Attached files
| file | filename |
|---|---|
| 8-K - LENS_8K_20160629 - Presbia PLC | lens-8k_20160630.htm |

July 2016 ROTH Capital Partners Investor Presentation Exhibit 99.1

Disclosure To the extent statements contained in this presentation are not descriptions of historical facts regarding Presbia PLC and its subsidiaries (collectively “Presbia,” “we,” “us,” or “our”), they are forward-looking statements reflecting management’s current beliefs and expectations. Forward-looking statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance, or achievements to be materially different from those anticipated by such statements. You can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “intends,” or “continue,” or the negative of these terms or other comparable terminology. Forward-looking statements contained in this presentation include, but are not limited to, statements regarding: (i) the initiation, timing, progress and results of our clinical trials, our regulatory submissions and our research and development programs; (ii) our ability to advance our products into, and successfully complete, clinical trials; (iii) our ability to obtain pre-market approvals; (iv) the commercialization of our products; (v) the implementation of our business model, strategic plans for our business, products and technology; (vi) the scope of protection we are able to establish and maintain for intellectual property rights covering our products and technology; (vii) estimates of our expenses, future revenues, growth of operations, capital requirements and our needs for additional financing; (viii) the timing or likelihood of regulatory filings and approvals; (ix) our financial performance; (x) developments relating to our competitors and our industry; and (xi) statements regarding our markets, including the estimated size and anticipated growth in those markets. Various factors may cause differences between our expectations and actual results, including those risks discussed under “Risk Factors” in our Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 28, 2016 and those risks discussed under “Risk Factors” in other reports we may file with the Securities and Exchange Commission. Except as required by law, we assume no obligation to update these forward-looking statements publicly or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future.

Todd Cooper, President & CEO NASDAQ: LENS

Designed to Eliminate the Need for Reading Glasses “Presbia… provides the patient with correction for nearsightedness (loss of near vision) 1. It marks a new era to turn back the clock and regain the near vision you enjoyed when younger2. The Presbia... Microlens is the solution for the future 3 with dogma defying outcomes...” 4 Prof. Ioannis Pallikaris (Greece) Wayne Crewe-Brown, MD (Ireland, UK) Mickey Gordon, MD (USA) Kerry Assil, MD (USA)

Loss of Near Vision Affects the Majority of People Starting at the Age of 40 Presbia and related research shows: The Inconvenience of Presbyopia “I can never find my glasses”* “Can’t read using my iPhone”* “The hassle of my glasses on and off all day”* Freedom and confidence Reading glasses are one of the most ubiquitous signs of aging Recent Bausch & Lomb survey found “almost half of women over the age of 40 admit to feeling embarrassed, frumpy, or annoyed when reaching for reading glasses”* “I feel younger”* “My friends notice I don’t have reading glasses”* “I feel free now that I don’t need them”* * Focus Groups, February, 2015, Dublin, Ireland
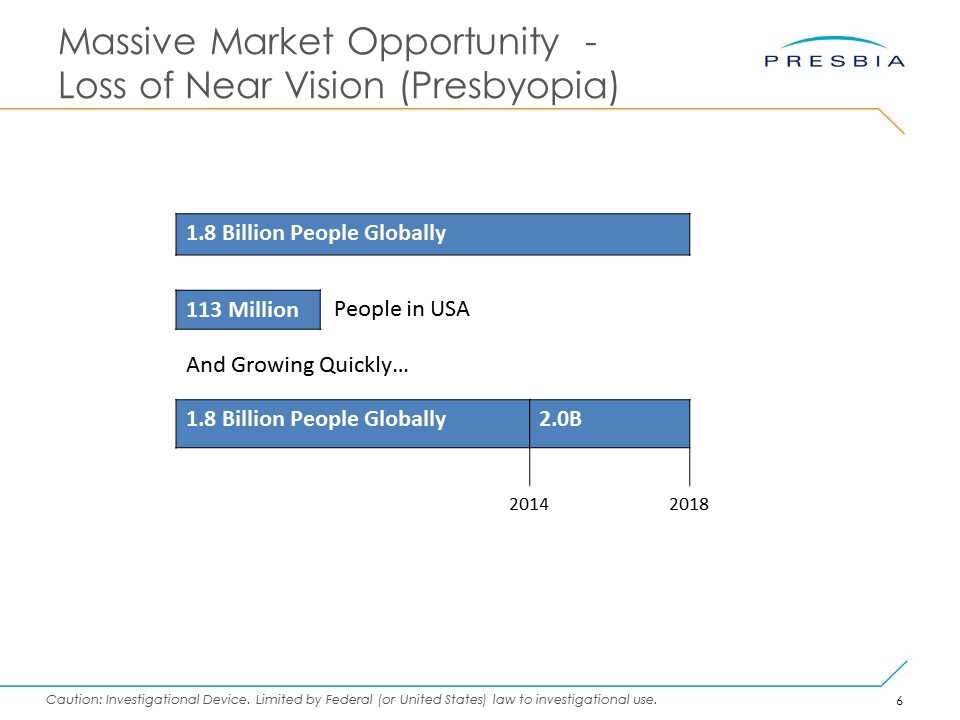
Massive Market Opportunity - Loss of Near Vision (Presbyopia) 1.8 Billion People Globally 113 Million People in USA 1.8 Billion People Globally And Growing Quickly… 2.0B 2014 2018
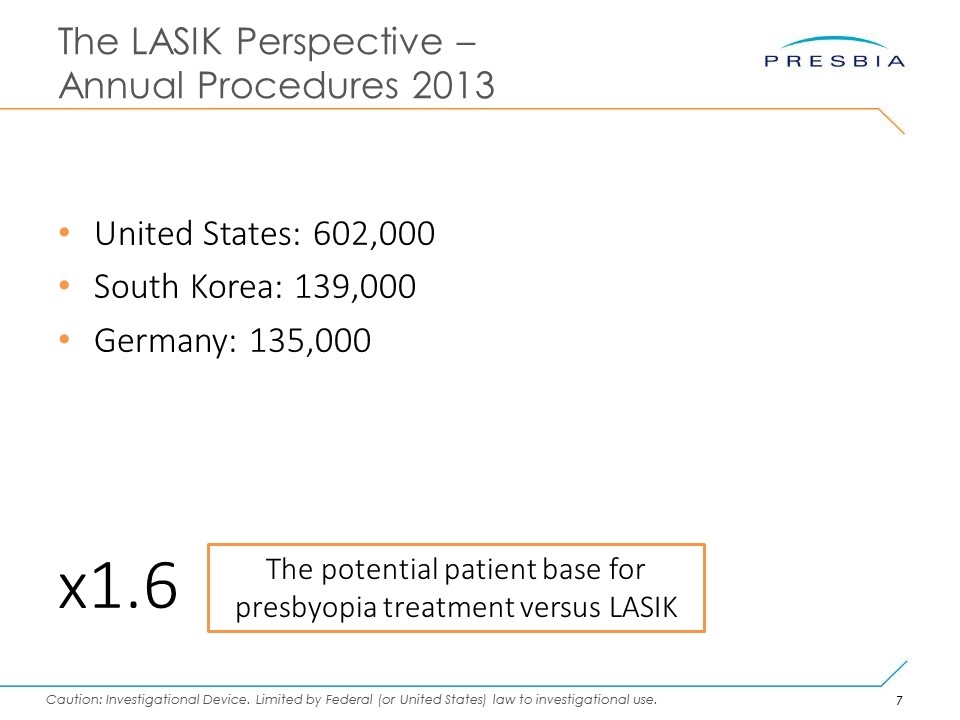
The LASIK Perspective – Annual Procedures 2013 United States: 602,000 South Korea: 139,000 Germany: 135,000 x1.6 The potential patient base for presbyopia treatment versus LASIK
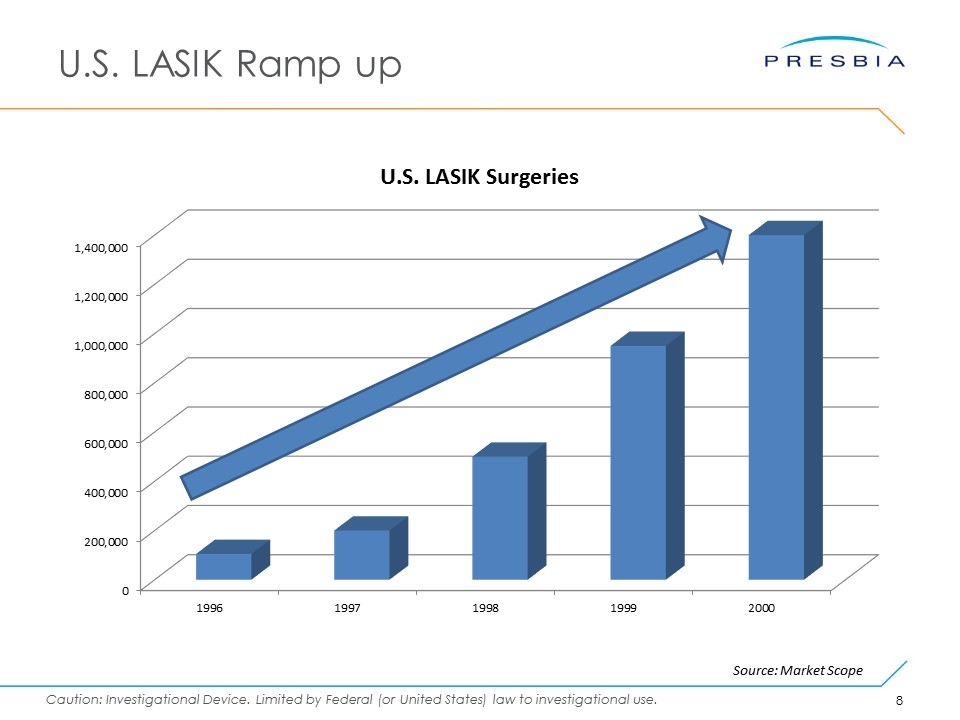
U.S. LASIK Ramp up Source: Market Scope
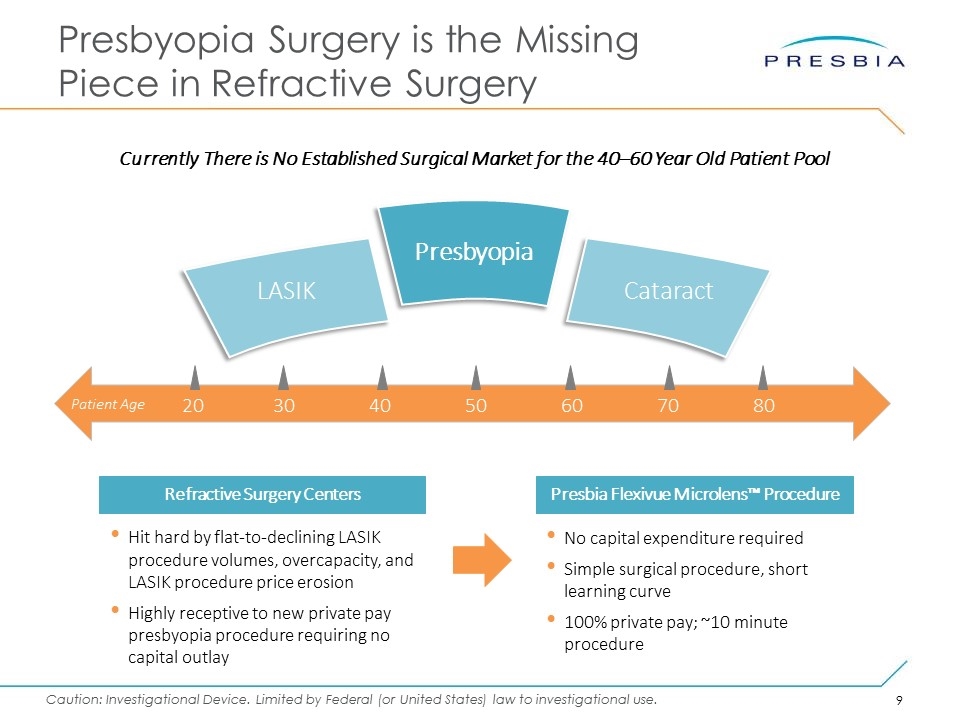
Presbyopia Surgery is the Missing Piece in Refractive Surgery Hit hard by flat-to-declining LASIK procedure volumes, overcapacity, and LASIK procedure price erosion Highly receptive to new private pay presbyopia procedure requiring no capital outlay Refractive Surgery Centers No capital expenditure required Simple surgical procedure, short learning curve 100% private pay; ~10 minute procedure Presbia Flexivue Microlens™ Procedure 20304050607080 Patient Age LASIK Cataract Presbyopia Currently There is No Established Surgical Market for the 40–60 Year Old Patient Pool
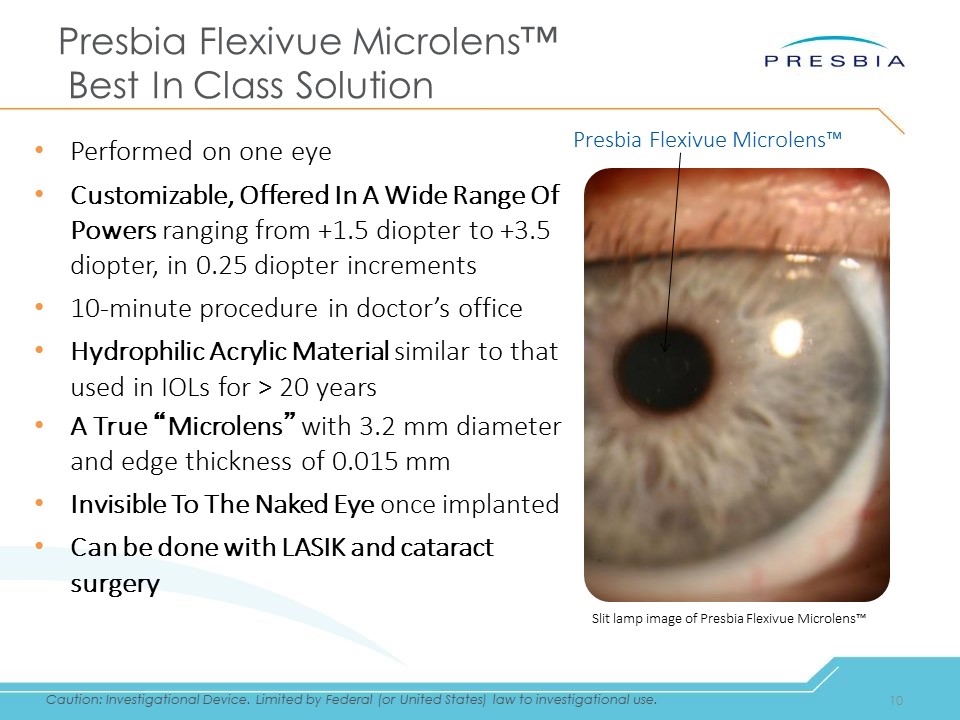
Presbia Flexivue Microlens™ Best In Class Solution Performed on one eye Customizable, Offered In A Wide Range Of Powers ranging from +1.5 diopter to +3.5 diopter, in 0.25 diopter increments 10-minute procedure in doctor’s office Hydrophilic Acrylic Material similar to that used in IOLs for > 20 years A True “Microlens” with 3.2 mm diameter and edge thickness of 0.015 mm Invisible To The Naked Eye once implanted Can be done with LASIK and cataract surgery Slit lamp image of Presbia Flexivue Microlens™ Presbia Flexivue Microlens™
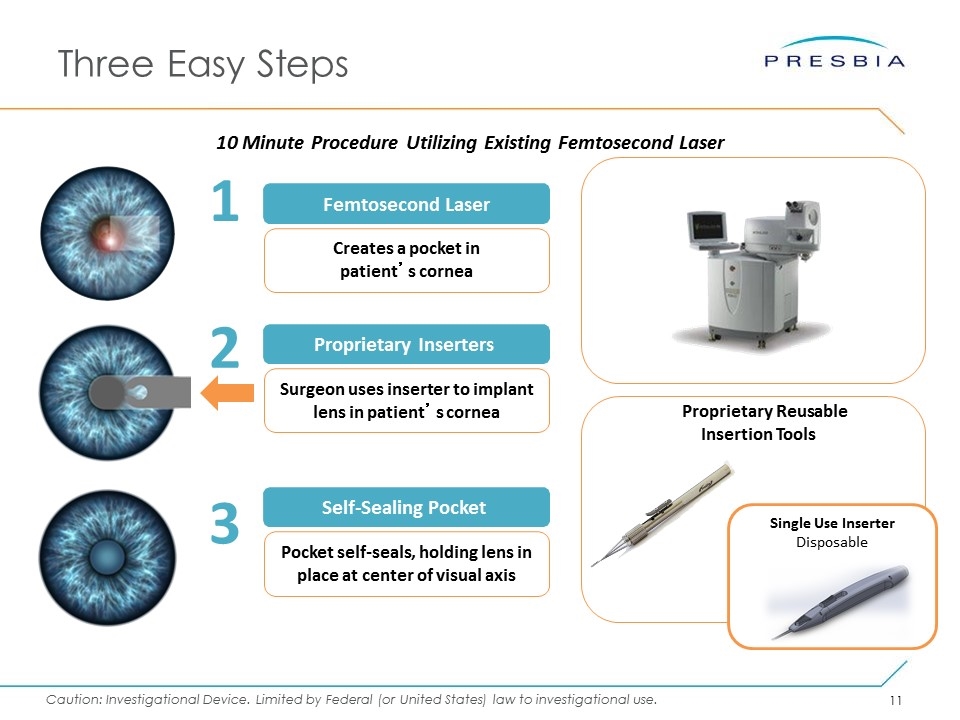
Three Easy Steps 10 Minute Procedure Utilizing Existing Femtosecond Laser Femtosecond Laser 1 Creates a pocket in patient’s cornea 3 2 Proprietary Inserters Surgeon uses inserter to implant lens in patient’s cornea Self-Sealing Pocket Pocket self-seals, holding lens in place at center of visual axis Proprietary Reusable Insertion Tools Single Use Inserter Disposable
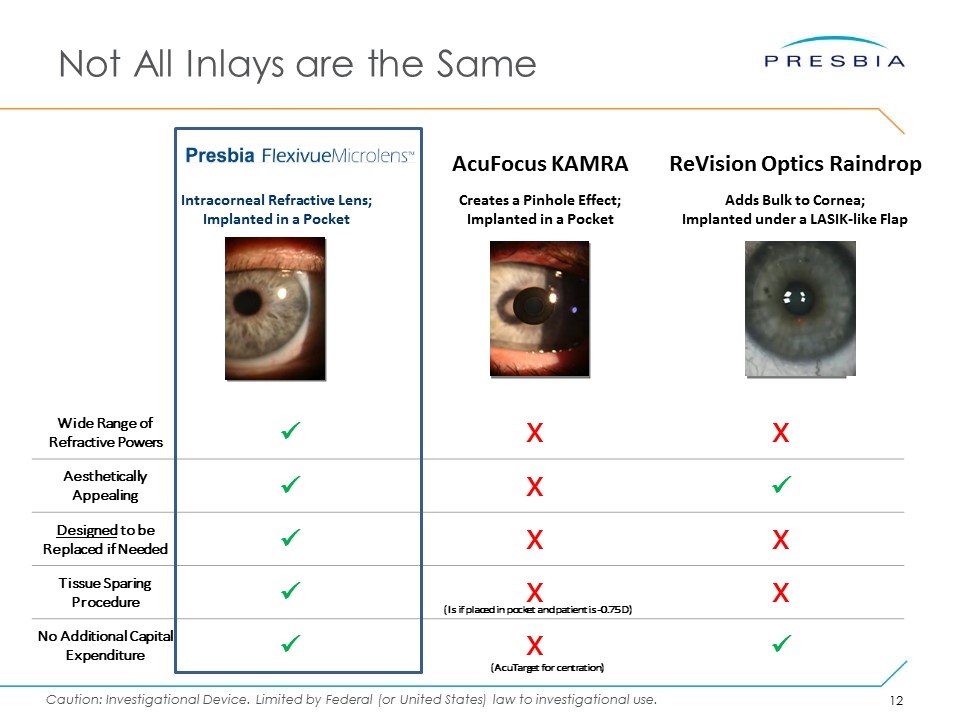
Not All Inlays are the Same Intracorneal Refractive Lens; Implanted in a Pocket Creates a Pinhole Effect; Implanted in a Pocket AcuFocus KAMRA Adds Bulk to Cornea; Implanted under a LASIK-like Flap ReVision Optics Raindrop Wide Range of Refractive Powers ü X X Aesthetically Appealing ü X ü Designed to be Replaced if Needed ü X X Tissue Sparing Procedure ü X X No Additional Capital Expenditure ü X ü (Is if placed in pocket and patient is -0.75 D) (AcuTarget for centration)
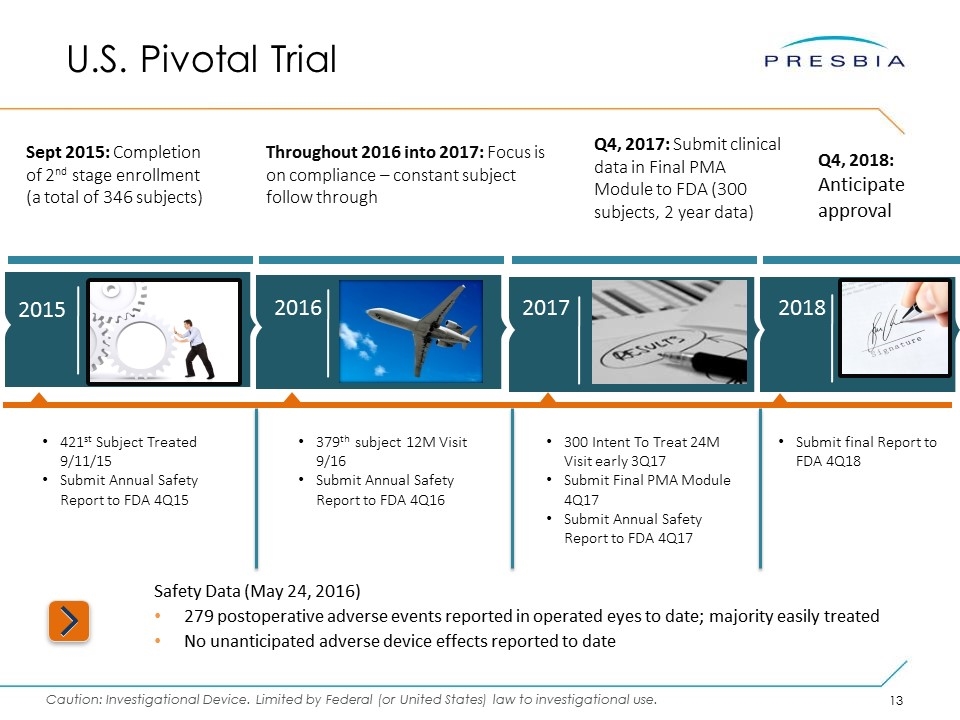
Safety Data (May 24, 2016) 279 postoperative adverse events reported in operated eyes to date; majority easily treated No unanticipated adverse device effects reported to date Sept 2015: Completion of 2nd stage enrollment (a total of 346 subjects) Throughout 2016 into 2017: Focus is on compliance – constant subject follow through Q4, 2017: Submit clinical data in Final PMA Module to FDA (300 subjects, 2 year data) U.S. Pivotal Trial 2017 2015 2016 2018 Q4, 2018: Anticipate approval 421st Subject Treated 9/11/15 Submit Annual Safety Report to FDA 4Q15 379th subject 12M Visit 9/16 Submit Annual Safety Report to FDA 4Q16 300 Intent To Treat 24M Visit early 3Q17 Submit Final PMA Module 4Q17 Submit Annual Safety Report to FDA 4Q17 Submit final Report to FDA 4Q18
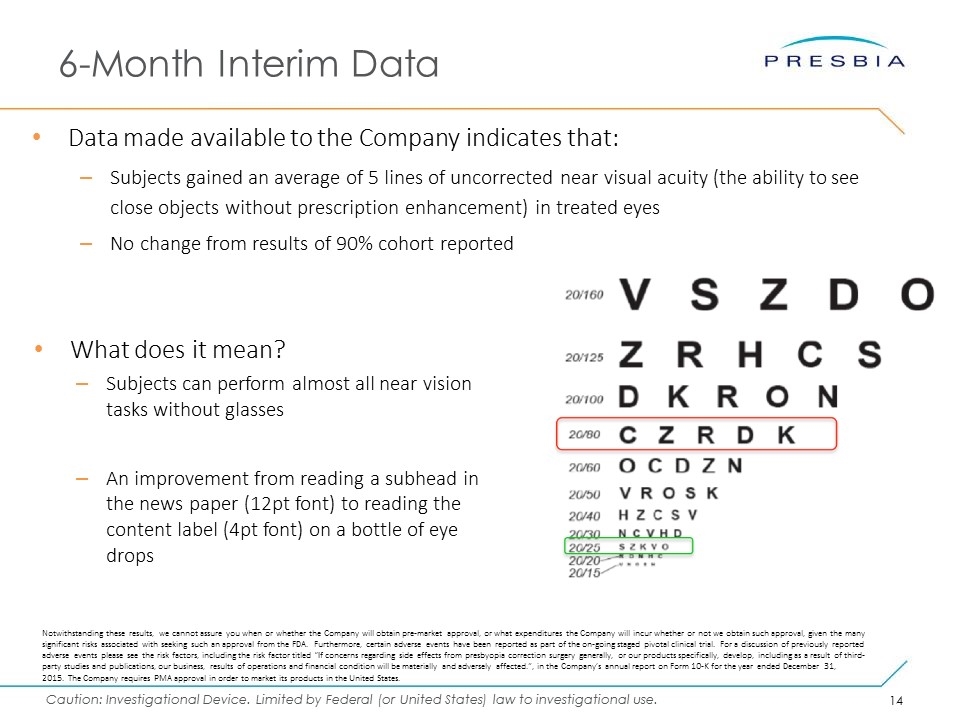
6-Month Interim Data Data made available to the Company indicates that: Subjects gained an average of 5 lines of uncorrected near visual acuity (the ability to see close objects without prescription enhancement) in treated eyes No change from results of 90% cohort reported Notwithstanding these results, we cannot assure you when or whether the Company will obtain pre-market approval, or what expenditures the Company will incur whether or not we obtain such approval, given the many significant risks associated with seeking such an approval from the FDA. Furthermore, certain adverse events have been reported as part of the on-going staged pivotal clinical trial. For a discussion of previously reported adverse events please see the risk factors, including the risk factor titled “If concerns regarding side effects from presbyopia correction surgery generally, or our products specifically, develop, including as a result of third-party studies and publications, our business, results of operations and financial condition will be materially and adversely affected.”, in the Company’s annual report on Form 10-K for the year ended December 31, 2015. The Company requires PMA approval in order to market its products in the United States. What does it mean? Subjects can perform almost all near vision tasks without glasses An improvement from reading a subhead in the news paper (12pt font) to reading the content label (4pt font) on a bottle of eye drops
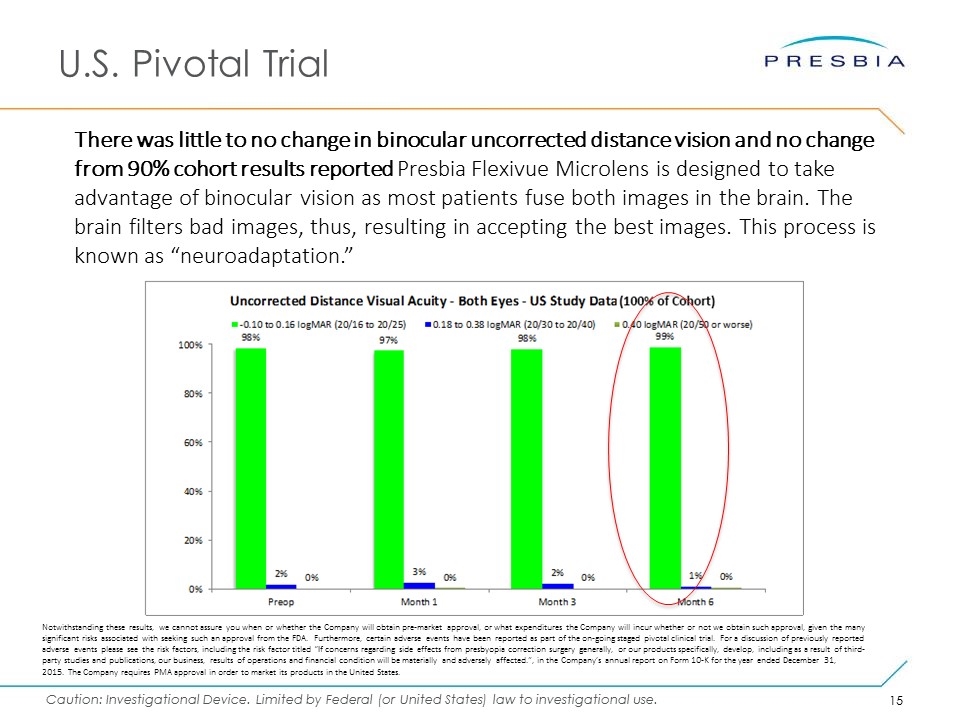
U.S. Pivotal Trial There was little to no change in binocular uncorrected distance vision and no change from 90% cohort results reported Presbia Flexivue Microlens is designed to take advantage of binocular vision as most patients fuse both images in the brain. The brain filters bad images, thus, resulting in accepting the best images. This process is known as “neuroadaptation.” Notwithstanding these results, we cannot assure you when or whether the Company will obtain pre-market approval, or what expenditures the Company will incur whether or not we obtain such approval, given the many significant risks associated with seeking such an approval from the FDA. Furthermore, certain adverse events have been reported as part of the on-going staged pivotal clinical trial. For a discussion of previously reported adverse events please see the risk factors, including the risk factor titled “If concerns regarding side effects from presbyopia correction surgery generally, or our products specifically, develop, including as a result of third-party studies and publications, our business, results of operations and financial condition will be materially and adversely affected.”, in the Company’s annual report on Form 10-K for the year ended December 31, 2015. The Company requires PMA approval in order to market its products in the United States.
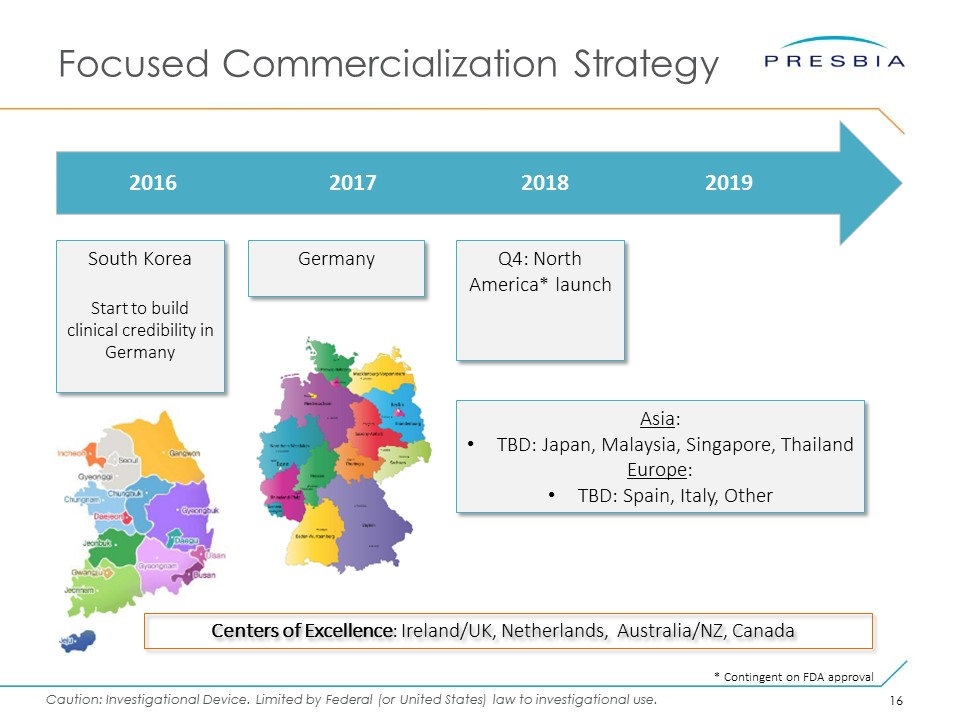
Focused Commercialization Strategy 2016 2017 2018 2019 South Korea Start to build clinical credibility in Germany Centers of Excellence: Ireland/UK, Netherlands, Australia/NZ, Canada Q4: North America* launch Asia: TBD: Japan, Malaysia, Singapore, Thailand Europe: TBD: Spain, Italy, Other Germany * Contingent on FDA approval

Focused Launch Strategy Selected countries based upon: how many people need reading glasses, LASIK procedures, surgeons, income Korea - First (Asia Pacific Hub) Most penetrated LASIK market in the world Big elective surgery market Many surgeons concentrated geographically Commercial and clinical validation Germany - Next (European Hub) Largest European market Clinically respected across Europe Commercial and clinical validation Centers of Excellence To support clinical and commercial expansion in initial Focus markets Ireland/UK, Netherlands, Australia, Canada, Italy, Greece

Experienced Leadership Team & Board Vanessa Tasso Vice President of Clinical Affairs John Strobel Vice President of Sales Todd Cooper President, Chief Executive Officer & Director Dr. Kerry K. Assil Founder and Medical Director of the Assil Eye Institute in Southern California EXECUTIVE MAB Vlad Feingold Chief Technology Officer & Director Jarett Fenton Chief Financial Officer Prof. Ioannis Pallikaris Founder and Director of the Vardinoyannion Eye Institute of Crete Dr. Mark S, Blumenkranz H.J. Smead Professor and Chairman of the Dept. of Ophthalmology at Stanford University Dr. Wayne Crewe-Brown Registered on Specialist Register of the General Medical Council of Great Britain, Irish Medical Council and Health Prof Council of RSA Dr. Marco Fantozzi Pescia, Italy – Performed 10,000 + refractive procedures and is an experienced Presbia certified surgeon Dr. Michael Gordon Founder of Gordon Schanzlin New Vision Institute in Southern California and recognized authority in refractive surgery Dr. Ronald R. Krueger Medical Director of Refractive surgery at Cleveland Clinic Cole Eye Institute in Ohio
Business Highlights Large, Underserved Presbyopia Opportunity Best-in-Class Near Vision Microlens Refractive corneal inlay restores reading vision, multiple lines of improvement Wide range of lens refractive powers to offer patients a customized therapy Can be done with LASIK and cataract surgery CE-marked with over 1000 lenses safely implanted globally Clear FDA Pathway Second and final FDA two-stage clinical trial enrollment completed: 421 U.S. patients received Presbia Flexivue Microlens™ Submission of final PMA module in Q4 2017 expected to lead to FDA approval Q4 2018 + 5 lines of uncorrected near visual acuity in treated eyes (100% of 6 month US study cohort) 113 million presbyopes in the U.S.; 1.8 billion worldwide (2014)* 4,000+ ophthalmic surgery centers with no effective treatment of presbyopia Refractive surgeons are highly motivated to develop this market to replace lost LASIK volumes and utilize installed base of expensive femtosecond lasers Compelling surgery center economics: 100% private pay, ~10 minute procedure Two beach-heads Now: Asia Pacific – South Korea Next: Europe – Germany Ongoing: Centers of Excellence – Ireland, Netherlands, Canada, Australia Commercial Strategy *Source: 2013 Market Scope.

Additional Materials
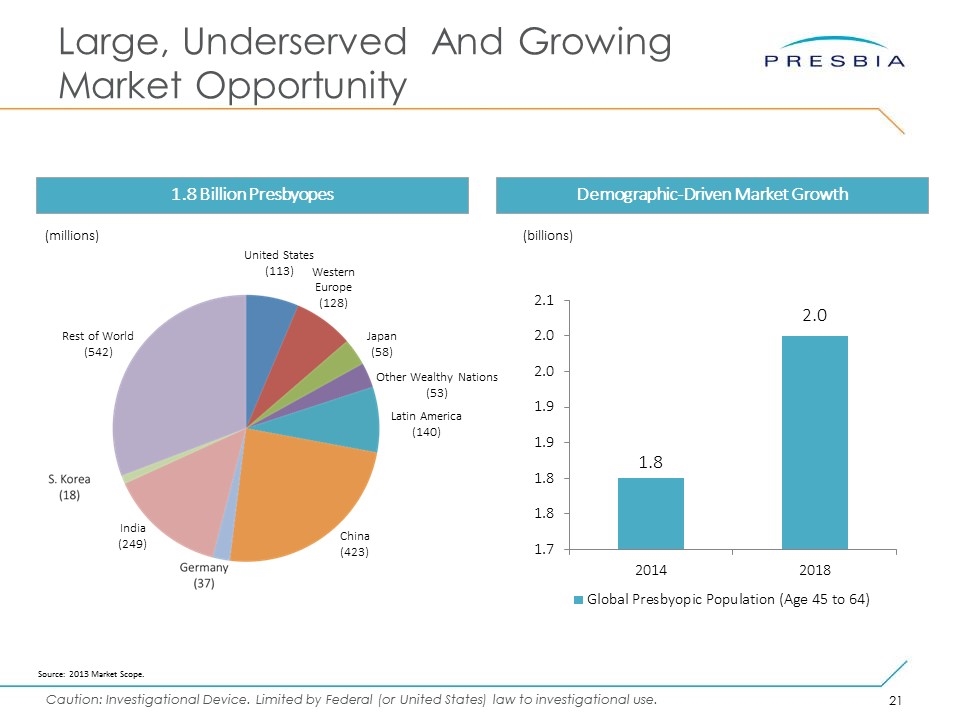
Large, Underserved And Growing Market Opportunity Demographic-Driven Market Growth 1.8 Billion Presbyopes (billions) Source: 2013 Market Scope. United States (113) Western Europe (128) Latin America (140) China (423) India (249) Rest of World (542) Other Wealthy Nations (53) Japan (58) (millions)
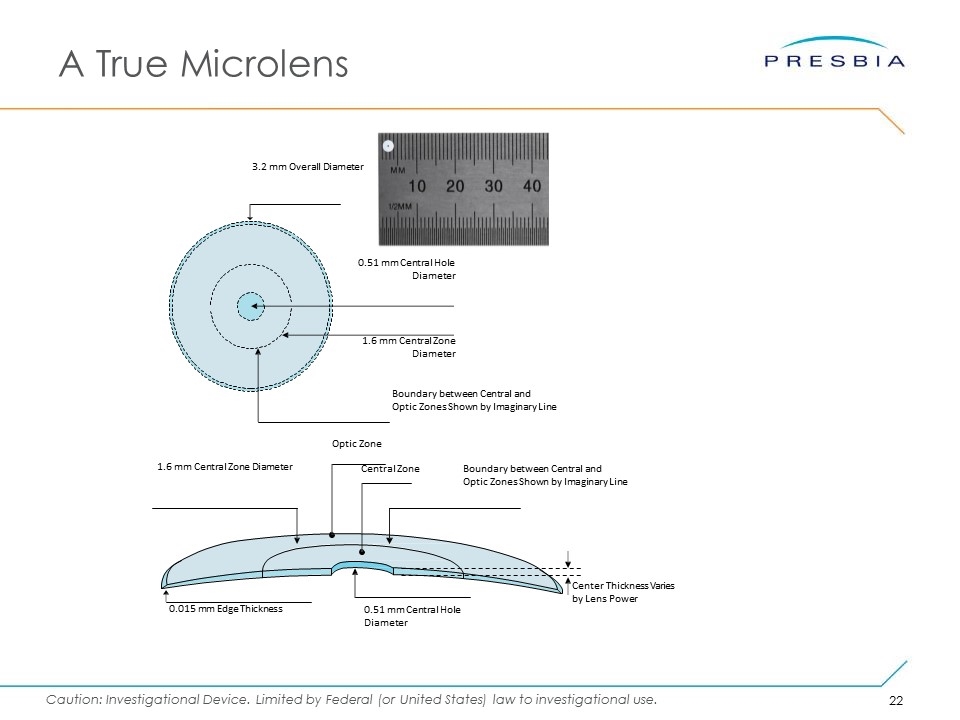
A True Microlens 1.6 mm Central Zone Diameter Boundary between Central and Optic Zones Shown by Imaginary Line 0.51 mm Central Hole Diameter 3.2 mm Overall Diameter Central Zone Boundary between Central and Optic Zones Shown by Imaginary Line Optic Zone Center Thickness Varies by Lens Power 1.6 mm Central Zone Diameter 0.51 mm Central Hole Diameter 0.015 mm Edge Thickness
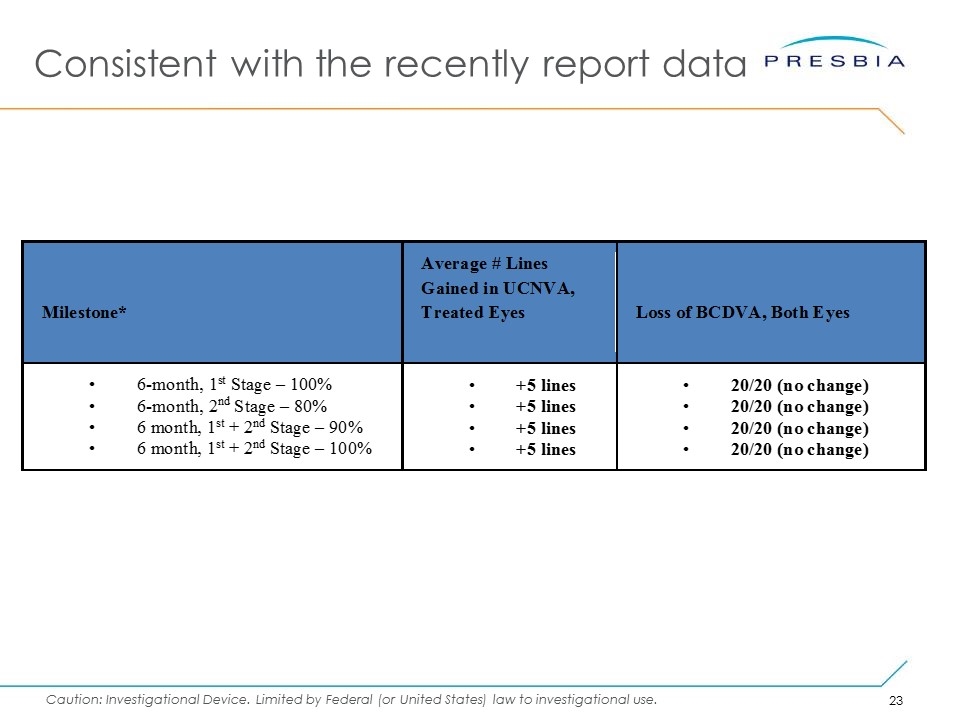
Consistent with the recently report data
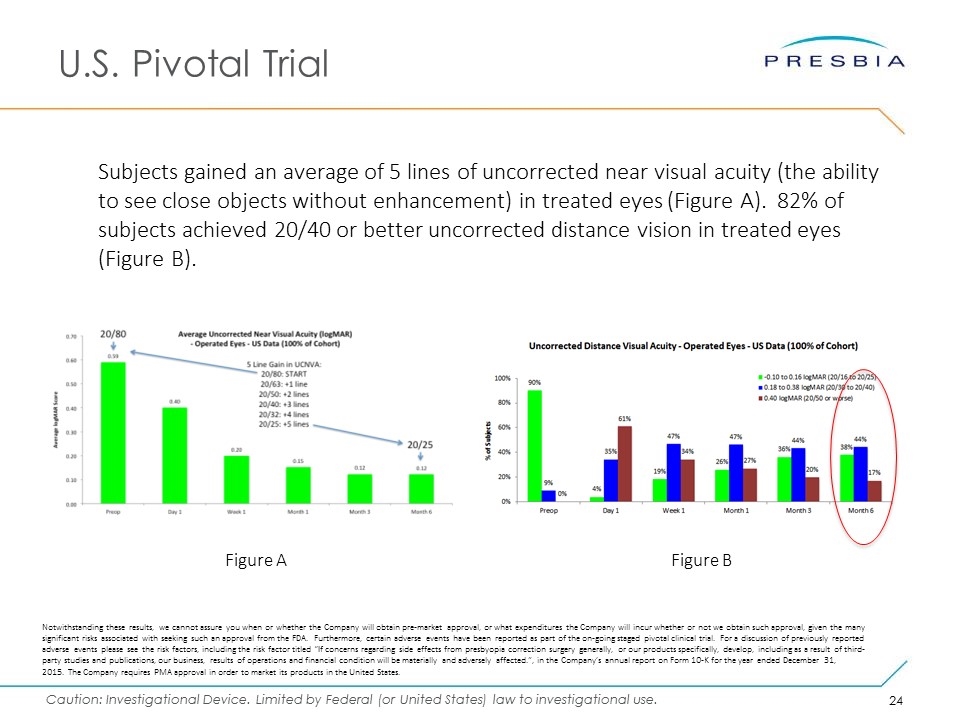
U.S. Pivotal Trial Subjects gained an average of 5 lines of uncorrected near visual acuity (the ability to see close objects without enhancement) in treated eyes (Figure A). 82% of subjects achieved 20/40 or better uncorrected distance vision in treated eyes (Figure B). Figure B Figure A Notwithstanding these results, we cannot assure you when or whether the Company will obtain pre-market approval, or what expenditures the Company will incur whether or not we obtain such approval, given the many significant risks associated with seeking such an approval from the FDA. Furthermore, certain adverse events have been reported as part of the on-going staged pivotal clinical trial. For a discussion of previously reported adverse events please see the risk factors, including the risk factor titled “If concerns regarding side effects from presbyopia correction surgery generally, or our products specifically, develop, including as a result of third-party studies and publications, our business, results of operations and financial condition will be materially and adversely affected.”, in the Company’s annual report on Form 10-K for the year ended December 31, 2015. The Company requires PMA approval in order to market its products in the United States.
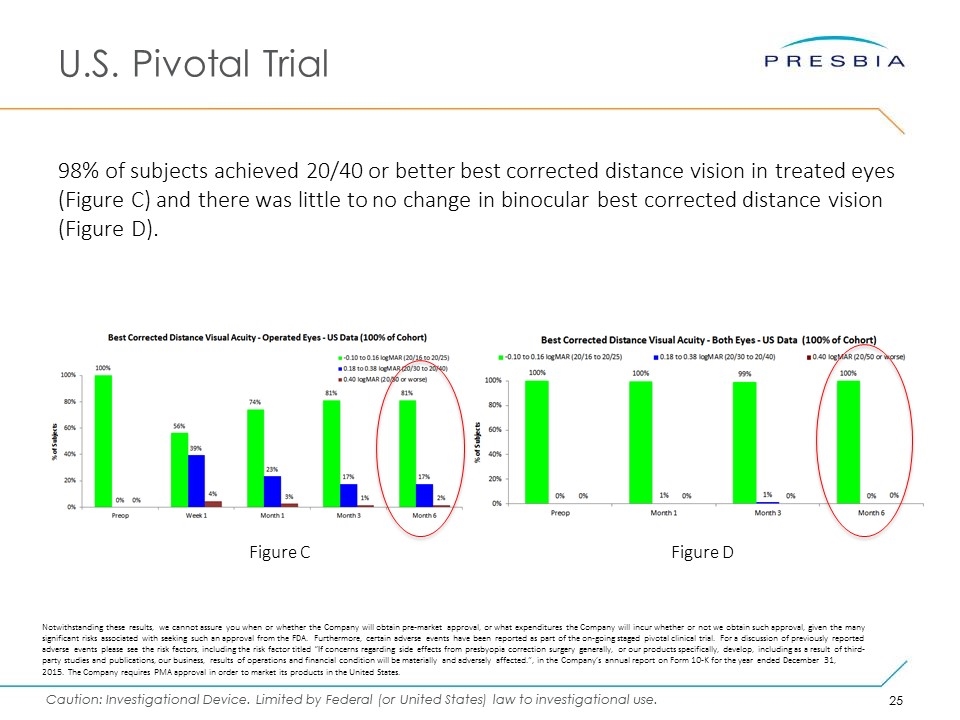
U.S. Pivotal Trial 98% of subjects achieved 20/40 or better best corrected distance vision in treated eyes (Figure C) and there was little to no change in binocular best corrected distance vision (Figure D). Figure C Figure D Notwithstanding these results, we cannot assure you when or whether the Company will obtain pre-market approval, or what expenditures the Company will incur whether or not we obtain such approval, given the many significant risks associated with seeking such an approval from the FDA. Furthermore, certain adverse events have been reported as part of the on-going staged pivotal clinical trial. For a discussion of previously reported adverse events please see the risk factors, including the risk factor titled “If concerns regarding side effects from presbyopia correction surgery generally, or our products specifically, develop, including as a result of third-party studies and publications, our business, results of operations and financial condition will be materially and adversely affected.”, in the Company’s annual report on Form 10-K for the year ended December 31, 2015. The Company requires PMA approval in order to market its products in the United States.
Intellectual Property Five patents issued: Lens Holder Apparatus and System Method (US 8,869,975 B2) Lens Inserter Apparatus and Method (US 9,017,401 B2) Lens Injector Apparatus and Method (US 8,454,687 B2) Lens Injector Apparatus System and Method (US 9,010,817 B2) Method for Laser Cutting a Corneal Pocket (US 9,168,175 B2) U.S. Patents Foreign Patents Lens Holder Apparatus and System Method Issued: Canada Allowed, waiting for issue: China Awaiting Examination: Australia, Europe, Israel, Japan, Korea, Russia, India, Brazil Lens Inserter Apparatus and Method Issued: Japan, China, Australia Allowed, waiting for issue: Israel Pending: Canada, Europe, Korea Two patents pending (patent applications): Lens Inserter Assembly System for Monitoring and Tracking Patient Outcomes After Surgical Implantation of an Intracorneal Lens Lens Injector Apparatus and Method Pending: Japan, Korea Method for Laser Cutting a Corneal Pocket Pending: Australia, Canada, China, Europe, Hong Kong, Israel, Japan, Korea

Manufacturing 4,000 square-foot, two-part (wet/dry) manufacturing facility Approved to manufacture devices for U.S. IDE by State of California FDA in 2013 Sufficient capacity to handle projected Presbia Flexivue Microlens™ volume through U.S. launch Approved to manufacture devices for OUS sale by Intertek (ISO 13485:2012 certified) Additional third-party manufacturing facility in Israel supplies product for all current OUS requirements Distribution facilities in Ireland and the Netherlands Irvine, CA Manufacturing Facility

Presbia PLC (Headquarters) 120-121 Lower Baggot St. Dublin 2, Ireland tel: +353-1-659-9446 Presbia Coöperatief U.A. Pieter Pauwstraat 2A – I · 1017 ZJ Amsterdam, Netherlands tel: +31-20-7084-556 · fax: +31-20-7084-556 PresbiBio LLC 8845 Irvine Center Dr., Suite 100 · Irvine, CA 92618 tel: +1-949‐502‐7010 · fax: +1-323-832-8447 info@presbia.com • www.presbia.com
