Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Fibrocell Science, Inc. | fcsc071216form8-k.htm |
Corporate Presentation
July 12, 2016

This presentation and our accompanying remarks contain “forward-looking statements” within the meaning of the
U.S. Private Securities Litigation Reform Act of 1995. All statements that are not historical facts are hereby identified
as forward-looking statements for this purpose and include, among others, statements relating to: the potential
advantages of our product candidates; the initiation, design and timing of pre-clinical studies and clinical trials and
activities and the reporting of the results thereof; the timing of regulatory submissions and actions; expected
milestones; and all other statements relating to our future operations, future financial performance, future financial
condition, prospects or other future events.
Forward-looking statements are based upon our current expectations and assumptions and are subject to a number
of known and unknown risks, uncertainties and other factors that could cause actual results to differ materially and
adversely from those expressed or implied by such statements. Factors that could cause or contribute to such
differences include, among others: our ability to obtain additional capital to fund our operations; FDA allowance to
treat pediatric subjects in the Phase II portion of our Phase I/II clinical trial of FCX-007; uncertainties relating to the
initiation and completion of clinical trials and whether the results will validate and support the safety and efficacy of
our product candidates; the risk that results seen in pre-clinical studies may not be replicated in humans; varying
interpretation of clinical and pre-clinical data; our ability to maintain our collaborations with Intrexon; and the other
factors discussed under the caption “Item 1A. Risk Factors” in our most recent annual report on Form 10-K which is
available through the “Investors—SEC Filings” page of our website at www.fibrocell.com. As a result, you should not
place undue reliance on forward-looking statements.
The forward-looking statements made in connection with this presentation represent our views only as of the date
of this presentation (or any earlier date indicated in such statement). While we may update certain forward-looking
statements from time to time, we specifically disclaim any obligation to do so, even if new information becomes
available in the future.
2
Forward-Looking Statements

Company Highlights
•Autologous cell and gene therapy company translating personalized biologics into
medical breakthroughs for diseases affecting the skin and connective tissue
• FCX-007 — gene therapy for Recessive Dystrophic Epidermolysis Bullosa (RDEB)
Actively recruiting adult subjects in Phase I/II clinical trial June 2016
Granted pediatric rare disease designation by FDA
• FCX-013 — gene therapy for Linear Scleroderma
Proof-of-concept completed January 2016; IND planned 2017
Received FDA orphan drug designation for treatment of localized scleroderma
• Gene therapy research program for Arthritis and related conditions
Deliver protein therapy locally to the joint providing sustained efficacy while avoiding key
side effects typically associated with systemic therapy
• Gene therapy portfolio being developed in collaboration with Intrexon (NYSE:
XON)
3

Autologous Fibroblasts as a Platform for Therapy
•Most common cell in skin and connective tissue
Ideal delivery vehicle to administer protein of interest locally
•Key advantages of our autologous fibroblast platform for creating cell and
gene therapies:
Localized administration avoids side effects typically associated with systemic therapy
Reduced rejection concerns because autologous fibroblasts are compatible with the
unique biology of each patient
Fibroblasts are genetically-modified ex vivo to enable testing for safety and
confirmation of protein expression prior to administration
•We are using our proprietary technology to create personalized biologics
Expertise in commercial-scale manufacturing of autologous cell therapy
4

Personalized Biologics Approach
5
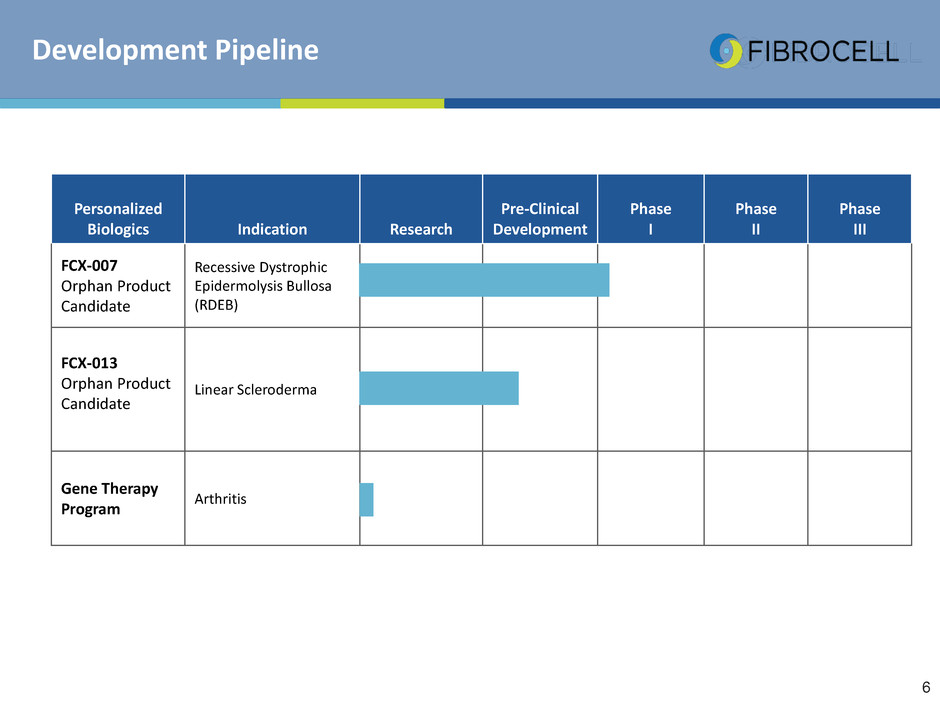
Development Pipeline
6
Personalized
Biologics Indication Research
Pre-Clinical
Development
Phase
I
Phase
II
Phase
III
FCX-007
Orphan Product
Candidate
Recessive Dystrophic
Epidermolysis Bullosa
(RDEB)
FCX-013
Orphan Product
Candidate
Linear Scleroderma
Gene Therapy
Program
Arthritis
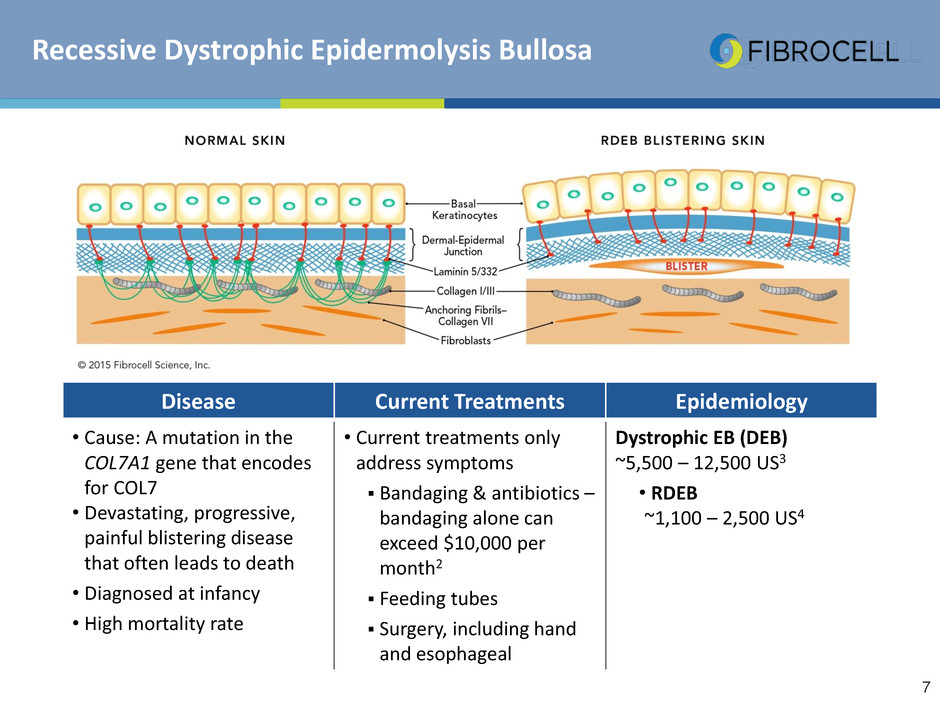
Recessive Dystrophic Epidermolysis Bullosa
7
Disease Current Treatments Epidemiology
• Cause: A mutation in the
COL7A1 gene that encodes
for COL7
• Devastating, progressive,
painful blistering disease
that often leads to death
• Diagnosed at infancy
• High mortality rate
• Current treatments only
address symptoms
Bandaging & antibiotics –
bandaging alone can
exceed $10,000 per
month2
Feeding tubes
Surgery, including hand
and esophageal
Dystrophic EB (DEB)
~5,500 – 12,500 US3
• RDEB
~1,100 – 2,500 US4
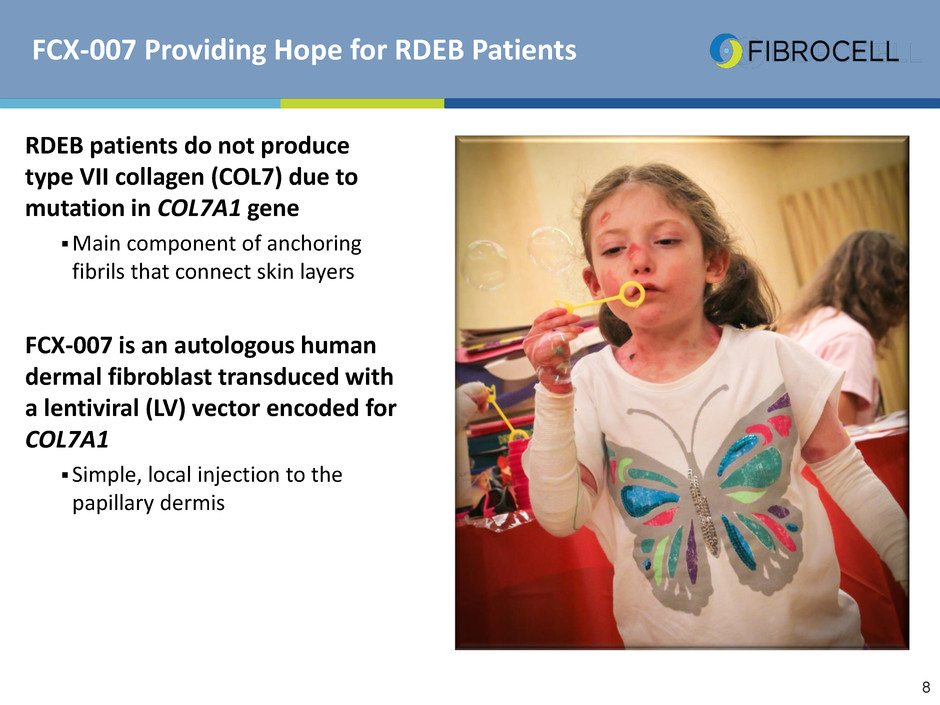
FCX-007 Providing Hope for RDEB Patients
8
RDEB patients do not produce
type VII collagen (COL7) due to
mutation in COL7A1 gene
Main component of anchoring
fibrils that connect skin layers
FCX-007 is an autologous human
dermal fibroblast transduced with
a lentiviral (LV) vector encoded for
COL7A1
Simple, local injection to the
papillary dermis
9
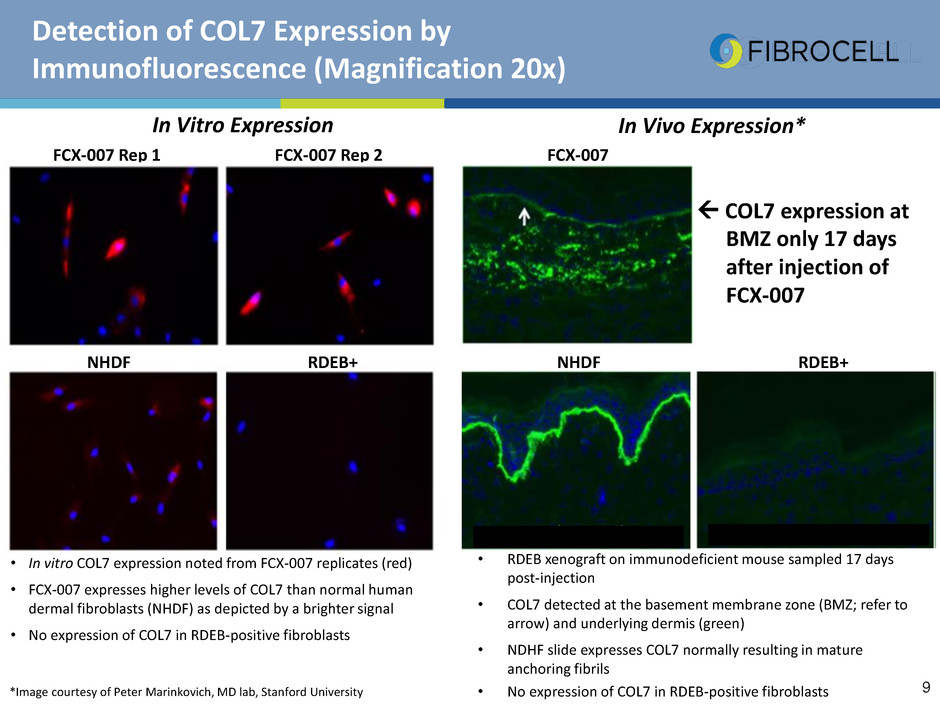
Detection of COL7 Expression by
Immunofluorescence (Magnification 20x)
• In vitro COL7 expression noted from FCX-007 replicates (red)
• FCX-007 expresses higher levels of COL7 than normal human
dermal fibroblasts (NHDF) as depicted by a brighter signal
• No expression of COL7 in RDEB-positive fibroblasts
FCX-007 Rep 1 FCX-007 Rep 2 FCX-007
In Vivo Expression*In Vitro Expression
NHDF RDEB+ NHDF RDEB+
• RDEB xenograft on immunodeficient mouse sampled 17 days
post-injection
• COL7 detected at the basement membrane zone (BMZ; refer to
arrow) and underlying dermis (green)
• NDHF slide expresses COL7 normally resulting in mature
anchoring fibrils
• No expression of COL7 in RDEB-positive fibroblasts*Image courtesy of Peter Marinkovich, MD lab, Stanford University
COL7 expression at
BMZ only 17 days
after injection of
FCX-007

COL7 Expression Confirmation
10
Culture supernatant evaluated for in vitro COL7 expression
• ELISA assay indicates virus dose-dependent protein expression
• Trimeric form of COL7 produced by RDEB patient fibroblasts
transduced with LV-COL7
•Must be trimeric to be functional
Reference: Bruckner-Tuderman, Leena. Can Type VII Collagen Injections Cure Dystrophic
Epidermolysis Bullosa? Molecular Therapy (2008) 17 1, 6–7.
RDE
B
+
C
o
n
tr
o
l
P
u
ri
fi
e
d
C
OL
7
FC
X
-0
0
7
-0
1
FC
X
-0
0
7
-0
2
Trimeric
COL7
(900kDa)
COL7 IP
Immunoprecipitation (IP)/Western Blot
COL7 Formation
Trimeric Form
(900kDa)

• Autologous cells having less rejection risk
• Direct administration of FCX-007 to the upper dermis ensures COL7 is expressed in
the basement membrane zone where anchoring fibrils are formed
• The integrative nature of the lentivirus allows the COL7A1 gene to be passed to
daughter cells as FCX-007 cells divide
• Academic research suggests that in vivo COL7 expression persists for at least one
year in RDEB skin tissue regenerated on immunodeficient mice5
• Even if COL7A1 transgene expression were silenced, COL7 anchoring fibers would
persist, perhaps for years, due to slow turnover rate and long life of molecules6
11
Long-lasting COL7 Persistence Expected

FCX-007 Phase I/II Clinical Trial Design
12
Title A Phase I/II Trial of FCX-007 (Genetically-Modified Autologous Human Dermal Fibroblasts) for
Recessive Dystrophic Epidermolysis Bullosa (RDEB)
Objectives Primary
1) To evaluate the safety of FCX-007
Secondary
1) To evaluate mechanism of action of FCX-007 at weeks 12, 25, 52 and unscheduled visits through
the evaluation of skin biopsies for COL7 expression and the presence of anchoring fibrils
2) To evaluate the efficacy of FCX-007 through an intra-subject paired analysis of target wound area
at weeks 4, 12, 25, 52 and unscheduled visits, comparing FCX-007 treated wounds to untreated
wounds in Phase I and to wounds administered sterile saline in Phase II through the evaluation
of digital imaging of wounds
Number of
Subjects
Twelve subjects consisting of six adults in the Phase I portion of the trial and, subject to FDA
allowance, six pediatrics in the Phase II portion of the trial
Status Actively recruiting adult subjects
Linear Scleroderma
13
Disease Epidemiology
• Excess production of collagen
I and III characterized by skin
fibrosis and linear scars
• The linear areas of skin
thickening may extend to
underlying tissue and muscle
in children which may impair
growth in affected legs and
arms or forehead
• Lesions appearing across
joints impair motion and may
be permanent
• Localized Scleroderma
~200,000 sufferers US7
comprised of many different
sub-types
Linear Scleroderma
Initial target for FCX-013 is
a group of ~40,000 patients
who have scleroderma over
a major joint and exhibit
severe joint pain8
Current Treatments
Current treatments only
address symptoms:
• Systemic or topical
corticosteroids
• UVA light therapy
• Physical therapy
Photo: Reprinted from the Journal of the American Academy of Dermatology, Volume 59, Issue 3, Stéphanie Christen-Zaech, Miriam D. Hakim, F. Sule Afsar, Amy S. Paller.
Pediatric morphea (localized scleroderma): Review of 136 patients, Figure 1, pp. 385-396. Copyright Sept 2008. Used with permission from Elsevier Ltd.

FCX-013 Development Progressing
14
•Product profile
Autologous fibroblasts genetically modified using lentivirus and encoded for a
protein responsible for breaking down excess collagen I and III
Incorporates Intrexon’s RheoSwitch Therapeutic System® (RTS®) to control protein
expression
•Proof-of-concept animal study data achieved January 2016
Demonstrated protein expression, reduced thickness of fibrotic tissue
• Scale-up manufacturing in process
•Received FDA orphan drug designation for treatment of localized
scleroderma
•Next steps include dose-ranging and toxicology/biodistribution studies
•IND submission expected 2017

FCX-013 Proof-of-Concept Study
15
• Study Design
Bleomycin treated SCID mouse model
N=30 mice over test and control groups
Assessed histologically for reduction of dermal
thickness and sub-dermal muscle in the presence
of FCX-013 and oral ligand
• Result
Bleomycin treatment resulted in skin fibrosis, measured by a significant increase in dermal thickness
Demonstrated that FCX-013 with ligand reduced the dermal thickness of fibrotic tissue to levels
similar to non-bleomycin (saline) with ligand treated skin
Further reduced the thickness of the sub-dermal muscle layer
Blecomycin treatments Ligand Treatment
D0 D28 D29 D39
Cell
injection
Harvest skin
samples
CONTROL:
Saline (no Bleo)
No Cells
TEST:
Bleomycin
FCX-013
CONTROL:
Bleomycin
Non-Modified Cells

• FCX-013 employs Intrexon’s RheoSwitch
Therapeutic System® (RTS®)
The RTS® biologic switch is activated by an orally-
administered activator ligand (AL) that provides the
ability to control level and timing of protein
expression
• Enhances the safety profile of FCX-013 by
providing control over the expression of the
protein
• In vitro studies support RTS® control over FCX-
013 protein expression:
Significant increase in expression of the target
protein in the presence of the AL
In the absence of the AL, the expression is reduced
to normal cellular production of the target protein
Cells transduced with RTS® and off-target gene
construct (RTS-GFP) and non-transduced fibroblasts
(mock) express normal cellular production levels
16
RheoSwitch® Control
RTS-GFP Control FCX-013 Mock Control
FCX-013 RTS® Control
no AL
+ AL
Ta
rg
et P
ro
tein
Conce
n
tr
ati
o
n

Gene Therapy Research Program for Arthritis
17
• Deliver protein therapy locally to the joint providing sustained efficacy while
avoiding key side effects typically associated with systemic therapy
• Combines Fibrocell’s autologous fibroblast technology with Intrexon’s cellular
engineering to develop localized gene therapies
• Focused on addressing chronic inflammation and degenerative diseases of
the joint
Arthritis (characterized by joint inflammation, pain and decreased range of motion) is
the leading cause of disability in the U.S. affecting >52 million adults and 300,000
children
Milestones & Corporate Information
18
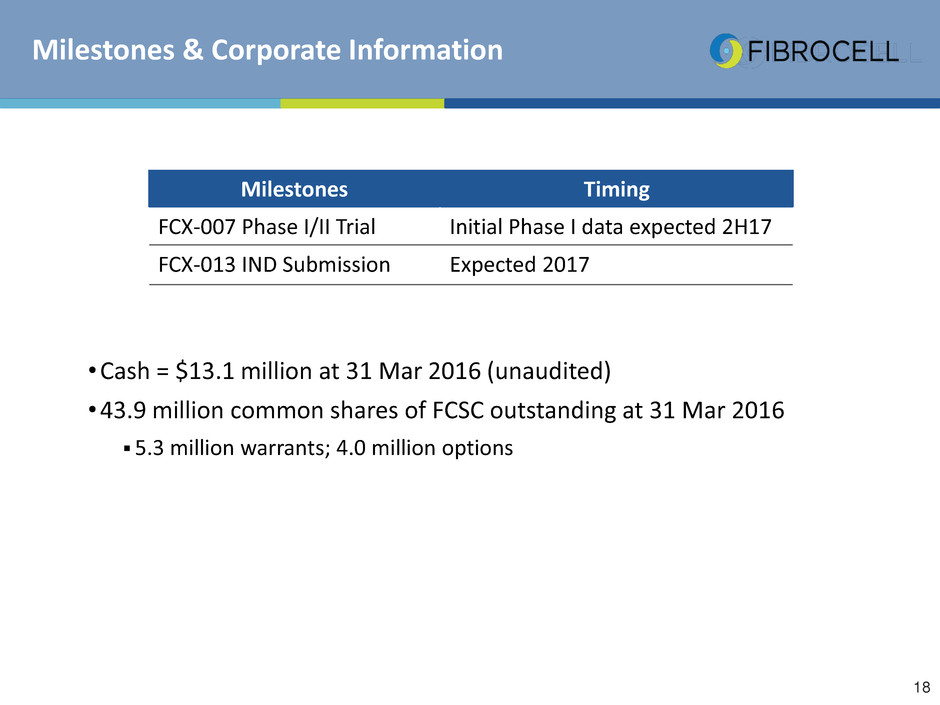
•Cash = $13.1 million at 31 Mar 2016 (unaudited)
•43.9 million common shares of FCSC outstanding at 31 Mar 2016
5.3 million warrants; 4.0 million options
Milestones Timing
FCX-007 Phase I/II Trial Initial Phase I data expected 2H17
FCX-013 IND Submission Expected 2017

Company Highlights
•Autologous cell and gene therapy company translating personalized biologics into
medical breakthroughs for diseases affecting the skin and connective tissue
• FCX-007 — gene therapy for Recessive Dystrophic Epidermolysis Bullosa (RDEB)
Actively recruiting adult subjects in Phase I/II clinical trial June 2016
Granted pediatric rare disease designation by FDA
• FCX-013 — gene therapy for Linear Scleroderma
Proof-of-concept completed January 2016; IND planned 2017
Received FDA orphan drug designation for treatment of localized scleroderma
• Gene therapy research program for Arthritis and related conditions
Deliver protein therapy locally to the joint providing sustained efficacy while avoiding key
side effects typically associated with systemic therapy
• Gene therapy portfolio being developed in collaboration with Intrexon (NYSE:
XON)
19

References
1Data on file. Fibrocell Science, Inc.
2The Dystrophic Epidermolysis Research Association of America (DebRA). DEB brochure, page 6:
http://www.debra.org/pdfs/Debra-of-America-Brochure.pdf; accessed 07/20/15.
3DEBRA International. What is EB Infographic: http://www.debra-international.org/epidermolysis-bullosa.html.;
accessed 10/06/2014.
4Petrof G., et al. Fibroblast cell therapy enhances initial healing in recessive dystrophic epidermolysis bullosa
wounds: results of a randomised, vehicle-controlled trial. Brit J Dermatol. 2013 Nov;169(5):1025-33.
5Siprashvili Z., et al. Long-term type VII collagen restoration to human epidermolysis bullosa skin tissue. Human
Gene Therapy. 2010 Oct;21(10): 1299-1310.
6Chen M., et al. Restoration of type VII collagen expression and function in dystrophic epidermolysis bullosa. Nat
Genet. 2002 Dec;32(4):670-5.
7The Scleroderma Foundation. What is Scleroderma?
http://www.scleroderma.org/site/PageServer?pagename=patients_whatis#.VaUwk7BFBMw, paragraph 6;
accessed 10/09/2014—states, “It’s estimated that about 300,000 Americans have scleroderma. About one third of
those people have the systemic form of scleroderma (i.e., 200,000 have a form of localized scleroderma).”
8The Scleroderma Foundation. “Localized Scleroderma” brochure, pages 4, 6-7:
http://www.scleroderma.org/site/DocServer/Localized.pdf?docID=317; accessed 07/20/15—states, “Some
patients with localized scleroderma, an estimated 10 to 20 percent (20% of 200,000 = 40,000 patients), develop
joint pain (arthralgia) during the course of their disease.”
20
