Attached files
| file | filename |
|---|---|
| EX-99.1 - PRESS RELEASE - LUMOS PHARMA, INC. | nlnk-20161101x8kxex991.htm |
| 8-K - 8-K - LUMOS PHARMA, INC. | nlnk-20161101x8k.htm |

NewLink Genetics Corporation
Nasdaq: NLNK
November 1, 2016
Third Quarter 2016 Financial Results

Cautionary Note Regarding Forward Looking Statements
This presentation contains forward-looking statements of NewLink that involve substantial risks and uncertainties. All
statements, other than statements of historical fact, contained in this press release are forward-looking statements, within
the meaning of The Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “estimate,” “expect,”
“intend,” “may,” “plan,” “target,” “potential,” “will,” “could,” “should,” “seek” or the negative of these terms or other similar
expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these
identifying words. These forward-looking statements include, among others, statements about NewLink Genetics’ financial
guidance for 2016; results of its clinical trials for product candidates; its timing of release of data from ongoing clinical
studies; its plans related to moving additional indications into clinical development; NewLink Genetics’ future financial
performance, results of operations, cash position and sufficiency of capital resources to fund its operating requirements; and
any other statements other than statements of historical fact. Actual results or events could differ materially from the plans,
intentions and expectations disclosed in the forward-looking statements that NewLink makes due to a number of important
factors, including those risks discussed in “Risk Factors” and elsewhere in NewLink Genetics’ Annual Report on Form 10-K
for the year ended December 31, 2015 and other reports filed with the U.S. Securities and Exchange Commission (SEC).
The forward-looking statements represent NewLink's views as of the date of this presentation. NewLink anticipates that
subsequent events and developments will cause its views to change. However, while it may elect to update these forward-
looking statements at some point in the future, it specifically disclaims any obligation to do so. You should, therefore, not
rely on these forward-looking statements as representing NewLink Genetics’ views as of any date subsequent to the date of
this presentation.
2

Investor Day – Distinguished Speakers
IDO Combinations with Checkpoint Inhibitors
George C. Prendergast, PhD, President & Chief Executive Officer, Lankenau Institute for Medical Research
(LIMR), Editor-in-Chief, Cancer Research
3
Immunoregulatory Role of Tryptophan Metabolism
David H. Munn, MD, Medical College of Georgia, Augusta University
Understanding Current Melanoma Clinical Data
Montaser Shaheen MD, Associate Professor, University of New Mexico Cancer Center
Indoximod in Treatment of Patients with Acute Myeloid Leukemia (AML)
Ashkan Emadi, MD, PhD, Associate Professor of Medicine, Pharmacology & Experimental Therapeutics,
University of Maryland

4
NewLink Genetics
Investor Day Takeaways
IDO pathway is central to immune escape
IDO pathway is becoming increasingly validated as a target for drugs
Two promising candidates that target the IDO pathway, with distinct mechanisms of action
GDC-0919, which targets the enzyme directly (partnered with Genentech)
Indoximod, which inhibits the effects of IDO by supplying a “tryptophan-sufficiency” signal
Proven track record in both in-and-out licensing
Strong balance sheet to advance current clinical programs
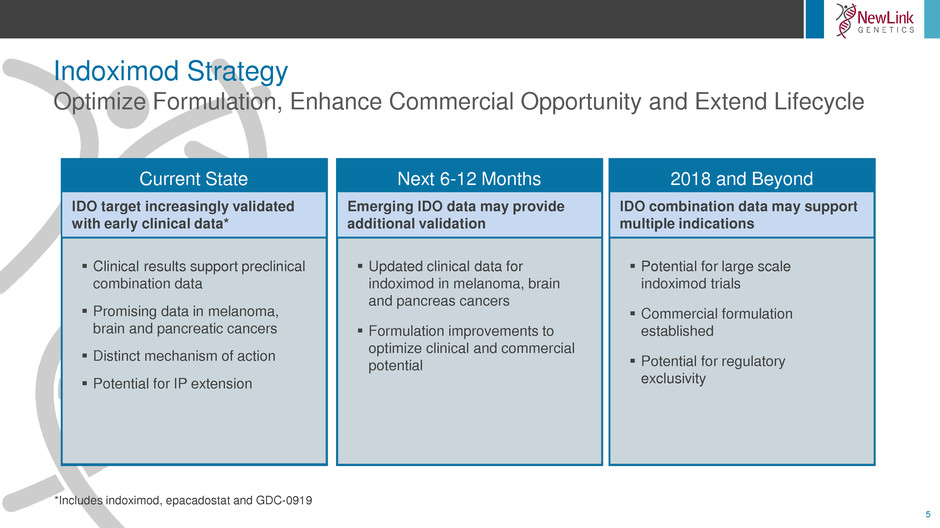
Indoximod Strategy
5
Optimize Formulation, Enhance Commercial Opportunity and Extend Lifecycle
*Includes indoximod, epacadostat and GDC-0919
Next 6-12 Months
Updated clinical data for
indoximod in melanoma, brain
and pancreas cancers
Formulation improvements to
optimize clinical and commercial
potential
Emerging IDO data may provide
additional validation
2018 and Beyond
Potential for large scale
indoximod trials
Commercial formulation
established
Potential for regulatory
exclusivity
IDO combination data may support
multiple indications
Current State
IDO target increasingly validated
with early clinical data*
Clinical results support preclinical
combination data
Promising data in melanoma,
brain and pancreatic cancers
Distinct mechanism of action
Potential for IP extension
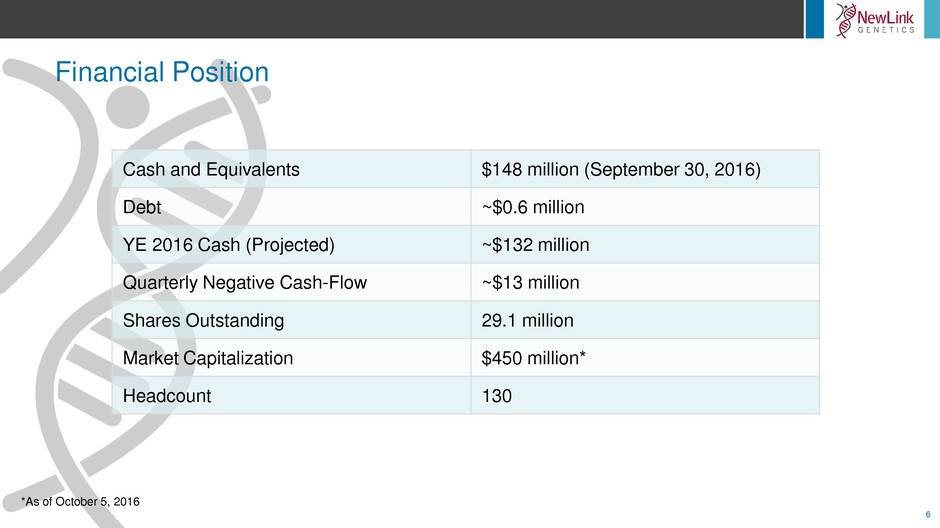
Financial Position
Cash and Equivalents $148 million (September 30, 2016)
Debt ~$0.6 million
YE 2016 Cash (Projected) ~$132 million
Quarterly Negative Cash-Flow ~$13 million
Shares Outstanding 29.1 million
Market Capitalization $450 million*
Headcount 130
6
*As of October 5, 2016
