Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - ZIOPHARM ONCOLOGY INC | d284050dex991.htm |
| 8-K - FORM 8-K - ZIOPHARM ONCOLOGY INC | d284050d8k.htm |
Exhibit 99.2

ZIOPHARM Oncology
Investor Update Call
November 17, 2016
21st Annual Scientific Meeting of the Society for Neuro-Oncology
1

Forward-looking statements
This presentation contains certain forward-looking information about ZIOPHARM Oncology, Inc. that is intended to be covered by the safe harbor for
“forward-looking statements” provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts, and in some cases can be identified by terms such as
“may,” “will,” “could,” “expects,” “plans,” “anticipates,” and “believes.” These statements include, but are not limited to, statements regarding the progress, timing and results
of preclinical and clinical trials involving the Company’s drug candidates, and the progress of the Company’s research and development programs. All of such statements are subject to certain risks and uncertainties, many of which are
difficult to predict and generally beyond the control of the Company, that could cause actual results to differ materially from those expressed in, or implied by, the forward-looking statements. These risks and uncertainties include, but are not
limited to: whether chimeric antigen receptor T cell (CAR T) approaches, Ad-RTS-IL-12, TCR and NK cell-based therapies, or any of our other therapeutic candidates will advance further in the pre-clinical or clinical trials process and whether and
when, if at all, they will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether chimeric antigen receptor T cell (CAR T) approaches, Ad-RTS-IL-12, TCR and NK
cell-based therapies, and our other therapeutic products will be successfully marketed if approved; the strength and enforceability of our intellectual property rights; competition from other pharmaceutical and biotechnology companies; and the other
risk factors contained in our periodic and interim SEC reports filed from time to time with the Securities and Exchange Commission, including but not limited to, our Annual Report on Form 10-K for the fiscal year ended December 31, 2015, and
our Quarterly Report on Form 10-Q for the quarter ended September 30,
2016. Readers are cautioned not to place undue reliance on these forward-looking
statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the
occurrence of or non-occurrence of any events.
2
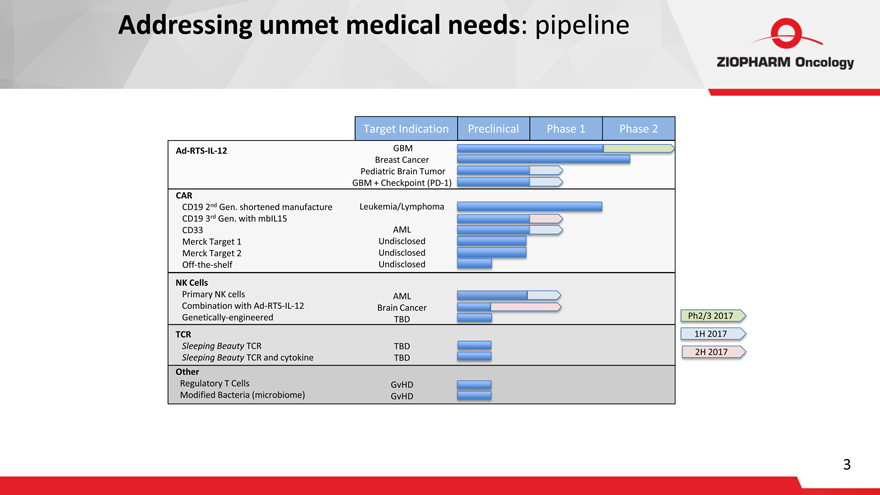
Addressing unmet medical needs: pipeline
Ad-RTS-IL-12
CAR
CD19 2nd Gen. shortened manufacture
CD19 3rd Gen. with mbIL15
CD33
Merck Target 1
Merck Target 2
Off-the-shelf
NK Cells
Primary NK cells
Combination with Ad-RTS-IL-12
Genetically-engineered
TCR
Sleeping Beauty TCR
Sleeping Beauty TCR and cytokine
Other
Regulatory T Cells
Modified Bacteria (microbiome)
Target Indication
GBM Breast Cancer Pediatric Brain Tumor
GBM + Checkpoint (PD-1) Leukemia/Lymphoma
AML
Undisclosed
Undisclosed
Undisclosed
AML Brain Cancer TBD
TBD TBD
GvHD GvHD
Preclinical Phase 1 Phase 2
Ph2/3 2017
1H 2017
2H 2017
3
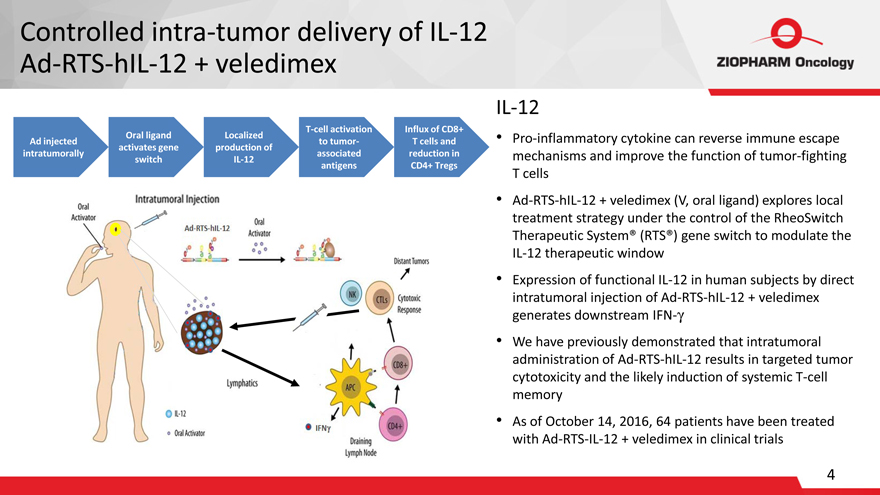
Controlled intra-tumor delivery of IL-12
Ad-RTS-hIL-12 + veledimex
IL-12
Ad injected intratumorally
Oral ligand activates gene switch
Localized production of IL-12
T-cell activation to tumor- associated antigens
Influx of CD8+ T cells and reduction in CD4+ Tregs
Pro-inflammatory cytokine can reverse
immune escape mechanisms and improve the function of tumor-fighting T cells
Ad-RTS-hIL-12 + veledimex (V, oral ligand) explores local treatment strategy under the
control of the RheoSwitch Therapeutic System® (RTS®) gene switch to modulate the IL-12 therapeutic window
Expression of functional IL-12 in human subjects
by direct intratumoral injection of Ad-RTS-hIL-12 + veledimex generates downstream IFN-?
We have previously demonstrated that intratumoral administration of
Ad-RTS-hIL-12 results in targeted tumor cytotoxicity and the likely induction of systemic T-cell memory
As of October 14, 2016, 64 patients have been treated
with Ad-RTS-IL-12 + veledimex in clinical trials
4
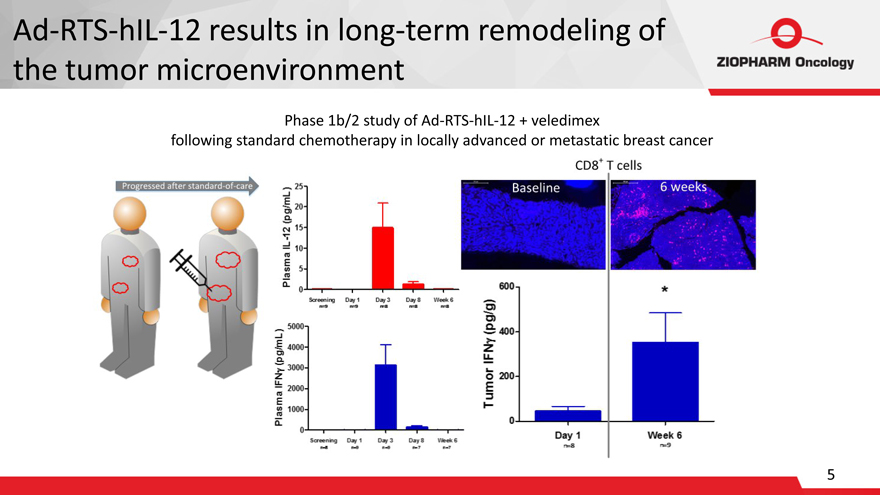
Ad-RTS-hIL-12 results in long-term remodeling of
the tumor microenvironment
Phase 1b/2 study of Ad-RTS-hIL-12 + veledimex
following standard chemotherapy in locally advanced or metastatic breast cancer
5
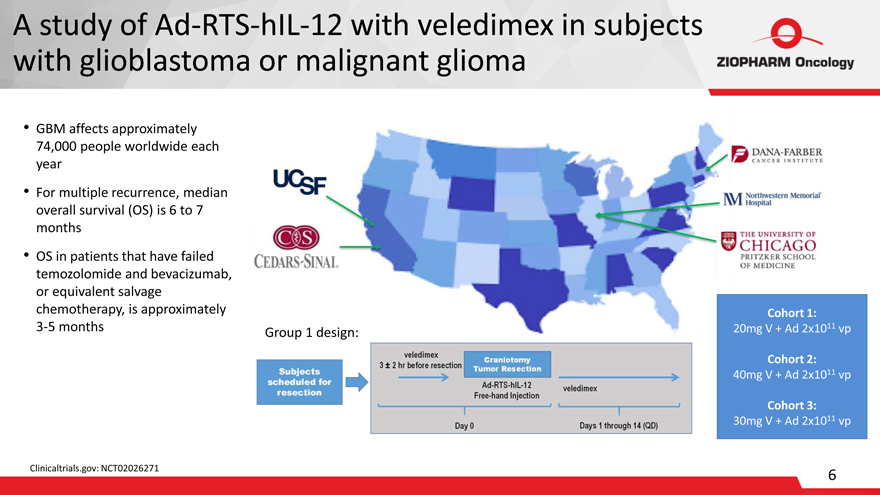
A study of Ad-RTS-hIL-12 with veledimex in subjects with glioblastoma or malignant glioma
GBM affects approximately
74,000 people worldwide each
year
For multiple recurrence, median overall survival (OS) is 6 to 7 months
OS in patients that have failed temozolomide and bevacizumab, or equivalent salvage chemotherapy, is approximately
3-5 months
Clinicaltrials.gov: NCT02026271
Group 1 design:
Cohort 1:
20mg V + Ad 2x1011 vp
Cohort 2:
40mg V + Ad 2x1011 vp
Cohort 3:
30mg V + Ad 2x1011 vp
6
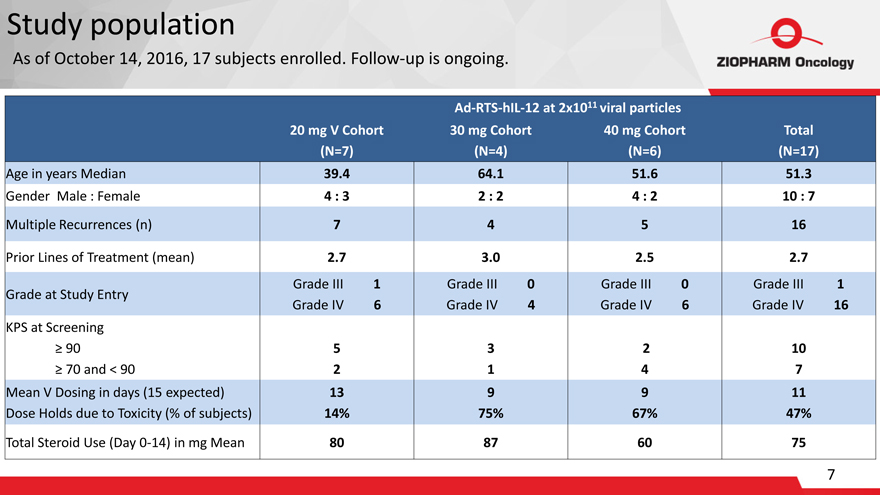
Study population
As of
October 14, 2016, 17 subjects enrolled. Follow-up is ongoing.
Ad-RTS-hIL-12 at 2x1011 viral particles
| 20 | mg V Cohort 30 mg Cohort 40 mg Cohort Total |
(N=7)(N=4)(N=6)(N=17)
Age in years Median-39.4-64.1-51.6-51.3
Gender Male : Female-4 : 3-2 : 2-4 : 2-10 : 7
Multiple Recurrences (n)- 7- 4-
5- 16 Prior Lines of Treatment (mean)-
2.7- 3.0- 2.5- 2.7
Grade at Study Entry-Grade III 1 Grade IV 6-Grade III 0
Grade IV 4-Grade III 0 Grade IV
6-Grade III 1 Grade IV 16 KPS at Screening
90
70 and < 90- 5 2- 3 1- 2 4-
10 7
Mean V Dosing in days (15 expected)
Dose Holds due to Toxicity (% of
subjects)-13
14%-9 75%-9 67%-11 47%
Total Steroid Use (Day 0-14) in mg Mean-
80- 87-
60-
75
| 7 |
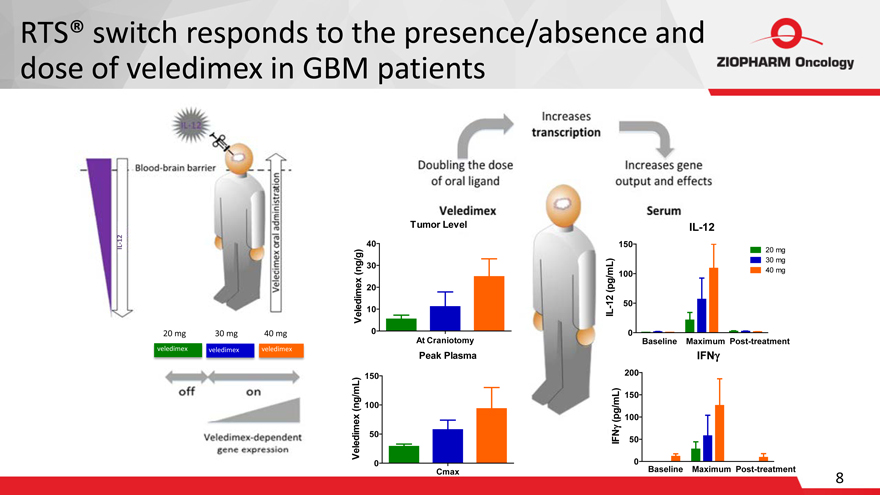
RTS® switch responds to the presence/absence and dose of veledimex in GBM patients
Tumor Level
40
30
20
10
150
100
50
IL-12
20 mg
30 mg
40 mg
20 mg 30 mg 40 mg
0
At Craniotomy
0
Baseline Maximum Post-treatment
veledimex
veledimex veledimex
150
Peak Plasma
200
IFN?
100
50
150
100
50
0
Cmax
0
Baseline Maximum Post-treatment
8
IL-12
Veledimex (ng/g)
IL-12 (pg/mL)
Veledimex (ng/mL)
20 mg
30 mg
40 mg
IFN? (pg/mL)
8
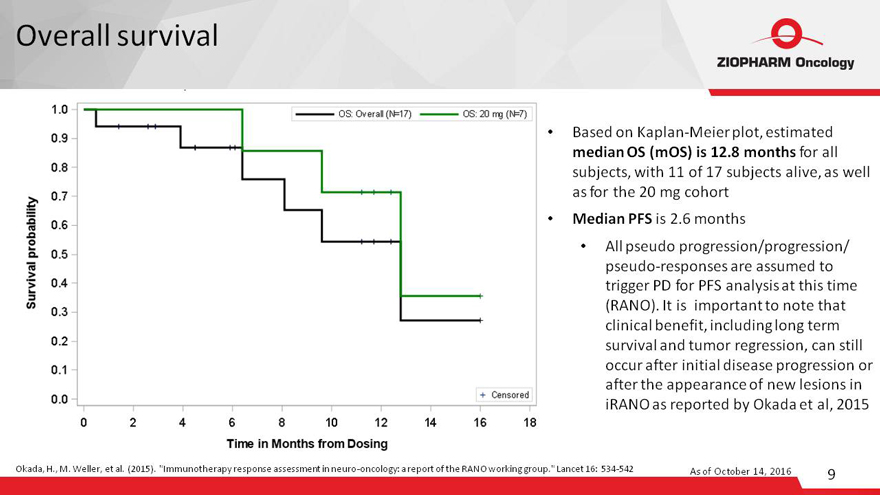
Overall survival
Based on
Kaplan-Meier plot, estimated median OS (mOS) is 12.8 months for all subjects, with 11 of 17 subjects alive, as well as for the 20 mg cohort
Median PFS is 2.6
months
All pseudo progression/progression/
pseudo-responses are assumed to
trigger PD for PFS analysis at this time (RANO). It is important to note that clinical benefit, including long term survival and tumor regression, can still occur after initial disease progression or after the appearance of new lesions in iRANO as
reported by Okada et al, 2015
Okada, H., M. Weller, et al. (2015). “Immunotherapy response assessment in neuro-oncology: a report of the RANO working
group.” Lancet 16: 534-542
As of October 14, 2016
9
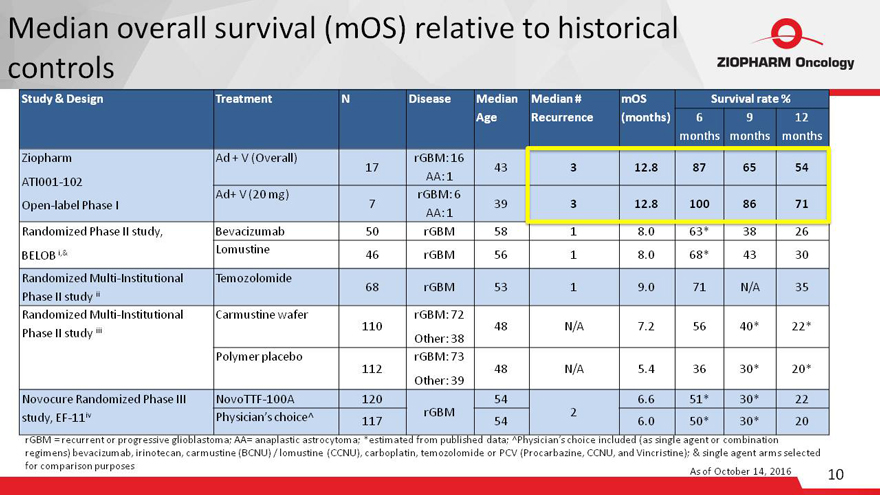
Median overall survival (mOS) relative to historical
controls
Study & Design Treatment N Disease Median
Age Median # Recurrence mOS (months) Survival rate %
6 months 9 months 12 months
Ziopharm
ATI001-102
Open-label Phase I Ad + V (Overall)
17 rGBM: 16
AA: 1
43
3
12.8
87
65
54
Ad+ V (20 mg)
7 rGBM: 6
AA: 1
39
3
12.8
100
86
71
Randomized Phase II study,
BELOB i,& Bevacizumab 50 rGBM 58 1 8.0 63* 38 26
Lomustine
46
rGBM
56
1
8.0
68*
43
30
Randomized Multi-Institutional
Phase II study ii Temozolomide
68
rGBM
53
1
9.0
71
N/A
35
Randomized Multi-Institutional
Phase II study iii Carmustine wafer
110 rGBM: 72
Other: 38
48
N/A
7.2
56
40*
22*
Polymer placebo
112 rGBM: 73
Other: 39
48
N/A
5.4
36
30*
20*
Novocure Randomized Phase III
study, EF-11iv NovoTTF-100A 120
rGBM 54
2 6.6 51* 30* 22
Physician’s choice^
117
54
6.0
50*
30*
20
rGBM = recurrent or progressive glioblastoma; AA= anaplastic astrocytoma; *estimated from published data; ^Physician’s choice included (as single agent
or combination
regimens) bevacizumab, irinotecan, carmustine (BCNU) / lomustine (CCNU), carboplatin, temozolomide or PCV (Procarbazine, CCNU, and
Vincristine); & single agent arms selected
for comparison purposes
As of October 14, 2016 11
10
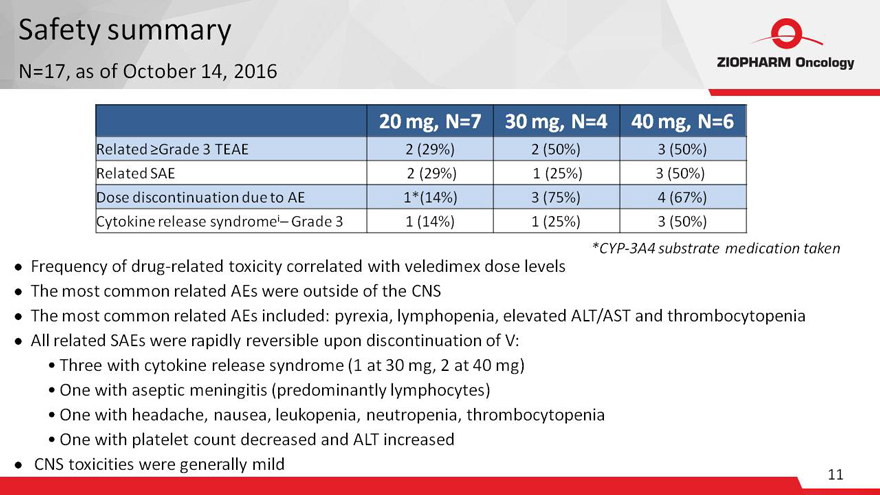
Safety summary
N=17, as
of October 14, 2016
20 mg, N=7 30 mg, N=4 40 mg, N=6
Related ?Grade 3
TEAE 2 (29%) 2 (50%) 3 (50%)
Related SAE 2 (29%) 1 (25%) 3 (50%)
Dose discontinuation due to AE 1*(14%) 3 (75%) 4 (67%)
Cytokine release syndromei–
Grade 3 1 (14%) 1 (25%) 3 (50%)
Frequency of drug-related toxicity correlated with veledimex dose levels
The most common related AEs were outside of the CNS
*CYP-3A4 substrate medication taken
The most common related AEs included: pyrexia, lymphopenia, elevated ALT/AST and thrombocytopenia
All related SAEs were rapidly reversible upon discontinuation of V:
Three with cytokine
release syndrome (1 at 30 mg, 2 at 40 mg)
One with aseptic meningitis (predominantly lymphocytes)
One with headache, nausea, leukopenia, neutropenia, thrombocytopenia
One with platelet count
decreased and ALT increased
CNS toxicities were generally mild
11
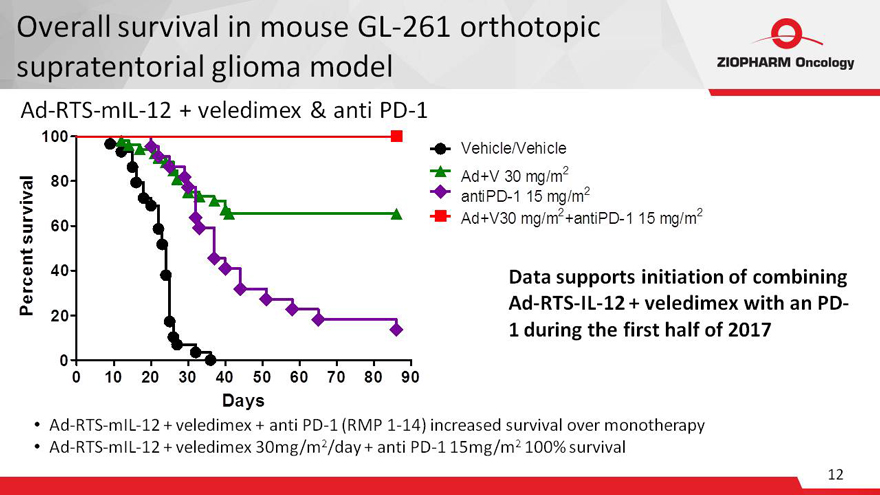
Overall survival in mouse GL-261 orthotopic
supratentorial glioma model
Ad-RTS-mIL-12 + veledimex & anti PD-1
100
Vehicle/Vehicle
80 Ad+V 30 mg/m2
antiPD-1 15 mg/m2
2 2
60 Ad+V30 mg/m
+antiPD-1 15 mg/m
40
20
0
0
10 20 30 40 50 60 70 80 90
Days
Percent survival
Data supports initiation of combining
Ad-RTS-IL-12 + veledimex with an PD-
1 during the first half of 2017
Ad-RTS-mIL-12 + veledimex + anti PD-1 (RMP 1-14) increased
survival over monotherapy
Ad-RTS-mIL-12 + veledimex 30mg/m2/day + anti PD-1 15mg/m2 100% survival
12
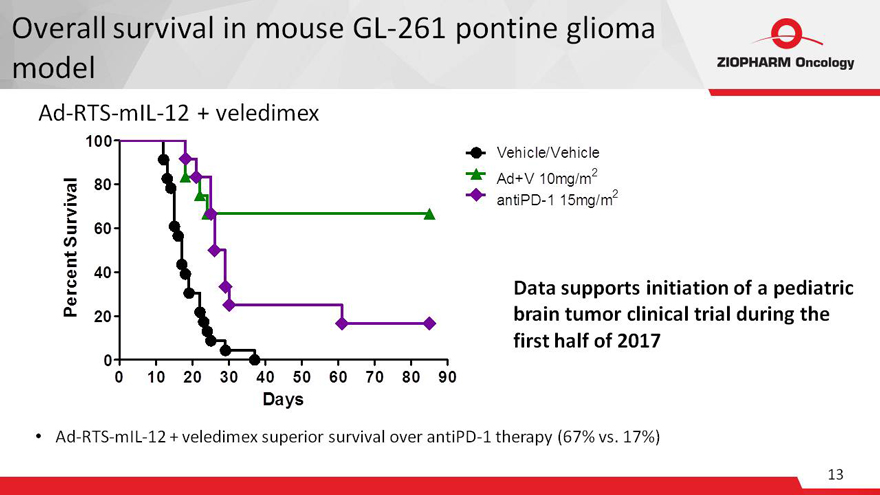
Overall survival in mouse GL-261 pontine glioma
model
Ad-RTS-mIL-12 + veledimex
100
Vehicle/Vehicle
80 Ad+V 10mg/m2
antiPD-1 15mg/m2
60
40
20
0
0 10 20 30 40 50 60 70 80 90
Days Percent Survival
Data supports initiation of a pediatric brain tumor clinical trial
during the first half of 2017
Ad-RTS-mIL-12 + veledimex superior survival over antiPD-1 therapy (67% vs. 17%)
13

Conclusions and next steps
Ad-RTS-hIL-12 + 20 mg veledimex is well tolerated and suggests a survival benefit over historical control at
6, 9 and 12 months
Based on tolerability and survival benefit, 20 mg has been selected for an
(ongoing) expansion cohort
Toxicities were tolerable, predictable and reversible upon discontinuing veledimex
There is a positive correlation between veledimex dose, blood-brain barrier penetration, IL-12 levels
These data demonstrate that the RTS® gene switch works in humans toggling not only as a switch to turn on and off the production of IL-12, but also as a rheostat to control the
level of IL-12
Next Steps
Continue study at 20 mg in an expansion cohort
Discuss with FDA to determine appropriate next steps for a registration-enabling study
Initiate an anti-PD1 combination study in the first half of 2017
Initiate a pediatric brain
tumor study in the first half of 2017
14

Corporate and financial highlights
Amended Exclusive Channel Collaborations with Intrexon
o All unpartnered programs divided
80:20 in favor of ZIOPHARM
– Provides greater reward for investing in development
– Increases attractiveness to potential strategic partners
o Sublicensed programs
continue to be divided 50:50*
o Consideration for new terms is dilutive only under certain developments
Well Capitalized
o Approximately $94.7M in cash as of 3Q16
o Cash runway through 4Q17
o Shares outstanding as of November 3, 2016: 131.7M
Highly Efficient Operations with a Headcount of 33
o Partnerships support
preclinical, clinical and commercial development
*Includes exclusive agreement with Intrexon for the development and commercialization of CAR-T products with Merck
KGaA, Darmstadt, Germany
15

Intra-tumoral IL-12 RheoSwitch® programs
o Initiate Phase 2/3 clinical trial for GBM in 2017
o Initiate combination study of
Ad-RTS-hIL-12 + veledimex with immune checkpoint inhibitor therapy (PD-1) during the first half of 2017
o Initiate Phase 1 study in the treatment of brain tumors
in children during the first half of 2017
CAR+ T programs
o Continuation of
CD19 CAR+ T clinical study
o Initiate a CD33 specific CAR+ T clinical study for relapsed or refractory AML in the first half of 2017
o Initiate CAR+ T-cell preclinical studies for other hematological malignancies and solid tumors in 2016
TCR-T programs
o Initiate TCR-modified T-cell preclinical studies in 2016
o Preclinical data to be presented at ASH 2016
NK cell programs
o Initiate a Phase 1 study of off-the-shelf NK cells for AML in 2017
GvHD programs
o Initiate preclinical studies in 2016
16

References
i: Taal, W, et
al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus
lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised
controlled phase 2
trial. Lancet Oncology, 2014, 15: 943–953.
ii:
Balmaceda C, et al. Multi-institutional phase II study of temozolomide administered twice daily in
the treatment of recurrent high-grade gliomas. Cancer.
2008;112(5): 1139–1146.
iii: Brem, H, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery
by biodegradable polymers of chemotherapy for recurrent gliomas. The Lancet 1995;345: 1008-
1012.
iv: Stupp R, et al. NovoTTF-100A versus physician’s choice
chemotherapy in recurrent glioblastoma:
A randomized phase III trial of a novel treatment modality. European J of Cancer. 2012;48:2192-
2202.
17

ZIOPHARM Oncology
Investor Update Call
November 17, 2016
21st Annual Scientific Meeting of the Society for Neuro-Oncology
