Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - SHINECO, INC. | v449246_ex99-1.htm |
| EX-32.2 - EXHIBIT 32.2 - SHINECO, INC. | v449246_ex32-2.htm |
| EX-32.1 - EXHIBIT 32.1 - SHINECO, INC. | v449246_ex32-1.htm |
| EX-31.2 - EXHIBIT 31.2 - SHINECO, INC. | v449246_ex31-2.htm |
| EX-31.1 - EXHIBIT 31.1 - SHINECO, INC. | v449246_ex31-1.htm |
| EX-14.1 - EXHIBIT 14.1 - SHINECO, INC. | v449246_ex14-1.htm |
| EX-10.60 - EXHIBIT 10.60 - SHINECO, INC. | v449246_ex10-60.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
| ☑ | annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
| For the fiscal year ended | June 30, 2016 |
or
| ☐ | Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
| For the transition period from | __________________________________ to _________________________________ |
| Commission File Number: | 001-37776 |

| SHINECO, INC. |
| (Exact name of issuer as specified in its charter) |
| Delaware | 52-2175898 | |
| (State or other jurisdiction of incorporation or | (I.R.S. employer identification number) | |
| organization) | ||
| 2nd Floor, Wanyuan Business Center | ||
| 10 N. Hongda Road | ||
| Daxing District, Beijing | ||
| People’s Republic of China | ||
| 100176 | ||
| (Address of principal executive offices) | (Zip Code) |
| Registrant’s telephone number, including area code | (+86) 10-87227366 |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |
| Common stock, $0.001 par value | NASDAQ Capital Market |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☑
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every, Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (Sec. 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one):
| Large Accelerated Filer ☐ | Accelerated Filer | ☐ | |
| Non-accelerated filer ☐ | Smaller reporting company | ☑ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☑
The aggregate market value of the voting stock of the registrant held by non-affiliates on December 31, 2015, the last business day of the registrant's second fiscal quarter, was approximately $50.1 million, based on the number of outstanding Shares held by non-affiliates on that date multiplied by the $4.50 offering price of our common stock. Until our initial public offering there was no public market for the shares of our common stock
As of September 27, 2016, the registrant had 21,034,072 shares of common stock outstanding.
TABLE OF CONTENTS
TO ANNUAL REPORT ON FORM 10-K
FOR YEAR ENDED JUNE 30, 2015
| i |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This annual report on Form 10-K (the “Report”) and other reports (collectively the “Filings”) filed by the registrant from time to time with the Securities and Exchange Commission (the “SEC”) contain or may contain forward looking statements and information that are based upon beliefs of, and information currently available to, the registrant’s management as well as estimates and assumptions made by the registrant’s management. When used in the filings the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan” or the negative of these terms and similar expressions as they relate to the registrant or the registrant’s management identify forward looking statements. Such statements reflect the current view of the registrant with respect to future events and are subject to risks, uncertainties, assumptions and other factors (including the risks contained in the section of this Report entitled “Risk Factors”) relating to the registrant’s industry, the registrant’s operations and results of operations and any businesses that may be acquired by the registrant. Should one or more of these risks or uncertainties materialize, or should the underlying assumptions prove incorrect, actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned.
Although the registrant believes that the expectations reflected in the forward looking statements are reasonable, the registrant cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, the registrant does not intend to update any of the forward-looking statements to conform these statements to actual results. The following discussion should be read in conjunction with the registrant’s financial statements and the related notes thereto included in this Report.
In this Report, the terms “we,” “us,” “our company,” “our” and “Shineco” refer to Shineco, Inc. (“TYHT” when referring solely to our Delaware company), including its consolidated subsidiaries and variable interest entities (“VIEs”), unless the context otherwise requires;
| ii |
| ITEM 1. | Business. |
General Overview
We are a Delaware holding company that uses our subsidiaries’ and variable interest entities’ vertically- and horizontally-integrated production, distribution and sales channels to provide health and well-being focused plant-based products. Our products are only sold domestically in China. We utilize modern engineering technologies and biotechnologies to produce, among other products, Chinese herbal medicines, organic agricultural produce and specialized textiles. Our health and well-being focused plant-based products business is divided into three major segments:
1. Processing and distributing traditional Chinese herbal medicine products as well as other pharmaceutical products. The companies of this segment, Ankang Longevity Group, operate 66 cooperative retail pharmacies throughout Ankang, a city in southern Shaanxi province, China, through which we sell directly to individual customers traditional Chinese medicinal products produced by us as well as by third parties. Ankang Longevity Group also owns a factory specializing in decoction, which is the process by which solid materials are heated or boiled in order to extract liquids, and distributes decoction products to wholesalers and pharmaceutical companies around China. This segment accounted for approximately 40% of our revenues for the fiscal year ending June 30, 2016.
2. Planting, processing and distributing green and organic agricultural produce as well as growing and cultivating yew trees (taxus media). We currently cultivate and sell yew mainly to large group and corporate customers, but do not currently process yew into Chinese or Western medicines. This segment is conducted through the Company’s variable interest entities: the Zhisheng Group, which comprises the following Chinese companies participating in our yew tree business: Shineco Zhisheng (Beijing) Bio-Technology Co. (“Zhisheng Bio-Tech”), Yantai Zhisheng International Freight Forwarding Co., Ltd (“Zhisheng Freight”), Yantai Zhisheng International Trade Co., Ltd (“Zhisheng Trade”), Yantai Mouping District Zhisheng Agricultural Produce Cooperative (“Zhisheng Agricultural”), and Qingdao Zhihesheng Agricultural Produce Services, Ltd (“Qingdao Zhihesheng”). This segment accounted for approximately 48% of our revenues for the fiscal year ending June 30, 2016.
3. Developing and distributing specialized fabrics, textiles and other byproducts derived from an indigenous Chinese plant Apocynum Venetum, grown in the Xinjiang region of China, and known in Chinese as “Luobuma” or “bluish dogbane”. Our Luobuma products are specialized textile and health supplement products designed to incorporate traditional Eastern medicines with modern scientific methods. These products are predicated on centuries-old traditions of Eastern herbal remedies derived from the Luobuma raw material. This segment is channeled through the Company’s directly-owned subsidiary, Beijing Tenet-Jove Technological Development Co., Ltd. (“Tenet-Jove”). This segment accounted for approximately 12% of our revenues for the fiscal year ending June 30, 2016.
We primarily market our health and wellbeing-focused products in China. At present, we do not sell any of our products in the United States or Canada. China’s domestic pharmaceutical and healthcare product market is fast-growing but, in our opinion, underdeveloped. We believe China’s healthcare sector has the capacity to develop even further. According to China’s National Health and Family Planning Commission, China’s healthcare spending in 2013 was only 5.5% of GDP, which is far below healthcare spending of developed countries. According to the World Bank, healthcare spending for 2012 for the United States, United Kingdom, France and Germany was 17.9%, 9.4%, 11.7% and 11.3%, respectively. According to a 2015 report by Deloitte, healthcare expenditures in China are projected to grow at an average rate of 11.8% annually from 2014-2018, reaching $892 billion by 2018. From pharmaceuticals to medical products to general consumer health, China remains among the world’s most attractive markets, and by far the fastest-growing of all the large emerging ones. This growth is being driven by China’s aging population, increased incidence of chronic diseases, and a material increase in investment from both domestic and foreign corporations. The growth also reflects the Chinese government’s focus on healthcare as both a social priority (as witnessed in its late 2000s healthcare reforms) and a strategic priority (as witnessed in the 12th five-year plan’s stated focus on growing the biomedical industry in the future).
| 1 |
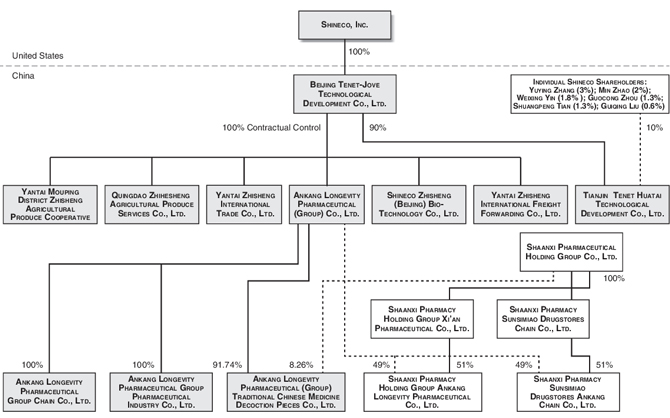
History and Corporate Structure
Shineco, Inc. was incorporated under the laws of the State of Delaware on August 20, 1997 as Supcor, Inc. From 1997 to 2004, the Company’s only activities were organizational ones, directed at developing its business plan and raising initial capital. On December 30, 2004, the Company acquired all of the issued and outstanding shares of Tenet-Jove, a company operating in the fabrics and textile business organized under the laws of the People’s Republic of China on December 15, 2003, in exchange for shares of Shineco’s common stock. At that point, the sole operating business of Tenet-Jove then became that of the Company. On June 9, 2005, we changed our name to Shineco, Inc.
Currently, Tenet-Jove, through a series of contractual relationships, effectively controls and manages:
| • | Ankang Longevity Pharmaceutical (Group) Co., Ltd. (“Ankang Longevity Group”), which owns a controlling interest in the following Chinese companies participating in our traditional Chinese medicine business: Ankang Longevity Pharmaceutical (Group) Traditional Chinese Medicine Decoction Pieces Co., Ltd. (“Ankang Longevity Decoction Pieces”), Ankang Longevity Pharmaceutical Group Chain Co., Ltd. (“Ankang Longevity Chain”), and Ankang Longevity Pharmaceutical Group Pharmaceutical Industry Co., Ltd. (“Ankang Longevity Industry”); and |
| • | Zhisheng Group, which comprises the following Chinese companies participating in our yew tree business: Shineco Zhisheng (Beijing) Bio-Technology Co., Yantai Zhisheng International Freight Forwarding Co., Ltd, Yantai Zhisheng International Trade Co., Ltd, Yantai Mouping District Zhisheng Agricultural Produce Cooperative, and Qingdao Zhihesheng Agricultural Produce Services, Ltd. |
The Company is also a majority shareholder of Tianjin Tenet Huatai Technological Development Co., Ltd. (“Tianjin Tenet Huatai”), and it owns 49% of Shaanxi Pharmacy Holding Group Ankang Longevity Pharmaceutical Co., Ltd. and Shaanxi Pharmacy Sunsimiao Drugstores Ankang Chain Co., Ltd. through a joint venture with Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd., a Chinese state-owned pharmaceutical enterprise.
Contractual Arrangements with Hongli Group and its Owners
We conduct our business through a combination of contractual arrangements with PRC operating companies and equity ownership of PRC subsidiaries. In some cases we have chosen to use contractual relationships because direct investment by foreign-owned companies like our Delaware company is prohibited or restricted. In other cases we have elected to do so in spite of being permitted to own such operating company directly. Where we operate our business through such contractual relationships, we are subject to risks related to such operation. See “Risk Factors— We rely on contractual arrangements with our variable interest entities in China for our business operations, which may not be as effective in providing operational control or enabling us to derive economic benefits as through ownership of controlling equity interests.”
The principal regulation governing foreign ownership of businesses in the PRC is the Foreign Investment Industrial Guidance Catalogue, effective as of April 10, 2015 (the “Catalogue”). The Catalogue classifies various industries into three categories: encouraged, restricted and prohibited. Shineco is engaged in business in industries where direct foreign investment is expressly prohibited: the preparation of traditional Chinese medicines in small pieces ready for decoction.
| 2 |
Due, in part, to the regulations on foreign ownership of PRC businesses, neither we nor our subsidiaries own any equity interest in Ankang Longevity Group or the Zhisheng Group. The foregoing companies are referred to herein as the “Controlled Companies.” Instead, we control and receive the economic benefits of the Controlled Companies’ business operations through a series of contractual arrangements. Tenet-Jove, each of the Controlled Companies and its shareholders have entered into a series of contractual arrangements, also known as VIE Agreements. The VIE agreements are designed to provide Tenet-Jove with the power, rights and obligations equivalent in all material respects to those it would possess as the sole equity holder of each Controlled Company, including absolute control rights and the rights to the assets, property and revenue of each Controlled Company. Based on a legal opinion issued by Dentons LLP to Tenet-Jove, the VIE agreements constitute valid and binding obligations of the parties to such agreements and are enforceable and valid in accordance with the laws of the PRC.
Each of the types of VIE Agreements is described below and consist of, for each of Ankang Longevity Group and the Zhisheng Group, (a) exclusive business cooperation agreements, (b) timely reporting agreements, (c) equity interest pledge agreements, (d) exclusive option agreements, and (e) powers of attorney. As an overview, these agreements taken together are designed to allow our Company to manage the operations of each of the Controlled Companies and to receive all of the net income of such Controlled Companies in return therefor. To secure our interest in the Controlled Companies, the equity interest pledges and option agreements and the powers of attorney are designed to allow us to step in and convert our contractual interest into an equity interest in the event we determine that doing so is warranted. Finally, the timely reporting agreement is designed to ensure that we have timely access to the financial and other information from the Controlled Companies that we require in order to prepare regulatory and other filings.
The following is a summary of the common contractual arrangements that provide us with effective control of our VIEs and that enable us to receive substantially all of the economic benefits from their operations.
Exclusive Business Cooperation Agreements
Pursuant to substantially identical Exclusive Business Cooperation Agreements between each Controlled Company and Tenet-Jove, Tenet-Jove provides such Controlled Company with technical support, consulting services and other management services relating to its day-to-day business operations and management, on an exclusive basis, utilizing its advantages in technology, human resources, and information. Additionally, each Controlled Company has granted an irrevocable and exclusive option to Tenet-Jove to purchase from such Controlled Company, any or all of its assets, to the extent permitted under applicable PRC law. Tenet-Jove may exercise, at its sole discretion, the option to purchase from each Controlled Company any or all of such Controlled Company’s assets at the lowest purchase price permitted by PRC law. Should Tenet-Jove exercise such option, the parties shall enter into a separate asset transfer or similar agreement. Tenet-Jove shall own all intellectual property rights that are developed during the course of each Exclusive Business Cooperation Agreement. For services rendered to each Controlled Company by Tenet-Jove under the agreement to which such Controlled Company is a party, Tenet-Jove is entitled to collect a service fee calculated based on the time of services rendered multiplied by the corresponding rate, which is approximately equal to the net income of such Controlled Company.
Each Exclusive Business Cooperation Agreement shall remain in effect for ten years until it is extended or terminated by Tenet-Jove (which may be done unilaterally). Such agreements may only be terminated by the Controlled Companies upon gross negligence or fraud by Tenet-Jove. Tenet-Jove entered into an Exclusive Business Cooperation Agreement with Zhisheng Bio-Tech, Ankang Longevity Group, and Qingdao Zhihesheng on February 24, 2014, December 31, 2008, and May 24, 2012, respectively, and with each of Zhisheng Freight, Zhisheng Trade, and Zhisheng Agricultural on June 16, 2011.
Tenet-Jove is currently managing each Controlled Company pursuant to the terms of an Exclusive Business Cooperation Agreement. Pursuant to each such agreement, Tenet-Jove has absolute authority relating to the management of each Controlled Company, including but not limited to decisions with regard to expenses, salary raises and bonuses, hiring, firing and other operational functions. Although the Exclusive Business Cooperation Agreements do not prohibit related party transactions, the audit committee of Shineco will be required to review and approve in advance any related party transactions, including transactions involving Tenet-Jove or any Controlled Company.
| 3 |
Timely Reporting Agreements
To ensure each Controlled Company promptly provides all of the information that Tenet-Jove and the Company need to file various reports with the SEC and other applicable regulatory authorities, a Timely Reporting Agreement was entered between each Controlled Company and Shineco on July 3, 2014.
Under the Timely Reporting Agreements, each Controlled Company agrees that it is obligated to make its officers and directors available to Shineco and promptly provide all information required by Shineco so that Shineco can file all necessary SEC and other regulatory reports as required.
Equity Interest Pledge Agreements
Under the Equity Interest Pledge Agreements among each Controlled Company (other than Zhisheng Agricultural, which is a cooperative and thus has no equity interests that can be pledged), the shareholders of each such Controlled Company and Tenet-Jove, the shareholders pledged all of their equity interests in each such Controlled Company to Tenet-Jove to guarantee the performance of such Controlled Company’s obligations under the respective Exclusive Business Cooperation Agreement. Under the terms of each agreement, in the event that the Controlled Company or its shareholders breach their respective contractual obligations under the Exclusive Business Cooperation Agreement to which they are a party, Tenet-Jove, as pledgee, will be entitled to certain rights, including, but not limited to, the right to collect dividends generated by the pledged equity interests. Each Controlled Company’s shareholders also agreed that upon occurrence of any event of default, as set forth in the applicable Equity Interest Pledge Agreement, Tenet-Jove is entitled to dispose of the pledged equity interest in accordance with applicable PRC laws. Each Controlled Company’s shareholders further agree not to dispose of the pledged equity interests or take any actions that would prejudice Tenet-Jove’s interest in the applicable Controlled Company.
Each Equity Interest Pledge Agreement shall be effective until all payments due under the related Exclusive Business Cooperation Agreement have been paid by the Controlled Company party thereto. Tenet-Jove shall cancel or terminate an Equity Interest Pledge Agreement upon a Controlled Company’s full payment of fees payable under its applicable Exclusive Business Cooperation Agreement.
Exclusive Option Agreements
Under the Exclusive Option Agreements, shareholders of each of the Controlled Companies (other than Zhisheng Agricultural, which is a cooperative and thus has no equity holders) irrevocably granted Tenet-Jove (or its designee) an exclusive option to purchase, to the extent permitted under PRC law, once or at multiple times, at any time, part or all of their equity interests in each Controlled Company. The option price is equal to the capital paid in by the applicable Controlled Company shareholders subject to any appraisal or restrictions required by applicable PRC laws and regulations. The option purchase price shall increase in case the applicable Controlled Company shareholders make additional capital contributions to such Controlled Company.
Each agreement remains effective for a term of ten years and may be unilaterally renewed at Tenet-Jove’s election.
Powers of Attorney
Under the Powers of Attorney, the shareholders of each Controlled Company authorize Tenet-Jove to act on their behalf as their exclusive agent and attorney with respect to all rights as shareholders of the respective Controlled Companies, including but not limited to: (a) attending shareholders’ meetings; (b) exercising all the shareholder’s rights, including voting, that shareholders are entitled to under the laws of China and the Articles of Association, including but not limited to the sale or transfer or pledge or disposition of shares in part or in whole; and (c) designating and appointing on behalf of shareholders the legal representative, the executive director, supervisor, the chief executive officer and other senior management members of the respective Controlled Companies.
| 4 |
Guarantee Agreement
As noted above, Zhisheng Agricultural is a cooperative and has no equity interests to pledge and no equity holders to grant options. Thus, in order to secure the obligations of Zhisheng Agricultural to pay certain service fees owing to Tenet-Jove under the Exclusive Business Cooperation Agreement to which it is a party, it has entered into a Guarantee Agreement among Zhisheng Agricultural, Tenet-Jove, and Wang Qiwei, a member of the Zhisheng Agricultural cooperative dated June 16, 2011. Pursuant to the terms of the Guarantee Agreement, Wang Qiwei has agreed to guarantee the payment of service fees under the Exclusive Business Cooperation Agreement should Zhisheng Agricultural breach its obligation to do so.
Our Current Corporate Structure
Shineco, Inc. was incorporated under the laws of the State of Delaware on August 20, 1997 as Supcor, Inc. On December 30, 2004, the Company acquired all of the issued and outstanding shares of Tenet-Jove, a company organized under the laws of the People’s Republic of China on December 15, 2003, in exchange for restricted shares of Shineco’s common stock, and the sole operating business of Shineco then became that of its subsidiary, Tenet-Jove.
Tenet-Jove, through a series of contractual relationships, effectively controls and manages the Zhisheng Group, comprising Zhisheng Bio-Tech, Zhisheng Freight, Zhisheng Trade, Zhisheng Agricultural, and Qingdao Zhihesheng, and Ankang Longevity Group, which substantially owns Ankang Longevity Decoction Pieces, Ankang Longevity Chain, and Ankang Longevity Industry. The Company is a majority shareholder of Tianjin Tenet Huatai.
The Company also owns 49% of Shaanxi Pharmacy Holding Group, Ankang Longevity Pharmaceutical Co., Ltd. and Shaanxi Pharmacy Sunsimiao Drugstores Ankang Chain Co., Ltd. through a joint venture with Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd., a Chinese state-owned pharmaceutical enterprise.
Our current corporate structure is as follows:
Our Products and Operations
Our health and well-being focused plant-based products business is divided into three major segments:
| 1. | Processing and distributing traditional Chinese herbal medicine products as well as other pharmaceutical products. This segment is conducted by the Company’s VIE, Ankang Longevity Group. |
| 2. | Planting, processing and distributing green and organic agricultural produce as well as growing and cultivating yew trees (taxus media). This segment is conducted through the Company’s VIEs, the Zhisheng Group. |
| 3. | Developing and distributing specialized fabrics, textiles and other byproducts derived from an indigenous Chinese plant Apocynum Venetum, known in Chinese as “Luobuma” or “bluish dogbane”. This segment is channeled through the Company’s directly-owned subsidiary, Tenet-Jove. |
The details of each business segment are described as follows:
Ankang Longevity Group
The companies of this segment, Ankang Longevity Group, operates 66 cooperative retail pharmacies throughout Ankang, a city in southern Shaanxi province, PRC, through which we sell directly to individual customers traditional Chinese medicinal products produced by us as well as by third parties. This group also processes more than 600 kinds of Chinese medicinal herbal products such as medicines for bone and joint pain, arthritis, respiratory infections and insomnia, and distribute such products through an established Chinese domestic sales and distribution network, including more than twenty pharmaceutical companies and more than fifty hospitals throughout China. Ankang Longevity Group has recently established a joint venture with a large state-owned domestic pharmacy chain group, Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd. (“Shaanxi Pharmaceutical Group”). As part of the joint venture, Ankang Longevity Group contributed 13 retail pharmacies— operating as Sunsimiao Pharmacies— to the joint venture while retaining 66 retail pharmacies whereby Ankang Longevity Group acts as a franchisor. We also provide evaluation and diagnostic services by on-site doctors at pharmacies to support and drive our Chinese medicine product sales. The operations of this segment are focused in the northwest region of Mainland China, particularly Shaanxi province. This segment accounts for approximately 40% of our revenues.
| 5 |
Ankang Longevity Group has built a scalable decocting-free Chinese herbal medicine manufacturing facility that passed the Good Manufacturing Practice (“GMP”) certification in 2009 and was recognized as a Key Agricultural Industrialization Companies by the Shaanxi Provincial Government. The facility covers an area of over 4,000 square meters and is equipped with an advanced toxic herbal medicine treatment production line and herbal medicine testing instruments.
Ankang Longevity Group has recently acquired land use rights to use 8,200 acres of selenium-rich farmland and woodland in Ziyang County, China to grow our Chinese medicine and other plant products.
Zhisheng Group
The Company’s other VIEs, the Zhisheng Group, which includes Zhisheng Bio-Tech, Zhisheng Freight, Zhisheng Trade, Zhisheng Agricultural and Qingdao Zhihesheng, engage in the business of organic agricultural products, principally yew trees, as well as providing logistics services for all of the agricultural products we produce. Since 2013, this segment is focusing its efforts on the growing and cultivation of yew trees (taxus media), small evergreen trees that can be used for the production of anti-cancer medication as well as ornamental bonsai trees, which are known to have the effect of purifying indoor air quality. We currently cultivate and sell yew but do not currently process yew into Chinese or Western medicines. The entities composing the Zhisheng Group are currently focusing on researching, developing and cultivating organic produce, yew ecological products and other native plants. The operations of this segment are focused in the East region of Mainland China, principally Shandong Province, and in Beijing where we have newly developed over 100 acres of modern greenhouses for cultivating yew and other plants. This segment accounts for approximately 47% of our revenues.
Tenet-Jove
Through Tenet-Jove, the Company develops and distributes specialized textiles and health supplements derived from a native Chinese plant Apocynum venetum, grown in the Xinjiang region of China and known in Chinese as “Luobuma” or “bluish dogbane” and referred to herein as Luobuma. This plant has traditionally been used in China both internally and externally for centuries to treat high blood pressure, depression, dizziness, pain, insomnia, and other common ailments. The stems of Luobuma serve as raw material for fiber used in textile production, and the leaves serve as raw material for pharmaceutical drugs. This segment accounts for approximately 12% of our revenues.
The companies of this segment, Tenet-Jove and Tianjin Tenet Huatai, specialize in Luobuma sourcing and developing Luobuma byproducts. With rich experience and broad channels in the Chinese domestic market, we believe that we are one of the leaders in Luobuma textile sales in China. This segment’s operations are focused in the north region of Mainland China, mostly carried out in Xinjiang and Tianjin. We are presently in preliminary negotiations to build a new facility in Hefei, Anhui Province, China to exploit our steam explosion Luobuma fiber production technology. Our Luobuma products are specialized textile and health supplement products designed to incorporate traditional Eastern medicines with modern scientific methods. These products are predicated on centuries-old traditions of Eastern herbal remedies derived from the Luobuma raw material.
In addition to developing textile products, we expect to use our high-pressure steam degumming process to extract other Luobuma byproducts we intend to commercialize and distribute: flavonoids, xylooligosaccharides (XOS), edible pectin, fiberboard, and organic fertilizer. The traditional method of degumming Luobuma only produces Luobuma fiber, whereas our high-pressure steam degumming process produces these five additional Luobuma byproducts. Flavonoids are organic compounds widely distributed in plants, and flavonoid-rich Luobuma extract can be used in the manufacture of many pharmaceuticals. Xylooligosaccharides, or XOS, is a sugar that can be used as a food additive that provides various health benefits like lowering glucose levels. Pectin is a thickener and stabilizer used in food, beverages and cosmetics, as well as a gelling agent for jellies. Fiberboard is a type of engineered wood alternative that is made out of Luobuma fibers; it is used widely for furniture manufacturing and packaging.
| 6 |
Product Descriptions
Traditional Chinese Medicines
Our Ankang Longevity Group develops and manufactures hundreds of Chinese medicinal herbal products and decoction pieces such as medicines for bone and joint pain, arthritis, respiratory infections, insomnia, as well as many other common ailments. We distribute such products through an established Chinese domestic sales and distribution network, including through more than twenty pharmaceutical wholesale companies and more than fifty hospitals throughout China, as well as through our own and cooperative retail pharmacies. In addition to distributing Chinese medicinal herbal products, we also distribute many popular Western medicines we purchase from outside parties through our wholesale and retail channels so that we can offer a good variety of products to meet customer demands. In the second quarter of 2013, this group entered the Pharmaceutical retailing industry through a joint venture with another large domestic pharmacy chain group, Shaanxi Pharmaceutical Group. Ankang’s main products include the following traditional Chinese medicines:
| • | Polygonum cuspidatum or Japanese knotweed, which is ingested to treat colds, digestive diseases, hepatitis and Cholecystitis (gallbladder inflammation); |
| • | Salvia mint, which is ingested to improve microcirculation; |
| • | Tianma, which is ingested to treat Rheumatoid arthritis; |
| • | Eucommia, which is ingested for bone and join pain; |
| • | Radix, which is ingested to treat respiratory infections; |
| • | Schisandra shrub, which is ingested to treat insomnia; and |
| • | Berberis shrub, which is used as a raw material for antibiotics. |
Yew Trees
Currently, through our Zhisheng Group VIEs, we sell ornamental yew trees and yew cuttings to third parties. We also rent ornamental yew trees to companies who desire the environmental benefits of natural plants in their workplaces. Until recently we were primarily engaged in the production, distribution and sale of agricultural products, including the planting and processing of organic fruits and vegetables, such as tomato, eggplants, string beans, peppers as well as certain popular fruits in China like blueberries and wine grapes, but those operations have been temporarily scaled back due to stiff competition and a change of our internal policy in favor of the expansion of our yew tree business.
As our inventories of young yew trees mature, our long-term goals are particularly focused on the extraction of paclitaxel or taxol, which is derived from certain species of yew trees including those we grow. Taxol, a broad-spectrum mitotic inhibitor used in cancer chemotherapy, can be extracted from mature yew trees. As a mitotic inhibitor, taxol adheres to rapidly dividing cancerous cells during mitosis (cell division) and interferes with the division process. It may suppress tumor growth through regulating microtubule stabilization, inducing apoptosis and adjusting immunologic mechanism. Taxol is also used for the prevention of restenosis, which is the narrowing of blood vessels. In the treatment of certain soft tissue cancers, such as breast cancer, taxol is given for early stage and metastatic breast cancer after combination anthracycline and cytoxan therapy and is also given as treatment to shrink a tumor before surgery. It can also be used together with a drug called Cisplatin to treat advanced ovarian cancer and non-small cell lung cancer, or “NSCLC.” The U.S. Food and Drug Administration approved taxol as the primary and secondary treatment for NSCLC. There are other generally accepted protocols for the use of taxol as a cancer drug alone or in combination with other drugs depending upon the diagnosis, staging and type of cancer, as well as a patient’s medical history, tolerances and allergies, among other relevant factors. Taxol is usually sold to large pharmaceutical companies to be used in their products, which can be used to treat patients with lung, ovarian, breast, head and neck cancer, and advanced forms of Kaposi’s sarcoma.
| 7 |
We believe that in three to five years at the earliest, the yew trees we are cultivating at present will begin to mature to a point where taxol extraction could be possible. We intend to invest in extraction equipment and facilities, as well as to increase planting and growing of yew trees, upon the successful launch of our initial public offering.
Tenet-Jove Textiles
Our company’s scientists and other Chinese researchers have brought modern scientific methods to the study of Luobuma, and have determined that Luobuma fibers have an increased tendency to radiate light at the “far infrared” end of the light spectrum, with wavelengths measuring between 8-15 microns (referred to as “FIR”). Based on Chinese scientific studies some believe that Luobuma’s FIR-radiating qualities exert a positive effect on various functions of the human body, including cellular metabolism. For this reason, we have marketed and sold these products utilizing such technology. These products are popular with Chinese customers seeking the perceived benefits of traditional Chinese medicine.
For example, according to a report by the College of Science of Tianjin University, tests conducted by the PRC’s National Institute of Metrology have reported that the radiance rate of far infrared light from Luobuma fiber is 84%, 2 to 4 times higher than that from cotton and other natural fibers. The same tests found that the FIR radiance rate from our proprietary bio-ceramic powder reaches 91%. Healthful benefits have been observed at radiance rate levels above 70%. Based on these observations about FIR radiance, we have developed textiles that our customers can wear and from which we believe they can receive those health benefits commonly associated with Chinese herbal remedies.
Tenet-Jove first commercially developed the natural FIR-radiant properties of the Luobuma plant in 1997. We refer to this natural Luobuma fiber as a “Second Generation” FIR textile. The “First Generation” of FIR-radiant textiles initially became popular in China around 1989, when manufacturers learned to add 3% of a FIR-radiant inorganic material to synthetic fibers comparable to nylon or polyester. This “First Generation” FIR material employs a relatively low level of technology and has relatively few perceived or measurable health benefits. The “Second Generation” FIR textiles we have developed are softer, smoother and more breathable natural fibers that are not as prone to static electricity as the low technology “First Generation” FIR-radiant textiles.
Our Luobuma fabrics have been a success in the Chinese domestic market and have also received numerous awards. The technology applied to our Luobuma-based FIR Therapeutic Clothing and Textile Products has received a “Special Golden Award” from the China National Intellectual Property Bureau at China’s National Patent and Brand Expo. Our products under the brand name of “Tenethealth®” have also been honored with the title of “Consumer’s Favorite Products” by the Chinese Consumer Association.
The fibers of natural Luobuma FIR materials can contain up to 32 medicinal compounds, many of which are familiar to practitioners of traditional Chinese medicine. In addition, our processes for manufacturing Luobuma textiles produce a fabric that is smooth, air-permeable, and soft. By combining a product that is familiar to Chinese consumers seeking the benefits of traditional Chinese medicine with quality and comfort, we believe we are well positioned in the Chinese textile market.
| 8 |
Tenet-Jove Product Development
We have developed what we term a “Third Generation” of FIR textiles under a contract with the Institute of Process Engineering at the Chinese Academy of Sciences, one of the leading scientific institutions in China. Our research and development has focused on adding nanotechnology enhancements to our Luobuma textile products, in which we use small-scale nanotechnology to embed or impregnate our Luobuma-fiber textiles with other FIR-radiant materials, bio-ceramic materials, or other Chinese herbal remedies. Using these nanotechnology methods, we have developed and marketed health-promoting textile goods that are impregnated with FIR-radiant materials or other Chinese herbal remedies, which are then absorbed through the wearer’s skin. We believe these “Third Generation” FIR textiles will better combine the health benefits of Luobuma with an even softer, more natural cotton-like fabric that will be popular with Chinese consumers.
The Company presently produces approximately one hundred “Third Generation” FIR textile products. These textile products include:
| • | Far Infrared bedding sets (including various pillows, comforters, and sheets); |
| • | Far Infrared underwear, T-shirts, and socks; |
| • | Far Infrared knee and shin pads, waist supports and other protective clothing; and |
| • | Far Infrared body wraps or protectors (for the ankle, elbow, wrist, and knee). |
All our textile products are made of Luobuma-based fibers and are impregnated with bio-ceramic powder, which contains various minerals such as halloysite. Both the fiber and the bio-ceramic powder are developed with the Company’s patented, proprietary techniques.
Manufacturing and Production Facilities
We have formed strategic alliances with several certified knitting and clothing manufacturers throughout China in order to produce our Luobuma products. We assign them limited manufacturing jobs and require certain conditions, including protecting our proprietary techniques and meeting our rigid quality standards. We are in preliminary negotiations to build a new facility in Hefei, Anhui Province, China to exploit our steam explosion Luobuma fiber production technology.
In 2013, we began large-scale operations in newly leased, energy efficient greenhouses aggregating 50,000 square meters in Beijing and have temporarily scaled back our operations in our former greenhouses located in Shandong Province. At this time, we rent our greenhouses in Shandong Province to local farmers. Our new greenhouse facilities are used primarily for the cultivation of yew trees and other decorative plants and trees. These state-of-the-art facilities allow us to better regulate light, temperate, humidity and other conditions within the greenhouse as well as to significantly increase the number of plants that can be housed in a particular greenhouse footprint. To a lesser degree, we expect these new greenhouses will reduce our manual labor costs associated with our greenhouse operations by approximately 25%. Rather than competing on price, which has become increasingly difficult in the Chinese market, we expect that technological and productivity advances from our new greenhouses will improve our competitive position within our agricultural segment, but there can be no guarantee that we will experience such advances, or if we do, that such advances would improve our competitive position.
Ankang Longevity Group has an approximately 4,000 square meter production factory and an approximately 2,000 square meter production facility in Minxing Village, Wuli Town, Hanbin District, Ankang City used for production of decoction pieces.
Our Research and Development
Our Research and Development Center for our products in Tianjin is owned by our subsidiary, Tenet-Jove. The center is managed by senior research fellows, who specialize in different but related scientific fields, such as biochemistry, electrical engineering, textile engineering, medical and clinical studies, and pharmaceuticals. We are engaged in research and development of new products under cooperation with universities and research institutions, including the Process Engineering Research Institute of Chinese Academy of Sciences, Chinese Academy of Forestry, East China Textile University, Tianjin University, Tianjin Research Institute of Knitting. In addition, we have partnered with five textile mills to testing spin and weaving of Luobuma fiber. Since inception, we have spent more than $800,000 on Luobuma research and development and $300,000 on Chinese medicinal product research and development.
| 9 |
Our Strategy for Research and Development
| • | To keep our products proprietary and patented; |
| • | To focus on our core existing product lines: Chinese herbal medicines and pharmaceutical sales, yew cultivation, Luobuma-based products, and FIR technology; |
| • | To commit to further development of our Luobuma byproducts, houpu magnolia products, and selenium-enriched herbs and plants; and |
| • | To build strategic alliances with universities and scientific institutions, which will allow us exposure to advanced technologies, excellent researchers and scientists and we believe will lower the costs and timing of the development of new products. |
Tenet-Jove specializes in developing Luobuma products and combining FIR technology with natural herbal medicines. We estimate that there are large supplies of Luobuma in China, especially Xinjiang Province. In China, Luobuma can grow as high as 3.6 meters. In the first year after planting, Luobuma can be harvested once during that year; thereafter, it can be harvested twice per year before or at the beginning of the flowering period in June and a second time around September. Currently, we believe China’s Luobuma supplies are largely undeveloped. The Company’s future success will depend on improving its techniques to industrialize Luobuma by developing new Luobuma-derived products such as improved Luobuma functional fiber and various Luobuma nutritional supplements, which can be marketed and distributed through the Company’s Ankang Longevity business segment.
High-Pressure Steam Degumming Process
We currently produce an extensive line of Luobuma-based textile products; we have an exclusive patent on a Luobuma fiber yarn spinning method. Large-scale production of Luobuma fiber products is generally difficult due to the limited yield of Luobuma fiber and the high cost of obtaining that fiber. The current mainstream technology used to produce Luobuma fiber mainly uses chemical agents to remove gum from Luobuma, which destroys Luobuma fiber and causes lower Luobuma fiber production and pollution and makes it very difficult to extract other valuable by-products. A central technical challenge is how to quickly and efficiently remove the gum and sap from the Luobuma plants so that only the natural fiber remains.
To solve this technical challenge, in August 2006 we signed a technology development contract with the Institute of Process Engineering at the Chinese Academy of Science, one of China’s leading scientific institutes. Pursuant to our contract, we and the Institute of Process Engineering worked together to develop a high-pressure steam degumming process for the mass production of Luobuma fiber, as well as its byproducts, which we completed in 2008. A literal translation of the project name is “The Stream Steam Explosion Project.” Essentially, the project will develop a modern material engineering method for quickly blasting a large amount of high-pressure steam into a large container filled with raw Luobuma plants. The steam will remove the gum and sap and leave the fiber for use in textile production. The gum and sap and other Luobuma byproducts will be washed out with the steam and collected in a separate place for other uses.
If we can successfully implement our high-pressure steam degumming process, we expect that our annual yield of Luobuma fiber can rise by over one hundredfold— from less than 200 tons to approximately 27,000 tons per year, and the cost of extracting the fiber will be reduced by approximately 50%. For example, the older method of production might require 150 laborers to produce one ton of Luobuma fiber in one day, whereas our high-pressure steam degumming process can utilize just 30 laborers to produce 15 tons of Luobuma fiber in one day. During the process, a certain amount of steam will be used, but unlike the traditional method of degumming, our high-pressure steam degumming process discharges no hazardous byproducts of any kind, because the fiber will be collected in one place, and the liquefied gum and sap and other material will be collected in another place to further separate flavonoids, xylooligosaccharides (XOS), and edible pectin through biological reverse osmosis membrane. The remaining material could be used to produce fiberboard and organic fertilizer, leaving no hazardous discharge or waste. We expect that this technique will be relatively simple and convenient for us to operate with our advanced technology. Luobuma fiber produced by this technique is more like cotton and more spinnable than before.
| 10 |
Ziyang Planting Base
Ankang Longevity Group has established long-term cooperative relationships with Shaanxi Chinese Medicine Research Institution, Shaanxi Normal University School of Life Science, and Shaanxi Chinese Medicine Association. Ankang Longevity Group has also entered into an agreement with the local government in Ziyang County for the use of an approximately 8,200 acre selenium-rich parcel of farmland and woodland for the cultivation of herbs and plants used in traditional Chinese medicine. Ankang Longevity Group has one of the largest Chinese medicine planting bases in China. The soil in Ankang City, especially in Ziyang County, which is located in Ziyang District of Ankang City, contains large quantities of selenium. Because the selenium content of raw plants is largely dependent on location and soil conditions, which can vary widely, the tea, traditional Chinese medicines and raw materials produced and cultivated in Ankang contain great amounts of selenium, which is a beneficial nutrient in foods and an important raw material for medicines. The innovative value of this project is the extraction and development of useful byproducts of medicinal houpu magnolia and eucommia. In China, traditionally only the barks of such trees have been used for medicinal purposes, and the remainder of the trees are treated as waste. We hope to utilize this parcel of farmland in Ziyang to extract valuable materials from the magnolia and eucommia we are cultivating while processing any waste into environmentally-friendly building materials, such as fiberboard.
Intellectual Property
Trademarks
We market our products under our brand name of “Tenethealth®” and “Tenet BojianTM.” Our subsidiary, Tenet-Jove, currently owns eight trademarks in China covering various categories of the company’s products. Other than “Sunsimiao,” which is trademarked by our joint venture with Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd, none of our other segments have trademarks.
Patents
Our core technology and its derivative applications for our Luobuma business are covered by the Company’s nine patents filed in China. Our patents relate to methods of extracting and processing the Luobuma fiber itself, as well as our formulations of the active medicinal constituents of the Luobuma plant.
Presently the Company holds a patent in the People’s Republic of China for Luobuma fiber yarn preparation and an application method (patent number: 201110429362.9). The Company also obtained the patents listed below in the People’s Republic of China, which patents have been suspended due to unintentional late payment of annual dues as of the date of this filing. The Company is working with the State Intellectual Property Office of the PRC and is in the process of reinstating such patents.
| • | Luobuma fiber blending method (patent number: ZL 941120295) |
| • | Luobuma four-season mat (patent number: ZL 2004 2 0029462.8) |
| • | Far-infrared ceramic material (patent number: ZL 981261701) |
| • | Luobuma cotton terry blanket (patent number: ZL 2004 2 0029465.1) |
| • | 40* fine Luobuma cloth (patent number: ZL 023381736) |
| • | Luobuma far-infrared healthcare socks with mineral Chinese medicine (patent number: ZL 2004 2 0029463.2) |
| 11 |
| • | Luobuma cushion with magnetic therapy (patent number: ZL 2004 2 0028883.9) |
| • | Heart-protecting card (patent number: ZL 2004 2 0028885.8) |
Distribution Network
We sell our products through various distribution networks. Our traditional Chinese medicinal products and Western medicines are largely sold through either our wholesale customers or our Ankang retail pharmacies — 13 pharmacies operating as Sunsimiao Pharmacies and 66 pharmacies operated by third parties as Ankang Longevity Group Pharmacy cooperatives. Additionally, we sell decoction pieces on the Anhui Bozhou Chinese medicine transaction market, to medical materials company, such as Qianhe Pharmaceutical Industry Co. and to Chinese patent medicine factories, such as Wanxi Pharmaceutical Factory.
Our Luobuma product distribution networks consists of four distributors who distributed our products to a total of approximately 21 outlets, including flagship stores, retail stores and sales counters. These distributors sell our products throughout mainland China. They all carried our proprietary brand name and “Tenethealth®” trademark. We also sell our Luobuma textile products online through third party e-commerce websites, such as Taobao, Tmall and 360buy. Our yew trees and agricultural products are primarily sold through our sales personnel and group and institutional sales. In 2013, the Company placed its Luobuma, traditional Chinese medicine and yew products in a total of 144 retail stores and sales counters throughout China and on four e-commerce websites.
Our sales and distribution strategy for our products focuses on expanding our distribution network of retail stores and sales counters into all major provinces and cities of China. We also plan to use our current distribution network to introduce our newly developed products into target markets more efficiently and effectively.
All of these certified outlets operate independently, but they prominently display our trade names “Tenethealth®” and “Tenet BojianTM.” These independent retailers sell our products as well as other products.
The location and number of retail stores and sales counters selling our products, including all sales outlets (both those connected with our major distributors (i.e., our most frequently used distributors) and other independent sellers) are listed below:
| Location | Number
of Major Distributors |
Number
of Sales Outlets | ||
| Liaoning | 1 | 1 | ||
| Jilin | 1 | 1 | ||
| Shandong | 2 | 16 | ||
| Jiangsu | 2 | 6 | ||
| Anhui | 2 | 4 | ||
| Shaanxi | 5 | 94 | ||
| Xinjiang | 1 | 2 | ||
| Sichuan | 1 | 5 | ||
| Guangdong | 1 | 3 | ||
| Tianjin | 1 | 2 | ||
| Beijing | 1 | 4 | ||
| Chongqing | 1 | 3 | ||
| Hubei | 1 | 3 | ||
| TOTALS | 20 | 144 |
| 12 |
Sales and Marketing
We market Luobuma to consumers primarily by highlighting its unique characteristics— the material is soft like cotton, breathable like hemp and is smooth to the touch like silk, and its FIR-radiating qualities are believed by some to exert a positive effect on various functions of the human body. Very few other companies in China are involved with Luobuma fiber production, so we are chiefly able to market our products against products of natural and man-made fibers that do not have the perceived advantages of Luobuma. The small number of companies that are involved in Luobuma fiber production are still using the traditional, outdated methods of producing Luobuma. We are the only company using advanced technologies. Tenet-Jove’s overall marketing strategy includes:
| • | Brand marketing strategy, primarily through media publicity, product- and market-oriented strategy; |
| • | Distinguishing Luobuma as a high-end, technologically advanced native Chinese product; and |
| • | Online advertising, which includes online advertisements appearing on the sites where we sell our products, as well as social media advertising, including Wechat, and direct e-mail solicitations. |
Ankang Longevity Group’s overall marketing strategy of its traditional Chinese medicine products focuses on promoting the Ankang district’s place in traditional Chinese medicine history and its geographic location. First, Ankang City is located in the Han River area between Qinling Mountain and Ba Mountain. Because of its geographic location and favorable climate, it is the principal production location of the ancient Qin medicine, which dates from the Qin dynasty, the first imperial dynasty of China. This area contains more than 1,200 types of herbs and plants used in traditional Chinese medicine and is home to 176 of the 282 types of herbal medicine set forth in The Pharmacopoeia of the People’s Republic of China (PPRC), compiled by the Pharmacopoeia Commission of the Ministry of Health of the PRC. The PPRC is the PRC’s official compendium of drugs, covering traditional Chinese and western medicines. Second, the soil in Ankang City, especially in Ziyang County, which is located in Ziyang District of Ankang City, contains large quantities of selenium. Because the selenium content of food is largely dependent on location and soil conditions, which can vary widely, the tea, traditional Chinese medicines and raw materials produced and cultivated in Ankang contain great amounts of selenium, which is a beneficial nutrient in foods and an important raw material for medicines. Ankang Longevity Group has entered into an agreement with the local government in Ziyang County for the use of an approximately 8,200 acre selenium-rich parcel of farmland and woodlands for the cultivation of herbs and plants — principally houpu magnolia and eucommia — used in traditional Chinese medicine. Selenium has also attracted attention because of its antioxidant properties; antioxidants protect cells from damage.
Ankang Longevity emphasizes the following marketing strategies:
| • | A focus on the excellent quality of its selenium Chinese herbal medicines; |
| • | Establishment of a long-term supply relationships with pharmaceutical companies and hospitals nationwide; and |
| • | Developing its Chinese herbal medicine wholesale market and e-commerce platform. |
Finally, the Zhisheng Group emphasizes the following marketing strategies:
| • | Focusing on the advanced growing conditions provided by our modern greenhouse operations and the potential pharmaceutical byproducts of yew, especially paclitaxel or taxol; and |
| • | Brand marketing to focus on our yew’s brand positioning. |
| 13 |
In October 2012, the Company, through its VIEs, Zhisheng Freight and Zhisheng Agricultural, entered into an agreement with an unrelated third party, Zhejiang Zhen’Ai Network Warehousing Services Co., Ltd. (“Zhen’Ai Network”), to invest RMB 14.5 million (Approximately $2.4 million) into the Tiancang Systematic Warehousing Project (“Tiancang Project”) operated by Zhen’Ai Network in exchange for a 60% equity interest in this project upon completion. The Tiancang Project is an online platform aiming to provide comprehensive warehousing and logistic solutions for various companies’ e-commerce needs. The Company expects to increase its distribution channels through this platform as well as to benefit from boosting its own revenue by providing synergetic logistic and warehousing services using its existing facilities and a service team in the Eastern port city of Qingdao.
Currently, the Company’s sales are generated through the following five major channels:
| 1. | Retail stores and sales counters. We mainly sell our Luobuma related products through sales counters and medicine through our pharmacy chain stores. |
| 2. | Sales to group or institutional customers. We mainly sell our organic agricultural products and yew trees to group or corporate customers. |
| 3. | Seminars and conferences. Because a majority of new consumers need to learn about our new products before buying them, it becomes very important and effective for us to organize or sponsor seminars and events to present healthcare knowledge while introducing and selling our products to new users. |
| 4. | Wholesale. We mainly sell our decocting-free Chinese herbal medicines to large Chinese medicine resellers and pharmaceutical companies. |
| 5. | E-commerce. We mainly sell the Luobuma related products through Tmall and Taobao to underdeveloped regions in China, Taiwan and Macau. We are currently one of only three certified online sellers of Luobuma textile products on China’s largest online sales platform, Tmall run by Alibaba. Selling through the Internet has become increasingly important to our sales in undeveloped regions and developed cities. |
The Market
We primarily market our health and wellbeing-focused products in China. At present, we do not sell any of our products in the United States or Canada. On the demand side, we believe that the following four forces drive market growth in all three of our business segments:
| 1. | The rapid growth of China’s economy, which has produced one of the largest groups of middle-class families in the world, with the largest collective purchasing power in the world. The Brookings Institution estimates that by 2030, over 70 percent of China’s population could be middle class, consuming approximately $10 trillion in goods and services. |
| 2. | The increase of China’s aging population. The China Census Bureau predicts that the majority of the China “baby boom” population (representing 40% of China’s total population) will be 65 or older by 2020, which represents over 500 million potential consumers of our pharmaceutical and healthcare products, the majority of which are sold to older customers. |
| 3. | Chinese people’s increasing attention and awareness to healthy and active lifestyles, especially in urban areas. |
| 4. | Chinese healthcare reforms. Due in part to national healthcare reforms in China, IMS Health has predicted that by 2015 China will surpass Japan to become the second largest pharmaceutical market in the world. |
We believe China’s healthcare sector has the capacity to grow in the coming years. According to China’s National Health and Family Planning Commission, China’s healthcare spending in 2013 was only 5.5% of GDP, which is far below healthcare spending of other developed countries. According to the World Bank, healthcare spending for 2012 for the United States, United Kingdom, France and Germany was 17.9%, 9.4%, 11.7% and 11.3%, respectively. Deloitte has projected that China’s healthcare spending could grow to nearly $900 billion by 2018. From pharmaceuticals to medical products to general consumer health, China remains among the world’s most attractive markets, and by far the fastest-growing of all the large emerging ones. This growth is being driven by China’s aging population, increased incidence of chronic diseases, and a material increase in investment from both domestic and foreign corporations.
| 14 |
China’s healthcare market is being shaped by positive economic and demographic trends, further healthcare reform efforts, and the policies set forth in the government’s 12th five-year plan. We believe that improvements in infrastructure, the broadening of insurance coverage, and government encouragement and support for innovation will have positive implications for us and other healthcare companies.
Strong growth in the Chinese healthcare sector has been fueled by favorable demographic trends, continued urbanization throughout China, the overall Chinese economy’s expansion, and income growth (which encourages a greater awareness of and access to healthcare among Chinese consumers). It also reflects the Chinese government’s focus on healthcare as both a social priority (as witnessed in its late 2000s healthcare reforms) and a strategic priority (as witnessed in the 12th five-year plan’s stated focus on growing the Chinese biomedical industry). According to a 2015 report by Deloitte, healthcare expenditures in China are projected to grow at an average rate of 11.8% annually from 2014 to 2018, reaching approximately $892 billion by 2018. From pharmaceuticals to medical devices to traditional Chinese medicine, almost every health sector has benefited.
A 2012 McKinsey & Co. report notes that China’s ten largest multinational pharmaceutical companies now have an aggregate total sales force numbering more than 25,000, even as many have downsized their sales forces in the United States and Europe. China has recently overtaken the United States in the total number of pharmaceutical medical sales reps employed by multinational corporations.
Competition
We compete with other top-tier pharmaceutical and healthcare companies in China. Many of them are more established than we are and have significantly greater financial, technical, marketing and other resources than we presently possess. Some of our competitors have greater name recognition and a larger customer base. Those competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers, and adopt more aggressive pricing policies. Some of our competitors have also developed similar products that compete with ours.
Our most prominent competitors in China’s textile products market are primarily large-scale textile companies, such as Luolai Home Textile Co., Fuanna Bedding and Furnishing Co., Ltd., Violet Home Textile Co., and Shuixing Home Textile Co., Ltd, as well as Bauerfeind Sports and Albert Medical, makers of protective clothing products similar to our protective clothing products. Our most prominent competitors in our pharmaceutical sales market include Ankang City Zhenning Chinese Medicine Decoction Pieces Co. Ltd. and Zhenping County Chinese Medicine Decoction Pieces Co. Ltd. Our most prominent competitors in China’s agricultural market are Beijing Jinfu Yinong Agricultural Technology Group Co., Ltd. for vegetables and other produce and Shenyang Xincheng Garden Engineering Co., Ltd. for yew trees. In the pharmaceutical sales market, we believe that our competitive position is strong because Shaanxi Pharmacy Holding in which Ankang Longevity Group has a 49% ownership stake, is a participant in the Ankang municipal government’s “Three Unities of Medicine” program, which is designed to facilitate the purchase, sale and delivery of pharmaceuticals. The “Three Unities of Medicine” program refers to “unified procurement price, unified selling price, and unified logistics”. This project is promoted by the Shaanxi Government to regulate the price of medicines in the local market. Under the program, the Shaanxi Pharmacy Holding joint venture directly sells medicines to local institutional purchasers, like hospitals; these institutional purchasers are only permitted to purchase pharmaceuticals from selected preferred providers. After a bidding process, Shaanxi Pharmacy Holding, along with other two companies, were selected by the Ankang municipal government as preferred providers under the program. However, Shaanxi Pharmacy Holding is entitled to cover all counties and districts of Ankang City, and the other two providers only cover portions of Ankang City. This program is valid until 2020, and the participants are subject to an ongoing review of their qualifications by the local pharmaceutical supervision authority every three years till 2020.
| 15 |
Ankang Longevity Group
Ankang Longevity Group competes within its traditional Chinese medicinal products and Western medicine wholesale and retail business primarily against Ankang City Zhenning Chinese Medicine Decoction Pieces Co. Ltd. and Zhenping County Chinese Medicine Decoction Pieces Co. Ltd., which conduct their wholesale pharmaceuticals business in the Ankang area, but Ankang Longevity Group has greater revenues than any of its competitors; its two main competitors’ output value and sales combined are just one-third of Ankang Longevity Group’s. The Ankang Longevity Group has formed a new pharmaceutical company and chain of drug stores with two entities wholly owned by the state-owned Shaanxi Pharmaceutical Group that has resulted in a favorable market position in the Ankang area.
Numerous competitors nationwide, including Ankang City Zhenning Chinese Medicine Decoction Pieces Co. Ltd. and Zhenping County Chinese Medicine Decoction Pieces Co. Ltd., participate in the sale of Chinese medicinal herbs and Chinese medicine decoction pieces; among them are some high-profile and large-scale companies along with some companies that have huge production and storage capacity to influence the market price. Because we believe we can control the natural resources for producing selenium Chinese herbal medicines, the Group is especially focusing on those products in order to gain market share.
Zhisheng Group
There are dozens of companies planting and cultivating yew trees in China, some of which are large-scale companies. Shenyang Xincheng Garden Engineering Co., Ltd. is a large agricultural competitor whose main product is yew. Their nurseries have the most mature yew in northeast China, and the average age of their yew trees is more than eleven years old. Another competitor, Chongqing Jiangjin District Mansheng Agricultural Development Co., Ltd., has the biggest nursery for young plants in Southwest China. And Jingyin City Hengtu Town Green Industry Yew Base specializes in cultivating, planting, gardening, and technological development of yew. They were the first company to introduce taxus media yew trees in China.
Tenet-Jove
There are few viable competitors producing advanced technology textile products with health benefits like our Luobuma textile products. Principally, our competitors are those that market and sell traditional textile products, such as Luolai Home Textile Co., Fuanna Bedding and Furnishing Co., Ltd., Violet Home Textile Co., and Shuixing Home Textile Co., Ltd, as well as those companies that market and sell protective clothing, like Bauerfeind Sports and Albert Medical. Luobuma is native to China, thus our ability to source raw materials locally greatly enhances our competitive position in the Chinese market for high quality textile products with perceived health benefits.
| 16 |
Employees
As of September 1, 2016, we employed a total of 389 full-time and no part-time employees in the following functions.
| Number of Employees | ||||||||||||||||
| Department | Sept. 1, 2016 | June 30, 2016 | June 30, 2014 | June 30, 2013 | ||||||||||||
| Senior Management | 28 | 28 | 28 | 28 | ||||||||||||
| Human Resource & Administration | 23 | 23 | 23 | 22 | ||||||||||||
| Finance | 34 | 34 | 34 | 32 | ||||||||||||
| Research & Development | 16 | 15 | 15 | 15 | ||||||||||||
| Production & Procurement | 144 | 144 | 142 | 140 | ||||||||||||
| Sales & Marketing | 148 | 148 | 144 | 140 | ||||||||||||
| Total | 389 | 388 | 386 | 377 | ||||||||||||
Our employees are not represented by a labor organization or covered by a collective bargaining agreement. We have not experienced any work stoppages.
The Company plans to hire additional employees as required. Its management and employees enjoy both compensation and welfare benefits pursuant to Chinese laws. We are required under PRC law to make contributions to employee benefit plans at specified percentages of our after-tax profit. In addition, we are required by PRC law to cover employees in China with various types of social insurance. In 2016, 2015 and 2014, we contributed approximately $128,763, $124,452 and $108,512, respectively, to employee social insurance. The effect on our liquidity by the payments for these contributions is immaterial. We believe that we are in material compliance with the relevant PRC employment laws.
Relevant PRC Regulations
Laws and Regulations in China Regarding Agriculture and Pharmaceutical Products and Distribution
Laws regulating pharmaceutical and healthcare products and agriculture cover a broad array of subjects. We must comply with numerous additional provincial and local laws relating to matters such as safe working conditions, manufacturing practices, environmental protection and fire hazard control. We believe we are in compliance with these laws and regulations in all material respects. We may be required to incur significant costs to comply with these laws and regulations. Unanticipated changes in existing regulatory requirements or adoption of new requirements could materially adversely affect our business, financial condition and results of operations.
| 17 |
Pharmaceutical Administration Law of The People’s Republic of China and its detailed implementation provisions
According to Pharmaceutical Administration Law of The People’s Republic of China effective on December 1, 2001, a Pharmaceutical Trade License is required in order to sell pharmaceutical products and a Pharmaceutical Manufacture License shall be obtained to manufacture pharmaceutical products. Our license currently expires in April 2019. The regulations indicate the specific procedure to obtain and maintain other licenses, packaging, price control, advertising and relevant penalty.
Notice on Supervision of Manufacture of Chinese Medicine in Pieces
According to Notice on Supervision of Manufacture of Chinese medicine in Pieces on February 1, 2008 and effective on June 1, 2008, companies processing raw Chinese medicine shall be a GMP certified company. Our GMP license currently expires on April 17, 2019.
Standards of Preparation for Chinese Medicine in Pieces
Shaanxi province has issued standards of preparation for Chinese Medicine in Pieces, which became effective on July 1, 2011. Our company is required to follow the procedures designated in the standards during the process of preparing the pieces of herbs.
Food Safety Law of the People’s Republic of China
The Food Safety Law of the People’s Republic of China as adopted at the 7th Session of the Standing Committee of the 11th National People’s Congress of the People’s Republic of China and effective on June 1, 2009, governs the food safety in food production and business operation activities. Pursuant to the Food Safety Law of the People’s Republic of China, food producers must establish an internal inspection and record system for raw materials and pre-delivery products, and food distributors must also establish internal systems to record and inspect food products procured from suppliers. In addition, any food additives that are not in the approved government catalog must not be used and no food products can be sold inspection-free.
Regulations on the Implementation of the Food Safety Law of the People’s Republic of China
The Regulations on the Implementation of the Food Safety Law of the People’s Republic of China as adopted at the 73rd Standing Committee Meeting of the State Council on July 8, 2009 and effective on July 20, 2009, are promulgated in accordance with the Food Safety Law of the People’s Republic of China. The Regulations require that the local People’s Government at or above the county level shall perform the responsibility specified in the Food Safety Law of the People’s Republic of China, improve the ability for supervision and administration of food safety, ensure supervision and administration of food safety; establish and improve the coordination mechanism between food safety regulatory authorities, integrate and improve the food safety information network, and realize the sharing of food safety and food inspection information and other technical resources.
Law of the People’s Republic of China on Quality and Safety of Agricultural Products
The Law of the People’s Republic of China on Quality and Safety of Agricultural Products was adopted at the 21st Meeting of the Standing Committee of the Tenth National People’s Congress on April 29, 2006. This Law was enacted in order to ensure the quality and safety of agricultural products, maintain the health of the general public, and promote the development agriculture and rural economy. Pursuant to this Law, agricultural products distribution enterprises shall establish a sound system of inspection and acceptance for their purchases. In addition, agricultural products that fail to pass the inspection based on the quality and safety standards of agricultural products cannot be marketed.
| 18 |
Regulation on Product Advertisements and Promotion
Article 5 of the Provisions for Health Food Management provides that foods claimed to have health function shall be approved by the Chinese Ministry of Health. The developer or manufacturer shall submit an application to the provincial level health administrative departments where such developer or manufacturer is located. After preliminary examination and approval by Ministry of Health, the Ministry of Health may issue a health food license to the qualified health food. Under Article 21, the label and package insert of health foods shall conform to national standards and requirements and indicate, among other things, its function and suitable users; dosage and administration; storage methods; and active ingredients.
When promoting health foods, the advertisement of health food shall conform to the other regulations. Article 19 of The Advertisement Law of People’s Republic of China provides that “an advertisement for foods, alcoholic drinks or cosmetics must meet requirements for public health, and shall not employ medical jargon or terms liable to confuse them with pharmaceuticals.” In Interim Provisions on Health Food Advertisements Review, Article 4 provides that prior to advertising health foods, developers or manufacturers should first submit an application to the food and drug administration departments on the provincial, autonomous, municipal level under the Central Government. Article 8 provides that publicizing of health functions, active ingredients, content, suitable users, dosage in health food advertisements shall be subject to prior review of the package insert ratified by the food and drug administration departments in the State Council and cannot be changed without permission. Certain content may not appear in health food advertisements, including: a guarantee of its functions; exaggerated claims; jargon, mysterious terms and technical content; promises such as “safe” or “no side effects”; or comprehensive assessment information such as efficiency, cure rate, ranking and awards.
Laws and Regulations Regarding Promotion and Advertisement of Health Textiles
In The Model Code of Health Textiles, “health textiles” refer to textiles without toxic side effects that have far infrared functions, magnetic functions and/or antibacterial effects, and which aim at regulating the body, but not healing illnesses. The Code requires that the effect of health textiles cannot be exaggerated in any form of advertising.
In addition, the promotion of health textiles should comply with The Advertisement Law of People’s Republic of China. Where there are statements in an advertisement on the performance, place of origin, usage, quality, price, producer or manufacturer, or on the items, forms, quality, price and promise of service, such statements shall be clear and explicit.
Regulation on Product Liability
Manufacturers and vendors of defective products in the PRC may incur liability for losses and injuries caused by such products. Under the General Principles of the Civil Laws of the PRC, which became effective on January 1, 1987 and were amended on August 27, 2009, manufacturers or retailers of defective products that cause property damage or physical injury to any person will be subject to civil liability.
In 1993, the General Principles of the PRC Civil Law were supplemented by the Product Quality Law of the PRC (as amended in 2000 and 2009) and the Law of the PRC on the Protection of the Rights and Interests of Consumers (as amended in 2009), which were enacted to protect the legitimate rights and interests of end-users and consumers and to strengthen the supervision and control of the quality of products. If our products are defective and cause any personal injuries or damage to assets, our customers have the right to claim compensation from us.
The PRC Tort Law was promulgated on December 26, 2009 and became effective from July 1, 2010. Under this law, a patient who suffers injury from a defective product can claim damages from either the hospital or medical institution or the manufacturer of the defective product. If our pharmaceutical products injure a patient, for example, and if the patient claims damages from the medical institution, the medical institution is entitled to claim repayment from us. Pursuant to the PRC Tort Law, where a personal injury is caused by a tort, the tortfeasor shall compensate the victim for the reasonable costs and expenses for treatment and rehabilitation, as well as death compensation and funeral costs and expenses if it causes the death of the victim. There is no cap on monetary damages the plaintiffs may seek under the PRC Tort Law.
| 19 |
Regulation of Work Safety
On June 29, 2002, the Work Safety Law of the PRC was adopted by the Standing Committee of the 9th National People’s Congress and came into effect on November 1, 2002, as amended on August 27, 2009. The Work Safety Law provides general work safety requirements for entities engaging in manufacturing and business activities within the PRC. Additionally, Regulation on Work Safety Licenses, as adopted by the State Council on January 7, 2004 and effective on January 13, 2004, requires enterprises engaging in the manufacture of dangerous chemicals to obtain a work safety license with a term of three years. If a work safety license needs to be extended, the enterprise must go through extension procedures with authorities three months prior to its expiration. In addition, on May 17, 2004, the Measures for Implementation of Work Safety Licenses of Dangerous Chemicals Production was promulgated as implementing measures to the Regulation on Work Safety Licenses which provides that entities producing dangerous chemicals are required to obtain work safety licenses pursuant to specific requirements. Without work safety licenses, no entity may engage in the formal manufacture of dangerous chemicals.
The Regulations on the Safety Administration of Dangerous Chemicals was promulgated by the State Council on January 26, 2002, and effective as of March 15, 2002. It sets forth general requirements for manufacturing and the storage of dangerous chemicals in China. The Regulations on the Safety Administration of Dangerous Chemicals requires that companies manufacturing dangerous chemicals establish and strengthen their internal regulations and rules on safety control and fulfill the national standards and other relevant provisions of the State. In addition, according to the Regulations on the Safety Administration of Dangerous Chemicals, companies that manufacture, store, transport or use dangerous chemicals shall be required to obtain corresponding approvals or licenses with the State Administration of Work Safety and its local branches and other proper authorities. Companies that manufacture or store dangerous chemicals without approval or registration with the proper authorities can be shut down, ordered to stop manufacturing or ordered to destroy the dangerous chemicals. Such companies can also be subject to fines. If criminal law is violated, the persons chiefly liable, along with other personnel directly responsible for such impropriety, shall be subject to relevant criminal liability.
Regulation of Foreign Currency Exchange
Foreign currency exchange in the PRC is governed by a series of regulations, including the Foreign Currency Administrative Rules (1996), as amended, and the Administrative Regulations Regarding Settlement, Sale and Payment of Foreign Exchange (1996), as amended. Under these regulations, the Renminbi is freely convertible for trade and service-related foreign exchange transactions, but not for direct investment, loans or investments in securities outside the PRC without the prior approval of China’s State Administration of Foreign Exchange. Pursuant to the Administrative Regulations Regarding Settlement, Sale and Payment of Foreign Exchange (1996), Foreign Investment Entities may purchase foreign exchange without the approval of the State Administration of Foreign Exchange for trade and service-related foreign exchange transactions by providing commercial documents evidencing these transactions. They may also retain foreign exchange, subject to a cap approved by the State Administration of Foreign Exchange, to satisfy foreign exchange liabilities or to pay dividends. However, the relevant Chinese government authorities may limit or eliminate the ability of foreign investment entities to purchase and retain foreign currencies in the future. In addition, foreign exchange transactions for direct investment, loan and investment in securities outside the PRC are still subject to limitations and require approvals from the State Administration of Foreign Exchange.
China has allowed for a more market-based exchange rate to influence the valuation of the Renmenbi versus global currencies, and supported devaluation consistently over the seven months prior to the date of this prospectus. To the extent any of our future revenues are denominated in Renmenbi or other currencies other than the United States dollar, we would be subject to increased risks relating to foreign currency exchange rate fluctuations which could have a material adverse effect on our financial condition and operating results since operating results are reported in United States dollars and significant changes in the exchange rate could materially impact our reported earnings.
| 20 |
Regulation of Foreign Exchange in Certain Onshore and Offshore Transactions
In October 2005, the State Administration of Foreign Exchange issued the Notice on Issues Relating to the Administration of Foreign Exchange in Fund-raising and Return Investment Activities of Domestic Residents Conducted via Offshore Special Purpose Companies, or the State Administration of Foreign Exchange Notice 75, which became effective as of November 1, 2005, and was further supplemented by three implementation notices issued by the State Administration of Foreign Exchange on November 24, 2005, May 29, 2007 and May 20, 2011, respectively. On July 4th, 2014, SAFE amended it as Circular 37 (i.e., SAFE Circular on Issues Relating to the Administration of Foreign Exchange in Offshore Investment and Financing and Round-trip Investment Activities of Domestic Residents Conducted via Offshore Special Purpose Companies issued by SAFE on July 4, 2014). The State Administration of Foreign Exchange Circular states that PRC residents, whether natural or legal persons, must register with the relevant local State Administration of Foreign Exchange branch prior to establishing or taking control of an offshore entity established for the purpose of overseas equity financing involving onshore assets or equity interests held by them. The term “PRC legal person residents” as used in the State Administration of Foreign Exchange Circular 37 refers to those entities with legal person status or other economic organizations established within the territory of the PRC. The term “PRC natural person residents” as used in the State Administration of Foreign Exchange Circular 37 includes all PRC citizens and all other natural persons, including foreigners, who habitually reside in the PRC for economic benefit.
PRC residents are required to complete amended registrations with the local State Administration of Foreign Exchange branch upon: (i) injection of equity interests or assets of an onshore enterprise to the offshore entity, or (ii) subsequent overseas equity financing by such offshore entity. PRC residents are also required to complete amended registrations or filing with the local State Administration of Foreign Exchange branch within 30 days of any material change in the his shareholding or capital of the offshore entity, such as changes in share capital, share transfers and long-term equity or debt investments and merger and split activities.
Regulation on Dividend Distributions
Our PRC subsidiaries are wholly foreign-owned and joint venture enterprises under the PRC law. The principal regulations governing the distribution of dividends paid by wholly foreign-owned enterprises include:
| • | Corporate Law (1993), as amended in 2005 and 2013; |
| • | The Wholly Foreign-Owned Enterprise Law (1986), as amended in 2000; |
| • | The Wholly Foreign-Owned Enterprise Law Implementation Regulations (1990), as amended in 2001; and |
| • | The Enterprise Income Tax Law (2007) and its Implementation Regulations (2007). |
Under these regulations, wholly foreign-owned and joint venture enterprises in China may pay dividends only out of their accumulated profits, if any, as determined in accordance with PRC accounting standards and regulations. In addition, an enterprise in China is required to set aside at least 10% of its after-tax profit based on PRC accounting standards each year to its general reserves until its cumulative total reserve funds reaches 50% of its registered capital. The board of directors of a wholly foreign-owned enterprise has the discretion to allocate a portion of its after-tax profits to its employee welfare and bonus funds. These reserve funds, however, may not be distributed as cash dividends.
On March 16, 2007, the National People’s Congress enacted the Enterprise Income Tax Law, and on December 6, 2007, the State Council issued the Implementation Regulations on the Enterprise Income Tax Law, both of which became effective on January 1, 2008. Under this law and its implementation regulations, dividends payable by a foreign-invested enterprise in the PRC to its foreign investor who is a non-resident enterprise will be subject to a 10% withholding tax, unless any such foreign investor’s jurisdiction of incorporation has a tax treaty with the PRC that provides for a lower withholding tax rate.
| 21 |
M&A Rules and Regulation on Overseas Listings
On August 8, 2006, six PRC regulatory agencies, the Ministry of Commerce, the State Assets Supervision and Administration Commission, the State Administration for Taxation, the State Administration for Industry and Commerce, the China Securities Regulatory Commission (“CSRC”) and the SAFE, jointly adopted the Regulation on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rules, which became effective on September 8, 2006. The M&A Rules purport, among other things, to require that offshore SPVs that are controlled by PRC companies or individuals and that have been formed for overseas listing purposes through acquisitions of PRC domestic interests held by such PRC companies or individuals, obtain the approval of the CSRC prior to publicly listing their securities on an overseas stock exchange. On September 21, 2006, the CSRC published a notice on its official website specifying documents and materials required to be submitted to it by SPVs seeking CSRC approval of their overseas listings.
While the application of the M&A Rules remains unclear, our PRC counsel have advised us that, based on their understanding of the current PRC laws and regulations as well as the notice announced on September 21, 2006:
| • | the CSRC currently has not issued any definitive rule or interpretation concerning whether offerings such as our initial public offering are subject to the CSRC approval procedures under the M&A Rules; |
| • | despite the lack of any definitive rule or interpretation from CSRC, the main purpose of the M&A rule is for national security and national industrial policy and so far none of the Chinese companies that have completed their public listing in the U.S. have obtained such approval; and |
| • | our Delaware company is not controlled by a Chinese citizen. Accordingly, although the purpose of Delaware incorporation is for overseas listing, the M&A rule should not apply to us. |
Our PRC counsel also advises us, however, that there is still uncertainty as to how the M&A Rules will be interpreted and implemented. If the CSRC or other PRC regulatory agencies, subsequently determine that CSRC approval was required for our initial public offering, we may need to apply for remedial approval from the CSRC and we may be subject to penalties and administrative sanctions administered by these regulatory agencies. These regulatory agencies may impose fines and penalties on our operations in the PRC, limit our operating privileges in the PRC, delay or restrict the repatriation of the proceeds from our initial public offering into the PRC, or take other actions that could materially adversely affect our business, financial condition, results of operations, reputation and prospects, as well as the trading price of our common stock.
In addition, if the CSRC later requires that we obtain its approval for our initial public offering, we may be unable to obtain a waiver of the CSRC approval requirements, if and when procedures are established to obtain such a waiver. Any uncertainties or negative publicity regarding the CSRC approval requirements could have a material adverse effect on the trading price of our common stock.
Restriction on Foreign Ownership
The Foreign Investment Industrial Catalogue jointly issued by the Ministry of Commerce for the People’s Republic of China and the National Development and Reform Commission in 2011 classified various industries/business into three different categories: (i) encouraged for foreign investment; (ii) restricted to foreign investment; and (iii) prohibited from foreign investment. For any industry/business not covered by any of these three categories, they will be deemed industries/business permitted to have foreign investment. Except for those expressly provided restrictions, encouraged and permitted industries/business are usually 100% open to foreign investment and ownership. With regard to those industries/business restricted to or prohibited from foreign investment, there is always a limitation on foreign investment and ownership. The decoction business conducted by Ankang Longevity Group falls under the industry categories that are prohibited from foreign investment and thus is subject to limitation on foreign investment and ownership. None of the other business activities conducted by us fall under industry categories that are restricted to, or prohibited from foreign investment.
| 22 |
Regulations on Offshore Parent Holding Companies’ Direct Investment in and Loans to Their PRC Subsidiaries
An offshore company may invest equity in a PRC company, which will become the PRC subsidiary of the offshore holding company after investment. Such equity investment is subject to a series of laws and regulations generally applicable to any foreign-invested enterprise in China, which include the Wholly Foreign Owned Enterprise Law, the Sino-foreign Equity Joint Venture Enterprise Law, the Sino-foreign Contractual Joint Venture Enterprise Law, all as amended from time to time, and their respective implementing rules; the Tentative Provisions on the Foreign Exchange Registration Administration of Foreign-Invested Enterprise; and the Notice on Certain Matters Relating to the Change of Registered Capital of Foreign-Invested Enterprises.
Under the aforesaid laws and regulations, the increase of the registered capital of a foreign-invested enterprise is subject to the prior approval by the original approval authority of its establishment. In addition, the increase of registered capital and total investment amount shall both be registered with the State Administration for Industry and Commerce (“SAIC”) and SAFE.
Shareholder loans made by offshore parent holding companies to their PRC subsidiaries are regarded as foreign debts in China for regulatory purposes, which debts are subject to a number of PRC laws and regulations, including the PRC Foreign Exchange Administration Regulations, the Interim Measures on Administration on Foreign Debts, the Tentative Provisions on the Statistics Monitoring of Foreign Debts and its implementation rules, and the Administration Rules on the Settlement, Sale and Payment of Foreign Exchange.
Under these regulations, the shareholder loans made by offshore parent holding companies to their PRC subsidiaries shall be registered with SAFE. Furthermore, the total amount of foreign debts that can be incurred by such PRC subsidiaries, including any shareholder loans, shall not exceed the difference between the total investment amount and the registered capital amount of the PRC subsidiaries, both of which are subject to governmental approval.
Regulations on Trademarks
Trademarks are protected by the PRC Trademark Law adopted in 1982, as subsequently amended, as well as the Implementation Regulations of the PRC Trademark Law adopted by the State Council in 2002 and 2013. The Trademark Office under the SAIC handles trademark registrations. Trademarks can be registered for a term of ten years and can be extended for another ten years if requested upon expiration of the first or any renewed ten-year term. The PRC Trademark Law has adopted a “first-to-file” principle with respect to trademark registration. Where a trademark for which a registration application has been made is identical or similar to another trademark which has already been registered or been subject to a preliminary examination and approval for use on the same type of or similar commodities or services, the application for such trademark registration may be rejected. Any person applying for the registration of a trademark may not prejudice the existing right first obtained by others, nor may any person register in advance a trademark that has already been used by another party and has already gained a “sufficient degree of reputation” through such other party’s use. Trademark license agreements must be filed with the Trademark Office or its regional offices.
Regulations on Patents
The PRC Patent Law provides for patentable inventions, utility models and designs, which must meet three conditions: novelty, inventiveness and practical applicability. The State Intellectual Property Office is responsible for examining and approving patent applications. A patent is valid for a term of twenty years in the case of an invention and a term of ten years in the case of utility models and designs. Presently the company has obtained a patent in the People’s Republic of China for Luobuma fiber yarn preparation and an application method (patent number: 201110429362.9), which patent is a critical patent used for our high-pressure steam degumming process project. The company also obtained the patents listed below in the People’s Republic of China, which patents have been suspended due to unintentional late payment of annual dues. The company is in the process of reinstating such suspended patents.
| 23 |
| • | Luobuma fiber blending method (patent number: ZL 941120295) |
| • | Luobuma four-season mat (patent number: ZL 2004 2 0029462.8) |
| • | Far-infrared ceramic material (patent number: ZL 981261701) |
| • | Luobuma cotton terry blanket (patent number: ZL 2004 2 0029465.1) |
| • | 40* fine Luobuma cloth (patent number: ZL 023381736) |
| • | Luobuma far-infrared healthcare socks with mineral Chinese medicine (patent number: ZL 2004 2 0029463.2) |
| • | Luobuma cushion with magnetic therapy (patent number: ZL 2004 2 0028883.9) |
| • | Heart-protecting card (patent number: ZL 2004 2 0028885.8) |
PRC Enterprise Income Tax Law and Individual Income Tax Law
Under the Enterprise Income Tax Law or EIT Law, enterprises are classified as resident enterprises and non-resident enterprises. PRC resident enterprises typically pay an enterprise income tax at the rate of 25%. An enterprise established outside of the PRC with its “de facto management bodies” located within the PRC is considered a “resident enterprise,” meaning that it can be treated in a manner similar to a PRC domestic enterprise for enterprise income tax purposes. The implementation rules of the EIT Law define “de facto management body” as a managing body that in practice exercises “substantial and overall management and control over the production and operations, personnel, accounting, and properties” of the enterprise.
The SAT Circular 82 issued by the SAT in April 2009 provides certain specific criteria for determining whether the “de facto management body” of a PRC-controlled offshore incorporated enterprise is located in China. Pursuant to the SAT Circular 82, a PRC-controlled offshore incorporated enterprise has its “de facto management body” in China only if all of the following conditions are met: (a) the senior management and core management departments in charge of its daily operations function have their presence mainly in the PRC; (b) its financial and human resources decisions are subject to determination or approval by persons or bodies in the PRC; (c) its major assets, accounting books, company seals, and minutes and files of its board and shareholders’ meetings are located or kept in the PRC; and (d) more than half of the enterprise’s directors or senior management with voting rights habitually reside in the PRC. The SAT Bulletin 45, in effect from September 2011, provides more guidance on the implementation of the SAT Circular 82 and provides for procedures and administration details on determining resident status and administration on post-determination matters. Although the SAT Circular 82 and the SAT Bulletin 45 only apply to offshore enterprises controlled by PRC enterprises or PRC enterprise groups, not those controlled by PRC individuals or foreign individuals, the determining criteria set forth there may reflect the SAT’s general position on how the “de facto management body” test should be applied in determining the tax resident status of offshore enterprises, regardless of whether they are controlled by PRC enterprises or PRC enterprise groups or by PRC or foreign individuals.
Due to the lack of applicable legal precedents, it remains unclear how the PRC tax authorities will determine the PRC tax resident treatment of a foreign company controlled by individuals. We may be classified as a PRC “resident enterprise” for PRC enterprise income tax purposes. Such classification would likely result in unfavorable tax consequences to us and our non-PRC shareholders, and would have a material adverse effect on our results of operations and the value of your investment.
| 24 |
PRC Value Added Tax
Pursuant to the Provisional Regulation of China on Value Added Tax, all entities and individuals that are engaged in the businesses of sales of goods, provision of repair and placement services and importation of goods into China are generally subject to a VAT at a rate of 17% (with the exception of certain goods which are subject to a rate of 13%) of the gross sales proceeds received, less any VAT already paid or borne by the taxpayer on the goods or services purchased by it and utilized in the production of goods or provisions of services that have generated the gross sales proceeds.
PRC Business Tax
Companies in China are generally subject to business tax and related surcharges by various local tax authorities at rates ranging from 3% to 20% on revenue generated from providing services and revenue generated from the transfer of intangibles.
Beginning May 1, 2016, all business tax was changed to the form of value added tax in China, and there is no longer business tax imposed in China.
Employment Laws
In accordance with the PRC National Labor Law and the PRC Labor Contract Law, employers must execute written labor contracts with full-time employees in order to establish an employment relationship. All employers must compensate their employees equal to at least the local minimum wage standards. All employers are required to establish a system for labor safety and sanitation, strictly abide by state rules and standards and provide employees with appropriate workplace safety training. In addition, employers in China are obliged to pay contributions to the social insurance plan and the housing fund plan for employees.
We have not entered into employment agreements with any of our executive officers. We have contributed to the basic and minimum social insurance plan. While we believe we have made adequate provision of such outstanding amounts of contributions to such plans in our audited financial statements, any failure to make sufficient payments to such plans would be in violation of applicable PRC laws and regulations and, if we are found to be in violation of such laws and regulations, we could be required to make up the contributions for such plans as well as to pay late fees and fines.
Regulations on Environmental Protection
According to the Prevention and Control of Water Pollution Law, China adopted a licensing system for pollutant discharge. Companies directly or indirectly responsible for discharge of industrial waste water or medical sewage to waters shall be required to obtain a pollutant discharge license. All companies are prohibited from discharging wastewater and sewage to waters without or in violation of the terms of the pollutant discharge license.
The Regulations on the Administration of Construction Projects Environmental Protection governs construction projects and the impact such projects will have on the environment. Pursuant to the Regulations on the Administration of Construction Projects Environmental Protection, the governing body is responsible for supervising the implementation of a three tiered system that includes (i) reviewing and approving a construction project, (ii) overseeing the construction project and (iii) to inspect the finished construction project and ensure that all harmful pollutants are disposed of correctly. Manufacturing companies are required to apply for inspection with environment protection authorities upon completion of a construction project.
| 25 |
The Company is not required to provide the information required by this Item because the Company is a smaller reporting company.
| Item 1b. | Unresolved Staff Comments |
The Company is not required to provide the information required by this Item because the Company is a smaller reporting company.
| Item 2. | Properties |
According to the Chinese laws and regulations regarding land usage rights, land in urban districts is owned by the State, while land in the rural areas and suburban areas, except otherwise provided for by the State, is collectively owned by individuals designated as resident farmers by the State. Also, in accordance with the legal principle that land ownership is separate from the right to the use of the land, the State assigns land usage rights to land users for a certain number of years’ period in return for the payment of fees. The maximum term with respect to the assigned land usage right is 50 years for industrial purposes and 40 years for commercial purposes.
Because the period of land usage is quite long, can be renewed, enables its users to transfer, lease, or mortgage the land usage right, or use it for other economic activities, and the lawful rights and interests are protected by the laws of the State, in common practice, we consider or refer to the right of land usage below for certain properties as an asset “owned” by the company. None of our properties are encumbered by debt, and we are not aware of any environmental concerns or limitations on the use of our properties for the purposes we currently use them or intend to use them in the future. Following is a list of our properties, all of which we lease or for which we have land use rights:
|
Property |
Address |
Rental Term |
Space | |||
| Office leased to an unrelated third party— Beijing Tenet-Jove Technological Development Co. | Room B-3106, Jianwai SOHO, 39 East Middle Third Ring Road, Chaoyang District, Beijing | Company-owned | 280 square meters | |||
| Office—owned by an unrelated third party | Eastern area of 2nd Floor, Wanyuan Business Center, 10 N Hongda Road, Daxing District, Beijing | 2 years (March 1, 2016 - February 28, 2018) | 943 square meters | |||
| Office and Warehouse— Subsidiary-Tianjin Tenet Huatai Technological Development Co., Ltd. |
3 Zhendong Road, Xuzhuang Village Tianjin |
1 year (September 17, 2016 - September 16, 2017) |
1,800 square meters | |||
| Branch office—Xuzhou Branch, Beijing Tenet-Jove Technological Development Co., Ltd. (Office) |
Room 412, Ximatai Commercial Building (N), One Xiangwang Road, Yunlong District, Xuzhou City Jiangsu Province |
1 year (March 1, 2016- |
119 square meters | |||
| Branch office—Xuzhou Branch, Beijing Tenet-Jove Technological Development Co., Ltd (Show room) |
Room 402, Ximatai Commercial Building (N), One Xiangwang Road, Yunlong District, Xuzhou City Jiangsu Province |
1 year (March 1, 2016- March 1, 2017) |
51 square meters
| |||
| 26 |
Sales outlet—Xuzhou Branch, Beijing Tenet-Jove Technological Development Co., Ltd. (Warehouse)
|
Room 13-101, Jinpeng Community Fengming Road, Quanshan District, Xuzhou City Jiangsu Province |
1 year (November 11, 2015 -
|
150 square meters |
| Office— Qingdao Zhihesheng Agricultural Produce Services Co., Ltd (General office); Yantai Zhisheng International Trade Co., Ltd. | 766-43 Wangsha Road, Chengyang District, Qingdao City | 5 years (March 1,2014- February 28, 2019) |
234.16 square meters | |||
| Office— Qingdao Zhihesheng Agricultural Produce Services Co., Ltd (General office); Yantai Zhisheng International Trade Co., Ltd. | 766-43 Wangsha Road, Chengyang District, Qingdao City | 5 years (March 1,2014- February 28, 2019) |
234.16 square meters | |||
| Office— Qingdao Zhihesheng Agricultural Produce Services Co., Ltd. (Farmland) | Qingfeng Community, Xifu Town Chengyang District, Qingdao City |
8 years (October 1, 2013 - September 30, 2018) |
282,141 square meters | |||
| Office— Yantai Zhisheng International Freight Forwarding Co., Ltd | Room 23A01, Yihe International Building A, Hongkong Central Road, Qingdao City | 1 year (February 1, 2016 - January 31, 2017) |
102 square meters | |||
| Factory— Yantai Mouping District Zhisheng Agricultural Produce Cooperative | Gaoling Village, Muping District, Yantai City | 30 years (April 27, 2011 - April 26, 2041) |
13,333 square meters | |||
| Office— Ankang Longevity Pharmaceutical (Group) Co., Ltd. | 31 Daqiao Road, Hanbin District, Ankang City | 1 year (June 20, 2016 – June 20, 2017) |
1,300 square meters | |||
| Warehouse— Ankang Longevity Pharmaceutical Group Pharmaceutical Industry Co., Ltd. (Medicine Logistics Warehouse) (Construction of medicine logistics and warehouse project) |
Chenjiagou Village, New City Office, Hanbin District, Ankang City | Company-owned | 5,530 square meters | |||
| Office— Ankang Longevity Pharmaceutical Group Pharmaceutical Industry Co., Ltd. | Second floor, 31 Daqiao Road, Hanbin District, Ankang City | 1 year (June 20, 2016 - June 20, 2017) |
400 square meters |
| 27 |
|
Office— Ankang Longevity Pharmaceutical Group Pharmaceutical Industry Co., Ltd.
|
Second floor, 31 Daqiao Road, Hanbin District, Ankang City
|
1 year (June 20, 2016 - June 20, 2017) |
400 square meters | |||
| Dormitory— Ankang Longevity Pharmaceutical Group Chain Co. Ltd. (Land) (General) | 15 East Xing’an Road, Ankang City | Company-owned | 2,750 square meters | |||
| Office— Ankang Longevity Pharmaceutical Group Chain Co. Ltd. (Land) (Commercial land) | 36 Shidi Street, Ankang City | Company-owned | 1,543 square meters | |||
| Office— Ankang Longevity Pharmaceutical Group Chain Co. Ltd. |
31 Daqiao Road, Hanbin District, Ankang City
|
1 year (June 20, 2016 - June 20, 2017) |
500 square meters | |||
| Office— Ankang Longevity Pharmaceutical (Group) Traditional Chinese Medicine Decoction Pieces Co., Ltd. (Offices) (Land) | Minxing Village, Wuli Town, Hanbin District, Ankang City | Company-owned | 1,733 square meters | |||
| Production facility— Ankang Longevity Pharmaceutical (Group) Traditional Chinese Medicine Decoction Pieces Co., Ltd. (Industrial use) (Land) | Minxing Village, Wuli Town, Hanbin District, Ankang City | Company-owned | 33,545 square meters | |||
| Office— Ankang Longevity Pharmaceutical (Group) Traditional Chinese Medicine Decoction Pieces Co., Ltd. (Offices) (Buildings) (Mixed type, five-story) | Minxing Village, Wuli Town, Hanbin District, Ankang City | Company-owned | 3,672 square meters | |||
| Production facility— Ankang Longevity Pharmaceutical (Group) Traditional Chinese Medicine Decoction Pieces Co., Ltd. (Industrial use) (Buildings) (Mixed type, one-story) | Minxing Village, Wuli Town, Hanbin District, Ankang City | Company owned | 3,596 square meters | |||
| Production facility— Qingdao Zhihesheng Agricultural Produce Services Co., Ltd., Yantai Mouping District Zhisheng Agricultural Produce Cooperative (Agricultural use) | Mafang Town, Pinggu District, Beijing |
18 years August 31, 2030) |
26,666 square meters | |||
| Production facility— Qingdao Zhihesheng Agricultural Produce Services Co., Ltd., Yantai Mouping District Zhisheng Agricultural Produce Cooperative (Agricultural use) | South of Bridge, Jixiang Temple, Xiangnaixi Village, Cuigezhuang, Chaoyang District, Beijing |
12 years July 31, 2024) |
73,333 square meters | |||
| 28 |
| Item 3. | Legal Proceedings |
We know of no material, existing or pending legal proceedings against us, nor are we involved as a plaintiff in any material proceeding or pending litigation. There are no proceedings in which any of our directors, officers or affiliates, or any registered or beneficial stockholder, is an adverse party or has a material interest adverse to our company.
| Item 4. | Mine Safety Disclosures |
The information required by Item 4 is not applicable to us, as we have no mining operations in the United States.
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchase of Equity Securities |
Market Information
On September 27, 2016, we completed an initial public offering of 1,713,190 shares of common stocks at a $4.50 offering price. Our common stocks started trading on the NASDAQ Capital Market under the symbol of TYHT on September 28, 2016. As such, the high and low common stock sales prices per share is not available as of the day of this report.
Holders
As of September 27, 2016, there were 304 registered holders of record of our common stock.
IPO Proceeds
Our registration statement on Form S-1 was declared effective on May 13, 2016. After several post-effective amendments, the registration statement was declared effective on September 20, 2016. We completed our initial public offering on September 27, 2016. Since the effective date of the registration statement, we have not used any proceeds with respect to our initial public offering.
| 29 |
Dividends
We anticipate that we will retain any earnings to support operations and to finance the growth and development of our business. Therefore, we do not expect to pay cash dividends in the foreseeable future. Any future determination relating to our dividend policy will be made at the discretion of our Board of Directors and will depend on a number of factors, including future earnings, capital requirements, financial conditions and future prospects and other factors the Board of Directors may deem relevant. Furthermore, our ability to pay dividends is limited by the Delaware General Corporation Law, which provides that a corporation may only pay dividends out of existing “surplus,” which is defined as the amount by which a corporation’s net assets exceeds its stated capital.
During the current fiscal year and the two most recent completed fiscal years, we did not declare or pay any cash dividends on our shares of common stock, and we do not expect to pay cash dividends in the foreseeable future. If we determine to pay dividends on any of our common stock in the future, as a holding company, we will be dependent principally on receipt of funds from our operating subsidiaries. Current PRC regulations permit our PRC subsidiaries to pay dividends to Shineco only out of their accumulated profits, if any, determined in accordance with Chinese accounting standards and regulations. In addition, each of our subsidiaries in China is required to set aside at least 10% of its after-tax profits each year, if any, to fund a statutory reserve until such reserve reaches 50% of its registered capital. Each of such entity in China is also required to further set aside a portion of its after-tax profits to fund the employee welfare fund, although the amount to be set aside, if any, is determined at the discretion of its board of directors. Although the statutory reserves can be used, among other ways, to increase the registered capital and eliminate future losses in excess of retained earnings of the respective companies, the reserve funds are not distributable as cash dividends except in the event of liquidation.
In addition, pursuant to the EIT Law and its implementation rules, dividends generated after January 1, 2008 and distributed to us by our PRC subsidiaries are subject to withholding tax at a rate of 10% unless otherwise exempted or reduced according to treaties or arrangements between the PRC central government and governments of other countries or regions where the non-PRC-resident enterprises are incorporated.
Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior approval of SAFE, by complying with certain procedural requirements. Specifically, under the existing exchange restrictions, without prior approval of SAFE, cash generated from the operations in China may be used to pay dividends to our company.
Securities Authorized for Issuance under Equity Compensation Plans
We approved a stock incentive plan prior to our initial public offering. We intend to file a registration statement on Form S-8 under the Securities Act as soon as practicable after the closing of our initial public offering to register up to 2,103,407 of our shares of common stock subject to outstanding stock options or reserved for issuance under a stock incentive plan, such amount being equal to ten percent (10%) of the number of shares of common stock issued and outstanding after the closing of the offering. This registration will permit the resale of these shares of common stock by nonaffiliates in the public market without restriction under the Securities Act, upon the completion of the lock-up period described below. Shares of common stock registered pursuant to the Form S-8 held by affiliates will be subject to Rule 144 volume limitations. As of the date of this report, we have not issued any options to purchase our shares of common stock.
Recent Sales of Unregistered Securities
On August 15, 2013, the Company effected a 5.5 for 1 stock split on all outstanding common stock.
On October 15, 2013, the Company issued convertible notes to a group of individuals and one institution in China in exchange for cash. The total amount of notes issued and outstanding was RMB 32,430,000 (approximately $5.1 million). The notes matured on June 30, 2014 and were convertible, in whole or in part, into shares of common stock at the option of the holders, at any time prior to the maturity date.
| 30 |
On June 29, 2014, all of the holders of the convertible notes chose to convert their notes, including any accrued interest on the notes, into shares of the Company’s common stock at the stipulated conversion price. The Company issued a total of 9,640,000 shares of common stock to the holders of the convertible notes for the principal and accrued interest.
On June 30, 2014, the Company effected a 1 for 4 reverse split of all of its outstanding common stock.
The above transactions were not registered under the Securities Act in reliance on an exemption from registration set forth in Section 4(a)(2) thereof, Regulation D and Regulation S promulgated hereunder as a transaction by the Registrant not involving any public offering, the purchasers met the “accredited investor” criteria and had adequate information about the Registrant as required by the rules and regulations promulgated under the Securities Act, and the transaction occurred outside the United States and no directed selling efforts were made in the United States. These securities may not be offered or sold in the United States in the absence of an effective registration statement, or exemption from the registration requirements under the Securities Act.
| Item 6. | Selected Financial Data |
The Company is not required to provide the information required by this Item because the Company is a smaller reporting company.
| Item 7. | Management’s Discussion and Analysis of Financial Conditions and Results of Operations |
The following discussion and analysis of the results of our operations and financial condition for the fiscal years ended June 30, 2016 and 2015 should be read in conjunction with our consolidated financial statements, and the notes to those consolidated financial statements that are included elsewhere in this Report. All monetary figures are presented in U.S. dollars, unless otherwise indicated.
Forward-Looking Statements
The statements in this discussion that are not historical facts are “forward-looking statements.” The words “may,” “will,” “expect,” “believe,” “anticipate,” “intend,” “could,” “estimate,” “continue,” the negative forms thereof, or similar expressions, are intended to identify forward-looking statements, although not all forward-looking statements are identified by those words or expressions. Forward-looking statements by their nature involve substantial risks and uncertainties, certain of which are beyond our control. Actual results, performance or achievements may differ materially from those expressed or implied by forward-looking statements depending on a variety of important factors, including, but not limited to, weather, local, regional, national and global coke and coal price fluctuations, levels of coal and coke production in the region, the demand for raw materials such as iron and steel which require coke to produce, availability of financing and interest rates, competition, changes in, or failure to comply with, government regulations, costs, uncertainties and other effects of legal and other administrative proceedings, and other risks and uncertainties. We are not undertaking to update or revise any forward-looking statement, whether as a result of new information, future events or circumstances or otherwise.
| 31 |
Business Overview
Shineco was incorporated in the State of Delaware on August 20, 1997. On December 30, 2004, the Company acquired all of the issued and outstanding shares of Tenet-Jove, a PRC company, in exchange for our restricted shares of common stock. Consequently, Tenet-Jove became our 100% owned subsidiary and its operating business became that of the Company. Tenet-Jove was incorporated on December 16, 2003 under the laws of China and was officially granted the status of a Wholly Foreign-Owned Entity (“WFOE”) by Chinese authorities on July 14, 2006. This transaction was accounted for as a recapitalization. Tenet-Jove owns a 90% interest of Tianjin Tenet Huatai Technological Development Co., Ltd. (“Tenet Huatai”). On June 9, 2005, we changed our name to Shineco, Inc.
On December 31, 2008, June 11, 2011 and May 24, 2012, respectively, Tenet-Jove entered into a series of contractual agreements with the owner of Ankang Longevity Pharmaceutical (Group) Co., Ltd. (“Ankang Longevity Group”), each of Yantai Zhisheng International Freight Forwarding Co., Ltd (“Zhisheng Freight”), Yantai Zhisheng International Trade Co., Ltd (“Zhisheng Trade”), Yantai Mouping District Zhisheng Agricultural Produce Cooperative (“Zhisheng Agricultural”) and Qingdao Zhihesheng Agricultural Produce Services., Ltd (“Qingdao Zhihesheng”). On February 24, 2014, Tenet-Jove also subsequently entered into the same series of contractual agreements with ShinecoZhisheng (Beijing) Bio-Technology Co., Ltd (“Zhisheng Bio-Tech”), which is a new company incorporated in 2014. Zhisheng Bio-Tech, Zhisheng Freight, Zhisheng Trade, Zhisheng Agricultural, and Qingdao Zhihesheng are collectively referred to herein as “Zhisheng Group.” These agreements include an Executive Business Cooperation Agreement; Timely Reporting Agreement; Equity Interest Pledge Agreement and Executive Option Agreement.
Pursuant to these agreements, Tenet-Jove has the exclusive right to provide to Zhisheng Group and Ankang Longevity Group consulting services related to business operation and management. All these contractual agreements obligate Tenet-Jove to absorb a majority of the risk of loss from Zhisheng Group and Ankang Longevity Group’s activities and entitle Tenet-Jove to receive a majority of their residual returns. In essence, Tenet-Jove has gained effective control over Zhisheng Group and Ankang Longevity Group. Based on these contractual arrangements, we believe that Zhisheng Group and Ankang Longevity should be considered as Variable Interest Entities (“VIEs”) under the Statement of Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 810 “Consolidation”. Accordingly, the accounts of these entities are consolidated with those of Tenet-Jove. We carry out all of our business in China through our PRC subsidiaries, our VIEs and their subsidiaries. Currently, we operate three main business segments: (i) Tenet-Jove is engaged in manufacturing and selling of Luobuma and related products, also known in Chinese as “Luobuma”, including therapeutic clothing and textile products made from Luobuma; (ii) Zhisheng Group is engaged in the business of planting, processing and distributing of green agricultural produce as well as providing domestic and international logistic services for agricultural products (“Agricultural Products”); (iii) Ankang Longevity develops and manufactures traditional Chinese herbal medicinal products as well as other retail pharmaceutical products. These different business activities and products can potentially be integrated and benefit from one and other.
| 32 |
Factors Affecting Financial Performance
We believe that the following factors will affect our financial performance:
Increasing demand for our products - The increasing demand for our Luobuma therapeutic products, our Chinese herbal medicinal products and our agricultural products, will have a positive impact on our financial position. We plan to develop new products and expand our distribution network as well as to grow our business through possible mergers and acquisitions of similar or synergetic businesses, all aimed at increasing awareness of our brand, developing customer loyalty, meeting customer demands in various markets and providing solid foundations for our continuous growth. We do not currently have any agreements, undertakings or understandings to acquire any such entity.
Expansion of our sources of supply, production capacity and sales network- To meet the increasing demand for our products, we need to expand our sources of supply and production capacity. We plan to make capital improvements in our existing production facilities, which would improve both their efficiency and capacity. In the short-run, we intend to increase our investment in our reliable supply network, personnel training, information technology applications and logistic system upgrades. We also participate in two non-equity investment opportunities through a VIE, both of which are expect to provide us with new network and platforms.
Maintaining effective control of our costs and expenses -Successful cost control depends upon our ability to obtain and maintain adequate material supplies as required by our operations at competitive prices. We will focus on improving our long-term cost control strategies including establishing long-term alliances with certain suppliers to ensure adequate supply is maintained. We will carry forward the economies of scale and advantages from our nationwide distribution network and diversified offerings. Moreover, we will step up our efforts in higher value added products of Luobuma by using an exclusive and patented technology, to optimize quality management, procurement processes and cost control, and give full play to the strong production capacity and trustworthy sales teams to maximize our profit and bring better long-term return for our shareholders.
Economic and Political Risks
Our operations are conducted primarily in the PRC. Accordingly, our business, financial conditions and results may be influenced by the political, economic and legal environment in the PRC, and by the general state of the PRC economy.
Our operations in the PRC are subject to special considerations and significant risks not typically associated with companies in North America and Western Europe. These include risks with, among others, the political, economic and legal environment and foreign currency exchange. Our Company’s results may be adversely affected by changes in the political and social conditions in the PRC, and by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversions, remittances abroad, and rates and methods of taxation, among other things.
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles (“U.S. GAAP”) requires the use of estimates and assumptions that affect the reported amounts of assets and liabilities, revenues and expenses, and related disclosures in the financial statements. Critical accounting policies are those accounting policies that may be material due to the levels of subjectivity and judgment necessary to account for highly uncertain matters or the susceptibility of such matters to change, and that have a material impact on financial condition or operating performance. While we base our estimates and judgments on our experience and on various other factors that we believe to be reasonable under the circumstances, actual results may differ from these estimates under different assumptions or conditions. We believe the following critical accounting policies used in the preparation of our financial statements require significant judgments and estimates. For additional information relating to these and other accounting policies, see Note 2 to our consolidated financial statements included elsewhere in our annual report.
| 33 |
Consolidation of Variable Interest Entities
In accordance with accounting standards regarding consolidation of VIEs, VIEs are generally entities that lack sufficient equity to finance their activities without additional financial support from other parties or whose equity holders lack adequate decision-making ability. All VIEs with which the Company is involved must be evaluated to determine the primary beneficiary of the risks and rewards of the VIE. The primary beneficiary is required to consolidate the VIE for financial reporting purposes.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements as well as the reported amounts of revenue and expenses during the reporting period. Significant estimates required to be made by management include, but are not limited to, useful lives of property, plant, and equipment, intangible assets, the recoverability of long-lived assets and the valuation of accounts receivable, deferred tax assets, accrued expenses and taxes payable and inventories. Actual results could differ from those estimates.
Inventories
Inventories, which are stated at the lower of cost or current market value, consisting of raw materials, work-in-progress, finished goods related to the Company’s products. Cost is determined using the first in first out (“FIFO”) method. Market is the lower of replacement cost or net realizable value. Agricultural products that the Company farms are recorded at cost, which includes direct costs such as seed selection, fertilizer, labor cost and contract fee that are spent in growing agricultural products on the leased farmland, and indirect costs which include amortization of prepayment of farmland lease fee and farmland development cost. All the costs are accumulated until the time of harvest and then allocated to harvested crops costs when they are sold. The Company periodically evaluates its inventory and records inventory reserve for certain inventories that may not be saleable.
Revenue Recognition
The Company recognizes revenue from sales of Luobuma products, Chinese medicinal herbal products and agricultural products, as well as providing logistic service and other processing service to external customers. The Company recognizes revenue when all of the following have occurred: (i) there is persuasive evidence of an arrangement with a customer, (ii) delivery has occurred or services have been rendered, (iii) the sales price is fixed or determinable, and (iv) the Company’s collection of such fees is reasonable assured. These criteria, as related to the Company’s revenue, are considered to have been met as follows:
Sales of products: the Company recognizes revenue on sale of products when the goods are delivered and title to the goods passes to the customer provided that there are no uncertainties regarding customer acceptance; persuasive evidence of the an arrangement exists; the sales price is fixed or determinable; and collectability is deemed probable.
Revenue from the Rendering of Services: Revenue from international freight forwarding, domestic air and overland freight forwarding services are recognized upon performance of services as stipulated in the underlying contract or when commodities are being released from the customer’s warehouse.
Fair Value of Financial Instruments
The Company adopted the provisions of ASC 820, “Fair Value Measurements and Disclosures.” ASC 820 clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
| 34 |
Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
The carrying value of accounts receivable, other current assets and prepaid expenses, short term loans, other payables and accrued expenses approximate their fair values because of the short-term nature of these instruments.
Equity Investment
An investment in which the Company has the ability to exercise significant influence, but does not have a controlling interest, is accounted for using the equity method. Significant influence is generally considered to exist when the Company has an ownership interest in the voting stock between 20% and 50%, and other factors, such as representation on the Board of Directors, voting rights and the impact of commercial arrangements, are considered in determining whether the equity method of accounting is appropriate.
Results of Operations for the Years Ended June 30, 2016 and 2015
Overview
The following table summarizes our results of operations for the years ended June 30, 2016 and 2015:
| Years End June 30, | Variance | |||||||||||||||
| 2016 | 2015 | Amount | % | |||||||||||||
| Revenue | $ | 35,206,852 | $ | 32,630,502 | $ | 2,576,350 | 7.90 | % | ||||||||
| Cost of revenue | 24,122,279 | 22,282,995 | 1,839,284 | 8.25 | % | |||||||||||
| Gross profit | 11,084,573 | 10,347,507 | 737,066 | 7.12 | % | |||||||||||
| General and administrative expense | 1,988,101 | 2,160,666 | (172,565 | ) | (7.99 | )% | ||||||||||
| Selling and distribution expense | 1,755,264 | 1,684,423 | 70,841 | 4.21 | % | |||||||||||
| Income from operations | 7,341,208 | 6,502,418 | 838,790 | 12.90 | % | |||||||||||
| Income from equity method investments | 1,796,527 | 1,788,288 | 8,239 | 0.46 | % | |||||||||||
| Other income | 197,390 | 231,908 | (34,518 | ) | (14.88 | )% | ||||||||||
| Interest income | 135,404 | 42,502 | 92,902 | 218.58 | % | |||||||||||
| Income before income tax provision | 9,470,529 | 8,565,116 | 905,413 | 10.57 | % | |||||||||||
| Provision for income taxes | 1,177,707 | 1,124,716 | 52,991 | 4.71 | % | |||||||||||
| Net income | $ | 8,292,822 | $ | 7,440,400 | $ | 852,422 | 11.46 | % | ||||||||
| Comprehensive income | $ | 4,301,466 | $ | 7,746,808 | $ | (3,445,342 | ) | (44.47 | )% | |||||||
Revenue
Currently, we have three types of revenue streams deriving from our three major business segments: First, developing, manufacturing and distributing specialized fabrics, textiles and other by-products derived from an indigenous Chinese plant Apocynum Venetum, known in Chinese as “Luobuma” or “Bluish Dogbane.” This segment is channeled through our directly owned subsidiary, Tenet-Jove. Second, processing and distributing traditional Chinese herbal medicine products as well as other pharmaceutical products. This segment is conducted by our Ankang Longevity Group VIEs. And third, planting, processing and distributing green and organic agricultural produce as well as growing and cultivation of yew trees. This segment is conducted through our VIEs, the Zhisheng Group.
| 35 |
The following table sets forth the breakdown of our revenue for the years ended June 30, 2016 and 2015, respectively:
| Years Ended June 30, | Variance | |||||||||||||||||||||||
| 2016 | % | 2015 | % | Amount | % | |||||||||||||||||||
| Sales of Luobuma products | $ | 4,387,060 | 12.46 | % | $ | 3,530,807 | 10.82 | % | $ | 856,253 | 24.25 | % | ||||||||||||
| Sales of Chinese medicinal herbal products | 14,031,955 | 39.86 | % | 13,858,949 | 42.47 | % | 173,006 | 1.25 | % | |||||||||||||||
| Sales of other agricultural products | 16,787,837 | 47.68 | % | 15,240,746 | 46.71 | % | 1,547,091 | 10.15 | % | |||||||||||||||
| Total Amount | $ | 35,206,852 | 100.00 | % | $ | 32,630,502 | 100.00 | % | $ | 2,576,350 | 7.90 | % | ||||||||||||
For the years ended June 30, 2016 and 2015, revenue from sales of Luobuma products was $4,387,060 and $3,530,807, respectively, which represented an increase of $856,253 or 24.25%. The increase of revenue from this segment was primarily due to increased sales volume of our health awareness related products. The sales volume increase was mainly due to the increase in our number of customers.
For the years ended June 30, 2016 and 2015, revenue from sales of Chinese medicinal herbal products was $14,031,955 and $13,858,949, respectively, representing a slight increase of $173,006 or 1.25%. The increase was primarily due to the fact that we fulfilled more sales orders from customers for the year ended June 30, 2016 than the same period in 2015.
For the years ended June 30, 2016 and 2015, revenue from other agricultural products was $16,787,837 and $15,240,746, respectively, representing an increase of $1,547,091 or 10.15%. The increase was mainly attributable to the increase in sales price of yew trees.
Cost of Revenue
The following table sets forth the breakdown of our cost of revenue for the years ended June 30, 2016 and 2015, respectively:
| Years Ended June 30, | Variance | |||||||||||||||||||||||
| 2016 | % | 2015 | % | Amount | % | |||||||||||||||||||
| Sales of Luobuma products | $ | 1,974,182 | 8.18 | % | $ | 1,349,520 | 6.06 | % | $ | 624,662 | 46.29 | % | ||||||||||||
| Sales of Chinese medicinal herbal products | 10,990,162 | 45.56 | % | 10,982,826 | 49.29 | % | 7,336 | 0.07 | % | |||||||||||||||
| Sales of other agricultural products | 11,072,897 | 45.91 | % | 9,872,387 | 44.30 | % | 1,200,510 | 12.16 | % | |||||||||||||||
| Business and sales related tax | 85,038 | 0.35 | % | 78,262 | 0.35 | % | 6,776 | 8.66 | % | |||||||||||||||
| Total Amount | $ | 24,122,279 | 100.00 | % | $ | 22,282,995 | 100.00 | % | $ | 1,839,284 | 8.25 | % | ||||||||||||
For the years ended June 30, 2016 and 2015, cost of revenue from sales of our Luobuma products were $1,974,182 and $1,349,520, respectively, representing an increase of $624,662 or 46.29%. The percentage of the increase in cost of revenue was higher than the increase in sales during this period, primarily due to the higher raw material and labor costs we incurred for the year ended June 30, 2016 as compared to the corresponding period in 2015.
For the years ended June 30, 2016 and 2015, cost of revenue from sales of Chinese medicinal herbal products were $10,990,162 and $10,982,826, respectively, representing an increase of $7,336 or 0.07 %. The percentage of the increase in costs was proportional to the percentage of the increase in sales due to the stable gross margin of our Chinese medicine herbal products.
| 36 |
For the years ended June 30, 2016 and 2015, cost of revenue from sales of other agricultural products was $11,072,897 and $9,872,387, respectively, representing an increase of $1,200,510 or 12.16%. The percentage of the increase in costs was proportional to the percentage of the increase in sales due to the stable gross margin of those products.
Gross Profit
The following table sets forth the breakdown of our gross profit for the years ended June 30, 2016 and 2015, respectively:
| Years Ended June 30, | Variance | |||||||||||||||||||||||
| 2016 | % | 2015 | % | Amount | % | |||||||||||||||||||
| Sales of Luobuma products | $ | 2,392,167 | 21.58 | % | $ | 2,168,663 | 20.96 | % | $ | 223,504 | 10.31 | % | ||||||||||||
| Sales of Chinese medicinal herbal products | 2,977,466 | 26.86 | % | 2,810,499 | 27.16 | % | 166,967 | 5.94 | % | |||||||||||||||
| Sales of other agricultural products | 5,714,940 | 51.56 | % | 5,368,345 | 51.88 | % | 346,595 | 6.46 | % | |||||||||||||||
| Total Amount | $ | 11,084,573 | 100.00 | % | $ | 10,347,507 | 100.00 | % | $ | 737,066 | 7.12 | % | ||||||||||||
Gross profit from Luobuma product sales increased by $223,504 and gross profit contribution percentage increased by 10.31% for the year ended June 30, 2016 as compared to the same period of 2015. The increase in gross profit was primarily because an increase in sales volume resulting from the sale of health products and sales expansion from our self-operated online E-commerce platform on Alibaba and JD.com. We expect a higher profit in this segment in the future with increasing sales through the online sales platform, and with new technology aiming to lower the costs of our products. The continual increase in the contribution percentage of Luobuma products is mainly because many of the Luobuma products are relatively new products to the market and the market is still gradually accepting these products and its benefits.
Gross profit from sales of Chinese herbal products had an increase of $166,967 for the year ended June 30, 2016 as compared to the same period of 2015, primarily due to the slightly increased sales orders. The gross profit contribution percentage increased by 5.94% for the year ended June 30, 2016 as compared to the same period of 2015, which an increase was mainly due to the relatively steady market of these Chinese herbal products and the increase in sales of our other products.
Gross profit from sales of other agricultural products increased by $346,595 for the year ended June 30, 2016 as compared to the same period of 2015. The increase was primarily a result of the combination of increased sales volume and higher selling prices as compared to the same period of 2015. The gross profit contribution percentage for the year ended June 30, 2016 was kept stable as compared to the same period of 2015.
Expenses
The following table sets forth the breakdown of our operating expenses for the years ended June 30, 2016 and 2015, respectively:
| Years Ended June 30, | Variance | |||||||||||||||||||||||
| 2016 | % | 2015 | % | Amount | % | |||||||||||||||||||
| General and administrative expenses | $ | 1,988,101 | 53.11 | % | $ | 2,160,666 | 56.19 | % | $ | (172,565 | ) | (7.99 | )% | |||||||||||
| Selling and distribution expense | 1,755,264 | 46.89 | % | 1,684,423 | 43.81 | % | 70,841 | 4.21 | % | |||||||||||||||
| Total Amount | $ | 3,743,365 | 100.00 | % | $ | 3,845,089 | 100.00 | % | $ | (101,724 | ) | (2.65 | )% | |||||||||||
General and Administrative Expenses
For the year ended June 30, 2016, our general and administrative expenses were $1,988,101, representing a decrease of $172,565 or 7.99%, as compared to the same period of 2015. The decrease was primarily attributable to of the gain on disposal of the investment in OU’Feng in the amount of RMB 1.5 million (approximately $0.23 million) resulting from collection of RMB 3.0 million (approximately $0.45 million) from Nanjing Kangtianyi and Tianjin Ou’Feng as return of the investments during the year ended June 30, 2016.
| 37 |
Selling and Distribution Expense
For the year ended June 30, 2016, our selling and distribution expenses were $1,755,264, representing an increase of $70,841 or 4.21%, as compared to the same period of 2015. The increase was primarily due to increased salary expense since more staff recruited and increased sales promotion on online platforms such as Alibaba and JD.com.
Income from Equity Method Investments
On September 27, 2012, Ankang Longevity Group entered into two equity investment agreements with a third party, Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd. (“Shaanxi Pharmaceutical Group”), a Chinese state-owned pharmaceutical enterprise, to invest a total of RMB 6.8 million (approximately $1.0 million) to form a joint venture pharmacy retail company called Shaanxi Pharmaceutical Sunsimiao Drugstores Ankang Retail Chain Co., Ltd. (“Sunsimiao Drugstores”), and a joint venture pharmaceutical wholesale distribution company named Shaanxi Pharmaceutical Holding Group Longevity Pharmacy Co., Ltd. (“Shaanxi Longevity Pharmacy”). Ankang Longevity Group obtained a 49% equity interest in each of these two new joint venture companies. We recorded net income of $532,320 and $599,118 from these equity method investments for the years ended June 30, 2016 and 2015, respectively. The decrease in net income was primarily due to the lower net profit in the two joint ventures in the current period.
In September 2013, Ankang Longevity Group entered into a supplemental agreement with Shaanxi Pharmaceutical Group. According to the supplemental agreement, the new joint venture companies established by Shaanxi Pharmaceutical and Ankang Longevity Group are required to purchase certain raw materials and drug products exclusively from Shaanxi Pharmaceutical Group. In return, Shaanxi Pharmaceutical Group has agreed to compensate Ankang Longevity Group with a preferred distribution equal to 7% of the total purchases made from Shaanxi Pharmaceutical Group. For the year ended June 30, 2016, total income of $1,124,258 was recognized by Ankang Longevity Group from this supplemental agreement, compared to $1,124,010 in the same period in 2015.
On October 21, 2013, the Company, through its controlled subsidiaries, Zhisheng Freight and Zhisheng Agricultural, entered into an agreement with an unrelated third party, Zhejiang Zhen’Ai Network Warehousing Services Co., Ltd. (“Zhen’Ai Network”), and invested RMB 14.5 million (approximately $2.2 million) into Tiancang Systematic Warehousing project (“Tiancang Project”) operated by Zhen’Ai Network. Tiancang Project is an online platform established to provide comprehensive warehousing and logistic solutions to companies involved in E-commerce. The Company is entitled to a profit sharing of 29% of Tiancang Project’s after-tax net income annually, less 30% statutory reserve and 10% employee welfare fund. When the amount of the accumulated statutory reserve reaches 30% of the total investment for the Tiancang Project, no additional appropriation of the statutory reserve is required. The Tiancang Project is currently up and running. For the year ended June 30, 2016, Zhisheng Freight received investment income distribution of $139,949 from Zhen’Ai Network.
Interest Income, net
For the year ended June 30, 2016, our net interest income was $135,404 as compared to interest income of $42,502 in the same period of 2015. The increase in net interest income was attributable to the lower average short term loan outstanding as compared to the corresponding period in 2015.
Provision for Income Taxes
For the years ended June 30, 2016 and 2015, the Company’s provision for income taxes increased by $52,991 or 4.71% from $1,124,716 for the year ended June 30, 2015 to $1,177,707 for the year ended June 30, 2016. The increase in the Company’s provision for income taxes was primarily due to increased taxable income of Ankang Longevity Group for the period indicated.
| 38 |
Net Income
Our net income increased by $852,422 or 11.46% for the year ended June 30, 2016 as compared to the same period of 2015. The increase in our net income was primarily a result of the increase in gross profit and the decrease in general and administrative expense.
Comprehensive Income
The foreign currency translation gain or loss resulting from translation of the financial statements expressed in RMB to US$ is reported in other comprehensive income (loss) in the consolidated statements of income and comprehensive income. Our comprehensive income decreased by $3,399,548 to $4,194,247 for the year ended June 30, 2016 from $7,593,795 for the year ended June 30, 2015 due to the Renminbi’s significant weakening against the U.S. dollar during the such period.
Liquidity and Capital Resources
We currently finance our business operations primarily through cash provided by operating activities and bank loans. Our current cash primarily consists of cash on hand, which is unrestricted as to withdrawal and use and is deposited with banks in China.
As of June 30, 2016 and 2015, we possessed cash of $22,009,374 and $6,056,105, respectively. Management believes that our current cash, cash flows provided by operating activities, and access to loans will be sufficient to meet our working capital needs for at least the next 12 months. We intend to continue to carefully execute our growth plans and manage market risk.
Treasury Policies
We have established treasury policies with the objectives of achieving effective control of treasury operations and of lowering cost of funds. Therefore, funding for all operations and foreign exchange exposure have been centrally reviewed and monitored from the top level. To manage our exposure to fluctuations in exchange rates and interest rates on specific transactions and foreign currency borrowings, currency structured instruments and other appropriate financial instruments will be used to hedge material exposure, if any.
Our policy precludes us from entering into any derivative contracts purely for speculative activities. Through our treasury policies, we aim to:
(a) Minimize interest risk
This is accomplished by loan re-financing and negotiation. We will continue to closely monitor the total loan portfolio and compare the loan margin spread under our existing agreements against the current borrowing interest rates under different currencies and new offers from banks.
(b) Minimize currency risk
In view of the current volatile currency market, we will closely monitor the foreign currency borrowings at company level. As of June 30, 2016 and 2015, we do not engage in any foreign currency borrowings or loan contracts.
Working Capital
The following table provides the information about our working capital at June 30, 2016 and 2015:
| June 30, 2016 | June 30, 2015 | |||||||
| Current Assets | $ | 35,529,801 | $ | 28,255,871 | ||||
| Current Liabilities | 6,538,024 | 5,922,826 | ||||||
| Working Capital | $ | 28,991,777 | $ | 22,333,045 | ||||
The working capital increased by $6,658,732 or 29.82% from June 30, 2015 to June 30, 2016, primarily as a result of an increase in cash due to better operating results during the year ended June 30, 2016. We believe that we currently have sufficient working capital to run our business.
| 39 |
As of June 30, 2016 and 2015, other major components of our working capital included inventory and accounts receivable. Our inventory balance decreased by 44.50% from $8,302,297 as of June 30, 2015 to $4,608,179 as of June 30, 2016, which was mainly due to the decreased work in process of yew trees. The average inventory turnover days of finished goods in our industry ranges from 40 days to 60 days. Our inventory turnover days for raw materials and finished goods were around the industry average, because we tried to manage and control our inventory stockpile.
The accounts receivable, including accounts receivable from unconsolidated entities, as of June 30, 2016 were $5,552,242, a decrease of approximately 23.82% from $7,288,042 as of June 30 2015, mainly due to an increase of profit distribution received from Shaanxi Pharmaceutical Group of Ankang Longevity Medicine. The turnover days of trade receivables were 65.6 days for the year ended June 30, 2016, compared to 54.5 days in the corresponding period last period. The average turnover days of accounts receivable in our industry is approximately 40 days. As of June 30, 2016, about 96.50% of our outstanding account receivables were aged within 90 days, which was in compliance with our credit terms.
Capital Commitments and Contingencies
Capital commitments refer to the allocation of funds for a possible liability in the near future arising out of capital expenditures. Contingency refers to a condition that arises from past transactions or events, the outcome of which will be confirmed only by the occurrence or non-occurrence of uncertain futures events.
As of June 30, 2016 and 2015 we had no material capital commitment or contingent liabilities.
Cash Flows
The following table provides detailed information about our net cash flows for the years ended June 30, 2016 and 2015.
| For the years ended June 30, | ||||||||
| 2016 | 2015 | |||||||
| Net cash provided by operating activities | $ | 13,290,365 | $ | 2,646,789 | ||||
| Net cash provided by (used in) investing activities | 3,952,681 | (1,230,987 | ) | |||||
| Net cash provided by (used in) financing activities | (255,922 | ) | 1,527,350 | |||||
| Effect of exchange rate changes on cash | (1,033,855 | ) | 23,108 | |||||
| Net increase in cash | 15,953,269 | 2,966,260 | ||||||
| Cash, beginning of period | 6,056,105 | 3,089,845 | ||||||
| Cash, end of period | $ | 22,009,374 | $ | 6,056,105 | ||||
Operating Activities
Net cash provided by operating activities during fiscal 2016 was approximately $13.3 million, consisting of net income of $8.3 million, net reduction in noncash adjustments of $0.7 million and net changes in our operating assets and liabilities, which mainly included repayments from advances to suppliers of $1.2 million, reduction in inventory of $2.8 million, an increase of $1 million from other payables, net of payments to related parties of $0.6 million. Net cash provided by operating activities during fiscal 2015 was approximately $2.6 million, consisting of net income of $7.4 million, net reduction in noncash adjustments of $0.6 million and net changes in our operating assets and liabilities, which mainly included additional accounts receivable, inventory and prepaid leases of $3.5 million and $2.8 million and $1.1 respectively, net of repayments from advances to suppliers of $1.9 million and collections from related parties of $1.1 million.
Investing Activities
For the year ended June 30, 2016, net cash provided by investing activities amounted to $3,952,681 as compared to net cash used in investing activities of $1,230,987 for the same period of 2015. The increases in net cash provided by investing activities were primarily because $2,428,894 investment income received from Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd and $1,366,787 proceeds from loans to related parties for the year ended June 30, 2016.
Financing Activities
For the year ended June 30, 2016, net cash used in financing activities amounted to $255,922, as opposed to net cash provided by financing activities of $1,527,350 for the same period of 2015. The decrease of $1,783,272 in net cash provided by financing activities was primarily due to net repayments of short-term bank loans of $3,526,423, offset by an increase in proceeds received from short term bank loans in the amount of $3,148,077 for the year ended June 30, 2016.
| 40 |
| Item 7a. | Quantitative and Qualitative Disclosures About Market Risk |
The Company is not required to provide the information required by this Item because the Company is a smaller reporting company.
| Item 8. | Financial Statements and Supplementary Data |
The consolidated financial statements are included in Part III, Item 15 (a) (1) and (2) of this Report.
| Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and ProcedureS |
| (a) | Evaluation of Controls and Procedures |
We maintain disclosure controls and procedures designed to provide reasonable assurance that material information required to be disclosed by us in the reports filed or submitted under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission rules and forms, and that the information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure. Based on our review, our management, including our Chief Executive Officer and Chief Financial Officer, concluded that the Company’s disclosure controls and procedures were not effective at the reasonable assurance level as of the end of the period covered by this report due to following material weaknesses:
· The Company does not have U.S. GAAP full-time qualified personnel in the accounting department to monitor the recording of the daily transactions;
· Lack of segregation of duties for accounting personnel who prepared and reviewed the journal entries.
In order to address the above material weaknesses, our management plans to take the following steps:
· Recruiting sufficient qualified professionals with appropriate levels of knowledge of U.S. GAAP and experience to assist in reviewing and resolving accounting issues in routine or complex transactions. To mitigate the reporting risks, we engaged an outside professional consulting firm to supplement our efforts to improve our internal control over financial reporting;
· Improving the communication between management, board of directors and the Chief Financial Officer; and
· Obtaining proper approval for other significant and non-routine transactions from the Board of Directors.
The Company believes the foregoing measures will remediate the identified material weaknesses in future periods. The Company is committed to monitoring the effectiveness of these measures and making any changes that are necessary and appropriate.
| 41 |
| (b) | Changes in Internal Control over Financial Reporting |
There have been no changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting during our fourth fiscal quarter of 2016. Because of its inherent limitations, a system of internal control over financial reporting can provide only reasonable assurance and may not prevent or detect misstatements. Further, because of changes in conditions, effectiveness of internal controls over financial reporting may vary over time. Our system contains self-monitoring mechanisms, and actions are taken to correct deficiencies as they are identified.
| (c) | Management’s Annual Report on Internal Control over Financial Reporting |
Management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) for the Company. These controls are designed and implemented under the supervision of our Chief Executive Officer and Chief Financial Officer to provide reasonable assurance to the management and our Board of Directors regarding the reliability of financial reporting and the preparation of our financial statements for external reporting purposes in accordance with U.S. GAAP. Our internal control over financial reporting includes those policies and procedures that:
| 1. | Pertain to the maintenance of records that in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; |
| 2. | Provide reasonable assurance that transactions are recorded properly to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorization of our management and directors; and |
| 3. | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisitions, use or disposition of our assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Because of the inherent limitations of internal control, there is a risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
As of June 30, 2016, management assessed the effectiveness of its internal control over financial reporting based on the criteria for effective internal control over financial reporting established in Internal Control—2013 Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) and SEC guidance on conducting such assessments. Based on such evaluation, management identified deficiencies that were determined to be material weaknesses.
| 42 |
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. Because of the following material weaknesses, management concluded that our internal controls over financial reporting were ineffective as of June 30, 2016:
| 1. | We did not have sufficient skilled accounting personnel that are either qualified as Certified Public Accountants in the United States or that have received education from U.S. institutions or other educational programs that would provide adequate relevant education relating to U.S. GAAP. Our Chief Financial Officer and Controller have limited experience with U.S. GAAP and are not U.S. Certified Public Accountants. Furthermore, our operating subsidiaries are based in China and are therefore required to comply with PRC GAAP, rather than U.S. GAAP. Thus, the accounting skills and understanding necessary to fulfill the requirements of U.S. GAAP-based reporting, including the preparation of consolidated financial statements, remain inadequate and thus constitute a material weakness. | |
| 2. |
Lack of segregation of duties for accounting personnel who prepared and reviewed the journal entries. | |
| 3. | In addition, since we only completed the design of our internal controls and assessments for all of our financial reporting cycles in March 2012, we are not yet able to declare our controls as effective over a sufficient period of time in order to demonstrate the operating effectiveness of our controls as of June 30, 2016. Therefore, we have determined that such lack of time to evaluate the design and operating effectiveness of our controls is also a material weakness. |
In an effort to remedy the foregoing material weaknesses in the future, we intend to do the following:
| · | Develop a comprehensive training and development plan for our finance, accounting and internal audit personnel, including our Chief Financial Officer and Controller, in the principles and rules of U.S. GAAP, SEC reporting requirements and the application thereof; | |
| · | Design and implement a program to provide ongoing company-wide training regarding our internal controls, with particular emphasis on our finance and accounting staff; | |
| · | Implement an internal review process over financial reporting to review all recent accounting pronouncements and to verify that any accounting treatment identified in such report has been fully implemented and confirmed by our third-party consultant, and to continue to improve our ongoing review and supervision of our internal control over financial reporting; and | |
| · | Hire a full-time employee who possesses the requisite U.S. GAAP experience and education. |
Despite the material weaknesses and deficiencies reported above, our management believes that our consolidated financial statements included in this report fairly present in all material respects our financial condition, results of operations and cash flows for the periods presented and that this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this annual report.
This annual report does not include an attestation report of the Company’s independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s independent registered public accounting firm pursuant to rules of the SEC that permit the Company to provide only management’s report in this annual report.
| 43 |
| Item 9b. | Other Information |
None.
Part III
| Item 10. | Directors, Executive Officers and Corporate Governance |
Executive Officers and Directors
The following table sets forth our executive officers and directors, their ages and the positions held by them:
| Name | Age | Role | Since | |||
| Yuying Zhang | 63 | Chair of the Board, Chief Executive Officer and Director | 2011 | |||
| Sam Wang | 30 | Chief Financial Officer and Director | 2015* | |||
| Jiping Chen | 66 | Director | 2011 | |||
| Ying (Teresa) Zhang | 37 | Director (Independent) | 2014 | |||
| Yajun Shi | 38 | Director (Independent) | 2014 | |||
| Leiger Yongmin Yang | 39 | Director (Independent) | 2015 | |||
| Hua Yang | 45 | Director (Independent) | 2016 |
| * | Mr. Sam Wang has been our CFO since 2015 and director since 2016. |
Yuying Zhang, age 63, has been Chairman of Shineco since 2011 and is the Chairman and CEO of the Company. He was the principal founder of Tenet-Jove, which was established in 1995 with his research and development of Luobuma functional fiber healthcare products. He has been the Chairman and CEO of Tenet-Jove since December 2003; under his leadership, the company has worked with more than 20 research institutions and enterprises and has obtained numerous national invention and new product patent rights for Luobuma product development. From April 2014, he has been the Chairman of the Board of Beijing Huiyin Ansheng Asset Management Co., Ltd., which is a related party to Shineco. He also serves as a director in Tianjin Tenet Huatai Technological Development Co., Ltd. since 2003. From 1995 until December 2003, he served as general manager of Tianjin Balas Technological Development Co., Ltd. Prior to starting Tenet-Jove in 1995, he was the deputy director at the Army Institute of Integrative Medicine. From 1991 to 1994, he was the Executive Director and Deputy General Manager at Shan Haidan Pharmaceutical Group, where he was responsible for strategic development planning and marketing. Mr. Zhang is a senior economist with a degree from China Central Radio and Television University in China. Mr. Zhang has been chosen as director because his knowledge and extensive experience in research and development and management.
| 44 |
Sam Wang, age 31, became our Chief Financial Officer in February 2015 and Director since 2016. Mr. Wang has worked for Shineco, Inc. since 2011 where he served as Financial Controller until his appointment as our Chief Financial Officer. Mr. Wang has been the supervisory director of Shineco Zhisheng (Beijing) Bio-Technology Co., Ltd. since 2014. He serves as the General Manager of Qingdao Yinghuanhai International Logistics Co., Ltd. since 2012. Prior to joining Shineco, he worked for Citibank in Shenzhen from 2008 till 2011, where he served as Manager of Corporate Finance. Mr. Wang obtained a Masters in Commerce with a concentration in applied finance from The University of Queensland in 2010. In 2008, he received a bachelor’s degree in Accounting from Griffith University in Australia. Mr. Wang has been chosen as a director because he has profound knowledge of our industry and he is experienced with our financial matters.
Jiping Chen, age 66, has been a director for Shineco since 2011. Mr. Chen has served as Chairman, General Manager and Legal Representative of Ankang Longevity Group since 2006. From 1985 to 1992, Mr. Chen worked at Ankang Longevity Group where he a marketing manager. He has also served as a supervisor of Ankang Longevity Pharmaceutical (Group) Traditional Chinese Medicine Management Co., Ltd. since 2015. Since 2014, he has been Executive Director of Ankang Longevity Pharmaceutical (Group) Ziyang Longevity Traditional Chinese Medicine Materials Planting Co., Ltd. Since 2011 he has served as a supervisor of Ankang Longevity Real Estate Development Co., Ltd. He has also served as the Chairman of Board and General Manager of Ankang Longevity Pharmaceutical Group Pharmaceutical Industry Co., Ltd. since 2008. During 2008 to 2014, he served as a director of Ankang Longevity Pharmaceutical Group Breeding and Planting Co., Ltd. Since 2004, he has been the legal representative and Chairman of Board of Ankang Longevity Pharmaceutical (Group) Traditional Chinese Medicine Decoction Pieces Co., Ltd. He serves as legal representative and General Manager of Ankang Longevity Pharmaceutical Group Chain Co., Ltd. since 2003. Since 2002, Mr. Chen has been the Chairman of Board and General Manager of Ankang Longevity Pharmaceutical Group Purchasing Station Co., Ltd. From February 1975 to September 1985, Mr. Chen worked at the Ankang Area Public Bus Company where he served as company staff. From February 1973 to January 1975, he worked for Ankang County. Mr. Chen completed his three-year college education in Chinese Traditional Medicine major in Shannxi College of Chinese Traditional Medicine in 1997. Mr. Chen has been chosen as director because of his experience in the traditional Chinese medicine business and his familiarity with Ankang Longevity Group’s operations.
Ying (Teresa) Zhang, age 37, has been a director for Shineco since October 2014. From 2014 to July 2016, she served as the director in Beijing Huiyin Ansheng Assets Management Co., Ltd., which is a related party to Shineco. Since October 2010, Ms. Zhang has served as a director for Mixbox Co. Ltd., an international chain store management company. From January 2010 through December 2010 she served as the chief financial officer and a director of Cleantech Solutions International, Inc., a U.S. public company (NASDAQ: CLNT) that manufactures wind power equipment in China. Ms. Zhang has served as a director in Shiqiao (Tianjin) Investment Consulting Co., Ltd. since 2009. Ms. Zhang was chosen to serve as a member of the board of directors because of her experience with U.S. GAAP, as well as her extensive prior work experience and educational background in the accounting field. Ms. Zhang was previously an auditing manager at GC Alliance HK CPA in Beijing from July 2005 until January 2010, where she provided auditing services to China-based companies. From January 2003 through June 2005, Ms. Zhang served as a liaison officer for the Australian-Chinese Friendship Business Association, a trade organization, and from July 2000 to September 2002 she was an auditor at Ernst & Young in Beijing. None of these companies that Ms. Zhang worked with is related to or affiliated with us. Ms. Zhang is a certified practicing accountant in Australia. She received a bachelor degree in international accounting from Renmin University in China in 1996 and a master’s degree in accounting from Macquarie University in Australia in 2005.
| 45 |
Yajun Shi, age 38, has been a director for Shineco since October 2014. He is an associate professor and supervisor of postgraduate education at Shaanxi University of Chinese Medicine, is currently the vice president of the College of Pharmacy of Shaanxi University of Chinese Medicine, and is the director of instrument center and committee member of the Traditional Chinese Medicine Chinese Drugs Pharmaceutics Association. Mr. Shi graduated from Shaanxi University of Chinese Medicine in June 1999, and has been engaging in new medicine development successively in The Fourth Military Medical University, the Pharmaceutical Research Institute and Shaanxi Tiansen Medicine Development Company. In July 2003, Mr. Shi received Master’s Degree from the College of Pharmacy of Shaanxi University of Chinese Medicine. In June 2012, Mr. Shi received Doctor of Medicine degree (M.D.) from Chengdu University of Traditional Chinese Medicine where he specialized in traditional Chinese medicine preparation. Mr. Shi’s major research efforts are focused on traditional Chinese medicine and health products, focusing on the basic study and application of a Chinese medicine nasal drug application system, the study of traditional Chinese medicine powder characterization, and the modification and the adaptability, as well as the research and development of, healthy foods. Mr. Shi has published more than 40 academic papers, and compiled and published six professional books. Mr. Shi was chosen as a director because of his extensive knowledge and research of traditional Chinese medicines.
Leiger Yongmin Yang, age 39, has been a director for Shineco since January 2015. He has served as a director in Beijing Lanzhong Time Internet Science and Technology Co., Ltd. since 2014. Mr. Yang founded in 2012 China Offshore Financial Group (COFG), a professional offshore financial services provider, focused on financial services for private equity and venture capital firms, outbound and inbound investments, international trading, family trusts, and tax planning. Prior to founding COFG, Yang served as the General Manager of Offshore Incorporations Limited Group & Vistra Group (OV Group) beginning in 2010. The OV Group focuses on the formation and maintenance of offshore companies, fund formation and fund administration, immigration and trust services. Mr. Yang has also worked as Chief Editor of the Chinese Asian Venture Capital Journal since 2006, where he is responsible for content of the weekly Chinese Asian Venture Capital Journal. From 2003 to 2011, he was the director of Zhiyuan Rongtong Investment Management Consulting (Beijing) Co., Ltd. Mr. Yang has spent the major part of his career in the private equity and venture capital sectors. Yang holds a Bachelor of Journalism degree from Jilin University in China. He was chosen as a director because of his hands-on experience in the private equity and venture capital sectors and his strong network with leading venture capital and private equity firms, law firms, accounting firms and banks in China.
Hua Yang, age 45, has been a director for Shineco since 2016. Ms. Yang has been a Partner and attorney in Grandall Law Firm in Beijing since March 2007. From 2006 to 2011, she served as a director of Beijing Shichen International Consulting Co., Ltd. Her practice area covers Corporation Law, foreign investments, international litigations and arbitrations. She provides services in the industries including civil aviation, agriculture biotechnology and nuclear power. From 2001 to 2007, Ms. Yang was a lawyer in Beijing Weiheng Law Firm. She was also a visiting attorney for Canada Fraser Milner Casgrain LLP and Pothier Delisle Law Office during 2002 to 2003. Before that, she was the Legal Manager of Weihai International Trust and Investment Corporation during 1995 to 1999. Ms. Yang received her degree of Master of Laws in Indiana University in 2006. She also graduated from Renmin University of China with a degree of Master of Laws in 2001. She obtained a Bachelor’s degree of Laws from Northwest University of Political Science and Law in 1995 in China. We have chosen Ms. Yang to serve as a director because of the perspective she brings to legal matters in China and her rich experience in Corporate Law and foreign investments.
Board of Directors and Board Committees
Our board of directors currently consists of seven directors, four of whom — Ying (Teresa) Zhang, Yajun Shi, Leiger Yongmin Yang and Hua Yang — are independent, as such term is defined by The NASDAQ Capital Market. We expect that all current directors will continue to serve after our initial public offering.
A director may vote in respect of any contract or transaction in which he is interested, provided, however that the nature of the interest of any director in any such contract or transaction shall be disclosed by him at or prior to its consideration and any vote on that matter. A general notice or disclosure to the directors or otherwise contained in the minutes of a meeting or a written resolution of the directors or any committee thereof of the nature of a director’s interest shall be sufficient disclosure and after such general notice it shall not be necessary to give special notice relating to any particular transaction. A director may be counted for a quorum upon a motion in respect of any contract or arrangement which he shall make with our company, or in which he is so interested and may vote on such motion.
| 46 |
Mr. Yuying Zhang currently holds both the positions of Chief Executive Officer and Chair of the Board. These two positions have not been consolidated into one position; Mr. Zhang simply holds both positions at this time. We do not have a lead independent director because of the foregoing reason and also because we believe our independent directors are encouraged to freely voice their opinions on a relatively small company board. We believe this leadership structure is appropriate because we are a smaller reporting company in the process of listing on a public exchange; as such we deem it appropriate to be able to benefit from the guidance of Mr. Zhang as both our principal executive officer and Chair of the Board. Our Board of Directors plays a significant role in our risk oversight. The Board of Directors makes all relevant Company decisions. As a smaller reporting company with a small board of directors, we believe it is appropriate to have the involvement and input of all of our directors in risk oversight matters.
Board Committees
We have established three standing committees under the board: the audit committee, the compensation committee and the nominating committee. Each committee has three members, and each member is independent, as such term is defined by The Nasdaq Capital Market. The audit committee is responsible for overseeing the accounting and financial reporting processes of our company and audits of the financial statements of our company, including the appointment, compensation and oversight of the work of our independent auditors. The compensation committee of the board of directors reviews and makes recommendations to the board regarding our compensation policies for our officers and all forms of compensation, and also administers our incentive compensation plans and equity-based plans (but our board retains the authority to interpret those plans). The nominating committee of the board of directors is responsible for the assessment of the performance of the board, considering and making recommendations to the board with respect to the nominations or elections of directors and other governance issues. The nominating committee considers diversity of opinion and experience when nominating directors.
The members of the audit committee, the compensation committee and the nominating committee are set forth below. All such members qualify as independent under the rules of The Nasdaq Capital Market.
| Director | Audit Committee |
Compensation Committee |
Nominating Committee |
|||||||||
| Ying Zhang | (1)(2)(3) | (1) | (1) | |||||||||
| Hua Yang | (1) | (1)(2) | ||||||||||
| Yongmin Yang | (1) | (1) | ||||||||||
| Yajun Shi | (1)(2) | (1) | ||||||||||
| (1) | Committee member |
| (2) | Committee chair |
| (3) | Audit committee financial expert |
| 47 |
Limitation on Liability and Other Indemnification Matters
Section 102 of the Delaware General Corporation Law, as amended (“DGCL”) allows a corporation to eliminate or limit the personal liability of directors to a corporation or its shareholders for monetary damages for breach of fiduciary duty as a director, except where the director breached his duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase or redemption in violation of Delaware corporate law or engaged in a transaction from which the director obtained an improper personal benefit. In accordance with Delaware law, our Certificate of Incorporation provides that no director shall be personally liable to us or any of our shareholders for monetary damages for breach of fiduciary duty as a director, except for the foregoing exceptions set forth in Section 102 of the DGCL.
Section 145 of the DGCL provides, among other things, that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than an action by or in the right of the corporation) by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the corporation’s request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with the action, suit or proceeding. The power to indemnify applies if (i) such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation, and with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful or, (ii) to the extent that such person is a present or former director or officer of a corporation, such person is successful on the merits or otherwise in defense of any action, suit or proceeding. The power to indemnify applies to actions brought by or in the right of the corporation as well, but only to the extent of defense expenses (including attorneys’ fees but excluding amounts paid in settlement) actually and reasonably incurred and not to any satisfaction of judgment or settlement of the claim itself, and with the further limitation that in such actions no indemnification shall be made in the event such person is adjusted to be liable to the corporation, unless a court determines that in light of all the circumstances indemnification should apply.
Section 174 of the DGCL provides, among other things, that a director who willfully and negligently approves of an unlawful payment of dividends or an unlawful stock purchase or redemption may be held liable for such actions to the full amount of the dividend unlawfully paid or the purchase or redemption of the corporation’s stock, with interest from the time such liability accrued. A director who was either absent when the unlawful actions were approved or dissented at the time may avoid liability by causing his or her dissent to such actions to be entered on the books containing the minutes of the meetings of the board of directors at the time the action occurred or immediately after the absent director receives notice of the unlawful acts.
Our Bylaws provide that we will indemnify, to the fullest extent permitted by the DGCL, any person made or threatened to be made a party to any action by reason of the fact that the person is or was our director or officer, or serves or served as a director or officer of any other enterprise at our request. Expenses incurred by a director or officer in defending against such legal proceedings are payable before the final disposition of the action, provided that the director or officer undertakes to repay us if it is later determined that he or she is not entitled to indemnification.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
We do not maintain policies of insurance under which coverage is provided (a) to our directors and officers against loss rising from claims made by reason of breach of duty or other wrongful act, and (b) to us with respect to payments which we may make to such officers and directors pursuant to the above indemnification provision or otherwise as a matter of law for a privately held company. We will consider modifying our coverage to address public company specific exposures in connection with the completion of our initial public offering.
| 48 |
Director Compensation
Historically, we have not paid our directors. Upon completion of our initial public offering, we plan to pay our independent directors an annual cash retainer of $10,000. We may also provide stock, option or other equity-based incentives to our directors for their service. We also plan to reimburse our directors for any out-of-pocket expenses incurred by them in connection with their services provided in such capacity.
Section 16(a) of the Exchange Act
Section 16(a) of the Exchange Act requires our directors, executive officers and persons who own more than 10% of our common stock to file reports of ownership and changes in ownership of our common stock with the SEC. Directors, executive officers and persons who own more than 10% of our common stock are required by SEC regulations to furnish to the Company copies of all Section 16(a) forms they file.
To our knowledge, based solely upon review of the copies of such reports received or written representations from the reporting persons, we believe that during our fiscal year ended June 30, 2016, our directors, executive officers and persons who owned more than 10% of our common stock complied with all Section 16(a) filing requirements except for Ms. Hua Yang. Ms. Hua Yang did not timely file a Form 3 after she was appointed as an independent director of the Company on June 21, 2016. However, the Company disclosed Ms. Hua Yang’s appointment as an independent director in the Company’s 8-K dated June 23, 2016. Ms. Hua Yang completed her Form 3 filing on September 28, 2016.
Code of Ethics
We have adopted a code of ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. We will provide a copy of our code of ethics to any person who requests a copy in writing to the Secretary of the Company, including the e-mail address or facsimile number of the requesting party. Any written requests should be mailed to us at Shineco, Inc., 2nd Floor, Wanyuan Business Center, 10 N. Hongda Road, Daxing District, Beijing, People’s Republic of China.
| Item 11. | Executive Compensation |
The following table shows the annual compensation paid by us for the years ended June 30, 2015, 2014 and 2013 to Yuying Zhang, our principal executive officer. No executive officers of our company have annual compensation exceeding $100,000.
Summary Compensation Table – Named Executive Officer
| Name and principal position | Year | Salary(1) | Bonus | All Other Compensation (2) |
Total | |||||||||||||
| Yuying Zhang, | 2016 | $ | 38,709.00 | $ | - | $ | 14,567.00 | $ | 53,276.00 | |||||||||
| principal executive officer | 2015 | $ | 39,012.00 | $ | - | $ | 14,973.51 | $ | 53,985.51 | |||||||||
| 2014 | $ | 38,745.00 | $ | - | $ | 13,076.54 | $ | 51,821.54 | ||||||||||
| (1) | Salaries were paid in RMB. |
| (2) | Mr. Zhang receives monthly payments for rent for his personal home and parking. |
Employment Agreements
Generally
Under Chinese law, we may only terminate employment agreements without cause and without penalty by providing notice of non-renewal one month prior to the date on which the employment agreement is scheduled to expire. If we fail to provide this notice or if we wish to terminate an employment agreement in the absence of cause, then we are obligated to pay the employee one month’s salary for each year we have employed the employee. We are, however, permitted to terminate an employee for cause without penalty to our company, where the employee has committed a crime or the employee’s actions or inactions have resulted in a material adverse effect to us. At this time, we have no employment agreements with any of our executive officers.
| 49 |
Outstanding Equity Awards
There was no equity awards granted to our officers or directors in the year ended June 30, 2016.
Retirement Plans
We currently have no plans that provide for the payment of retirement benefits, or benefits that will be paid primarily following retirement, including but not limited to tax-qualified defined benefit plans, supplemental executive retirement plans, tax-qualified defined contribution plans and nonqualified defined contribution plans.
Potential Payments upon Termination or Change-in-Control
We currently have no contract, agreement, plan or arrangement, whether written or unwritten, that provides for payments to a named executive officer at, following, or in connection with any termination, including without limitation resignation, severance, retirement or a constructive termination of a named executive officer, or a change in control of the Company or a change in the named executive officer’s responsibilities, with respect to each named executive officer.
Director Compensation
Historically, we have not paid our directors. Upon completion of our initial public offering, we plan to pay our independent directors an annual cash retainer of $10,000. We may also provide stock, option or other equity-based incentives to our directors for their service. We also plan to reimburse our directors for any out-of-pocket expenses incurred by them in connection with their services provided in such capacity.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The following table sets forth information, as of September 27, 2016, regarding the beneficial ownership of our common stock by any person known to us to be the beneficial owner of more than 5% of the outstanding common stock, by directors and certain executive officers, and by all of our directors and executive officers as a group. Unless otherwise noted, our officers and directors utilize the following address for correspondence purposes: 2nd Floor, Wanyuan Business Center, 10 N Hongda Road, Daxing District, Beijing, 100176 100022, People’s Republic of China.
| Name and Address | Title of | Amount and Nature of
Beneficial Ownership | Percent (%) of Class | |||||||
| Yuying Zhang | common | 1,143,140 | (1) | 5.435 | % | |||||
| Sai (Sam) Wang | common | 749,645 | 3.564 | % | ||||||
| Jiping Chen | common | 2,194,115 | 10.431 | % | ||||||
| Ying (Teresa) Zhang | common | - | - | |||||||
| Leiger Yongmin Yang | common | - | - | |||||||
| Hua Yang | common | - | - | |||||||
| Yajun Shi | common | - | - | |||||||
| All Officers and Directors as a Group (7 total) | common | 4,086,900 | 19.43 | % | ||||||
| 5% Shareholders Not Mentioned Above: | ||||||||||
| Xiaoyan Chen | common | 1,088,067 | 5.173 | % | ||||||
| Qiwei Wang | common | 1,109,908 | 5.277 | % | ||||||
| (1) | Includes 878,018 shares owned by Min Zhao, the wife of Yuying Zhang. By virtue of this relationship, each of Ms. Zhao and Mr. Zhang may be deemed to share beneficial ownership of the shares of our company held by each of them. Mr. Zhang disclaims beneficial ownership of these shares. |
| 50 |
| Item 13. | Certain Relationships and Related Transactions |
Receivables from Related Parties
As of September 1, 2016, the Company temporarily advanced a total of $1,608,027 to (i) Shanxi Pharmacy Holding Group Longevity Pharmacy Co., Ltd., which is owned by one of our shareholders, (ii) XinyangYifangyuan Garden Technology Co., Ltd., which is owned by one of our shareholders, (iii) Qi Qiuchi, one of our shareholders, (iv) Yang Bin, one of our shareholders, (v) Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd., which owns 51% of two of our equity investments, (vi) Zhang Xin, one of our shareholders, (vii) Chang Song, one of our shareholders, (viii) Qiwei Wang, one of our shareholders, and (ix) Tian Shuangpeng, one of our shareholders. All the advances were short-term loans for the requirements of the related parties and were made interest-free.
As of June 30, 2016, the Company temporarily advanced a total of $1,671,435 to (i) Shanxi Pharmacy Holding Group Longevity Pharmacy Co., Ltd., which is owned by one of our shareholders, (ii) XinyangYifangyuan Garden Technology Co., Ltd., which is owned by one of our shareholders, (iii) Qi Qiuchi, one of our shareholders, (iv) Yang Bin, one of our shareholders, (v) Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd., which owns 51% ownership of two of our equity investments, (vi) Zhang Xin, one of our shareholders, (vii) Chang Song, one of our shareholders, (viii) Qiwei Wang, one of our shareholders, and (ix) Tian Shuangpeng, one of our shareholders. All the advances were short-term loans for the requirements of the related parties and were made interest-free.
As of June 30, 2015, the Company temporarily advanced a total of $2,623,169 to (i) Shanxi Pharmacy Holding Group Longevity Pharmacy Co., Ltd., which is owned by one of our shareholders, (ii) XinyangYifangyuan Garden Technology Co., Ltd., which is owned by one of our shareholders, (iii) Qi Qiuchi, one of our shareholders, (iv) Yang Bin, one of our shareholders, (v) KuerLe Tenet-Jove Business & Trading Co., Ltd., which is owned by a family member of our Chief Executive Officer and director, Yuying Zhang, (vi) Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd., which owns 51% ownership of two of our equity investments, (vii) Zhang Xin, one of our shareholders, (viii) Chang Song, one of our shareholders, (ix) HuiyinAnsheng, which is partially owned by one of our shareholders, (x) Qiwei Wang, one of our shareholders, and (xi) Yuying Zhang, our Chief Executive Officer and director. As of date of this prospectus, all outstanding amounts due from those related parties have been collected. All the advances were short-term loans for the requirements of the related parties and were made interest-free, and the balances were settled in cash.
As of June 30, 2014, the Company temporarily advanced a total of $2,690,273 to (i) XinyangYifangyuan Garden Technology Co., Ltd., which is owned by one of our shareholders (ii)Yang Bin, one of our shareholders, (iii) KuerLe Tenet-Jove Business & Trading Co., Ltd., which is owned by a family member of our Chief Executive Officer and director, Yuying Zhang, (iv) Zhang Xin, one of our shareholders, (v) HuiyinAnsheng, which is partially owned by one of our shareholders, (vi) Qiwei Wang, one of our shareholders, (vii) Longevity Pharmaceutical Group Real Estate Co., Ltd., which is owned by our director Jiping Chen, (viii) Li Qiang, one of our shareholders, (ix) Beijing Juxing Cultural Communication Center, which is owned by one of our shareholders, (x) Beijing Juxingzuo Advertising Co., Ltd., which is owned by one of our shareholders, (xi) Song Dandan, one of our shareholders, and (xii) Zhao Min, one of our shareholders. As of date of this prospectus, all outstanding amounts due from those related parties have been collected. All the advances were short-term loans for the requirements of the related parties and were made interest-free, and the balances were settled in cash.
The detailed amounts due from related parties consist of the following:
| Sept. 1, 2016 | June 30, 2016 |
June 30, 2015 |
June 30, 2014 |
|||||||||||||
| Shanxi Pharmacy Holding Group Longevity Pharmacy Co., Ltd. | $ | - | - | $ | 1,158,764 | $ | - | |||||||||
| XinyangYifangyuan Garden Technology Co., Ltd. | 481,786 | 500,784 | 593,413 | 507,813 | ||||||||||||
| Qi Qiuchi | 1,448 | 1,505 | 171,885 | - | ||||||||||||
| Yang Bin | 144,794 | 150,504 | 163,700 | 422,500 | ||||||||||||
| KuerLe Tenet-Jove Business & Trading Co., Ltd. | 163,700 | 162,500 | ||||||||||||||
| Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd. | 789,593 | 820,728 | 144,830 | - | ||||||||||||
| Zhang Xin | 89,772 | 93,312 | 101,494 | 100,750 | ||||||||||||
| Chang Song | 81,809 | 85,035 | 64,662 | - | ||||||||||||
| HuiyinAnsheng | 48,092 | 61,451 | ||||||||||||||
| Wang Qiwei | 7,965 | 8,279 | 9,005 | 361,827 | ||||||||||||
| Zhang Yuying | - | - | 3,624 | - | ||||||||||||
| Longevity Pharmaceutical Group Real Estate Co., Ltd. | - | - | - | 827,303 | ||||||||||||
| Li Qiang | - | - | - | 81,250 | ||||||||||||
| Beijing Juxing Cultural Communication Center | - | - | - | 68,250 | ||||||||||||
| Beijing Juxingzuo Advertising Co., Ltd. | - | - | - | 68,058 | ||||||||||||
| Song Dandan | - | - | - | 18,688 | ||||||||||||
| Zhao Min | - | - | - | 9,883 | ||||||||||||
| Tian Shuangpeng | 10,860 | 11,288 | - | - | ||||||||||||
| Total | $ | 1,608,027 | $ | 1,671,435 | $ | 2,623,169 | $ | 2,690,273 | ||||||||
| 51 |
We have a policy under which we are prohibited from making or renewing any personal loan to our executive officers or directors in accordance with Section 13(k) of the Exchange Act. The related party transactions with Yuying Zhang, our Chief Executive Officer and director, described in this section occurred prior to adoption of this policy, and as such, these transactions were not subject to such prohibition. As of date of this report, all outstanding amounts due from any loans to executive officers or directors have been collected in full.
Payables to Related Parties
As of September 1, 2016, June 30, 2016, June 30, 2015 and 2014, the Company has related party payables of $235,624, $244,915, $137,508 and $0, respectively, mainly due to Wu Yang who lend funds for the Company’s operations. Wu Yang is the wife of Yin Weixing, one of the directors of the Company. The payables are unsecured, non-interest bearing and due on demand.
The detailed amount due to related parties consists of the following:
| Sept. 1, 2016 | June 30, 2016 |
June 30, 2015 |
June 30, 2014 |
|||||||||||||
| Wu Yang | $ | 92,741 | 96,398 | $ | 137,508 | $ | - | |||||||||
| Huiyin Ansheng | 860 | 894 | - | - | ||||||||||||
| Wang Sai | 116,269 | 120,854 | - | - | ||||||||||||
| Zhang Yuying | 25,753 | 26,769 | ||||||||||||||
| Total | $ | 235,624 | 244,915 | $ | 137,508 | $ | - | |||||||||
Sales and Purchases From Related Parties
For the period from July 1, 2016 to September 1, 2016, the Company recorded sales to related parties of $1,058,795, and no purchases from related parties. For the year ended June 30, 2016, the Company recorded sales to related parties of $3,014,198 and no purchases from related parties. For the year ended June 30, 2015, the Company recorded sales to related parties of $3,176,386 and no purchases from related parties. For the year ended June 30, 2014, the Company recorded sales to related parties of $2,708,502 and no purchases from related parties. All sales to related parties were sales of traditional Chinese herbal products to one of our affiliates, Shaanxi Pharmacy Holding Group Ankang Longevity Pharmaceutical Co., Ltd., an entity partially owned by the Company through a joint venture with Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd., a Chinese state-owned pharmaceutical enterprise.
| 52 |
| Item 14. | Principal Accounting Fees and Services |
The following table shows the fees that were billed for audit and other services provided by Friedman LLP, our independent accountant, during the following fiscal years:
| Fiscal Year Ended June 30, | ||||||||
| 2016 | 2015 | |||||||
| Audit Fees(1) | $ | 300,000 | $ | 290,000 | ||||
| Audit-related Fees(2) | - | - | ||||||
| Tax Fees(3) | - | - | ||||||
| All Other Fees(4) | - | - | ||||||
| Total | $ | 300,000 | $ | 290,000 | ||||
| (1) | Audit Fees – This category includes the audit of our annual financial statements, review of financial statements included in our Quarterly Reports on Form 10-Q, and services that are normally provided by independent auditors in connection with statutory and regulatory filings or the engagement for fiscal years. This category also includes advice on audit and accounting matters that arose during, or as a result of, the audit or the review of interim financial statements. |
| (2) | Audit-Related Fees – This category consists of assurance and related services by our independent auditor that are reasonably related to the performance of the audit or review of our financial statements and are not reported above under "Audit Fees." The services for the fees disclosed under this category include consultation regarding our correspondence with the SEC. |
| (3) | Tax Fees – This category consists of professional services rendered by our independent auditors for tax compliance and tax advice. The services for the fees disclosed under this category include tax return preparation and technical tax advice. |
| (4) | All Other Fees – This category consists of fees for other miscellaneous items such as travel and out-of-pocket expenses. |
Pre-Approval Policies and Procedures of the Audit Committee
Our Audit Committee approves the engagement of our independent auditors and is also required to pre-approve all audit and non-audit expenses. Prior to engaging its accountants to perform particular services, our Audit Committee obtains an estimate for the service to be performed. All of the services described above were approved by the Audit Committee in accordance with its procedure.
| Item 15. | Exhibits and Financial Statement Schedules |
(1) Financial Statements
The following consolidated financial statements for the years ended June 30, 2016 and 2015 are included in Part II, Item 8 of this Report:
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets
Consolidated Statements of Income and Comprehensive Income
| 53 |
Consolidated Statements of Equity
Consolidated Statements of Cash Flows
Notes to Consolidated Financial Statements
(2) Financial Statement Schedules
Schedules are omitted because the required information is not present or is not present in amounts sufficient to require submission of the schedule or because the information required is given in the consolidated financial statements or the notes thereto.
(3) Exhibits
EXHIBIT INDEX
The following documents are filed herewith:
| Number | Exhibit | |
| 3.1† | Certificate of Incorporation of Shineco, Inc. (1) | |
| 3.2† | Amended and Restated Bylaws of Shineco, Inc.(1) | |
| 4.1† | Specimen Common Stock Share Certificate(1) | |
| 10.1† | Exclusive Business Cooperation Agreement between Beijing Tenet-Jove Technological Development Co., Ltd. and Shineco Zhisheng (Beijing) Bio-Technology Co., Ltd. dated February 24, 2014. (1) | |
| 10.2† | Timely Reporting Agreement between Shineco Inc. and Shineco Zhisheng (Beijing) Bio-Technology Co., Ltd. dated July 3, 2014. (1) | |
| 10.3† | Equity Interest Pledge Agreement among Beijing Tenet Jove Technological Development Co., Ltd., Wang Qiwei, Wang Sai, Yin Weixing, Liu Yu, Zhou Qi, Yang Chunhong, and Shineco Zhisheng (Beijing) Bio-Technology Co., Ltd. dated February 24, 2014. (1) | |
| 10.4† | Exclusive Option Agreement among Beijing Tenet Jove Technological Development Co., Ltd., Wang Qiwei, Wang Sai, Yin Weixing, Liu Yu, Zhou Qi, Yang Chunhong (Shareholders from Shineco Zhisheng (Beijing) Bio-Technology Co., Ltd.), and Shineco Zhisheng (Beijing) Bio-Technology Co., Ltd. dated February 24, 2014. (1) | |
| 10.5† | Power of Attorney by and between Yang Chunhong and Beijing Tenet Jove Technological Development Co., Ltd. regarding shareholding of Shineco Zhisheng (Beijing) Bio-Technology Co., Ltd. dated February 24, 2014. (1) | |
| 10.6† | Power of Attorney by and between Yin Weixing and Beijing Tenet Jove Technological Development Co., Ltd. regarding shareholding of Shineco Zhisheng (Beijing) Bio-Technology Co., Ltd. dated February 24, 2014. (1) | |
| 10.7† | Power of Attorney by and between Liu Yu and Beijing Tenet Jove Technological Development Co., Ltd. regarding shareholding of Shineco Zhisheng (Beijing) Bio-Technology Co., Ltd. dated February 24, 2014. (1) | |
| 10.8† | Power of Attorney by and between Wang Qiwei and Beijing Tenet Jove Technological Development Co., Ltd. regarding shareholding of Shineco Zhisheng (Beijing) Bio-Technology Co., Ltd. dated February 24, 2014. (1) | |
| 10.9† | Power of Attorney by and between Wang Sai and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Shineco Zhisheng (Beijing) Bio-Technology Co., Ltd. dated February 24, 2014. (1) | |
| 10.10† | Power of Attorney by and between Zhou Qi and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Shineco Zhisheng (Beijing) Bio-Technology Co., Ltd. dated February 24, 2014. (1) | |
| 10.11† | Exclusive Business Cooperation Agreement between Beijing Tenet-Jove Technological Development Co., Ltd. and Yantai Zhisheng International Freight Forwarding Co., Ltd. dated June 16, 2011. (1) | |
| 10.12† | Timely Reporting Agreement between Shineco Inc. and Yantai Zhisheng International Freight Forwarding Co., Ltd. dated July 3, 2014. (1) | |
| 10.13† | Equity Interest Pledge Agreement among Beijing Tenet-Jove Technological Development Co., Ltd., Wang Qiwei, Wang Sai, Yin Weixing, Zhang Weisheng, Zhou Qi, Yang Chunhong, and Yantai Zhisheng International Freight Forwarding Co., Ltd. dated June 16, 2011. (1) | |
| 10.14† | Exclusive Option Agreement among Beijing Tenet-Jove Technological Development Co., Ltd., Wang Qiwei, Wang Sai, Yin Weixing, Zhang Weisheng, Zhou Qi, Yang Chunhong, and Yantai Zhisheng International Freight Forwarding Co., Ltd. dated June 16, 2011. (1) | |
| 10.15† | Power of Attorney by and between Zhou Qi and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Yantai Zhisheng International Freight Forwarding Co., Ltd. dated June 16, 2011. (1) | |
| 10.16† | Power of Attorney by and between Zhang Weisheng and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Yantai Zhisheng International Freight Forwarding Co., Ltd. dated June 16, 2011. (1) |
| 54 |
| 10.17† | Power of Attorney by and between Yang Chunhong and Beijing Tenet Jove Technological Development Co., Ltd. regarding shareholding of Yantai Zhisheng International Freight Forwarding Co., Ltd. dated June 16, 2011. (1) | |
| 10.18† | Power of Attorney by and between Wang Qiwei and Beijing Tenet Jove Technological Development Co., Ltd. regarding shareholding of Yantai Zhisheng International Freight Forwarding Co., Ltd. dated June 16, 2011. (1) | |
| 10.19† | Power of Attorney by and between Wang Sai and Beijing Tenet Jove Technological Development Co., Ltd. regarding shareholding of Yantai Zhisheng International Freight Forwarding Co., Ltd. dated June 16, 2011. (1) | |
| 10.20† | Power of Attorney by and between Yin Weixing and Beijing Tenet Jove Technological Development Co., Ltd. regarding shareholding of Yantai Zhisheng International Freight Forwarding Co., Ltd. dated June 16, 2011. (1) | |
| 10.21† | Exclusive Business Cooperation Agreement between Beijing Tenet Jove Technological Development Co., Ltd. and Yantai Zhisheng International Trade Co., Ltd. dated June 16, 2011. (1) | |
| 10.22† | Timely Reporting Agreement between Shineco Inc. and Yantai Zhisheng International Trade Co., Ltd. dated July 3, 2014. (1) | |
| 10.23† | Equity Interest Pledge Agreement among Beijing Tenet Jove Technological Development Co., Ltd., Wang Qiwei, Wang Sai, Yin Weixing, Zhang Weisheng, Zhou Qi, Yang Chunhong, and Yantai Zhisheng International Trade Co., Ltd. dated June 16, 2011. (1) | |
| 10.24† | Exclusive Option Agreement among Beijing Tenet Jove Technological Development Co., Ltd., Wang Qiwei, Wang Sai, Yin Weixing, Zhang Weisheng, Zhou Qi, Yang Chunhong, and Yantai Zhisheng International Trade Co., Ltd. dated June 16, 2011. (1) | |
| 10.25† | Power of Attorney by and between Zhang Weisheng and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Yantai Zhisheng International Trade Co., Ltd. dated June 16, 2011. (1) | |
| 10.26† | Power of Attorney by and between Zhou Qi and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Yantai Zhisheng International Trade Co., Ltd. dated June 16, 2011. (1) | |
| 10.27† | Power of Attorney by and between Wang Qiwei and Beijing Tenet Jove Technological Development Co., Ltd. regarding shareholding of Yantai Zhisheng International Trade Co., Ltd. dated June 16, 2011. (1) | |
| 10.28† | Power of Attorney by and between Yin Weixing and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Yantai Zhisheng International Trade Co., Ltd. dated June 16, 2011. (1) | |
| 10.29† | Power of Attorney by and between Wang Sai and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Yantai Zhisheng International Trade Co., Ltd. dated June 16, 2011. (1) | |
| 10.30† | Power of Attorney by and between Yang Chunhong and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Yantai Zhisheng International Trade Co., Ltd. dated June 16, 2011. (1) | |
| 10.31† | Exclusive Business Cooperation Agreement between Beijing Tenet-Jove Technological Development Co., Ltd. and Qingdao Zhihesheng Agricultural Produce Services, Co., Ltd. dated May 24, 2012. (1) | |
| 10.32† | Timely Reporting Agreement between Shineco Inc. and Qingdao Zhihesheng Agricultural Produce Services, Co., Ltd. dated July 3, 2014. (1) | |
| 10.33† | Equity Interest Pledge Agreement among Beijing Tenet-Jove Technological Development Co., Ltd., Wang Qiwei, Wang Sai, Yin Weixing, Zhang Weisheng, Zhou Qi, Yang Chunhong, and Qingdao Zhihesheng Agricultural Produce Services, Co., Ltd. dated May 24, 2012. (1) | |
| 10.34† | Exclusive Option Agreement among Beijing Tenet-Jove Technological Development Co., Ltd., Wang Qiwei, Wang Sai, Yin Weixing, Zhang Weisheng, Zhou Qi, Yang Chunhong, and Qingdao Zhihesheng Agricultural Produce Services, Co., Ltd. dated May 24, 2012. (1) | |
| 10.35† | Power of Attorney by and between Wang Sai and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Qingdao Zhihesheng Agricultural Produce Services Co., Ltd. dated May 24, 2012. (1) | |
| 10.36† | Power of Attorney by and between Wang Qiwei and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Qingdao Zhihesheng Agricultural Produce Services Co., Ltd. dated May 24, 2012. (1) | |
| 10.37† | Power of Attorney by and between Yin Weixing and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Qingdao Zhihesheng Agricultural Produce Services, Co., Ltd. dated May 24, 2012. (1) | |
| 10.38† | Power of Attorney by and between Zhang Weisheng and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Qingdao Zhihesheng Agricultural Produce Services, Co., Ltd. dated May 24, 2012. (1) | |
| 10.39† | Power of Attorney by and between Zhou Qi and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Qingdao Zhihesheng Agricultural Produce Services, Co., Ltd. dated May 24, 2012. (1) | |
| 10.40† | Power of Attorney by and between Yang Chunhong and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Qingdao Zhihesheng Agricultural Produce Services, Co., Ltd. dated May 24, 2012. (1) | |
| 10.41† | Exclusive Business Cooperation Agreement between Beijing Tenet-Jove Technological Development Co., Ltd. and Yantai Mouping District Zhisheng Agricultural Produce Cooperative dated June 16, 2011. (1) | |
| 10.42† | Timely Reporting Agreement between Shineco Inc. and Yantai Mouping District Zhisheng Agricultural Produce Cooperative dated July 3, 2014. (1) | |
| 10.43† | Guarantee Agreement among Beijing Tenet-Jove Technological Development Co., Ltd., Wang Qiwei, and Yantai Mouping District Zhisheng Agricultural Produce Cooperative dated June 16, 2011. (1) | |
| 10.44† | Power of Attorney by and between Zhang Weisheng and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Yantai Mouping District Zhisheng Agricultural Produce Cooperative dated June 16, 2011. (1) |
| 55 |
| 10.45† | Power of Attorney by and between Yin Weixing and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Yantai Mouping District Zhisheng Agricultural Produce Cooperative dated June 16, 2011. (1) | |
| 10.46† | Power of Attorney by and between Wang Sai and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Yantai Mouping District Zhisheng Agricultural Produce Cooperative dated June 16, 2011. (1) | |
| 10.47† | Power of Attorney by and between Wang Qiwei and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Yantai Mouping District Zhisheng Agricultural Produce Cooperative dated June 16, 2011. (1) | |
| 10.48† | Exclusive Business Cooperation Agreement between Beijing Tenet-Jove Technological Development Co., Ltd. and Ankang Longevity Pharmaceutical (Group) Co., Ltd. dated December 31, 2008. (1) | |
| 10.49† | Timely Reporting Agreement between Shineco Inc. and Ankang Longevity Pharmaceutical (Group) Co., Ltd. dated July 3, 2014. (1) | |
| 10.50† | Equity Interest Pledge Agreement among Beijing Tenet-Jove Technological Development Co., Ltd., Chen Jiping, Chen Xiaoyan, and Ankang Longevity Pharmaceutical (Group) Co., Ltd. dated December 31, 2008. (1) | |
| 10.51† | Exclusive Option Agreement among Beijing Tenet-Jove Technological Development Co., Ltd., Chen Jiping, Chen Xiaoyan, and Ankang Longevity Pharmaceutical (Group) Co., Ltd. dated December 31, 2008. (1) | |
| 10.52† | Power of Attorney by and between Chen Xiaoyan and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Ankang Longevity Pharmaceutical (Group) Co., Ltd. dated December 31, 2008. (1) | |
| 10.53† | Power of Attorney by and between Chen Jiping and Beijing Tenet-Jove Technological Development Co., Ltd. regarding shareholding of Ankang Longevity Pharmaceutical (Group) Co., Ltd. dated December 31, 2008. (1) | |
| 10.54† | Summary translation of Cooperation Agreement between Shaanxi Pharmacy Sunsimiao Drugstore Chain Co., Ltd. and Ankang Longevity Pharmaceutical (Group) Co., Ltd. dated September 27, 2012. (1) | |
| 10.55† | Summary translation of Cooperation Agreement between Shaanxi Pharmacy Holding Group Xi’an Pharmaceutical Co., Ltd. and Ankang Longevity Pharmaceutical (Group) Co., Ltd. dated September 27, 2012. (1) | |
| 10.56† | Summary translation of Loan Contract between Beijing Tenet-Jove Technological Development Co., Ltd. and Beijing Rural Commercial Bank Co., Ltd. Tiantongyuan Branch dated December 31, 2009. (1) | |
| 10.57† | Summary translation of Project Shares Purchase Contract among Yantai Zhisheng International Freight Forwarding Co., Ltd., Yantai Mouping District Zhisheng Agricultural Produce Cooperative and Zhejiang Zhen’Ai Network Warehousing Services Co., Ltd. dated October 21, 2013. (1) | |
| 10.58† | Summary translation of Contractual Management/Operation Agreement between Ankang Longevity Pharmaceutical Group Chain Co., Ltd. and Qiu Haiyin dated March 1, 2013. (1) | |
| 10.59† | Summary translation of Supplementary Agreement between Ankang Longevity Pharmaceutical Group Chain Co., Ltd. and Qiu Haiyin dated February 28, 2014. (1) | |
| 10.60* | Form of Independent Director Engagement Letter | |
| 14.1* | Code of Ethics of the Company. | |
| 21.1† | List of subsidiaries of the Company. (1) | |
| 31.1* | Certification Pursuant to Rule 13a-14(a) and 15d-14(a) (4) of Chief Executive Officer | |
| 31.2* | Certification Pursuant to Rule 13a-14(a) and 15d-14(a) (4) of Chief Financial Officer | |
| 32.1* | Certification Pursuant to Section 1350 of Title 18 of the United States Code of Chief Executive Officer | |
| 32.2* | Certification Pursuant to Section 1350 of Title 18 of the United States Code of Chief Financial Officer | |
| 99.1* | 2016 Share Incentive Plan | |
| 101.INS* | XBRL Instance Document | |
| 101.SCH* | XBRL Taxonomy Extension Schema Document | |
| 101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB* | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document |
| * | Filed herewith. |
| † | Previously filed. |
| (1) | Incorporated by reference to the Company’s Registration Statement on Form S-1, Registration No. 333-202803. |
| 56 |
SIGNATURES
Pursuant to the requirements of section 13 or 15 (d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
SHINECO, INC. (Registrant) |
||||
| Date: September 28, 2016 | By: | /s/ Yuying Zhang | ||
| Yuying Zhang | ||||
| Chief Executive Officer | ||||
| Date: September 28, 2016 | By: | /s/ Sam Wang | ||
| Sam Wang | ||||
| Chief Financial Officer |
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
|
Signature |
Title |
Date | ||
| /s/ Yuying Zhang | Chief Executive Officer and Director | September 28, 2016 | ||
| Yuying Zhang | (Principal Executive Officer) | |||
| * | Chief Financial Officer and Director | September 28, 2016 | ||
| Sam Wang | (Principal Accounting and Financial Officer) | |||
| * | Director | September 28, 2016 | ||
| Jiping Chen | ||||
| * | Director | September 28, 2016 | ||
| Ying Zhang | ||||
| * | Director | September 28, 2016 | ||
| Yajun Shi | ||||
| * | Director | September 28, 2016 | ||
| Leiger Yongmin Yang | ||||
| * | Director | September 28, 2016 | ||
| Hua Yang | ||||
| */s/ Yuying Zhang | Attorney-in-Fact | September 28, 2016 | ||
| Yuying Zhang | ||||
| 57 |
SHINECO, INC.
CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED JUNE 30, 2016 AND 2015
AND
REPORT OF INDEPENDENT REGISTERED
PUBLIC ACCOUNTING FIRM
| F-1 |
SHINECO, INC.
TABLE OF CONTENTS
| Page | |
| F-3 | |
| Consolidated Financial Statements | |
| Consolidated Balance Sheets | F-4 |
| Consolidated Statements of Income and Comprehensive Income | F-5 |
| Consolidated Statements of Changes in Equity | F-6 |
| Consolidated Statements of Cash Flows | F-7 |
| Notes to Consolidated Financial Statements | F-8 |
| F-2 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Shineco, Inc.
We have audited the accompanying consolidated balance sheets of Shineco, Inc. and subsidiaries (the “Company”) as of June 30, 2016 and 2015, and the related consolidated statements of income and comprehensive income, changes in equity and cash flows for each of the years in the two-year period ended June 30, 2016. The Company’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of June 30, 2016 and 2015, and the results of their operations and their cash flows for each of the years in the two-year period ended June 30, 2016, in conformity with accounting principles generally accepted in the United States of America.
/s/ Friedman LLP
Friedman LLP
New York, NY
September 28, 2016

| F-3 |
CONSOLIDATED BALANCE SHEETS
| June 30, | June 30, | |||||||
| 2016 | 2015 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 22,009,374 | $ | 6,056,105 | ||||
| Accounts receivable, net - third parties | 4,464,098 | 4,878,378 | ||||||
| - unconsolidated entity | 1,088,144 | 2,409,664 | ||||||
| Due from related parties | 1,671,435 | 2,623,169 | ||||||
| Inventories | 4,608,179 | 8,302,297 | ||||||
| Advances to suppliers, net | 53,024 | 1,383,934 | ||||||
| Loans to third parties, net | 560,234 | 1,130,139 | ||||||
| Other receivables, net | 463,361 | 663,193 | ||||||
| Short-term deposit | 100,270 | 87,453 | ||||||
| Prepaid leases - current, net | 478,565 | 521,235 | ||||||
| Prepaid expenses | 33,117 | 200,304 | ||||||
| TOTAL CURRENT ASSETS | 35,529,801 | 28,255,871 | ||||||
| Property and equipment at cost, net of accumulated depreciation and amortization | 11,035,199 | 5,805,965 | ||||||
| Land use right, net of accumulated amortization | 1,408,765 | 1,568,701 | ||||||
| Investments | 4,766,847 | 11,417,953 | ||||||
| Long-term deposit and other noncurrent assets | 120,357 | 135,242 | ||||||
| Prepaid leases-non current, net | 3,860,327 | 4,719,320 | ||||||
| Deferred tax assets | 327,492 | 229,072 | ||||||
| TOTAL ASSETS | $ | 57,048,788 | $ | 52,132,124 | ||||
| LIABILITIES AND EQUITY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Short-term loans-banks | $ | 2,745,945 | $ | 3,385,006 | ||||
| Accounts payable | 259,803 | 171,642 | ||||||
| Advances from customers | 9,597 | 32,826 | ||||||
| Due to related parties | 244,915 | 137,508 | ||||||
| Other payables and accrued expenses | 1,999,622 | 1,119,591 | ||||||
| Taxes payable | 1,278,142 | 1,076,253 | ||||||
| TOTAL LIABILITIES | 6,538,024 | 5,922,826 | ||||||
| Commitments and contingencies | ||||||||
| EQUITY: | ||||||||
| Common stock; par value $0.001, 100,000,000 shares authorized; 19,320,882 issued and outstanding | 19,321 | 19,321 | ||||||
| Additional paid-in capital | 17,344,466 | 17,344,466 | ||||||
| Statutory reserve | 3,242,139 | 2,924,921 | ||||||
| Retained earnings | 30,837,399 | 23,017,721 | ||||||
| Accumulated other comprehensive income (loss) | (1,887,929 | ) | 2,054,720 | |||||
| Total Stockholders' equity of Shineco, Inc. | 49,555,396 | 45,361,149 | ||||||
| Non-controlling interest | 955,368 | 848,149 | ||||||
| TOTAL EQUITY | 50,510,764 | 46,209,298 | ||||||
| TOTAL LIABILITIES AND EQUITY | $ | 57,048,788 | $ | 52,132,124 | ||||
The accompanying notes are an integral part of these consolidated financial statements
| F-4 |
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
| For the Years Ended June 30, | ||||||||
| 2016 | 2015 | |||||||
| REVENUE | $ | 35,206,852 | $ | 32,630,502 | ||||
| COST OF REVENUE | ||||||||
| Cost of product and services | 24,037,241 | 22,204,733 | ||||||
| Business and sales related tax | 85,038 | 78,262 | ||||||
| GROSS PROFIT | 11,084,573 | 10,347,507 | ||||||
| OPERATING EXPENSES | ||||||||
| General and administrative expenses | 1,988,101 | 2,160,666 | ||||||
| Selling expenses | 1,755,264 | 1,684,423 | ||||||
| Total operating expense | 3,743,365 | 3,845,089 | ||||||
| INCOME FROM OPERATIONS | 7,341,208 | 6,502,418 | ||||||
| OTHER INCOME | ||||||||
| Income from equity method investments | 1,796,527 | 1,788,288 | ||||||
| Other income | 197,390 | 231,908 | ||||||
| Interest income, net | 135,404 | 42,502 | ||||||
| Total other income | 2,129,321 | 2,062,698 | ||||||
| INCOME BEFORE INCOME TAX PROVISION | 9,470,529 | 8,565,116 | ||||||
| PROVISIONS FOR INCOME TAXES | 1,177,707 | 1,124,716 | ||||||
| NET INCOME | 8,292,822 | 7,440,400 | ||||||
| Less: net income attributable to non-controlling interest | (155,926 | ) | (160,122 | ) | ||||
| NET INCOME ATTRIBUTABLE TO SHINECO, INC. | $ | 8,136,896 | $ | 7,280,278 | ||||
| COMPREHENSIVE INCOME | ||||||||
| Net income | 8,292,822 | 7,440,400 | ||||||
| Other comprehensive income (loss): foreign currency translation gain (loss) | (3,991,356 | ) | 306,408 | |||||
| Total comprehensive income | 4,301,466 | 7,746,808 | ||||||
| Comprehensive income attributable to non-controlling interest | (107,219 | ) | (153,013 | ) | ||||
| COMPREHENSIVE INCOME ATTRIBUTABLE TO SHINECO, INC. | $ | 4,194,247 | $ | 7,593,795 | ||||
| Weighted average number of shares basic and diluted | 19,320,882 | 19,320,882 | ||||||
| Basic and diluted earnings per common share | $ | 0.42 | $ | 0.38 | ||||
The accompanying notes are an integral part of these consolidated financial statements
| F-5 |
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
FOR THE YEARS ENDED JUNE 30, 2016 AND 2015
| ACCUMULATED OTHER | NON- | |||||||||||||||||||||||||||||||
| COMMON STOCK | ADDITIONAL | STATUTORY | RETAINED | COMPREHENSIVE | CONTROLLING | TOTAL | ||||||||||||||||||||||||||
| SHARES | AMOUNT | PAID-IN CAPITAL | RESERVE | EARNINGS | INCOME (LOSS) | INTEREST | EQUITY | |||||||||||||||||||||||||
| Balance at June 30, 2014 | 19,320,882 | $ | 19,321 | $ | 17,344,466 | $ | 2,040,382 | $ | 16,621,982 | $ | 1,741,203 | $ | 695,136 | $ | 38,462,490 | |||||||||||||||||
| Net income for the year | - | - | - | - | 7,280,278 | - | 160,122 | 7,440,400 | ||||||||||||||||||||||||
| Appropriation of statutory reserve | - | - | - | 884,539 | (884,539 | ) | - | - | - | |||||||||||||||||||||||
| Foreign currency translation gain (loss) | - | - | - | - | - | 313,517 | (7,109 | ) | 306,408 | |||||||||||||||||||||||
| Balance at June 30, 2015 | 19,320,882 | $ | 19,321 | $ | 17,344,466 | $ | 2,924,921 | $ | 23,017,721 | $ | 2,054,720 | $ | 848,149 | $ | 46,209,298 | |||||||||||||||||
| Net income for the year | - | - | - | - | 8,136,896 | - | 155,926 | 8,292,822 | ||||||||||||||||||||||||
| Appropriation of statutory reserve | - | - | - | 317,218 | (317,218 | ) | - | - | - | |||||||||||||||||||||||
| Foreign currency translation loss | - | - | - | - | - | (3,942,649 | ) | (48,707 | ) | (3,991,356 | ) | |||||||||||||||||||||
| Balance at June 30, 2016 | 19,320,882 | $ | 19,321 | $ | 17,344,466 | $ | 3,242,139 | $ | 30,837,399 | $ | (1,887,929 | ) | $ | 955,368 | $ | 50,510,764 | ||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
| F-6 |
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended June 30, | ||||||||
| 2016 | 2015 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net income | $ | 8,292,822 | $ | 7,440,400 | ||||
| Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||
| Depreciation and amortization | 871,099 | 811,135 | ||||||
| Provision for doubtful accounts | 324,515 | 111,303 | ||||||
| Change of inventory reserve | 290,515 | 62,447 | ||||||
| Deferred tax benefit | (120,765 | ) | (64,419 | ) | ||||
| Income from equity method investments | (1,796,527 | ) | (1,788,288 | ) | ||||
| Impairment of investment | - | 244,350 | ||||||
| Gain on disposal of investment | (233,249 | ) | - | |||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (8,395 | ) | (3,481,607 | ) | ||||
| Advances to suppliers | 1,249,897 | 1,916,195 | ||||||
| Inventories | 2,834,739 | (2,809,027 | ) | |||||
| Other receivables | 172,176 | (277,087 | ) | |||||
| Prepaid expense and other assets | 135,528 | (43,287 | ) | |||||
| Due from related parties | (601,940 | ) | 1,056,256 | |||||
| Long-term deposit and other noncurrent assets | - | 9,298 | ||||||
| Prepaid leases | 495,122 | (1,139,092 | ) | |||||
| Accounts payable | 105,382 | 133,055 | ||||||
| Advances from customers | (21,266 | ) | 20,281 | |||||
| Other payables | 1,008,132 | 92,962 | ||||||
| Taxes payable | 292,580 | 351,914 | ||||||
| NET CASH PROVIDED BY OPERATING ACTIVITIES | 13,290,365 | 2,646,789 | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Acquisitions of property and equipment | (498,772 | ) | (101,620 | ) | ||||
| Proceeds from withdrawal of investments | 466,497 | - | ||||||
| Proceeds from (repayment of) loans to third parties | 189,275 | (224,816 | ) | |||||
| Proceeds from (repayment of) loans to related parties | 1,366,787 | (969,711 | ) | |||||
| Distribution received from investments in unconsolidated entities | 2,428,894 | 65,160 | ||||||
| NET CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES | 3,952,681 | (1,230,987 | ) | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from short-term bank loans | 3,148,077 | 3,583,800 | ||||||
| Repayment of short-term bank loans | (3,526,423 | ) | (2,893,104 | ) | ||||
| Proceeds from private equity | - | 699,818 | ||||||
| Proceeds from advances from related parties | 122,424 | 136,836 | ||||||
| NET CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES | (255,922 | ) | 1,527,350 | |||||
| EFFECT OF EXCHANGE RATE CHANGE ON CASH | (1,033,855 | ) | 23,108 | |||||
| NET INCREASE IN CASH | 15,953,269 | 2,966,260 | ||||||
| CASH - Beginning of the Year | 6,056,105 | 3,089,845 | ||||||
| CASH - End of Year | $ | 22,009,374 | $ | 6,056,105 | ||||
| SUPPLEMENTAL CASH FLOW DISCLOSURES: | ||||||||
| Cash paid for income tax | $ | 991,599 | $ | 913,288 | ||||
| Cash paid for interest | $ | 366,844 | $ | 223,933 | ||||
| SUPPLEMENTAL NON-CASH INVESTING ACTIVITY: | ||||||||
| Long-term investment converted to fixed assets | $ | 6,219,960 | $ | - | ||||
The accompanying notes are an integral part of these consolidated financial statements
| F-7 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 1 - ORGANIZATION AND NATURE OF OPERATIONS
Shineco, Inc. ("Shineco" or the “Company”) was incorporated in the State of Delaware on August 20, 1997. The Company is a holding company whose primary purpose is to develop business opportunities in the People’s Republic of China (“PRC” or “China”).
On December 30, 2004, the Company acquired all of the issued and outstanding shares of Beijing Tenet-Jove Technological Development Corp., Ltd. (“Tenet-Jove”), a PRC company, in exchange for restricted shares of the Company’s common stock, and the sole operating business of the Company became that of its subsidiary, Tenet-Jove. Tenet-Jove was incorporated on December 15, 2003 under the laws of China. Consequently, Tenet-Jove became a 100% owned subsidiary of Shineco and was officially granted the status of a Wholly Foreign-Owned Entity (“WFOE”) by Chinese authority on July 14, 2006. This transaction was accounted for as a recapitalization. Tenet-Jove owns 90% interest of Tianjin Tenet Huatai Technological Development Co., Ltd. (“Tenet Huatai”).
On December 31, 2008, June 11, 2011 and May 24, 2012, respectively, Tenet-Jove entered into a series of contractual agreements with the owners of Ankang Longevity Pharmaceutical (Group) Co., Ltd. (“Ankang Longevity Group”), each of Yantai Zhisheng International Freight Forwarding Co., Ltd (“Zhisheng Freight”), Yantai Zhisheng International Trade Co., Ltd (“Zhisheng Trade”), Yantai Mouping District Zhisheng Agricultural Produce Cooperative (“Zhisheng Agricultural”)and Qingdao Zhihesheng Agricultural Produce Services., Ltd (“Qingdao Zhihesheng”). On February 24, 2014, Tenet-Jove also subsequently entered into the same series of contractual agreements with Shineco Zhisheng (Beijing) Bio-Technology Co., Ltd (“Zhisheng Bio-Tech”), which is a new company incorporated in 2014. Zhisheng Bio-Tech, Zhisheng Freight, Zhisheng Trade, Zhisheng Agricultural, and Qingdao Zhihesheng are collectively referred to herein as “Zhisheng Group”. These agreements include an Executive Business Cooperation Agreement; Timely Reporting Agreements; Equity Interest Pledge Agreement and Executive Option Agreements.
Pursuant to the above agreements, Tenet-Jove has the exclusive right to provide to Zhisheng Group and Ankang Longevity Group consulting services related to business operation and management. All the above contractual agreements obligate Tenet-Jove to absorb a majority of the risk of loss from Zhisheng Group and Ankang Longevity Group’s activities and entitle Tenet-Jove to receive a majority of their residual returns. In essence, Tenet-Jove has gained effective control over Zhisheng Group and Ankang Longevity Group. Therefore, the Company believes that Zhisheng Group and Ankang Longevity Group should be considered as Variable Interest Entities (“VIEs”) under the Statement of Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 810 “Consolidation”. Accordingly, the accounts of these entities are consolidated with those of Tenet-Jove.
Since Shineco is effectively controlled by the majority shareholders of Zhisheng Group and Ankang Longevity Group. Shineco owns 100% equity interest of Tenet-Jove. Accordingly, Shineco, Tenet-Jove, and its VIEs, Zhisheng Group and Ankang Longevity Group are effectively controlled by the same majority shareholders. Therefore, Shineco, Tenet-Jove and its VIEs are considered under common control. The consolidation of Tenet-Jove and its VIEs into Shineco has been accounted for at historical cost and prepared on the basis as if the aforementioned exclusive contractual agreements between Tenet-Jove and its VIES had become effective as of the beginning of the first period presented in the accompanying consolidated financial statements.
The Company, its subsidiaries, its VIEs and its VIEs’ subsidiaries (collectively the “Group”) operate three main business segments: 1) Tenet-Jove is engaged in planting, manufacturing and selling of Bluish Dogbane and related products, also known in Chinese as “Luobuma”, including therapeutic clothing and textile products made from Luobuma; 2) Zhisheng Group is engaged in the business of planting, processing and distributing of green agricultural produce as well as providing domestic and international logistic services for agricultural products (“Agricultural Products”); 3) Ankang Longevity Group develops and manufactures traditional Chinese herbal medicinal products as well as other retail pharmaceutical products. These different business activities and products can potentially be integrated and benefit from one and other.
| F-8 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation and Principles of Consolidation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”).
The consolidated financial statements of the Company reflect the principal activities of the Company, its subsidiaries, its VIEs and its VIEs’ subsidiaries. The non-controlling interest represents the minority shareholders’ interest in the Company’s majority owned subsidiaries. All intercompany transactions have been eliminated.
Consolidation of Variable Interest Entities
In accordance with accounting standards regarding consolidation of variable interest entities (“VIEs”), VIEs are generally entities that lack sufficient equity to finance their activities without additional financial support from other parties or whose equity holders lack adequate decision making ability. All VIEs with which the Company is involved must be evaluated to determine the primary beneficiary of the risks and rewards of the VIE. The primary beneficiary is required to consolidate the VIE for financial reporting purposes.
The carrying amount of the VIEs and their subsidiaries’ consolidated assets and liabilities are as following:
| June 30, 2016 | June 30, 2015 | |||||||
| Current assets | $ | 30,560,208 | $ | 21,054,168 | ||||
| Plant and equipment, net | 9,595,357 | 4,187,397 | ||||||
| Other noncurrent assets | 10,136,632 | 17,559,402 | ||||||
| Total assets | 50,292,197 | 42,800,967 | ||||||
| Total liabilities | (4,398,424 | ) | (4,804,296 | ) | ||||
| Net assets | $ | 45,893,773 | $ | 37,996,671 | ||||
Non-controlling Interests
US GAAP requires that non-controlling interests in subsidiaries and affiliates be reported in the equity section of a company’s balance sheet. In addition, the amounts attributable to the net income (loss) of those subsidiaries are reported separately in the consolidated statements of income and comprehensive income.
Risks and Uncertainties
The operations of the Company are located in the PRC. Accordingly, the Company’s business, financial condition, and results of operations may be influenced by the political, economic, and legal environments in the PRC, as well as by the general state of the PRC economy. The Company’s operations in the PRC are subject to special considerations and significant risks not typically associated with companies in North America and Western Europe. These include risks associated with, among other factors, the political, economic and legal environment and foreign currency exchange. The Company’s results may be adversely affected by changes in the political, regulatory and social conditions in the PRC, and by changes in governmental policies or interpretations with respect to laws and regulations, anti-inflationary measures, currency conversion, remittances abroad, and rates and methods of taxation, among other things. Although the Company has not experienced losses from these situations and believes that it is in compliance with existing laws and regulations, this may not be indicative of future results.
| F-9 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Members of the current management team own controlling interests in the Company and are also the owners of the VIEs in the PRC. The Company only controls the VIEs through contractual arrangements which obligate it to absorb the risk of loss and to receive the residual expected returns. As such, the controlling shareholders of the Company and the VIEs could cancel these agreements or permit them to expire at the end of the agreement terms, as a result of which the Company would not retain control of the VIEs.
Use of Estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements as well as the reported amounts of revenue and expenses during the reporting period. Significant estimates required to be made by management include, but are not limited to, useful lives of property, plant, and equipment, intangible assets, the recoverability of long-lived assets and the valuation of accounts receivable, deferred tax assets, accrued expenses, taxes payable and inventories. Actual results could differ from those estimates.
Revenue Recognition
The Company recognizes revenue from sales of bluish dogbane products, Chinese medicinal herbal products and agricultural products, as well as providing logistic service and other processing service to external customers. The Company recognizes revenue when all of the following have occurred: (i) there is persuasive evidence of an arrangement with a customer (ii) delivery has occurred or services have been rendered (iii) the sales price is fixed or determinable and (iv) the Company’s collection of such fees is reasonable assured. These criteria, as related to the Company’s revenue, are considered to have been met as follows:
Sales of products: The Company recognizes revenue on sale of products when the goods are delivered and title to the goods passes to the customer provided that there are no uncertainties regarding customer acceptance; persuasive evidence of an arrangement exists; the sales price is fixed or determinable; and collectability is deemed probable.
Revenue from the rendering of services: Revenue from international freight forwarding, domestic air and overland freight forwarding services are recognized upon performance of services as stipulated in the underlying contract or when commodities are being released from the customer’s warehouse; the service price is fixed or determinable; and collectability is deemed probable.
Cash and Cash Equivalents
Cash and cash equivalents consist of cash on hand, cash on deposits and other highly liquid investments which are unrestricted as to withdrawal or use, and which have maturities of three months or less when purchased. The Company maintains cash and cash equivalents with various financial institutions mainly in the PRC. Balances in banks in the PRC are uninsured.
Accounts Receivable
Accounts receivable are recorded at net realizable value consisting of carrying amount less an allowance for uncollectible accounts, as necessary. The Company reviews the accounts receivable on a periodic basis and makes general and specific allowance when there is doubt as to the collectability of individual balances. In evaluating the collectability of individual receivable balances, the Company considers many factors, including the age of the balance, customers’ historical payment history, their current credit-worthiness and current economic trends. As of June 30, 2016 and 2015, the allowances for doubtful accounts were $103,968 and $81,374, respectively. Accounts are written off against the allowance after efforts at collection prove unsuccessful.
| F-10 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Accounts receivable-unconsolidated entity represents the amount due from Shanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd. (“Shaanxi Pharmaceutical Group”), with whom the Company entered into a supplemental agreement in September 2013. According to the supplemental agreement, the joint-venture company established by Shaanxi Pharmaceutical and the Company are required to exclusively purchase certain raw materials and drug products from Shaanxi Pharmaceutical Group. In return, Shaanxi Pharmaceutical Group has agreed to compensate Ankang Longevity Group with a preferred distribution that equals to 7% of the total purchases made from Shaanxi Pharmaceutical Group. The accounts receivable mainly represents the preferred distribution due from Shaanxi Pharmaceutical Group. As of June 30, 2016 and 2015, the balance of accounts receivable – unconsolidated entity was $1,088,144 and $2,409,664, respectively.
Inventories
Inventories, which are stated at the lower of cost or current market value, consisting of raw materials, work-in-progress, finished goods related to the Company’s products. Cost is determined using the first in first out (FIFO) method. Market is the lower of replacement cost or net realizable value. Agricultural products that the Company farms are recorded at cost, which includes direct costs such as seed selection, fertilizer, labor cost and contract fee that are spent in growing agricultural products on the leased farmland, and indirect costs which include amortization of prepayment of farmland lease fee and farmland development costs. All the costs are accumulated until the time of harvest and then allocated to harvested crops costs when they are sold. The Company periodically evaluates its inventory and records inventory reserve for certain inventories that may not be saleable. As of June 30, 2016 and 2015, the Company recorded inventory reserves of $819,285 and $585,283, respectively.
Advances to Suppliers
Advances to suppliers consist of balance paid to suppliers for materials that have not been received. Advances to suppliers are reviewed periodically to determine whether their carrying value has become impaired. As of June 30, 2016 and 2015, the Company had an allowance for uncollectible advances to suppliers in the amount of $10,118 and $561, respectively.
Loans to Third Parties
Loans to third parties consist of various cash advances to unrelated companies and individuals, with whom the Company has business relationships. The loans are due within one year with no interest rate. Loans to third parties are reviewed periodically as to whether their carrying values remain realizable.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation and amortization. Expenditures for additions, major renewal and betterments are capitalized, and expenditures for maintenance and repairs are charged to expense as incurred. Depreciation is provided on a straight-line basis, less estimated residual value, over an asset’s estimated useful life, Farmland leasehold improvements are amortized over the shorter of lease term or remaining lease period of the underlying assets. Following are the estimated useful lives of the Company’s property and equipment:
| Estimated useful lives | ||
| Buildings | 20-50 years | |
| Machinery equipment | 5-10 years | |
| Motor vehicles | 5-10 years | |
| Office equipment | 5-10 years | |
| Farmland leasehold improvements | 12-18 years |
| F-11 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Land Use Right
Under PRC law, all land in the PRC is owned by the government and cannot be sold to an individual or company. The government grants individuals and companies the rights to use parcels of land for a specified period of time. Land use rights are stated at cost less accumulated amortization. Amortization is provided over the respective useful lives, using the straight-line method. Estimated useful life is 50-years, based on the term of the land use rights.
Long-lived Assets
The Company accounts for long-lived assets under FASB Codification Topic 360 (ASC Topic 360) “Accounting for Goodwill and Other Intangible Assets” and “Accounting for Impairment or Disposal of Long-Lived Assets.” Finite-lived assets and intangibles are also reviewed for impairment testing when circumstances require. For purposes of evaluating the recoverability of long-lived assets, when undiscounted future cash flows will not be sufficient to recover an asset’s carrying amount, the asset is written down to its fair value. The long-lived assets of the Company that are subject to evaluation consist primarily of property, plant and equipment, land use rights, investments and long-term prepaid lease. For the years ended June 30, 2016 and 2015, the Company did not recognize any impairment of its long-lived assets.
Fair Value of Financial Instruments
The Company adopted the provisions of ASC 820, “Fair Value Measurements and Disclosures.” ASC 820 clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
The carrying value of accounts receivable, other current assets, short term loans, other payables and accrued expenses approximate their fair values because of the short-term nature of these instruments.
Income Tax
The Company accounts for income taxes under FASB Codification Topic 740 (ASC 740). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
The provisions of ASC 740-10-25, “Accounting for Uncertainty in Income Taxes,” prescribe a more-likely-than-not threshold for consolidated financial statement recognition and measurement of a tax position taken (or expected to be taken) in a tax return. This interpretation also provides guidance on the recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, and related disclosures. The Company does not have any uncertain tax positions at June 30, 2016 and 2015.
| F-12 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
The statute of limitations for the Company’s U.S. federal income tax returns and certain state income tax returns remains open for tax years 2013 and after. As of June 30, 2016, the tax years ended June 30, 2007 through June 30, 2016 for the Company’s People’s Republic of China (“PRC”) subsidiaries remain open for statutory examination by PRC tax authorities.
Value Added Tax
Sales revenue represents the invoiced value of goods, net of a Value-Added Tax (“VAT”). All of the Company’s products that are sold in the PRC are subject to a Chinese value-added tax at a rate of 17% of the gross sales price. This VAT may be offset by VAT paid by the Company on raw materials and other materials included in the cost of producing their finished product or acquiring its finished products. The Company recorded a VAT payable or VAT receivable net of payment in the accompanying consolidated financial statements.
Foreign Currency Translation
The Company uses the United States dollar (“U.S. dollars” or “USD”) for financial reporting purposes. The Company’s subsidiaries and VIEs maintain their books and records in their functional currency of Renminbi (“RMB”), the currency of the PRC.
In general, for consolidation purposes, the Company translates the assets and liabilities of its subsidiaries and VIEs into U.S. dollars using the applicable exchange rates prevailing at the balance sheet date, and the statements of income and cash flows are translated at average exchange rates during the reporting period. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheet. Equity accounts are translated at historical rates. Adjustments resulting from the translation of the financial statements of the subsidiaries and VIEs are recorded as accumulated other comprehensive income (loss).
The balance sheet amounts, with the exception of equity, at June 30, 2016 and 2015 were translated at 1 RMB to $0.1505 USD and at 1 RMB to $0.1637 USD, respectively. The average translation rates applied to income and cash flow statement amounts for the years ended June 30, 2016 and 2015 were at 1 RMB to $0.1555 USD and at 1 RMB to $0.1629 USD, respectively.
Comprehensive Income
Comprehensive income consists of two components, net income and other comprehensive income. The foreign currency translation gain or loss resulting from translation of the financial statements expressed in RMB to US$ is reported in other comprehensive income (loss) in the consolidated statements of income and comprehensive income.
Equity Investment
An investment in which the Company has the ability to exercise significant influence, but does not have a controlling interest, is accounted for using the equity method. Significant influence is generally considered to exist when the Company has an ownership interest in the voting stock between 20% and 50%, and other factors, such as representation on the Board of Directors, voting rights and the impact of commercial arrangements, are considered in determining whether the equity method of accounting is appropriate.
Earnings per Share
The Company computes earnings per share (“EPS”) in accordance with ASC 260, “Earnings per Share” (“ASC 260”). ASC 260 requires companies with complex capital structures to present basic and diluted EPS. Basic EPS is measured as net income divided by the weighted average common shares outstanding for the period. Diluted EPS is similar to basic EPS but presents the dilutive effect on a per share basis of potential common shares (e.g., convertible securities, options and warrants) as if they had been converted at the beginning of the periods presented, or issuance date, if later. Potential common shares that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS. There is no anti-dilutive effect for the years ended June 30, 2016 and 2015.
| F-13 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Reclassification
Certain prior year amounts have been reclassified to conform to the current period presentation. These reclassifications had no effect on net income or cash flow as previously prepared.
New Accounting Pronouncements
In November 2015, the FASB has issued Accounting Standards Update (ASU) No. 2015-17, Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes, which changes how deferred taxes are classified on organizations’ balance sheets. The ASU eliminates the current requirement for organizations to present deferred tax liabilities and assets as current and noncurrent in a classified balance sheet. Instead, organizations will be required to classify all deferred tax assets and liabilities as noncurrent. The amendments apply to all organizations that present a classified balance sheet. For public companies, the amendments are effective for financial statements issued for annual periods beginning after December 15, 2016, and interim periods within those annual periods. The Company early adopted this update as permitted and, accordingly, has presented deferred tax assets as long-term on the Company's consolidated balance sheets.
In February 2016, the FASB issued ASU No. 2016-02, "Leases (Topic 842)" ("ASU 2016-02"), to increase the transparency and comparability about leases among entities. The new guidance requires lessees to recognize a lease liability and a corresponding lease asset for virtually all lease contracts. It also requires additional disclosures about leasing arrangements. ASU 2016-02 is effective for interim and annual periods beginning after December 15, 2018, and requires a modified retrospective approach to adoption. Early adoption is permitted. The Company is currently evaluating the impact of this new standard on its consolidated financial statements and related disclosures.
In March 2016, the FASB issued Accounting Standards Update No. 2016-07, Investments - Equity Method and Joint Ventures (Topic 323): Simplifying the Transition to the Equity Method of Accounting. The amendments affect all entities that have an investment that becomes qualified for the equity method of accounting as a result of an increase in the level of ownership interest or degree of influence. The amendments eliminate the requirement that when an investment qualifies for use of the equity method as a result of an increase in the level of ownership interest or degree of influence, an investor must adjust the investment, results of operations, and retained earnings retroactively on a step-by-step basis as if the equity method had been in effect during all previous periods that the investment had been held. The amendments require that the equity method investor add the cost of acquiring the additional interest in the investee to the current basis of the investor’s previously held interest and adopt the equity method of accounting as of the date the investment becomes qualified for equity method accounting. Therefore, upon qualifying for the equity method of accounting, no retroactive adjustment of the investment is required. The amendments require that an entity that has an available-for-sale equity security that becomes qualified for the equity method of accounting recognize through earnings the unrealized holding gain or loss in accumulated other comprehensive income at the date the investment becomes qualified for use of the equity method. The amendments are effective for all entities for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2016. The amendments should be applied prospectively upon their effective date to increases in the level of ownership interest or degree of influence that result in the adoption of the equity method. Earlier application is permitted. The Company is currently evaluating the impact of this new standard on its consolidated financial statements.
In April 2016, FASB issued Accounting Standards Update No. 2016-10, Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing. The amendments clarify the following two aspects of Topic 606: (a) identifying performance obligations; and (b) the licensing implementation guidance. The amendments do not change the core principle of the guidance in Topic 606. The effective date and transition requirements for the amendments are the same as the effective date and transition requirements in Topic 606. Public entities should apply the amendments for annual reporting periods beginning after December 15, 2017, including interim reporting periods therein (i.e., January 1, 2018, for a calendar year entity). Early application for public entities is permitted only as of annual reporting periods beginning after December 15, 2016, including interim reporting periods within that reporting period. The Company is currently evaluating the impact of this new standard on its consolidated financial statements.
| F-14 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
In May 2016, the FASB issued ASU 2016-11, “Revenue Recognition (Topic 605) and Derivatives and Hedging (Topic 815): Rescission of SEC Guidance Because of Accounting Standards Updates 2014-09 and 2014-16 Pursuant to Staff Announcements at the March 3, 2016 EITF Meeting”, The amendments rescinds SEC paragraphs pursuant to two SEC Staff Announcements at the March 3, 2016 Emerging Issues Task Force (EITF) meeting. Specifically, registrants should not rely on the following SEC Staff Observer comments upon adoption of Topic 606: 1) Revenue and Expense Recognition for Freight Services in Process, which is codified in paragraph 605-20-S99-2; 2) Accounting for Shipping and Handling Fees and Costs, which is codified in paragraph 605-45-S99-1; 3) Accounting for Consideration Given by a Vendor to a Customer (including Reseller of the Vendor's Products), which is codified in paragraph 605-50-S99-1; 4) Accounting for Gas-Balancing Arrangements (i.e., use of the "entitlements method"), which is codified in paragraph 932-10-S99-5, which is effective upon adoption of ASU 2014-09. The Company is currently in the process of evaluating the impact of the adoption on its consolidated financial statements.
In May 2016, the FASB issued ASU 2016-12, "Revenue from Contracts with Customers (Topic 606): Narrow-Scope Improvements and Practical Expedients". The amendments, among other things: (1) clarify the objective of the collectability criterion for applying paragraph 606-10-25-7; (2) permit an entity to exclude amounts collected from customers for all sales (and other similar) taxes from the transaction price; (3) specify that the measurement date for noncash consideration is contract inception; (4) provide a practical expedient that permits an entity to reflect the aggregate effect of all modifications that occur before the beginning of the earliest period presented when identifying the satisfied and unsatisfied performance obligations, determining the transaction price, and allocating the transaction price to the satisfied and unsatisfied performance obligations; (5) clarify that a completed contract for purposes of transition is a contract for which all (or substantially all) of the revenue was recognized under legacy US GAAP before the date of initial application, and (6) clarify that an entity that retrospectively applies the guidance in Topic 606 to each prior reporting period is not required to disclose the effect of the accounting change for the period of adoption. The effective date of these amendments is at the same date that Topic 606 is effective. The Company is currently in the process of evaluating the impact of the adoption on its consolidated financial statements.
In August 2016, the FASB has issued Accounting Standards Update (ASU) No. 2016-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments, to address diversity in how certain cash receipts and cash payments are presented and classified in the statement of cash flows. The amendments provide guidance on the following eight specific cash flow issues: (1) Debt Prepayment or Debt Extinguishment Costs; (2) Settlement of Zero-Coupon Debt Instruments or Other Debt Instruments with Coupon Interest Rates That Are Insignificant in Relation to the Effective Interest Rate of the Borrowing; (3) Contingent Consideration Payments Made after a Business Combination; (4)Proceeds from the Settlement of Insurance Claims; (5) Proceeds from the Settlement of Corporate-Owned Life Insurance Policies, including Bank-Owned; (6) Life Insurance Policies; (7) Distributions Received from Equity Method Investees; (8) Beneficial Interests in Securitization Transactions; and Separately Identifiable Cash Flows and Application of the Predominance Principle. The amendments are effective for public business entities for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. Early adoption is permitted, including adoption in an interim period. The amendments should be applied using a retrospective transition method to each period presented. If it is impracticable to apply the amendments retrospectively for some of the issues, the amendments for those issues would be applied prospectively as of the earliest date practicable. The Company is currently evaluating the impact of this new standard on its consolidated financial statements and related disclosures.
| F-15 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 3- INVENTORIES
The inventories consist of the following:
| June 30, 2016 | June 30, 2015 | |||||||
| Raw materials | $ | 825,028 | $ | 920,973 | ||||
| Work-in-process | 3,230,729 | 6,674,890 | ||||||
| Finished goods | 1,354,176 | 1,268,681 | ||||||
| Packing materials | 17,531 | 23,036 | ||||||
| Less: inventory reserve | (819,285 | ) | (585,283 | ) | ||||
| $ | 4,608,179 | $ | 8,302,297 | |||||
Work-in-process includes direct costs such as seed selection, fertilizer, labor cost and subcontractor fee that are spent in growing agricultural products on the leased farmland, and indirect costs which include amortization of prepayment of farmland lease fee and farmland development cost. All the costs are accumulated until the time of harvest and then allocated to harvested crops costs when they are sold.
NOTE 4- PROPERTY AND EQUIPMENT, NET
Property and equipment consist of the following:
| June 30, 2016 | June 30, 2015 | |||||||
| Buildings | $ | 10,726,872 | * | $ | 5,113,662 | |||
| Building improvement | 52,834 | 57,467 | ||||||
| Machinery equipment | 443,846 | 476,044 | ||||||
| Motor vehicles | 231,434 | 251,726 | ||||||
| Construction in progress | 451,512 | - | ||||||
| Office equipment | 208,022 | 206,134 | ||||||
| Farmland leasehold improvement | 3,164,943 | 3,442,441 | ||||||
| 15,279,463 | 9,547,474 | |||||||
| Less: accumulated depreciation and amortization | (4,244,264 | ) | (3,741,509 | ) | ||||
| Property, plant and equipment, net | $ | 11,035,199 | $ | 5,805,965 | ||||
*: $6,219,960 was converted from long-term investment to building, details see Note 6.
Depreciation and amortization expense charged to operations were $831,057 and $766,705 for the years ended June 30, 2016 and 2015, respectively.
Farmland leasehold improvements consist of following:
| June 30, 2016 | June 30, 2015 | |||||||
| Blueberry farmland leasehold reconstruction | $ | 2,431,450 | $ | 2,644,636 | ||||
| Yew tree planting base reconstruction | 272,412 | 296,297 | ||||||
| Greenhouse renovation | 461,081 | 501,508 | ||||||
| Total farmland leasehold improvement | $ | 3,164,943 | $ | 3,442,441 | ||||
| F-16 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 5- LAND USE RIGHTS, NET
The Company states land use right at cost less accumulated amortization. All land in the People’s Republic of China is government owned and cannot be sold to any individual or company. However, the government grants the user a “land use right” (the “Right”) to use the land. The Company has the Right to use the land for 50 years and amortizes the Right on a straight-line basis over the period of 50 years.
| June 30, 2016 | June 30, 2015 | |||||||
| Land use rights | $ | 1,674,053 | $ | 1,820,832 | ||||
| Less: accumulated amortization | (265,288 | ) | (252,131 | ) | ||||
| Land use rights, net | $ | 1,408,765 | $ | 1,568,701 | ||||
For the years ended June 30, 2016 and 2015, the Company incurred amortization expense of $34,592 and $36,239, respectively.
The estimated future amortization expenses are as follows:
| Twelve months ending June 30: | ||||
| 2017 | $ | 33,481 | ||
| 2018 | 33,481 | |||
| 2019 | 33,481 | |||
| 2020 | 33,481 | |||
| 2021 | 33,481 | |||
| Thereafter | 1,241,360 | |||
| Total | $ | 1,408,765 | ||
NOTE 6- INVESTMENTS
| A) | Investments in unconsolidated entities |
On September 27, 2012, Ankang Longevity Group entered into two equity investment agreements with a third party, Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd., a Chinese state-owned pharmaceutical enterprise to invest a total of RMB 6.8 million (approximately $1.0 million) to form a joint venture pharmacy retail company called Shaanxi Pharmaceutical Sunsimiao Drugstores Ankang Retail Chain Co., Ltd. (“Sunsimiao Drugstores”), and a joint venture pharmaceutical wholesale distribution company named Shaanxi Pharmaceutical Holding Group Longevity Pharmacy Co., Ltd. (“Shaanxi Longevity Pharmacy”). Ankang Longevity Group obtained 49% interest in each of these two new joint venture companies. These two joint ventures are formed as new business entities to collaborate with Shaanxi Pharmaceutical Group to expand sales to regional hospitals and clinics and to establish the presence of retail pharmacies under the Brand name “Sunsimiao”. These two companies started business operations in May 2013. The investments were accounted for using the equity method because Ankang Longevity Group has significant influence, but no control of these two entities. Ankang Longevity Group recorded a net income of $532,320 and $599,118 for the years ended June 30, 2016 and 2015, respectively, from the investment, which was included in “Income from equity method investments” in the consolidated statements of operations and comprehensive income.
In September 2013, Ankang Longevity Group entered into a supplemental agreement with Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd. (“Shaanxi Pharmaceutical Group”). According to the supplemental agreement, the new joint-venture companies established by Shaanxi Pharmaceutical and Ankang Longevity Group are required to exclusively purchase certain raw materials and drug products from Shaanxi Pharmaceutical Group. In return, Shaanxi Pharmaceutical Group has agreed to compensate Ankang Longevity Group with a preferred distribution equal to 7% of the total purchases made from Shaanxi Pharmaceutical Group. For the years ended June 30, 2016 and 2015, a total of $1,124,258 and $1,124,010 were recognized by Ankang Longevity Group from this supplemental agreement in addition to its 49% share of the net income of the joint ventures, respectively.
| F-17 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 6 - INVESTMENTS (Continued)
On each of April 6, 2013 and June 2, 2013, Tenet-Jove entity invested RMB 1.5 million each (approximately $0.23 million) into two new companies: Nanjing Kang Tian Yi Dressing & Adornment Co., Ltd (“Nanjing Kangtianyi”) and Tianjin Ou Feng Biotechnology Co., Ltd (“Tianjin Ou’Feng”), to obtain non-controlling interests of 30% and 40%. Both companies have not been able to generate profits. Tianjin Ou’Feng has gone out of business as of June 30, 2015. As a result, the Company recorded impairment of $244,350 on these investments in unconsolidated entities for the year ended June 30, 2015. In November 2015, Tenet-Jove entity collected RMB 100,000 ($16,370) from Nanjing Kangtianyi as investment income. In June 2016, the Company withdrew its investments in both companies and received RMB 3.0 million (approximately $0.45 million) of its original investment amount. As a result of the withdrawal, the Company recognized a gain of approximately $233,000 for the year ended June 30, 2016.
On October 21, 2013, the Company, through its controlled subsidiaries, Zhisheng Freight and Zhisheng Agricultural, entered into an agreement with an unrelated third party, Zhejiang Zhen’Ai Network Warehousing Services Co., Ltd. (“Zhen’Ai Network”), and invested RMB 14.5 million (approximately $2.2 million) into Tiancang Systematic Warehousing project (“Tiancang Project”) operated by Zhen’Ai Network. Tiancang Project is an online platform established to provide comprehensive warehousing and logistic solutions to companies involved in E-commerce. The Company is entitled to a profit sharing of 29% of Tiancang Project’s after-tax net income annually, less 30% statutory reserve and 10% employee welfare fund. When the amount of the accumulated statutory reserve reaches 30% of the total investment for the Tiancang Project, no additional appropriation of the statutory reserve is required. The Tiancang Project is currently up and running. For the year ended June 30, 2016, Zhisheng Freight received investment income distribution of $139,949 from Zhen’Ai Network.
The Company’s investments in unconsolidated entities consist of the following:
| June 30, 2016 | June 30, 2015 | |||||||
| Shaanxi Pharmacy Holding Group Longevity Pharmacy Co., Ltd ( Ankang Longevity Pharmacy ) | $ | 2,091,531 | $ | 1,797,814 | ||||
| Shaanxi Pharmacy Sunsimiao Drugstores Ankang Chain Co., Ltd | 493,008 | 452,939 | ||||||
| Nanjing Kang Tian Yi Dressing & Adornment Co., Ltd | - | 245,550 | ||||||
| Zhejiang Zhen’Ai Network Warehousing Services Co., Ltd. | 2,182,308 | 2,373,650 | ||||||
| Total | $ | 4,766,847 | $ | 4,869,953 | ||||
Summarized financial information of unconsolidated entities is as follows:
| June 30, 2016 | June 30, 2015 | |||||||
| Current assets | $ | 28,450,106 | $ | 26,768,724 | ||||
| Noncurrent assets | 386,764 | 547,736 | ||||||
| Current liabilities | 23,577,799 | 22,739,953 | ||||||
| Noncurrent liabilities | - | - | ||||||
| For the years ended June 30, | ||||||||
| 2016 | 2015 | |||||||
| Net sales | $ | 25,456,718 | $ | 35,891,304 | ||||
| Gross profit | 3,382,468 | 3,233,879 | ||||||
| Income from operations | 1,071,742 | 1,857,819 | ||||||
| Net income | 1,086,377 | 1,856,794 | ||||||
| F-18 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 6 - INVESTMENTS (Continued)
B) Investments in real estate project
On January 28, 2014, the Company, through one of its VIEs, Ankang Longevity Group, entered into an agreement (“Agreement”) with an unrelated third party, Shaanxi Xunyang Hongye Real Estate Co., Ltd. (“Xunyang Hongye”), to jointly invest a total of RMB 60.0 million (approximately $9.0 million) to build a site for planned Chinese herbal medicine exchange market (the “Exchange”) in Ankang City, China. The Exchange intends to provide services to Chinese herbal medicine traders and wholesalers by incorporating physical trading spaces, an online trading platform and logistic services into one-stop shop. Ankang Longevity Group contributed RMB 40.0 million (approximately $6.0 million) for this project, which, in return, entitles Ankang Longevity Group to 60% ownership of the real estate properties that are being constructed. Xunyang Hongye is an experienced real estate developer which is contributing its expertise and license to develop this project. The building has been completed and put in use during the last quarter of the fiscal 2016 after being granted the Certificate of Occupancy. Pursuant to the allocation agreement signed between Ankang Longevity Group and Xunyang Hongye initially signed on October 30, 2015, the Chinese herbal medicine trading hall on the 1st and 2nd floor of the building (total area of 1,720 square meter), the apartments on 3rd floors (total area of 820 square meter) and 4 underground parking spots were allocated and transferred to the Company. Thus the Company reclassified RMB 40.0 million investment in real estate project (approximately $6.0 million) to property and equipment.
The Company’s investments in real estate project consist of the following:
| June 30, 2016 | June 30, 2015 | |||||||
| Shanxi XunyangHongye Real Estate Co., Ltd | $ | - | $ | 6,548,000 | ||||
| Total investments (A+B) | $ | 4,766,847 | $ | 11,417,953 | ||||
NOTE 7 - PREPAID LEASES
One of the Company’s controlled subsidiaries, Zhisheng Group entered into several farmland lease contracts with farmer cooperatives to lease farmland in order to plant and grow organic vegetable, fruit and Chinese yew trees. The lease term varies from 5 years to 24 years. The aggregate lease payments on these leases amounted to RMB 36.8 million (approximately $5.54 million). Zhisheng Group paid off the entire required lease amount plus transfer fees at the beginning of the lease.
These leases are accounted for as operating leases and will be amortized each year on a straight-line basis over the lease terms. The amortization expense is initially recorded as work in process under inventory account during the growing period and then transferred to harvested crops costs at the time of harvest and then allocated to cost of sales when they are sold.
The prepaid leases consist of the following:
| June 30, 2016 | June 30, 2015 | |||||||
| Current | $ | 478,565 | $ | 521,235 | ||||
| Non-current | 3,860,327 | 4,719,320 | ||||||
| Total | $ | 4,338,892 | $ | 5,240,555 | ||||
| F-19 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 7 - PREPAID LEASES (Continued)
Further amortization expense is as follows:
| Twelve months ending June 30: | ||||
| 2017 | $ | 478,565 | ||
| 2018 | 475,304 | |||
| 2019 | 475,304 | |||
| 2020 | 475,304 | |||
| 2021 | 475,304 | |||
| Thereafter | 1,959,111 | |||
| Total | $ | 4,338,892 | ||
NOTE 8 - SHORT-TERM LOANS
Short-term loans consist of the following:
| Lender | June 30, 2016 | Maturity Date | Int. Rate/Year | |||||||||
| Chongqing Alibaba Micro-Credit Company-a | $ | 36,873 | 2016-9-9 | * | 15.21 | % | ||||||
| Agricultural Bank of China-b | 301,008 | 2016-9-24 | * | 5.52 | % | |||||||
| Agricultural Bank of China-d | 451,512 | 2016-8-9 | * | 5.82 | % | |||||||
| Agricultural Bank of China-c | 451,512 | 2016-10-27 | 5.27 | % | ||||||||
| Agricultural Bank of China-c | 1,204,032 | 2016-11-18 | 5.22 | % | ||||||||
| Agricultural Bank of China-c | 301,008 | 2017-4-7 | 5.22 | % | ||||||||
| Total | $ | 2,745,945 | ||||||||||
| Lender | June 30, 2015 | Maturity Date | Int. Rate/Year | |||||||||
| Beijing Rural Commercial Bank Donghua Branch | $ | 111,006 | Due on demand | * | 7.97 | % | ||||||
| Agricultural Bank of China-b | 327,400 | 2015-8-25 | * | 7.20 | % | |||||||
| Agricultural Bank of China-c | 491,100 | 2015-7-29 | * | 7.20 | % | |||||||
| Agricultural Bank of China-c | 327,400 | 2015-10-14 | * | 7.80 | % | |||||||
| Agricultural Bank of China-c | 491,100 | 2015-10-16 | * | 7.20 | % | |||||||
| Agricultural Bank of China-c | 1,309,600 | 2015-10-30 | * | 7.20 | % | |||||||
| Agricultural Bank of China-c | 327,400 | 2016-2-16 | * | 6.42 | % | |||||||
| Total | $ | 3,385,006 | ||||||||||
The loans outstanding were guaranteed by the following properties, entities or individuals:
a. Not collateralized or guaranteed.
b. Collateralized by the building owned by Xiaoyan Chen and Jing Chen, who are both the related parties of the company. Xiaoyan Chen is one of the shareholders of Ankang Longevity Pharmaceutical (Group) Co., Ltd. Jing Chen is the sister of the Xiaoyan Chen but not a shareholder of Ankang Longevity Group.
c. Guaranteed by commercial credit guaranty companies unrelated to the Company.
d. Guaranteed by a commercial credit guaranty company, unrelated to the Company and also the loan guaranteed by Jiping Chen, a shareholder of the Company.
| * | The Company repaid the loans in full on maturity date. |
| F-20 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 8 - SHORT-TERM LOANS (Continued)
The Company recorded interest expense of $171,827 and $238,265 for the years ended June 30, 2016 and 2015, respectively. The annual weighted average interest rates are 5.96% and 7.24% for the years ended June 30, 2016 and 2015, respectively.
NOTE 9 - RELATED PARTY TRANSACTIONS
DUE FROM RELATED PARTIES
The Company had previously made temporary advances to certain shareholders of the Company as well as several other entities that are either owned by directors or family members of those directors. Those advances are due on demand, non-interest bearing, except for advance to Xinyang Yifangyuan Garden Technology Co., Ltd., which bears fixed monthly interest rate of 4.17% and advance to Shanxi Pharmacy Holding Group Longetive Pharmacy Co., Ltd., which bore a fixed annual interest rate of 5.1%, and was paid in full in December, 2015.
As of June 30, 2016 and 2015, the outstanding amounts due from related parties consist of the following:
| June 30, 2016 | June 30, 2015 | |||||||
| Shanxi Pharmacy Holding Group Longetive Pharmacy Co., Ltd | $ | - | $ | 1,158,764 | ||||
| Xinyang Yifangyuan Garden Technology Co., Ltd | 500,784 | 593,413 | ||||||
| Shaanxi Pharmaceutical Group Pai’ang Medicine Co. Ltd | 820,728 | 144,830 | ||||||
| Yang Bin | 150,504 | 163,700 | ||||||
| Zhang Xin | 93,312 | 101,494 | ||||||
| Chang Song | 85,035 | 64,662 | ||||||
| Wang Qi Wei | 8,279 | 9,005 | ||||||
| Tian Shuangpeng | 11,288 | - | ||||||
| Zhang Yuying | - | 3,624 | ||||||
| Qi Qiuchi | 1,505 | 171,885 | ||||||
| KuerLe Tenet Jove Business & Trading Co., Ltd | - | 163,700 | ||||||
| Huiyin Ansheng | - | 48,092 | ||||||
| $ | 1,671,435 | $ | 2,623,169 | |||||
For the year ended June 30, 2016, interest income of $261,015 recognized on these loans was included in “Interest income, net” on the consolidated statements of income and comprehensive income. For the year ended June 30, 2015, interest income of $260,874 recognized on these loans was included in “Interest income, net” on the consolidated statements of income and comprehensive income.
DUE TO RELATED PARTIES
As of June 30, 2016 and 2015, the Company has related party payables of $244,915 and $137,508, respectively, mainly due to the principal shareholders or certain relative of the shareholder of the Company who lend funds for the Company’s operations. The payables are unsecured, non-interest bearing and due on demand.
| June 30, 2016 | June 30, 2015 | |||||||
| Wang Sai | 120,854 | - | ||||||
| Wu Yang | 96,398 | 137,508 | ||||||
| Zhang Yuying | 26,769 | - | ||||||
| Huiyin Ansheng | 894 | - | ||||||
| $ | 244,915 | $ | 137,508 | |||||
| F-21 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 9 - RELATED PARTY TRANSACTIONS (Continued)
SALES TO AND PURCHASES FROM RELATED PARTIES
For the year ended June 30, 2016 and 2015, the Company recorded sales to Shaanxi Pharmaceutical Group Pai’ang Medicine Co., Ltd, a related party, of $3,014,198 and $3,176,386, respectively. There were no purchases from related parties for years ended June 30, 2016 and 2015.
NOTE 10 - TAXES
| (a) | Corporate Income Taxes |
The Company is subject to income taxes on an entity basis on income arising in or derived from the location in which each entity is domiciled.
Shineco is incorporated in the United States and has no operating activities. Tenet-Jove and its VIEs entities are governed by the Income Tax Laws of the PRC, and are currently subject to tax at a statutory rate of 25% on net income reported after appropriated tax adjustment. Two VIE entities receive a full income tax exemption from the local tax authority of PRC as agricultural enterprises as long as the favorable tax policy remains unchanged.
| i) | The components of the income tax (benefit) expense are as follows: |
| For the years ended June 30, | ||||||||
| 2016 | 2015 | |||||||
| Current income tax provision | $ | 1,298,472 | $ | 1,189,135 | ||||
| Deferred income tax benefit | (120,765 | ) | (64,419 | ) | ||||
| Total | $ | 1,177,707 | $ | 1,124,716 | ||||
| ii) | The following table summarizes deferred tax assets resulting from differences between the financial reporting basis and tax basis of assets and liabilities: |
| June 30, 2016 | June 30, 2015 | |||||||
| Allowance for doubtful accounts | $ | 123,818 | $ | 22,612 | ||||
| Inventory reserve | 203,674 | 145,073 | ||||||
| Impairment of investment | - | 61,387 | ||||||
| Net operating loss carry-forwards | 114,122 | 108,932 | ||||||
| Total | 441,614 | 338,004 | ||||||
| Valuation allowance | (114,122 | ) | (108,932 | ) | ||||
| Deferred tax assets, net | $ | 327,492 | $ | 229,072 | ||||
As of June 30, 2016 and 2015, net operating loss carry-forwards allowed by PRC tax authorities was $456,477 and $435,728, respectively. These carryforwards will expire, if not utilized by June 2021 and June 2020, respectively.
Movement of valuation allowance:
| June 30, 2016 | June 30, 2015 | |||||||
| Beginning balance | $ | 108,932 | $ | 313,342 | ||||
| Current year addition | 13,971 | - | ||||||
| Current year reversal | - | (206,724 | ) | |||||
| Exchange difference | (8,781 | ) | 2,314 | |||||
| Ending balance | $ | 114,122 | $ | 108,932 | ||||
| F-22 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 10 - TAXES (Continued)
| iii) | The following table reconciles the PRC statutory rates to the Company's effective tax rate for the years ended June 30, 2016 and 2015: |
| For the years ended June 30, | ||||||||
| 2016 | 2015 | |||||||
| PRC statutory tax rate | 25.00 | % | 25.00 | % | ||||
| Exemption rendered by local tax authorities | (12.56 | )% | (11.87 | )% | ||||
| Effective tax rate | 12.44 | % | 13.13 | % | ||||
(b) Value Added Tax
The Company is subject to a value added tax (“VAT”) for selling merchandise. The applicable VAT rate is 17% for products sold in the PRC. The amount of VAT liability is determined by applying the applicable tax rate to the invoiced amount of goods sold (output VAT) less VAT paid on purchases made with the relevant supporting invoices (input VAT). Under the commercial practice of the PRC, the Company pays VAT based on tax invoices issued. The tax invoices may be issued subsequent to the date on which revenue is recognized, and there may be a considerable delay between the date on which the revenue is recognized and the date on which the tax invoice is issued.
In the event that the PRC tax authorities dispute the date on which revenue is recognized for tax purposes, the PRC tax office has the right to assess a penalty based on the amount of the taxes which are determined to be late or deficient, and will be expensed in the period if and when a determination is made by the tax authorities.
(c) Taxes Payable
Taxes payable consists of the following:
| June 30, 2016 | June 30, 2015 | |||||||
| Income tax payable | $ | 1,201,641 | $ | 982,532 | ||||
| Value added tax payable | 69,955 | 93,292 | ||||||
| Business tax and other taxes payable | 6,546 | 429 | ||||||
| $ | 1,278,142 | $ | 1,076,253 | |||||
NOTE 11 - STATUTORY RESERVE
The Company is required to make appropriations to reserve funds, comprising the statutory surplus reserve and discretionary surplus reserve, based on after-tax net income determined in accordance with generally accepted accounting principles of the PRC (“PRC GAAP”).
Appropriations to the statutory surplus reserve is required to be at least 10% of the after tax net income determined in accordance with PRC GAAP until the reserve is equal to 50% of the entities’ registered capital. Appropriations to the discretionary surplus reserve are made at the discretion of the Board of Directors.
| F-23 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 12- EARNINGS PER SHARE
The following table presents a reconciliation of basic and diluted net income per share:
| For the years ended June 30, | ||||||||
| 2016 | 2015 | |||||||
| Net income | $ | 8,292,822 | $ | 7,440,400 | ||||
| Net income attributable to common stock holders | 8,136,896 | 7,280,278 | ||||||
| Weighted average shares used in basic computation | 19,320,882 | 19,320,882 | ||||||
| Diluted effect of contingently redeemable convertible preferred stock | - | - | ||||||
| Weighted average shares used in diluted computation | 19,320,882 | 19,320,882 | ||||||
| Earnings per share – Basic and diluted: | ||||||||
| Net income | 0.43 | 0.39 | ||||||
| Less: Net income attributable to non-controlling interest | (0.01 | ) | (0.01 | ) | ||||
| Net income attributable to controlling interest | 0.42 | 0.38 | ||||||
NOTE 13 - CONCENTRATION AND RISKS
The Company maintains certain bank accounts in the PRC which are not protected by Federal Deposit Insurance Corporation (“FDIC”) insurance or other insurance. The cash balance held in the PRC bank accounts was $21,986,817 and $4,683,945 as of June 30, 2016 and 2015, respectively.
During the years ended June 30, 2016 and 2015, almost 100% of the Company's assets were located in the PRC and 100% of the Company's revenues were derived from its subsidiaries located in the PRC.
For the year ended June 30, 2016, one customer accounted for approximately 40% of the Company’s total sales. For the year ended June 30, 2015, two customers accounted for approximately 33% and 10% of the Company’s total sales, respectively.
For the year ended June 30, 2016, three vendors accounted for approximately 25%, 13% and 11% of the Company’s total purchases, respectively. For the year ended June 30, 2015, one vendor accounted for approximately 42% of the Company’s total purchases.
NOTE 14 - COMMITMENTS AND CONTINGENCIES
The Company leases eight main office spaces under non-cancelable operating lease agreements through February 28, 2019. The Company also leases farmland under non-cancelable operating lease agreement through April 26, 2041. Most of those operating lease payments are scheduled on a quarterly basis. The future minimum rental payments are as follows:
Twelve months ending June 30:
| 2017 | $ | 563,084 | ||
| 2018 | 467,832 | |||
| 2019 | 240,501 | |||
| 2020 | 223,920 | |||
| 2021 | 223,920 | |||
| Thereafter | 4,441,080 | |||
| Total | $ | 6,160,337 |
Rent expense totaled $434,475 and $388,544 for the years ended June 30, 2016 and 2015, respectively.
In addition, due to the shift of business focus, the Company sublets the above-mentioned farmland to a third party under non-cancelable operating lease agreement through May 31, 2020. The future minimum sublease rental income to be received are as follows:
Twelve months ending June 30:
| 2017 | $ | 233,920 | ||
| 2018 | 233,920 | |||
| 2019 | 233,920 | |||
| 2020 | 205,260 | |||
| Total | $ | 907,020 |
Sublease rent income totaled $233,920 and $234,576 for the years ended June 30, 2016 and 2015, respectively.
| F-24 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 15 - SEGMENT REPORTING
ASC 280, “Segment Reporting”, establishes standards for reporting information about operating segments on a basis consistent with the Group's internal organizational structure as well as information about geographical areas, business segments and major customers in financial statements for details on the Group's business segments.
The Company's chief operating decision maker has been identified as the Chief Executive Officer who reviews the financial information of separate operating segments when making decisions about allocating resources and assessing performance of the Group. Based on management's assessment, the Company has determined that it has three operating segments according to the major products and locations as follows:
| Ø | Developing, manufacturing and distributing of specialized fabrics, textile products and other by-products derived from an indigenous Chinese plant called Apocynum Venetum, commonly known as “Bluish Dogbane” or known in Chinese as “Luobuma” (referred to herein as Luobuma): |
The operating companies of this segment, namely Tenet-Jove and Tenet Huatai, specialize in Luobuma developing and manufacturing of relevant products. With rich experience and broad channels in domestic market, the Group is leading in Luobuma textile production and other by-products.
This segment’s operations are focused in the north region of Mainland China, mostly carried out in Beijing and Tianjin City.
| Ø | Planting, processing and distributing of traditional Chinese medicinal herbal products as well as other pharmaceutical products (“Herbal products”): |
The operating companies of this segment, namely AnKang Longevity Group and its subsidiaries, plant and process more than 600 kinds of Chinese medicinal herbal products with an established domestic sales and distribution network.
Ankang Longevity Group is also engaged in retail pharmacy business and the operating revenue is also included in this segment due to immaterial amount. Ankang Longevity Group also started a joint-venture pharmacy retail and wholesale distribution business in the second quarter of 2013 with a large Chinese pharmaceutical company, Shaanxi Pharmaceutical Holdings Group, aiming to expand its pharmacy retail and wholesale business. The operations of this segment are mainly located in the Mid-western region of Mainland China.
| Ø | Planting, processing and distributing of green and organic agricultural produce as well as growing and cultivating of Chinese Yew trees (“Agricultural products”): |
The operating companies of this segment, the Zhisheng Group, engage in the business of growing and distributing green and organic vegetables and fruits as well as providing logistics services for distributing agricultural products. This segment has been focusing its efforts on the growing and cultivating of Chinese yew tree (formally known as “taxus media”), a small evergreen tree whose branches can be used for the production of anti-cancer medication and tree itself can be used for ornamental indoor bonsai tree, which are known to have the effect of purifying air quality.
The operations of this segment are located in the East and North regions of Mainland China, mostly carried out in Shandong Province and in Beijing where the Zhisheng Group has newly developed over 100 acres of modern greenhouses for cultivating yew trees and other plants.
| F-25 |
| Shineco, Inc. |
| Notes to Consolidated Financial Statements |
NOTE 15 - SEGMENT REPORTING (Continued)
The following table presents summarized information by segment for the year ended June 30, 2016:
| For the year ended June 30, 2016 | ||||||||||||||||
| Bluish | Herbal | Agricultural | ||||||||||||||
| dogbane | products | products | Total | |||||||||||||
| Segment revenue | $ | 4,387,060 | $ | 14,031,955 | $ | 16,787,837 | $ | 35,206,852 | ||||||||
| Cost of goods | 1,974,182 | 10,990,162 | 11,072,897 | 24,037,241 | ||||||||||||
| Business and sales related tax | 20,711 | 64,327 | - | 85,038 | ||||||||||||
| Gross profit | 2,392,167 | 2,977,466 | 5,714,940 | 11,084,573 | ||||||||||||
| Gross profit contribution % | 21.6 | % | 26.9 | % | 51.5 | % | 100.0 | % | ||||||||
The following table presents summarized information by segment for year ended June 30, 2015:
| For the year ended June 30, 2015 | ||||||||||||||||
| Bluish | Herbal | Agricultural | ||||||||||||||
| dogbane | products | products | Total | |||||||||||||
| Segment revenue | $ | 3,530,807 | $ | 13,858,949 | $ | 15,240,746 | $ | 32,630,502 | ||||||||
| Cost of goods | 1,349,520 | 10,982,826 | 9,872,387 | 22,204,733 | ||||||||||||
| Business and sales related tax | 12,624 | 65,624 | 14 | 78,262 | ||||||||||||
| Gross profit | 2,168,663 | 2,810,499 | 5,368,345 | 10,347,507 | ||||||||||||
| Gross profit contribution % | 21.0 | % | 27.2 | % | 51.8 | % | 100.0 | % | ||||||||
Total Assets as of
| June 30, 2016 | June 30, 2015 | |||||||
| Bluish Dogbane or “Luobuma” | $ | 6,963,093 | $ | 6,308,727 | ||||
| Herbal products | 28,088,515 | 25,001,487 | ||||||
| Agricultural products | 21,997,180 | 20,821,910 | ||||||
| $ | 57,048,788 | $ | 52,132,124 | |||||
NOTE 16 - SUBSEQUENT EVENTS
On September 23, 2016, the Board of Directors of the Company approved a stock incentive plan (“Plan”). Pursuant to the Plan, the Company plans to file a registration statement on Form S-8 as soon as practicable after the closing of its initial public offering to register up to 2,103,407 of the Company’s shares of common stock. As of the date of this report, the Company has not issued any options to purchase the Company's shares of common stock.
On September 27, 2016, the Company completed an initial public offering of 1,713,190 shares of common stocks at a $4.50 offering price. Our common stocks started trading on the NASDAQ Capital Market under the symbol of TYHT on September 28, 2016.
| F-26 |
