certain brand-name pharmaceutical companies and others have objected to the FDA’s interpretation of Section 505(b)(2). If the FDA’s interpretation of Section 505(b)(2) is successfully challenged in court, the FDA may be required to change its interpretation of Section 505(b)(2) which could delay or even prevent the FDA from approving any Section 505(b)(2) NDA that we submit. The pharmaceutical industry is highly competitive, and it is not uncommon for a manufacturer of an approved product to file a citizen petition with the FDA seeking to delay approval of, or impose additional approval requirements for, pending competing products. If successful, such petitions can significantly delay, or even prevent, the approval of the new product. Moreover, even if the FDA ultimately denies such a petition, the FDA may substantially delay approval while it considers and responds to the petition.
Marketing Exclusivity
Market exclusivity provisions under the FDCA can delay the submission or the approval of Section 505(b)(2) NDAs, thereby delaying a Section 505(b)(2) product from entering the market. The FDCA provides five-year marketing exclusivity to the first applicant to gain approval of an NDA for an NCE, meaning that the FDA has not previously approved any other drug containing the same active moiety. This exclusivity prohibits the submission of a Section 505(b)(2) NDA for any drug product containing the active ingredient during the five-year exclusivity period. However, submission of a Section 505(b)(2) NDA that certifies that a listed patent is invalid, unenforceable, or will not be infringed, as discussed above, is permitted after four years, but if a patent infringement lawsuit is brought within 45 days after such certification, FDA approval of the Section 505(b)(2) NDA may automatically be stayed until 7½ years after the NCE approval date. The FDCA also provides three years of marketing exclusivity for the approval of new and supplemental NDAs for product changes, including, among other things, new indications, dosage forms, routes of administration or strengths of an existing drug, or for a new use, if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by FDA to be essential to the approval of the application. Five-year and three-year exclusivity will not delay the submission or approval of another full NDA; however, as discussed above, an applicant submitting a full NDA under Section 505(b)(1) would be required to conduct or obtain a right of reference to all of the nonclinical and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Other types of exclusivity in the United States include orphan drug exclusivity and pediatric exclusivity. The FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for this type of disease or condition will be recovered from sales in the United States for that drug. Seven-year orphan drug exclusivity is available to a product that has orphan drug designation and that receives the first FDA approval for the indication for which the drug has such designation. Orphan drug exclusivity prevents approval of another application for the same drug for the same orphan indication, for a period of seven years, regardless of whether the application is a full NDA or a Section 505(b)(2) NDA, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity. Pediatric exclusivity, if granted, provides an additional six months to an existing exclusivity or statutory delay in approval resulting from a patent certification. This six-month exclusivity, which runs from the end of other exclusivity protection or patent delay, may be granted based on the voluntary completion of a pediatric study in accordance with an FDA-issued “Written Request” for such a study.
| 19 |
Section 505(b)(2) NDAs are similar to full NDAs filed under Section 505(b)(1) in that they are entitled to any of these forms of exclusivity if they meet the qualifying criteria. They also are entitled to the patent protections described above, based on patents that are listed in the FDA’s Orange Book in the same manner as patents claiming drugs and uses approved for NDAs submitted as full NDAs.
Breakthrough Therapy Designation
On July 9, 2012, the Food and Drug Administration Safety and Innovation Act, or FDASIA, was signed. FDASIA Section 902 provides for a new drug designation –Breakthrough Therapy. A Breakthrough Therapy is a drug:
| • | intended alone or in combination with one or more other drugs to treat a serious or life threatening disease or condition; and |
| • | preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. |
If a drug is designated as Breakthrough Therapy, the FDA will expedite the development and review of such drug. In the event that our AtEase study of TNX-102 SL in PTSD is successful, we will request Breakthrough Therapy designation for TNX-102 SL. The Breakthrough Therapy designation process is relatively new, and the majority of requests for designation have been denied. We cannot predict the likelihood of success in seeking Breakthrough Therapy designation.
Other Regulatory Requirements
Maintaining substantial compliance with appropriate federal, state and local statutes and regulations requires the expenditure of substantial time and financial resources. Drug manufacturers are required to register their establishments with the FDA and certain state agencies, and after approval, the FDA and these state agencies conduct periodic unannounced inspections to ensure continued compliance with ongoing regulatory requirements, including cGMPs. In addition, after approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further FDA review and approval. The FDA may require post-approval testing and surveillance programs to monitor safety and the effectiveness of approved products that have been commercialized. Any drug products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including:
| • | record-keeping requirements; |
| • | reporting of adverse experiences with the drug; |
| • | providing the FDA with updated safety and efficacy information; |
| • | reporting on advertisements and promotional labeling; |
| • | drug sampling and distribution requirements; and |
| • | complying with electronic record and signature requirements. |
In addition, the FDA strictly regulates labeling, advertising, promotion and other types of information on products that are placed on the market. There are numerous regulations and policies that govern various means for disseminating information to health-care professionals as well as consumers, including to industry sponsored scientific and educational activities, information provided to the media and information provided over the Internet. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label.
The FDA has very broad enforcement authority and the failure to comply with applicable regulatory requirements can result in administrative or judicial sanctions being imposed on us or on the manufacturers and distributors of our approved products, including warning letters, refusals of government contracts, clinical holds, civil penalties, injunctions, restitution and disgorgement of profits, recall or seizure of products, total or partial suspension of production or distribution, withdrawal of approvals, refusal to approve pending applications, and criminal prosecution resulting in fines and incarceration. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability. In addition, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market.
| 20 |
Food and Drug Administration Amendments Act of 2007
In September 2007, the Food and Drug Administration Amendments Act of 2007, or FDAAA, became law. This legislation grants significant new powers to the FDA, many of which are aimed at improving drug safety and assuring the safety of drug products after approval. In particular, the new law authorizes the FDA to, among other things, require post-approval studies and clinical trials, mandate changes to drug labeling to reflect new safety information, and require risk evaluation and mitigation strategies for certain drugs, including certain currently approved drugs. In addition, the new law significantly expands the federal government’s clinical trial registry and results databank and creates new restrictions on the advertising and promotion of drug products. Under the FDAAA, companies that violate these and other provisions of the new law are subject to substantial civil monetary penalties.
The FDA has not yet implemented many of the provisions of the FDAAA, so we cannot predict the impact of the new legislation on the pharmaceutical industry or our business. However, the requirements and changes imposed by the FDAAA may make it more difficult, and more costly, to obtain and maintain approval for new pharmaceutical products, or to produce, market and distribute existing products. In addition, the FDA’s regulations, policies and guidance are often revised or reinterpreted by the agency or the courts in ways that may significantly affect our business and our products. It is impossible to predict whether additional legislative changes will be enacted, or FDA regulations, guidance or interpretations changed, or what the impact of such changes, if any, may be.
Employees
As of March 1, 2016, we had 28 full-time employees, of whom eight hold M.D. or Ph.D. degrees. We have 15 employees dedicated to research and development. Our research and development operations are located in New York, NY, San Diego and San Jose, CA, Dublin, Ireland and Montreal, Canada. We have used, and expect to continue to use, third parties to conduct our nonclinical and clinical studies as well as part-time employees. None of our employees are represented by a collective bargaining agreement, and we believe that our relations with our employees are good.
Corporate Information
Our principal executive offices are located at 509 Madison Avenue, Suite 306, New York, New York 10022, and our telephone number is (212) 980-9155. Our website addresses are www.tonixpharma.com, www.tonix.com, and www.krele.com. We do not incorporate the information on our websites into this annual report, and you should not consider such information part of this annual report.
We were incorporated on November 16, 2007 under the laws of the State of Nevada as Tamandare Explorations Inc. On October 11, 2011, we changed our name to Tonix Pharmaceuticals Holding Corp.
RISKS RELATED TO OUR BUSINESS
We have a history of operating losses and expect to incur losses for the foreseeable future. We may never generate revenues or, if we are able to generate revenues, achieve profitability.
We are focused on product development, and we have not generated any revenues to date. We have incurred losses in each year of our operations, and we expect to continue to incur operating losses for the foreseeable future. These operating losses have adversely affected and are likely to continue to adversely affect our working capital, total assets and shareholders’ equity.
The Company and its prospects should be examined in light of the risks and difficulties frequently encountered by new and early-stage companies in new and rapidly evolving markets. These risks include, among other things, the speed at which we can scale up operations, our complete dependence upon development of products that currently have no market acceptance, our ability to establish and expand our brand name, our ability to expand our operations to meet the commercial demand of our clients, our development of and reliance on strategic and customer relationships and our ability to minimize fraud and other security risks.
The process of developing our products requires significant clinical development and laboratory testing and clinical trials. In addition, commercialization of our product candidates will require that we obtain necessary regulatory approvals and establish sales, marketing and manufacturing capabilities, either through internal hiring or through contractual relationships with others. We expect to incur substantial losses for the foreseeable future as a result of anticipated increases in our research and development costs, including costs associated with conducting preclinical and nonclinical testing and clinical trials, and regulatory compliance activities.
| 21 |
Our ability to generate revenues and achieve profitability will depend on numerous factors, including success in:
| • | developing and testing product candidates; | |
| • | receiving regulatory approvals; | |
| • | commercializing our products; and | |
| • | establishing a favorable competitive position. |
Many of these factors will depend on circumstances beyond our control. We cannot assure you that we will ever have a product approved by the FDA, that we will bring any product to market or, if we are successful in doing so, that we will ever become profitable.
We expect to incur substantial additional operating expenses over the next several years as our research, development, preclinical and nonclinical testing, and clinical trial activities increase. The amount of future losses and when, if ever, we will achieve profitability are uncertain. We have no products that have generated any commercial revenue, do not expect to generate revenues from the commercial sale of products in the near future, and might never generate revenues from the sale of products. Our ability to generate revenue and achieve profitability will depend on, among other things, successful completion of the development of our product candidates; obtaining necessary regulatory approvals from the FDA; establishing manufacturing, sales, and marketing arrangements with third parties; and raising sufficient funds to finance our activities. We might not succeed at any of these undertakings. If we are unsuccessful at some or all of these undertakings, our business, prospects, and results of operations may be materially adversely affected.
We have a limited operating history and we expect a number of factors to cause our operating results to fluctuate on a quarterly and annual basis, which may make it difficult to predict our future performance.
We are a development-stage biopharmaceutical company with a limited operating history. Our operations to date have been primarily limited to developing our technology and undertaking preclinical and nonclinical testing and clinical trials of our clinical-stage product candidates, TNX-102 SL for FM and PTSD. We have not yet obtained regulatory approvals for TNX-102 SL or any of our other product candidates. Consequently, any predictions made about our future success or viability may not be as accurate as they could be if we had a longer operating history or commercialized products. Our financial condition has varied significantly in the past and will continue to fluctuate from quarter-to-quarter or year-to-year due to a variety of factors, many of which are beyond our control. Factors relating to our business that may contribute to these fluctuations include other factors described elsewhere in this annual report and also include:
| • | our ability to obtain additional funding to develop our product candidates; | |
| • | delays in the commencement, enrollment and timing of clinical trials; | |
| • | the success of our clinical trials through all phases of clinical development, including trials of our product candidate TNX-102 SL; | |
| • | any delays in regulatory review and approval of product candidates in clinical development; | |
| • | our ability to obtain and maintain regulatory approval for our product candidate TNX-102 SL for FM and PTSD or any of our other product candidates in the United States and foreign jurisdictions; | |
| • | potential nonclinical toxicity and/or side effects of our product candidates that could delay or prevent commercialization, limit the indications for any approved drug, require the establishment of risk evaluation and mitigation strategies, or cause an approved drug to be taken off the market; | |
| • | our dependence on third party contract manufacturing organizations, or CMOs, to supply or manufacture our products; | |
| • | our dependence on third party contract research organizations, or CROs, to conduct our clinical trials and nonclinical research; | |
| • | our ability to establish or maintain collaborations, licensing or other arrangements; |
| 22 |
| • | market acceptance of our product candidates; | |
| • | our ability to establish and maintain an effective sales and marketing infrastructure, either through the creation of a commercial infrastructure or through strategic collaborations; | |
| • | competition from existing products or new products that may emerge; | |
| • | the ability of patients or healthcare providers to obtain coverage of or sufficient reimbursement for our products; | |
| • | our ability to leverage our proprietary technology platform to discover and develop additional product candidates; | |
| • | our ability and our licensors’ abilities to successfully obtain, maintain, defend and enforce intellectual property rights important to our business; | |
| • | our ability to attract and retain key personnel to manage our business effectively; | |
| • | our ability to build our finance infrastructure and improve our accounting systems and controls; | |
| • | potential product liability claims; | |
| • | potential liabilities associated with hazardous materials; and | |
| • | our ability to obtain and maintain adequate insurance policies. |
Accordingly, the results of any quarterly or annual periods should not be relied upon as indications of future operating performance.
We have no approved products on the market and therefore do not expect to generate any revenues from product sales in the foreseeable future, if at all.
To date, we have no approved product on the market and have generated no product revenues. We have funded our operations primarily from sales of our securities. We have not received, and do not expect to receive for at least the next couple of years, if at all, any revenues from the commercialization of our product candidates. To obtain revenues from sales of our product candidates, we must succeed, either alone or with third parties, in developing, obtaining regulatory approval for, manufacturing and marketing drugs with commercial potential. We may never succeed in these activities, and we may not generate sufficient revenues to continue our business operations or achieve profitability.
We are largely dependent on the success of our clinical-stage product candidate, TNX-102 SL for FM and PTSD, and we cannot be certain that this product candidate will receive regulatory approval or be successfully commercialized.
We currently have no products for sale, and we cannot guarantee that we will ever have any drug products approved for sale. We and our product candidates are subject to extensive regulation by the FDA and comparable regulatory authorities in other countries governing, among other things, research, testing, clinical trials, manufacturing, labeling, promotion, selling, adverse event reporting and recordkeeping. We are not permitted to market any of our product candidates in the United States until we receive approval of an NDA for a product candidate from the FDA or the equivalent approval from a foreign regulatory authority. Obtaining FDA approval is a lengthy, expensive and uncertain process. We currently have one product candidate in clinical stages of development for two indications: TNX-102 SL for the management of FM and PTSD, and the success of our business currently depends on its successful development, approval and commercialization. Any projected sales or future revenue predictions are predicated upon FDA approval and market acceptance of TNX-102 SL. If projected sales do not materialize for any reason, it would have a material adverse effect on our business and our ability to continue operations.
TNX-102 SL has not completed the clinical development process; therefore, we have not yet submitted an NDA or foreign equivalent or received marketing approval for these product candidates anywhere in the world. The clinical development programs for TNX-102 SL for FM or PTSD may not lead to commercial products for a number of reasons, including if we fail to obtain necessary approvals from the FDA or foreign regulatory authorities because our clinical trials fail to demonstrate to their satisfaction that these product candidates are safe and effective or a clinical program may be put on hold due to unexpected safety issues. We may also fail to obtain the necessary approvals if we have inadequate financial or other resources to advance our product candidates through the clinical trial process. Any failure or delay in completing clinical trials or obtaining regulatory approvals for TNX-102 SL for FM or PTSD in a timely manner would have a material adverse impact on our business and our stock price.
| 23 |
We may use our financial and human resources to pursue a particular research program or product candidate and fail to capitalize on programs or product candidates that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and human resources, we are currently focusing on the regulatory approval of TNX-102 SL for FM and PTSD. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on existing and future product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through strategic alliance, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate, or we may allocate internal resources to a product candidate in a therapeutic area in which it would have been more advantageous to enter into a partnering arrangement.
We may need additional capital. If additional capital is not available or is available at unattractive terms, we may be forced to delay, reduce the scope of or eliminate our research and development programs, reduce our commercialization efforts or curtail our operations.
In order to develop and bring our product candidates to market, we must commit substantial resources to costly and time-consuming research, preclinical and nonclinical testing, clinical trials and marketing activities. We anticipate that our existing cash and cash equivalents will enable us to maintain our current operations for at least the next 12 months. We anticipate using our cash and cash equivalents to fund further research and development with respect to our lead product candidates. We may, however, need to raise additional funding sooner if our business or operations change in a manner that consumes available resources more rapidly than we anticipate. Our requirements for additional capital will depend on many factors, including:
| • | successful commercialization of our product candidates; | |
| • | the time and costs involved in obtaining regulatory approval for our product candidates; | |
| • | costs associated with protecting our intellectual property rights; | |
| • | development of marketing and sales capabilities; | |
| • | payments received under future collaborative agreements, if any; and | |
| • | market acceptance of our products. |
To the extent we raise additional capital through the sale of equity securities, the issuance of those securities could result in dilution to our shareholders. In addition, if we obtain debt financing, a substantial portion of our operating cash flow may be dedicated to the payment of principal and interest on such indebtedness, thus limiting funds available for our business activities. If adequate funds are not available, we may be required to delay, reduce the scope of or eliminate our research and development programs, reduce our commercialization efforts or curtail our operations. In addition, we may be required to obtain funds through arrangements with collaborative partners or others that may require us to relinquish rights to technologies, product candidates or products that we would otherwise seek to develop or commercialize ourselves or license rights to technologies, product candidates or products on terms that are less favorable to us than might otherwise be available.
We will require substantial additional funds to support our research and development activities, and the anticipated costs of preclinical and nonclinical testing and clinical trials, regulatory approvals and eventual commercialization. Such additional sources of financing may not be available on favorable terms, if at all. If we do not succeed in raising additional funds on acceptable terms, we may be unable to commence clinical trials or obtain approval of any product candidates from the FDA and other regulatory authorities. In addition, we could be forced to discontinue product development, forego sales and marketing efforts and forego attractive business opportunities. Any additional sources of financing will likely involve the issuance of our equity securities, which will have a dilutive effect on our shareholders.
There is no assurance that we will be successful in raising the additional funds needed to fund our business plan. If we are not able to raise sufficient capital in the near future, our continued operations will be in jeopardy and we may be forced to cease operations and sell or otherwise transfer all or substantially all of our remaining assets.
| 24 |
We face intense competition in the markets targeted by our product candidates. Many of our competitors have substantially greater resources than we do, and we expect that all of our product candidates under development will face intense competition from existing or future drugs.
We expect that all of our product candidates under development, if approved, will face intense competition from existing and future drugs marketed by large companies. These competitors may successfully market products that compete with our products, successfully identify drug candidates or develop products earlier than we do, or develop products that are more effective, have fewer side effects or cost less than our products.
Additionally, if a competitor receives FDA approval before we do for a drug that is similar to one of our product candidates, FDA approval for our product candidate may be precluded or delayed due to periods of non-patent exclusivity and/or the listing with the FDA by the competitor of patents covering its newly-approved drug product. Periods of non-patent exclusivity for new versions of existing drugs such as our current product candidates can extend up to three and one-half years.
These competitive factors could require us to conduct substantial new research and development activities to establish new product targets, which would be costly and time consuming. These activities would adversely affect our ability to commercialize products and achieve revenue and profits.
Competition and technological change may make our product candidates and technologies less attractive or obsolete.
We compete with established pharmaceutical and biotechnology companies that are pursuing other forms of treatment for the same indications we are pursuing and that have greater financial and other resources. Other companies may succeed in developing products earlier than us, obtaining FDA approval for products more rapidly, or developing products that are more effective than our product candidates. Research and development by others may render our technology or product candidates obsolete or noncompetitive, or result in treatments or cures superior to any therapy we develop. We face competition from companies that internally develop competing technology or acquire competing technology from universities and other research institutions. As these companies develop their technologies, they may develop competitive positions that may prevent, make futile, or limit our product commercialization efforts, which would result in a decrease in the revenue we would be able to derive from the sale of any products.
There can be no assurance that any of our product candidates will be accepted by the marketplace as readily as these or other competing treatments. Furthermore, if our competitors' products are approved before ours, it could be more difficult for us to obtain approval from the FDA. Even if our products are successfully developed and approved for use by all governing regulatory bodies, there can be no assurance that physicians and patients will accept our product(s) as a treatment of choice.
Furthermore, the pharmaceutical research industry is diverse, complex, and rapidly changing. By its nature, the business risks associated therewith are numerous and significant. The effects of competition, intellectual property disputes, market acceptance, and FDA regulations preclude us from forecasting revenues or income with certainty or even confidence.
If we fail to protect our intellectual property rights, our ability to pursue the development of our technologies and products would be negatively affected.
Our success will depend in part on our ability to obtain patents and maintain adequate protection of our technologies and products. If we do not adequately protect our intellectual property, competitors may be able to use our technologies to produce and market drugs using our technologies and patents in direct competition with us and erode our competitive advantage. Some foreign countries lack rules and methods for defending intellectual property rights and do not protect proprietary rights to the same extent as the United States. Many companies have had difficulty protecting their proprietary rights in these foreign countries. We may not be able to prevent misappropriation of our proprietary rights and intellectual property rights in these and other countries.
We have received, and are currently seeking, patent protection for numerous compounds and methods of treating diseases. However, the patent process is subject to numerous risks and uncertainties, and there can be no assurance that we will be successful in protecting our products by obtaining and defending patents related to them. These risks and uncertainties include the following: patents that may be issued or licensed may be challenged, invalidated, or circumvented, or otherwise may not provide us any competitive advantage; our competitors, many of which have substantially greater resources than we and many of which have made significant investments in competing technologies, may seek, or may already have obtained, patents that will limit, interfere with, or eliminate our ability to make, use, and sell our potential products either in the United States or in international markets; there may be significant pressure on the United States government and other international governmental bodies to limit the scope of patent protection both inside and outside the United States for treatments that prove successful as a matter of public policy regarding worldwide health concerns; and countries other than the United States may have less robust patent laws than those upheld by United States courts, allowing foreign competitors the ability to exploit these laws to create, develop, and market competing products using our technologies and patents.
| 25 |
Moreover, any patents issued to us may not provide us with meaningful protection, or others may challenge, circumvent or narrow our patents. Third parties may also independently develop products similar to our products, duplicate our unpatented products or design around any patents or propriety technologies on products we develop. Additionally, extensive time is required for development, testing and regulatory review of a potential product. While extensions of patent term due to regulatory delays may be available, it is possible that, before any of our product candidates can be commercialized, any related patent, even with an extension, may expire or remain in force for only a short period following commercialization, thereby reducing any advantages to us of the patent.
In addition, the United States Patent and Trademark Office, or USPTO, and patent offices in other jurisdictions have often required that patent applications concerning pharmaceutical and/or biotechnology-related inventions be limited or narrowed substantially to cover only the innovations specifically exemplified in the patent application, thereby limiting the scope of protection against competitive challenges. Thus, even if we or our licensors are able to obtain patents, the patents may be substantially narrower than anticipated.
Our success depends on our patents and patent applications that may be licensed exclusively to us and other patents and patent applications to which we may obtain assignment or licenses. We may not be aware, however, of all patents, published applications or published literature that may affect our business either by blocking our ability to commercialize our product candidates, by preventing the patentability of our product candidates to us or our licensors, or by covering the same or similar technologies. These patents, patent applications, and published literature may limit the scope of our future patent claims or adversely affect our ability to market our product candidates.
In addition to patents, we rely on a combination of trade secrets, confidentiality, nondisclosure and other contractual provisions, and security measures to protect our confidential and proprietary information. These measures may not adequately protect our trade secrets or other proprietary information. If they do not adequately protect our rights, third parties could use our technology, and we could lose any competitive advantage we may have. In addition, others may independently develop similar proprietary information or techniques or otherwise gain access to our trade secrets, which could impair any competitive advantage we may have.
Patent protection and other intellectual property protection is crucial to the success of our business and prospects, and there is a substantial risk that such protections will prove inadequate.
We may be involved in lawsuits to protect or enforce our patents, which could be expensive and time consuming.
The pharmaceutical industry has been characterized by extensive litigation regarding patents and other intellectual property rights, and companies have employed intellectual property litigation to gain a competitive advantage. We may become subject to infringement claims or litigation arising out of present and future patents and other proceedings of our competitors. The defense and prosecution of intellectual property suits are costly and time-consuming to pursue, and their outcome is uncertain. Litigation may be necessary to determine the enforceability, scope, and validity of the proprietary rights of others. An adverse determination in litigation to which we may become a party could subject us to significant liabilities, require us to obtain licenses from third parties, or restrict or prevent us from selling our products in certain markets. Although patent and intellectual property disputes might be settled through licensing or similar arrangements, the costs associated with such arrangements may be substantial and could include our paying large fixed payments and ongoing royalties. Furthermore, the necessary licenses may not be available on satisfactory terms or at all.
Competitors may infringe our patents, and we may file infringement claims to counter infringement or unauthorized use. Third parties may assert that our patents are invalid and/or unenforceable in these proceedings. Such litigation can be expensive, particularly for a company of our size, and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover its technology. An adverse determination of any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly.
Third parties may also assert that our patents are invalid in patent office administrative proceedings. These proceedings include oppositions in the European Patent Office and inter partes review and post-grant review proceedings in the USPTO. The success rate of these administrative challenges to patent validity in the United States is higher than it is for validity challenges in litigation.
Interference or derivation proceedings brought before the USPTO may be necessary to determine priority of invention with respect to innovations disclosed in our patents or patent applications. During these proceedings, it may be determined that we do not have priority of invention for one or more aspects in our patents or patent applications and could result in the invalidation in part or whole of a patent or could put a patent application at risk of not issuing. Even if successful, an interference or derivation proceeding may result in substantial costs and distraction to our management.
| 26 |
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or interference or derivation proceedings, there is a risk that some of our confidential information could be compromised by disclosure. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If investors perceive these results to be negative, the price of our common stock could be adversely affected.
There are no unresolved communications, allegations, complaints or threats of litigation related to the possibility that our patents are invalid or unenforceable. Any litigation or claims against us, whether or not merited, may result in substantial costs, place a significant strain on our financial resources, divert the attention of management and harm our reputation. An adverse decision in litigation or administrative proceedings could result in inadequate protection for our product candidates and/or reduce the value of any license agreements we have with third parties.
If we infringe the rights of third parties we could be prevented from selling products, forced to pay damages, and defend against litigation.
If our products, methods, processes and other technologies infringe the proprietary rights of other parties, we could incur substantial costs and we may have to: obtain licenses, which may not be available on commercially reasonable terms, if at all; abandon an infringing product candidate; redesign our products or processes to avoid infringement; stop using the subject matter claimed in the patents held by others; pay damages; and/or defend litigation or administrative proceedings which may be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources.
If preclinical and nonclinical testing or clinical trials for our product candidates are unsuccessful or delayed, we will be unable to meet our anticipated development and commercialization timelines.
We rely and expect to continue to rely on third parties, including CROs and outside consultants, to conduct, supervise or monitor some or all aspects of preclinical and nonclinical testing and clinical trials involving our product candidates. We have less control over the timing and other aspects of these preclinical and nonclinical testing activities and clinical trials than if we performed the monitoring and supervision entirely on our own. Third parties may not perform their responsibilities for our preclinical and nonclinical testing and clinical trials on our anticipated schedule or, for clinical trials, consistent with a clinical trial protocol. Delays in preclinical and nonclinical testing, and clinical trials could significantly increase our product development costs and delay product commercialization. In addition, many of the factors that may cause, or lead to, a delay in the clinical trials may also ultimately lead to denial of regulatory approval of a product candidate.
The commencement of clinical trials can be delayed for a variety of reasons, including delays in:
| • | demonstrating sufficient safety and efficacy to obtain regulatory approval to commence a clinical trial; | |
| • | reaching agreement on acceptable terms with prospective contract research organizations and trial sites; | |
| • | developing a stable formulation of a product candidate; | |
| • | manufacturing sufficient quantities of a product candidate; and | |
| • | obtaining institutional review board approval to conduct a clinical trial at a prospective site. |
Once a clinical trial has begun, it may be delayed, suspended or terminated by us or the FDA or other regulatory authorities due to a number of factors, including:
| • | ongoing discussions with the FDA or other regulatory authorities regarding the scope or design of our clinical trials; | |
| • | failure to conduct clinical trials in accordance with regulatory requirements; | |
| • | lower than anticipated recruitment or retention rate of patients in clinical trials; | |
| • | inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities resulting in the imposition of a clinical hold; | |
| • | lack of adequate funding to continue clinical trials; | |
| • | negative results of clinical trials; | |
| • | investigational drug product out-of-specification; or | |
| • | nonclinical or clinical safety observations, including adverse events and serious adverse events. |
If clinical trials are unsuccessful, and we are not able to obtain regulatory approvals for our product candidates under development, we will not be able to commercialize these products, and therefore may not be able to generate sufficient revenues to support our business.
| 27 |
We rely on third parties to conduct, supervise and monitor our clinical trials, and if those third parties perform in an unsatisfactory manner, it may harm our business.
We rely on CROs and clinical trial sites to ensure the proper and timely conduct of our clinical trials. While we have agreements governing their activities, we will have limited influence over their actual performance. We will control only certain aspects of our CROs’ activities. Nevertheless, we will be responsible for ensuring that our clinical trials are conducted in accordance with the applicable protocol, legal, regulatory and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities.
We and our CROs are required to comply with the FDA’s current good clinical practices requirements, or cGCP, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of clinical trial participants are protected. The FDA enforces these cGCPs through periodic inspections of trial sponsors, principal investigators and clinical trial sites. If we or our CROs fail to comply with applicable cGCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA may require us to perform additional clinical trials before approving any marketing applications. Upon inspection, the FDA may determine that our clinical trials did not comply with cGCPs. In addition, our clinical trials, including our planned Phase 3 trial of TNX-102 SL in FM, will require a sufficiently large number of test subjects to evaluate the effectiveness and safety of TNX-102 SL. Accordingly, if our CROs fail to comply with these regulations or fail to recruit a sufficient number of patients, our clinical trials may be delayed or we may be required to repeat such clinical trials, which would delay the regulatory approval process.
Our CROs are not our employees, and we are not able to control whether or not they devote sufficient time and resources to our clinical trials. These CROs may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials, or other drug development activities which could harm our competitive position. If our CROs do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements, or for any other reasons, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize our product candidates. As a result, our financial results and the commercial prospects for such product candidates would be harmed, our costs could increase, and our ability to generate revenues could be delayed.
We also rely on other third parties to store and distribute drug products for our clinical trials. Any performance failure on the part of our distributors could delay clinical development or marketing approval of our product candidates or commercialization of our products, if approved, producing additional losses and depriving us of potential product revenue.
We have never conducted a Phase 3 clinical trial or submitted an NDA before, and may be unable to do so for, TNX-102 SL or other product candidates we are developing.
In addition to our ongoing AFFIRM trial of TNX-102 SL in FM, we plan to initiate a second Phase 3 confirmatory trial in support of product registration prior to completion of the ongoing AFFIRM trial. As these trials are intended to provide evidence to support marketing approval by the FDA, they are considered pivotal, or registration, trials. The conduct of pivotal clinical trials and the submission of a successful NDA is a complicated process. Although members of our management team have extensive industry experience, including in the development, clinical testing and commercialization of drug candidates, our company has never conducted a pivotal clinical trial before (other than the ongoing AFFIRM trial), has limited experience in preparing, submitting and prosecuting regulatory filings, and has not submitted an NDA before. Consequently, we may be unable to successfully and efficiently execute and complete these planned clinical trials in a way that leads to NDA submission and approval of TNX-102 SL and other product candidates we are developing. We may require more time and incur greater costs than our competitors and may not succeed in obtaining regulatory approvals of product candidates that we develop. Failure to commence or complete, or delays in, our planned clinical trials would prevent or delay commercialization of TNX-102 SL and other product candidates we are developing.
| 28 |
Our product candidates may cause serious adverse events or undesirable side effects which may delay or prevent marketing approval, or, if approval is received, require them to be taken off the market, require them to include safety warnings or otherwise limit their sales.
Serious adverse events or undesirable side effects from TNX-102 SL or any of our other product candidates could arise either during clinical development or, if approved, after the approved product has been marketed. The results of future clinical trials, including TNX-102 SL, may show that our product candidates cause serious adverse events or undesirable side effects, which could interrupt, delay or halt clinical trials, resulting in delay of, or failure to obtain, marketing approval from the FDA and other regulatory authorities.
If TNX-102 SL or any of our other product candidates cause serious adverse events or undesirable side effects or suffer from quality control issues:
| • | regulatory authorities may impose a clinical hold or risk evaluation and mitigation strategies, or REMS, which could result in substantial delays, significantly increase the cost of development, and/or adversely impact our ability to continue development of the product; | |
| • | regulatory authorities may require the addition of statements, specific warnings, or contraindications to the product label, or restrict the product’s indication to a smaller potential treatment population; | |
| • | we may be required to change the way the product is administered or conduct additional clinical trials; | |
| • | we may be required to implement a risk minimization action plan, which could result in substantial cost increases and have a negative impact on our ability to commercialize the product; | |
| • | we may be required to limit the patients who can receive the product; | |
| • | we may be subject to limitations on how we promote the product; | |
| • | we may, voluntarily or involuntarily, initiate field alerts for product recall, which may result in shortages; | |
| • | sales of the product may decrease significantly; | |
| • | regulatory authorities may require us to take our approved product off the market; | |
| • | we may be subject to litigation or product liability claims; and | |
| • | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product or could substantially increase commercialization costs and expenses, which in turn could delay or prevent us from generating significant revenues from the sale of our products.
If we are unable to file for approval of TNX-102 SL under Section 505(b)(2) of the FDCA or if we are required to generate additional data related to safety and efficacy in order to obtain approval under Section 505(b)(2), we may be unable to meet our anticipated development and commercialization timelines.
Our current plans for filing NDAs for our product candidates include efforts to minimize the data we will be required to generate in order to obtain marketing approval for our product candidates and therefore reduce the development time. We held an End-of-Phase 2 meeting with the FDA in February 2013 to discuss our most advanced development program, in which we are developing TNX-102 SL for the management of FM. In late 2014, following the results of the BESTFIT trial, we corresponded with the FDA to further discuss our Phase 3 registration program plan. We held a pre-IND meeting with the FDA in October 2012 to discuss the development of TNX-102 SL in PTSD. Although our interactions with the FDA have encouraged our efforts to continue to develop TNX-102 SL for FM and PTSD, there is no assurance that we will satisfy the FDA’s requirements for approval in these indications. The timeline for filing and review of our NDAs for TNX-102 SL for FM and PTSD is based on our plan to submit those NDAs under Section 505(b)(2) of the FDCA, which would enable us to rely in part on data in the public domain or elsewhere. We have not yet filed an NDA under Section 505(b)(2) for any of our product candidates. Depending on the data that may be required by the FDA for approval, some of the data may be related to products already approved by the FDA. If the data relied upon is related to products already approved by the FDA and covered by third-party patents we would be required to certify that we do not infringe the listed patents or that such patents are invalid or unenforceable. As a result of the certification, the third-party would have 45 days from notification of our certification to initiate an action against us. In the event that an action is brought in response to such a certification, the approval of our NDA could be subject to a stay of up to 30 months or more while we defend against such a suit. Approval of our product candidates under Section 505(b)(2) may therefore be delayed until patent exclusivity expires or until we successfully challenge the applicability of those patents to our product candidates. Alternatively, we may elect to generate sufficient additional clinical data so that we no longer rely on data which triggers a potential stay of the approval of our product candidates. Even if no exclusivity periods apply to our applications under Section 505(b)(2), the FDA has broad discretion to require us to generate additional data on the safety and efficacy of our product candidates to supplement third-party data on which we may be permitted to rely. In either event, we could be required, before obtaining marketing approval for any of our product candidates, to conduct substantial new research and development activities beyond those we currently plan to engage in order to obtain approval of our product candidates. Such additional new research and development activities would be costly and time consuming.
| 29 |
We may not be able to realize shortened development timelines for TNX-102 SL for FM or PTSD, and the FDA may not agree that any of our products qualify for marketing approval. If CBP-containing products are withdrawn from the market by the FDA for any safety reason, we may not be able to reference such products to support a 505(b)(2) NDA for TNX-102 SL, and we may need to fulfill the more extensive requirements of Section 505(b)(1). If we are required to generate additional data to support approval, we may be unable to meet our anticipated development and commercialization timelines, may be unable to generate the additional data at a reasonable cost, or at all, and may be unable to obtain marketing approval of our product candidates.
We will need to expand our operations and increase the size of our company, and we may experience difficulties in managing growth.
As we advance our product candidates through preclinical and nonclinical testing and clinical trials, and develop new product candidates, we will need to increase our product development, scientific, regulatory and compliance and administrative headcount to manage these programs. In addition, to meet our obligations as a public company, we will need to increase our general and administrative capabilities. Our management, personnel and systems currently in place may not be adequate to support this future growth. Our need to effectively manage our operations, growth and various projects requires that we:
| • | successfully attract and recruit new employees with the expertise and experience we will require; | |
| • | manage our clinical programs effectively, which we anticipate being conducted at numerous clinical sites; | |
| • | develop a marketing, distribution and sales infrastructure in addition to a post-marketing surveillance program if we seek to market our products directly; and | |
| • | continue to improve our operational, manufacturing, quality assurance, financial and management controls, reporting systems and procedures. |
If we are unable to successfully manage this growth and increased complexity of operations, our business may be adversely affected.
Our executive officers and other key personnel are critical to our business, and our future success depends on our ability to retain them.
Our success depends to a significant extent upon the continued services of Dr. Seth Lederman, our President and Chief Executive Officer. Dr. Lederman has overseen Tonix Pharmaceuticals, Inc., a wholly-owned subsidiary, since inception and provides leadership for our growth and operations strategy as well as being an inventor on many of our patents. Loss of the services of Dr. Lederman would have a material adverse effect on our growth, revenues, and prospective business. The loss of any of our key personnel, or the inability to attract and retain qualified personnel, may significantly delay or prevent the achievement of our research, development or business objectives and could materially adversely affect our business, financial condition and results of operations.
Any employment agreement we enter into will not ensure the retention of the employee who is a party to the agreement. In addition, we have only limited ability to prevent former employees from competing with us. Furthermore, our future success will also depend in part on the continued service of our key scientific and management personnel and our ability to identify, hire, and retain additional personnel. We experience intense competition for qualified personnel and may be unable to attract and retain the personnel necessary for the development of our business. Moreover, competition for personnel with the scientific and technical skills that we seek is extremely high and is likely to remain high. Because of this competition, our compensation costs may increase significantly.
If we are unable to hire additional qualified personnel, our ability to grow our business may be harmed.
Over time we will need to hire additional qualified personnel with expertise in drug development, product registration, clinical, preclinical and nonclinical research, quality compliance, government regulation, formulation and manufacturing, financial matters and sales and marketing. We compete for qualified individuals with numerous biopharmaceutical companies, universities and other research institutions. Competition for such individuals is intense, and we cannot be certain that our search for such personnel will be successful. Attracting and retaining qualified personnel will be critical to our success.
| 30 |
We rely on third parties to manufacture the compounds used in our trials, and we intend to rely on them for the manufacture of any approved products for commercial sale. If these third parties do not manufacture our product candidates in sufficient quantities and at an acceptable cost, clinical development and commercialization of our product candidates could be delayed, prevented or impaired.
We have no manufacturing facilities, and we have no experience in the clinical or commercial-scale manufacture of drugs or in designing drug manufacturing processes. We intend to rely on CMOs to manufacture some or all of our product candidates in clinical trials and our products that reach commercialization. Completion of our clinical trials and commercialization of our product candidates requires the manufacture of a sufficient supply of our product candidates. We have contracted with outside sources to manufacture our development compounds, including TNX-102 SL. If, for any reason, we become unable to rely on our current sources for the manufacture of our product candidates, either for clinical trials or, at some future date, for commercial quantities, then we would need to identify and contract with additional or replacement third-party manufacturers to manufacture compounds for nonclinical, preclinical, clinical, and commercial purposes. Although we are in discussions with other manufacturers we have identified as potential alternative CMOs of TNX-102 SL, we may not be successful in negotiating acceptable terms with any of them.
We believe that there are a variety of manufacturers that we may be able to retain to produce these products. However, once we retain a manufacturing source, if our manufacturers do not perform in a satisfactory manner, we may not be able to develop or commercialize potential products as planned. Certain specialized manufacturers are expected to provide us with modified and unmodified pharmaceutical compounds, including finished products, for use in our preclinical and nonclinical testing and clinical trials. Some of these materials are available from only one supplier or vendor. Any interruption in or termination of service by such sole source suppliers could result in a delay or interruption in manufacturing until we locate an alternative source of supply. Any delay or interruption in manufacturing operations (or failure to locate a suitable replacement for such suppliers) could materially adversely affect our business, prospects, or results of operations. We do not have any short-term or long-term manufacturing agreements with many of these manufacturers. If we fail to contract for manufacturing on acceptable terms or if third-party manufacturers do not perform as we expect, our development programs could be materially adversely affected. This may result in delays in filing for and receiving FDA approval for one or more of our products. Any such delays could cause our prospects to suffer significantly.
Failure by our third-party manufacturers to comply with the regulatory guidelines set forth by the FDA with respect to our product candidates could delay or prevent the completion of clinical trials, the approval of any product candidates or the commercialization of our products.
Such third-party manufacturers must be inspected by FDA for current Good Manufacturing Practice, or cGMP, compliance before they can produce commercial product. We may be in competition with other companies for access to these manufacturers' facilities and may be subject to delays in manufacture if the manufacturers give other clients higher priority than they give to us. If we are unable to secure and maintain third-party manufacturing capacity, the development and sales of our products and our financial performance may be materially affected.
Manufacturers are obligated to operate in accordance with FDA-mandated requirements. A failure of any of our third-party manufacturers to establish and follow cGMP requirements and to document their adherence to such practices may lead to significant delays in the availability of material for clinical trials, may delay or prevent filing or approval of marketing applications for our products, and may cause delays or interruptions in the availability of our products for commercial distribution following FDA approval. This could result in higher costs to us or deprive us of potential product revenues.
Complying with cGMP and non-U.S. regulatory requirements will require that we expend time, money, and effort in production, recordkeeping, and quality control to assure that the product meets applicable specifications and other requirements. We, or our contracted manufacturing facility, must also pass a pre-approval inspection prior to FDA approval. Failure to pass a pre-approval inspection may significantly delay FDA approval of our products. If we fail to comply with these requirements, we would be subject to possible regulatory action and may be limited in the jurisdictions in which we are permitted to sell our products. As a result, our business, financial condition, and results of operations may be materially harmed.
Drug manufacturers are subject to ongoing periodic unannounced inspections by the FDA, the Drug Enforcement Agency and corresponding state and foreign agencies to ensure strict compliance with cGMP requirements and other requirements under Federal drug laws, other government regulations and corresponding foreign standards. If we or our third-party manufacturers fail to comply with applicable regulations, sanctions could be imposed on us, including fines, injunctions, civil penalties, failure by the government to grant marketing approval of drugs, delays, suspension or withdrawal of approvals, seizures or recalls of product, operating restrictions and criminal prosecutions.
| 31 |
Corporate and academic collaborators may take actions to delay, prevent, or undermine the success of our products.
Our operating and financial strategy for the development, clinical testing, manufacture, and commercialization of drug candidates is heavily dependent on our entering into collaborations with corporations, academic institutions, licensors, licensees, and other parties. Our current strategy assumes that we will successfully establish these collaborations, or similar relationships; however, there can be no assurance that we will be successful establishing such collaborations. Some of our existing collaborations are, and future collaborations may be, terminable at the sole discretion of the collaborator. Replacement collaborators might not be available on attractive terms, or at all. The activities of any collaborator will not be within our control and may not be within our power to influence. There can be no assurance that any collaborator will perform its obligations to our satisfaction or at all, that we will derive any revenue or profits from such collaborations, or that any collaborator will not compete with us. If any collaboration is not pursued, we may require substantially greater capital to undertake development and marketing of our proposed products and may not be able to develop and market such products effectively, if at all. In addition, a lack of development and marketing collaborations may lead to significant delays in introducing proposed products into certain markets and/or reduced sales of proposed products in such markets.
Data provided by collaborators and others upon which we rely that has not been independently verified could turn out to be false, misleading, or incomplete.
We rely on third-party vendors, scientists, and collaborators to provide us with significant data and other information related to our projects, clinical trials, and our business. If such third parties provide inaccurate, misleading, or incomplete data, our business, prospects, and results of operations could be materially adversely affected.
Our product candidates are novel and still in development.
We are a clinical-stage pharmaceutical company focused on the development of drug product candidates, all of which are still in development. Our drug development methods may not lead to commercially viable drugs for any of several reasons. For example, we may fail to identify appropriate targets or compounds, our drug candidates may fail to be safe and effective in clinical trials, or we may have inadequate financial or other resources to pursue development efforts for our drug candidates. Our drug candidates will require significant additional development, clinical trials, regulatory clearances and additional investment by us or our collaborators before they can be commercialized.
Successful development of our products is uncertain.
Our development of current and future product candidates is subject to the risks of failure and delay inherent in the development of new pharmaceutical products, including: delays in product development, clinical testing, or manufacturing; unplanned expenditures in product development, clinical testing, or manufacturing; failure to receive regulatory approvals; emergence of superior or equivalent products; inability to manufacture on its own, or through any others, product candidates on a commercial scale; and failure to achieve market acceptance.
Because of these risks, our research and development efforts may not result in any commercially viable products. If a significant portion of these development efforts are not successfully completed, required regulatory approvals are not obtained or any approved products are not commercially successfully, our business, financial condition, and results of operations may be materially harmed.
Clinical trials required for our product candidates are expensive and time-consuming, and their outcome is uncertain.
In order to obtain FDA approval to market a new drug product, we must demonstrate proof of safety and effectiveness in humans. To meet these requirements, we must conduct "adequate and well controlled" clinical trials. Conducting clinical trials is a lengthy, time-consuming, and expensive process. The length of time may vary substantially according to the type, complexity, novelty, and intended use of the product candidate, and often can be several years or more per trial. Delays associated with products for which we are directly conducting clinical trials may cause us to incur additional operating expenses. The commencement and rate of completion of clinical trials may be delayed by many factors, including, for example: inability to manufacture sufficient quantities of stable and qualified materials under cGMP, for use in clinical trials; slower than expected rates of patient recruitment; failure to recruit a sufficient number of patients; modification of clinical trial protocols; changes in regulatory requirements for clinical trials; the lack of effectiveness during clinical trials; the emergence of unforeseen safety issues; delays, suspension, or termination of the clinical trials due to the institutional review board responsible for overseeing the study at a particular study site; and government or regulatory delays or "clinical holds" requiring suspension or termination of the trials.
The results from early clinical trials are not necessarily predictive of results obtained in later clinical trials. Accordingly, even if we obtain positive results from early clinical trials, we may not achieve the same success in future clinical trials. Clinical trials may not demonstrate sufficient safety and effectiveness to obtain the requisite regulatory approvals for product candidates.
| 32 |
Our clinical trials may be conducted in patients with CNS conditions, and in some cases, our product candidates are expected to be used in combination with approved therapies that themselves have significant adverse event profiles. During the course of treatment, these patients could suffer adverse medical events or die for reasons that may or may not be related to our product candidates. We cannot ensure that safety issues will not arise with respect to our product candidates in clinical development.
The failure of clinical trials to demonstrate safety and effectiveness for the desired indications could harm the development of that product candidate and other product candidates. This failure could cause us to abandon a product candidate and could delay development of other product candidates. Any delay in, or termination of, our clinical trials would delay the filing of our NDAs with the FDA and, ultimately, our ability to commercialize our product candidates and generate product revenues. Any change in, or termination of, our clinical trials could materially harm our business, financial condition, and results of operations.
We are subject to extensive and costly government regulation.
Product candidates employing our technology are subject to extensive and rigorous domestic government regulation including regulation by the FDA, the Centers for Medicare and Medicaid Services, other divisions of the United States Department of Health and Human Services, the United States Department of Justice, state and local governments, and their respective foreign equivalents. The FDA regulates the research, development, preclinical and nonclinical testing and clinical studies, manufacture, safety, effectiveness, record-keeping, reporting, labeling, storage, approval, advertising, promotion, sale, distribution, import, and export of biopharmaceutical products. The FDA regulates small molecule chemical entities as drugs, subject to an NDA under the FDCA. If products employing our technologies are marketed abroad, they will also be subject to extensive regulation by foreign governments, whether or not they have obtained FDA approval for a given product and its uses. Such foreign regulation may be equally or more demanding than corresponding United States regulation.
Government regulation substantially increases the cost and risk of researching, developing, manufacturing, and selling our products. The regulatory review and approval process, which includes preclinical and nonclinical testing and clinical trials of each product candidate, is lengthy, expensive, and uncertain. We or our collaborators must obtain and maintain regulatory authorization to conduct clinical trials. We or our collaborators must obtain regulatory approval for each product we intend to market, and the manufacturing facilities used for the products must be inspected and meet legal requirements. Securing regulatory approval requires the submission of extensive preclinical, nonclinical and clinical data and other supporting information for each proposed therapeutic indication in order to establish the product's safety and efficacy, and in the case of biologics also potency and purity, for each intended use. The development and approval process takes many years, requires substantial resources, and may never lead to the approval of a product.
Even if we are able to obtain regulatory approval for a particular product, the approval may limit the indicated medical uses for the product, may otherwise limit our ability to promote, sell, and distribute the product, may require that we conduct costly post-marketing surveillance, and/or may require that we conduct ongoing post-marketing studies. Material changes to an approved product, such as, for example, manufacturing changes or revised labeling, may require further regulatory review and approval. Once obtained, any approvals may be withdrawn, including, for example, if there is a later discovery of previously unknown problems with the product, such as a previously unknown safety issue.
If we, our collaborators, or our contract manufacturers fail to comply with applicable regulatory requirements at any stage during the regulatory process, such noncompliance could result in, among other things delays in the approval of applications or supplements to approved applications; refusal of a regulatory authority, including the FDA, to review pending market approval applications or supplements to approved applications; warning letters; fines; import and/or export restrictions; product recalls or seizures; injunctions; total or partial suspension of production; civil penalties; withdrawals of previously approved marketing applications or licenses; recommendations by the FDA or other regulatory authorities against governmental contracts; and/or criminal prosecutions.
We do not have, and may never obtain, the regulatory approvals we need to market our product candidates.
Following completion of clinical trials, the results are evaluated and, depending on the outcome, submitted to the FDA in the form of an NDA in order to obtain FDA approval of the product and authorization to commence commercial marketing. In responding to an NDA, the FDA may require additional testing or information, may require that the product labeling be modified, may impose post-approval study and other commitments or reporting requirements or other restrictions on product distribution, or may deny the application. The FDA has established performance goals for review of NDAs: six months for priority applications and ten months for standard applications. However, the FDA is not required to complete its review within these time periods. The timing of final FDA review and action varies greatly, but can take years in some cases and may involve the input of an FDA advisory committee of outside experts. Product sales in the United States may commence only when an NDA is approved.
| 33 |
To date, we have not applied for or received the regulatory approvals required for the commercial sale of any of our products in the United States or in any foreign jurisdiction. None of our product candidates have been determined to be safe and effective, and we have not submitted an NDA to the FDA or an equivalent application to any foreign regulatory authorities for any of our product candidates.
It is possible that none of our product candidates will be approved for marketing. Failure to obtain regulatory approvals, or delays in obtaining regulatory approvals, may adversely affect the successful commercialization of any drugs or biologics that we or our partners develop, may impose additional costs on us or our collaborators, may diminish any competitive advantages that we or our partners may attain, and/or may adversely affect our receipt of revenues or royalties.
Even if approved, our products will be subject to extensive post-approval regulation.
Once a product is approved, numerous post-approval requirements apply. Among other things, the holder of an approved NDA is subject to periodic and other FDA monitoring and reporting obligations, including obligations to monitor and report adverse events and instances of the failure of a product to meet the specifications in the NDA. Application holders must submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling, or manufacturing process. Application holders must also submit advertising and other promotional material to the FDA and report on ongoing clinical trials.
Depending on the circumstances, failure to meet these post-approval requirements can result in criminal prosecution, fines, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of pre-marketing product approvals, or refusal to allow us to enter into supply contracts, including government contracts. In addition, even if we comply with FDA and other requirements, new information regarding the safety or effectiveness of a product could lead the FDA to modify or withdraw product approval.
Even if we obtain regulatory approval to market our product candidates, our product candidates may not be accepted by the market.
Even if the FDA approves one or more of our product candidates, physicians and patients may not accept it or use it. Even if physicians and patients would like to use our products, our products may not gain market acceptance among healthcare payors such as managed care formularies, insurance companies or government programs such as Medicare or Medicaid. Acceptance and use of our products will depend upon a number of factors including: perceptions by members of the health care community, including physicians, about the safety and effectiveness of our drug or device product; cost-effectiveness of our product relative to competing products; availability of reimbursement for our product from government or other healthcare payors; and effectiveness of marketing and distribution efforts by us and our licensees and distributors, if any.
The degree of market acceptance of any pharmaceutical product that we develop will depend on a number of factors, including:
| • | cost-effectiveness; | |
| • | the safety and effectiveness of our products, including any significant potential side effects (including drowsiness and dry mouth), as compared to alternative products or treatment methods; | |
| • | the timing of market entry as compared to competitive products; | |
| • | the rate of adoption of our products by doctors and nurses; | |
| • | product labeling or product insert required by the FDA for each of our products; | |
| • | reimbursement policies of government and third-party payors; | |
| • | effectiveness of our sales, marketing and distribution capabilities and the effectiveness of such capabilities of our collaborative partners, if any; and | |
| • | unfavorable publicity concerning our products or any similar products. |
Our product candidates, if successfully developed, will compete with a number of products manufactured and marketed by major pharmaceutical companies, biotechnology companies and manufacturers of generic drugs. Our products may also compete with new products currently under development by others. Physicians, patients, third-party payors and the medical community may not accept and utilize any of our product candidates. If our products do not achieve market acceptance, we will not be able to generate significant revenues or become profitable.
| 34 |
Because we expect sales of our current product candidates, if approved, to generate substantially all of our product revenues for the foreseeable future, the failure of these products to find market acceptance would harm our business and could require us to seek additional financing.
If we fail to establish marketing, sales and distribution capabilities, or fail to enter into arrangements with third parties, we will not be able to create a market for our product candidates.
Our strategy with our product candidates is to control, directly or through contracted third parties, all or most aspects of the product development process, including marketing, sales and distribution. Currently, we do not have any sales, marketing or distribution capabilities. In order to generate sales of any product candidates that receive regulatory approval, we must either acquire or develop an internal marketing and sales force with technical expertise and with supporting distribution capabilities or make arrangements with third parties to perform these services for us. The acquisition or development of a sales and distribution infrastructure would require substantial resources, which may divert the attention of our management and key personnel and defer our product development efforts. To the extent that we enter into marketing and sales arrangements with other companies, our revenues will depend on the efforts of others. These efforts may not be successful. If we fail to develop sales, marketing and distribution channels, or enter into arrangements with third parties, we will experience delays in product sales and incur increased costs.
Sales of pharmaceutical products largely depend on the reimbursement of patients' medical expenses by government health care programs and private health insurers. Without the financial support of the government or third-party payors, the market for our products will be limited. These third-party payors are increasingly challenging the price and examining the cost effectiveness of medical products and services. Recent proposals to change the health care system in the United States have included measures that would limit or eliminate payments for medical products and services or subject the pricing of medical treatment products to government control. Significant uncertainty exists as to the reimbursement status of newly approved health care products. Third-party payors may not reimburse sales of our products or enable our collaborators to sell them at profitable prices.
Our business strategy might involve out-licensing product candidates to or collaborating with larger firms with experience in marketing and selling pharmaceutical products. There can be no assurance that we will be able to successfully establish marketing, sales, or distribution relationships; that such relationships, if established, will be successful; or that we will be successful in gaining market acceptance for our products. To the extent that we enter into any marketing, sales, or distribution arrangements with third parties, our product revenues will be lower than if we marketed and sold our products directly, and any revenues we receive will depend upon the efforts of such third-parties. If we are unable to establish such third-party sales and marketing relationships, or choose not to do so, we will have to establish and rely on our own in-house capabilities.
We, as a company, have no experience in marketing or selling pharmaceutical products and currently have no sales, marketing, or distribution infrastructure. To market any of our products directly, we would need to develop a marketing, sales, and distribution force that both has technical expertise and the ability to support a distribution capability. The establishment of a marketing, sales, and distribution capability would significantly increase our costs, possibly requiring substantial additional capital. In addition, there is intense competition for proficient sales and marketing personnel, and we may not be able to attract individuals who have the qualifications necessary to market, sell, and distribute our products. There can be no assurance that we will be able to establish internal marketing, sales, or distribution capabilities. If we are unable to, or choose not to establish these capabilities, or if the capabilities we establish are not sufficient to meet our needs, we will be required to establish collaborative marketing, sales, or distribution relationships with third parties.
In the event that we are successful in bringing any products to market, our revenues may be adversely affected if we fail to obtain acceptable prices or adequate reimbursement for our products from third-party payors.
Our ability to commercialize pharmaceutical products successfully may depend in part on the availability of reimbursement for our products from:
| • | government and health administration authorities; | |
| • | private health insurers; and | |
| • | other third party payors, including Medicare and Medicaid. |
| 35 |
We cannot predict the availability of reimbursement for health care products to be approved in the future. Third-party payors, including Medicare and Medicaid, are challenging the prices charged for medical products and services. Government and other third-party payors increasingly are limiting both coverage and the level of reimbursement for new drugs whether approved under Section 505(b)(1), 505(b)(2), or 505(j) of the FDCA, through direct payment mechanisms and through cost containment programs such as the Medicaid Drug Rebate Program. Third-party insurance coverage may not be available to patients for any of our products.
The continuing efforts of government and third-party payors to contain or reduce the costs of health care may limit our commercial opportunity. If government and other third-party payors do not provide adequate coverage and reimbursement for any prescription product we bring to market, doctors may not prescribe them or patients may ask to have their physicians prescribe competing drugs with more favorable reimbursement. In some foreign markets, pricing and profitability of prescription pharmaceuticals are subject to government control. In the United States, we expect that there will continue to be federal and state proposals for similar controls. In addition, we expect that increasing emphasis on managed care in the United States will continue to put pressure on the pricing of pharmaceutical products. Cost control initiatives could decrease the price that we receive for any products in the future. Further, cost control initiatives could impair our ability to commercialize our products and our ability to earn revenues from this commercialization.
If we obtain approval to commercialize any approved products outside of the United States, a variety of risks associated with international operations could materially adversely affect our business.
If TNX-102 SL or any of our other product candidates are approved for commercialization outside of the United States, we intend to enter into agreements with third parties to market them on a worldwide basis or in more limited geographical regions. We expect that we will be subject to additional risks related to entering into international business relationships, including:
| • | different regulatory requirements for drug approvals; | |
| • | reduced protection for intellectual property rights, including trade secret and patent rights; | |
| • | unexpected changes in tariffs, trade barriers and regulatory requirements; | |
| • | economic weakness, including inflation, or political instability in particular foreign economies and markets; | |
| • | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; | |
| • | foreign taxes, including withholding of payroll taxes; | |
| • | foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country; | |
| • | workforce uncertainty in countries where labor unrest is more common than in the United States; | |
| • | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; | |
| • | business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters including earthquakes, hurricanes, floods and fires; and | |
| • | difficulty in importing and exporting clinical trial materials and study samples. |
We face the risk of product liability claims and may not be able to obtain insurance.
Our business exposes us to the risk of product liability claims that are inherent in the development of drugs. If the use of one or more of our or our collaborators' drugs harms people, we may be subject to costly and damaging product liability claims brought against us by clinical trial participants, consumers, health care providers, pharmaceutical companies or others selling our products. Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of pharmaceutical products we develop, alone or with collaborators. While we currently carry clinical trial insurance and product liability insurance, we cannot predict all of the possible harms or side effects that may result and, therefore, the amount of insurance coverage we hold now or in the future may not be adequate to cover all liabilities we might incur. We intend to expand our insurance coverage to include the sale of commercial products if we obtain marketing approval for our drug candidates in development, but we may be unable to obtain commercially reasonable product liability insurance for any products approved for marketing. If we are unable to obtain insurance at an acceptable cost or otherwise protect against potential product liability claims, we will be exposed to significant liabilities, which may materially and adversely affect our business and financial position. If we are sued for any injury allegedly caused by our or our collaborators' products, our liability could exceed our total assets and our ability to pay the liability. A product liability claim or series of claims brought against us would decrease our cash and could cause our stock price to fall.
| 36 |
We use hazardous chemicals in our business. Potential claims relating to improper handling, storage or disposal of these chemicals could affect us and be time consuming and costly.
Our research and development processes and/or those of our third party contractors may involve the controlled use of hazardous materials and chemicals. These hazardous chemicals are reagents and solvents typically found in a chemistry laboratory. Our operations also produce hazardous waste products. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of hazardous materials. While we attempt to comply with all environmental laws and regulations, including those relating to the outsourcing of the disposal of all hazardous chemicals and waste products, we cannot eliminate the risk of contamination from or discharge of hazardous materials and any resultant injury. In the event of such an accident, we could be held liable for any resulting damages and any liability could materially adversely affect our business, financial condition and results of operations.
Compliance with environmental laws and regulations may be expensive. Current or future environmental regulations may impair our research, development or production efforts. We might have to pay civil damages in the event of an improper or unauthorized release of, or exposure of individuals to, hazardous materials. We are not insured against these environmental risks.
If we enter into collaborations with third parties, they might also work with hazardous materials in connection with our collaborations. We may agree to indemnify our collaborators in some circumstances against damages and other liabilities arising out of development activities or products produced in connection with these collaborations.
In addition, the federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous or radioactive materials and waste products may require us to incur substantial compliance costs that could materially adversely affect our business, financial condition and results of operations.
Our insurance policies are expensive and protect us only from some business risks, which will leave us exposed to significant uninsured liabilities.
We carry insurance for most categories of risk that our business may encounter, however, we may not have adequate levels of coverage. We currently maintain general liability, clinical trial, property, workers’ compensation, products liability and directors’ and officers’ insurance, along with an umbrella policy, which collectively costs approximately $300,000 per annum. We cannot provide any assurances that we will be able to maintain existing insurance at current or adequate levels of coverage. Any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our cash position and results of operations.
If we retain collaborative partners and our partners do not satisfy their obligations, we will be unable to develop our partnered product candidates.
In the event we enter into any collaborative agreements, we may not have day-to-day control over the activities of our collaborative partners with respect to any of these product candidates. Any collaborative partner may not fulfill its obligations under these agreements. If a collaborative partner fails to fulfill its obligations under an agreement with us, we may be unable to assume the development of the products covered by that agreement or enter into alternative arrangements with a third party. In addition, we may encounter delays in the commercialization of the product candidate that is the subject of the agreement. Accordingly, our ability to receive any revenue from the product candidates covered by these agreements will be dependent on the efforts of our collaborative partner. We could also become involved in disputes with a collaborative partner, which could lead to delays in or termination of our development and commercialization programs and time-consuming and expensive litigation or arbitration. In addition, any such dispute could diminish our collaborators’ commitment to us and reduce the resources they devote to developing and commercializing our products. Conflicts or disputes with our collaborators, and competition from them, could harm our relationships with our other collaborators, restrict our ability to enter future collaboration agreements and delay the research, development or commercialization of our product candidates. If any collaborative partner terminates or breaches its agreement, or otherwise fails to complete its obligations in a timely manner, our chances of successfully developing or commercializing these product candidates would be materially and adversely affected. We may not be able to enter into collaborative agreements with partners on terms favorable to us, or at all. Our inability to enter into collaborative arrangements with collaborative partners, or our failure to maintain such arrangements, would limit the number of product candidates that we could develop and ultimately, decrease our sources of any future revenues.
| 37 |
RISKS RELATED TO OUR STOCK
Sales of additional shares of our common stock could cause the price of our common stock to decline.
Sales of substantial amounts of our common stock in the public market, or the availability of such shares for sale, by us or others, including the issuance of common stock upon exercise of outstanding options and warrants, could adversely affect the price of our common stock. We and our directors and officers may sell shares into the market, which could adversely affect the market price of shares of our common stock.
The market price for our common stock may be volatile, and your investment in our common stock could decline in value.
The stock market in general has experienced extreme price and volume fluctuations. The market prices of the securities of biotechnology and specialty pharmaceutical companies, particularly companies like ours without product revenues and earnings, have been highly volatile and may continue to be highly volatile in the future. This volatility has often been unrelated to the operating performance of particular companies. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our common stock:
| • | announcements of technological innovations or new products by us or our competitors; | |
| • | announcement of FDA approval or disapproval of our product candidates or other product-related actions; | |
| • | developments involving our discovery efforts and clinical trials; | |
| • | developments or disputes concerning patents or proprietary rights, including announcements of infringement, interference or other litigation against us or our potential licensees; | |
| • | developments involving our efforts to commercialize our products, including developments impacting the timing of commercialization; | |
| • | announcements concerning our competitors, or the biotechnology, pharmaceutical or drug delivery industry in general; | |
| • | public concerns as to the safety or efficacy of our product candidates or our competitors’ products; | |
| • | changes in government regulation of the pharmaceutical or medical industry; | |
| • | changes in the reimbursement policies of third party insurance companies or government agencies; | |
| • | actual or anticipated fluctuations in our operating results; | |
| • | changes in financial estimates or recommendations by securities analysts; | |
| • | developments involving corporate collaborators, if any; | |
| • | changes in accounting principles; and | |
| • | the loss of any of our key scientific or management personnel. |
In the past, securities class action litigation has often been brought against companies that experience volatility in the market price of their securities. Whether or not meritorious, litigation brought against us could result in substantial costs and a diversion of management’s attention and resources, which could adversely affect our business, operating results and financial condition.
We do not anticipate paying dividends on our common stock and, accordingly, shareholders must rely on stock appreciation for any return on their investment.
We have never declared or paid cash dividends on our common stock and do not expect to do so in the foreseeable future. The declaration of dividends is subject to the discretion of our board of directors and will depend on various factors, including our operating results, financial condition, future prospects and any other factors deemed relevant by our board of directors. You should not rely on an investment in our company if you require dividend income from your investment in our company. The success of your investment will likely depend entirely upon any future appreciation of the market price of our common stock, which is uncertain and unpredictable. There is no guarantee that our common stock will appreciate in value.
| 38 |
We expect that our quarterly results of operations will fluctuate, and this fluctuation could cause our stock price to decline.
Our quarterly operating results are likely to fluctuate in the future. These fluctuations could cause our stock price to decline. The nature of our business involves variable factors, such as the timing of the research, development and regulatory pathways of our product candidates, which could cause our operating results to fluctuate.
Due to the possibility of fluctuations in our revenues and expenses, we believe that quarter-to-quarter comparisons of our operating results are not a good indication of our future performance.
The rights of the holders of common stock may be impaired by the potential issuance of preferred stock.
Our articles of incorporation give our board of directors the right to create new series of preferred stock. As a result, the board of directors may, without stockholder approval, issue preferred stock with voting, dividend, conversion, liquidation or other rights which could adversely affect the voting power and equity interest of the holders of common stock. Preferred stock, which could be issued with the right to more than one vote per share, could be utilized as a method of discouraging, delaying or preventing a change of control. The possible impact on takeover attempts could adversely affect the price of our common stock. Although we have no present intention to issue any shares of preferred stock or to create a series of preferred stock, we may issue such shares in the future.
If we fail to comply with the rules under the Sarbanes-Oxley Act of 2002 related to accounting controls and procedures, or if we discover material weaknesses and deficiencies in our internal control and accounting procedures, our stock price could decline significantly and raising capital could be more difficult.
If we fail to comply with the rules under the Sarbanes-Oxley Act of 2002 related to disclosure controls and procedures, or, if we discover material weaknesses and other deficiencies in our internal control and accounting procedures, our stock price could decline significantly and raising capital could be more difficult. Section 404 of the Sarbanes-Oxley Act requires annual management assessments of the effectiveness of our internal control over financial reporting and a report by our independent auditors addressing these assessments. If material weaknesses or significant deficiencies are discovered or if we otherwise fail to achieve and maintain the adequacy of our internal control, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act. Moreover, effective internal controls are necessary for us to produce reliable financial reports and are important to helping prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock could drop significantly.
Because certain of our stockholders control a significant number of shares of our common stock, they may have effective control over actions requiring stockholder approval.
As of March 2, 2016, our directors, executive officers and principal stockholders (those beneficially owning in excess of 5%), and their respective affiliates, beneficially own approximately 30.5% of our outstanding shares of common stock. As a result, these stockholders, acting together, would have the ability to control the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets. In addition, these stockholders, acting together, would have the ability to control the management and affairs of our company. Accordingly, this concentration of ownership might harm the market price of our common stock by:
| • | delaying, deferring or preventing a change in corporate control; | |
| • | impeding a merger, consolidation, takeover or other business combination involving us; or | |
| • | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. |
If securities or industry analysts do not publish research or reports about our business, or if they change their recommendations regarding our stock adversely, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. Our research coverage by industry and financial analysts is currently limited. Even if our analyst coverage increases, if one or more of the analysts who cover us downgrade our stock, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
| 39 |
ITEM 1B – UNRESOLVED STAFF COMMENTS
There are no unresolved staff comments at December 31, 2015.
We maintain our principal office at 509 Madison Avenue, Suite 306, New York, New York 10022. Our telephone number at that office is (212) 980-9155 and our fax number is (212) 923-5700. On February 11, 2014, we entered into a lease amendment and expansion agreement, whereby we agreed to lease additional premises for office space, commencing May 1, 2014 and expiring on April 30, 2019. In connection therewith, the original letter of credit was increased by $72,354 to $132,417 and we deposited an additional $72,354 into the restricted cash account maintained at the bank that issued the letter of credit. Including the additional premises, the total square footage of our principal office space is approximately 4,800.
On April 28, 2014, we entered into a lease for approximately 3,578 square feet of office space in San Jose, California, whereby we agreed to lease premises, commencing August 1, 2014 and expiring on October 31, 2018. In connection therewith, we paid a security deposit of $44,546.
On June 19, 2015, we entered into a lease for approximately 2,450 square feet of office space in Dublin, Ireland, whereby we agreed to lease premises, commencing June 1, 2015 and expiring on May 31, 2018.
On July 27, 2015, we entered into a lease for approximately 132 square feet of office space in Montreal, Canada, whereby we agreed to lease premises, commencing August 1, 2015 and expiring on July 31, 2016. In connection therewith, we paid a security deposit of $800.
On August 24, 2015, we entered into a lease for approximately 2,762 square feet of office space in San Diego, California, whereby we agreed to lease premises, commencing September 1, 2015 and expiring on August 31, 2019. In connection therewith, we paid a security deposit of $11,272.
Future minimum lease payments are as follows (in thousands):
| Year Ending December 31, | ||||
| 2016 | $ | 670 | ||
| 2017 | 683 | |||
| 2018 | 607 | |||
| 2019 | 181 | |||
| $ | 2,141 | |||
We believe that our existing facilities are suitable and adequate to meet our current business requirements.
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business. We are currently not aware of any such legal proceedings or claims that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition or operating results.
ITEM 4 – MINE SAFETY DISCLOSURES
Not applicable.
| 40 |
ITEM 5 - MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Price Range of Common Stock
Our common stock has been listed on The NASDAQ Global Market under the symbol “TNXP” since August 11, 2014. Previous to that date, our common stock was listed on The NASDAQ Capital Market under the symbol “TNXP.” Prior to August 9, 2013, our common stock was traded on the OTCQB under the symbol “TNXP.” The following table sets forth, for the periods indicated, the high and low sales prices per share of our common stock as reported by The NASDAQ Stock Market.
| Fiscal Year 2015 | ||||||||
| High | Low | |||||||
| First Quarter | $ | 8.65 | $ | 5.61 | ||||
| Second Quarter | $ | 10.72 | $ | 5.88 | ||||
| Third Quarter | $ | 9.89 | $ | 5.14 | ||||
| Fourth Quarter | $ | 7.84 | $ | 5.05 | ||||
| Fiscal Year 2014 | ||||||||
| High | Low | |||||||
| First Quarter | $ | 21.00 | $ | 9.15 | ||||
| Second Quarter | $ | 14.43 | $ | 8.14 | ||||
| Third Quarter | $ | 15.21 | $ | 5.85 | ||||
| Fourth Quarter | $ | 8.14 | $ | 5.33 | ||||
On March 2, 2016, the closing sale price of our common stock, as reported by The NASDAQ Stock Market, was $2.38 per share. On March 2, 2016, there were 115 holders of record of our common stock. Because many of our shares of common stock are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of stockholders represented by these record holders.
Dividend Policy
We have never paid any cash dividends on our capital stock and do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain future earnings to fund ongoing operations and future capital requirements of our business. Any future determination to pay cash dividends will be at the discretion of the Board and will be dependent upon our financial condition, results of operations, capital requirements and such other factors as the Board deems relevant.
Equity Compensation Information
The following table summarizes information about our equity compensation plans as of December 31, 2015.
| Plan Category | Number of Shares of Common Stock to be Issued upon Exercise of Outstanding Options (a) | Weighted-Average Exercise Price of Outstanding Options (b) | Number of Options Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column (a)) (c) | |||||||||
| Equity compensation plans approved by stockholders | 1,656,643 | 10.64 | 651,357 | |||||||||
| Equity compensation plans not approved by stockholders | — | — | — | |||||||||
| Total | 1,656,643 | 10.64 | 651,357 | |||||||||
| 41 |
Stock Performance Graph
The following stock performance graph illustrates a comparison of the total cumulative stockholder return on our common stock since August 9, 2013, which is the date our common stock first began trading on the NASDAQ Global Market, to three indices: S&P 500 Index, the NASDAQ Composite Index and the NASDAQ Biotechnology Index. An investment of $100 (with reinvestment of all dividends) is assumed to have been made in Tonix’s common stock and in each index since August 9, 2013, and its relative performance is tracked through December 31, 2015. The returns shown are based on historical results and are not intended to suggest future performance.
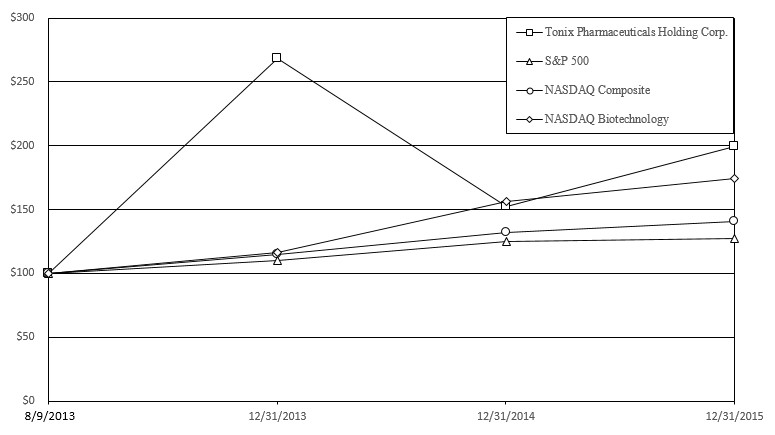
| As of December 31, | |||||||||||
| August 9, 2013 | 2013 | 2014 | 2015 | ||||||||
| Tonix Pharmaceuticals Holding Corp. | $ | 100.00 | $ | 268.49 | $ | 152.08 | $ | 199.74 | |||
| S&P 500 | 100.00 | 110.19 | 125.26 | 126.98 | |||||||
| NASDAQ Composite | 100.00 | 114.71 | 131.71 | 141.08 | |||||||
| NASDAQ Biotechnology | 100.00 | 116.26 | 156.25 | 174.64 | |||||||
Recent Sales of Unregistered Securities
None.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
We did not purchase any of our registered securities during the period covered by this Annual Report.
| 42 |
ITEM 6 – SELECTED FINANCIAL DATA
The following Selected Consolidated Financial Data should be read in conjunction with our Consolidated Financial Statements and the related Notes thereto, Management's Discussion and Analysis of Financial Condition and Results of Operations and other financial information included elsewhere in this Annual Report on Form 10-K. The data set forth below with respect to our Consolidated Statements of Income for the years ended December 31, 2015, 2014 and 2013 and the Consolidated Balance Sheet data as of December 31, 2015 and 2014 are derived from our Consolidated Financial Statements which are included elsewhere in this Annual Report on Form 10-K and are qualified by reference to such Consolidated Financial Statements and related Notes thereto. The data set forth below with respect to our Consolidated Statements of Income for the years ended December 31, 2012 and 2011 and the Consolidated Balance Sheet data as of December 31, 2013, 2012 and 2011 are derived from our Consolidated Financial Statements, which are not included elsewhere in this Annual Report on Form 10-K. The weighted average number of shares of common stock outstanding for the years ended December 31, 2012 and 2011 reflect a 20:1 reverse stock split that was effective on May 1, 2013.
TONIX PHARMACEUTICALS HOLDING CORP.
| Year ended December 31, | ||||||||||||||||||||
| 2015 | 2014 | 2013 | 2012 | 2011 | ||||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||||||
| COSTS AND EXPENSES: | ||||||||||||||||||||
| Research and development | $ | 35,504 | $ | 18,617 | $ | 4,650 | $ | 2,584 | $ | 1,158 | ||||||||||
| General and administrative | 12,658 | 9,039 | 6,238 | 4,078 | 2,220 | |||||||||||||||
| 48,162 | 27,656 | 10,888 | 6,662 | 3,378 | ||||||||||||||||
| Operating Loss | (48,162 | ) | (27,656 | ) | (10,888 | ) | (6,662 | ) | (3,378 | ) | ||||||||||
| Other income | - | - | - | 2 | - | |||||||||||||||
| Change in fair value of warrant liability | - | - | - | (1,177 | ) | - | ||||||||||||||
| Interest and other financing costs, net | 108 | 40 | 4 | (1,613 | ) | (92 | ) | |||||||||||||
| NET LOSS | $ | (48,054 | ) | $ | (27,616 | ) | $ | (10,884 | ) | $ | (9,450 | ) | $ | (3,470 | ) | |||||
| Net loss per common share, basic and diluted | $ | (2.86 | ) | $ | (2.77 | ) | $ | (3.37 | ) | $ | (5.58 | ) | $ | (3.24 | ) | |||||
| Weighted average common shares outstanding, basic and diluted | 16,791,059 | 9,985,515 | 3,231,311 | 1,693,416 | 1,071,295 | |||||||||||||||
| As of December 31, | ||||||||||||||||||||
| 2015 | 2014 | 2013 | 2012 | 2011 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Current assets: | ||||||||||||||||||||
| Cash, cash equivalents and marketable securities | $ | 43,016 | $ | 38,184 | $ | 8,202 | $ | 1,785 | $ | 41 | ||||||||||
| Working capital | 39,709 | 35,654 | 6,420 | 872 | (782 | ) | ||||||||||||||
| Total assets | 47,018 | 39,542 | 8,736 | 2,117 | 425 | |||||||||||||||
| Accumulated deficit | (102,398 | ) | (54,344 | ) | (26,728 | ) | (15,844 | ) | (6,395 | ) | ||||||||||
| Total stockholders' equity | 40,262 | 36,092 | 6,512 | 959 | (2,454 | ) | ||||||||||||||
| 43 |
ITEM 7 - MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This Management's Discussion and Analysis of Financial Condition and Results of Operations includes a number of forward-looking statements that reflect Management's current views with respect to future events and financial performance. You can identify these statements by forward-looking words such as “may” “will,” “expect,” “anticipate,” “believe,” “estimate” and “continue,” or similar words. Those statements include statements regarding the intent, belief or current expectations of us and members of its management team as well as the assumptions on which such statements are based. Prospective investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risk and uncertainties, and that actual results may differ materially from those contemplated by such forward-looking statements.
Readers are urged to carefully review and consider the various disclosures made by us in this report and in our other reports filed with the Securities and Exchange Commission. Important factors known to us could cause actual results to differ materially from those in forward-looking statements. We undertake no obligation to update or revise forward-looking statements to reflect changed assumptions, the occurrence of unanticipated events or changes in the future operating results over time. We believe that its assumptions are based upon reasonable data derived from and known about our business and operations and the business and operations of the Company. No assurances are made that actual results of operations or the results of our future activities will not differ materially from its assumptions. Factors that could cause differences include, but are not limited to, expected market demand for the Company’s services, fluctuations in pricing for materials, and competition.
Business Overview
We are a clinical-stage pharmaceutical company dedicated to the invention and development of next-generation medicines. Our clinical-stage product candidates, TNX-102 SL and TNX-201, are directed toward conditions affecting the CNS. In the second quarter of 2015, we initiated a Phase 3 clinical trial of our most advanced candidate, TNX-102 SL, for the treatment of FM. We are also developing TNX-102 SL as a potential treatment for PTSD, and we commenced a Phase 2 trial for this indication in the first quarter of 2015. We completed a Phase 2 trial of TNX-201 in ETTH in the first quarter of 2016. When the drug failed to show efficacy, development was terminated. Our pipeline includes a preclinical program for the treatment of AUD as well as two biodefense development programs for protection from smallpox virus and from radiation injury. We hold worldwide development and commercialization rights to all of our candidates.
Our therapeutic strategy in FM is supported by results from the randomized, double-blind, placebo-controlled Phase 2b BESTFIT trial of TNX-102 SL in FM. Although the BESTFIT trial demonstrated only a positive trend and did not achieve statistical significance for TNX-102 SL in the primary efficacy analysis of change in mean pain intensity at week 12, it did demonstrate statistical significance (p<0.05) in a 30% responder analysis of the primary pain data, a declared secondary endpoint in which a responder is defined as a subject for whom pain intensity was reduced by at least 30% at week 12 as compared to baseline. The BESTFIT trial also showed statistically significant improvements with TNX-102 SL in the declared secondary analyses of the Patient Global Impression of Change (p<0.05) and the Fibromyalgia Impact Questionnaire-Revised, or FIQ-R (p<0.05). In addition, the study showed statistically significant improvement with TNX-102 SL on measures of sleep quality as well as on several FIQ-R items. TNX-102 SL was well tolerated in the BESTFIT trial, and the most common adverse events were local in nature, with transient tongue or mouth numbness occurring in 44% of participants on TNX-102 SL vs. 2% on placebo, and bitter taste in 8% on TNX-102 SL compared to none on placebo. These local adverse events did not appear to affect either rates of retention of study participants or their compliance with taking TNX-102 SL. Systemic adverse events were similar between TNX-102 SL and placebo. No serious adverse events were reported. Among subjects randomized to the active and control arms, 86% and 83%, respectively, completed the 12-week dosing period. In August 2015, we completed a 12-month open-label extension study of TNX-102 SL, into which patients who completed the BESTFIT study were eligible to enroll.
On the basis of our discussions with the FDA, we believe that positive results from two adequate, well-controlled efficacy and safety studies and long-term (six- and 12-month) safety exposure studies would provide sufficient evidence of efficacy and safety to support FDA approval of TNX-102 SL for the management of FM. Following the BESTFIT study, we received written guidance from the FDA which accepted our proposal to use a 30% pain responder analysis as the primary efficacy endpoint in our Phase 3 program to support the approval of TNX-102 SL for the management of FM. We initiated the randomized, double-blind, placebo-controlled, 12-week Phase 3 AFFIRM trial of TNX-102 SL in 500 patients with FM in the second quarter of 2015, from which we expect to report top line results from this trial in the third quarter of 2016. We plan to initiate a second Phase 3 trial of TNX-102 SL in FM in the second quarter of 2016. An End-of-Phase 2 Chemistry, Manufacturing and Controls meeting was held in February 2016 to discuss the quality data requirement for the TNX-102 SL NDA submission. As of the date of this filing, we have not received the FDA official minutes from the meeting.
| 44 |
We are evaluating TNX-102 SL for the treatment of military-related PTSD in the randomized, double-blind, placebo-controlled Phase 2 AtEase study, from which we expect to report top line results in the second quarter of 2016. The primary objective of the AtEase trial is to evaluate the efficacy of TNX-102 SL 2.8 mg as compared to placebo sublingual tablet following 12 weeks of treatment using the Clinician-Administered PTSD Scale. Based on our communications with the FDA to date, we believe that positive results from two adequate, well-controlled efficacy and safety studies and long-term (six- and 12-month) safety exposure studies would support FDA approval of TNX-102 SL for the management of PTSD. If we achieve our primary outcome measure in the AtEase study, it could qualify as one of the two studies required to support the NDA. We expect that we can use the data generated by our clinical development of TNX-102 SL in FM to supplement the long-term safety exposure data required for the PTSD NDA.
We were developing TNX-201 for the treatment of ETTH. We completed a 150-patient Phase 2 study in ETTH and announced results of the study on February 16, 2016. In that study patients were randomized at approximately 10 U.S. centers to receive TNX-201 140 mg (4 x 35 mg) or placebo capsules. The primary efficacy endpoint was the difference between the two study arms in the number of subjects who report complete relief from their headache pain at two hours following a dose of study medication. We reported top line results from this study on February 16, 2016. Since TNX-201 failed to show efficacy, we abandoned development of TNX-201.
We also have a pipeline of other product candidates, including TNX-301. TNX-301 is a fixed dose CDP, containing two FDA-approved drugs, disulfiram and selegiline. We intend to develop TNX-301 CDP under Section 505(b)(2) of the FDCA as a potential treatment for AUD, and we have commenced development work on TNX-301 formulations. We have initiated pre-IND consultation with the FDA to discuss the clinical development program of TNX-301 for AUD. A pre-IND meeting was held in February 2016. As of the date of this filing, we have not received the FDA official minutes from the meeting. In addition, we own rights to intellectual property on two biodefense development programs for protection from smallpox virus and from radiation injury. We have begun non-clinical research and development on these programs. The FDA Animal Efficacy Rule provides a mechanism for product licensure when human efficacy studies are not feasible or ethical. As a result, the licensure of these biodefense products in the U.S. may not require human efficacy studies, which we believe will reduce our development costs and risks compared to the development of other NCEs or new biologic candidates.
Current Operating Trends
Our current research and development efforts are focused on developing TNX-102 SL for FM and PTSD, but we also expend increasing effort on our other pipeline programs, including TNX-301. Our research and development expenses consist of manufacturing work and the cost of drug ingredients used in such work, fees paid to consultants for work related to clinical trial design and regulatory activities, fees paid to providers for conducting various clinical studies as well as for the analysis of the results of such studies, and for other medical research addressing the potential efficacy and safety of our drugs. We believe that significant investment in product development is a competitive necessity, and we plan to continue these investments in order to be in a position to realize the potential of our product candidates and proprietary technologies.
We are currently conducting a Phase 3 clinical trial of TNX-102 SL in FM and a Phase 2 clinical trial of TNX-102 SL in PTSD. In the second quarter of 2016, we plan to begin a second Phase 3 trial of TNX-102 SL in FM. Clinical trials can be very expensive. If these and additional necessary clinical trials are successful, we plan to prepare and submit applications to the FDA for marketing approval for our drug candidates. This process entails significant costs. As a result of these and other factors, we expect our research and development expenses to increase significantly over the next 12 to 24 months.
We expect that all of our research and development expenses in the near-term future will be incurred in support of our current and future preclinical and clinical development programs rather than technology development. These expenditures are subject to numerous uncertainties relating to timing and cost to completion. We test compounds in numerous preclinical studies for safety, toxicology and efficacy. At the appropriate time, subject to the approval of regulatory authorities, we expect to conduct early-stage clinical trials for each drug candidate. We anticipate funding these trials ourselves, and possibly with the assistance of federal grants. As we obtain results from trials, we may elect to discontinue or delay clinical trials for certain products in order to focus our resources on more promising products. Completion of clinical trials may take several years, and the length of time generally varies substantially according to the type, complexity, novelty and intended use of a product candidate.
The commencement and completion of clinical trials for our products may be delayed by many factors, including lack of efficacy during clinical trials, unforeseen safety issues, slower than expected patient recruitment, or government delays. In addition, we may encounter regulatory delays or rejections as a result of many factors, including results that do not support the intended safety or efficacy of our product candidates, perceived defects in the design of clinical trials and changes in regulatory policy during the period of product development. As a result of these risks and uncertainties, we are unable to accurately estimate the specific timing and costs of our clinical development programs or the timing of material cash inflows, if any, from our product candidates. Our business, financial condition and results of operations may be materially adversely affected by any delays in, or termination of, our clinical trials or a determination by the FDA that the results of our trials are inadequate to justify regulatory approval, insofar as cash in-flows from the relevant drug or program would be delayed or would not occur.
| 45 |
Results of Operations (in thousands except per share data)
We anticipate that our results of operations will fluctuate for the foreseeable future due to several factors, such as the progress of our research and development efforts and the timing and outcome of regulatory submissions. Due to these uncertainties, accurate predictions of future operations are difficult or impossible to make.
Fiscal year Ended December 31, 2015 Compared to Fiscal year Ended December 31, 2014
Revenues and Cost of Goods Sold. We had no revenues or cost of goods sold during the fiscal years ended December 31, 2015 and 2014.
Research and Development Expenses. Research and development expenses for the fiscal year ended December 31, 2015 were $35,504, an increase of $16,887, or 91%, from $18,617 for the fiscal year ended December 31, 2014. This increase is primarily due to increased development work related to TNX-102 SL and TNX-201, including formulation development, manufacturing, human safety and efficacy trials as well as pharmacokinetic studies. In 2015, we incurred $14,596, $4,963, $4,111 and $2,991 in clinical, non-clinical, manufacturing and medical research, respectively, as compared to $5,948, $1,501, $3,743 and $1,560 in 2014, respectively. Costs related to product development increased to $884 for the fiscal year ended December 31, 2015 from $727 for the fiscal year ended December 31, 2014, an increase of $157, or 22%. The increase is primarily due to additional trials conducted in 2015. During the year ended December 31, 2014, we acquired intellectual property rights for $858, as compared to $0 in the current period.
Compensation-related expenses increased to $4,085 for the fiscal year ended December 31, 2015, from $2,014 for the fiscal year ended December 31, 2014, an increase of $2,071, or 103%. We incurred $1,231 in stock-based compensation in connection with the vesting of stock options in 2015, which were previously issued to officers and consultants, as compared to $655 in stock-based compensation in 2014. The increase in cash compensation-related costs of $1,495 was primarily a result of annual salary increases and added personnel. Regulatory and legal costs increased to $1,763 for the fiscal year ended December 31, 2015, from $1,403 for the fiscal year ended December 31, 2014, an increase of $360, or 26%. The increase in regulatory and legal costs is primarily due to the increase in active trials.
Travel, meals and entertainment costs increased to $1,353 for the fiscal year ended December 31, 2015, from $580 for the fiscal year ended December 31, 2014, an increase of $773, or 133%. Travel, meals and entertainment costs include travel related to clinical development, including investigator meetings and medical-related conferences, which primarily accounted for the increase from 2014. Other research and development costs increased to $758 for the fiscal year ended December 31, 2015, from $283 for the fiscal year ended December 31, 2014, an increase of $475, or 168%. Other research and development costs include rent, insurance and other office-related expenses.
General and Administrative Expenses. General and administrative expenses for the fiscal year ended December 31, 2015 were $12,658, an increase of $3,619, or 40%, from $9,039 incurred in the fiscal year ended December 31, 2014. This increase is primarily due to compensation-related expenses and professional services.
Compensation-related expenses increased to $5,824 for the fiscal year ended December 31, 2015, from $4,511 for the fiscal year ended December 31, 2014, an increase of $1,313, or 29%. We incurred $3,158 in stock-based compensation in connection with the 2014 employee stock purchase plan and the vesting of restricted stock units and stock options in 2015, which were previously issued to board members, officers and a consultant, as compared to $2,434 in stock-based compensation in 2014. The increase in cash compensation-related costs of $589 was primarily a result of annual salary increases and added personnel.
Professional services for the fiscal year ended December 31, 2015 totaled $4,247, an increase of $1,683, or 66%, over the $2,564 incurred for the fiscal year ended December 31, 2014. Of professional services, legal fees totaled $1,756 for the fiscal year ended December 31, 2015, an increase of $753, or 75%, from $1,003 incurred for the fiscal year ended December 31, 2014. Of the legal fees incurred, $1,173 were patent-related costs in 2015, as compared to $554 in 2014. Audit and accounting fees incurred in the fiscal years ended December 31, 2015 and 2014 amounted to $513 and $515, respectively, a decrease of $2, or 0%. Investor and public relations fees totaled $1,333 for the fiscal year ended December 31, 2015, an increase of $459, or 53%, from $874 incurred in the fiscal year ended December 31, 2014. The increase is due to additional non-deal roadshows and attending investor-related conferences. Other consulting fees and other professional fees totaled $645 for the fiscal year ended December 31, 2015, an increase of $473, or 275%, from $172 for the fiscal year ended December 31, 2014. Other professional fees include human resources, finance and corporate consultants.
Travel, meals and entertainment costs for the fiscal year ended December 31, 2015 were $872, an increase of $471, or 117%, from $401 incurred in the fiscal year ended December 31, 2014. Travel, meals and entertainment costs include travel related to business development and investor relations activities, which accounted for the primary increase from 2014. Office and other administrative expenses for the fiscal year ended December 31, 2015 totaled $1,715, an increase of $191, or 13%, as compared to of $1,524 for the year ended December 31, 2014. Office and other administrative expenses include rent, depreciation, insurance, business taxes, dues and subscriptions and other office related expenses.
| 46 |
Net Loss. As a result of the foregoing, the net loss for the year ended December 31, 2015 was $48,054, compared to a net loss of $27,616 for the year ended December 31, 2014.
Fiscal year Ended December 31, 2014 Compared to Fiscal year Ended December 31, 2013
Revenues and Cost of Goods Sold. We had no revenues or cost of goods sold during the fiscal years ended December 31, 2014 and 2013.
Research and Development Expenses. Research and development expenses for the fiscal year ended December 31, 2014 were $18,617, an increase of $13,967, or 300%, from $4,650 for the fiscal year ended December 31, 2013. This increase is primarily due to increased development work related to TNX-102 SL, including formulation development, manufacturing, human safety and efficacy trials as well as pharmacokinetic studies. In 2014, we incurred $3,743, $5,948 and $1,501 in manufacturing cost, clinical activities and cost, and non-clinical activities and cost, respectively, as compared to $1,161, $1,733 and $432 in 2013, respectively. During the year ended December 31, 2014, we acquired intellectual property rights for $858 as compared to $0 in the year ended December 31, 2013. In addition, beginning in 2014, we began classifying certain salaries, bonuses, and stock-based compensation to research and development expenses based on individuals’ responsibilities. Included in the year ended December 31, 2014 was $655 related to stock-based compensation in connection with the vesting of stock options and cash compensation of $1,310, primarily as a result of added personnel.
General and Administrative Expenses. General and administrative expenses for the fiscal year ended December 31, 2014 were $9,039, an increase of $2,801, or 45%, from $6,238 incurred in the fiscal year ended December 31, 2013. This increase is primarily due to compensation related expenses and professional services.
Compensation related expenses increased to $4,511 for the fiscal year ended December 31, 2014 from $3,248 for the fiscal year ended December 31, 2013, an increase of $1,263, or 39%. We incurred $2,434 in stock-based compensation in connection with the vesting of stock options in 2014 previously issued to board members, officers and a consultant as compared to $1,717 in stock-based compensation in 2013. The increase in cash compensation related costs of $546 was primarily a result of annual salary increases and added personnel, net with classification of wages and benefits related to research and development from general and administrative expenses.
Professional services for the fiscal year ended December 31, 2014 totaled $2,564, an increase of $682, or 36%, over the $1,882 incurred for the fiscal year ended December 31, 2013. Of professional services, legal fees totaled $1,003 for the fiscal year ended December 31, 2014, an increase of $100, or 11%, from $903 incurred for the fiscal year ended December 31, 2013. Of the legal fees incurred, $554 were patent related costs in the 2014 year as compared to $458 in 2013. Audit and accounting fees incurred in the fiscal years ended December 31, 2014 and 2013 amounted to $515 and $244, respectively, an increase of $271, or 111%. The increase is due to additional work required in 2014 related to Sarbanes Oxley as well additional audit and accounting fees related to our additional subsidiaries. Investor and public relations fees totaled $874 for the fiscal year ended December 31, 2014, an increase of $219 or 33%, from $655 incurred in fiscal year ended December 31, 2013. The increase is due to expenses incurred during an analyst day as well as costs incurred related to brand awareness and drug name development. Other consulting fees and other professional fees totaled $172 for the fiscal year ended December 31, 2014, an increase of $92, or 115%, from $80 for the fiscal year ended December 31, 2013. Other professional fees include human resources, finance and corporate consultants.
Travel, meals and entertainment costs for the fiscal year ended December 31, 2014 were $401, an increase of $87, or 28%, from $314 incurred in the fiscal year ended December 31, 2013. Travel, meals and entertainment costs include travel related to investor relations activities, which accounted for the primary increase from 2013. Rent for the fiscal years ended December 31, 2014 and 2013 totaled $246 and $124, respectively. In 2014, we increased the size of our corporate headquarters in New York and opened a satellite office in California. Market- related materials and analysis for the fiscal year ended December 31, 2014 was $210, an increase of $162, or 338%, from $48 incurred in the fiscal year ended December 31, 2013.The increase is mainly due to updated company materials presented at investor relations events. Depreciation expense in fiscal 2014 totaled $36, an increase of $19, or 112%, over the expense of $17 incurred in fiscal 2013, as a result of the purchase of new office computers.
Net Loss. As a result of the foregoing, the net loss for the year ended December 31, 2014 was $27,616, compared to a net loss of $10,884 for the year ended December 31, 2013.
| 47 |
Liquidity and Capital Resources
As of December 31, 2015, we had working capital of $39.7 million, comprised primarily of cash and cash equivalents of $19.2 million, short-term investments of $23.8 million and prepaid expenses and other of $3.3 million, offset by $3.0 million of accounts payable and $3.6 million of accrued expenses. A significant portion of the accounts payable and accrued expenses are due to work performed in relation to our ongoing clinical trials of TNX-102 SL in FM and PTSD. For the years ended December 31, 2015 and 2014, we used approximately $42.5 million and $22.8 million of cash in operating activities, respectively, which represents cash outlays for research and development and general and administrative expenses in such periods. Increases in cash outlays principally resulted from clinical, non-clinical, manufacturing, medical research, regulatory cost, and payroll. For the year ended December 31, 2015, net proceeds from financing activities were $47.7 million, predominately from the sale of our common stock. In the comparable 2014 period, approximately $47.8 million was raised through the sale of shares of common stock and $5.7 million from the exercise of warrants, net offset by the repayments of related party promissory notes of $0.3 million. At December 31, 2014, we had cash of $38.2 million. Our cash and cash equivalents consisted of bank deposit accounts and money market funds.
Cash used in investing activities for the year ended December 31, 2015 was approximately $24.2 million, of which $28.6 million related to the purchase of marketable securities, $0.1 million related to the purchase of equipment and leasehold improvements and $0.1 million related to the purchase of an intangible asset, offset by maturities of marketable securities of $4.7 million, as compared to cash used for the year ended December 31, 2014 of approximately $0.3 million, reflecting the purchase of equipment.
August 2013 financing
On August 9, 2013, we entered into an underwriting agreement (the “Underwriting Agreement”) with Roth Capital Partners, LLC (“Roth”), as representative of the underwriters named therein (the “First Underwriters”), pursuant to which we agreed to offer to the public through the First Underwriters an aggregate of 2,680,000 units (each a “Unit”, and collectively, the “Units”) at a public offering price of $4.25 per Unit in an underwritten public offering (the “August 2013 Financing”). Each Unit consisted of (i) one share of common stock and (ii) one Series A Warrant (the “Warrants”) to purchase one share of common stock. The Warrants are exercisable at an exercise price of $4.25 per share, subject to anti-dilutive adjustment, and expire on the fifth anniversary of the date of issuance. The Warrants will be exercisable on a “cashless” basis in certain circumstances. Pursuant to the Underwriting Agreement, the Company also granted the First Underwriters an option for a period of 45 days to purchase up to (i) 402,000 additional Units or (ii) 402,000 additional shares of common stock and/or additional Warrants to purchase up to 402,000 shares of common stock, on the same terms, to cover over-allotments, if any.
The August 2013 Financing closed on August 14, 2013. The First Underwriters purchased the Units at an eight percent discount to the public offering price, for an aggregate discount of approximately $0.9 million (or $0.34 per unit). We received net cash proceeds of $10.0 million after deducting underwriting discounts and commissions and offering expenses of $0.4 million. On August 14, 2013, the First Underwriters exercised their over-allotment option by purchasing for $4,000 additional Warrants to purchase 402,000 shares of common stock.
The First Underwriters received warrants to purchase up to an aggregate of 107,200 shares of common stock, or four percent of the total number of shares included in the Units, which warrants have an exercise price of $4.25.
January 2014 financing
On January 24, 2014, we entered into an underwriting agreement with Roth, as representative of several underwriters (collectively, the “Second Underwriters”), relating to the issuance and sale of 2,898,550 shares of our common stock in an underwritten public offering (the “January 2014 Financing”). The public offering price for each share of common stock was $15.00. We granted the Second Underwriters a 45-day option to purchase up to an additional 434,782 shares of common stock to cover over-allotments, if any.
The January 2014 Financing closed on January 29, 2014. The Second Underwriters purchased the shares at a six percent discount to the public offering price, for an aggregate discount of approximately $2.6 million (or $0.90 per share). We also paid offering expenses of approximately $0.2 million. We received net proceeds of approximately $40.7 million. The over-allotment option expired unexercised.
July 2014 financing
On July 11, 2014, we entered into subscription agreements with investors, relating to the issuance and sale of 657,000 shares of our common stock in a registered direct offering. The purchase price for each share of common stock was $11.90.
| 48 |
Roth acted as the exclusive placement agent in this offering pursuant to the terms of a placement agent agreement, dated July 11, 2014, between us and Roth. Pursuant to the placement agent agreement, we agreed to pay Roth a placement agent fee equal to six percent of the gross proceeds of the offering.
The registered direct offering closed on July 16, 2014 and we received net proceeds of approximately $7.2 million, after deducting placement agent fees and offering expenses of approximately $0.6 million.
February 2015 financing
On February 4, 2015, we entered into an underwriting agreement with Roth and Oppenheimer & Co Inc. (collectively, the “Representatives”), as representatives of several underwriters (collectively, the “Third Underwriters”), relating to the issuance and sale of 4,900,000 shares of our common stock, in an underwritten public offering (the “February 2015 Financing”). The public offering price for each share of common stock was $5.85. We granted the Third Underwriters a 45-day option to purchase up to an additional 735,000 shares of common stock to cover over-allotments, if any.
The February 2015 Financing closed on February 9, 2015. The Third Underwriters purchased the shares at a six percent discount to the public offering price, for an aggregate discount of $1.7 million (or $0.35 per share). We also paid offering expenses of approximately $0.3 million. We received net proceeds of approximately $26.7 million. On February 24, 2015, the Third Underwriters partially exercised the over-allotment option and purchased 418,700 shares of common stock for net proceeds of approximately $2.3 million, net of an aggregate discount of $0.1 million (or $0.35 per share).
July 2015 financing
On July 14, 2015, we entered into an underwriting agreement with the Representatives of the Third Underwriters, relating to the issuance and sale of 2,325,000 shares of our common stock, in an underwritten public offering (the “July 2015 Financing”). The public offering price for each share of common stock was $7.50. We granted the Third Underwriters a 45-day option to purchase up to an additional 348,750 shares of common stock to cover over-allotments, if any.
The July 2015 Financing closed on July 17, 2015. The Third Underwriters purchased the shares at a six percent discount to the public offering price, for an aggregate discount of $1.0 million (or $0.45 per share). We also paid offering expenses of approximately $0.2 million. We received net proceeds of approximately $16.2 million. On July 17, 2015, the Third Underwriters fully exercised the over-allotment option and purchased 348,750 shares of common stock for net proceeds of approximately $2.5 million, net of an aggregate discount of $0.2 million (or $0.45 per share).
Future liquidity requirements
We expect to incur losses and net cash outflows from operations for the near future. We expect to incur increasing research and development expenses, including expenses related to additional clinical trials. We expect that our general and administrative expenses will increase in the future as we expand our business development, add infrastructure and incur additional costs related to being a public company, including incremental audit fees, investor relations programs and increased professional services.
Our future capital requirements will depend on a number of factors, including the progress of our research and development of product candidates, the timing and outcome of regulatory approvals, the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims and other intellectual property rights, the status of competitive products, the availability of financing and our success in developing markets for our product candidates. We believe our existing cash is sufficient to fund our operating expenses and planned clinical trials for at least the next 12 months.
We presently do not have any available credit, bank financing or other external sources of liquidity. Due to our history and historical operating losses, our operations have not been a source of liquidity. We may need to obtain additional capital in order to fund future research and development activities. Future financing may include the issuance of equity or debt securities, obtaining credit facilities, or other financing mechanisms. Even if we are able to raise the funds required, it is possible that we could incur unexpected costs and expenses, fail to collect significant amounts owed to us, or experience unexpected cash requirements that would force us to seek alternative financing. Furthermore, if we issue additional equity or debt securities, shareholders may experience additional dilution or the new equity securities may have rights, preferences or privileges senior to those of existing holders of our common stock.
If additional financing is not available or is not available on acceptable terms, we may be required to delay, reduce the scope of or eliminate our research and development programs, reduce our commercialization efforts or obtain funds through arrangements with collaborative partners or others that may require us to relinquish rights to certain product candidates that we might otherwise seek to develop or commercialize independently.
| 49 |
Contractual Obligations
The following table sets forth our contractual obligations as of December 31, 2015 (in thousands):
| Payment Due By Period | ||||||||||||||||||||
| Less than 1 Year | 1 to 3 Years | 3 to 5 Years | More than 5 Years | Total | ||||||||||||||||
| Research and Development Obligations | $ | 19,687 | $ | 380 | $ | - | $ | - | $ | 21,067 | ||||||||||
| Operating Lease Obligations | 665 | 1,290 | 181 | - | 2,136 | |||||||||||||||
| Total | $ | 20,352 | $ | 1,670 | $ | 181 | $ | - | $ | 23,203 | ||||||||||
We are a party to research and development agreements in the normal course of business with CROs for clinical trials and clinical manufacturing, with vendors for preclinical research studies and for other services and products for operating purposes. We have included as purchase obligations our commitments under agreements to the extent they are quantifiable and are not cancelable.
Transactions with Related Parties
We have entered into an agreement with Lederman & Co., LLC (“Lederman & Co”), a company under the control of Dr. Seth Lederman, our Chief Executive Officer and Chairman of the Board of Directors. Effective October 15, 2013, Lederman & Co received $0.3 million per annum for its consulting services. On February 11, 2014, the agreement with Lederman & Co was terminated, and we simultaneously entered into an employment agreement with Dr. Lederman.
On July 31 and August 1, 2013, we sold three promissory notes in the aggregate principal face amount of $0.3 million to two related parties in exchange for $0.3 million. The notes were payable on demand at any time after one year from issuance and bore no interest. On July 31, 2014 and August 1, 2014, we repaid $0.2 million and $0.1 million, respectively.
On March 18, 2014, Tonix Barbados entered into an asset purchase agreement (the “Starling Agreement”) with Starling Pharmaceuticals, Inc. (“Starling”) and an asset purchase agreement (the “Leder Agreement”) with Leder Laboratories, Inc. (“Leder”). Seth Lederman, the Company’s Chairman and Chief Executive Officer, is the Chairman, CEO and majority owner (through majority-owned entities) of Starling and Leder.
Pursuant to the Starling Agreement, Tonix Barbados acquired from Starling rights to a United States patent application for radio- and chemo-protective agents and related intellectual property rights, in exchange for $0.1 million and 25,000 shares of our common stock.
Pursuant to the Leder Agreement, Tonix Barbados acquired from Leder rights to a United States patent application for novel smallpox vaccines and related intellectual property rights, in exchange for $0.1 million and 25,000 shares of our common stock.
Stock Compensation
Stock Options
In February 2012, we approved the 2012 Incentive Stock Options Plan, which was amended and restated in February 2013 (“2012 Plan”). The 2012 Plan provides for the issuance of options to purchase up to 550,000 shares of our common stock to officers, directors, employees and consultants. Under the terms of the 2012 Plan, we may issue Incentive Stock Options, as defined by the Internal Revenue Code, and nonstatutory options. The Board of Directors determines the exercise price, vesting and expiration period of the options granted under the 2012 Plan. However, the exercise price of an Incentive Stock Option must be at least 100% of fair value of the common stock at the date of the grant (or 110% for any shareholder that owns 10% or more of our common stock). The fair market value of the common stock determined based on quoted market price or in absence of such quoted market price, by the Board of Directors in a good faith. Additionally, the vesting period of the grants under the 2012 Plan should not be more than five years and expiration period not more than ten years. We reserved 550,000 shares of our common stock for future issuance under the terms of the 2012 Plan.
We measure the fair value of stock options on the date of grant, based on a Binomial option pricing model using certain assumptions discussed in the following paragraph, and the closing market price of our common stock on the date of the grant. For employees and directors, the fair value of the award is measured on the grant date and for non-employees, the fair value of the award is generally re-measured on vesting dates and interim financial reporting dates until the service period is complete. Stock options granted pursuant to the Plans typically vest 1/3rd 12 months from the date of grant and 1/36th each month thereafter for 24 months and expire ten years from the date of grant. Share-based compensation expense related to awards is amortized over the applicable vesting period using the straight-line method.
| 50 |
In May 2012, we issued options to purchase 175,000 shares of common stock pursuant to the 2012 Plan having an exercise price of $30.00. In February 2013, we issued options to purchase 226,500 shares of common stock pursuant to the 2012 Plan having an exercise price of $10.20. In February 2014, we issued options to purchase 173,500 shares of common stock pursuant to the 2012 Plan having an exercise price of $15.88.
On June 9, 2014, we approved the Tonix Pharmaceuticals Holding Corp. 2014 Stock Incentive Plan (the “2014 Plan” and together with the 2012 Plan, the “Plans”). Under the terms of the 2014 Plan, the Company may issue (1) stock options (incentive and nonstatutory), (2) restricted stock, (3) stock appreciation rights, or SARs, (4) restricted stock units, or RSUs, (5) other stock-based awards, and (6) cash-based awards. The 2014 Plan provides for the issuance of up to 1,800,000 shares of common stock, provided, however, that, of the aggregate number of 2014 Plan shares authorized, no more than 200,000 of such shares may be issued pursuant to stock-settled awards other than options (that is, restricted stock, RSUs, SARs, performance awards, other stock-based awards and dividend equivalent awards, in each case to the extent settled in shares of common stock). The Board of Directors determines the exercise price, vesting and expiration period of the grants under the 2014 Plan. However, the exercise price of an incentive stock option may not be less than 110% of fair value of the common stock at the date of the grant for a 10% or more shareholder and 100% of fair value for a grantee who is not a 10% shareholder. The fair value of the common stock is determined based on quoted market price or in absence of such quoted market price, by the Board of Directors in good faith. Additionally, the vesting period of the grants under the 2014 Plan may not be more than five years and expiration period not more than ten years. The Company reserved 1,800,000 shares of its common stock for future issuance under the terms of the 2014 Plan.
On June 17, 2014, 295,100 and 60,000 options were granted to employees/directors and consultants, respectively, under the 2014 Plan (of which 255,300 and 60,000 were outstanding at December 31, 2015), with an exercise price of $9.87, a 10 year life and fair value of $8.76. As of December 31, 2015, the fair value related to consultant grants was $5.78.
On October 29, 2014, 321,700 options were granted to employees and directors under the 2014 Plan (of which 281,900 were outstanding at December 31, 2015), with an exercise price of $6.68, a 10 year life and fair value of $5.80.
On February 25, 2015, 419,500 and 30,000 options were granted to employees/directors and consultants, respectively, under the 2014 Plan (of which 415,700 employee/director options and 30,000 consultant options were outstanding at December 31, 2015), with an exercise price of $5.95, a 10 year life and fair value of $4.69. Additionally, we granted options to purchase 7,143 shares of our common stock to Seth Lederman as a non-cash bonus, with an exercise price of $5.95, a 10 year life and fair value of $4.43. As of December 31, 2015, the fair value related to consultant grants was $6.34.
On April 14, 2015, 7,600 options were granted to employees under the 2014 Plan (all of which were outstanding at December 31, 2015), with an exercise price of $6.34, a 10 year life and fair value of $4.56.
On July 27, 2015, 4,000 options were granted to an employee under the 2014 Plan (all of which were outstanding at December 31, 2015), with an exercise price of $8.25, a 10 year life and fair value of $5.71.
On October 21, 2015, 38,000 options were granted to employees under the 2014 Plan (all of which were outstanding at December 31, 2015), with an exercise price of $6.33, a 10 year life and fair value of $4.57.
On November 30, 2015, 7,000 options were granted to an employee under the 2014 Plan (all of which were outstanding at December 31, 2015), with an exercise price of $6.77, a 10 year life and fair value of $5.11.
On February 9, 2016, 411,125 options were granted to employees with an exercise price of $5.03. Additionally, 200,000 options were granted to employees with an exercise price of $5.03, exercisable for a period of ten years, vesting 1/3 each upon the Corporation’s common stock having an average closing sale price equal to or exceeding each of $6.00, $7.00 and $8.00 per share for 20 consecutive trading days, subject to a one year minimum service period prior to vesting.
Employee Stock Purchase Plan
On June 9, 2014, we approved the Tonix Pharmaceuticals Holdings Corp. 2014 Employee Stock Purchase Plan (the “2014 ESPP”). The 2014 ESPP allows eligible employees to purchase up to an aggregate of 300,000 shares of our common stock. Under the 2014 ESPP, on the first day of each offering period, each eligible employee for that offering period has the option to enroll for that offering period, which allows the eligible employees to purchase shares of our common stock at the end of the offering period. Each offering period under the 2014 ESPP is for six months, which can be modified from time-to-time. Subject to limitations, each participant will be permitted to purchase a number of shares determined by dividing the employee’s accumulated payroll deductions for the offering period by the applicable purchase price, which is equal to 85 percent of the fair market value of our common stock at the beginning or end of each offering period, whichever is less. A participant must designate in his or her enrollment package the percentage (if any) of compensation to be deducted during that offering period for the purchase of stock under the 2014 ESPP, subject to the statutory limit under the Code.
| 51 |
The 2014 ESPP is considered a compensatory plan with the related compensation cost written off over the six month offering period. In February 2015, 13,978 shares that were purchased as of December 31, 2014, were issued under the 2014 ESPP, and approximately $70,000 of employee payroll deductions accumulated at December 31, 2014, related to acquiring such shares, were transferred from accrued expenses to additional paid in capital. In July 2015, 18,021 shares that were purchased as of June 30, 2015, were issued under the 2014 ESPP, and approximately $0.1 million of employee payroll deductions accumulated at June 30, 2015, related to acquiring such shares, was transferred from accrued expenses to additional paid in capital. The compensation expense related to the 2014 ESPP for the year ended December 31, 2015 was $0.1 million. As of December 31, 2015, approximately $0.1 million of employee payroll deductions, which had been withheld since July 1, 2015, the commencement of the offering period ended December 31, 2015, are included in accrued expenses in the accompanying balance sheet. In January 2016, 17,595 shares that were purchased as of December 31, 2015 were issued under the 2014 ESPP, and the employee payroll deductions accumulated at December 31, 2015, related to acquiring such shares, were transferred from accrued expenses to additional paid in capital. As of December 31, 2015, after giving effect to shares purchased as described above, there were 250,406 shares available for future purchase under the 2014 ESPP.
On February 25, 2015, we granted an aggregate of 42,000 RSUs with a fair value of $6.24 per unit to our non-employee directors for board services in 2015, in lieu of cash, which vest one year from the grant date.
Stock-based compensation expense related to RSUs of $0.2 million was recognized during the year ended December 31, 2015. As of December 31, 2015, 42,000 unvested RSUs were outstanding and stock-based compensation relating to such RSUs of $43,680 remains unamortized and is expected to be amortized over the remaining period of approximately two months. As of February 25, 2016, the RSUs for the non-employee directors vested and the non-employee directors were entitled to receive their stock awards and the remaining related compensation has been recognized.
On February 9, 2016, we granted an aggregate of 56,250 RSUs with a fair value of $3.81 per unit to our non-employee directors for board services in 2016, in lieu of cash, which vest one year from the grant date.
Lease Commitments
On February 11, 2014, we entered into a lease amendment and expansion agreement, whereby we agreed to lease additional premises for office space, commencing May 1, 2014 and expiring on April 30, 2019. In connection therewith, the original letter of credit was increased by $72,354 to $132,417 and we deposited an additional $72,354 into the restricted cash account maintained at the bank that issued the letter of credit.
On April 28, 2014, we entered into a lease for approximately 3,578 square feet of office space in San Jose, California, whereby we agreed to lease premises, commencing August 1, 2014 and expiring on October 31, 2018. In connection therewith, we paid a security deposit of $44,546.
On June 19, 2015, we entered into a lease for approximately 2,450 square feet of office space in Dublin, Ireland, whereby we agreed to lease premises, commencing June 1, 2015 and expiring on May 31, 2018.
On July 27, 2015, we entered into a lease for approximately 132 square feet of office space in Montreal, Canada, whereby we agreed to lease premises, commencing August 1, 2015 and expiring on July 31, 2016. In connection therewith, we paid a security deposit of $800.
On August 24, 2015, we entered into a lease for approximately 2,762 square feet of office space in San Diego, California, whereby we agreed to lease premises, commencing September 1, 2015 and expiring on August 31, 2019. In connection therewith, we paid a security deposit of $11,272.
Future minimum lease payments are as follows (in thousands):
| Year Ending December 31, | ||||
| 2016 | $ | 670 | ||
| 2017 | 683 | |||
| 2018 | 607 | |||
| 2019 | 181 | |||
| $ | 2,141 | |||
| 52 |
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. We evaluate our estimates and judgments on an ongoing basis. We base our estimates on historical experience and on assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates.
We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our consolidated financial statements.
Research and Development. We outsource our research and development efforts and expenses related costs as incurred, including the cost of manufacturing product for testing, licensing fees and costs associated with planning and conducting clinical trials. The value ascribed to patents and other intellectual property acquired was expensed as research and development costs, as it related to particular research and development projects and had no alternative future uses.
We estimate our accrued expenses. Our clinical trial accrual process is designed to account for expenses resulting from our obligations under contracts with vendors, consultants and clinical research organizations and clinical site agreements in connection with conducting clinical trials. The financial terms of these contracts are subject to negotiations, which vary from contract to contract and may result in payment flows that do not match the periods over which materials or services are provided to us under such contracts. We account for trial expenses according to the progress of the trial as measured by patient progression and the timing of various aspects of the trial. We determine accrual estimates that take into account discussions with applicable personnel and outside service providers as to the progress or state of completion of trials, or the services completed. During the course of a clinical trial, we adjust our clinical expense recognition if actual results differ from our estimates. We make estimates of our accrued expenses as of each balance sheet date based on the facts and circumstances known to us at that time. Our clinical trial accruals and prepaid assets are dependent upon the timely and accurate reporting of contract research organizations and other third-party vendors.
Stock-Based Compensation. All stock-based payments to employees and to nonemployee directors for their services as directors consisted of grants of restricted stock and stock options, which are measured at fair value on the grant date and recognized in the consolidated statements of operations as compensation expense over the relevant vesting period. Restricted stock payments to nonemployees are recognized as an expense over the period of performance. Such payments are measured at fair value at the earlier of the date a performance commitment is reached or the date performance is completed. In addition, for awards that vest immediately and are nonforfeitable, the measurement date is the date the award is issued.
Income Taxes. Deferred income tax assets and liabilities are determined based on the estimated future tax effects of net operating loss and credit carryforwards and temporary differences between the tax basis of assets and liabilities and their respective financial reporting amounts measured at the current enacted tax rates. We record an estimated valuation allowance on its deferred income tax assets if it is not more likely than not that these deferred income tax assets will be realized. We recognizes a tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by taxing authorities, based on the technical merits of the position. The tax benefits recognized in the consolidated financial statements from such a position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement.
Recent Accounting Pronouncements
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-02, Leases (Topic 842). Under the new guidance, lessees will be required to recognize the following for all leases (with the exception of short-term leases) at the commencement date: a lease liability, which is a lessee‘s obligation to make lease payments arising from a lease, measured on a discounted basis; and a right-of-use asset, which is an asset that represents the lessee’s right to use, or control the use of, a specified asset for the lease term. Public business entities should apply the amendments in ASU 2016-02 for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. Early application is permitted. Lessees (for capital and operating leases) must apply a modified retrospective transition approach for leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements. The modified retrospective approach would not require any transition accounting for leases that expired before the earliest comparative period presented. Lessees may not apply a full retrospective transition approach. We are currently evaluating the impact of adopting this guidance.
| 53 |
ITEM 7A – QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Risk
Our cash and cash equivalents primarily consist of securities issued by the U.S. government, deposits, and money market deposits managed by commercial banks. The goals of our investment policy are preservation of capital, fulfillment of liquidity needs and fiduciary control of cash and investments. We also seek to maximize income from our investments without assuming significant risk. As of December 31, 2015, we had cash and cash equivalents and marketable securities of $43.0 million consisting of cash and highly liquid investments deposited in highly rated financial institutions in the United States.
Our portfolio of marketable securities includes certificates of deposit, corporate notes and U.S. Treasury and government agency bonds classified as available-for-sale securities, with no security having a maturity in excess of two years. Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of interest rates. Due to the short-term duration of our investment portfolio and the relatively low risk profile of our investments, we believe that our exposure to interest rate risk is not significant and a 1% movement in market interest rates would not have a significant impact on the total value of our investment portfolio.
Foreign Currency Risk
We do not hold more than a de minimus amount of foreign currency denominated financial instruments.
Effects of Inflation
Inflation generally affects us by increasing our cost of labor and clinical trial costs. We do not believe that inflation and changing prices during the years ended December 31, 2015, 2014 and 2013 had a significant impact on our results of operations.
| 54 |
ITEM 8 - FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
TONIX PHARMACEUTICALS HOLDING CORP.
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Tonix Pharmaceuticals Holding Corp.
We have audited the accompanying consolidated balance sheets of Tonix Pharmaceuticals Holding Corp. (the "Company") as of December 31, 2015 and 2014, and the related consolidated statements of operations, comprehensive loss, stockholders' equity, and cash flows for the each of the three years in the period ended December 31, 2015. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Tonix Pharmaceuticals Holding Corp. as of December 31, 2015 and 2014, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2015, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Tonix Pharmaceuticals Holding Corp.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 3, 2016 expressed an unqualified opinion thereon.
| /s/ EisnerAmper LLP | |
| New York, New York | |
| March 3, 2016 |
| F-2 |
TONIX PHARMACEUTICALS HOLDING CORP.
CONSOLIDATED BALANCE SHEETS
DECEMBER 31, 2015 AND 2014
(Dollars In Thousands)
| 2015 | 2014 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 19,175 | $ | 38,184 | ||||
| Marketable securities – available for sale, at fair value | 23,841 | - | ||||||
| Prepaid expenses and other | 3,343 | 852 | ||||||
| Total current assets | 46,359 | 39,036 | ||||||
| Property and equipment, net | 350 | 328 | ||||||
| Restricted cash | 132 | 133 | ||||||
| Intangible asset | 120 | |||||||
| Security deposits | 57 | 45 | ||||||
| Total assets | $ | 47,018 | $ | 39,542 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 3,049 | $ | 1,487 | ||||
| Accrued expenses | 3,601 | 1,895 | ||||||
| Total current liabilities | 6,650 | 3,382 | ||||||
| Deferred rent payable | 106 | 68 | ||||||
| Total liabilities | 6,756 | 3,450 | ||||||
| Commitments (Note 10) | - | - | ||||||
| Stockholders' equity: | ||||||||
| Preferred stock, $0.001 par value; 5,000,000 shares authorized, none issued or outstanding | - | - | ||||||
| Common stock, $0.001 par value; 150,000,000 shares authorized; 18,831,669 and 10,805,220 shares issued and outstanding as of December 31, 2015 and 2014, respectively and 17,595 and 13,978 shares to be issued as of December 31, 2015 and 2014, respectively | 19 | 11 | ||||||
| Additional paid in capital | 142,658 | 90,423 | ||||||
| Accumulated deficit | (102,398 | ) | (54,344 | ) | ||||
| Accumulated other comprehensive (loss) income | (17 | ) | 2 | |||||
| Total stockholders' equity | 40,262 | 36,092 | ||||||
| Total liabilities and stockholders' equity | $ | 47,018 | $ | 39,542 | ||||
See the accompanying notes to the consolidated financial statements
| F-3 |
TONIX PHARMACEUTICALS HOLDING CORP.
CONSOLIDATED STATEMENTS OF OPERATIONS
(Dollars In Thousands Except Per Share Amounts)
| Year ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| COSTS AND EXPENSES: | ||||||||||||
| Research and development | $ | 35,504 | $ | 18,617 | $ | 4,650 | ||||||
| General and administrative | 12,658 | 9,039 | 6,238 | |||||||||
| 48,162 | 27,656 | 10,888 | ||||||||||
| Operating Loss | (48,162 | ) | (27,656 | ) | (10,888 | ) | ||||||
| Interest income, net | 108 | 40 | 4 | |||||||||
| NET LOSS | $ | (48,054 | ) | $ | (27,616 | ) | $ | (10,884 | ) | |||
| Net loss per common share, basic and diluted | $ | (2.86 | ) | $ | (2.77 | ) | $ | (3.37 | ) | |||
| Weighted average common shares outstanding, basic and diluted | 16,791,059 | 9,985,515 | 3,231,311 | |||||||||
See the accompanying notes to the consolidated financial statements
| F-4 |
TONIX PHARMACEUTICALS HOLDING CORP.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(Dollars In Thousands)
| Year ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Net loss | $ | (48,054 | ) | $ | (27,616 | ) | $ | (10,884 | ) | |||
| Other comprehensive loss: | ||||||||||||
| Foreign currency translation gain (loss) | 8 | 3 | (1 | ) | ||||||||
| Unrealized loss on available for sale securities | (27 | ) | - | - | ||||||||
| Total other comprehensive (loss) gain | (19 | ) | 3 | (1 | ) | |||||||
| Comprehensive loss | $ | (48,073 | ) | $ | (27,613 | ) | $ | (10,885 | ) | |||
See the accompanying notes to the consolidated financial statements
| F-5 |
TONIX PHARMACEUTICALS HOLDING CORP.
CONSOLIDATED STATEMENT OF STOCKHOLDERS' EQUITY
(Dollars In Thousands Except Per Share Amounts)
| Accumulated | ||||||||||||||||||||||||||||||||
| Additional | Other | |||||||||||||||||||||||||||||||
| Preferred stock | Common stock | Paid in | Comprehensive | Accumulated | ||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Gain (loss) | Deficit | Total | |||||||||||||||||||||||||
| Balance at December 31, 2012 | - | $ | - | 2,159,159 | $ | 2 | $ | 16,801 | $ | - | $ | (15,844 | ) | $ | 959 | |||||||||||||||||
| Stock-based compensation | - | - | - | - | 1,717 | - | - | 1,717 | ||||||||||||||||||||||||
| Issuance of common stock in exchange for exercise of warrants in April 2013 ($8.00 per share) | - | - | 38,334 | - | 307 | - | - | 307 | ||||||||||||||||||||||||
| Issuance of common stock and warrants in August 2013 ($4.25 per share) net of transaction expenses of $1,352 | - | - | 2,680,000 | 3 | 10,039 | - | - | 10,042 | ||||||||||||||||||||||||
| Issuance of common stock in exchange for exercise of warrants in December 2013 ($4.25 per share) | - | - | 884,885 | 1 | 3,760 | - | - | 3,761 | ||||||||||||||||||||||||
| Issuance of common stock in exchange for exercise of warrants in December 2013 ($8.00 per share) | - | - | 70,031 | - | 560 | - | - | 560 | ||||||||||||||||||||||||
| Issuance of common stock in exchange for 3,185 warrants exercised on a cashless basis | - | - | 1,672 | - | - | - | - | - | ||||||||||||||||||||||||
| Warrants issued for services rendered | - | - | - | - | 51 | - | - | 51 | ||||||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | (1 | ) | - | (1 | ) | ||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | (10,884 | ) | (10,884 | ) | ||||||||||||||||||||||
| Balance at December 31, 2013 | - | - | 5,834,081 | 6 | 33,235 | (1 | ) | (26,728 | ) | 6,512 | ||||||||||||||||||||||
| Issuance of common stock in exchange for exercise of warrants ($4.25 per share) | - | - | 1,331,911 | 1 | 5,660 | - | - | 5,661 | ||||||||||||||||||||||||
| Issuance of common stock in January 2014 ($15.00 per share) net of transaction expenses of $2,824 | - | - | 2,898,550 | 3 | 40,651 | - | - | 40,654 | ||||||||||||||||||||||||
| Issuance of common stock in July 2014 ($11.90 per share) net of transaction expenses of $636 | - | - | 657,000 | 1 | 7,181 | 7,182 | ||||||||||||||||||||||||||
| Issuance of common stock to acquire intellectual property rights from related party in March 2014 ($12.15 per share) | - | - | 50,000 | - | 608 | - | 608 | |||||||||||||||||||||||||
| Issuance of common stock in exchange for 48,240 warrants exercised on a cashless basis | - | - | 33,678 | - | - | - | - | - | ||||||||||||||||||||||||
| Employee stock purchase plan | - | - | - | - | 35 | - | - | 35 | ||||||||||||||||||||||||
| Stock-based compensation | - | - | - | - | 3,053 | - | - | 3,053 | ||||||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | 3 | - | 3 | ||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | (27,616 | ) | (27,616 | ) | ||||||||||||||||||||||
| Balance, December 31, 2014 | - | $ | - | 10,805,220 | $ | 11 | $ | 90,423 | $ | 2 | $ | (54,344 | ) | $ | 36,092 | |||||||||||||||||
| F-6 |
| Accumulated | ||||||||||||||||||||||||||||||||
| Additional | Other | |||||||||||||||||||||||||||||||
| Preferred stock | Common stock | Paid in | Comprehensive | Accumulated | ||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Gain (loss) | Deficit | Total | |||||||||||||||||||||||||
| Balance, December 31, 2014 | - | $ | - | 10,805,220 | $ | 11 | $ | 90,423 | $ | 2 | $ | (54,344 | ) | $ | 36,092 | |||||||||||||||||
| Issuance of common stock in February 2015 ($5.85 per share) net of transaction expenses of $2,115 | - | - | 5,318,700 | 5 | 28,995 | - | - | 29,000 | ||||||||||||||||||||||||
| Issuance of common stock in July 2015 ($7.50 per share) net of transaction expenses of $1,369 | - | - | 2,673,750 | 3 | 18,682 | 18,685 | ||||||||||||||||||||||||||
| Issuance of common stock in exchange for exercise of warrants ($4.25 per share) | - | - | 2,000 | - | 9 | - | - | 9 | ||||||||||||||||||||||||
| Employee stock purchase plan | - | - | 31,999 | - | 160 | - | - | 160 | ||||||||||||||||||||||||
| Stock-based compensation | - | - | 4,389 | - | - | 4,389 | ||||||||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | 8 | - | 8 | ||||||||||||||||||||||||
| Unrealized loss on available for sale securities | (27 | ) | (27 | ) | ||||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | (48,054 | ) | (48,054 | ) | ||||||||||||||||||||||
| Balance, December 31, 2015 | - | $ | - | 18,831,669 | $ | 19 | $ | 142,658 | $ | (17 | ) | $ | (102,398 | ) | $ | 40,262 | ||||||||||||||||
See the accompanying notes to the consolidated financial statements
| F-7 |
| TONIX PHARMACEUTICALS HOLDING CORP. |
| CONSOLIDATED STATEMENTS OF CASH FLOWS |
| (Dollars in Thousands) |
| Year ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | (48,054 | ) | $ | (27,616 | ) | $ | (10,884 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation and amortization | 161 | 36 | 17 | |||||||||
| Warrants issued for services rendered | - | - | 51 | |||||||||
| Stock-based compensation | 4,389 | 3,088 | 1,717 | |||||||||
| Common stock issued in exchange for intellectual property | - | 608 | - | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Prepaid expenses and other | (2,524 | ) | (423 | ) | (204 | ) | ||||||
| Accounts payable | 1,583 | 728 | (60 | ) | ||||||||
| Accrued interest | - | - | (3 | ) | ||||||||
| Accrued expenses | 1,874 | 729 | 856 | |||||||||
| Security deposit | (11 | ) | (45 | ) | - | |||||||
| Deferred rent payable | 54 | 55 | (7 | ) | ||||||||
| Net cash used in operating activities | (42,528 | ) | (22,840 | ) | (8,517 | ) | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||||||
| Purchase of furniture and fixtures | (118 | ) | (319 | ) | (15 | ) | ||||||
| Purchase of intangible asset | (120 | ) | - | - | ||||||||
| Maturities of marketable securities | 4,710 | - | - | |||||||||
| Purchase of marketable securities | (28,643 | ) | - | - | ||||||||
| Increase in restricted cash balance | - | (73 | ) | - | ||||||||
| Net cash used in investing activities | (24,171 | ) | (392 | ) | (15 | ) | ||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||||||
| Proceeds from related party promissory notes | - | - | 280 | |||||||||
| Proceeds from exercise of warrants | 9 | 5,661 | 4,628 | |||||||||
| Proceeds, net of expenses of $3,484, $3,460 and $3,454 from sale of common stock | 47,685 | 47,836 | 10,042 | |||||||||
| Repayments of related party promissory notes | - | (280 | ) | - | ||||||||
| Net cash provided by financing activities | 47,694 | 53,217 | 14,950 | |||||||||
| Effect of currency rate change on cash | (4 | ) | (3 | ) | (1 | ) | ||||||
| Net (decrease) increase in cash | (19,009 | ) | 29,982 | 6,417 | ||||||||
| Cash, beginning of the period | 38,184 | 8,202 | 1,785 | |||||||||
| Cash, end of period | $ | 19,175 | $ | 38,184 | $ | 8,202 | ||||||
| Supplemental disclosures of cash flow information: | ||||||||||||
| Interest paid | $ | - | $ | - | $ | 3 | ||||||
| Non cash financing activities: | ||||||||||||
| Issuance of common stock under employee benefit plan | $ | 160 | $ | - | $ | - | ||||||
See the accompanying notes to consolidated financial statements
| F-8 |
TONIX PHARMACEUTICALS HOLDING CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BUSINESS
Tonix Pharmaceuticals Holding Corp., through its wholly owned subsidiary Tonix Pharmaceuticals, Inc. (“Tonix Sub”), is a clinical-stage pharmaceutical company dedicated to the identification and development of novel pharmaceutical products for challenging disorders of the central nervous system (“CNS”). All drug product candidates are still in development.
On May 15, 2015, Tonix Sub formed Tonix Medicines, Inc. (“Tonix Medicines”) for the purpose of manufacturing and distributing pharmaceutical products in the U.S.
On October 29, 2014, Tonix Sub formed Tonix Pharma Holdings Limited (“Tonix International Holding”), which was incorporated under the laws of Ireland and is a tax resident in Bermuda, for the purpose of acquiring the rights to develop and commercialize Tonix products. Tonix International Holding formed Tonix Pharma Limited (“Tonix Ireland”) for the purpose of manufacturing, trading and developing Tonix products. On December 15, 2014, Tonix Sub and Tonix International Holding entered into an intercompany license agreement whereby Tonix Sub granted Tonix International Holding a non-exclusive right to exercise certain product technologies and related intangible rights. As consideration, Tonix International Holding paid licensing fees to Tonix Sub.
On October 24, 2013, Tonix Sub formed Tonix Pharmaceuticals (Barbados) Ltd. (“Tonix Barbados”). Tonix Barbados had previously entered into a license agreement and a cost-sharing agreement with Tonix Sub, pursuant to which Tonix Barbados acquired the rights to develop and commercialize certain products for non-U.S. markets. Tonix Barbados was liquidated and dissolved during the year ended December 31, 2015. All assets have been transferred to, and liabilities were assumed by, Tonix International Holding.
On April 23, 2013, Tonix Sub formed a wholly owned subsidiary, Tonix Pharmaceuticals (Canada), Inc. (“Tonix Canada”), in the province of New Brunswick, Canada for the purpose of obtaining research and development credits from the Canadian government for any research and development studies performed in Canada.
On August 16, 2010, Tonix Sub formed Krele LLC ("Krele") in the state of Delaware. Krele is a limited liability corporation whose sole member is Tonix Pharmaceuticals Inc. Krele was established to commercialize products that are generic versions of predicate new drug application products or versions of drug efficacy study implementation products. Tonix Sub expects that its relationship to Krele will be similar to that of several other pharmaceutical companies and their subsidiaries that market generic versions of the parent's branded products at different periods in their product life-cycle.
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES
Consolidation
The consolidated financial statements include the accounts of Tonix Pharmaceuticals Holding Corp. and its direct and indirect wholly owned subsidiaries referred to in Note 1 (hereafter referred to as the “Company” or “Tonix”).
All significant intercompany balances and transactions have been eliminated in consolidation.
Recent accounting pronouncements
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-02, Leases (Topic 842). Under the new guidance, lessees will be required to recognize the following for all leases (with the exception of short-term leases) at the commencement date: a lease liability, which is a lessee‘s obligation to make lease payments arising from a lease, measured on a discounted basis; and a right-of-use asset, which is an asset that represents the lessee’s right to use, or control the use of, a specified asset for the lease term. Public business entities should apply the amendments in ASU 2016-02 for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. Early application is permitted. Lessees (for capital and operating leases) must apply a modified retrospective transition approach for leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements. The modified retrospective approach would not require any transition accounting for leases that expired before the earliest comparative period presented. Lessees may not apply a full retrospective transition approach. The company is currently evaluating the impact of adopting this guidance.
| F-9 |
TONIX PHARMACEUTICALS HOLDING CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Risks and uncertainties
The Company's primary efforts are devoted to conducting research and development for the treatment of disorders of the central nervous system. The Company has experienced net losses and negative cash flows from operations since inception and expects these conditions to continue for the foreseeable future. Further, the Company does not have any commercial products available for sale and has not generated revenues and there is no assurance that if the Food and Drug Administration (“FDA”) approval of their products is received that the Company will be able to generate cash flow to fund operations. In addition, there can be no assurance that the Company's research and development will be successfully completed or that any product will be FDA-approved or commercially viable.
At December 31, 2015, the Company had working capital of $39.7 million, after raising $47.7 million through the sale of common stock during 2015. Management believes that the Company has sufficient funds to meet its research and development and other funding requirements for at least the next 12 months. The Company expects that cash used in operations for research and development will increase significantly over the next several years. In the event the funding obtained is not sufficient to complete the development and commercialization of its current product candidates, the Company intends to raise additional funds through equity or debt financing. If the Company is unsuccessful in raising additional financing, it will need to reduce costs and operations in the future.
Use of estimates
The preparation of financial statements in accordance with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates include the useful life of fixed assets, assumptions used in the fair value of stock-based compensation and other equity instruments, and the percent of completion of research and development contracts.
Cash equivalents
The Company considers cash equivalents to be those investments which are highly liquid, readily convertible to cash and have an original maturity of three months or less when purchased. At December 31, 2015, cash equivalents, which consisted of money market funds, amounted to $7.6 million.
Marketable securities
Marketable securities consist primarily of certificates of deposit and corporate, U.S. agency, and U.S. treasury bonds with maturities greater than three months and up to two years at the time of purchase. These securities, which are classified as available for sale, are carried at fair value, with unrealized gains and losses, net of any tax effect, reported in stockholders’ equity as accumulated other comprehensive (loss) income. As investments are available for current operations, they are classified as current irrespective of their maturities. Amortization of premiums is included in interest income. For the year ended December 31, 2015, the amortization of bond premiums totaled $65,000. As of December 31, 2015, amortized cost basis of the securities approximate their fair value. The values of these securities may fluctuate as a result of changes in market interest rates and credit risk. The schedule of maturities at December 31, 2015 is as follows (in thousands):
| Maturities as of December 31, 2015 | ||||||||
| 1 Year or Less | 1 to 2 Years | |||||||
| U.S. Treasury bonds | $ | - | $ | 2,750 | ||||
| U.S agency bonds | 1,248 | 2,531 | ||||||
| Corporate bonds | 6,142 | - | ||||||
| Certificates of deposit | 7,994 | 3,176 | ||||||
| Total | $ | 15,384 | $ | 8,457 | ||||
Intangible asset with indefinite lives
During the year ended December 31, 2015, the Company purchased certain internet domain rights, which were determined to have an indefinite life. Identifiable intangibles with indefinite lives are not amortized but are reviewed for impairment whenever events or changes in circumstances indicate that its carrying amount may not be recoverable, or at least annually.
| F-10 |
TONIX PHARMACEUTICALS HOLDING CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Research and development costs
The Company outsources its research and development efforts and expenses related costs as incurred, including the cost of manufacturing product for testing, as well as licensing fees and costs associated with planning and conducting clinical trials. The value ascribed to patents and other intellectual property acquired is expensed as research and development costs, as such property related to particular research and development projects and had no alternative future uses (see note 12).
The Company estimates its expenses resulting from its obligations under contracts with vendors, clinical research organizations and consultants and under clinical site agreements in connection with conducting clinical trials. The financial terms of these contracts are subject to negotiations, which vary from contract to contract and may result in payment flows that do not match the periods over which materials or services are provided under such contracts. The Company accounts for trial expenses according to the timing of various aspects of the trial. The Company determines accrual estimates taking into account discussion with applicable personnel and outside service providers as to the progress or state of consummation of trials, or the services completed. During the course of a clinical trial, the Company adjusts its clinical expense recognition if actual results differ from its estimates. The Company makes estimates of its accrued expenses as of each balance sheet date based on the facts and circumstances known to it at that time. The Company’s clinical trial accruals are dependent upon the timely and accurate reporting of contract research organizations and other third-party vendors.
Property and equipment
Property and equipment are stated at cost, less accumulated depreciation. Depreciation is calculated using the straight-line method over the asset's estimated useful life, which is three years for computer assets, five years for furniture and all other equipment and term of lease for leasehold improvements. Expenditures for maintenance and repairs are expensed as incurred. Depreciation and amortization expense for the years ended December 31, 2015, 2014 and 2013 was $96,000, $36,000 and $17,000, respectively. All property and equipment is located in the United States.
Income taxes
Deferred income tax assets and liabilities are determined based on the estimated future tax effects of net operating loss and credit carryforwards and temporary differences between the tax basis of assets and liabilities and their respective financial reporting amounts measured at the current enacted tax rates. The Company records an estimated valuation allowance on its deferred income tax assets if it is not more likely than not that these deferred income tax assets will be realized.
The Company recognizes a tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by taxing authorities, based on the technical merits of the position. The tax benefits recognized in the consolidated financial statements from such a position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement. As of December 31, 2015, the Company has not recorded any unrecognized tax benefits.
Stock-based compensation
All stock-based payments to employees and to nonemployee directors for their services as directors, including grants of restricted stock units (“RSUs”), and stock options, are measured at fair value on the grant date and recognized in the consolidated statements of operations as compensation or other expense over the relevant service period. Stock-based payments to nonemployees are recognized as an expense over the period of performance. Such payments are measured at fair value at the earlier of the date a performance commitment is reached or the date performance is completed. In addition, for awards that vest immediately and are non-forfeitable, the measurement date is the date the award is issued.
Foreign currency translation
Operations of the Canadian subsidiary are conducted in local currency which represents its functional currency. The U.S. dollar is the functional currency of the other foreign subsidiaries. Balance sheet accounts of the Canadian subsidiary were translated from foreign currency into U.S. dollars at the exchange rate in effect at the balance sheet date and income statement accounts were translated at the average rate of exchange prevailing during the period. Translation adjustments resulting from this process, were included in accumulated other comprehensive loss on the consolidated balance sheet.
| F-11 |
TONIX PHARMACEUTICALS HOLDING CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Comprehensive income (loss)
Comprehensive income (loss) is defined as the change in equity of a business during a period from transactions and other events and circumstances from non-owners sources. It includes all changes in equity during a period except those resulting from investments by owners and distributions to owners. Other comprehensive income (loss) represents foreign currency translation adjustments and unrealized gains or losses from available for sale securities.
Per share data
Basic and diluted net loss per common share is calculated by dividing net loss, by the weighted average number of outstanding shares of common stock, adjusted to give effect to the 20-for-1 reverse stock split, which was effected on May 1, 2013 (see Note 7).
As of December 31, 2015, 2014 and 2013, there were outstanding warrants to purchase an aggregate of 1,729,217, 1,745,755 and 3,126,656 shares, respectively, of the Company’s common stock (see Note 9). The Company has issued to employees, directors and consultants, options to acquire shares of the Company’s common stock of which 1,656,643, 1,226,800 and 376,500 were outstanding at December 31, 2015, 2014 and 2013, respectively. In addition at December 31, 2015, there were outstanding, 42,000 unvested RSUs. In computing diluted net loss per share for the years ended December 31, 2015, 2014 and 2013, no effect has been given to such options, warrants and RSUs as their effect would be anti-dilutive.
NOTE 3 – RESTRICTED CASH
Restricted cash at December 31, 2015 and 2014 of approximately $132,000, collateralizes a letter of credit issued in connection with the lease of office space in New York City (see Note 10).
NOTE 4 – OTHER BALANCE SHEET INFORMATION
Components of selected captions in the consolidated balance sheets consist of:
| December 31, | ||||||||
| 2015 | 2014 | |||||||
| (in thousands) | ||||||||
| Property, plant and equipment, net: | ||||||||
| Office furniture and equipment | $ | 351 | $ | 240 | ||||
| Leasehold improvements | 179 | 172 | ||||||
| 530 | 412 | |||||||
| Less: Accumulated depreciation and amortization | (180 | ) | (84 | ) | ||||
| $ | 350 | $ | 328 | |||||
| Prepaid expenses and other: | ||||||||
| Contract-related | $ | 2,826 | $ | 519 | ||||
| Professional fees and other | 517 | 333 | ||||||
| $ | 3,343 | $ | 852 | |||||
| Accrued expenses: | ||||||||
| Contract-related | $ | 2,246 | $ | 604 | ||||
| Compensation and compensation-related | 1,128 | 868 | ||||||
| Professional fees and other | 227 | 423 | ||||||
| $ | 3,601 | $ | 1,895 | |||||
| F-12 |
TONIX PHARMACEUTICALS HOLDING CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – FAIR VALUE MEASUREMENTS
Fair value measurements affect the Company’s accounting for certain of its financial assets. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date and is measured according to a hierarchy that includes:
Level 1: Observable inputs, such as quoted prices in active markets.
Level 2: Inputs, other than quoted prices in active markets, that are observable either directly or indirectly. Level 2 assets and liabilities include debt securities with quoted market prices that are traded less frequently than exchange-traded instruments. This category includes U.S. government agency-backed debt securities and corporate-debt securities.
Level 3: Unobservable inputs in which there is little or no market data.
The following table summarizes the Company’s financial assets measured at fair value on a recurring basis as of December 31, 2015 (in thousands):
| Description | December 31, 2015 | Quoted Prices in Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | |||||||||
| Assets: | ||||||||||||
| Cash equivalents | $ | 7,649 | $ | 7,649 | $ | — | ||||||
| Marketable securities – available for sale | 23,841 | 13,920 | 9,921 | |||||||||
| Total assets | $ | 31,490 | $ | 21,569 | $ | 9,921 | ||||||
NOTE 6 – SALE OF COMMON STOCK
February 2015 financing
On February 4, 2015, the Company entered into an underwriting agreement with Roth Capital Partners, LLC (“Roth”), and Oppenheimer & Co Inc. (collectively, the “Representatives”), as representatives of several underwriters (collectively, the “Underwriters”), relating to the issuance and sale of 4,900,000 shares of the Company’s common stock, in an underwritten public offering (the “February 2015 Financing”). The public offering price for each share of common stock was $5.85. The Company granted the Underwriters a 45-day option to purchase up to an additional 735,000 shares of common stock to cover over-allotments, if any.
The February 2015 Financing closed on February 9, 2015. The Underwriters purchased the shares at a six percent discount to the public offering price, for an aggregate discount of $1.7 million (or $0.35 per share). The Company also paid offering expenses of approximately $0.3 million. The Company received net proceeds of approximately $26.7 million. On February 24, 2015, the Underwriters partially exercised the over-allotment option and purchased 418,700 shares of common stock for net proceeds of approximately $2.3 million, net of an aggregate discount of $0.1 million (or $0.35 per share).
July 2015 financing
On July 14, 2015, the Company entered into an underwriting agreement with the Representatives of the Underwriters, relating to the issuance and sale of 2,325,000 shares of the Company’s common stock, in an underwritten public offering (the “July 2015 Financing”). The public offering price for each share of common stock was $7.50. The Company granted the Underwriters a 45-day option to purchase up to an additional 348,750 shares of common stock to cover over-allotments, if any.
The July 2015 Financing closed on July 17, 2015. The Underwriters purchased the shares at a six percent discount to the public offering price, for an aggregate discount of $1.0 million (or $0.45 per share). The Company also paid offering expenses of approximately $0.2 million. The Company received net proceeds of approximately $16.2 million. On July 17, 2015, the Underwriters fully exercised the over-allotment option and purchased 348,750 shares of common stock for net proceeds of approximately $2.5 million, net of an aggregate discount of $0.2 million (or $0.45 per share).
| F-13 |
TONIX PHARMACEUTICALS HOLDING CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
January 2014 financing
On January 24, 2014, the Company entered into an underwriting agreement with Roth, as representative of several underwriters (collectively, the “Second Underwriters”), relating to the issuance and sale of 2,898,550 shares of its common stock in an underwritten public offering (the “January 2014 Financing”). The public offering price for each share of common stock was $15.00. The Company granted the Second Underwriters a 45-day option to purchase up to an additional 434,782 shares of common stock to cover over-allotments, if any.
The January 2014 Financing closed on January 29, 2014. The Second Underwriters purchased the shares at a six percent discount to the public offering price, for an aggregate discount of $2,608,695 (or $0.90 per share). The Company also paid offering expenses of $215,756. The Company received net proceeds of $40,653,799. The over-allotment option expired unexercised.
July 2014 financing
On July 11, 2014, the Company entered into subscription agreements with investors, relating to the issuance and sale of 657,000 shares of the Company’s common stock in a registered direct offering. The purchase price for each share of common stock was $11.90.
Roth acted as the exclusive placement agent in this offering pursuant to the terms of a placement agent agreement, dated July 11, 2014, between the Company and Roth. Pursuant to the placement agent agreement, the Company agreed to pay Roth a placement agent fee equal to six percent of the gross proceeds of the offering.
The registered direct offering closed on July 16, 2014 and the Company received net proceeds of $7,182,670, after deducting placement agent fees and offering expenses of approximately $0.6 million.
August 2013 financing
On August 9, 2013, the Company entered into an underwriting agreement (the “Underwriting Agreement”) with Roth, as representative of the underwriters named therein (the “Third Underwriters”), pursuant to which the Company agreed to offer to the public through the Third Underwriters an aggregate of 2,680,000 units (each a “Unit”, and collectively, the “Units”) at a public offering price of $4.25 per Unit in an underwritten public offering (the “August 2013 Financing”). Each Unit consisted of (i) one share of common stock and (ii) one Series A Warrant (the “Warrants”) to purchase one share of common stock. The Warrants are exercisable at an exercise price of $4.25 per share, subject to anti-dilutive adjustment, and expire on the fifth anniversary of the date of issuance. The Warrants will be exercisable on a “cashless” basis in certain circumstances. Pursuant to the Underwriting Agreement, the Company also granted the Third Underwriters an option for a period of 45 days to purchase up to (i) 402,000 additional Units or (ii) 402,000 additional shares of common stock and/or additional Warrants to purchase up to 402,000 shares of common stock, on the same terms, to cover over-allotments, if any.
The August 2013 Financing closed on August 14, 2013. The Third Underwriters purchased the Units at an eight percent discount to the public offering price, for an aggregate discount of approximately $0.9 million (or $0.34 per unit). The Company received net cash proceeds of $10 million after deducting underwriting discounts and commissions and offering expenses of $0.4 million. On August 14, 2013, the Third Underwriters exercised their over-allotment option by purchasing for $4,000 additional Warrants to purchase 402,000 shares of common stock.
The Third Underwriters received warrants to purchase up to an aggregate of 107,200 shares of common stock, or four percent of the total number of shares included in the Units, which warrants have an exercise price of $4.25.
NOTE 7 – STOCKHOLDERS' EQUITY
On May 1, 2013, the Company filed an amendment to its Articles of Incorporation and effected a 20-for-1 reverse stock split of its issued and outstanding shares of common stock, $0.001 par value, whereby 43,182,599 outstanding shares of the Company’s common stock were exchanged for 2,159,159 shares of the Company's common stock. All per share amounts and number of shares in the consolidated financial statements and related notes have been retroactively restated to reflect the reverse stock split, resulting in the transfer of $41.0 million from common stock to additional paid in capital at December 31, 2012.
| F-14 |
TONIX PHARMACEUTICALS HOLDING CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 8 – SHARE-BASED COMPENSATION
2012 incentive stock option plan
In April, 2012, the Company’s stockholders approved the 2012 Incentive Stock Option Plan (the “2012 Plan”). The 2012 Plan provides for the issuance of options to purchase up to 200,000 shares of the Company’s common stock to officers, directors, employees and consultants of the Company. Under the terms of the 2012 Plan, the Company may issue incentive stock options as defined by the Internal Revenue Code of 1986, as amended (the “Code”) to employees of the Company and may also issue nonstatutory options to employees and others. The Company’s board of directors (“Board of Directors”) determines the exercise price, vesting and expiration period of the grants under the 2012 Plan. However, the exercise price of an incentive stock option may not be less than 110% of fair value of the common stock at the date of the grant for a 10% or more shareholder and 100% of fair value for a grantee who is not a 10% shareholder. The fair value of the common stock is determined based on quoted market price or in absence of such quoted market price, by the Board of Directors in good faith. Additionally, the vesting period of the grants under the 2012 Plan may not be more than five years and expiration period not more than ten years. The Company reserved 200,000 shares of its common stock for future issuance under the terms of the 2012 Plan. On February 12, 2013, the 2012 Plan was amended and restated to increase the number of shares reserved under the plan to 550,000. At December 31, 2015, all reserved shares under the 2012 Plan were subject to granted awards outstanding.
2014 incentive stock option plan
On June 9, 2014, the Company’s stockholders approved the Tonix Pharmaceuticals Holding Corp. 2014 Stock Incentive Plan (the “2014 Plan” and together with the 2012 Plan, the “Plans”).
Under the terms of the 2014 Plan, the Company may issue (1) stock options (incentive and nonstatutory), (2) restricted stock, (3) stock appreciation rights (“SARs”), (4) RSUs, (5) other stock-based awards, and (6) cash-based awards. The 2014 Plan provides for the issuance of up to 1,800,000 shares of common stock, provided, however, that, of the aggregate number of 2014 Plan shares authorized, no more than 200,000 of such shares may be issued pursuant to stock-settled awards other than options (that is, restricted stock, RSUs, SARs, performance awards, other stock-based awards and dividend equivalent awards, in each case to the extent settled in shares of common stock). The Board of Directors determines the exercise price, vesting and expiration period of the grants under the 2014 Plan. However, the exercise price of an incentive stock option may not be less than 110% of fair value of the common stock at the date of the grant for a 10% or more shareholder and 100% of fair value for a grantee who is not a 10% shareholder. The fair value of the common stock is determined based on quoted market price or in absence of such quoted market price, by the Board of Directors in good faith. Additionally, the vesting period of the grants under the 2014 Plan may not be more than five years and expiration period not more than ten years. The Company reserved 1,800,000 shares of its common stock for future issuance under the terms of the 2014 Plan. As of December 31, 2015, 651,357 shares were available for future grants under the 2014 Plan.
General
A summary of the stock option activity and related information for the Plans for the years ended December 31, 2015, 2014 2013 is as follows:
| Shares | Weighted-Average Exercise Price | Weighted-Average Remaining Contractual Term | Aggregate Intrinsic | |||||||||||||
| Outstanding at January 1, 2013 | 150,000 | $ | 30.00 | $ | - | |||||||||||
| Grants | 226,500 | $ | 10.20 | - | ||||||||||||
| Exercised | - | |||||||||||||||
| Forfeitures or expirations | - | |||||||||||||||
| Outstanding at January 1, 2014 | 376,500 | $ | 18.09 | $ | - | |||||||||||
| Grants | 850,300 | $ | 9.53 | $ | - | |||||||||||
| Exercised | - | |||||||||||||||
| Forfeitures or expirations | - | |||||||||||||||
| Outstanding at January 1, 2015 | 1,226,800 | $ | 12.40 | $ | - | |||||||||||
| Grants | 513,243 | $ | 6.01 | $ | - | |||||||||||
| Exercised | - | |||||||||||||||
| Forfeitures or expirations | (83,400 | ) | 8.17 | |||||||||||||
| Outstanding at December 31, 2015 | 1,656,643 | $ | 10.64 | 8.35 | $ | 1,125,299 | ||||||||||
| Vested and expected to vest at December 31, 2015 | 1,656,643 | $ | 10.64 | 8.35 | $ | 1,125,299 | ||||||||||
| Exercisable at December 31, 2015 | 744,385 | $ | 14.37 | 7.66 | $ | 120,817 | ||||||||||
| F-15 |
TONIX PHARMACEUTICALS HOLDING CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The aggregate intrinsic value in the preceding tables represents the total pretax intrinsic value, based on options with an exercise price less than the Company’s closing stock price at the respective dates of issuance.
The Company measures the fair value of stock options on the date of grant, based on a Binomial option pricing model using certain assumptions discussed in the following paragraph, and the closing market price of the Company's common stock on the date of the grant. For employees and directors, the fair value of the award is measured on the grant date and for non-employees, the fair value of the award is generally re-measured on vesting dates and interim financial reporting dates until the service period is complete. Stock options granted pursuant to the Plans vest 1/3rd 12 months from the date of grant and 1/36th each month thereafter for 24 months and expire ten years from the date of grant. Share-based compensation expense related to awards is amortized over the applicable vesting period using the straight-line method.
The weighted-average grant-date fair value of stock options granted was $4.74 in 2015, $8.20 in 2014 and $7.83 in 2013.
The assumptions used in the valuation of stock options granted during the years ended December 31, 2015, 2014 and 2013 were as follows:
| 2015 | 2014 | 2013 | |||
| Risk-free interest rate | 1.47% to 2.35% | 2.03% to 2.52% | 2.02% | ||
| Expected term of option | 6.0 to 9.91 years | 6.0 to 9.72 years | 6.0 years | ||
| Expected stock price volatility | 80.91% to 92.13% | 92.87% to 100.73% | 99.96% |
The risk-free interest rate is based on the yield of Daily U.S. Treasury Yield Curve Rates with terms equal to the expected term of the options as of the grant date. The expected term of options is determined using the simplified method, as provided in an SEC Staff Accounting Bulletin, and the expected stock price volatility is based on comparable companies’ historical stock price volatility since the Company does not have sufficient historical exercise or volatility data because its equity shares have been publicly traded for only a limited period of time.
Share-based compensation expense relating to options granted of $4.1 million, $3.1 million and $1.7 million was recognized for the years ended December 31, 2015, 2014 and 2013, respectively.
As of December 31, 2015, the Company had approximately $4.6 million of total unrecognized compensation cost related to non-vested awards granted under the Plans, which the Company expects to recognize over a weighted average period of 1.76 years.
2014 employee stock purchase plan
On June 9, 2014, the Company’s stockholders approved the Tonix Pharmaceuticals Holdings Corp. 2014 Employee Stock Purchase Plan (the “2014 ESPP”). The 2014 ESPP allows eligible employees to purchase up to an aggregate of 300,000 shares of the Company’s common stock. Under the 2014 ESPP, on the first day of each offering period, each eligible employee for that offering period has the option to enroll for that offering period, which allows the eligible employees to purchase shares of the Company’s common stock at the end of the offering period. Each offering period under the 2014 ESPP is for six months, which can be modified from time-to-time. Subject to limitations, each participant will be permitted to purchase a number of shares determined by dividing the employee’s accumulated payroll deductions for the offering period by the applicable purchase price, which is equal to 85 percent of the fair market value of our common stock at the beginning or end of each offering period, whichever is less. A participant must designate in his or her enrollment package the percentage (if any) of compensation to be deducted during that offering period for the purchase of stock under the 2014 ESPP, subject to the statutory limit under the Code. As of December 31, 2015, after giving effect to shares purchased as described below, there were 250,406 shares available for future issuance under the 2014 ESPP.
The 2014 ESPP is considered a compensatory plan with the related compensation cost written off over the six month offering period. In February 2015, 13,978 shares that were purchased as of December 31, 2014, were issued under the 2014 ESPP, and approximately $0.1 million of employee payroll deductions accumulated at December 31, 2014, related to acquiring such shares, were transferred from accrued expenses to additional paid in capital. In July 2015, 18,021 shares that were purchased as of June 30, 2015, were issued under the 2014 ESPP, and approximately $0.1 million of employee payroll deductions accumulated at June 30, 2015, related to acquiring such shares, was transferred from accrued expenses to additional paid in capital. The compensation expense related to the 2014 ESPP for the year ended December 31, 2015 was $0.1 million. As of December 31, 2015, approximately $0.1 million of employee payroll deductions, which had been withheld since July 1, 2015, the commencement of the offering period ended December 31, 2015, are included in accrued expenses in the accompanying balance sheet. In January 2016, 17,595 shares that were purchased as of December 31, 2015, were issued under the 2014 ESPP, and the employee payroll deductions accumulated at December 31, 2015, related to acquiring such shares, were transferred from accrued expenses to additional paid in capital.
| F-16 |
TONIX PHARMACEUTICALS HOLDING CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Restricted stock units
On February 25, 2015, the Company granted an aggregate of 42,000 RSUs with a fair value of $6.24 per unit to its non-employee directors for board services in 2015, in lieu of cash, which vest one year from the grant date.
Stock-based compensation expense related to RSUs of $0.2 million was recognized during the year ended December 31, 2015. As of December 31, 2015, 42,000 unvested RSUs were outstanding and stock-based compensation relating to such RSUs of $44,000 remains unamortized and is being amortized over the remaining period of approximately two months. As of February 25, 2016, the RSUs for the non-employee directors vested.
NOTE 9 – STOCK WARRANTS
The following table summarizes information with respect to outstanding warrants to purchase common stock of the Company, all of which were vested and exercisable, at December 31, 2015:
| Exercise | Number | Expiration | ||||||
| Price | Outstanding | Date | ||||||
| $ | 4.25 | 918,979 | August 2018 | |||||
| $ | 12.00 | 456,009 | December 2017 to February 2018 | |||||
| $ | 25.00 | 354,229 | January 2017 to February 2019 | |||||
| 1,729,217 | ||||||||
On January 1, 2013, the Company issued warrants to non-employees to purchase 10,800 shares of the Company's common stock at an exercise price of $12.00 per share expiring five years from the date of issuance vesting ratably over twelve months beginning January 1, 2013 in connection with services. Compensation of $51,000 related to outstanding warrants was recognized for the year ended December 31, 2013.
In connection with the August 2013 Financing, the Company issued to investors Warrants to purchase 2,680,000 shares of the Company's common stock. The Warrants are exercisable at $4.25 per share, expire five years from the date of issuance, and may be exercised on a cashless basis under certain circumstances. In addition, the Company issued to the Third Underwriters warrants to purchase 509,200 shares of the Company's common stock. The warrants are exercisable at $4.25 per share, expire five years from the date of issuance, and may be exercised on a cashless basis.
The Company measures the fair value of the vested portion of the issued warrants based on a Binomial option pricing model using certain assumptions discussed in the following paragraph, and the closing market price of the Company's common stock on the date of the fair value determination.
The assumptions used in the valuation of warrants, which vested during the year ended December 31, 2013, were as follows:
| Risk-free interest rate | 0.77 to 1.75 | % | ||
| Life of warrant | 4.75 to 4.01 years | |||
| Expected stock price volatility | 91.31% to 102.46 | % | ||
| Expected dividend yield | $ | 0.0 |
The risk-free interest rate is based on the yield of Daily U.S. Treasury Yield Curve Rates with terms equal to the expected term of the options as of the grant date. The expected stock price volatility is based on comparable companies’ historical stock price volatility since the Company does not have sufficient historical exercise or volatility data because its equity shares have been publicly traded for only a limited period of time.
In April 2013, the Company issued an aggregate of 38,334 shares of its common stock upon the exercise of warrants at $8.00 per share.
| F-17 |
TONIX PHARMACEUTICALS HOLDING CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In December 2013, the Company issued an aggregate of 884,885 and 70,031 shares of its common stock upon the exercise of warrants at $4.25 and $8.00 per share, respectively.
In December 2013, the Company issued 1,672 shares of its common stock upon the exercise of 3,185 warrants exercisable at $4.25 per share on a cashless basis.
In January 2014, 750 warrants with an exercise price of $20.00 expired.
In August 2014, the Company issued 33,678 shares of its common stock upon the exercise of 48,240 warrants exercisable at $4.25 per share on a cashless basis.
In January 2015, 14,538 warrants with an exercise price of $20.00 expired.
During the years ended December 31, 2015 and 2014, the Company issued an aggregate of 2,000 and 1,331,911 shares of its common stock upon the exercise of warrants at $4.25 per share.
NOTE 10 – COMMITMENTS
Operating leases
On February 11, 2014, the Company entered into a lease amendment and expansion agreement, whereby the Company agreed to lease additional premises for office space in New York City, commencing May 1, 2014 and expiring on April 30, 2019. In connection therewith, the original letter of credit was increased by $72,000 to $132,000 and the Company deposited an additional $72,354 into the restricted cash account maintained at the bank that issued the letter of credit.
As of December 31, 2015, future minimum lease payments for office space are as follows (in thousands):
| Year Ending December 31, | ||||
| 2016 | $ | 670 | ||
| 2017 | 683 | |||
| 2018 | 607 | |||
| 2019 | 181 | |||
| $ | 2,141 | |||
Rent expense charged to operations, which differs from rent paid due to rent credits and to increasing amounts of base rent, is calculated by allocating total rental payments on a straight-line basis over the term of the lease. During the years ended December 31, 2015 and 2014, rent expense was $0.6 million and $0.2 million, respectively and as of December 31, 2015 and 2014, deferred rent payable was $112,000 and $52,000, respectively, including the unamortized reimbursement referred to below and the current portion, which at December 31, 2015 and 2014, is $6,000 and $17,000, which is included in accrued expenses in 2015 and in prepaid expenses and other in 2014. In December 2015, the Company received a reimbursement of approximately $53,000 for leasehold improvements the Company incurred from the lessor of our San Diego facility. The reimbursement is being accounted for as deferred rent and is being amortized as a reduction to rent expense over the remaining term of the lease. As of December 31, 2015, the remaining unamortized amount is $34,000.
Research and development agreements
During 2015 and 2014, the Company entered into contracts with various contract research organizations for which there are outstanding commitments aggregating approximately $20.1 million at December 31, 2015 for future work to be performed.
Lederman employment agreement
On February 11, 2014, the Company entered into an employment agreement (the “Agreement”) with Dr. Seth Lederman (“Lederman”) to continue to serve as President, Chief Executive Officer and Chairman of the Board of Directors of the Company. Previously, the Company entered into a consulting agreement with Lederman & Co, pursuant to which Lederman received compensation for serving as the Company’s President and Chief Executive Officer. On February 11, 2014, the consulting agreement was terminated.
| F-18 |
TONIX PHARMACEUTICALS HOLDING CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The Agreement, which has an initial term of one year and automatically renews for successive one year terms unless either party delivers written notice not to renew at least 60 days prior to the end of the current term, provides for various payments and benefits to Lederman in the event Lederman’s employment is terminated without cause (as defined therein), Lederman resigns for Good Reason (as defined therein) or in the event employment is terminated as a result of death or permanent disability.
Defined contribution plan
Effective April 1, 2014, the Company established a qualified defined contribution plan (the “401(k) Plan”) pursuant to Section 401(k) of the Code, whereby all eligible employees may participate. Participants may elect to defer a percentage of their annual pretax compensation to the 401(k) plan, subject to defined limitations. The Company is required to make contributions to the 401(k) Plan equal to 100 percent of each participant’s pretax contributions of up to 19 percent of his or her eligible compensation, and the Company is also required to make a contribution equal to six percent of each participant’s salary, on an annual basis, subject to limitations under the Code. For the years ended December 31, 2015 and 2014, the Company charged operations $0.4 million for both periods for contributions under the 401(k) Plan.
NOTE 11 – INCOME TAXES
Components of the Net Loss consist of the following (in thousands):
| Year Ended | ||||||||||||
| December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Foreign | (45,303 | ) | (14,693 | ) | (502 | ) | ||||||
| Domestic | (2,751 | ) | (12,923 | ) | (10,382 | ) | ||||||
| (48,054 | ) | (27,616 | ) | (10,884 | ) | |||||||
In 2015, the foreign losses were primarily comprised of $43.9 million related to the Bermudan operations of Tonix International Holding, which included a licensing fee of $4.0 million charged by Tonix Sub pursuant to a licensing agreement with Tonix Sub. In 2014, the foreign losses are comprised of $9.0 million related to the Bermudan operations of Tonix International Holding, which included a licensing fee of $8.0 million charged by Tonix Sub and $5.7 million related to Tonix Barbados pursuant to a cost sharing agreement with Tonix Sub. In 2013, the foreign losses are primarily comprised of $0.5 million related to Tonix Canada.
The operations and management of Tonix International Holding are located in Bermuda, and accordingly, are not subject to income taxes in Ireland, which is its country of incorporation. The operations of Tonix International Holding are not subject to income tax in Bermuda.
A reconciliation of the effect of applying the federal statutory rate to the net loss and the effective income tax rate used to calculate the Company's income tax provision is as follows:
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Statutory federal income tax | (35.0 | )% | (35.0 | )% | (34.0 | )% | ||||||
| State income tax, net of federal tax effect | (0.6 | )% | (10.2 | )% | (10.5 | )% | ||||||
| Permanent difference | 0.2 | % | 0.3 | % | 6.7 | % | ||||||
| Change in valuation allowance | 4.6 | % | 22.0 | % | 37.8 | % | ||||||
| Foreign loss not subject to income tax | 32.7 | % | 24.0 | % | 0.0 | % | ||||||
| Other | (1.9 | )% | (1.1 | )% | 0.0 | % | ||||||
| Income tax provision | 0 | % | 0 | % | 0 | % | ||||||
| F-19 |
TONIX PHARMACEUTICALS HOLDING CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Deferred tax assets and liabilities and related valuation allowance as of December 31, 2015 and 2014 are as follows (in thousands):
| December 31, | ||||||||
| 2015 | 2014 | |||||||
| Deferred tax assets: | ||||||||
| Research and development credit carryforward (1) | 6 | 6 | ||||||
| Net operating loss carryforwards | 11,645 | 11,320 | ||||||
| Stock-based compensation | 3,186 | 1,336 | ||||||
| Accrued bonuses | 388 | 200 | ||||||
| Other | 224 | 364 | ||||||
| Total deferred tax assets | 15,449 | 13,226 | ||||||
| Valuation allowance | (15,449 | ) | (13,226 | ) | ||||
| Net deferred tax assets | $ | 0 | $ | 0 | ||||
| (1) | The Company has incurred research and development (“R&D”) expenses, a portion of which may qualify for tax credits. The Company has not conducted an R&D credit study to quantify the amount of credits and has not claimed an R&D credit on its federal tax returns filed except for $6,000 in 2007. The Company may conduct the study in future years and may establish the R&D credit carryforward for prior years. In such event, the net operating loss carryforward will be correspondingly reduced by the amount of the credit. |
At December 31, 2015, the Company had available unused net operating loss (“NOL”) carryforwards of approximately $24 million that expire from 2027 to 2035 for federal tax purposes. The Company also has approximately $27 million of NOL carryforwards for New York State and New York City purposes expiring from 2030 to 2035. Additionally, the Company has $0.2 million of foreign NOL balances in various jurisdictions with various expiration periods. At December 31, 2015, the Company has a research and development carryforward of $6,000 for federal tax purposes that expires in 2027. A portion of these NOL and research and development credit carryforwards are subject to annual limitations in their use in accordance with Internal Revenue Code (“IRC”) section 382. The NOL carryforwards at December 31, 2015 have been reduced to reflect IRC section 382 ownership changes through December 31, 2014 and the resultant inability due to annual limitations, to utilize a portion of the NOL prior to its expiration. Additional adjustments may be required based on ownership activity during 2015.
Management assesses the available positive and negative evidence to estimate if sufficient future taxable income will be generated to use the existing deferred tax assets. A significant piece of objective negative evidence evaluated was the cumulative loss incurred over the three-year period ended December 31, 2015. Such objective evidence limits the ability to consider other subjective evidence such as our projections for future growth. As such, the Company has determined that it is not more likely than not that the deferred tax assets will be realized and accordingly, has provided a full valuation allowance against its gross deferred tax assets. The increase in the valuation allowance for the years ended December 31, 2015, 2014 and 2013 was $2.2 million, $3.8 million and $4.1 million, respectively.
The Company recognizes interest accrued related to unrecognized tax benefits and penalties as income tax expense. However, as of December 31, 2015 there are no unrecognized tax benefits recorded. The Company is subject to taxation in the United States and various states and foreign jurisdictions. As of December 31, 2015, the Company's tax returns remain open and subject to examination by the tax authorities for the tax years 2012 and after.
NOTE 12 – RELATED PARTY TRANSACTIONS
Dr. Seth Lederman, the Company’s Chief Executive Officer and Chairman of the Board is one of the primary founders of the Company. We previously entered into an agreement with a company under his control, Lederman & Co. Total expenses paid under this agreement were $38,000 and $0.3 million during the years ended December 31, 2014 and 2013, respectively.
On July 31 and August 1, 2013, the Company sold three promissory notes in the aggregate principal face amount of $0.3 million to two related parties in exchange for $0.3 million. The notes were payable on demand at any time after one year from issuance and bore no interest. On July 31, 2014 and August 1, 2014, the Company repaid $0.2 million and $0.1 million, respectively.
| F-20 |
TONIX PHARMACEUTICALS HOLDING CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Intellectual property acquired
On March 18, 2014, Tonix Barbados entered into an agreement with Leder Laboratories, Inc. (“Leder”), to acquire intellectual property related to novel smallpox vaccines. As consideration, $0.1 million was paid in cash and 25,000 shares of the Company’s common stock valued at $0.3 million were issued to Leder.
On March 18, 2014, Tonix Barbados entered into an agreement with Starling Pharmaceuticals, Inc. (“Starling”), to acquire intellectual property related to radio- and chemo-protective agents. As consideration, $0.1 million was paid in cash and 25,000 shares of the Company’s common stock valued at $0.3 million were issued to Starling.
Seth Lederman, the Company’s Chairman and Chief Executive Officer, is the Chairman, CEO and majority owner (through majority-owned entities) of Starling and Leder.
NOTE 13 – SUMMARY QUARTERLY DATA (unaudited)
Unaudited quarterly financial data for fiscal 2015 and 2014 is summarized as follows:
| 2015 Quarter Ended | ||||||||||||||||
| March 31, | June 30, | September 30, | December 31, | |||||||||||||
| (in thousands, except per share amounts) | ||||||||||||||||
| Operating Loss | $ | (9,696 | ) | $ | (11,784 | ) | $ | (13,280 | ) | $ | (13,402 | ) | ||||
| NET LOSS | $ | (9,681 | ) | $ | (11,763 | ) | $ | (13,250 | ) | $ | (13,360 | ) | ||||
| Net loss per common share, basic and diluted | $ | (0.71 | ) | $ | (0.73 | ) | $ | (0.72 | ) | $ | (0.71 | ) | ||||
| 2014 Quarter Ended | ||||||||||||||||
| March 31, | June 30, | September 30, | December 31, | |||||||||||||
| (in thousands, except per share amounts) | ||||||||||||||||
| Operating Loss | $ | (5,169 | ) | $ | (6,049 | ) | $ | (7,434 | ) | $ | (9,004 | ) | ||||
| NET LOSS | $ | (5,164 | ) | $ | (6,044 | ) | $ | (7,419 | ) | $ | (8,989 | ) | ||||
| Net loss per common share, basic and diluted | $ | (0.59 | ) | $ | (0.61 | ) | $ | (0.71 | ) | $ | (0.83 | ) | ||||
Because loss per share amounts are calculated using the weighted average number of common shares outstanding during each quarter, the sum of the per share amounts for the four quarters does not equal the total loss per share amount for the year.
NOTE 14 – SUBSEQUENT EVENTS
On February 9, 2016, the Company granted options to purchase an aggregate of 411,125 shares of the Company’s common stock to employees with an exercise price of $5.03, exercisable for a period of ten years, vesting 1/3 on the first anniversary and 1/36th each month thereafter for 24 months. Additionally, the Company granted options to purchase 200,000 shares of the Company’s common stock to employees with an exercise price of $5.03, exercisable for a period of ten years, vesting 1/3 each upon the Company’s common stock having an average closing sale price equal to or exceeding each of $6.00, $7.00 and $8.00 per share for 20 consecutive trading days, subject to a one year minimum service period prior to vesting.
On February 9, 2016, the Company granted an aggregate of 56,250 RSUs with a fair value of $3.81 per unit to its non-employee directors for board services in 2016, in lieu of cash, which vest one year from the grant date.
| F-21 |
ITEM 9 - CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES
None.
ITEM 9A – CONTROLS AND PROCEDURES
Evaluation of disclosure controls and procedures.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures pursuant to Rule 13a-15 under the Exchange Act. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply its judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Based on management’s evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures are designed at a reasonable assurance level and are effective to provide reasonable assurance that information we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms, and that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Changes in internal control over financial reporting.
There were no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2015 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s report on internal control over financial reporting.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting for our company. Internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) promulgated under the Exchange Act, as a process designed by, or under the supervision of, a company’s principal executive and principal financial officer and effected by the our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| (1) | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; |
| (2) | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made in accordance with authorizations of management and directors of the company; and |
| (3) | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible enhancements to controls and procedures.
We conducted an evaluation of the effectiveness of internal control over financial reporting based on the framework in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our principal executive officer and principal financial officer conclude that, at December 31, 2015, our internal control over financial reporting was effective.
| 55 |
The effectiveness of our internal control over financial reporting as of December 31, 2015 has been audited by EisnerAmper LLP, an independent registered public accounting firm, as stated in its report below.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Tonix Pharmaceuticals Holding Corp.
We have audited the internal controls over financial reporting of Tonix Pharmaceuticals Holding Corp. (the "Company") as of December 31, 2015, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO"). The Company's management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management's report on internal control over financial reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Tonix Pharmaceuticals Holding Corp. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on criteria established in the Internal Control - Integrated Framework (2013) issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements of Tonix Pharmaceuticals Holding Corp. as of and for the year ended December 31, 2015 and our report dated March 3, 2016 expressed an unqualified opinion thereon.
| /s/ EisnerAmper LLP | |
| New York, New York | |
| March 3, 2016 |
None.
| 56 |
ITEM 10 – DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The names of our executive officers and directors and their age, title, and biography as of March 2, 2016 are set forth below:
| Name | Age | Title | ||
| Seth Lederman | 58 | President, CEO and Chairman of the Board of Directors | ||
| Bradley Saenger | 42 | Chief Financial Officer | ||
| Bruce Daugherty | 58 | Chief Scientific Officer, Controller and Secretary | ||
| Gregory Sullivan | 50 | Chief Medical Officer | ||
| Stuart Davidson | 59 | Director | ||
| Patrick Grace | 60 | Director | ||
| Donald W. Landry | 61 | Director | ||
| Ernest Mario | 77 | Director | ||
| Charles E. Mather IV | 56 | Director | ||
| John Rhodes | 59 | Lead Director | ||
| Samuel Saks | 61 | Director |
Directors are elected annually and hold office until the next annual meeting of the stockholders of the Company and until their successors are elected. Officers are elected annually and serve at the discretion of the Board of Directors.
Seth Lederman, MD became our President, Chief Executive Officer, Chairman of the Board and a Director in October 2011. Dr. Lederman founded Tonix Pharmaceuticals, Inc., a wholly-owned subsidiary of the Company (“Tonix Sub”) in June of 2007 and has acted as its Chairman of the Board of Directors since its inception and as President since June 2010. Dr. Lederman is an inventor on key patents and patent applications underlying our programs including: TNX-102 SL fibromyalgia; TNX-102 SL for post-traumatic stress disorder; TNX-201 for episodic tension-type headache; and TNX-301 for alcoholism. Dr. Lederman has been the Chairman of Krele since its inception in August 2010. Dr. Lederman has also been the President and a director of Tonix Pharmaceuticals (Canada), Inc. since its inception in April 2013, a director of Tonix Pharmaceuticals (Barbados), Ltd. from December 2013 until it was dissolved in 2015. Lederman served as a director of Tonix Pharma Limited between December 2014 and September 2015 and Tonix Pharma Holdings Limited between December 2014 and November 2015. Since 1996, Dr. Lederman has been an Associate Professor at Columbia University, but has been on leave since November 2015. As an Assistant Professor at Columbia, Dr. Lederman discovered and characterized the CD40-ligand and invented therapeutic candidates to treat autoimmune diseases and transplant rejection. Dr. Lederman has been a Manager of L&L Technologies LLC, or L&L, since 1996. In addition, Dr. Lederman has been the Managing Member of Seth Lederman Co, LLC since January 2007 and the Managing Member of Lederman & Co, LLC, or Lederman & Co, since 2002, both of which are biopharmaceutical consulting and investing companies. Dr. Lederman has also been the Managing Member of Targent Pharmaceuticals, LLC, or Targent, since 2000, and Managing Member of Plumbline LLC since 2002. Targent was a founder of Targent Pharmaceuticals Inc. on which Board of Directors Dr. Lederman served from inception in 2001 until the sale of its assets to Spectrum Pharmaceuticals Inc. in 2006. Between January 2007 and November 2008, Dr. Lederman was a Managing Partner of Konanda Pharma Partners, LLC, a Director of Konanda Pharma Fund I, LP, and a Managing Partner of Konanda General Partner, LLC, which were related private growth equity fund entities. As well, between January 2007 and November 2008, Dr. Lederman was Chairman of Validus Pharmaceuticals, Inc. and Fontus Pharmaceuticals, Inc., which were portfolio companies of the Konanda private growth equity funds. Since December 2011, Dr. Lederman has served as CEO and Chairman of Leder Laboratories Inc., or Leder Labs, and Starling Pharmaceuticals Inc., or Starling, which are biopharmaceutical development companies. Since March 2013, Dr. Lederman has been the chairman of Leder Laboratories, Ltd., a wholly-owned subsidiary of Leder Laboratories Inc. Since 2015, Dr. Lederman has served as a member of the US – Japan Business Council. Between 2006 and 2011, Dr. Lederman was a director of Research Corporation, a New York-based non-profit organization. Dr. Lederman received his BA degree in Chemistry from Princeton University in 1979 and his MD from Columbia University in 1983. Dr. Lederman has been a New York State licensed physician since 1985. Dr. Lederman’s significant experience with our patent portfolio and his experience as an entrepreneur, seed capital investor, fund manager, and director of start-up biopharmaceutical companies were instrumental in his selection as a member of the board of directors.
Bradley Saenger, CPA became our Chief Financial Officer in February 2016. Mr. Saenger has worked for Tonix since May 2014, as the Director of Accounting (May 2014 – December 2015) and VP of Accounting (January 2016 – February 2016). Between June 2013 and March 2014, Mr. Saenger worked for Shire Pharmaceuticals as a consultant in the financial analyst research and development group. Since November 2015, Mr. Saenger has been a director of Tonix Pharma Holdings Limited. Between February 2013 and May 2013, Mr. Saenger worked for Stewart Health Care System as a financial consultant. Between October 2011 and December 2012, Mr. Saenger was an Associate Director of Accounting at Vertex Pharmaceuticals, Inc. Between January 2005 and September 2011, Mr. Saenger worked for Alere Inc., as a Manager of Corporate Accounting and Consolidations (2007 – 2011) and Manager of Financial Reporting (2005 – 2006). Mr. Saenger also worked for PricewaterhouseCoopers LLP, Shifren Hirsowitz, public accountants and auditors in Johannesburg, South Africa, Investec Bank in Johannesburg, South Africa and Norman Sifris and Company, public accountants and auditors in Johannesburg, South Africa. Mr. Saenger received his Bachelor’s and Honors’ degrees in Accounting Science from the University of South Africa. Mr. Saenger is a Chartered Accountant in South Africa and a Certified Public Accountant in the Commonwealth of Massachusetts.
| 57 |
Bruce Daugherty, PhD became our Controller in April 2012, our Secretary in November 2012 and our Chief Scientific Officer in August 2013. Between April 2012 and August 2013, Dr. Daugherty was our Senior Director of Drug Development. Dr. Daugherty has also been the Secretary and a director of Tonix Pharmaceuticals (Canada), Inc. since its inception in April 2013. Since September 2015, Dr. Daugherty has been a director of Tonix Pharma Limited. Since January 2009, Dr. Daugherty has worked as a consultant to academia and biotechnology companies in drug discovery/development and licensing through his consulting company, LeClair Pharma Consulting, LLC. Dr. Daugherty was a consultant to our company between November 2011 and March 2012. In 2009, Dr. Daugherty was employed at Assumption College in Mendham, New Jersey, where he was a lecturer in Biology for freshman students. From 1987 to 2008, Dr. Daugherty was employed at Merck & Co., where he was a scientist in drug discovery and development. Dr. Daugherty earned his MBA from Emory University’s Goizueta Business School, his PhD in Molecular Genetics and Microbiology from Rutgers University-Robert Wood Johnson Medical School, his MS in Zoology from Rutgers University and his BA in Biology from Washington University in St. Louis.
Gregory Sullivan, MD became our Chief Medical Officer on June 3, 2014. Prior to that date, he served on our Scientific Advisory Board since October 2010, and had also provided ad hoc consulting services. Previously, Dr. Sullivan had been a member of the faculty of Columbia University since July 1999, where he served as an Assistant Professor of Psychiatry in the Department of Psychiatry at Columbia University Medical Center (CUMC) until June 2014. Between June 1997 and August 2014, Dr. Sullivan maintained a part-time psychiatry practice. He served as a Research Scientist at the New York State Psychiatric Institute (NYSPI) from December 2006 to June 2014. He also served as a member of the Institutional Review Board of the NYSPI from January 2009 to June 2014. As Principal Investigator and Co-Investigator on several human studies of posttraumatic stress disorder (PTSD), Dr. Sullivan has administered the recruitment, biological assessments, treatment, and safety of participants with PTSD in clinical trials of the disorder. He has published more than 50 articles and chapters on research topics ranging from stress and anxiety disorders to abnormal serotonin receptor expression in depression, PTSD and panic disorder. He is a recipient of grants from the National Institute of Mental Health (NIMH), the Anxiety Disorders Association of America, NARSAD, the Dana Foundation, and the American Foundation for Suicide Prevention. Dr. Sullivan received a BA in Biology from the University of California, Berkeley, and received his MD from the College of Physicians & Surgeons at Columbia University. He completed his residency training in psychiatry at CUMC, and then a two-year NIMH-sponsored research fellowship in anxiety and affective disorders before joining the faculty at Columbia.
Stuart Davidson became a Director in October 2011. Between July 2010 and October 2011, Mr. Davidson served as a director of Tonix Sub. Since 2011, Mr. Davidson has been a Managing Director of Sonen Capital. Since 1994, Mr. Davidson has been a Managing Partner of Labrador Ventures. Prior to Labrador, Mr. Davidson founded and served as CEO of Combion, Inc., which was acquired by Incyte. He also served as President of Alkermes, Inc., a biotechnology company focused on drug delivery. Mr. Davidson received his Bachelor’s Degree from Harvard College in 1978 and his MBA from Harvard Business School in 1984. Mr. Davidson’s prior experience as a venture capital investor, entrepreneur, and biotechnology industry executive experience in the leadership of pharmaceutical companies was instrumental in his selection as a member of our board of directors.
Patrick Grace became a Director in October 2011. Between June 2007 and October 2011, Mr. Grace served as a director of Tonix Sub. Mr. Grace was the co-founder of and served as the Managing Partner of Apollo Philanthropy Partners, L.L.C. from October 2008 until October 2012. He has also been President of MLP Capital, Inc., an investment holding company, since 1996. Mr. Grace served in various senior management roles with W. R. Grace & Co. from 1977 to 1995, and was last President and CEO of Grace Logistics Services, Inc. From January 2002 to August 2002, Mr. Grace was also President and Chief Executive Officer of Kingdom Group, LLC (“Kingdom”), a provider of turnkey compressed natural gas fueling systems, and he was Executive Vice President of Kingdom from August 1999 to December 2000. Since 1996, he has been a director of Chemed Corporation. Mr. Grace was a liberal arts major at the University of Notre Dame and earned a MBA in finance from Columbia University. Mr. Grace’s extensive executive experience, along with his membership on the board of directors of a public company, was instrumental in his selection as a member of our board of directors.
Donald W. Landry, MD, PhD became a Director in October 2011. Between June 2007 and October 2011, Dr. Landry served as a director of Tonix Sub. Dr. Landry has been a member of the faculty of Columbia University since 1986, and has served as the Samuel Bard Professor of Medicine, Chair of the Department of Medicine and Physician-in-Chief at New York Presbyterian Hospital/Columbia University since 2008. Since 1996, he has been a director of Sensient Technologies Corp. Dr. Landry was a co-founder and has been a member of L&L since 1996. Dr. Landry received his BS degree in Chemistry from Lafayette College in 1975, his PhD in Organic Chemistry from Harvard University in 1979 and his M.D. from Columbia University in 1983. Dr. Landry has been a New York State licensed physician since 1985. In 2008, Dr. Landry was awarded the Presidential Citizens Medal, the second-highest award that the President can confer upon a civilian. Dr. Landry’s significant medical and scientific background was instrumental in his selection as a member of the board of directors.
| 58 |
Ernest Mario, PhD became a Director in October 2011. Between September 2010 and October 2011, Dr. Mario served as a director of Tonix Sub. Dr. Mario is a former Deputy Chairman and Chief Executive of Glaxo Holdings plc and a former Chairman and Chief Executive Officer of ALZA Corporation. Since April 2014, Dr. Mario has served as Chairman of Capnia, Inc., a specialty pharmaceutical company in Palo Alto, CA. Between August 2007 and February 2014, Dr. Mario served as the Chief Executive Officer and Chairman of Capnia, Inc. and between February 2014 and April 2014, Dr. Mario served as Executive Chairman. From 2003 to 2007, he was Chairman and Chief Executive of Reliant Pharmaceuticals, Inc. Dr. Mario is currently a director of Boston Scientific Corp. (since 2001), Capnia Inc. (since 2007), Celgene Corp. (since 2007), Chimerix, Inc. (since February 2013) and Kindred Biosciences, Inc. (since February 2013). Dr. Mario is also Chairman of Chimerix. Between 2012 and 2015, Dr. Mario served as a director of XenoPort Inc. Between 2001 and 2013, Dr. Mario was a director of Maxygen Inc. He is Chairman of the American Foundation for Pharmaceutical Education and serves as an advisor to The Ernest Mario School of Pharmacy at Rutgers University. In 2007, Dr. Mario was awarded the Remington Medal by the American Pharmacists’ Association, pharmacy’s highest honor. Dr. Mario received a PhD and an MS in physical sciences from the University of Rhode Island and a BS in pharmacy from Rutgers University. Dr. Mario brings to his service as a director his significant executive leadership experience, including his experience leading several pharmaceutical companies, as well as his membership on public company boards and foundations. He also has extensive experience in financial and operations management, risk oversight, and quality and business strategy.
Charles E. Mather IV became a Director in October 2011. Between April and October 2011, Mr. Mather served as a director of Tonix Sub. Mr. Mather has been a Managing Director of Equity Capital Markets at BTIG since March 2015. From December 2009 to February 2015 he was the Head of Private and Alternative Capital and Co-Head of Equity Capital Markets at Janney Montgomery Scott. Between May 2007 and September 2008, Mr. Mather was the head of the Structured Equity Group at Jefferies Group Inc. Prior to that, Mr. Mather held various senior investment banking positions at Cowen and Company, including as Co-Head of the Private Equity Group. Since July 2015, Mr. Mather has served as a director of the Finance Company of Pennsylvania. Mr. Mather received a BA in History from Brown University and an MBA in Finance from The Wharton School, University of Pennsylvania. Mr. Mather’s extensive experience advising life science companies as an investment banker was instrumental in his selection as a member of our board of directors.
John Rhodes became a Director in October 2011 and Lead Director in February 2014. Mr. Rhodes has served as President and CEO of the New York State Energy Research and Development Authority since September 2013. Between October 2010 and October 2011, Mr. Rhodes served as a director of Tonix Sub. Between 2005 and 2013, Mr. Rhodes was a director of Dewey Electronics Company, a manufacturer of electronic and electromechanical systems for the military and commercial markets. Between January 2013 and September 2013, he served as director of the Center for Market Innovation at Natural Resources Defense Council. Between April 2007 and June 2010, Mr. Rhodes was a Senior Advisor to Good Energies, Inc., a renewable energy company. Mr. Rhodes is a former Vice President of Booz Allen Hamilton, Inc. Mr. Rhodes is a graduate of Princeton University and the Yale School of Management. Mr. Rhodes’ extensive business and consulting experience, along with his membership on the board of directors of a public company was instrumental in his selection as a member of our board of directors.
Samuel Saks, MD became a Director in May 2012. Between 2003 and April 2009, Dr. Saks was the chief executive officer and a director of Jazz Pharmaceuticals, Inc., a publicly-held biopharmaceutical company, which he co-founded in 2003. From April 2011 until February 2012, Dr. Saks served as interim Chief Medical Officer of Threshold Pharmaceuticals, a publicly-held biopharmaceutical company. Between November 2013 and May 2015, Dr. Saks served as the Chief Development Officer of Auspex Pharmaceuticals, Inc., a publicly-held biopharmaceutical company. From 2001 until 2003, Dr. Saks was company group chairman of ALZA Corporation and a member of the Johnson & Johnson Pharmaceuticals Operating Committee. From 1992 until 2001, Dr. Saks held various positions at ALZA, including Chief Medical Officer and Group Vice President, where he was responsible for clinical, regulatory and commercial activities. Previously, Dr. Saks held clinical research and development management positions with Schering-Plough, Xoma and Genentech. Dr. Saks formerly served as a scientific advisor to ArQule Pharmaceuticals, CMEA Ventures and ProQuest Investments. Dr. Saks is currently a director of Velocity Pharmaceutical Development LLC (since 2011), Depomed (since 2012), Bullet Biotechnology, Inc. (since 2012), NuMedii (since 2013) and PDL BioPharma, Inc. (since September 2015). Between 2009 and May 2015, Dr. Saks was a director of Auspex Pharmaceuticals. From September 2005 until October 2010, Dr. Saks served on the board of directors of Trubion Pharmaceuticals, a publicly-held biopharmaceutical company. Dr. Saks has also served on the board of directors of Corixa, Coulter and Ribozyme. Dr. Saks is board certified in oncology and received a B.S. and an M.D. from the University of Illinois. Mr. Saks’ extensive scientific and medical expertise and experience in formulating partnering and business development strategies, including those involving larger pharmaceutical companies, was instrumental in his selection as a member of our board of directors.
Family Relationships
None.
| 59 |
Board Independence
The board of directors has determined that (i) Seth Lederman, has a relationship which, in the opinion of the board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and is not an “independent director” as defined in the Marketplace Rules of The NASDAQ Stock Market and (ii) Stuart Davidson, Patrick Grace, Donald Landry, Ernest Mario, Charles Mather, John Rhodes and Samuel Saks are each an independent director as defined in the Marketplace Rules of The NASDAQ Stock Market.
Meetings and Committees of the Board of Directors
During the fiscal year ended December 31, 2015, the Board of Directors held four meetings, the Audit Committee held five meetings, the Compensation Committee held three meetings and the Nominating and Corporate Governance Committee held three meetings. The Board and Board committees also approved certain actions by unanimous written consent.
Board Committees
The Board of Directors has standing Audit, Compensation, and Governance and Nominating Committees. Information concerning the membership and function of each committee is as follows:
| Board Committee Membership | ||||||||||||
| Name | Audit Committee |
Compensation Committee | Nominating and Corporate Governance Committee |
|||||||||
| Seth Lederman | ||||||||||||
| Stuart Davidson | ** | |||||||||||
| Patrick Grace | ** | * | ||||||||||
| Donald W. Landry | ||||||||||||
| Ernest Mario | * | |||||||||||
| Charles E. Mather IV | * | * | ||||||||||
| John Rhodes | * | ** | ||||||||||
| Samuel Saks | * | |||||||||||
* Member of Committee
** Chairman of Committee
Audit Committee
Our Audit Committee consists of Patrick Grace, Charles Mather and John Rhodes, with Mr. Grace elected as Chairman of the Committee. Our Board of Directors has determined that each of Messrs. Grace, Mather and Rhodes are “independent” as that term is defined under applicable SEC rules and under the current listing standards of the NASDAQ Stock Market. Mr. Grace is our audit committee financial expert.
Our Audit Committee’s responsibilities include: (i) reviewing the independence, qualifications, services, fees, and performance of the independent auditors, (ii) appointing, replacing and discharging the independent auditor, (iii) pre-approving the professional services provided by the independent auditor, (iv) reviewing the scope of the annual audit and reports and recommendations submitted by the independent auditor, and (v) reviewing our financial reporting and accounting policies, including any significant changes, with management and the independent auditor. The Audit Committee reviewed and discussed with management the Company’s audited financial statements for the year ended December 31, 2015.
Compensation Committee
Our Compensation Committee consists of Stuart Davidson, Ernest Mario and Samuel Saks, with Mr. Davidson elected as Chairman of the Committee. Our Board of Directors has determined that all of the members are “independent” under the current listing standards of the NASDAQ Stock Market. Our Board of Directors has adopted a written charter setting forth the authority and responsibilities of the Compensation Committee.
Our Compensation Committee has responsibility for, among other things, evaluating and making decisions regarding the compensation of our executive officers and directors, assuring that the executive officers are compensated effectively in a manner consistent with our stated compensation strategy, producing an annual report on executive compensation in accordance with the rules and regulations promulgated by the SEC, periodically evaluating and administering the terms and administration of our incentive plans and benefit programs and monitoring of compliance with the legal prohibition on loans to our directors and executive officers.
| 60 |
Nominating Corporate Governance Committee
Our Nominating and Corporate Governance Committee consists of Donald Landry, Charles Mather and John Rhodes, with Mr. Rhodes elected as Chairman of the Committee. The Board of Directors has determined that all of the members are “independent” under the current listing standards of the NASDAQ Stock Market.
Our Nominating and Corporate Governance Committee has responsibility for assisting the Board in, among other things, effecting the organization, membership and function of the Board and its committees. The Nominating and Corporate Governance Committee shall identify and evaluate the qualifications of all candidates for nomination for election as directors. In addition, the Nominating and Corporate Governance Committee is responsible for developing, recommending and evaluating corporate governance standards and a code of business conduct and ethics.
Nomination of Directors
As provided in its charter and our company’s corporate governance principles, the Nominating and Corporate Governance Committee is responsible for identifying individuals qualified to become directors. The Nominating and Corporate Governance Committee seeks to identify director candidates based on input provided by a number of sources, including (1) the Nominating and Corporate Governance Committee members, (2) our other directors, (3) our shareholders, (4) our Chief Executive Officer or Chairman, and (5) third parties such as professional search firms. In evaluating potential candidates for director, the Nominating and Corporate Governance Committee considers the entirety of each candidate’s credentials.
Qualifications for consideration as a director nominee may vary according to the particular areas of expertise being sought as a complement to the existing composition of the Board of Directors. However, at a minimum, candidates for director must possess:
| · | high personal and professional ethics and integrity; |
| · | the ability to exercise sound judgment; |
| · | the ability to make independent analytical inquiries; |
| · | a willingness and ability to devote adequate time and resources to diligently perform Board and committee duties; and |
| · | the appropriate and relevant business experience and acumen. |
In addition to these minimum qualifications, the Nominating and Corporate Governance Committee also takes into account when considering whether to nominate a potential director candidate the following factors:
| · | whether the person possesses specific industry expertise and familiarity with general issues affecting our business; |
| · | whether the person’s nomination and election would enable the Board to have a member that qualifies as an “audit committee financial expert” as such term is defined by the SEC in Item 401 of Regulation S-K; |
| · | whether the person would qualify as an “independent” director under the listing standards of the Nasdaq Stock Market; |
| · | the importance of continuity of the existing composition of the Board of Directors to provide long term stability and experienced oversight; and |
| · | the importance of diversified Board membership, in terms of both the individuals involved and their various experiences and areas of expertise. |
The Nominating and Corporate Governance Committee will consider director candidates recommended by shareholders provided such recommendations are submitted in accordance with the procedures set forth below. In order to provide for an orderly and informed review and selection process for director candidates, the Board of Directors has determined that shareholders who wish to recommend director candidates for consideration by the Nominating and Corporate Governance Committee must comply with the following:
| 61 |
| · | The recommendation must be made in writing to the Corporate Secretary at Tonix Pharmaceuticals Holding Corp.; |
| · | The recommendation must include the candidate's name, home and business contact information, detailed biographical data and qualifications, information regarding any relationships between the candidate and the Company within the last three years and evidence of the recommending person's ownership of the Company’s common stock; |
| · | The recommendation shall also contain a statement from the recommending shareholder in support of the candidate; professional references, particularly within the context of those relevant to board membership, including issues of character, judgment, diversity, age, independence, expertise, corporate experience, length of service, other commitments and the like; and personal references; and |
| · | A statement from the shareholder nominee indicating that such nominee wants to serve on the Board and could be considered "independent" under the Rules and Regulations of the Nasdaq Stock Market and the SEC, as in effect at that time. |
All candidates submitted by shareholders will be evaluated by the Nominating and Corporate Governance Committee according to the criteria discussed above and in the same manner as all other director candidates.
Code of Ethics
We have adopted a Code of Business Conduct and Ethics that applies to all of our directors, officers and employees. A copy of the Code of Business Conduct and Ethics is incorporated by reference as an exhibit.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our directors, executive officers and holders of more than 10% of our common stock to file with the SEC reports regarding their ownership and changes in ownership of our securities. We believe that, during fiscal 2015, our directors, executive officers and 10% stockholders complied with all Section 16(a) filing requirements.
Involvement in Certain Legal Proceedings
Our Directors and Executive Officers have not been involved in any of the following events during the past ten years:
| 1. | any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| 2. | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| 3. | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from or otherwise limiting his involvement in any type of business, securities or banking activities or to be associated with any person practicing in banking or securities activities; |
| 4. | being found by a court of competent jurisdiction in a civil action, the Securities and Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| 5. | being subject of, or a party to, any federal or state judicial or administrative order, judgment decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| 6. | being subject of or party to any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
| 62 |
ITEM 11 – EXECUTIVE COMPENSATION
The information required by this item is incorporated by reference to our Proxy Statement for the 2016 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission (SEC) within 120 days of the fiscal year ended December 31, 2015.
ITEM 12 – SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information regarding beneficial ownership of our common stock as of March 2, 2016:
| · | by each person who is known by us to beneficially own more than 5% of our common stock; |
| · | by each of our officers and directors; and |
| · | by all of our officers and directors as a group. |
Unless otherwise indicated in the footnotes to the following table, each person named in the table has sole voting and investment power and that person’s address is c/o Tonix Pharmaceuticals Holding Corp., 509 Madison Avenue, Suite 306, New York New York 10022.
| NAME OF OWNER | TITLE OF CLASS | NUMBER OF SHARES OWNED (1) | PERCENTAGE OF COMMON STOCK (2) | |||||||
| Seth Lederman | Common Stock | 854,268 | (3) | 4.42 | % | |||||
| Bradley Saenger | Common Stock | 21,327 | (4) | * | ||||||
| Bruce Daugherty | Common Stock | 237,231 | (5) | 1.25 | % | |||||
| Gregory Sullivan | Common Stock | 62,940 | (6) | * | ||||||
| Stuart Davidson | Common Stock | 130,645 | (7) | * | ||||||
| Patrick Grace | Common Stock | 46,025 | (8) | * | ||||||
| Donald Landry | Common Stock | 122,990 | (9) | * | ||||||
| Ernest Mario | Common Stock | 309,284 | (10) | 1.64 | % | |||||
| Charles Mather IV | Common Stock | 52,809 | (11) | * | ||||||
| John Rhodes | Common Stock | 150,431 | (12) | * | ||||||
| Samuel Saks | Common Stock | 84,746 | (13) | * | ||||||
| Officers and Directors as a Group (11 persons) | Common Stock | 2,021,572 | (14) | 10.18 | % | |||||
| Kingdon Capital Management, L.L.C. (15) | Common Stock | 1,467,858 | 7.78 | % | ||||||
| Deerfield Special Situations Fund, L.P. (16) | Common Stock | 1,315,551 | 6.97 | % | ||||||
| Broadfin Capital, LLC (17) | Common Stock | 1,243,748 | 6.59 | % | ||||||
* Denotes less than 1%
(1) Beneficial Ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Shares of common stock subject to options or warrants currently exercisable or convertible, or exercisable or convertible within 60 days of March 2, 2016 are deemed outstanding for computing the percentage of the person holding such option or warrant but are not deemed outstanding for computing the percentage of any other person.
(2) Percentage based upon 18,873,264 shares of common stock issued and outstanding as of March 2, 2016.
(3) Includes 345,546 shares of common stock underlying options which are currently exercisable or become exercisable within 60 days, 184,628 shares of common stock and 54,500 shares of common stock underlying warrants owned by Lederman & Co, 32,457 shares of common stock and 12,667 shares of common stock underlying warrants owned by L&L, 58,972 shares of common stock and 8,250 shares of common stock underlying warrants owned by Targent, 29,167 shares of common stock and 4,167 shares of common stock underlying warrants owned by Leder Labs and 29,167 shares of common stock and 4,167 shares of common stock underlying warrants owned by Starling. Seth Lederman, as the Managing Member of Lederman & Co and Targent, the Manager of L&L and the Chairman of Leder Labs and Starling, has investment and voting control over the shares held by these entities.
| 63 |
(4) Includes 17,288 shares of common stock underlying options which are currently exercisable or become exercisable within 60 days.
(5) Includes 116,062 shares of common stock underlying options which are currently exercisable or become exercisable within 60 days and 55,392 shares of common stock underlying warrants.
(6) Includes 39,755 shares of common stock underlying options which are currently exercisable or become exercisable within 60 days.
(7) Includes 38,729 shares of common stock underlying options and restricted stock units which are currently exercisable or vested or become exercisable within 60 days, 74,536 shares of common stock and 10,834 shares of common stock underlying warrants owned by Lysander, LLC and 6,546 shares owned by Oystercatcher Trust. Stuart Davidson, as the Member of Lysander, LLC and Trustee of Oystercatcher Trust, has investment and voting control over the shares held by these entities.
(8) Includes 39,479 shares of common stock underlying options and restricted stock units which are currently exercisable or vested or become exercisable within 60 days.
(9) Includes 31,979 shares of common stock underlying options which are currently exercisable or become exercisable within 60 days and 32,457 shares of common stock and 12,667 shares of common stock underlying warrants owned by L&L. Donald Landry, as a Member of L&L, has investment and voting control over the shares held by this entity.
(10) Includes 31,979 shares of common stock underlying options which are currently exercisable or become exercisable within 60 days and 10,833 shares of common stock underlying warrants.
(11) Includes 31,979 shares of common stock underlying options which are currently exercisable or become exercisable within 60 days and 3,000 shares of common stock underlying warrants.
(12) Includes 36,470 shares of common stock underlying options which are currently exercisable or become exercisable within 60 days and 26,765 shares of common stock underlying warrants.
(13) Includes 37,979 shares of common stock underlying options and restricted stock units which are currently exercisable or vested or become exercisable within 60 days and 14,217 shares of common stock underlying warrants.
(14) Includes 767,245 shares of common stock underlying options and restricted stock units which are currently exercisable or vested or become exercisable within 60 days, 184,628 shares of common stock and 54,500 shares of common stock underlying warrants owned by Lederman & Co, 32,457 shares of common stock and 12,667 shares of common stock underlying warrants owned by L&L, 58,972 shares of common stock and 8,250 shares of common stock underlying warrants owned by Targent, 29,167 shares of common stock and 4,167 shares of common stock underlying warrants owned by Leder Labs, 29,167 shares of common stock and 4,167 shares of common stock underlying warrants owned by Starling, 74,536 shares of common stock and 10,834 shares of common stock underlying warrants owned by Lysander, LLC, 6,546 shares owned by Oystercatcher Trust and 123,222 shares of common stock underlying warrants owned directly by the executive officers and directors.
(15) Based upon a Schedule 13G/A filed with the SEC on February 16, 2016. The mailing address for this beneficial owner is 152 West 57th Street, 50th Floor, New York, NY 10019. Mark Kingdon is the managing member and has voting and investment power over the securities owned by it.
(16) Based upon a Schedule 13G/A filed with the SEC on February 16, 2016. The mailing address for this beneficial owner is 780 Third Avenue, 37th Floor, New York, New York 10017. Deerfield Mgmt, L.P. is the general partner of Deerfield Special Situations Fund, L.P. Deerfield Management Company, L.P. is the investment manager of Deerfield Special Situations Fund, L.P. Mr. James E. Flynn is the sole member of the general partner of Deerfield Mgmt, L.P. and Deerfield Management Company, L.P. Each of Deerfield Mgmt, L.P., Deerfield Management Company, L.P. and Mr. Flynn may be deemed to beneficially own the securities held by Deerfield Special Situations Fund, L.P.
(17) Based upon a Schedule 13G/A filed with the SEC on February 12, 2016. The mailing address for this beneficial owner is 300 Park Avenue, 25th Floor, New York, New York 10022. Kevin Kotler is the managing member and has voting and investment power over the securities owned by it.
| 64 |
ITEM 13 – CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
We have adopted a written related-person transactions policy that sets forth our policies and procedures regarding the identification, review, consideration and oversight of “related-party transactions.” For purposes of our policy only, a “related-party transaction” is a transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we and any “related party” are participants involving an amount that exceeds $120,000.
Transactions involving compensation for services provided to us as an employee, consultant or director are not considered related-person transactions under this policy. A related party is any executive officer, director or a holder of more than five percent of our common stock, including any of their immediate family members and any entity owned or controlled by such persons.
Under the policy, where a transaction has been identified as a related-party transaction, our Chief Compliance Officer must present information regarding the proposed related-party transaction to our Nominating and Corporate Governance Committee for review. The presentation must include a description of, among other things, the material facts, the direct and indirect interests of the related parties, the benefits of the transaction to us and whether any alternative transactions are available. To identify related-party transactions in advance, we rely on information supplied by our executive officers, directors and certain significant stockholders. In considering related-party transactions, our Nominating and Corporate Governance Committee will take into account the relevant available facts and circumstances including, but not limited to:
| • | whether the transaction was undertaken in the ordinary course of our business; | |
| • | whether the related party transaction was initiated by us or the related party; | |
| • | whether the transaction with the related party is proposed to be, or was, entered into on terms no less favorable to us than terms that could have been reached with an unrelated third party; | |
| • | the purpose of, and the potential benefits to us from the related party transaction; | |
| • | the approximate dollar value of the amount involved in the related party transaction, particularly as it relates to the related party; | |
| • | the related party’s interest in the related party transaction, and | |
| • | any other information regarding the related party transaction or the related party that would be material to investors in light of the circumstances of the particular transaction. |
The Nominating and Corporate Governance Committee shall then make a recommendation to the board of directors, who will determine whether or not to approve of the related party transaction, and if so, upon what terms and conditions. In the event a director has an interest in the proposed transaction, the director must recuse himself or herself from the deliberations and approval.
Other than as disclosed below, during the last two fiscal years, there have been no related party transactions.
We previously entered into an agreement with Lederman & Co, a company under the control of Dr. Seth Lederman, our Chief Executive Officer and Chairman of the Board of Directors. Effective October 15, 2013, Lederman & Co received $0.3 million per annum for its consulting services. On February 11, 2014, the agreement with Lederman & Co was terminated, and we simultaneously entered into an employment agreement with Dr. Lederman.
On July 31 and August 1, 2013, we sold three promissory notes in the aggregate principal face amount of $0.3 million to two related parties in exchange for $0.3 million. The notes were payable on demand at any time after one year from issuance and bear no interest, and were included in current liabilities on the consolidated balance sheet at December 31, 2013. On July 31, 2014 and August 1, 2014, we repaid $0.2 million and $0.1 million, respectively.
On March 18, 2014, Tonix Barbados entered into the Starling Agreement with Starling and the Leder Agreement with Leder. Seth Lederman, the Company’s Chairman and Chief Executive Officer, is the Chairman, CEO and majority owner (through majority-owned entities) of Starling and Leder. Pursuant to the Starling Agreement, Tonix Barbados acquired from Starling rights to a United States patent application for radio- and chemo-protective agents and related intellectual property rights, in exchange for $0.1 million and 25,000 shares of our common stock. Pursuant to the Leder Agreement, Tonix Barbados acquired from Leder rights to a United States patent application for novel smallpox vaccines and related intellectual property rights, in exchange for $0.1 million and 25,000 shares of our common stock.
| 65 |
On February 3, 2015, we entered into an underwriting agreement for an offering of common stock with a group of underwriters, including Janney Montgomery Scott LLC. Charles Mather, one of our directors, was a Managing Director of Janney until February 2015.
ITEM 14 – PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this item is incorporated by reference to our Proxy Statement for the 2016 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission (SEC) within 120 days of the fiscal year ended December 31, 2015.
| 66 |
ITEM 15 – EXHIBITS, FINANCIAL STATEMENT SCHEDULES
| (a) | List of Documents Filed as a Part of This Report: |
| Index to Consolidated Financial Statements | F-1 | |
| Report of Independent Registered Public Accounting Firm | F-2 | |
| Consolidated balance sheets as of December 31, 2015 and 2014 | F-3 | |
| Consolidated statements of operations for the years ended December 31, 2015, 2014 and 2013 | F-4 | |
| Consolidated statements of comprehensive loss for the years ended December 31, 2015, 2014 and 2013 | F-5 | |
| Consolidated statements of stockholders’ equity for the years ended December 31, 2015, 2014 and 2013 | F-6 | |
| Consolidated statements of cash flows for the years ended December 31, 2015, 2014 and 2013 | F-8 | |
| Notes to consolidated financial statements | F-9 |
| (b) | Index to Financial Statement Schedules: |
All schedules have been omitted because the required information is included in the consolidated financial statements or the notes thereto, or is not applicable or required.
| (c) | Index to Exhibits |
The Exhibits listed below are identified by numbers corresponding to the Exhibit Table of Item 601 of Regulation S-K. The Exhibits designated by an asterisk (*) are management contracts or compensatory plans or arrangements required to be filed pursuant to Item 15.
| Exhibit No. | Description | |
| 3.01 | Articles of Incorporation, filed as an exhibit to the Registration Statement on Form S-1, filed with the Securities and Exchange Commission (the “Commission”) on April 9, 2008 and incorporated herein by reference. | |
| 3.02 | Articles of Merger between Tamandare Explorations Inc. and Tonix Pharmaceuticals Holding Corp., effective October 11, 2011, filed as an exhibit to the Current Report on Form 8-K, filed with the Commission on October 17, 2011 and incorporated herein by reference. | |
| 3.03 | Amended and Restated Bylaws, filed as an exhibit to the Current Report on Form 8-K, filed with the Commission on June 19, 2014 and incorporated herein by reference. | |
| 10.01 | Lease Agreement, dated as of September 28, 2010, by and between 509 Madison Avenue Associates, L.P. and Tonix Pharmaceuticals, Inc., filed as an exhibit to the amended Current Report on Form 8-K/A, filed with the Commission on February 3, 2012 and incorporated herein by reference. | |
| 10.02 | Form of Class A Warrant, filed as an exhibit to the Current Report on Form 8-K, filed with the Commission on January 23, 2012 and incorporated herein by reference. | |
| 10.03 | Form of Class A Warrant, dated December 4, 2012, filed as an exhibit to the Current Report on Form 8-K filed with the Commission on December 5, 2012 and incorporated herein by reference. | |
| 10.04 | Form of Class A Warrant, dated December 21, 2012, filed as an exhibit to the Current Report on Form 8-K filed with the Commission on December 27, 2012 and incorporated herein by reference. | |
| 10.05 | Employment Agreement, between Tonix Pharmaceuticals Holding Corp. and Seth Lederman, dated February 11, 2014, filed as an exhibit to the Current Report on Form 8-K filed with the Commission on February 14, 2014 and incorporated herein by reference.* |
| 67 |
| 10.06 | Letter of Termination, between Tonix Pharmaceuticals Holding Corp. and Lederman & Co., LLC, dated February 11, 2014, filed as an exhibit to the Current Report on Form 8-K filed with the Commission on February 14, 2014 and incorporated herein by reference. | |
| 10.07 | Employment Agreement, between Tonix Pharmaceuticals Holding Corp. and Bruce Daugherty, dated March 14, 2014, filed as an exhibit to the Current Report on Form 8-K filed with the Commission on March 19, 2014 and incorporated herein by reference.* | |
| 10.08 | Employment Agreement, between Tonix Pharmaceuticals Holding Corp. and Leland Gershell, dated March 19, 2014, filed as an exhibit to the Current Report on Form 8-K filed with the Commission on March 19, 2014 and incorporated herein by reference.* | |
| 10.09 | Employment Agreement, between Tonix Pharmaceuticals Holding Corp. and Donald Kellerman, dated April 1, 2014, filed as an exhibit to the Current Report on Form 8-K filed with the Commission on April 1, 2014 and incorporated herein by reference.* | |
| 10.10 | Employment Agreement, between Tonix Pharmaceuticals Holding Corp. and Gregory Sullivan, dated June 3, 2014, filed as an exhibit to the Current Report on Form 8-K filed with the Commission on June 3, 2014 and incorporated herein by reference.* | |
| 10.11 | Form of Subscription Agreement, dated July 11, 2014 between Tonix Pharmaceuticals Holding Corp. and the investors named therein, filed as an exhibit to the Current Report on Form 8-K filed with the Commission on July 11, 2014 and incorporated herein by reference. | |
| 10.12 | Placement Agent Agreement, dated July 11, 2014 between Tonix Pharmaceuticals Holding Corp. and Roth Capital Partners, LLC, filed as an exhibit to the Current Report on Form 8-K filed with the Commission on July 11, 2014 and incorporated herein by reference. | |
| 10.13 | Lease Amendment and Expansion Agreement, dated February 11, 2014, by and between 509 Madison Avenue Associates, L.P. and Tonix Pharmaceuticals, Inc., filed as an exhibit to the Annual Report on Form 10-K filed with the Commission on February 27, 2015 and incorporated herein by reference. | |
| 14.01 | Code of Business Conduct and Ethics for Employees, Executive Officers and Directors, filed as an exhibit to the Current Report on Form 8-K, filed with the Commission on February 16, 2016 and incorporated herein by reference. | |
| 21.01 | List of Subsidiaries, filed herewith. | |
| 23.01 | Consent of Independent Registered Public Accounting Firm, filed herewith. | |
| 31.01 | Certification of Chief Executive Officer pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 31.02 | Certification of Chief Financial Officer pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 32.01 | Certifications of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 101 | The following materials from Tonix Pharmaceuticals Holding Corp.’s Annual Report on Form 10-K for the year ended December 31, 2015, formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Operations, (iii) the Consolidated Statements of Comprehensive Loss, (iv) the Consolidated Statements of Stockholders' Equity, (v) the Consolidated Statements of Cash Flows, and (vi) Notes to Consolidated Financial Statements. |
| 68 |
In accordance with the requirements of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| TONIX PHARMACEUTICALS HOLDING CORP. | |||
| Date: March 3, 2016 | By: | /s/ SETH LEDERMAN | |
| Seth Lederman | |||
| Chief Executive Officer (Principal Executive Officer) |
|||
| Date March 3, 2016 | By: | /s/ BRADLEY SAENGER | |
| Bradley Saenger | |||
| Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name | Position | Date | ||
| /s/ SETH LEDERMAN | Director | March 3, 2016 | ||
| Seth Lederman | ||||
| /s/ STUART DAVIDSON | Director | March 3, 2016 | ||
| Stuart Davidson | ||||
| /s/ PATRICK GRACE | Director | March 3, 2016 | ||
| Patrick Grace | ||||
| /s/ DONALD W. LANDRY | Director | March 3, 2016 | ||
| Donald W. Landry | ||||
| /s/ ERNEST MARIO | Director | March 3, 2016 | ||
| Ernest Mario | ||||
| /s/ CHARLES MATHER IV | Director | March 3, 2016 | ||
| Charles Mather IV | ||||
| /s/ JOHN RHODES | Director | March 3, 2016 | ||
| John Rhodes | ||||
| /s/ SAMUEL SAKS | Director | March 3, 2016 | ||
| Samuel Saks |
| 69 |
