Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - KERYX BIOPHARMACEUTICALS INC | d286746dex991.htm |
| 8-K - FORM 8-K - KERYX BIOPHARMACEUTICALS INC | d286746d8k.htm |

Third Quarter 2016 Financial Results Conference Call November 9, 2016 Keryx Biopharmaceuticals, Inc. Exhibit 99.2

Safe Harbor Statement Some of the statements included in this presentation, particularly those regarding future revenues and expenses and the commercialization and ongoing clinical development of Auryxia, including those statements related to the interruption in the supply of Auryxia and when Auryxia may be available to patients again as well as the submission of an sNDA to the FDA to expend the label of ferric citrate to include the treatment of IDA in adults with stage 3-5 NDD-CKD, may be forward-looking statements that involve a number of risks and uncertainties. For those statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. Among the factors that could cause our actual results to differ materially are the following: our ability to quickly and successfully identify and resolve the production-related issue; our ability to quickly and successfully identify and engage secondary suppliers of finished drug product; our ability to receive FDA approval of any secondary suppliers of finished drug product including approval of our second manufacturer by the assigned PDUFA action date; whether we can increase adoption of Auryxia in patients with CKD on dialysis; whether we can maintain our operating expenses to projected levels while continuing our current clinical, regulatory and commercial activities; whether we will able to identify and negotiate acceptable terms with a commercialization partner in the E.U.; whether we or a partner can successfully launch Fexeric® in the E.U.; whether Riona will be successfully marketed in Japan by our Japanese partner, Japan Tobacco, Inc. and its subsidiary Torii Pharmaceutical Co., Ltd; the risk that the FDA may not concur with our interpretation of our Phase 3 study results in NDD-CKD, supportive data, conduct of the studies, or any other part of our regulatory submission and could ultimately deny approval of ferric citrate for the treatment of IDA in adults with stage 3-5 NDD-CKD; the risk that if approved for use in NDD-CKD that we may not be able to successfully market Auryxia for use in this indication; and other risk factors identified from time to time in our reports filed with the Securities and Exchange Commission. Any forward looking statements set forth in this press release speak only as of the date of this press release. We do not undertake to update any of these forward-looking statements to reflect events or circumstances that occur after the date hereof. This press release and prior releases are available at http://www.keryx.com. The information found on our website is not incorporated by reference into this press release and is included for reference purposes only.
Third Quarter 2016 Financial Results - Agenda Topic Speaker Introduction Amy Sullivan, VP Strategic Operations and Corporate Affairs Opening Remarks Greg Madison, President and CEO Third Quarter Financial Results Scott Holmes, Chief Financial Officer sNDA submission and ASN 2016 John Neylan, M.D., Chief Medical Officer Plans to make Auryxia available to patients Doug Jermasek, VP, Marketing & Strategy Question & Answer All
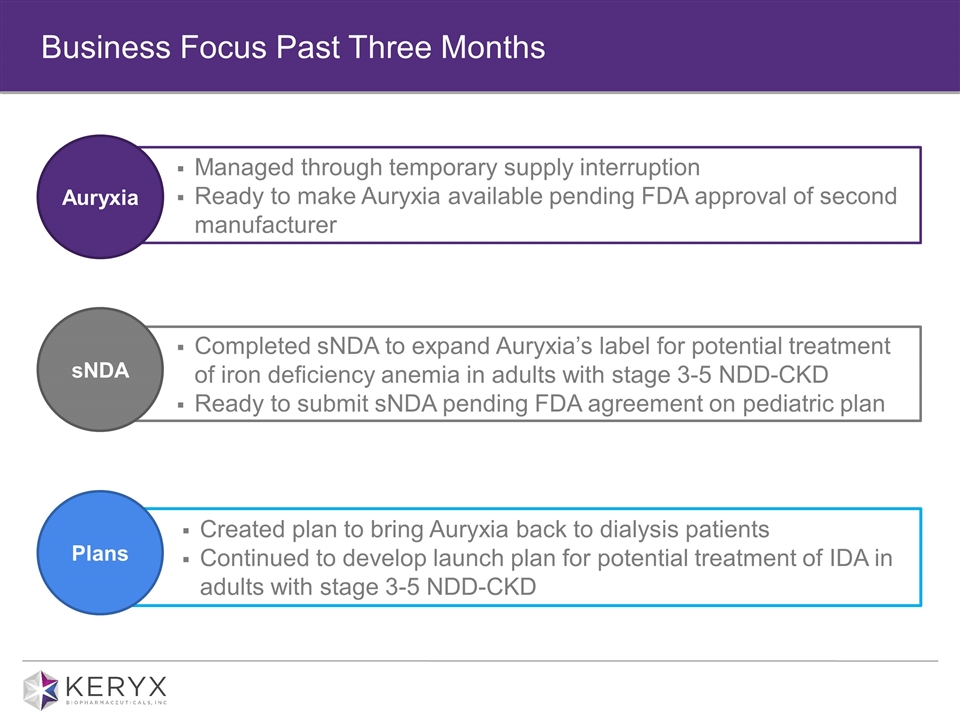
Business Focus Past Three Months Managed through temporary supply interruption Ready to make Auryxia available pending FDA approval of second manufacturer Completed sNDA to expand Auryxia’s label for potential treatment of iron deficiency anemia in adults with stage 3-5 NDD-CKD Ready to submit sNDA pending FDA agreement on pediatric plan Created plan to bring Auryxia back to dialysis patients Continued to develop launch plan for potential treatment of IDA in adults with stage 3-5 NDD-CKD Plans Auryxia sNDA
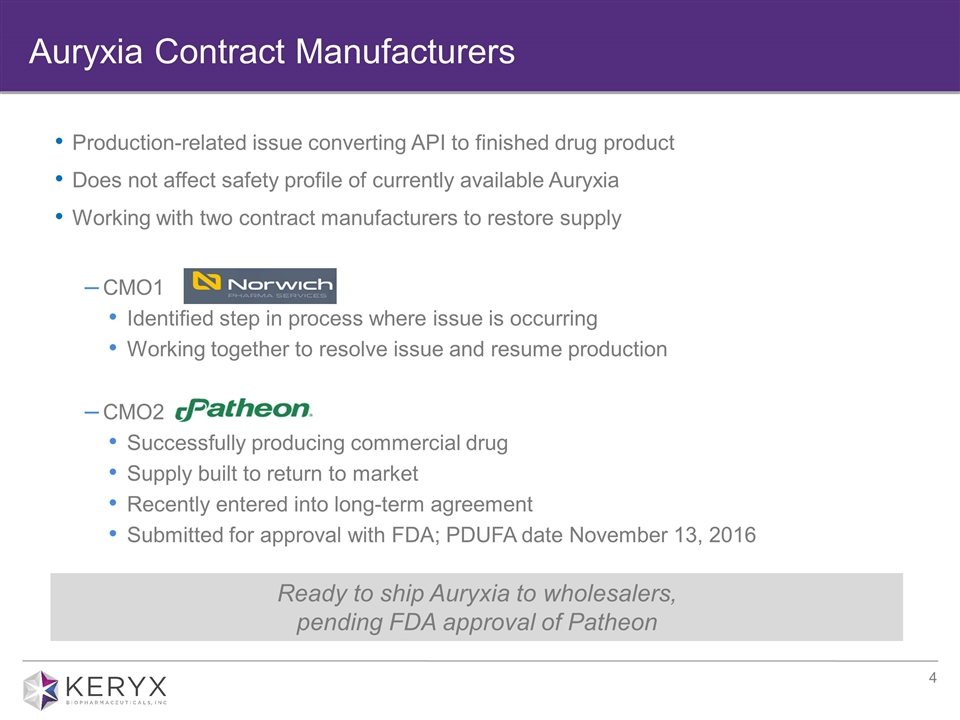
Production-related issue converting API to finished drug product Does not affect safety profile of currently available Auryxia Working with two contract manufacturers to restore supply CMO1 Identified step in process where issue is occurring Working together to resolve issue and resume production CMO2 Successfully producing commercial drug Supply built to return to market Recently entered into long-term agreement Submitted for approval with FDA; PDUFA date November 13, 2016 Auryxia Contract Manufacturers Ready to ship Auryxia to wholesalers, pending FDA approval of Patheon

Financial Highlights Scott Holmes Chief Financial Officer
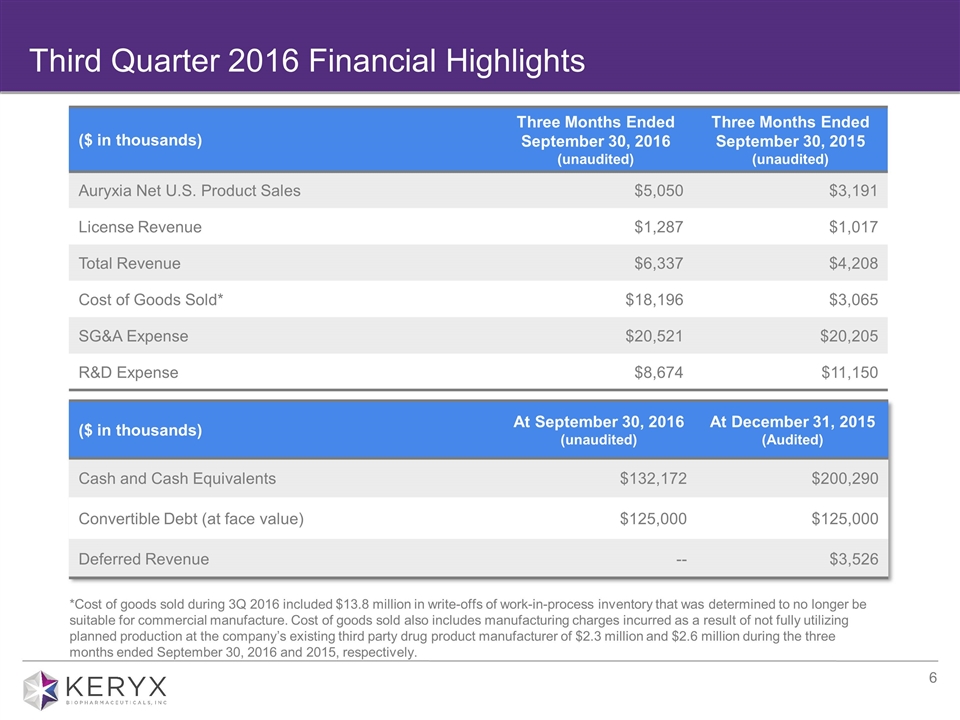
Third Quarter 2016 Financial Highlights ($ in thousands) Three Months Ended September 30, 2016 (unaudited) Three Months Ended September 30, 2015 (unaudited) Auryxia Net U.S. Product Sales $5,050 $3,191 License Revenue $1,287 $1,017 Total Revenue $6,337 $4,208 Cost of Goods Sold* $18,196 $3,065 SG&A Expense $20,521 $20,205 R&D Expense $8,674 $11,150 ($ in thousands) At September 30, 2016 (unaudited) At December 31, 2015 (Audited) Cash and Cash Equivalents $132,172 $200,290 Convertible Debt (at face value) $125,000 $125,000 Deferred Revenue -- $3,526 *Cost of goods sold during 3Q 2016 included $13.8 million in write-offs of work-in-process inventory that was determined to no longer be suitable for commercial manufacture. Cost of goods sold also includes manufacturing charges incurred as a result of not fully utilizing planned production at the company’s existing third party drug product manufacturer of $2.3 million and $2.6 million during the three months ended September 30, 2016 and 2015, respectively.

sNDA for Potential Label Expansion & ASN 2016 John Neylan, M.D. Chief Medical Officer
Potential Label Expansion of Auryxia Anemia Management Phase 3 study for IDA in adults with stage 3-5 NDD-CKD demonstrated statistically significant differences between the ferric citrate and placebo groups for the primary and all pre-specified secondary endpoints sNDA complete; ready to submit application, pending agreement with FDA on pediatric plan Four IDA-related abstracts accepted for poster presentations at 2016 ASN Plan to submit Phase 3 data for publication in a peer reviewed medical journal Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; Ferric citrate is being evaluated for use in non-dialysis dependent CKD patients as a treatment for iron deficiency anemia.
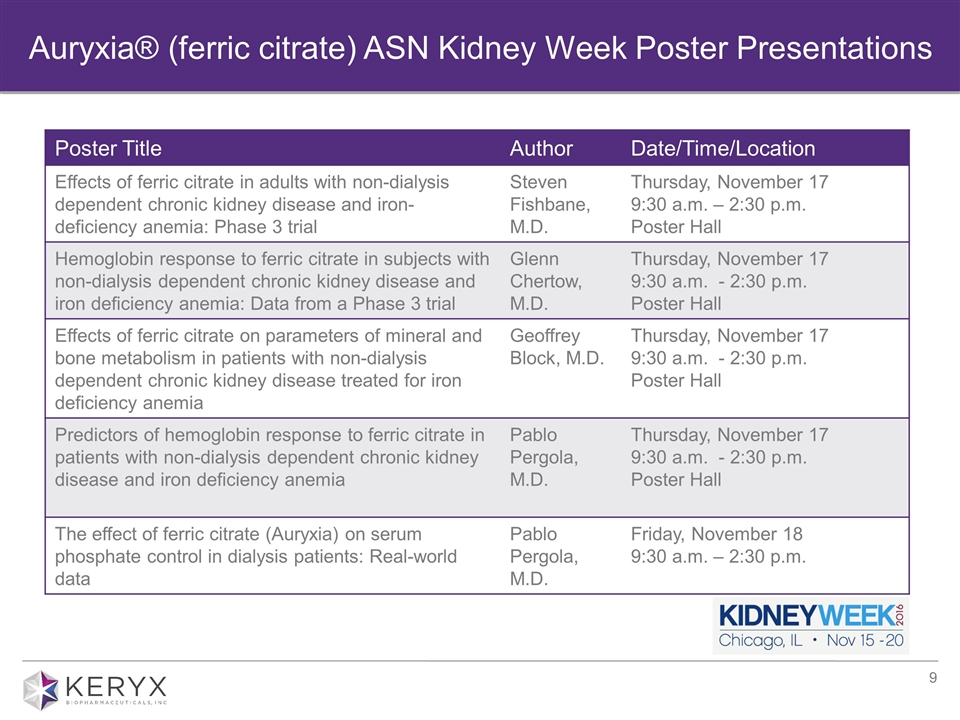
Auryxia® (ferric citrate) ASN Kidney Week Poster Presentations Poster Title Author Date/Time/Location Effects of ferric citrate in adults with non-dialysis dependent chronic kidney disease and iron-deficiency anemia: Phase 3 trial Steven Fishbane, M.D. Thursday, November 17 9:30 a.m. – 2:30 p.m. Poster Hall Hemoglobin response to ferric citrate in subjects with non-dialysis dependent chronic kidney disease and iron deficiency anemia: Data from a Phase 3 trial Glenn Chertow, M.D. Thursday, November 17 9:30 a.m. - 2:30 p.m. Poster Hall Effects of ferric citrate on parameters of mineral and bone metabolism in patients with non-dialysis dependent chronic kidney disease treated for iron deficiency anemia Geoffrey Block, M.D. Thursday, November 17 9:30 a.m. - 2:30 p.m. Poster Hall Predictors of hemoglobin response to ferric citrate in patients with non-dialysis dependent chronic kidney disease and iron deficiency anemia Pablo Pergola, M.D. Thursday, November 17 9:30 a.m. - 2:30 p.m. Poster Hall The effect of ferric citrate (Auryxia) on serum phosphate control in dialysis patients: Real-world data Pablo Pergola, M.D. Friday, November 18 9:30 a.m. – 2:30 p.m.

Auryxia Return to Patients Doug Jermasek V.P. Marketing and Strategy

Renal Community Support
Three types of patients upon resupply: Patients who have been switched to another binder Patients who have been maintained on Auryxia New patients who could benefit from Auryxia treatment Market research suggests: Physicians and dietitians who have had patients on Auryxia have a willingness to return patients to Auryxia Variability around the timing of Auryxia use Sales force has remained engaged with the renal care teams during the supply interruption and is ready for resupply Commercial Readiness for Auryxia Resupply Goal: Recapture sales momentum as soon as possible and ensure that patients once again have access to the potential benefits of Auryxia
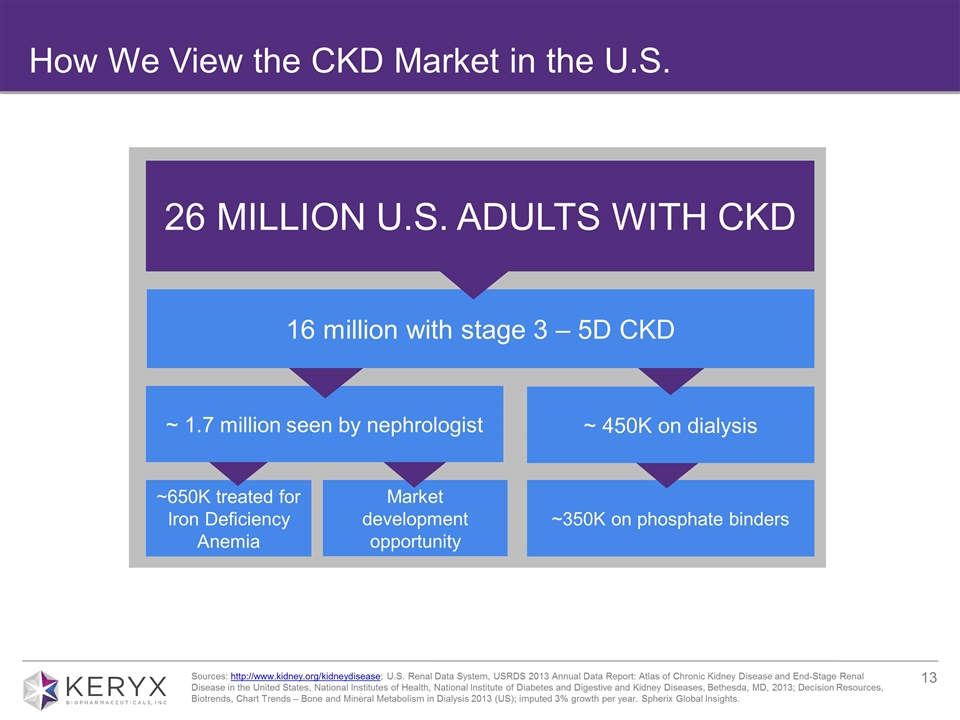
How We View the CKD Market in the U.S. Sources: http://www.kidney.org/kidneydisease; U.S. Renal Data System, USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013; Decision Resources, Biotrends, Chart Trends – Bone and Mineral Metabolism in Dialysis 2013 (US); imputed 3% growth per year. Spherix Global Insights. 26 MILLION U.S. ADULTS WITH CKD ~650K treated for Iron Deficiency Anemia ~350K on phosphate binders ~ 450K on dialysis Market development opportunity ~ 1.7 million seen by nephrologist 16 million with stage 3 – 5D CKD

Closing Remarks and Q&A

Key Takeaways Supply of Auryxia rebuilt; prepared to make Auryxia available to people on dialysis, pending FDA approval of Patheon Completed sNDA for potential label expansion of Auryxia; ready to submit application pending FDA agreement on pediatric plan Presenting more detailed IDA/CKD Phase 3 data at ASN Kidney Week Developing go-to-market strategy for IDA/NDD-CKD opportunity Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; Ferric citrate is being evaluated for use in non-dialysis dependent CKD patients as a treatment for iron deficiency anemia.

Third Quarter 2016 Financial Results Conference Call November 9, 2016 Keryx Biopharmaceuticals, Inc.
